Isolation, Identification and Molecular Mechanism Analysis of the Nematicidal Compound Spectinabilin from Newly Isolated Streptomyces sp. DT10
Abstract
1. Introduction
2. Results
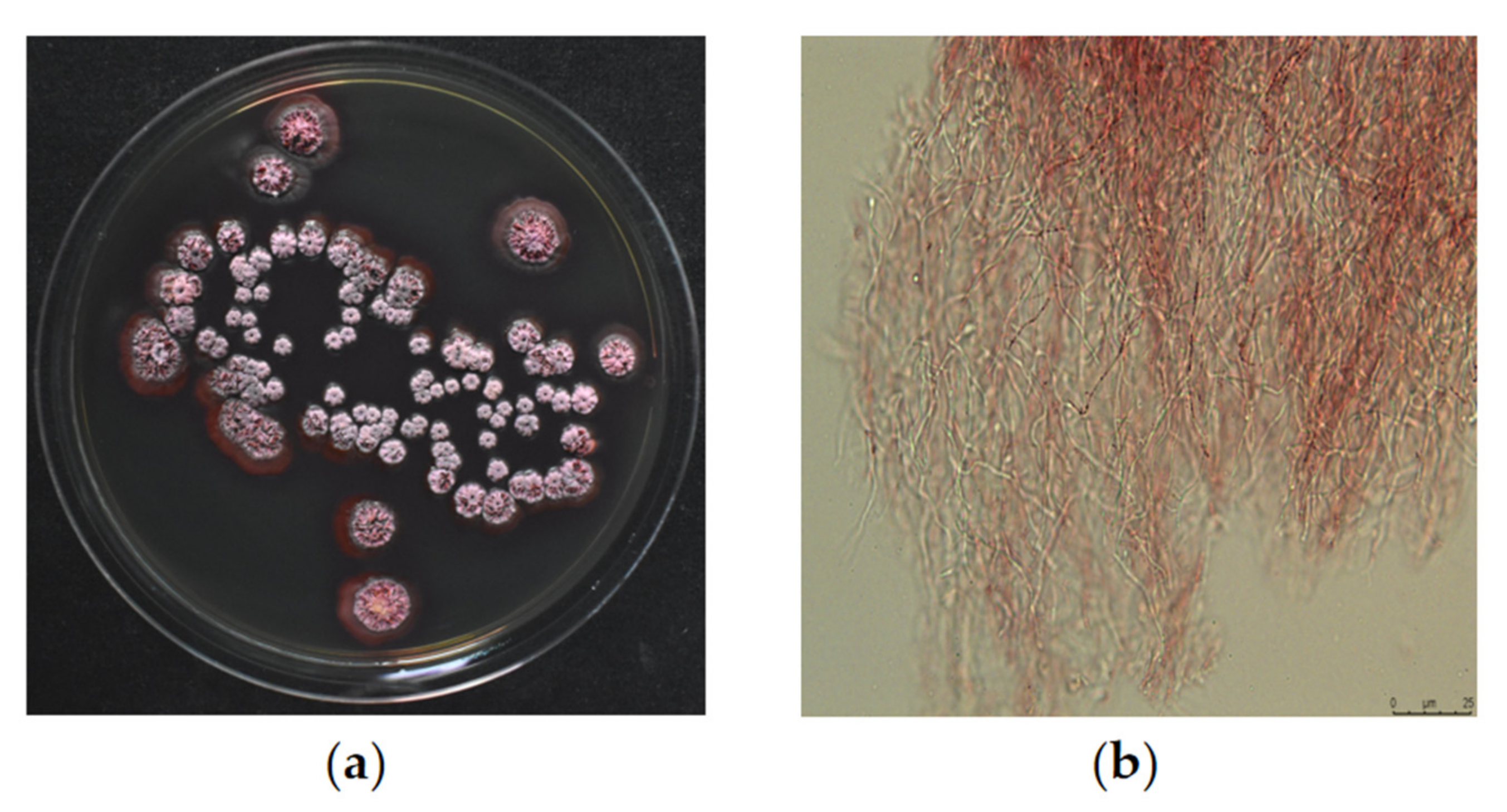
2.1. Screening and Identification of Strains with Nematicidal Activity
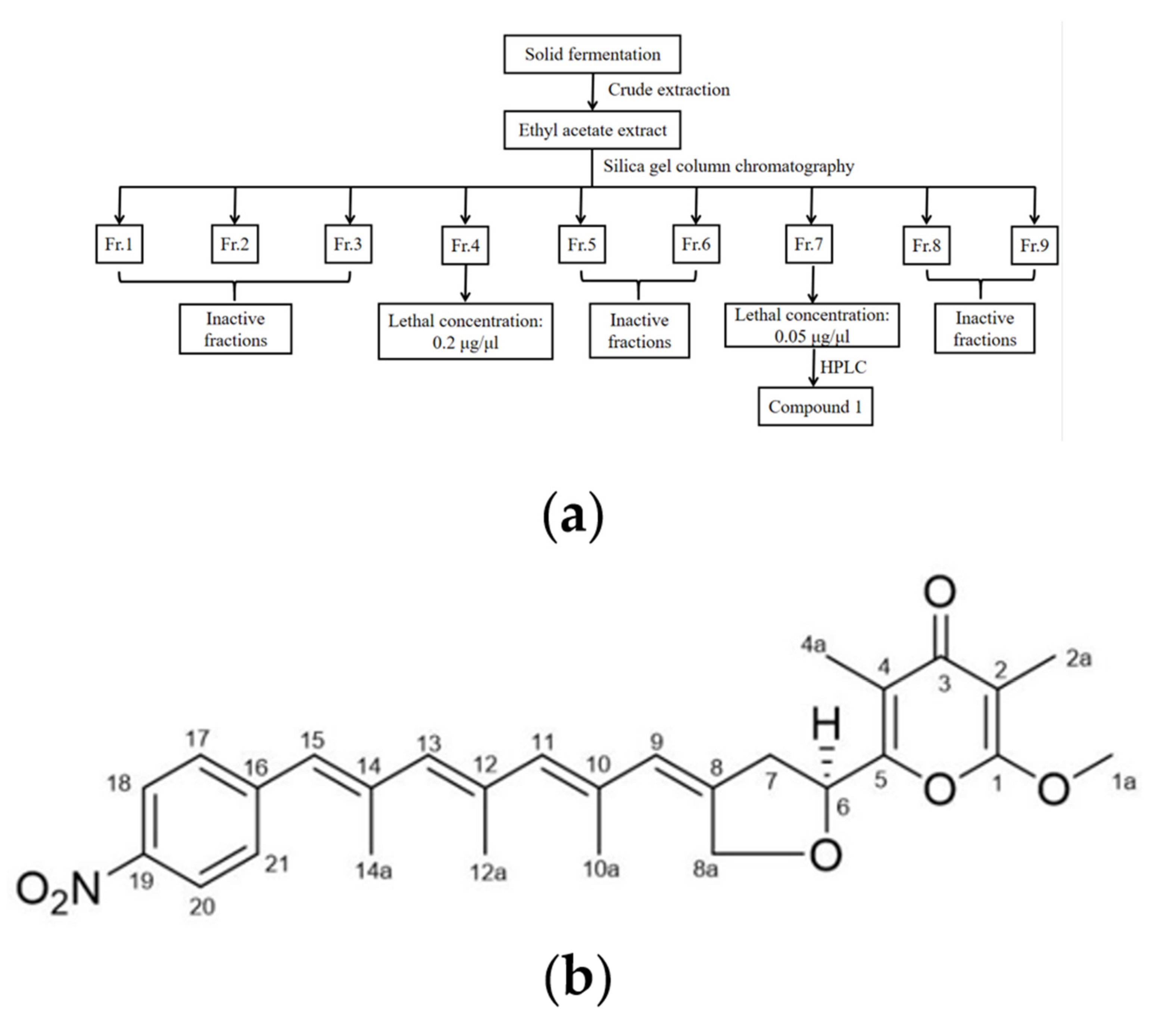
2.2. Nematicidal Active Compound 1 Extracted from DT10 Fermentation Product and Identification of Compound 1 as Spectinabilin
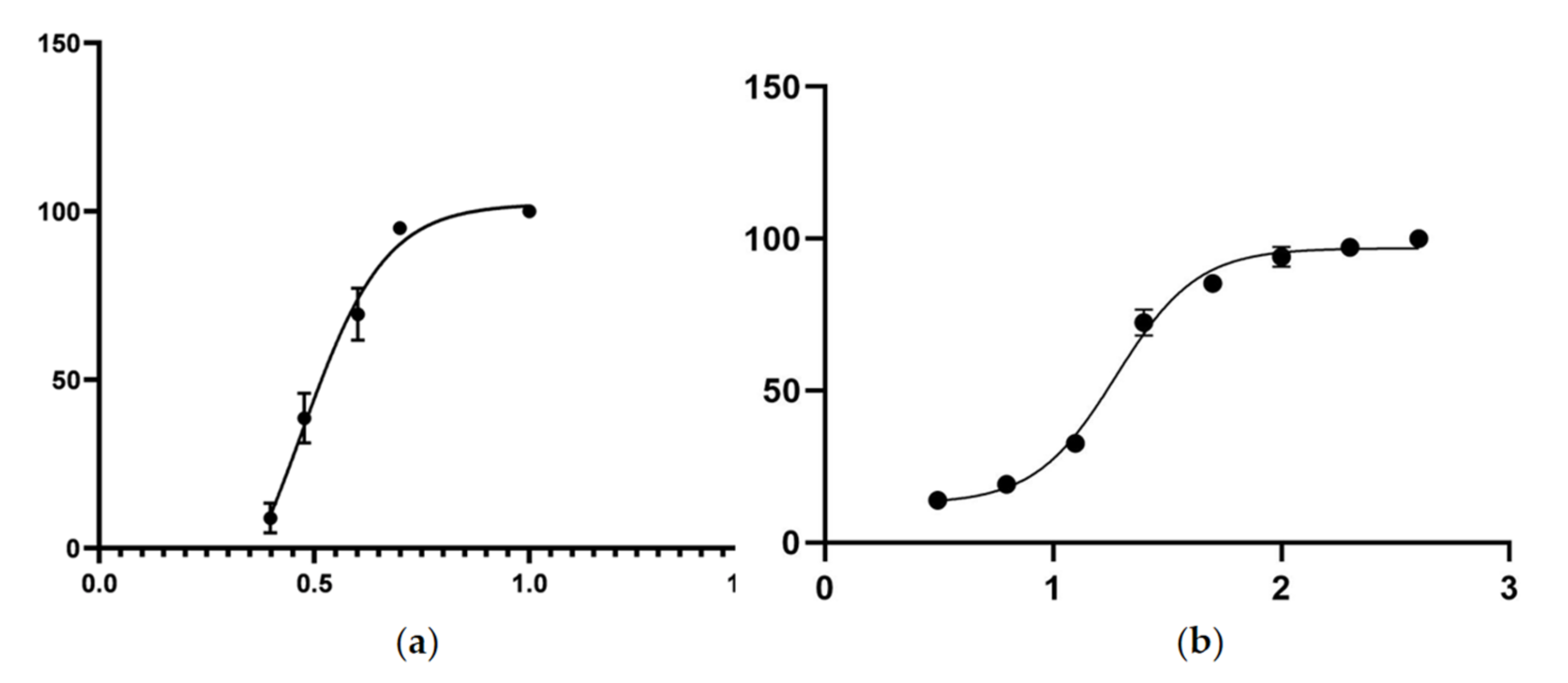
2.3. Nematicidal Effect of Spectinabilin
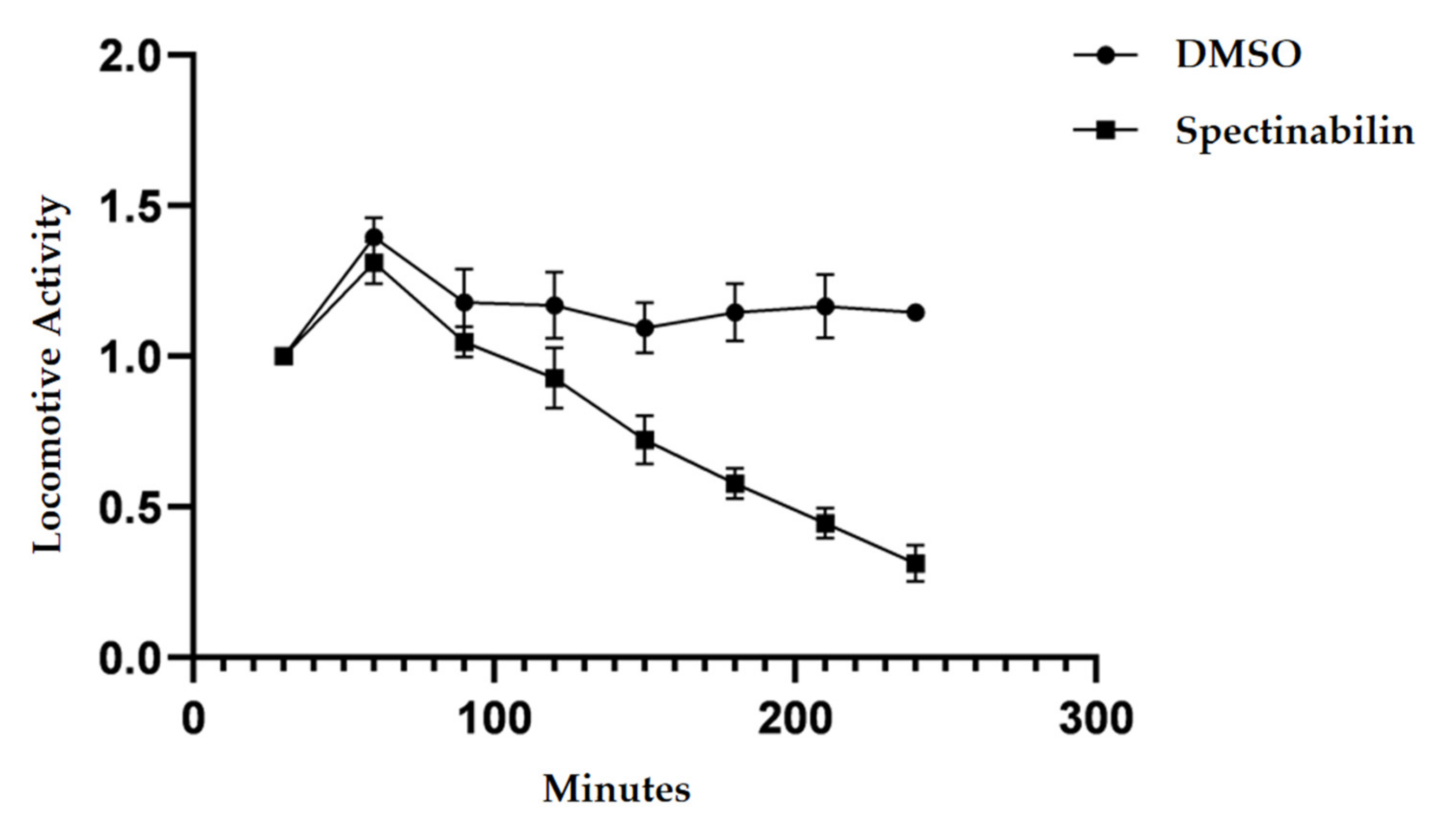
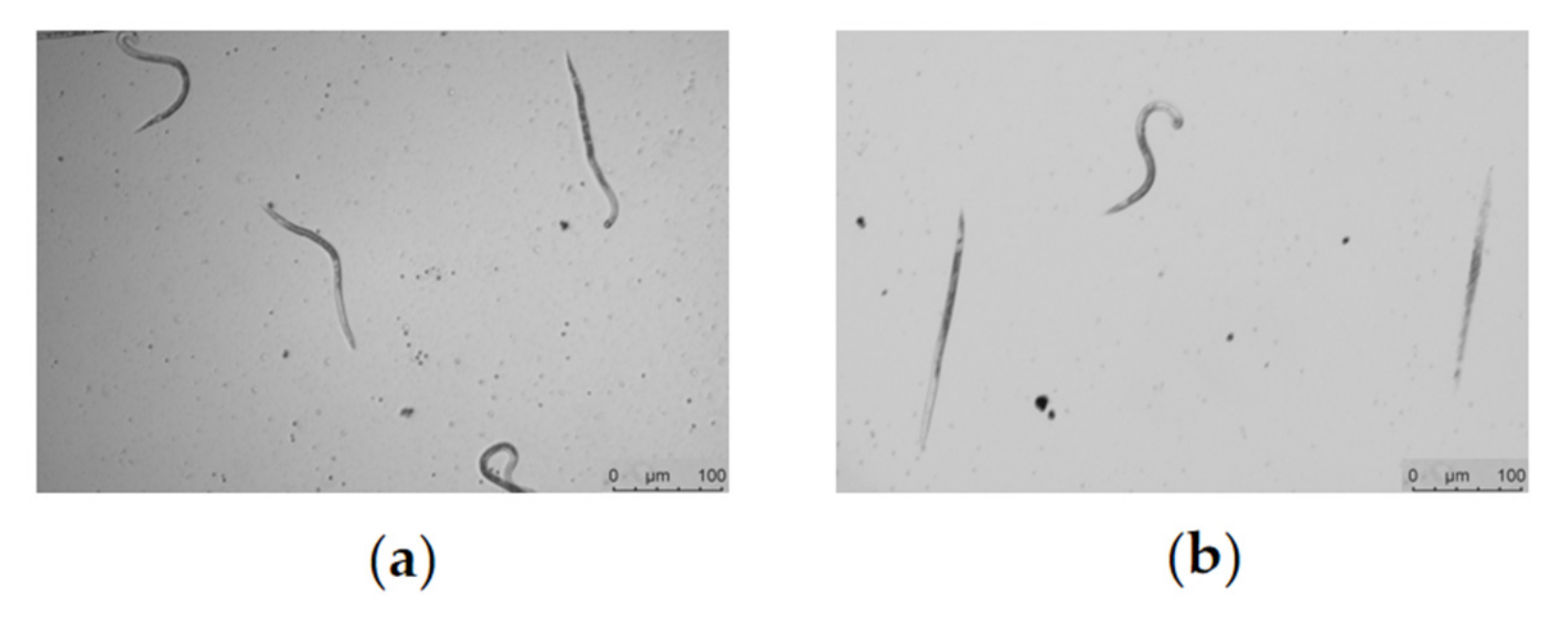
2.4. Spectinabilin Reduces the Motility of C. elegans L4 Worms
2.5. Analysis of Spectinabilin on Known Nematicidal Molecular Targets
2.6. Spectinabilin Exhibited Nematicidal Activity against Meloidogyne incognita J2s
3. Discussion
4. Materials and Methods
4.1. Bacterial Material
4.2. Nematode Strain and Maintenance
4.3. Screening of Strains with Nematicidal Activity
4.4. Identification of Strain DT10
4.5. Cultivation, Extraction and Isolation
4.6. Structure Identification of Compound 1
4.7. Effect of Spectinabilin on Survival of C. elegans
4.8. Effect of Spectinabilin on Survival of M. incognita J2s
4.9. Detection of Locomotor Ability of C. elegans L4 Worms
4.10. Analysis of Potential Drug Targets for Spectinabilin
4.11. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Abd-Elgawad, M.M.M. Optimizing safe approaches to manage plant-parasitic nematodes. Plants 2021, 10, 1191. [Google Scholar] [CrossRef] [PubMed]
- Tileubayeva, Z.; Avdeenko, A.; Avdeenko, S.; Stroiteleva, N.; Kondrashev, S. Plant-parasitic nematodes affecting vegetable crops in greenhouses. Saudi J. Biol. Sci. 2021, 28, 5428–5433. [Google Scholar] [CrossRef] [PubMed]
