Abstract
P. maritimum L., belonging to the Amaryllidaceae family, is a species that grows on beaches and coastal sand dunes mainly on both sides of the Mediterranean Sea and Black Sea, the Middle East, and up to the Caucasus region. It has been largely investigated due to its several interesting biological properties. With the aim of providing new insights into the phytochemistry and pharmacology of this species, the ethanolic extract of the bulbs from a local accession, not previously studied, growing in Sicily (Italy), was investigated. This chemical analysis, performed by mono- and bi-dimensional NMR spectroscopy, as well as LC-DAD-MSn, allowed to identify several alkaloids, three of which were never detected in the genus Pancratium. Furthermore, the cytotoxicity of the preparation was assessed in differentiated human Caco-2 intestinal cells by trypan blue exclusion assay, and its antioxidant potential was evaluated using the DCFH-DA radical scavenging method. The results obtained demonstrate that P. maritimum bulbs’ extract exerts no cytotoxic effect and is able to remove free radicals at all the concentrations tested.
1. Introduction
The family of Amaryllidaceae includes about sixty genera with more than 850 species, and it is largely spread all over the world, with South America and South Africa being the regions with major diversity [1], whereas in the Mediterranean region, only eight genera are present.
Among all the genera of Amaryllidaceae, Pancratium is the most widespread [2], distributed in Africa, South Europe, the Middle East, the Arabian Peninsula, and the Indian area [3]. The genus takes its name from the Greek word “pagkration”, meaning almighty, due to the numerous medicinal properties of some species, and, according to POWO [3], twenty-four taxa have the rank of accepted species.
Pancratium maritimum L. (marine narcissus, sea daffodil) (Figure 1) is a bulbous perennial plant with a long neck and glaucous, broadly linear leaves, evergreen, but the leaves often die back during hot summers. The white flowers (3–15 in an umbel) are long, up to 15 cm, with a corona two-thirds as long as the tepals. It flowers from August up to October [4,5].

Figure 1.
P.maritimum L. (left) and its bulbs (right).
It mainly grows on beaches and coastal sand dunes, often with some leaves in the sand, and it is native to both sides of the Mediterranean Sea and Black Sea, the Middle East, and up to the Caucasus region [3]. However, recently, due to urbanization, tourism development, alteration, and destruction of dune systems, its populations have drastically declined [6].
The first phytochemical study on this plant dates from 1954 [7] and is concerned with the identification of some alkaloids. Since then, several other studies have shown the occurrence of many alkaloids belonging to the lycorine-, lycorenine-, montaninetazettine-, galanthamine-, haemanthidine-, crinine-, isocarbostiryl-, narciclasine-, and pancratistatin groups, all biogenetically formed from norbelladines [8]. Alkaloids with these skeletons have been identified in plants, mainly bulbs, collected in Egypt [9,10,11,12,13,14,15,16], Bulgaria [17], Turkey [18,19,20,21,22], Tunisia [23], Portugal [24], Spain [25,26], and Italy (Calabria) [27]. The occurrence of the alkaloids in the bulbs of the different accessions is reported in Table 1.

Table 1.
Alkaloids in the bulbs of other accessions of Pancratium maritimum L. (P. maritimum).
Apart from alkaloids, other metabolites have been identified in the bulbs or aerial parts of Pancratium maritimum L. (P. maritimum) such as chromones, [30,31,32], flavonoids [30,31,33], phenylpropanoids [16], phenolic acids [33,34], and alginates [23,35].
Furthermore, the extracts of different accessions of P. maritimum, as well as the pure isolated compounds, have been investigated for their cytotoxic [14,16,24,27,36,37,38,39], antimicrobial [14,15,40,41], antiviral [27], antioxidant [34,38], antinociceptive [42], amoebicidal [43], antimalarial [22], and acetylcholinesterase inhibitory properties [18,44,45].
In the frame of the ongoing research on Mediterranean plants with antioxidant properties [46,47,48,49,50], we describe here for the first time the chemical composition of a not previously studied accession of P. maritimum growing wild in Sicily. HPLC-DAD analysis is frequently applied for the detection of such compounds in Amarillidaceae and allows the separation of different derivatives and their quantification. LC-MS and GC-MS have also been used [51] to detect such constituents in different plant extracts, offering the opportunity for their quali/quantitative analysis also in low concentrations. In this work, a simple HPLC-DAD-MS method using reverse-phase chromatography was used. In addition, its cytotoxic and antioxidant activities were evaluated on differentiated Caco-2 cells. Caco-2 cell cultures, even if cancerous in origin, undergo a gradual differentiation process in enterocytes that takes place spontaneously once confluence has been reached. Thus, they represent an established “in vitro” test system for the evaluation of the biological activity of natural compounds [52].
2. Results and Discussion
2.1. Chemical Characterization
2.1.1. Nuclear Magnetic Resonance Analysis of Bulbs’ Extract from P. maritimum
To establish the presence of main constituents, 1H-NMR, HSQC-DEPT, HMBC, COSY, TOCSY, and NOESY spectra (Supplementary Materials) were acquired and allowed to recognize the presence of different constituents. 1H-NMR spectra (Supplementary Materials) showed a crowded region due to carbohydrate constituents, and, due to the presence of broad peaks, at least part of these compounds can be related to the presence of oligo or polysaccharides. Several signals can be diagnostic of Amaryllidaceae alkaloids, namely a series of N-linked CH presenting δH 3.20–3.60 and carbon resonances at δC 52.0–58.0. Furthermore, aromatic signals and singlets support the presence of the methylenedioxy group. In particular, some diagnostic signals can be ascribed to the presence of lycorin-type alkaloids and are highlighted in the Figure 2 that present the HSQC-DEPT spectrum of the extract. Main signals are the N-linked CH2 assigned to the positions C-4 (δH 2.42; δC 33.72), C-5 (δH 3.32; δC 52.97), the CHOH C-1 at (δH 4.57; δC 7.051), C-2 (δH 4.27; δC 71.8), and the sp2 CH in position C-3 (δH 5.64; δC 117.3). H-2, H-3 coupling is observed in the COSY spectrum; furthermore, in the TOCSY spectrum, we can observe cross peaks that allow to establish connections between H-5, H-4, H-3, and H-2. Other diagnostic signals can be related to the aromatic part of the compound, namely the C-8 (δH 6.74; δC 107.8), C-11 (δH 6.98; δC 104.8), and the methylene dioxy C-12 (δH 6.02; δC 101.07) that are part of one aromatic ring, as demonstrated by the HMBC correlation observed from the H-12 and from the H-11 and H-8 to the oxygenated carbons C-9 and C-10. The obtained information suggests the presence of the pyrrolo [d,e] phenanthridine ring system and is in agreement with literature data for lycorine-type alkaloids [53,54,55,56]. Signals also suggest the presence of crinamidine-type alkaloids [53,54,55,56], namely due to the observation of CH linked to epoxy groups ascribable to C-1 (δH 3.77; δC 54.3) and C-2 (δH 3.26; δC 53.3) and the latter coupling with CHOH at C-3 (δH 4.46; δC 70.8). Furthermore, N-linked CH2 C-6 (δH 4.19–3.53; δC 56.4) and C-12 (δH 3.31–2.43; δC 53.1) can be observed. The presence of lycorenine or oduline-type alkaloids can be suggested due to the resonance observed at δH 5.93; δC 97.10 that appears as a singlet ascribable to position C-6 that is emiacetalic, and the C-3 observed at δH 5.53; δC 117.3 being in good agreement with the assignment reported from Codina et al. [57].
