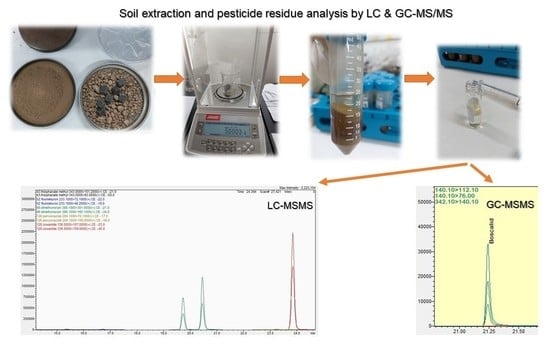
Validation and Simultaneous Monitoring of 311 Pesticide Residues in Loamy Sand Agricultural Soils by LC-MS/MS and GC-MS/MS, Combined with QuEChERS-Based Extraction
Abstract
1. Introduction
2. Results
2.1. Determination of Compounds
2.1.1. Optimization of MS/MS Parameters
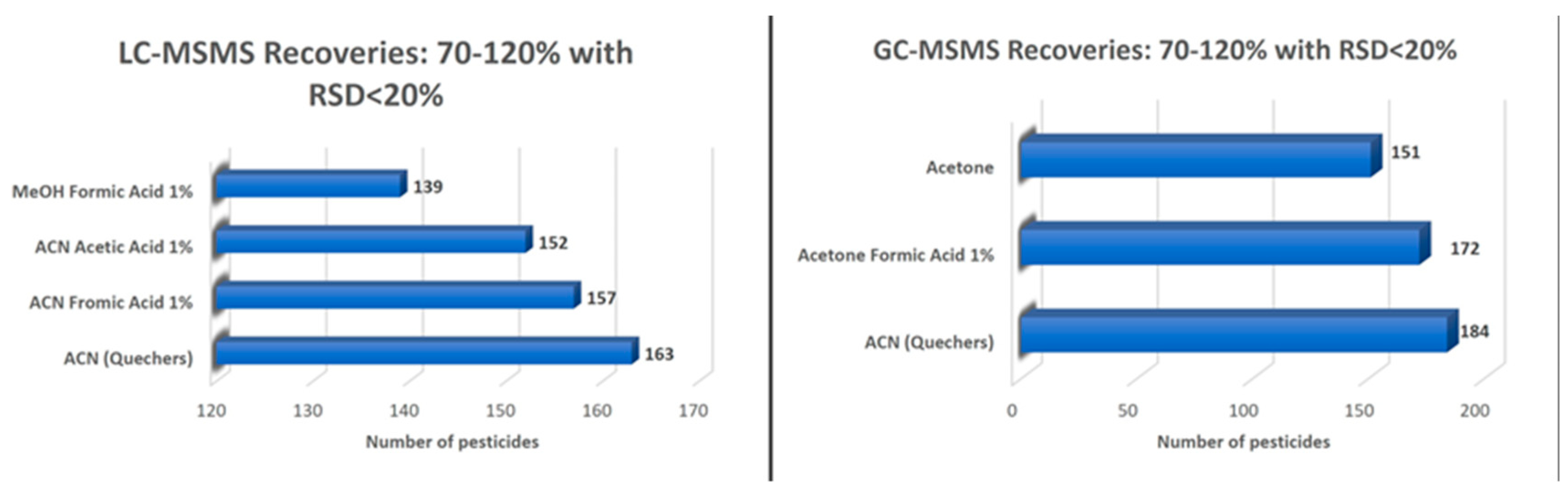
2.1.2. Optimization of the Extraction Efficiency
2.2. Validation of the Analytical Method
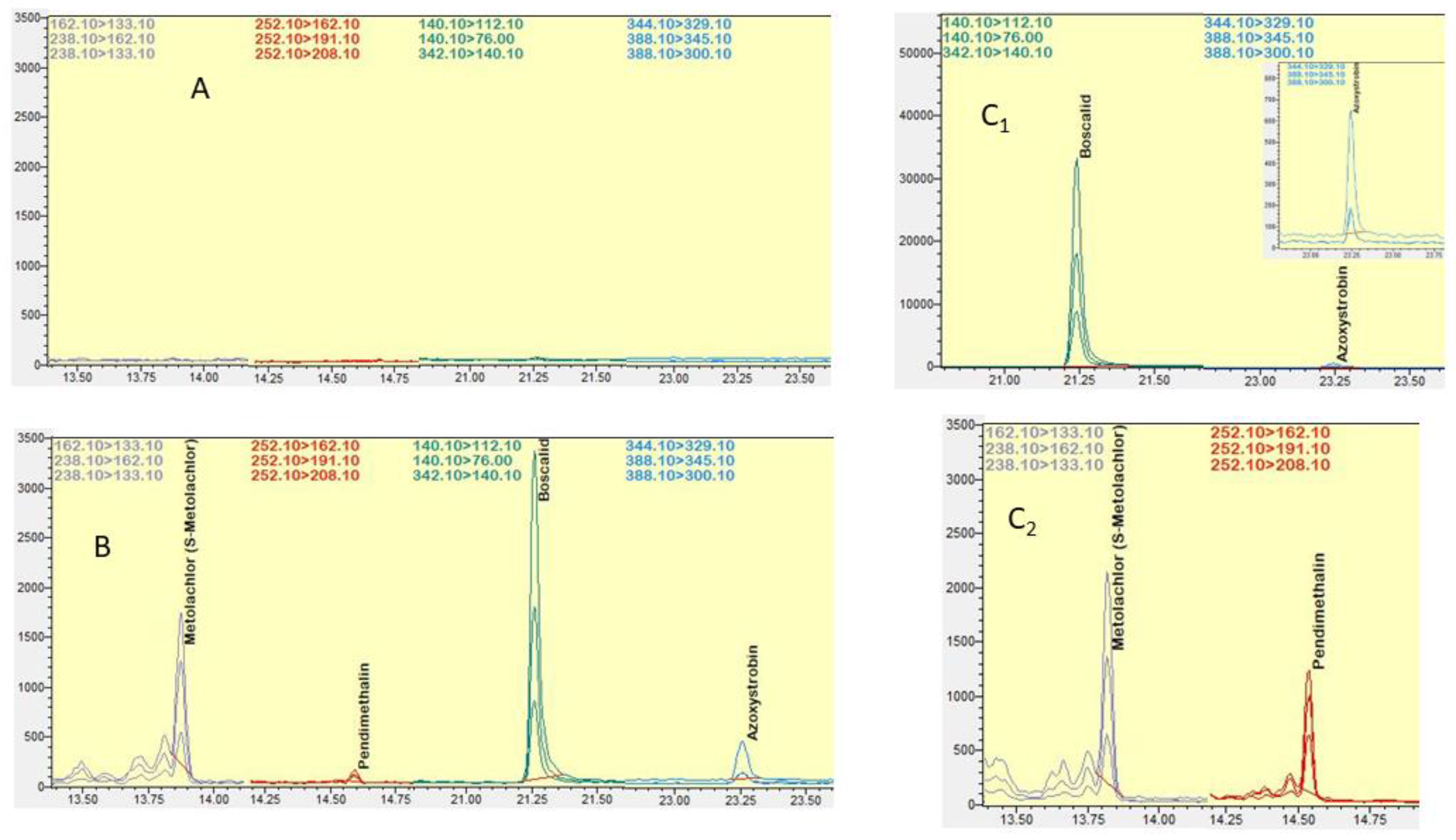
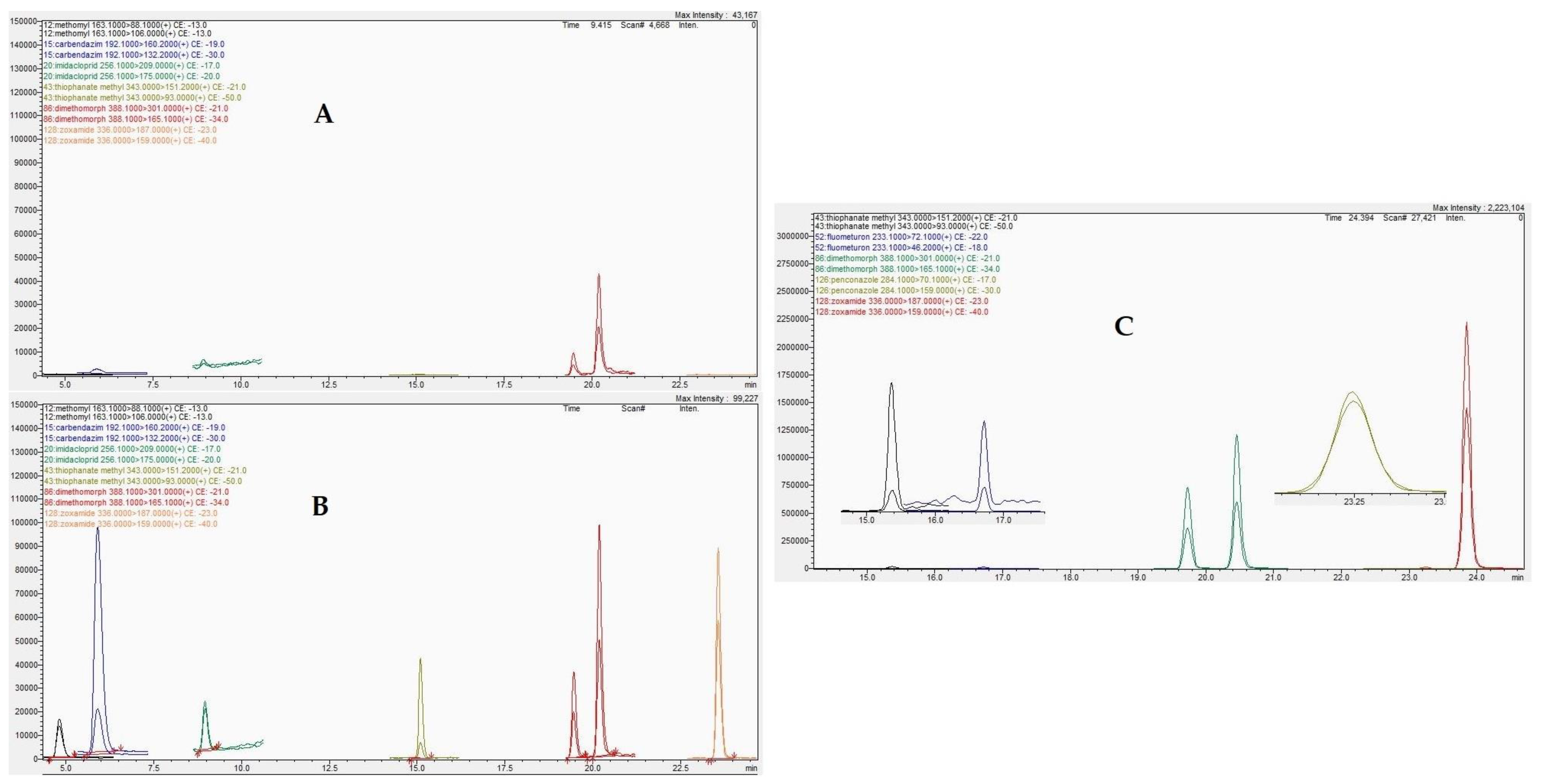
2.2.1. Method Selectivity
2.2.2. Accuracy and Precision
2.2.3. Linearity
2.2.4. Limit of Quantification and Limit of Detection
2.2.5. Matrix Effects (ME)
2.2.6. Participation in Proficiency Testing Scheme
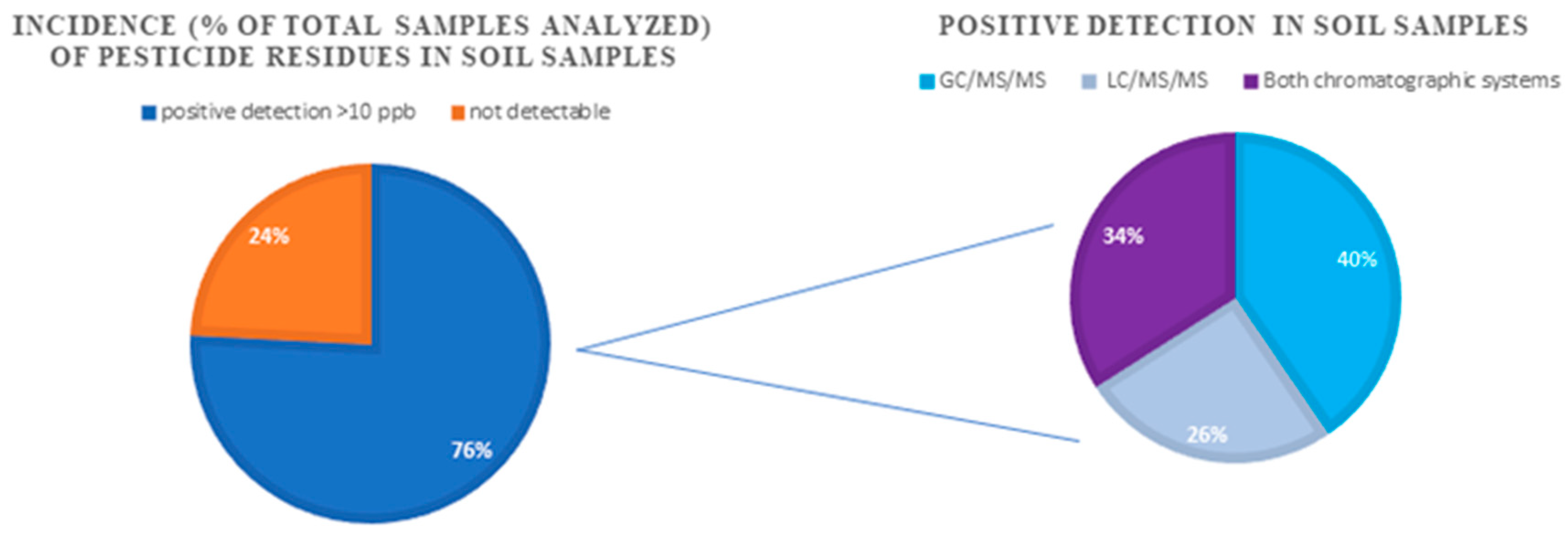
2.3. Method Application
3. Materials and Methods
3.1. Studied Area and Sampling of Soil
3.2. Selection of Analyzed Pesticides
3.3. Analytical Methodology
3.3.1. Chemicals and Solvents
3.3.2. Standard Solutions
3.3.3. Experimental Design
3.3.4. Sample Preparation
Optimization of the Extraction Efficiency
