Vernonia amygdalina Ethanol Extract Protects against Doxorubicin-Induced Cardiotoxicity via TGFβ, Cytochrome c, and Apoptosis
Abstract
1. Introduction
2. Results
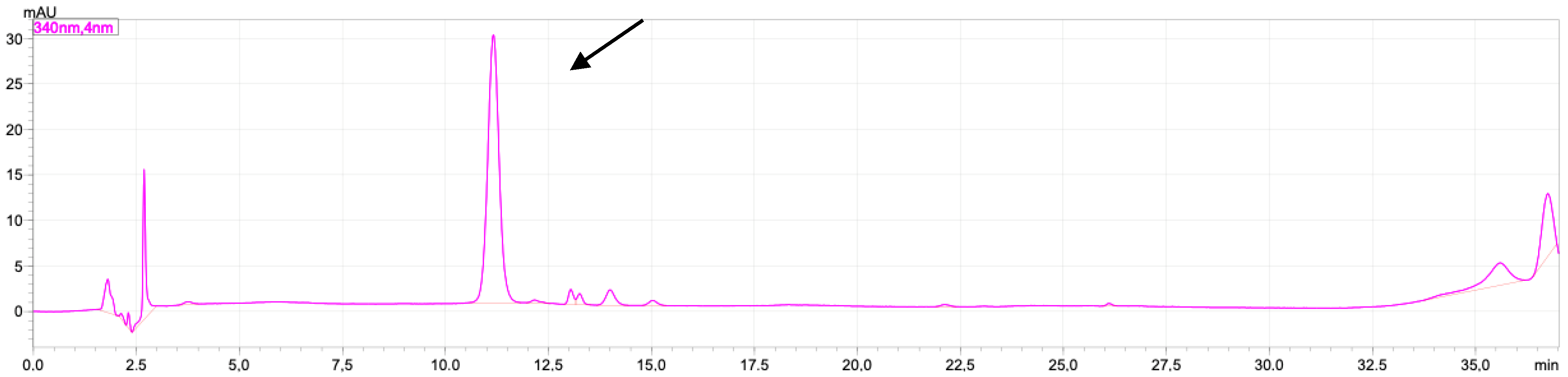
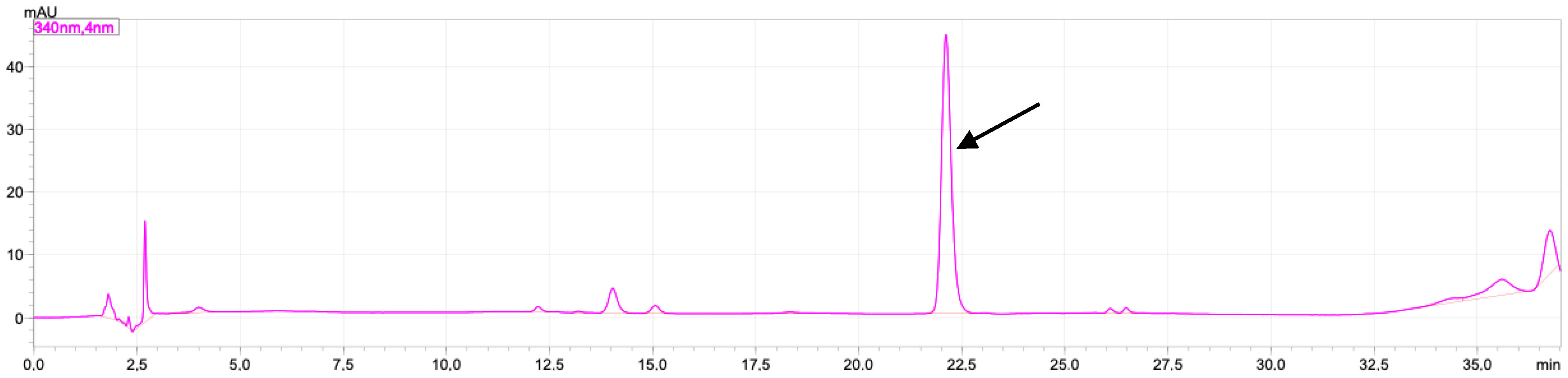
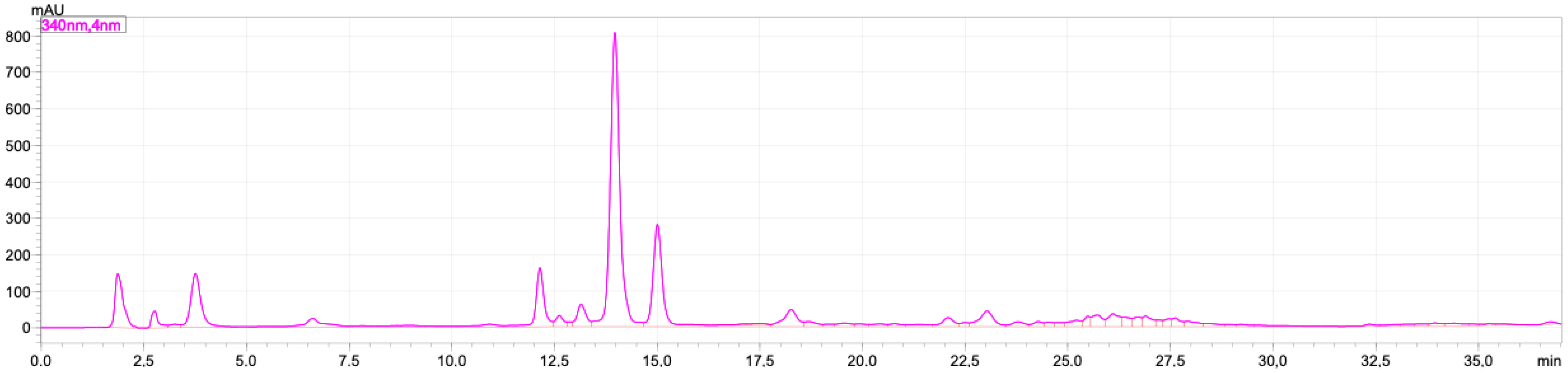
2.1. Rutin and Luteolin Content
2.2. Doxorubicinol
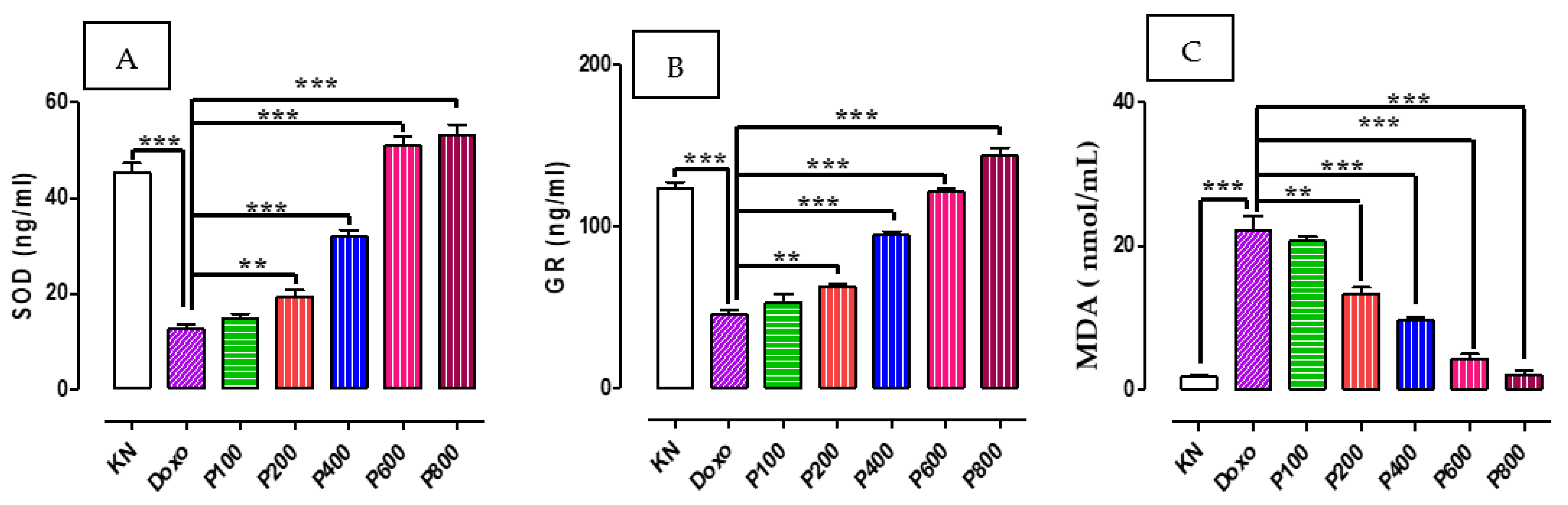
2.3. SOD, GR, and MDA Concentration
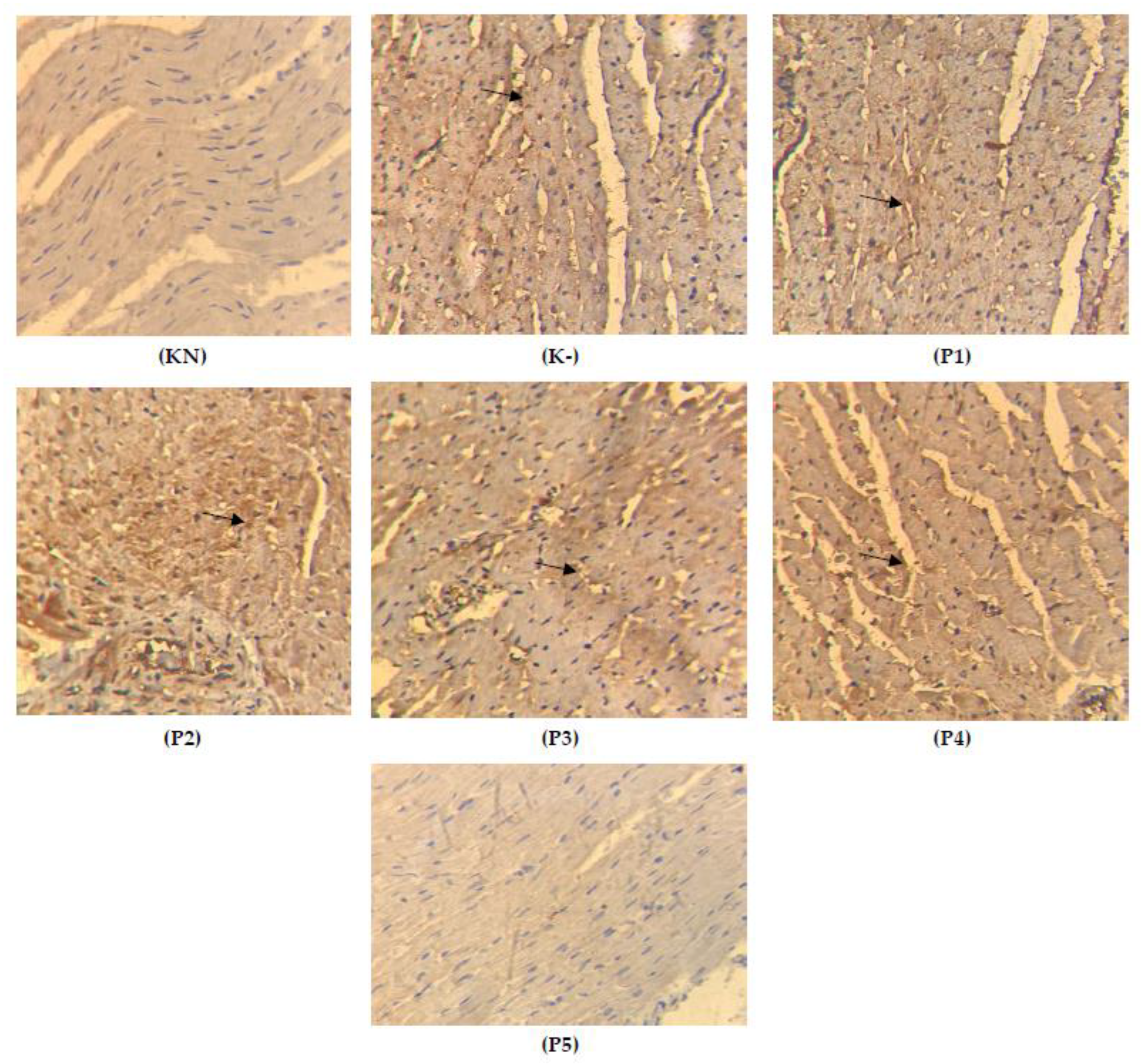
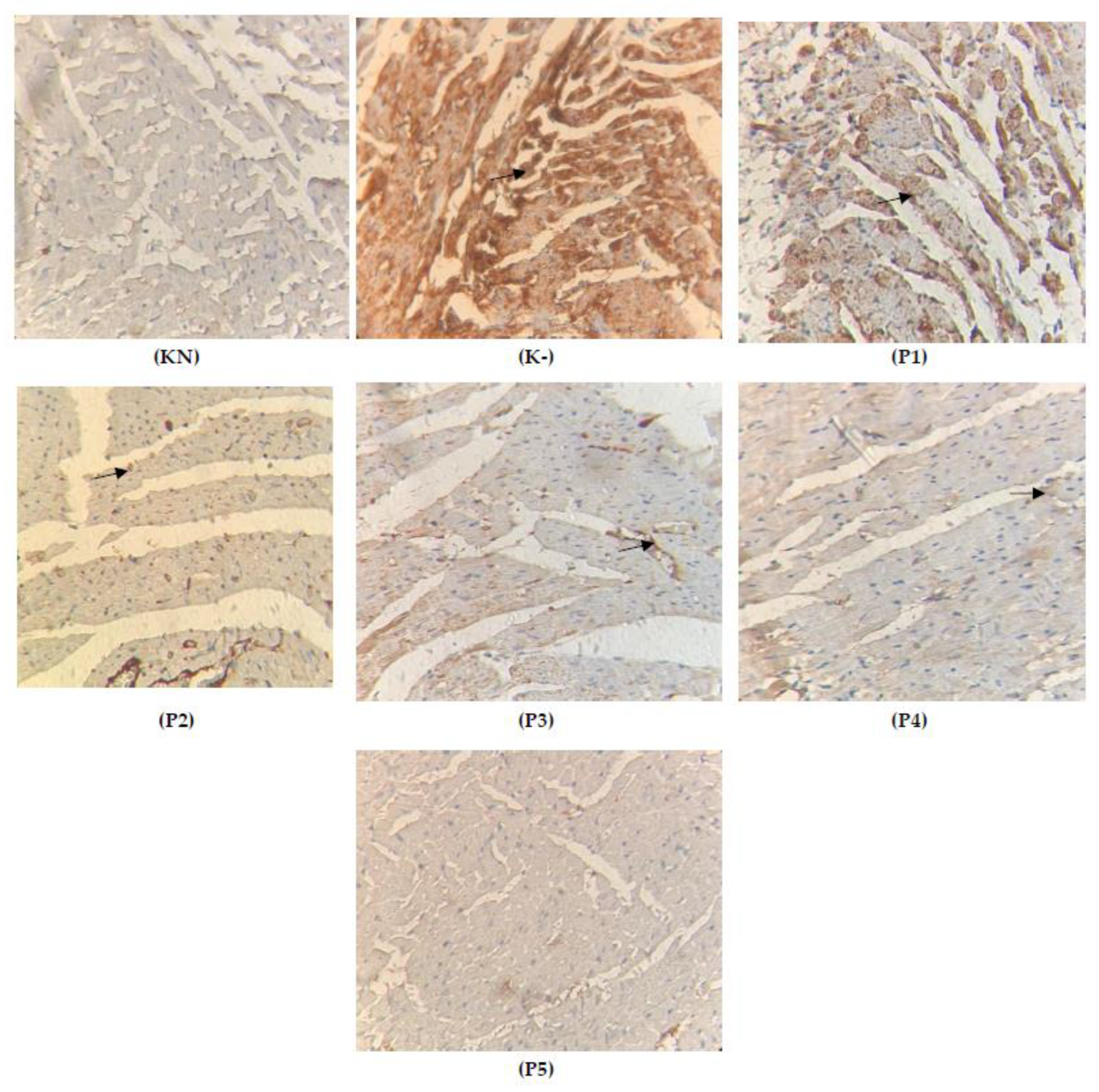
2.4. Expression of TGFβ on the Cardiac Tissue
2.5. Apoptosis on the Cardiac Tissue
2.6. Expression of Cytochrome c on the Cardiac Tissue
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Animals
4.3. Extract Preparation
4.4. Determination of Luteolin and Rutin
4.5. Experimental Design
4.6. ELISA Analysis
4.7. Doxorubicinol Analysis
4.7.1. Preparation of Solutions and Standards
4.7.2. Blood Preparations
4.7.3. Sample Preparations
4.8. TUNEL Assay
4.9. Imunohistochemistry of TGFβ and Cytochrome c
4.10. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Mei, S.; Hong, L.; Cai, X.; Xiao, B.; Zhang, P.; Shao, L. Oxidative stress injury in doxorubicin-induced cardiotoxicity. Toxicol. Lett. 2019, 307, 41–48. [Google Scholar] [CrossRef]
- Christidi, E.; Brunham, L.R. Regulated cell death pathways in doxorubicin-induced cardiotoxicity. Cell Death Dis. 2021, 12, 339. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Liu, X.; Bawa-Khalfe, T.; Lu, L.-S.; Lyu, Y.L.; Liu, L.F.; Yeh, E.T.H. Identification of the molecular basis of doxorubicin-induced cardiotoxicity. Nat. Med. 2012, 18, 1639–1642. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.-W.; Shi, J.; Li, Y.-J.; Wei, L. Cardiomyocyte death in doxorubicin-induced cardiotoxicity. Arch. Immunol. Ther. Exp. 2009, 57, 435–445. [Google Scholar] [CrossRef]
