A Mechanism Study of Redox Reactions of the Ruthenium-oxo-polypyridyl Complex
Abstract
1. Introduction
2. Results
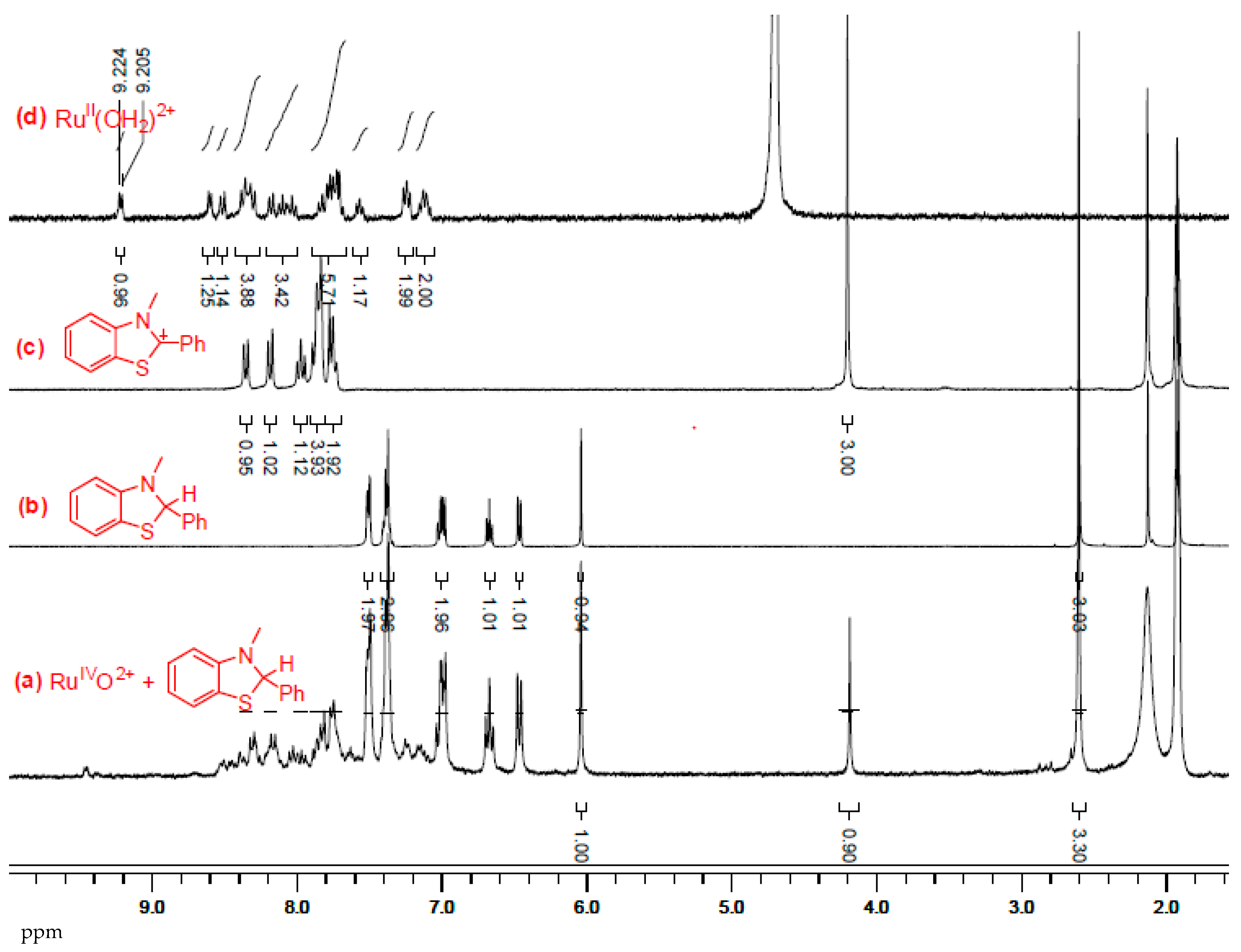
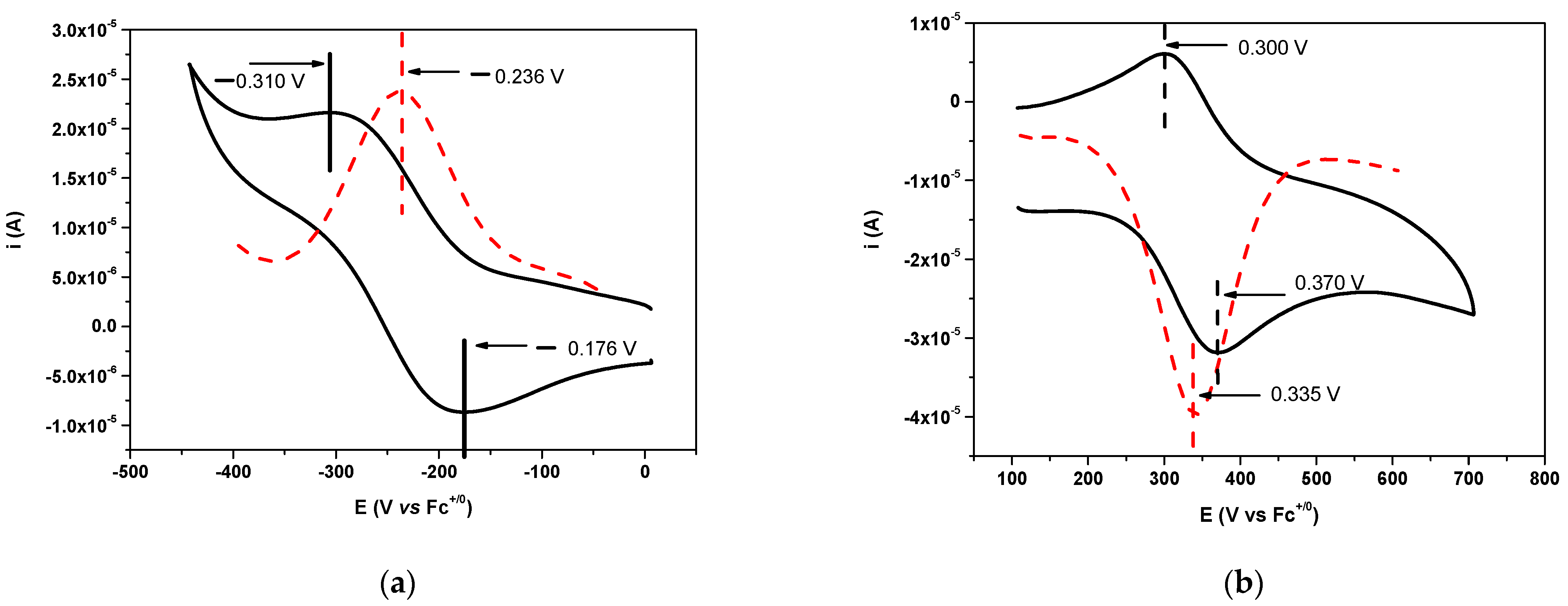
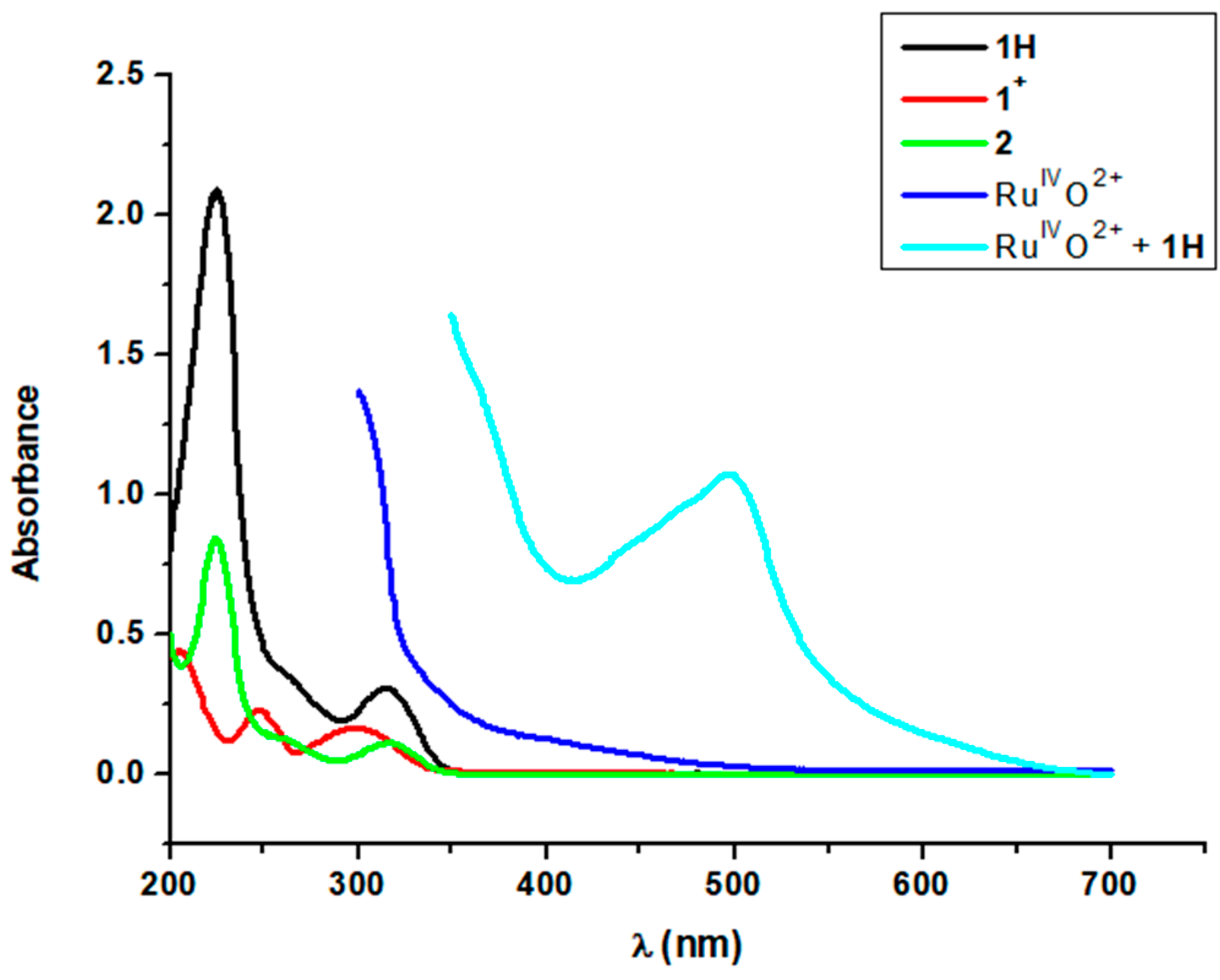
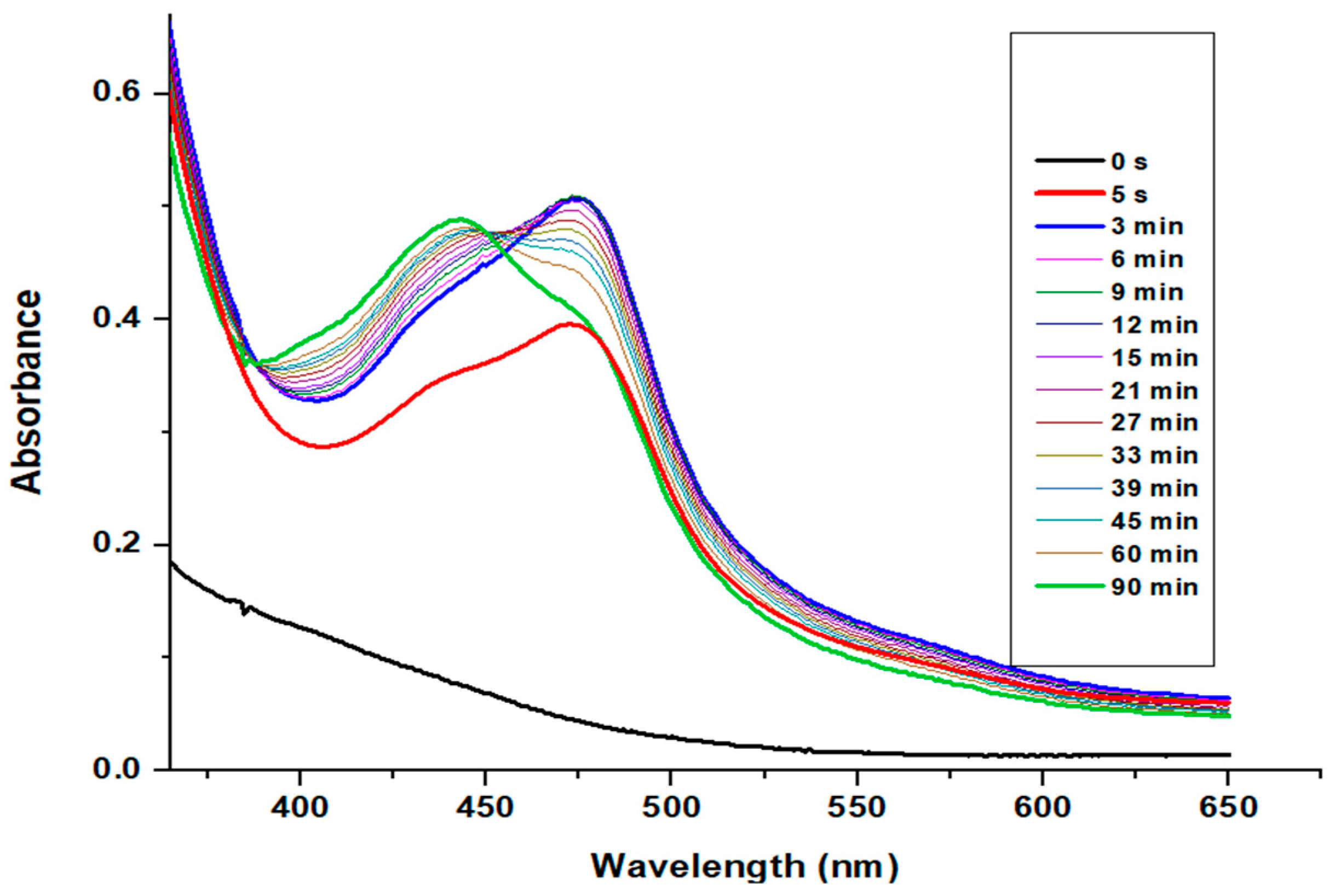
2.1. Results for the Reaction of Ruthenium-oxo-polypyridyl Compounds and 1H
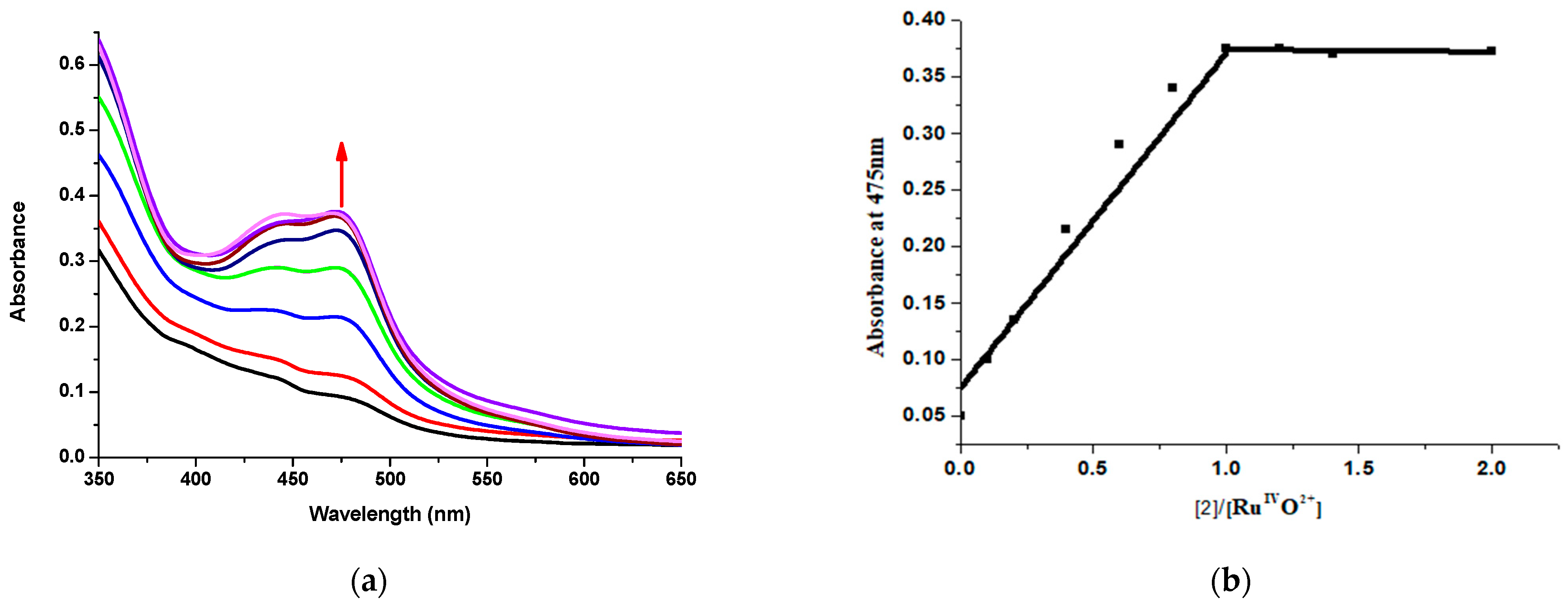
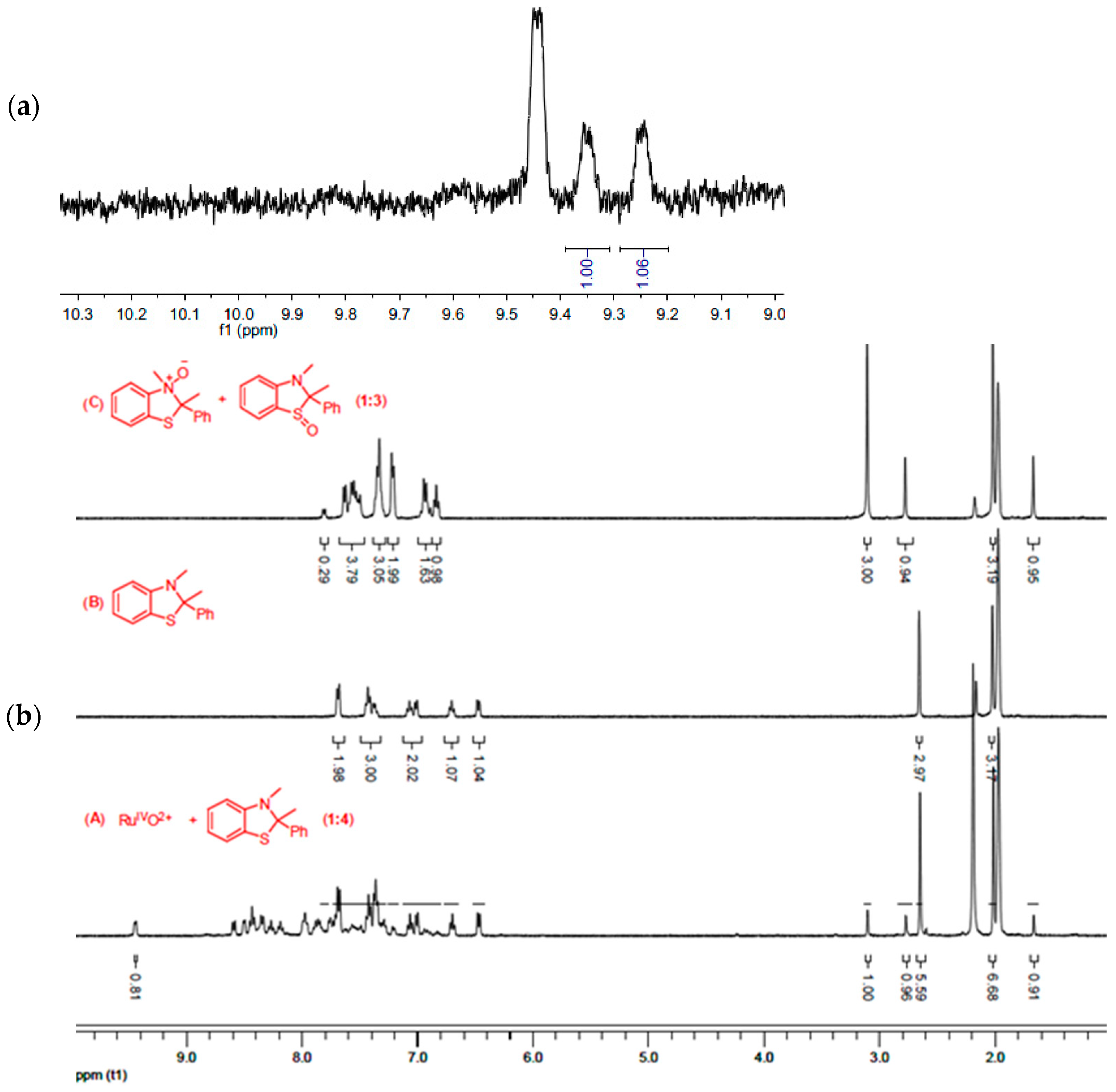
2.2. Results for the Reaction of Ruthenium-oxo-polypyridyl Compounds and 2
2.3. Results for the Reaction of Ruthenium-oxo-polypyridyl Compounds and 3H
3. Discussion
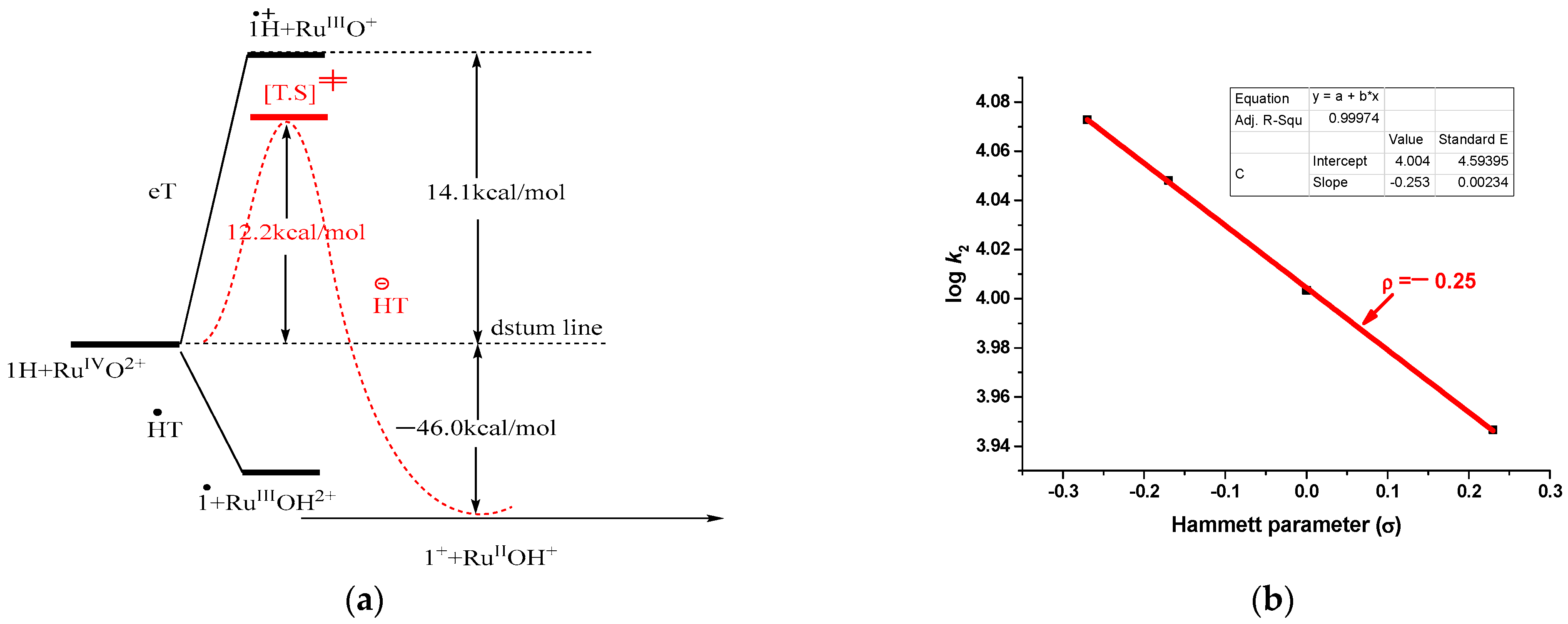
3.1. Considerations Regarding the Reaction of Ruthenium-oxo-polypyridyl Compounds and 1H
3.2. Considerations Regarding the Reaction of Ruthenium-oxo-polypyridyl Compounds and 2
3.3. Considerations Regarding the Reaction of Ruthenium-oxo-polypyridyl Compounds and 3H
3.4. Considerations Regarding the Novelty of This Work
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Doe, J.S.; Smith, J.; Roe, P. Syntheses and biological activities of rebeccamycin analogues. Introduction of a halogenoacetyl substituent. J. Am. Chem. Soc. 1968, 90, 8234–8241. [Google Scholar]
