Applications of BiOX in the Photocatalytic Reactions
Abstract
1. Introduction
2. The Difference of Band Gap among BiOX Series Photocatalysts
3. Recent Application of BiOX in the Field of Photocatalytic Reactions
3.1. Splitting H2O to Produce H2 and O2
3.2. Degrading Organic Pollutants
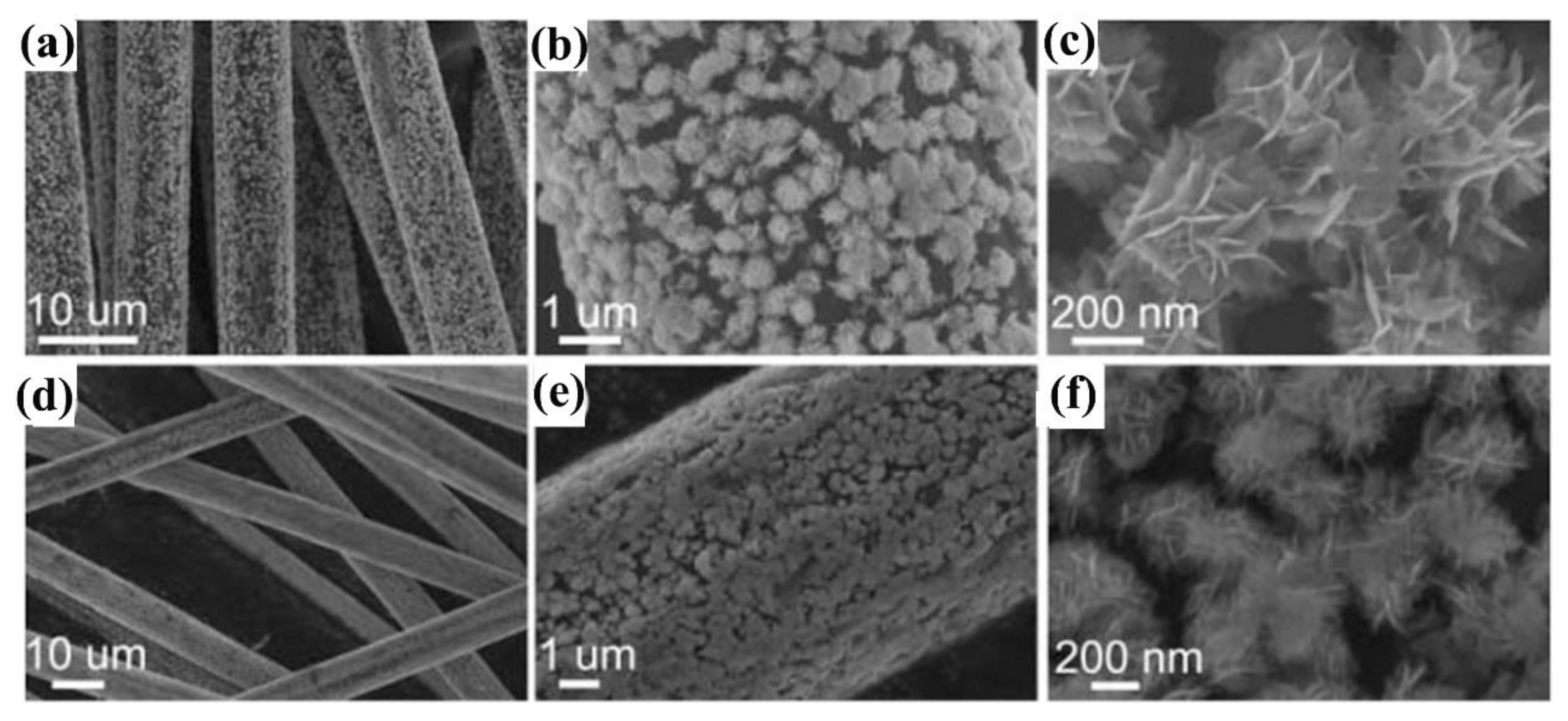
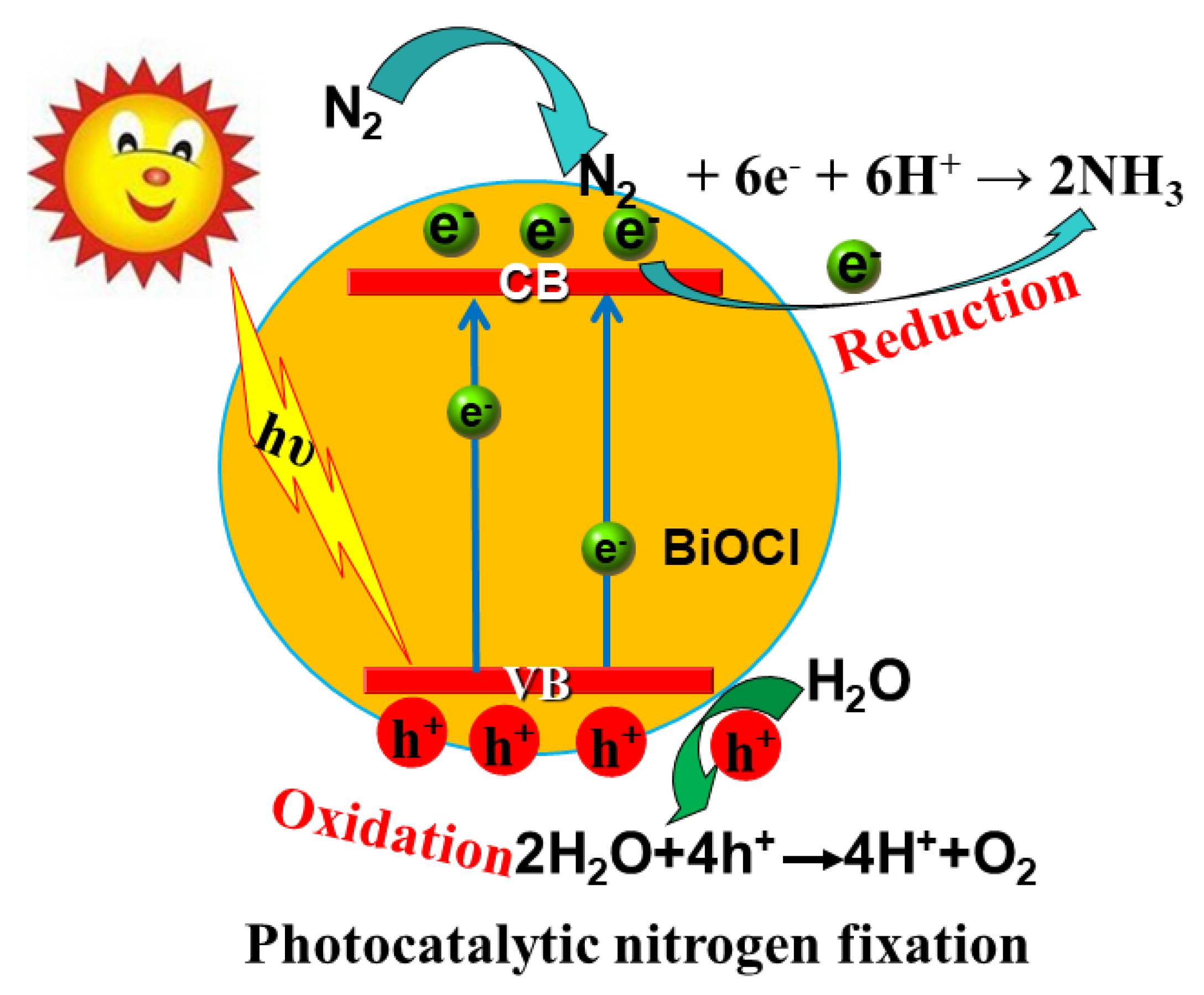
3.3. Photocatalytic Nitrogen Fixation
3.4. Degrading of Inorganics (Hexavalent Chromium Ions)
3.5. Reducing Carbon Dioxide to Organic Carbon Resources
3.6. Killing Bacteria
4. Future Perspectives and Summary
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Zhao, W.; Chen, Z.; Yang, X.R.; Qian, X.X.; Liu, C.X.; Zhou, D.T.; Sun, T.; Zhang, M.; Wei, G.Y.; Dissanayake, P.D.; et al. Recent advances in photocatalytic hydrogen evolution with high-performance catalysts without precious metals. Renew. Sust. Energ. Rev. 2020, 132, 110040. [Google Scholar] [CrossRef]
- Nunes, B.N.; Lopes, O.F.; Patrocinio, A.O.T.; Bahnemann, D.W. Recent Advances in Niobium-Based Materials for Photocatalytic Solar Fuel Production. Catalysts 2020, 10, 126. [Google Scholar] [CrossRef]
- Ma, K.; Dong, Y.; Zhang, M.; Xu, C.; Ding, Y. A homogeneous Cu-based polyoxometalate coupled with mesoporous TiO2 for efficient photocatalytic H2 production. J. Colloid Interface Sci. 2021, 587, 613–621. [Google Scholar] [CrossRef]
- Jiang, Z.; Zhang, X.; Sun, W.; Yang, D.; Duchesne, P.N.; Gao, Y.; Wang, Z.; Yan, T.; Yuan, Z.; Yang, G.; et al. Building a Bridge from Papermaking to Solar Fuels. Angew. Chem. Int. Ed. 2019, 58, 14850–14854. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.; Li, X.; Li, J.; Wei, B. Boosting photocatalytic hydrogen production from water by photothermally induced biphase systems. Nat. Commun. 2021, 12, 1343. [Google Scholar] [CrossRef]
- Chen, Z.; Yao, D.; Chu, C.; Mao, S. Photocatalytic H2O2 production Systems: Design strategies and environmental applications. Chem. Eng. J. 2023, 451, 138489. [Google Scholar] [CrossRef]
- Jiang, Z.; Sun, W.; Miao, W.; Yuan, Z.; Yang, G.; Kong, F.; Yan, T.; Chen, J.; Huang, B.; An, C.; et al. Living Atomically Dispersed Cu Ultrathin TiO2 Nanosheet CO2 Reduction Photocatalyst. Adv. Sci. 2019, 6, 1900289. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Mueses, M.A.; Marquez, J.A.C.; Machuca-Martinez, F.; Grcic, I.; Peralta Muniz Moreira, R.; Li Puma, G. Engineering and modeling perspectives on photocatalytic reactors for water treatment. Water Res. 2021, 202, 117421. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Cheng, Y.; Zhou, N.; Chen, P.; Wang, Y.; Li, K.; Huo, S.; Cheng, P.; Peng, P.; Zhang, R.; et al. Photocatalytic degradation of organic pollutants using TiO2-based photocatalysts: A review. J. Clean. Prod. 2020, 268, 121725. [Google Scholar] [CrossRef]
- Jiang, Z.Y.; Miao, W.K.; Zhu, X.L.; Yang, G.H.; Yuan, Z.M.; Chen, J.C.; Ji, X.X.; Kong, F.G.; Huang, B.B. Modifying lewis base on TiO2 nanosheets for enhancing CO2 adsorption and the separation of photogenerated charge carriers. Appl. Catal. B Environ. 2019, 256, 117881. [Google Scholar] [CrossRef]
- Xu, J.; Zhong, W.; Gao, D.; Wang, X.; Wang, P.; Yu, H. Phosphorus-enriched platinum diphosphide nanodots as a highly efficient cocatalyst for photocatalytic H2 evolution of CdS. Chem. Eng. J. 2022, 439, 135758. [Google Scholar] [CrossRef]
- Xie, Y.; Chen, J.; Wu, X.; Wen, J.; Zhao, R.; Li, Z.; Tian, G.; Zhang, Q.; Ning, P.; Hao, J. Frustrated Lewis Pairs Boosting Low-Temperature CO2 Methanation Performance over Ni/CeO2 Nanocatalysts. ACS Catal. 2022, 12, 10587–10602. [Google Scholar] [CrossRef]
- Zhu, J.; Cannizzaro, F.; Liu, L.; Zhang, H.; Kosinov, N.; Filot, I.A.W.; Rabeah, J.; Bruckner, A.; Hensen, E.J.M. Ni-In Synergy in CO2 Hydrogenation to Methanol. ACS Catal. 2021, 11, 11371–11384. [Google Scholar] [CrossRef]