- Tokarska-Schlattner, M.; Zaugg, M.; Zuppinger, C.; Wallimann, T.; Schlattner, U. New insights into doxorubicin-induced cardiotoxicity: The critical role of cellular energetics. J. Mol. Cell. Cardiol. 2006, 41, 389–405. [Google Scholar] [CrossRef]
- Khan, M.; Shobha, J.C.; Mohan, I.K.; Naidu, M.U.R.; Sundaram, C.; Singh, S.; Kuppusamy, P.; Kutala, V.K. Protective effect of Spirulina against doxorubicin-induced cardiotoxicity. Phytother. Res. 2005, 19, 1030–1037. [Google Scholar] [CrossRef]
- Dirks-Naylor, A.J. The role of autophagy in doxorubicin-induced cardiotoxicity. Life Sci. 2013, 93, 913–916. [Google Scholar] [CrossRef]
- dos Santos, D.S.; dos Santos Goldenberg, R.C. Doxorubicin-induced cardiotoxicity: From mechanisms to development of efficient therapy. In Cardiotoxicity; IntechOpen: London, UK, 2018; pp. 3–24. [Google Scholar]
- Syahputra, R.A.; Harahap, U.; Dalimunthe, A.; Nasution, M.P.; Satria, D. The Role of Flavonoids as a Cardioprotective Strategy against Doxorubicin-Induced Cardiotoxicity: A Review. Molecules 2022, 27, 1320. [Google Scholar] [CrossRef]
- Hayek, E.R.; Speakman, E.; Rehmus, E. Acute Doxorubicin Cardiotoxicity. N. Engl. J. Med. 2005, 352, 2456–2457. [Google Scholar] [CrossRef]
- Syahputra, R.A.; Harahap, U.; Dalimunthe, A.; Pandapotan, M.; Satria, D. Protective effect of Vernonia amygdalina Delile against doxorubicin-induced cardiotoxicity. Heliyon 2021, 7, e07434. [Google Scholar] [CrossRef]
- Syahputra, R.; Harahap, U.; Dalimunthe, A.; Nasution, P.; Haro, G.; Widodo, D.H.; Satria, D. In-silico toxicity prediction of bioactive compounds of Vernonia amygdalina delile. And digoxin. Rasayan J. Chem. 2020, 13, 1220–1224. [Google Scholar] [CrossRef]
