Synthesis and Characterization of New-Type Soluble β-Substituted Zinc Phthalocyanine Derivative of Clofoctol
Abstract
1. Introduction
2. Results
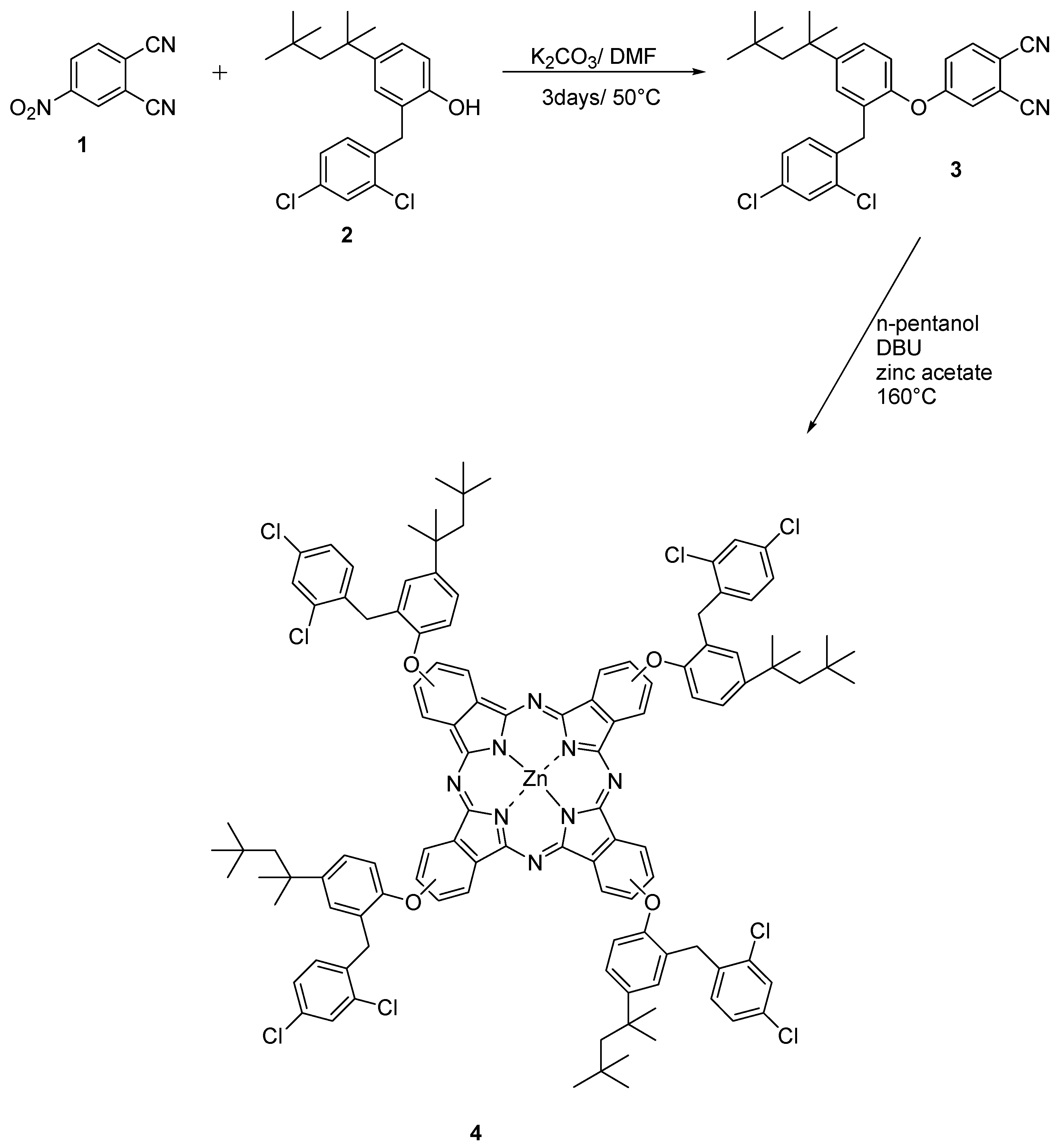
2.1. Synthesis and Characterization
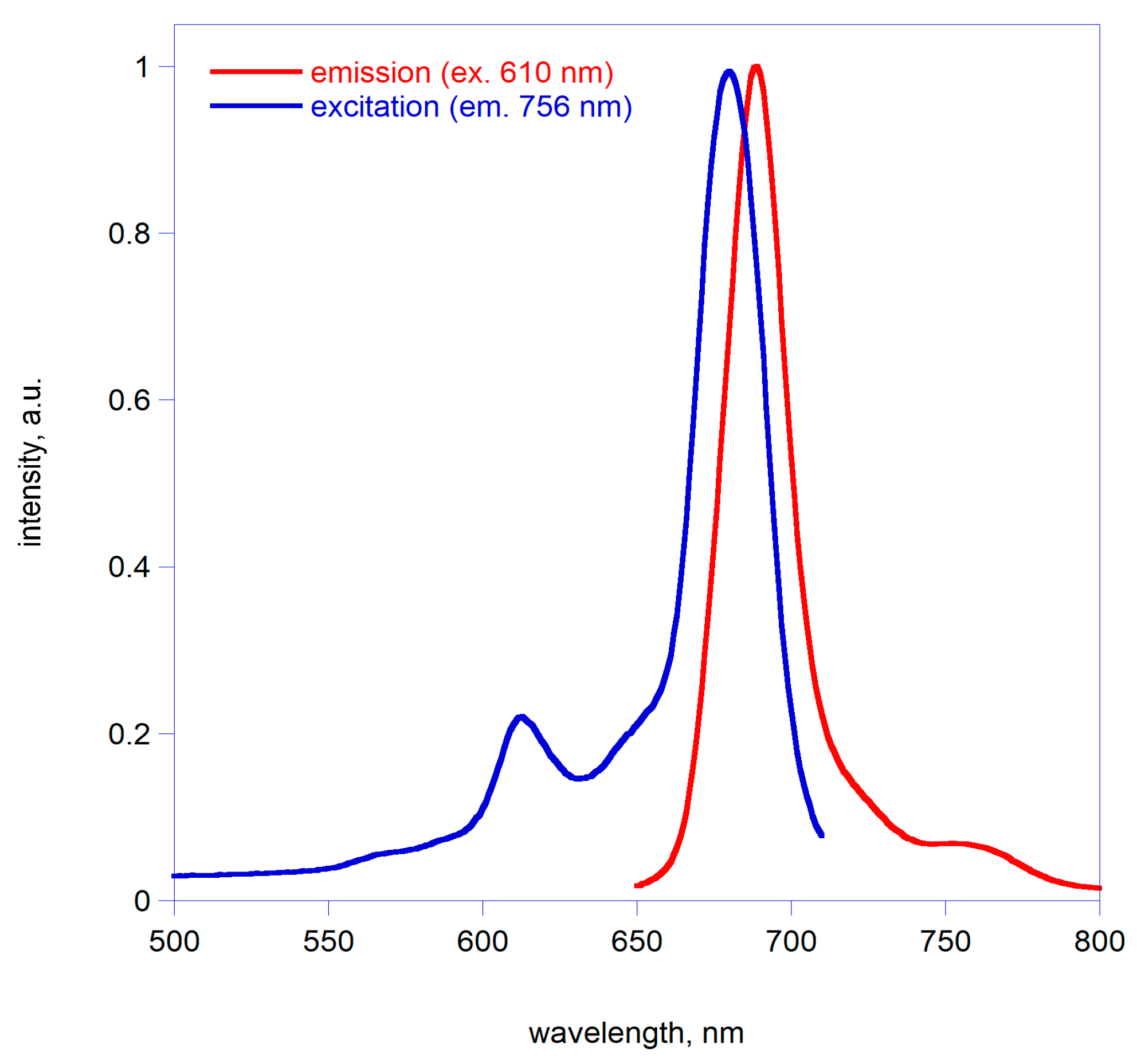
2.2. Spectroscopic Characterization
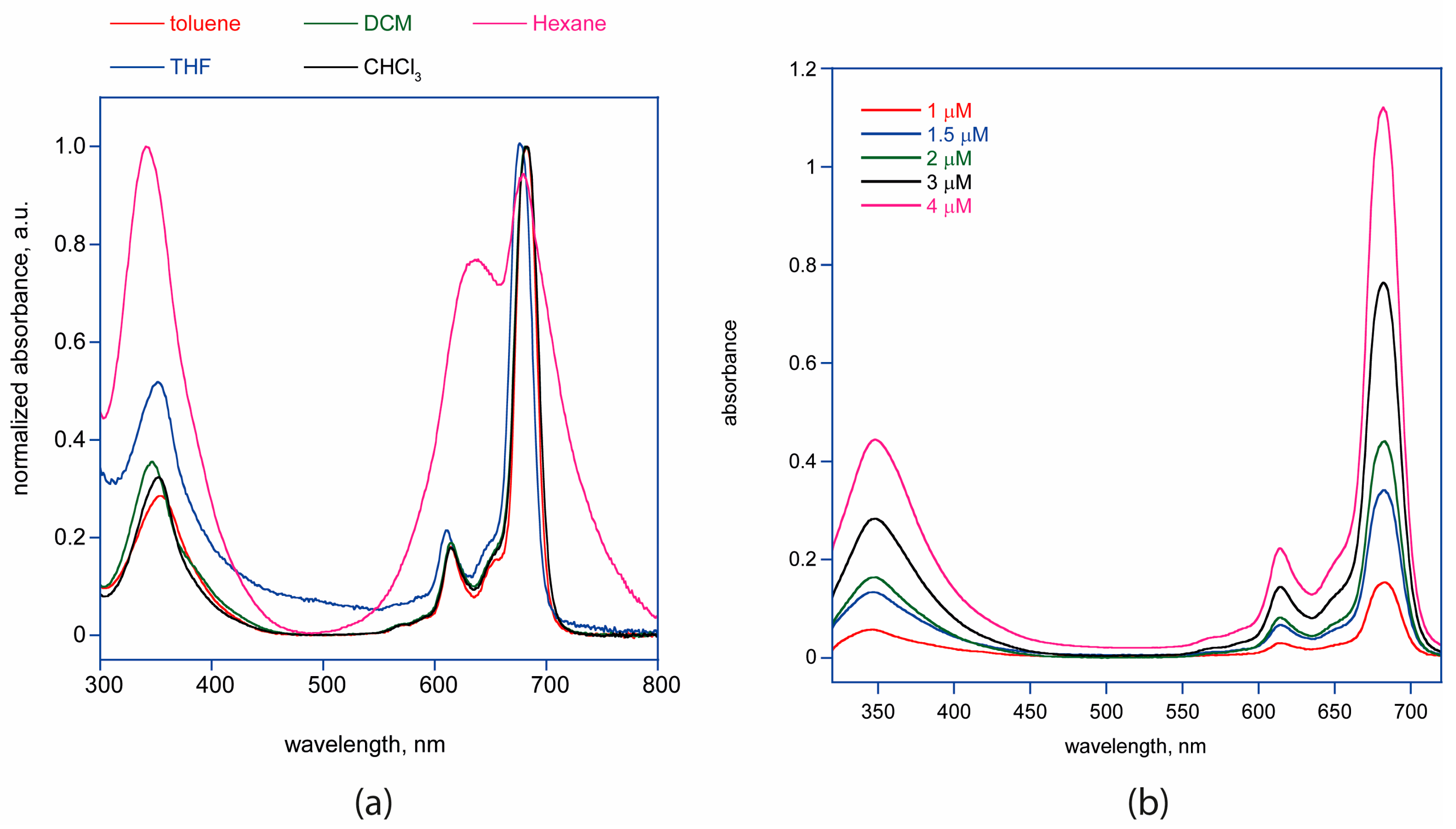
2.3. UV-Vis Characterization
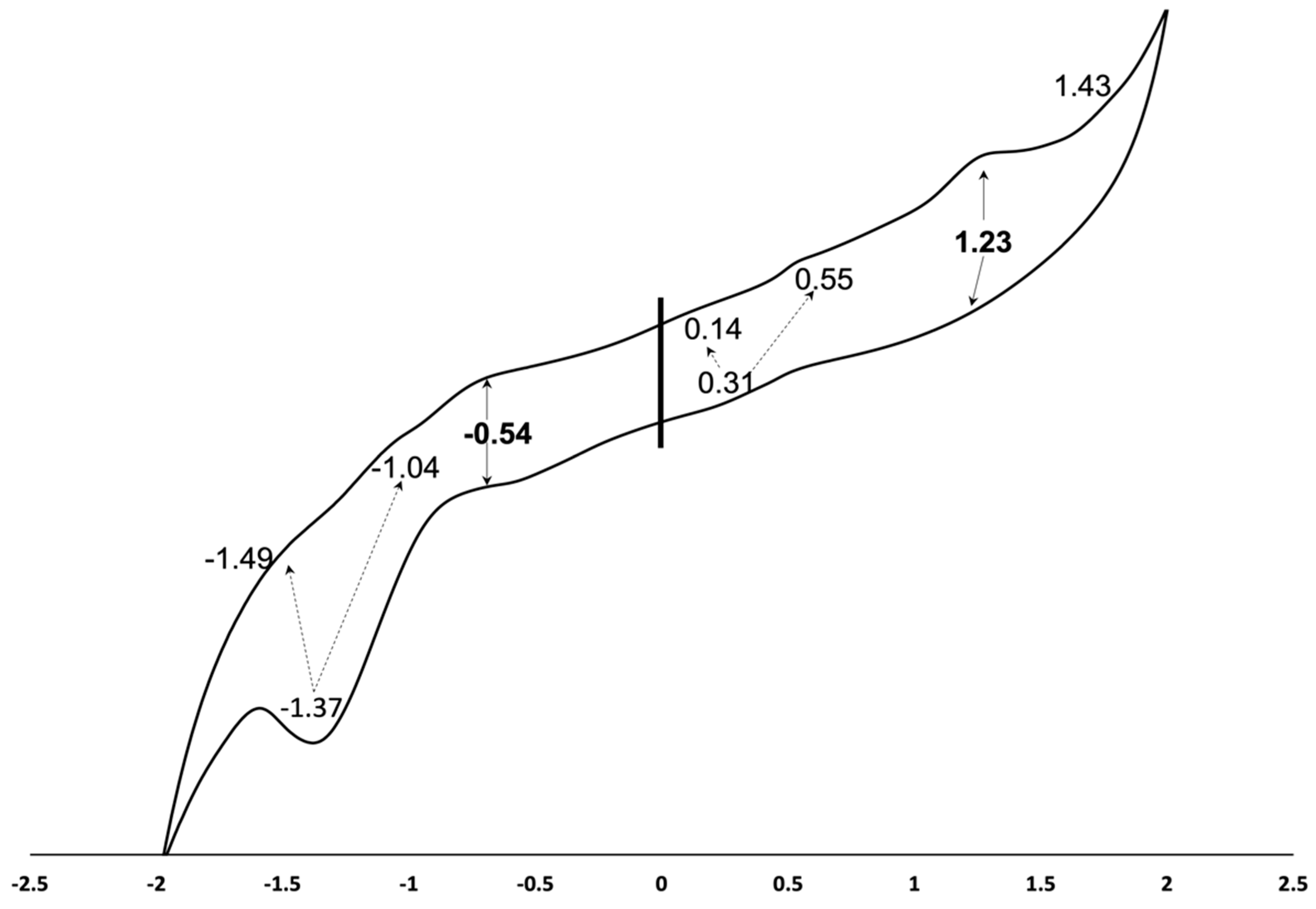
2.4. Electrochemical Studies
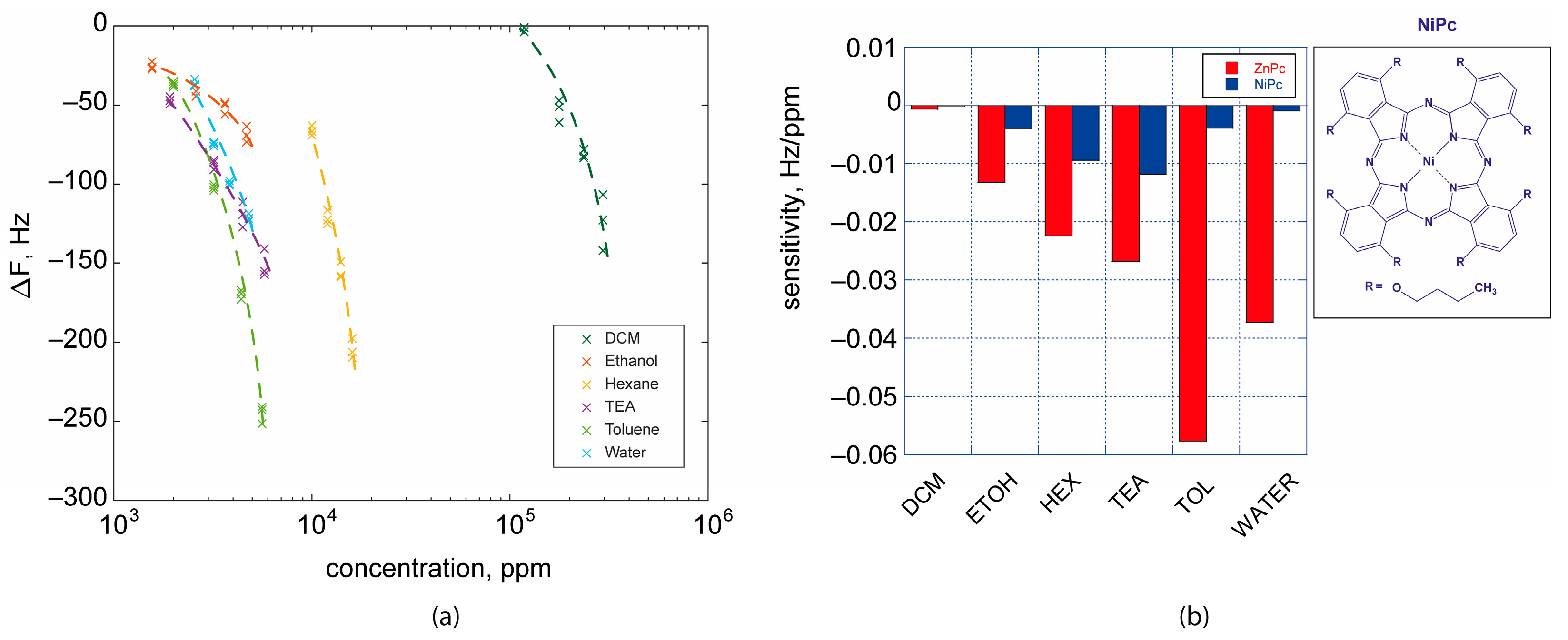
2.5. Gas Sensing Properties
3. Materials and Methods
3.1. Equipment
3.2. Sensors Preparation and Characterization
3.3. Synthesis
3.3.1. 4-[2-(2,4-Dichloro-benzyl)-4-(1,1,3,3-tetramethyl-butyl)-phenoxy]-phthalonitrile (3)
3.3.2. 2(3),9(10),16(17),23(24)-Tetrakis 2-(2,4-dichloro-benzyl)-4-(1,1,3,3-tetramethyl-butyl)-phenoxy zinc (II) phthalocyanine (4)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Zheng, B.D.; He, Q.X.; Li, X.; Yoon, J.; Huang, J.D. Phthalocyanines as contrast agents for photothermal therapy. Coord. Chem. Rev. 2021, 426, 213548. [Google Scholar] [CrossRef]
- Demirbaş, Ü.; Akçay, H.T.; Koca, A.; Kantekin, H. Synthesis, characterization and investigation of electrochemical and spectroelectrochemical properties of peripherally tetra 4-phenylthiazole-2-thiol substituted metal-free, zinc(II), copper(II) and cobalt(II) phthalocyanines. J. Mol. Struct. 2017, 1141, 643–649. [Google Scholar] [CrossRef]