- Li, Y.; Li, B.; Zhang, D.; Cheng, L.; Xiang, Q. Crystalline Carbon Nitride Supported Copper Single Atoms for Photocatalytic CO2 Reduction with Nearly 100% CO Selectivity. ACS Nano 2020, 14, 10552–10561. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Lai, C.; Li, B.; Xu, F.; Huang, D.; Liu, S.; Qin, L.; Fu, Y.; Liu, X.; Yi, H.; et al. Unravelling the role of dual quantum dots cocatalyst in 0D/2D heterojunction photocatalyst for promoting photocatalytic organic pollutant degradation. Chem. Eng. J. 2020, 396, 125343. [Google Scholar] [CrossRef]
- Liang, C.; Niu, H.Y.; Guo, H.; Niu, C.G.; Huang, D.W.; Yang, Y.Y.; Liu, H.Y.; Shao, B.B.; Feng, H.P. Insight into photocatalytic nitrogen fixation on graphitic carbon nitride: Defect-dopant strategy of nitrogen defect and boron dopant. Chem. Eng. J. 2020, 396, 125395. [Google Scholar] [CrossRef]
- Li, Z.; Wang, L.; Qin, L.; Lai, C.; Wang, Z.; Zhou, M.; Xiao, L.; Liu, S.; Zhang, M. Recent advances in the application of water-stable metal-organic frameworks: Adsorption and photocatalytic reduction of heavy metal in water. Chemosphere 2021, 285, 131432. [Google Scholar] [CrossRef]
- Baaloudj, O.; Assadi, I.; Nasrallah, N.; El Jery, A.; Khezami, L.; Assadi, A.A. Simultaneous removal of antibiotics and inactivation of antibiotic-resistant bacteria by photocatalysis: A review. J. Water Process. Eng. 2021, 42, 102089. [Google Scholar] [CrossRef]
- Han, L.; Jing, F.; Zhang, J.; Luo, X.-Z.; Zhong, Y.-L.; Wang, K.; Zang, S.-H.; Teng, D.-H.; Liu, Y.; Chen, J.; et al. Environment friendly and remarkably efficient photocatalytic hydrogen evolution based on metal organic framework derived hexagonal/cubic In2O3 phase-junction. Appl. Catal. B Environ. 2021, 282, 119602. [Google Scholar] [CrossRef]
- Zhu, X.; Wang, Y.; Guo, Y.; Wan, J.; Yan, Y.; Zhou, Y.; Sun, C. Environmental-friendly synthesis of heterojunction photocatalysts g-C3N4/BiPO4 with enhanced photocatalytic performance. Appl. Surf. Sci. 2021, 544, 148872. [Google Scholar] [CrossRef]
- Kumar, A.; Kumar, A.; Krishnan, V. Perovskite Oxide Based Materials for Energy and Environment-Oriented Photocatalysis. ACS Catal. 2020, 10, 10253–10315. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, Q.; Ma, D. Advances in 2D/2D Z-Scheme Heterojunctions for Photocatalytic Applications. Sol. RRL 2020, 5, 2000397. [Google Scholar] [CrossRef]
- Zhang, X.H.; Yuan, Z.M.; Chen, J.C.; Yang, G.H.; Dionysiou, D.D.; Huang, B.B.; Jiang, Z.Y. Enhanced CO2 photoconversion activity of TiO2 via double effect of CoPi as hole traps and high CO2 capture. Catal. Today 2020, 340, 204–208. [Google Scholar] [CrossRef]
- Goktas, S.; Goktas, A. A comparative study on recent progress in efficient ZnO based nanocomposite and heterojunction photocatalysts: A review. J. Alloys Compd. 2021, 863, 158734. [Google Scholar] [CrossRef]
- Yerli Soylu, N.; Soylu, A.; Dikmetas, D.N.; Karbancioglu-Guler, F.; Kucukbayrak, S.; Erol Taygun, M. Photocatalytic and Antimicrobial Properties of Electrospun TiO2–SiO2–Al2O3–ZrO2–CaO–CeO2 Ceramic Membranes. ACS Omega 2023, 8, 10836–10850. [Google Scholar] [CrossRef] [PubMed]
- Mu, J.; Chen, B.; Zhang, M.; Guo, Z.; Zhang, P.; Zhang, Z.; Sun, Y.; Shao, C.; Liu, Y. Enhancement of the visible-light photocatalytic activity of In2O3-TiO2 nanofiber heteroarchitectures. ACS Appl. Mater. Interfaces 2012, 4, 424–430. [Google Scholar] [CrossRef]
- Cheng, S.W.; Sun, Z.H.; Lim, K.H.; Zhang, T.X.; Hondo, E.; Du, T.; Liu, L.Y.; Judd, M.; Cox, N.; Yin, Z.Y.; et al. BiOCl Nanoflowers with High Levels of Oxygen Vacancy for Photocatalytic CO2 Reduction. ACS Appl. Nano Mater. 2023, 6, 3608–3617. [Google Scholar] [CrossRef]
- Xu, M.L.; Jiang, X.J.; Li, J.R.; Wang, F.J.; Li, K.; Cheng, X. Self-Assembly of a 3D Hollow BiOBr@Bi-MOF Heterostructure with Enhanced Photocatalytic Degradation of Dyes. ACS Appl. Mater. Interfaces 2021, 13, 56171–56180. [Google Scholar] [CrossRef]
