Investigation on Hydrazonobenzenesulfonamides as Human Carbonic Anhydrase I, II, IX and XII Inhibitors
Abstract
1. Introduction
2. Results
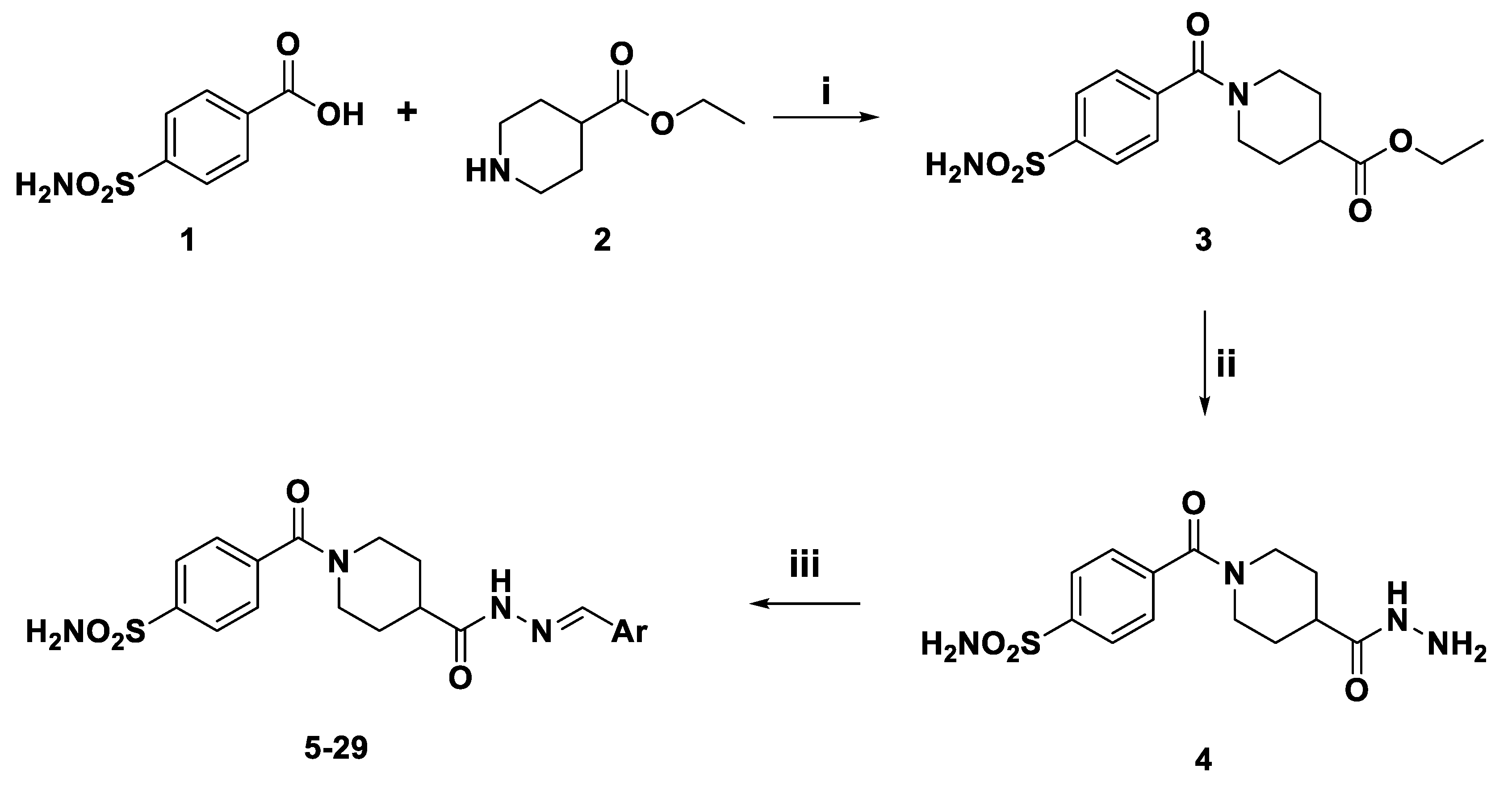
2.1. Chemistry
2.2. Carbonic Anhydrase Inhibition
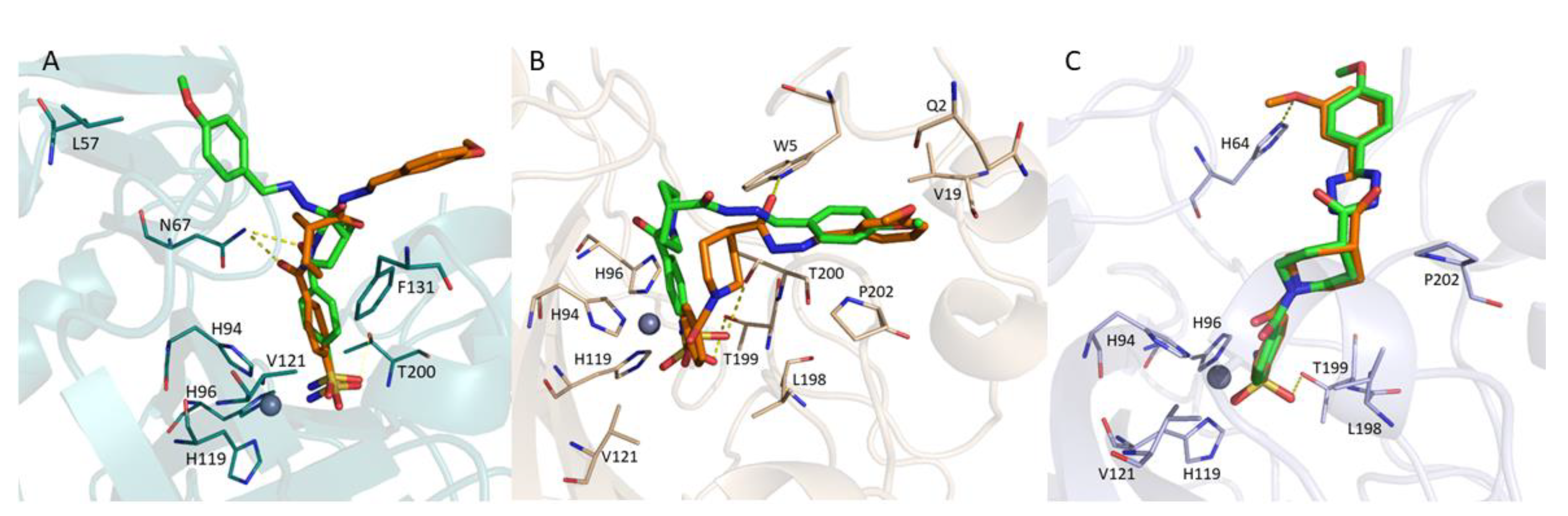
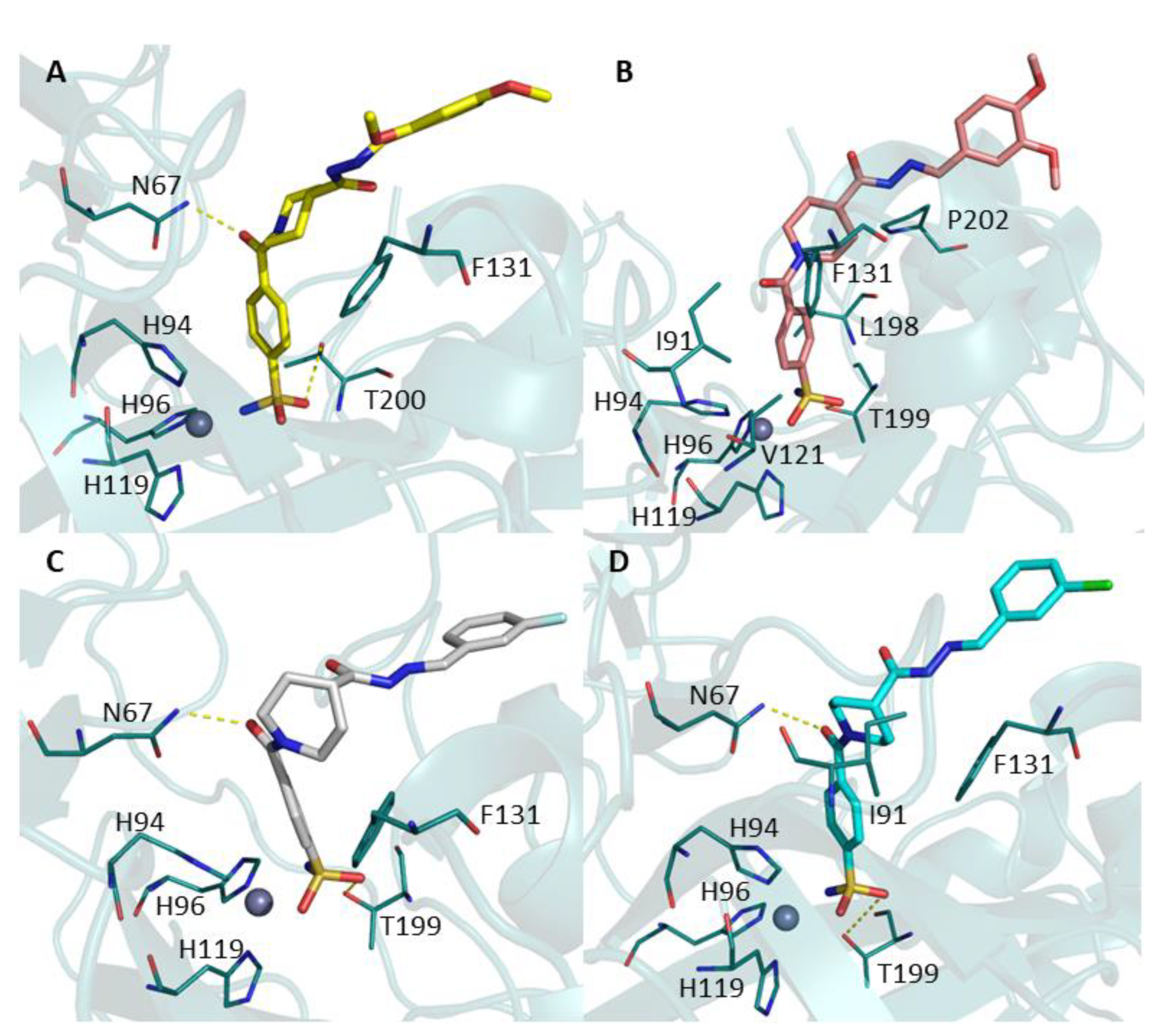
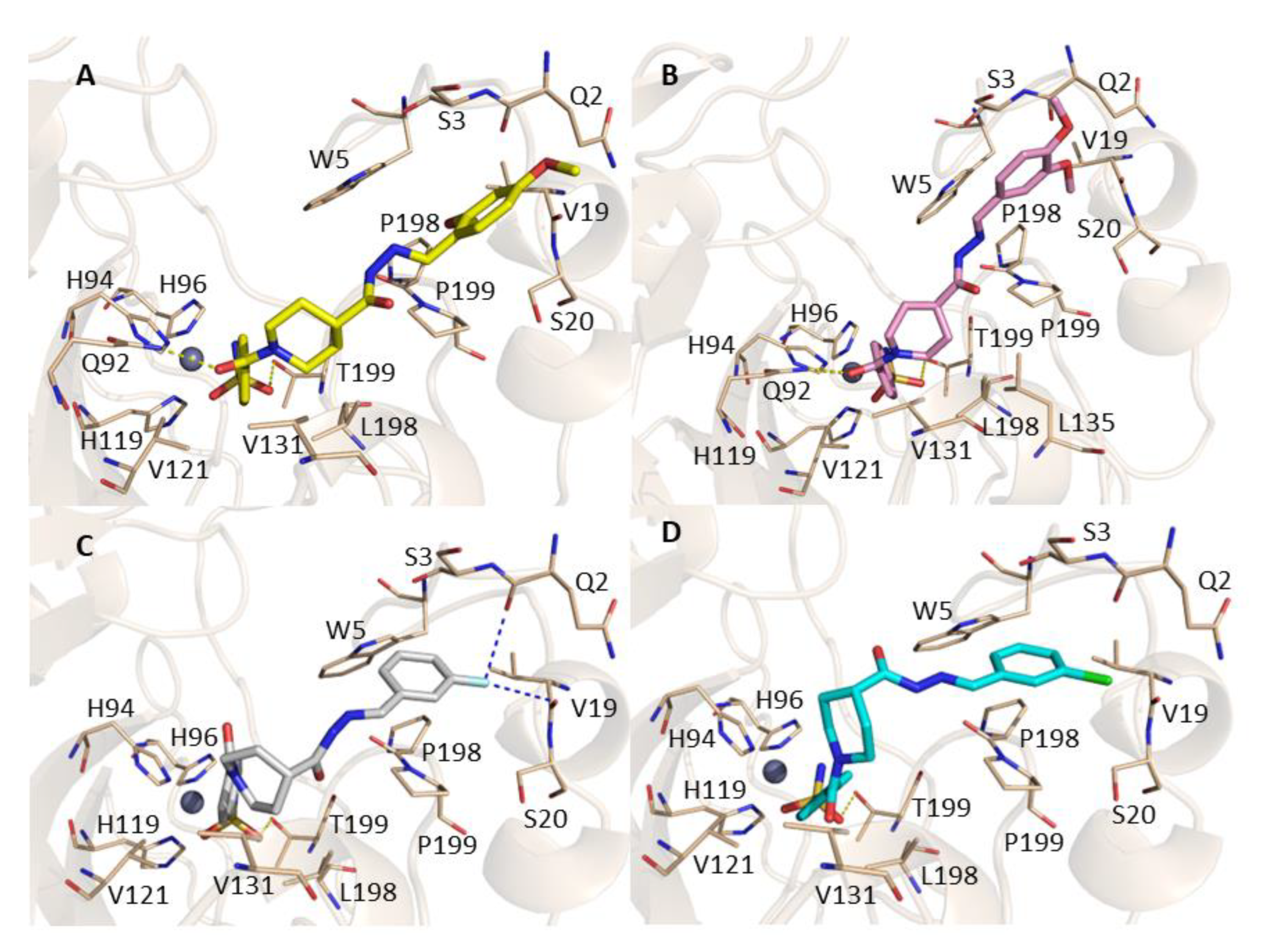
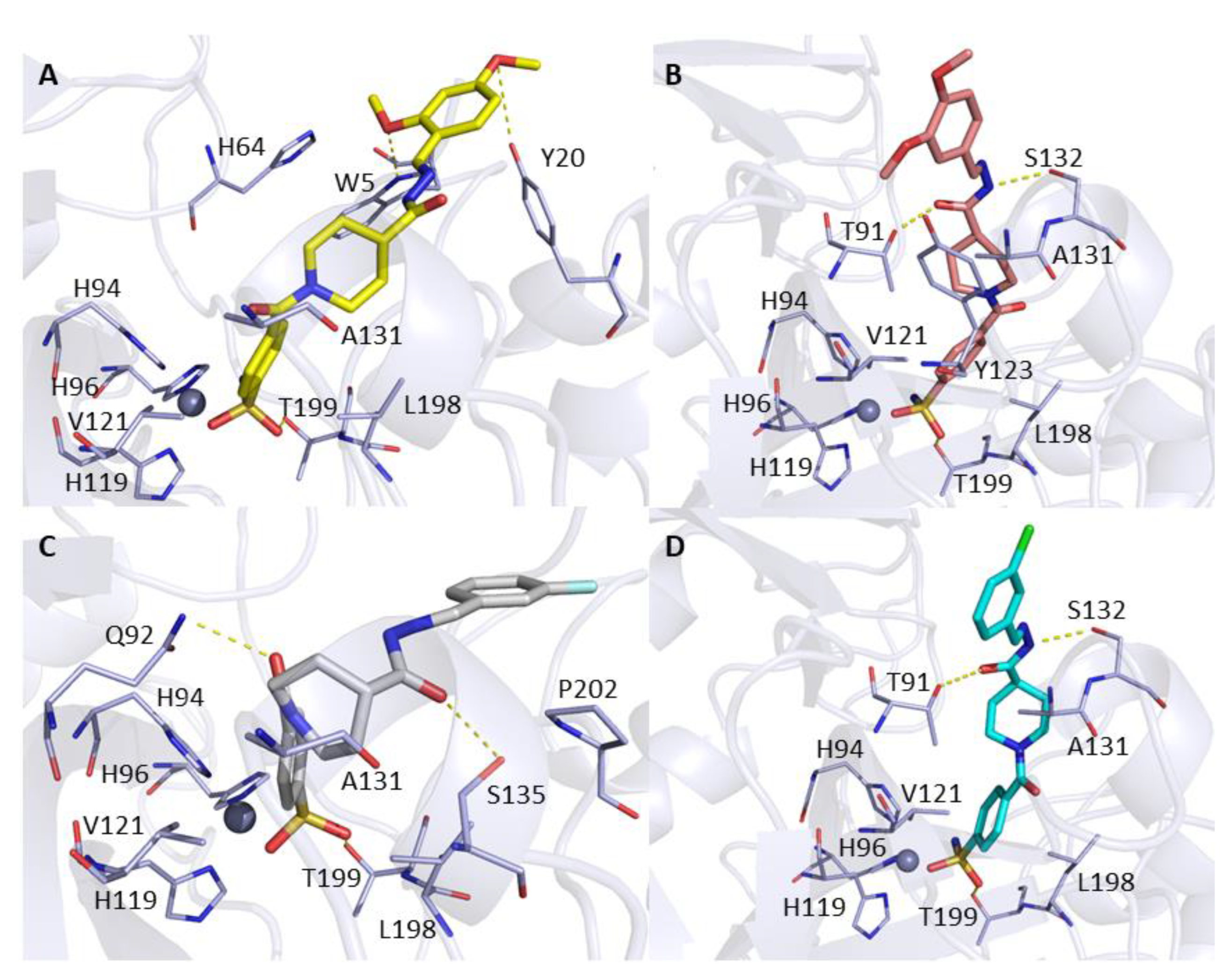
2.3. Molecular Docking
3. Materials and Methods
3.1. Chemistry
3.1.1. General Procedure for the Preparation of (E)-4-(4-(2-benzylidenehydrazinecarbonyl)piperidine-1-carbonyl)benzenesulfonamides (5-25)
(E)-4-(4-(2-benzylidenehydrazinecarbonyl)piperidine-1-carbonyl)benzenesulfonamide (5)
(E)-4-(4-(2-(4-methylbenzylidene)hydrazinecarbonyl)piperidine-1-carbonyl)benzenesulfonamide (6)
(E)-4-(4-(2-(2-methoxybenzylidene)hydrazinecarbonyl)piperidine-1-carbonyl)benzenesulfonamide (7)
(E)-4-(4-(2-(3-methoxybenzylidene)hydrazinecarbonyl)piperidine-1-carbonyl)benzenesulfonamide (8)
(E)-4-(4-(2-(4-methoxybenzylidene)hydrazinecarbonyl)piperidine-1-carbonyl)benzenesulfonamide (9)
(E)-4-(4-(2-(2,4-dimethoxybenzylidene)hydrazinecarbonyl)piperidine-1-carbonyl)benzenesulfonamide (10)
(E)-4-(4-(2-(3,4-dimethoxybenzylidene)hydrazinecarbonyl)piperidine-1-carbonyl)benzenesulfonamide (11)
(E)-4-(4-(2-(2,5-dimethoxybenzylidene)hydrazinecarbonyl)piperidine-1-carbonyl)benzenesulfonamide (12)
(E)-4-(4-(2-(2,4,5-trimethoxybenzylidene)hydrazinecarbonyl)piperidine-1-carbonyl)benzenesulfonamide (13)
(E)-4-(4-(2-(3,4,5-trimethoxybenzylidene)hydrazinecarbonyl)piperidine-1-carbonyl)benzenesulfonamide (14)
(E)-4-(4-(2-(2-bromo-3,4-dimethoxybenzylidene)hydrazinecarbonyl)piperidine-1-carbonyl)benzenesulfonamide (15)
(E)-4-(4-(2-((naphthalen-2-yl)methylene)hydrazinecarbonyl)piperidine-1-carbonyl)benzenesulfonamide (16)
(E)-4-(4-(2-((1-methoxynaphthalen-2-yl)methylene)hydrazinecarbonyl)piperidine-1-carbonyl)benzenesulfonamide (17)
(E)-4-(4-(2-(4-(trifluoromethyl)benzylidene)hydrazinecarbonyl)piperidine-1-carbonyl)benzenesulfonamide (18)
(E)-4-(4-(2-(3-fluorobenzylidene)hydrazinecarbonyl)piperidine-1-carbonyl)benzenesulfonamide (19)
(E)-4-(4-(2-(4-fluorobenzylidene)hydrazinecarbonyl)piperidine-1-carbonyl)benzenesulfonamide (20)
(E)-4-(4-(2-(3-nitrobenzylidene)hydrazinecarbonyl)piperidine-1-carbonyl)benzenesulfonamide (21)
(E)-4-(4-(2-(4-nitrobenzylidene)hydrazinecarbonyl)piperidine-1-carbonyl)benzenesulfonamide (22)
(E)-4-(4-(2-(2-chlorobenzylidene)hydrazinecarbonyl)piperidine-1-carbonyl)benzenesulfonamide (23)
(E)-4-(4-(2-(3-chlorobenzylidene)hydrazinecarbonyl)piperidine-1-carbonyl)benzenesulfonamide (24)
