Abstract
The continuous search for novel biobased polymers with high-performance properties has highlighted the role of monofuranic-based polyesters as some of the most promising for future plastic industry but has neglected the huge potential for the polymers’ innovation, relatively low cost, and synthesis easiness of 5,5′-isopropylidene bis-(ethyl 2-furoate) (DEbF), obtained from the platform chemical, worldwide-produced furfural. In this vein, poly(1,12-dodecylene 5,5′-isopropylidene -bis(ethyl 2-furoate)) (PDDbF) was introduced, for the first time, as a biobased bisfuranic long-chain aliphatic polyester with an extreme flexibility function, competing with fossil-based polyethylene. This new polyester in-depth characterization confirmed its expected structure (FTIR, 1H, and 13C NMR) and relevant thermal features (DSC, TGA, and DMTA), notably, an essentially amorphous character with a glass transition temperature of −6 °C and main maximum decomposition temperature of 340 °C. Furthermore, PDDbF displayed an elongation at break as high as 732%, around five times higher than that of the 2,5-furandicarboxylic acid counterpart, stressing the unique features of the bisfuranic class of polymers compared to monofuranic ones. The enhanced ductility combined with the relevant thermal properties makes PDDbF a highly promising material for flexible packaging.
1. Introduction
In the past decades, an increasing social, environmental, legislative, and ultimately economic demand for a more sustainable model of development [1] has witnessed a growing interest in renewable-based polymers to replace their analogous from fossil origin, namely the highly consumed polyethylene (PE), polypropylene (PP), poly(ethylene terephthalate) (PET), and polystyrene (PS), among other thermoplastics [2,3,4,5,6].
In this vein, ubiquitous biomass fractions such as cellulose, lignin, polysaccharides, proteins, and vegetable oils have emerged as an inexhaustible source of raw materials to prepare a wide portfolio of valuable, and, in many cases, innovative building-block chemicals for polymer synthesis. Among them, 2,5-furandicarboxylic acid (FDCA) and various alkyl 2-furoates (including bisfuranic derivatives) are some of the most important [7,8,9,10,11]. While FDCA has been extensively used [11,12], mainly due to its structural similarity to fossil-based terephthalic acid and a set of unique feature it can impart to polymers thereof (e.g., enhanced barrier properties) [13,14,15], opposite, bisfuran monomers have been widely overlooked.
FDCA has been exploited in both fully aromatic polymers (mainly polyesters) [16,17,18,19] as well as in aliphatic-furanic ones [20,21,22,23,24]. The most popular class is undoubtedly the linear-aliphatic polyesters [12], with poly(ethylene 2,5-furandicarboxylate) (PEF) and poly(1,3-propylene 2,5-furandicarboxylate) (PPF) occupying a preeminent place as biobased alternatives to the petrochemical PET in films, fibers, and packaging [5]. Additionally, other poly(alkylene 2,5-furandicarboxylate)s with longer alkyl chain-length have also been extensively investigated to modulate the flexibility, crystallization kinetics, and (bio)degradability, and, hence, to expand their overall use in, for example, packaging [25,26].
Of particular interest are those with very long alkyl-chains (≥C8) due to the combination of rigidity imparted by the furan ring and the extreme softness associated with the aliphatic moieties. Despite this feature, there are only a few reports on this type of polyesters, focusing mostly on the optimization of their synthesis or in the study of their thermal properties [25,27,28,29,30,31]. The high boiling points of the very long-chain diols is one of the serious problems that hinders their synthesis by the classical polycondensation procedures via the evaporation of the excess of diols [30]. To circumvent this issue, in 2015, the Bikiaris group, developed an elegant approach to synthesize high-molecular-weight polyesters from these type of monomers, simply by reacting dimethyl 2,5-furandicarboxylate (DMFDC) with the long-chain diols to form the correspondent bis(hydroxyalkyl)-2,5-furandicarboxylates, followed by the addition of a slight excess of DMFDC (1.05 eqv.) [27]. Later, in 2017, Li et al. [25] proposed a different strategy based on the use of asymmetric 2-(hydroxyalkyl)-5-methyl 2,5-furandicarboxylate monomer, prepared from FDCA, but following a highly unsustainable approach using acyl chloride intermediate.
Other works focused instead on the assessment of properties of such polyesters [25,26,28]; for example, Feherenbacher et al. [32] early reported, in 2009, on the thermal characterization of poly(1,6-hexylene 2,5-furandicarboxylate) (PHF), poly(1,12-dodecylene 2,5-furandicarboxylate) (PDDF), and poly(1,18-octadecylene 2,5-furandicarboxylate) (PODF). Importantly, they found that these polyesters are semi-crystalline polymers with a melting feature decreasing with the increasing chain length. These results were later corroborated by the work of Bikiaris and Papageorgiou [27]. Notably, PDDF displayed a melting temperature (Tm) of 111 °C and a sub-ambient glass transition temperature (Tg~−22 °C). Despite the relatively moderate melting and glass transition temperatures, FDCA-based long-chain aliphatic polyesters display a combination of (bio)degradation and mechanical properties, which makes them suitable for a broad range of applications [24,26]. More recently, nanocomposite films prepared by blending poly(lactic acid) (PLA), PDDF (10 wt%), and reduced graphene oxide as filler were studied as new promising biobased materials for packaging [31]. Fibers prepared from PLA/PDDF blends for textile applications have also been reported [33].
