Exploration of the Transglycosylation Activity of Barley Limit Dextrinase for Production of Novel Glycoconjugates
Abstract
1. Introduction
2. Results and Discussion
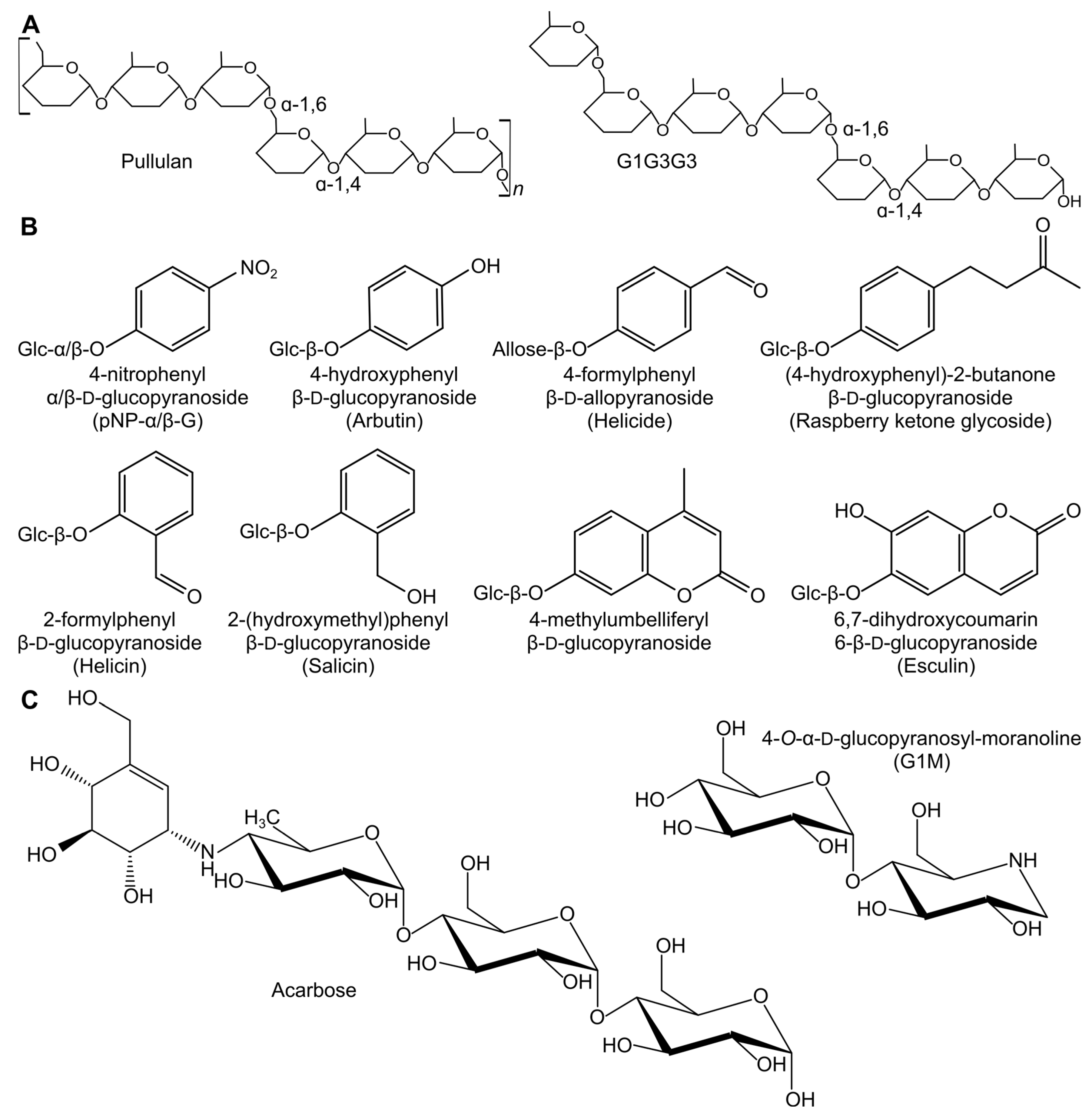
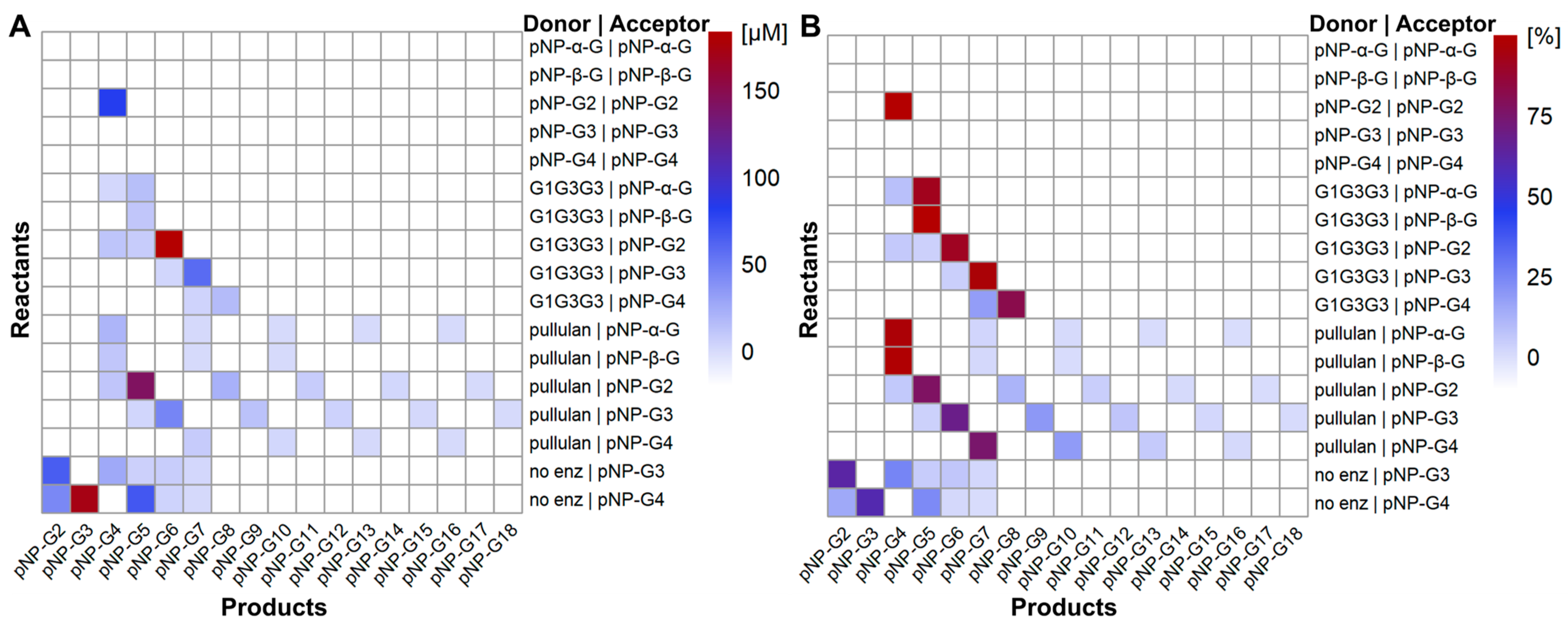
2.1. Transglycosylation Reactions with Natural Substrates and pNP-Sugars
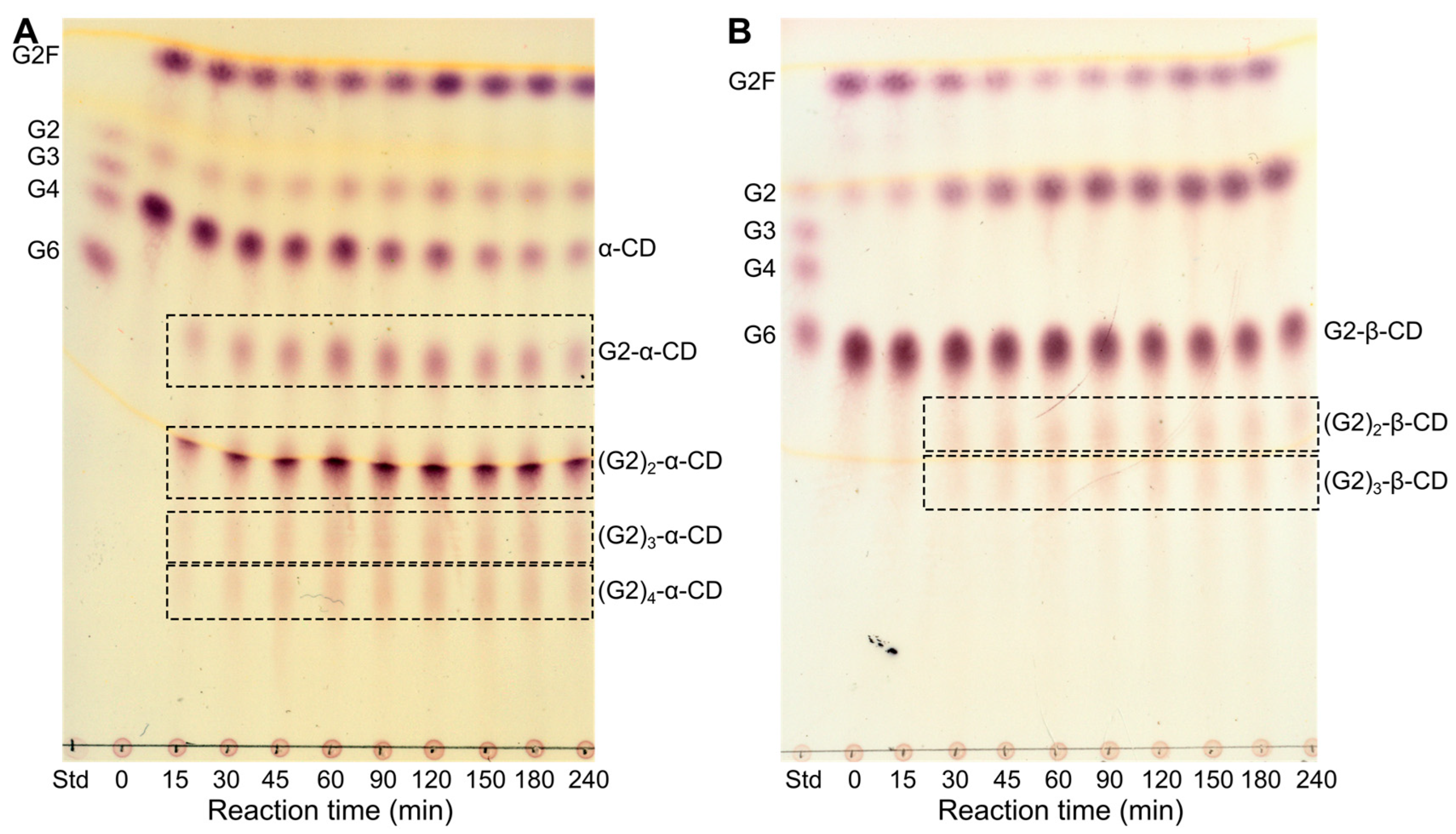
2.2. Alternative Compounds as Acceptors
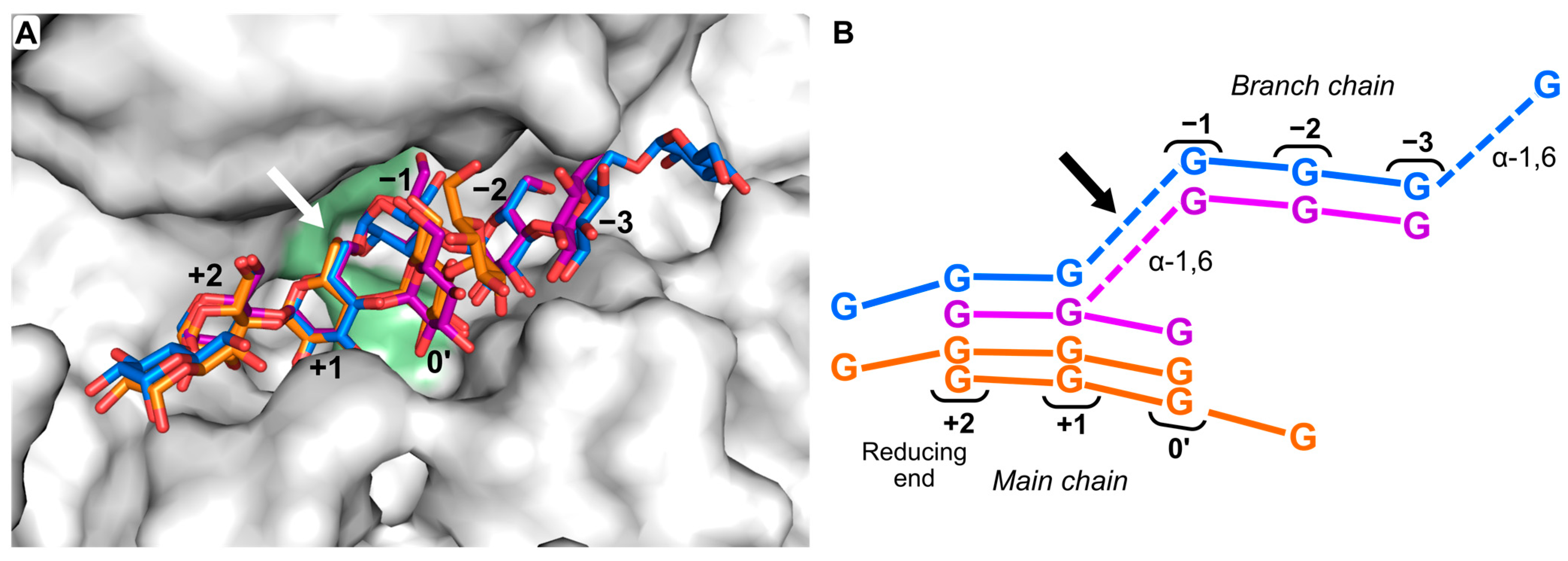
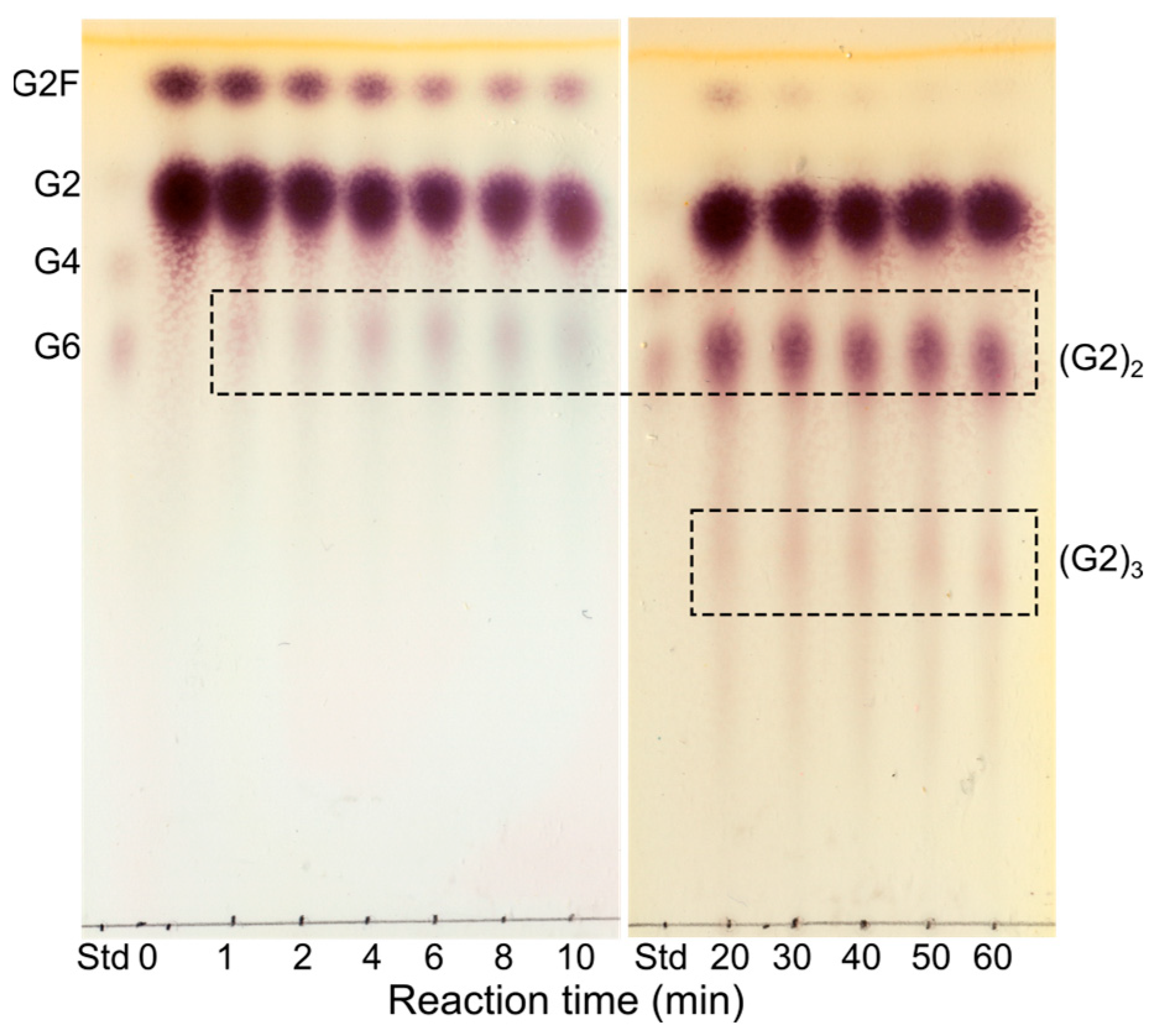
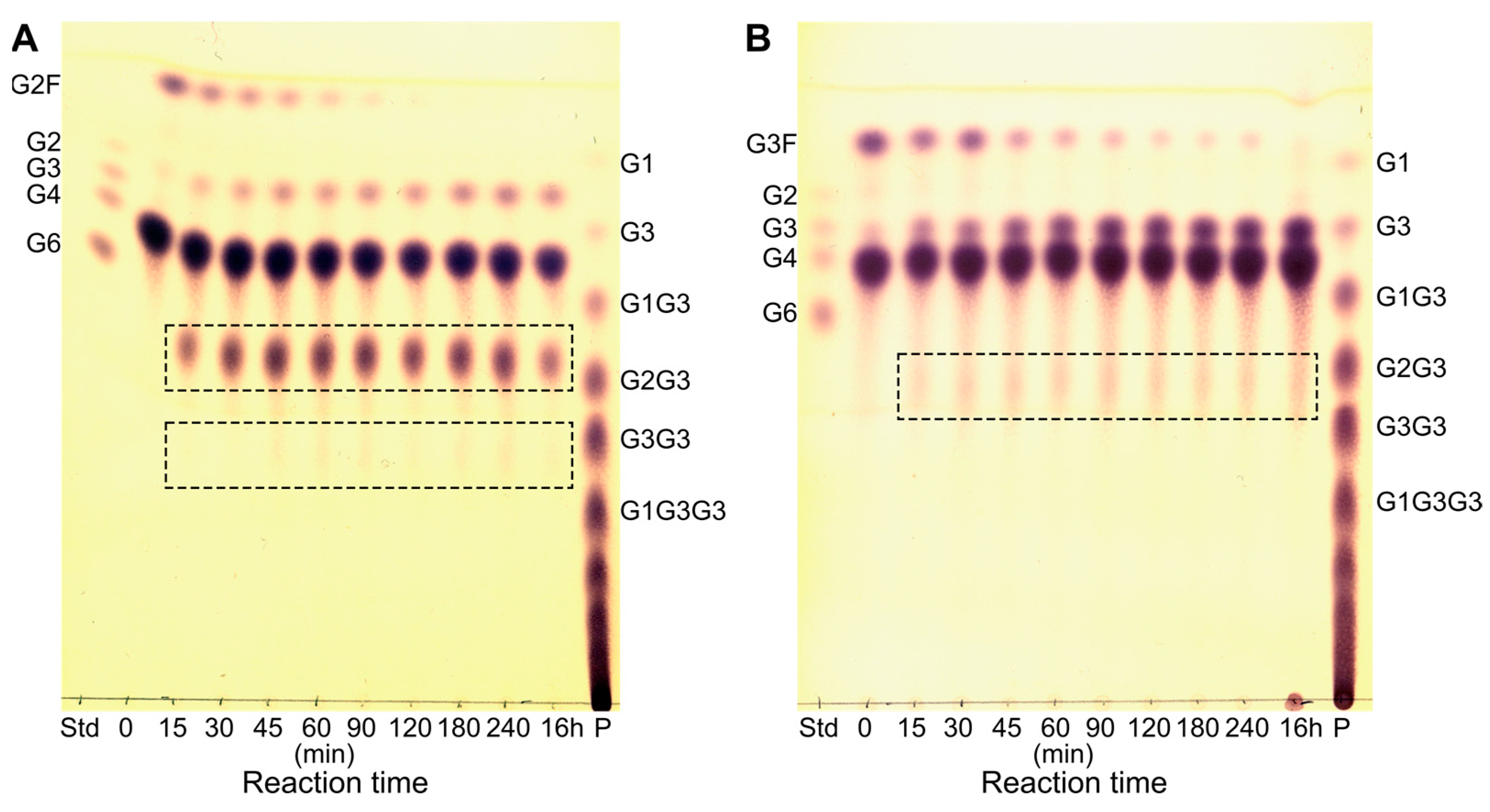
2.3. Probing the Acceptor and Donor Length Preferences Using Fluoride Maltooligosaccharides
2.4. Production of Potential GH Inhibitors from Known GH Inhibitors
3. Materials and Methods
3.1. Materials
3.2. Analysis of Transglycosylation Activity with Glucoside Compounds and Natural Substrates
3.3. Transglycosylation Reactions with Fluoride Donors
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Drula, E.; Garron, M.-L.; Dogan, S.; Lombard, V.; Henrissat, B.; Terrapon, N. The carbohydrate-active enzyme database: Functions and literature. Nucleic Acids Res. 2022, 50, D571–D577. [Google Scholar] [CrossRef]
- Stam, M.R.; Danchin, E.G.J.; Rancurel, C.; Coutinho, P.M.; Henrissat, B. Dividing the large glycoside hydrolase family 13 into subfamilies: Towards improved functional annotations of alpha-amylase-related proteins. Protein Eng. Des. Sel. 2006, 19, 555–562. [Google Scholar] [CrossRef]
- Zhao, W.; Li, T.; Woodward, R.; Xia, C.; Wang, P.G.; Guan, W. Enzymatic synthesis of complex carbohydrates. In Comprehensive Natural Products II; Elsevier: Amsterdam, The Netherlands, 2010; Volume 6, pp. 5–54. [Google Scholar]
