Application of Alkali Lignin and Spruce Sawdust for the Effective Removal of Reactive Dyes from Model Wastewater
Abstract
1. Introduction
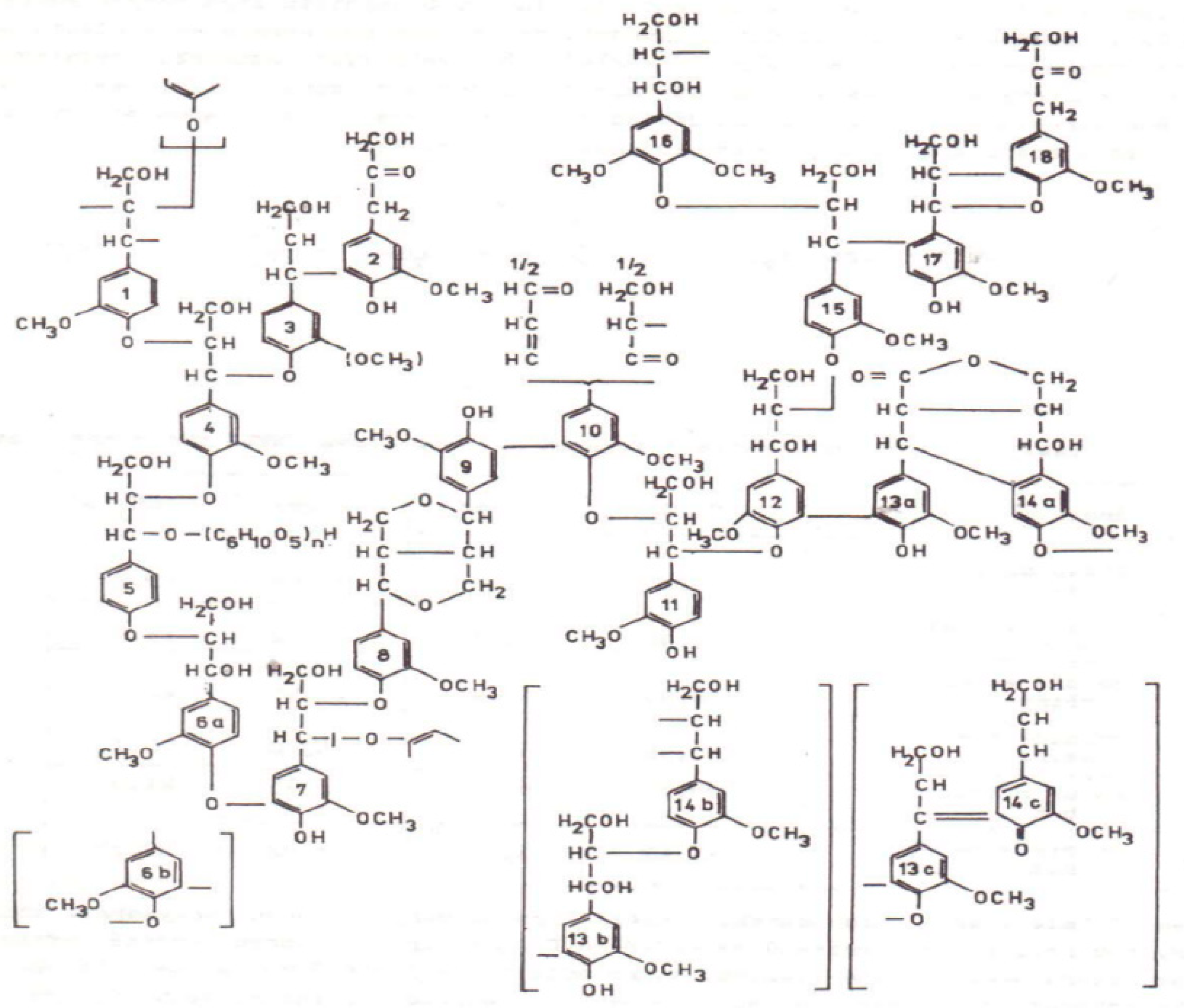
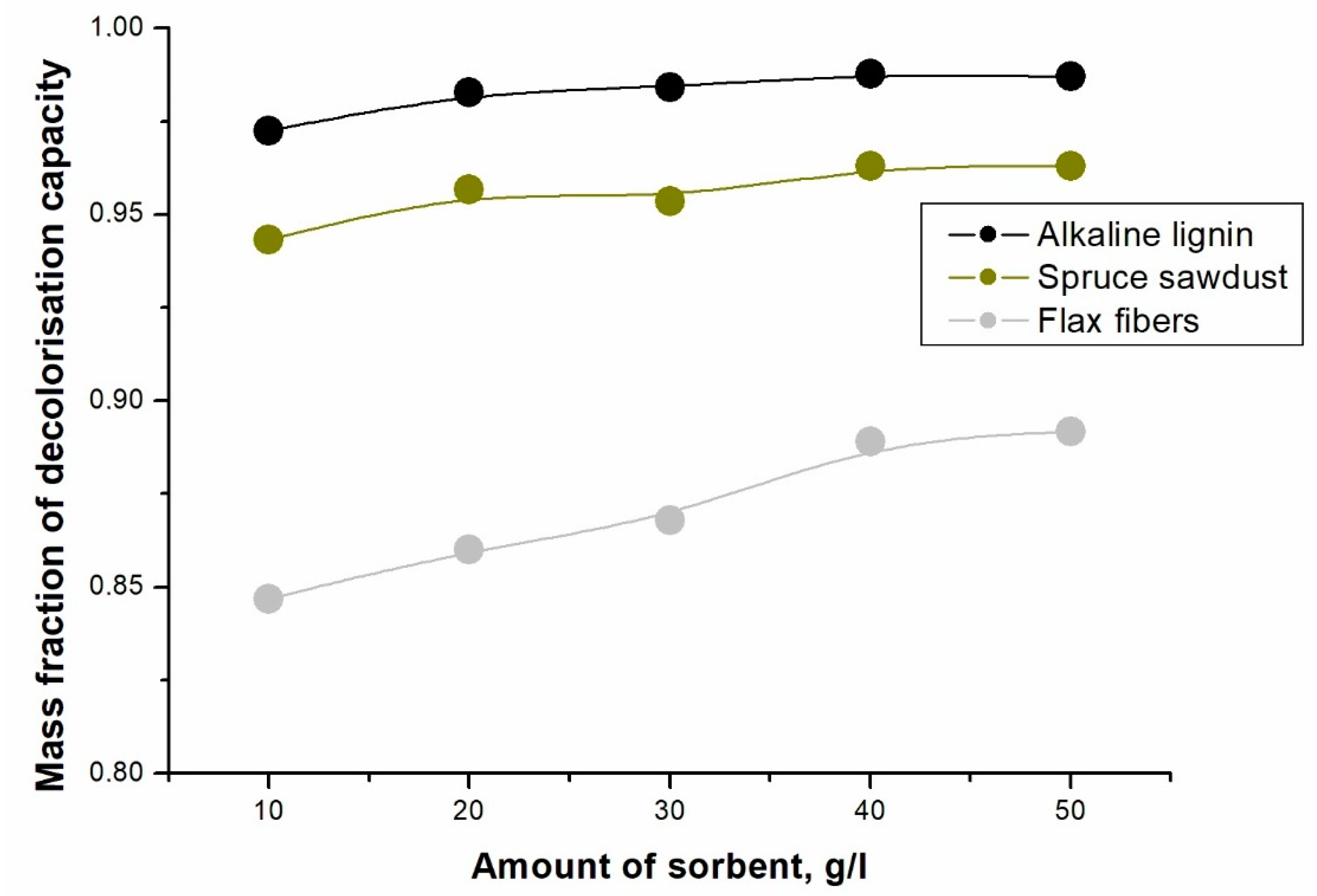
2. Results and Discussion
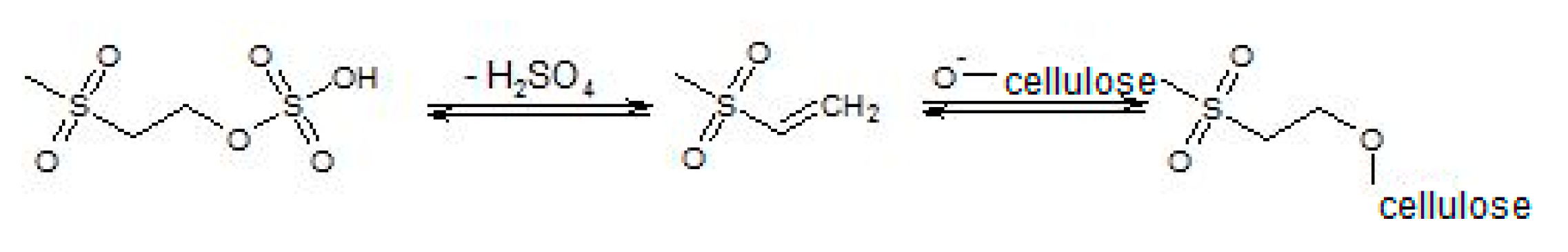
3. Materials and Method
3.1. Materials
3.1.1. Spruce Sawdust
3.1.2. Black Liquor
3.1.3. Dyes
3.2. Experimental Methods
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Dorieh, A.; Khan, A.; Selakjani, P.P.; Pizzi, A.; Hasankhah, A.; Meraj, M.; Pirouzram, O.; Abatari, M.N.; Movahed, S.G. Influence of wood leachate industrial waste as a novel catalyst for the synthesis of UF resins and MDF bonded with them. Int. J. Adhes. Adhes. 2021, 111, 102985. [Google Scholar] [CrossRef]
- Robinson, T.; McMullan, G.; Marchant, R.; Nigam, P. Remediation of dyes in textile effluent: A critical review on current treatment technologies with a proposed alternative. Bioresour. Technol. 2001, 77, 247–255. [Google Scholar] [CrossRef]
- Bassyouni, D.G.; Hamad, H.A.; El-Ashtoukhy, E.S.Z.; Amin, N.K.; Abd El-Latif, M.M. Comparative performance of anodic oxidation and electrocoagulation as clean processes for electrocatalytic degradation of diazo dye Acid Brown 14 in aqueous medium. J. Hazard. Mater. 2017, 335, 178–187. [Google Scholar] [CrossRef] [PubMed]
- Yakubu, I.S.; Muhammad, A.R.; Lawan, U. Determination of heavy metals in tannery effluent. Int. J. Adv. Acad. Res. 2018, 4, 132–136. [Google Scholar]
- Bassat, I.M.; Nigam, P.; Singh, D.; Marchant, R. Microbial decolorization of textile-dye-containing effluents: A review. Bioresour. Technol. 1996, 58, 217–227. [Google Scholar]