- Odabaş, Z.; Orman, E.B.; Durmuş, M.; Dumludağ, F.; Özkaya, A.R.; Bulut, M. Novel alpha-7-oxy-4-(4-methoxyphenyl)-8-methylcoumarin substituted metal-free, Co(II) and Zn(II) phthalocyanines: Photochemistry, photophysics, conductance and electrochemistry. Dye. Pigment. 2012, 95, 540–552. [Google Scholar] [CrossRef]
- Acar, E.T.; Tabakoglu, T.A.; Atilla, D.; Yuksel, F.; Atun, G. Synthesis, electrochemistry and electrocatalytic activity of cobalt phthalocyanine complexes–effects of substituents for oxygen reduction reaction. Polyhedron 2018, 152, 114–124. [Google Scholar] [CrossRef]
- Ertem, B.; Yalazan, H.; Güngör, Ö.; Sarkı, G.; Durmuş, M.; Saka, E.T.; Kantekin, H. Synthesis, structural characterization, and investigation on photophysical and photochemical features of new metallophthalocyanines. J. Lumin. 2018, 204, 464–471. [Google Scholar] [CrossRef]
- Günsel, A.; Kobyaoğlu, A.; Bilgicli, A.T.; Tüzün, B.; Tosun, B.; Arabaci, G.; Yarasir, M.N. Novel biologically active metallophthalocyanines as promising antioxidant-antibacterial agents: Synthesis, characterization and computational properties. J. Mol. Struct. 2020, 1200, 127127. [Google Scholar] [CrossRef]