Less extensive research has been dedicated to bisfurans, such as 5,5′-isopropylidene bis-(ethyl 2-furoate) (DEbF), obtained with high purity, through a simple and cost-effective process from the platform chemical furfural [34,35], albeit their huge potential for the polymers’ innovation, relatively low cost [36], and synthesis easiness compared to FDCA [37]. DEbF has been readily prepared in optimized conditions, simply from the condensation of biobased ethyl 2-furoate with acetone in acidic medium [38]. Entirely aromatic polyesters bearing bisfuranic and pyridinic units in the main chain display high Tg and amorphous character [39], opposite to FDCA-based counterparts, which are typically semi-crystalline materials. Ionic polyesters containing sulfonated functions and bisfuranic moieties have sorption and hydrolytic degradation features [40,41]. High molecular weight aliphatic-bisfuranic polyesters, obtained through the interfacial polycondensation of the bisfuranic dichloride derivative 5,5′-isopropylidene bis-(2-furoyl chloride), with C2 to C6 diols (ethylene glycol, 1,4-butanediol and 1,6-hexandiol), typically exhibited good stability and moderate Tg, showing the same typical trend already mentioned for FDCA-based polymers decreasing with the increasing number of methylene groups [6]. To the best of our knowledge, the mechanical properties of this type of polymer have not been reported before, despite the importance of this functional property for the assessment of potential applications.
Additionally, to the best of our knowledge, bisfuranic long-chain aliphatic polyesters have never been reported before, albeit their anticipated enhanced flexibility, imparted by the combination of a long-aliphatic chain spacer and the less-rigid DEbF bisfuranic structure, with a symmetrical carbon between the two furan rings. This kind of polyester will be of interest for flexible packaging, an ever-growing sector [42]. Therefore, in the current study, the unclosed potential of biobased long-chain aliphatic-bisfuranic polyesters was exploited for the first time, by synthesizing the novel poly(1,12-dodecylene 5,5′-isopropylidene-bis(ethyl 2-furoate)) (PDDbF), using a straightforward polycondensation approach. PDDbF in-depth structural (FTIR and 1H and 13C NMR), thermal (TGA, DSC), and mechanical (tensile testing and DMTA) properties were assessed.
2. Results and Discussions
In order to evaluate the unexploited influence of the chain length on bisfuranic polymers properties, this study addresses, for the first time, the synthesis and characterization of biobased poly(1,12-dodecylene 5,5′-isopropylidene-bis(ethyl 2-furoate)). PDDbF comprises both stiff units (5,5′-isopropylidene-bis(ethyl 2-furoate)) and soft aliphatic chain (1,12-dodecylene), promptly synthesized by applying a two-step melt polycondensation procedure, as described in the experimental section and depicted in Scheme 1. The PDDbF isolation yield after purification was over 75% and the high intrinsic viscosity ( dL.g−1) was found to be in accordance with a high molecular weight polymer ( of 40,000). Highly supple films, with c.a. 700–800 μm thickness, prepared by polyester melting/cooling process, pave the way to assess, for the first-time, the bisfuranic-based materials’ mechanical properties.
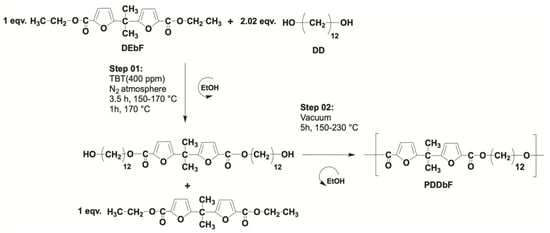
Scheme 1.
Synthesis of PDDbF.
2.1. Structural Characterization
The detailed chemical structure of PDDbF was studied using FTIR, 1H and 13C NMR spectroscopies (Figure 1 and Figure 2) and compared to the monomers’ counterparts (Figures S1–S3). The FTIR spectrum of Figure 1 displays all the typical characteristic bands for furan rings. Namely, a small band at 3128 cm−1 assigned to =C-H stretching vibration, an intense peak near 2922 cm−1 characteristic of the stretching mode of the methyl groups, a band at 1590 cm−1 related to the C=C stretching, a very intense peak near 1127 cm−1 arising from the C-O-C furan ring vibration, and a band at 1020 cm−1 assigned to the furan ring breathing and several bands (971, 803 and 759 cm−1) related to 2,5-disubstituted furans. In addition, the FTIR spectrum also exhibits a band at 2852 cm−1 associated to the C-H stretching of the methylene groups of the aliphatic diol moiety and two very intense bands near 1716 and 1295 cm−1 assigned to the C=O and C-O-C stretching vibrations of ester groups, respectively.
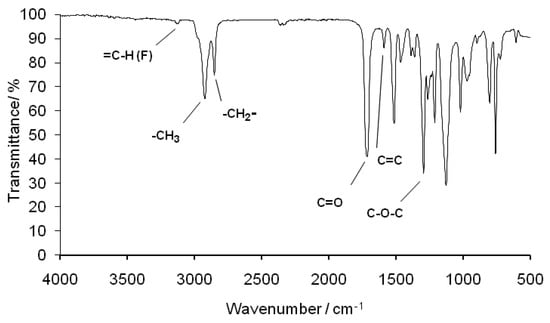
Figure 1.
PDDbF FTIR spectrum.
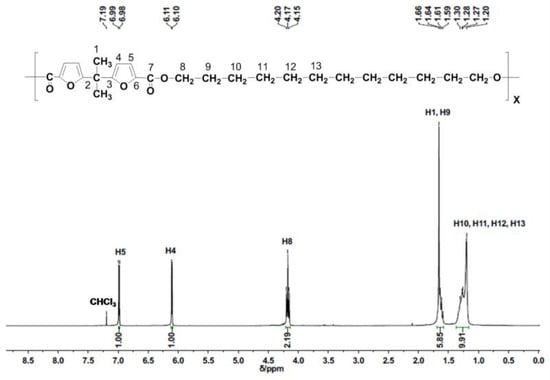
Figure 2.