- La Farre, M.; Pe’rez, S.; Kantiani, L.; Barcelo’, D. Fate and toxicity of emerging pollutants, their metabolites and transformation products in the aquatic environment. Trend Anal. Chem. 2008, 11, 991–1007. [Google Scholar] [CrossRef]
- Akceylan, E.; Bahadir, M.; Yılmaz, M. Removal efficiency of a calix[4]arene-based polymer for water-soluble carcinogenic direct azo dyes and aromatic amines. J. Hazard. Mater. 2009, 162, 960–966. [Google Scholar] [CrossRef]
- Weber, J.; Stickney, V.C. Hydrolysis kinetics of reactive blue 19-vinyl sulfone. Water Res. 1993, 27, 63–67. [Google Scholar] [CrossRef]
- Chung, T.; Fulk, G.E.; Andres, A.W. Mutagenicity testing of some commonly used dyes. Appl. Environ. Microbiol. 1981, 42, 641–648. [Google Scholar] [CrossRef]
- Kavitha, D.; Namasivayam, C. Capacity of activated carbon in the removal of acid brilliant blue: Determination of equilibrium and kinetic model parameters. Chem. Eng. J. 2008, 139, 453–461. [Google Scholar] [CrossRef]
- Dai, S.; Zhuang, Y.; Chen, Y.; Chen, L. Study on the relationship between structure of synthetic organic chemicals and their biodegradability. Environ. Chem. 1995, 14, 354–367. [Google Scholar]
- Koprivanac, N.; Bozic, A.L.; Papic, S. Cleaner production processes in the synthesis of blue anthraquinone reactive dyes. Dye. Pigment. 2000, 44, 33–40. [Google Scholar] [CrossRef]
- Vaidya, A.A.; Datye, K.V. Environmental pollution during chemical processing of synthetic fibres. Colorage 1982, 14, 3–10. [Google Scholar]
- Soleymani Lashkenrai, A.; Najafi, M.; Peyravi, M.; Jahanshahi, M.; Mosavian, M.T.H.; Amiri, A.; Shahavi, M.H. Direct filtration procedure to attain antibacterial TFC membrane: A facile developing route of membrane surface properties and fouling resistance. Chem. Eng. Res. Des. 2009, 149, 158–168. [Google Scholar] [CrossRef]