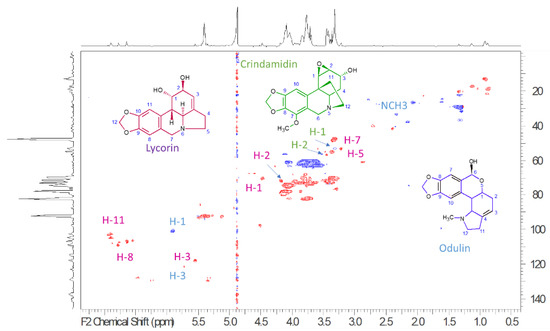
Figure 2.
HSQC-DEPT of ethanol bulbs’ extract of P. maritimum, blue peaks are representing the CH2, while red peaks are the CH and CH3. In the spectrum the diagnostic positions assigned to the most abundant alkaloid derivatives are reported in different colour. Lycorin diagnostic signals are highlighted in purple color, crindamidin in green color, and odulin in blue color.
2.1.2. LC-DAD-MSn Analysis of Bulbs’ Extract from P. maritimum
The DAD trace is reported in Figure 3 and revealed the presence of seven main peaks that present a UV/visible spectrum compatible with lycorine, oduline, and crindamine-type alkaloids. In order to obtain structural information on the eluted compounds, mass spectrometry in positive ion mode using an electrospray ion source was used. An ion trap was the analyzer, and multiple-stage fragmentation mass spectrometry was used to generate fragmentation schemes for each eluted species. The experimental data (see Supplementary Materials) were compared with the literature [58,59,60,61,62,63], allowing tentative identification of the compounds.
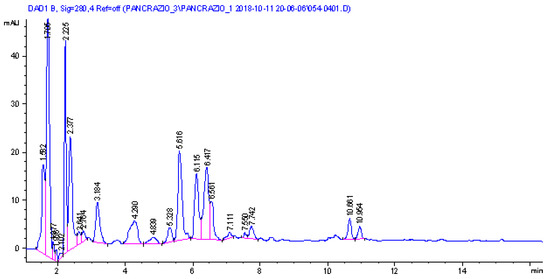
Figure 3.
LC-DAD of the methanol bulbs extract of P. maritimum.
Main ions ascribable to alkaloids were observed at m/z 334, 288, 302, 318, and 306. Two main peaks presenting m/z 334 were observed at 1.8 and 2.3 min, and their fragmentation allows for a tentative assignment to 2-hydroxy-3-dihydro-6-O-methyl-oduline and one isomer. Four peaks presenting [M + H]+ at m/z 288 and a similar fragmentation pattern were assigned to lycorine isomers. Due to the presence of the two different OH groups in Lycorine, the four different peaks with identical MS/MS fragmentation suggest the occurrence in the sample of the four isomers due to the orientation of positions 1 and 2. Six different peaks present [M + H]+ at m/z 302, and we can observe two different schemes of fragmentation ascribable to oduline for the peaks with retention times at minutes 3.33, 4.08, and 4.40, while tentatively assigned to methoxy lycorine for the peaks at retention times 6.05, 7.38, and 7.84 min. More in detail, the oduline diagnostic fragments were the ions at m/z 284, 266, 255, while for the methoxy lycorine, the ions were at m/z 270, 266, 252, 227, and 182. One relevant peak was observed at 5.6 min, presenting [M + H]+ at m/z 318, and it was assigned to crinamidine. Two peaks were observed presenting [M + H]+ at m/z 306 and were assigned to crinamabine and its isomer.
For quantitative purposes, galanthamine was used as a reference compound due to its commercial availability and relative similarity in structure. Galanthamine was not detectable in the analyzed extract. The amount of alkaloids detected in the bulbs’ extract is reported in Table 2.

Table 2.
LC-DAD-ESI-MSn data and putative identification and semiquantitative amount % of metabolites in the bulbs’ extract of P. maritimum.
The structure of the most abundant organic constituents of the bulbs’ extract of P. maritimum is reported in Figure 4.
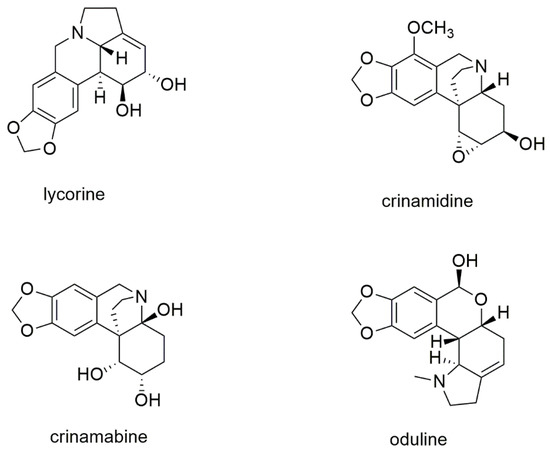
Figure 4.
Structure of the most abundant alkaloids in the bulbs’ extract of P. maritimum.
The main constituent of the extract was lycorine, an alkaloid found practically in all the other accessions of P. maritimun studied so far (Table 1). On the other hand, it is very interesting to point out that the other three main alkaloids, crinamidine, crinamabine, and oduline, have never been detected in any other extract of P. maritimun previously investigated, nor in any other species of the genus Pancratium.
Crinamabine was first isolated from the bulbs of Crinum amabile Donn. (Amaryllidaceae), collected in Vietnam [64], and successively identified only in the leaves of Crinum asiaticum L. var. sinicum from Taipei [65]. Oduline has been identified in the bulbs of Narcissus pseudonarcissus subsp. pseudonarcissus cv. Carlton (Amaryllidaceae) [66] and Narcissus poeticus cv. Golden Ducat [67], from plants cultivated in Holland and the Czech Republic, respectively, and in the bulbs of two Chinese species, Lycoris aurea Herb. (Amaryllidaceae) [68] and Lycoris radiata Herb. [69]. The distribution of crinamidine is quite large. In fact, it has been detected in the bulbs of several Amaryllidaceae species such as Crinum bulbispermum Milne-Redhead et Schweickerdt [70], C. latifolium (L.) [71], C. macowanii Baker [72], Nerine bowdenii W. Wats [73,74], N. flexuasa Herb. [75], Brunsvigia gregaria R. A. [76], B. orientalis (L.) Ait ex Eckl [77], Ammocharis tinneana (Kotschy & Peyr.) Milne-Redh. & Schweick [78], and Zephyranthes grandiflora [79], as well as, recently, in the leaves of Carica papaya L. [80], belonging to the Caricaceae family.
