Abstract
Ammonia (NH3) synthesis is one of the most important catalytic reactions in energy and chemical fertilizer production, which is of great significance to the sustainable development of society and the economy. The electrochemical nitrogen reduction reaction (eNRR), especially when driven by renewable energy, is generally regarded as an energy-efficient and sustainable process to synthesize NH3 in ambient conditions. However, the performance of the electrocatalyst is far below expectations, with the lack of a high-efficiency catalyst being the main obstacle. Herein, by means of comprehensive spin-polarized density functional theory (DFT) computations, the catalytic performance of MoTM/C2N (TM = 3d transition metal) for use in eNRR was systematically evaluated. Among the results, MoFe/C2N can be considered the most promising catalyst due to its having the lowest limiting potential (−0.26 V) and high selectivity in the context of eNRR. Compared with its homonuclear counterparts, MoMo/C2N and FeFe/C2N, MoFe/C2N can balance the first protonation step and the sixth protonation step synergistically, showing outstanding activity regarding eNRR. Our work not only opens a new door to advancing sustainable NH3 production by tailoring the active sites of heteronuclear diatom catalysts but also promotes the design and production of novel low-cost and efficient nanocatalysts.
1. Introduction
Ammonia (NH3) is one of the most productive inorganic compounds in the world, with an annual output of about 150 million tons. It is used for the manufacture of many important chemicals, particularly fertilizers [1]. At present, industrial NH3 synthesis still adopts a high-temperature and high-pressure Haber–Bosch process invented more than a century ago [2]. The Haber–Bosch method of ammonia synthesis has successfully altered the history of food production, fed explosive population growth, and laid the foundation for heterogeneous catalysis and chemical engineering as well. However, this synthetic method consumes 1–2% of the world’s energy supply and about 2% of the natural gas supply and also produces 1.44% of global carbon dioxide (CO2) emissions [1,3,4,5,6]. Therefore, it is necessary to find more sustainable and environmentally friendly alternatives as soon as possible to replace the energy- and carbon-intensive Haber–Bosch process [1,3,7,8]. Making full use of the abundant nitrogen (N2) in the Earth’s atmosphere and using water as the proton source, an electrochemical nitrogen reduction reaction (eNRR), especially when driven by renewable energy, is generally regarded as an energy-efficient and sustainable process to synthesize NH3 in ambient conditions [9,10]. However, the performance of the electrocatalyst is far below expectations, and the lack of a high-efficiency catalyst is the main obstacle to large-scale NH3 synthesis.
Ever since Zhang Tao and co-workers first proposed the concept of a single-atom catalyst (SAC) in 2011 [11], single-atom catalysts (SACs), considered a hot spot in the field of heterogeneous catalysis in recent years, have been widely and exhaustively studied for their high atom utilization rate and excellent catalytic performance. In the context of theory, many SACs, such as Fe-N3/graphene [12], W-C3/graphene [13], Ti-N4/graphene [14], Mo/BN [15], Mo/C2N [16], Ru/g-C3N4 [17], and single-B catalysts [18,19,20], were predicted to be high-performance eNRR catalysts. Experimental research shows that some SACs can suppress the hydrogen evolution reaction (HER) to some extent and, thus, are capable of improving Faradaic efficiency (FE) [21,22,23,24,25,26]. SACs contain only one metal center, while eNRR involves several reaction steps and intermediates. This contradiction makes it difficult for SACs to break the linear adsorption relationship of reaction intermediates and balance several reaction steps simultaneously. In order to solve this problem, a wise strategy is to introduce a second metal atom near the original single metal atom to form a diatomic active center, which helps to tune the adsorption properties of the target intermediate [27]. Such diatom catalysts (DACs) have more flexible active sites and synergistic interatomic interactions, which can maximize the potential of SACs for multistep reactions. In addition, DACs offer more possibilities than SACs, which also provide rich candidates for screening advanced catalysts [3,27,28,29].
In 2015, Baek et al. first fabricated a novel two-dimensional layered material of C2N with uniform hole distribution [30]. The unique porous structure provides ideal support for the metallic active center, while the high surface-to-volume ratio ensures the sufficient exposure of transition metal (TM) atoms to interact with the reactant molecules [31]. Therefore, C2N, as a two-dimensional base material loaded with transition metals or nonmetallic elements, has attracted more and more attention. More importantly, the well-ordered large holes in C2N offer the opportunity to anchor two TM atoms tightly together, which efficiently suppresses the diffusion and aggregation of TM atoms [32,33,34]. For example, Jiang et al. identified that Co2/C2N, Ni2/C2N, and Cu2/C2N exhibit high adsorption energies and extremely low dissociation barriers for O2 [32]. Zhou et al. designed a series of catalysts of single or double transition metal atoms anchored on a C2N monolayer and studied their catalytic performance regarding NRR. Among them, Mo2/C2N exhibits the best catalytic performance [34]. An’s research group investigated the synergy of a metallic NiCo dimer anchored on a C2N graphene matrix for promoting the CO2 reduction reaction. It was found that heterometallic NiCo/C2N outperforms homometallic Co2/C2N and Ni2/C2N in terms of catalyzing the CO2 reduction reaction toward CH4 formation, owing to synergy within the dimer [35].
So far, most research efforts have primarily focused on homonuclear DACs. However, in principle, heteronuclear DACs have more possibilities waiting for us to explore. Previous theoretical studies showed that the single-atom catalyst Mo/C2N [16] and the double-atom catalyst Mo2/C2N [34] offer good performance regarding eNRR. Considering the regular change of 3d elements from Sc to Ni (the number of 3d electrons gradually increases, the atomic radius gradually decreases, and the electronegativity gradually increases), we constructed a series of heteronuclear diatom catalysts MoTM/C2N (TM = 3d transition metal) to investigate the eNRR mechanism and performance. Through the regular tuning of 3d transition metals, it is expected that researchers will obtain better eNRR catalysts and reveal the essence of tuning. The results show that the heteronuclear diatom catalyst MoFe/C2N is the best eNRR catalyst and has the lowest limiting potential (−0.26 V), which is superior to its homonuclear counterparts, MoMo/C2N and FeFe/C2N. This work not only opens a new door to advancing sustainable NH3 production by tailoring the active sites of heteronuclear DACs but also promotes the design and production of novel low-cost and efficient nanocatalysts.