- Dobson, J.C.; Seok, W.K.; Meyer, T.J. Epoxidation and catalytic oxidation of olefins based on a RuIV:O/RuII-OH2 couple. Inorg. Chem. 1986, 25, 1513–1514. [Google Scholar]
- Roecker, L.E.; Dobson, J.C.; Vining, W.J.; Meyer, T.J. Oxygen atom transfer in the oxidations of dimethyl sulfide and dimethyl sulfoxide by bis(bipyridine)oxo(pyridine) ruthenium(2+). Inorg. Chem. 1987, 26, 779–781. [Google Scholar] [CrossRef]
- Seok, W.K. The Reaction of Polypyridyl Ru Mono-oxo Complexes Toward Sulfur Compounds. Bull. Korean Chem. Soc. 2001, 22, 244. [Google Scholar]
- Roecker, L.E.; Meyer, T.J. Hydride transfer in the oxidation of alcohols by [(bpy)2(py)Ru(Q)]2+. A kH/kD kinetic isotope effect of 50. J. Am. Chem. Soc. 1987, 109, 746–754. [Google Scholar] [CrossRef]
- Thompson, M.S.; Meyer, T.J. Mechanisms of oxidation of 2-propanol by polypyridyl complexes of ruthenium(III) and ruthenium(IV). J. Am. Chem. Soc. 1982, 104, 4106–4115. [Google Scholar] [CrossRef]
- Bryant, J.R.; Mayer, J.M. Oxidation of C−H Bonds by [(bpy)2(py)RuIVO]2+ Occurs by Hydrogen Atom Abstraction. J. Am. Chem. Soc. 2003, 125, 10351–10361. [Google Scholar] [CrossRef]
