Optimization and Antibacterial Evaluation of Novel 3-(5-Fluoropyridine-3-yl)-2-oxazolidinone Derivatives Containing a Pyrimidine Substituted Piperazine
Abstract
1. Introduction
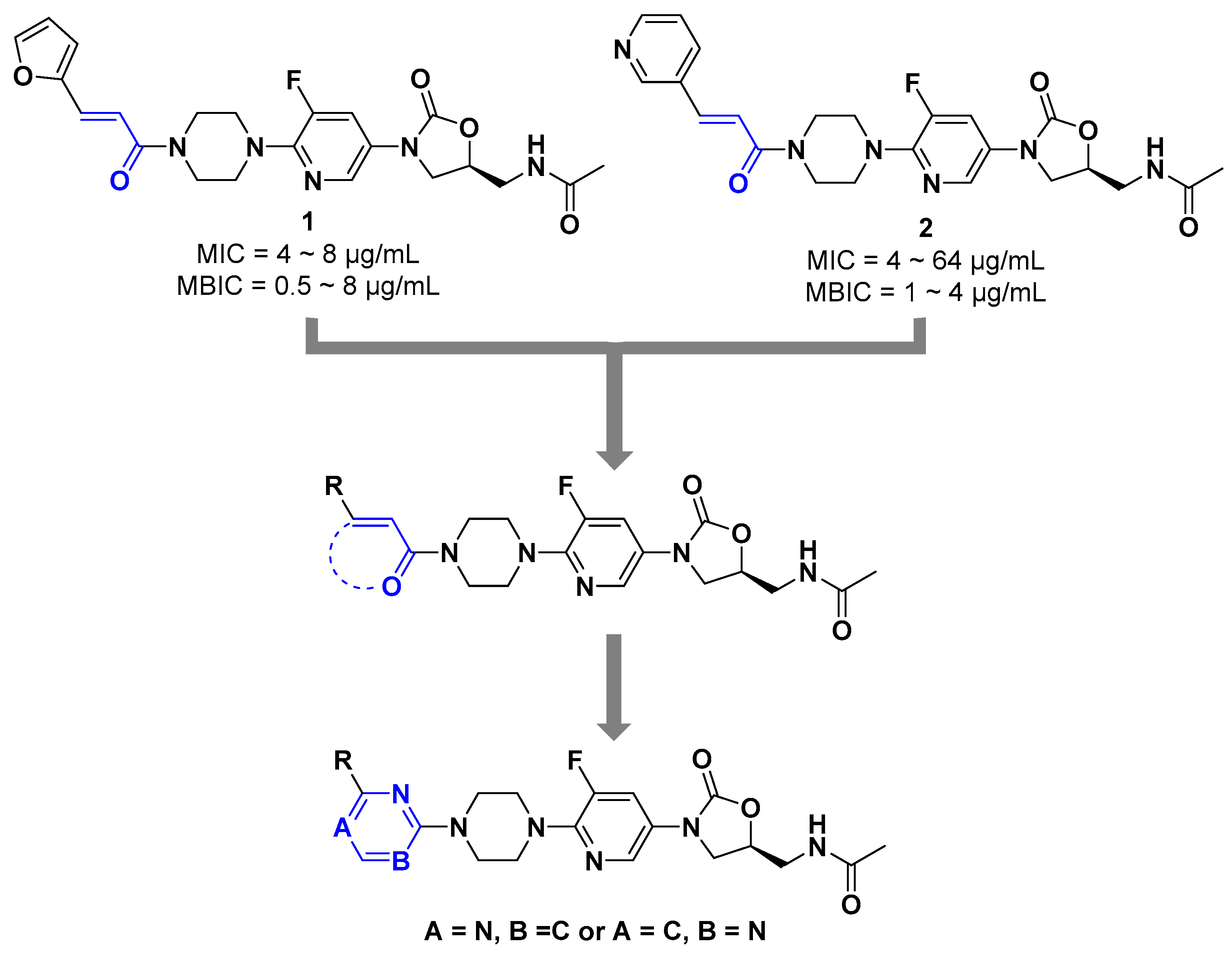
2. Results and Discussion
2.1. Chemistry
2.2. Antibacterial Activity Assay
2.2.1. Minimum Inhibitory Concentration against Standard Strains
2.2.2. Minimum Inhibitory Concentration against Drug-Resistant Strains
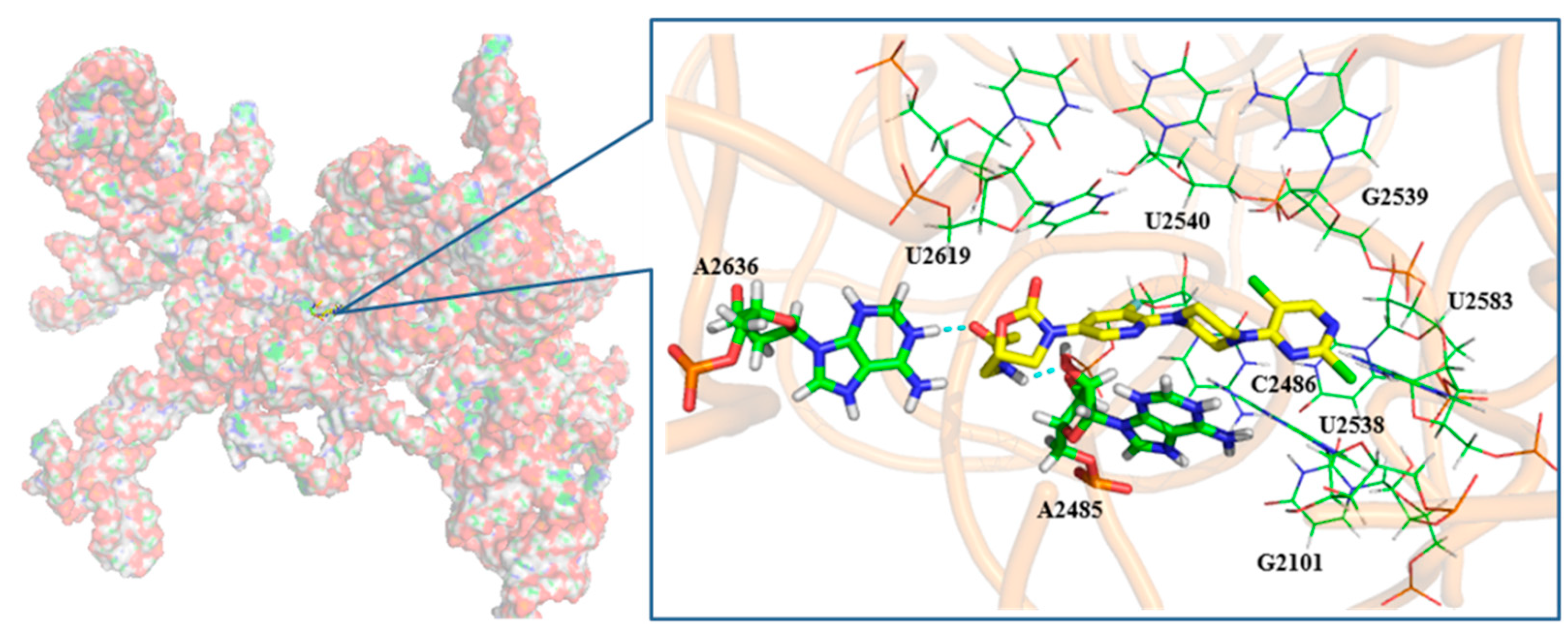
2.3. Molecular Docking Study
2.4. Inhibition of Biofilm Formation
2.5. Cytotoxicity Determination
3. Experimental Section
3.1. Materials and Methods
3.2. Chemistry
3.2.1. Synthesis of (S)-N-((3-(6-(4-(2-chloropyrimidin-4-yl)piperazin-1-yl)-5-fluoropyridin-3-yl)-2-oxooxazolidin-5-yl)methyl)acetamide(5)and(S)-N-((3-(6-(4-(4-chloropyrimidin-2-yl)piperazin-1-yl)-5-fluoropyridin-3-yl)-2-oxooxazolidin-5-yl)methyl)acetamide (7b)
3.2.2. General Procedure for the Synthesis of 6a and 6b
3.2.3. General Procedure for the Synthesis of 6c–m
3.2.4. General Procedure for the Synthesis of 7a and 7c-n
3.3. MIC Determination
3.4. Molecular Docking Studies
3.5. Inhibition of Biofilm Formation Assay
3.6. Cytotoxicity Assay
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Yan, X.; Schouls, L.M.; Pluister, G.N.; Tao, X.; Yu, X.; Yin, J.; Song, Y.; Hu, S.; Luo, F.; Hu, W.; et al. The population structure of Staphylococcus aureus in China and Europe assessed by multiple-locus variable number tandem repeat analysis; clues to geographical origins of emergence and dissemination. Clin. Microbiol. Infect. 2016, 22, 60.e1–60.e8. [Google Scholar] [CrossRef] [PubMed]
- Zaman, S.B.; Hussain, M.A.; Nye, R.; Mehta, V.; Mamun, K.T.; Hossain, N. A Review on Antibiotic Resistance: Alarm Bells are Ringing. Cureus 2017, 9, e1403. [Google Scholar] [CrossRef]
- Parrino, B.; Schillaci, D.; Carnevale, I.; Giovannetti, E.; Diana, P.; Cirrincione, G.; Cascioferro, S. Synthetic small molecules as anti-biofilm agents in the struggle against antibiotic resistance. Eur. J. Med. Chem. 2019, 161, 154–178. [Google Scholar] [CrossRef]