(E)-4-(4-(2-(2,4-dichlorobenzylidene)hydrazinecarbonyl)piperidine-1-carbonyl)benzenesulfonamide (25)
(E)-4-(4-(2-(2,5-dichlorobenzylidene)hydrazinecarbonyl)piperidine-1-carbonyl)benzenesulfonamide (26)
(E)-4-(4-(2-(2,6-dichlorobenzylidene)hydrazinecarbonyl)piperidine-1-carbonyl)benzenesulfonamide (27)
(E)-4-(4-(2-(4-(dimethylamino)benzylidene)hydrazinecarbonyl)piperidine-1-carbonyl)benzenesulfonamide (28)
(E)-4-(4-(2-(4-(diethylamino)benzylidene)hydrazinecarbonyl)piperidine-1-carbonyl)benzenesulfonamide (29)
3.2. Carbonic Anhydrase Inhibition
3.3. Molecular Docking
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Alterio, V.; Di Fiore, A.; D’Ambrosio, K.; Supuram, C.T.; De Simone, G. Multiple binding modes of inhibitors to carbonic anhydrases: How to design specific drugs targeting 15 different isoforms? Chem. Rev. 2012, 112, 4421–4468. [Google Scholar] [CrossRef]
- Supuran, C.T. Structure and function of carbonic anhydrases. Biochem. J. 2016, 473, 2023–2032. [Google Scholar] [CrossRef]
- Mishra, C.B.; Tiwari, M.; Supuran, C.T. Progress in the development of human carbonic anhydrase inhibitors and their pharmacological applications: Where are we today? Med. Res. Rev. 2020, 40, 2485–2565. [Google Scholar] [CrossRef] [PubMed]
- Supuran, C.T. Novel carbonic anhydrase inhibitors. Future Med. Chem. 2021, 13, 1935–1937. [Google Scholar] [CrossRef]
- Carta, F.; Supuran, C.T.; Scozzafava, A. Novel therapies for glaucoma: A patent review 2007–2011. Expert Opin. Ther. Pat. 2012, 22, 79–88. [Google Scholar] [CrossRef] [PubMed]
- Aggarwal, M.; Boone, C.D.; Kondeti, B. Structural annotation of human carbonic anhydrases. J. Enz. Inhib. Med. Chem. 2013, 28, 267–277. [Google Scholar] [CrossRef] [PubMed]
- Carta, F.; Scozzafava, A.; Supuran, C.T. Sulfonamides: A patent review (2008–2012). Expert Opin. Ther. Pat. 2012, 22, 747–758. [Google Scholar] [CrossRef] [PubMed]
- Pollard, A.; Shephard, F.; Freed, J.; Liddell, S.; Chakrabarti, L. Mitochondrial proteomic profiling reveals increased carbonic anhydrase II in aging and neurodegeneration. Aging-Us. 2016, 8, 2425–2436. [Google Scholar] [CrossRef]
- Jarvinen, P.; Kivela, A.J.; Nummela, P.; Lepisto, A.; Ristimaki, A.; Parkkila, S. Carbonic anhydrase II: A novel biomarker for pseudomyxoma peritonei. APMIS 2017, 125, 207–212. [Google Scholar] [CrossRef]
- Pastorekova, S.; Parkkila, S.; Parkkila, A.K.; Opavsky, R.; Zelnik, V.; Saarnio, J.; Pastorek, J. Carbonic anhydrase IX, MN/CA IX: Analysis of stomach complementary DNA sequence and expression in human and rat alimentary tracts. Gastroenterology 1997, 112, 398–408. [Google Scholar] [CrossRef]
