Abstract
An N-heterocyclic carbene (NHC)-catalyzed atroposelective annulation reaction is disclosed for quick and efficient access to thiazine derivatives. A series of axially chiral thiazine derivatives bearing various substituents and substitution patterns were produced in moderate to high yields with moderate to excellent optical purities. Preliminary studies revealed that some of our products exhibit promising antibacterial activities against Xanthomonas oryzae pv. oryzae (Xoo) that causes rice bacterial blight.
1. Introduction
Thiazine is a privileged structure that exists in a good number of natural products, medicines, and agrochemicals with proven biological activities (Figure 1a) [1,2,3,4,5]. For example, thiazine-containing cephalosporins such as cefalexin [6] have been used to cure or prevent bacterial infections. Dazomet [7] is a commercially available insecticide used to protect crops from various pests. Omonasteine [8] bearing a hydrothiazine fragment can be used in the curing of coronary heart diseases. Some molecules containing thiazine moiety have also shown potential antifungal or antibacterial activities in the field of pesticides [9,10,11]. Therefore, the development of quick and efficient strategies to construct thiazine scaffolds from readily available chemicals is of particular interest and has certainly attracted considerable attention in organic synthesis [12,13,14,15,16,17,18,19]. On the other hand, axial chirality is a common phenomenon in modern organic synthesis and living systems. Axially chiral molecules have found wieldy applications in catalysts [20,21], ligands [22,23], and medicines [24,25]. The incorporation of thiazine moiety into the axial skeletons would result in unique physical and chemical properties and will certainly bring wide potential applications.
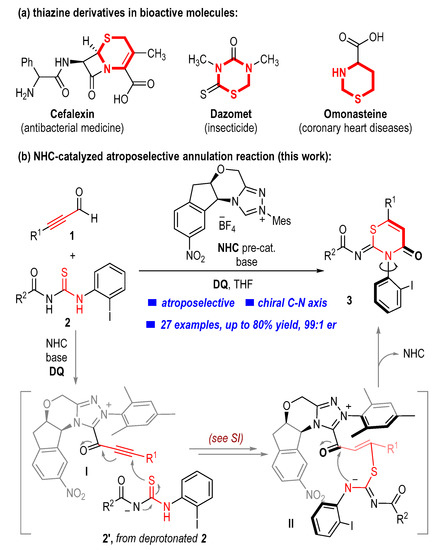
Figure 1.
Bioactive molecules containing thiazine rings and our project design.
N-heterocyclic carbenes (NHCs) have emerged as powerful organocatalysts for the asymmetric synthesis of structurally diverse heterocyclic molecules due to their unique Lewis basicity and nucleophilicity [26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49]. However, most of the chiral compounds are provided by the introduction of point-chiral centers. On the contrary, the chiral molecules with axial chirality obtained from asymmetric synthesis enabled by NHC organocatalysis are still relatively scarce and challenging [50,51,52,53]. Based on the continuous interest in harnessing NHC catalysis for asymmetric construction of bioactive chiral molecules, we herein report a carbene-catalyzed atroposelective annulation of alkynyl aldehyde and benzoylthiourea to produce axially chiral thiazine derivatives (Figure 1b). Key steps of our protocol include the nucleophilic addition of the deprotonated benzoylthiourea 2 to the acetylenic acylazolium intermediate I (generated from alkynyl aldehyde 1 and NHC catalyst) to forge the C-S bond. Subsequent proton transfer and tautomerization of the intermediate I afford an NHC-bound acyl azolium intermediate II, which then undergoes intramolecular nucleophilic addition and elimination steps to deliver the desired axially chiral product and regenerate the NHC catalyst (see SI for detailed proposed reaction mechanism). A series of axially chiral thiazine derivatives bearing various substituents and substitution patterns were produced by using this method in moderate to high yields with moderate to excellent optical purities. Compared to our previously reported atroposelective annulation between alkyl-substituted thioureas and ynals [54], the incorporation of iodine atoms into the axially chiral thiazine derivatives results in better antibacterial activities. For example, some of such axially chiral products exhibit promising antibacterial activities against Xanthomonas oryzae pv. oryzae (Xoo) that causes rice bacterial blight.
2. Results and Discussion
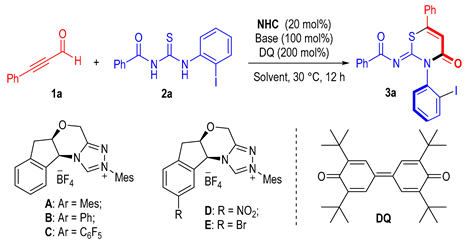
The alkynyl aldehyde 1a and benzoylthiourea 2a were selected as model substrates to evaluate the feasibility of the axially selective cyclization. A typical reaction mixture contained 1a (2 equivalents), 2a (1 equivalent), an NHC (20 mol%) as a catalyst, tetra-tert- butyldiphenylquinone (DQ, 2 equivalents) as the oxidant, and 4-dimethylaminopyridine (DMAP, 1.0 equivalent) as a base in furan as the solvent. Various aminoindanol-derived NHC precatalysts were evaluated (e.g., Table 1, entries 1–5). It was found that the NHC precatalysts bearing electron-rich N-aryl groups [55,56,57] could deliver the thiazine derivative 3a in lower yields with moderate optical purities (entries 1–2). On the contrary, the NHC precatalyst C [58] bearing an electron-poor N-C6F5 group gave only a trace amount of the desired product 3a (entry 3). Installation of a NO2 group (D) [59] or a Br group (E) [60] resulted in similar yields but with slightly improved enantioselectivity (entries 4–5). Then, the effects of base and solvent were explored by using D as the optimal NHC precatalyst. Replacing the DMAP with various organic or inorganic bases resulted in decreased yields, albeit with similar or slightly increased enantioselectivities (entries 6–8). The solvent was proved to have a significant impact on both the reaction outcome and enantioselectivity (entries 9–11). An acceptable yield and high enantioselectivity were obtained when tetrahydrofuran was used as a solvent (entry 9).

Table 1.
Condition optimization [a].
With the optimized reaction conditions in hand, we turned to evaluate the scope of our axially selective annulation reaction. The scope of alkynyl aldehyde (1) was first evaluated by using benzoylthiourea 2a as the model reaction partner (Scheme 1). Various functional groups on the 4-position of the phenyl group of 1a, such as methoxyl (3b), methyl (3c), and halides (3d–3f), were tolerated, giving the corresponding axially chiral products in moderate to good yields with maintained or slightly decreased enantioselectivities, regardless of their electronic nature. Substrates bearing a substituent on the 3-position of the benzene ring proceeded to smoothly produce the target products in moderate yields with good to excellent enantioselectivities. For example, 3h–3i were obtained in 53% and 65% yields, respectively, and a 97:3 enantiomeric ratio. The structure of 3h was further characterized by X-ray single crystal diffraction analysis (Figure 2). The steric effect on the benzene ring has little influence on our reaction since alkynyl aldehydes bearing methyl (3j) or halides (3k–3l) on the 2-position of the benzene ring were all converted to the corresponding products in acceptable yields and with good optical purities. Other aromatic systems such as naphthalene and thiophene were also tolerated under the standard conditions affording 3m–3n in good yields with excellent optical purities.
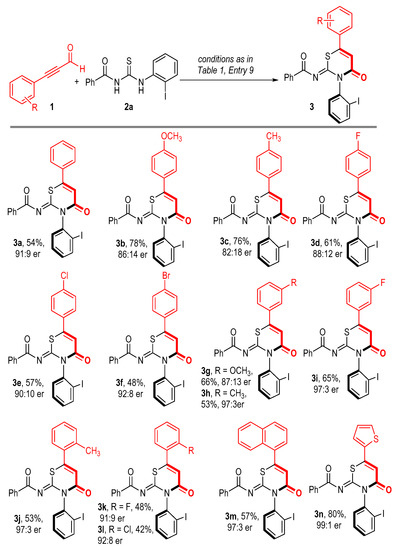
Scheme 1.
Scope of ynals 1 [a]. [a] All reactions were carried out at 30 °C using 1 (0.2 mmol), 2a (0.1 mmol), pre-NHC D (20 mol%), DMAP (1.0 equiv), DQ (0.2 mmol), and 2.0 mL THF for 12 h.
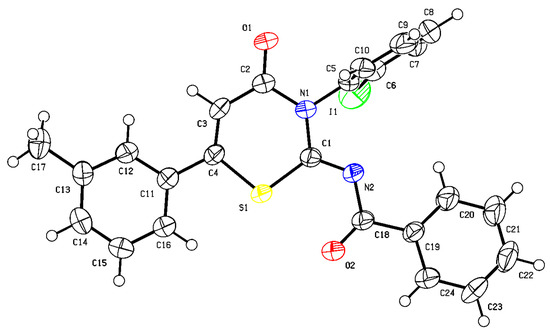
Figure 2.
X-ray crystallography of 3h (black for carbon atoms, green for iodine atom, yellow for sulfur atom, dark blue for nitrogen atoms, and red for oxygen atoms), CCDC number: 2255058.