- Moyer, B.A.; Thompson, M.S.; Meyer, T.J. Chemically catalyzed net electrochemical oxidation of alcohols, aldehydes, and unsaturated hydrocarbons using the system (trpy)(bpy)Ru(OH2)2+/(trpy)(bpy)RuO2+. J. Am. Chem. Soc. 1980, 102, 2310–2323. [Google Scholar] [CrossRef]
- Thompson, M.S.; Giovani, W.; Moyer, B.A.; Meyer, T.J. Novel electrocatalytic procedure for the oxidation of alcohols, aldehydes, cyclic ketones, and carbon-hydrogen bonds adjacent to olefinic or aromatic groups. J. Org. Chem. 1984, 49, 4972–4977. [Google Scholar] [CrossRef]
- Laia, F.; Roc, M.; James, R.D. Kinetic Analysis of an Efficient Molecular Light-Driven Water Oxidation System. ACS Catal. 2017, 7, 5142–5150. [Google Scholar]
- Yan, Z.-Y.; Chen, J.; Zhu, B.-Z. The cell-impermeable Ru(II) polypyridyl complex as a potent intracellular photosensitizer under visible light irradiation via ion-pairing with suitable lipophilic counter-anions. Free Radic. Biol. Med. 2021, 171, 69–79. [Google Scholar] [CrossRef]
- Priscila, P.; Antonio, C.; Elene, C. Photocytotoxic Activity of Ruthenium(II) Complexes with Phenanthroline-Hydrazone Ligands. Molecules 2021, 26, 2084. [Google Scholar]