- Costerton, J.W.; Stewart, P.S.; Greenberg, E.P. Bacterial Biofilms: A Common Cause of Persistent Infections. Science 1999, 284, 1318–1322. [Google Scholar] [CrossRef] [PubMed]
- Liang, J.; Sun, D.; Yang, Y.; Li, M.; Li, H.; Chen, L. Discovery of metal-based complexes as promising antimicrobial agents. Eur. J. Med. Chem. 2021, 224, 113696. [Google Scholar] [CrossRef] [PubMed]
- Ling, H.; Lou, X.; Luo, Q.; He, Z.; Sun, M.; Sun, J. Recent advances in bacteriophage-based therapeutics: Insight into the post-antibiotic era. Acta Pharm. Sin. B 2022, 12, 4348–4364. [Google Scholar] [CrossRef]
- Pisoschi, A.M.; Pop, A.; Cimpeanu, C.; Turcuş, V.; Predoi, G.; Iordache, F. Nanoencapsulation techniques for compounds and products with antioxidant and antimicrobial activity—A critical view. Eur. J. Med. Chem. 2018, 157, 1326–1345. [Google Scholar] [CrossRef]
- Wang, Y.; Lv, Q.; Chen, Y.; Xu, L.; Feng, M.; Xiong, Z.; Li, J.; Ren, J.; Liu, J.; Liu, B. Bilayer hydrogel dressing with lysozyme-enhanced photothermal therapy for biofilm eradication and accelerated chronic wound repair. Acta Pharm. Sin. B 2022, 13, 284–297. [Google Scholar] [CrossRef]
- Malla, T.R.; Brewitz, L.; Muntean, D.G.; Aslam, H.; Owen, C.D.; Salah, E.; Tumber, A.; Lukacik, P.; Strain-Damerell, C.; Mikolajek, H.; et al. Penicillin Derivatives Inhibit the SARS-CoV-2 Main Protease by Reaction with Its Nucleophilic Cysteine. J. Med. Chem. 2022, 65, 7682–7696. [Google Scholar] [CrossRef]
- Naclerio, G.A.; Abutaleb, N.S.; Onyedibe, K.I.; Karanja, C.; Eldesouky, H.E.; Liang, H.W.; Dieterly, A.; Aryal, U.K.; Lyle, T.; Seleem, M.N.; et al. Mechanistic Studies and In Vivo Efficacy of an Oxadiazole-Containing Antibiotic. J. Med. Chem. 2022, 65, 6612–6630. [Google Scholar] [CrossRef]
- Xie, Y.P.; Sangaraiah, N.; Meng, J.P.; Zhou, C.H. Unique Carbazole-Oxadiazole Derivatives as New Potential Antibiotics for Combating Gram-Positive and -Negative Bacteria. J. Med. Chem. 2022, 65, 6171–6190. [Google Scholar] [CrossRef] [PubMed]