- Zhou, C.; Jiang, C.; Wang, R.; Chen, J.; Wang, G. CdS Microparticles Decorated with Bi0/BiOI Nanosheets for Visible Light Photocatalytic Hydrogen Evolution. ACS Appl. Nano Mater. 2021, 4, 4939–4947. [Google Scholar] [CrossRef]
- Li, Y.; Ding, L.; Guo, Y.; Liang, Z.; Cui, H.; Tian, J. Boosting the Photocatalytic Ability of g-C3N4 for Hydrogen Production by Ti3C2 MXene Quantum Dots. ACS Appl. Mater. Interfaces 2019, 11, 41440–41447. [Google Scholar] [CrossRef] [PubMed]
- Kang, M.; Yu, S.H.; Baek, K.-Y.; Sung, M.M.; Cho, S. MIL-101-NH2(Fe)-Coated Nylon Microfibers for Immobilized Photocatalysts in RhB and Cr(VI) Removal. ACS Omega 2023, 8, 15298–15305. [Google Scholar] [CrossRef]
- Lu, J.; Zhou, W.; Zhang, X.; Xiang, G. Electronic Structures and Lattice Dynamics of Layered BiOCl Single Crystals. J. Phys. Chem. Lett. 2020, 11, 1038–1044. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Yang, X.; Peng, Y.; Li, Z.; Yu, J.; Zhang, Y. Regulating the Built-In Electric Field of BiOBr by a Piezoelectric Mineral Tourmaline and the Enhanced Photocatalytic Property. Ind. Eng. Chem. Res. 2022, 61, 1704–1714. [Google Scholar] [CrossRef]
- Wang, Z.; Chu, Z.; Dong, C.; Wang, Z.; Yao, S.; Gao, H.; Liu, Z.; Liu, Y.; Yang, B.; Zhang, H. Ultrathin BiOX (X = Cl, Br, I) Nanosheets with Exposed {001} Facets for Photocatalysis. ACS Appl. Nano Mater. 2020, 3, 1981–1991. [Google Scholar] [CrossRef]
- Zhang, H.; Yang, Y.; Zhou, Z.; Zhao, Y.; Liu, L. Enhanced Photocatalytic Properties in BiOBr Nanosheets with Dominantly Exposed (102) Facets. J. Phys. Chem. C 2014, 118, 14662–14669. [Google Scholar] [CrossRef]
- Shi, M.; Li, G.; Li, J.; Jin, X.; Tao, X.; Zeng, B.; Pidko, E.A.; Li, R.; Li, C. Intrinsic Facet-Dependent Reactivity of Well-Defined BiOBr Nanosheets on Photocatalytic Water Splitting. Angew. Chem. Int. Ed. Engl. 2020, 59, 6590–6595. [Google Scholar] [CrossRef]
- Li, X.; Hu, Y.; Dong, F.; Huang, J.; Han, L.; Deng, F.; Luo, Y.; Xie, Y.; He, C.; Feng, Z.; et al. Non-noble-metallic Ni2P nanoparticles modified OV-BiOBr with boosting photoelectrochemical hydrogen evolution without sacrificial agent. Appl. Catal. B Environ. 2023, 325, 122341. [Google Scholar] [CrossRef]
- Guan, C.; Hou, T.; Nie, W.; Zhang, Q.; Duan, L.; Zhao, X. Sn4+ doping enhanced inner electric field for photocatalytic performance promotion of BiOCl based nanoflowers. Appl. Surf. Sci. 2022, 604, 154498. [Google Scholar] [CrossRef]
- Zhang, H.; Chen, X.; Zhang, Z.; Yu, K.; Zhu, W.; Zhu, Y. Highly-crystalline Triazine-PDI Polymer with an Enhanced Built-in Electric Field for Full-Spectrum Photocatalytic Phenol Mineralization. Appl. Catal. B Environ. 2021, 287, 119957. [Google Scholar] [CrossRef]
- Zhang, K.; Liu, C.; Huang, F.; Zheng, C.; Wang, W. Study of the electronic structure and photocatalytic activity of the BiOCl photocatalyst. Appl. Catal. B Environ. 2006, 68, 125–129. [Google Scholar] [CrossRef]
- Huang, W.L.; Zhu, Q.S. Electronic structures of relaxed BiOX (X = F, Cl, Br, I) photocatalysts. Comput. Mater. Sci. 2008, 43, 1101–1108. [Google Scholar] [CrossRef]
- Singh, S.; Sharma, R.; Khanuja, M. A review and recent developments on strategies to improve the photocatalytic elimination of organic dye pollutants by BiOX (X = Cl, Br, I, F) nanostructures. Korean J. Chem. Eng. 2018, 35, 1955–1968. [Google Scholar] [CrossRef]
- Hu, X.; Guo, R.T.; Chen, X.; Bi, Z.X.; Wang, J.; Pan, W.G. Bismuth-based Z-scheme structure for photocatalytic CO2 reduction: A review. J. Environ. Chem. Eng. 2022, 10, 108582. [Google Scholar] [CrossRef]
- Ahmad, I.; Shukrullah, S.; Yasin Naz, M.; Ullah, S.; Ali Assiri, M. Designing and modification of bismuth oxyhalides BiOX (X = Cl, Br and I) photocatalysts for improved photocatalytic performance. J. Ind. Eng. Chem. 2022, 105, 1–33. [Google Scholar] [CrossRef]
- Cheng, H.; Huang, B.; Dai, Y. Engineering BiOX (X = Cl, Br, I) nanostructures for highly efficient photocatalytic applications. Nanoscale 2014, 6, 2009–2026. [Google Scholar] [CrossRef]
