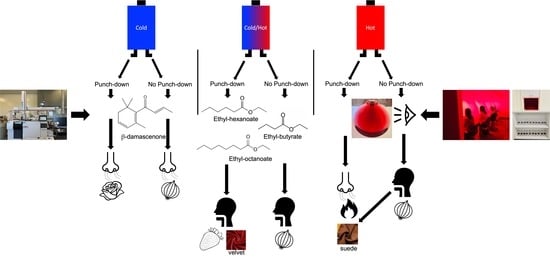
The Impact of Fermentation Temperature and Cap Management on Selected Volatile Compounds and Temporal Sensory Characteristics of Grenache Wines from the Central Coast of California
Abstract
1. Introduction
2. Results and Discussion
2.1. Perceived Color
2.2. Orthonasal Aroma Attributes
2.3. Taste, Mouthfeel, and Retronasal Aroma
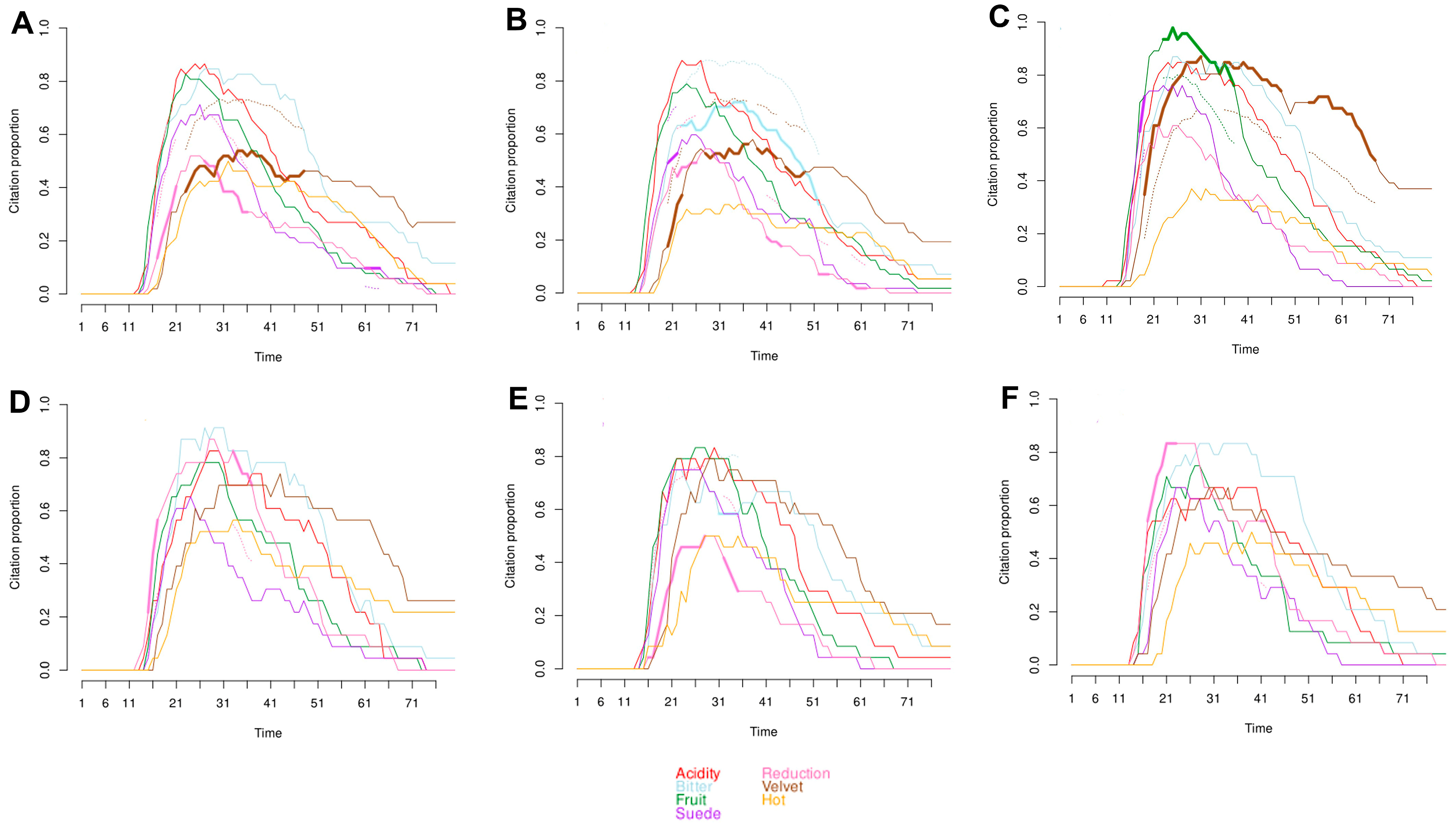
2.4. Salivary Flow Rate and Temporal Sensory Perception
3. Materials and Methods
3.1. Winemaking
3.2. Basic Chemistry and Phenolic Measurements
3.3. Volatile Compound Analysis
3.3.1. Solid-Phase Microextraction
3.3.2. Stir Bar Sorptive Extraction
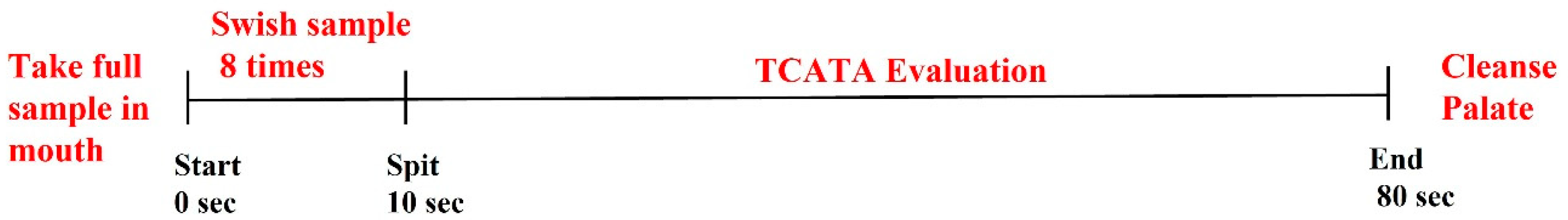
3.4. Descriptive Analysis and TCATA Sensory Panel
3.4.1. Panel Training
3.4.2. Formal Evaluations
3.5. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Rentzsch, M.; Schwarz, M.; Winterhalter, P.; Hermosín-Gutiérrez, I. Formation of hydroxyphenyl-pyranoanthocyanins in Grenache wines: Precursor levels and evolution during aging. J. Agric. Food. Chem. 2007, 55, 4883–4888. [Google Scholar] [CrossRef] [PubMed]
- Leriche, C.; Molinier, C.; Caillé, S.; Razungles, A.; Symoneaux, R.; Coulon-Leroy, C. Development of a methodology to study typicity of PDO wines with professionals of the wine sector. J. Sci. Food. Agric. 2020, 100, 3866–3877. [Google Scholar] [CrossRef] [PubMed]
- López-Giral, N.; González-Arenzana, L.; González-Ferrero, C.; López, R.; Santamaría, P.; López-Alfaro, I.; Garde-Cerdán, T. Pulsed electric field treatment to improve the phenolic compound extraction from Graciano, Tempranillo and Grenache grape varieties during two vintages. Innov. Food Sci. Emerg. Technol. 2015, 28, 31–39. [Google Scholar] [CrossRef]
- Ferreira, V.; Ortín, N.; Escudero, A.; López, R.; Cacho, J. Chemical characterization of the aroma of Grenache rosé wines: Aroma extract dilution analysis, quantitative determination, and sensory reconstitution studies. J. Agric. Food Chem. 2002, 50, 4048–4054. [Google Scholar] [CrossRef]
- Petretto, G.L.; Mercenaro, L.; Urgeghe, P.P.; Fadda, C.; Valentoni, A.; Del Caro, A. Grape and wine composition in Vitis vinifera l. cv. Cannonau explored by GC-MS and sensory analysis. Foods 2021, 10, 101. [Google Scholar] [CrossRef] [PubMed]
- Segurel, M.A.; Razungles, A.J.; Riou, C.; Salles, M.; Baumes, R.L. Contribution of dimethyl sulfide to the aroma of Syrah and Grenache noir wines and estimation of its potential in grapes of these varieties. J. Agric. Food Chem. 2004, 52, 7084–7093. [Google Scholar] [CrossRef] [PubMed]
- Caillé, S.; Samson, A.; Wirth, J.; Diéval, J.B.; Vidal, S.; Cheynier, V. Sensory characteristics changes of red Grenache wines submitted to different oxygen exposures pre and post bottling. Anal. Chim. Acta 2010, 660, 35–42. [Google Scholar] [CrossRef]
- Castura, J.C.; Antúnez, L.; Giménez, A.; Ares, G. Temporal Check-All-That-Apply (TCATA): A novel dynamic method for characterizing products. Food Qual. Prefer. 2016, 47, 79–90. [Google Scholar] [CrossRef]
- Ares, G.; Jaeger, S.R.; Antúnez, L.; Vidal, L.; Giménez, A.; Coste, B.; Picallo, A.; Castura, J.C. Comparison of TCATA and TDS for dynamic sensory characterization of food products. Food Res. Int. 2015, 78, 148–158. [Google Scholar] [CrossRef]
- Meyners, M.; Castura, J.C. The analysis of Temporal Check-All-That-Apply (TCATA) data. Food Qual. Prefer. 2018, 67, 67–76. [Google Scholar] [CrossRef]
- Berget, I.; Castura, J.C.; Ares, G.; Næs, T.; Varela, P. Exploring the common and unique variability in TDS and TCATA data—A comparison using canonical correlation and orthogonalization. Food Qual. Prefer. 2020, 79, 103790. [Google Scholar] [CrossRef]
- Varela, P.; Antúnez, L.; Carlehög, M.; Alcaire, F.; Castura, J.C.; Berget, I.; Giménez, A.; Næs, T.; Ares, G. What Is dominance? An exploration of the concept in TDS Tests with trained assessors and consumers. Food Qual. Prefer. 2018, 64, 72–81. [Google Scholar] [CrossRef]
- Jaeger, S.R.; Alcaire, F.; Hunter, D.C.; Jin, D.; Castura, J.C.; Ares, G. Number of terms to use in Temporal Check-All-That-Apply Studies (TCATA and TCATA fading) for sensory product characterization by consumers. Food Qual. Prefer. 2018, 64, 154–159. [Google Scholar] [CrossRef]
- Jaeger, S.R.; Beresford, M.K.; Hunter, D.C.; Alcaire, F.; Castura, J.C.; Ares, G. Does a familiarization step influence results from a TCATA task? Food Qual. Prefer. 2017, 55, 91–97. [Google Scholar] [CrossRef]
- Pineau, N.; Schlich, P.; Cordelle, S.; Mathonnière, C.; Issanchou, S.; Imbert, A.; Rogeaux, M.; Etiévant, P.; Köster, E. Temporal Dominance of Sensations: Construction of the TDS curves and comparison with Time-Intensity. Food Qual. Prefer. 2009, 20, 450–455. [Google Scholar] [CrossRef]
- Ares, G.; Castura, J.C.; Antúnez, L.; Vidal, L.; Giménez, A.; Coste, B.; Picallo, A.; Beresford, M.K.; Cheang, S.L.; Jaeger, S.R. Comparison of two TCATA variants for dynamic sensory characterization of food products. Food Qual. Prefer. 2016, 54, 160–172. [Google Scholar] [CrossRef]