- Zurenko, G.E.; Yagi, B.H.; Schaadt, R.D.; Allison, J.W.; Kilburn, J.O.; Glickman, S.E.; Hutchinson, D.K.; Barbachyn, M.R.; Brickner, S.J. In vitro activities of U-100592 and U-100766, novel oxazolidinone antibacterial agents. Antimicrob. Agents Chemother. 1996, 40, 839–845. [Google Scholar] [CrossRef] [PubMed]
- Lei, H.; Jiang, Y.; Wang, D.; Gong, P.; Li, Y.; Dong, Y.; Dong, M. In vitro activity of novel oxazolidinone analogs and 13 conventional antimicrobial agents against clinical isolates of Staphylococcus aureus in Beijing, China. Jpn. J. Infect. Dis. 2014, 67, 402–404. [Google Scholar] [CrossRef]
- Lin, A.H.; Murray, R.W.; Vidmar, T.J.; Marotti, K.R. The oxazolidinone eperezolid binds to the 50S ribosomal subunit and competes with binding of chloramphenicol and lincomycin. Antimicrob. Agents Chemother. 1997, 41, 2127–2131. [Google Scholar] [CrossRef]
- Swaney, S.M.; Aoki, H.; Ganoza, M.C.; Shinabarger, D.L. The oxazolidinone linezolid inhibits initiation of protein synthesis in bacteria. Antimicrob. Agents Chemother. 1998, 42, 3251–3255. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.C.; Swaney, S.M.; Shinabarger, D.L.; Stockman, B.J. 1H nuclear magnetic resonance study of oxazolidinone binding to bacterial ribosomes. Antimicrob. Agents Chemother. 2002, 46, 625–629. [Google Scholar] [CrossRef]
- Foti, C.; Piperno, A.; Scala, A.; Giuffrè, O. Oxazolidinone Antibiotics: Chemical, Biological and Analytical Aspects. Molecules 2021, 26, 4280. [Google Scholar] [CrossRef]
- Pandit, N.; Singla, R.K.; Shrivastava, B. Current updates on oxazolidinone and its significance. Int. J. Med. Chem. 2012, 2012, 159285. [Google Scholar] [CrossRef]
- Yuan, S.; Shen, D.D.; Bai, Y.R.; Zhang, M.; Zhou, T.; Sun, C.; Zhou, L.; Wang, S.Q.; Liu, H.M. Oxazolidinone: A promising scaffold for the development of antibacterial drugs. Eur. J. Med. Chem. 2023, 250, 115239. [Google Scholar] [CrossRef]
- Vinh, D.C.; Rubinstein, E. Linezolid: A review of safety and tolerability. J. Infect. 2009, 59 (Suppl. S1), S59–S74. [Google Scholar] [CrossRef]
- Bai, P.-Y.; Qin, S.-S.; Chu, W.-C.; Yang, Y.; Cui, D.-Y.; Hua, Y.-G.; Yang, Q.-Q.; Zhang, E. Synthesis and antibacterial bioactivities of cationic deacetyl linezolid amphiphiles. Eur. J. Med. Chem. 2018, 155, 925–945. [Google Scholar] [CrossRef]
