Triethylamine-Promoted Oxidative Cyclodimerization of 2H-Azirine-2-carboxylates to Pyrimidine-4,6-dicarboxylates: Experimental and DFT Study
Abstract
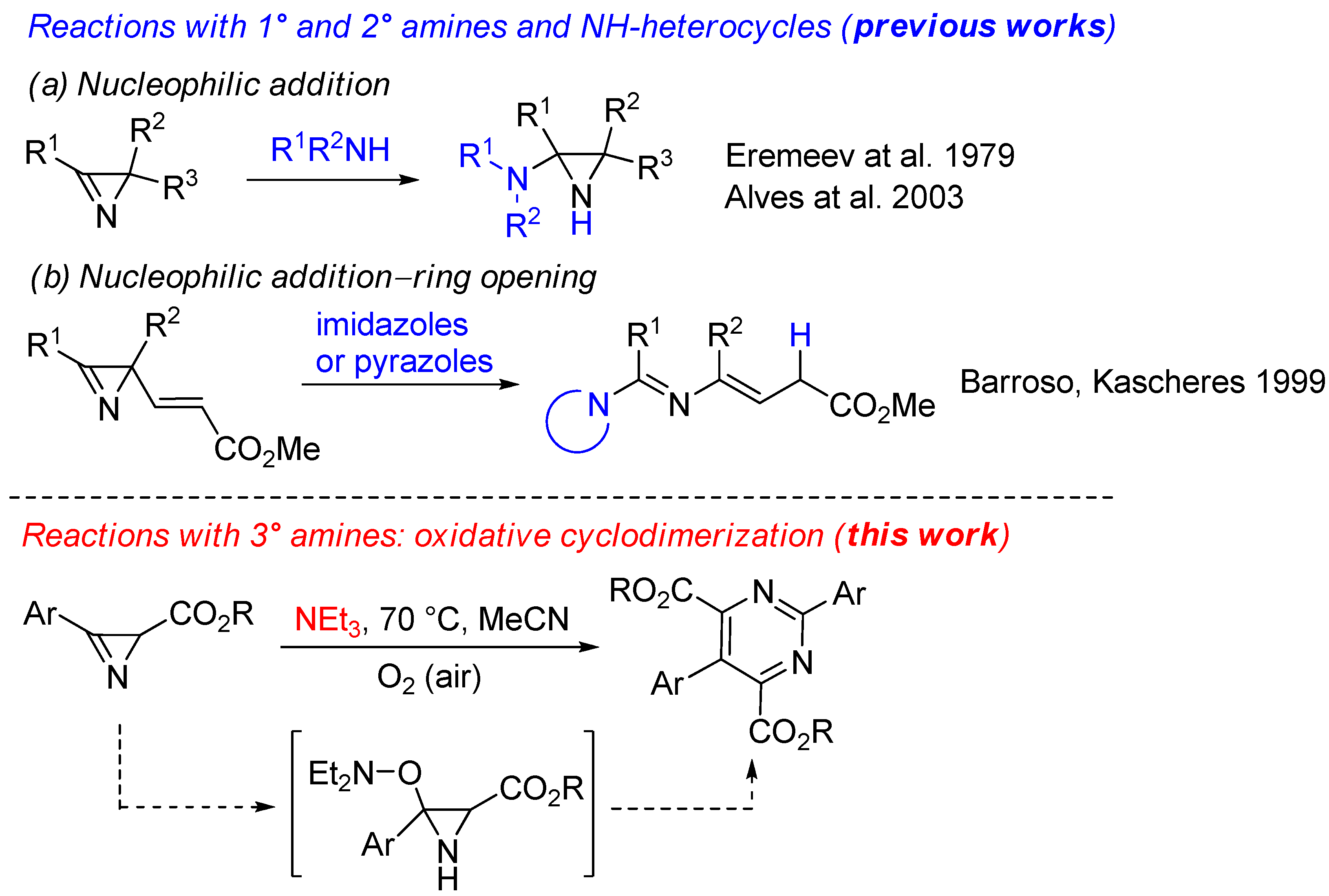
1. Introduction
2. Results and Discussion
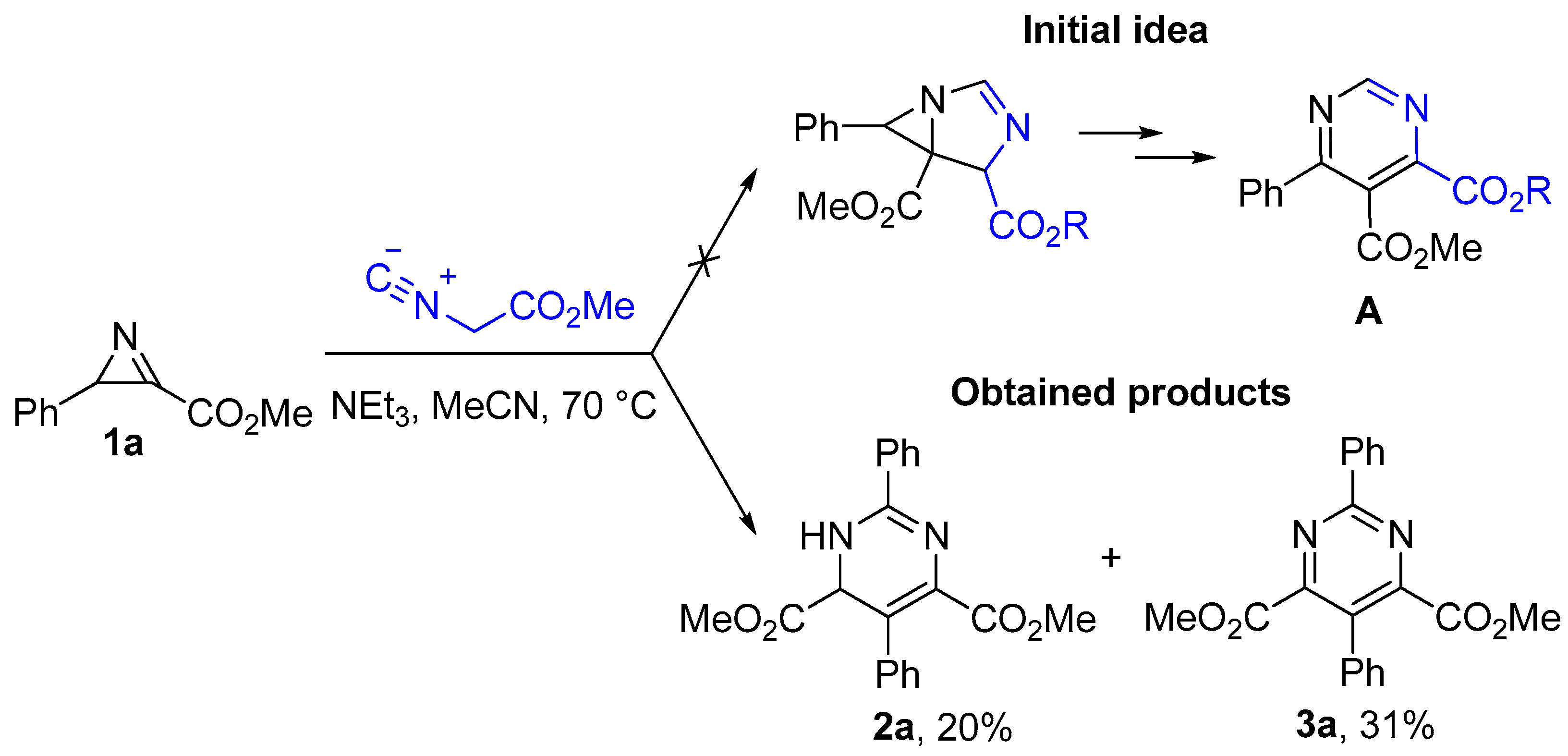
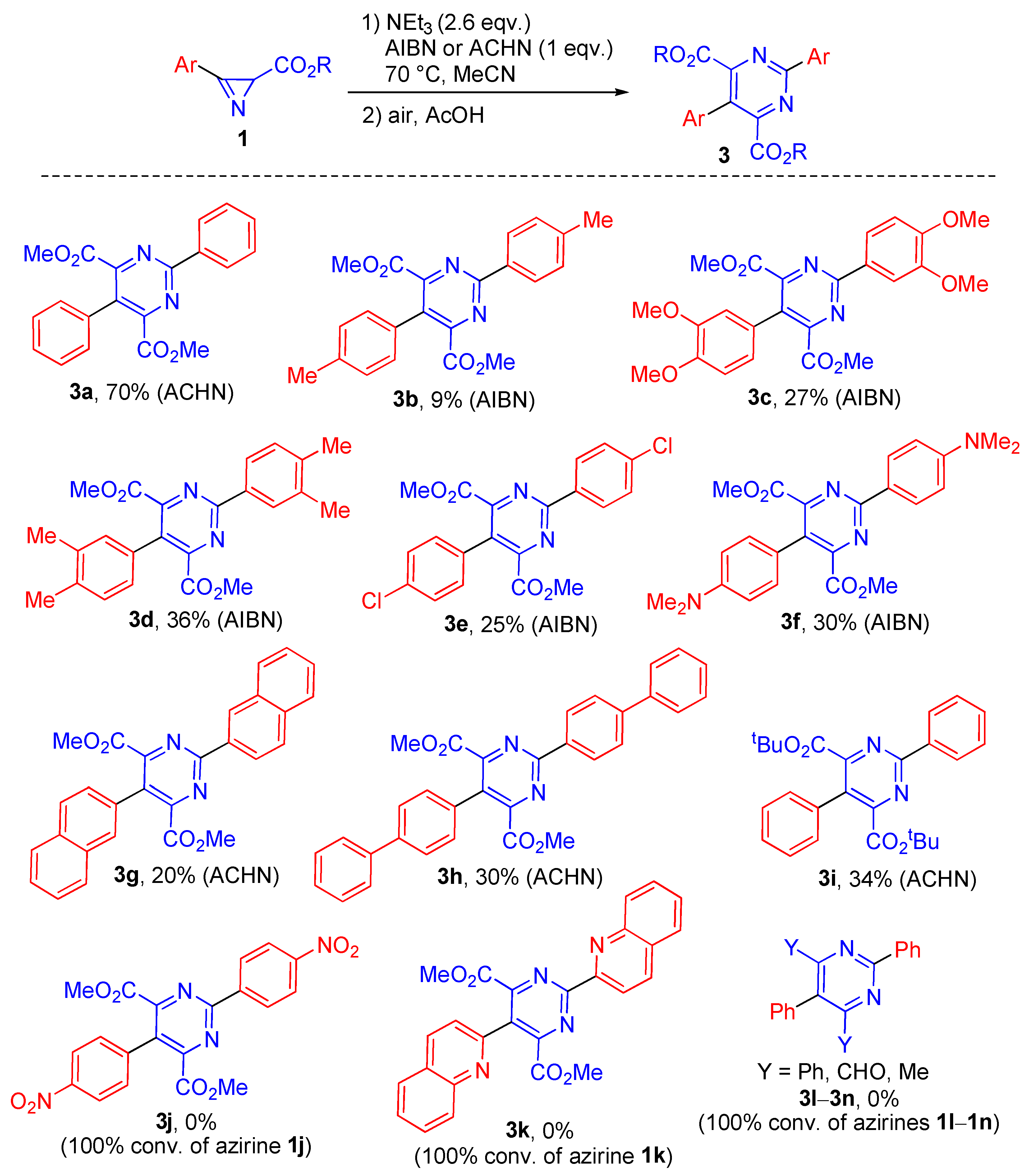
2.1. Synthesis of Pyrimidines