- Baker, A.K.; Castura, J.C.; Ross, C.F. Temporal Check-All-That-Apply characterization of Syrah wine. J. Food Sci. 2016, 81, S1521–S1529. [Google Scholar] [CrossRef]
- McMahon, K.M.; Culver, C.; Castura, J.C.; Ross, C.F. Perception of carbonation in sparkling wines using Descriptive Analysis (DA) and Temporal Check-All-That-Apply (TCATA). Food Qual. Prefer. 2017, 59, 14–26. [Google Scholar] [CrossRef]
- Kemp, B.; Trussler, S.; Willwerth, J.; Inglis, D. Applying Temporal Check-All-That-Apply (TCATA) to mouthfeel and texture properties of red wines. J. Sens. Stud. 2019, 34, e12503. [Google Scholar] [CrossRef]
- McMahon, K.M.; Culver, C.; Ross, C.F. The production and consumer perception of sparkling wines of different carbonation levels. J. Wine Res. 2017, 28, 123–134. [Google Scholar] [CrossRef]
- Charters, S.; Pettigrew, S. The dimensions of wine quality. Food Qual. Prefer. 2007, 18, 997–1007. [Google Scholar] [CrossRef]
- Francis, I.L.; Williamson, P.O. Application of consumer sensory science in wine research. Aust. J. Grape Wine Res. 2015, 21, 554–567. [Google Scholar] [CrossRef]
- Sacchi, K.L.; Bisson, L.F.; Adams, D.O. A review of the effect of winemaking techniques on phenolic extraction in red wines. Am. J. Enol. Vitic. 2005, 56, 197–206. [Google Scholar] [CrossRef]
- Şener, H. Effect of temperature and duration of maceration on colour and sensory properties of red wine: A review. S. Afr. J. Enol. Vitic. 2018, 39, 227–234. [Google Scholar] [CrossRef]
- Casassa, L.F. Flavonoid Phenolics in Red Winemaking. In Phenolic Compounds—Natural Sources, Importance and Applications; InTech: London, UK, 2017; pp. 153–196. [Google Scholar]
- Casassa, L.F.; Harbertson, J.F. Extraction, evolution, and sensory impact of phenolic compounds during red wine maceration. Annu. Rev. Food Sci. Technol. 2014, 5, 83–109. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, J.C.; Meusel, R.C.; Catania, A.A.; Casassa, L.F. Chemical and chromatic effects of fermentation temperature on three clones of Pinot noir over two consecutive vintages. Am. J. Enol. Vitic. 2022, 73, 75–92. [Google Scholar] [CrossRef]
- Pérez-Navarro, J.; García Romero, E.; Gómez-Alonso, S.; Izquierdo Cañas, P.M. Comparison between the phenolic composition of Petit Verdot wines elaborated at different maceration/fermentation temperatures. Int. J. Food Prop. 2018, 21, 996–1007. [Google Scholar] [CrossRef]
- Reynolds, A.; Cliff, M.; Girard, B.; Kopp, T. Influence of fermentation on properties of Semillon and Shiraz wines. Am. J. Enol. Vitic. 2001, 52, 235–240. [Google Scholar] [CrossRef]
- Massera, A.; Assof, M.; Sari, S.; Ciklic, I.; Mercado, L.; Jofré, V.; Combina, M. Effect of low temperature fermentation on the yeast-derived volatile aroma composition and sensory profile in Merlot wines. LWT 2021, 142, 111069. [Google Scholar] [CrossRef]
- Lerno, L.A.; Panprivech, S.; Ponangi, R.; Hearne, L.; Blair, T.; Oberholster, A.; Block, D.E. Effect of pump-over conditions on the extraction of phenolic compounds during Cabernet Sauvignon fermentation. Am. J. Enol. Vitic. 2018, 69, 295–301. [Google Scholar] [CrossRef]
- Wimalasiri, P.M.; Zhan, J.; Tian, B. Characterisation of tannin and aroma profiles of Pinot noir wines made with or without grape pomace. Fermentation 2022, 8, 718. [Google Scholar] [CrossRef]
- Frost, S.C.; Blackman, J.W.; Ebeler, S.E.; Heymann, H. Analysis of Temporal Dominance of Sensation data using correspondence analysis on Merlot wine with differing maceration and cap management regimes. Food Qual. Prefer. 2018, 64, 245–252. [Google Scholar] [CrossRef]
- Frost, S.C.; Blackman, J.W.; Hjelmeland, A.K.; Ebeler, S.E.; Heymann, H. Extended maceration and cap management impacts on the phenolic, volatile, and sensory profiles of Merlot wine. Am. J. Enol. Vitic. 2018, 69, 360–370. [Google Scholar] [CrossRef]
- Lesschaeve, I.; Noble, A.C. Polyphenols: Factors influencing their sensory properties and their effects on food and beverage preferences. Am. J. Clin. Nutr. 2005, 81, 330S–335S. [Google Scholar] [CrossRef] [PubMed]
- Fischer, U.; Boulton, R.B.; Noble, A. Physiological factors contributing to the variability of sensory assessment: Relationship between salivary flow rate and temporal perception of gustatory stimuli. Food Qual. Prefer. 1994, 5, 55–64. [Google Scholar] [CrossRef]
- Ishikawa, T.; Noble, A.C. Temporal perception of astringency and sweetness in red wine. Food Qual. Prefer. 1995, 6, 27–33. [Google Scholar] [CrossRef]
- Baker, A.K.; Ross, C.F. Wine finish in red wine: The effect of ethanol and tannin concentration. Food Qual. Prefer. 2014, 38, 65–74. [Google Scholar] [CrossRef]