- De Rosa, M.; Zanfardino, A.; Notomista, E.; Wichelhaus, T.A.; Saturnino, C.; Varcamonti, M.; Soriente, A. Novel promising linezolid analogues: Rational design, synthesis and biological evaluation. Eur. J. Med. Chem. 2013, 69, 779–785. [Google Scholar] [CrossRef] [PubMed]
- Fortuna, C.G.; Bonaccorso, C.; Bulbarelli, A.; Caltabiano, G.; Rizzi, L.; Goracci, L.; Musumarra, G.; Pace, A.; Palumbo Piccionello, A.; Guarcello, A.; et al. New linezolid-like 1,2,4-oxadiazoles active against Gram-positive multiresistant pathogens. Eur. J. Med. Chem. 2013, 65, 533–545. [Google Scholar] [CrossRef]
- Gadekar, P.K.; Roychowdhury, A.; Kharkar, P.S.; Khedkar, V.M.; Arkile, M.; Manek, H.; Sarkar, D.; Sharma, R.; Vijayakumar, V.; Sarveswari, S. Design, synthesis and biological evaluation of novel azaspiro analogs of linezolid as antibacterial and antitubercular agents. Eur. J. Med. Chem. 2016, 122, 475–487. [Google Scholar] [CrossRef]
- Naresh, A.; Venkateswara Rao, M.; Kotapalli, S.S.; Ummanni, R.; Venkateswara Rao, B. Oxazolidinone derivatives: Cytoxazone-linezolid hybrids induces apoptosis and senescence in DU145 prostate cancer cells. Eur. J. Med. Chem. 2014, 80, 295–307. [Google Scholar] [CrossRef] [PubMed]
- Palumbo Piccionello, A.; Musumeci, R.; Cocuzza, C.; Fortuna, C.G.; Guarcello, A.; Pierro, P.; Pace, A. Synthesis and preliminary antibacterial evaluation of Linezolid-like 1,2,4-oxadiazole derivatives. Eur. J. Med. Chem. 2012, 50, 441–448. [Google Scholar] [CrossRef]
- Wei, H.; Mao, F.; Ni, S.; Chen, F.; Li, B.; Qiu, X.; Hu, L.; Wang, M.; Zheng, X.; Zhu, J.; et al. Discovery of novel piperonyl derivatives as diapophytoene desaturase inhibitors for the treatment of methicillin-, vancomycin- and linezolid-resistant Staphylococcus aureus infections. Eur. J. Med. Chem. 2018, 145, 235–251. [Google Scholar] [CrossRef]
