Abstract
One of the most important constituents of the cell membrane is arachidonic acid. Lipids forming part of the cellular membrane can be metabolized in a variety of cellular types of the body by a family of enzymes termed phospholipases: phospholipase A2, phospholipase C and phospholipase D. Phospholipase A2 is considered the most important enzyme type for the release of arachidonic acid. The latter is subsequently subjected to metabolization via different enzymes. Three enzymatic pathways, involving the enzymes cyclooxygenase, lipoxygenase and cytochrome P450, transform the lipid derivative into several bioactive compounds. Arachidonic acid itself plays a role as an intracellular signaling molecule. Additionally, its derivatives play critical roles in cell physiology and, moreover, are involved in the development of disease. Its metabolites comprise, predominantly, prostaglandins, thromboxanes, leukotrienes and hydroxyeicosatetraenoic acids. Their involvement in cellular responses leading to inflammation and/or cancer development is subject to intense study. This manuscript reviews the findings on the involvement of the membrane lipid derivative arachidonic acid and its metabolites in the development of pancreatitis, diabetes and/or pancreatic cancer.
1. Introduction
This work is an invited review that covers interesting findings on the involvement of the membrane lipid derivative arachidonic acid and its metabolites in the development of pancreatic diseases such as pancreatitis, diabetes and/or pancreatic cancer. Released from the constituent lipids of the cellular membrane, arachidonic acid is metabolized by major enzymes, which yields a subfamily of derivatives with important functions in cell physiology and pathophysiology, either in an autocrine, paracrine or endocrine manner. In this regard, the so-called tumor microenvironment (TME) has been signaled as an important cause of pancreatic disease. Specifically, the TME plays a critical role in cancer proliferation, invasion, metastasis and resistance to radiotherapy and chemotherapy. Because the relationship between inflammation and cancer is close, the role of the stroma and the putative cell-to-cell intercommunication in the pathophysiology of the pancreatic gland has been extensively analyzed. This research tries to summarize the advances made in the involvement of membrane lipid derivatives in pancreatic physiology and focuses its attention on the usefulness of arachidonic acid (AA) metabolism for the diagnosis, prevention and/or treatment of pancreatic disorders.
2. Arachidonic Acid Metabolism in the Pancreas
Lipid-derived messengers are well-known mediators of intracellular signaling. One of the most important constituents of biological membranes is AA, a polyunsaturated 20 carbon fatty acid (PUFA). Due to the presence of four double bonds in its molecule, AA can interact with oxygen molecules in order to generate several bioactive compounds such as eicosanoids and isoprostanes [1]. Normally, membrane-embedded lipids can be metabolized in a variety of cellular types of the body by a family of enzymes termed phospholipases. These enzymes are mostly responsible for the release of AA from glycerophospholipids located within the cellular membrane. Three types of phospholipases have been described: phospholipase A2 (PLA2), phospholipase C (PLC) and phospholipase D (PLD). PLA2 is considered the most important enzyme type for the metabolization of AA. It recognizes the sn-2 acyl bond within the membrane phospholipids and releases AA and lysophospholipids in a single step [2,3,4]. The mentioned enzyme comprises six subtypes, which are known as cytosolic PLA2, calcium-independent PLA2, secreted PLA2, lysosomal PLA2, platelet-activating factor acetyl-hydrolases and adipose-specific PLA2 [5,6]. AA exerts a pivotal role as a messenger in the cell physiology [7]. In addition to its role as an intracellular signal, AA also acts as a precursor for eicosanoid synthesis. The latter, in turn, also plays biological roles in the body, including the pancreas [8,9]. In this context, AA can be metabolized via three possible enzymatic pathways, through the enzymes cyclooxygenase (COX), lipoxygenase (LOX) and cytochrome P450 (CYP450) [10].
COX is also known as a prostaglandin endoperoxide synthase and is a type of oxidoreductase enzyme. Two isoforms of COX exist, termed COX-1 and COX-2. COX-1 is constitutively expressed in nearly all tissue and carries out normal (physiological) functions. COX-2 is inducible. The inducing stimuli include pro-inflammatory cytokines and growth factors. Consequently, COX-2 has been signaled to play a key role in inflammation and the control of cell growth [11]. COX-2 is highly expressed in a variety of cancers, where it exerts pleiotropic and multifaceted functions, which are considered responsible for, or critical contributors to, the genesis and/or promotion of carcinogenesis and cancer cell resistance [12]. COX generate prostanoids (20-carbon fatty acids), including prostaglandins (PGs) and thromboxanes (TXs). AA is firstly cyclized and oxygenated by COX. This step forms a cyclic endoperoxide derivative, prostaglandin G2 (PGG2). The hydroperoxyl group of PGG2 is also rapidly reduced by COX to produce hydroxyl-prostaglandin H2 (PGH2) [13]. PGH2 is later metabolized by various downstream enzymes, such as prostaglandin synthases and isomerases, leading to the generation of other specific PGs in the pancreas, such as prostaglandin E2 (PGE2—the most abundant PG in humans), prostaglandin D2 (PGD2), prostaglandin F1 alpha (PGF1α) and prostacyclin or PGI2. COX isoform derivatives have a preference for downstream PG synthases/isomerases. However, this coupling is not exclusive. For example, COX-1-derived PGH2 pairs mainly with PGF synthase, thromboxane synthase and cytosolic PGE synthase. Meanwhile, COX-2-derived PGH2 feeds mostly with PGI synthase and microsomal PGE synthase [14,15,16]. PGs exert their effects by activating membrane-linked G-protein receptors and have several different effects, depending on the specific PG and the cell type [17]. Another COX isoform has been found, COX-3, which is produced by the splicing of COX-1. However, its expression in the pancreas has not been properly studied yet. The only information that we could find refers to the study by Persaud et al. [18], who signal that this form of COX is not expressed in pancreatic β cells. Likewise, PGH2 can be metabolized to TXA2 by the TXA2 synthase. The latter is a highly unstable compound; its half-life is approximately 30 s. Thus, it is spontaneously hydrolyzed to thromboxane B2 (TXB2) [15,19]. TXB2 or its derivates, 2,3-dinor-TXB2 and 11-dehydro-TXB2, can be used as parameters for TXA2 production, whose increase is associated with pancreatic diseases [20,21,22].
LOX comprises a group of dioxygenases that catalyze the peroxidation of PUFAs, mainly linoleic acid (LA) or AA, in the presence of molecular oxygen [23]. The LOX family is classified according to the carbon atom that is oxygenated. Four types of LOX have described: 5-lipoxygenase (5-LOX), 8-lipoxygenase (8-LOX), 12-lipoxygenase (12-LOX) and 15-lipoxygenase type 1 and 2 (15-LOX-1 and 15-LOX-2). However, the main enzymes expressed in humans are 5-LOX (called leukocyte 5-LOX), 12-LOX (also known as platelet 12-LOX and leukocyte 12-LOX, respectively) and 15-LOX [3,24]. Generally, these enzymes synthesize active metabolites such as leukotrienes (LTs), lipoxins (LXs), hepoxillins (HOs) and hydroxy-eicosatetraenoic acids (HETEs) [25], which could act in an autocrine, paracrine and/or endocrine manner [26]. LOX enzymes generate the forms 5-, 8-, 12- or 15- hydroperoxyl-eicosatetraenoic acids (HpETEs), which are reduced by glutathione peroxidase (GPx) to the hydroxy forms (5-, 8-, 12-, 15-HETE), respectively [3,25]. However, the major sites on which AA is oxygenated are represented by the 5-, 12- and 15-positions. Therefore, in this review, we will focus on 5-LOX, 12-LOX and 15-LOX, which have been indicated to play important roles in the development and progression of human cancers, including pancreatic cancer, while 8-LOX has not been properly studied in the pancreas yet [10,24,27].
Firstly, 5-LOX generates 5-HpEPES from AA, which is a precursor for the synthesis of leukotrienes A4 (LTA4), 5-HEPEs and lipoxins (LXA4 and LXB4) [14]. LTA4 is an unstable metabolite that might be converted into LTB4 by LTA4 hydrolase. Then, LTB4 is conjugated with glutathione by glutathione S-transferase (GST) in order to produce LTC4, LTD4 and LTE4 [24]. With regard to the 12-LOX pathway, the metabolite generated from AA is termed 12-HpETES, which is further reduced to the more stable form 12-HETE. In addition, 12-HETE could lead to the generation of HO forms, such as 8-hydroxy-11,12-epoxy-eicosatetraenoic acid (HxA3) and 10-hydroxy-11,12-epoxy-eicosatrienoic acid (HxB3). Finally, the 15-LOX enzyme is subdivided into two subtypes, 15-LOX-1 and 15-LOX-2, which may have pro- or antitumorigenic activity depending on the isoforms. Moreover, 15-LOX-1 metabolizes AA to 3-hydroxy-octadecadienoic acid (13-HODE) and 15-HETE, while 15-LOX-2 mainly produces 15-HETE. Then, 15-HETE, which is a substrate for 5-LOX, is rapidly converted to LXA4 and LXB4 [28].
CYP450 consists of a group of membrane-bound hemoproteins with enzymatic activity that detoxify xenobiotics and exert key roles in cellular metabolism and homeostasis. CYP450 can be transcriptionally activated by different xenobiotics and endogenous substrates and its expression can be modulated by hormones and growth factors [29]. The CYP family is composed of numerous subclasses, giving rise to more than 6000 different enzymes. However, the CYP enzymes that catalyze AA are those with omega-hydroxylases and epoxidase activity. The former enzymes provide HETEs from AA and the latter produce epoxides and epoxyeicosatrienoic acids (EETs). The best known HETEs are 6-, 12-, 17-, 18-, 19- and 20-HETE. Some HETEs synthesized by CYP450 can be transformed subsequently by LOX [30]. CYP450 epoxygenases generate four EETs: 5,6-EET; 8,9-EET; 11,12-EET; and 14,15-EET. Stereoisomers exist for each of the four EET regioisomers, each of which may exert different effects [31]. CYP450 2J2 (CYP2J2) is the primary extrahepatic enzyme that processes AA to produce EETs in human [32]. These EETs have functions of their own, such as promoting angiogenesis and cell migration and cancer. In addition, EETs are rapidly metabolized by epoxide hydrolases to form dihydroxyeicosatrienoic acids (DHETs), which also exhibit diverse cellular functions [33,34,35]. The rapid transformation of EETs into DHETs has made it necessary to inhibit or delete epoxide hydroxylase to analyze the effects of EETs [36].
In summary, all these enzymes convert AA into different metabolites that will be involved in cellular fates. Among them, PGs, TXs, LTs and HETEs are the major metabolites generated from AA that are responsible for cellular responses [3]. Figure 1 provides a schematic representation of the bioactive eicosanoids derived from the AA pathway.
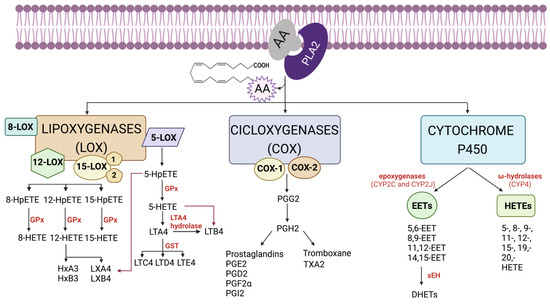
Figure 1.