2.2. Absence of Cytotoxic Effect by P. maritimum Bulbs’ Extract on Human Intestinal Cells
The first step in evaluating the suitability of plant extracts for further applications is the assessment of their potential cytotoxicity (Figure 5). In our study, the trypan blue exclusion method on Caco-2 differentiated cells was used. Cells were treated for 24 h with concentrations of P. maritimum bulbs’ extract ranging from 0.05 to 0.2 mg/mL. As shown in Figure 6, no significant effect on cell viability was observed at all the concentrations tested. In particular, the maximal decrease in cell viability observed, down to 84% compared to controls, was detected at a concentration of 0.2 mg/mL. Although cytotoxic effects of P. maritimum and its alkaloid components have been reported in different tumor cell lines [16,39,81,82], our data are in agreement with the observations indicating the absence of anti-proliferative and death-inducing effects in normal cell culture, as in human lymphocytes [39].
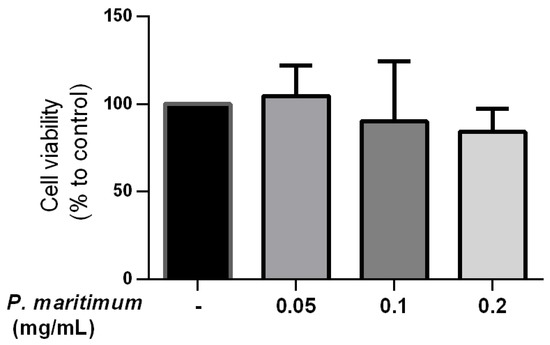
Figure 5.
Effect of P. maritimum bulbs’ extract on cell viability in differentiated Caco-2. The cytotoxic effects of exposure to P. maritimum bulbs’ extract (0.05–0.2 mg/mL) for 24 h were assessed by trypan blue assay. The data were represented as means ± SEM of three independent experiments.
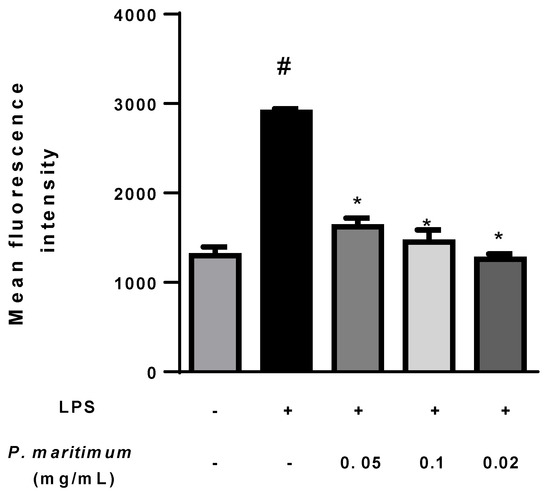
Figure 6.
Effect of P. maritimum bulbs’ extract on intracellular reactive oxygen species (ROS) production in lipopolysaccharide (LPS)-stimulated differentiated Caco-2 cells. Intracellular ROS was analyzed using fluorescent probe H2DCF-DA in cells untreated (negative control (−)), treated with only 0.1 mg/mL LPS ((+) positive control), or co-treated with P. maritimum bulbs’ extract (0.05–0.2 mg/mL) and with LPS (0.1 mg/mL) for 24 h. The ROS production was determined as mean fluorescence intensity of dichlorofluorescein (DCF). The data were represented as means ± SEM of three independent experiments. Confidence intervals calculated by one-way ANOVA and Tukey post hoc test: # significant vs. untreated control cells: p < 0.001; * significant vs. LPS-treated cells: p < 0.001.
2.3. Down-Regulation of Reactive Oxygen Species (ROS) Production in LPS-Induced Cells by P. maritimum Bulbs’ Extract
Many studies have shown that ROS production, which leads to oxidative stress, plays a major role in the hyperactivation of inflammatory processes [83].
The inflammatory mediators, such as cytokines, accelerate the accumulation of intracellular ROS that, as secondary messengers, would participate in the inflammatory signaling pathway [84].
Several studies report the antioxidant potential of P. maritimum stems, flowers, and bulbs [38,44,85]. These effects appear to be related to the high contents of phenols and flavonoids [33] and also to the lycorine alkaloid [86].
The effect of our bulb extracts on intracellular ROS levels was investigated using a cell-based assay in which H2DCF-DA, a fluorescent probe, is used as an indicator of ROS and oxidative stress. The H2DCF-DA probe passively diffuses into the cells and is hydrolyzed by intracellular esterases to form the non-fluorescent 2′,7′-dichlorofluorescein (H2DCF). In the presence of ROS, intracellular oxidases, and oxidants, H2DCF is oxidized to fluorescent 2′,7′-dichlorofluorescein (DCF), remaining in the cells [87]. Antioxidants would prevent the generation of DCF.
To induce inflammation, Caco-2 cells were stimulated with lipopolysaccharide (LPS) in order to produce pro-inflammatory cytokines and other inflammatory mediators, including ROS [88]. As expected, we observed in differentiated Caco-2 cells exposed to 0.1 mg/mL of LPS for 24 h an enhanced ROS production of about 130% compared to the control (Figure 6).
The treatment with increasing concentrations of P. maritimum bulbs’ extract down-regulated ROS production compared to the positive control, suggesting an antioxidant effect.
We can conceivably suppose a pivotal role in the down-regulation of ROS production played by alkaloids such as lycorine and lycorine-like compounds present in the composition of Sicilian P. maritimum bulbs’ extract. However, further studies are needed to solve this issue. Moreover, it will be interesting to investigate whether the inhibition of ROS generation induced by P. maritimum extract may have the potential to inhibit the expression of pro-inflammatory mediators and cytokines.
3. Materials and Methods
3.1. Plant Material
Bulbs of P. maritimum L. (see Figure 1) were collected from plants at their full flowering stage in September 2021 at Salinelle (38°01′48″ N, 13°18′40″ E, 16 m s/l), Lascari (Palermo), in Sicily, Italy, on a sandy soil. Typical specimens, identified by Mr. Emanuele Schimmenti, have been deposited in the Department STEBICEF, University of Palermo, Palermo, Italy (voucher No. MB 385/21).
3.2. Extraction of Plant Material
The ethanolic extract of P. maritimum was obtained from bulbs according to the following procedure. After washing and air-drying, the bulbs’ parts were pulverized by using an electric blender. About 100 g of the dried sample was soaked in 99% ethanol in a 1000 mL volumetric flask. The flask was covered and allowed to stand for 48 h at room temperature. After filtration, the excess solvent was removed by using a rotary evaporator. The dried residue (7.23% yield) was stored in an air-sealed analytical container at 4 °C.
3.3. Chemical Analysis
Bulbs’ extract was dissolved in deuterated methanol (10 mg/mL) and used for the 1H NMR and 2D NMR (HSQC, HMBC, and COSY) experiments in order to assess the presence of the main constituents.