- Ivanov, S.; Liao, S.Y.; Ivanova, A.; Danilkovitch-Miagkova, A.; Tarasova, N.; Weirich, G.; Merrill, M.J.; Proescholdt, M.A.; Oldfield, E.H.; Lee, J.; et al. Expression of hypoxia-inducible cell-surface transmembrane carbonic anhydrases in human cancer. Am. J. Pathol. 2001, 158, 905–919. [Google Scholar] [CrossRef] [PubMed]
- Ondriskova, E.; Debreova, M.; Pastorekova, S. Tumor-associated carbonic anhydrases IX and XII. In Carbonic Anhydrases as Biocatalysts: From Theory to Medical and Industrial Applications; Supuran, C.T., Simone, G., Eds.; Elsevier: Amsterdam, the Netherlands, 2015; pp. 169–206. [Google Scholar]
- Türeci, O.; Sahin, U.; Vollmar, E.; Siemer, S.; Göttert, E.; Seitz, G.; Parkkila, A.K.; Shah, G.N.; Grubb, J.H.; Pfreundschuh, M.; et al. Human carbonic anhydrase XII: cDNA cloning, expression, and chromosomal localization of a carbonic anhydrase gene that is overexpressed in some renal cell cancers. Proc. Natl. Acad. Sci. USA 1998, 95, 7608–7613. [Google Scholar] [CrossRef] [PubMed]
- Ivanov, S.V.; Kuzmin, I.; Wei, M.H.; Pack, S.; Geil, L.; Johnson, B.E.; Stanbridge, E.J.; Lerman, M.I. Down-regulation of transmembrane carbonic anhydrases in renal cell carcinoma cell lines by wild-type von Hippel-Lindau transgenes. Proc. Natl. Acad. Sci. USA 1998, 95, 12596–12601. [Google Scholar] [CrossRef]
- Karhumaa, P.; Kaunisto, K.; Parkkila, S.; Waheed, A.; Pastorekova, S.; Pastorek, J.; Sly, W.S.; Rajaniemi, H. Expression of the transmembrane carbonic anhydrases, CA IX and CA XII, in the human male excurrent ducts. Mol. Hum. Reprod. 2000, 7, 611–616. [Google Scholar] [CrossRef]
- Parkkila, S.; Parkkila, A.K.; Saarnio, J.; Kivela, J.; Karttunen, T.; Kaunisto, K.; Waheed, A.; Sly, W.S.; Tureci, O.; Virtanen, I.; et al. Expression of the membrane-associated carbonic anhydrase isozyme XII in the human kidney and renal tumors. Histochem. Cytochem. 2000, 48, 1601–1608. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Lomelino, C.L.; Mboge, M.Y.; Frost, S.C.; McKenna, R. Cancer drug development of carbonic anhydrase inhibitors beyond the active site. Molecules 2018, 23, 1045. [Google Scholar] [CrossRef] [PubMed]
- Pastorek, J.; Pastorekova, S. Hypoxia-induced carbonic anhydrase IX as a target for cancer therapy: From biology to clinical use. Semin. Cancer. Biol. 2015, 31, 52–64. [Google Scholar] [CrossRef]
- Gieling, R.G.; Williams, K.J. Carbonic anhydrase IX as a target for metastatic disease. Bioorg. Med. Chem. 2013, 21, 1470–1476. [Google Scholar] [CrossRef]
- Gemma, S.; Kukreja, G.; Fattorusso, C.; Persico, M.; Romano, M.P.; Altarelli, M.; Savini, L.; Campiani, G.; Fattorusso, E.; Basilico, N. Synthesis of N1-arylidene-N2-quinolyl-and N2-acrydinylhydrazones as potent antimalarial agents active against CQ-resistant P. falciparum strains. Bioorg. Med. Chem. Lett. 2006, 16, 5384–5388. [Google Scholar] [CrossRef]
