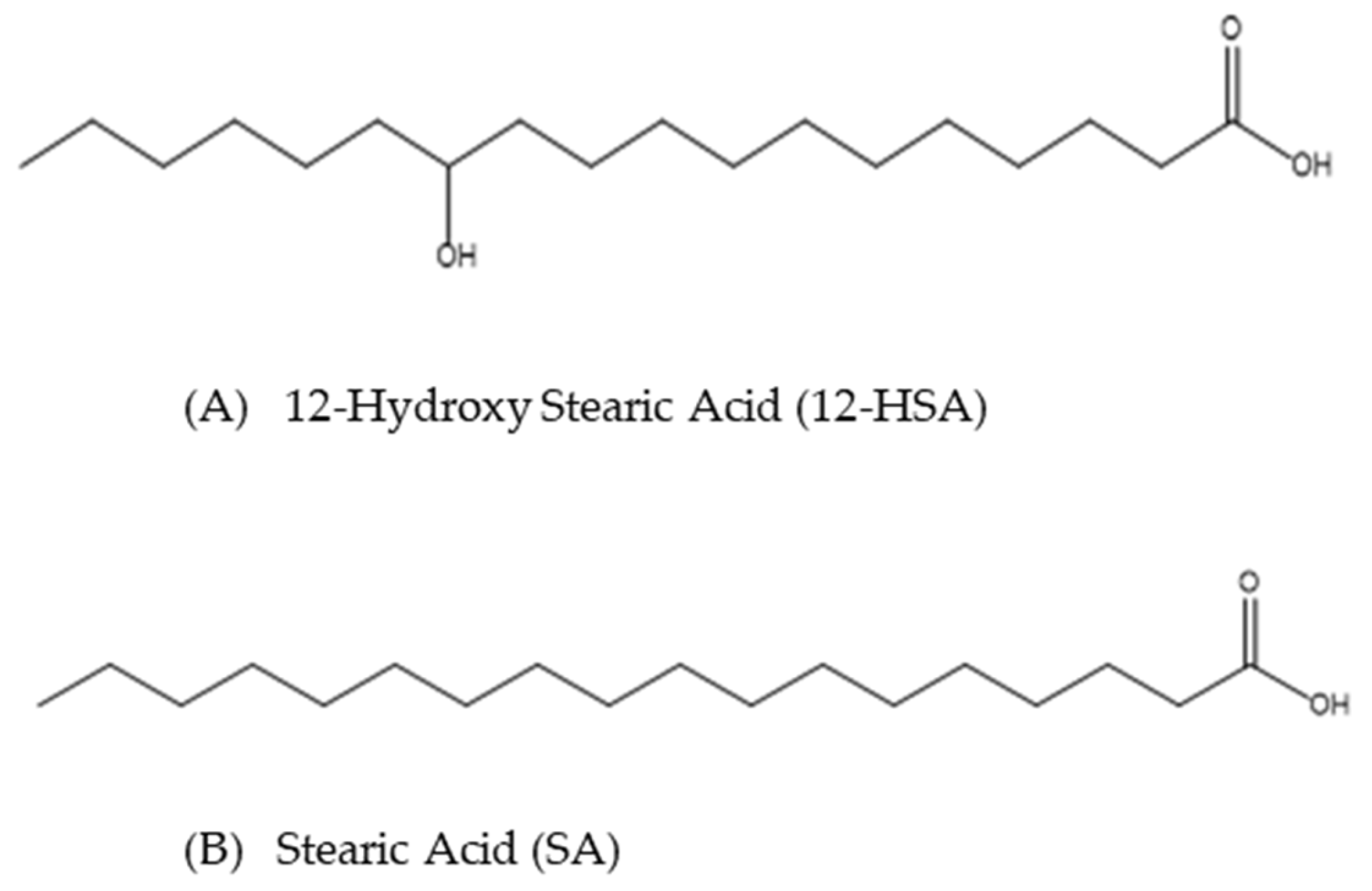
2.2. Confocal Microscopy
Confocal microscopy of samples has been performed on the whole range of ratio R using a hydrophobic dye, Nile Red. The Nile Red concentration is negligible with respect to the fatty acid concentration (see
Section 4), so that we assume that the morphology of the self-assembled structures is not modified by its introduction.
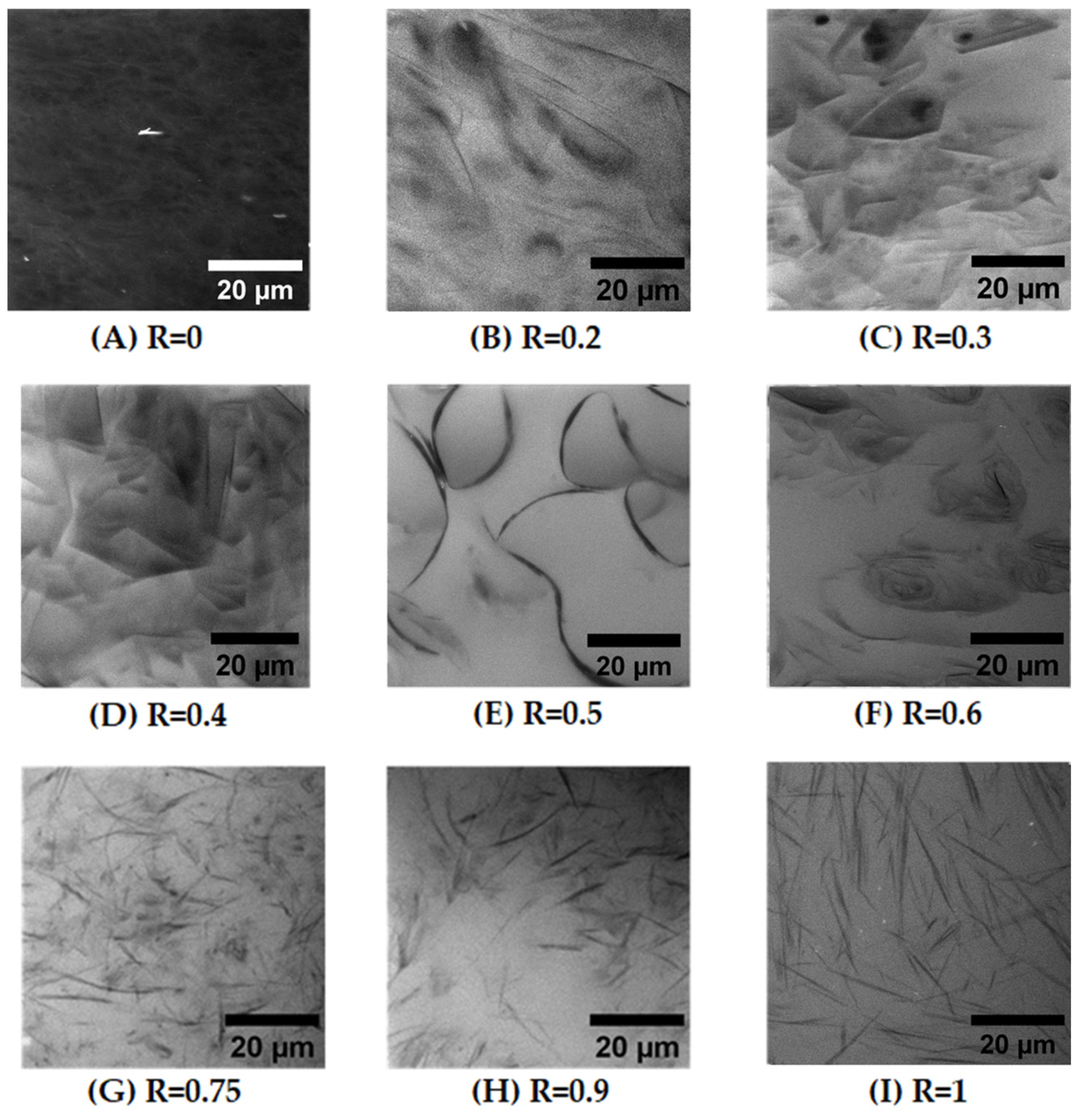
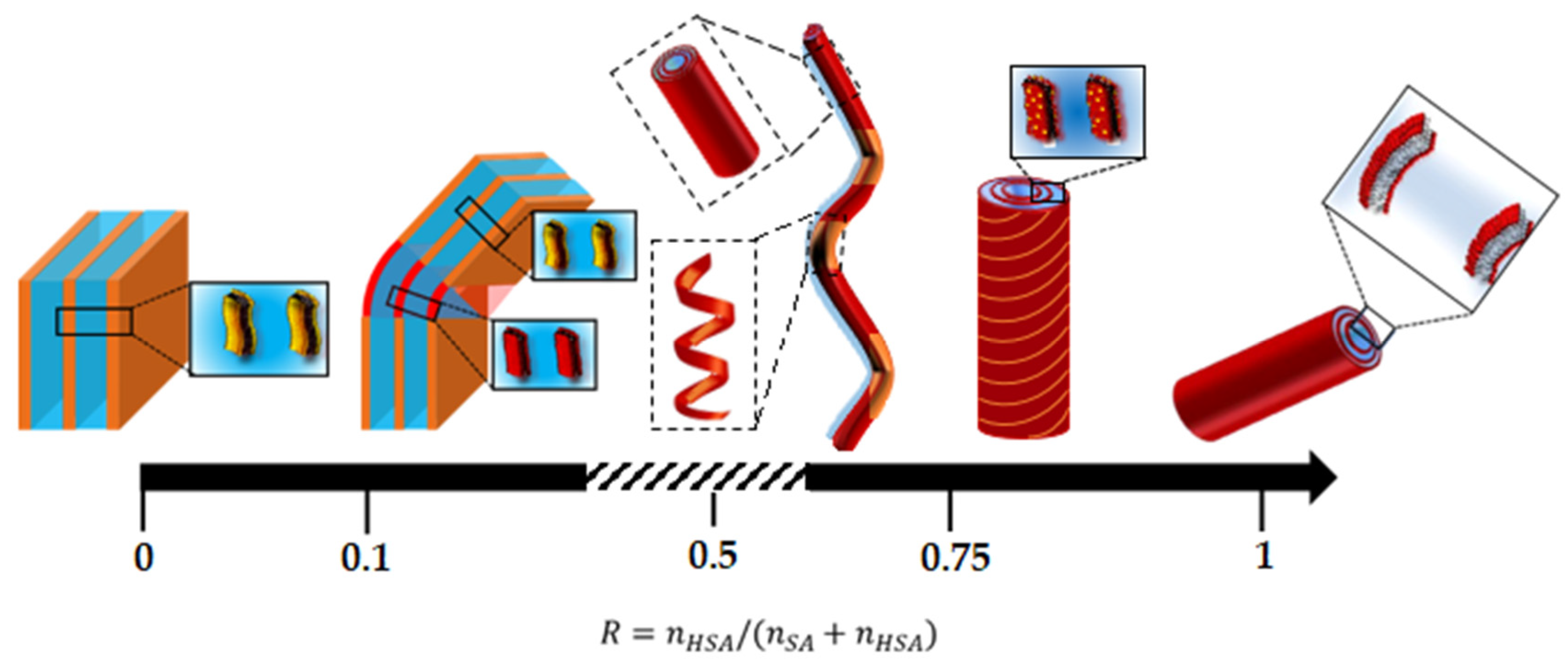
Figure 3 shows some representative images of samples for all R ratios examined using ×100 magnification. On the images, Nile Red is concentrated in the black areas that do correspond to the hydrophobic regions of the self-assembled structures made of the alkyl chains of the fatty acids, whereas the white domains do not contain the fluorophores.
Figure 3I shows the image obtained for R = 1 (pure 12-has), which reveals the presence of disordered thin and rigid rods with a length of around 20 μm. These rods correspond to the multilamellar tubes resulting from the self-assembly of 12-HSA molecules [
39]. The organization of these tubes appears disordered, without the formation of any nematic order. When R decreases from 1 to 0.75 upon the introduction of a small amount of SA (