- Lubis, M.F.; Hasibuan, P.A.Z.; Syahputra, H.; Astyka, R.; Baruna, I. Phytochemical Profile and Pharmacological Activity of Vernonia amygdalina Delile Stem Bark Extracts Using Different Solvent Extraction. Open Access Maced. J. Med. Sci. 2022, 10, 860–866. [Google Scholar] [CrossRef]
- Hasibuan, P.; Harahap, U.; Sitorus, P.; Lubis, M.F.; Satria, D. In-silico analysis of vernonioside d and vernonioside e from Vernonia amygdalina delile. Leaves as inhibitor of epidermal growth factor receptor (egfr) and mammalian target of rapamycin (mTOR). Rasayan J. Chem. 2021, 14, 1539–1543. [Google Scholar] [CrossRef]
- Igile, G.O.; Oleszek, W.; Jurzysta, M.; Burda, S.; Fafunso, M.; Fasanmade, A.A. Flavonoids from Vernonia amygdalina and their antioxidant activities. J. Agric. Food Chem. 1994, 42, 2445–2448. [Google Scholar] [CrossRef]
- Koval’skii, I.V.; Krasnyuk, I.I.; Nikulina, O.I.; Belyatskaya, A.V.; Kharitonov, Y.Y.; Feldman, N.B.; Lutsenko, S.V. Mechanisms of Rutin Pharmacological Action (Review). Pharm. Chem. J. 2014, 48, 73–76. [Google Scholar] [CrossRef]
- Taheri, Y.; Sharifi-Rad, J.; Antika, G.; Yılmaz, Y.B.; Tumer, T.B.; Abuhamdah, S.; Chandra, S.; Saklani, S.; Kılıç, C.S.; Sestito, S.; et al. Paving Luteolin Therapeutic Potentialities and Agro-Food-Pharma Applications: Emphasis on In Vivo Pharmacological Effects and Bioavailability Traits. Oxidative Med. Cell. Longev. 2021, 2021, 1987588. [Google Scholar] [CrossRef] [PubMed]
- Olson, R.D.; Mushlin, P.S.; Brenner, D.E.; Fleischer, S.; Cusack, B.J.; Chang, B.K.; Boucek, R.J., Jr. Doxorubicin cardiotoxicity may be caused by its metabolite, doxorubicinol. Proc. Natl. Acad. Sci. USA 1988, 85, 3585–3589. [Google Scholar] [CrossRef]