2. Results and Discussion
2.1. Geometric Structures, Stabilities, and Electronic Properties of the MoTM/C2N (TM = 3d Transition Metal)
First, the geometric structure of the C2N monolayer was optimized and the lattice parameters were calculated to be a = b = 8.32 Å, which matches well with the experimental data and previous DFT reports (Figure 1a) [30,34]. In the C2N monolayer, benzene rings are bridged by pyrazine rings to form six-membered nitrogen holes, in which the distance between two opposite nitrogen atoms is 5.51 Å [33].
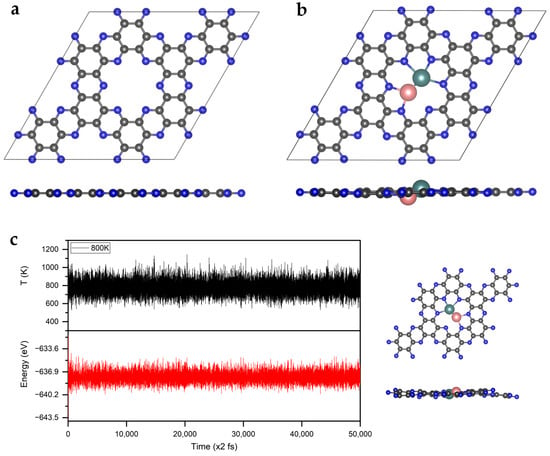
Figure 1.
The optimized structures of (a) C2N and (b) MoFe/C2N. The C, N, Mo, and Fe atoms are labeled as gray, blue, teal, and pink balls, respectively. (c) Variations in temperature and energy, shown against the time for AIMD simulations of MoFe/C2N; the inserts show the top and side views of the snapshot of the atomic configuration. The simulation is run under 800 K for 100 ps, with a time step of 2 fs.
Then, we investigated the geometric structures and the stability of MoTM/C2N (TM = 3d transition metal). The optimized structures of MoTM/C2N are presented in Figure S1 and Table S1 in the Supplementary Materials. Clearly, the framework structure of C2N can be maintained well with the decoration of Mo and TM atoms. Except for the larger atomic radius of Sc, which slightly protrudes out of the C2N plane, all the other metal atoms can be embedded into the central cavity of C2N holes and form almost coplanar structures. The bond lengths of Mo-TM are in the range of 1.93 Å (for MoV/C2N) and 2.48 Å (for MoSc/C2N). Compared with the length of the metal bond in the corresponding bulk phase, the bond lengths of Mo-TM in the system of MoTM/C2N are shorter. Such interconnections enable the heteronuclear diatom to respond cooperatively to the adsorbate, with communicative structural self-adaption and electronic transformation, which induces a different catalytic performance from that of its single-atom counterparts [3,27,36]. The binding energy (Eb) of MoTM and the Bader charge of anchored Mo and TM were calculated to examine structural stability, as shown in Table S1 in the Supplementary Materials. The smallest Eb of MoTM on C2N is −9.10 eV, suggesting strong coupling and good stability. Meanwhile, the Bader charge analysis showed that the atomic charge of the anchored TM atoms (from Sc to Ni) decreased gradually from +2.32 to +0.43, which is due to the increasing electronegativity of TM atoms (from Sc to Ni). Conversely, the atomic charge of anchored Mo atoms gradually increased.
In order to evaluate the thermal stability of MoFe/C2N, we simulated MoFe/C2N using the ab initio molecular dynamics (AIMD) method. As shown in Figure 1c, the energy fluctuation oscillates around the equilibrium state according to time evolution. Even at 800 K, the structure of MoFe/C2N remained stable after the 100 ps simulation, showing high thermal stability. This high stability stems from the strong bonding between Mo and Fe, as well as between the MoFe and N atoms of the C2N substrate. In addition, the AIMD results also show that the anchored Mo and Fe atoms cannot escape from the holes in the substrate, thus excluding the problems of diffusion and agglomeration.
2.2. Adsorption of N2
It is well known that the adsorption of reactants on the catalyst surface is the key step to initializing a reaction. In order to comprehensively study the adsorption of N2 on the catalyst’s surface, we considered seven possible adsorption configurations, in which N2 is adsorbed on the top site of a single metal or bimetallic bridge site via the end-on or side-on mode, as shown in Figure S2 in the Supplementary Materials. After full structural relaxation, the most favorable adsorption configurations of N2 on MoTM/C2N are presented in Figure S3 in the Supplementary Materials. The adsorption energies of N2 were calculated and are displayed in Figure 2a.
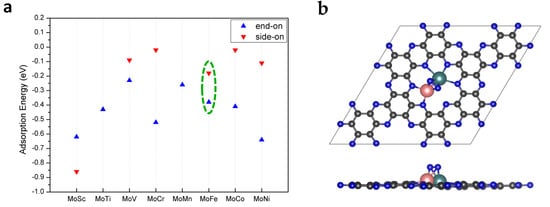
Figure 2.
(a) The adsorption energies of N2 on MoTM/C2N, with the end-on and side-on modes. (b) Adsorption of N2 on the bimetallic site of MoFe/C2N in the side-on mode. The C, N, Mo, and Fe atoms are labeled as gray, blue, teal, and pink balls, respectively.
From the point of view of adsorption energy, N2 tends to be adsorbed onto MoTM/C2N (except MoSc/C2N) via the end-on mode. Because of the geometric constraint that the Sc atom protrudes out of the catalyst surface, N2 is preferably adsorbed on MoSc/C2N via the side-on mode instead of the end-on mode. It can be clearly seen from Figure S3 in the Supplementary Materials that the site where N2 is adsorbed onto MoTM/C2N via the end-on mode is the top site of the Mo atom. However, there are two kinds of sites where N2 is adsorbed onto the catalyst surface via the side-on mode: one (MoTM/C2N, TM = Cr and Ni) is the top site of the Mo atom, and the other (MoTM/C2N, TM = Sc, V, Fe, and Co) is a bimetallic bridge site. The side-on adsorption configuration of N2 on MoFe/C2N as a representative is displayed in Figure 2b. According to previous studies [27], the moderate adsorption strengths and small differences in N2 adsorption energies between side-on and end-on adsorption configurations suggest that MoFe/C2N may be a good eNRR catalyst.