- Kırbaç, E.; Erdoğmuş, A. New non-peripherally substituted zinc phthalocyanines; synthesis, and comparative photophysicochemical properties. J. Mol. Struct. 2020, 1202, 127392. [Google Scholar] [CrossRef]
- Bilgiçli, A.T.; Kandemir, T.; Tüzün, B.; Arıduru, R.; Günsel, A.; Abak, Ç.; Yarasir, M.N.; Arabaci, G. Octa-substituted Zinc(II), Cu(II), and Co(II) phthalocyanines with 1-(4-hydroxyphenyl) propane-1-one: Synthesis, sensitive protonation behaviors, Ag(I) induced H-type aggregation properties, antibacterial–antioxidant activity, and molecular docking studies. Appl. Organomet. Chem. 2021, 35, e6353. [Google Scholar] [CrossRef]
- Bian, Y.; Chen, J.; Xu, S.; Zhou, Y.; Zhu, L.; Xiang, Y.; Xia, D. The effect of a hydrogen bond on the supramolecular self-aggregation mode and the extent of metal-free benzoxazole-substituted phthalocyanines. New J. Chem. 2015, 39, 5750–5758. [Google Scholar] [CrossRef]
- Gonzalez, A.C.; Damas, L.; Aroso, R.T.; Tome, V.A.; Dias, L.D.; Pina, J.; Carrilho, R.M.B.; Pereira, M.M. Monoterpene-based metallophthalocyanines: Sustainable synthetic approaches and photophysical studies. J. Porphyr. Phthalocyanines 2020, 24, 947–958. [Google Scholar] [CrossRef]