- Le Roy, J.; Huss, B.; Creach, A.; Hawkins, S.; Neutelings, G. Glycosylation is a major regulator of phenylpropanoid availability and biological activity in plants. Front. Plant. Sci. 2016, 7, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Dias, C.; Matos, A.M.; Rauter, A.P. Chemical approaches towards neurodegenerative disease prevention: The role of coupling sugars to phenolic biomolecular entities. In Coupling and Decoupling of Diverse Molecular Units in Glycosciences; Witczak, Z.J., Bielski, R., Eds.; Springer: Cham, Switzerland, 2018; pp. 167–194. ISBN 978-3-319-65586-4. [Google Scholar]
- Sugimoto, K.; Nomura, K.; Nishimura, T.; Kiso, T.; Sugimoto, K.; Kuriki, T. Syntheses of α-arbutin-α-glycosides and their inhibitory effects on human tyrosinase. J. Biosci. Bioeng. 2005, 99, 272–276. [Google Scholar] [CrossRef] [PubMed]
- Best, R.B.; Jackson, G.E.; Naidoo, K.J. Molecular dynamics and NMR study of the α(1→4) and α(1→6) glycosidic linkages: Maltose and isomaltose. J. Phys. Chem. B 2001, 105, 4742–4751. [Google Scholar] [CrossRef]
- Kaneko, T.; Kohomoto, T.; Kikuchi, H.; Shiota, M.; Iino, H.; Mitsuoka, T. Effects of isomaltooligosaccharides with different degrees of polymerization on human fecal bifidobacteria. Biosci. Biotechnol. Biochem. 1994, 58, 228–2290. [Google Scholar] [CrossRef]
- Goffin, D.; Delzenne, N.; Blecker, C.; Hanon, E.; Deroanne, C.; Paquot, M. Will isomalto-oligosaccharides, a well-established functional food in Asia, break through the European and American market? The status of knowledge on these prebiotics. Crit. Rev. Food Sci. Nutr. 2011, 51, 394–409. [Google Scholar] [CrossRef] [PubMed]