- Guo, J.; Li, X.; Liang, J.; Yuan, X.; Jiang, L.; Yu, H.; Sun, H.; Zhu, Z.; Ye, S.; Tang, N.; et al. Fabrication and regulation of vacancy-mediated bismuth oxyhalide towards photocatalytic application: Development status and tendency. Coord. Chem. Rev. 2021, 443, 214033. [Google Scholar] [CrossRef]
- Yang, Y.; Zhang, C.; Lai, C.; Zeng, G.; Huang, D.; Cheng, M.; Wang, J.; Chen, F.; Zhou, C.; Xiong, W. BiOX (X = Cl, Br, I) photocatalytic nanomaterials: Applications for fuels and environmental management. Adv. Colloid Interface Sci. 2018, 254, 76–93. [Google Scholar] [CrossRef]
- Zhao, L.; Zhang, X.; Fan, C.; Liang, Z.; Han, P. First-principles study on the structural, electronic and optical properties of BiOX (X = Cl, Br, I) crystals. Physica B Condens. Matter 2012, 407, 3364–3370. [Google Scholar] [CrossRef]
- Fujishima, A.; Honda, K. Electrochemical Photolysis of Water at a Semiconductor Electrode. Nature 1972, 238, 37–38. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.M.; Zhu, S.L.; Kong, X.C.; Liang, Y.Q.; Li, Z.Y.; Wu, S.L.; Luo, S.Y.; Chang, C.T.; Cui, Z.D. Rutile-Coated B-Phase TiO2 Heterojunction Nanobelts for Photocatalytic H2 Evolution. ACS Appl. Nano Mater. 2020, 3, 10349–10359. [Google Scholar] [CrossRef]
- Trang, T.N.Q.; Phan, T.B.; Nam, N.D.; Thu, V.T.H. In Situ Charge Transfer at the Ag@ZnO Photoelectrochemical Interface toward the High Photocatalytic Performance of H2 Evolution and RhB Degradation. ACS Appl. Mater. Interfaces 2020, 12, 12195–12206. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Wang, Z.; Zhang, Y.; Wei, P.; Xu, W.; Wang, H.; Yu, H.; Jia, J.; Zhang, K.; Peng, C. S-Scheme and Schottky Junction Synchronous Regulation Boost Hierarchical CdS@Nb2O5/Nb2CTx (MXene) Heterojunction for Photocatalytic H2 Production. ACS Appl. Mater. Interfaces 2023, 15, 20027–20039. [Google Scholar] [CrossRef] [PubMed]
- Sun, G.T.; Shi, J.W.; Mao, S.M.; Ma, D.D.; He, C.; Wang, H.K.; Cheng, Y.H. Dodecylamine coordinated tri-arm CdS nanorod wrapped in intermittent ZnS shell for greatly improved photocatalytic H2 evolution. Chem. Eng. J. 2022, 429, 132382. [Google Scholar] [CrossRef]
- Li, J.; Yang, W.; Wu, A.; Zhang, X.; Xu, T.; Liu, B. Band-Gap Tunable 2D Hexagonal (GaN)1-x(ZnO)x Solid-Solution Nanosheets for Photocatalytic Water Splitting. ACS Appl. Mater. Interfaces 2020, 12, 8583–8591. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Z.Y.; Liu, Y.Y.; Jing, T.; Huang, B.B.; Wang, Z.Y.; Zhang, X.Y.; Qin, X.Y.; Dai, Y. One-pot solvothermal synthesis of S doped BiOCl for solar water oxidation. RSC Adv. 2015, 5, 47261–47264. [Google Scholar] [CrossRef]
- Lee, G.J.; Zheng, Y.C.; Wu, J.J. Fabrication of hierarchical bismuth oxyhalides (BiOX, X = Cl, Br, I) materials and application of photocatalytic hydrogen production from water splitting. Catal. Today 2018, 307, 197–204. [Google Scholar] [CrossRef]
- Liu, Y.; Yang, B.; He, H.; Yang, S.; Duan, X.; Wang, S. Bismuth-based complex oxides for photocatalytic applications in environmental remediation and water splitting: A review. Sci. Total Environ. 2022, 804, 150215. [Google Scholar] [CrossRef]
- Wang, J.J.; Zhang, M.; Meng, J.; Li, Q.X.; Yang, J.L. Single- and few-layer BiOI as promising photocatalysts for solar water splitting. RSC Adv. 2017, 7, 24446–24452. [Google Scholar] [CrossRef]
- Bai, L.; Ye, F.; Li, L.; Lu, J.; Zhong, S.; Bai, S. Facet Engineered Interface Design of Plasmonic Metal and Cocatalyst on BiOCl Nanoplates for Enhanced Visible Photocatalytic Oxygen Evolution. Small 2017, 13, 1701607. [Google Scholar] [CrossRef]
- Ning, S.B.; Shi, X.Q.; Zhang, H.W.; Lin, H.X.; Zhang, Z.Z.; Long, J.L.; Li, Y.; Wang, X.X. Reconstructing Dual-Induced {0 0 1} Facets Bismuth Oxychloride Nanosheets Heterostructures: An Effective Strategy to Promote Photocatalytic Oxygen Evolution. Sol. RRL 2019, 3, 1900059. [Google Scholar] [CrossRef]