1H NMR spectrum of PDDbF.
The structure of PDDbF was also studied by 1H NMR (Figure 2 and Table S1 of the Supplementary Materials). As expected for (hetero-)aromatic-based polyesters, the most deshielded protons of PDDbF are those from the furanic rings [39,43,44], namely two doublets at 6.98 and 6.10 ppm attributed to the protons of furan heterocycle (H5 and H4, respectively). The resonance assigned to the methylene protons (H8) directly attached to the carbonyl group appears at 4.15–4.20 ppm as a triplet. The other proton resonances of the aliphatic segments are displayed at: 1.59–1.64 ppm (H9, multiplet), partially overlapped with the singlet at 1.66 ppm (H1), and between 1.20–1.30 ppm (H10, H11, H12, H13). Notably, the 1H NMR spectrum shows the high purity of PDDbF and the equivalence in terms of integration. Additionally, the PDDbF 13C NMR spectrum (Figure S4 and Table S2 of the Supplementary Materials) corroborates the previous assessment, with the detection of the following typical carbon resonances: 163.1 ppm arising from the carbonyl carbon (C7); several resonances at 158.9, 134.7, 118.6, 107.3, 38.3, and 25.9 ppm assigned to the furan ring carbons (C6, C3, C5, C4, C2 and C1, respectively); and at 64.9, 29.6, 29.5, 29.3, 28.7, and 26.0 ppm for the 1,12-dodecylene chain carbon resonances (C8, C9, C10, C11, C12, and C13).
2.2. Thermal and X-ray Diffraction Properties
The thermal stability of the as prepared PDDbF was evaluated by TGA. The thermogram of Figure 3 exhibits a major characteristic feature, at a maximum decomposition temperature (Td,max) of 341 °C (c.a. 83% weight loss), followed by a smaller peak near 430 °C. Comparing with linear low density poly(ethylene) (LLDPE), the thermal stability of PDDbF is much higher (Td,max, c.a. 341 vs. 250 °C) [44].
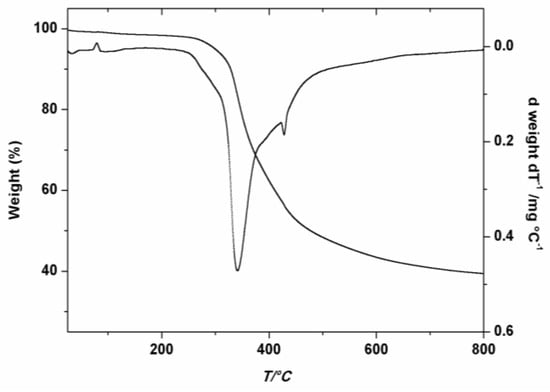
Figure 3.
TGA and DTG thermograms of PDDbF.
The thermal behavior of the PDDbF below the degradation temperature was studied by DSC analysis. The heating thermogram (Figure S5 of the Supplementary Materials) agrees with an essentially amorphous character, displaying accordingly, only one step in the baseline, ascribed to the glass transition temperature (Tg) at −6 °C (also in agreement with DMTA results described below). This moderately low Tg is due to the high number of methylene groups in the aliphatic chain, which is associated to the high flexibility of the macromolecular chain. Nevertheless, this Tg is still higher than that of LLDPE [45]. This effect has never been exploited for the promising family of poly(5,5′-isopropylidene-bis(2-furoate))s, although long aliphatic chain FDCA derivatives, such as poly(1,12-dodecylene 2,5-furandicarboxylate) homologous (PDDF) [27] or poly(1,20-eicosanediyl 2,5-furandicarboxylate) (PM20F) [29], also have low Tgs (Table 1). The DSC thermogram also shows a small melting peak at 81.6 °C. However, the enthalpy associated to such transition is much lower than that of PDDF with the same number of methylene groups (9 J g−1 vs. 50 J g−1), which is indicative of a lower crystallinity degree and that the crystallization of PDDbF is hampered by the bisfuranic moiety [27].

Table 1.
Thermal properties of the synthesized PDDbF compared to PDDF, PM20F, and LLDPE.
The XRD diffractogram (Figure S6 of the Supplementary Materials) of PDDbF presents only a halo centered at 2θ~19.2°. Polymers bearing the bisfuran motif follow a similar trend, being typically amorphous materials [6,39,41], opposite to the FDCA-based counterpart, poly(1,12-dodecylene 2,5-furandicarboxylate), which as mentioned above, is semi-crystalline [27]. The amorphous nature of bisfuranic polymers is most likely related to the myriad of different spatial arrangements of both furan rings hampering crystallization, opposite to FDCA-based ones.
2.3. DMTA and Mechanical Properties
Dynamic mechanical behavior of PDDbF was investigated by DMTA and its storage modulus (E′) (Figure 4a), loss modulus (E″) (Figure S7 of the Supplementary Materials), and tan δ (Figure 4b) traces were recorded. At sub-ambient temperatures, c.a. −24 °C, the E’ trace shows a first small decrease of the modulus typically associated with an increase of chain degrees of freedom and most probably ascribed to the β relaxation, followed by a rapid drop attributed to an α relaxation, the Tg (c.a. 6 °C).