- Møller, M.S.; Fredslund, F.; Majumder, A.; Nakai, H.; Poulsen, J.-C.N.; Lo Leggio, L.; Svensson, B.; Abou Hachem, M. Enzymology and structure of the GH13_31 glucan 1,6-α-glucosidase that confers isomaltooligosaccharide utilization in the probiotic Lactobacillus acidophilus NCFM. J. Bacteriol. 2012, 194, 4249–4259. [Google Scholar] [CrossRef] [PubMed]
- Petrova, P.; Petrov, K. Prebiotic–probiotic relationship: The genetic fundamentals of polysaccharides conversion by Bifidobacterium and Lactobacillus genera. In Food Bioconversion; Elsevier: Amsterdam, The Netherlands, 2017; Volume 2, pp. 237–278. ISBN 9780128114131. [Google Scholar]
- Palaniappan, A.; Emmambux, M.N. The challenges in production technology, health-associated functions, physico-chemical properties and food applications of isomaltooligosaccharides. Crit. Rev. Food Sci. Nutr. 2021, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Kitahata, S.; Yoshimura, Y.; Okada, S. Formation of 6-O-α-maltosylcyclomalto-oligosaccharides from α-maltosyl fluoride and cyclomalto-oligosaccharides by pullulanase. Carbohydr. Res. 1987, 159, 303–313. [Google Scholar] [CrossRef]
- Yoshimura, Y.; Kitahata, S.; Okada, S. Effects of temperature on 6-O-α-maltosyl cyclodextrin production from α-maltosylfluoride and cyclodextrins. Agric. Biol. Chem. 1988, 52, 1655–1659. [Google Scholar] [CrossRef]
- Kitahata, S.; Hashimoto, H.; Koizumi, K. Syntheses of various branched cyclodextrins by transglycosylation. J. Appl. Glycosci. 1994, 41, 449–456. [Google Scholar] [CrossRef]
- Okada, Y.; Koizumi, K.; Kitahata, S. Separation and characterization of five positional isomers of trimaltosyl-cyclomaltoheptaose (trimaltosyl-β-cyclodextrin). Carbohydr. Res. 1994, 254, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Williams, S.J.; Withers, S.G. Glycosyl fluorides in enzymatic reactions. Carbohydr. Res. 2000, 327, 27–46. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.; Park, K.-M.; Choi, K.-H.; Park, C.-S.; Kim, G.-E.; Kim, D.; Cha, J. Molecular cloning and biochemical characterization of a heat-stable type I pullulanase from Thermotoga neapolitana. Enzyme Microb. Technol. 2011, 48, 260–266. [Google Scholar] [CrossRef] [PubMed]
- Lobov, S.V.; Kasai, R.; Ohtani, K.; Tanaka, O.; Yamasaki, K. Enzymic production of sweet stevioside derivatives: Transglucosylation by glucosidases. Agric. Biol. Chem. 1991, 55, 2959–2965. [Google Scholar] [CrossRef]
- McDougall, G.J.; Ross, H.A.; Swanston, J.S.; Davies, H.V. Limit dextrinase from germinating barley has endotransglycosylase activity, which explains its activation by maltodextrins. Planta 2004, 218, 542–551. [Google Scholar] [CrossRef]
- MacGregor, A.W.; Bazin, S.L.; Schroeder, S. Effect of starch hydrolysis products on the determination of limit dextrinase and limit dextrinase inhibitors in barley and malt. J. Cereal Sci. 2002, 35, 17–28. [Google Scholar] [CrossRef]
- Mangan, D.; McCleary, B.V.; Cornaggia, C.; Ivory, R.; Rooney, E.; McKie, V. Colourimetric and fluorimetric substrates for the assay of limit dextrinase. J. Cereal Sci. 2015, 62, 50–57. [Google Scholar] [CrossRef]
- Watanabe, Y.; Makino, Y.; Omichi, K. Fluorogenic substrates of glycogen debranching enzyme for assaying debranching activity. Anal. Biochem. 2005, 340, 279–286. [Google Scholar] [CrossRef]
- Wallenfels, K.; Földi, P.; Niermann, H.; Bender, H.; Linder, D. The enzymic synthesis, by transglucosylation of a homologous series of glycosidically substituted malto-oligosaccharides, and their use as amylase substrates. Carbohydr. Res. 1978, 61, 359–368. [Google Scholar] [CrossRef]