3.3.5. Determination of Compounds—Instrumentation
Analysis of Samples with Gas Chromatography
Analysis of Samples with Liquid Chromatography
Optimization of MS/MS Parameters
3.3.6. Validation Procedure
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Ogura, A.P.; Lima, J.Z.; Marques, J.P.; Sousa, L.M.; Rodrigues, V.G.S.; Espíndola, E.L.G. A review of pesticides sorption in biochar from maize, rice, and wheat residues: Current status and challenges for soil application. J. Environ. Manag. 2021, 300, 113753. [Google Scholar] [CrossRef] [PubMed]
- Singh, K.P.; Mohan, D.; Sinha, S.; Dalwani, S. Impact assessment of treated/untreated wastewaters toxicants discharged by sewage treatment plants on health, agricultural and environmental quality in the wastewater disposal area. Chemosphere 2004, 55, 227–255. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Lonappan, L.; Brar, S.K.; Yang, S. Impact of biochar amendment in agricultural soils on the sorption, desorption, and degradation of pesticides: A review. Sci. Total Environ. 2018, 645, 60–70. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Zou, W.; Chen, J.; Chen, H.; Yu, Z.; Huang, J.; Tang, H.; Wei, X.; Gao, B. Biochar amendment improves crop production in problem soils: A review. J. Environ. Manag. 2019, 232, 8–21. [Google Scholar] [CrossRef] [PubMed]
- Agnihotri, N.P.; Vijay, P.; Kumar, T.; Mohapatra, M.; Salja, P. Organochlorine insecticide residue in Ganga River, water near Farrukhabad. Environ. Monit. Assess. 1994, 30, 105–112. [Google Scholar] [CrossRef]
- Cedergreen, N. Quantifying synergy: A systematic review of mixture toxicity studies within environmental toxicology. PLoS ONE 2014, 9, e96580. [Google Scholar] [CrossRef]
- FAO. Food and Agriculture Organization of the United Nations. FAOSTAT, Agri-Environmental Indicators/Pesticides; FAOSTAT Analytical Brief 46; FAO: Rome, Italy, 2020. [Google Scholar]
- FAO. The Future of Food and Agriculture—Alternative Pathways to 2050. Licence: CC BY-NC-SA 3.0 IGO. 2018, p. 224. Available online: http://www.fao.org/3/I8429EN/i8429en.pdf (accessed on 15 December 2022).