- Inam, A.; Siddiqui, S.M.; Macedo, T.S.; Moreira, D.R.M.; Leite, A.C.L.; Soares, M.B.P.; Azam, A. Design, synthesis and biological evaluation of 3-[4-(7-chloro-quinolin-4-yl)-piperazin-1-yl]-propionic acid hydrazones as antiprotozoal agents. Eur. J. Med. Chem. 2014, 75, 67–76. [Google Scholar] [CrossRef]
- Terzioglu, N.; Gürsoy, A. Synthesis and anticancer evaluation of some new hydrazone derivatives of 2, 6-dimethylimidazo [2,1-b][1,3,4] thiadiazole-5-carbohydrazide. Eur. J. Med. Chem. 2003, 38, 781–786. [Google Scholar] [CrossRef] [PubMed]
- Cocco, M.T.; Congiu, C.; Lilliu, V.; Onnis, V. Synthesis and in vitro antitumoral activity of new hydrazinopyrimidine-5-carbonitrile derivatives. Bioorg. Med. Chem. 2006, 14, 366–372. [Google Scholar] [CrossRef] [PubMed]
- Nasr, T.; Bondock, S.; Youns, M. Anticancer activity of new coumarin substituted hydrazide–hydrazone derivatives. Eur. J. Med. Chem. 2014, 76, 539–548. [Google Scholar] [CrossRef] [PubMed]
- Easmon, J.; Pürstinger, G.; Thies, K.-S.; Heinisch, G.; Hofmann, J. Synthesis, structure- activity relationships, and antitumor studies of 2-benzoxazolyl hydrazones derived from alpha-(N)-acyl heteroaromatics. J. Med. Chem. 2006, 49, 6343–6350. [Google Scholar] [CrossRef]
- Baldisserotto, A.; Demurtas, M.; Lampronti, I.; Tacchini, M.; Moi, D.; Balboni, G.; Vertuani, S.; Manfredini, S.; Onnis, V. In-vitro evaluation of antioxidant, antiproliferative and photo-protective activities of benzimidazolehydrazone derivatives. Pharmauceticals 2020, 13, 68. [Google Scholar] [CrossRef] [PubMed]
- Koc, H.C.; Atlihan, I.; Mega-Tiber, P.; Orun, O.; Kucukguzel, S.G. Synthesis of some novel hydrazide-hydrazones derived from etodolac as potential anti-prostate cancer agents. J. Res.Pharm. 2022, 26, 1018–1029. [Google Scholar] [CrossRef]
- Güzel-Akdemir, Ö.; Angeli, A.; Supuran, C.T.; Akdemir, A. Novel Indole-Based Hydrazones as Potent Inhibitors of the α-class Carbonic Anhydrase from Pathogenic Bacterium Vibrio cholerae. Int. J. Mol. Sci. 2020, 21, 3131. [Google Scholar] [CrossRef]
- Demir-Yazıcı, K.; Bua, S.; Nurgül Mutlu Akgüneş, N.M.; Akdemir, A.; Supuran, C.T.; Güzel-Akdemir, O. Indole-Based Hydrazones Containing A Sulfonamide Moiety as Selective Inhibitors of Tumor-Associated Human Carbonic Anhydrase Isoforms IX and XII. Int. J. Mol. Sci. 2019, 20, 2354. [Google Scholar] [CrossRef]