- Alencar, W.S.; Lima, E.C.; Royer, B.; dos Santos, B.D.; Calvete, T.; da Silva, E.A.; Alves, C.N. Application of aqai stalks as biosorbents for the removal of the dye Procion Blue MX-R from aqueous solution. Sep. Sci. Technol. 2012, 47, 513–526. [Google Scholar] [CrossRef]
- Calvete, T.; Lima, E.C.; Cardoso, N.F.; Dias, S.L.P.; Pavan, F.A. Application of carbon adsorbents prepared from the Brazilian-pine fruit shell for removal of Procion Red MX 3B from aqueous solution—Kinetic, equilibrium, and thermodynamic studies. Chem. Eng. J. 2009, 155, 627–636. [Google Scholar] [CrossRef]
- Cardoso, N.F.; Lima, E.C.; Pinto, I.S.; Amavisca, C.V.; Royer, B.; Pinto, R.B.; Alencar, W.S.; Pereira, S.F.P. Application of cupuassu shell as biosorbent for the removal of textile dyes from aqueous solution. J. Environ. Manag. 2011, 92, 1237–1247. [Google Scholar] [CrossRef] [PubMed]
- Cardoso, N.F.; Lima, E.C.; Royer, B.; Bach, M.V.; Dotto, G.L.; Pinto, L.A.A.; Calvete, T. Comparison of Spirulina platensis microalgae and commercial activated carbon as adsorbents for the removal of Reactive Red 120 dye from aqueous effluents. J. Hazard. Mater. 2012, 241–242, 146–153. [Google Scholar] [CrossRef] [PubMed]
- Zaidi, N.A.H.M.; Lim, L.B.L.; Usman, A.; Kooh, M.R.R. Efficient adsorption of malachite green dye using Artocarpus odoratissimus leaves with artificial neural network modelling. Desalination Water Treat. 2018, 101, 313–324. [Google Scholar] [CrossRef]
- Lu, Y.C.; Kooh, M.R.R.; Lim, L.B.L.; Priyantha, N. Effective and Simple NaOH-Modification Method to Remove Methyl Violet Dye via Ipomoea aquatic Roots. Adsorpt. Sci. Technol. 2021, 2021, 593222. [Google Scholar] [CrossRef]