- McDougall, P. Evolution of the Crop Protection Industry Since 1960. 2018. Available online: https://croplife.org/wp-content/uploads/2018/11/Phillips-McDougall-Evolution-ofthe-Crop-Protection-Industry-since-1960-FINAL.pdf (accessed on 24 November 2022).
- Chiaia-Hernandez, A.C.; Keller, A.; Wächter, D.; Steinlin, C.; Camenzuli, L.; Hollender, J.; Krauss, M. Long-term persistence of pesticides and TPs in archived agricultural soil samples and comparison with pesticide application. Environ. Sci. Technol. 2017, 51, 10642–10651. [Google Scholar] [CrossRef][Green Version]
- Gamon, M.; Saez, E.; Gil, J.; Boluda, R. Direct and indirect exogenous contamination by pesticides of rice-farming soils in a Mediterranean Wetland. Arch. Environ. Contam. Toxicol. 2003, 44, 141–151. [Google Scholar] [CrossRef]
- Hvezdova, M.; Kosubova, P.; Kosíkova, M.; Scherr, K.E.; Simek, Z.; Brodský, L.; Sudoma, M.; Skulcova, L.; Sanka, M.; Svobodova, M.; et al. Currently and recently used pesticides in Central European arable soils. Sci. Total Environ. 2018, 613–614, 361–370. [Google Scholar] [CrossRef]
- Karasali, H.; Marousopoulou, A.; Machera, K. Pesticide residue concentration in soil following conventional and Low-Input Crop Management in a Mediterranean agro-ecosystem, in Central Greece. Sci. Total Environ. 2016, 541, 130–142. [Google Scholar] [CrossRef]
- Markovic, M.; Cupac, S.; Đurovic, R.; Milinovic, J.; Kljajic, P. Assessment of heavy metal and pesticide levels in soil and plant products from agricultural area of Belgrade. Serbia. Arch Environ. Contam Toxicol. 2010, 58, 341–351. [Google Scholar] [CrossRef] [PubMed]
- Silva, V.; Mol, H.G.J.; Zomer, P.; Tienstra, M.; Ritsema, C.J.; Geissen, V. Pesticide residues in European agricultural soils—A hidden reality unfolded. Sci. Total Environ. 2019, 653, 1532–1545. [Google Scholar] [CrossRef] [PubMed]
- Suszter, G.K.; Ambrus, A. Distribution of pesticide residues in soil and uncertainty of sampling. J. Environ. Sci. Health B 2017, 52, 557–563. [Google Scholar] [CrossRef]
- Zhao, P.; Wang, Z.; Li, K.; Guo, X.; Zhao, L. Multi-residue enantiomeric analysis of 18 chiral pesticides in water, soil and river sediment using magnetic solid-phase extraction based on amino modified multiwalled carbon nanotubes and chiral liquid chromatography coupled with tandem mass spectrometry. J. Chromatogr. A 2018, 1568, 8–21. [Google Scholar] [CrossRef]
- Perez, D.J.; Iturburu, F.G.; Calderon, G.; Oyesqui, L.A.E.; De Geronimo, E.; Aparicio, V.C. Ecological risk assessment of current-use pesticides and biocides in soils, sediments and surface water of a mixed land-use basin of the Pampas region, Argentina. Chemosphere 2021, 263, 128061. [Google Scholar] [CrossRef] [PubMed]
- Ene, A.; Bogdevich, O.; Sion, A. Levels and distribution of organochlorine pesticides (OCPs) and polycyclic aromatic hydrocarbons (PAHs) in top soils from SE Romania. Sci. Total Environ. 2012, 439, 76–86. [Google Scholar] [CrossRef] [PubMed]
- Łozowicka, B.; Jankowska, M.; Rutkowska, E.; Kaczyn’ski, P.; Hrynko, I. Comparison of extraction techniques by matrix solid phase dispersion and liquid-liquid for the screening of 150 pesticides from soil and determination by gas chromatography. Pol. J. Environ. Stud. 2012, 21, 973–992. [Google Scholar]
- Skrbic, B.; Cvejanov, J.; Mladenovic, N.D. Organochlorine pesticides and polychlorinated biphenyls in surface soils of Novi Sad and bank sediment of the Danube River. J. Environ. Sci. Health Part B 2007, 42, 311–319. [Google Scholar] [CrossRef]
- Covaci, A.; Hurab, C.; Schepensa, P. Selected persistent organochlorine pollutants in Romania. Sci. Total Environ. 2001, 280, 143–152. [Google Scholar] [CrossRef]
- Manjarres-López, D.P.; Andrades, M.S.; Sánchez-González, S.; Rodríguez-Cruz, M.S.; Sánchez-Martín, M.J.; Herrero-Hernández, E. Assessment of pesticide residues in waters and soils of a vineyard region and its temporal evolution. Environ. Pollut. 2021, 284, 117463. [Google Scholar] [CrossRef]
- Khan, N.; Yaqub, G.; Hafeez, T.; Tariq, M. Assessment of Health Risk due to Pesticide Residues in Fruits, Vegetables, Soil, and Water. Hindawi J. Chem. 2020, 2020, 5497952. [Google Scholar] [CrossRef]
- Picó, Y.; Alvarez-Ruiz, R.; Alfarhan, A.H.; El-Sheikh, M.A.; Alobaid, S.M.; Barceló, D. Uptake and accumulation of emerging contaminants in soil and plant treated with wastewater under real-world environmental conditions in the Al Hayer area (Saudi Arabia). Sci. Total Environ. 2019, 652, 562–572. [Google Scholar] [CrossRef] [PubMed]