- Harahap, Y.; Ardiningsih, P.; Corintias Winarti, A.; Purwanto, D.J. Analysis of the Doxorubicin and Doxorubicinol in the Plasma of Breast Cancer Patients for Monitoring the Toxicity of Doxorubicin. Drug Des. Dev. Ther. 2020, 14, 3469–3475. [Google Scholar] [CrossRef] [PubMed]
- Kalyanaraman, B.; Joseph, J.; Kalivendi, S.; Wang, S.; Konorev, E.; Kotamraju, S. Doxorubicin-induced apoptosis: Implications in cardiotoxicity. Mol. Cell. Biochem. 2002, 234, 119–124. [Google Scholar] [CrossRef]
- Ma, Y.; Yang, L.; Ma, J.; Lu, L.; Wang, X.; Ren, J.; Yang, J. Rutin attenuates doxorubicin-induced cardiotoxicity via regulating autophagy and apoptosis. Biochim. Biophys. Acta BBA Mol. Basis Dis. 2017, 1863, 1904–1911. [Google Scholar] [CrossRef]
- Zare, M.F.R.; Rakhshan, K.; Aboutaleb, N.; Nikbakht, F.; Naderi, N.; Bakhshesh, M.; Azizi, Y. Apigenin attenuates doxorubicin induced cardiotoxicity via reducing oxidative stress and apoptosis in male rats. Life Sci. 2019, 232, 116623. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, M.; Shiojima, I.; Ikeda, H.; Komuro, I. Chronic doxorubicin cardiotoxicity is mediated by oxidative DNA damage-ATM-p53-apoptosis pathway and attenuated by pitavastatin through the inhibition of Rac1 activity. J. Mol. Cell. Cardiol. 2009, 47, 698–705. [Google Scholar] [CrossRef] [PubMed]
- Merten, K.E.; Feng, W.; Zhang, L.; Pierce, W.; Cai, J.; Klein, J.B.; Kang, Y.J. Modulation of Cytochrome c Oxidase-Va Is Possibly Involved in Metallothionein Protection from Doxorubicin Cardiotoxicity. Experiment 2005, 315, 1314–1319. [Google Scholar] [CrossRef]