Besides the adsorption energy, the Bader charge, charge density difference, and bond length of the adsorbed N2 were also measured and are shown in Figure S3 in the Supplementary Materials. Charge density difference plots indicate that the charge transfer between the adsorbed N2 and the anchored MoTM is bidirectional, and charge accumulation and depletion can be observed on both sides. This phenomenon is in perfect accordance with the “push-pull” hypothesis, in which the anchored MoTM can “push” the d electrons into the antibonding orbitals of N2 and simultaneously “pull” the lone-pair electrons from N2. More importantly, we found that there is a good correlation between the N-N bond length and the Bader charge of N2; that is, the more negative the Bader charge of N2 is, the longer the N-N bond length is. For the side-on adsorption configurations of N2 on the bimetallic bridge site of MoTM/C2N (TM = Sc, V, Fe and Co), the Bader charge of N2 decreases to between −0.82 |e| and −1.05 |e|, with their N-N bond lengths obviously elongated to 1.213~1.293 Å (1.12 Å in the free gas phase). In the case of the side-on adsorption configurations of N2 on the top site of the Mo atom of the MoTM/C2N (TM = Cr and Ni), the Bader charge of N2 decreases to between −0.46 |e| and −0.49 |e|, with their N-N bond lengths elongated to 1.176–1.178 Å. In comparison, the end-on adsorption configurations of N2 on the top site of the Mo-atom of MoTM/C2N, the Bader charge of N2 decreases to between −0.34 |e| and 0.42 |e| with their N-N bond lengths slightly elongated to 1.138~1.143 Å. As is consistent with previous reports [27,37,38], this means that the activation of adsorbed N2 with the side-on mode is stronger than that with the end-on mode.
2.3. Catalytic Activity for eNRR
Next, we move to the assessment of the catalytic performance of MoTM/C2N for the reduction of N2 into NH3. Four possible associative NRR catalytic mechanisms, namely, distal, alternative, enzymatic, and mixed mechanisms, are possible with MoTM/C2N catalysts, as is schematically illustrated in Figure 3a. In the case of end-on adsorption, the conversion of N2 to NH3 follows a distal or alternative or mixed mechanism. In the case of side-on adsorption, the conversion of N2 to NH3 follows either an enzymatic or mixed mechanism.
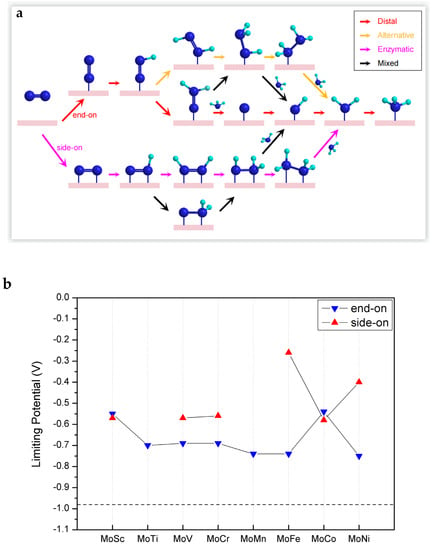
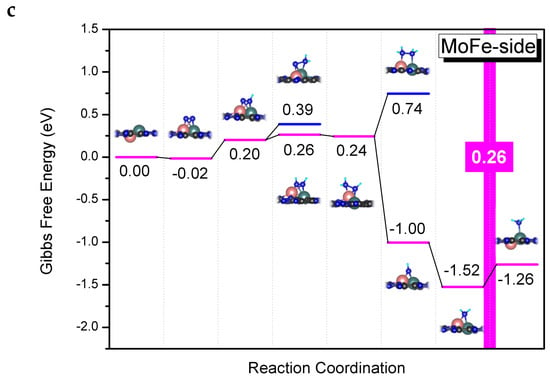
Figure 3.
(a) Schematic illustration of the possible reaction mechanisms during the N2 reduction reaction. (b) Limiting potentials for eNRR on MoTM/C2N. (c) Gibbs free energy diagrams for eNRR on MoFe/C2N in the side-on mode.
In principle, the intrinsic activity of electrocatalysts can be evaluated by limiting potential (UL) or overpotential. Considering that overpotential can be affected by electrolyte conditions, we choose the limiting potential (UL) as the evaluation standard in this paper. Among all the protonation steps, the protonation step with the largest positive Gibbs free energy difference is defined as the potential-determination step (PDS), while the lowest negative potential that promotes PDS to become exothermic is defined as the limiting potential (UL = −ΔGPDS/e). As shown in Figure 3b, the UL of all catalysts MoTM/C2N (TM = 3d transition metal) were marked, and the catalytic activity of the widely reported Ru (0001) stepped surface (−0.98 V) was taken as a benchmark. As can be seen, the catalytic activity of all candidate catalysts of MoTM/C2N is superior to that of the Ru (0001) stepped surface.
Detailed Gibbs free energy diagrams of eNRR on MoTM/C2N are given in Figures S4–S11. In the case of end-on adsorption, the PDS of eNRR is generally the first protonation step, that is, the generation of *NNH (where * stands for end-on adsorption), owing to the relatively low charge transfer from the substrate to N2. In the case of side-on adsorption, the PDS of the eNRR is the sixth protonation step, that is, the generation of the second ammonia, owing to the effective activation of N2. Among them, the limiting potential of eNRR on MoFe/C2N with side-on adsorption is the lowest (−0.26 V). Therefore, we now focus on analyzing the reaction path of eNRR on MoFe/C2N.