- Abad, P.; Gouzy, J.; Aury, J.M.; Castagnone-Sereno, P.; Danchin, E.G.; Deleury, E.; Perfus-Barbeoch, L.; Anthouard, V.; Artiguenave, F.; Blok, V.C.; et al. Genome sequence of the metazoan plant-parasitic nematode Meloidogyne incognita. Nat. Biotechnol. 2008, 26, 909–915. [Google Scholar] [CrossRef]
- Palomares-Rius, J.E.; Hasegawa, K.; Siddique, S.; Vicente, C.S.L. Editorial: Protecting our crops-approaches for plant parasitic nematode control. Front. Plant Sci. 2021, 12, 726057. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Servat, S.; Pinyol-Escala, L.; Daura-Pich, O.; Almazan, M.; Hernandez, I.; Lopez-Garcia, B.; Fernandez, C. Characterization of Lysobacter enzymogenes B25, a potential biological control agent of plant-parasitic nematodes, and its mode of action. AIMS Microbiol. 2023, 9, 151–176. [Google Scholar] [CrossRef]
- Caboni, P.; Ntalli, N.G.; Aissani, N.; Cavoski, I.; Angioni, A. Nematicidal activity of (E, E)-2,4-decadienal and (E)-2-decenal from Ailanthus altissima against Meloidogyne javanica. J. Agric. Food. Chem. 2012, 60, 1146–1151. [Google Scholar] [CrossRef]
- Geary, T.G. Ivermectin 20 years on: Maturation of a wonder drug. Trends Parasitol. 2005, 21, 530–532. [Google Scholar] [CrossRef]
- Lahm, G.P.; Desaeger, J.; Smith, B.K.; Pahutski, T.F.; Rivera, M.A.; Meloro, T.; Kucharczyk, R.; Lett, R.M.; Daly, A.; Smith, B.T.; et al. The discovery of fluazaindolizine: A new product for the control of plant parasitic nematodes. Bioorganic Med. Chem. Lett. 2017, 27, 1572–1575. [Google Scholar] [CrossRef]
- Chen, J.; Song, B. Natural nematicidal active compounds: Recent research progress and outlook. J. Integr. Agric. 2021, 20, 2015–2031. [Google Scholar] [CrossRef]
- Wang, L.; Yang, B.; Li, C. A review of biological control of root-knot nematodes. J. Nanjing Fores. U. 2002, 26, 64–68. (In Chinese) [Google Scholar]
- János, B. Bioactive Microbial Metabolites. J. Antibiot. 2005, 58, 1–26. [Google Scholar]