- Domon, B.; Costello, C.E. A systematic nomenclature for carbohydrate fragmentations in FAB-MS/MS spectra of glycoconjugates. Glycoconj. J. 1988, 5, 397–409. [Google Scholar] [CrossRef]
- Čmelík, R.; Štikarovská, M.; Chmelík, J. Different behavior of dextrans in positive-ion and negative-ion mass spectrometry. J. Mass Spectrom. 2004, 39, 1467–1473. [Google Scholar] [CrossRef] [PubMed]
- Abdullah, M.; French, D. Substrate specificity of pullulanase. Arch. Biochem. Biophys. 1970, 137, 483–493. [Google Scholar] [CrossRef] [PubMed]
- Kitahata, S.; Tanimoto, T.; Ikuta, A.; Tanaka, A.; Fujita, K.; Hashimoto, H.; Murakami, H.; Nakano, H.; Koizumi, K. Synthesis of novel heterobranched β-cyclodextrins from 4^2-O-β-D-galactosyl-maltose and β-cyclodextrin by the reverse action of pullulanase and isolation and characterization of the products. Biosci. Biotechnol. Biochem. 2000, 64, 1223–1229. [Google Scholar] [CrossRef] [PubMed]
- Desmet, T.; Soetaert, W.; Bojarová, P.; Kařen, V.; Dijkhuizen, L.; Eastwick-Field, V.; Schiller, A. Enzymatic glycosylation of small molecules: Challenging substrates require tailored catalysts. Chem. A Eur. J. 2012, 18, 10786–10801. [Google Scholar] [CrossRef]
- Lammerts van Bueren, A.; Ficko-Blean, E.; Pluvinage, B.; Hehemann, J.-H.; Higgins, M.A.; Deng, L.; Ogunniyi, A.D.; Stroeher, U.H.; El Warry, N.; Burke, R.D.; et al. The conformation and function of a multimodular glycogen-degrading pneumococcal virulence factor. Structure 2011, 19, 640–651. [Google Scholar] [CrossRef]
- Danby, P.M.; Withers, S.G. Advances in enzymatic glycoside synthesis. ACS Chem. Biol. 2016, 11, 1784–1794. [Google Scholar] [CrossRef]