- Wu, Y.; Ding, X.; Ding, L.; Zhang, Y.; Cui, L.; Sun, L.; Li, W.; Wang, D.; Zhao, Y. Synthesis and antibacterial activity evaluation of novel biaryloxazolidinone analogues containing a hydrazone moiety as promising antibacterial agents. Eur. J. Med. Chem. 2018, 158, 247–258. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Ding, X.; Yang, Y.; Li, Y.; Qi, Y.; Hu, F.; Qin, M.; Liu, Y.; Sun, L.; Zhao, Y. Optimization of biaryloxazolidinone as promising antibacterial agents against antibiotic-susceptible and antibiotic-resistant gram-positive bacteria. Eur. J. Med. Chem. 2020, 185, 111781. [Google Scholar] [CrossRef]
- Jin, B.; Chen, J.Y.; Sheng, Z.L.; Sun, M.Q.; Yang, H.L. Synthesis, Antibacterial and Anthelmintic Activity of Novel 3-(3-Pyridyl)-oxazolidinone-5-methyl Ester Derivatives. Molecules 2022, 27, 1103. [Google Scholar] [CrossRef]
- Jin, B.; Wang, T.; Chen, J.Y.; Liu, X.Q.; Zhang, Y.X.; Zhang, X.Y.; Sheng, Z.L.; Yang, H.L. Synthesis and Biological Evaluation of 3-(Pyridine-3-yl)-2-Oxazolidinone Derivatives as Antibacterial Agents. Front. Chem. 2022, 10, 949813. [Google Scholar] [CrossRef]
- Yang, H.-l.; Jin, B.; Chen, J.-q.; Sheng, Z.-l. Synthesis and Biological Activity of Pyridinyl-4,5-2H-isoxazole Heterocyclic Derivatives. Fine Chem. 2019, 36, 487. [Google Scholar]
- Yang, H.-L.; Xu, G.-X.; Bao, M.-Y.; Zhang, D.-P.; Li, Z.-W.; Pei, Y.-Z. Design and Synthesis of Pyridinylisoxazoles and Their Anticancer Activities. Chem. J. Chin. Univ. 2014, 35, 2584. [Google Scholar]
- Yang, H.-l.; Xu, G.-x.; Pei, Y.-z. Synthesis, preliminary structure-activity relationships and biological evaluation of pyridinyl-4,5-2H-isoxazole derivatives as potent antitumor agents. Chem. Res. Chin. Univ. 2017, 33, 61–69. [Google Scholar] [CrossRef]
- Elattar, K.M.; Mert, B.D.; Monier, M.; El-Mekabaty, A. Advances in the chemical and biological diversity of heterocyclic systems incorporating pyrimido[1,6-a]pyrimidine and pyrimido[1,6-c]pyrimidine scaffolds. RSC Adv. 2020, 10, 15461–15492. [Google Scholar] [CrossRef] [PubMed]
- Albratty, M.; Alhazmi, H.A. Novel pyridine and pyrimidine derivatives as promising anticancer agents: A review. Arab. J. Chem. 2022, 15, 103846. [Google Scholar] [CrossRef]
- Tao, Y.; Chen, J.X.; Fu, Y.; Chen, K.; Luo, Y. Exploratory Process Development and Kilogram-Scale Synthesis of a Novel Oxazolidinone Antibacterial Candidate. Org. Process Res. Dev. 2014, 18, 511–519. [Google Scholar] [CrossRef]