- Kotani, H.; Shimomura, H.; Horimoto, M.; Ishizuka, T.; Shiota, Y. Fundamental electron- -transfer and proton-coupled electron-transfer properties of Ru(IV)-oxo complexes. Dalton Trans. 2019, 48, 13154–13161. [Google Scholar] [CrossRef]
- Mijatovic, A.M.; Jelic, R.M.; Bogojeski, J.; Bugarcic, Z.D.; Petrovic, B. Kinetics, mechanism, and equilibrium studies of the reactions between a ruthenium(II) complex and some nitrogen- and sulfur-donor nucleophiles. Mon. Fuer Chem. 2013, 144, 1489–1498. [Google Scholar] [CrossRef]
- Jo, M.R.; Seok, W.K. Oxidation of benzyl alcohols with extraordinarily high kinetic isotope effects. Bull. Korean Chem. Soc. 2011, 32, 3003–3008. [Google Scholar]
- Shi, J.; Guo, Y.-H.; Xie, F.; Chen, Q.-F.; Zhang, M.-T. Redox-Active Ligand Assisted Catalytic Water Oxidation by a RuIV=O Intermediate. Angew. Chem. Int. Ed. 2020, 59, 4000–4008. [Google Scholar] [CrossRef]
- Chen, J.-J.; Zhu, Y.-F.; Kaskel, S. Porphyrin-Based Metal-Organic Frameworks for Biomedical Applications. Angew. Chem. Int. Ed. 2021, 60, 5010–5035. [Google Scholar] [CrossRef]