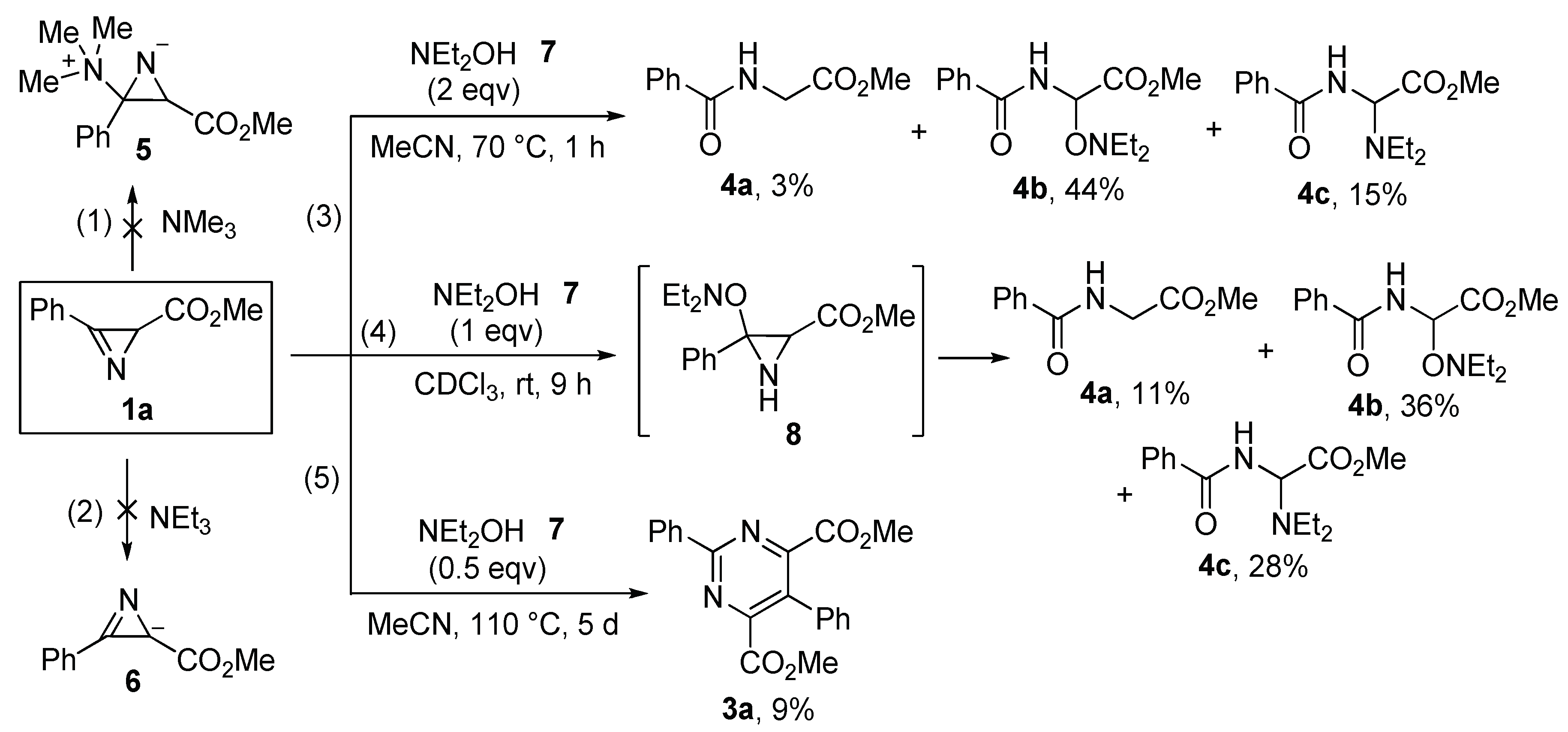
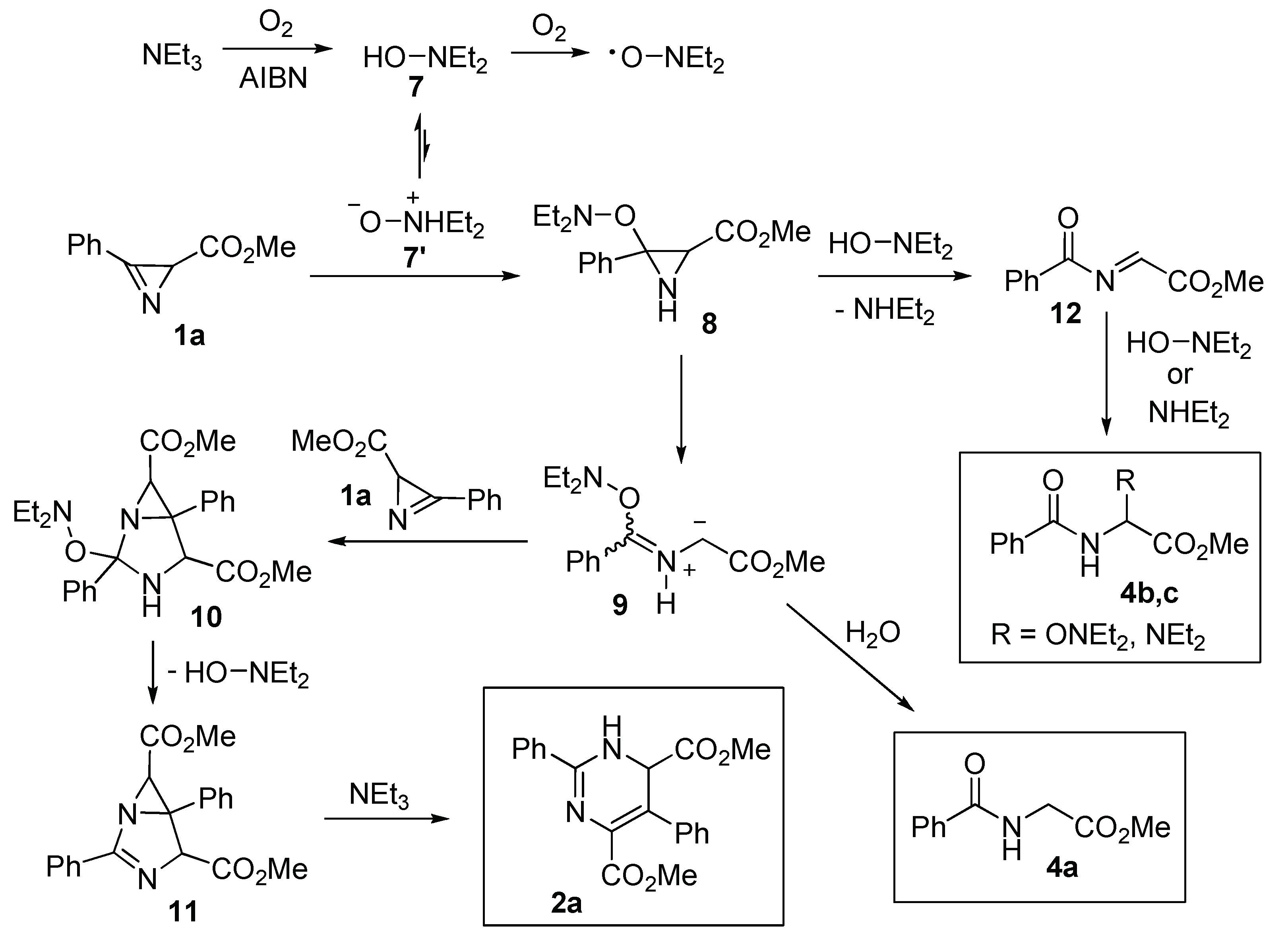
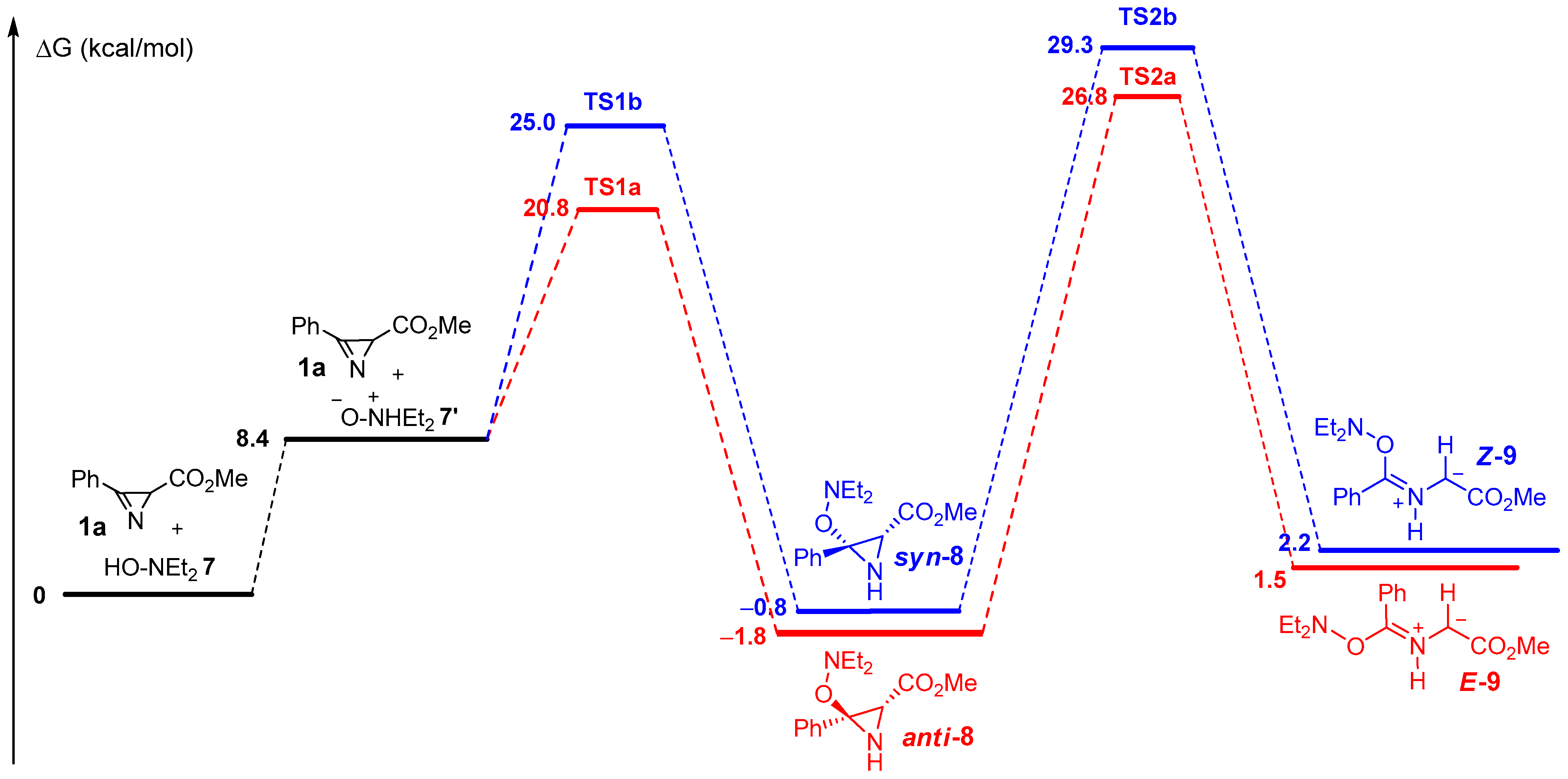
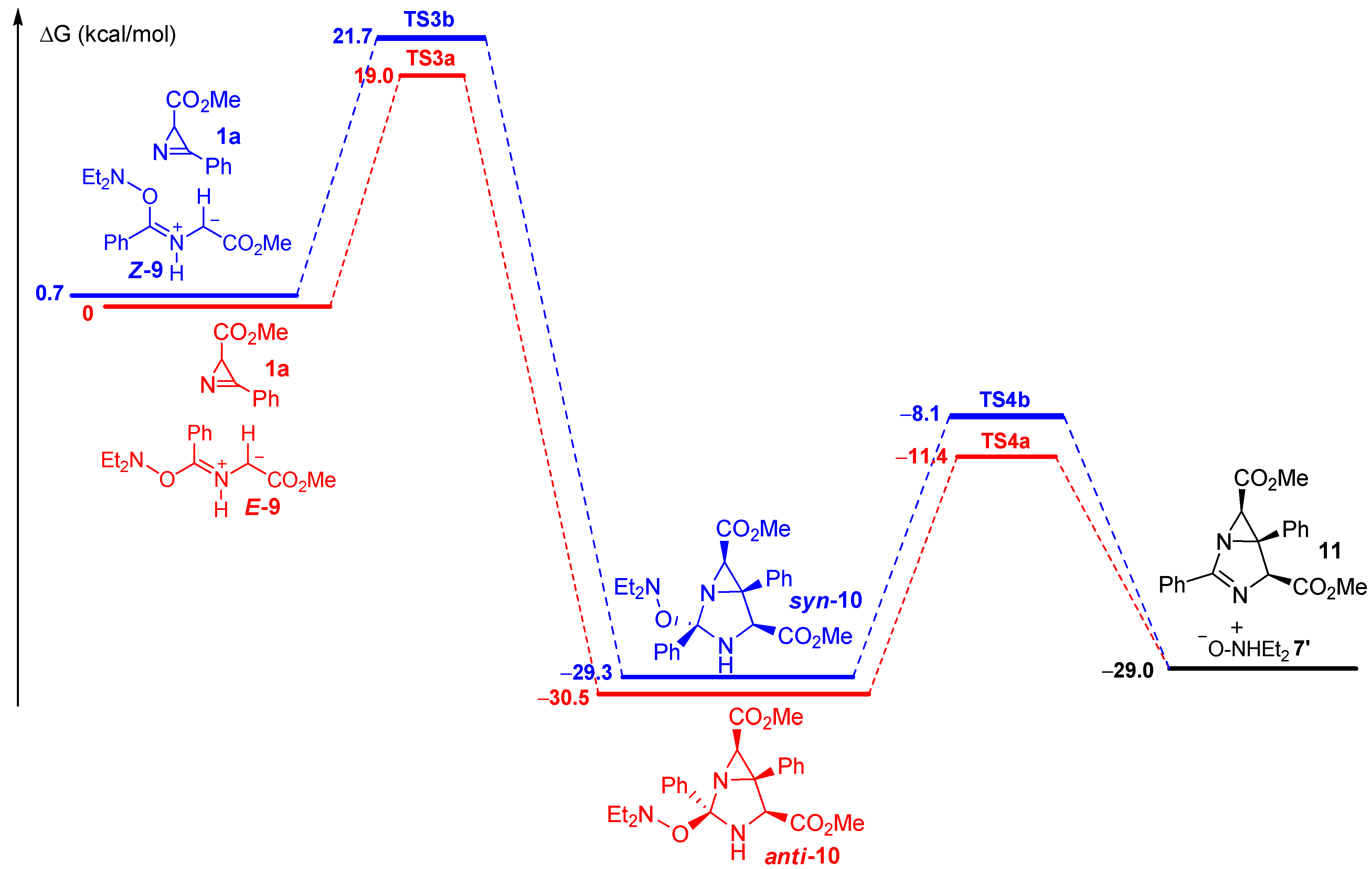
2.2. Study of the Reaction Mechanism
3. Materials and Methods
3.1. General Instrumentation
3.2. Synthesis and Characterization of 5-Methoxyisoxazoles
- 5-Methoxy-3-(naphthalen-2-yl)isoxazole [32]. Obtained as a pink solid (320 mg, 31%) according to the general procedure. Mp: 129–130 °C (lit. 128–129 °C [32]). 1H NMR (400 MHz, CDCl3), δ, ppm: 8.21 (s, 1H), 8.00–7.84 (m, 4H), 7.63–7.50 (m, 2H), 5.69 (s, 1H), 4.09 (s, 3H). 13C{1H} NMR (100 MHz, CDCl3), δ, ppm: 174.6, 164.3, 134.1, 133.2, 128.6, 128.5, 127.8, 127.0 (2C), 126.6, 126.3, 123.5, 75.5, 58.9.