Heteronuclear correlation spectroscopy gives signals based on coupling between nuclei of two different types. Heteronuclear single-quantum correlation spectroscopy (HSQC) detects correlations between nuclei of two different types that are separated by one bond. Heteronuclear multiple-bond correlation spectroscopy (HMBC) detects heteronuclear correlations over longer ranges of about 2–4 bonds. The homonuclear correlation spectroscopy (COSY) sequence is used to identify spins that are coupled to each other.
NMR data were considered a preliminary investigation, and then, in order to assess the presence of the compounds in the extract and also to perform quantitative analysis, the extract was subjected to LC-DAD-MS/MS in an ion trap in positive ion mode.
Qualitative characterization and quantitative analysis of metabolites from bulbs’ extract of P. maritimum were achieved by liquid chromatography hyphenated with photodiode-array detection and tandem electrospray ionization mass spectrometry (LC-DAD-ESI-MSn) in an ion trap in positive ion mode. The extract of the bulbs was dissolved in methanol and sonicated, obtaining a solution at 5 mg/mL. After centrifugation, the solution was used for analysis by means of an Agilent 1260 chromatograph equipped with a diode array (DAD) 1260 series and an MS 500 ion trap mass spectrometer. Spectra were acquired in positive ion mode in the range of m/z 50–1000. Fragmentation of the main ionic species was obtained using the turbo data-dependent scan (tdds) function of the instrument. UV spectra were obtained in the DAD detector in the range of 200–400 nm, and galanthamine chloridrate was used as a reference compound and also as a reference for the semiquantification of alkaloids. A stock solution of galantamine chloridrate was prepared in methanol at a concentration of 50 µg/mL, diluted to 20 µg/mL, 10 µg/mL, 5 µg/mL, and 1 µg/mL, and used to perform a calibration curve.
3.4. Cell Culture, Differentiation and Treatments
The Caco-2 cells, taken from laboratory stocks, were cultured in a high-glucose Dulbecco’s Modified Eagle’s Medium (DMEM) (Sigma-Aldrich, Inc., St. Louis, MO, USA) supplemented with 10% fetal bovine serum (Life Technologies, Carlsbad, CA, USA) and antibiotics (100 U/mL penicillin and 100 μg/mL streptomycin) (Sigma-Aldrich, Inc., St. Louis, MO, USA) at 37 °C in a humidified 5% CO2 incubator. For the experimental studies, 3.5 × 104 cells/cm2 were grown in 24-well plates and used after 21 days, when fully differentiated. For this purpose, once cells reached 100% confluence four days after seeding, the medium was changed every other day. The dome formation, as a hallmark of the differentiated phenotype, was observed under the microscope [89].
The dry extract was dissolved in DMSO and added to the cell medium at concentrations ranging from 0.05 to 0.2 mg/mL, as previously reported [38,44].
3.5. Trypan Blue Exclusion Assay
Cell viability was assessed using the trypan blue exclusion assay [90]. Briefly, the differentiated Caco-2 cells were treated with different concentrations of the extract (0.05–0.2 mg/mL) and DMSO vehicle for 24 h, trypsinized with a 1:30 dilution of standard trypsin-EDTA solution, resuspended in DMEM, and stained with a 0.4% trypan blue dye solution. The number of unstained viable cells was quantitated in a Burker hemocytometer under a phase contrast microscope. The percentage of cell viability was calculated as the number of treated viable cells/number of control viable cells × 100. Each experiment was performed in triplicate.
3.6. Assay for Cellular Antioxidant Activity
The intracellular accumulation of ROS was evaluated using the ROS Detection Assay Kit (Canvax Biotech, Cordoba, Spain), which uses the cell-permeant reagent dichlorodihydrofluorescein-diacetate (H2DCF-DA), a fluorogenic dye that measures hydroxyl, peroxyl, and other ROS activity within the cell. After diffusion into the cell, the acetyl groups on H2DCF-DA are cleaved by intracellular esterase to yield the non-fluorescent compound, which is rapidly oxidized to highly fluorescent 2′,7′-dichlorodihydrofluorescein by ROS. The fluorescence intensity is proportional to the ROS levels within the cell cytosol.
The Caco-2 cells, differentiated in 24-well cell culture plates, were exposed to 0.1 mg/mL LPS supplemented with the extract at concentrations of 0.05, 0.1, and 0.2 mg/mL. The negative control (DMSO vehicle only) and the positive control (0.1 mg/mL LPS only) were included in the assay.
ROS production was analyzed by flow cytometry [91]. Three assays were performed on treated and control cells using a FACSCanto instrument (BD Biosciences, Franklin Lakes, NJ, USA) in the FL1 channel (Ex/Em = 485/530). Ten thousand events were assessed, and the obtained data were analyzed with the Floreada analysis tool available at https://floreada.io (accessed on 10 December 2022). Results are expressed as the mean intensity fluorescence of DCF (λ = 495 nm).
3.7. Statistical Analyses
Data are reported as the mean ± SEM. A one-way analysis of variance (ANOVA) followed by Tukey’s test was performed in order to highlight possible significant differences between groups (p < 0.001) using the GraphPad Prism 6 software (GraphPad Software, San Diego, CA, USA).
4. Conclusions
In conclusion, our findings suggest that Sicilian P. maritimum L. is a good source of Amaryllidaceae alkaloids, namely lycorine, oduline, and crindamine. The obtained data revealed that this sample presents a peculiar alkaloid profile with respect to the other accessions studied so far. This suggests the need for further studies on Sicilian P. maritimum L. to deeply investigate the chemical diversity of this plant material that can become a valuable source of bioactive constituents. Furthermore, the obtained data indicate that this plant can supply antioxidant compounds as potent radical scavengers by inhibiting ROS production while conversely exerting no cytotoxic activity on differentiated Caco-2 cells as a model of the gut epithelium at the range of concentrations tested under the experimental conditions used in the present work.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/molecules28103986/s1. NMR and LCMS data of Pancratium bulb extract. Figure S1: H-NMR of Pancratium bulb extract. Figure S2: HSQC-DEPT of Pancratium bulb extract. Figure S3: HMBC of Pancratium bulb extract. Figure S4: COSY of Pancratium bulb extract. Figure S5: TOCSY of Pancratium bulb extract. Figure S6: NOESY of Pancratium bulb extract. Table S1: Principal assignments of identified alkaloids in Bulb extract as obtained from NMR spectra. Figure S7: chromatogram related to specie at m/z 334. Figure S8: specie at m/z 334, RT 1.7 min. Figure S9: specie at m/z 334, RT 2.3 min. Figure S10: chromatogram related to specie at m/z 288. Figure S11: specie at m/z 288 lycorine RT 1,9 min. Figure S12: specie at m/z 288 epilycorine RT 2.45 min. Figure S13: specie at m/z 288 lycorine type RT 3.8 min. Figure S14: specie at m/z 288 lycorine type RT 3.9 min. Figure S15: chromatogram related to specie at m/z 302. Figure S16: specie at m/z 302 oduline RT 3.3 min. Figure S17: specie at m/z 302 RT 4.4 min. Figure S18: specie at m/z 302 RT 4.5 min. Figure S19: specie at m/z 302 RT 6.0 min lycorine derivatives. Figure S20: specie at m/z 302 RT 7.3 min. Figure S21: specie at m/z 302 RT 7.84 min. Figure S22: chromatogram related to specie at m/z 318. Figure S23: specie at m/z 318 crinamidine RT 5.6 min. Figure S24: chromatogram related to specie at m/z 306. Figure S25: specie at m/z 306 RT 5 min. Figure S26: specie at m/z 306 RT 7 min. Figure S27: chromatogram related to specie at m/z 494. Figure S28: specie at m/z 494 RT 4.5 min.