- Boerjan, W.; Ralph, J.; Baucher, M. Lignin biosynthesis. Annu. Rev. Plant Biol. 2003, 54, 519–546. [Google Scholar] [CrossRef]
- Ralph, J.; Lundquist, K.; Brunow, G.; Lu, F.; Kim, H.; Schatz, P.F.; Marita, J.M.; Hatfield, R.D.; Ralph, S.A.; Christensen, J.H. Lignins: Natural polymers from oxidative coupling of 4-hydroxyphenylpropanoids. Phytochem. Rev. 2004, 3, 29–60. [Google Scholar] [CrossRef]
- Vanholme, R.; Demedts, B.; Morreel, K.; Ralph, J.; Boerjan, W. Lignin Biosynthesis and Structure. Plant Physiol. 2010, 153, 895–905. [Google Scholar] [CrossRef] [PubMed]
- Pieter, J.; Fredrik, B.; Riwu Kaho, M. Valuasi ekonomi ekowisata terhadap pengembangan objek wisata kawasan pesisir pantai. J. Ilmu Lingkung. 2015, 13, 55–64. [Google Scholar] [CrossRef]
- Červenka, E.; Král, Z.; Tomis, B. Wood and Cellulose Chemistry; University of Chemistry and Technology Pardubice: Pardubice, Czech Republic, 1980; p. 228. (In Czech) [Google Scholar]
- Wu, X.; Jiang, J.; Wang, C.; Liu, J.; Pu, Y.; Ragauskas, A.; Li, S.; Yang, B. Lignin-derived electrochemical energy materials and system. Biofuels Bioprod. Biorefin. 2020, 14, 650–672. [Google Scholar] [CrossRef]
- Kulas, D.G.; Thies, M.C.; Shonnard, D.R. Techno-economic analysis and life cycle assessment of waste lignin fractionantion and valorization using the ALPHA process. ACS Sustain. Chem. Eng. 2021, 9, 5388–5395. [Google Scholar] [CrossRef]
- Kazzaz, A.E.; Fatehi, P. Technical lignin and its potential modification routes: A mini-review. Ind. Crops Prod. 2020, 154, 112732. [Google Scholar] [CrossRef]
- Gondaliya, A.; Nejad, M. Lignin as a partial polyol replacement in polyurethane flexible foam. Molecules 2021, 26, 2302. [Google Scholar] [CrossRef]