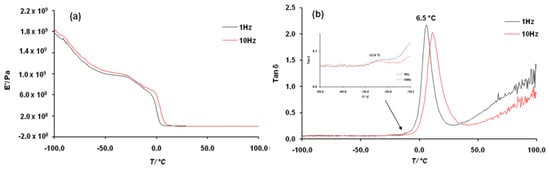
Figure 4.
DMTA storage modulus (E′) (a) and tan δ (b) traces of PDDbF.
Importantly, the E’ value, measured at room temperature (20 °C) and in the region of the glass transition (4.07 × 106 and 1.86 × 107 Pa, respectively), allowed to study the elastic behavior of PDDbF. Typically, this polyester had a lower E’ than other aromatic polymers incorporating shorter aliphatic moieties (e.g., PET and PEF), hence being less stiff [48].
The tan δ trace (Figure 4b) obtained in the single cantilever mode agrees with the transitions mentioned above, displaying two peaks centered at approximately −24 and 6 °C attributed to Tβ and Tg, respectively. After the glass transition, the chain segments movement began to be easier, reaching a viscous liquid, which explain the loose increase of the tan δ value.
Additionally, PDDbF compression molded films were subjected to stress–strain measurements (Figure 5) in order to explore their mechanical properties and assess their suitability to practical applications in, for example, flexible packaging [49]. Despite the evident importance of mechanical properties for the applications’ evaluation, to the best of our knowledge, this is the first study reporting these features for bisfuranic polyesters.
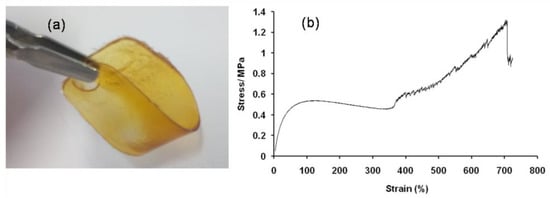
Figure 5.
Digital image of a PDDbF film (a) and corresponding stress–strain curve (b).
The elongation at break, tensile stress at break, tensile stress at yield, and Young’s modulus of PDDbF, and of similar FDCA-based polyesters, are summarized in Table 2.

Table 2.
Mechanical properties of PDDbF compared with similar FDCA-based polyesters.
PDDbF exhibits a low Young’s modulus, above 2 MPa, but the elongation at break reached 732%, a value much higher than that of its FDCA counterpart (130%) with similar molecular weight [27], which is indicative of the role played by the bisfuranic moiety in the very flexible and easily deformable nature of the material obtained compared to the FDCA-based counterparts (PDDF and PDeF, Table 2) or even to PE [50]. Thus, considering also the PDDF values (Table 2), the explanation for the high elongation at break for PDDbF is not only due to the long-chain diol spacer but also due to the lower rigidity of the 5,5′-isopropylidene-bis(ethyl 2-furoate) moiety, most probably related to the symmetric carbon between the two furan rings, which results in a greater unfolding stretchability during deformation. Furthermore, the typical high stress at yield, high stress at break, and low elongation at break of furanoate-based polyesters compared to those of PDDbF can be explained by fact that the crystallites appear to serve a stress-bearing phase during deformation [51].
3. Materials and Methods
3.1. Materials
1,12-Dodecandiol (DD, 99%), ethyl 2-furoate (99%), titanium (IV) tert-butoxide (TBT, 97%), deuterated dimethyl sulfoxide (DMSO-d6, 99.5% D), and deuterated chloroform (CHCl3-d, 99.8% D) were purchased from Merck (Darmstadt, Germany). All solvents used were analytical grade. All chemicals were used as received, without further purification.
3.2. Preparation of 5,5′-Isopropylidene-bis(ethyl 2-furoate) (DEbF)
DEbF was synthesized according to a procedure described elsewhere [44]. Briefly, DEbF was prepared by reacting ethyl 2-furoate (0.214 mol) with acetone under acidic conditions, at 60 °C for 8 h. The monomer was then isolated as fine crystals after extraction with ethyl ether, followed by distillation and recrystallization from hexane. The structure and purity were confirmed by FTIR and 1H and 13C NMR spectroscopic techniques (Figures S1–S3 of the Supplementary Materials).
3.3. Synthesis of Poly(1,12-dodecylene 5,5′-isopropylidene-bis(ethyl 2-furoate)) (PDDbF)
PDDbF was synthesized by melt polycondensation, according to a previously reported procedure [29]. In the first step, stoichiometric amounts of DEbF (5 g, 15.6 mmol), an excess of DD (6.4 g, 31.5 mmol) and TBT (6.5 mg, 19.2 × 10−3 mmol) were charged into the glass reactor. The reaction started by heating progressively from 150 to 170 °C, during 3.5 h, and kept at that maximum temperature, under a nitrogen atmosphere, for 1 h. Before starting the second step, the reaction temperature was decreased to 150 °C, and an additional quantity of DEbF (5 g, 15.6 mmol) was added and the temperature was slowly increased again until 170 °C under vacuum for 3 h. Afterwards, the temperature was raised to 210 °C, and then, during 2 h, progressively increased up to 230 °C and kept at that maximum temperature for at least half hour. Finally, the obtained product was dissolved in chloroform, precipitated in an excess of cold methanol, filtered, and dried. PDDbF film specimens were prepared by melting procedure at c.a. 165 °C, placing the samples in a Teflon rectangular mold (50 × 10 × 1 mm3).
3.4. Characterization
Attenuated total reflection Fourier transform infrared (ATR FTIR) spectra were obtained using a PARAGON 1000 PerkinElmer FTIR spectrophotometer equipped with a single horizontal Golden Gate ATR cell. The resolution was 8 cm−1 after 128 scans in the range of 500–4000 cm−1.