- Garabedian, S.; Van Gundy, S.D. Use of avermectins for the control of Meloidogyne incognita on tomatoes. J. Nematol. 1983, 15, 503–510. [Google Scholar] [PubMed]
- Sun, Y.; Zhou, X.; Liu, J.; Bao, K.; Zhang, G.; Tu, G.; Kieser, T.; Deng, Z. ‘Streptomyces nanchangensis’, a producer of the insecticidal polyether antibiotic nanchangmycin and the antiparasitic macrolide meilingmycin, contains multiple polyketide gene clusters. Microbiology 2002, 148, 361–371. [Google Scholar] [CrossRef]
- Engle, P. Plasmid transformation of Streptomyces tendae after heat attenuation of restriction. Appl. Environ. Microbiol. 1987, 53, 1–3. [Google Scholar] [CrossRef]
- Kakinuma, K.; Hanson, C.A.; Rinehart, K.L., Jr. Spectinabilin, a new nitro-containing metabolite isolated from Streptomyces Spectabilis. Tetrahedron 1976, 32, 217–222. [Google Scholar] [CrossRef]
- Otoguro, K.; Liu, Z.; Fukuda, K.; Li, Y.; Iwai, Y.; Tanaka, H.; Omura, S. Screening for new nematocidal substances of microbial origin by a new method using the pine wood nematode. J. Antibiot. 1987, 41, 573–575. [Google Scholar] [CrossRef]
- Traitcheva, N.; Jenke-Kodama, H.; He, J.; Dittmann, E.; Hertweck, C. Non-colinear polyketide biosynthesis in the aureothin and neoaureothin pathways: An evolutionary perspective. Chembiochem 2007, 8, 1841–1849. [Google Scholar] [CrossRef]
- Liu, M.; Hwang, B.; Jin, C.; Li, W.; Park, D.J.; Seo, S.T.; Kim, C.J. Screening, isolation and evaluation of a nematicidal compound from actinomycetes against the pine wood nematode, Bursaphelenchus xylophilus. Pest Manag. Sci. 2019, 75, 1585–1593. [Google Scholar] [CrossRef]
- Rajasekharan, S.K.; Lee, J. Hydropic anthelmintics against parasitic nematodes. PLoS Pathog. 2020, 16, e1008202. [Google Scholar] [CrossRef]
- Holden-Dye, L.; Walker, R.J. Anthelmintic drugs and nematicides: Studies in Caenorhabditis elegans. WormBook 2014, 16, 1–29. [Google Scholar] [CrossRef]