- Criado, C.; Chaya, C.; Fernández-Ruíz, V.; Álvarez, M.D.; Herranz, B.; Pozo-Bayón, M.Á. Effect of saliva composition and flow on inter-individual differences in the temporal perception of retronasal aroma during wine tasting. Food Res. Int. 2019, 126, 108677. [Google Scholar] [CrossRef]
- Meilgaard, M.C.; Civille, G.V.; Carr, T.B. Sensory Evaluation Techniques, 5th ed.; CRC Press: Boca Raton, FL, USA, 2016. [Google Scholar]
- Stoffel, E.S.; Lesniauskas, R.O.; Anderson, S.R.; Krystoff, C.T.; Casassa, L.F. Temporal evaluation of retronasal and mouthfeel sensations in cofermented and blended red wines from California. Am. J. Enol. Vitic. 2023, 74, 1–15. [Google Scholar] [CrossRef]
- Gao, L.; Girard, B.; Mazza, G.; Reynolds, A.G. Changes in anthocyanins and color characteristics of Pinot noir wines during different vinification processes. J. Agric. Food Chem. 1997, 45, 2003–2008. [Google Scholar] [CrossRef]
- Morel-Salmi, C.; Souquet, J.M.; Bes, M.; Cheynier, V. Effect of flash release treatment on phenolic extraction and wine composition. J. Agric. Food Chem. 2006, 54, 4270–4276. [Google Scholar] [CrossRef] [PubMed]
- Rinaldi, A.; Picariello, L.; Soares, S.; Brandão, E.; de Freitas, V.; Moio, L.; Gambuti, A. Effect of oxidation on color parameters, tannins, and sensory characteristics of Sangiovese wines. Eur. Food Res. Technol. 2021, 247, 2977–2991. [Google Scholar] [CrossRef]
- Franco-Luesma, E.; Ferreira, V. Reductive off-odors in wines: Formation and release of H2S and methanethiol during the accelerated anoxic storage of wines. Food Chem. 2016, 199, 42–50. [Google Scholar] [CrossRef] [PubMed]
- Smith, M.E.; Bekker, M.Z.; Smith, P.A.; Wilkes, E.N. Sources of volatile sulfur compounds in wine. Aust. J. Grape Wine Res. 2015, 21, 705–712. [Google Scholar] [CrossRef]
- Bekker, M.Z.; Day, M.P.; Holt, H.; Wilkes, E.; Smith, P.A. Effect of oxygen exposure during fermentation on volatile sulfur compounds in Shiraz wine and a comparison of strategies for remediation of reductive character. Aust. J. Grape Wine Res. 2016, 22, 24–35. [Google Scholar] [CrossRef]
- Ugliano, M.; Kolouchova, R.; Henschke, P.A. Occurrence of hydrogen sulfide in wine and in fermentation: Influence of yeast strain and supplementation of yeast available nitrogen. J. Ind. Microbiol. Biotechnol. 2011, 38, 423–429. [Google Scholar] [CrossRef] [PubMed]
- Bohlscheid, J.C.; Osborne, J.P.; Ross, C.F.; Edwards, C.G. Interactive effects of selected nutrients and fermentation temperature on H2S production by wine strains of Saccharomyces. J. Food Qual. 2011, 34, 51–55. [Google Scholar] [CrossRef]
- Fang, Y.; Qian, M.C. Sensitive quantification of sulfur compounds in wine by headspace solid-phase microextraction technique. J. Chromatogr. A 2005, 1080, 177–185. [Google Scholar] [CrossRef]
- Siebert, T.E.; Solomon, M.R.; Pollnitz, A.P.; Jeffery, D.W. Selective determination of volatile sulfur compounds in wine by gas chromatography with sulfur chemiluminescence detection. J. Agric. Food Chem. 2010, 58, 9454–9462. [Google Scholar] [CrossRef]
- Pineau, B.; Barbe, J.C.; Van Leeuwen, C.; Dubourdieu, D. Examples of perceptive interactions involved in specific “red-” and “black-berry” aromas in red wines. J. Agric. Food Chem. 2009, 57, 3702–3708. [Google Scholar] [CrossRef]
- Lorrain, B.; Tempere, S.; Iturmendi, N.; Moine, V.; De Revel, G.; Teissedre, P.L. Influence of phenolic compounds on the sensorial perception and volatility of red wine esters in model solution: An insight at the molecular level. Food Chem. 2013, 140, 76–82. [Google Scholar] [CrossRef] [PubMed]
- Cometto-Muñiz, J.E.; Cain, W.S.; Abraham, M.H.; Gil-Lostes, J. Concentration-detection functions for the odor of homologous n-acetate esters. Physiol. Behav. 2008, 95, 658–667. [Google Scholar] [CrossRef] [PubMed]
- Cameleyre, M.; Lytra, G.; Tempere, S.; Barbe, J.C. Olfactory Impact of higher alcohols on red wine fruity ester aroma expression in model solution. J. Agric. Food Chem. 2015, 63, 9777–9788. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Míguez, M.J.; Cacho, J.F.; Ferreira, V.; Vicario, I.M.; Heredia, F.J. Volatile components of Zalema white wines. Food Chem. 2007, 100, 1464–1473. [Google Scholar] [CrossRef]
- Rocha, S.M.; Rodrigues, F.; Coutinho, P.; Delgadillo, I.; Coimbra, M.A. Volatile composition of Baga red wine: Assessment of the identification of the would-be impact odourants. Anal. Chim. Acta 2004, 513, 257–262. [Google Scholar] [CrossRef]
- Arias-Pérez, I.; Sáenz-Navajas, M.P.; de-la-Fuente-Blanco, A.; Ferreira, V.; Escudero, A. Insights on the role of acetaldehyde and other aldehydes in the odour and tactile nasal perception of red wine. Food Chem. 2021, 361, 130081. [Google Scholar] [CrossRef]
- Zalacain, A.; Marín, J.; Alonso, G.L.; Salinas, M.R. Analysis of wine primary aroma compounds by stir bar sorptive extraction. Talanta 2007, 71, 1610–1615. [Google Scholar] [CrossRef]
- Bowen, A.J.; Reynolds, A.G. Odor potency of aroma compounds in Riesling and Vidal Blanc table wines and icewines by gas chromatography-olfactometry-mass spectrometry. J. Agric. Food Chem. 2012, 60, 2874–2883. [Google Scholar] [CrossRef]