Author Contributions
Conceptualization, A.C., M.B. and M.G.Z.; methodology, A.C., S.D. and M.G.Z.; formal analysis, A.C., S.S. and S.D.; investigation, A.C., S.S. and S.D; data curation, A.C., S.S., S.D., C.L., R.S. and M.G.Z.; writing—original draft preparation, A.C., S.D. and M.B.; writing—review and editing, A.C., S.D., M.B., C.L., R.S. and M.G.Z.; supervision, M.B. and R.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data will be made available from the corresponding author on reasonable request. are available in section “MDPI Research Data Policies” at https://www.mdpi.com/ethics.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Meerow, A.D.; Snijman, D. The Families and Genera of Vascular Plants III. Flowering Plants Monocotyledons; Kubitzki, K., Ed.; Springer: Berlin/Heidelberg, Germany, 1998; pp. 83–110. [Google Scholar]
- Meerow, A.; Francisco-Ortega, J.; Kuhn, D.; Schnell, R. Phylogenetic relationships and biogeography within the Eurasian Clade of Amaryllidaceae based on plastid ndhF and nrDNA ITS sequences: Lineage sorting in a reticulate area? Syst. Bot. 2006, 31, 42–60. [Google Scholar] [CrossRef]
- Plants of the World Online. Available online: https://powo.science.kew.org/ (accessed on 12 January 2023).
- Davis, P.H. Flora of Turkey and the East Aegean Islands; Edinburgh University Press: Edinburgh, UK, 1984; Volume 8, pp. 1–632. [Google Scholar]
- Press Syndicate of the University of Cambridge. Flora Europaea; Tutin, T.G., Ed.; Cambridge University Press: Cambridge, UK, 1980; Volume 5, pp. 1–452. [Google Scholar]
- Perrone, B.; Salmeri, C.; Brullo, S.; Colombo, P.; De Castro, O. What do leaf anatomy and micro-morphology tell us about the psammophilous P. maritimum L. (Amaryllidaceae) in response to sand dune conditions? Flora 2015, 213, 20–31. [Google Scholar] [CrossRef]
- Du Merac, M.L. Systematics and biochemistry of the Amaryllidaceae—Alkaloid content of P. maritimum. Compt. Rend. 1954, 239, 300–302. [Google Scholar]
- Cedrón, J.C.; Del Arco-Aguilar, M.; Estévez-Braun, A.; Ravelo, Á.G. Chemistry and biology of Pancratium Alkaloids. In The Alkaloids: Chemistry and Biology; Cordell, G.A., Ed.; Elesevier: Amsterdam, The Netherlands, 2010; Volume 68, pp. 1–37. [Google Scholar]
- Abou-Donia, A.H.; De Giulio, A.; Evidente, A.; Gaber, M.; Habib, A.A.; Lanzetta, R.; El Din, A.A.S. Narciclasine-4-O-β-D-glucopyranoside, a glucosyloxy amidic phenanthridone derivative from P. maritimum. Phytochemistry 1991, 30, 3445–3448. [Google Scholar] [CrossRef]
- Abou-Donia, A.H.; Abib, A.A.; El Din, A.S.; Evidente, A.; Gaber, M.; Scopa, A. Two betaine-type alkaloids from Egyptian P. maritimum. Phytochemistry 1992, 31, 2139–2141. [Google Scholar] [CrossRef]
- Ibrahim, S.R.; Mohamed, G.A.; Shaala, L.A.; Youssef, D.T.; El Sayed, K.A. New alkaloids from P. maritimum. Planta Med. 2013, 79, 1480–1484. [Google Scholar] [CrossRef]
- Youssef, D.T.A.; Frahm, A.W. Alkaloids of the flowers of P. maritimum. Planta Med. 1998, 64, 669–670. [Google Scholar] [CrossRef]
- Youssef, D.T.A. Further alkaloids from the flowers of P. maritimum. Pharmazie 1999, 54, 535–537. [Google Scholar]
- Hetta, M.H.; Shafei, A.A. Comparative cytotoxic and antimicrobial activities of the alkaloid content of Egyptian P. maritimum L. fruits and flowers. J. Am. Sci. 2013, 9, 104–109. [Google Scholar]
- Schrader, K.K.; Avolio, F.; Andolfi, A.; Cimmino, A.; Evidente, A. Ungeremine and its hemisynthesized analogues as bactericides against Flavobacterium columnare. J. Agric. Food Chem. 2013, 61, 1179–1183. [Google Scholar] [CrossRef]
- Youssef, D.T.A.; Shaala, L.A.; Altyar, A.E. Cytotoxic phenylpropanoid derivatives and alkaloids from the flowers of P. maritimum L. Plants 2022, 11, 476. [Google Scholar] [CrossRef] [PubMed]
- Berkov, S.; Evstatieva, L.; Popov, S. Alkaloids in Bulgarian P. maritimum L. Z. Naturforsch. C 2004, 59, 65–69. [Google Scholar] [CrossRef] [PubMed]
- Bozkurt, B.; Kaya, G.I.; Somer, N.U. Chemical composition and enzyme inhibitory activities of Turkish P. maritimum bulbs. Nat. Prod. Commun. 2019, 14, 1–4. [Google Scholar] [CrossRef]
- Şener, B.; Konukol, S.; Kruk, C.; Pandit, U.K. New crinine-type alkaloids from P. maritimum L- growing in Turkey. Nat. Prod. Lett. 1993, 1, 287–291. [Google Scholar] [CrossRef]
- Şener, B.; Konukol, S.; Kruk, C.; Pandit, U.K. Alkaloids from Amaryllidaceae: IV. Alkaloids from the aerial parts of P. maritimum L. Gazi Univ. Eczaci. Fak. Derg. 1993, 10, 83–86. [Google Scholar]
- Şener, B.; Konukol, S.; Kruk, C.; Pandit, U.K. Alkaloids from Amaryllidaceae III. Alkaloids from the bulbs of P. maritimum. Nat. Prod. Sci. 1998, 4, 148–152. [Google Scholar]
- Şener, B.; Orhan, I.; Satayavivad, J. Antimalarial activity screening of some alkaloids and the plant extracts from Amaryllidaceae. Phytother. Res. 2003, 17, 1220–1223. [Google Scholar] [CrossRef]
- Sanaa, A.; Boussaid, M.; Ben Fadhel, N. Alkaloids and sodium alginate in Tunisian P. maritimum L. Acta Hortic. 2010, 853, 215–218. [Google Scholar] [CrossRef]