- Zhang, L.; Han, Z.; Wang, W.; Li, X.; Su, Y.; Jiang, D.; Lei, X.; Sun, S. Solar-Light-Driven Pure Water Splitting with Ultrathin BiOCl Nanosheets. Chemistry 2015, 21, 18089–18094. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Liu, X.; Dong, Y.M.; Xia, Y.M.; Wang, H.J. A special synthesis of BiOCl photocatalyst for efficient pollutants removal: New insight into the band structure regulation and molecular oxygen activation. Appl. Catal. B Environ. 2019, 256, 117872. [Google Scholar] [CrossRef]
- Yang, J.F.; Su, H.; Wu, Y.Y.; Li, D.G.; Zhang, D.; Sun, H.; Yin, S.Y. Facile synthesis of kermesinus BiOI with oxygen vacancy for efficient hydrogen generation. Chem. Eng. J. 2021, 420, 127607. [Google Scholar] [CrossRef]
- Carey, J.H.; Lawrence, J.; Tosine, H.M. Photodechlorination of PCB’s in the presence of titanium dioxide in aqueous suspensions. Bull. Environ. Contam. Toxicol. 1976, 16, 697–701. [Google Scholar] [CrossRef]
- Akpan, U.G.; Hameed, B.H. Parameters affecting the photocatalytic degradation of dyes using TiO2-based photocatalysts: A review. J. Hazard. Mater. 2009, 170, 520–529. [Google Scholar] [CrossRef] [PubMed]
- Tang, X.; Tang, R.; Xiong, S.; Zheng, J.; Li, L.; Zhou, Z.; Gong, D.; Deng, Y.; Su, L.; Liao, C. Application of natural minerals in photocatalytic degradation of organic pollutants: A review. Sci. Total Environ. 2022, 812, 152434. [Google Scholar] [CrossRef]
- Sano, T.; Puzenat, E.; Guillard, C.; Geantet, C.; Matsuzawa, S. Degradation of C2H2 with modified-TiO2 photocatalysts under visible light irradiation. J. Mol. Catal. A Chem. 2008, 284, 127–133. [Google Scholar] [CrossRef]
- Xie, Y.; Chang, F.; Li, C.; Chen, J.; Luo, J.; Li, L.; Hu, X. One-Pot Polyvinyl Alcohol-Assisted Hydrothermal Synthesis of Hierarchical Flower-Like BiOCl Nanoplates with Enhancement of Photocatalytic Activity for Degradation of Rhodamine B. Clean-Soil Air Water 2014, 42, 521–527. [Google Scholar] [CrossRef]
- Su, Q.; Zhu, L.; Zhang, M.; Li, Y.; Liu, S.; Lin, J.; Song, F.; Zhang, W.; Zhu, S.; Pan, J. Construction of a Bioinspired Hierarchical BiVO4/BiOCl Heterojunction and Its Enhanced Photocatalytic Activity for Phenol Degradation. ACS Appl. Mater. Interfaces 2021, 13, 32906–32915. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Du, C.; Li, M.; Zhang, S.; Bai, H.; Yang, L.; Zhang, S. One-Pot Hydrothermal Synthesis of SnO2/BiOBr Heterojunction Photocatalysts for the Efficient Degradation of Organic Pollutants Under Visible Light. ACS Appl. Mater. Interfaces 2018, 10, 28686–28694. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, A.; Kar, P.; Wulferding, D.; Lemmens, P.; Pal, S.K. Flower-Like BiOI Microspheres Decorated with Plasmonic Gold Nanoparticles for Dual Detoxification of Organic and Inorganic Water Pollutants. ACS Appl. Nano Mater. 2020, 3, 2733–2744. [Google Scholar] [CrossRef]
- Yu, Y.; Yang, Z.X.; Shang, Z.C.; Wang, X.H. One-step solution combustion synthesis of Bi/BiOCl nanosheets: Reaction mechanism and photocatalytic RhB degradation. J. Phys. Chem. Solids 2023, 174, 111172. [Google Scholar] [CrossRef]
- Jiang, Z.; Huang, B.; Lou, Z.; Wang, Z.; Meng, X.; Liu, Y.; Qin, X.; Zhang, X.; Dai, Y. Immobilization of BiOX (X = Cl, Br) on activated carbon fibers as recycled photocatalysts. Dalton Trans. 2014, 43, 8170–8173. [Google Scholar] [CrossRef]
- Guo, T.Y.; Fan, X.R.; Jiang, X.Y.; Qi, Y.; Du, J.P.; Zhang, A.M.; Wang, H.T. Engineering shape of BiOCl nanosheets with improved visible-light response for superior photocatalytic degradation of Rhodamine B. J. Alloys Compd. 2023, 948, 169586. [Google Scholar] [CrossRef]
- Wu, Z.H.; Li, W.L.; Xu, J.Y.; Jing, J.F.; Li, J.S.; Shen, J.; Yang, L.; Feng, W.H.; Zhang, S.Y.; Zhu, Y.F. Internal Electric Field Enhancement by the I-Rich Surface of Highly Crystallized BiOI Nanosheets for Boosted Photocatalytic Degradation of Phenol. Small Struct. 2023, 2200380. [Google Scholar] [CrossRef]