- Teze, D.; Zhao, J.; Wiemann, M.; Kazi, Z.G.A.; Lupo, R.; Zeuner, B.; Vuillemin, M.; Rønne, M.E.; Carlström, G.; Duus, J.; et al. Rational enzyme design without structural knowledge: A sequence-based approach for efficient generation of transglycosylases. Chem. A Eur. J. 2021, 27, 10323–10334. [Google Scholar] [CrossRef] [PubMed]
- Arreola-Barroso, R.A.; Llopiz, A.; Olvera, L.; Saab-Rincón, G. Modulating glycoside hydrolase activity between hydrolysis and transfer reactions using an evolutionary approach. Molecules 2021, 26, 6586. [Google Scholar] [CrossRef] [PubMed]
- Tovborg, M. Structure and Function of Barley Limit Dextrinase & Mutational Analysis of Barley α-amylase Isozyme Differences and Interaction with Branched Oligosaccharides. Ph.D. Thesis, University of Southern Denmark, Odense, Denmark, 2002. [Google Scholar]
- Møller, M.S.; Windahl, M.S.; Sim, L.; Bøjstrup, M.; Abou Hachem, M.; Hindsgaul, O.; Palcic, M.; Svensson, B.; Henriksen, A. Oligosaccharide and substrate binding in the starch debranching enzyme barley limit dextrinase. J. Mol. Biol. 2015, 427, 1263–1277. [Google Scholar] [CrossRef] [PubMed]
- Manners, D.J.; Yellowlees, D. Studies on carbohydrate metabolising enzymes. Part XXVI. The limit dextrinase from germinated barley. Starch Stärke 1971, 136, 228–234. [Google Scholar] [CrossRef]
- Vester-Christensen, M.B.; Abou Hachem, M.; Naested, H.; Svensson, B. Secretory expression of functional barley limit dextrinase by Pichia pastoris using high cell-density fermentation. Protein Expr. Purif. 2010, 69, 112–119. [Google Scholar] [CrossRef] [PubMed]