- Sancha, S.A.R.; Gomes, V.A.; Loureiro, J.B.; Saraiva, L.; Ferreira, M.J.U. Amaryllidaceae-type alkaloids from Pancratium maritimum: Apoptosis-inducing effect and cell cycle arrest on triple-negative breast cancer cells. Molecules 2022, 27, 5759. [Google Scholar] [CrossRef]
- Suau, R.; Gómez, A.I.; Rico, R.; Tato, M.P.V.; Castedo, L.; Riguera, R. Alkaloid N-oxides of Amaryllidaceae. Phytochemistry 1998, 27, 3285–3287. [Google Scholar] [CrossRef]
- Tato, M.P.V.; Castedo, L.; Riguera, R. New alkaloids from P. maritimum L. Heterocycles 1988, 27, 2833–2838. [Google Scholar] [CrossRef]
- Masi, M.; Di Lecce, R.; Mérindol, N.; Girard, M.P.; Berthoux, L.; Desgagné-Penix, I.; Calabrò, V.; Evidente, A. Cytotoxicity and antiviral properties of alkaloids isolated from P. maritimum. Toxins 2022, 14, 262. [Google Scholar] [CrossRef] [PubMed]
- Boit, H.G.; Ehmke, H. Alkaloide von Nerine bowdenii, Crinum powellii, Amaryllis belladonna und P. maritimum (XII. Mitteil. Über Amaryllidaceen-Alkaloide). Chem. Ber. 1956, 89, 2093–2097. [Google Scholar] [CrossRef]
- Petit, G.R.; Petit, G.R., III; Groszek, G.; Backhaus, R.A.; Doubek, D.L.; Barr, R.J. Antineoplastic agents, 301. An investigation of the Amaryllidaceae genus Hymenocallis. J. Nat. Prod. 1995, 58, 756–759. [Google Scholar] [CrossRef] [PubMed]
- Ali, A.A.; Makboul, M.A.; Attia, A.A.; Ali, D.T. Chromones and flavans from P. maritimum. Phytochemistry 1990, 29, 625–627. [Google Scholar] [CrossRef]
- Youssef, D.T.A.; Ramadan, M.A.; Khalifa, A.A. Acetophenones, a chalcone, a chromone and flavonoids from P. maritimum. Phytochemistry 1998, 49, 2579–2583. [Google Scholar] [CrossRef]
- Ibrahim, S.R.M.; Mohamed, G.A.; Shaala, L.A.; Youssef, D.T.A. Non-alkaloidal compounds from the bulbs of the Egyptian plant P. maritimum. Z. Naturforsch. C 2014, 69, 92–98. [Google Scholar] [CrossRef]
- Rokbeni, N.; M’rabet, Y.; Cluzet, S.; Richard, T.; Krisa, S.; Boussaid, M.; Boulila, A. Determination of phenolic composition and antioxidant activities of P. maritimum L. from Tunisia. Ind. Crop. Prod. 2016, 94, 505–513. [Google Scholar] [CrossRef]
- Nikolova, M.; Gevrenova, R. Determination of phenolic acids in Amaryllidaceae species by high performance liquid chromatography. Pharm. Biol. 2005, 43, 289–291. [Google Scholar] [CrossRef]
- Sanaa, A.; Boulila, A.; Boussaid, M.; Fadhel, N.B. Alginic acid and derivatives, new polymers from the endangered P. maritimum L. Ind. Crop. Prod. 2013, 44, 290–293. [Google Scholar] [CrossRef]
- Kaya, G.I.; Sarikaya, B.; Çiçek, D.; Somer, N.U. In vitro cytotoxic activity of Sternbergia sicula, S. lutea and P. maritimum extracts. Hacet. Univ. J. Fac. Pharm. 2010, 30, 41–48. [Google Scholar]
- Calderón-Montaño, J.M.; Martínez-Sánchez, S.M.; Jiménez-González, V.; Burgos-Morón, E.; Guillén-Mancina, E.; Jiménez-Alonso, J.J.; Díaz-Ortega, P.; García, F.; Aparicio, A.; López-Lázaro, M. Screening for selective anticancer activity of 65 extracts of plants collected in western Andalusia, Spain. Plants 2021, 10, 2193. [Google Scholar] [CrossRef] [PubMed]
- Leporini, M.; Catinella, G.; Bruno, M.; Falco, T.; Tundis, R.; Loizzo, M.R. Investigating the antiproliferative and antioxidant properties of P. maritimum L. (Amaryllidaceae) stems, flowers, bulbs, and fruits extracts. Evid. Based Complem. Altern. Med. 2018, 2018, 9301247. [Google Scholar] [CrossRef] [PubMed]
- Tayoub, G.; Al-Odat, M.; Amer, A.; Aljapawe, A.; Ekhtiar, A. Antiproliferative effects of P. maritimum extracts on normal and cancerous cells. Iran. J. Med. Sci. 2018, 43, 52–64. [Google Scholar]
- Sür-Altiner, D.; Gürkan, E.; Mutlu, G.; Tuzlaci, E.; Ang, O. The antifungal activity of P. maritimum. Fitoterapia 1999, 70, 187–189. [Google Scholar] [CrossRef]
- Tosun, F.; Kizilay, C.A.; Şener, B.; Vural, M.; Palittapongarnpim, P. Antimycobacterial activity of some Turkish plants. Pharm. Biol. 2004, 42, 39–43. [Google Scholar] [CrossRef]
- Çakici, I.; Ulug, H.Y.; Inci, S.; Tunçtan, B.; Abacioglu, N.; Kanzik, I.; Sener, B. Antinociceptive effect of some Amaryllidaceae plants in mice. J. Pharm. Pharmacol. 1997, 49, 828–830. [Google Scholar] [CrossRef]
- El-Sayed, N.M.; Ismail, K.A.; Ahmed, S.A.-E.-G.; Hetta, M.H. In vitro amoebicidal activity of ethanol extracts of Arachis hypogaea L., Curcuma longa L. and P. maritimum L. on Acanthamoeba castellanii cysts. Parasitol. Res. 2012, 110, 1985–1992. [Google Scholar] [CrossRef]
- Soltan, M.M.; Hamed, A.R.; Hetta, M.H.; Hussein, A.A. Egyptian P. maritimum L. flowers as a source of anti-Alzheimer’s agents. Bull. Fac. Pharm. Cairo Univ. 2015, 53, 19–22. [Google Scholar] [CrossRef]
- Orhan, I.; Sener, B. Bioactivity-directed fractionation of alkaloids from some Amaryllidaceae plants and their anticholinesterase activity. Chem. Nat. Comp. 2003, 39, 383–386. [Google Scholar] [CrossRef]
- Badalamenti, N.; Ilardi, V.; Rosselli, S.; Bruno, M.; Maggi, F.; Leporini, M.; Falco, T.; Loizzo, M.R.; Tundis, R. Ferulago nodosa subsp. geniculata (Guss.) Troia & Raimondo from Sicily (Italy): Isolation of essential oil and evaluation of its bioactivity. Molecules 2020, 25, 3249. [Google Scholar] [CrossRef] [PubMed]