- Brichet, J.; Rodrigo Arancibia, R.; Berrino, E.; Supuran, C.T. Bioorganometallic derivatives of 4-hydrazino-benzenesulphonamide as carbonic anhydrase inhibitors: Synthesis, characterisation and biological evaluation. J. Enz. Inhib. Med. Chem. 2020, 35, 622–628. [Google Scholar] [CrossRef]
- Kucukoglu, K.; Gul, H.I.; ParhamTaslimi, P.; Gulcin, I.; Supuran, C.T. Investigation of inhibitory properties of some hydrazone compounds on hCA I, hCA II and AChE enzymes. Bioorg. Chem. 2019, 86, 316–321. [Google Scholar] [CrossRef]
- Sharma, V.; Kumar, R.; Bua, S.; Supuran, C.T.; Sharma, P.K. Synthesis of novel benzenesulfonamide bearing 1,2,3-triazole linked hydroxy-trifluoromethylpyrazolines and hydrazones as selective carbonic anhydrase isoforms IX and XII inhibitors. Bioorg. Chem. 2019, 85, 198–208. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Aziz, A.A.-M.; El-Azab, A.S.; El-Enin, M.A.A.; Almehizia, A.A.; Supuran, C.T.; Nocentini, A. Synthesis of novel isoindoline-1,3-dione-based oximes and benzenesulfonamide hydrazones as selective inhibitors of the tumor-associated carbonic anhydrase IX. Bioorg. Chem. 2018, 80, 706–713. [Google Scholar] [CrossRef] [PubMed]
- Celebioglu, H.U.; Erden, Y.; Hamurcu, F.; Taslimi, P.; Şentürk, O.S.; Özmen, Ü.Ö.; Tuzun, B.; Gulçin, İ. Cytotoxic effects, carbonic anhydrase isoenzymes, α-glycosidase and acetylcholinesterase inhibitory properties, and molecular docking studies of heteroatom-containing sulfonyl hydrazone derivatives. J. Biomol. Struct. Dyn. 2021, 3, 5539–5550. [Google Scholar] [CrossRef] [PubMed]
- Yamali, C.; Sakagami, H.; Uesawa, Y.; Kurosaki, K.; Satoh, K.; Masuda, Y.; Yokose, S.; Ece, A.; Bua, S.; Angeli, A.; et al. Comprehensive study on potent and selective carbonic anhydrase inhibitors: Synthesis, bioactivities and molecular modelling studies of 4-(3-(2-arylidenehydrazine-1-carbonyl)-5-(thiophen-2-yl)-1H-pyrazole-1-yl) benzenesulfonamides. Eur. J. Med. Chem. 2021, 217, 113351. [Google Scholar] [CrossRef] [PubMed]
- Queen, A.; Khan, P.; Idrees, D.; Azama, A.; Hassan, I. Biological evaluation of p-toluene sulphonylhydrazone as carbonic anhydrase IX inhibitors: An approach to fight hypoxia-induced tumors. Int. J. Biol. Mac. 2018, 106, 840–850. [Google Scholar] [CrossRef] [PubMed]
- Moi, D.; Deplano, A.; Angeli, A.; Balboni, G.; Supuran, C.T.; Onnis, V. Synthesis of Sulfonamides Incorporating Piperidinyl-Hydrazidoureido and Piperidinyl Hydrazidothioureido Moieties and Their Carbonic Anhydrase I, II, IX and XII Inhibitory Activity. Molecules 2022, 27, 5370. [Google Scholar] [CrossRef]
- Nocentini, A.; Moi, D.; Deplano, A.; Osman, S.M.; AlOthman, Z.A.; Balboni, G.; Supuran, C.T.; Onnis, V. Sulfonamide/sulfamate switch with a series of piperazinylureido derivatives: Synthesis, kinetic and in silico evaluation as carbonic anhydrase isoforms I, II, IV, and IX inhibitors. Eur. J. Med. Chem. 2020, 186, 111896. [Google Scholar] [CrossRef]