Schematic representation of the bioactive eicosanoids derived from arachidonic acid (AA) pathway. AA is mainly metabolized by three types of enzymes: cyclooxygenases (COX), lipoxygenases (LOX) and cytochrome P450 (CYP450). First, AA, which forms part of the phospholipids of the biological membrane, is released by phospholipase A2 (PLA2). Then, COX, LOX and/or CYP450 enzymes act on the free AA to generate a cascade of prostaglandins (PGs), thromboxanes (TXs), a series of hydroxyeicosatetraenoic acids (HETEs), leukotrienes (LTs), lipoxins (LXs) hepoxilins (HOs) and epoxyeicosatrienoics acids (EETs). Finally, the effect of each bioactive eicosanoid will depend on the specific receptor that it binds to (HpETE: hydroperoxy-eicosatetraenoic acid; GPx: glutathione peroxidase; GST: glutathione S-transferase; HxA3: 8-hydroxy-11,12-epoxy-eicosatetraenoic acid; HxB3: 10-hydroxy-11,12-epoxy-eicosatrienoic acid; sEH: soluble epoxidehydrolase; DHETs: dihydroxyepoxyeicosatrienoic acid). Created with BioRender.com (accessed on 17 May 2023).
Finally, the activation of cell membrane receptors for pancreatic secretagogues, such as cholecystokinin, is linked to PLA2 and to AA metabolism and, hence, modulates pancreatic function [18,37]. Furthermore, the overstimulation of pancreatic cells with secretagogues has been related to pancreatic disease [38,39]. Thus, either unbalanced secretagogue stimulation or the arrival of noxious agents may alter signaling in pancreatic cells, which has been related to the onset of pancreatic diseases such as inflammation and cancer [40,41,42,43,44,45].
In line with the above-reported information, AA and its metabolites, as well as associated genes, could be used for prognostication and therapeutic aspects of pancreatic diseases. For example, an impaired serum fatty acid composition has been associated with primary insulin autoimmunity, on the basis that higher palmitoleic acid, cis-vaccenic, arachidonic, docosapentaenoic and docosahexaenoic acids decrease the risk, whereas higher α-linoleic acid and arachidonic:docosahexaenoic and n-6:n-3 acid ratios increase the risk [46]. Similarly, the lipidomic profiling of serum and pancreatic fluid can be used for the detection of chronic pancreatitis, a disease in which the serum and/or pancreatic fluid levels of oxidized fatty acids are elevated [47]. With regard to prognosis and therapy, signaling via 12-lipoxygenase-Gpr31 has been proposed to be required for pancreatic organogenesis in the zebrafish. This was based on the observation that 12-LOX-generated metabolites of AA increased sharply during organogenesis stages and that either the depletion or inhibition of 12-LOX impaired exocrine pancreas growth and the generation of insulin-producing β cells [48]. Additionally, the feedback modulation of glucose-induced insulin secretion by AA metabolites has been reported. In this context, the stimulation of insulin release by glucose may trigger a negative feedback loop via the local release of an inhibitor of β cell function. One or more metabolites of AA could be involved. Hence, the development of selective inhibitors of AA metabolism might be used in the therapy of impaired β cell function [49]. Moreover, the endocannabinoid system, which is reported as a lipid signaling system, comprises endogenous cannabis-like ligands that are derived from AA—for example, 2-arachidonoylglycerol. These signaling molecules bind to G-protein-coupled receptors, termed CB1 and CB2. The receptor for CB1 is widely distributed in the body, including the pancreas, where it could be coupled to the functional regulation of the gland and could represent a therapeutical approach [50]. Last but not least, suppression of the 5-LOX gene could be involved in the activation of apoptosis in pancreatic tumor cell lines in response to triptolide. These results suggest that the inhibition of AA metabolism by the 5-LOX pathway is associated with the antiproliferation activity of the mentioned drug, exhibiting clinical therapeutic value for patients with pancreatic cancer [51].
3. Arachidonic Acid and Calcium Signaling in the Pancreas
Calcium (Ca2+) is a universal intracellular messenger that, in addition, exhibits enormous versatility. As such, Ca2+ serves as a powerful tool to control a wide array of processes—for example, proliferation, growth and development, learning and memory, muscle contraction, fertilization and/or secretion (enzymes and hormones). Nevertheless, impairment of its control may lead to undesirable effects, which might result in cell transformation and/or cell death [52].
As in other tissues and organs, Ca2+ is a critical intracellular messenger in pancreatic cell physiology [53]. Moreover, impairment of Ca2+ homeostasis is of critical relevance for pancreatic diseases, such as inflammation and cancer [54,55]. Attention has been paid to how genetic alterations can conduct Ca2+ signaling pathways, in the sense that mutations or the impaired expression of critical proteins that are involved in the control of the intracellular Ca2+ concentration could lead to the deregulation of Ca2+-dependent effectors and, as a consequence, to the impairment of the mechanisms that control the cell’s fate. Of major relevance is the way in which the impairment of Ca2+ homeostasis might influence a cell´s behavior to promote enhanced proliferation, survival and invasion, which are all pathophysiological hallmarks of cancer [56,57].
Interestingly, the involvement of AA metabolism and Ca2+ signaling in the pancreas has been reported. The release of Ca2+ from its intracellular stores depends on the activation of different intracellular pathways by the gut hormone cholecystokinin, which may bind to low- and/or high-affinity receptors. The latter would be linked to the activation of PLA2 cascades. The products of the mentioned enzyme, AA and/or its metabolites, might modulate Ca2+ signals and, hence, the pancreatic physiology. Specifically, AA modulated the propagation of Ca2+ waves in pancreatic acinar cells, reducing the speed of propagation of Ca2+ signals [37,58,59]. Moreover, endogenously generated AA inhibited polyphosphoinositide synthesis and blocked agonist-induced inositol trisphosphate synthesis and Ca2+ mobilization in healthy cells [8].
On the contrary, AA might behave differentially in tumoral/transformed cells. In the study by Wu et al., the authors signaled that the AA pathway was involved in the increase in the intracellular Ca2+ concentration in pancreatic cancer cells. In the research, it was suggested that the metabolites of arachidonic acid were not involved in AA-mediated Ca2+ release. Rather, AA itself was responsible for their observation [60]. In rat pancreatic β cells, AA released Ca2+ from intracellular stores, an effect that was not observed in response to the analogue eicosatetraynoic acid. This further confirmed that AA metabolism was not required for Ca2+ mobilization [9]. Similarly, the analogue of AA arachidonyltrifluoromethyl ketone increased cytosolic Ca2+ in HIT insulinoma cells, which contributed to insulin secretion [61]. Thus, the effects of AA on cell physiology and its consequences might depend on the cellular type and on its status.
4. Role of Arachidonic Acid in Pancreatitis and Diabetes
As mentioned above, not only AA itself but its metabolization into different derivatives might be involved in major inflammatory responses in different tissues and organs, including the pancreas. Pancreatitis is a multifactorial disease that may be caused by the activation of different inflammatory mediators. Gallstones, smoking and alcohol consumption are well-established risk factors that may induce pancreatitis [62,63], but there are other factors contributing to the development of inflammation within the gland, including certain drugs such as mesalazine, azathioprine and simvastatine [64]. Additionally, post-endoscopic retrograde cholangiopancreatography has also been considered a potential risk factor that might induce pancreatitis as a common complication [65], in a similar way to pancreaticoduodenectomy [66]. Alteration of the immune response induced by gene mutations and/or environmental factors has also been considered a determinant of pancreatic damage [67]. All these factors might be related to the inappropriate intrapancreatic activation and release of pancreatic hydrolases. This could represent a pathogenetic mechanism of autodigestion of the gland that might lead to the onset of pancreatic inflammation [68]. The underlying cause responsible for the impairment of enzyme secretion could involve intracellular Ca2+ accumulation and concomitant oxidative stress [69,70,71]. The relationship between AA and Ca2+ signaling has been studied (see above).
In general, a major consensus points towards AA derivatives as being responsible for inflammation. COX, LOX and/or CYP450 produce metabolites that, to a variable extent, are involved in cell damage [72,73]. In fact, the levels of leukotriene B4 (LTB4), 15 hydroxyeicosatetraenoic acid (15-HETE), 6-keto prostaglandin F1 alpha (6-keto PGF1α), thromboxane B2 (TXB2) and prostaglandin E2 (PGE2) were increased in pancreatic tissue upon the induction of acute pancreatitis [20].
As previously reported, there are two genes encoding two COX isoenzymes. COX-1 is expressed constitutively in the cell and appears to regulate many normal physiologic functions. Conversely, COX-2 is inducible. Various growth factors, endotoxins, mitogens and tumor agents lead to an increase in its expression [74]. Activation of COX-2 has been signaled to mediate the inflammatory response. As such, COX-2 might be involved in the progression of inflammatory disease and in the development of chronic pancreatitis and/or diabetes [75]. COX-1 and COX-2 catalyze the formation of prostaglandins (PGs), thromboxanes (TXs) and levuloglandins [12]. The work carried out by Huang et al. showed that the expression of COX-2 in transgenic mice induced progressive changes in the pancreas, which included pancreas enlargement, inflammation, collagen deposition and acinar-to-ductal metaplasia [76]. Indeed, the success of anti-inflammatory drugs has been linked to their ability to inhibit COX-2 at the sites of damage and to a decrease in inflammation [77,78].
The generation of PGE2 through the COX pathway was considered a significant factor involved in β cell dysfunction and destruction and contributed to the pathogenesis of diabetes [3]. PGE2 is upregulated in diabetes and induces cell damage. Blockade of its receptor EP3 promoted β cell proliferation and survival via activation of the transcription factor nuclear factor E2-related factor 2 (Nrf2) [24]. AA metabolization led to the generation of PGE2 and TXB2, which activated the pro-inflammatory pathways nuclear factor kappa-light-chain-enhancer of activated B cells (NFκB) and Janus kinase (JAK)/signal transducer and activator of transcription 3 (STAT3) [79].
The induction of pancreatitis was related to increases in the levels of PGE2, PGD2 and TBX2, among others, in the gland [80]. High levels of PGE2 were detected in Sprague-Dawley rats upon the induction of acute necrotizing pancreatitis. Lipid peroxidation, together with edema, inflammation, bleeding and necrosis, was observed. The histopathologic severity was decreased by n-3 fatty acids, which was explained by the inhibition of PGE2 [81]. PGE2 modulated the activation of tumor necrosis factor alpha (TNF-α) in rat pancreatic lobules, an effect probably mediated by the activation of protein kinase A. This was interpreted as a putative mechanism that might explain the COX-2-dependent propagation of pancreatic inflammation [82]. Similarly, increased levels of PGE2 and TNF-α were detected in a cell line and animal models of severe acute pancreatitis. Meanwhile, 2-acetoxy-5-(2-4-(trifluoromethyl)-phenethylamino)-benzoic acid exhibited antioxidative and anti-inflammatory activity and diminished serum amylase and lipase levels and pancreatic wet weights, thereby inducing significant tissue-protective effects [83]. Rofecoxib, a selective COX-2 inhibitor, diminished PGE2 levels and collagen and transforming growth factor β (TGFβ) synthesis in an animal model of chronic pancreatitis. All the effects reported were related to the diminished infiltration of macrophages [84].
Apart from prostaglandins, leukotrienes also may be involved in the onset of pancreatic damage. LOX is a family of iron-containing dioxygenases that catalyze the formation of bioactive HETE metabolites from polyunsaturated fatty acids such as linoleic acid and AA [85]. These enzymes comprise six isoforms, which are expressed in different types of cells and tissues, such as immune, epithelial and tumor cells. LOX displays a wide range of functions, which include inflammation and tumorigenesis. The major end products of their activity are termed leukotrienes [86]. It should be pointed out that lipoxins have an important role in cancer cells. Lipoxins have anti-inflammatory effects, which decrease the chronic inflammation in the damaged tissue. This action takes place when these molecules bind to G-protein-coupled lipoxin A4 receptor (ALX)/formyl peptide receptor (FPR2). On the one hand, lipoxins may interact with many cells of the immune system, such as neutrophils, macrophages and T and B cells. On the other hand, these molecules also might regulate the levels of several transcription factors, such as NFκB factor [87]. Specifically, it has been shown that LXA4 is able to reduce cell proliferation, to inhibit cell invasion and to suppress tumor growth, therefore exhibiting anti-inflammatory properties in cancer. For this reason, it is important to evaluate its putative use in the treatment of cancer [25].