Figure 3H,G), and the self-assembled structures conserve their rodlike shapes. It appears, however, that the overall length of the rods decreases progressively, with a distribution of lengths that become more polydisperse when R decreases. We did not perform a statistical study of these variations in tube length in this study of large R ratios because the images are transversal cross-sections of a 3D sample.
At R = 0.75, it also appears that a very small number of curved objects coexist with the long tubes. This trend is confirmed when decreasing R in the regime of intermediate R (R = 0.6, R = 0.5). Indeed, for R = 0.6 (
Figure 3F), the rigid thin polydisperse rods now coexist with an important number of objects with a large curvature. For R = 0.5, during stoichiometry, fatty acids self-assemble into long, bent tubes that are able to reach a much longer length that the 20 μm obtained at R = 1 and extend to up to 60 μm (
Figure 3E). These bent tubes appear to be made up of two types of domains, with rigid parts bound to each other by curved parts. In this regime of intermediate R, the structure of fatty acid self-assemblies thus evolves continuously from bent tubes to straight ones through R. In their seminal study, where they introduced a significant amount of SA into aqueous solutions of 12-HSA, Fameau et al. [
11] observed, through phase contrast microscopy, the formation of twisted ribbons that are the precursors of multilamellar tubes. Confocal microscopy does not allow the discrimination between multilamellar tubes and ribbons; however, we hypothesize that the rigid parts correspond to the tubes and the bent ones to ribbons.