- Moi, D.; Nocentini, A.; Deplano, A.; Balboni, G.; Supuran, C.T.; Onnis, V. Structure-activity relationship with pyrazoline-based aromatic sulfamates as carbonic anhydrase isoforms I, II, IX and XII inhibitors: Synthesis and biological evaluation. Eur. J. Med. Chem. 2019, 182, 111638. [Google Scholar] [CrossRef]
- Nocentini, A.; Moi, D.; Deplano, A.; Balboni, G.; Salvadori, S.; Onnis, V.; Supuran, C.T. Synthesis and biological evaluation of novel pyrazoline-based aromatic sulfamates with potent carbonic anhydrase isoforms II, IV and IX inhibitory efficacy. Bioorg. Chem. 2018, 77, 633–639. [Google Scholar] [CrossRef]
- Nocentini, A.; Moi, D.; Deplano, A.; Balboni, G.; Onnis, V.; Supuran, C.T. Discovery of thiazolin-4-one-based aromatic sulfamates as a new class of carbonic anhydrase isoforms I, II, IV, and IX inhibitors. Bioorg. Chem. 2018, 77, 293–299. [Google Scholar] [CrossRef]
- Nocentini, A.; Moi, D.; Deplano, A.; Osman, S.M.; AlOthman, Z.A.; Piras, V.; Balboni, G.; Supuran, C.T.; Onnis, V. Appliance of the piperidinyl-hydrazidoureido linker to benzenesulfonamide compounds: Synthesis, in vitro and in silico evaluation of potent carbonic anhydrase II, IX and XII inhibitors. Bioorg. Chem. 2020, 98, 103728. [Google Scholar] [CrossRef]
- Lima, P.C.; Lima, L.M.; da Silva, K.C.M.; Léda, P.H.O.; de Miranda, A.L.P.; Fraga, C.A.M.; Barreiro, E.J. Synthesis and analgesic activity of novel N-acylarylhydrazones and isosters, derived from natural safrole. Eur. J. Med. Chem. 2020, 35, 187–203. [Google Scholar] [CrossRef] [PubMed]
- Khalifah, R.G. The carbon dioxide hydration activity of carbonic anhydrase. I. Stop-flow kinetic studies on the native human isoenzymes B and C. J. Biol. Chem. 1971, 246, 2561–2573. [Google Scholar] [CrossRef] [PubMed]
- Kiss, L.E.; Ferreira, H.S.; Torrão, L.; Bonifácio, M.J.; Palma, P.N.; Soares-da-Silva, P.; Learmonth, D.A. Discovery of a long-acting, peripherally selective inhibitor of catechol-O-methyltransferase. J. Med. Chem. 2010, 53, 3396–3411. [Google Scholar] [CrossRef] [PubMed]
- Vullo, D.; Del Prete, S.; Nocentini, A.; Osman, S.M.; AlOthman, Z.A.; Capasso, C.; Bozdag, M.; Carta, F.; Gratteri, P.; Supuran, C.T. Dithiocarbamates effectively inhibit the β-carbonic anhydrase from the dandruff-producing fungus Malassezia Globose Bioorg. Med. Chem. 2017, 25, 1260–1265. [Google Scholar] [CrossRef]