- Cantu, R.; Fabian, G.; Lin, Y.-T.; Stanczak, A.; de Visse, S.P. Bioengineering of cytochrome P450 OleTJE: How does substrate positioning affect the product distributions? Molecules 2020, 25, 2675. [Google Scholar] [CrossRef]
- Wayner, D.D.M.; Parker, V.D. Bond energies in solution from electrode potentials and thermochemical cycles. A simplified and general approach. Acc. Chem. Res. 1993, 26, 287–294. [Google Scholar] [CrossRef]
- Dobson, J.C.; Helms, J.H.; Doppelt, P.; Sullivan, B.P.; Hatfield, W.E.; Meyer, T.J. Electronic structure of the oxidation catalyst cis(bipyridine)oxo(pyridine) ruthenium(IV) diperchlorate. Inorg. Chem. 1989, 28, 2200–2204. [Google Scholar] [CrossRef]
- Zhu, X.-Q.; Zhang, M.-T.; Yu, A.; Wang, C.-H.; Cheng, J.-P. Hydride, Hydrogen Atom, Proton, and Electron Transfer Driving Forces of Various Five-Membered Heterocyclic Organic Hydrides and Their Reaction Intermediates in Acetonitrile. J. Am. Chem. Soc. 2008, 130, 2501–2516. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.-Q.; Deng, F.-H.; Yang, J.-D.; Li, X.-T.; Chen, Q.; Lei, N.-P.; Meng, F.-K.; Zhao, X.-P.; Han, S.-H.; Hao, E.-J.; et al. A Classical but New Kinetic Equation for Hydride Transfer Reactions. Org. Biomol. Chem. 2013, 11, 6071–6089. [Google Scholar] [CrossRef] [PubMed]