- Badalamenti, N.; Modica, A.; Ilardi, V.; Bruno, M.; Maresca, V.; Zanfardino, A.; Di Napoli, M.; Castagliuolo, G.; Varcamonti, M.; Basile, A. Daucus carota subsp. maximus (Desf.) Ball from Pantelleria, Sicily (Italy): Isolation of essential oils and evaluation of their bioactivity. Nat. Prod. Res. 2021, 19, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Cicio, A.; Serio, R.; Zizzo, M.G. Anti-inflammatory potential of Brassicaceae-derived phytochemicals: In vitro and in vivo evidence for a putative role in the prevention and treatment of IBD. Nutrients 2023, 15, 31. [Google Scholar] [CrossRef] [PubMed]
- Cicio, A.; Badalamenti, N.; Bruno, M. The ethnobotany, phytochemistry, and biological properties of genus Phagnalon (Asteraceae): A review. Nat. Prod. Res. 2023, in press. [Google Scholar] [CrossRef] [PubMed]
- Napolitano, A.; Di Napoli, M.; Castagliuolo, G.; Badalamenti, N.; Cicio, A.; Bruno, M.; Piacente, S.; Maresca, V.; Cianciullo, P.; Capasso, L.; et al. The chemical composition of the aerial parts of Stachys spreitzenhoferi (Lamiaceae) extract growing in Kythira Island (Greece) and its antioxidant, antimicrobial, anti-inflammatory and antiproliferative properties. Phytochemistry 2022, 203, 113373. [Google Scholar] [CrossRef] [PubMed]
- Ptak, A.; El Tahchy, A.; Dupire, F.; Boisbrun, M.; Henry, M.; Chapleur, Y.; Moś, M.; Laurain-Mattar, D. LCMS and GCMS for the screening of alkaloids in natural and in vitro extracts of Leucojum aestivum. J. Nat. Prod. 2009, 72, 142–147. [Google Scholar] [CrossRef]
- Ponce de León-Rodríguez, M.C.; Guyot, J.P.; Laurent-Babot, C. Intestinal in vitro cell culture models and their potential to study the effect of food components on intestinal inflammation. Crit. Rev. Food Sci. Nutr. 2019, 59, 3648–3666. [Google Scholar] [CrossRef]
- Evidente, A.; Cicala, M.R.; Giudicianni, I.; Randazzo, G.; Riccio, R. 1H and 13C NMR analysis of lycorine and α-dihydrolycorine. Phytochemistry 1983, 22, 581–584. [Google Scholar] [CrossRef]
- Berkov, S.; Reyes-Chilpa, R.; Codina, C.; Viladomat, F.; Batista, J. Revised NMR data for incartine: An alkaloid from Galanthus elwesii. Molecules 2007, 12, 1430–1435. [Google Scholar] [CrossRef]
- Şener, B.; Könükol, S.; Kruk, C.; Pandit, U.K. Alkaloids from Amaryllidaceae, I: Alkaloids of lycorine and lycorenine class from P. maritimum L. Arch. Pharm. 1993, 326, 61–62. [Google Scholar] [CrossRef]
- Bastida, J.; Codina, C.; Viladomat, F.; Rubiralta, M.; Quirion, J.C.; Husson, H.P.; Ma, G. Narcissus alkaloids, XIII. Complete assignment of the NMR Spectra of papyramine and 6-epi-papyramine by two-dimensional NMR Spectroscopy. J. Nat. Prod. 1990, 56, 1456–1462. [Google Scholar] [CrossRef]
- Codina, C.; Viladomat, F.; Bastida, J.; Rubiralta, M.; Quirion, J.-C. 2d Nmr studies of Lycorenine as a model for the structural assignment of Lycorenine-Type Alkaloids. Nat. Prod. Lett. 1992, 1, 85–92. [Google Scholar] [CrossRef]
- Makhkamova, A.U.; Safanova, E.V. Method for quantitative determination of lycorine in leaves of Ungernia sewerzowi. Chem. Nat. Comp. 1995, 30, 529–530. [Google Scholar] [CrossRef]
- Evidente, A.; Iasiello, I.; Randazzo, G. Rapid quantitative analysis of lycorine by reversed-phase high-performance liquid chromatography. J. Chromatogr. A 1983, 281, 362–366. [Google Scholar] [CrossRef]
- Konukol, S.; Sener, B. High-pressure liquid chromatographic analysis of some Amaryllidaceae alkaloids from P. maritimum L. J. Fac. Pharm. Gazi Univ. 1992, 9, 89–95. [Google Scholar]
- Kaya, G.I.; Fillik, A.; Hısıl, Y.; Unver, N. High pressure liquid chromatographic analysis of lycorine in four Galanthus species growing in Turkey. Turk. J. Pharm. Sci. 2004, 1, 105–114. [Google Scholar]
- Çitoğlu, G.S.; Yılmaz, B.S.; Bahadır, O. Quantitative analysis of lycorine in Sternbergia species growing in Turkey. Chem. Nat. Comp. 2008, 44, 826–828. [Google Scholar] [CrossRef]
- Kaya, G.I.; Cicek, D.; Sarıkaya, B.; Onur, M.A.; Somer, N.U. HPLC-DAD analysis of lycorine in Amaryllidaceae species. Nat. Prod. Commun. 2010, 5, 873–876. [Google Scholar] [CrossRef]
- Pham, L.H.; Döpke, W.; Wagner, J.; Mügge, C. Alkaloids from Crinum amabile. Phytochemistry 1998, 48, 371–376. [Google Scholar] [CrossRef]
- Thi Ngoc Tram, N.; Titorenkova, T.V.; Bankova, V.S.; Handjieva, N.V.; Popov, S.S. Crinum L. (Amaryllidaceae). Fitoterapia 2002, 73, 183–208. [Google Scholar] [CrossRef]
- Kreh, M.; Matusch, R. O-Methyloduline and N-demethylmasonine, alkaloids from Narcissus pseudonarcissus. Phytochemistry 1995, 38, 1533–1535. [Google Scholar] [CrossRef]
- Cahlíková, L.; Ločárek, M.; Benešová, N.; Kučera, R.; Chlebek, J.; Novák, Z.; Opletal, L. Isolation and cholinesterase inhibitory activity of Narcissus extracts and Amaryllidaceae alkaloid. Nat. Prod. Commun. 2013, 8, 781–785. [Google Scholar] [CrossRef]