- Vester-Christensen, M.B.; Abou Hachem, M.; Svensson, B.; Henriksen, A. Crystal structure of an essential enzyme in seed starch degradation: Barley limit dextrinase in complex with cyclodextrins. J. Mol. Biol. 2010, 403, 739–750. [Google Scholar] [CrossRef] [PubMed]
- Challa, R.; Ahuja, A.; Ali, J.; Khar, R.K. Cyclodextrins in drug delivery: An updated review. AAPS PharmSciTech 2005, 6, 329–357. [Google Scholar] [CrossRef]
- Szente, L.; Szejtli, J. Cyclodextrins as food ingredients. Trends Food Sci. Technol. 2004, 15, 137–142. [Google Scholar] [CrossRef]
- Sharma, N.; Baldi, A. Exploring versatile applications of cyclodextrins: An overview. Drug. Deliv. 2016, 23, 739–757. [Google Scholar] [CrossRef]
- Oudjeriouat, N.; Moreau, Y.; Santimone, M.; Svensson, B.; Marchis-Mouren, G.; Desseaux, V. On the mechanism of α-amylase. Acarbose and cyclodextrin inhibition of barley amylase isozymes. Eur. J. Biochem. 2003, 270, 3871–3879. [Google Scholar] [CrossRef]
- Borges de Melo, E.; da Silveira Gomes, A.; Carvalho, I. α- and β-glucosidase inhibitors: Chemical structure and biological activity. Tetrahedron 2006, 62, 10277–10302. [Google Scholar] [CrossRef]
- Lillelund, V.H.; Jensen, H.H.; Liang, X.; Bols, M. Recent developments of transition-state analogue glycosidase inhibitors of non-natural product origin. Chem. Rev. 2002, 102, 515–553. [Google Scholar] [CrossRef]
- Woo, E.-J.; Lee, S.; Cha, H.; Park, J.-T.; Yoon, S.-M.; Song, H.-N.; Park, K.-H. Structural insight into the bifunctional mechanism of the glycogen-debranching enzyme TreX from the archaeon Sulfolobus solfataricus. J. Biol. Chem. 2008, 283, 28641–28648. [Google Scholar] [CrossRef] [PubMed]