- Belouzard, S.; Machelart, A.; Sencio, V.; Vausselin, T.; Hoffmann, E.; Deboosere, N.; Rouillé, Y.; Desmarets, L.; Se’ron, K.; Danneels, A.; et al. Clofoctol inhibits SARS-CoV-2 replication and reduces lung pathology in mice. PLoS Pathog. 2022, 18, e1010498. [Google Scholar] [CrossRef]
- Demirbaş, Ü.; Kobak, R.Z.U.; Barut, B.; Bayrak, R.; Koca, A.; Kantekin, H. Synthesis and electrochemical characterization of tetra-(5-chloro-2-(2, 4-dichlorophenoxy) phenol) substituted Ni (II), Fe (II) and Cu (II) metallophthalocyanines. Synth. Met. 2016, 215, 7–13. [Google Scholar] [CrossRef]
- Breloy, L.; Yavuz, O.; Yilmaz, I.; Yagci, Y.; Versace, D.L. Design, synthesis and use of phthalocyanines as a new class of visible-light photoinitiators for free-radical and cationic polymerizations. Polym. Chem. 2021, 12, 4291–4316. [Google Scholar] [CrossRef]
- Bayrak, R.; Akçay, H.T.; Pişkin, M.; Durmuş, M.; Değirmencioğlu, İ. Azine-bridged binuclear metallophthalocyanines functioning photophysical and photochemical-responsive. Dye. Pigment. 2012, 95, 330–337. [Google Scholar] [CrossRef]
- Amitha, G.S.; Yoosuf Ameen, M.; Sivaji Reddy, V.; Vasudevan, S. Synthesis of peripherally tetra substituted neutral azophenoxy zinc phthalocyanine and its application in bulk hetero junction solar cells. J. Mol. Struct. 2019, 1185, 425–431. [Google Scholar] [CrossRef]