- Phillips, O.A.; D’Silva, R.; Bahta, T.O.; Sharaf, L.H.; Udo, E.E.; Benov, L.; Eric Walters, D. Synthesis and biological evaluation of novel 5-(hydroxamic acid)methyl oxazolidinone derivatives. Eur. J. Med. Chem. 2015, 106, 120–131. [Google Scholar] [CrossRef]
- Wang, Y.; Shen, J.K.; Schroeder, S.J. Nucleotide Dynamics at the A-Site Cleft in the Peptidyltransferase Center of H. marismortui 50S Ribosomal Subunits. J. Phys. Chem. Lett. 2012, 3, 1007–1010. [Google Scholar] [CrossRef]
- Kotb, A.; Abutaleb, N.S.; Seleem, M.A.; Hagras, M.; Mohammad, H.; Bayoumi, A.; Ghiaty, A.; Seleem, M.N.; Mayhoub, A.S. Phenylthiazoles with tert-Butyl side chain: Metabolically stable with anti-biofilm activity. Eur. J. Med. Chem. 2018, 151, 110–120. [Google Scholar] [CrossRef]
- Ding, R.; Wang, X.; Fu, J.; Chang, Y.; Li, Y.; Liu, Y.; Liu, Y.; Ma, J.; Hu, J. Design, synthesis and antibacterial activity of novel pleuromutilin derivatives with thieno[2,3-d]pyrimidine substitution. Eur. J. Med. Chem. 2022, 237, 114398. [Google Scholar] [CrossRef] [PubMed]
- Wiegand, I.; Hilpert, K.; Hancock, R.E. Agar and broth dilution methods to determine the minimal inhibitory concentration (MIC) of antimicrobial substances. Nat. Protoc. 2008, 3, 163–175. [Google Scholar] [CrossRef] [PubMed]
- Ippolito, J.A.; Kanyo, Z.F.; Wang, D.; Franceschi, F.J.; Moore, P.B.; Steitz, T.A.; Duffy, E.M. Crystal Structure of the Oxazolidinone Antibiotic Linezolid Bound to the 50S Ribosomal Subunit. J. Med. Chem. 2008, 51, 3353–3356. [Google Scholar] [CrossRef] [PubMed]
- Noolvi, M.N.; Patel, H.M.; Kamboj, S.; Kaur, A.; Mann, V. 2,6-Disubstituted imidazo[2,1-b][1,3,4]thiadiazoles: Search for anticancer agents. Eur. J. Med. Chem. 2012, 56, 56–69. [Google Scholar] [CrossRef]
- Vaarla, K.; Kesharwani, R.K.; Santosh, K.; Vedula, R.R.; Kotamraju, S.; Toopurani, M.K. Synthesis, biological activity evaluation and molecular docking studies of novel coumarin substituted thiazolyl-3-aryl-pyrazole-4-carbaldehydes. Bioorg. Med. Chem. Lett. 2015, 25, 5797–5803. [Google Scholar] [CrossRef]