The Gibbs free energy diagrams of eNRR on the MoFe/C2N catalyst with side-on adsorption are presented in Figure 3c. In the reduction process, **NNH (where ** stands for side-on adsorption) is formed by the interaction between the **N2 and H+/e− pair, and the first protonation step (**N2 + H+ + e− → **NNH) is 0.22 eV uphill in the free energy profile. When **NNH is protonated further, the H+/e− pair can continue to attack the same N atom to form **NNH2 or attack another N atom to form **NHNH. Comparing the Gibbs free energy changes of these two elementary steps, we found that it prefers to go through a step (**NNH + H+ + e− → **NHNH) with a Gibbs free energy uphill of 0.06 eV. Subsequently, the **NHNH can be protonated to **NH2NH easily, and this step (**NHNH + H+ + e− → **NH2NH) is exothermic at 0.02 eV. Once the **NH2NH is formed, the first NH3 molecule can be readily desorbed from the catalyst surface, leaving *NH (the symbol * here only represents the adsorption state) on the catalyst surface and releasing 1.24 eV of heat. The fifth protonation step (*NH + H+ + e− → *NH2) is exothermic at 0.52 eV. The sixth protonation step (*NH2 + H+ + e− → *NH3) is endothermic at 0.26 eV. Among all these elementary steps, the sixth protonation step has the largest positive Gibbs free energy difference of 0.26 eV; therefore, it is considered the PDS. To sum up, the eNRR reaction on MoFe/C2N follows the mixed mechanism, and the UL is −0.26 V.
For comparison, we also investigated the catalytic performance of the homonuclear diatomic catalysts, MoMo/C2N and FeFe/C2N, with N2 side-on adsorption for eNRR, as shown in Figure 4. Note that the sixth protonation step (*NH2 + H+ + e− → *NH3) on MoMo/C2N is the PDS with a maximum free energy change of 0.77 eV, and the first protonation step (**N2 + H+ + e− → **NNH) on FeFe/C2N is the PDS with a maximum free energy change of 0.71 eV. As mentioned in the literature [38], the hydrogenation of *NH2 species to *NH3 is identified as the PDS on Mo-based catalysts, while the formation step of * NNH species is identified as the PDS on Fe-based catalysts. However, the energy changes of the first protonation step and the sixth protonation step on the heteronuclear diatomic catalyst MoFe/C2N are both small (0.22 eV and 0.26 eV, respectively). Notably, compared with MoMo/C2N and FeFe/C2N, MoFe/C2N can balance the first protonation step and the sixth protonation step synergistically, showing outstanding activity for eNRR. From this perspective, the heteronuclear diatomic catalyst is more effective than its homonuclear counterparts. Moreover, except for their high catalytic activity, MoFe-based catalysts also possess other merits, such as nontoxicity and a low price [39,40,41,42].
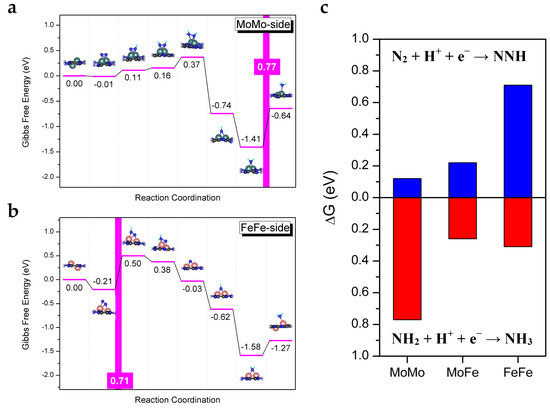
Figure 4.
(a,b) Gibbs free energy diagrams of eNRR with N2 side-on adsorption on MoMo/C2N and FeFe/C2N, respectively. (c) Comparison diagram of Gibbs free energy change of the first protonation step and the sixth protonation step on the MoMo/C2N, MoFe/C2N, and FeFe/C2N.
2.4. Selectivity of MoFe/C2N
As we all know, the hydrogen evolution reaction (HER) is the major competing reaction in eNRR and significantly affects the Faradaic efficiency of eNRR. It is necessary for an excellent eNRR catalyst to effectively suppress HER. In order to test the selectivity of MoFe/C2N for eNRR, we calculated the adsorption free energy of H on MoFe/C2N and used it to estimate the overpotential of HER. The *H structure of the adsorption site and corresponding free energy diagram are depicted in Figure 5a. The free energy barrier (0.45 eV) is considerably larger than the PDS barrier for eNRR (0.26 eV) on heteronuclear MoFe/C2N. Thus, MoFe/C2N exhibits an excellent suppression effect on HER during eNRR. In summary, as we expected, the heteronuclear diatom catalyst MoFe/C2N exhibits superior catalytic activity for eNRR, with a limiting potential of −0.26 V, and possesses high selectivity toward eNRR.
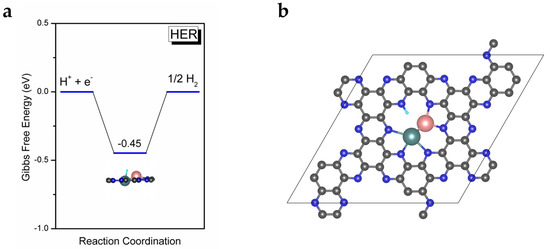
Figure 5.
(a) Gibbs free energy diagram of HER on MoFe/C2N. (b) The *H adsorption on the N site of MoFe/C2N.
Because the catalyst MoFe/C2N itself contains the N element, another problem to be solved is to exclude the nitrogen source of produced NH3 from the nitrogen-containing catalyst, that is, to judge whether the well-known Mars–van Krevelen (MvK) pathway [43,44] will occur on MoFe/C2N. It is worth noting that in the MvK pathway, a lattice N atom of the catalyst is reduced to NH3; the catalyst is subsequently regenerated with gaseous N2, in which the adsorption of *H species on the N site of the catalyst is the first and most critical step. In order to solve this problem, we examined the *H adsorption on the N site of the MoFe/C2N (Figure 5b). The result showed that the Ead of *H on the N site of MoFe/C2N is almost zero. Thus, the N site of MoFe/C2N is unlikely to be the active site for eNRR, implying that the reactant N2 molecule is the only N source for the NH3 product.
2.5. Electronic Structures
To reveal the origin of MoFe/C2N as an efficient catalyst for eNRR, the electronic structure was investigated. As can be seen from Figure 6a, before N2 adsorption on MoFe/C2N, the d orbital hybridized peaks between Mo and Fe are observed, which means that the bond of Mo and Fe is formed in the C2N substrate. After N2 adsorption with the side-on mode, the energy levels of MoFe d orbitals and N2 π* orbitals are well matched, leading to an anti-bonding orbital d-π*(unocc) above the Fermi level (located at 2.88 eV and 4.01 eV) and a bonding orbital d-π*(occ) below the Fermi level (located at −0.98 eV and −0.58 eV). This strong interaction between N2 and MoFe metal atoms leads to the formation of N-Mo and N-Fe, as well as the weakening of the N-N bond, which facilitates the subsequent reduction reaction of N2. Meanwhile, this is also clearly confirmed by the charge density difference diagram (Figure 6b). The charge accumulation (shown in yellow) is mainly distributed between N and Mo, as well as between N and Fe, indicating the formation of N-Mo bonds and N-Fe bonds. In contrast, charge depletion (shown in cyan) is mainly distributed in the N-N bond, indicating that the N-N bond is weakened.