- 3-(Biphenyl-4-yl)-5-methoxyisoxazole. Obtained as a colorless solid (740 mg, 64%) according to the general procedure. Mp: 147–148 °C. 1H NMR (400 MHz, CDCl3), δ, ppm: 7.88–7.83 (m, 2H), 7.73–7.68 (m, 2H), 7.67–7.63 (m, 2H), 7.52–7.46 (m, 2H), 7.43–7.38 (m, 1H), 5.59 (s, 1H), 4.09 (s, 3H). 13C{1H} NMR (100 MHz, CDCl3), δ, ppm: 174.5, 163.9, 142.9, 140.3, 128.9, 128.5, 127.8, 127.5, 127.1, 126.9, 75.4, 58.8. HRMS (ESI-TOF) calculated for C16H13NO2 [M + Na]+ 274.0838; found 274.0843.
- 5-Methoxy-3-(quinolin-2-yl)isoxazole. Obtained as a colorless solid (730 mg, 70%) according to the general procedure. Mp: 109–110 °C. 1H NMR (400 MHz, CDCl3), δ, ppm: 8.25 (d, J = 8.6 Hz, 1H), 8.20–8.04 (m, 2H), 7.87 (d, J = 8.1, 1H), 7.83–7.71 (m, 1H), 7.66–7.54 (m, 1H), 6.13 (s, 1H), 4.12 (s, 3H). 13C{1H} NMR (100 MHz, CDCl3), δ, ppm: 174.9, 165.3, 148.9, 148.0, 136.8, 129.9, 129.7, 128.4, 127.7, 127.3, 118.5, 76.3, 59.2. HRMS (ESI-TOF) calculated for C13H10N2NaO2 [M + Na]+ 249.0634; found 249.0638.
3.3. Synthesis and Characterization of 2H-Azirines 1
- Methyl 3-(3,4-dimethoxyphenyl)-2H-azirine-2-carboxylate (1c). Obtained as a colorless solid (97 mg, yield 97%) from 3-(3,4-dimethoxyphenyl)-5-methoxyisoxazole [33] according to the general procedure (40 mol% FeCl2·4H2O, 1.5 mL of acetonitrile). Mp: 96–97 °C. 1H NMR (400 MHz, CDCl3), δ, ppm: 7.46–7.42 (m, 2H), 7.02 (d, J = 8.8 Hz, 1H), 3.98 (s, 3H), 3.97 (s, 3H), 3.75 (s, 3H), 2.84 (s, 1H). 13C{1H} NMR (100 MHz, CDCl3), δ, ppm: 172.3, 157.5, 153.8, 149.7, 125.4, 114.6, 111.6, 111.1, 56.18, 56.17, 52.2, 29.6. HRMS (ESI-TOF) calculated for C12H13NNaO4 [M + Na]+ 258.0737; found 258.0743.
- Methyl 3-(4-chlorophenyl)-2H-azirine-2-carboxylate (1e) [34]. Obtained as a colorless solid (49 mg, yield 98%) from 3-(4-chlorophenyl)-5-methoxyisoxazole [35] according to the general procedure (10 mol% FeCl2·4H2O, 1.0 mL of acetonitrile). Mp: 66–67 °C (lit. 63.4–64.2 °C [34]). 1H NMR (400 MHz, CDCl3), δ, ppm: 7.88–7.82 (m, 2H), 7.61–7.56 (m, 2H), 3.77 (s, 3H), 2.89 (s, 1H).
- Methyl 3-(4-(dimethylamino)phenyl)-2H-azirine-2-carboxylate (1f). Obtained as a yellow solid (256 mg, yield 80%) from 3-(4-(dimethylamino)phenyl)-5-methoxyisoxazole [36] according to the general procedure (10 mol% FeCl2·4H2O, 5 mL of acetonitrile). Mp: 120–121 °C. 1H NMR (400 MHz, CDCl3), δ, ppm: 7.76–7.71 (m, 2H), 6.81–6.76 (m, 2H), 3.74 (s, 3H), 3.11 (s, 6H), 2.76 (s, 1H). 13C{1H} NMR (100 MHz, CDCl3), δ, ppm: 173.1, 156.2, 153.8, 132.4, 111.6, 108.4, 52.1, 40.1, 28.9. HRMS (ESI-TOF) calculated for C12H14N2NaO2 [M + Na]+ 241.0947; found 241.0951.
- Methyl 3-(naphthalen-2-yl)-2H-azirine-2-carboxylate (1g) [37]. Obtained as a colorless solid (600 mg, yield 90%) from 5-methoxy-3-(naphthalen-2-yl)isoxazole according to the general procedure (40 mol% FeCl2·4H2O, 15 mL of acetonitrile). Mp: 67–68 °C (lit. 69–70 °C [37]). 1H NMR (400 MHz, CDCl3), δ, ppm: 8.33 (s, 1H), 8.04–7.93 (m, 4H), 7.69–7.60 (m, 2H), 3.79 (s, 3H), 2.97 (s, 1H). 13C{1H} NMR (100 MHz, CDCl3), δ, ppm: 172.1, 158.5, 135.8, 132.9, 132.6, 129.4, 129.12, 129.08, 128.1, 127.3, 124.7, 119.5, 52.3, 29.7. HRMS (ESI-TOF) calculated for C14H11NNaO2 [M + Na]+ 248.0682; found 248.0685.
- Methyl 3-(biphenyl-4-yl)-2H-azirine-2-carboxylate (1h). Obtained as a colorless solid (195 mg, yield 99%) from 3-(biphenyl-4-yl)-5-methoxyisoxazole according to the general procedure (40 mol% FeCl2·4H2O, 6 mL of acetonitrile). Mp: 104–105 °C. 1H NMR (400 MHz, CDCl3), δ, ppm: 8.03–7.93 (m, 2H), 7.87–7.79 (m, 2H), 7.72–7.62 (m, 2H), 7.55–7.49 (m, 2H), 7.49–7.39 (m, 1H), 3.78 (s, 3H), 2.91 (s, 1H). 13C{1H} NMR (100 MHz, CDCl3), δ, ppm: 172.1, 158.2, 146.8, 139.5, 131.0, 129.1, 128.6, 128.0, 127.3, 120.9, 52.3, 29.5. HRMS (ESI-TOF) calculated for C16H13NNaO2 [M + Na]+ 274.0838; found 274.0839.
- Methyl 3-(quinolin-2-yl)-2H-azirine-2-carboxylate (1k). Obtained as a brown solid (50 mg, yield 40%) from 5-methoxy-3-(quinolin-2-yl)isoxazole according to the general procedure (10 mol% FeCl2·4H2O, 3 mL of acetonitrile). Mp: 110–111 °C. 1H NMR (400 MHz, CDCl3), δ, ppm: 8.40 (d, J = 8.4 Hz, 1H), 8.28 (d, J = 8.5 Hz, 1H), 8.16 (d, J = 8.4 Hz, 1H), 7.94 (d, J = 8.1 Hz, 1H), 7.87–7.83 (m, 1H), 7.74–7.70 (m, 1H), 3.80 (s, 3H), 3.17 (s, 1H). 13C{1H} NMR (100 MHz, CDCl3), δ, ppm: 171.6, 160.9, 148.5, 142.7, 137.5, 130.8, 130.6, 129.4, 129.3, 127.8, 121.7, 52.5, 31.5. HRMS (ESI-TOF) calculated for C13H10N2NaO2 [M + Na]+ 249.0634; found 249.0632.
3.4. Reaction of Azirine 1a with Triethylamine
- Dimethyl 2,5-diphenyl-1,6-dihydropyrimidine-4,6-dicarboxylate (2a). Colorless oil. 1H NMR (400 MHz, CDCl3), δ, ppm: 7.90–7.86 (m, 2H), 7.76 (br.s, 1H), 7.54–7.46 (m, 3H), 7.40–7.28 (m, 5H), 5.29 (br.s, 1H), 3.71 (s, 3H), 3.63 (s, 3H). 13C{1H} NMR (100 MHz, CDCl3), δ, ppm: 171.0, 163.6, 153.9, 137.2, 133.6, 131.1, 128.8 (2C), 128.0, 127.8, 126.8, 124.3, 118.9, 65.8, 52.41, 52.36. HRMS (ESI-TOF) calculated for C20H19N2O4 [M + H]+ 351.1339; found 351.1350.
- Dimethyl 2,5-diphenylpyrimidine-4,6-dicarboxylate (3a) [38]. Mp: 160–161 °C (lit. 161–163 °C [38]). 1H NMR (400 MHz, CDCl3), δ, ppm: 8.58–8.52 (m, 2H), 7.55–7.50 (m, 3H), 7.49–7.42 (m, 3H), 7.37–7.32 (m, 2H), 3.76 (s, 6H). 13C{1H} NMR (100 MHz, CDCl3), δ, ppm: 165.4, 163.5, 158.6, 135.8, 132.8, 131.6, 128.9, 128.7, 128.61, 128.58, 128.4, 127.8, 52.8.