We then examined the scope of benzoylthiourea 2 using 1a as the model substrate. As disclosed in Scheme 2, both electron-donating (4a–4d) and electron-withdrawing (4e–4m) functional groups can be decorated on the benzene ring of benzoylthiourea 2 to produce the corresponding axially chiral thiazine-containing products with acceptable to good yields and enantioselectivities. Halide atoms including fluorine, chlorine, and bromine were all tolerated to afford halide atom-containing products, regardless of their substituted position (4e–4m). Such halide atom-substituted products provide opportunities for further transformations.
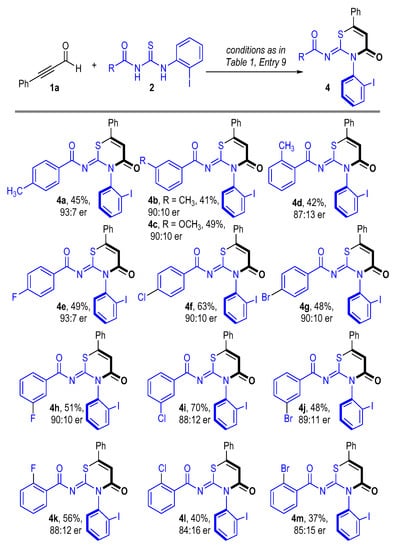
Scheme 2.
Scope of thioureas 2 [a]. [a] All reactions were carried out at 30 °C using 1a (0.2 mmol), 2 (0.1 mmol), pre-NHC D (20 mol%), DMAP (1.0 equiv), DQ (0.2 mmol), and 2.0 mL THF for 12 h.
To test the potential utility of this carbene-catalyzed atroposelective annulation reaction, the model reaction was conducted in 2.0 mmol scale. To our delight, the desired axially chiral 3a was isolated in a maintained yield (52%) without any loss of the enantioselectivity (Figure 3).
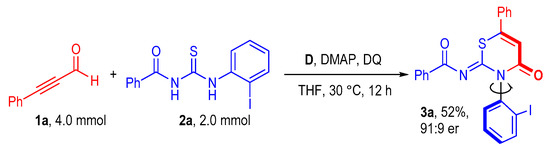
Figure 3.
Up-scaled reaction.
Based on the fantastic bioactivities of the thiazine derivatives and our continuous interest in searching for unique small molecules bearing antiviral and antibacterial activities in agricultural applications, the antibacterial activities of our axially chiral thiazine derivatives against Xanthomonas oryzae pv. oryzae (Xoo) that cause rice bacterial blight [61] were evaluated and are summarized in Table 2. To our delight, many of our chiral products exhibited superior bioactivities to the commercial thiodiazole copper (TC) and bismerthiazol (BT).

Table 2.
Antibacterial activities of the products.
3. Materials and Methods
3.1. General Information
Commercially available materials and dry solvents purchased from Energy Chemical and J&K were used as received. Unless otherwise specified, all reactions were prepared using 4 mL vials. NMR spectra were measured on a Bruker ASCEND (AVANCE III HD 400 MHz) spectrometer. The chemical shift values were corrected to 7.26 ppm (1H NMR) and 77.23 ppm (13C NMR) for CDCl3 or 3.33 ppm (1H NMR) and 39.51 ppm (13C NMR) for DMSO-d6. 1H NMR splitting patterns were designated as singlet (s), double (d), triplet (t), quartet (q), doublet of doublets (dd), multiplets (m), etc. All first-order splitting patterns were assigned on the basis of the appearance of the multiplet. Splitting patterns that could not be easily interpreted are designated as multiplet (m) or broad (br). High-resolution mass spectrometer analysis (HRMS) was performed on a Thermo Fisher Q Exactive mass spectrometer (QTOF mass analyzer). HPLC analyses were measured on Shimadzu Model SIL-20AC220V instruments. Chiralcel brand chiral columns from Daicel Chemical Industries were used with models IA, IB, ODH in 4.6 × 250 mm size. UPLC analyses were measured on Waters systems with an Empower3 system controller, Waters UPLC H-Class, and Waters ACQUITY UPLC PDA detector. Chiralcel brand chiral columns from Daicel Chemical Industries were used with models AD-3, OD-3 in 3.0 × 100 mm size. The racemic products used to determine the er values were synthesized using a racemic catalyst. Optical rotations were measured on an Insmark IP-digi Polarimeter in a 1 dm cuvette. The concentration (c) is given in g/100 mL. Melting points were measured on an uncorrected Beijing Tech Instrument X-4 digital display micro melting point apparatus. Single-crystal X-ray diffraction was recorded at Xcalibur, Eos, Gemini. Analytical thin-layer chromatography (TLC) was carried out on precoated silica gel plates (0.2 mm thickness). Visualization was performed using a UV lamp.
3.2. General Procedure for the Synthesis of Target Compounds 3 or 4
To a 4 mL vial equipped with a magnetic stir bar, chiral NHC precatalyst D (0.02 mmol, 20 mol%, 9.28 mg), DMAP (0.1 mmol, 100 mol%, 12.22 mg), DQ (0.2 mmol, 200 mol%, 81.73 mg), and substituted thiourea 2 (0.1 mmol) were added. After that, THF (2.0 mL) and ynal 1 (0.2 mmol) were added and the reaction mixture was allowed to stir for 12 h at 30 °C. The solution was then concentrated under reduced pressure, and the residue was subjected to column chromatography (petroleum ether/ethyl acetate = 50/1 to 20/1) directly to obtain the products 3 or 4.
- (Z)-N-(3-(2-iodophenyl)-4-oxo-6-phenyl-3,4-dihydro-2H-1,3-thiazin-2-ylidene)benzamide (3a), white solid, 54% yield, 27.5 mg, m.p. 134–136 °C; = +54.3 (c = 0.5 in CHCl3); 1H NMR (400 MHz, CDCl3) δ 8.04 (dd, J = 8.0, 1.4 Hz, 1H), 7.81–7.78 (m, 2H), 7.72–7.69 (m, 2H), 7.58–7.45 (m, 5H), 7.35–7.31 (m, 3H), 7.22 (td, J = 7.6, 1.6 Hz, 1H), 6.94 (s, 1H); 13C NMR (101 MHz, CDCl3) δ 175.6, 161.8, 160.4, 151.6, 141.4, 140.0, 135.0, 134.4, 133.1, 131.8, 130.13, 130.11, 129.7, 129.4, 129.1, 128.3, 126.8, 115.8, 98.0; HRMS (ESI-TOF) m/z: [M + Na]+ calcd for C23H15IN2O2SNa 532.9791, found 532.9786; UPLC analysis: 91:9 er (OD-3 column, 25 °C, hexane/iPrOH = 80/20, 0.5 mL/min, λ = 254 nm), Rt (major) = 25.9 min, Rt (minor) = 33.1 min.
- (Z)-N-(3-(2-iodophenyl)-6-(4-methoxyphenyl)-4-oxo-3,4-dihydro-2H-1,3-thiazin-2-ylidene)benzamide (3b), white solid, 78% yield, 42.3 mg, m.p. 118–120 °C; = +52.5 (c = 1.0 in CHCl3); 1H NMR (400 MHz, CDCl3) δ 8.03 (dd, J = 8.0, 1.4 Hz, 1H), 7.82–7.79 (m, 2H), 7.69–7.65 (m, 2H), 7.55 (td, J = 7.6, 1.4 Hz, 1H), 7.49–7.45 (m, 1H), 7.35–7.31 (m, 3H), 7.21 (td, J = 7.6, 1.6 Hz, 1H), 7.03–7.0 (m, 2H), 6.88 (s, 1H), 3.88 (s, 3H); 13C NMR (101 MHz, CDCl3) δ 175.6, 162.6, 162.0, 160.4, 150.9, 141.5, 139.8, 135.1, 133.0, 130.11, 130.05, 129.6, 129.2, 128.4, 128.3, 126.5, 114.8, 113.9, 98.1, 55.6; HRMS (ESI-TOF) m/z: [M + Na]+ calcd for C24H17IN2O3SNa 562.9897, found 562.9892; HPLC analysis: 86:14 er (AD-3 column, 25 °C, hexane/iPrOH = 80/20, 0.5 mL/min, λ = 254 nm), Rt (major) = 35.2 min, Rt (minor) = 42.3 min.
- (Z)-N-(3-(2-iodophenyl)-4-oxo-6-(p-tolyl)-3,4-dihydro-2H-1,3-thiazin-2-ylidene)benzamide (3c), white solid, 76% yield, 40.0 mg, m.p. 156–158 °C; = +17.3 (c = 1.0 in CHCl3); 1H NMR (400 MHz, CDCl3) δ 8.04 (dd, J = 8.0, 1.4 Hz, 1H), 7.78–7.76 (m, 2H), 7.57 (td, J = 7.6, 1.4 Hz, 1H), 7.49–7.44 (m, 1H), 7.39–7.29 (m, 7H), 7.22 (td, J = 7.6, 1.6 Hz, 1H), 6.64 (s, 1H), 2.46 (s, 3H); 13C NMR (101 MHz, CDCl3) δ 175.6, 161.5, 153.0, 141.5, 139.9, 135.6, 135.0, 134.1, 133.1, 131.1, 130.6, 130.1, 129.7, 129.2, 128.6, 128.3, 126.4, 119.2, 98.0, 19.9; HRMS (ESI-TOF) m/z: [M + Na]+ calcd for C24H17IN2O2SNa 546.9948, found 546.9941; HPLC analysis: 82:18 er (IB column, 25 °C, hexane/iPrOH = 80/20, 0.5 mL/min, λ = 254 nm), Rt (major) = 24.4 min, Rt (minor) = 25.9 min.