At low R, where SA is in excess, the structure is completely different and images are less simple to interpret (see
Figure 3B–D, which corresponds, respectively, to R = 0.4, R = 0.3 and R = 0.2). There are no longer thin black lines in which all the hydrophobic Nile Red molecules concentrate, as was the case for higher R, but instead, 2D domains of variable levels of grey are extant. Most of these domains have straight sides, suggesting the formation of faceted objects, connected to each other by either edges or by curved parts. Some other domains display a more spherical shape. These domains are all made of multilamellar bilayers, as demonstrated by SANS, as we will show in a later section. Thus, they have different distributions of thicknesses and orientations, which give rise to this variation in the grey level from one domain to another. The fact that the majority of their sides are either straight or circular suggests that they are made of planar lamellar phases connected by spherical lamellar phases, which are possibly parts of multilamellar tubes. The images also suggest that these domains are strongly entangled. Indeed, when following the temporal evolution of the system during the experiment, it appeared that these domains do not move individually but move collectively. This collective motion of domains is consistent with the fact that they are either connected to each other and/or strongly entangled.
Finally, for R = 0 (pure SA), there are only small fluctuations in the color of the images, with a typical size of fluctuations of the order of a few microns, although SANS experiments shown later will unambiguously demonstrate that this sample contains planar lamellae. Since all lamellar phase domains contain the same level of fluorophores on average, the only variations of the grey level within the images come from the orientation of the 2D lamellar domains. The image is thus consistent with a structural organization of the planar lamellar phase domains with random orientations that occupy all space, in agreement with the literature [
15].
2.3. Cryogenic Transmission Electron Microscopy (Cryo-TEM)
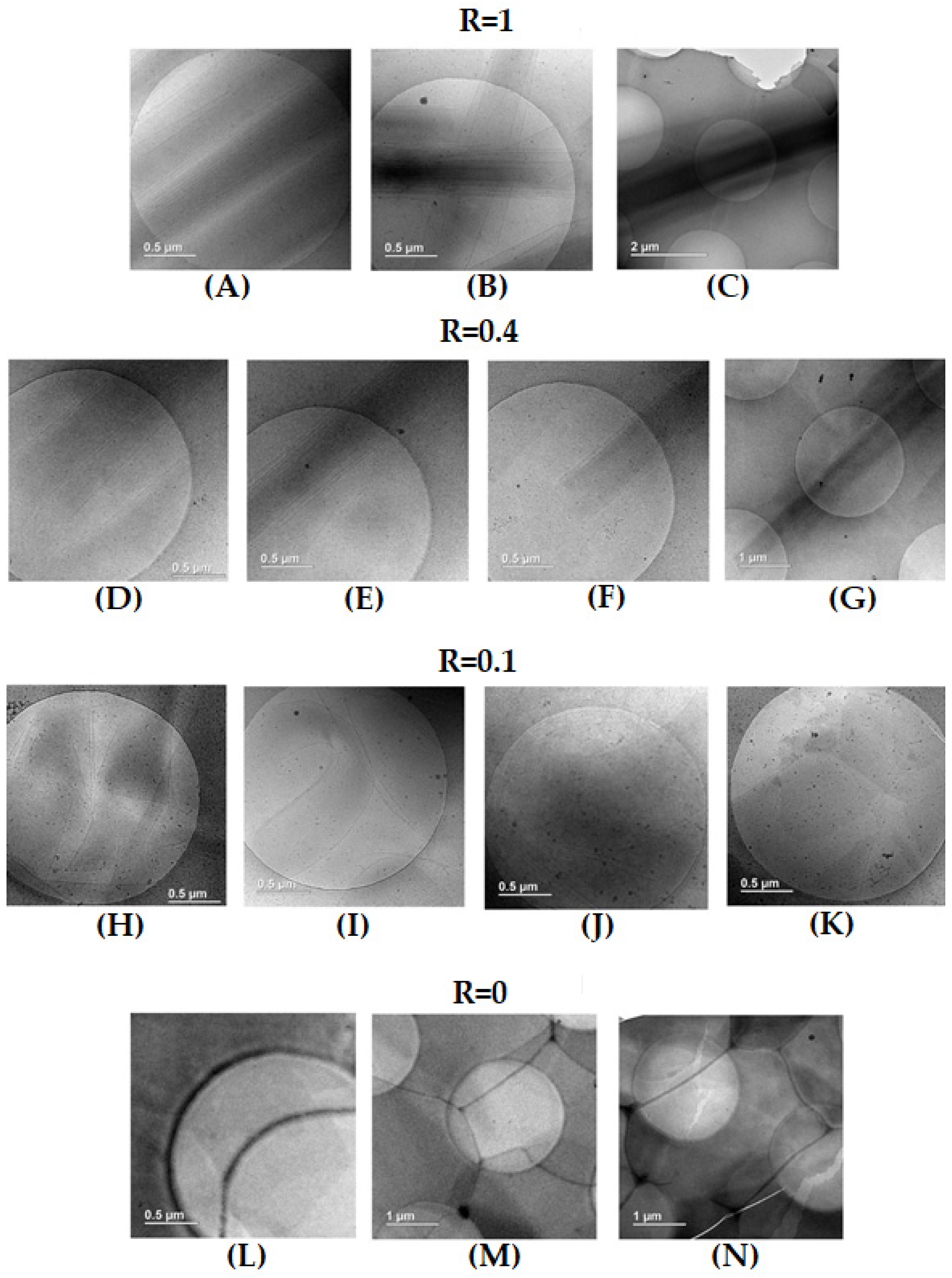
The pictures of samples were obtained through cryogenic transmission electron microscopy for pure 12-HSA (R = 1), pure SA (R = 0), the intermediate content of both kinds of molecules (R = 0.4) and the low content of SA (R = 0.1). Samples were left at room temperature (around 20 °C) for 24 h to equilibrate before the freezing step. The final thickness of the frozen cut sample is around one micrometer, which is much lower than the length of the tubes of the order of 20 μm, observed by confocal microscopy. This forces them to lie on the grid’s surface.