Caerulein, a pancreatic secretagogue, or intraductal bile acids, are common tools used to induce pancreatitis. Both of them increased the production of LTB4 in mice pancreatic acinar cells. In the study, a marked increase in the level of 5-LOX was observed [88]. The administration of LTB4 induced pancreatic damage, evidenced by pancreatic edema, neutrophil infiltration and necrosis [89]. LTB4 induced polymorphonuclear leucocyte accumulation in response to the generation of oxygen free radicals in an experimental rat model of pancreatic inflammation. The LTB4 inhibitor MK-886 exhibited protective effects [90]. The 5-LOX inhibitor zileuton repressed blood biomarkers of neutrophil activation and attenuated pancreatic tissue damage in a rat model [91]. Zafirlukast, a leukotriene receptor antagonist, improved histopathological parameters in the pancreas of rats subjected to acute pancreatitis [92].
Moreover, 12-lipoxygenase (12-LOX) has also been involved in the development of diabetes [26]. Increases in the levels of 12- and 15-HETE followed increases in the expression of 12/15-LOX and were accompanied by islet dysfunction and insulin resistance [93]. Leukotrienes inhibited glucose-induced insulin release, thereby altering endocrine pancreas functioning [94]. Zafirlukast also proved to be a potential candidate for a therapeutic intervention in diabetes, since it enhanced insulin secretion and prompted the activation of Ca2+/calmodulin-dependent protein kinase II and extracellular signal-regulated kinase signaling. Zafirlukast treatment further resulted in a significant drop in glucose levels [95].
CYP450 is a group of enzymes that are differentiated by a number for the isoform or individual enzyme (e.g., CYP1A1, CYP2D6) [96]. The major site of CYP expression is the liver [97]. These enzymes convert AA to four EETs that exhibit various biological effects, especially in the cardiovascular system [32]. There is some evidence for the involvement of CYP in pancreatic β cell dysfunction. CYP1A1 and CYP1B1 have been signaled to be involved in glucose homeostasis, insulin resistance and diabetes development [98]. EETs play an important role in insulin and glucagon secretion. Moreover, 5,6-EET was found to increase insulin release, while 8,9-, 11,12- or 14,15-EET stimulated glucagon production [99]. Loss of epoxide hydroxylase, an enzyme that degrades EETs, significantly reduced hyperglycemia in streptozotocin-treated mice and increased glucose-dependent insulin secretion and reduced apoptosis in pancreatic β cells [36]. An association between the CYP27B1 and CYP24A1 gene polymorphisms and type 1 diabetes has been reported [100]. However, to our knowledge, studies on the involvement of CYP in pancreatitis are currently lacking.
In general, it is accepted that the inhibition of the AA pathway and/or blockade of its metabolites will undoubtedly protect the pancreas against inflammation. In this context, knockout of the co-chaperone protein St13 exacerbated fatty replacement and fibrosis in a model of chronic pancreatitis. Therefore, it was suggested that St13 was functionally activated in acinar cells and exerted protection against inflammation, via regulation of the AA pathway [101].
Although the majority of the studies reviewed point toward the deleterious actions of AA metabolism, protective actions of AA have been reported in the pancreas. AA restored the antioxidant status to a normal range in an experimental animal model of diabetes mellitus. The study suggested that AA exerted protective actions in pancreatic β cells against alloxan-induced diabetes by attenuating oxidative stress [102]. The AA present in ARASCO oil was related to protective actions in the pancreas. Indeed, ARASCO oil, which was identified as a source of AA, diminished hyperglycemia, restored insulin sensitivity, suppressed inflammation and reversed the altered antioxidant status in streptozotocin-induced diabetes mellitus [103]. In a similar way, a decrease in PGE2 was correlated with an increase in tissue damage in alcohol-fed rats. Conversely, there was an inverse correlation between PGE2 levels and fibrogenesis. Because of these observations, the authors of this research suggested that endogenous PGE2 plays a protective role in alcohol-induced injury in the pancreas [104]. All this suggests the possibility, mentioned earlier in this manuscript, that the effects of AA on cell physiology and its consequences may vary from cell to cell and also may depend on the cellular state.
5. Arachidonic Acid’s Involvement in Pancreatic Cancer Development
The relationship between inflammation and cancer is widely accepted [105], including in pancreatic cancer [106]. In fact, both COX-2 and 5-lipoxygenase (5-LOX) are upregulated in different types of cancer, including pancreatic cancer. It is generally accepted that the metabolization of AA by these two enzymes leads to the formation of eicosanoids that directly contribute to pancreatic cancer cell proliferation, whereas the inhibition of the mentioned enzymes abrogates cancer cell proliferation [107].
The COX-2 enzyme appears to be overexpressed in a number of cancers and, as such, its products might play critical roles in carcinogenesis [108]. In fact, COX-2 activation has been related to an increase in cell proliferation and survival and the inhibition of the pro-apoptotic pathway, thereby resulting in tumor angiogenesis, invasion and metastasis [109]. Thus, the inhibition of COX-2 has received increasing attention as a useful tool for the prevention and treatment of cancer [110,111,112]. Drugs that blocked COX enzymes inhibited pancreatic cancer growth both in vitro and in vivo and induced cell death through the activation of apoptosis [113]. Omura et al. [114] suggested that COX-2-derived PG are used by cancer cells to proliferate and that the inhibition of its import from the extracellular medium via the blockade of multidrug-resistance-associated proteins abrogated pancreatic cancer growth. Likewise, the expression of PG biosynthetic pathway enzymes in mucinous pancreatic cysts has been detected. Additionally, the levels of COX-2 and cPLA2 were increased in the epithelia of mucinous pancreatic cysts [115]. Concomitant activation of COX-2 and K-Ras(G12D) accelerated the progression of pancreatic intraepithelial lesions, which involved components of the neurogenic locus notch homolog protein 1 (Notch1) [116]. The expression of COX-2 was increased in a number of resection specimens of pancreatic ductal adenocarcinoma (PDCA). Synergistic increases were also detected in the expression of the tumor protein p53 [117]. The latter consists of a gene that codes a protein (p53) that plays a key role in controlling cell division and cell death and, hence, is pivotal to cancer cell growth and the development of the disease [118]. Therefore, the inhibition of COX-2 and/or PLA2 might help to prevent the progression of tumor cell growth and cancer development [115]. A decrease in both cell proliferation and PG levels was related to the improved turnover of preneoplastic lesions in the pancreas [119]. The COX inhibitor ibuprofen exerted modulating effects in pancreatic cancers experimentally induced in hamsters [4].
With respect to LOX enzymes, evidence also exists that signals their involvement in pancreatic cancer development. Expression of both 5-LOX and 12-LOX has been detected in pancreatic cancer tissue [113] and cell lines, including PANC-1, AsPC-1 and MiaPaCa2 cells. The expression of the receptor of the downstream 5-LOX metabolite, leukotriene B4, was also increased [120]. LOX metabolites stimulated the growth of the tumor cells, whereas their inhibition markedly inhibited pancreatic cancer cell proliferation [113]. Similarly, growth inhibition and apoptosis in human pancreatic cell lines were noticed upon the downregulation of 5-LOX expression, as well as in the production of its downstream product LTB4 [51]. Meanwhile, 15-LOX-1 expression and activity were suggested to exert antitumorigenic effects in pancreatic cancer. Interestingly, 15-LOX-1 was found to be strongly present in normal ductal cells, tubular complexes and centro-acinar cells, whereas no staining was noted in tumor cells. These observations suggested that 15-LOX-1 expression might be lost during pancreatic cancer development [121]. The lichen (symbiotic partnership of a fungus and an alga) metabolites protolichesterinic acid, lobaric acid and baeomycesic acid exhibited antiproliferative effects against twelve different human cancer cell lines, including the pancreatic cancer cell lines Capan-1, Capan-2 and PANC-1. All three compounds had in vitro 5-LOX inhibitory activity, whereas protolichesterinic and lobaric acid inhibited 12-LOX [122]. Zyflo, a selective inhibitor of 5-LOX, diminished the incidence and the sizes of carcinomas in a model of pancreatic cancer induced in Syrian hamsters. Additionally, the activity of several antioxidant enzymes was increased and the concentration of products of lipid peroxidation was decreased. Thus, it was suggested that the inhibition of 5-LOX might be useful to decrease tumor growth in advanced pancreatic cancer [123]. Moreover, Zyflo also diminished liver metastasis in pancreatic cancer [124]. Another compound, termed triptolide, induced apoptosis that was related to the inhibition of the 5-LOX pathway in SW1990 pancreatic cancer cells in vitro. Conversely, overexpression of 5-LOX or the exogenous administration of LTB4 made cells more resistant [51]. The work carried out by Tersey et al. showed that 12-LOX promoted inflammation and increased the production of reactive oxygen species, through the p38 mitogen-activated protein kinase (p38 MAPK) and NFκB pathway [125]. Therefore, 5-HETE and 12-HETE metabolites might promote cancer growth via activation of the p44/42 and PI3/Akt mitogen-activated protein kinase pathways [27].
CYP450s are considered another group of enzymes related to AA metabolism that play a key role in carcinogenesis. They represent a superfamily of enzymes that catalyze the oxidation of lipids, steroids and drugs [126]. Several CYP enzymes metabolically activate procarcinogens [127]. The expression of the form CYP2J2 is increased in various human tumor cells and its metabolites are suggested to be involved in the development of human cancers [32]. CYP2J2 is overexpressed in PDAC, and its metabolites downstream of AA, specifically 8,9-EET, could inhibit ferroptosis in the pancreatic tumor line PANC-1 [34]. CYP450-2E1 (CYP2E1) was induced by ethanol consumption and was related to consequent toxicity, including carcinogenesis in the gastrointestinal tract [128]. The expression of certain CYP4 isoforms has been detected at significantly high levels in PDAC tissue, suggesting a role in the development of the disease [126]. The role of this enzyme system in the pathogenesis of chronic inflammatory and malignant pancreatic diseases has been confirmed by other studies. Compared to the normal pancreas, the expression of CYPs was increased in a number of pancreatic cancer samples [129]. CYP2A6, a metabolic enzyme that activates several procarcinogens, which include dietary and tobacco-specific nitrosamines, has been linked to pancreatic cancer. Consistent with this, high levels of CYP2A6 activity were detected in patients suffering from pancreatic cancer [130].
6. Arachidonic Acid and Stroma: Interplay in the Tissue Microenvironment
The stroma comprises the cells, components and structures that support and give form to organs, glands and/or tissues in the body. The stroma is mainly composed of connective tissue, blood vessels, lymphatic vessels and nerves. It provides the conditions for the normal functioning of the cells present in a certain tissue or organ [131]. Replacement of the exocrine parenchyma (i.e., the tissue formed by the cells that carry out an essential function) by fibrous tissue is a major characteristic of chronic pancreatitis and pancreatic cancer [76].
The tumor microenvironment (TME) is defined as the environment and/or conditions that surround a population of cells that form a tumor [132]. This environment includes blood vessels, immune cells, fibroblasts, stellate cells, signaling molecules, respiratory gases and the extracellular matrix, all of which participate as constituents of the stroma [133]. In cancer, the stroma plays an important role in contributing to the development of malignant cells [131]. The cells and their surroundings establish a close relationship, i.e., a bidirectional interaction is constantly ongoing “indoors”. This creates favorable conditions that will confer upon tumor cells the capability for active proliferation and growth, invasion and metastasis, which are all hallmarks of malignancy [134].
As previously reported, inflammation within the tumor microenvironment is a hallmark of cancer and is accepted as a major characteristic of carcinogens. The participation of AA´s metabolites has been investigated. Its products, and the metabolism of related fatty acids, which include prostaglandins, leukotrienes, lipoxins and epoxyeicosanoids, exhibit a critical ability to regulate inflammation. It is therefore expected that the resolution of inflammation might be a valuable tool to prevent malignant transformation [135]. Moreover, it has been suggested that targeting the enzymes, such as PLA2, COX and LOX, would lead to the achievement of beneficial outcomes in cancer therapy [136].