1H and 13C Nuclear Magnetic Resonance spectroscopy (1H, 13C NMR) analyses of samples dissolved in CDCl3 were recorded using a Bruker AMX 300 spectrometer operating at 300 or 75 MHz. All chemical shifts were expressed in parts per million (ppm) and reported downfield from 0.00 ppm using tetramethylsilane (TMS) as an internal reference.
Intrinsic viscosity
measurements were performed using an Ubbelohde-type viscometer, in a water bath at 25 °C. A mixture of phenol/1,1,2,2-tetrachloroethane (50/50) (wt%/wt%) was used as solvent and at a polymer concentration of 0.1 g per 20 mL of solution. The intrinsic viscosity was determined by the ratio of specific viscosity and sample solution concentration: , where and and are the solvent mixture and polyester solution elution time, respectively. The viscosity-average molecular weight () of PDDbF was calculated by the Mark–Houwink equation using the K and α parameters for PET viscosity [52,53,54].
Thermogravimetric analyses (TGA) were carried out with a Shimadzu TGA50 analyzer equipped with a platinum cell using platinum pans to encapsulate the samples (ca. 7 mg). Thermograms were recorded under a nitrogen flow of 20 mL min−1 at a constant heating rate of 10 °C min−1 from 25 to 800 °C.
DSC thermograms were obtained with a Setaram DSC92 calorimeter. Scans were carried out under dry nitrogen, with a heating rate of 10 °C min−1 in the temperature range of −50 to 250 °C. Two heating/cooling cycles were performed. The first heating scan was used to erase the thermal history of the polymer, and the second heating scan was performed after rapid cooling from the melted polymer.
X-ray diffraction (XRD) patterns were acquired using a Philips X’pert MPD diffractometer operating with CuKα radiation (λ = 1.5405980 Å) at 45 kV and 40 mA. Samples were scanned in the 2θ range of 3 to 60 °C, with a step size of 0.026° and a time per step of 69 s.
Dynamical mechanical thermal analysis (DMTA) of thick samples (5.0 × 3.6 × 0.8 mm3) were recorded with a Tritec 2000 DMTA Triton equipment operating in the single cantilever bending geometry. Tests were performed in multifrequency mode (1 and 10 Hz), from −100 to 200 °C, at 2 °C.min−1.
Tensile mechanical tests were performed on an Instron 5966 Series machine. A load cell of 500 N was used and operated at a deformation rate of 10 mm.min−1, under ambient conditions. For each sample, at least 5 specimens were tested. Young’s modulus, tensile strength, and elongation at break were calculated using the Bluehill 3 material testing software.
4. Conclusions
This study highlights the possibility of preparing a new biobased homopolyester from the bisfuranic monomer DEbF and a long-chain aliphatic diol spacer adopting a very straightforward approach based on the two-step polymerization approach. However, most importantly, this study uncloses these monomers’ ability to impart enhanced flexibility to the ensuing polymer chain compared to FDCA counterparts, but still having higher thermal stability and glass transition compared to polyolefins. In fact, the in-depth characterization of PDDbF showed that this polyester is amorphous, with a sub-ambient glass transition temperature (around −6 °C), and a maximum decomposition temperature of 341.4 °C, much higher than the same parameter for LLDPE (341 vs. 250 °C, respectively) [44]. All in all, PDDbF is a promising biobased polyester from the bisfuranic family and shows an enhanced elongation at break value of about 732%, thus opening their wider exploration among, for example, flexible packaging, an ever-growing sector.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/molecules28104124/s1, Figure S1: FTIR spectrum of DEbF, Figure S2: 1H NMR spectrum of DEbF, Figure S3: 13C NMR spectrum of DEbF, Table S1: 1H NMR resonances [300 MHz, CDCl3-d] of PDDbF, Figure S4: 13C NMR spectrum of PDDbF, Table S2: 13C NMR chemical shifts assignments of PDDbF, Figure S5: DSC thermogram for PDDbF, Figure S6: XRD pattern of PDDbF, Figure S7: Loss modulus trace of PDDbF.
Author Contributions
Conceptualization, A.F.S.; methodology, A.F.S. and S.Z.; formal analysis, S.Z., A.F.S., A.B. and A.J.D.S.; investigation, all authors; writing—original draft preparation, S.Z. and A.F.S.; writing—review and editing, all authors; supervision, A.F.S., A.J.D.S., A.B., M.A. and S.A.; funding acquisition, A.F.S. and A.J.D.S. All authors have read and agreed to the published version of the manuscript.
Funding
This work is based upon work from the COST Action FUR4Sustain-European network of FURan based chemicals and materials FOR a Sustainable development, CA18220, supported by COST (European Cooperation in Science and Technology), and also developed within the scope of the ERASMUS + ICM project (Ref. 2017-1-PTO1-KA107-035493) between the University of Sfax and the University of Aveiro. This work was developed within the scope of the project CICECO-Aveiro Institute of Materials, UIDB/50011/2020, UIDP/50011/2020 & LA/P/0006/2020, financed by national funds through the FCT/MCTES (PIDDAC). This research is also sponsored by FEDER funds through the program COMPETE—Programa Operacional Factores de Competitividade—and by national funds through the FCT under the project UID/EMS/00285/2020. The FCT is also acknowledged for the research contract under Scientific Employment Stimulus to AFS (CEECIND/02322/2020). The authors would also like to acknowledge the financial support of the Ministry of Higher Education and Scientific Research in Tunisia.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available in this article and supplementary material.