- Sevim, A.M.; Yüzeroğlu, M.; Gül, A. Novel metallophthalocyanines with bulky 4-[3, 4-bis (benzyloxy) benzylidene] aminophenoxy substituents. Mon. Chem. Chem. Mon. 2020, 151, 1059–1068. [Google Scholar] [CrossRef]
- Kabay, N.; Baygu, Y.; Metin, A.K.; İzzet, K.A.R.A.; EsraNur, K.A.Y.A.; Durmu, Ş.M.; Yaşar, G.Ö.K. Novel nonperipheral octa-3-hydroxypropylthio substituted metallo-phthalocyanines: Synthesis, characterization, and investigation of their electrochemical, photochemical and computational properties. Turk. J. Chem. 2021, 45, 143. [Google Scholar] [CrossRef]
- Nas, A.; Biyiklioglu, Z.; Fandaklı, S.; Sarkı, G.; Yalazan, H.; Kantekin, H. Tetra (3-(1, 5-diphenyl-4, 5-dihydro-1H-pyrazol-3-yl) phenoxy) substituted cobalt, iron and manganese phthalocyanines: Synthesis and electrochemical analysis. Inorg. Chim. Acta 2017, 466, 86–92. [Google Scholar] [CrossRef]
- Hanabusa, K.; Shirai, H. Catalytic functions and application of metallophthalocyanine polymers. ChemInform 1993, 24. Available online: https://onlinelibrary.wiley.com/action/showCitFormats?doi=10.1002%2Fchin.199343294 (accessed on 1 April 2023). [CrossRef]
- Bouvet, M. Phthalocyanine-based field-effect transistors as gas sensors. Anal. Bioanal. Chem. 2006, 384, 366–373. [Google Scholar] [CrossRef]