- Del Prete, S.; Angeli, A.; Ghobril, C.; Hitce, J.; Clavaud, C.; Marat, X.; Supuran, C.T.; Capasso, C. Sulfonamide Inhibition Profile of the β-Carbonic Anhydrase from Malassezia restricta, An Opportunistic Pathogen Triggering Scalp Conditions. Metabolites 2020, 10, 39. [Google Scholar] [CrossRef]
- Nocentini, A.; Bonardi, A.; Gratteri, P.; Cerra, B.; Gioiello, A.; Supuran, C.T. Steroids interfere with human carbonic anhydrase activity by using alternative binding mechanisms. J. Enzyme Inhib. Med. Chem. 2018, 33, 1453–1459. [Google Scholar] [CrossRef]
- Bonardi, A.; Vermelho, A.B.; da Silva Cardoso, V.; de Souza Pereira, M.C.; da Silva Lara, L.; Selleri, S.; Gratteri, P.; Supuran, C.T.; Nocentini, A. N-Nitrosulfonamides as Carbonic Anhydrase Inhibitors: A Promising Chemotype for Targeting Chagas Disease and Leishmaniasis. ACS Med. Chem. Lett. 2018, 10, 413–418. [Google Scholar] [CrossRef]
- Sippel, K.H.; Robbins, A.H.; Domsic, J.; Genis, C.; Agbandje-McKenna, M.; McKenna, R. High-resolution structure of human carbonic anhydrase II complexed with acetazolamide reveals insights into inhibitor drug design. Acta Crystallogr. Sect. F Struct. Biol. Cryst. Commun. 2009, 65, 992–995. [Google Scholar] [CrossRef]
- Alterio, V.; Hilvo, M.; Di Fiore, A.; Supuran, C.T.; Pan, P.; Parkkila, S.; Scaloni, A.; Pastorek, J.; Pastorekova, S.; Pedone, C.; et al. Crystal structure of the catalytic domain of the tumor-associated human carbonic anhydrase IX. Proc. Natl. Acad. Sci. USA 2009, 106, 16233–16238. [Google Scholar] [CrossRef]
- Whittington, D.A.; Waheed, A.; Ulmasov, B.; Shah, G.N.; Grubb, J.H.; Sly, W.S.; Christianson, D.V. Crystal structure of the dimeric extracellular domain of human carbonic anhydrase XII, a bitopic membrane protein overexpressed in certain cancer tumor cells. Proc. Natl. Acad. Sci. USA 2001, 98, 9545–9550. [Google Scholar] [CrossRef] [PubMed]
- Baron, G.; Borella, S.; Della Vedova, L.; Vittorio, S.; Vistoli, G.; Carini, M.; Aldini, G.; Altomare, A. An integrated metabolomic and proteomic approach for the identification of covalent inhibitors of the main protease (Mpro) of SARS-CoV-2 from crude natural extracts. Talanta 2022, 252, 123824.1–123824.13. [Google Scholar] [CrossRef] [PubMed]
- Pedretti, A.; Mazzolari, A.; Gervasoni, S.; Fumagalli, L.; Vistoli, G. The VEGA suite of programs: An versatile platform for cheminformatics and drug design projects. Bioinformatics 2021, 37, 1174–1175. [Google Scholar] [CrossRef]
- Dewar, M.J.S.; Walter Thiel, W. Ground states of molecules. 38. The MNDO method. Approximations and parameters. J. Am. Chem. Soc. 1977, 99, 4899–4907. [Google Scholar] [CrossRef]
- Jones, G.; Willett, P.; Glen, R.C.; Leach, A.R.; Taylor, R. Development and validation of a genetic algorithm for flexible docking. J. Mol. Biol. 1997, 267, 727–748. [Google Scholar] [CrossRef]