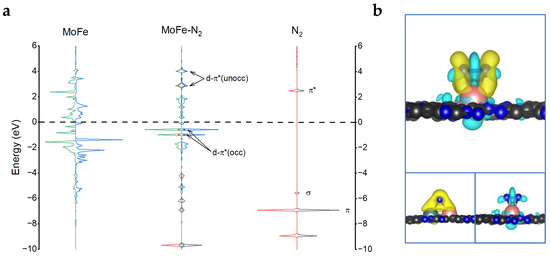
Figure 6.
(a) Projected electronic densities of states (pDOS) of MoFe (d orbitals) within the MoFe/C2N and N2 gas molecule before and after side-on adsorption. (b) Charge density differences in N2 adsorption on MoFe/C2N with the side-on mode. The charge accumulation and depletion are depicted in yellow and cyan, respectively. The isosurface value is 0.003 e/Å3.
3. Computational Methods
In this work, all the computations were carried out using the spin-polarized DFT method, which includes van der Waals (vdW) corrections, as implemented in the Vienna ab-initio simulation package (VASP) [45,46,47]. The projector-augmented-wave (PAW) method and the generalized gradient approximation (GGA) with a Perdew–Burke–Ernzerhof (PBE) exchange-correlation function were used [48,49,50]. Grimme’s semiempirical DFT-D3 scheme of dispersion correction was employed to account for the weak interactions [51,52]. Lattice parameters were fully relaxed, and the crystal geometry was fully optimized before electron structure and total energy calculations. A plane-wave cutoff energy of 600 eV was set to evaluate the core-electron interaction, and the total energy and force converged within 10−6 eV and 0.02 eV·Å−1, respectively. The convergence standard for the calculation of the vibrational frequency was set at 10−7 eV. The integration of the Brillouin zones was sampled with 2 × 2 × 1 Monkhorst–Pack meshes [53]. In order to prevent any artificial interaction between periodically repeated images, a vacuum space of 15 Å was inserted along the z direction to the monolayers. The atomic charge was computed using Bader’s charge population analysis [54]. To evaluate the stability of the catalysts, ab initio molecular dynamics (AIMD) simulations were performed in an NVT ensemble. The AIMD simulations lasted 100 ps, with a time step of 2.0 fs, and the temperature was controlled using the Nosé–Hoover method [55].
The binding energy (Eb) of the MoTM atoms was computed from the following equation:
where EMoTM/C2N, EC2N, EMo, and ETM represent the total energy of MoTM/C2N, the energy of the substrate C2N, the energy of the gas-phase Mo atom, and the energy of the gas-phase TM atom, respectively.
Eb = EMoTM/C2N − EC2N − EMo − ETM
The adsorption energy (Ead) of the adsorbates was computed according to the following equation:
where Eadsorbate + EMoTM/C2N, EMoTM/C2N, and Eadsorbate represent the total energy of the catalyst MoTM/C2N and the adsorbate, the energy of the catalyst MoTM/C2N, and the energy of free adsorbate, respectively. According to this definition, the larger the negative value of Ead is, the more stable the adsorption state is.
Ead = Eadsorbate + EMoTM/C2N − EMoTM/C2N − Eadsorbate
The computational hydrogen electrode (CHE) model proposed by Nørskov et al. was used to compute the free energy change of each elementary step for the N2 reduction reaction [56]. According to the CHE model, the chemical potential of the H+/e− pair is equal to half that of H2 with the standard conditions of pH = 0 and p(H2) = 1 bar: . The free energy change (ΔG) of each protonation step was computed according to the following equation:
where ΔE is the reaction energy obtained from DFT computations, ΔEZPE is the change of zero-point energy, T is the temperature (298.15 K), and ΔS is the entropy change. The entropies of the gas phase were taken from the NIST database. The entropies and zero-point energy of the adsorbates were calculated based on their vibrational frequencies; the entropy can be determined as follows [57,58]:
where R is the universal gas constant, h is Planck’s constant, kB represents Boltzmann’s constant, NA indicates Avogadro’s number, νi represents the frequency of the normal mode, and N is the number of adsorbed atoms. ΔGU could be computed using ΔGU = −eU, where e represents the charge transferred and U represents the potential at the electrode. ΔGpH is the correction of the H+ free energy by the concentration and can be expressed as ΔGpH = kBT ln 10 × pH.
ΔG = ΔE + ΔEZPE − TΔS + ΔGU + ΔGpH
4. Conclusions
In order to obtain more efficient electrocatalysts with higher Faradaic efficiency, we have constructed a series of heteronuclear diatomic catalysts of MoTM/C2N (TM = 3d transition metal) for eNRR by tuning the interaction between Mo and TM. Then, the stability, activity, and selectivity of MoTM/C2N catalysts have been comprehensively evaluated using the spin-polarized DFT method. Among them, MoFe/C2N has been identified as the most promising catalyst, due to its low value of UL (−0.26 V) and high selectivity toward the eNRR. In order to reach a deeper understanding, we have compared MoFe/C2N with its homonuclear counterparts, MoMo/C2N and FeFe/C2N. The results show that MoFe/C2N can balance the first protonation step and the sixth protonation step synergistically, which is the origin of its excellent activity. We hope that our work will inspire more experimental and theoretical studies to further explore the potential of heteronuclear DACs in other chemical reactions.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/molecules28104003/s1, Figure S1: Optimized structures of the most stable MoTM/C2N (TM = 3d transition metal). The C, N and Mo atoms are labeled as gray, blue and teal balls, respectively. Figure S2: Possible adsorption configurations diagram of N2 on MoTM/C2N catalysts (take MoTi/C2N as an example). The C, N, Mo and Ti atoms are labeled as gray, blue, teal and red balls, respectively. Figure S3: Optimized adsorption configurations and charge density differences of N2 chemisorbed on MoTM/C2N (TM = 3d transition metal). The charge accumulation and depletion were depicted by yellow and cyan, respectively. The isosurface value is 0.003 e/Å3. Figure S4: Gibbs free energy diagrams for NRR on MoSc/C2N. Figure S5: Gibbs free energy diagram for NRR on MoTi/C2N. Figure S6: Gibbs free energy diagrams for NRR on MoV/C2N. Figure S7: Gibbs free energy diagrams for NRR on MoCr/C2N. Figure S8: Gibbs free energy diagram for NRR on MoMn/C2N. Figure S9: Gibbs free energy diagrams for NRR on MoFe/C2N. Figure S10: Gibbs free energy diagrams for NRR on MoCo/C2N. Figure S11: Gibbs free energy diagrams for NRR on MoNi/C2N. Figure S12: Gibbs free energy diagrams for NRR on MoMo/C2N and FeFe/C2N, respectively. Figure S13: Gibbs free energy diagram of HER on MoFe/C2N. Figure S14: The *H adsorption on the N site of MoFe/C2N. Table S1: The bond length of Mo-TM (dMo-TM), the binding energy (Eb) of MoTM and the atomic charge of anchored Mo and TM in the system of MoTM/C2N (TM = 3d transition metal), and the bond length of TM-TM in their bulk phase (DTM-TM).