- Clementi, M.E.; Giardina, B.; Di Stasio, E.; Mordente, A.; Misiti, F. Doxorubicin-derived metabolites induce release of cytochrome c and inhibition of respiration on cardiac isolated mitochondria. Anticancer Res. 2003, 23, 2445–2450. [Google Scholar] [PubMed]
- Childs, A.C.; Phaneuf, S.L.; Dirks, A.J.; Phillips, T.; Leeuwenburgh, C. Doxorubicin treatment in vivo causes cytochrome c release and cardiomyocyte apoptosis, as well as increased mitochondrial efficiency, superoxide dismutase activity, and Bcl-2:Bax ratio. Cancer Res. 2002, 62, 4592–4598. [Google Scholar] [PubMed]
- Chandran, K.; Aggarwal, D.; Migrino, R.Q.; Joseph, J.; McAllister, D.; Konorev, E.A.; Antholine, W.E.; Zielonka, J.; Srinivasan, S.; Avadhani, N.G.; et al. Doxorubicin Inactivates Myocardial Cytochrome c Oxidase in Rats: Cardioprotection by Mito-Q. Biophys. J. 2009, 96, 1388–1398. [Google Scholar] [CrossRef]
- An, J.; Li, P.; Li, J.; Dietz, R.; Donath, S. ARC is a critical cardiomyocyte survival switch in doxorubicin cardiotoxicity. J. Mol. Med. 2009, 87, 401–410. [Google Scholar] [CrossRef]
- Wei, Y.; Zhao, J.; Xiong, J.; Chai, J.; Yang, X.; Wang, J.; Chen, J.; Wang, J. Wogonin reduces cardiomyocyte apoptosis from mitochondrial release of cytochrome c to improve doxorubicin-induced cardiotoxicity. Exp. Ther. Med. 2022, 23, 1–8. [Google Scholar] [CrossRef]
- Hasinoff, B.B.; Davey, J.P. The iron(III)-adriamycin complex inhibits cytochrome c oxidase before its inactivation. Biochem. J. 1988, 250, 827–834. [Google Scholar] [CrossRef]
- Pecoraro, M.; Sorrentino, R.; Franceschelli, S.; Del Pizzo, M.; Pinto, A.; Popolo, A. Doxorubicin-Mediated Cardiotoxicity: Role of Mitochondrial Connexin 43. Cardiovasc. Toxicol. 2015, 15, 366–376. [Google Scholar] [CrossRef]
- Gustafson, D.L.; Swanson, J.D.; Pritsos, C.A. Role of Xanthine Oxidase in the Potentiation of Doxorubicin-Induced Cardiotoxicity by Mitomycin C. Cancer Commun. 1991, 3, 299–304. [Google Scholar] [CrossRef]