- Geary, T.G.; Thompson, D.P. Caenorhabditis elegans: How good a model for veterinary parasites? Vet. Parasitol. 2001, 101, 371–386. [Google Scholar] [CrossRef]
- Hahnel, S.R.; Dilks, C.M.; Heisler, I.; Andersen, E.C.; Kulke, D. Caenorhabditis elegans in anthelmintic research—Old model, new perspectives. Int. J. Parasitol. Drugs Drug Resist. 2020, 14, 237–248. [Google Scholar] [CrossRef]
- Sepulveda-Crespo, D.; Reguera, R.M.; Rojo-Vazquez, F.; Balana-Fouce, R.; Martinez-Valladares, M. Drug discovery technologies: Caenorhabditis elegans as a model for anthelmintic therapeutics. Med. Res. Rev. 2020, 40, 1715–1753. [Google Scholar] [CrossRef] [PubMed]
- Holden-Dye, L.; Crisford, A.; Welz, C.; von Samson-Himmelstjerna, G.; Walker, R.J.; O’connor, V. Worms take to the slo lane: A perspective on the mode of action of emodepside. Invertebr. Neurosci. 2012, 12, 29–36. [Google Scholar] [CrossRef] [PubMed]
- Lu, C.; Zhang, Z.Q.; Zhang, X.C.; Kang, Q.J. New spectinabilin and hexadienamide derivatives from Streptomyces sp. S012. Rec. Nat. Prod. 2020, 14, 312–318. [Google Scholar] [CrossRef]
- Sanadhya, P.; Bucki, P.; Liarzi, O.; Ezra, D.; Gamliel, A.; Braun Miyara, S. Caenorhabditis elegans susceptibility to Daldinia cf. concentrica bioactive volatiles is coupled with expression activation of the stress-response transcription factor daf-16, a part of distinct nematicidal action. PLoS ONE 2018, 13, e0196870. [Google Scholar]
- Yan, J.; Wang, X.; Tian, M.; Liu, C.; Zhang, K.; Li, G. Chemical constituents from the fungus Stereum sp. YMF1.04183. Phytochem. Lett. 2017, 22, 6–8. [Google Scholar] [CrossRef]
- Rupcic, Z.; Chepkirui, C.; Hernandez-Restrepo, M.; Crous, P.W.; Luangsa-Ard, J.J.; Stadler, M. New nematicidal and antimicrobial secondary metabolites from a new species in the new genus Pseudobambusicola thailandica. MycoKeys 2018, 33, 1–23. [Google Scholar] [CrossRef]