- Fenelon, E.; Bui, D.P.; Tran, H.H.; You, S.J.; Wang, Y.F.; Cao, T.M.; Van Pham, V. Straightforward Synthesis of SnO2/Bi2S3/BiOCl-Bi24O31Cl10 Composites for Drastically Enhancing Rhodamine B Photocatalytic Degradation under Visible Light. ACS Omega 2020, 5, 20438–20449. [Google Scholar] [CrossRef]
- Chen, J.G.; Crooks, R.M.; Seefeldt, L.C.; Bren, K.L.; Bullock, R.M.; Darensbourg, M.Y.; Holland, P.L.; Hoffman, B.; Janik, M.J.; Jones, A.K.; et al. Beyond fossil fuel-driven nitrogen transformations. Science 2018, 360, eaar6611. [Google Scholar] [CrossRef]
- Gao, X.; Wen, Y.J.; Qu, D.; An, L.; Luan, S.L.; Jiang, W.S.; Zong, X.P.; Liu, X.Y.; Sun, Z.C. Interference Effect of Alcohol on Nessler’s Reagent in Photocatalytic Nitrogen Fixation. ACS Sustain. Chem. Eng. 2018, 6, 5342–5348. [Google Scholar] [CrossRef]
- Liao, Y.; Lin, J.N.; Cui, B.H.; Xie, G.; Hu, S. Well-dispersed ultrasmall ruthenium on TiO2(P25) for effective photocatalytic N2 fixation in ambient condition. J. Photochem. Photobiol. A 2020, 387, 112100. [Google Scholar] [CrossRef]
- Schrauzer, G.N.; Guth, T.D. Photolysis of Water and Photoreduction of Nitrogen on Titanium Dioxide. J. Am. Chem. Soc. 1977, 99, 7189–7193. [Google Scholar] [CrossRef]
- Medford, A.J.; Hatzell, M.C. Photon-Driven Nitrogen Fixation: Current Progress, Thermodynamic Considerations, and Future Outlook. ACS Catal. 2017, 7, 2624–2643. [Google Scholar] [CrossRef]
- Chen, X.Z.; Li, N.; Kong, Z.Z.; Ong, W.J.; Zhao, X.J. Photocatalytic fixation of nitrogen to ammonia: State-of-the-art advancements and future prospects. Mater. Horizons 2018, 5, 9–27. [Google Scholar] [CrossRef]
- Li, H.; Shang, J.; Ai, Z.; Zhang, L. Efficient Visible Light Nitrogen Fixation with BiOBr Nanosheets of Oxygen Vacancies on the Exposed {001} Facets. J. Am. Chem. Soc. 2015, 137, 6393–6399. [Google Scholar] [CrossRef]
- Xue, X.; Chen, R.; Chen, H.; Hu, Y.; Ding, Q.; Liu, Z.; Ma, L.; Zhu, G.; Zhang, W.; Yu, Q.; et al. Oxygen Vacancy Engineering Promoted Photocatalytic Ammonia Synthesis on Ultrathin Two-Dimensional Bismuth Oxybromide Nanosheets. Nano Lett. 2018, 18, 7372–7377. [Google Scholar] [CrossRef]
- Gao, K.; Zhang, C.; Zhu, H.; Xia, J.; Chen, J.; Xie, F.; Zhao, X.; Tang, Z.; Wang, X. Unique Tubular BiOBr/g-C3N4 Heterojunction with Efficient Separation of Charge Carriers for Photocatalytic Nitrogen Fixation. Chemistry 2023, e202300616. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, R.A.; Pereira, W.; Souza, E.S.; Ramos, S.J.; Dias, Y.N.; Lima, M.W.; de Souza Neto, H.F.; Oliveira, E.S.; Fernandes, A.R. Artisanal gold mining in the eastern Amazon: Environmental and human health risks of mercury from different mining methods. Chemosphere 2021, 284, 131220. [Google Scholar] [CrossRef]
- Hoang, H.G.; Chiang, C.F.; Lin, C.; Wu, C.Y.; Lee, C.W.; Cheruiyot, N.K.; Tran, H.T.; Bui, X.T. Human health risk simulation and assessment of heavy metal contamination in a river affected by industrial activities. Environ. Pollut. 2021, 285, 117414. [Google Scholar] [CrossRef] [PubMed]
- Ding, Z.; Sun, G.; Fu, F.; Ye, C. Phase transformation of Cr(VI)-adsorbed ferrihydrite in the presence of Mn(II): Fate of Mn(II) and Cr(VI). J. Environ. Sci. 2022, 113, 251–259. [Google Scholar] [CrossRef]
- Bonola, B.; Sosa-Rodríguez, F.S.; García-Pérez, U.M.; Romero-Ibarra, I.; Henquin, E.R.; Vazquez-Arenas, J. The influence of cathode material, current density and pH on the rapid Cr(III) removal from concentrated tanning effluents via electro-precipitation. J. Hazard. Mater. Adv. 2021, 2, 100008. [Google Scholar] [CrossRef]
- Li, T.; Zhang, G.; Lan, H.C.; Liu, H.J.; Qu, J.H. Enhanced Photoreduction of Chromium(VI) Intercalated Ion Exchange in BiOBr0.75I0.25 Layers Structure by Bulk Charge Transfer. ACS Sustain. Chem. Eng. 2018, 7, 2429–2436. [Google Scholar] [CrossRef]
- Luo, Z.; Ye, X.; Zhang, S.; Xue, S.; Yang, C.; Hou, Y.; Xing, W.; Yu, R.; Sun, J.; Yu, Z.; et al. Unveiling the charge transfer dynamics steered by built-in electric fields in BiOBr photocatalysts. Nat. Commun. 2022, 13, 2230. [Google Scholar] [CrossRef]