- Brown, G.M.; Callahan, R.W.; Meyer, T.J. Thermal and light-induced decomposition of azido(bis-2,2′-bipyridine) complexes of ruthenium(III). Inorg. Chem. 1975, 14, 1915. [Google Scholar] [CrossRef]
- Xu, W.-L.; Li, Y.-Z.; Zhang, Q.-S.; Zhu, H.-S. A Selective, Convenient, and Efficient Conversion of Sulfides to Sulfoxides. Synthesis 2004, 2, 227–232. [Google Scholar] [CrossRef]
- Sullivan, B.P.; Salmon, D.J.; Meyer, T.J. Mixed phosphine 2,2′-bipyridine complexes of ruthenium. Inorg. Chem. 1978, 17, 3334. [Google Scholar] [CrossRef]
- Sprintschnik, G.; Sprintschnik, H.W.; Kirsch, P.P.; Whitten, D.G. Photochemical reactions in organized monolayer assemblies. 6. Preparation and photochemical reactivity of surfactant ruthenium(II) complexes in monolayer assemblies and at water-solid interfaces. J. Am. Chem. Soc. 1977, 99, 4947–4953. [Google Scholar] [CrossRef]
- Moyer, B.A.; Meyer, T.J. Properties of the oxo/aqua system (bpy)2(py)RuO2+/(bpy)2(py)Ru(OH2)2+. Inorg. Chem. 1981, 20, 436–444. [Google Scholar] [CrossRef]
- Jacob, P.; Richter, W.; Ugi, I. 1,3,2λ5-Benzothiazaphosphole 2-Oxide and 1,3,2h5-Benzoxazaphosphole 2-Oxide Derivatives, New and Versatile Phosphorylating Reagents. Liebigs Ann. Chem. 1991, 13, 519–522. [Google Scholar] [CrossRef]