For R = 1, long straight rectangular objects are visible with a width of ~500 nm and a length that is at least 8 μm, as they exceed the size that corresponds to the image section at the lowest magnification utilized, in agreement with the length of 20 μm determined via confocal microscopy (
Figure 4A–C). In all of these rectangles, there is a continuous gradient within the grey scale from the outer of the edges of the rectangle, which are lighter, to the inner areas of the rectangle, which are darker, demonstrating a continuous inward variation in their thickness from the exterior to the interior. Even though the images are 2D, it is demonstrated that these rectangular shapes correspond to cylindrical tubes. There are some parallel dark lines within the tubes, which are parallel to their edges, which is caused by the presence of lamellas. Although SANS experiments demonstrate that the inter-lamellar distance is constant (see later), such a distance between lamellas does not appear to be strictly constant in the images, but this is an effect of projection from the 3D self-assemblies to the 2D images. Moreover, the dark centers of the rectangles tubes do not display any lamella. This is also consistent with the formation of multilamellar tubes, as such a dark center corresponds to the top of the adsorbed cylinders, where lamella are parallel to the surface. Thus, the exact estimate of the number of lamella is not possible from these cryo-TEM images, yet they reveal that there are at least five or six lamella per tube. Since the tubes lie on the surface, there are no images of the cross-sections of the tubes, but they would resemble onions. Similar pictures have been obtained via cryo-TEM on the multilamellar tubes of SDS@2β-CD [
42]. In summary, these cryo-TEM images are fully consistent with the structure proposed in the literature for 12-HSA self-assemblies as long rigid multilamellar tubes. They also show that the radius of these tubes are rather monodispersed, since their widths are fairly constant from one tube to another. However, a more interesting feature concerns their ends, which are open and not capped, as it is unambiguously visible in some pictures (
Figure 4B). This had not previously been established in the literature.
For pure SA (R = 0), while turbidity and SANS (see later) unambiguously demonstrate that large self-assembled lamellar aggregates do exist in the solution, they are not visible at first sight on the grid. There are areas of various size, which are more or less dark, separated by straight edges that presumably correspond to steps between domains of different thicknesses (
Figure 4L,M). Such areas derive from the covering of the surface by surfactants molecules, as proven by the presence of cracks, which are depleted in molecules, since their light grey level corresponds to the pure grid, as shown in
Figure 4N. It is likely that planar lamellar phases with a variable number of lamellae, but probably a low number, are deposited on the surface and lay parallel to it. The images thus depict a top-down view of such lamella and the steps correspond to a change in lamella number from one domain to another. The darker the area, the larger the number of lamella. Similar pictures have also been obtained via cryo-TEM on the Lα lamella phase on fatty acids with an excess of alkali [
15].
For R = 0.4, there are also long rigid multilamellar tubes with open ends, as for the R = 1 case with the same order of magnitude of the radius (
Figure 4D–F) and a length that still exceeds 10 microns (
Figure 4G). However, compared to the R = 1 case, the number of lamella seems to be slightly reduced on average and varies more significantly from one tube to another. It also appears that parts of the tubes are slightly curved. This is in line with what was observed via confocal microscopy at R = 0.5 with long 1D objects with rigid and soft domains. This suggests that the parts of the objects analyzed via cryo-TEM are twisted ribbons and not multilamellar tubes, although the cryo-TEM images do not allow the arbitration between both structures as they provide the same image due to projection. The curved parts would result from the ribbons with the lowest rigidity.
For R = 0.1, there are no longer multilamellar tubes, but the images still reveal the existence of lamellar structures with different morphologies and a varying number of lamella. The most striking feature concerning the curvature of these lamella is that they appear either straight, which may account for planar lamella or pieces of large tubes, or spherical, suggesting truncated onion vesicles (
Figure 4H,J). Such straight and spherical lamella eventually merge to form a single multilamellar objects (
Figure 4I). Regarding the number of lamella, it ranges from a single lamella up to multiple with the same order of magnitudes as the tubes at R = 1. Additionally, images depict the dark areas and the steps, revealing the presence of planar lamellas sitting on the surface, as seen in the R = 0 case (
Figure 4K). The samples are thus composed of different forms of self-assemblies that partially fuse with different curvatures. It is thus likely that these different kinds of self-assembled aggregates do not all contain the same amounts of SA and 12-HSA molecules, i.e., a partitioning between the two surfactants exists at the local scale.
2.4. Structure at Local Scale via SANS
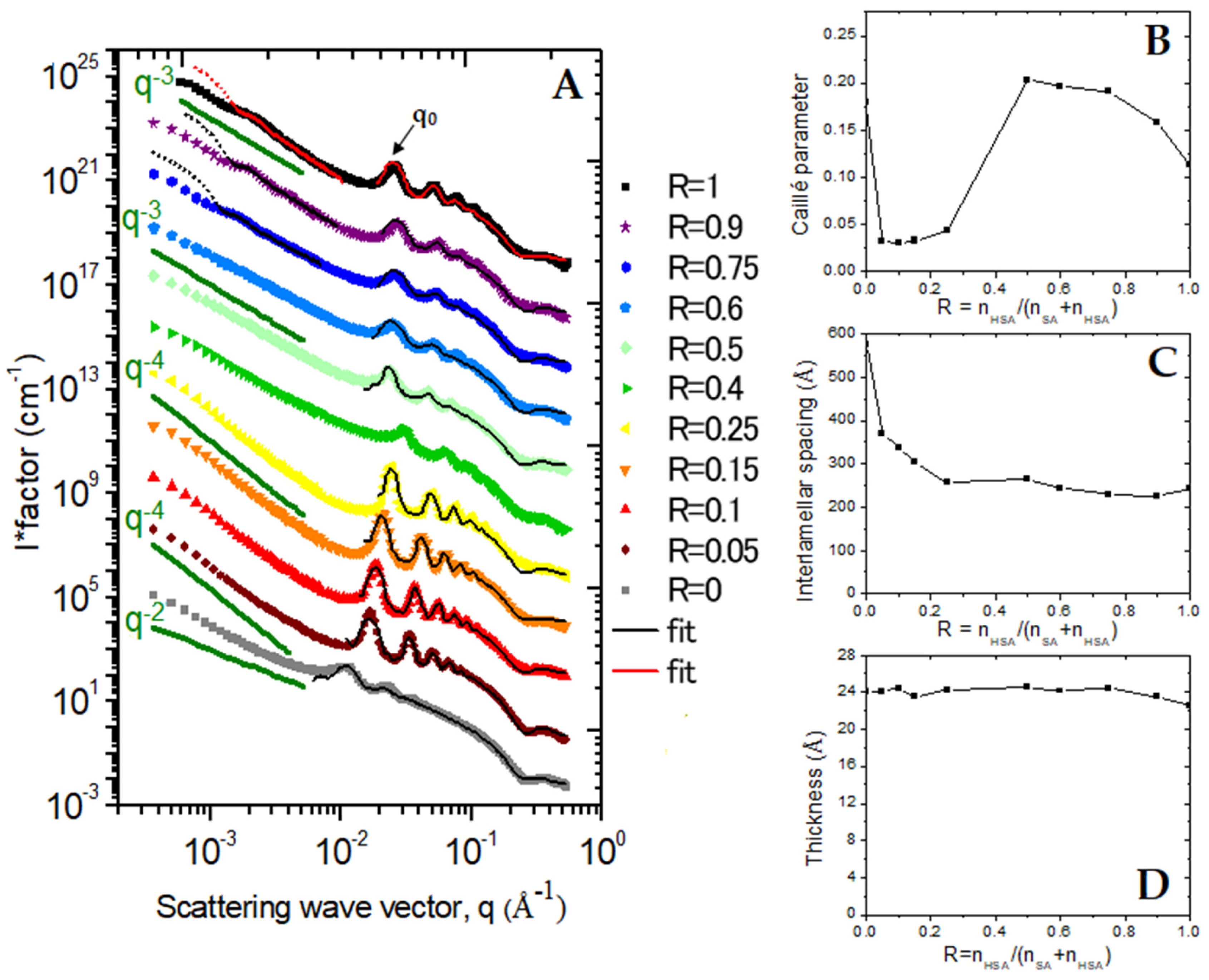
The structure of self-assembled surfactants was determined via SANS for the whole range of R (
Figure 5A). SANS examines a range of distances from ca. 10 Å to 1 µm in the solution, with no artifacts from deposition on a 2D surface and no addition of an extra probe. It does require to work in heavy water D