- Acosta-Dacal, A.; Rial-Berriel, C.; Díaz-Díaz, D.; Bernal-Suárez, M.; Luzardo, O.P. Optimization and validation of a QuEChERS-based method for the simultaneous environmental monitoring of 218 pesticide residues in clay loam soil. Sci. Total Environ. 2021, 753, 142015. [Google Scholar] [CrossRef]
- Jo, H.-W.; Park, M.-G.; Jeon, H.-J.; Moon, J.-K.; Lee, S.-E. Analysis of multiresidue pesticides in agricultural paddy soils near industrial areas in Korea by GC-MS/MS and LC-MS/MS using QuEChERS extraction with dSPE clean-up. Appl. Sci. 2021, 11, 8415. [Google Scholar] [CrossRef]
- Wang, F.; Li, X.; Yu, S.; He, S.; Cao, D.; Yao, S.; Fang, H.; Yu, Y. Chemical factors affecting uptake and translocation of six pesticides in soil by maize (Zea mays L.). J. Hazard. Mater. 2021, 405, 124269. [Google Scholar] [CrossRef]
- Zhao, L.; Lia, Y.; Ren, W.; Huang, Y.; Wang, X.; Fu, Z.; Ma, W.; Teng, Y.; Luo, Y. Pesticide residues in soils planted with Panax notoginseng in south China, and their relationships in Panax notoginseng and soil. Ecotoxicol. Environ. Saf. 2020, 201, 110783. [Google Scholar] [CrossRef]
- Fu, Y.; Dou, X.; Lu, Q.; Qin, J.; Luo, J.; Yang, M. Comprehensive assessment for the residual characteristics and degradation kinetics of pesticides in Panax notoginseng and planting soil. Sci. Total Environ. 2020, 714, 136718. [Google Scholar] [CrossRef]
- Neuwirthová, N.; Trojan, M.; Svobodová, M.; Vašíčková, J.; Šimek, Z.; Hofman, J.; Bielská, L. Pesticide residues remaining in soils from previous growing season(s)—Can they accumulate in non-target organisms and contaminate the food web? Sci. Total Environ. 2019, 646, 1056–1062. [Google Scholar] [CrossRef]
- Zaidon, S.Z.; Ho, Y.B.; Hamsan, H.; Hashim, Z.; Saari, N.; Praveena, S.M. Improved QuEChERS and solid phase extraction for multi-residue analysis of pesticides in paddy soil and water using ultra-high performance liquid chromatography tandem mass spectrometry. Microchem. J. 2019, 145, 614–621. [Google Scholar] [CrossRef]
- Socas-Rodríguez, B.; González-Sálamo, J.; Herrera-Herrera, A.V.; Javier Hernández-Borges, J.; Rodríguez-Delgado, M.A. Chapter Eleven—Recent Advances and Developments in the QuEChERS Method. Compr. Anal. Chem. 2017, 76, 319–374. [Google Scholar] [CrossRef]
- Yu, Y.; Liu, X.; He, Z.; Wang, L.; Luo, M.; Peng, Y.; Zhou, Q. Development of a multi-residue method for 58 pesticides in soil using QuEChERS and gas chromatography-tandem mass spectrometry. Anal. Methods 2016, 8, 2463–2470. [Google Scholar] [CrossRef]
- Gonzalez-Curbelo, M.A.; Socas-Rodríguez, B.; Herrera-Herrera, A.V.; Gonzalez- Salamo, J.; Hernandez-Borges, J.; Rodríguez-Delgado, M.A. Evolution and applications of the QuEChERS method. Trac. Trends Anal. Chem. 2015, 71, 169–185. [Google Scholar] [CrossRef]
- Masia, A.; Vasquez, K.; Campo, J.; Pico, Y. Assessment of two extraction methods to determine pesticides in soils, sediments and sludges. Application to the Túria River Basin. J. Chromatogr. A 2016, 1378, 19–31. [Google Scholar] [CrossRef]
- Homazava, N.; Gachet Aquillon, C.; Vermeirssen, E.; Werner, I. Simultaneous multi-residue pesticide analysis in soil samples with ultra-high-performance liquid chromatography-tandem mass spectrometry using QuEChERS and pressurised liquid extraction methods. Int. J. Environ. Anal. Chem. 2014, 94, 1085–1099. [Google Scholar] [CrossRef]
- Perestrelo, R.; Silva, P.; Porto-Figueira, P.; Pereira, J.A.M.; Silva, C.; Medina, S.; Camara, J.S. QuEChERS-fundamentals, relevant improvements, applications and future trends. Anal. Chim. Acta 2019, 1070, 1–28. [Google Scholar] [CrossRef] [PubMed]
- Moder, M.; Popp, P.; Eisert, R.; Pawliszyn, J. Determination of polar pesticides in soil by solid phase microextraction coupled to high-performance liquid chromatography-electrospray/mass spectrometry. Fresenius J. Anal. Chem. 1999, 363, 680. [Google Scholar] [CrossRef]
- Bouaid, L.; Ramos, M.J.; Gonzalez, P.; Fernandez, C.; Camara, J. Solid-phase microextraction method for the determination of atrazine and four organophosphorus pesticides in soil samples by gas chromatography. J. Chromatogr. A 2001, 939, 13. [Google Scholar] [CrossRef]
- Herbert, P.; Morais, S.; Paíga, P.; Alves, A.; Santos, L. Development and Validation of a Novel Method for the Analysis of Chlorinated Pesticides in Soils Using Microwave-Assisted Extraction-Headspace Solid Phase Microextraction and Gas Chromatography-Tandem Mass Spectrometry. Anal. Bioanal. Chem. 2006, 384, 810–816. [Google Scholar] [CrossRef][Green Version]