- (Z)-N-(6-(4-fluorophenyl)-3-(2-iodophenyl)-4-oxo-3,4-dihydro-2H-1,3-thiazin-2-ylidene)benzamide (3d), white solid, 61% yield, 32.2 mg, m.p. 216–218 °C; = +24.0 (c = 1.0 in CHCl3); 1H NMR (400 MHz, CDCl3) δ 8.04 (dd, J = 8.0, 1.4 Hz, 1H), 7.81–7.78 (m, 2H), 7.73–7.68 (m, 2H), 7.56 (td, J = 7.6, 1.4 Hz, 1H), 7.50–7.46 (m, 1H), 7.35–7.31 (m, 3H), 7.24–7.19 (m, 3H), 6.89 (s, 1H); 13C NMR (101 MHz, CDCl3) δ 175.6, 164.8 (d, J = 255.3 Hz), 161.7, 160.0 150.4, 141.3, 139.9, 134.9, 133.2, 130.58, 130.6 (d, J = 3.1 Hz), 130.1, 129.7, 129.1 (d, J = 4.2 Hz), 129.0, 128.3, 116.7 (d, J = 22.4 Hz), 115.8, 98.0; 19F NMR (377 MHz, CDCl3) δ -107.0; HRMS (ESI-TOF) m/z: [M + Na]+ calcd for C23H14FIN2O2SNa 550.9697, found 550.9698; HPLC analysis: 88:12 er (IA column, 25 °C, hexane/iPrOH = 80/20, 0.5 mL/min, λ = 254 nm), Rt (major) = 45.6 min, Rt (minor) = 48.8 m.
- (Z)-N-(6-(4-chlorophenyl)-3-(2-iodophenyl)-4-oxo-3,4-dihydro-2H-1,3-thiazin-2-ylidene)benzamide (3e), white solid, 57% yield, 31.2 mg, m.p. 180–182 °C; = +22.6 (c = 1.0 in CHCl3); 1H NMR (400 MHz, CDCl3) δ 8.04 (dd, J = 8.0, 1.4 Hz, 1H), 7.80–7.78 (m, 2H), 7.66–7.63 (m, 2H), 7.56 (td, J = 7.8, 1.4 Hz, 1H), 7.51–7.46 (m, 3H), 7.35–7.31 (m, 3H), 7.22 (td, J = 7.8, 1.6 Hz, 1H), 6.91 (s, 1H); 13C NMR (101 MHz, CDCl3) δ 175.6, 161.6, 160.0, 150.3, 141.3, 139.9, 138.2, 134.9, 133.2, 132.8, 130.2, 130.1, 129.8, 129.7, 129.1, 128.3, 128.1, 116.0, 98.0; HRMS (ESI-TOF) m/z: [M + Na]+ calcd for C23H14ClIN2O2SNa 566.9401, found 566.9391; HPLC analysis: 90:10 er (IB column, 25 °C, hexane/iPrOH = 80/20, 0.5 mL/min, λ = 254 nm), Rt (major) = 36.7 min, Rt (minor) = 40.1 min.
- (Z)-N-(6-(4-bromophenyl)-3-(2-iodophenyl)-4-oxo-3,4-dihydro-2H-1,3-thiazin-2-ylidene)benzamide (3f), white solid, 48% yield, 28.2 mg, m.p. 94–96 °C; = +10.0 (c =1.0 in CHCl3); 1H NMR (400 MHz, CDCl3) δ 8.04 (dd, J = 8.0, 1.4 Hz, 1H), 7.80–7.78 (m, 2H), 7.67–7.64 (m, 2H), 7.59–7.54 (m, 3H), 7.50–7.46 (m, 1H), 7.35–7.31 (m, 3H), 7.22 (td, J = 7.6, 1.6 Hz, 1H), 6.91 (s, 1H); 13C NMR (101 MHz, CDCl3) δ 175.6, 161.6, 160.0, 150.4, 141.3, 139.9, 134.9, 133.3, 133.2, 132.7, 130.2, 130.1, 129.7, 129.1, 128.3, 128.2, 126.6, 116.0, 98.0; HRMS (ESI-TOF) m/z: [M + Na]+ calcd for C23H14BrIN2O2SNa 610.8896, found 610.8893; UPLC analysis: 92:8 er (AD-3 column, 25 °C, hexane/iPrOH = 80/20, 0.5 mL/min, λ = 254 nm), Rt (major) = 46.2 min, Rt (minor) = 49.9 min.
- (Z)-N-(3-(2-iodophenyl)-6-(3-methoxyphenyl)-4-oxo-3,4-dihydro-2H-1,3-thiazin-2-ylidene)benzamide (3g), white solid, 66% yield, 35.8 mg, m.p. 118–120 °C; = +15.5 (c = 1.0 in CHCl3); 1H NMR (400 MHz, CDCl3) δ 8.04 (dd, J = 8.0, 1.4 Hz, 1H), 7.81–7.78 (m, 2H), 7.55 (dd, J = 7.8, 1.4 Hz, 1H), 7.50–7.45 (m, 1H), 7.42 (t, J = 8.0 Hz, 1H), 7.37–7.27 (m, 4H), 7.24–7.18 (m, 2H), 7.10–7.07 (m, 1H), 6.93 (s, 1H), 3.88 (s, 3H); 13C NMR (101 MHz, CDCl3) δ 175.5, 161.8, 160.34, 160.25, 151.6, 141.4, 139.9 (2C), 135.7, 135.0, 133.1, 130.5, 130.1, 129.7, 129.1, 128.3, 119.2, 117.7, 115.9, 111.9, 98.0, 55.6; HRMS (ESI-TOF) m/z: [M + Na]+ calcd for C24H17IN2O3SNa 562.9897, found 562.9886; HPLC analysis: 87:13 er (IB column, 25 °C, hexane/iPrOH = 80/20, 0.5 mL/min, λ = 254 nm), Rt (major) = 27.0 min, Rt (minor) = 32.6 min.
- (Z)-N-(3-(2-iodophenyl)-4-oxo-6-(m-tolyl)-3,4-dihydro-2H-1,3-thiazin-2-ylidene)benzamide (3h), white solid, 53% yield, 27.8 mg, m.p. 206–208 °C; = +24.0 (c = 1.0 in CHCl3); 1H NMR (400 MHz, CDCl3) δ 8.04 (dd, J = 8.0, 1.4 Hz, 1H), 7.82–7.79 (m, 2H), 7.56 (td, J = 7.8, 1.4 Hz, 1H), 7.51–7.45 (m, 3H), 7.39–7.31 (m, 5H), 7.21 (td, J = 7.8, 1.6 Hz, 1H), 6.92 (s, 1H), 2.44 (s, 3H); 13C NMR (101 MHz, CDCl3) δ 175.6, 161.8, 160.5, 151.8, 141.5, 139.9, 139.4, 135.0, 134.3, 133.1, 132.6, 130.12, 130.09, 129.7, 129.3, 129.1, 128.3, 127.4, 123.9, 115.6, 98.1, 21.4; HRMS (ESI-TOF) m/z: [M + Na]+ calcd for C24H17IN2O2SNa 546.9947, found 546.9944; HPLC analysis: 97:3 er (ODH column, 25 °C, hexane/iPrOH = 80/20, 0.5 mL/min, λ = 254 nm), Rt (major) = 35.4 min, Rt (minor) = 38.5 min.
- (Z)-N-(6-(3-fluorophenyl)-3-(2-iodophenyl)-4-oxo-3,4-dihydro-2H-1,3-thiazin-2-ylidene)benzamide (3i), white solid, 65% yield, 34.4 mg, m.p. 162–164 °C; = +10.0 (c = 1.0 in CHCl3); 1H NMR (400 MHz, CDCl3) δ 8.04 (dd, J = 8.0, 1.4 Hz, 1H), 7.80–7.78 (m, 2H), 7.57 (td, J = 7.8, 1.4 Hz, 1H), 7.51–7.46 (m, 3H), 7.43–7.39 (m, 1H), 7.35–7.31 (m, 3H), 7.25–7.20 (m, 2H), 6.92 (s, 1H); 13C NMR (101 MHz, CDCl3) δ 175.6, 163.0 (d, J = 250.7, Hz), 161.5, 160.0, 150.2, 141.3, 139.9, 136.4 (d, J = 7.9 Hz), 134.9, 133.2, 131.2 (d, J = 4.2 Hz), 130.19, 130.15, 129.7, 129.1, 128.3, 122.6 (d, J = 3.0 Hz), 118.8 (d, J = 21.1 Hz), 116.5, 114.0 (d, J = 23.9 Hz), 98.0; 19F NMR (377 MHz, CDCl3) δ -110.3; HRMS (ESI-TOF) m/z: [M + Na]+ calcd for C23H14FIN2O2SNa 550.9697, found 550.9687; HPLC analysis: 97:3 er (ODH column, 25 °C, hexane/iPrOH = 80/20, 0.5 mL/min, λ = 254 nm), Rt (minor) = 45.0 min, Rt (major) = 47.1 min.