2O to create a contrast with the hydrogen-rich molecules and decrease the large incoherent scattering of
1H, which acts as a constant background for SANS. Data are presented as intensity versus q, the magnitude of the wavevector, which is expressed at a reciprocal length scale. In an initial approximation, intensity features at a given q-value can be associated with the characteristic dimensions
d based on the Bragg relationship
d~2π/q.
The SANS scattering spectra of the pure SA sample (R = 0) display all the characteristic features of a 2D lamellar phase: (
i) a q
−2 scattering decay in the low q region, typical of the scattering of a 2D object and accounts for the planar shape of lamella; (
ii) a strong correlation peak at q
0 = 0.0109 Å
−1, followed by its harmonics at n·q
0 (
n = 2, 3, 4…), which accounts for the Bragg peak associated with the interlamellar distance
dinter, whose order of magnitude is ~575 Å (2π/q
0); and (
iii) a so-called form factor oscillation at around 0.25 Å
−1, originating from the cross-section of the lamellae and enabling the determination of its thickness at ~25 Å (2π/0.25). Such scattering spectra unambiguously demonstrate that the sample is made of a lamellar phase, in agreement with previous results [
14], which deduced the formation of a lamellar phase through birefringence measurements, even though they were not visible on the cryo-TEM pictures.
The pure 12-HSA system (R = 1) also displays the characteristic features of lamellar phases at the intermediate and large q, (i.e., a correlation peak followed by its harmonics, oscillation at large q). It was immediately obvious, however, that interlamellar thickness is strongly reduced compared to that in the pure SA case and interactions between lamella are different from those in the pure SA case, since q
0_R = 1 is strongly shifted towards low q with respect to q
0_R = 0 at ~0.025 Å
−1 (
dinter = 240 Å). The correlation peak is narrower, its harmonics are visible up to the sixth order, indicating a more regular periodicity, i.e., bilayers fluctuate less in the R = 1 case and/or comprise more stacked bilayers in the tube wall than those forming the SA lamellar phase. The thickness of the lamella is similar to that of the pure SA case, since the form factor oscillation is also at 0.25 Å
−1. The main difference to the SA case appears in the low q region, where the overall scattering decays as q
−3 and no longer as q
−2 and shows a well-defined marked oscillation at 1.2 10
−3 Å
−1, resulting from the form factor of the tube diameter. The highlighting of such an oscillation was made possible by the extended low q-range of the D11 diffractometer and was not yet reported on the scattering curves of the same system in the literature as they were all limited to a reduced q-range. The overall q
−3 decay has, however, already been obtained on the scattering of hollow tubes and multilamellar tubes on other systems [
42,
43,
45,
52]. Assuming that tubes are rigid, such q
−3 can be explained by a decoupling approximation from a tube in its length (∝ 1/q) and by its 2D discotic section (∝ 1/q
2). In the case of an imogolite hollow tube, Paineau et al. [
52], proposed fitting the scattering form factor through the equation
, where Δ
ρ is the difference in scattering density between the tubes and the solvent; R
ext and R
int are, respectively, the external and internal diameters of the hollow tubes; and F is the Fourier transform of the projection or a full cylinder of radius R along its axis
, with J
1 being the first-order cylindrical Bessel function. The extension to orientationally averaged multilamellar tubes was proposed in Reference [
42] for the fitting of the scattering of multilamellar tubes made of SDS@2β-CD complexes. It is difficult to fit by such a model here because the number of lamella is unknown. We therefore fitted the low q part of the scattering spectra with a model of hollow tube with a single bilayer of fatty acids, which allows the fitting of the minima and the recovery of the q
−3 decay (
Figure 5A). The tube radius R
tube was estimated at 200 nm. Such a model, however, overestimates the intensity in the very low q part of the spectra with respect to the experimental data. This is due to the high scattering intensity in this very low q region at ~10
6–10
7 cm
−1, resulting in multiple scattering events that, in practice, lower the experimental measured pattern from its true value.
For the other R ratio examined, the SANS scattering demonstrate the formation of lamellar phases in every case, yet several systems can be clearly evidenced.
In the instance of the low content of SA molecules, in which rigid tubes were observed through confocal microscopy (1 > R ≥ 0.75), the scattering spectra are very similar to those of the pure 12-HSA system at R = 1. The oscillation arising from the form factor of the tube radius and an overall q−3 scattering decay at low q, as well as the correlation peak of the interlamellar phase and its harmonics, are present at the same positions and their amplitudes and widths display the same orders of magnitude. The mixture of molecules thus self-associated into multilamellar tubes have a very similar structures, as seen in the pure 12-HSA system, revealing that these tubes are able to accommodate a significant number of SA molecules. These SA molecules insert themselves into the 12-HSA self-assemblies without disturbing the structures of the tubes. The tube radius increases slightly with respect to the R = 1 case, with 210 nm for R = 0.9 and 250 nm for R = 0.75.