The role of stromal sources of PG for pancreatic cancers has been demonstrated. Overexpression of COX-2 is a factor that links chronic inflammation with metaplastic and neoplastic changes in various tissues, including the exocrine pancreas. Indeed, elevated expression is associated with worse outcomes. The COX-2 product PGE2 acts through a receptor termed EP4, which has been found to be upregulated in cancer. Activation of this receptor supports cell proliferation, migration, invasion and metastasis through the activation of multiple signaling pathways, including extracellular signal-regulated kinase (ERK), cyclic adenosine monophosphate/protein kinase A (cAMP/PKA), phosphoinositide 3-kinase/protein kinase B (PI3K/AKT) and NFκB [137]. Stimulation of pancreatic cancer cells with PGE2 led to the secretion of fibroblast growth factor 1 (FGF1). This was related to the enhanced proliferation of cancer-associated fibroblasts and increased expression of vascular endothelial growth factor A (VEGF-A) [138]. In a transgenic murine model, cellular atypia and a loss of normal cell/tissue organization were observed. A diet containing celecoxib, a COX-2 inhibitor, prevented the development of an abnormal pancreatic phenotype [139]. Profibrogenic factors were upregulated in transgenic mice expressing COX-2 [76]. Another study reported that COX-1 expression was not detected in various pancreatic cancer cells and some of them also lacked COX-2 expression. This led the researchers to suspect that such cancers rely on exogenous sources of PG. Interestingly, fibroblasts expressing COX-1 and COX-2 might be a source of PG, which would be used by pancreatic cancer cells, allowing them to grow and to proliferate [114]. Pancreatic stellate cells (PSC) contribute, in addition to fibroblasts, as a source of fibrotic stroma [140]. Moreover, other components of the TME, such as the peripherical blood mononuclear cells, are able to produce PG [141]. A correlation between the levels of COX-2 and its product PGE2 and the extent of pancreatic fibrosis has been suggested. PGE2 stimulated the proliferation, migration and invasion of PSC. Furthermore, PGE2 increased the expression of extracellular matrix genes [142]. The peroxisome proliferator-activated receptor gamma (PPAR-γ) is a ligand-activated transcription factor that controls inflammation, in addition to growth and differentiation. In PSC, activation of this transcription factor inhibited cell proliferation and the synthesis of collagen [143]. Melatonin, the main product of the pineal gland, exhibits several pharmacological properties, including anti-inflammatory effects. In this context, this indolamine reduced the expression of COX-2 though the inhibition of NFκB signaling in pancreatic stellate cells subjected to hypoxia [144]. Melatonin has shown a significant antifibrotic role in the pancreas and some of its effects may be mediated by its anti-inflammatory actions exerted by modulating COX-2 expression. One of the characteristics of the pancreatic tumor microenvironment is the exclusion or poor infiltration of T cells [145]. This is one of the main reasons that pancreatic tumors are often refractory to immunotherapy. The production of PGE2 by the metabolization of AA via COX-2 appears to have an immunosuppressive effect, by which T cells are excluded from the tumor focus [146]. A similar observation was made by Zhang et al., who observed that the inhibition of COX-2 by apricoxib alone or in combination with anti-VEGF therapy increased the infiltration of CD8+ T cells in PDAC in vivo models [147].
Among the six different isoforms of LOX that have been described, 5-LOX is the most vital enzyme for the synthesis of leukotrienes. Evidence exists that relates 5-LOX to tumors [23]. Leukotriene signaling contributes to the active tumor microenvironment, promoting tumor growth and resistance to therapy [148]. Metabolites generated by 5-LOX from AA, such as 5-hydroxyeicosatetraenoic acid (5-HETE) and a variety of leukotrienes, have been suggested to act as mediators of inflammation in pathological states, leading to cancer. Furthermore, upregulation of the expression of 5-LOX has been associated with increased tumorigenesis. Pathways activated by 5-LOX may interact with the tumor microenvironment and can participate actively during the development and progression of a tumor [149]. Moreover, 5-LOX and the prostaglandin E synthase-1 (PGES-1) play an important role in the immune evasion evoked by tumor associated macrophages, which constrains the action of the antitumoral natural killer T cells [150].
Increased expression of 12-LOX and elevated levels of its metabolite 12-(S)-HETE were found in fibroblasts, one of the major nonmalignant cell types present in the stroma of pancreatic ductal adenocarcinoma (PDAC). This fact conferred upon fibroblasts the capability to transfer AA derivatives to tumor cells, which would be used for their proliferative needs [151]. However, not all metabolites of LOX exhibit fibrogenic effects. LXA4, a derivative metabolite of AA generated via LOX, inhibited the differentiation of PSC into a myofibroblast phenotype and reduced the proliferation and migration of pancreatic cancer cells evoked by PSC [152].
In addition to AA metabolism, CYP450 enzymes also are involved in the metabolism of drugs, foreign chemicals, cholesterol, steroids and other major lipids. This primarily takes place in the liver and in the gastrointestinal tract. However, evidence also exists that suggests that this occurs within the tumor microenvironment [153]. CYP450-derived AA epoxides, termed epoxyeicosatrienoic acids (EETs), also play a certain role in the promotion of the growth of certain tumors [154].
7. Discussion and Conclusions
As stated above, the relationship between inflammation and cancer is close. The existing literature covers a wide range of studies that show that isoenzymes of both COX and LOX are upregulated in different types of cancer, including pancreatic cancer. Less is known about the role of CYP450. Table 1 contains information about the functions of different metabolites of AA in pancreatitis, diabetes and pancreatic cancer The mentioned enzymes mainly convert AA into PGs, TXs, LTs and HETEs. These lipid-derived messengers are well-known mediators of intra- and intercellular signaling and are involved in cellular responses and fates. In this context, the role of the stroma and the putative cell-to-cell intercommunication in the pathophysiology of pancreatic diseases exhibits major relevance. The effect of certain inhibitors of the enzymes involved in AA metabolism is summarized in Table 2.

Table 1.
AA metabolites and its effects related to pancreatic diseases.

Table 2.
Inhibitors of AA pathway and their effects on cell physiology.
The stroma provides the conditions for the normal functioning of the cells present in a certain tissue or organ. Inflammation within the TME is a hallmark of cancer and is accepted as a major characteristic of carcinogens. Consequently, the stroma plays an important role in terms of a contribution to the development of malignant cells. In this context, the modulation of AA and the pathways regulated by its metabolites might be a valuable tool to prevent malignant transformation.
In conclusion, AA exhibits a role as an intracellular signal. Moreover, AA also acts as a precursor for eicosanoid synthesis, which further amplifies the actions of AA in terms of extending its actions to the surrounding cells. Cell communication within the TME depicts major relevance for cancer development and growth, and AA’s derivatives exhibit potential roles in the development of the disease. A summary of the main findings on the involvement of AA metabolization in the pancreatic tumor microenvironment is given in Figure 2.
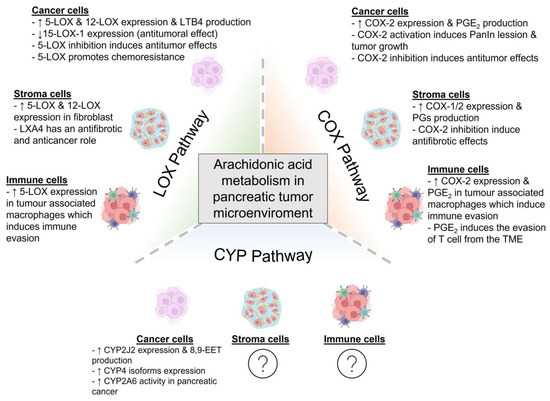
Figure 2.
Metabolism of arachidonic acid in the pancreatic tumor microenvironment. The cells that form the pancreatic tumor microenvironment can metabolize arachidonic acid (AA) by the action of cyclooxygenases (COX), lipoxygenases (LOX) or cytochrome P450 (CYP450). These pathways give rise to different metabolites that can promote or diminish tumor development and growth. In this figure, the main findings on AA metabolization within the pancreatic tumor microenvironment are summarized (8,9-EET: 8,9-epoxyeicosatrienoic acid; COX: cyclooxygenase; LOX: lipoxygenase; CYP: cytochrome, LTB4: leukotriene B4; LXA4: lipoxin A4; PanIN: pancreatic intraepithelial neoplasia; PGE2: prostaglandin E2; PGs: prostaglandins; TME: tumor microenvironment). Created with BioRender.com (accessed on 17 May 2023).
8. Future Directions
In contrast to the wide variety of research focused on the involvement of COX and LOX in cancer development, fewer studies exist regarding the functions in carcinogenesis of CYP450. Therefore, further studies will be needed to shed more light on the role of CYP450 in pancreatic cancer development and/or progression. Additionally, although the majority of the studies reviewed point toward the deleterious actions of AA metabolism, protective actions of AA in the pancreas have been suggested, which need to be explored.
Author Contributions
C.O.-P. wrote parts of the manuscript; designed the figures; and revised, corrected and approved the submitted version of the manuscript. A.C.-R. wrote parts of the manuscript; designed the figures; and revised, corrected and approved the submitted version of the manuscript. M.E. wrote parts of the manuscript; designed the figures; and revised, corrected and approved the submitted version of the manuscript. A.G. designed the first draft; wrote parts of the manuscript; and revised, corrected and approved the submitted version of the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
Funding for the studies was provided by Junta de Extremadura—FEDER (GR21037). Matias Estaras was awarded a grant from the Valhondo Calaff Foundation. Candido Ortiz was awarded a grant from the Spanish Association Against Cancer (AECC). The contract for Alba Castillejo-Rufo was funded by Servicio Extremeño de Empleo (SEXPE, TE-0028-21). The funding sources had no role in the study design; in the collection, analysis and interpretation of data; in the writing of the report; or in the decision to submit the paper for publication.
Conflicts of Interest
The authors declare that there is no conflict of interest. All authors contributed to the preparation of this work and revised and approved the published version of the manuscript.