- (Z)-N-(3-(2-iodophenyl)-4-oxo-6-(o-tolyl)-3,4-dihydro-2H-1,3-thiazin-2-ylidene)benzamide (3j), white solid, 53% yield, 28.0 mg, m.p. 160–162 °C; = +19.3 (c = 1.0 in CHCl3); 1H NMR (400 MHz, CDCl3) δ 8.04 (dd, J = 8.0, 1.4 Hz, 1H), 7.78–7.76 (m, 2H), 7.57 (td, J = 7.4, 1.2 Hz, 1H), 7.49–7.44 (m, 1H), 7.38–7.31 (m, 7H), 7.22 (td, J = 7.8, 1.6 Hz, 1H), 6.64 (s, 1H), 2.46 (s, 3H); 13C NMR (101 MHz, CDCl3) δ 175.6, 161.5, 161.2, 153.0, 141.5, 139.9 (2C), 135.6, 135.0, 134.1, 133.1, 131.1, 130.6, 130.1, 129.7, 129.2, 128.6, 128.3, 126.4, 119.2, 98.0, 19.9; HRMS (ESI-TOF) m/z: [M + Na]+ calcd for C24H17IN2O2SNa 546.9948, found 546.9941; UPLC analysis: 97:3 er (OD-3 column, 25 °C, hexane/iPrOH = 80/20, 0.5 mL/min, λ = 254 nm), Rt (minor) = 14.7 min, Rt (major) = 17.3 min.
- (Z)-N-(6-(2-fluorophenyl)-3-(2-iodophenyl)-4-oxo-3,4-dihydro-2H-1,3-thiazin-2-ylidene)benzamide (3k), white solid, 48% yield, 25.8 mg, m.p. 114–116 °C; = +10.2 (c = 1.0 in CHCl3); 1H NMR (400 MHz, CDCl3) δ 8.04 (dd, J = 8.0, 1.4 Hz, 1H), 7.79–7.77 (m, 2H), 7.63–7.45 (m, 4H), 7.36–7.29 (m, 4H), 7.25–7.20 (m, 2H), 6.95 (d, J = 1.0 Hz, 1H); 13C NMR (101 MHz, CDCl3) δ 175.6, 161.4, 160.6, 159.4 (d, J = 258.5 Hz), 146.0, 141.4, 139.9, 134.9, 133.1, 133.0, 130.1, 129.7, 129.1, 128.3, 125.0 (d, J = 3.6 Hz), 124.2, 123.6, 122.4 (d, J = 6.55 Hz), 119.8 (d, J = 5.6 Hz), 117.0 (d, J = 21.9 Hz), 98.0; 19F NMR (377 MHz, CDCl3) δ -112.4; HRMS (ESI-TOF) m/z: [M + Na]+ calcd for C23H14FIN2O2SNa 550.9697, found 550.9682; UPLC analysis: 91:9 er (AD-3 column, 25 °C, hexane/iPrOH = 80/20, 0.5 mL/min, λ = 254 nm), Rt (minor) = 35.4 min, Rt (major) = 47.6 min.
- (Z)-N-(6-(2-chlorophenyl)-3-(2-iodophenyl)-4-oxo-3,4-dihydro-2H-1,3-thiazin-2-ylidene)benzamide (3l), yellow solid, 42% yield, 23.0 mg, m.p. 168–170 °C; = +9.0 (c = 1.0 in CHCl3); 1H NMR (400 MHz, CDCl3) δ 8.05 (dd, J = 8.0, 1.4 Hz, 1H), 7.78–7.75 (m, 2H), 7.60–7.30 (m, 9H), 7.23 (td, J = 7.8, 1.6 Hz, 1H), 6.77 (s, 1H); 13C NMR (101 MHz, CDCl3) δ 175.6, 161.4, 161.2, 150.0, 141.4, 139.9, 134.9, 133.3, 133.1, 132.4, 131.8, 130.7, 130.24, 130.16, 130.1, 129.7, 129.1, 128.3, 127.4, 120.5, 98.0; HRMS (ESI-TOF) m/z: [M + Na]+ calcd for C23H14ClIN2O2SNa 566.9401, found 566.9395; HPLC analysis: 92:8 er (IB column, 25 °C, hexane/iPrOH = 80/20, 0.5 mL/min, λ = 254 nm), Rt (minor) = 18.4 min, Rt (major) = 21.1 min.
- (Z)-N-(3-(2-iodophenyl)-6-(naphthalen-1-yl)-4-oxo-3,4-dihydro-2H-1,3-thiazin-2-ylidene)benzamide (3m), white solid, 57% yield, 32.0 mg, m.p. 185–187 °C; = +24.0 (c = 1.0 in CHCl3); 1H NMR (400 MHz, CDCl3) δ 8.14–8.12 (m, 1H), 8.07 (dd, J = 8.0, 1.4 Hz, 1H), 7.99 (d, J = 8.2 Hz, 1H), 7.95–7.93 (m, 1H), 7.78–7.76 (m, 2H), 7.66–7.54 (m, 5H), 7.48–7.41 (m, 2H), 7.32 (t, J = 7.8 Hz, 2H), 7.23 (dd, J = 7.8, 1.6 Hz, 1H), 6.87 (s, 1H); 13C NMR (101 MHz, CDCl3) δ 175.6, 161.5, 161.3, 151.8, 141.5, 139.9, 135.0, 133.8, 133.1, 132.0, 131.3, 130.1(2C), 130.0, 129.7, 129.2, 128.8, 128.3, 127.7, 127.0, 126.9, 125.1, 124.5, 120.2, 98.1; HRMS (ESI-TOF) m/z: [M + Na]+ calcd for C27H17IN2O2SNa 582.9948, found 582.9943; HPLC analysis: 97:3 er (ODH column, 25 °C, hexane/iPrOH = 80/20, 0.5 mL/min, λ = 254 nm), Rt (major) = 35.4 min, Rt (minor) = 38.5 min.
- (Z)-N-(3-(2-iodophenyl)-4-oxo-6-(thiophen-2-yl)-3,4-dihydro-2H-1,3-thiazin-2-ylidene)benzamide (3n), white solid, 80% yield, 41.8 mg, m.p. 144–146 °C; = +7.6 (c = 1.0 in CHCl3); 1H NMR (400 MHz, CDCl3) δ 8.03 (dd, J = 8.0, 1.4 Hz, 1H), 7.81–7.78 (m, 2H), 7.64 (dd, J = 3.8, 1.2 Hz, 1H), 7.60–7.53 (m, 2H), 7.50–7.46 (m, 1H), 7.35–7.31 (m, 3H), 7.23–7.18 (m, 2H), 6.93 (s, 1H); 13C NMR (101 MHz, CDCl3) δ 175.6, 161.6, 160.0, 144.1, 141.5, 139.8, 136.9, 134.9, 133.1, 130.6, 130.13, 130.10, 129.6, 129.1, 128.9, 128.3, 113.2, 98.0; HRMS (ESI-TOF) m/z: [M + Na]+ calcd for C21H13IN2O2S2Na 538.9355, found 538.9376; HPLC analysis: 99:1 er (ODH column, 25 °C, hexane/iPrOH = 80/20, 0.5 mL/min, λ = 254 nm), Rt (minor) = 40.9 min, Rt (major) = 46.7 min.
- (Z)-N-(3-(2-iodophenyl)-4-oxo-6-phenyl-3,4-dihydro-2H-1,3-thiazin-2-ylidene)-4-methylbenzamide (4a), yellow solid, 45% yield, 23.4 mg, m.p. 156–158 °C; = +16.1 (c = 1.0 in CHCl3); 1H NMR (400 MHz, CDCl3) δ 8.03 (dd, J = 8.0, 1.4 Hz, 1H), 7.71–7.68 (m, 4H), 7.57–7.49 (m, 4H), 7.33 (dd, J = 8.0, 1.6 Hz, 1H), 7.20 (td, J = 7.8, 1.6 Hz, 1H), 7.13 (d, J = 8.0 Hz, 2H), 6.92 (s, 1H), 2.36 (s, 3H); 13C NMR (101 MHz, CDCl3) δ 175.6, 161.9, 159.9, 151.7, 144.0, 141.4, 139.8, 134.4, 132.4, 131.8, 130.2, 130.1, 129.7, 129.4, 129.1, 126.8, 115.7, 98.1, 21.8; HRMS (ESI-TOF) m/z: [M + Na]+ calcd for C24H17IN2O2SNa 546.9948, found 546.9949; UPLC analysis: 93:7 er (OD-3 column, 25 °C, hexane/iPrOH = 80/20, 0.5 mL/min, λ = 254 nm), Rt (major) = 24.1 min, Rt (minor) = 32.6 min.