Conflicts of Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Sample Availability
Samples of the compounds are available from the authors.
References
- UN. Sustainable Development Goals. 2020. Available online: https://www.un.org/sustainabledevelopment/sustainable-development-goals/ (accessed on 2 August 2022).
- Niaounakis, M. Introduction to Biopolymers. Biopolym. Reuse Recycl. Dispos. 2013, 5, 1–75. [Google Scholar] [CrossRef]
- Narancic, T.; Cerrone, F.; Beagan, N.; O’Connor, K.E. Recent Advances in Bioplastics: Application and Biodegradation. Polymers 2020, 12, 920. [Google Scholar] [CrossRef]
- Zia, K.M.; Akram, N.; Tabasum, S.; Noreen, A.; Akbar, M.U. Future trends in the bio-based polymer processing industry. In Processing Technology for Bio-Based Polymers; Elsevier: Amsterdam, The Netherlands, 2021; pp. 267–279. [Google Scholar] [CrossRef]
- Sousa, A.F.; Patrício, R.; Terzopoulou, Z.; Bikiaris, D.N.; Stern, T.; Wenger, J.; Loos, K.; Lotti, N.; Siracusa, V.; Szymczyk, A.; et al. Recommendations for replacing PET on packaging, fiber, and film materials with biobased counterparts. Green Chem. 2021, 23, 8795–8820. [Google Scholar] [CrossRef]
- Gharbi, S.; Andreolety, J.; Gandini, A. Polyesters bearing furan moieties: IV. Solution and interfacial polycondensation of 2,2′-bis(5-chloroformyl-2-furyl)propane with various diols and bisphenols. Eur. Polym. J. 2000, 36, 463–472. [Google Scholar] [CrossRef]
- Zhu, Y.; Romain, C.; Williams, C.K. Sustainable polymers from renewable resources. Nature 2016, 540, 354–362. [Google Scholar] [CrossRef]
- Yao, K.; Tang, C. Controlled Polymerization of Next-Generation Renewable Monomers and Beyond. Macromolecules 2013, 46, 1689–1712. [Google Scholar] [CrossRef]
- Marshall, A.-L.; Alaimo, P.J. Useful products from complex starting materials: Common chemicals from biomass feedstocks. Chemistry 2010, 16, 4970–4980. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Yin, G. Catalytic Transformation of the Furfural Platform into Bifunctionalized Monomers for Polymer Synthesis. ACS Catal. 2021, 11, 10058–10083. [Google Scholar] [CrossRef]
- Terzopoulou, Z.; Papadopoulos, L.; Zamboulis, A.; Papageorgiou, D.G.; Papageorgiou, G.Z.; Bikiaris, D.N. Tuning the properties of furandicarboxylic acid-based polyesters with copolymerization: A review. Polymers 2020, 12, 1209. [Google Scholar] [CrossRef] [PubMed]
- Sousa, A.F.; Vilela, C.; Fonseca, A.C.; Matos, M.; Freire, C.S.R.; Gruter, G.-J.M.; Coelho, J.F.J.; Silvestre, A.J.D. Biobased polyesters and other polymers from 2,5-furandicarboxylic acid: A tribute to furan excellency. Polym. Chem. 2015, 6, 5961–5983. [Google Scholar] [CrossRef]
- Burgess, S.K.; Kriegel, R.M.; Koros, W.J. Carbon dioxide sorption and transport in amorphous poly(ethylene furanoate). Macromolecules 2015, 48, 2184–2193. [Google Scholar] [CrossRef]
- Burgess, S.K.; Karvan, O.; Johnson, J.R.; Kriegel, R.M.; Koros, W.J. Oxygen sorption and transport in amorphous poly(ethylene furanoate). Polymer 2014, 55, 4748–4756. [Google Scholar] [CrossRef]
- Burgess, S.K.; Wenz, G.B.; Kriegel, R.M.; Koros, W.J. Penetrant transport in semicrystalline poly(ethylene furanoate). Polymer 2016, 98, 305–310. [Google Scholar] [CrossRef]
- Zaidi, S.; Soares, M.J.; Bougarech, A.; Thiyagarajan, S.; Guigo, N.; Abid, S.; Abid, M.; Silvestre, A.J.D.; Sousa, A.F. Unravelling the para- and ortho-benzene substituent effect on the glass transition of renewable wholly (hetero-)aromatic polyesters bearing 2,5-furandicarboxylic moieties. Eur. Polym. J. 2021, 150, 110413. [Google Scholar] [CrossRef]
- Wilsens, C.H.R.M.; Noordover, B.A.J.; Rastogi, S. Aromatic thermotropic polyesters based on 2,5-furandicarboxylic acid and vanillic acid. Polymer 2014, 55, 2432–2439. [Google Scholar] [CrossRef]
- Wilsens, C.H.R.M.; Verhoeven, J.M.G.A.; Noordover, B.A.J.; Hansen, M.R.; Auhl, D.; Rastogi, S. Thermotropic polyesters from 2,5-furandicarboxylic acid and vanillic acid: Synthesis, thermal properties, melt behavior, and mechanical performance. Macromolecules 2014, 47, 3306–3316. [Google Scholar] [CrossRef]
- Fei, X.; Zhang, X.; Liu, J.; Wang, J.; Liu, X. Synthesis of a fire-retardant and high Tg biobased polyester from 2,5-furandicarboxylic acid. Polym. J. 2022, 54, 995–1008. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, Y.; Qi, Z.; He, L.; Peng, L. Progress in the synthesis and properties of 2,5-furan dicarboxylate based polyesters. BioResources 2020, 15, 4502–4527. [Google Scholar] [CrossRef]