- Schumacher, M.A.; Wörmann, M.E.; Henderson, M.; Salinas, R.; Latoscha, A.; Al-Bassam, M.M.; Singh, K.S.; Barclay, E.; Gunka, K.; Tschowri, N. Allosteric regulation of glycogen breakdown by the second messenger cyclic di-GMP. Nat. Commun. 2022, 13, 5834. [Google Scholar] [CrossRef] [PubMed]
- Pelletier, G.; Zwicker, A.; Allen, C.L.; Schepartz, A.; Miller, S.J. Aqueous glycosylation of unprotected sucrose employing glycosyl fluorides in the presence of calcium ion and trimethylamine. J. Am. Chem. Soc. 2016, 138, 3175–3182. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vester-Christensen, M.B.; Holck, J.; Rejzek, M.; Perrin, L.; Tovborg, M.; Svensson, B.; Field, R.A.; Møller, M.S. Exploration of the Transglycosylation Activity of Barley Limit Dextrinase for Production of Novel Glycoconjugates. Molecules 2023, 28, 4111. https://doi.org/10.3390/molecules28104111
Vester-Christensen MB, Holck J, Rejzek M, Perrin L, Tovborg M, Svensson B, Field RA, Møller MS. Exploration of the Transglycosylation Activity of Barley Limit Dextrinase for Production of Novel Glycoconjugates. Molecules. 2023; 28(10):4111. https://doi.org/10.3390/molecules28104111
Chicago/Turabian StyleVester-Christensen, Malene Bech, Jesper Holck, Martin Rejzek, Léa Perrin, Morten Tovborg, Birte Svensson, Robert A. Field, and Marie Sofie Møller. 2023. "Exploration of the Transglycosylation Activity of Barley Limit Dextrinase for Production of Novel Glycoconjugates" Molecules 28, no. 10: 4111. https://doi.org/10.3390/molecules28104111
APA StyleVester-Christensen, M. B., Holck, J., Rejzek, M., Perrin, L., Tovborg, M., Svensson, B., Field, R. A., & Møller, M. S. (2023). Exploration of the Transglycosylation Activity of Barley Limit Dextrinase for Production of Novel Glycoconjugates. Molecules, 28(10), 4111. https://doi.org/10.3390/molecules28104111