- Mobaraki, M.; Faraji, A.; Zare, M.; Dolati, P.; Ataei, M.; Dehghan Manshadi, H.R. Molecular Mechanisms of Cardiotoxicity: A Review on Major Side-effect of Doxorubicin. Indian J. Pharm. Sci. 2017, 79, 335–344. [Google Scholar] [CrossRef]
- Green, P.S.; Leeuwenburgh, C. Mitochondrial dysfunction is an early indicator of doxorubicin-induced apoptosis. Biochim. Biophys. Acta (BBA) Mol. Basis Dis. 2002, 1588, 94–101. [Google Scholar] [CrossRef]
- Mantawy, E.M.; El-Bakly, W.M.; Esmat, A.; Badr, A.M.; El-Demerdash, E. Chrysin alleviates acute doxorubicin cardiotoxicity in rats via suppression of oxidative stress, inflammation and apoptosis. Eur. J. Pharmacol. 2014, 728, 107–118. [Google Scholar] [CrossRef]
- Lim, H.; Zhu, Y.Z. Role of transforming growth factor-β in the progression of heart failure. Cell. Mol. Life Sci. 2006, 63, 2584–2596. [Google Scholar] [CrossRef]
- Segura, A.M.; Frazier, O.H.; Buja, L.M. Fibrosis and heart failure. Heart Fail. Rev. 2014, 19, 173–185. [Google Scholar] [CrossRef] [PubMed]
- Okada, H.; Takemura, G.; Kosai, K.-I.; Li, Y.; Takahashi, T.; Esaki, M.; Yuge, K.; Miyata, S.; Maruyama, R.; Mikami, A.; et al. Postinfarction Gene Therapy against Transforming Growth Factor-β Signal Modulates Infarct Tissue Dynamics and Attenuates Left Ventricular Remodeling and Heart Failure. Circulation 2005, 111, 2430–2437. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.; Schriewer, J.; Tang, M.; Marlin, J.; Taylor, F.; Shohet, R.V.; Konorev, E.A. The TGF-β pathway mediates doxorubicin effects on cardiac endothelial cells. J. Mol. Cell. Cardiol. 2016, 90, 129–138. [Google Scholar] [CrossRef]