3.5. Synthesis and Characterization of Pyrimidines 3
- Dimethyl 2,5-diphenylpyrimidine-4,6-dicarboxylate (3a) [38]. Obtained as a colorless solid (42 mg, yield 70%) according to the general procedure (initiator: ACHN, 3 days, eluent: benzene–ethylacetate, 5:1).
- Dimethyl 2,5-di(p-tolyl)pyrimidine-4,6-dicarboxylate (3b) [38]. Obtained as a colorless solid (6 mg, yield 9%) according to the general procedure (initiator: AIBN, 7 days, eluent: hexane–ethylacetate, 2:1). Mp: 145–146 °C (lit. 144–146 °C [38]). 1H NMR (400 MHz, CDCl3), δ, ppm: 8.47–8.40 (m, 2H), 7.36–7.30 (m, 2H), 7.27–7.19 (m, 4H), 3.78 (s, 6H), 2.46 (s, 3H), 2.43 (s, 3H). 13C{1H} NMR (100 MHz, CDCl3), δ, ppm: 165.7, 163.4, 158.6, 142.0, 138.8, 133.2, 129.8, 129.4, 129.2, 128.7, 128.5, 127.5, 52.8, 21.6, 21.4. HRMS (ESI-TOF) calculated for C22H20N2NaO4 [M + Na]+ 399.1315; found 399.1318.
- Dimethyl 2,5-di(3,4-dimethoxyphenyl)pyrimidine-4,6-dicarboxylate (3c) [38]. Obtained as a pale yellow solid (20 mg, yield 27%) according to the general procedure (initiator: AIBN, 7 days, eluent: hexane–ethylacetate, 3:1). Mp: 163–165 °C (lit. 164–166 °C [38]). 1H NMR (400 MHz, CDCl3), δ, ppm: 8.18 (dd, J = 8.5, 2.0 Hz, 1H), 8.05 (d, J = 2.0 Hz, 1H), 6.98 (d, J = 8.5 Hz, 1H), 6.95–6.86 (m, 3H), 4.03 (s, 3H), 3.99 (s, 3H), 3.95 (s, 3H), 3.90 (s, 3H), 3.79 (s, 6H). 13C{1H} NMR (100 MHz, CDCl3), δ, ppm: 165.8, 162.9, 158.7, 152.2, 149.5, 149.1, 148.9, 128.7, 126.3, 125.0, 122.6, 121.5, 111.8, 111.1, 111.0, 110.8, 56.1, 56.00, 55.96, 55.8, 52.9.
- Dimethyl 2,5-di(3,4-dimethylphenyl)pyrimidine-4,6-dicarboxylate (3d). Obtained as a colorless solid (25 mg, yield 36%) according to the general procedure (initiator: AIBN, 7 days, eluent: benzene–ethylacetate, 5:1). Mp: 173–174 °C. 1H NMR (400 MHz, CDCl3), δ, ppm: 8.30 (s, 1H), 8.29–8.24 (m, 1H), 7.31–7.25 (m, 1H), 7.19 (d, J = 7.7 Hz, 1H), 7.10 (s, 1H), 7.09–7.04 (m, 1H), 3.79 (s, 6H), 2.39 (s, 3H), 2.36 (s, 3H), 2.33 (s, 3H), 2.31 (s, 3H). 13C{1H} NMR (125 MHz, CDCl3), δ, ppm: 165.8, 163.4, 158.6, 140.7, 137.4, 136.9, 136.8, 133.5, 130.2, 130.0, 129.8, 129.7, 129.6, 127.3, 126.3, 126.0, 52.8, 19.9, 19.80, 19.76, 19.7. HRMS (ESI-TOF) calculated for C24H24N2NaO4 [M + Na]+ 427.1628; found 427.1631.
- Dimethyl 2,5-di(4-chlorophenyl)pyrimidine-4,6-dicarboxylate (3e) [38]. Obtained as a bright-yellow solid (18 mg, yield 25%) according to the general procedure (initiator: AIBN, 4 days, eluent: hexane–ethylacetate, 3:1). Mp: 137–138 °C (lit. 139–140 °C [38]). 1H NMR (400 MHz, CDCl3), δ, ppm: 8.52–8.49 (m, 2H), 7.51–7.49 (m, 2H), 7.46–7.44 (m, 2H), 7.28–7.26 (m, 2H), 3.80 (s, 6H).
- Dimethyl 2,5-di(4-dimethylaminophenyl)pyrimidine-4,6-dicarboxylate (3f). Obtained as a bright-yellow solid (22 mg, yield 30%) according to the general procedure (initiator: AIBN, 7 days, eluent: hexane–ethylacetate, 2:1). Mp: 216–217 °C. 1H NMR (400 MHz, CDCl3), δ, ppm: 8.41–8.38 (m, 2H), 7.19–7.17 (m, 2H), 6.77–6.73 (m, 4H), 3.79 (s, 6H), 3.08 (s, 6H), 3.02 (s, 6H). 13C{1H} NMR (125 MHz, CDCl3), δ, ppm: 166.4, 162.9, 158.5, 152.5, 150.3, 130.1, 129.5, 125.8, 123.7, 120.2, 112.0, 111.4, 52.7, 40.24, 40.29. HRMS (ESI-TOF) calculated for C24H26N4NaO4 [M + Na]+ 457.1846; found 457.1852.
- Dimethyl 2,5-di(naphthalen-2-yl)pyrimidine-4,6-dicarboxylate (3g) [38]. Obtained as a pale-yellow solid (15 mg, yield 20%) according to the general procedure (initiator: ACHN, 6 days, eluent: benzene–ethylacetate, 5:1). Mp: 196–197 °C (lit. 197–198 °C [38]). 1H NMR (400 MHz, CDCl3), δ, ppm: 9.15 (s, 1H), 8.66 (dd, J = 8.6, 1.8 Hz, 1H), 8.10–8.03 (m, 1H), 8.00 (d, J = 8.6 Hz, 1H), 7.97–7.88 (m, 4H), 7.84 (s, 1H), 7.63–7.53 (m, 4H), 7.48 (dd, J = 8.6, 1.8 Hz, 1H), 3.74 (s, 6H). 13C{1H} NMR (125 MHz, CDCl3), δ, ppm: 165.6, 163.6, 158.9, 135.2, 133.2, 133.14, 133.09, 133.0, 130.4, 129.7, 129.5, 128.5, 128.3 (2C), 128.0, 127.89, 127.87, 127.8, 127.7, 127.0, 126.8, 126.5, 126.3, 125.2, 53.0. HRMS (ESI-TOF) calculated for C28H20N2NaO4 [M + Na]+ 471.1315; found 471.1307.
- Dimethyl 2,5-di(4-biphenyl)pyrimidine-4,6-dicarboxylate (3h). Obtained as a colorless solid (30 mg, yield 36%) according to the general procedure (initiator: ACHN, 6 days, eluent: benzene–ethylacetate, 1:1). Mp: 222–223 °C. 1H NMR (400 MHz, CDCl3), δ, ppm: 8.67–8.61 (m, 2H), 7.80–7.75 (m, 2H), 7.74–7.66 (m, 6H), 7.54–7.48 (m, 4H), 7.46–7.39 (m, 4H), 3.81 (s, 6H). 13C{1H} NMR (125 MHz, CDCl3), δ, ppm: 165.6, 163.3, 158.7, 144.3, 141.6, 140.3, 140.0, 134.7, 131.7, 129.3, 129.1, 128.92, 128.88, 127.9, 127.8, 127.5, 127.4, 127.2, 127.13, 127.09, 53.0. HRMS (ESI-TOF) calculated for C32H24N2NaO4 [M + Na]+ 523.1628; found 523.1616.
- Di-tert-butyl 2,5-diphenylpyrimidine-4,6-dicarboxylate (3i) [38]. Obtained as a colorless solid (25 mg, yield 34%) according to the general procedure (initiator: ACHN, 3 days, eluent: benzene–ethylacetate, 5:1). Mp: 128–129 °C (lit. 124–125 °C [38]). 1H NMR (400 MHz, CDCl3), δ, ppm: 8.61–8.57 (m, 2H), 7.56–7.49 (m, 3H), 7.49–7.43 (m, 3H), 7.41–7.34 (m, 2H), 1.28 (s, 18H). 13C{1H} NMR (100 MHz, CDCl3), δ, ppm: 164.3, 163.6, 159.5, 136.2, 133.7, 131.3, 129.5, 128.8, 128.53, 128.49, 128.2, 126.6, 83.8, 27.6.
3.6. Reaction of Azirine 1a with N,N-Diethylhydroxylamine
- Methyl 2-benzamidoacetate (4a) [39]. 1H NMR (400 MHz, CDCl3), δ, ppm: 7.86–7.82 (m, 2H), 7.58–7.52 (m, 1H), 7.50–7.44 (m, 2H), 6.69 (br.s, 1H), 4.28 (d, J = 5.1 Hz, 2H), 3.83 (s, 3H).