- (Z)-N-(3-(2-iodophenyl)-4-oxo-6-phenyl-3,4-dihydro-2H-1,3-thiazin-2-ylidene)-3-methylbenzamide (4b), white solid, 41% yield, 21.7 mg, m.p. 128–130 °C; = +17.5 (c = 1.0 in CHCl3); 1H NMR (400 MHz, CDCl3) δ 8.04 (dd, J = 8.0, 1.4 Hz, 1H), 7.71–7.69 (m, 2H), 7.62–7.49 (m, 6H), 7.35 (dd, J = 8.0, 1.4 Hz, 1H), 7.30–7.28 (m, 1H), 7.24–7.19 (m, 2H), 6.93 (s, 1H), 2.30 (s, 3H); 13C NMR (101 MHz, CDCl3) δ 175.7, 161.8, 159.7, 151.5, 141.5, 139.8, 138.0, 134.9, 134.4, 133.9, 131.8, 130.9, 130.0, 129.7, 129.4, 129.2, 129.1, 128.2, 127.2, 126.8, 115.7, 98.1, 21.3; HRMS (ESI-TOF) m/z: [M + Na]+ calcd for C24H17IN2O2SNa 546.9948, found 546.9941; UPLC analysis: 90:10 er (AD-3 column, 25 °C, hexane/iPrOH = 80/20, 0.5 mL/min, λ = 254 nm), Rt (major) = 32.5 min, Rt (minor) = 36.2 min.
- (Z)-N-(3-(2-iodophenyl)-4-oxo-6-phenyl-3,4-dihydro-2H-1,3-thiazin-2-ylidene)-3-methoxybenzamide (4c), white solid, 49% yield, 26.6 mg, m.p. 131–133 °C; = +14.3 (c = 1.0 in CHCl3); 1H NMR (400 MHz, CDCl3) δ 8.03 (dd, J = 8.0, 1.4 Hz, 1H), 7.73–7.70 (m, 2H), 7.59–7.50 (m, 4H), 7.45 (dt, J = 7.6, 1.2 Hz, 1H), 7.35 (dd, J = 8.0, 1.6 Hz, 1H), 7.29–7.28 (m, 1H), 7.24–7.18 (m, 2H), 7.04–7.01 (m, 1H), 6.94 (s, 1H), 3.70 (s, 3H); 13C NMR (101 MHz, CDCl3) δ 175.4, 161.8, 160.8, 159.4, 151.7, 141.6, 139.8, 136.4, 134.3, 131.9, 130.0, 129.7, 129.5, 129.4, 129.1, 126.8, 122.6, 120.7, 115.8, 113.4, 98.1, 55.3; HRMS (ESI-TOF) m/z: [M + Na]+ calcd for C24H17IN2O3SNa 562.9897, found 562.9893; UPLC analysis: 90:10 er (AD-3 column, 25 °C, hexane/iPrOH = 80/20, 0.5 mL/min, λ = 254 nm), Rt (minor) = 34.3 min, Rt (major) = 43.5 min.
- (Z)-N-(3-(2-iodophenyl)-4-oxo-6-phenyl-3,4-dihydro-2H-1,3-thiazin-2-ylidene)-2-methylbenzamide (4d), light yellow solid, 42% yield, 22.1 mg, m.p. 139–141 °C; = +10.7 (c = 1.0 in CHCl3); 1H NMR (400 MHz, CDCl3) δ 8.01 (dd, J = 8.0, 1.4 Hz, 1H), 7.72–7.69 (m, 2H), 7.62 (dd, J = 8.0, 1.5 Hz, 1H), 7.58–7.49 (m, 4H), 7.34–7.30 (m, 2H), 7.20–7.15 (m, 2H), 7.09 (td, J = 7.6, 1.4 Hz, 1H), 6.92 (s, 1H), 2.50 (s, 3H); 13C NMR (101 MHz, CDCl3) δ 177.2, 161.9, 159.5, 151.7, 141.5, 141.2, 139.9, 134.5, 133.7, 132.3, 132.2, 131.9, 131.8, 130.1, 129.7, 129.4, 129.1, 126.9, 125.6, 115.8, 98.2, 22.3; HRMS (ESI-TOF) m/z: [M + Na]+ calcd for C24H17IN2O2SNa 546.9948, found 546.9937; UPLC analysis: 87:13 er (AD-3 column, 25 °C, hexane/iPrOH = 80/20, 0.5 mL/min, λ = 254 nm), Rt (minor) = 17.4 min, Rt (major) = 25.5 min.
- (Z)-4-fluoro-N-(3-(2-iodophenyl)-4-oxo-6-phenyl-3,4-dihydro-2H-1,3-thiazin-2-ylidene)benzamide (4e), yellow solid, 49% yield, 26.1 mg, m.p. 147–149 °C; = +17.0 (c = 1.0 in CHCl3); 1H NMR (400 MHz, CDCl3) δ 8.04 (dd, J = 8.0, 1.4 Hz, 1H), 7.82–7.77 (m, 2H), 7.72–7.69 (m, 2H), 7.59–7.50 (m, 4H), 7.33 (dd, J = 8.0, 1.6 Hz, 1H), 7.22 (td, J = 7.8, 1.6 Hz, 1H), 7.02–6.96 (m, 2H), 6.94 (s, 1H); 13C NMR (101 MHz, CDCl3) δ 174.4, 165.9 (d, J = 255.4 Hz), 161.7, 161.0, 151.6, 141.4, 139.9, 134.3, 132.7 (d, J = 9.5 Hz), 131.9, 131.4 (d, J = 2.6 Hz), 130.2, 129.7, 129.5, 129.0, 126.8, 115.8, 115. 4 (d, J = 21.9 Hz), 98.0; 19F NMR (377 MHz, CDCl3) δ -105.5; HRMS (ESI-TOF) m/z: [M + Na]+ calcd for C23H14FIN2O2SNa 550.9697, found 550.9694; UPLC analysis: 93:7 er (OD-3 column, 25 °C, hexane/iPrOH = 80/20, 0.5 mL/min, λ = 254 nm), Rt (major) = 21.3 min, Rt (minor) = 33.9 min.
- (Z)-4-chloro-N-(3-(2-iodophenyl)-4-oxo-6-phenyl-3,4-dihydro-2H-1,3-thiazin-2-ylidene)benzamide (4f), white solid, 63% yield, 34.3 mg, m.p. 160–163 °C; = +17.4 (c = 1.0 in CHCl3); 1H NMR (400 MHz, CDCl3) δ 8.03 (dd, J = 8.0, 1.4 Hz, 1H), 7.72–7.69 (m, 4H), 7.59–7.50 (m, 5H), 7.34–7.28 (m, 3H), 7.22 (td, J = 7.8, 1.6 Hz, 1H), 6.95 (s, 1H); 13C NMR (101 MHz, CDCl3) δ 174.5, 161.6, 161.2, 151.6, 141.4, 139.9, 139.5, 134.3, 133.6, 131.9, 131.5, 130.6, 129.7, 129.5, 129.1, 128.6, 126.8, 115.9, 97.9; HRMS (ESI-TOF) m/z: [M + Na]+ calcd for C23H14ClIN2O2SNa 566.9401, found 566.9392; UPLC analysis: 90:10 er (AD-3 column, 25 °C, hexane/iPrOH = 80/20, 0.5 mL/min, λ = 254 nm), Rt (major) = 36.2 min, Rt (minor) = 56.0 min.
- (Z)-4-bromo-N-(3-(2-iodophenyl)-4-oxo-6-phenyl-3,4-dihydro-2H-1,3-thiazin-2-ylidene)benzamide (4g), white solid, 48% yield, 28.3 mg, m.p. 166–168 °C; = +20.4 (c = 1.0 in CHCl3); 1H NMR (400 MHz, CDCl3) δ 8.03 (dd, J = 8.0, 1.4 Hz, 1H), 7.72–7.69 (m, 2H), 7.64–7.61 (m, 2H), 7.59–7.49 (m, 4H), 7.48–7.45 (m, 2H), 7.32 (dd, J = 8.0, 1.6 Hz, 1H), 7.21 (td, J = 7.8, 1.6 Hz, 1H), 6.94 (s, 1H); 13C NMR (101 MHz, CDCl3) δ 174.7, 161.6, 161.3, 151.6, 141.4, 139.9, 134.3, 134.0, 131.9, 131.63, 131.59, 130.2, 129.7, 129.5, 129.1, 128.3, 126.8, 115.9, 97.9; HRMS (ESI-TOF) m/z: [M + Na]+ calcd for C23H14BrIN2O2SNa 610.8896, found 610.8890; HPLC analysis: 90:10 er (IA column, 25 °C, hexane/iPrOH = 80/20, 0.5 mL/min, λ = 254 nm), Rt (major) = 37.0 min, Rt (minor) = 48.5 min.