- Fan, Z.; Zhao, Y.B.; Zhai, W.; Qiu, L.; Li, H.; Hoffmann, M.R. Facet-dependent performance of BiOBr for photocatalytic reduction of Cr(VI). RSC Adv. 2016, 6, 2028–2031. [Google Scholar] [CrossRef]
- Peng, Y.; Mao, Y.G.; Kan, P.F.; Liu, J.Y.; Fang, Z. Controllable synthesis and photoreduction performance towards Cr(VI) of BiOCl microrods with exposed (110) crystal facets. New J. Chem. 2018, 42, 16911–16918. [Google Scholar] [CrossRef]
- Hussain, M.B.; Khan, M.S.; Loussala, H.M.; Bashir, M.S. The synthesis of a BiOClxBr1-x nanostructure photocatalyst with high surface area for the enhanced visible-light photocatalytic reduction of Cr(VI). RSC Adv. 2020, 10, 4763–4771. [Google Scholar] [CrossRef]
- Yu, F.; Jin, M.; Zhang, Y.; Lei, C.; Zhou, L.; Zhu, H.; Yu, B. Visible-Light-Driven Zr-MOF/BiOBr Heterojunction for the Efficient Synchronous Removal of Hexavalent Chromium and Rhodamine B from Wastewater. ACS Omega 2022, 7, 25066–25077. [Google Scholar] [CrossRef] [PubMed]
- Shahid, M.Z.; Mehmood, R.; Athar, M.; Hussain, J.; Wei, Y.; Khaliq, A. BiOCl Nanoplates Doped with Fe3+ Ions for the Visible-Light Degradation of Aqueous Pollutants. ACS Appl. Nano Mater. 2020, 4, 746–758. [Google Scholar] [CrossRef]
- Xiong, J.; Zeng, H.Y.; Xu, S.; Peng, J.F.; Liu, F.Y.; Wang, L.H. Enhancing the intrinsic properties of flower-like BiOI by S-doping toward excellent photocatalytic performances. J. Mater. Sci. Technol. 2022, 118, 181–189. [Google Scholar] [CrossRef]
- Fu, Z.Y.; Yang, Q.; Liu, Z.; Chen, F.; Yao, F.B.; Xie, T.; Zhong, Y.; Wang, D.B.; Li, J.; Li, X.M.; et al. Photocatalytic conversion of carbon dioxide: From products to design the catalysts. J. CO2 Util. 2019, 34, 63–73. [Google Scholar] [CrossRef]
- Mustafa, A.; Lougou, B.G.; Shuai, Y.; Wang, Z.J.; Tan, H.P. Current technology development for CO2 utilization into solar fuels and chemicals: A review. J. Energy Chem. 2020, 49, 96–123. [Google Scholar] [CrossRef]
- Chen, G.; Waterhouse, G.I.N.; Shi, R.; Zhao, J.; Li, Z.; Wu, L.Z.; Tung, C.H.; Zhang, T. From Solar Energy to Fuels: Recent Advances in Light-Driven C1 Chemistry. Angew. Chem. Int. Ed. Engl. 2019, 58, 17528–17551. [Google Scholar] [CrossRef]
- Zhao, X.Z.; Xia, Y.G.; Li, H.P.; Wang, X.; Wei, J.; Jiao, X.L.; Chen, D.R. Oxygen vacancy dependent photocatalytic CO2 reduction activity in liquid-exfoliated atomically thin BiOCl nanosheets. Appl. Catal. B Environ. 2021, 297, 120426. [Google Scholar] [CrossRef]
- Zhu, J.Y.; Li, Y.P.; Wang, X.J.; Zhao, J.; Wu, Y.S.; Li, F.T. Simultaneous Phosphorylation and Bi Modification of BiOBr for Promoting Photocatalytic CO2 Reduction. ACS Sustain. Chem. Eng. 2019, 7, 14953–14961. [Google Scholar] [CrossRef]
- Sun, N.C.; Zhou, M.; Ma, X.X.; Cheng, Z.H.; Wu, J.; Qi, Y.F.; Sun, Y.J.; Zhou, F.H.; Shen, Y.X.; Lu, S.Y. Self-assembled spherical In2O3/BiOI heterojunctions for enhanced photocatalytic CO2 reduction activity. J. CO2 Util. 2022, 65, 102220. [Google Scholar] [CrossRef]
- Wu, J.; Li, X.; Shi, W.; Ling, P.; Sun, Y.; Jiao, X.; Gao, S.; Liang, L.; Xu, J.; Yan, W.; et al. Efficient Visible-Light-Driven CO2 Reduction Mediated by Defect-Engineered BiOBr Atomic Layers. Angew. Chem. Int. Ed. Engl. 2018, 57, 8719–8723. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Shen, J.; Sun, K.H.; Tang, H.; Liu, Q.Q. Enhancement in photocatalytic activity of CO2 reduction to CH4 by 0D/2D Au/TiO2 plasmon heterojunction. Appl. Surf. Sci. 2019, 493, 1142–1149. [Google Scholar] [CrossRef]
- Di, J.; Chen, C.; Zhu, C.; Song, P.; Xiong, J.; Ji, M.; Zhou, J.; Fu, Q.; Xu, M.; Hao, W.; et al. Bismuth Vacancy-Tuned Bismuth Oxybromide Ultrathin Nanosheets toward Photocatalytic CO2 Reduction. ACS Appl. Mater. Interfaces 2019, 11, 30786–30792. [Google Scholar] [CrossRef]