- Vega Moreno, D.; Sosa Ferrera, Z.; Santana Rodrıguez, J. JDetermination of Organochlorine Pesticides from Agricultural Soils Using Pressurized Liquid Extraction. Anal. Chim. Acta 2006, 571, 51. [Google Scholar]
- De Geronimo, E.; Botero-Coy, A.M.; Marin, J.M.; Aparicio, V.C.; Costa, J.L.; Sancho, J.V.; Hernandez, F. A simple and rapid analytical methodology based on liquid chromatography-tandem mass spectrometry for monitoring pesticide residues in soils from Argentina. Anal. Methods. 2015, 7, 9504–9512. [Google Scholar] [CrossRef]
- Perez, D.J.; Okada, E.; De Geronimo, E.; Aparicio, V.C.; Costa, J.L. Spatial and temporal trends and flow dynamics of glyphosate and other pesticides within an agricultural watershed in Argentina. Environ. Toxicol. Chem. 2017, 36, 3206–3216. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Zhang, K.; Wong, J.W.; Yang, P.; Tech, K.; Dibenedetto, A.L.; Lee, N.S.; Hayward, D.G.; Makovi, C.M.; Krynitsky, A.J.; Banerjee, K.; et al. Multiresidue pesticide analysis of agricultural commodities using acetonitrile salt-out extraction, dispersive solid-phase sample cleanup, and high-performance liquid chromatography-tandem mass spectrometry. J. Agric. Food Chem. 2011, 59, 7636–7646. [Google Scholar] [CrossRef] [PubMed]
- Pastor-Belda, M.; Garrido, I.; Campillo, N.; Vinas, P.; Hellin, P.; Flores, P.; Fenoll, J. Dispersive liquid-liquid microextraction for the determination of new generation pesticides in soils by liquid chromatography and tandem mass spectrometry. J. Chromatogr. A 2015, 1394, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Salemi, A.; Rasoolzadeh, R.; Nejad, M.M.; Vosough, M. Ultrasonic assisted headspace single drop micro-extraction and gas chromatography with nitrogen-phosphorus detector for determination of organophosphorus pesticides in soil. Anal. Chim. Acta 2013, 769, 121–126. [Google Scholar] [CrossRef]
- Redondo, M.J.; Ruiz, M.J.; Boluda, R.; Font, G. Optimization of a solid-phase extraction technique for the extraction of pesticides from soil samples. J. Chromatogr. A 1996, 719, 69–76. [Google Scholar] [CrossRef]
- EPA. Persistent Organic Pollutants: A Global Issue, A Global Response. 2009. Available online: https://www.epa.gov/international-cooperation/persistent-organic-pollutants-global-issue-global-response) (accessed on 2 December 2022).
- Abhilash, P.C.; Singh, N. Pesticide use and application: An Indian scenario. J. Hazard. Mater. 2009, 165, 1–12. [Google Scholar] [CrossRef]
- Zhang, W.J.; Jiang, F.B.; Ou, J.F. Global pesticide consumption and pollution: With China as a focus. Proc. Int. Acad. Ecol. Environ. Sci. 2011, 1, 125–144. [Google Scholar]
- Cremlyn, R.J. Agrochemicals Preparation and Mode of Action; John Wiley & Sons: New York, NY, USA, 1991. [Google Scholar]
- Yadav, I.C.; Devi, N.L.; Syed, J.H.; Cheng, Z.; Li, J.; Zhang, G.; Jones, C. Current status of persistent organic pesticides residues in air, water, and soil, and their possible effect on neighboring countries: A comprehensive review of India. Sci. Total Environ. 2015, 511, 123–137. [Google Scholar] [CrossRef]
- Tanabe, S.; Hidaka, H.; Tatsukawa, R. PCBs and chlorinated hydrocarbon pesticides in Antarctic atmosphere and hydrosphere. Chemosphere 1983, 12, 227–288. [Google Scholar] [CrossRef]
- Tzanetou, E.N.; Karasali, H. A Comprehensive Review of Organochlorine Pesticide Monitoring in Agricultural Soils: The Silent Threat of a Conventional Agricultural Past. Agriculture 2022, 12, 728. [Google Scholar] [CrossRef]
- Ganie, A.S.; Bano, S.; Khan, N.; Sultana, S.; Rehman, Z.; Rahman, M.M.; Sabir, S.; Coulon, F.; Khan, M.Z. Nanoremediation technologies for sustainable remediation of contaminated environments: Recent advances and challenges. Chemosphere 2021, 275, 130065. [Google Scholar] [CrossRef]
- Morillo, E.; Villaverde, J. Advanced technologies for the remediation of pesticide contaminated soils. Sci. Total Environ. 2017, 586, 576–597. [Google Scholar] [CrossRef][Green Version]
- Rani, L.; Thapa, K.; Kanojia, N.; Sharma, N.; Singh, S.; Grewal, A.S.; Srivastav, A.L.; Kaushal, J. An extensive review on the consequences of chemical pesticides on human health and environment. J. Clean. Prod. 2021, 283, 124657. [Google Scholar] [CrossRef]
- Saleh, I.A.; Zouari, N.; Al-Ghouti, M.A. Removal of pesticides from water and wastewater: Chemical, physical and biological treatment approaches. Environ. Technol. Innov. 2020, 19, 101026. [Google Scholar] [CrossRef]
- SANTE/11312/2021; Guidance SANTE/11312/2021—Guidance Document on Analytical Quality Control and Method Validation Procedures for Pesticides Residues Analysis in Food and Feed. European Commission: Brussels, Belgium, 2021.