- Methyl 2-benzamido-2-((diethylamino)oxy)acetate (4b). Yellow oil. 1H NMR (400 MHz, CDCl3), δ, ppm: 7.93–7.82 (m, 2H), 7.59–7.53 (m, 1H), 7.51–7.44 (m, 2H), 7.23 (br.d, J = 9.3 Hz, 1H), 6.07 (d, J = 9.3 Hz, 1H), 3.85 (s, 3H), 2.86 (m, 4H), 1.12 (t, J = 7.1 Hz, 6H). 13C{1H} NMR (100 MHz, CDCl3), δ, ppm: 168.8 (C(O)O), 166.6 (C(O)N), 133.5 (ipso-C), 132.2 (para-C), 128.7 (meta-C), 127.2 (ortho-C), 79.6 (CH), 52.6 (CH3O), 52.2 (CH2N), 11.7 (CH3). HRMS (ESI-TOF) calculated for C14H20N2NaO4 [M + Na]+ 303.1315; found 303.1311.
- Methyl 2-benzamido-2-(diethylamino)acetate (4c). Yellow oil. 1H NMR (400 MHz, CDCl3), δ, ppm: 7.85–7.80 (m, 2H), 7.58–7.51 (m, 1H), 7.50–7.42 (m, 2H), 6.99 (br.d, J = 8.3 Hz, 1H), 5.71 (d, J = 8.3 Hz, 1H), 3.83 (s, 3H), 2.76–2.60 (m, 4H), 1.18 (t, J = 7.1 Hz, 6H). 13C{1H} NMR (125 MHz, CDCl3), δ, ppm: 171.2 (C(O)O), 167.7 (C(O)N), 133.9 (ipso-C), 131.8 (para-C), 128.6 (meta-C), 127.1 (ortho-C), 66.5 (CH), 52.8 (CH3O), 44.1 (CH2N), 13.4 (CH3). HRMS (ESI-TOF) calculated for C14H20N2NaO3 [M + Na]+ 287.1366; found 287.1371.
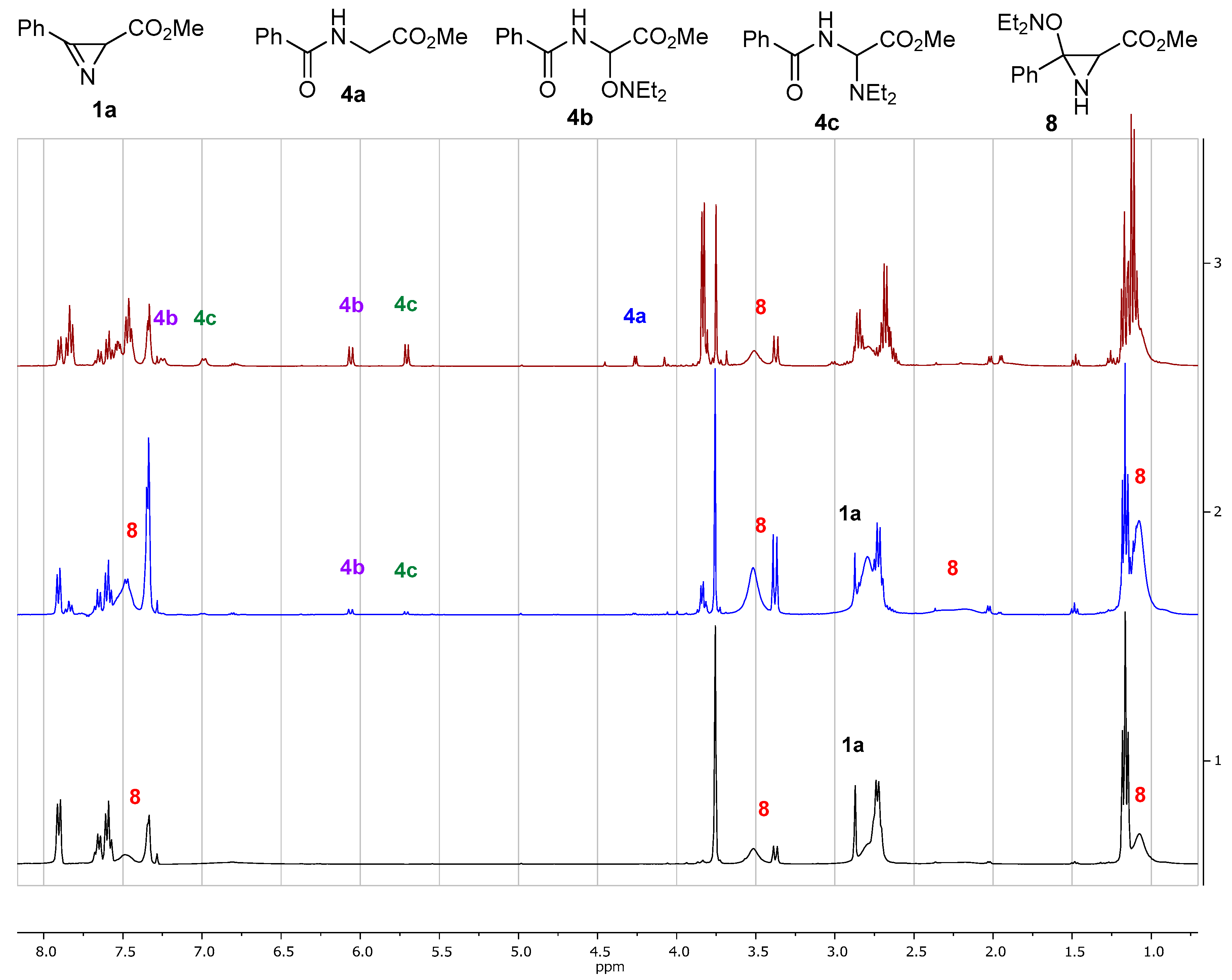
3.7. NMR Detection of Aziridine 8
- Methyl 3-(diethylaminooxy)-3-phenylaziridine-2-carboxylate (8). 1H NMR (500 MHz, CDCl3,–40 °C), δ, ppm: 7.58–7.53 (m, 2.5H, ortho-H, dia-2), 7.45–7.33 (m, 8.5H), 3.61 (s, 3H, CH3O, dia-1), 3.45 (s, 3.5H, CH3O, dia-2), 3.43 (d, J = 8.9 Hz, 1H, CH, dia-1), 3.36 (d, J = 10.2 Hz, 1.2H, CH, dia-2), 2.85–2.56 (m), 2.44 (d, J = 10.2 Hz, 1.2H, NH, dia-2), 2.19 (d, J = 8.9 Hz, 1H, NH, dia-1), 1.24–1.07 (m), 0.99 (t, J = 7.1 Hz, 3H, CH3, dia-1). 13C{1H} NMR (125 MHz, CDCl3,–40 °C), δ, ppm: aziridine carbons 78.0 (C, dia-1), 77.8 (C, dia-2), 38.7 (CH, dia-1), 37.2 (CH, dia-2).