At intermediate R, for R = 0.6 and R = 0.5, all of the scattering features of multilamellar tubes are recovered, except for the low q oscillation of the tube form factor that is no longer present, the scattering decays similarly to q
−3 over the whole low q-range examined. It this thus likely that the distribution of the outer diameters of the tubes becomes very broad, which leads to the disappearance of the oscillation. This likely results from the fact that the self-assemblies are made of both multilamellar tubes and twisted ribbons, and the respective rigid and soft domains of the tubes were observed through confocal and cryo-TEM microscopies, in agreement with Reference [
11]. Even if the interlamellar distance is well defined, it is also possible that both rigid and curved domains do not contain the same number of lamella, as this depends on the degree to which the lamella rolled into the ribbons.
At R = 0.4, the behavior is different. Although the scattering features are similar to those of the intermediate R case, the correlation peak and harmonics are strongly attenuated and shifted towards a larger q, which demonstrates that interactions between the lamella within tubes are largely different for such R. However, it should be kept in mind that sample phases undergo a partial phase separation at the macroscopic scale for R and that the two phases are present in the measurement cuvette, since it is filled by an homogenous solution at 70 °C that has been cooled down. Given that the neutron beam illuminates both phases (with a beam cross-section of around 1 cm−2), it is difficult to go deeper into SANS analysis in such a sample.
For R ≤ 0.25, with a large content in the SA molecules, the scattering curves markedly differ from the other cases. First, the correlation peak and its harmonics are much narrower than in the other cases, demonstrating either stronger repulsions between lamella, thus leading to a more regular periodicity, or a larger number of lamella per stack. Second, the scattering decays similarly to q−4 in the low q regime and no longer similarly to q−3, even if cryo-TEM images show that there are still tubes in sample. This is the sample where confocal microscopy shows faceted objects and cryo-TEM straight rigid lamella and spherically curved lamella joined to each other. Neutrons thus interact with the surfaces of very different orientations within the typical scale of the coherence length of neutrons (a few micrometers), which enables the recovery of the q−4 Porod’s law. We postulate that in this regime, where SA molecules are in excess, there is a partitioning between the two types of surfactants within the self-assemblies with 2D lamellar phases of SA that are capped by partial pieces of vesicles (unilamellar or multilamellar), made up of 12-HSA molecules.
These first qualitative descriptions pointed out that there is a significant evolution of the broadening of correlation peaks as a function of R. This may occur either because bilayers fluctuate with different amplitudes or because the number of stacked bilayers in the tube walls/lamellar phase changes. In order to obtain a refined quantitative description, all scattering curves in the intermediate and large q-region were fitted using a model proposed by Nallet et al. [
53], which considers a form factor of the lamella and a structure factor between lamella; both the number of stacked bilayers N
lam and the Caillé parameter (η) were adjusted, accounting for the thermal fluctuations of the bilayers. Such a model allows us to determine the structural parameters of the lamella (thickness, d-spacing
dinter, rigidity from the Caillé parameter). η is essentially determined from the region of the spectrum where the Bragg peaks arise at intermediate q and the thickness of the lamella from the oscillation at large q, which enables us to decouple these parameters fairly confidently in the fitting process. It is, however, difficult to unambiguously decouple N
lam from the Caillé parameter since both parameters play on the Bragg peak amplitudes. We thus varied both parameters when minimizing χ
2 during the fitting procedure, with a range of N
lam varying from 3 to 8. This maximal tested N
lam was chosen from geometrical constraints, given that N
lam multiplied by the interlamellar spacing
dinter cannot exceed the tube radius. The values of N
lam, corresponding to the best fits, are shown in
Table S1. For samples with a large R, N
lam has a value of 3 or 4, in agreement with cryo-TEM images and previous data reported in the literature of four stacked bilayers for a pure 12-HSA system [
37] and reaches a maximal value of 7 at low R.
This model is able to satisfactorily fit all the data of which multilamellar objects were formed at 20 °C for all R ratios (see
Figure 5A). The low q part of the curves was not fitted as it displays different behaviors from one sample to another (q
−2 versus q
−3 versus q
−4), which is derived from either planar objects, tubes or ribbons or faceted objects. These different behaviors at low q are highlighted in
Figure S1 in the Supplementary Materials, which show the experimental data with a I(q)q
α versus q representation, with α = 2, α = 3 and α = 4, respectively). In the case of tubes that have a monodisperse radius, the radius was obtained from the fit of the low q part via a model of a hollow tube, as explained preivously. The quantitative values obtained for the fits of
dinter, η and the bilayer thickness as a function of R are shown in
Table S1 in the Supplementary Materials.
The bilayer thickness has an almost constant value of ~24 Å in all cases—the slight differences from one sample to another are within the fit precision range—which is slightly larger than the chain lengths of the fatty acids (21 Å) [
38], which are similar regardless of whether 12-HSA or SA molecules are present. Such a low value of bilayer thickness demonstrates that fatty acid chains are strongly interdigitated.
dinter is much larger for pure SA (dinter = 575 Å at R = 0) than for pure 12-HSA (dinter = 240 Å at R = 1) and varies almost continuously with R between these two values with two clear-cut regimes: (i) at a large R (>0.5), in the system where multilamellar tubes are formed, this interlamellar distance varies only very slightly from one R to another, (ii) while at a lower R, it decreases continuously with R as a linear decrease in the low R system where partitioning between two types of fatty acids occurs (0.1 ≤ R ≤ 0.25). In this last low R system, one may hypothesize that this experimentally obtained dinter is simply effective interlamellar spacing, resulting from the linear combination of the pure planar lamellar spacing of large dinter and the pieces of multilamellar tubes of low dinter. Such a coexistence of zones of very different dinter is, however, very unlikely because it would have led to the broadening of the peak, and the reverse is observed, up to the appearance of two separate correlation peaks. Moreover, the objects depicted in cryo-TEM and confocal microscopy appear continuously. Finally, dinter is much smaller than for the other samples at R = 0.4, but there are uncertainties regarding the measurement of such a sample, as noted previously.