- Calbiani, F.; Careri, M.; Elviri, L.; Mangia, A.; Pistar, L.; Zagnoni, I. Development and in-House Validation of a Liquid Chromatography-Electrospray-Tandem Mass Spectrometry Method for the Simultaneous Determination of Sudan I, Sudan II, Sudan III and Sudan IV in Hot Chilli Products. J. Chromatogr. A 2004, 1042, 123–130. [Google Scholar] [CrossRef]
- Zhou, W.; Han, G.; Liu, M.; Li, X. Effects of soil pH and texture on soil carbon and nitrogen in soil profiles under different land uses in Mun River Basin, Northeast Thailand. PeerJ 2019, 7, e7880. [Google Scholar] [CrossRef][Green Version]
- Kuśmierz, S.; Skowrońska, M.; Tkaczyk, P.; Lipiński, W.; Mielniczuk, J. Soil Organic Carbon and Mineral Nitrogen Contents in Soils as Affected by Their pH, Texture and Fertilization. Agronomy 2023, 13, 267. [Google Scholar] [CrossRef]
- European Commission. EU Pesticides Database. European Commission. 2020. Available online: https://food.ec.europa.eu/plants/pesticides/eu-pesticides-database_en (accessed on 1 September 2022).
- Kosubová, P.; Škulcová, L.; Poláková, S.; Hofman, J.; Bielská, L. Spatial and temporal distribution of the currently-used and recently-banned pesticides in arable soils of the Czech Republic. Chemosphere 2020, 254, 126902. [Google Scholar] [CrossRef]
- Al-Wabel, M.I.; El-Saeid, M.H.; Al-Turki, A.M.; Abdel-Nasser, G. Monitoring of pesticide residues in Saudi Arabia agricultural soils. Res. J. Environ. Sci. 2011, 5, 269–278. [Google Scholar]
- Devi, N.L.; Chakraborty, P.; Shihua, Q.; Zhang, G. Selected organochlorine pesticides (OCPs) in surface soils from three major states from the northeastern part of India. Environ. Monit. Assess. 2013, 185, 6667–6676. [Google Scholar] [CrossRef]
- Hoai, P.M.; Ngoc, N.T.; Minh, N.H.; Virt, P.H.; Berg, M.; Alder, A.C.; Giger, W. Recent levels of organochlorine pesticides and polychlorinated biphenyls in sediments of the sewer system in Hanoi, Vietnam. Environ. Pollut. 2010, 158, 913–920. [Google Scholar] [CrossRef]
- Kata, M.; Rao, S.S.; Mohan, K.R. Spatial distribution, ecological risk evaluation and potential sources of organochlorine pesticides from soils in India. Environ. Earth Sci. 2014, 74, 4031–4038. [Google Scholar] [CrossRef]
- Kumar, B.; Verma, V.K.; Mishra, M.; Gaur, R.; Kumar, S.; Sharma, C.S. DDT and HCH (organochlorine pesticides) in residential soils and health assessment for human populations in Korba, India. Hum. Ecol. Risk. Assess. 2014, 20, 1538–1549. [Google Scholar] [CrossRef]
- Kumari, B.; Madan, V.K.; Kathpal, T.S. Status of insecticide contamination of soil and water in Haryana, India. Environ. Monit. Assess. 2008, 136, 239–244. [Google Scholar] [CrossRef]
- Liu, J.; Cui, Z.J.; Xu, H.Y.; Tan, F.X. Dioxin-like polychlorinated biphenyls contamination and distribution in soils from the Modern Yellow River delta, China. Soil Sediment Contam. 2009, 18, 144–154. [Google Scholar] [CrossRef]
- Senthilkumar, K.; Priya, M.; Sajwan, K.S.; Kolli, R.; Roots, O. Residues of persistent organic pollutants in Estonian soil. Est. J. Earth Sci. 2009, 58, 109–123. [Google Scholar] [CrossRef]
- Mwevura, H.; Othman, O.C.; Mhehe, G.L. Organochlorine pesticide residues in sediments and biota from the coastal area of Dar es Salaam city, Tanzania. Mar. Pollut. Bull. 2002, 45, 262–267. [Google Scholar] [CrossRef]
- Mwevura, H.; Othman, O.C.; Mhehe, G.L. Organochlorine pesticides in residues in waters from the coastal area of Dar es Salaam and their effect on aquatic biota. Tanz. J. Sci. 2002, 28, 117–130. [Google Scholar] [CrossRef][Green Version]
- Mmochi, A. Levels and Chemo Dynamics of Pesticide Residues and Metabolites in the Zanzibar Coastal Marine Environment; University of Dar es Salaam: Dar es Salaam, Tanzania, 2005. [Google Scholar]
- Mwevura, H.; Bouwman, H.; Kylin, H.; Vogt, T.; Issa, M.A. Organochlorine pesticides and polycyclic aromatic hydrocarbons in marine sediments and polychaete worms from the west coast of Unguja island, Tanzania. Reg. Stud. Mar. Sci. 2020, 36, 101287. [Google Scholar] [CrossRef]
- Wandiga, S.; Yugi, P.; Barasa, M.; Jumba, I.O.; Lalah, J. The distribution of organochlorine pesticides in marine samples along the Indian Ocean coast of Kenya. Environ. Technol. 2002, 23, 1235–1246. [Google Scholar] [CrossRef]
- SANTE/2017/10632; Technical Guideline on the Evaluation of Extraction Efficiency of Residue Analytical Methods. Rev. 4, 23 February 2022. European Commission Directorate—General for Health and Food Safety: Brussels, Belgium, 2022.