- (Z)-3-fluoro-N-(3-(2-iodophenyl)-4-oxo-6-phenyl-3,4-dihydro-2H-1,3-thiazin-2-ylidene)benzamide (4h), white solid, 51% yield, 26.7 mg, m.p. 132–134 °C; = +23.6 (c = 1.0 in CHCl3); 1H NMR (400 MHz, CDCl3) δ 8.05 (dd, J = 8.0, 1.4 Hz, 1H), 7.72–7.69 (m, 2H), 7.60–7.50 (m, 5H), 7.43–7.39 (m, 1H), 7.34–7.27 (m, 2H), 7.25–7.14 (m, 2H), 6.95 (s, 1H); 13C NMR (101 MHz, CDCl3) δ 174.3, 162.7 (d, J = 247.9 Hz), 161.6, 161.5, 151.6, 141.3, 139.9, 137.4 (d, J = 7.3 Hz), 134.3, 131.9, 130.2, 129.9 (d, J = 7.9 Hz), 129.7, 129.5, 129.0, 126.8, 125.7 (d, J = 2.9 Hz), 120.0 (d, J = 21.8 Hz), 116.8 (d, J = 23.1 Hz), 115.9, 97.9; 19F NMR (377 MHz, CDCl3) δ -112.6; HRMS (ESI-TOF) m/z: [M + Na]+ calcd for C23H14FIN2O2SNa 550.9697, found 550.9694; UPLC analysis: 90:10 er (AD-3 column, 25 °C, hexane/iPrOH = 80/20, 0.5 mL/min, λ = 254 nm), Rt (minor) = 29.5 min, Rt (major) = 33.9 min.
- (Z)-3-chloro-N-(3-(2-iodophenyl)-4-oxo-6-phenyl-3,4-dihydro-2H-1,3-thiazin-2-ylidene)benzamide (4i), white solid, 70% yield, 38.5 mg, m.p. 124–126 °C; = +32.7 (c = 1.0 in CHCl3); 1H NMR (400 MHz, CDCl3) δ 8.05 (dd, J =7.6, 1.2 Hz, 1H), 7.71–7.66 (m, 4H), 7.60–7.49 (m, 4H), 7.44–7.41 (m, 1H), 7.33 (dd, J = 7.6, 1.6 Hz, 1H), 7.27 (d, J = 7.8 Hz, 1H), 7.25–7.21 (m, 1H), 6.95 (s, 1H); 13C NMR (101 MHz, CDCl3) δ 174.1, 161.6, 161.4, 151.5, 141.3, 139.9, 136.9, 134.4, 134.3, 132.9, 131.9, 130.4, 130.2, 129.7, 129.6, 129.5, 129.0, 128.0, 126.8, 115.9, 97.9; HRMS (ESI-TOF) m/z: [M + Na]+ calcd for C23H14ClIN2O2SNa 566.9401, found 566.9395; HPLC analysis: 88:12 er (IB column, 25 °C, hexane/iPrOH = 80/20, 0.5 mL/min, λ = 254 nm), Rt (major) = 27.6 min, Rt (minor) = 38.8 min.
- (Z)-3-bromo-N-(3-(2-iodophenyl)-4-oxo-6-phenyl-3,4-dihydro-2H-1,3-thiazin-2-ylidene)benzamide (4j), white solid, 48% yield, 28.5 mg, m.p. 126–128 °C; = +25.1 (c = 1.0 in CHCl3); 1H NMR (400 MHz, CDCl3) δ 8.06 (dd, J = 8.0, 1.4 Hz, 1H), 7.85 (t, J = 1.8 Hz, 1H), 7.75–7.69 (m, 3H), 7.60–7.50 (m, 5H), 7.33 (dd, J = 7.8, 1.4 Hz, 1H), 7.24–7.19 (m, 2H), 6.96 (s, 1H); 13C NMR (101 MHz, CDCl3) δ 174.0, 161.6, 161.5, 151.5, 141.3, 140.0, 137.0, 135.8, 134.2, 133.4, 132.0, 130.3, 129.9, 129.8, 129.5, 129.0, 128.4, 126.8, 122.5, 116.0, 97.9; HRMS (ESI-TOF) m/z: [M + Na]+ calcd for C23H14BrIN2O2SNa 610.8896, found 610.8890; UPLC analysis: 89:11 er (AD-3 column, 25 °C, hexane/iPrOH = 80/20, 0.5 mL/min, λ = 254 nm), Rt (minor) = 31.0 min, Rt (major) = 35.4 min.
- (Z)-2-fluoro-N-(3-(2-iodophenyl)-4-oxo-6-phenyl-3,4-dihydro-2H-1,3-thiazin-2-ylidene)benzamide (4k), yellow solid, 56% yield, 29.6 mg, m.p. 131–133 °C; = +8.4 (c = 1.0 in CHCl3); 1H NMR (400 MHz, CDCl3) δ 8.02 (dd, J = 8.0, 1.4 Hz, 1H), 7.72–7.69 (m, 2H), 7.59–7.49 (m, 5H), 7.46–7.40 (m, 1H), 7.32 (dd, J = 8.0, 1.4 Hz, 1H), 7.20 (td, J = 7.8, 1.6 Hz, 1H), 7.08–7.00 (m, 2H), 6.95 (s, 1H); 13C NMR (101 MHz, CDCl3) δ 173.2, 162.8 (d, J = 263.2 Hz), 161.7, 160.9, 151.7, 141.3, 139.8, 134.7 (d, J = 9.4 Hz), 134.3, 133.0, 131.9, 130.1, 129.7, 129.5, 129.1, 126.8, 123.7 (d, J = 4.3 Hz), 123.2 (d, J = 6.9 Hz), 117.0 (d, J = 22.2 Hz), 115.8, 98.0; 19F NMR (377 MHz, CDCl3) δ -110.2; HRMS (ESI-TOF) m/z: [M + Na]+ calcd for C23H14FIN2O2SNa 550.9697, found 550.9692; UPLC analysis: 88:12 er (AD-3 column, 25 °C, hexane/iPrOH = 80/20, 0.5 mL/min, λ = 254 nm), Rt (minor) = 28.8 min, Rt (major) = 51.5 min.
- (Z)-2-chloro-N-(3-(2-iodophenyl)-4-oxo-6-phenyl-3,4-dihydro-2H-1,3-thiazin-2-ylidene)benzamide (4l), yellow solid, 40% yield, 21.6 mg, m.p. 141–143 °C; = +7.1 (c = 1.0 in CHCl3); 1H NMR (400 MHz, CDCl3) δ 8.00 (dd, J = 8.0, 1.4 Hz, 1H), 7.73–7.70 (m, 2H), 7.59–7.50 (m, 5H), 7.39–7.29 (m, 3H), 7.20–7.12 (m, 2H), 6.95 (s, 1H); 13C NMR (101 MHz, CDCl3) δ 174.5, 161.7, 161.2, 151.7, 141.4, 139.8, 134.7, 134.3, 133.4, 133.0, 132.7, 132.0, 131.4, 130.1, 129.6, 129.5, 129.1, 126.9, 126.4, 115.9, 98.0; HRMS (ESI-TOF) m/z: [M + Na]+ calcd for C23H14ClIN2O2SNa 566.9401, found 566.9392; HPLC analysis: 84:16 er (IB column, 25 °C, hexane/iPrOH = 80/20, 0.5 mL/min, λ = 254 nm), Rt (minor) = 28.2 min, Rt (major) = 31.9 min.
- (Z)-2-bromo-N-(3-(2-iodophenyl)-4-oxo-6-phenyl-3,4-dihydro-2H-1,3-thiazin-2-ylidene)benzamide (4m), yellow solid, 37% yield, 21.7 mg, m.p. 138–140 °C; = +10.2 (c = 0.8 in CHCl3); 1H NMR (400 MHz, CDCl3) δ 8.00 (dd, J = 8.0, 1.4 Hz, 1H), 7.73–7.70 (m, 2H), 7.61–7.50 (m, 6H), 7.31 (dd, J = 8.0, 1.6 Hz, 1H), 7.25–7.15 (m, 3H), 6.95 (s, 1H); 13C NMR (101 MHz, CDCl3) δ 174.9, 161.7, 161.3, 151.7, 141.3, 139.8, 135.1, 134.8, 134.3, 133.1, 132.7, 131.9, 130.1, 129.7, 129.5, 129.2, 127.0, 126.9, 122.7, 115.9, 98.0; HRMS (ESI-TOF) m/z: [M + Na]+ calcd for C23H14BrIN2O2SNa 610.8896, found 610.8890; UPLC analysis: 85:15 er (AD-3 column, 25 °C, hexane/iPrOH = 80/20, 0.5 mL/min, λ = 254 nm), Rt (minor) = 25.2 min, Rt (major) = 47.3 min.
4. Conclusions
In summary, we have developed an NHC-catalyzed atroposelective annulation reaction for facile synthesis of axially chiral thiazine derivatives from readily available starting materials. The reaction conditions are very mild with various well-tolerated functional groups. A series of axially chiral thiazine derivatives can be readily produced using our method. Preliminary bioactive studies revealed that some of our products exhibit promising antibacterial activities against Xanthomonas oryzae pv. oryzae (Xoo) that causes rice bacterial blight. Further applications of such axially chiral thiazine derivatives in the development of novel green pesticides are in progress in our laboratories.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/molecules28104052/s1. Table S1. The effects of NHCs, bases, solvents and additives.; Figure S1. Proposed Reaction Mechanism. Table S2. Antibacterial activities of the products. Reference [62] is cited in the Supplementary Materials.