- Gupta, R.; Singh, A.; Ajayakumar, P.V.; Pandey, R. Microbial interference mitigates Meloidogyne incognita mediated oxidative stress and augments bacoside content in Bacopa monnieri L. Microbiol. Res. 2017, 199, 67–78. [Google Scholar] [CrossRef]
- Gomez-Escribano, J.P.; Bibb, M.J. Heterologous expression of natural product biosynthetic gene clusters in Streptomyces coelicolor: From genome mining to manipulation of biosynthetic pathways. J. Ind. Microbiol. Biotechnol. 2014, 41, 425–431. [Google Scholar] [CrossRef]
- Law, J.W.; Law, L.N.; Letchumanan, V.; Tan, L.T.; Wong, S.H.; Chan, K.G.; Ab Mutalib, N.S.; Lee, L.H. Anticancer drug discovery from microbial sources: The unique mangrove Streptomycetes. Molecules 2020, 25, 5365. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Li, S.; Tan, Z.; Xie, B.; Wu, C.; Yang, Y. Progress of study on endophytic actinomycetes in plant. Biotech. Bull. 2008, 1, 42–46. (In Chinese) [Google Scholar]
- Kaur, T.; Manhas, R.K. Antifungal, insecticidal, and plant growth promoting potential of Streptomyces hydrogenans DH16. J. Basic. Microbiol. 2014, 54, 1175–1185. [Google Scholar] [CrossRef] [PubMed]
- Ruanpanun, P.; Laatsch, H.; Tangchitsomkid, N.; Lumyong, S. Nematicidal activity of fervenulin isolated from a nematicidal actinomycete, Streptomyces sp. CMU-MH021, on Meloidogyne incognita. World J. Microbiol. Biotechnol. 2011, 27, 1373–1380. [Google Scholar] [CrossRef] [PubMed]
- Sharma, M.; Jasrotia, S.; Ohri, P.; Manhas, R.K. Nematicidal potential of Streptomyces antibioticus strain M7 against Meloidogyne incognita. AMB Express 2019, 9, 168. [Google Scholar] [CrossRef] [PubMed]
- Kang, M.K.; Kim, M.H.; Li, M.J.; Ji, C.Z.; Park, S.H.; Lee, J.M.; Kim, J.; Park, D.J.; Park, H.R.; Kim, Y.H.; et al. Nematicidal activity of teleocidin B4 isolated from Streptomyces sp. against pine wood nematode, Bursaphelenchus xylophilus. Pest Manag. Sci. 2021, 77, 1607–1615. [Google Scholar] [CrossRef]