- Arefmanesh, M.; Vuong, T.V.; Nikafshar, S.; Wallmo, H.; Nejad, M.; Master, E.R. Enzymatic synthesis of kraft lignin-acrylate copolymers using an alkaline tolerant laccase. Appl. Microbiol. Biotechnol. 2022, 106, 2969–2979. [Google Scholar] [CrossRef]
- Cifuentes, A.R.; Avila, K.; García, J.C.; Daza, C.E. The pyrolysis of rose stems to obtain activated carbons: A study on the adsorption of Ni(II). Ind. Eng. Chem. Res. 2013, 52, 16197–16205. [Google Scholar] [CrossRef]
- Can, M. Investigation of the factors affecting acid blue 256 adsorption from aqueous solutions onto red pine sawdust: Equilibrium, kinetics, process design, and spectroscopic analysis. Desalination Water Treat. 2015, 57, 5636–5653. [Google Scholar] [CrossRef]
- Wahab, M.A.; Jellali, S.; Jedidi, N. Ammonium biosorption onto sawdust: FTIR analysis, kinetics and adsorption isotherms modelling. Bioresour. Technol. 2010, 101, 5070–5075. [Google Scholar] [CrossRef]
- Zou, W.; Bai, H.; Gao, S.; Li, K. Characterization of modified sawdust, kinetic and equilibrium study about methylene blue adsorption in batch mode. Korean J. Chem. Eng. 2013, 30, 111–122. [Google Scholar] [CrossRef]
- Weidlich, T. Use of Organic-Based Reactants Halogen Derivatives for Obtaining Chemical Specialties Produced in the Czech Republic and New Options for Limiting Them Emissions. Proffesor’s Thesis, University of Pardubice, Pardubice, Czech Republic, 2013. (In Czech). [Google Scholar]
- Filipi, M.; Dušek, L. The use of spruce, birch, flax and rapeseed for the removal of vinyl sulfone dyes from wastewater. Rostlinolékař 2018, 3, 26–29. (In Czech) [Google Scholar]
- Alkan, M.; Dogan, M. Adsorption kinetics of Victoria blue onto perlite. Fresenius Environ. Bull. Adv. Food Sci. 2003, 125, 418–428. [Google Scholar]