Author Contributions
Conceptualization, X.Y., Z.J. and S.-C.R.; methodology, Z.J. and S.-C.R.; software, X.Y., Z.J. and S.-C.R.; validation, X.Y., T.L., J.C. and Y.H.; formal analysis, X.Y., Y.H., T.S., S.L., Z.J. and S.-C.R.; investigation, X.Y., T.L., Z.J. and S.-C.R.; data curation, X.Y.; writing—original draft preparation, Z.J. and S.-C.R.; writing—review and editing, X.Y., Z.J. and S.-C.R.; visualization, Z.J. and S.-C.R.; supervision, Z.J. and S.-C.R.; project administration, Z.J. and S.-C.R.; funding acquisition, Z.J. and S.-C.R. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the National Natural Science Foundation of China (21732002, 21961006, 32172459), the Science and Technology Department of Guizhou Province (Qiankehejichu-ZK[2021]Key033), the Program of Introducing Talents of Discipline to Universities of China (111 Program, D20023) at Guizhou University, Frontiers Science Center for Asymmetric Synthesis and Medicinal Molecules, Department of Education, Guizhou Province (Qianjiaohe KY (2020)004).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available in the Supporting information.
Acknowledgments
We acknowledge the Instrumental Analysis and Research Platform of Guizhou University.
Conflicts of Interest
The authors declare no conflict of interest.
Sample Availability
Samples of the compounds are not available from the authors.
References
- De Pestel, D.D.; Benninger, M.S.; Danziger, L.; LaPlante, K.L.; May, C.; Luskin, A.; Pichichero, M.; Hadley, J.A. Cephalosporin use in treatment of patients with penicillin allergies. J. Am. Pharm. Assoc. 2008, 48, 530–540. [Google Scholar] [CrossRef] [PubMed]
- Zhanel, G.G.; Lam, A.; Schweizer, F.; Thomson, K.; Walkty, A.; Rubinstein, E.; Gin, A.S.; Hoban, D.J.; Noreddin, A.M.; Karlowsky, J.A. Ceftobiprole: A review of a broad-spectrum and anti-MRSA cephalosporin. Am. J. Clin. Dermatol. 2008, 9, 245–254. [Google Scholar] [CrossRef] [PubMed]
- Choudhary, S.; Silakari, O.; Singh, P.K. Key updates on the chemistry and biological roles of thiazine scaffold: A review. Mini-Rev. Med. Chem. 2018, 18, 1452–1478. [Google Scholar] [CrossRef] [PubMed]
- Shah, M.I.A.; Khan, R.; Arfan, M.; Wadood, A.; Ghufran, M. Synthesis, in vitro urease inhibitory activity and molecular docking of 3,5-disubstituted thiadiazine-2-thiones. J. Heterocycl. Chem. 2019, 56, 3073–3080. [Google Scholar] [CrossRef]
- Asif, M.; Imran, M.; Abida. Antimicrobial activities of various thiazine based heterocyclic compounds: A mini-review. Mini-Rev. Org. Chem. 2022, 19, 166–172. [Google Scholar] [CrossRef]
- Salarian, A.A.; Mollamahale, Y.B.; Hami, Z.; Soltani-Rezaee-Rad, M. Cephalexin nanoparticles: Synthesis, cytotoxicity and their synergistic antibacterial study in combination with silver nanoparticles. Mater. Chem. Phys. 2017, 198, 125–130. [Google Scholar] [CrossRef]
- Hartwig, J.; Sommer, H.; Mueller, F. Nematicides. In Ullmann’s Encyclopedia of Industrial Chemistry; Wiley Online Library: New York, NY, USA, 2001; pp. 17–125. [Google Scholar]
- Mansour, R.; Belzunces, L.P.; Suma, P.; Zappalà, L.; Mazzeo, G.; Grissa-Lebdi, K.; Russo, A.; Biondi, A. Vine and citrus mealybug pest control based on synthetic chemicals. A review. Agron. Sustain. Dev. 2018, 38, 37. [Google Scholar] [CrossRef]
- Behalo, M.S. Synthesis of some novel thiazolo[3,2-a]pyrimidine and pyrimido[2,1-b][1,3]thiazine derivatives and their antimicrobial evaluation. J. Heterocycl. Chem. 2015, 55, 1391–1397. [Google Scholar] [CrossRef]
- Ishak, E.A. Microwave-assisted green synthesis of 1,3-thiazines as potential antifungal agents using lemon juice. J. Mater. Environ. Sci. 2019, 10, 54–59. [Google Scholar]
- Chen, Y.; Li, T.; Jin, Z.; Chi, Y.R. New axially chiral molecular scaffolds with antibacterial activities against Xanthomonas oryzae pv. oryzae for protection of rice. J. Agric. Food Chem. 2022, 70, 6050–6058. [Google Scholar] [CrossRef]
- Mizutani, N.; Chiou, W.-H.; Ojima, I. New and efficient synthesis of azabicyclo[4.4.0]alkane amino acids by Rh-catalyzed cyclohydrocarbonylation. Org. Lett. 2002, 4, 4575–4578. [Google Scholar] [CrossRef]
- Evans, D.A.; Fandrick, K.R.; Song, H.-J.; Scheidt, K.A.; Xu, R. Enantioselective Friedel-Crafts alkylations catalyzed by bis(oxazolinyl)pyridine-scandium(III) triflate complexes. J. Am. Chem. Soc. 2007, 129, 10029–10041. [Google Scholar] [CrossRef]
- La-Venia, A.; Ventosa-Andrés, P.; Hradilová, L.; Krchňák, V. From amino acids to nature-inspired molecular scaffolds: Incorporation of medium-sized bridged heterocycles into a peptide backbone. J. Org. Chem. 2014, 79, 10378–10389. [Google Scholar] [CrossRef]
- Unsworth, W.P.; Coulthard, G.; Kitsiou, C.; Taylor, R.J.K. Direct imine acylation for molecular diversity in heterocyclic synthesis. J. Org. Chem. 2014, 79, 1368–1376. [Google Scholar] [CrossRef]
- Xiong, J.; Zhong, G.; Liu, Y. Domino reactions initiated by copper-catalyzed aryl-I bond thiolation for the switchable synthesis of 2,3-dihydrobenzothiazinones and benzoisothiazolones. Adv. Synth. Catal. 2019, 361, 550–555. [Google Scholar] [CrossRef]
- Nosova, E.V.; Lipunova, G.N.; Charushin, V.N.; Chupakhin, O.N. Synthesis and biological activity of 2-amino- and 2-aryl (heteryl) substituted 1,3-benzothiazin-4-ones. Mini-Rev. Med. Chem. 2019, 19, 999–1014. [Google Scholar] [CrossRef]
- Luo, Z.; Bhavanarushi, S.; Sreenivas, A.; Reddy, N.S.; Valeru, A.; Khan, I.; Xu, Y.; Liu, B.; Xie, J. Trifluoroborane catalyzed chemoselective synthesis of highly functionalized 1,3-thiazin-2-ylidenes. J. Heterocycl. Chem. 2020, 57, 3334–3341. [Google Scholar] [CrossRef]
- Wang, H.; Gu, S.; Yan, Q.; Ding, L.; Chen, F.-E. Asymmetric catalysis in synthetic strategies for chiral benzothiazepines. Green Synth. Catal. 2020, 1, 12–25. [Google Scholar] [CrossRef]
- Giacalone, F.; Gruttadauria, M.; Agrigento, P.; Noto, R. Low-loading asymmetric organocatalysis. Chem. Soc. Rev. 2012, 41, 2406–2447. [Google Scholar] [CrossRef]
- Akiyama, T.; Mori, K. Stronger brønsted acids: Recent progress. Chem. Rev. 2015, 115, 9277–9306. [Google Scholar] [CrossRef]
- Tang, W.; Zhang, X. New chiral phosphorus ligands for enantioselective hydrogenation. Chem. Rev. 2003, 103, 3029–3070. [Google Scholar] [CrossRef] [PubMed]
- Parmar, D.; Sugiono, E.; Raja, S.; Rueping, M. Complete field guide to asymmetric BINOL-phosphate derived brønsted acid and metal catalysis: History and classification by mode of activation; brønsted acidity, hydrogen bonding, ion pairing, and metal phosphates. Chem. Rev. 2014, 114, 9047–9153. [Google Scholar] [CrossRef] [PubMed]
- LaPlante, S.R.; Fader, L.D.; Fandrick, K.R.; Fandrick, D.R.; Hucke, O.; Kemper, R.; Miller, S.P.F.; Edwards, P.J. Assessing atropisomer axial chirality in drug discovery and development. J. Med. Chem. 2011, 54, 7005–7022. [Google Scholar] [CrossRef] [PubMed]
- LaPlante, S.R.; Edwards, P.J.; Fader, L.D.; Jakalian, A.; Hucke, O. Revealing atropisomer axial chirality in drug discovery. ChemMedChem 2011, 6, 505–513. [Google Scholar] [CrossRef] [PubMed]