References
- Hanna, V.S.; Hafez, E.A.A. Synopsis of arachidonic acid metabolism: A review. J. Adv. Res. 2018, 11, 23–32. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Xiang, R.; Glorieux, C.; Huang, P. PLA2G2A Phospholipase Promotes Fatty Acid Synthesis and Energy Metabolism in Pancreatic Cancer Cells with K-ras Mutation. Int. J. Mol. Sci. 2022, 23, 11721. [Google Scholar] [CrossRef] [PubMed]
- Luo, P.; Wang, M.-H. Eicosanoids, β-cell function, and diabetes. Prostaglandins Other Lipid Mediat. 2011, 95, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Schuller, H.M.; Zhang, L.; Weddle, D.L.; Castonguay, A.; Walker, K.; Miller, M.S. The cyclooxygenase inhibitor ibuprofen and the FLAP inhibitor MK886 inhibit pancreatic carcinogenesis induced in hamsters by transplacental exposure to ethanol and the tobacco carcinogen NNK. J. Cancer Res. Clin. Oncol. 2002, 128, 525–532. [Google Scholar] [CrossRef]
- Murakami, M.; Nakatani, Y.; Atsumi, G.-I.; Inoue, K.; Kudo, I. Regulatory Functions of Phospholipase A2. Crit. Rev. Immunol. 2017, 37, 127–195. [Google Scholar] [CrossRef] [PubMed]
- Peng, Z.; Chang, Y.; Fan, J.; Ji, W.; Su, C. Phospholipase A2 superfamily in cancer. Cancer Lett. 2021, 497, 165–177. [Google Scholar] [CrossRef]
- Turunen, P.M.; Putula, J.; Kukkonen, J.P. Filtration assay for arachidonic acid release. Anal. Biochem. 2010, 407, 233–236. [Google Scholar] [CrossRef]
- Chaudhry, A.; Rubin, R.P. Mediators of Ca2(+)-dependent secretion. Environ. Health Perspect. 1990, 84, 35–39. [Google Scholar] [CrossRef]
- Yeung-Yam-Wah, V.; Lee, A.K.; Tse, A. Arachidonic acid mobilizes Ca2+ from the endoplasmic reticulum and an acidic store in rat pancreatic β cells. Cell Calcium 2012, 51, 140–148. [Google Scholar] [CrossRef]
- Ding, X.-Z.; Hennig, R.; Adrian, T.E. Lipoxygenase and cyclooxygenase metabolism: New insights in treatment and chemoprevention of pancreatic cancer. Mol. Cancer 2003, 2, 10. [Google Scholar] [CrossRef]
- Vane, J.R.; Bakhle, Y.S.; Botting, R.M. Cyclooxygenases 1 and 2. Annu. Rev. Pharmacol. Toxicol. 1998, 38, 97–120. [Google Scholar] [CrossRef] [PubMed]
- Fitzpatrick, F.A. Cyclooxygenase enzymes: Regulation and function. Curr. Pharm. Des. 2004, 10, 577–588. [Google Scholar] [CrossRef] [PubMed]
- Simmons, D.L.; Botting, R.M.; Hla, T. Cyclooxygenase isozymes: The biology of prostaglandin synthesis and inhibition. Pharmacol. Rev. 2004, 56, 387–437. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Wu, L.; Chen, J.; Dong, L.; Chen, C.; Wen, Z.; Hu, J.; Fleming, I.; Wang, D.W. Metabolism pathways of arachidonic acids: Mechanisms and potential therapeutic targets. Signal Transduct. Target. Ther. 2021, 6, 94. [Google Scholar] [CrossRef] [PubMed]
- Rong, Y.; Ren, J.; Song, W.; Xiang, R.; Ge, Y.; Lu, W.; Fu, T. Resveratrol Suppresses Severe Acute Pancreatitis-Induced Microcirculation Disturbance through Targeting SIRT1-FOXO1 Axis. Oxid. Med. Cell. Longev. 2021, 2021, 8891544. [Google Scholar] [CrossRef] [PubMed]
- Park, J.Y.; Pillinger, M.H.; Abramson, S.B. Prostaglandin E2 synthesis and secretion: The role of PGE2 synthases. Clin. Immunol. 2006, 119, 229–240. [Google Scholar] [CrossRef] [PubMed]
- Narumiya, S.; Fitzgerald, G.A. Genetic and pharmacological analysis of prostanoid receptor function. J. Clin. Investig. 2001, 108, 25–30. [Google Scholar] [CrossRef]
- Persaud, S.J.; Muller, D.; Belin, V.D.; Kitsou-Mylona, I.; Asare-Anane, H.; Papadimitriou, A.; Burns, C.J.; Huang, G.C.; Amiel, S.A.; Jones, P.M. The role of arachidonic acid and its metabolites in insulin secretion from human islets of langerhans. Diabetes 2007, 56, 197–203. [Google Scholar] [CrossRef]
- Ashton, A.W.; Zhang, Y.; Cazzolli, R.; Honn, K.V. The Role and Regulation of Thromboxane A2 Signaling in Cancer-Trojan Horses and Misdirection. Molecules 2022, 27, 6234. [Google Scholar] [CrossRef]
- Closa, D.; Rosello-Catafau, J.; Hotter, G.; Bulbena, O.; Fernandez-Cruz, L.; Gelpi, E. Cyclooxygenase and lipoxygenase metabolism in sodium taurocholate induced acute hemorrhagic pancreatitis in rats. Prostaglandins 1993, 45, 315–322. [Google Scholar] [CrossRef]
- Katori, M.; Kawamura, M.; Sawada, M. 11-Dehydro-TXB2 and 2,3-dinor-TXB2 as new parameters of TXA2 generation. Nihon Rinsho 1992, 50, 349–354. [Google Scholar] [PubMed]
- Lasserre, B.; Navarro-Delmasure, C.; Chanh, A.P.H.; Catala, J.; Hollande, E. Modifications in the TXA2and PGI2plasma levels and some other biochemical parameters during the initiation and development of non-insulin-dependent diabetes mellitus (NIDDM) syndrome in the rabbit. Prostaglandins Leukot. Essent. Fat. Acids 2000, 62, 285–291. [Google Scholar] [CrossRef] [PubMed]
- Merchant, N.; Bhaskar, L.V.K.S.; Momin, S.; Sujatha, P.; Reddy, A.B.M.; Nagaraju, G.P. 5-Lipoxygenase: Its involvement in gastrointestinal malignancies. Crit. Rev. Oncol. Hematol. 2018, 127, 50–55. [Google Scholar] [CrossRef] [PubMed]
- Bosma, K.J.; Andrei, S.R.; Katz, L.S.; Smith, A.A.; Dunn, J.C.; Ricciardi, V.F.; Ramirez, M.A.; Baumel-Alterzon, S.; Pace, W.A.; Carroll, D.T.; et al. Pharmacological blockade of the EP3 prostaglandin E2 receptor in the setting of type 2 diabetes enhances β-cell proliferation and identity and relieves oxidative damage. Mol. Metab. 2021, 54, 101347. [Google Scholar] [CrossRef]
- Borin, T.F.; Angara, K.; Rashid, M.H.; Achyut, B.R.; Arbab, A.S. Arachidonic Acid Metabolite as a Novel Therapeutic Target in Breast Cancer Metastasis. Int. J. Mol. Sci. 2017, 18, 2661. [Google Scholar] [CrossRef]
- Dobrian, A.D.; Morris, M.A.; Taylor-Fishwick, D.A.; Holman, T.R.; Imai, Y.; Mirmira, R.G.; Nadler, J.L. Role of the 12-lipoxygenase pathway in diabetes pathogenesis and complications. Pharmacol. Ther. 2019, 195, 100–110. [Google Scholar] [CrossRef] [PubMed]
- Zhou, G.-X.; Ding, X.-L.; Wu, S.-B.; Zhang, H.-F.; Cao, W.; Qu, L.-S.; Zhang, H. Inhibition of 5-lipoxygenase triggers apoptosis in pancreatic cancer cells. Oncol. Rep. 2015, 33, 661–668. [Google Scholar] [CrossRef]
- Wang, T.; Fu, X.; Chen, Q.; Patra, J.K.; Wang, D.; Wang, Z.; Gai, Z. Arachidonic Acid Metabolism and Kidney Inflammation. Int. J. Mol. Sci. 2019, 20, 3683. [Google Scholar] [CrossRef]
- Manikandan, P.; Nagini, S. Cytochrome P450 Structure, Function and Clinical Significance: A Review. Curr. Drug Targets 2018, 19, 38–54. [Google Scholar] [CrossRef]
- Gubbala, V.B.; Jytosana, N.; Trinh, V.Q.; Maurer, H.C.; Naeem, R.F.; Lytle, N.K.; Ma, Z.; Zhao, S.; Lin, W.; Han, H.; et al. Eicosanoids in the pancreatic tumor microenvironment—A multicellular, multifaceted progression. Gastro Hep Adv. 2022, 1, 682–697. [Google Scholar] [CrossRef]
- Kiss, L.; Bier, J.; Röder, Y.; Weissmann, N.; Grimminger, F.; Seeger, W. Direct and simultaneous profiling of epoxyeicosatrienoic acid enantiomers by capillary tandem column chiral-phase liquid chromatography with dual online photodiode array and tandem mass spectrometric detection. Anal. Bioanal. Chem. 2008, 392, 717–726. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Ju, W.; Hao, H.; Wang, G.; Li, P. Cytochrome P450 2J2: Distribution, function, regulation, genetic polymorphisms and clinical significance. Drug Metab. Rev. 2013, 45, 311–352. [Google Scholar] [CrossRef] [PubMed]
- Panigrahy, D.; Greene, E.R.; Pozzi, A.; Wang, D.W.; Zeldin, D.C. EET signaling in cancer. Cancer Metastasis Rev. 2011, 30, 525–540. [Google Scholar] [CrossRef] [PubMed]
- Tao, P.; Jiang, Y.; Wang, H.; Gao, G. CYP2J2-produced epoxyeicosatrienoic acids contribute to the ferroptosis resistance of pancreatic ductal adenocarcinoma in a PPARγ-dependent manner. Zhong Nan Da Xue Xue Bao Yi Xue Ban J. Cent. South Univ. Med. Sci. 2021, 46, 932–941. [Google Scholar] [CrossRef]
- Frömel, T.; Naeem, Z.; Pirzeh, L.; Fleming, I. Cytochrome P450-derived fatty acid epoxides and diols in angiogenesis and stem cell biology. Pharmacol. Ther. 2022, 234, 108049. [Google Scholar] [CrossRef]
- Luo, P.; Chang, H.-H.; Zhou, Y.; Zhang, S.; Hwang, S.H.; Morisseau, C.; Wang, C.-Y.; Inscho, E.W.; Hammock, B.D.; Wang, M.-H. Inhibition or deletion of soluble epoxide hydrolase prevents hyperglycemia, promotes insulin secretion, and reduces islet apoptosis. J. Pharmacol. Exp. Ther. 2010, 334, 430–438. [Google Scholar] [CrossRef]
- Schulz, I.; Krause, E.; González, A.; Göbel, A.; Sternfeld, L.; Schmid, A. Agonist-stimulated pathways of calcium signaling in pancreatic acinar cells. Biol. Chem. 1999, 380, 903–908. [Google Scholar] [CrossRef]
- Nadella, S.; Ciofoaia, V.; Cao, H.; Kallakury, B.; Tucker, R.D.; Smith, J.P. Cholecystokinin Receptor Antagonist Therapy Decreases Inflammation and Fibrosis in Chronic Pancreatitis. Dig. Dis. Sci. 2020, 65, 1376–1384. [Google Scholar] [CrossRef]
- Smith, J.P.; Solomon, T.E. Cholecystokinin and pancreatic cancer: The chicken or the egg? Am. J. Physiol. Gastrointest. Liver Physiol. 2014, 306, G91–G101. [Google Scholar] [CrossRef]
- Del Castillo-Vaquero, A.; Salido, G.M.; González, A. Increased calcium influx in the presence of ethanol in mouse pancreatic acinar cells. Int. J. Exp. Pathol. 2010, 91, 114–124. [Google Scholar] [CrossRef]