- Yıldız, B.; Baygu, Y.; Kara, İ.; Dal, H.; Gök, Y. The synthesis, characterization and computional investigation of new metalloporphyrazine containing 15-membered S4 donor macrocyclic moieties. Tetrahedron 2016, 72, 6972–6981. [Google Scholar] [CrossRef]
- Bayrak, R.; Ataşen, S.K.; Yılmaz, I.; Yalçın, İ.; Erman, M.; Ünver, Y.; Değirmencioğlu, İ. Synthesis and Spectro-Electrochemical Properties of New Metallophthalocyanines Having High Electron Transfer Capability. J. Mol. Struct. 2021, 1231, 129677. [Google Scholar] [CrossRef]
- Günay, İ.; Orman, E.B.; Altındal, A.; Salih, B.; Özer, M.; Özkaya, A.R. Novel tetrakis 4-(hydroxymethyl)-2, 6-dimethoxyphenoxyl substituted metallophthalocyanines: Synthesis, electrochemical redox, electrocatalytic oxygen reducing, and volatile organic compounds sensing and adsorption properties. Dye. Pigment. 2018, 154, 172–187. [Google Scholar] [CrossRef]
- Rana, M.K.; Sinha, M.; Panda, S. Gas sensing behavior of metal-phthalocyanines: Effects of electronic structure on sensitivity. Chem. Phys. 2018, 513, 23–34. [Google Scholar] [CrossRef]
- Magna, G.; Nardis, S.; Di Natale, C.; Perdigon, V.M.; Torres, T.; Paolesse, R. The Skeleton Counts! A study of the porphyrinoid structure’s influence on sensing properties. J. Porphyr. Phthalocyanines 2023, 27, 655–660. [Google Scholar] [CrossRef]