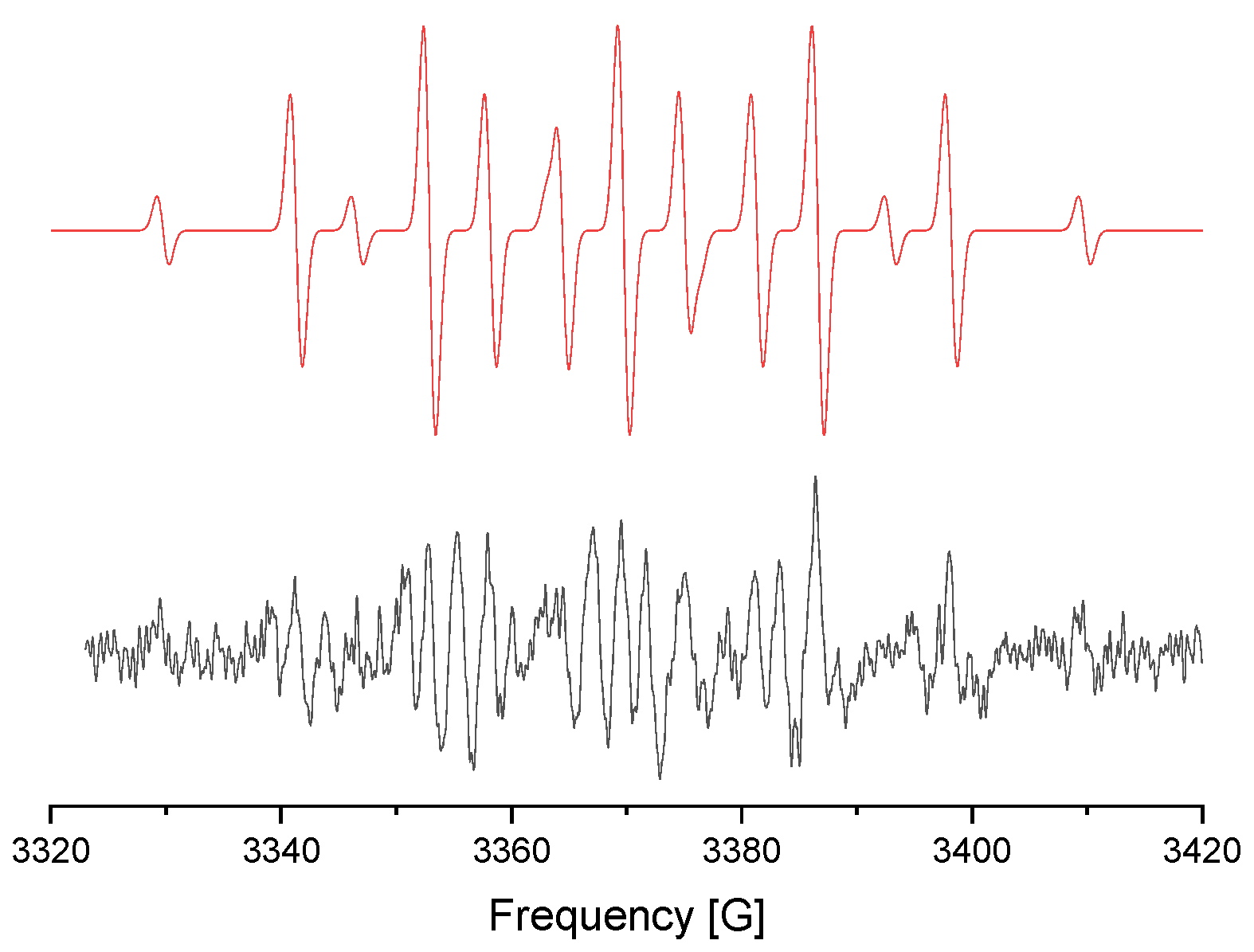
3.8. EPR Detection of Nitroxyl Radical Et2N-O·
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Khlebnikov, A.F.; Novikov, M.S.; Rostovskii, N.V. Advances in 2H-Azirine Chemistry: A Seven-Year Update. Tetrahedron 2019, 75, 2555–2624. [Google Scholar] [CrossRef]
- Darbandizadeh, S.A.; Amiri, K.; Rominger, F.; Balalaie, S. Synthesis of Naphthyridine and Azepine Backbones through Formal [4 + 3] and [4 + 2] Annulation via Cascade Ring-Opening/Cyclization Reaction of 2H-Azirines. Eur. J. Org. Chem. 2023, 26, e202201109. [Google Scholar] [CrossRef]
- Liu, Y.; He, Z.; Ma, W.; Bao, G.; Li, Y.; Yu, C.; Li, J.; E, R.; Xu, Z.; Wang, R.; et al. Copper(I)-Catalyzed Late-Stage Introduction of Oxime Ethers into Peptides at the Carboxylic Acid Site. Org. Lett. 2022, 24, 9248–9253. [Google Scholar] [CrossRef]
- Jiao, L.; Wang, Y.; Ding, L.; Zhang, C.; Wang, X.-N.; Chang, J. Synthesis of 2-Aminopyrroles Via Metal-Free Annulation of Ynamides with 2H-Azirines. J. Org. Chem. 2022, 87, 15564–15570. [Google Scholar] [CrossRef] [PubMed]
- Teng, Y.; Fang, T.; Lin, Z.; Qin, L.; Jiang, M.; Wu, W.; You, Y.; Weng, Z. Ring-expansion reaction for the synthesis of 2-(trifluoromethyl)oxazoles and 3-(trifluoromethyl)-1,2,4-triazines. Tetrahedron Lett. 2022, 107, 154100. [Google Scholar] [CrossRef]
- Sakharov, P.A.; Rostovskii, N.V.; Khlebnikov, A.F.; Novikov, M.S. Copper(II)-Catalyzed (3+2) Cycloaddition of 2H-Azirines to Six-Membered Cyclic Enols as a Route to Pyrrolo[3,2-c]quinolone, Chromeno[3,4-b]pyrrole, and Naphtho[1,8-ef]indole Scaffolds. Molecules 2022, 27, 5681. [Google Scholar] [CrossRef]
- Alves, M.J.; Teixeira e Costa, F. 2H-Azirines as Electrophiles in Heterocyclic Targets in Advanced Organic Synthesis; Research Signpost: Trivandrum, India, 2011; pp. 145–172. [Google Scholar]
- Eremeev, A.V.; Él’kinson, R.S.; Myagi, M.Y.; Liepin’sh, É.É. Reactions of 2,2-dimethyl-3-phenylazirine with amines. Chem. Heterocycl. Compd. 1979, 15, 1088–1090. [Google Scholar] [CrossRef]
- Alves, M.J.; Gil Fortes, A.; Gonçalves, L.F. Optically active aziridine esters by nucleophilic addition of nitrogen heterocycles to a chiral 2H-azirine-2-carboxylic ester. Tetrahedron Lett. 2003, 44, 6277–6279. [Google Scholar] [CrossRef]
- Nakamura, S. Enantioselective Reaction of 2H-Azirines. Chem. Asian J. 2019, 14, 1323–1330. [Google Scholar] [CrossRef]
- Callebaut, G.; Meiresonne, T.; De Kimpe, N.; Mangelinckx, S. Synthesis and Reactivity of 2-(Carboxymethyl)aziridine Derivatives. Chem. Rev. 2014, 114, 7954–8015. [Google Scholar] [CrossRef]
- Rotstein, B.H.; Zaretsky, S.; Rai, V.; Yudin, A.K. Small Heterocycles in Multicomponent Reactions. Chem. Rev. 2014, 114, 8323–8359. [Google Scholar] [CrossRef]
- Singh, G.S.; D’hooghe, M.; De Kimpe, N. Synthesis and Reactivity of C-Heteroatom-Substituted Aziridines. Chem. Rev. 2007, 107, 2080–2135. [Google Scholar] [CrossRef] [PubMed]
- Sakharov, P.A.; Rostovskii, N.V.; Khlebnikov, A.F.; Khoroshilova, O.V.; Novikov, M.S. Transition Metal-Catalyzed Synthesis of 3-Coumaranone-Containing NH-Aziridines from 2H-Azirines: Nickel(II) versus Gold(I). Adv. Synth. Catal. 2019, 361, 3359–3372. [Google Scholar] [CrossRef]
- Hu, H.; Xu, J.; Liu, W.; Dong, S.; Lin, L.; Feng, X. Copper-Catalyzed Asymmetric Addition of Tertiary Carbon Nucleophiles to 2H-Azirines: Access to Chiral Aziridines with Vicinal Tetrasubstituted Stereocenters. Org. Lett. 2018, 20, 5601–5605. [Google Scholar] [CrossRef] [PubMed]
- Vélez del Burgo, A.; Ochoa de Retana, A.M.; de los Santos, J.M.; Palacios, F. Reaction of 2H-Azirine-Phosphine Oxides and -Phosphonates with Enolates Derived from β-Keto Esters. J. Org. Chem. 2016, 81, 100–108. [Google Scholar] [CrossRef]
- Nakamura, S.; Hayama, D. Enantioselective Reaction of 2H-Azirines with Phosphite Using Chiral Bis(imidazoline)/Zinc(II) Catalysts. Angew. Chem. Int. Ed. 2017, 56, 8785–8789. [Google Scholar] [CrossRef]
- Pusch, S.; Kowalczyk, D.; Opatz, T. A Photoinduced Cobalt-Catalyzed Synthesis of Pyrroles through in Situ-Generated Acylazirines. J. Org. Chem. 2016, 81, 4170–4178. [Google Scholar] [CrossRef]
- Galenko, A.V.; Khlebnikov, A.F.; Novikov, M.S.; Avdontseva, M.S. Synthesis of 3-(1,2-dioxoethyl)- and 2,3-dicarbonyl-containing pyrroles. Tetrahedron 2015, 71, 1940–1951. [Google Scholar] [CrossRef]
- Barroso, M.T.; Kascheres, A. Electronically Mediated Selectivity in Ring Opening of 1-Azirines. The 3-Z Mode: Convenient Route to 2-Aza-1,3-dienes. J. Org. Chem. 1999, 64, 49–53. [Google Scholar] [CrossRef]
- Auricchio, S.; Grassi, S.; Malpezzi, L.; Sartori, A.S.; Truscello, A.M. New Cleavage of the Azirine Ring by Single Electron Transfer: The Synthesis of 2H-Imidazoles, Pyridazines and Pyrrolines. Eur. J. Org. Chem. 2001, 2001, 1183–1187. [Google Scholar] [CrossRef]
- Hossain, A.; Pagire, S.K.; Reiser, O. Visible-Light-Mediated Synthesis of Pyrazines from Vinyl Azides Utilizing a Photocascade Process. Synlett 2017, 28, 1707–1714. [Google Scholar] [CrossRef]
- Okamoto, K.; Mashida, A.; Watanabe, M.; Ohe, K. An unexpected disproportional reaction of 2H-azirines giving (1E,3Z)-2-aza-1,3-dienes and aromatic nitriles in the presence of nickel catalysts. Chem. Commun. 2012, 48, 3554–3556. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.K.; Titov, G.D.; Khoroshilova, O.V.; Kinzhalov, M.A.; Rostovskii, N.V. Light-induced one-pot synthesis of pyrimidine derivatives from vinyl azides. Org. Biomol. Chem. 2020, 18, 4971–4982. [Google Scholar] [CrossRef] [PubMed]
- Kascheres, A.; Oliveira, C.M.A.; De Azevedo, M.B.M.; Nobre, C.M.S. Reaction of methyl (E)-2-phenyl-1-azirine-3-acrylates with hydrazines and amidines. Synthetic and mechanistic implications. J. Org. Chem. 1991, 56, 7–9. [Google Scholar] [CrossRef]