Author Contributions
Conceptualization, J.J.; Software, R.W.; Investigation, X.Y.; Writing—original draft, X.Y.; Writing—review & editing, X.Y. and J.J.; Visualization, P.A.; Supervision, J.J. All authors have read and agreed to the published version of the manuscript.
Funding
This work is financially supported by the National Natural Science Foundation of China (21571119) and 1331 Engineering of Shanxi Province.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Sample Availability
Not applicable.
References
- Chen, J.G.; Crooks, R.M.; Seefeldt, L.C.; Bren, K.L.; Bullock, R.M.; Darensbourg, M.Y.; Holland, P.L.; Hoffman, B.; Janik, M.J.; Jones, A.K.; et al. Beyond fossil fuel-driven nitrogen transformations. Science 2018, 360, eaar6611. [Google Scholar] [CrossRef] [PubMed]
- Haber, F.; Oordt, G.V. Über die Bildung von Ammoniak den Elementen. Anorg. Chem. 1905, 44, 341–378. [Google Scholar] [CrossRef]
- Guo, X.; Gu, J.; Lin, S.; Zhang, S.; Chen, Z.; Huang, S. Tackling the Activity and Selectivity Challenges of Electrocatalysts toward the Nitrogen Reduction Reaction via Atomically Dispersed Biatom Catalysts. J. Am. Chem. Soc. 2020, 142, 5709–5721. [Google Scholar] [CrossRef]
- Li, K.; Andersen, S.Z.; Statt, M.J.; Saccoccio, M.; Bukas, V.J.; Krempl, K.; Sažinas, R.; Pedersen, J.B.; Shadravan, V.; Zhou, Y.; et al. Enhancement of lithium-mediated ammonia synthesis by addition of oxygen. Science 2021, 374, 1593–1597. [Google Scholar] [CrossRef] [PubMed]
- Capdevila-Cortada, M. Electrifying the Haber-Bosch. Nat. Catal. 2019, 2, 1055. [Google Scholar] [CrossRef]
- Winter, L.R.; Chen, J.G. N2 Fixation by Plasma-Activated Processes. Joule 2021, 5, 300–315. [Google Scholar] [CrossRef]
- Cherkasov, N.; Ibhadon, A.O.; Fitzpatrick, P. A review of the existing and alternative methods for greener nitrogen fixation. Chem. Eng. Process. Process Intensif. 2015, 90, 24–33. [Google Scholar] [CrossRef]
- Ertl, G. Reactions at surfaces: From atoms to complexity (Nobel Lecture). Angew. Chem. Int. Ed. Engl. 2008, 47, 3524–3535. [Google Scholar] [CrossRef]
- Van der Ham, C.J.; Koper, M.T.; Hetterscheid, D.G. Challenges in reduction of dinitrogen by proton and electron transfer. Chem. Soc. Rev. 2014, 43, 5183–5191. [Google Scholar] [CrossRef]
- Seh, Z.W.; Kibsgaard, J.; Dickens, C.F.; Chorkendorff, I.; Norskov, J.K.; Jaramillo, T.F. Combining theory and experiment in electrocatalysis: Insights into materials design. Science 2017, 355, eaad4998. [Google Scholar] [CrossRef]
- Qiao, B.; Wang, A.; Yang, X.; Allard, L.F.; Jiang, Z.; Cui, Y.; Liu, J.; Li, J.; Zhang, T. Single-atom catalysis of CO oxidation using Pt1/FeOx. Nat. Chem. 2011, 3, 634–641. [Google Scholar] [CrossRef]
- Li, X.F.; Li, Q.K.; Cheng, J.; Liu, L.; Yan, Q.; Wu, Y.; Zhang, X.H.; Wang, Z.Y.; Qiu, Q.; Luo, Y. Conversion of Dinitrogen to Ammonia by FeN3-Embedded Graphene. J. Am. Chem. Soc. 2016, 138, 8706–8709. [Google Scholar] [CrossRef]
- Ling, C.; Ouyang, Y.; Li, Q.; Bai, X.; Mao, X.; Du, A.; Wang, J. A General Two-Step Strategy-Based High-Throughput Screening of Single Atom Catalysts for Nitrogen Fixation. Small Methods 2018, 3, 1800376. [Google Scholar] [CrossRef]
- Choi, C.; Back, S.; Kim, N.-Y.; Lim, J.; Kim, Y.-H.; Jung, Y. Suppression of Hydrogen Evolution Reaction in Electrochemical N2 Reduction Using Single-Atom Catalysts: A Computational Guideline. ACS Catal. 2018, 8, 7517–7525. [Google Scholar] [CrossRef]
- Zhao, J.; Chen, Z. Single Mo Atom Supported on Defective Boron Nitride Monolayer as an Efficient Electrocatalyst for Nitrogen Fixation: A Computational Study. J. Am. Chem. Soc. 2017, 139, 12480–12487. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Yu, Z.; Zhao, J. Computational screening of a single transition metal atom supported on the C2N monolayer for electrochemical ammonia synthesis. Phys. Chem. Chem. Phys. 2018, 20, 12835–12844. [Google Scholar] [CrossRef]
- Liu, X.; Jiao, Y.; Zheng, Y.; Jaroniec, M.; Qiao, S.Z. Building Up a Picture of the Electrocatalytic Nitrogen Reduction Activity of Transition Metal Single-Atom Catalysts. J. Am. Chem. Soc. 2019, 141, 9664–9672. [Google Scholar] [CrossRef]
- Ling, C.; Niu, X.; Li, Q.; Du, A.; Wang, J. Metal-Free Single Atom Catalyst for N2 Fixation Driven by Visible Light. J. Am. Chem. Soc. 2018, 140, 14161–14168. [Google Scholar] [CrossRef]