- Chikashita, H.; Komazawa, S.; Ishimoto, N.; Inoue, K.; Itoh, K. Nonacidic and Highly Chemoselective Protection of the Carbonyl Function. 3-Methylbenzothiazolines as a Base- and Acid-Resistant Protected Form for the Carbonyl Groups. Bull. Chem. Soc. Jpn. 1989, 62, 1215–1225. [Google Scholar] [CrossRef]
- Zhu, X.-Q.; Li, Q.; Hao, W.-F.; Cheng, J.-P. Dissociation Energies and Charge Distribution of the Co? NO Bond for Nitrosyl-α,β,γ,δ-tetraphenylporphinatocobalt(II) and Nitrosyl-α,β,γ,δtetraphenyl-orphinatocobalt(III) in Benzonitrile Solution. J. Am. Chem. Soc. 2002, 124, 9887–9893. [Google Scholar] [CrossRef]
- Zhu, X.-Q.; Hao, W.-F.; Tang, H.; Wang, C.-H.; Cheng, J.-P. Determination of N-NO Bond Dissociation Energies of N-Methyl-N-Nitrosobenzenesulfonamindes in Acetonitrile and Application in the Mechanism Analyses on NO Transfer. J. Am. Chem. Soc. 2005, 127, 2696–2708. [Google Scholar] [CrossRef]


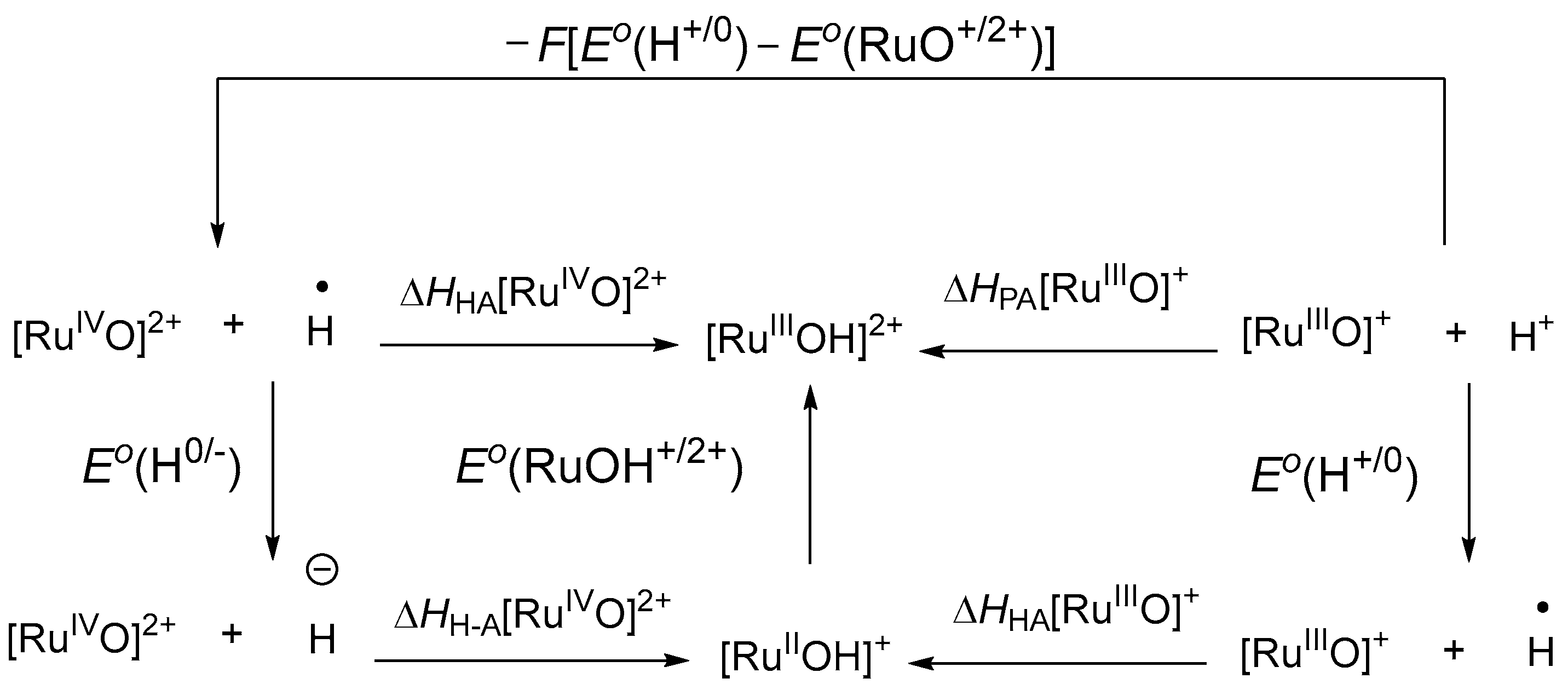
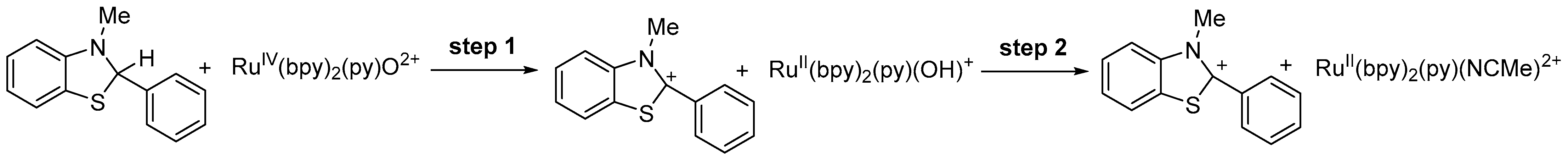
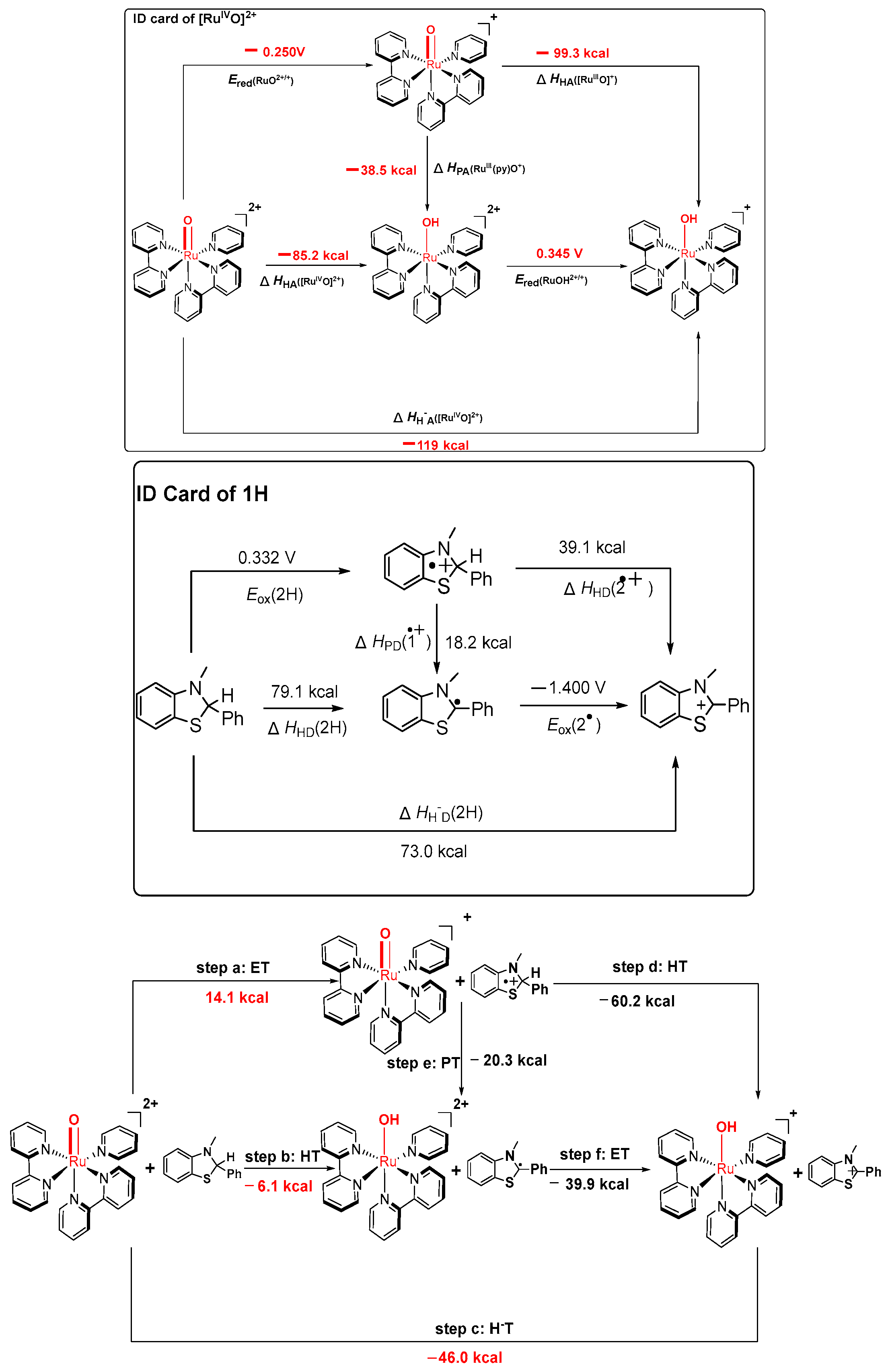
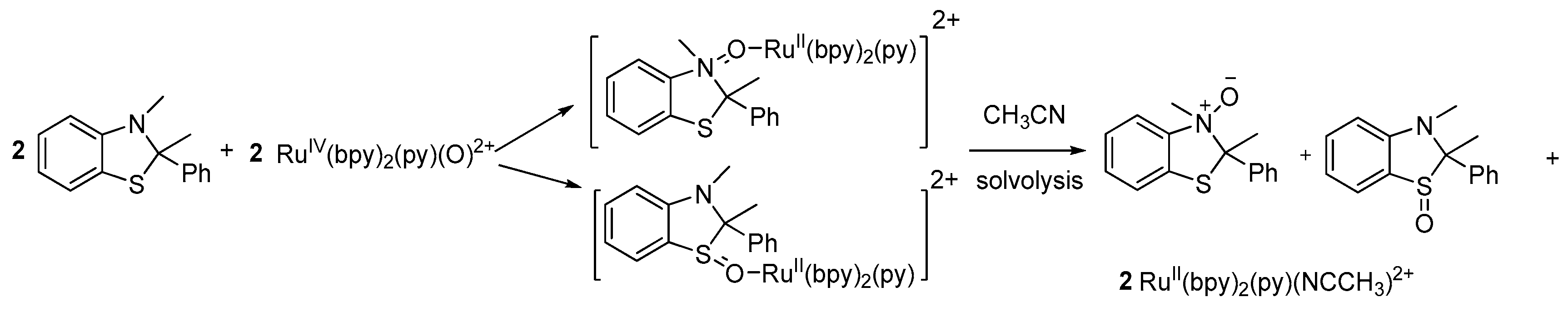