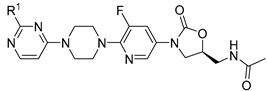 | ||||||||
| Compound | R1 | Sa a | Sp b | Ef c | Bs d | Sx e | Lm f | Ec g |
|---|---|---|---|---|---|---|---|---|
| 5 | -Cl | 1 | 1 | 1 | 1 | 1 | 2 | >128 |
| 6a |  | 8 | 16 | 8 | 8 | 8 | 32 | >128 |
| 6b | 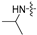 | 16 | 16 | 16 | 16 | 8 | 16 | >128 |
| 6c |  | 8 | 8 | 8 | 8 | 8 | 8 | >128 |
| 6d | 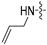 | 8 | 8 | 8 | 4 | 8 | 32 | >128 |
| 6e |  | 8 | 8 | 16 | 8 | 16 | 64 | >128 |
| 6f | 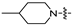 | 16 | 32 | 32 | 32 | 16 | 128 | >128 |
| 6g | 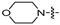 | 16 | 16 | 16 | 16 | 16 | 32 | >128 |
| 6h |  | 8 | 8 | 8 | 8 | 8 | 16 | >128 |
| 6i |  | 8 | 16 | 8 | 8 | 8 | 64 | >128 |
| 6j | 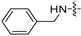 | 8 | 8 | 8 | 8 | 8 | 16 | >128 |
| 6k | 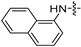 | 32 | 16 | 32 | 32 | 8 | 32 | >128 |
| 6l | 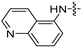 | 8 | 8 | 8 | 8 | 8 | 16 | >128 |
| 6m | 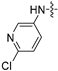 | 2 | 2 | 8 | 4 | 4 | 32 | >128 |
| linezolid | - | 2 | 2 | 2 | 2 | 2 | 2 | >128 |
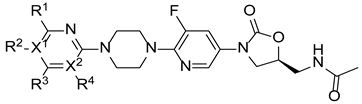 | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Compound | X1 | X2 | R1 | R2 | R3 | R4 | Sa a | Sp b | Ef c | Bs d | Sx e | Lm f | Ec g |
| 7a | N | C | H | - | Cl | H | 2 | 4 | 2 | 2 | 2 | 2 | >128 |
| 7b | C | N | Cl | H | H | - | 2 | 1 | 2 | 2 | 2 | 8 | >128 |
| 7c | C | N | H | CH3 | H | - | 2 | 2 | 4 | 2 | 1 | 1 | >128 |
| 7d | C | N | H | Br | H | - | 2 | 4 | 2 | 4 | 2 | 2 | >128 |
| 7e | N | C | NH2 | - | H | H | 0.5 | 0.5 | 1 | 1 | 1 | 0.5 | >128 |
| 7f | C | N | NH2 | H | H | - | 2 | 1 | 1 | 1 | 2 | 4 | >128 |
| 7g | C | N | Cl | H | Cl | - | 2 | 4 | 8 | 16 | 2 | 2 | >128 |
| 7h | N | C | Cl | - | Cl | H | 2 | 2 | 1 | 2 | 1 | 2 | >128 |
| 7i | N | C | Cl | - | H | F | 0.5 | 0.5 | 1 | 1 | 1 | 0.5 | >128 |
| 7j | N | C | Cl | - | H | Cl | 1 | 0.25 | 1 | 1 | 1 | 0.25 | >128 |
| 7k | N | C | Cl | - | H | Br | 1 | 0.5 | 0.5 | 1 | 2 | 0.5 | >128 |