- Zhang, Z.; Wu, X.; Song, R.; Zhang, J.; Wang, H.; Zhu, J.; Lu, C.; Shen, Y. Ansavaricins F–I, new DNA topoisomerase inhibitors produced by Streptomyces sp. S012. RSC Advances 2017, 7, 14857–14867. [Google Scholar] [CrossRef]
- Nair, M.G.; Chandra, A.; Thorogod, D.L.; Davis, R.M.G. Nematocidal and mosquitocidal aromatic nitro-compounds produced by Streptomyces spp. Pestic. Sci. 1995, 43, 361–365. [Google Scholar]
- Zhan, B.; Zeng, Q.; Wu, X.; Zhu, J.; Bao, S.; Huang, H. Identification of nematicidal actinomycetes DA09202 and active compounds. Microbiolo. China 2010, 37, 1283–1286. (In Chinese) [Google Scholar]
- Zheng, Y.; Pang, H.; Wang, J.; Chen, D.; Shi, G.; Huang, J. Novel diketopiperazine and ten-membered macrolides from the entomogenous fungus Paecilomyces tenuipes. Chem. J. Chin. U. 2014, 35, 1665–1669. (In Chinese) [Google Scholar]
- Isaka, M.; Jaturapat, A.; Kramyu, J.; Tanticharoen, M.; Thebtaranonth, Y. Potent in vitro antimalarial activity of metacycloprodigiosin isolated from Streptomyces spectabilis BCC 4785. Antimicrob. Agents Chemother. 2002, 46, 1112–1113. [Google Scholar] [CrossRef] [PubMed]
- Cully, D.F.; Vassilatis, D.K.; Liu, K.K.; Paress, P.S.; Van der Ploeg, L.H.T.; Schaeffer, J.M.; Arena, J.P. Cloning of an avermectin-sensitive glutamate-gated chloride channel from Caenorhabditis elegans. Nature 1994, 371, 707–711. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhang, W.; Wang, Y.; Liu, H.; Zhang, S.; Ji, X.; Qiao, K. Oxidative stress, intestinal damage, and cell apoptosis: Toxicity induced by fluopyram in Caenorhabditis elegans. Chemosphere 2022, 3, 131830. [Google Scholar] [CrossRef] [PubMed]
- Vang, L.E.; Opperman, C.H.; Schwarz, M.R.; Davis, E.L. Spirotetramat causes an arrest of nematode juvenile development. Nematology 2016, 18, 121–131. [Google Scholar] [CrossRef]