The evolution of η is shown in
Figure 5B. The bilayers are as rigid as the Caillé parameter is low. For such a parameter, we refrain from discussing slight differences from one sample to another too deeply, since N
lam and η are coupled parameters during fitting, which gives an intrinsic uncertainty regarding the quantitative fitted values of η. To assess whether such quantitative values were strongly influenced by N
lam, we also fitted all scattering curves of
Figure 5B with a fixed number of N
lam that was equal to 4. It appears that the values of η obtained in this latter case are always very close to those obtained when N
lam is a floating parameter during fitting (see
Table S1). The large variations of η observed in
Figure 5B are thus representative of variations in the rigidity of layers. It appears that the partial doping of the 12-HSA multilamellar tubes through SA induces a progressive decrease in the rigidity of the bilayer since η progressively increases from 0.11 at R = 1 to η = 0.18 at R = 0.75 when progressively introducing small amounts of 12-HSA molecules. A constant value of ~0.18 at the intermediate R is thus retained, which is also the rigidity obtained for pure SA lamellar phases at R = 0. Surprisingly, in the low R system (0.1 ≤ R ≤ 0.25), the bilayers are much more rigid, since the value of the Caillé parameter is around five times smaller, with an almost constant value of 0.03.
2.5. Wide Angle X-ray Scattering
WAXS measurements were performed to determine whether the fatty acids chains within the bilayers were fluid or in a gelled state for the whole range of 12-HSA/SA mixtures (
Figure 6), as well as for a reference sample of pure 12-HSA molecules at
r = 0.5. Indeed, the value of the bilayer thickness of 24 Å that we obtained through SANS is exactly similar to that obtained in References [
3,
39] in the case of pure 12-HSA at the
r = 0.2, which we consider here, i.e., with an excess of counterions. Such a state does not correspond to the L
β gel case, where the bilayer had a much larger value of around 42 Å, exactly twice the fatty acid length, as obtained when ethanolamine and 12-HSA had equimolarity for
r = 0.5. [
38] Moreover, when counterions are in excess, it has also been shown through coupled DSC and structural measurements that the multilamellar tube/micelle transitions matched the fluid/gel transition of the chains in the case of 12-HAS [
3,
39]. Thus, we hypothesize that fatty chains are present in a crystalline gel state in pure 12-HSA system at every
r, but with very different crystalline structures, regardless of whether ethanolamine and 12-HSA have equimolarity or an excess of ethanolamine, with a strong interdigitation of the fatty acid chains within the gelled bilayer in this latter case. All spectra show some Bragg diffraction peaks at the large q, demonstrating that the fatty acids are in a crystalline gel state in all cases; the main correlation peak of the lamellar phase and its harmonics, as already evidenced using SANS, are also visible at low q. For the pure suspensions of 12-HSA molecules, the crystalline structure of the bilayer at
r = 0.2 (R = 0) is strikingly different from the likely L
β gel phase reference at
r = 0.5, confirming our hypothesis that the 12-HSA/ethanolamine ratio tunes the crystalline structure. The
r = 0.2 solution indeed displays an intense peak at 1.495 Å
−1 and a less marked one at 1.58 Å
−1 within the q-window we examined, while the r = 0.5 shows an intense peak at 1.391 Å
−1 and two others of lower intensity at 1.572 Å
−1 and 1.597 Å
−1. The pure SA system (R = 0) has another crystalline structure with a single rather broad Bragg peak at 1.53 Å
−1.
The mixtures show four distinct behaviors. With the large excess of 12-HSA molecules (R ≥ 0.75), the suspension displays the same Bragg peaks as seen in in the R = 1 case. Hence, the introduction of a limited content of doping SA molecules does not prevent the system from crystallizing within the structure of the recovered pure 12-HSA solution. The amplitude of the peaks, however, decreases with an increase in SA content, suggesting that the size of the crystalline domains is reduced. Symmetrically, with a large excess of SA molecules (R = 0.05), the influence of the introduction of a small amount of 12-HSA doping molecules is minute since the structure is similar to that of pure 12-HSA. With an excess of SA fatty acids (0.1 ≤ R ≤ 0.5), the main crystalline structure recalls that of the pure SA sample: the Bragg peak of the SA remains and those of the 12-HSA crystalline structure at r = 0.2 are lacking. The intensity of such an SA Bragg peak decreases with an increase in R, showing the progressive decrease in the size of the crystalline phase in the lamellar domains of SA molecules. At the same time, a peak of low intensity appears at ~1.39 Å−1 whose amplitude increases concomitantly with the decrease in the main broad peak of SA with increasing R. At R = 0.5, both peaks have almost the same intensity. The origin of this new Bragg peak is likely the formation of crystal domains that contain the 12-HSA molecules and coexist with domains of pure SA molecules. Such a local phase separation between small domains that would have variable 12-HSA/SA ratios is consistent with the confocal microscopy and cryo-TEM experiments. The structure of the new crystalline phase that contains the 12-HSA molecules is difficult to assess, but it likely contains some SA molecules embedded in the 12-HSA molecules since some of the Bragg peaks obtained for the pure solutions of 12-HSA are not present on the diffractogram, whether for r = 0.2 or r = 0.5. Finally, at R = 0.6, the spectra can be viewed as a linear combination of the spectra of both pure systems of 12-HSA at r = 0.2 and SA, because it shows nicely all the Bragg peaks associated with their respective crystalline structures, while the Bragg peak of low intensity arising at lower R at 1.39 Å−1 is not any longer present. Moreover, the intensities of the main peaks of both structures are similar. This suggests the coexistence of similarly sized local domains of either pure 12-HSA or pure SA.
In summary, the crystalline structure of the gelled bilayers shifts continuously from that of the pure SA molecules to that of the pure 12-HSA molecules with the progressive introduction of 12-HSA molecules and a partial demixion between two types of molecule at intermediate R.
2.6. Rheology
Rheology was used to correlate the variety of structures observed as a function of R in terms of their mechanical properties. Shear–stress–amplitude sweep measurements were carried out at a frequency of 1 Hz for each ratio R from 0 to 1. All results are reported in
Figure S2. Those measurements allow for the determination of the storage G′ and the loss G″ modulus of the samples, which, respectively, represent the solid/elastic and viscous components of the samples’ mechanical responses. Hereafter, we focus on the properties in the viscoelastic linear domain (
Figure 7A,B and
Figure S1).