- Ferreira, V.; De la Fuente, A.; Sáenz-Navajas, M.P. Wine Aroma Vectors and Sensory Attributes. In Managing Wine Quality: Volume One: Viticulture and Wine Quality; Elsevier: Amsterdam, The Netherlands, 2021; pp. 3–39. ISBN 9780081020678. [Google Scholar]
- Sabon, I.; De Revel, G.; Kotseridis, Y.; Bertrand, A. Determination of volatile compounds in Grenache wines in relation with different terroirs in the Rhone Valley. J. Agric. Food Chem. 2002, 50, 6341–6345. [Google Scholar] [CrossRef]
- Delwiche, J. The impact of perceptual interactions on perceived flavor. Food Qual. Prefer. 2004, 15, 137–146. [Google Scholar] [CrossRef]
- Welke, J.E.; Zanus, M.; Lazzarotto, M.; Alcaraz Zini, C. Quantitative analysis of headspace volatile compounds using comprehensive two-dimensional gas chromatography and their contribution to the aroma of Chardonnay wine. Food Res. Int. 2014, 59, 85–99. [Google Scholar] [CrossRef]
- Bosso, A.; Panero, L.; Petrozziello, M.; Follis, R.; Motta, S.; Guaita, M. Influence of submerged-cap vinification on polyphenolic composition and volatile compounds of Barbera wines. Am. J. Enol. Vitic. 2011, 62, 503–511. [Google Scholar] [CrossRef]
- Franco-Luesma, E.; Sáenz-Navajas, M.P.; Valentin, D.; Ballester, J.; Rodrigues, H.; Ferreira, V. Study of the effect of H2S, MeSH and DMS on the sensory profile of wine model solutions by Rate-All-That-Apply (RATA). Food Res. Int. 2016, 87, 152–160. [Google Scholar] [CrossRef]
- Medel-Marabolí, M.; López-Solís, R.; Valenzuela-Prieto, D.; Vargas-Silva, S.; Obreque-Slier, E. Limited relationship between temporality of sensory perception and phenolic composition of red wines. LWT 2021, 142, 111028. [Google Scholar] [CrossRef]
- Casassa, L.F.; Bolcato, E.A.; Sari, S.E. Chemical, chromatic, and sensory attributes of 6 red wines produced with prefermentative cold soak. Food Chem. 2015, 174, 110–118. [Google Scholar] [CrossRef]
- Ugliano, M.; Travis, B.; Francis, I.L.; Henschke, P.A. Volatile composition and sensory properties of shiraz wines as affected by nitrogen supplementation and yeast species: Rationalizing nitrogen modulation of wine aroma. J. Agric. Food Chem. 2010, 58, 12417–12425. [Google Scholar] [CrossRef]
- Franco-Luesma, E.; Ferreira, V. Quantitative analysis of free and bonded forms of volatile sulfur compouds in wine. basic methodologies and evidences showing the existence of reversible cation-complexed forms. J. Chromatogr. A 2014, 1359, 8–15. [Google Scholar] [CrossRef] [PubMed]
- Ugliano, M.; Fedrizzi, B.; Siebert, T.; Travis, B.; Magno, F.; Versini, G.; Henschke, P.A. Effect of nitrogen supplementation and saccharomyces species on hydrogen sulfide and other volatile sulfur compounds in Shiraz fermentation and wine. J. Agric. Food Chem. 2009, 57, 4948–4955. [Google Scholar] [CrossRef] [PubMed]
- Harbertson, J.F.; Picciotto, E.A.; Adams, D.O. Measurement of polymeric pigments in grape berry extracts and wines using a protein precipitation assay combined with bisulfite bleaching. Am. J. Enol. Vitic. 2003, 54, 301–306. [Google Scholar] [CrossRef]
- Harbertson, J.F.; Kennedy, J.A.; Adams, D.O. Tannin in skins and seeds of Cabernet Sauvignon, Syrah, and Pinot noir berries during ripening. Am. J. Enol. Vitic. 2002, 53, 54–59. [Google Scholar] [CrossRef]
- Hjelmeland, A.K.; King, E.S.; Ebeler, S.E.; Heymann, H. Characterizing the chemical and sensory profiles of United States Cabernet Sauvignon wines and blends. Am. J. Enol. Vitic. 2013, 64, 169–179. [Google Scholar] [CrossRef]
- Castro, L.F.; Ross, C.F. Determination of flavour compounds in beer using stir-bar sorptive extraction and solid-phase microextraction. J. Inst. Brew. 2015, 121, 197–203. [Google Scholar] [CrossRef]
- Alves, R.F.; Nascimento, A.M.D.; Nogueira, J.M.F. Characterization of the aroma profile of Madeira wine by sorptive extraction techniques. Anal. Chim. Acta 2005, 546, 11–21. [Google Scholar] [CrossRef]
- Tepper, B.J.; Christensen, C.M.; Cao, J. Development of brief methods to classify individuals by PROP taster status. Physiol. Behav. 2001, 73, 571–577. [Google Scholar] [CrossRef]
- Pineau, N.; de Bouillé, A.G.; Lepage, M.; Lenfant, F.; Schlich, P.; Martin, N.; Rytz, A. Temporal Dominance of Sensations: What is a good attribute list? Food Qual. Prefer. 2012, 26, 159–165. [Google Scholar] [CrossRef]
- Noble, A.C.; Ebeler, S.E. Use of multivariate statistics in understanding wine flavor. Food Rev. Int. 2002, 18, 1–21. [Google Scholar] [CrossRef]
- Husson, F.; Lê, S.; Pagès, J. Confidence ellipse for the sensory profiles obtained by principal component analysis. Food Qual. Prefer. 2005, 16, 245–250. [Google Scholar] [CrossRef]
- Castura, J.C. tempR: Temporal Sensory Data Analysis 2022. Available online: https://cran.r-project.org/web/packages/tempR/tempR.pdf (accessed on 15 April 2023).