- Gunderson, E.; Bulman, C.; Luo, M.; Sakanari, J. In Vitro Screening Methods for Parasites: The WMicroTracker & the WormAssay. MicroPubl. Biol. 2020, 3, 7–9. [Google Scholar]
- Opperman, C.H.; Chang, S. Nematode acetylcholinesterases: Molecular forms and their potential role in nematode behavior. Parasitol. Today 1992, 8, 406. [Google Scholar] [CrossRef]
- Jorgensen, E.M.; Mango, S.E. The art and design of genetic screens: Caenorhabditis elegans. Nat. Rev. Genet. 2002, 3, 356–369. [Google Scholar] [CrossRef]
- Waaijers, S.; Boxem, M. Engineering the Caenorhabditis elegans genome with CRISPR/Cas9. Methods 2014, 68, 381–388. [Google Scholar] [CrossRef]
- Chen, Y.; Huang, H.; Liu, M.; Bao, S. Isolation and identification of an actinomycetes strain against rootknot nematode from mangrove soil. Biotech. Bull. 2014, 10, 174–178. (In Chinese) [Google Scholar]
- Nguyen, V.T.; Park, A.R.; Duraisamy, K.; Vo, D.D.; Kim, J.C. Elucidation of the nematicidal mode of action of grammicin on Caenorhabditis elegans. Pestic. Biochem. Physiol. 2022, 188, 105244. [Google Scholar] [CrossRef]
- Sloan, M.A.; Reaves, B.J.; Maclean, M.J.; Storey, B.E.; Wolstenholme, A.J. Expression of nicotinic acetylcholine receptor subunits from parasitic nematodes in Caenorhabditis elegans. Mol. Biochem. Parasitol. 2015, 204, 44–50. [Google Scholar] [CrossRef]
- Câmara, D.F.; Machado, M.L.; Arantes, L.P.; Silva, T.C.; Silveira, T.L.; Leal, J.G.; Dornelles, L.; Stefanello, S.T.; Soares, F.A. MPMT-OX up-regulates GABAergic transmission and protects against seizure-like behavior in Caenorhabditis elegans. Neurotoxicology 2019, 74, 272–281. [Google Scholar] [CrossRef] [PubMed]
- WormBase. Available online: https://wormbase.org/species/c_elegans/strain/WBStrain00004682#035--10 (accessed on 2 May 2023).