| 7l | N | C | Cl | - | H | CH3 | 0.5 | 1 | 4 | 4 | 2 | 0.5 | >128 |
| 7m | N | C | CH3S | - | H | C2H5OCO | 32 | 32 | 32 | 32 | 32 | 32 | >128 |
| 7n | N | C | Cl | - | Cl | Cl | 2 | 4 | 2 | 2 | 2 | 2 | >128 |
| linezolid | - | - | - | - | - | - | 2 | 2 | 2 | 2 | 2 | 2 | >128 |
| Compound | MRSA a | VRE b | LRSA c | LRSP d |
|---|---|---|---|---|
| 7i | 1 | 1 | >128 | >128 |
| 7j | 1 | 1 | >128 | >128 |
| 7k | 1 | 1 | >128 | >128 |
| 7l | 1 | 1 | >128 | >128 |
| linezolid | 2 | 2 | >128 | >128 |
| Compound | MRSA a | VRE b | LRSA c | LRSP d |
|---|---|---|---|---|
| 7i | 0.5 | 0.5 | 2 | 4 |
| 7j | 0.5 | 0.5 | 1 | 4 |
| 7k | 0.5 | 0.5 | 1 | 4 |
| 7l | 0.5 | 0.5 | 2 | 4 |
| Linezolid | 64 | 16 | 128 | 128 |
| Concentration (µg/mL) | 32 | 64 | 128 | 256 | 500 | 1000 |
|---|---|---|---|---|---|---|
| cell viability (%) | 100 | 97.5 | 96.9 | 93.5 | 33.3 | 7.3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, X.; Jin, B.; Han, Y.; Wang, T.; Sheng, Z.; Tao, Y.; Yang, H. Optimization and Antibacterial Evaluation of Novel 3-(5-Fluoropyridine-3-yl)-2-oxazolidinone Derivatives Containing a Pyrimidine Substituted Piperazine. Molecules 2023, 28, 4267. https://doi.org/10.3390/molecules28114267
Wang X, Jin B, Han Y, Wang T, Sheng Z, Tao Y, Yang H. Optimization and Antibacterial Evaluation of Novel 3-(5-Fluoropyridine-3-yl)-2-oxazolidinone Derivatives Containing a Pyrimidine Substituted Piperazine. Molecules. 2023; 28(11):4267. https://doi.org/10.3390/molecules28114267
Chicago/Turabian StyleWang, Xin, Bo Jin, Yutong Han, Tong Wang, Zunlai Sheng, Ye Tao, and Hongliang Yang. 2023. "Optimization and Antibacterial Evaluation of Novel 3-(5-Fluoropyridine-3-yl)-2-oxazolidinone Derivatives Containing a Pyrimidine Substituted Piperazine" Molecules 28, no. 11: 4267. https://doi.org/10.3390/molecules28114267
APA StyleWang, X., Jin, B., Han, Y., Wang, T., Sheng, Z., Tao, Y., & Yang, H. (2023). Optimization and Antibacterial Evaluation of Novel 3-(5-Fluoropyridine-3-yl)-2-oxazolidinone Derivatives Containing a Pyrimidine Substituted Piperazine. Molecules, 28(11), 4267. https://doi.org/10.3390/molecules28114267