- Liao, N.; Ao, M.; Zhang, P.; Yu, L. Extracts of Lycoris aurea induce apoptosis in murine sarcoma S180 cells. Molecules 2012, 17, 3723–3735. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.-D.; Zhang, Y.; He, H.-P.; Li, S.-F.; Tang, G.-H.; Chen, D.-Z.; Cao, M.-M.; Di, Y.-T.; Hao, X.-J. A new amaryllidaceae alkaloid from the bulbs of Lycoris radiata. Chin. J. Nat. Med. 2013, 11, 406–410. [Google Scholar] [CrossRef]
- Kobayashi, S.; Tokumoto, T.; Kihara, M.; Imakura, Y.; Shingu, T.; Taira, Z. Alkaloidal constituents of Crinum latifolium and Crinum bulbispermum (Amaryllidaceae). Chem. Pharm. Bull. 1984, 32, 3015–3022. [Google Scholar] [CrossRef]
- Hanh, T.T.H.; Anh, D.H.; Huong, P.T.T.; Thanh, N.V.; Trung, N.Q.; Cuong, T.V.; Mai, N.T.; Cuong, N.T.; Cuong, N.X.; Nam, N.H.; et al. Crinane, augustamine, and β-carboline alkaloids from Crinum latifolium. Phytochem. Lett. 2018, 24, 27–30. [Google Scholar] [CrossRef]
- Nair, J.J.; Machocho, A.K.; Campbell, W.E.; Brun, R.; Viladomat, F.; Codina, C.; Bastida, J. Alkaloids from Crinum macowani. Phytochemistry 2000, 54, 945–950. [Google Scholar] [CrossRef]
- Lyle, R.E.; Kielar, E.A.; Crowder, J.R.; Wildman, W.C. The alkaloids of Nerine bowdenii W. Wats, and Crinum moorei J. D. Hook. J. Am. Chem. Soc. 1960, 82, 2620–2625. [Google Scholar] [CrossRef]
- Cahlíková, L.; Zavadil, S.; Macaḱová, K.; Valterová, I.; Kulhańková, A.; Hošt’álková, A.; Kuneš, J.; Opletal, L. Isolation and cholinesterase activity of amaryllidaceae alkaloids from Nerine bowdenii. Nat. Prod. Commun. 2011, 6, 1827–1830. [Google Scholar] [CrossRef]
- Fales, H.M.; Wildman, W.C. Alkaloids of the Amaryllidaceae. XIX. On the structures of crinamidine, flexinine, and nerbowdine. J. Org. Chem. 1961, 26, 181–187. [Google Scholar] [CrossRef]
- Queckenberg, O.R.; Frahm, A.W.; Muller-Doblies, D.; Muller-Doblies, U. Alkaloids from Brunsvigia gregari. Planta Med. 1995, 61, 581. [Google Scholar] [CrossRef] [PubMed]
- Viladomat, F.; Almanza, G.R.; Codina, C.; Bastida, J.; Campbell, W.E.; Mathee, S. Alkaloids from Brunsvigia orientalis. Phytochemistry 1996, 43, 1379–1384. [Google Scholar] [CrossRef]
- Machocho, A.; Chhabra, S.C.; Viladomat, F.; Codina, C.; Bastida, J. Alkaloids from Ammocharis tinneana. Phytochemistry 1999, 51, 1185–1191. [Google Scholar] [CrossRef]
- Katoch, D.; Kumar, S.; Kumar, N.; Singh, B. Simultaneous quantification of Amaryllidaceae alkaloids from Zephyranthes grandiflora by UPLC-DAD/ESI-MS/MS. J. Pharm. Biomed. Anal. 2012, 71, 187–192. [Google Scholar] [CrossRef] [PubMed]
- Adedayo, B.C.; Oyeleye, S.I.; Okeke, B.M.; Oboh, G. Anti-cholinesterase and antioxidant properties of alkaloid and phenolic-rich extracts from pawpaw (Carica papaya) leaf: A comparative study. Flav. Fragr. J. 2021, 36, 47–54. [Google Scholar] [CrossRef]
- He, M.; Qu, C.; Gao, O.; Hu, X.; Hong, X. Biological and pharmacological activities of Amaryllidaceae alkaloids. RSC Adv. 2015, 5, 16562–16574. [Google Scholar] [CrossRef]
- Nair, J.J.; Van Staden, J. Cytotoxicity studies of lycorine alkaloids of the Amaryllidaceae. Nat. Prod. Commun. 2014, 9, 1193–1210. [Google Scholar] [CrossRef]
- Schieber, M.; Chandel, N.S. ROS function in redox signaling and oxidative stress. Curr. Biol. 2014, 24, R453–R462. [Google Scholar] [CrossRef]
- Forrester, S.J.; Kikuchi, D.S.; Hernandes, M.S.; Xu, Q.; Griendling, K.K. Reactive oxygen species in metabolic and inflammatory signaling. Circ. Res. 2018, 122, 877–902. [Google Scholar] [CrossRef]
- Taie, H.; Esawy, M.A.T.; Mohamed, M.A.E.M. Antioxidant and antimicrobial activities of different parts of P. maritimum. Planta Med. 2015, 81, 166–167. [Google Scholar] [CrossRef]
- Cao, Z.; Yang, P.; Zhou, Q. Multiple biological functions and pharmacological effects of lycorine. Sci. China Chem. 2013, 56, 1382–1391. [Google Scholar] [CrossRef] [PubMed]
- Wan, H.; Liu, D.; Yu, X.; Sun, H.; Li, Y. A Caco-2 cell-based quantitative antioxidant activity assay for antioxidants. Food Chem. 2015, 175, 601–608. [Google Scholar] [CrossRef] [PubMed]
- Elke, C.; Rosenberg, I.M.; Brandwein, S.L.; Beck, P.L.; Reinecker, H.C.; Podolsky, D.K. Lipopolysaccharide activates distinct signaling pathways in intestinal epithelial cell lines expressing toll-like receptors. J. Immunol. 2000, 164, 966–972. [Google Scholar] [CrossRef]
- Tunçer, S.; Banerjee, S. Determination of autophagy in the Caco-2 spontaneously differentiating model of intestinal epithelial cells. Methods Mol. Biol. 2019, 1854, 55–70. [Google Scholar] [CrossRef] [PubMed]
- Luparello, C.; Asaro, D.M.L.; Cruciata, I.; Hassell-Hart, S.; Sansook, S.; Spencer, J.; Caradonna, F. Cytotoxic activity of the histone deacetylase 3-selective inhibitor pojamide on MDA-MB-231 triple-negative breast cancer Cells. Int. J. Mol. Sci. 2019, 20, 804. [Google Scholar] [CrossRef]
- Luparello, C.; Branni, R.; Abruscato, G.; Lazzara, V.; Drahos, L.; Arizza, V.; Mauro, M.; Di Stefano, V.; Vazzana, M. Cytotoxic capability and the associated proteomic profile of cell-free coelomic fluid extracts from the edible sea cucumber Holothuria tubulosa on HepG2 liver cancer cells. EXCLI J. 2022, 21, 722–743. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).