Figure 7A displays the evolution of the elastic modulus G
0′and viscous modulus G
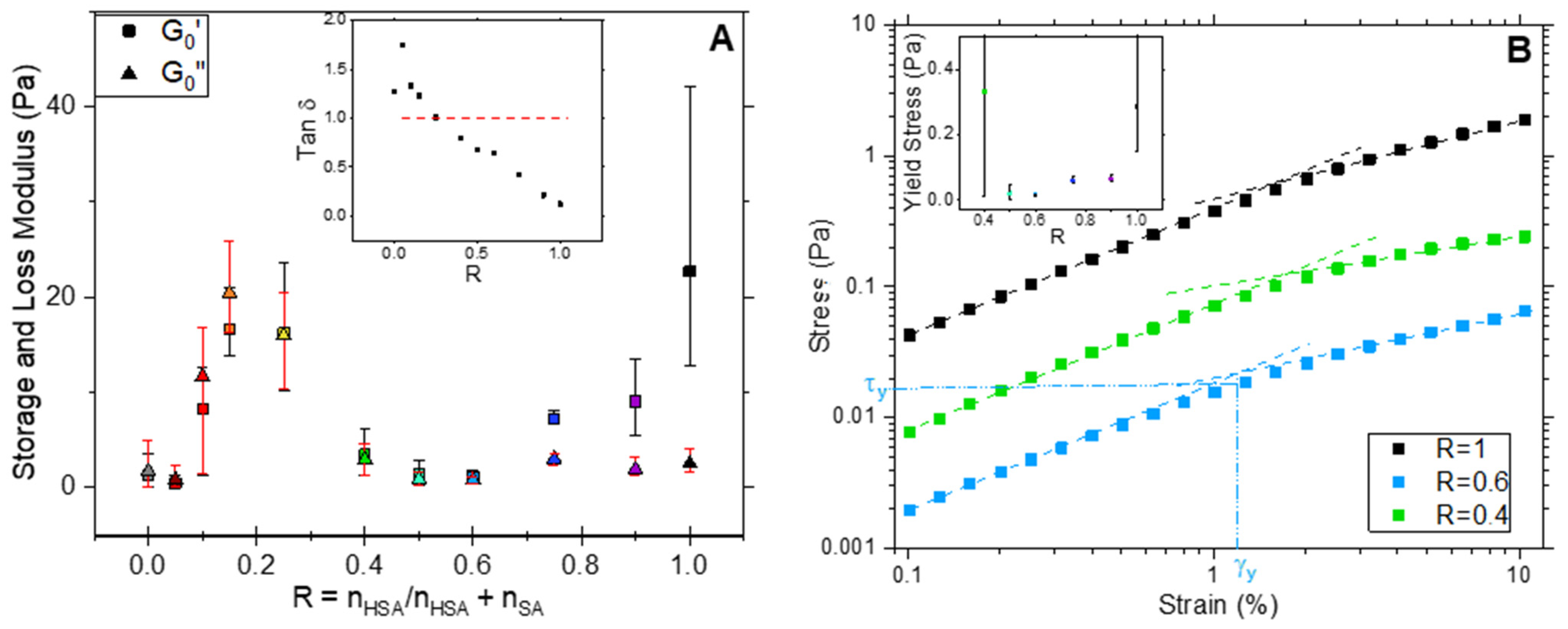
0″ determined for the linear domain as a function of R. The loss factor tan δ is shown as an inset for the sake of clarity. Three domains of R with distinctive behaviors can be distinguished and correlate well with the main structures of the mixture self-assemblies.
For R = 1, the elastic modulus G′
0 of the pure 12-HSA sample is significantly higher than the loss modulus and the loss factor tan δ = G″/G′ is low (<<1). Furthermore, two asymptotic behaviors can be identified for the stress τ as function of strain γ. At low γ, τ varies linearly with γ, and then as γ
α, with α < 1, once a critical stress τ
y is reached. τ
y is determined from the crossing point of the two asymptotic curves, as exemplified in
Figure 7B for R = 0.6. These results indicate that, at 1 Hz, a pure 12-HSA sample at 2 wt% is an elastic gel at low stress and begins to flow under large strain, as can be observed in
Figure S2a. Similar behavior was reported for the same system with multilamellar tubes but with a different ethanolamine/12-HSA ratio of
r = 0.5 [
47]. A solution of tubes can develop a yield stress at sufficiently high concentrations [
47], which is different from the jamming of spherical objects, which occurs during random close packing.
For R ≥ 0.75, the introduction of a few amount SA molecules in tubes causes a decrease in G′0 compared to the R = 1 samples while G″0 stays unchanged. Thus, the loss factor increases accordingly but always remains strictly below 1, which indicates a gel-like behavior. As for R = 1, the samples display the features of yield stress gels, with a linear variation of τ(γ) at low γ, but the yield stress τy decreases. This evolution is consistent with the shortening of the multilamellar tubes when decreasing R that is identified through confocal microscopy. Given that the number of fatty acids in a solution is kept constant, the decrease in the length of multilamellar tubes in turn decreases the number of tube entanglements and, subsequently, G′0.
From 0.6 ≥ R ≥ 0.4, G′0 and G″0 are almost constant, with G′0 being slightly larger than G″0. The samples are then also found in a gel state in this R system, which is confirmed by the loss factor value that remains below 1. Similarly to the system with a large R, samples display the characteristics of yield stress gels, with a linear variation of τ(γ) at low γ. A decrease in R leads to both an increase in tan δ, i.e., a less solid-like behavior, and an increase in τy. This specific behavior arises from the fact that part of the rigid tubes turns into more flexible ribbons that bind the tubes to form longer objects, as observed via confocal microscopy. The self-assembled objects are then more flexible than at larger R ratios but their overall length creates more entanglements.
Finally, in the system with a low content of SA (from 0.25 ≥ R ≥ 0), the behavior is completely different. Samples are no longer gels but viscous fluids, since G″ is larger than G′, the loss factor consequently rises above 1 (inset in
Figure 7A). However, they have G′
0 values that are much larger than in the system with a larger R where tubes are formed, with values that can reach 20 times those obtained for R ≥ 0.4 in some ratios (R = 0.15, R = 0.2 and R = 0.25), the maximum being at R = 0.25. This may come from the increased rigidity of the bilayers in this system, as revealed via SANS. In this system, planar bilayers are formed, whether they are pure 2D lamella at R = 0 or faceted objects where the planar part is bound by curved parts at other R ratios. These objects can entangle and organize themselves at a mesoscopic scale in large domains that are able to move collectively (as observed through confocal microscopy) and slide one on the top of the other. Such a slippage would then explain the viscous fluid behavior of the solution.
For all samples, oscillatory measurements were completed with a frequency sweep between 0.05 and 5 Hz, with a constant strain of 0.1%. They all display a shear thinning behavior (see
Figure S2). This is consistent with the yield stress fluid aspect in the case of gels. Regarding the fluid viscous samples made of planar lamella, this may result from an induced transition into multi lamellar vesicles under shear stress. The monitoring of the moduli did not enable us to determinate any relaxation time in the explored frequency range.
In summary, the mechanical properties of the samples display two main behaviors: at low content in SA, the samples are viscous fluids, with large G′0, and the samples turn into gels at higher R ratios of lower G′0, with a transition located between R = 0.25 and R = 0.4.