- Shi, Y.; Zhan, G.; Li, H.; Wang, X.; Liu, X.; Shi, L.; Wei, K.; Ling, C.; Li, Z.; Wang, H.; et al. Simultaneous Manipulation of Bulk Excitons and Surface Defects for Ultrastable and Highly Selective CO2 Photoreduction. Adv. Mater. 2021, 33, e2100143. [Google Scholar] [CrossRef]
- Wang, Q.L.; Miao, Z.R.; Zhang, Y.F.; Yan, T.J.; Meng, L.P.; Wang, X.X. Photocatalytic Reduction of CO2 with H2O Mediated by Ce-Tailored Bismuth Oxybromide Surface Frustrated Lewis Pairs. ACS Catal. 2022, 12, 4016–4025. [Google Scholar] [CrossRef]
- Wang, W.; Huang, G.; Yu, J.C.; Wong, P.K. Advances in photocatalytic disinfection of bacteria: Development of photocatalysts and mechanisms. J. Environ. Sci. 2015, 34, 232–247. [Google Scholar] [CrossRef]
- Zhou, Z.; Li, B.; Liu, X.; Li, Z.; Zhu, S.; Liang, Y.; Cui, Z.; Wu, S. Recent Progress in Photocatalytic Antibacterial. ACS Appl. Bio. Mater. 2021, 4, 3909–3936. [Google Scholar] [CrossRef]
- Ye, L.; Pelton, R.; Brook, M.A.; Filipe, C.D.; Wang, H.; Brovko, L.; Griffiths, M. Targeted disinfection of E. coli via bioconjugation to photoreactive TiO2. Bioconjug Chem. 2013, 24, 448–455. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Huang, S.; Ravisankar, P.; Zhu, H. Two-Dimensional Nanomaterials for Photoinduced Antibacterial Applications. ACS Appl. Bio. Mater. 2020, 3, 8188–8210. [Google Scholar] [CrossRef] [PubMed]
- Tipplook, M.; Panomsuwan, G.; Sudare, T.; Teshima, K. Graphitic Carbon Nitride Nanoflakes Decorated on Multielement-Doped Carbon as Photocatalysts for Bacterial Disinfection under Visible and Near-Infrared Light. ACS Appl. Nano Mater. 2022, 5, 3422–3433. [Google Scholar] [CrossRef]
- Ye, L.Q.; Chen, J.N.; Tian, L.H.; Liu, J.Y.; Peng, T.Y.; Deng, K.J.; Zan, L. BiOI thin film via chemical vapor transport: Photocatalytic activity, durability, selectivity and mechanism. Appl. Catal. B Environ. 2013, 130–131, 1–7. [Google Scholar] [CrossRef]
- Hao, R.; Xiao, X.; Zuo, X.; Nan, J.; Zhang, W. Efficient adsorption and visible-light photocatalytic degradation of tetracycline hydrochloride using mesoporous BiOI microspheres. J. Hazard. Mater. 2012, 209–210, 137–145. [Google Scholar] [CrossRef]
- Senasu, T.; Chankhanittha, T.; Hemavibool, K.; Nanan, S. Solvothermal synthesis of BiOBr photocatalyst with an assistant of PVP for visible-light-driven photocatalytic degradation of fluoroquinolone antibiotics. Catal. Today 2022, 384, 209–227. [Google Scholar] [CrossRef]
- Attri, P.; Garg, P.; Chauhan, M.; Singh, R.; Sharma, R.K.; Kumar, S.; Lim, D.-K.; Chaudhary, G.R. Metal doped BiOCl nano-architectures (M-BiOCl, M = Ni, Mo, Cd, Co) for efficient visible light photocatalytic and antibacterial behaviour. J. Environ. Chem. Eng. 2023, 11, 109498. [Google Scholar] [CrossRef]
- Jiang, Z.Y.; Liang, X.Z.; Liu, Y.Y.; Jing, T.; Wang, Z.Y.; Zhang, X.Y.; Qin, X.Y.; Dai, Y.; Huang, B.B. Enhancing visible light photocatalytic degradation performance and bactericidal activity of BiOI via ultrathin-layer structure. Appl. Catal. B Environ. 2017, 211, 252–257. [Google Scholar] [CrossRef]




| BiOX Six Applications in the Photocatalytic Field |
|---|
| Photocatalytic CO2 reduction |
| Photocatalytic degrading inorganics |
| Photocatalytic killing bacteria |
| Photocatalytic N2 fixation |
| Photocatalytic degrading organics |
| Photocatalytic splitting H2O |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yuan, Z.; Jiang, Z. Applications of BiOX in the Photocatalytic Reactions. Molecules 2023, 28, 4400. https://doi.org/10.3390/molecules28114400
Yuan Z, Jiang Z. Applications of BiOX in the Photocatalytic Reactions. Molecules. 2023; 28(11):4400. https://doi.org/10.3390/molecules28114400
Chicago/Turabian StyleYuan, Zhimin, and Zaiyong Jiang. 2023. "Applications of BiOX in the Photocatalytic Reactions" Molecules 28, no. 11: 4400. https://doi.org/10.3390/molecules28114400
APA StyleYuan, Z., & Jiang, Z. (2023). Applications of BiOX in the Photocatalytic Reactions. Molecules, 28(11), 4400. https://doi.org/10.3390/molecules28114400