- Castura, J.C.; Baker, A.K.; Ross, C.F. Using contrails and animated sequences to visualize uncertainty in dynamic sensory profiles obtained from Temporal Check-All-That-Apply (TCATA) data. Food Qual. Prefer. 2016, 54, 90–100. [Google Scholar] [CrossRef]
- Carrau, F.M.; Medina, K.; Boido, E.; Farina, L.; Gaggero, C.; Dellacassa, E.; Versini, G.; Henschke, P.A. De novo synthesis of monoterpenes by Saccharomyces cerevisiae wine yeasts. FEMS Microbiol. Lett. 2005, 243, 107–115. [Google Scholar] [CrossRef] [PubMed]
- Yilmaztekin, M.; Kocabey, N.; Hayaloglu, A.A. Effect of maceration time on free and bound volatiles of red wines from Cv. Karaoğlan (Vitis vinifera l.) grapes grown in Arapgir, Turkey. J. Food Sci. 2015, 80, C556–C563. [Google Scholar] [CrossRef]
- Dennis, E.G.; Keyzers, R.A.; Kalua, C.M.; Maffei, S.M.; Nicholson, E.L.; Boss, P.K. Grape contribution to wine aroma: Production of hexyl acetate, octyl acetate, and benzyl acetate during yeast fermentation is dependent upon precursors in the must. J. Agric. Food Chem. 2012, 60, 2638–2646. [Google Scholar] [CrossRef]
- Pittari, E.; Moio, L.; Piombino, P. Interactions between polyphenols and volatile compounds in wine: A literature review on physicochemical and sensory insights. Appl. Sci. 2021, 11, 1157. [Google Scholar] [CrossRef]
- Girard, B.; Yuksel, D.; Cliff, M.A.; Delaquis, P.; Reynolds, A.G. Vinification effects on the sensory, colour and gc profiles of Pinot noir wines from British Columbia. Food Res. Int. 2001, 34, 483–499. [Google Scholar] [CrossRef]
- Rodríguez-Bencomo, J.J.; Muñoz-González, C.; Andújar-Ortiz, I.; Martín-Álvarez, P.J.; Moreno-Arribas, M.V.; Pozo-Bayón, M.Á. Assessment of the effect of the non-volatile wine matrix on the volatility of typical wine aroma compounds by headspace solid phase microextraction/gas chromatography analysis. J. Sci. Food Agric. 2011, 91, 2484–2494. [Google Scholar] [CrossRef] [PubMed]
- Oliva, J.; Martínez-Gil, A.M.; Lorenzo, C.; Cámara, M.A.; Salinas, M.R.; Barba, A.; Garde-Cerdán, T. Influence of the use of fungicides on the volatile composition of Monastrell red wines obtained from inoculated fermentation. Food Chem. 2015, 170, 401–406. [Google Scholar] [CrossRef] [PubMed]
- Tomasino, E.; Harrison, R.; Breitmeyer, J.; Sedcole, R.; Sherlock, R.; Frost, A. Aroma composition of 2-year-old New Zealand Pinot noir wine and its relationship to sensory characteristics using canonical correlation analysis and addition/omission tests. Aust. J. Grape Wine Res. 2015, 21, 376–388. [Google Scholar] [CrossRef]
- Furdíková, K.; Machyňáková, A.; Drtilová, T.; Špánik, I. Comparison of different categories of Slovak Tokaj wines in terms of profiles of volatile organic compounds. Molecules 2020, 25, 669. [Google Scholar] [CrossRef]
- Ferreira, V.; López, R.; Cacho, J.F. Quantitative determination of the odorants of young red wines from different grape varieties. J. Sci. Food Agric. 2000, 80, 1659–1667. [Google Scholar] [CrossRef]
- Uthurry, C.A.; Varela, F.; Colomo, B.; Suárez Lepe, J.A.; Lombardero, J.; García Del Hierro, J.R. Ethyl carbamate concentrations of typical Spanish red wines. Food Chem. 2004, 88, 329–336. [Google Scholar] [CrossRef]
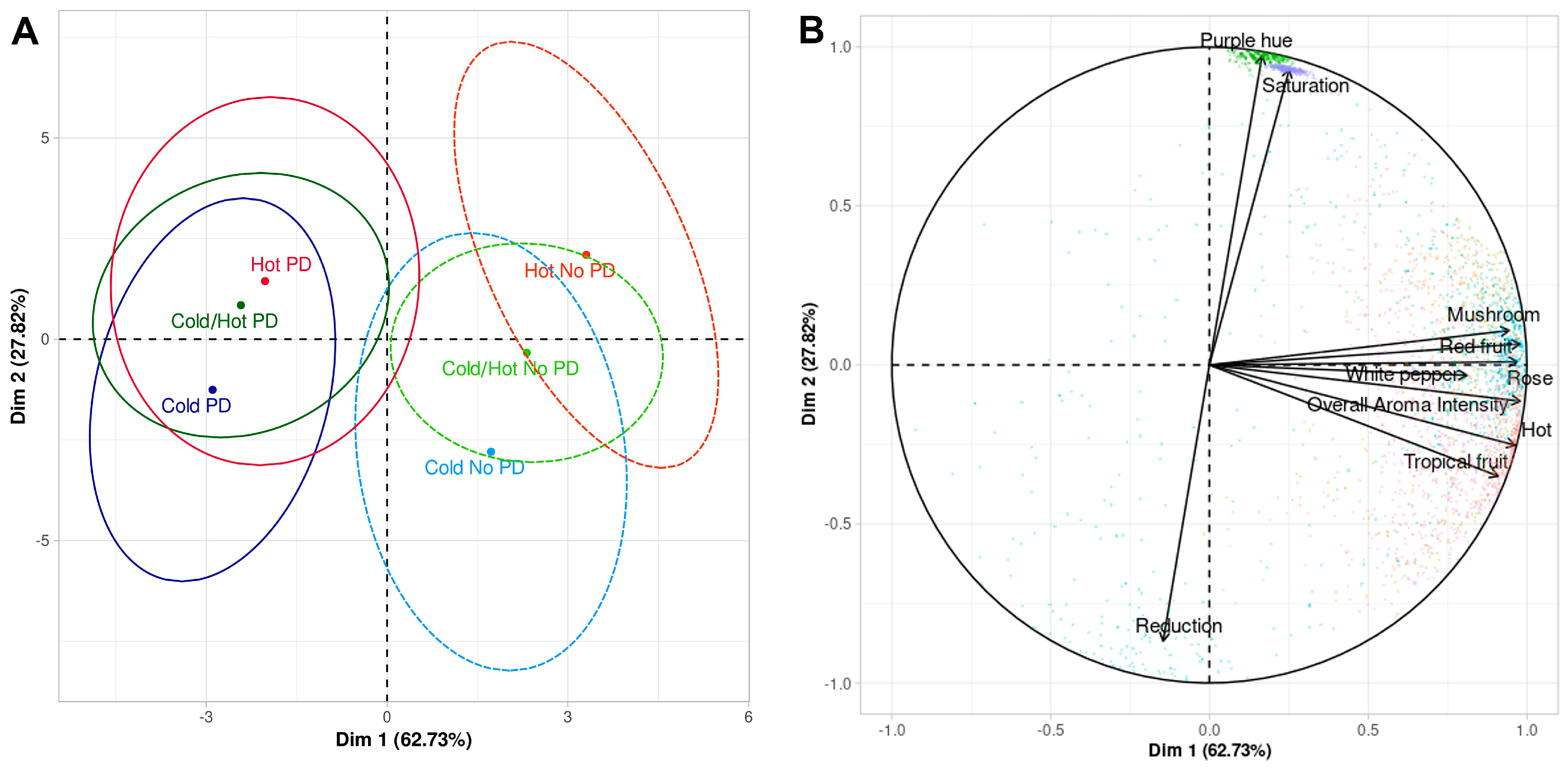
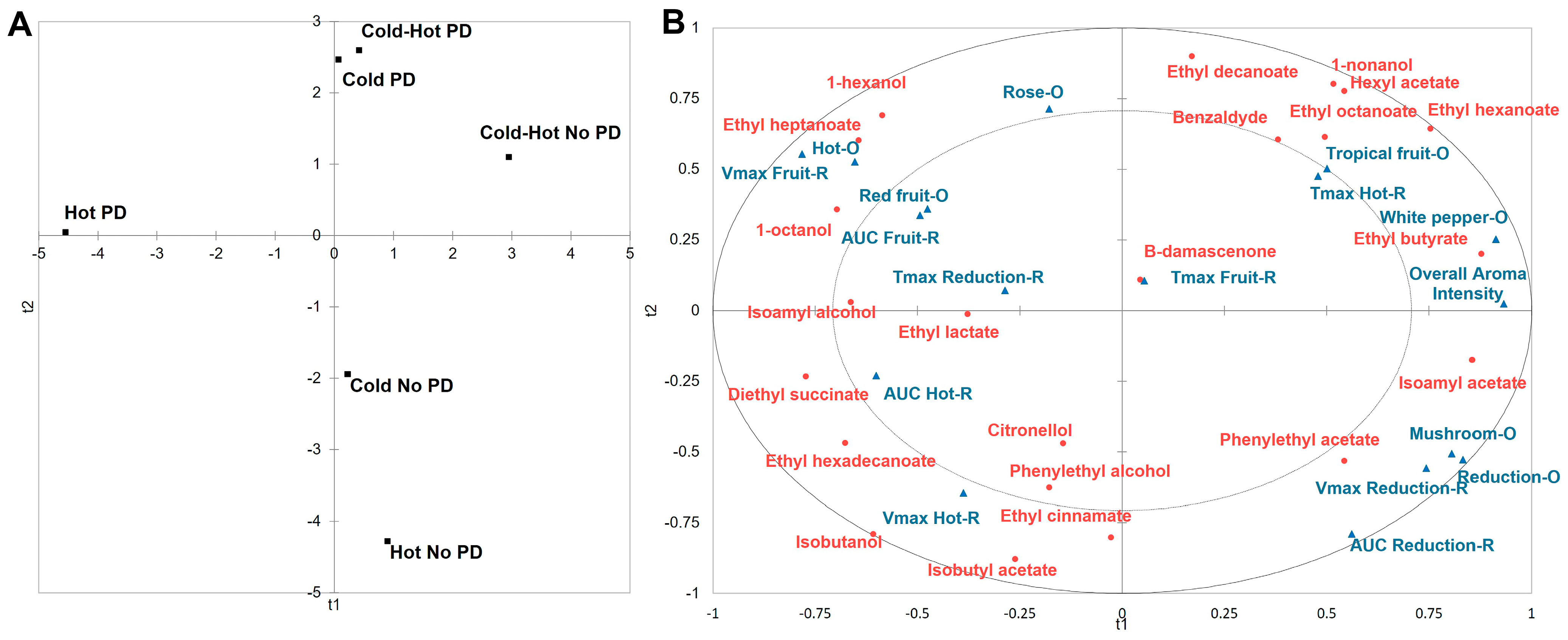
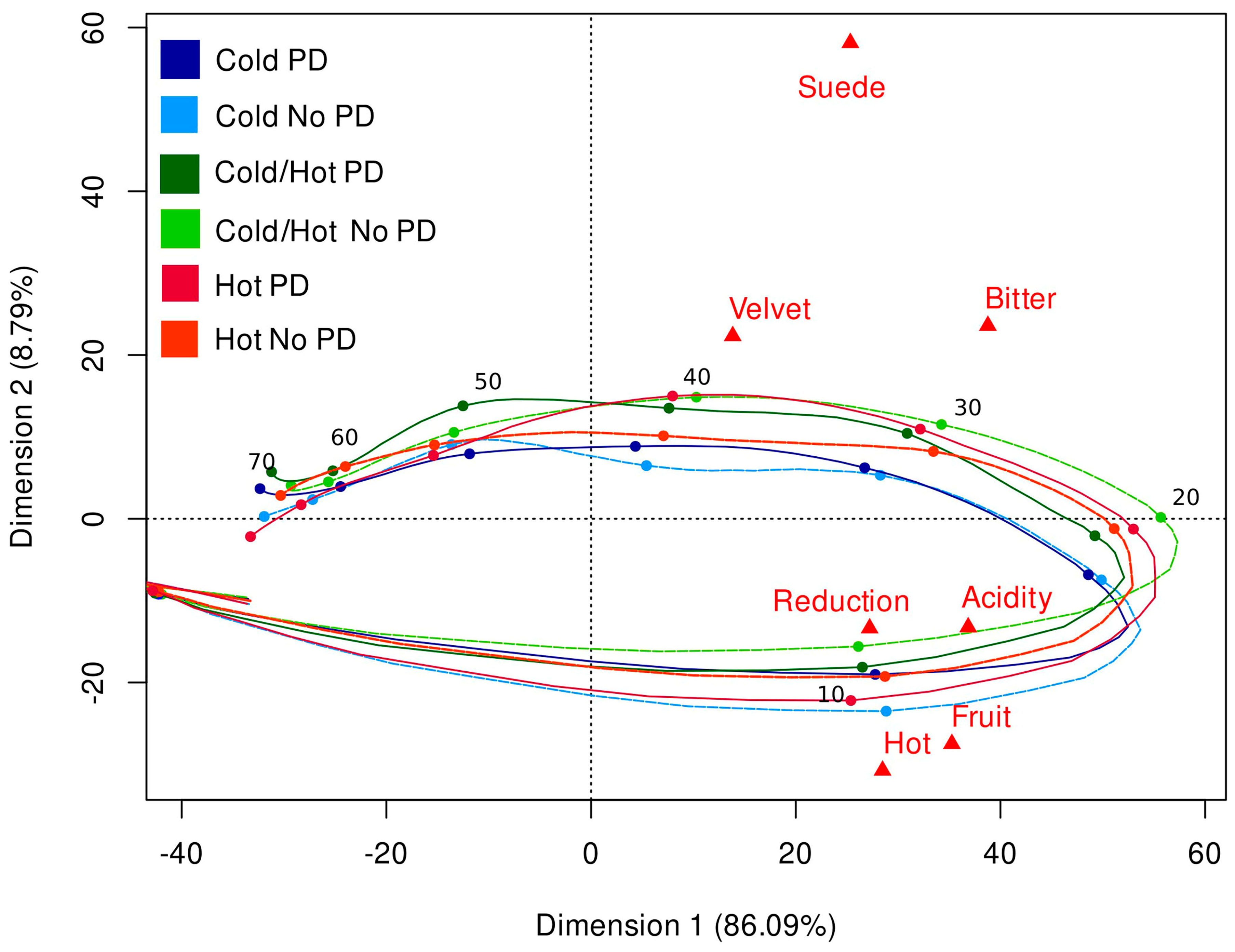
| Treatments | Saturation | Purple Hue | Overall Aroma Intensity | Reduction | Rose | Red Fruit | Tropical Fruit | White Pepper | Mushroom | Hot |
|---|---|---|---|---|---|---|---|---|---|---|
| Cold PD | 3.49 c 1 | 5.02 c | 7.61 ab | 3.53 bc | 5.67 a | 5.57 a | 5.06 ab | 3.23 ab | 2.70 ab | 6.55 a |