- Salleh, M.A.M.; Mahmoud, D.K.; Karim, W.A.; Idris, A. Cationic and anionic dye adsorption by agricultural solid wastes: A comprehensive review. Desalination 2011, 280, 1–13. [Google Scholar] [CrossRef]
- Senthilkumaar, S.; Kalaamani, P.; Subburaam, C.V. Liquid phase adsorption of crystal violet onto activated carbons derivated from male flowers of coconut tree. J. Hazard. Mater. 2006, 136, 800–808. [Google Scholar] [CrossRef]
- Hema, M.; Arivoli, S. Comparative study on the adsorption kinetics and thermodynamics of dyes onto acid activated low-cost carbon. Int. J. Phys. Sci. 2007, 2, 10–17. [Google Scholar]
- Tappi Test Methods; Tappi Press Atlanta: Atlanta, Georgia, 2004.
- Bharathi, K.S.; Ramesh, S.T. Removal of dyes using agriculture waste as low-cost adsorbents: A review. Appl. Water Sci. 2013, 3, 773–790. [Google Scholar] [CrossRef]
- Ofomaja, A.E.; Ho, Y.S. Equilibrium sorption of anionic dye from aqueous solution by palm kernel fibre as sorbent. Dye. Pigment. 2007, 74, 60–66. [Google Scholar] [CrossRef]
- Prola, L.D.T.; Machado, F.M.; Bergmann, C.P.; de Souza, F.E.; Gally, C.R.; Lima, E.C.; Adebyao, M.A.; Dias, S.L.P.; Calvete, T. Adsorption of Direct Blue 53 dye from aqueous solutions by multi-walled carbon nanotubes and activated carbon. J. Environ. Manag. 2013, 130, 166–175. [Google Scholar] [CrossRef] [PubMed]
- Filipi, M.; Milichovský, M. Adsorption Organic Cationic Dyes on Oxycelluloses and Linters. J. Encapsulation Adsorpt. Sci. 2014, 4, 43450. [Google Scholar] [CrossRef]

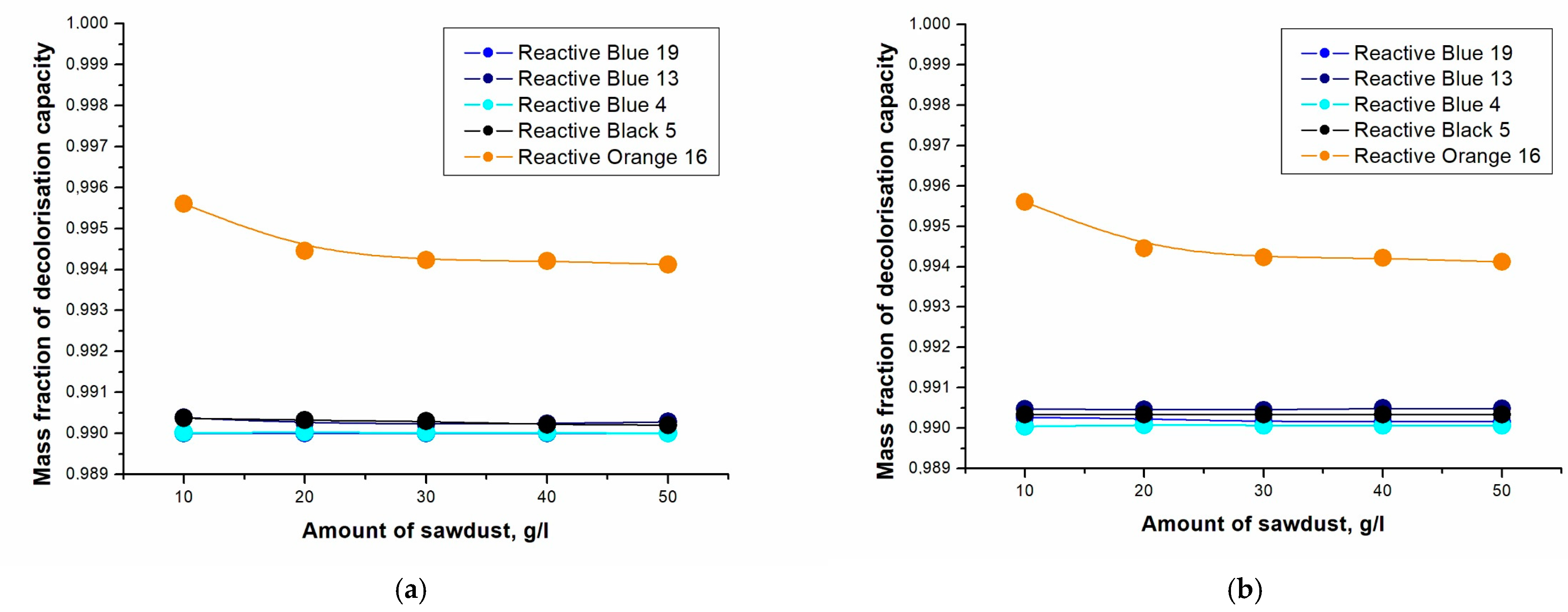
| Sorbent | Dyes | Decolorization Equation |
|---|---|---|