- Lv, X.; Wei, W.; Li, F.; Huang, B.; Dai, Y. Metal-Free B@g-CN: Visible/Infrared Light-Driven Single Atom Photocatalyst Enables Spontaneous Dinitrogen Reduction to Ammonia. Nano Lett. 2019, 19, 6391–6399. [Google Scholar] [CrossRef]
- Liu, C.; Li, Q.; Wu, C.; Zhang, J.; Jin, Y.; MacFarlane, D.R.; Sun, C. Single-Boron Catalysts for Nitrogen Reduction Reaction. J. Am. Chem. Soc. 2019, 141, 2884–2888. [Google Scholar] [CrossRef]
- Wang, M.; Liu, S.; Qian, T.; Liu, J.; Zhou, J.; Ji, H.; Xiong, J.; Zhong, J.; Yan, C. Over 56.55% Faradaic efficiency of ambient ammonia synthesis enabled by positively shifting the reaction potential. Nat. Commun. 2019, 10, 341. [Google Scholar] [CrossRef] [PubMed]
- Han, L.; Liu, X.; Chen, J.; Lin, R.; Liu, H.; Lu, F.; Bak, S.; Liang, Z.; Zhao, S.; Stavitski, E.; et al. Atomically Dispersed Molybdenum Catalysts for Efficient Ambient Nitrogen Fixation. Angew. Chem. Int. Ed. Engl. 2019, 58, 2321–2325. [Google Scholar] [CrossRef] [PubMed]
- He, C.; Wu, Z.-Y.; Zhao, L.; Ming, M.; Zhang, Y.; Yi, Y.; Hu, J.-S. Identification of FeN4 as an Efficient Active Site for Electrochemical N2 Reduction. ACS Catal. 2019, 9, 7311–7317. [Google Scholar] [CrossRef]
- Hui, L.; Xue, Y.; Yu, H.; Liu, Y.; Fang, Y.; Xing, C.; Huang, B.; Li, Y. Highly Efficient and Selective Generation of Ammonia and Hydrogen on a Graphdiyne-Based Catalyst. J. Am. Chem. Soc. 2019, 141, 10677–10683. [Google Scholar] [CrossRef]
- Geng, Z.; Liu, Y.; Kong, X.; Li, P.; Li, K.; Liu, Z.; Du, J.; Shu, M.; Si, R.; Zeng, J. Achieving a Record-High Yield Rate of 120.9 μgNH3 mgcat.−1 h−1 for N2 Electrochemical Reduction over Ru Single-Atom Catalysts. Adv. Mater. 2018, 30, e1803498. [Google Scholar] [CrossRef]
- Wang, X.; Wang, W.; Qiao, M.; Wu, G.; Chen, W.; Yuan, T.; Xu, Q.; Chen, M.; Zhang, Y.; Wang, X.; et al. Atomically dispersed Au1 catalyst towards efficient electrochemical synthesis of ammonia. Sci. Bull. 2018, 63, 1246–1253. [Google Scholar] [CrossRef]
- Wang, S.; Shi, L.; Bai, X.; Li, Q.; Ling, C.; Wang, J. Highly Efficient Photo-/Electrocatalytic Reduction of Nitrogen into Ammonia by Dual-Metal Sites. ACS Cent. Sci. 2020, 6, 1762–1771. [Google Scholar] [CrossRef]
- Jiao, J.; Lin, R.; Liu, S.; Cheong, W.C.; Zhang, C.; Chen, Z.; Pan, Y.; Tang, J.; Wu, K.; Hung, S.F.; et al. Copper atom-pair catalyst anchored on alloy nanowires for selective and efficient electrochemical reduction of CO2. Nat. Chem. 2019, 11, 222–228. [Google Scholar] [CrossRef]
- Cao, N.; Chen, Z.; Zang, K.; Xu, J.; Zhong, J.; Luo, J.; Xu, X.; Zheng, G. Doping strain induced bi-Ti3+ pairs for efficient N2 activation and electrocatalytic fixation. Nat. Commun. 2019, 10, 2877. [Google Scholar] [CrossRef]
- Mahmood, J.; Lee, E.K.; Jung, M.; Shin, D.; Jeon, I.Y.; Jung, S.M.; Choi, H.J.; Seo, J.M.; Bae, S.Y.; Sohn, S.D.; et al. Nitrogenated holey two-dimensional structures. Nat. Commun. 2015, 6, 6486. [Google Scholar] [CrossRef]
- Mahmood, J.; Jung, S.M.; Kim, S.J.; Park, J.; Yoo, J.W.; Baek, J.B. Cobalt Oxide Encapsulated in C2N-h2D Network Polymer as a Catalyst for Hydrogen Evolution. Chem. Mater. 2015, 27, 4860–4864. [Google Scholar] [CrossRef]
- Li, X.; Zhong, W.; Cui, P.; Li, J.; Jiang, J. Design of Efficient Catalysts with Double Transition Metal Atoms on C2N Layer. J. Phys. Chem. Lett. 2016, 7, 1750–1755. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.W.; Yan, J.M.; Jiang, Q. Single or Double: Which Is the Altar of Atomic Catalysts for Nitrogen Reduction Reaction? Small Methods 2018, 3, 1800291. [Google Scholar] [CrossRef]
- Zhang, X.; Chen, A.; Zhang, Z.; Zhou, Z. Double-atom catalysts: Transition metal dimer-anchored C2N monolayers as N2 fixation electrocatalysts. J. Mater. Chem. A 2018, 6, 18599–18604. [Google Scholar] [CrossRef]
- Huang, Q.; Liu, H.; An, W.; Wang, Y.; Feng, Y.; Men, Y. Synergy of a Metallic NiCo Dimer Anchored on a C2N-Graphene Matrix Promotes the Electrochemical CO2 Reduction Reaction. ACS Sustain. Chem. Eng. 2019, 7, 19113–19121. [Google Scholar] [CrossRef]
- Kresse, G.; Furthmüller, J. Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys. Rev. B 1996, 54, 11169–11186. [Google Scholar] [CrossRef]
- Hafner, J. Ab-initio simulations of materials using VASP: Density-functional theory and beyond. J. Comput. Chem. 2008, 29, 2044–2078. [Google Scholar] [CrossRef] [PubMed]