- Tapia, J.A.; Salido, G.M.; González, A. Ethanol consumption as inductor of pancreatitis. World J. Gastrointest. Pharmacol. Ther. 2010, 1, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Gitto, S.B.; Nakkina, S.P.; Beardsley, J.M.; Parikh, J.G.; Altomare, D.A. Induction of pancreatitis in mice with susceptibility to pancreatic cancer. Methods Cell Biol. 2022, 168, 139–159. [Google Scholar] [CrossRef] [PubMed]
- Minaga, K.; Watanabe, T.; Kamata, K.; Kudo, M.; Strober, W. A Mouse Model of Acute and Chronic Pancreatitis. Curr. Protoc. 2022, 2, e422. [Google Scholar] [CrossRef] [PubMed]
- Trulsson, L.M.; Gasslander, T.; Svanvik, J. Cholecystokinin-8-induced hypoplasia of the rat pancreas: Influence of nitric oxide on cell proliferation and programmed cell death. Basic Clin. Pharmacol. Toxicol. 2004, 95, 183–190. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.H.; Portincasa, P.; Wang, D.Q.-H. Update on the Molecular Mechanisms Underlying the Effect of Cholecystokinin and Cholecystokinin-1 Receptor on the Formation of Cholesterol Gallstones. Curr. Med. Chem. 2019, 26, 3407–3423. [Google Scholar] [CrossRef]
- Niinistö, S.; Takkinen, H.-M.; Erlund, I.; Ahonen, S.; Toppari, J.; Ilonen, J.; Veijola, R.; Knip, M.; Vaarala, O.; Virtanen, S.M. Fatty acid status in infancy is associated with the risk of type 1 diabetes-associated autoimmunity. Diabetologia 2017, 60, 1223–1233. [Google Scholar] [CrossRef]
- Stevens, T.; Berk, M.P.; Lopez, R.; Chung, Y.-M.; Zhang, R.; Parsi, M.A.; Bronner, M.P.; Feldstein, A.E. Lipidomic profiling of serum and pancreatic fluid in chronic pancreatitis. Pancreas 2012, 41, 518–522. [Google Scholar] [CrossRef]
- Hernandez-Perez, M.; Kulkarni, A.; Samala, N.; Sorrell, C.; El, K.; Haider, I.; Aleem, A.M.; Holman, T.R.; Rai, G.; Tersey, S.A.; et al. A 12-lipoxygenase-Gpr31 signaling axis is required for pancreatic organogenesis in the zebrafish. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2020, 34, 14850–14862. [Google Scholar] [CrossRef]
- Metz, S.A. Feedback modulation of glucose-induced insulin secretion by arachidonic acid metabolites: Possible molecular mechanisms and relevance to diabetes mellitus. Prostaglandins Med. 1981, 7, 581–589. [Google Scholar] [CrossRef]
- Mouslech, Z.; Valla, V. Endocannabinoid system: An overview of its potential in current medical practice. Neuro Endocrinol. Lett. 2009, 30, 153–179. [Google Scholar]
- Zhou, G.X.; Ding, X.L.; Huang, J.F.; Zhang, H.; Wu, S.B. Suppression of 5-lipoxygenase gene is involved in triptolide-induced apoptosis in pancreatic tumor cell lines. Biochim. Biophys. Acta 2007, 1770, 1021–1027. [Google Scholar] [CrossRef] [PubMed]
- Berridge, M.J.; Lipp, P.; Bootman, M.D. The versatility and universality of calcium signalling. Nat. Rev. Mol. Cell Biol. 2000, 1, 11–21. [Google Scholar] [CrossRef] [PubMed]
- Schulz, I.; Stolze, H.H. The exocrine pancreas: The role of secretagogues, cyclic nucleotides, and calcium in enzyme secretion. Annu. Rev. Physiol. 1980, 42, 127–156. [Google Scholar] [CrossRef] [PubMed]
- Crottès, D.; Lin, Y.-H.T.; Peters, C.J.; Gilchrist, J.M.; Wiita, A.P.; Jan, Y.N.; Jan, L.Y. TMEM16A controls EGF-induced calcium signaling implicated in pancreatic cancer prognosis. Proc. Natl. Acad. Sci. USA 2019, 116, 13026–13035. [Google Scholar] [CrossRef]
- Yang, F.; Wang, Y.; Sternfeld, L.; Rodriguez, J.A.; Ross, C.; Hayden, M.R.; Carriere, F.; Liu, G.; Schulz, I. The role of free fatty acids, pancreatic lipase and Ca2+ signalling in injury of isolated acinar cells and pancreatitis model in lipoprotein lipase-deficient mice. Acta Physiol. 2009, 195, 13–28. [Google Scholar] [CrossRef]
- Bettaieb, L.; Brulé, M.; Chomy, A.; Diedro, M.; Fruit, M.; Happernegg, E.; Heni, L.; Horochowska, A.; Housseini, M.; Klouyovo, K.; et al. Ca2+ Signaling and Its Potential Targeting in Pancreatic Ductal Carcinoma. Cancers 2021, 13, 3085. [Google Scholar] [CrossRef]
- Kutschat, A.P.; Johnsen, S.A.; Hamdan, F.H. Store-Operated Calcium Entry: Shaping the Transcriptional and Epigenetic Landscape in Pancreatic Cancer. Cells 2021, 10, 966. [Google Scholar] [CrossRef]
- Siegel, G.; Sternfeld, L.; Gonzalez, A.; Schulz, I.; Schmid, A. Arachidonic acid modulates the spatiotemporal characteristics of agonist-evoked Ca2+ waves in mouse pancreatic acinar cells. J. Biol. Chem. 2001, 276, 16986–16991. [Google Scholar] [CrossRef]
- González, A.; Schmid, A.; Sternfeld, L.; Krause, E.; Salido, G.M.; Schulz, I. Cholecystokinin-evoked Ca2+ waves in isolated mouse pancreatic acinar cells are modulated by activation of cytosolic phospholipase A2, phospholipase D, and protein kinase C. Biochem. Biophys. Res. Commun. 1999, 261, 726–733. [Google Scholar] [CrossRef]
- Wu, L.; Katz, S.; Brown, G.R. Inositol 1,4,5-trisphosphate-, GTP-, arachidonic acid- and thapsigargin-mediated intracellular calcium movement in PANC-1 microsomes. Cell Calcium 1994, 15, 228–240. [Google Scholar] [CrossRef]
- Stickle, D.; Ramanadham, S.; Turk, J. Effects of arachidonyltrifluoromethyl ketone on cytosolic [Ca2+] in HIT insulinoma cells. J. Lipid Mediat. Cell Signal. 1997, 17, 65–70. [Google Scholar] [CrossRef] [PubMed]
- Lankisch, P.G.; Apte, M.; Banks, P.A. Acute pancreatitis. Lancet 2015, 386, 85–96. [Google Scholar] [CrossRef] [PubMed]
- Ye, X.; Lu, G.; Huai, J.; Ding, J. Impact of smoking on the risk of pancreatitis: A systematic review and meta-analysis. PLoS ONE 2015, 10, e0124075. [Google Scholar] [CrossRef] [PubMed]
- Nitsche, C.J.; Jamieson, N.; Lerch, M.M.; Mayerle, J.V. Drug induced pancreatitis. Best Pract. Res. Clin. Gastroenterol. 2010, 24, 143–155. [Google Scholar] [CrossRef]
- Pekgöz, M. Post-endoscopic retrograde cholangiopancreatography pancreatitis: A systematic review for prevention and treatment. World J. Gastroenterol. 2019, 25, 4019–4042. [Google Scholar] [CrossRef]
- Yen, H.-H.; Ho, T.-W.; Wu, C.-H.; Kuo, T.-C.; Wu, J.-M.; Yang, C.-Y.; Tien, Y.-W. Late acute pancreatitis after pancreaticoduodenectomy: Incidence, outcome, and risk factors. J. Hepatobiliary Pancreat. Sci. 2019, 26, 109–116. [Google Scholar] [CrossRef]
- Uomo, G.; Manes, G. Risk factors of chronic pancreatitis. Dig. Dis. 2007, 25, 282–284. [Google Scholar] [CrossRef]
- Geokas, M.C.; Baltaxe, H.A.; Banks, P.A.; Silva, J.J.; Frey, C.F. Acute pancreatitis. Ann. Intern. Med. 1985, 103, 86–100. [Google Scholar] [CrossRef]
- González, A.; Schmid, A.; Salido, G.M.; Camello, P.J.; Pariente, J.A. XOD-catalyzed ROS generation mobilizes calcium from intracellular stores in mouse pancreatic acinar cells. Cell. Signal. 2002, 14, 153–159. [Google Scholar] [CrossRef]
- González, A.; Granados, M.P.; Salido, G.M.; Pariente, J.A. H2O2-induced changes in mitochondrial activity in isolated mouse pancreatic acinar cells. Mol. Cell. Biochem. 2005, 269, 165–173. [Google Scholar] [CrossRef]
- Rivera-Barreno, R.; del Castillo-Vaquero, A.; Salido, G.M.; Gonzalez, A. Effect of cinnamtannin B-1 on cholecystokinin-8-evoked responses in mouse pancreatic acinar cells. Clin. Exp. Pharmacol. Physiol. 2010, 37, 980–988. [Google Scholar] [CrossRef] [PubMed]
- Nevalainen, T.J.; Hietaranta, A.J.; Gronroos, J.M. Phospholipase A2 in acute pancreatitis: New biochemical and pathological aspects. Hepato-Gastroenterology 1999, 46, 2731–2735. [Google Scholar] [PubMed]
- Lei, X.; Barbour, S.E.; Ramanadham, S. Group VIA Ca2+-independent phospholipase A2 (iPLA2beta) and its role in beta-cell programmed cell death. Biochimie 2010, 92, 627–637. [Google Scholar] [CrossRef] [PubMed]
- Süleyman, H.; Demircan, B.; Karagöz, Y. Anti-inflammatory and side effects of cyclooxygenase inhibitors. Pharmacol. Rep. 2007, 59, 247–258. [Google Scholar]
- Schlosser, W.; Schlosser, S.; Ramadani, M.; Gansauge, F.; Gansauge, S.; Beger, H.-G. Cyclooxygenase-2 is overexpressed in chronic pancreatitis. Pancreas 2002, 25, 26–30. [Google Scholar] [CrossRef]
- Huang, H.; Chen, J.; Peng, L.; Yao, Y.; Deng, D.; Zhang, Y.; Liu, Y.; Wang, H.; Li, Z.; Bi, Y.; et al. Transgenic expression of cyclooxygenase-2 in pancreatic acinar cells induces chronic pancreatitis. Am. J. Physiol. Gastrointest. Liver Physiol. 2019, 316, G179–G186. [Google Scholar] [CrossRef]
- Polito, F.; Bitto, A.; Irrera, N.; Squadrito, F.; Fazzari, C.; Minutoli, L.; Altavilla, D. Flavocoxid, a dual inhibitor of cyclooxygenase-2 and 5-lipoxygenase, reduces pancreatic damage in an experimental model of acute pancreatitis. Br. J. Pharmacol. 2010, 161, 1002–1011. [Google Scholar] [CrossRef]
- Warner, T.D.; Mitchell, J.A. Cyclooxygenases: New forms, new inhibitors, and lessons from the clinic. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2004, 18, 790–804. [Google Scholar] [CrossRef]
- Mateu, A.; Ramudo, L.; Manso, M.A.; De Dios, I. Cross-talk between TLR4 and PPARγ pathways in the arachidonic acid-induced inflammatory response in pancreatic acini. Int. J. Biochem. Cell Biol. 2015, 69, 132–141. [Google Scholar] [CrossRef]
- Zhou, W.; Levine, B.A.; Olson, M.S. Lipid mediator production in acute and chronic pancreatitis in the rat. J. Surg. Res. 1994, 56, 37–44. [Google Scholar] [CrossRef]