- Miglioranza, K.S.B.; Sagrario, M.; Sagrario, M.d.l.A.G.; Aizpún de Moreno, J.E.; Moreno, V.J.; Escalante, A.H.; Osterrieth, M.L. Agricultural soil as a potential source of input of organochlorine pesticides into a nearby pond. Environ. Sci. Pollut. Res. 2002, 9, 250–256. [Google Scholar] [CrossRef]
- Mwevura, H.; Kylin, H.; Vogt, T.; Bouwman, H. Dynamics of organochlorine and organophosphate pesticide residues in soil, water, and sediment from the Rufiji River Delta, Tanzania. Reg. Stud. Mar. Sci. 2021, 41, 101607. [Google Scholar] [CrossRef]
- Gosetti, F.; Mazzucco, E.; Zampieri, D.; Gennaro, M.C. Signal suppression/enhancement in high-performance liquid chromatography tandem mass spectrometry. J. Chromatogr. A 2010, 1217, 3929–3937. [Google Scholar] [CrossRef]
- Ferrer, C.; Lozano, A.; Aguera, A.; Jimenez, A.R.; Fernandez, J. Overcoming matrix effects using the dilution approach in multiresidue methods for fruits and vegetables. J. Chromatogr. A 2011, 1218, 7634–7639. [Google Scholar] [CrossRef]
| Pesticide | Type | Frequency (%) | Max Concentration (mg/kg) | Approval (EC, 2022) |
|---|---|---|---|---|
| Azoxystrobin | F | 16 | 0.047 | Yes |
| Boscalid | F | 56 | 0.11 | Yes |
| Carbendazim | F | 4 | 0.037 | No |
| Carfentrazon ethyl | H | 8 | 0.03 | Yes |
| Clothianidin | I | 4 | 0.01 | No |
| Dimethomorph | F | 20 | 1.225 | Yes |
| Epoxiconazole | F | 8 | 0.01 | No |
| Fludioxonil | F | 4 | 0.01 | Yes |
| Fluometuron | H | 12 | 0.016 | Yes |
| Imidacloprid | I | 20 | 0.024 | No |
| Metconazole | F | 8 | 0.01 | Yes |
| Methomyl | I | 32 | 0.147 | No |
| Metolachlor | H | 4 | 0.021 | No |
| Metribuzin | H | 8 | 0.015 | Yes |
| Myclobutanil | F | 16 | 0.02 | No |
| p,p′-DDD | I | 12 | 0.023 | No |
| p,p′-DDE | I | 28 | 0.675 | No |
| p,p′-DDT | I | 16 | 0.084 | No |
| Penconazole | F | 32 | 0.032 | Yes |
| Pendimethalin | H | 36 | 0.341 | Yes |
| Pyraclostrobin | F | 4 | 0.014 | Yes |
| Spinosyn A | I | 4 | 0.01 | Yes |
| Tebuconazole | F | 8 | 0.025 | Yes |
| Thiophanate methyl | F | 12 | 4.074 | No |
| Zoxamide | F | 20 | 1.278 | Yes |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tsiantas, P.; Bempelou, E.; Doula, M.; Karasali, H. Validation and Simultaneous Monitoring of 311 Pesticide Residues in Loamy Sand Agricultural Soils by LC-MS/MS and GC-MS/MS, Combined with QuEChERS-Based Extraction. Molecules 2023, 28, 4268. https://doi.org/10.3390/molecules28114268
Tsiantas P, Bempelou E, Doula M, Karasali H. Validation and Simultaneous Monitoring of 311 Pesticide Residues in Loamy Sand Agricultural Soils by LC-MS/MS and GC-MS/MS, Combined with QuEChERS-Based Extraction. Molecules. 2023; 28(11):4268. https://doi.org/10.3390/molecules28114268
Chicago/Turabian StyleTsiantas, Petros, Eleftheria Bempelou, Maria Doula, and Helen Karasali. 2023. "Validation and Simultaneous Monitoring of 311 Pesticide Residues in Loamy Sand Agricultural Soils by LC-MS/MS and GC-MS/MS, Combined with QuEChERS-Based Extraction" Molecules 28, no. 11: 4268. https://doi.org/10.3390/molecules28114268
APA StyleTsiantas, P., Bempelou, E., Doula, M., & Karasali, H. (2023). Validation and Simultaneous Monitoring of 311 Pesticide Residues in Loamy Sand Agricultural Soils by LC-MS/MS and GC-MS/MS, Combined with QuEChERS-Based Extraction. Molecules, 28(11), 4268. https://doi.org/10.3390/molecules28114268