- Kresse, G.; Furthmüller, J. Efficiency of ab-initio total energy calculations for metals and semiconductors using a plane-wave basis set. Comput. Mater. Sci. 1996, 6, 15–50. [Google Scholar] [CrossRef]
- Blochl, P.E. Projector augmented-wave method. Phys. Rev. B Condens. Matter. 1994, 50, 17953–17979. [Google Scholar] [CrossRef]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized Gradient Approximation Made Simple. Phys. Rev. Lett. 1996, 77, 3865–3868. [Google Scholar] [CrossRef]
- Kresse, G.; Joubert, D. From ultrasoft pseudopotentials to the projector augmented-wave method. Phys. Rev. B 1999, 59, 1758–1775. [Google Scholar] [CrossRef]
- Grimme, S.; Antony, J.; Ehrlich, S.; Krieg, H. A consistent and accurate ab initio parametrization of density functional dispersion correction (DFT-D) for the 94 elements H-Pu. J. Chem. Phys. 2010, 132, 154104. [Google Scholar] [CrossRef]
- Grimme, S.; Ehrlich, S.; Goerigk, L. Effect of the damping function in dispersion corrected density functional theory. J. Comput. Chem. 2011, 32, 1456–1465. [Google Scholar] [CrossRef]
- Monkhorst, H.J.; Pack, J.D. Special points for Brillouin-zone integrations. Phys. Rev. B 1976, 13, 5188–5192. [Google Scholar] [CrossRef]
- Sanville, E.; Kenny, S.D.; Smith, R.; Henkelman, G. Improved grid-based algorithm for Bader charge allocation. J. Comput. Chem. 2007, 28, 899–908. [Google Scholar] [CrossRef]
- Martyna, G.J.; Klein, M.L.; Tuckerman, M. Nosé–Hoover chains: The canonical ensemble via continuous dynamics. J. Chem. Phys. 1992, 97, 2635–2643. [Google Scholar] [CrossRef]
- Nørskov, J.K.; Rossmeisl, J.; Logadottir, A.; Lindqvist, L.R.K.J.; Kitchin, J.R.; Bligaard, T.; Jonsson, H. Origin of the Overpotential for Oxygen Reduction at a Fuel-Cell Cathode. J. Phys. Chem. B 2004, 108, 17886–17892. [Google Scholar] [CrossRef]
- Wang, V.; Xu, N.; Liu, J.-C.; Tang, G.; Geng, W.-T. VASPKIT: A user-friendly interface facilitating high-throughput computing and analysis using VASP code. Comput. Phys. Commun. 2021, 267, 108033. [Google Scholar] [CrossRef]
- Zhu, Y.-A.; Chen, D.; Zhou, X.-G.; Yuan, W.-K. DFT studies of dry reforming of methane on Ni catalyst. Catal. Today 2009, 148, 260–267. [Google Scholar] [CrossRef]
- Li, Q.K.; Li, X.F.; Zhang, G.; Jiang, J. Cooperative Spin Transition of Monodispersed FeN3 Sites within Graphene Induced by CO Adsorption. J. Am. Chem. Soc. 2018, 140, 15149–15152. [Google Scholar] [CrossRef]
- Hu, R.; Li, Y.; Zeng, Q.; Wang, F.; Shang, J. Bimetallic Pairs Supported on Graphene as Efficient Electrocatalysts for Nitrogen Fixation: Search for the Optimal Coordination Atoms. ChemSusChem 2020, 13, 3636–3644. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Zhao, Z.; Cai, Q.; Yin, L.; Zhao, J. Nitrogen electroreduction performance of transition metal dimers embedded into N-doped graphene: A theoretical prediction. J. Mater. Chem. A 2020, 8, 4533–4543. [Google Scholar] [CrossRef]
- Ashida, Y.; Arashiba, K.; Nakajima, K.; Nishibayashi, Y. Molybdenum-catalysed ammonia production with samarium diiodide and alcohols or water. Nature 2019, 568, 536–540. [Google Scholar] [CrossRef]
- Cheng, H.; Ding, L.X.; Chen, G.F.; Zhang, L.; Xue, J.; Wang, H. Molybdenum Carbide Nanodots Enable Efficient Electrocatalytic Nitrogen Fixation under Ambient Conditions. Adv. Mater. 2018, 30, e1803694. [Google Scholar] [CrossRef]
- Piascik, A.D.; Li, R.; Wilkinson, H.J.; Green, J.C.; Ashley, A.E. Fe-Catalyzed Conversion of N2 to N(SiMe3)3 via an Fe-Hydrazido Resting State. J. Am. Chem. Soc. 2018, 140, 10691–10694. [Google Scholar] [CrossRef]
- Qin, B.; Li, Y.; Zhang, Q.; Yang, G.; Liang, H.; Peng, F. Understanding of nitrogen fixation electro catalyzed by molybdenum-iron carbide through the experiment and theory. Nano Energy 2020, 68, 104374. [Google Scholar] [CrossRef]
- Abghoui, Y.; Skúlason, E. Onset potentials for different reaction mechanisms of nitrogen activation to ammonia on transition metal nitride electro-catalysts. Catal. Today 2017, 286, 69–77. [Google Scholar] [CrossRef]
- Abghoui, Y.; Skúlason, E. Electrochemical synthesis of ammonia via Mars-van Krevelen mechanism on the (111) facets of group III–VII transition metal mononitrides. Catal. Today 2017, 286, 78–84. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).