| Cold No PD | 2.42 d | 4.36 c | 7.81 ab | 5.10 a | 4.58 b | 5.03 a | 4.42 b | 3.30 ab | 2.99 ab | 6.07 a |
| Cold/Hot PD | 6.29 b | 7.00 b | 7.99 a | 4.20 ab | 4.83 b | 5.41 a | 4.96 ab | 3.49 a | 2.84 ab | 6.94 a |
| Cold/Hot No PD | 6.65 b | 6.84 b | 8.03 a | 4.88 a | 4.95 ab | 5.44 a | 5.26 a | 3.51 a | 3.09 a | 6.10 a |
| Hot PD | 6.53 b | 8.21 a | 7.17 b | 2.77 c | 5.05 ab | 5.71 a | 4.72 ab | 2.80 b | 2.35 b | 6.90 a |
| Hot No PD | 7.98 a | 8.67 a | 7.83 ab | 5.08 a | 4.54 b | 5.43 a | 4.88 ab | 3.22 ab | 3.30 a | 6.25 a |
| p-value 2 | <0.0001 | <0.0001 | 0.291 | <0.0001 | 0.058 | 0.744 | 0.386 | 0.326 | 0.144 | 0.261 |
| Treatment | Anthocyanins (mg/L Malvidin-3-Glucoside) | SPP | LPP | TPP | Total Tannins (mg/L CE) | Total Phenolics (mg/L CE) |
|---|---|---|---|---|---|---|
| Cold PD | 187 ± 9.23 b 1 | 0.480 ± 0.03 c | 0.026 ± 0.02 bc | 0.505 ± 0.05 c | 26.4 ± 3.70 b | 487 ± 36.4 b |
| Cold No PD | 178 ± 6.98 b | 0.432 ± 0.01 c | 0.004 ± 0.01 c | 0.436 ± 0.01 c | 22.8 ± 4.11 b | 509 ± 17.1 b |
| Cold/Hot PD | 280 ± 4.61 a | 0.656 ± 0.02 b | 0.070 ± 0.02 bc | 0.727 ± 0.01 b | 94.8 ± 7.55 a | 800 ± 19.4 a |
| Cold/Hot No PD | 309 ± 8.27 a | 0.680 ± 0.01 b | 0.022 ± 0.02 bc | 0.702 ± 0.03 b | 87.7 ± 14.7 a | 862 ± 44.4 a |
| Hot PD | 220 ± 5.39 b | 0.718 ± 0.02 b | 0.209 ± 0.01 a | 0.927 ± 0.02 a | 96.8 ± 13.0 a | 735 ± 19.6 a |
| Hot No PD | 299 ± 41.2 a | 0.793 ± 0.04 a | 0.085 ± 0.04 b | 0.878 ± 0.07 a | 81.0 ± 18.8 a | 893 ± 112 a |
| p-value 2 | 0.001 | <0.0001 | 0.001 | <0.0001 | 0.001 | 0.000 |
| Compounds | Cold PD | Cold No PD | Cold/Hot PD | Cold/Hot No PD | Hot PD | Hot No PD | p-Value | Source 1 |
|---|---|---|---|---|---|---|---|---|
| Esters | ||||||||
| Isobutyl acetate | n.d. 2 (0.00) | 34.1 (0.016) bc 3 | n.d. (0.00) | n.d. (0.00) | 69.5 (0.033) b | 141 (0.067) a | 0.002 4 | [52] |
| Ethyl butyrate | 176 (3.42) bc | 156 (3.02) cd | 197 (3.83) ab | 229 (4.46) a | 137 (2.67) d | 191 (3.71) bc | 0.002 | [53] |
| Hexyl acetate | 6.44 (2.22) a | 2.77 (0.955) b | 4.79 (1.65) ab | 5.20 (1.79) a | n.d. (0.00) | n.d. (0.00) | 0.000 | [54] |
| Isoamyl acetate | 727 (19.7) cd | 752 (20.3) cd | 846 (22.9) bc | 1138 (30.7) a | 611 (16.5) d | 998 (27.0) ab | 0.001 | [53] |
| Ethyl hexanoate | 631 (3.16) a | 523 (2.62) bc | 613 (3.06) ab | 687 (3.44) a | 440 (2.20) c | 490 (2.45) c | 0.002 | [55] |
| Ethyl lactate | 22,125 (0.143) a | 26,695 (0.173) a | 26,704 (0.173) a | 33,023 (0.214) a | 35,740 (0.231) a | 26,006 (0.168) a | 0.365 | [56] |
| Ethyl heptanoate | 0.994 (-) a 5 | n.d. (-) | 0.464 (-) a | n.d. (-) | 0.855 (-) a | n.d. (-) | 0.165 | - 5 |
| Ethyl octanoate | 356 (2.21) b | 289 (1.80) b | 418 (2.60) ab | 525 (3.26) a | 336 (2.09) b | 294 (1.83) b | 0.021 | [53] |
| Ethyl decanoate | 10.7 (0.010) a | 8.33 (0.007) a | 10.8 (0.010) a | 12.3 (0.011) a | 9.49 (0.009) a | 5.49 (0.005) a | 0.771 | [57] |
| Diethyl succinate | 337 (0.002) b | 316 (0.002) b | 354 (0.002) b | 419 (0.002) b | 892 (0.004) a | 546 (0.003) ab | 0.043 | [56] |
| Ethyl hexadecanoate | 396 (-) ab | 614 (-) ab | 163 (-) b | 439 (-) ab | 871 (-) a | 520 (-) ab | 0.234 | - |
| Phenylethyl acetate | 8.15 (0.033) bc | 11.0 (0.044) abc | 4.65 (0.019) c | 16.7 (0.067) a | 8.20 (0.033) bc | 14.3 (0.057) ab | 0.081 | [57] |
| Ethyl cinnamate | 1.12 (1.02) b | 1.89 (1.72) ab | 1.49 (1.35) ab | 1.98 (1.80) ab | 2.01 (1.82) ab | 2.27 (2.07) a | 0.142 | [58] |
| Total Esters | 24,776 a | 29,403 a | 29,317 a | 36,497 a | 39,118 a | 29,209 a | 0.340 | |
| Nor-isoprenoids | ||||||||
| β-damascenone | 0.757 (15.1) a | 0.680 (13.6) a | n.d. (0.00) | n.d. (0.00) | n.d. (0.00) | n.d. (0.00) | 0.050 | [59] |
| Terpenes | ||||||||