- Hbaieb, S.; Kammoun, W.; Delaite, C.; Abid, M.; Abid, S.; Gharbi, R. El New copolyesters containing aliphatic and bio- based furanic units by bulk copolycondensation. J. Macromol. Sci. Part A Pure Appl. Chem. 2015, 52, 365–373. [Google Scholar] [CrossRef]
- Guidotti, G.; Soccio, M.; García-Gutiérrez, M.C.; Gutiérrez-Fernández, E.; Ezquerra, T.A.; Siracusa, V.; Munari, A.; Lotti, N. Evidence of a 2D-ordered structure in biobased poly(pentamethylene furanoate) responsible for Its outstanding barrier and mechanical properties. ACS Sustain. Chem. Eng. 2019, 7, 17863–17871. [Google Scholar] [CrossRef]
- Soccio, M.; Costa, M.; Lotti, N.; Gazzano, M.; Siracusa, V.; Salatelli, E.; Manaresi, P.; Munari, A. Novel fully biobased poly(butylene 2,5-furanoate/diglycolate) copolymers containing ether linkages: Structure-property relationships. Eur. Polym. J. 2016, 81, 397–412. [Google Scholar] [CrossRef]
- Chebbi, Y.; Kasmi, N.; Majdoub, M.; Papageorgiou, G.Z.; Achilias, D.S.; Bikiaris, D.N. Solid-state polymerization of poly(ethylene furanoate) biobased polyester, III: Extended study on effect of catalyst type on molecular weight increase. Polymers 2019, 11, 438. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Yao, B.; Yu, G.; Fu, Y.; Liu, F.; Li, Z. Facile preparation of bio-based polyesters from furandicarboxylic acid and long chain diols via asymmetric monomer strategy. Green Chem. 2017, 19, 4930–4938. [Google Scholar] [CrossRef]
- Guidotti, G.; Soccio, M.; García-Gutiérrez, M.C.; Ezquerra, T.; Siracusa, V.; Gutiérrez-Fernández, E.; Munari, A.; Lotti, N. Fully Biobased Superpolymers of 2,5-Furandicarboxylic Acid with Different Functional Properties: From Rigid to Flexible, High Performant Packaging Materials. ACS Sustain. Chem. Eng. 2020, 8, 9558–9568. [Google Scholar] [CrossRef]
- Tsanaktsis, V.; Papageorgiou, G.Z.; Bikiaris, D.N. A facile method to synthesize high-molecular-weight biobased polyesters from 2, 5-furandicarboxylic acid and long-chain diols. J. Polym. Sci. Part A Polym. Chem. 2015, 53, 2617–2632. [Google Scholar] [CrossRef]
- Terzopoulou, Z.; Tsanaktsis, V.; Nerantzaki, M.; Achilias, D.S.; Vaimakis, T.; Papageorgiou, G.Z.; Bikiaris, D.N. Thermal degradation of biobased polyesters: Kinetics and decomposition mechanism of polyesters from 2,5-furandicarboxylic acid and long-chain aliphatic diols. J. Anal. Appl. Pyrolysis 2016, 117, 162–175. [Google Scholar] [CrossRef]
- Soares, M.J.; Dannecker, P.-K.K.; Vilela, C.; Bastos, J.; Meier, M.A.R.; Sousa, A.F. Poly(1,20-eicosanediyl 2,5-furandicarboxylate), a biodegradable polyester from renewable resources. Eur. Polym. J. 2017, 90, 301–311. [Google Scholar] [CrossRef]
- Terzopoulou, Z.; Tsanaktsis, V.; Nerantzaki, M.; Papageorgiou, G.Z.; Bikiaris, D.N. Decomposition mechanism of polyesters based on 2,5-furandicarboxylic acid and aliphatic diols with medium and long chain methylene groups. Polym. Degrad. Stab. 2016, 132, 127–136. [Google Scholar] [CrossRef]
- Fredi, G.; Karimi Jafari, M.; Dorigato, A.; Bikiaris, D.N.; Checchetto, R.; Favaro, M.; Brusa, R.S.; Pegoretti, A. Multifunctionality of reduced graphene oxide in bioderived polylactide/poly(dodecylene furanoate) nanocomposite films. Molecules 2021, 26, 2938. [Google Scholar] [CrossRef]
- Fehrenbacher, U.; Grosshardt, O.; Kowollik, K.; Tübke, B.; Dingenouts, N.; Wilhelm, M. Synthese und charakterisierung von polyestern und polyamiden auf der basis von furan-2,5-dicarbonsäure. Chem. Ing. Tech. 2009, 81, 1829–1835. [Google Scholar] [CrossRef]
- Perin, D.; Rigotti, D.; Fredi, G.; Papageorgiou, G.Z.; Bikiaris, D.N.; Dorigato, A. Innovative bio-based poly(lactic acid)/poly(alkylene furanoate)s fiber blends for sustainable textile applications. J. Polym. Environ. 2021, 29, 3948–3963. [Google Scholar] [CrossRef]
- Werpy, T.; Petersen, G. Top Value Added Chemicals from Biomass. Volume I—Results of Screening for Potential Candidates from Sugars and Synthesis Gas; US Department of Energy: Washington, DC, USA, 2004. [Google Scholar]
- Bozell, J.; Petersen, G.R. Technology development for the production of biobased products from biorefinery carbohydrates—The US Department of Energy’s “top 10” revisited. Green Chem. 2010, 12, 539–554. [Google Scholar] [CrossRef]
- Furfural Market Size, Share & Trends Analysis Report by Process (Quaker Batch Process), by Raw Material (Corn Cob), by Application (Furfuryl Alcohol), by End-Use, by Region, and Segment Forecasts, 2023–2030. 2023. Available online: https://www.grandviewresearch.com/industry-analysis/furfural-market (accessed on 5 April 2023).