- Pinho e Melo, T.M.V.D.; Cardoso, A.L.; Gomes, C.S.B.; Rocha Gonsalves, A.M. d’A. 2H-Azirines as dipolarophiles. Tetrahedron Lett. 2003, 44, 6313–6315. [Google Scholar] [CrossRef]
- Sun, S.; Huang, J.; Yuan, C.; Wang, G.; Guo, D.; Wang, J. Switchable assembly of substituted pyrimidines and 2H-imidazoles via Cu(i)-catalysed ring expansion of 2 methoxyl-2H-azirines. Org. Chem. Front. 2022, 9, 3006–3011. [Google Scholar] [CrossRef]
- Muzart, J. DBU: A Reaction Product Component. ChemistrySelect 2020, 5, 11608–11620. [Google Scholar] [CrossRef]
- Grossi, L. Base-catalyzed autoxidation of trialkylamines. An e.s.r study. Tetrahedron Lett. 1987, 28, 3387–3390. [Google Scholar] [CrossRef]
- Motyakin, M.V.; Wasserman, A.M.; Stott, P.E.; Zaikov, G.E. Possible mediators of the “living” radical polymerization. Spectrochim. Acta A 2006, 63, 802–815. [Google Scholar] [CrossRef]
- Funt, L.D.; Tomashenko, O.A.; Novikov, M.S.; Khlebnikov, A.F. An Azirine Strategy for the Synthesis of Alkyl 4-Amino-5-(trifluoromethyl)-1H-pyrrole-2-carboxylates. Synthesis 2018, 50, 4809–4822. [Google Scholar]
- Purkayastha, M.L.; Bhat, L.; Ila, H.; Junjappa, H. 4-Alkoxy-3-cyano-2(1H)-pyridones and 5-Alkoxyisoxazoles and Their Aryl Substituted and Annulated Derivatives from Acylketene O,S-Acetals. Synthesis 1995, 6, 641–643. [Google Scholar] [CrossRef]
- Agafonova, A.V.; Smetanin, I.A.; Rostovskii, N.V.; Khlebnikov, A.F.; Novikov, M.S. Easy Access to 2-Fluoro- and 2-Iodo-2H-azirines via the Halex Reaction. Synthesis 2019, 51, 4582–4589. [Google Scholar] [CrossRef]
- An, D.; Guan, X.; Guan, R.; Jin, L.; Zhang, G.; Zhang, S. Organocatalyzed nucleophilic addition of pyrazoles to 2H-azirines: Asymmetric synthesis of 3,3-disubstituted aziridines and kinetic resolution of racemic 2H-azirines. Chem. Commun. 2016, 52, 11211–11214. [Google Scholar] [CrossRef] [PubMed]
- Smetanin, I.A.; Novikov, M.S.; Agafonova, A.V.; Rostovskii, N.V.; Khlebnikov, A.F.; Kudryavtsev, I.V.; Terpilowski, M.A.; Serebriakova, M.K.; Trulioff, A.S.; Goncharov, N.V. A novel strategy for the synthesis of thermally stable and apoptosis-inducing 2,3-dihydroazetes. Org. Biomol. Chem. 2016, 14, 4479–4487. [Google Scholar] [CrossRef] [PubMed]
- Galenko, E.E.; Galenko, A.V.; Khlebnikov, A.F.; Novikov, M.S. Domino transformation of isoxazoles to 2,4-dicarbonylpyrroles under Fe/Ni relay catalysis. RSC Adv. 2015, 5, 18172–18176. [Google Scholar] [CrossRef]
- Zhang, G.; Wang, Y.; Xu, J.; Sun, J.; Sun, F.; Zhang, Y.; Zhang, C.; Du, Y. A new hypervalent iodine(iii/v) oxidant and its application to the synthesis of 2H-azirines. Chem. Sci. 2020, 11, 947–953. [Google Scholar] [CrossRef]
- Zhou, N.; Xie, T.; Li, Z.; Xie, Z. CuII/TEMPO-Promoted One-Pot Synthesis of Highly Substituted Pyrimidines from Amino Acid Esters. Chem. Eur. J. 2014, 20, 17311–17314. [Google Scholar] [CrossRef]
- Mei, C.; Hu, Y.; Lu, W. Visible-Light-Driven Oxidation of N-Alkylamides to Imides Using Oxone/H2O and Catalytic KBr. Synthesis 2018, 50, 2999–3005. [Google Scholar]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. (Eds.) Gaussian 09, Revision C.01 & D.01; Gaussian: Wallingford, CT, USA, 2013. [Google Scholar]
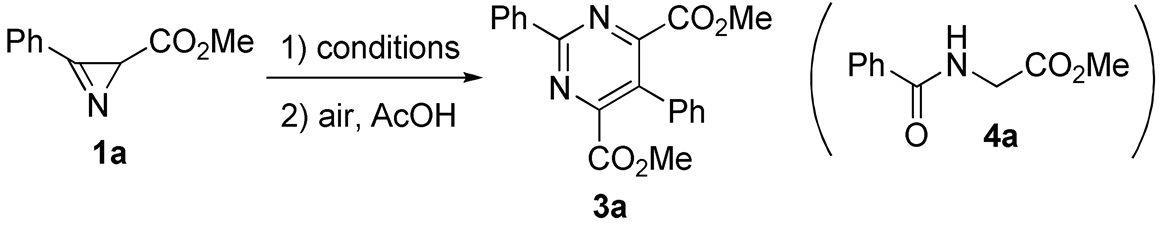 | ||||||
|---|---|---|---|---|---|---|
| Entry | Solvent | Base | Additive | T, °C | Time b | Yield of 3a, %/ Conversion of 1a, % |
| 1 | MeCN | NEt3 (2 eqv) | - | 70 | 4.5 days | 48/100 |
| 2 | MeCN | NEt3 (2 eqv) | - | 100 | 34 h | 22/100 |
| 3 | MeCN | NEt3 (2 eqv) | - | 40 | 5 days | 0/0 |
| 4 | MeCN | - | - | 70 | 4.5 days | 0/0 |
| 5 | MeCN | NEt3 (1 eqv) | - | 70 | 3 weeks | 41/100 |
| 6 | MeCN | DBU (1 eqv) | - | 70 | 18 h | 27/100 |
| 7 | MeCN | tBuOK (1 eqv) | - | rt | 1 h | 0/100 |
| 8 | PhMe | NEt3 (2 eqv) | - | 70 | 6 days | 0/85 (1H NMR) |
| 9 | Acetone | NEt3 (2 eqv) | - | 70 | 1 week | 0/19 (1H NMR) |
| 10 | DCE | NEt3 (2 eqv) | - | 70 | 8 days | 0/66 (1H NMR) |
| 11 | 1,4-Dioxane | NEt3 (2 eqv) | - | 70 | 5 days | 0/0 |
| 12 | MeCN | NEt3 (2.6 eqv) | TEMPO (1 eqv) | 70 | 5 days | 0/0 |
| 13 | MeCN | NEt3 (2.6 eqv) | AIBN (1 eqv) | 70 | 5 days | 70/100 |
| 14 | MeCN | NEt3 (2.6 eqv) | ACHN (1 eqv) | 70 | 3 days | 70/100 |
| 15 | MeCN | - | AIBN (1 eqv) | 70 | 24 h | 0/0 |
| 16 | MeCN | NEt3 (2.6 eqv) | (BzO)2 (1 eqv) | 70 | 3 days | 0/100 |
| 17 | MeCN | NEt3 (2 eqv) | under Ar | 70 | 5 days | 0/0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zakharov, T.N.; Sakharov, P.A.; Novikov, M.S.; Khlebnikov, A.F.; Rostovskii, N.V. Triethylamine-Promoted Oxidative Cyclodimerization of 2H-Azirine-2-carboxylates to Pyrimidine-4,6-dicarboxylates: Experimental and DFT Study. Molecules 2023, 28, 4315. https://doi.org/10.3390/molecules28114315
Zakharov TN, Sakharov PA, Novikov MS, Khlebnikov AF, Rostovskii NV. Triethylamine-Promoted Oxidative Cyclodimerization of 2H-Azirine-2-carboxylates to Pyrimidine-4,6-dicarboxylates: Experimental and DFT Study. Molecules. 2023; 28(11):4315. https://doi.org/10.3390/molecules28114315
Chicago/Turabian StyleZakharov, Timofei N., Pavel A. Sakharov, Mikhail S. Novikov, Alexander F. Khlebnikov, and Nikolai V. Rostovskii. 2023. "Triethylamine-Promoted Oxidative Cyclodimerization of 2H-Azirine-2-carboxylates to Pyrimidine-4,6-dicarboxylates: Experimental and DFT Study" Molecules 28, no. 11: 4315. https://doi.org/10.3390/molecules28114315
APA StyleZakharov, T. N., Sakharov, P. A., Novikov, M. S., Khlebnikov, A. F., & Rostovskii, N. V. (2023). Triethylamine-Promoted Oxidative Cyclodimerization of 2H-Azirine-2-carboxylates to Pyrimidine-4,6-dicarboxylates: Experimental and DFT Study. Molecules, 28(11), 4315. https://doi.org/10.3390/molecules28114315