- Şenoğlu, S.; Özer, M.; Dumludağ, F.; Acar, N.; Salih, B.; Bekaroğlu, Ö. Synthesis, characterization, DFT study, conductivity and effects of humidity on CO2 sensing properties of the novel tetrakis-[2-(dibenzylamino) ethoxyl] substituted metallophthalocyanines. Sens. Actuators B Chem. 2020, 310, 127860. [Google Scholar] [CrossRef]
- Su, H.C.; Tran, T.T.; Bosze, W.; Myung, N.V. Chemiresistive sensor arrays for detection of air pollutants based on carbon nanotubes functionalized with porphyrin and phthalocyanine derivatives. Sens. Actuators Rep. 2020, 2, 100011. [Google Scholar] [CrossRef]
- Sarıoğulları, H.; Sengul, I.F.; Gürek, A.G. Comparative study on sensing and optical properties of carbazole linked novel zinc (II) and cobalt (II) phthalocyanines. Polyhedron 2022, 227, 116139. [Google Scholar] [CrossRef]
- Young, J.G.; Onyebuagu, W. Synthesis and characterization of di-disubstituted phthalocyanines. J. Org. Chem. 1990, 55, 2155–2159. [Google Scholar] [CrossRef]
- Magna, G.; Belugina, R.; Mandoj, F.; Catini, A.; Legin, A.V.; Paolesse, R.; Di Natale, C. Experimental determination of the mass sensitivity of quartz microbalances coated by an optical dye. Sens. Actuators B Chem. 2020, 320, 1373. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dridi, S.; Khiari, J.E.; Magna, G.; Stefanelli, M.; Lvova, L.; Mandoj, F.; Khezami, K.; Durmuş, M.; Di Natale, C.; Paolesse, R. Synthesis and Characterization of New-Type Soluble β-Substituted Zinc Phthalocyanine Derivative of Clofoctol. Molecules 2023, 28, 4102. https://doi.org/10.3390/molecules28104102
Dridi S, Khiari JE, Magna G, Stefanelli M, Lvova L, Mandoj F, Khezami K, Durmuş M, Di Natale C, Paolesse R. Synthesis and Characterization of New-Type Soluble β-Substituted Zinc Phthalocyanine Derivative of Clofoctol. Molecules. 2023; 28(10):4102. https://doi.org/10.3390/molecules28104102
Chicago/Turabian StyleDridi, Sabrine, Jamel Eddine Khiari, Gabriele Magna, Manuela Stefanelli, Larisa Lvova, Federica Mandoj, Khaoula Khezami, Mahmut Durmuş, Corrado Di Natale, and Roberto Paolesse. 2023. "Synthesis and Characterization of New-Type Soluble β-Substituted Zinc Phthalocyanine Derivative of Clofoctol" Molecules 28, no. 10: 4102. https://doi.org/10.3390/molecules28104102
APA StyleDridi, S., Khiari, J. E., Magna, G., Stefanelli, M., Lvova, L., Mandoj, F., Khezami, K., Durmuş, M., Di Natale, C., & Paolesse, R. (2023). Synthesis and Characterization of New-Type Soluble β-Substituted Zinc Phthalocyanine Derivative of Clofoctol. Molecules, 28(10), 4102. https://doi.org/10.3390/molecules28104102