- Kilian, M.; Gregor, J.I.; Heukamp, I.; Wagner, C.; Walz, M.K.; Schimke, I.; Kristiansen, G.; Wenger, F.A. Early inhibition of prostaglandin synthesis by n-3 fatty acids determinates histologic severity of necrotizing pancreatitis. Pancreas 2009, 38, 436–441. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.-K.; Reding, T.; Bain, M.; Heikenwalder, M.; Bimmler, D.; Graf, R. Prostaglandin E2 modulates TNF-alpha-induced MCP-1 synthesis in pancreatic acinar cells in a PKA-dependent manner. Am. J. Physiol. Gastrointest. Liver Physiol. 2007, 293, G1196–G1204. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; An, C.S.; Yun, B.S.; Kang, K.S.; Lee, Y.A.; Won, S.M.; Gwag, B.J.; Cho, S.I.; Hahm, K.-B. Prevention effects of ND-07, a novel drug candidate with a potent antioxidative action and anti-inflammatory action, in animal models of severe acute pancreatitis. Eur. J. Pharmacol. 2012, 687, 28–38. [Google Scholar] [CrossRef] [PubMed]
- Reding, T.; Bimmler, D.; Perren, A.; Sun, L.-K.; Fortunato, F.; Storni, F.; Graf, R. A selective COX-2 inhibitor suppresses chronic pancreatitis in an animal model (WBN/Kob rats): Significant reduction of macrophage infiltration and fibrosis. Gut 2006, 55, 1165–1173. [Google Scholar] [CrossRef]
- Luci, D.K.; Jameson, J.B., 2nd; Yasgar, A.; Diaz, G.; Joshi, N.; Kantz, A.; Markham, K.; Perry, S.; Kuhn, N.; Yeung, J.; et al. Synthesis and structure-activity relationship studies of 4-((2-hydroxy-3-methoxybenzyl)amino)benzenesulfonamide derivatives as potent and selective inhibitors of 12-lipoxygenase. J. Med. Chem. 2014, 57, 495–506. [Google Scholar] [CrossRef] [PubMed]
- Mashima, R.; Okuyama, T. The role of lipoxygenases in pathophysiology; new insights and future perspectives. Redox Biol. 2015, 6, 297–310. [Google Scholar] [CrossRef]
- Chandrasekharan, J.A.; Sharma-Walia, N. Lipoxins: Nature’s way to resolve inflammation. J. Inflamm. Res. 2015, 8, 181–192. [Google Scholar] [CrossRef]
- Shahid, R.A.; Vigna, S.R.; Layne, A.C.; Romac, J.M.-J.; Liddle, R.A. Acinar Cell Production of Leukotriene B4 Contributes to Development of Neurogenic Pancreatitis in Mice. Cell. Mol. Gastroenterol. Hepatol. 2015, 1, 75–86. [Google Scholar] [CrossRef]
- Vigna, S.R.; Shahid, R.A.; Nathan, J.D.; McVey, D.C.; Liddle, R.A. Leukotriene B4 mediates inflammation via TRPV1 in duct obstruction-induced pancreatitis in rats. Pancreas 2011, 40, 708–714. [Google Scholar] [CrossRef]
- Hotter, G.; Closa, D.; Prats, N.; Pi, F.; Gelpí, E.; Roselló-Catafau, J. Free radical enhancement promotes leucocyte recruitment through a PAF and LTB4 dependent mechanism. Free Radic. Biol. Med. 1997, 22, 947–954. [Google Scholar] [CrossRef]
- Liao, D.; Qian, B.; Zhang, Y.; Wu, K.; Xu, M. Inhibition of 5-lipoxygenase represses neutrophils activation and activates apoptosis in pancreatic tissues during acute necrotizing pancreatitis. Biochem. Biophys. Res. Commun. 2018, 498, 79–85. [Google Scholar] [CrossRef] [PubMed]
- Oruc, N.; Yukselen, V.; Ozutemiz, A.O.; Yuce, G.; Celik, H.A.; Musoglu, A.; Batur, Y. Leukotriene receptor antagonism in experimental acute pancreatitis in rats. Eur. J. Gastroenterol. Hepatol. 2004, 16, 383–388. [Google Scholar] [CrossRef] [PubMed]
- Dobrian, A.D.; Huyck, R.W.; Glenn, L.; Gottipati, V.; Haynes, B.A.; Hansson, G.I.; Marley, A.; McPheat, W.L.; Nadler, J.L. Activation of the 12/15 lipoxygenase pathway accompanies metabolic decline in db/db pre-diabetic mice. Prostaglandins Other Lipid Mediat. 2018, 136, 23–32. [Google Scholar] [CrossRef] [PubMed]
- Pek, S.B.; Nathan, M.H. Role of eicosanoids in biosynthesis and secretion of insulin. Diabete Metab. 1994, 20, 146–149. [Google Scholar]
- Hwang, H.-J.; Park, K.-S.; Choi, J.H.; Cocco, L.; Jang, H.-J.; Suh, P.-G. Zafirlukast promotes insulin secretion by increasing calcium influx through L-type calcium channels. J. Cell. Physiol. 2018, 233, 8701–8710. [Google Scholar] [CrossRef]
- McDonnell, A.M.; Dang, C.H. Basic review of the cytochrome p450 system. J. Adv. Pract. Oncol. 2013, 4, 263–268. [Google Scholar] [CrossRef]
- Sarlis, N.J.; Gourgiotis, L. Hormonal effects on drug metabolism through the CYP system: Perspectives on their potential significance in the era of pharmacogenomics. Curr. Drug Targets Immune Endocr. Metab. Disord. 2005, 5, 439–448. [Google Scholar] [CrossRef]
- Sayed, T.S.; Maayah, Z.H.; Zeidan, H.A.; Agouni, A.; Korashy, H.M. Insight into the physiological and pathological roles of the aryl hydrocarbon receptor pathway in glucose homeostasis, insulin resistance, and diabetes development. Cell. Mol. Biol. Lett. 2022, 27, 103. [Google Scholar] [CrossRef]
- Falck, J.R.; Manna, S.; Moltz, J.; Chacos, N.; Capdevila, J. Epoxyeicosatrienoic acids stimulate glucagon and insulin release from isolated rat pancreatic islets. Biochem. Biophys. Res. Commun. 1983, 114, 743–749. [Google Scholar] [CrossRef]
- Bailey, R.; Cooper, J.D.; Zeitels, L.; Smyth, D.J.; Yang, J.H.M.; Walker, N.M.; Hyppönen, E.; Dunger, D.B.; Ramos-Lopez, E.; Badenhoop, K.; et al. Association of the vitamin D metabolism gene CYP27B1 with type 1 diabetes. Diabetes 2007, 56, 2616–2621. [Google Scholar] [CrossRef]
- Cao, R.-C.; Yang, W.-J.; Xiao, W.; Zhou, L.; Tan, J.-H.; Wang, M.; Zhou, Z.-T.; Chen, H.-J.; Xu, J.; Chen, X.-M.; et al. St13 protects against disordered acinar cell arachidonic acid pathway in chronic pancreatitis. J. Transl. Med. 2022, 20, 218. [Google Scholar] [CrossRef] [PubMed]
- Suresh, Y.; Das, U.N. Differential effect of saturated, monounsaturated, and polyunsaturated fatty acids on alloxan-induced diabetes mellitus. Prostaglandins, Leukot. Essent. Fat. Acids 2006, 74, 199–213. [Google Scholar] [CrossRef] [PubMed]
- Gundala, N.K.V.; Das, U.N. Arachidonic acid–rich ARASCO oil has anti-inflammatory and antidiabetic actions against streptozotocin + high fat diet induced diabetes mellitus in Wistar rats. Nutrition 2019, 66, 203–218. [Google Scholar] [CrossRef] [PubMed]
- Siegmund, E.; Weber, H.; Kasper, M.; Jonas, L. Role of PGE2 in the development of pancreatic injury induced by chronic alcohol feeding in rats. Pancreatol. Off. J. Int. Assoc. Pancreatol. 2003, 3, 26–35. [Google Scholar] [CrossRef] [PubMed]
- Shadhu, K.; Xi, C. Inflammation and pancreatic cancer: An updated review. Saudi J. Gastroenterol. Off. J. Saudi Gastroenterol. Assoc. 2019, 25, 3–13. [Google Scholar] [CrossRef]
- Ling, S.; Feng, T.; Jia, K.; Tian, Y.; Li, Y. Inflammation to cancer: The molecular biology in the pancreas (Review). Oncol. Lett. 2014, 7, 1747–1754. [Google Scholar] [CrossRef]
- Knab, L.M.; Grippo, P.J.; Bentrem, D.J. Involvement of eicosanoids in the pathogenesis of pancreatic cancer: The roles of cyclooxygenase-2 and 5-lipoxygenase. World J. Gastroenterol. 2014, 20, 10729–10739. [Google Scholar] [CrossRef]
- Subongkot, S.; Frame, D.; Leslie, W.; Drajer, D. Selective cyclooxygenase-2 inhibition: A target in cancer prevention and treatment. Pharmacotherapy 2003, 23, 9–28. [Google Scholar] [CrossRef]
- Sarkar, F.H.; Adsule, S.; Li, Y.; Padhye, S. Back to the future: COX-2 inhibitors for chemoprevention and cancer therapy. Mini Rev. Med. Chem. 2007, 7, 599–608. [Google Scholar] [CrossRef]
- Grossman, H.B. Selective COX-2 inhibitors as chemopreventive and therapeutic agents. Drugs Today 2003, 39, 203–212. [Google Scholar] [CrossRef]
- Anderson, W.F.; Umar, A.; Hawk, E.T. Cyclooxygenase inhibition in cancer prevention and treatment. Expert Opin. Pharmacother. 2003, 4, 2193–2204. [Google Scholar] [CrossRef] [PubMed]
- Stratton, M.S.; Alberts, D.S. Current application of selective COX-2 inhibitors in cancer prevention and treatment. Oncology 2002, 16, 37–51. [Google Scholar] [PubMed]
- Ding, X.-Z.; Tong, W.-G.; Adrian, T.E. Cyclooxygenases and lipoxygenases as potential targets for treatment of pancreatic cancer. Pancreatol. Off. J. Int. Assoc. Pancreatol. 2001, 1, 291–299. [Google Scholar] [CrossRef] [PubMed]
- Omura, N.; Griffith, M.; Vincent, A.; Li, A.; Hong, S.-M.; Walter, K.; Borges, M.; Goggins, M. Cyclooxygenase-deficient pancreatic cancer cells use exogenous sources of prostaglandins. Mol. Cancer Res. 2010, 8, 821–832. [Google Scholar] [CrossRef]
- Mensah, E.T.; Smyrk, T.; Zhang, L.; Bick, B.; Wood-Wentz, C.M.; Buttar, N.; Chari, S.T.; Gleeson, F.C.; Kendrick, M.; Levy, M.; et al. Cyclooxygenase-2 and Cytosolic Phospholipase A2 Are Overexpressed in Mucinous Pancreatic Cysts. Clin. Transl. Gastroenterol. 2019, 10, e00028. [Google Scholar] [CrossRef]
- Chiblak, S.; Steinbauer, B.; Pohl-Arnold, A.; Kucher, D.; Abdollahi, A.; Schwager, C.; Höft, B.; Esposito, I.; Müller-Decker, K. K-Ras and cyclooxygenase-2 coactivation augments intraductal papillary mucinous neoplasm and Notch1 mimicking human pancreas lesions. Sci. Rep. 2016, 6, 29455. [Google Scholar] [CrossRef]
- Hermanova, M.; Trna, J.; Nenutil, R.; Dite, P.; Kala, Z. Expression of COX-2 is associated with accumulation of p53 in pancreatic cancer: Analysis of COX-2 and p53 expression in premalignant and malignant ductal pancreatic lesions. Eur. J. Gastroenterol. Hepatol. 2008, 20, 732–739. [Google Scholar] [CrossRef]
- Kim, M.P.; Li, X.; Deng, J.; Zhang, Y.; Dai, B.; Allton, K.L.; Hughes, T.G.; Siangco, C.; Augustine, J.J.; Kang, Y.; et al. Oncogenic KRAS Recruits an Expansive Transcriptional Network through Mutant p53 to Drive Pancreatic Cancer Metastasis. Cancer Discov. 2021, 11, 2094–2111. [Google Scholar] [CrossRef]