| Citronellol | 15.1 (0.838) a | 14.5 (0.805) a | 11.5 (0.636) b | 11.6 (0.647) b | 13.0 (0.723) ab | 14.6 (0.809) a | 0.057 | [59] |
| Alcohols | ||||||||
| 1-hexanol | 2293 (2866) a | 1462 (1828) b | 2550 (3188) a | 1664 (2080) b | 2519 (3145) a | 1592 (1990) b | 0.000 | [59] |
| 1-octanol | n.d. (0.00) | n.d. (0.00) | 1.74 (0.016) a | n.d. (0.00) | 1.98 (0.018) a | n.d. (0.00) | 0.570 | [60] |
| 1-nonanol | 0.972 a (-) | n.d. (-) | 0.677 a (-) | 0.916 a (-) | n.d. (-) | n.d. (-) | 0.692 | - |
| Isobutanol | 7982 (0.200) bcd | 11,602 (0.290) abc | 7710 (0.193) cd | 7013 (0.175) d | 12,682 (0.317) a | 12,451 (0.311) ab | 0.047 | [56] |
| Isoamyl alcohol | 49,280 (1.64) a | 50,106 (1.67) a | 40,457 (1.35) a | 55,908 (1.86) a | 71,490 (2.38) a | 44,475 (1.48) a | 0.494 | [58] |
| Phenylethyl alcohol | 15,660 (1.12) de | 15,223 (1.09) e | 19,369 (1.38) cd | 20,646 (1.47) c | 25,698 (1.84) b | 32,479 (2.32) a | <0.0001 | [58] |
| Total Alcohols | 75,216 ab | 78,395 ab | 70,088 b | 85,230 ab | 112,387 a | 90,997 ab | 0.251 | |
| Aldehydes | ||||||||
| Benzaldehyde | 20.0 (0.001) a | 18.0 (0.001) a | 13.6 (0.001) a | 32.2 (0.002) a | 12.4 (0.001) a | n.d. (0.00) | 0.767 | [58] |
| Treatments | Ethanol (v/v%) | pH | Titratable Acidity (g/L) | Acetic Acid (g/L) | Glucose + Fructose (g/L) | Lactic Acid (g/L) | Malic Acid (g/L) |
|---|---|---|---|---|---|---|---|
| Cold PD | 13.3 ± 0.17 a 1 | 3.61 ± 0.04 ab | 5.52 ± 0.12 d | 0.267 ± 0.04 b | 0.140 ± 0.03 ab | 1.24 ± 0.03 ab | 0.060 ± 0.01 a |
| Cold No PD | 13.1 ± 0.23 ab | 3.69 ± 0.01 a | 5.67 ± 0.06 cd | 0.380 ± 0.03 a | 0.127 ± 0.01 ab | 1.18 ± 0.02 ab | 0.060 ± 0.01 a |
| Cold/Hot PD | 13.1 ± 0.11 ab | 3.54 ± 0.01 c | 5.74 ± 0.04 bcd | 0.180 ± 0.01 c | 0.113 ± 0.02 ab | 1.25 ± 0.01 a | 0.050 ± 0.01 a |
| Cold/Hot No PD | 12.8 ± 0.04 b | 3.55 ± 0.01 bc | 5.97 ± 0.02 ab | 0.177 ± 0.01 c | 0.150 ± 0.01 a | 1.26 ± 0.01 a | 0.040 ± 0.01 a |
| Hot PD | 13.1 ± 0.16 ab | 3.53 ± 0.02 c | 5.87 ± 0.06 bc | 0.287 ± 0.02 b | 0.097 ± 0.01 b | 1.15 ± 0.03 bc | 0.043 ± 0.01 a |
| Hot No PD | 12.8 ± 0.17 ab | 3.62 ± 0.03 ab | 6.19 ± 0.12 a | 0.277 ± 0.03 b | 0.120 ± 0.01 ab | 1.09 ± 0.05 c | 0.047 ± 0.01 a |
| p-value 2 | 0.300 | 0.004 | 0.001 | 0.001 | 0.194 | 0.011 | 0.318 |
| Attribute | AUC 1 | Tmax (Seconds) | Vmax (Citation Proportion) | Tfirst (Seconds) | Tlast (Seconds) | Length (Seconds) |
|---|---|---|---|---|---|---|
| Acidity | 24.2 b 2 | 24.1 d | 0.875 a | 14.8 d | 69.4 b | 54.7 bc |
| Bitter | 28.6 a | 28.4 bc | 0.861 ab | 15.3 cd | 76.7 a | 61.4 a |
| Fruit | 19.4 c | 23.9 d | 0.781 c | 14.6 d | 66.1 b | 51.5 c |
| Hot | 14.5 d | 22.4 d | 0.778 c | 15.2 d | 53.1 d | 37.9 d |
| Reduction | 15.3 d | 25.2 cd | 0.660 d | 16.1 c | 58.6 c | 42.5 d |
| Suede | 28.9 a | 30.1 ab | 0.785 bc | 17.6 b | 78.9 a | 61.3 a |
| Velvet | 19.2 c | 32.9 a | 0.576 e | 18.6 a | 75.8 a | 57.2 ab |
| p-value 3 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 |
| Attribute | Standard Composition | |
|---|---|---|
| Color | Purple Hue | High: L* = 39.93, C* = 61.92, a* = 60.82, b* = 11.63 1 |
| Low: L* = 86.40, C* = 15.57, a* = 15.13, b* = 3.66 2 | ||
| Saturation | High: L* = 39.93, C* = 61.92, h = 10.83 1 | |
| Low: L* = 86.40, C* = 15.57, h = 13.60 2 | ||
| Aroma 3 | Reduction | 100 mL of base reduction solution; 116.94 g raw white onion (Signature Farms, Better Living Brands LLC, Pleasanton, CA, USA), 112.02 g cooked, white onion (Signature Farms, Better Living Brands LLC, Pleasanton, CA, USA) on medium until browned and soaked in 1000 mL of base wine overnight. |
| Rose | 80 mL rose water (Fee Brothers, Fee Brothers, Rochester, NY, USA), 99.2 mL rose syrup (Monin, Monin Inc., Clearwater, FL, USA) | |