- Cole, M.; Chaiswing, L.; Oberley, T.; Edelmann, S.; Piascik, M.; Lin, S.; Kiningham, K.; Stclair, D. The protective roles of nitric oxide and superoxide dismutase in adriamycin-induced cardiotoxicity. Cardiovasc. Res. 2006, 69, 186–197. [Google Scholar] [CrossRef]
- Iqbal, M.; Dubey, K.; Anwer, T.; Ashish, A.; Pillai, K.K. Protective effects of telmisartan against acute doxorubicin-induced cardiotoxicity in rats. Pharmacol. Rep. 2008, 60, 382–390. [Google Scholar]
- Olson, R.D.; Boerth, R.C.; Gerber, J.G.; Nies, A.S. Mechanism of adriamycin cardiotoxicity: Evidence for oxidative stress. Life Sci. 1981, 29, 1393–1401. [Google Scholar] [CrossRef] [PubMed]

| Group | Mean | Kruskal—Wallis | KN | K- | P100 | P200 | P400 | P600 | P800 |
|---|---|---|---|---|---|---|---|---|---|
| KN | 46.200 | 0.000 | 0.007 ** | 0.008 ** | 0.008 ** | 0.008 ** | 0.001 ** | 0.002 ** | |
| K- | 81.700 | 0.189 ns | 0.015 * | 0.012 * | 0.006 ** | 0.006 ** | |||
| P100 | 78.100 | 0.343 ns | 0.192 | 0.008 ** | 0.008 ** | ||||
| P200 | 61.200 | 0.343 | 0.006 ** | 0.006 ** | |||||
| P400 | 58.900 | 0.008 ** | 0.008 ** | ||||||
| P600 | 50.100 | 0.180 ns | |||||||
| P800 | 48.100 |
| Group | Mean | Kruskal—Wallis | KN | K- | P100 | P200 | P300 | P600 | P800 |
|---|---|---|---|---|---|---|---|---|---|
| KN | 20.100 | 0.000 | 0.002 ** | 0.005 ** | 0.001 ** | 0.005 ** | 0.004 ** | 0.001 ** | |
| K- | 71.700 | 0.236 ns | 0.032 * | 0.018 * | 0.001 ** | 0.005 ** | |||
| P100 | 70.000 | 0.453 ns | 0.210 | 0.003 ** | 0.002 ** | ||||
| P200 | 58.100 | 0.543 | 0.002 ** | 0.001 ** | |||||
| P400 | 30.500 | 0.001 ** | 0.002 ** | ||||||
| P600 | 25.780 | 0.250 ns | |||||||
| P800 | 19.910 |
| Group | Mean | Kruskal—Wallis | KN | K- | P100 | P200 | P400 | P600 | P800 |