- Appel, M.J.; Woutersen, R.A. Modulation of growth and cell turnover of preneoplastic lesions and of prostaglandin levels in rat pancreas by dietary fish oil. Carcinogenesis 1994, 15, 2107–2112. [Google Scholar] [CrossRef]
- Hennig, R.; Ding, X.-Z.; Tong, W.-G.; Schneider, M.B.; Standop, J.; Friess, H.; Büchler, M.W.; Pour, P.M.; Adrian, T.E. 5-Lipoxygenase and leukotriene B4 receptor are expressed in human pancreatic cancers but not in pancreatic ducts in normal tissue. Am. J. Pathol. 2002, 161, 421–428. [Google Scholar] [CrossRef]
- Hennig, R.; Kehl, T.; Noor, S.; Ding, X.-Z.; Rao, S.M.; Bergmann, F.; Fürstenberger, G.; Büchler, M.W.; Friess, H.; Krieg, P.; et al. 15-lipoxygenase-1 production is lost in pancreatic cancer and overexpression of the gene inhibits tumor cell growth. Neoplasia 2007, 9, 917–926. [Google Scholar] [CrossRef] [PubMed]
- Haraldsdóttir, S.; Guolaugsdóttir, E.; Ingólfsdóttir, K.; Ogmundsdóttir, H.M. Anti-proliferative effects of lichen-derived lipoxygenase inhibitors on twelve human cancer cell lines of different tissue origin in vitro. Planta Med. 2004, 70, 1098–1100. [Google Scholar] [CrossRef] [PubMed]
- Wenger, F.A.; Kilian, M.; Achucarro, P.; Heinicken, D.; Schimke, I.; Guski, H.; Jacobi, C.A.; Müller, J.M. Effects of Celebrex and Zyflo on BOP-induced pancreatic cancer in Syrian hamsters. Pancreatol. Off. J. Int. Assoc. Pancreatol. 2002, 2, 54–60. [Google Scholar] [CrossRef] [PubMed]
- Gregor, J.I.; Kilian, M.; Heukamp, I.; Kiewert, C.; Kristiansen, G.; Schimke, I.; Walz, M.K.; Jacobi, C.A.; Wenger, F.A. Effects of selective COX-2 and 5-LOX inhibition on prostaglandin and leukotriene synthesis in ductal pancreatic cancer in Syrian hamster. Prostaglandins Leukot. Essent. Fat. Acids 2005, 73, 89–97. [Google Scholar] [CrossRef] [PubMed]
- Tersey, S.A.; Bolanis, E.; Holman, T.R.; Maloney, D.J.; Nadler, J.L.; Mirmira, R.G. Minireview: 12-Lipoxygenase and Islet β-Cell Dysfunction in Diabetes. Mol. Endocrinol. 2015, 29, 791–800. [Google Scholar] [CrossRef] [PubMed]
- Gandhi, A.V.; Saxena, S.; Relles, D.; Sarosiek, K.; Kang, C.Y.; Chipitsyna, G.; Sendecki, J.A.; Yeo, C.J.; Arafat, H.A. Differential expression of cytochrome P450 omega-hydroxylase isoforms and their association with clinicopathological features in pancreatic ductal adenocarcinoma. Ann. Surg. Oncol. 2013, 20, S636–S643. [Google Scholar] [CrossRef]
- Agundez, J.A.G. Cytochrome P450 gene polymorphism and cancer. Curr. Drug Metab. 2004, 5, 211–224. [Google Scholar] [CrossRef]
- Song, B.-J.; Abdelmegeed, M.A.; Cho, Y.-E.; Akbar, M.; Rhim, J.S.; Song, M.-K.; Hardwick, J.P. Contributing Roles of CYP2E1 and Other Cytochrome P450 Isoforms in Alcohol-Related Tissue Injury and Carcinogenesis. Adv. Exp. Med. Biol. 2019, 1164, 73–87. [Google Scholar] [CrossRef]
- Standop, J.; Schneider, M.; Ulrich, A.; Büchler, M.W.; Pour, P.M. Differences in immunohistochemical expression of xenobiotic-metabolizing enzymes between normal pancreas, chronic pancreatitis and pancreatic cancer. Toxicol. Pathol. 2003, 31, 506–513. [Google Scholar] [CrossRef]
- Kadlubar, S.; Anderson, J.P.; Sweeney, C.; Gross, M.D.; Lang, N.P.; Kadlubar, F.F.; Anderson, K.E. Phenotypic CYP2A6 variation and the risk of pancreatic cancer. JOP 2009, 10, 263–270. [Google Scholar]
- De Wever, O.; Mareel, M. Role of tissue stroma in cancer cell invasion. J. Pathol. 2003, 200, 429–447. [Google Scholar] [CrossRef] [PubMed]
- Gola, M.; Sejda, A.; Godlewski, J.; Cieślak, M.; Starzyńska, A. Neural Component of the Tumor Microenvironment in Pancreatic Ductal Adenocarcinoma. Cancers 2022, 14, 5246. [Google Scholar] [CrossRef] [PubMed]
- Hughes, R.; Snook, A.E.; Mueller, A.C. The poorly immunogenic tumor microenvironment of pancreatic cancer: The impact of radiation therapy, and strategies targeting resistance. Immunotherapy 2022, 14, 1393–1405. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Iovanna, J.; Santofimia-Castaño, P. Stroma-targeting strategies in pancreatic cancer: A double-edged sword. J. Physiol. Biochem. 2023, 79, 213–222. [Google Scholar] [CrossRef]
- Fishbein, A.; Hammock, B.D.; Serhan, C.N.; Panigrahy, D. Carcinogenesis: Failure of resolution of inflammation? Pharmacol. Ther. 2021, 218, 107670. [Google Scholar] [CrossRef]
- Kim, W.; Son, B.; Lee, S.; Do, H.; Youn, B. Targeting the enzymes involved in arachidonic acid metabolism to improve radiotherapy. Cancer Metastasis Rev. 2018, 37, 213–225. [Google Scholar] [CrossRef]
- Ching, M.M.; Reader, J.; Fulton, A.M. Eicosanoids in Cancer: Prostaglandin E2 Receptor 4 in Cancer Therapeutics and Immunotherapy. Front. Pharmacol. 2020, 11, 819. [Google Scholar] [CrossRef]
- Bu, L.; Yonemura, A.; Yasuda-Yoshihara, N.; Uchihara, T.; Ismagulov, G.; Takasugi, S.; Yasuda, T.; Okamoto, Y.; Kitamura, F.; Akiyama, T.; et al. Tumor microenvironmental 15-PGDH depletion promotes fibrotic tumor formation and angiogenesis in pancreatic cancer. Cancer Sci. 2022, 113, 3579–3592. [Google Scholar] [CrossRef]
- Colby, J.K.; Klein, R.D.; McArthur, M.J.; Conti, C.J.; Kiguchi, K.; Kawamoto, T.; Riggs, P.K.; Pavone, A.I.; Sawicki, J.; Fischer, S.M. Progressive metaplastic and dysplastic changes in mouse pancreas induced by cyclooxygenase-2 overexpression. Neoplasia 2008, 10, 782–796. [Google Scholar] [CrossRef]
- Estaras, M.; Ortiz-Placin, C.; Castillejo-Rufo, A.; Fernandez-Bermejo, M.; Blanco, G.; Mateos, J.M.; Vara, D.; Gonzalez-Cordero, P.L.; Chamizo, S.; Lopez, D.; et al. Melatonin controls cell proliferation and modulates mitochondrial physiology in pancreatic stellate cells. J. Physiol. Biochem. 2023, 79, 235–249. [Google Scholar] [CrossRef]
- Grekova, S.P.; Angelova, A.; Daeffler, L.; Raykov, Z. Pancreatic Cancer Cell Lines Can Induce Prostaglandin E2 Production from Human Blood Mononuclear Cells. J. Oncol. 2011, 2011, 741868. [Google Scholar] [CrossRef] [PubMed]
- Charo, C.; Holla, V.; Arumugam, T.; Hwang, R.; Yang, P.; Dubois, R.N.; Menter, D.G.; Logsdon, C.D.; Ramachandran, V. Prostaglandin E2 Regulates Pancreatic Stellate Cell Activity via the EP4 Receptor. Pancreas 2013, 42, 467–474. [Google Scholar] [CrossRef] [PubMed]
- Masamune, A.; Kikuta, K.; Satoh, M.; Sakai, Y.; Satoh, A.; Shimosegawa, T. Ligands of peroxisome proliferator-activated receptor-gamma block activation of pancreatic stellate cells. J. Biol. Chem. 2002, 277, 141–147. [Google Scholar] [CrossRef] [PubMed]
- Estaras, M.; Gonzalez-Portillo, M.R.; Martinez, R.; Garcia, A.; Estevez, M.; Fernandez-Bermejo, M.; Mateos, J.M.; Vara, D.; Blanco-Fernández, G.; Lopez-Guerra, D.; et al. Melatonin Modulates the Antioxidant Defenses and the Expression of Proinflammatory Mediators in Pancreatic Stellate Cells Subjected to Hypoxia. Antioxidants 2021, 10, 577. [Google Scholar] [CrossRef]
- Morrison, A.H.; Byrne, K.T.; Vonderheide, R.H. Immunotherapy and Prevention of Pancreatic Cancer. Trends Cancer 2018, 4, 418–428. [Google Scholar] [CrossRef]
- Markosyan, N.; Li, J.; Sun, Y.H.; Richman, L.P.; Lin, J.H.; Yan, F.; Quinones, L.; Sela, Y.; Yamazoe, T.; Gordon, N.; et al. Tumor cell–intrinsic EPHA2 suppresses antitumor immunity by regulating PTGS2 (COX-2). J. Clin. Investig. 2019, 129, 3594–3609. [Google Scholar] [CrossRef]
- Zhang, Y.; Kirane, A.; Huang, H.; Sorrelle, N.B.; Burrows, F.J.; Dellinger, M.T.; Brekken, R.A. Cyclooxygenase-2 Inhibition Potentiates the Efficacy of Vascular Endothelial Growth Factor Blockade and Promotes an Immune Stimulatory Microenvironment in Preclinical Models of Pancreatic Cancer. Mol. Cancer Res. 2019, 17, 348–355. [Google Scholar] [CrossRef]
- Tian, W.; Jiang, X.; Kim, D.; Guan, T.; Nicolls, M.R.; Rockson, S.G. Leukotrienes in Tumor-Associated Inflammation. Front. Pharmacol. 2020, 11, 1289. [Google Scholar] [CrossRef]
- Moore, G.Y.; Pidgeon, G.P. Cross-Talk between Cancer Cells and the Tumour Microenvironment: The Role of the 5-Lipoxygenase Pathway. Int. J. Mol. Sci. 2017, 18, 236. [Google Scholar] [CrossRef]
- Janakiram, N.B.; Mohammed, A.; Bryant, T.; Ritchie, R.; Stratton, N.; Jackson, L.; Lightfoot, S.; Benbrook, D.M.; Asch, A.S.; Lang, M.L.; et al. Loss of natural killer T cells promotes pancreatic cancer in LSL-KrasG12D/+ mice. Immunology 2017, 152, 36–51. [Google Scholar] [CrossRef]
- Sarsour, E.H.; Son, J.M.; Kalen, A.L.; Xiao, W.; Du, J.; Alexander, M.S.; O’Leary, B.R.; Cullen, J.J.; Goswami, P.C. Arachidonate 12-lipoxygenase and 12-hydroxyeicosatetraenoic acid contribute to stromal aging-induced progression of pancreatic cancer. J. Biol. Chem. 2020, 295, 6946–6957. [Google Scholar] [CrossRef] [PubMed]
- Schnittert, J.; Heinrich, M.A.; Kuninty, P.R.; Storm, G.; Prakash, J. Reprogramming tumor stroma using an endogenous lipid lipoxin A4 to treat pancreatic cancer. Cancer Lett. 2018, 420, 247–258. [Google Scholar] [CrossRef] [PubMed]
- Rooney, P.H.; Telfer, C.; McFadyen, M.C.E.; Melvin, W.T.; Murray, G.I. The role of cytochrome P450 in cytotoxic bioactivation: Future therapeutic directions. Curr. Cancer Drug Targets 2004, 4, 257–265. [Google Scholar] [CrossRef] [PubMed]
- Guo, Z.; Johnson, V.; Barrera, J.; Porras, M.; Hinojosa, D.; Hernández, I.; McGarrah, P.; Potter, D.A. Targeting cytochrome P450-dependent cancer cell mitochondria: Cancer associated CYPs and where to find them. Cancer Metastasis Rev. 2018, 37, 409–423. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).