| [RuIVO]2+ | ∆Hr a | ∆HH−A[RuIVO]2+ b | E°red(RuO2+/+) c | E°ox(RuOH+/2+) c | ||
|---|---|---|---|---|---|---|
| CV | OSWV | CV | OSWV | |||
| −64.9 | −119 | −0.243 | −0.250 | 0.335 | 0.335 | |
| [RuIVO]2+ | ∆HHA[RuIVO]2+ b | ∆HPA[RuIIIO]2+ b | ∆HHA[RuIIIO]2+ b |
|---|---|---|---|
| H | −85.2 | −38.5 | −99.3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, B.-L.; Yan, S.-Y.; Zhu, X.-Q. A Mechanism Study of Redox Reactions of the Ruthenium-oxo-polypyridyl Complex. Molecules 2023, 28, 4401. https://doi.org/10.3390/molecules28114401
Chen B-L, Yan S-Y, Zhu X-Q. A Mechanism Study of Redox Reactions of the Ruthenium-oxo-polypyridyl Complex. Molecules. 2023; 28(11):4401. https://doi.org/10.3390/molecules28114401
Chicago/Turabian StyleChen, Bao-Long, Sheng-Yi Yan, and Xiao-Qing Zhu. 2023. "A Mechanism Study of Redox Reactions of the Ruthenium-oxo-polypyridyl Complex" Molecules 28, no. 11: 4401. https://doi.org/10.3390/molecules28114401
APA StyleChen, B.-L., Yan, S.-Y., & Zhu, X.-Q. (2023). A Mechanism Study of Redox Reactions of the Ruthenium-oxo-polypyridyl Complex. Molecules, 28(11), 4401. https://doi.org/10.3390/molecules28114401