- Enders, D.; Niemeier, O.; Henseler, A. Organocatalysis by N-heterocyclic carbenes. Chem. Rev. 2007, 107, 5606–5655. [Google Scholar] [CrossRef]
- Biju, A.T.; Kuhl, N.; Glorius, F. Extending NHC-catalysis: Coupling aldehydes with unconventional reaction partners. Acc. Chem. Res. 2011, 44, 1182–1195. [Google Scholar] [CrossRef]
- Bugaut, X.; Glorius, F. Organocatalytic umpolung: N-heterocyclic carbenes and beyond. Chem. Soc. Rev. 2012, 41, 3511–3522. [Google Scholar] [CrossRef]
- Cohen, D.T.; Scheidt, K.A. Cooperative Lewis acid/N-heterocyclic carbene catalysis. Chem. Sci. 2012, 3, 53–57. [Google Scholar] [CrossRef]
- André Grossmann, D.-C.; Enders, D. N-heterocyclic carbene catalyzed domino reactions. Angew. Chem. Int. Ed. 2012, 51, 314–325. [Google Scholar] [CrossRef]
- Ryan, S.J.; Candisha, L.; Lupton, D.W. Acyl anion free N-heterocyclic carbene organocatalysis. Chem. Soc. Rev. 2013, 42, 4906–4917. [Google Scholar] [CrossRef]
- Connon, S.J. Diaminocyclopropenylidene organocatalysts: Beyond N-heterocyclic carbenes. Angew. Chem. Int. Ed. 2014, 53, 1203–1205. [Google Scholar] [CrossRef]
- Hopkinson, M.N.; Richter, C.; Schedler, M.; Glorius, F. An overview of N-heterocyclic carbenes. Nature 2014, 510, 485–496. [Google Scholar] [CrossRef]
- Mahatthananchai, J.; Bode, J.W. On the mechanism of N-heterocyclic carbene-catalyzed reactions involving acyl azoliums. Acc. Chem. Res. 2014, 47, 696–707. [Google Scholar] [CrossRef]
- Flanigan, D.M.; Romanov-Michailidis, F.; White, N.A.; Rovis, T. Organocatalytic reactions enabled by N-heterocyclic carbenes. Chem. Rev. 2015, 115, 9307–9387. [Google Scholar] [CrossRef]
- Menon, R.S.; Biju, A.T.; Nair, V. Recent advances in employing homoenolates generated by N-heterocyclic carbene (NHC) catalysis in carbon–carbon bond-forming reactions. Chem. Soc. Rev. 2015, 44, 5040–5052. [Google Scholar] [CrossRef]
- Wang, M.H.; Scheidt, K.A. Cooperative catalysis and activation with N-heterocyclic carbenes. Angew. Chem. Int. Ed. 2016, 55, 14912–14922. [Google Scholar] [CrossRef]
- Zhang, C.; Hooper, J.F.; Lupton, D.W. N-heterocyclic carbene catalysis via the α,β-unsaturated acyl azolium. ACS Catal. 2017, 7, 2583–2596. [Google Scholar] [CrossRef]
- Murauski, K.J.R.; Jaworskia, A.A.; Scheidt, K.A. A continuing challenge: N-heterocyclic carbene-catalyzed syntheses of γ-butyrolactones. Chem. Soc. Rev. 2018, 47, 1773–1782. [Google Scholar] [CrossRef]
- Smith, C.A.; Narouz, M.R.; Lummis, P.A.; Singh, I.; Nazemi, A.; Li, C.-H.; Crudden, C.M. N-Heterocyclic carbenes in materials chemistry. Chem. Rev. 2019, 119, 4986–5056. [Google Scholar] [CrossRef]
- Biju, A.T. N-Heterocyclic Carbenes in Organocatalysis; Wiley-VCH: Weinheim, Germany, 2019. [Google Scholar]
- Kim, Y.; Li, C.-J. Perspectives on green synthesis and catalysis. Green Synth. Catal. 2020, 1, 1–11. [Google Scholar] [CrossRef]
- Chen, X.; Wang, H.; Jin, Z.; Chi, Y.R. N-heterocyclic carbene organocatalysis: Activation modes and typical reactive intermediates. Chin. J. Chem. 2020, 38, 1167–1202. [Google Scholar] [CrossRef]
- Zhao, C.; Blaszczyk, S.A.; Wang, J. Asymmetric reactions of N-heterocyclic carbene (NHC)-based chiral acyl azoliums and azolium enolates. Green Synth. Catal. 2021, 2, 198–215. [Google Scholar] [CrossRef]
- Bellotti, P.; Koy, M.; Glorius, F.; Hopkinson, M.N. Recent advances in the chemistry and applications of N-heterocyclic carbenes. Nat. Rev. Chem. 2021, 5, 711–725. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Wang, H.; Jin, Z.; Chi, Y.R. Development of green and low-cost chiral oxidants for asymmetric catalytic hydroxylation of enals. Green Synth. Catal. 2021, 2, 295–298. [Google Scholar] [CrossRef]
- Li, T.; Jin, Z.; Chi, Y.R. N-heterocyclic carbene-catalyzed arene formation reactions. Sci. China Chem. 2022, 65, 210–223. [Google Scholar] [CrossRef]
- Wang, K.; Fan, R.; Wei, X.; Fang, W. Palladacyclic N-heterocyclic carbene precatalysts for transition metal catalysis. Green Synth. Catal. 2022, 3, 327–338. [Google Scholar] [CrossRef]
- Risi, C.D.; Brandolese, A.; Carmine, G.D.; Ragno, D.; Massi, A.; Bortolini, O. Oxidative N-heterocyclic carbene catalysis. Chem. Eur. J. 2023, 29, e202202467. [Google Scholar]
- Zhang, C.; Gao, Y.; Wang, H.-Y.; Zhou, B.-A.; Ye, S. Enantioselective synthesis of axially chiral benzothiophene/benzofuran-fused biaryls by N-heterocyclic carbene catalyzed arene formation. Angew. Chem. Int. Ed. 2021, 60, 13918–13922. [Google Scholar] [CrossRef]
- Feng, J.; Du, D. Asymmetric synthesis of atropisomers enabled by N-heterocyclic carbene catalysis. Tetrahedron 2021, 100, 132456. [Google Scholar] [CrossRef]
- Barik, S.; Das, R.C.; Balanna, K.; Biju, A.T. Kinetic resolution approach to the synthesis of C–N axially chiral N-aryl aminomaleimides via NHC-catalyzed [3 + 3] annulation. Org. Lett. 2022, 24, 5456–5461. [Google Scholar] [CrossRef]
- Wu, Y.-J.; Liao, G.; Shi, B.-F. Stereoselective construction of atropisomers featuring a C-N chiral axis. Green Synth. Catal. 2022, 3, 117–136. [Google Scholar] [CrossRef]
- Li, T.; Mou, C.; Qi, P.; Peng, X.; Jiang, S.; Hao, G.; Xue, W.; Yang, S.; Hao, L.; Chi, Y.R.; et al. N-heterocyclic carbene-catalyzed atroposelective annulation for access to thiazine derivatives with C-N axial chirality. Angew. Chem. Int. Ed. 2021, 60, 9362–9367. [Google Scholar] [CrossRef]
- Kerr, M.S.; Read de Alaniz, J.; Rovis, T. A highly enantioselective catalytic intramolecular Stetter reaction. J. Am. Chem. Soc. 2002, 124, 10298–10299. [Google Scholar] [CrossRef]
- He, M.; Struble, J.R.; Bode, J.W. Highly enantioselective azadiene Diels−Alder reactions catalyzed by chiral N-heterocyclic carbenes. J. Am. Chem. Soc. 2006, 128, 8418–8420. [Google Scholar] [CrossRef]
- Cardinal-David, B.; Raup, D.E.A.; Scheidt, K.A. Cooperative N-heterocyclic carbene/Lewis acid catalysis for highly stereoselective annulation reactions with homoenolates. J. Am. Chem. Soc. 2010, 132, 5345–5347. [Google Scholar] [CrossRef]
- Kerr, M.S.; Rovis, T. Enantioselective synthesis of quaternary stereocenters via a catalytic asymmetric Stetter reaction. J. Am. Chem. Soc. 2004, 126, 8876–8877. [Google Scholar] [CrossRef]
- Zhao, C.; Li, F.; Wang, J. N-heterocyclic carbene catalyzed dynamic kinetic resolution of pyranones. Angew. Chem. Int. Ed. 2016, 55, 1852–1856. [Google Scholar] [CrossRef]
- Kuwano, S.; Harada, S.; Kang, B.; Oriez, R.; Yamaoka, Y.; Takasu, K.; Yamada, K. Enhanced rate and selectivity by carboxylate salt as a basic cocatalyst in chiral N-heterocyclic carbene-catalyzed asymmetric acylation of secondary alcohols. J. Am. Chem. Soc. 2013, 135, 11485–11488. [Google Scholar] [CrossRef]
- Mew, T.W. Focus on bacterial blight of rice. Plant Pathol. 1993, 77, 5–12. [Google Scholar] [CrossRef]
- Rodl, C.B.; Vogt, D.; Kretschmer, S.B.; Ihlefeld, K.; Barzen, S.; Bruggerhoff, A.; Achenbach, J.; Proschak, E.; Steinhilber, D.; Stark, H.; et al. Multi-dimensional target profiling of N,4-diaryl-1,3-thiazole-2-amines as potent inhibitors of eicosanoid metabolism. Eur. J. Med. Chem. 2014, 84, 302–311. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).