| Alkaline lignin unheated sample | Reactive blue 13 | X = 0.9803ρ + 0.0036 |
| Reactive blue 19 | X = 0.9900ρ + 0.0002 | |
| Reactive blue 4 | X = 0.9801ρ + 0.0004 | |
| Reactive black 5 | X = 0.9804ρ + 0.0026 | |
| Reactive orange 16 | X = 0.9945ρ + 0.0203 | |
| Alkaline lignin heated sample | Reactive blue 13 | X = 0.9902ρ + 0.0025 |
| Reactive blue 19 | X = 0.9900ρ + 0.0001 | |
| Reactive blue 4 | X = 0.9900ρ + 0.0001 | |
| Reactive black 5 | X = 0.9901ρ + 0.0036 | |
| Reactive orange 16 | X = 0.9940ρ + 0.0202 | |
| Spruce sawdust unheated sample | Reactive blue 13 | X = 0.9903ρ + 0.0000 |
| Reactive blue 19 | X = 0.9900ρ + 0.0000 | |
| Reactive blue 4 | X = 0.9900ρ + 0.0000 | |
| Reactive black 5 | X = 0.9902ρ + 0.0001 | |
| Reactive orange 16 | X = 0.9938ρ + 0.0008 | |
| Spruce sawdust heated sample | Reactive blue 13 | X = 0.9905ρ + 0.0000 |
| Reactive blue 19 | X = 0.9901ρ + 0.0000 | |
| Reactive blue 4 | X = 0.9901ρ + 0.0000 | |
| Reactive black 5 | X = 0.9903ρ + 0.0000 | |
| Reactive orange 16 | X = 0.9938ρ + 0.0008 |
| Properties of Black Liquor | Amount in Black Liquor |
|---|---|
| Total dry matter content, % | 42.0 |
| Ash content of total dry matter, % | 27.3 |
| Content of organic substances from total dry matter, % | 14.7 |
| Concentration of alkali lignin, g∙dm−3 | 45.0 |
| Sodium concentration, g∙dm−3 | 39.0 |
| Chemical oxygen consumption ρ(O2), g∙dm−3 | 170.0 |
| Density, kg∙m−3 | 1062.0 |
| Viscosity, mPa∙s | 1.4 |
| Surface tension, mN∙m−1 | 55.2 |
| pH value | 12.1 |
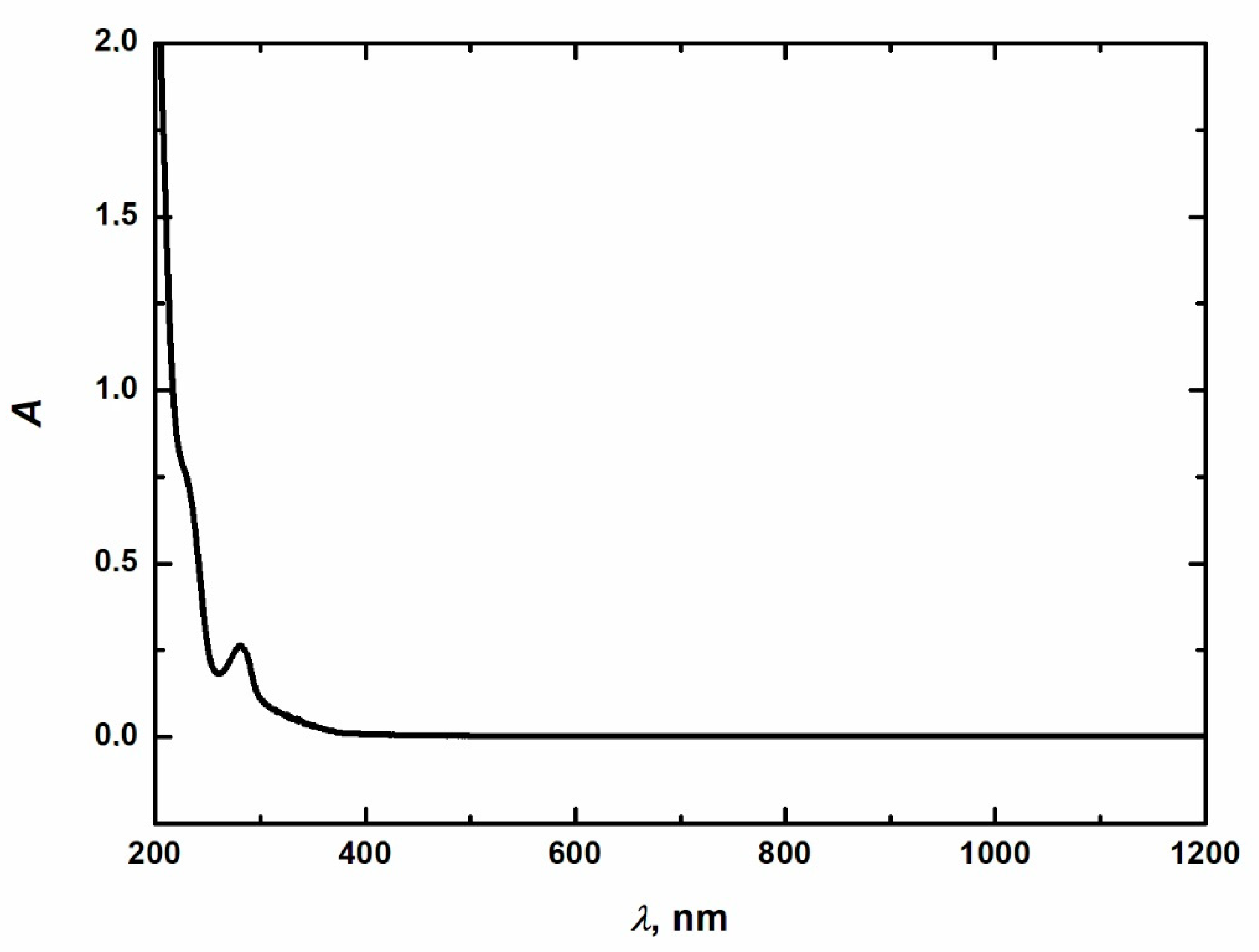
| Reactive blue 13 | Dye content 100 wt.%, Amax = 682 nm | 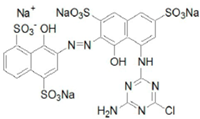 |
| Reactive blue 19 | Dye content 50 wt.%, Amax = 684 nm | 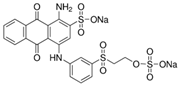 |