|---|---|---|---|---|---|---|---|---|---|
| KN | 23.400 | 0.000 | 0.007 ** | 0.008 ** | 0.008 ** | 0.008 ** | 0.381 | 0.501 | |
| K- | 39.600 | 0.189 | 0.015 * | 0.012 * | 0.006 ** | 0.006 ** | |||
| P100 | 38.000 | 0.343 | 0.192 | 0.008 ** | 0.008 ** | ||||
| P200 | 37.200 | 0.343 | 0.006 ** | 0.006 ** | |||||
| P400 | 36.800 | 0.008 ** | 0.008 ** | ||||||
| P600 | 24.000 | 0.180 | |||||||
| P800 | 22.000 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Syahputra, R.A.; Harahap, U.; Harahap, Y.; Gani, A.P.; Dalimunthe, A.; Ahmed, A.; Zainalabidin, S. Vernonia amygdalina Ethanol Extract Protects against Doxorubicin-Induced Cardiotoxicity via TGFβ, Cytochrome c, and Apoptosis. Molecules 2023, 28, 4305. https://doi.org/10.3390/molecules28114305
Syahputra RA, Harahap U, Harahap Y, Gani AP, Dalimunthe A, Ahmed A, Zainalabidin S. Vernonia amygdalina Ethanol Extract Protects against Doxorubicin-Induced Cardiotoxicity via TGFβ, Cytochrome c, and Apoptosis. Molecules. 2023; 28(11):4305. https://doi.org/10.3390/molecules28114305
Chicago/Turabian StyleSyahputra, Rony Abdi, Urip Harahap, Yahdiana Harahap, Andayana Puspitasari Gani, Aminah Dalimunthe, Amer Ahmed, and Satirah Zainalabidin. 2023. "Vernonia amygdalina Ethanol Extract Protects against Doxorubicin-Induced Cardiotoxicity via TGFβ, Cytochrome c, and Apoptosis" Molecules 28, no. 11: 4305. https://doi.org/10.3390/molecules28114305
APA StyleSyahputra, R. A., Harahap, U., Harahap, Y., Gani, A. P., Dalimunthe, A., Ahmed, A., & Zainalabidin, S. (2023). Vernonia amygdalina Ethanol Extract Protects against Doxorubicin-Induced Cardiotoxicity via TGFβ, Cytochrome c, and Apoptosis. Molecules, 28(11), 4305. https://doi.org/10.3390/molecules28114305