- Brenner, S. The genetics of Caenorhabditis elegans. Genetics 1974, 77, 71–94. [Google Scholar] [CrossRef]
- Sulston, J.; Hodgkin, J. The Nematode Caenorhabditis Elegans; Cold Spring Harbor Lab Press: New York, NY, USA, 1988. [Google Scholar]
- Singh, P.; Xie, J.; Qi, Y.; Qin, Q.; Jin, C.; Wang, B.; Fang, W. A thermotolerant marine Bacillus amyloliquefaciens S185 producing iturin A5 for antifungal activity against Fusarium oxysporum f. sp. cubense. Mar. Drugs 2021, 19, 516. [Google Scholar] [CrossRef]
- Zheng, W.; Li, D.; Zhao, J.; Liu, C.; Zhao, Y.; Xiang, W.; Wang, X. Promicromonospora soli sp. nov., a novel actinomycete isolated from soil. Int. J. Syst. Evol. Microbiol. 2017, 67, 3829–3833. [Google Scholar] [CrossRef] [PubMed]
- Tamura, K.; Stecher, G.; Kumar, S. MEGA11: Molecular Evolutionary Genetics Analysis Version 11. Mol. Biol. Evol. 2021, 38, 3022–3027. [Google Scholar] [CrossRef]
- Shanley, H.T.; Taki, A.C.; Byrne, J.J.; Jabbar, A.; Wells, T.N.C.; Samby, K.; Boag, P.R.; Nguyen, N.; Sleebs, B.E.; Gasser, R.B. A high-throughput phenotypic screen of the ‘Pandemic Response Box’ identifies a quinoline derivative with significant anthelmintic activity. Pharmaceuticals 2022, 15, 257. [Google Scholar] [CrossRef] [PubMed]


| Concentration (μg/mL) | Mortality (%) |
|---|---|
| 2.5 | 8.92 ± 4.45 |
| 3 | 38.56 ± 7.42 |
| 4 | 69.45 ± 7.70 |
| 5 | 95.02 ± 2.78 |
| 10 | 100 |
| Concentration (μg/mL) | Mortality (%) |
|---|---|
| 6.25 | 0 |
| 12.5 | 12.31 ± 1.72 |
| 25 | 19.20 ± 3.73 |
| 50 | 22.97 ± 2.67 |
| 100 | 40.81 ± 4.44 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, Y.; Xie, J.; Tang, L.; Odiba, A.S.; Chen, Y.; Fang, W.; Wu, X.; Wang, B. Isolation, Identification and Molecular Mechanism Analysis of the Nematicidal Compound Spectinabilin from Newly Isolated Streptomyces sp. DT10. Molecules 2023, 28, 4365. https://doi.org/10.3390/molecules28114365
Sun Y, Xie J, Tang L, Odiba AS, Chen Y, Fang W, Wu X, Wang B. Isolation, Identification and Molecular Mechanism Analysis of the Nematicidal Compound Spectinabilin from Newly Isolated Streptomyces sp. DT10. Molecules. 2023; 28(11):4365. https://doi.org/10.3390/molecules28114365
Chicago/Turabian StyleSun, Yuchen, Jin Xie, Lihua Tang, Arome Solomon Odiba, Yanlu Chen, Wenxia Fang, Xiaogang Wu, and Bin Wang. 2023. "Isolation, Identification and Molecular Mechanism Analysis of the Nematicidal Compound Spectinabilin from Newly Isolated Streptomyces sp. DT10" Molecules 28, no. 11: 4365. https://doi.org/10.3390/molecules28114365
APA StyleSun, Y., Xie, J., Tang, L., Odiba, A. S., Chen, Y., Fang, W., Wu, X., & Wang, B. (2023). Isolation, Identification and Molecular Mechanism Analysis of the Nematicidal Compound Spectinabilin from Newly Isolated Streptomyces sp. DT10. Molecules, 28(11), 4365. https://doi.org/10.3390/molecules28114365