| Reactive blue 4 | Dye content 35 wt.%, Amax = 595 nm | 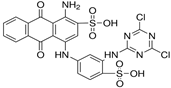 |
| Reactive black 5 | Dye content 50 wt.%, Amax = 597 nm | 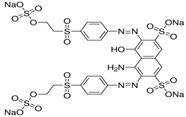 |
| Reactive orange 16 | Dye content 70 wt.%, Amax = 494 nm | 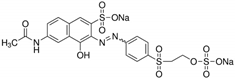 |
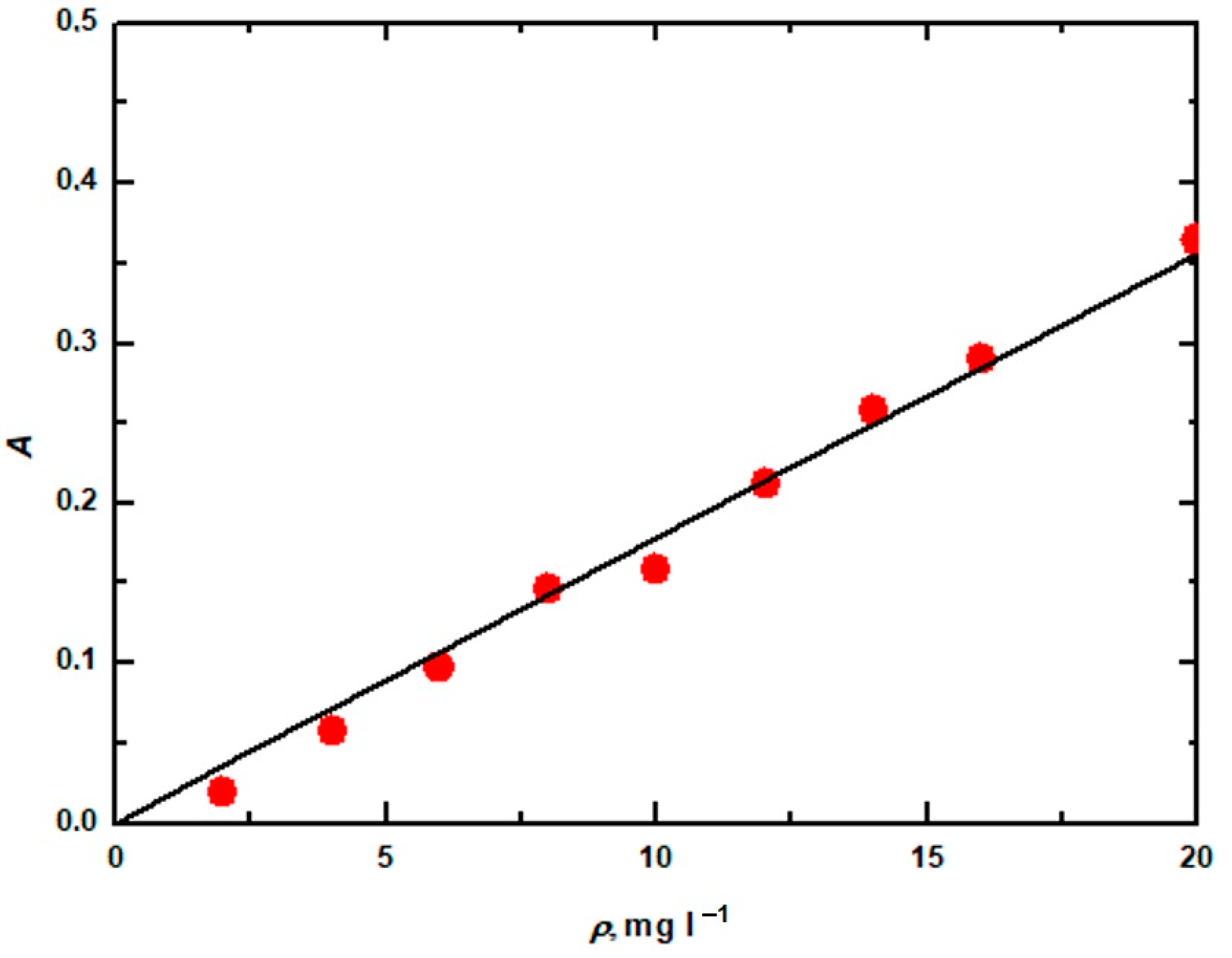
| Dyes | Concentration, mol∙L−1 | Calibration Curve Equation |
|---|---|---|
| Reactive blue 13 | 0.01 | ρ = 8317.7A + 0.184 |
| Reactive blue 19 | 0.01 | ρ = 11,686A + 0.498 |
| Reactive blue 4 | 0.01 | ρ = 20,886A + 0.511 |
| Reactive black 5 | 0.01 | ρ = 2712A + 0.019 |
| Reactive orange 16 | 0.007 | ρ = 2086.6A + 0.435 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hájková, K.; Filipi, M.; Fojtík, R.; Dorieh, A. Application of Alkali Lignin and Spruce Sawdust for the Effective Removal of Reactive Dyes from Model Wastewater. Molecules 2023, 28, 4114. https://doi.org/10.3390/molecules28104114
Hájková K, Filipi M, Fojtík R, Dorieh A. Application of Alkali Lignin and Spruce Sawdust for the Effective Removal of Reactive Dyes from Model Wastewater. Molecules. 2023; 28(10):4114. https://doi.org/10.3390/molecules28104114
Chicago/Turabian StyleHájková, Kateřina, Michaela Filipi, Roman Fojtík, and Ali Dorieh. 2023. "Application of Alkali Lignin and Spruce Sawdust for the Effective Removal of Reactive Dyes from Model Wastewater" Molecules 28, no. 10: 4114. https://doi.org/10.3390/molecules28104114
APA StyleHájková, K., Filipi, M., Fojtík, R., & Dorieh, A. (2023). Application of Alkali Lignin and Spruce Sawdust for the Effective Removal of Reactive Dyes from Model Wastewater. Molecules, 28(10), 4114. https://doi.org/10.3390/molecules28104114