- Abid, M.; Abid, S.; El Gharbi, R. Polyterephthalates Bearing Bio-based Moieties. J. Macromol. Sci. Part A Pure Appl. Chem. 2012, 49, 37–41. [Google Scholar] [CrossRef]
- Khrouf, A.; Abid, M.; Boufi, S.; Gharbi, R.E.; Gandini, A. Polyesters bearing furan moieties, 2. A detailed investigation of the polytransesterification of bisfuranic diesters with different diols. Macromol. Chem. Phys. 1998, 199, 2755–2765. [Google Scholar]
- Bougarech, A.; Abid, M.; Abid, S.; Fleury, E. Synthesis, characterization and thermal, hydrolytic and oxidative degradation study of biobased (BisFuranic-Pyridinic) copolyesters. Polym. Degrad. Stab. 2016, 133, 283–292. [Google Scholar] [CrossRef]
- Bougarech, A.; Abid, M.; Gouanvé, F.; Espuche, E.; Abid, S.; EL Gharbi, R.; Fleury, E. Synthesis, characterization and water sorption study of new biobased (furanic-sulfonated) copolyesters. Polymer 2013, 54, 5482–5489. [Google Scholar] [CrossRef]
- Bougarech, A.; Abid, M.; Dacruz-Boisson, F. Modulation of furanic-sulfonated isophthalic copolyesters properties through diols units control. Eur. Polym. J. 2014, 58, 207–217. [Google Scholar] [CrossRef]
- Flexible Packaging Market Size, Share & Trends Analysis Report. 2020. Available online: https://www.grandviewresearch.com/industry-analysis/global-flexible-packaging-market (accessed on 5 April 2023).
- Bougarech, A.; Zaidi, S.; Sousa, A.F.; Abid, S.; Silvestre, A.J.D.; Abid, M. Bisfuranic copolyesters bearing nitrated units: Synthesis, thermal properties and degradation essays. J. Polym. Res. 2022, 29, 215. [Google Scholar] [CrossRef]
- Singh, G.; Bhunia, H.; Rajor, A.; Choudhary, V. Thermal properties and degradation characteristics of polylactide, linear low density polyethylene, and their blends. Polym. Bull. 2011, 66, 939–953. [Google Scholar] [CrossRef]
- Dikobe, D.G.; Luyt, A.S. Comparative study of the morphology and properties of PP/LLDPE/wood powder and MAPP/LLDPE/wood powder polymer blend composites. Express Polym. Lett. 2010, 4, 729–741. [Google Scholar] [CrossRef]
- Papageorgiou, D.G.; Guigo, N.; Tsanaktsis, V.; Exarhopoulos, S.; Bikiaris, D.N.; Sbirrazzuoli, N.; Papageorgiou, G.Z. Fast Crystallization and Melting Behavior of a Long-Spaced Aliphatic Furandicarboxylate Biobased Polyester, Poly(dodecylene 2,5-furanoate). Ind. Eng. Chem. Res. 2016, 55, 5315–5326. [Google Scholar] [CrossRef]
- Run, M.; Gao, J.; Li, Z. Nonisothermal crystallization and melting behavior of mPE/LLDPE/LDPE ternary blends. Thermochim. Acta 2005, 429, 171–178. [Google Scholar] [CrossRef]
- Sousa, A.F.; Matos, M.; Freire, C.S.R.; Silvestre, A.J.D.; Coelho, J.F.J. New copolyesters derived from terephthalic and 2,5-furandicarboxylic acids: A step forward in the development of biobased polyesters. Polymer 2013, 54, 513–519. [Google Scholar] [CrossRef]
- Wang, G.; Song, J. Synthesis and characterization of bio-based polyesters derived from 1,10-decanediol. J. Appl. Polym. Sci. 2021, 138, 51163. [Google Scholar] [CrossRef]
- Grulke, E.A. Polymer Handbook, 4th ed.; Brandrup, J., Immergut, E.H., Grulke, E.A., Eds.; Wiley: New York, NY, USA, 2004; 2336p. [Google Scholar]
- Kong, X.; Qi, H.; Curtis, J.M. Synthesis and characterization of high-molecular weight aliphatic polyesters from monomers derived from renewable resources. J. Appl. Polym. Sci. 2014, 40579, 4–10. [Google Scholar] [CrossRef]
- Farah, S.; Kunduru, K.R.; Basu, A.; Domb, A.J. Molecular weight determination of polyethylene terephthalate. In Poly(ethylene terephthalate) Based Blends, Composites and Nanocomposites; Visakh, P.M., Liang, M., Eds.; Elsevier Inc.: Oxford, UK, 2015; pp. 143–165. [Google Scholar]
- Wang, K.; Huang, H.; Sheng, J. Determination of the Mark-Houwink equation parameters and their interrelationship. J. Liq. Chromatogr. Relat. Technol. 1998, 21, 1457–1470. [Google Scholar] [CrossRef]
- Jabarin, S.A. Crystallization kinetics of polyethylene terephthalate. I. Isothermal crystallization from the melt. J. Appl. Polym. Sci. 1987, 34, 85–96. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).