| Red Fruit | 51.54 g mashed, fresh raspberries (Fresh Kampo, Meridian Fruits, Periban Los Reyes, SN 60440, Mexico), 89.08 g cut, fresh strawberries (Signature Farms, Better Living Brands LLC, Pleasanton, CA, USA) | |
| Tropical Fruit | 23.62 g crème de banana syrup (Torani, R. Torre & Company, San Leandro, CA, USA), 12.98 g mango syrup (Torani, R. Torre & Company, San Leandro, CA, USA) | |
| White Pepper | 6.60 g white pepper (First Street, Amerifoods Trading Co, Los Angeles, CA, USA) | |
| Mushroom | 52.34 g chopped, raw Baby Bello mushrooms (Signature Farms, Better Living Brands LLC, Pleasanton, CA, USA) | |
| Hot | 444 mL of vodka (New Amsterdam, New Amsterdam Spirits Company, Modesto, CA, USA) | |
| Retronasal Aromas | Reduction | 100 mL of base reduction solution; 116.94 g raw white onion (Signature Farms, Better Living Brands LLC, Pleasanton, CA, USA), 112.02 g cooked, white onion (Signature Farms, Better Living Brands LLC, Pleasanton, CA, USA) on medium until browned and soaked in 1000 mL of base wine overnight. |
| Fruit | 61.26 g cut, fresh strawberries (Signature Farms, Better Living Brands LLC, Pleasanton, CA, USA), 13.26 g strawberry preserves (Bonne Maman, Andros, France), 16.31 g wild blueberry preserves (Bonne Maman, Andros, France), 18.39 g cherry preserves (Bonne Maman, Andros, France), 13.95 g blackberry syrup (Torani, R. Torre & Company, San Leandro, CA, USA), 15.29 g cherry syrup (Torani, R. Torre & Company, San Leandro, CA, USA) | |
| Hot | 444 mL of vodka (New Amsterdam, New Amsterdam Spirits Company, Modesto, CA, USA) | |
| Taste | Acidity 4 | 1.23 g tartaric acid (LD Carlson Company, LD Carlson Company, Kent, OH, USA) |
| Bitter 4 | 1.32 g caffeine, anhydrous (Sigma Aldrich, 1003363509, Sigma-Aldrich Co., St. Louis, MO, USA) | |
| Mouthfeel | Suede 5 | Suede dress (Willow Ridge) |
| Velvet 5 | Velvet jacket (Chico’s) | |
| Time Parameter | Definition |
|---|---|
| Vmax | Maximum citation proportion |
| Tmax | Time of maximum citation proportion |
| Area Under Curve | Total intensity response |
| Tfirst | Time of first citation proportion per attribute |
| Tlast | Time of last citation proportion per attribute |
| Length | Total time attribute was perceived (Tfirst–Tlast) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stoffel, E.S.; Robertson, T.M.; Catania, A.A.; Casassa, L.F. The Impact of Fermentation Temperature and Cap Management on Selected Volatile Compounds and Temporal Sensory Characteristics of Grenache Wines from the Central Coast of California. Molecules 2023, 28, 4230. https://doi.org/10.3390/molecules28104230
Stoffel ES, Robertson TM, Catania AA, Casassa LF. The Impact of Fermentation Temperature and Cap Management on Selected Volatile Compounds and Temporal Sensory Characteristics of Grenache Wines from the Central Coast of California. Molecules. 2023; 28(10):4230. https://doi.org/10.3390/molecules28104230
Chicago/Turabian StyleStoffel, Emily S., Taylor M. Robertson, Anibal A. Catania, and L. Federico Casassa. 2023. "The Impact of Fermentation Temperature and Cap Management on Selected Volatile Compounds and Temporal Sensory Characteristics of Grenache Wines from the Central Coast of California" Molecules 28, no. 10: 4230. https://doi.org/10.3390/molecules28104230
APA StyleStoffel, E. S., Robertson, T. M., Catania, A. A., & Casassa, L. F. (2023). The Impact of Fermentation Temperature and Cap Management on Selected Volatile Compounds and Temporal Sensory Characteristics of Grenache Wines from the Central Coast of California. Molecules, 28(10), 4230. https://doi.org/10.3390/molecules28104230