- De Luca, L.; Germanò, M.P.; Fais, A.; Pintus, F.; Buemi, M.R.; Vittorio, S.; Mirabile, S.; Rapisarda, A.; Gitto, R. Discovery of a new potent inhibitor of mushroom tyrosinase (Agaricus bisporus) containing 4-(4-hydroxyphenyl)piperazin-1-yl moiety. Bioorg. Med. Chem. 2020, 28, 115497. [Google Scholar] [CrossRef]
- Vittorio, S.; Ielo, L.; Mirabile, S.; Gitto, R.; Fais, A.; Floris, S.; Rapisarda, A.; Germanò, M.P.; De Luca, L. 4-Fluorobenzylpiperazine-Containing Derivatives as Efficient Inhibitors of Mushroom Tyrosinase. ChemMedChem 2020, 15, 1757–1764. [Google Scholar] [CrossRef]
| Cmpd | Ar | KI (nM) 1 | ||||||
|---|---|---|---|---|---|---|---|---|
| CAI | CAII | CAIX | CAXII | CAII/CAI Selectivity | CAII/CAIX Selectivity | CAII/CAXII Selectivity | ||
| 5 | 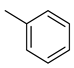 | 18.5 | 4.41 | 34.3 | 18.0 | 4.2 | 7.8 | 4.1 |
| 6 | 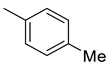 | 3235 | 5.62 | 48.1 | 13.9 | 575.6 | 8.6 | 2.5 |
| 7 | 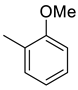 | 45.5 | 2.94 | 55.1 | 8.55 | 15.5 | 18.7 | 2.9 |
| 8 | 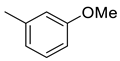 | 382.5 | 44.3 | 38.4 | 19.4 | 8.6 | 0.9 | 0.4 |
| 9 | 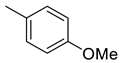 | 389.5 | 4.48 | 320 | 102 | 86.9 | 71,4 | 22.8 |
| 10 | 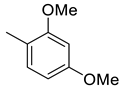 | 1885 | 159.6 | 15.4 | 9.21 | 11.8 | 0.1 | 0.06 |
| 11 | 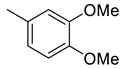 | 344 | 55.1 | 19.6 | 8.05 | 6.2 | 0.4 | 0.1 |
| 12 | 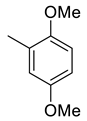 | 24.3 | 1.75 | 44.9 | 25.8 | 13.9 | 25.7 | 14.7 |
| 13 | 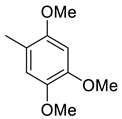 | 41.8 | 3.78 | 52.1 | 26.1 | 11.1 | 13.8 | 6.9 |
| 14 | 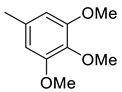 | 384.5 | 67.6 | 39.7 | 32.4 | 5.7 | 0.6 | 0.5 |
| 15 | 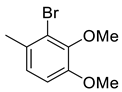 | 1535 | 65.5 | 58.9 | 36.5 | 23.4 | 0.9 | 0.6 |
| 16 | 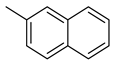 | 4000 | 209 | 123 | 9.36 | 19.1 | 0.6 | 0.04 |
| 17 | 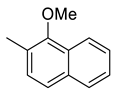 | 334 | 25.2 | 16.4 | 15.3 | 13.3 | 0.6 | 0.6 |
| 18 | 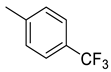 | 1750 | 37.4 | 67.5 | 55.7 | 46.8 | 1.8 | 1.5 |
| 19 | 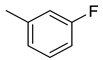 | 392 | 92.4 | 19.7 | 10.2 | 4.2 | 0.2 | 0.1 |
| 20 | 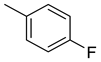 | 340 | 17.1 | 41.6 | 24.8 | 19.9 | 2.4 | 1.4 |
| 21 | 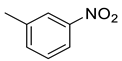 | 1825 | 46.2 | 149 | 29.4 | 39.5 | 3.3 | 0.6 |
| 22 | 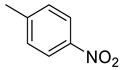 | 383.5 | 4.92 | 64.2 | 53.1 | 77.9 | 13.0 | 8.2 |
| 23 | 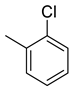 | 482.5 | 6.51 | 23.4 | 14.4 | 74.1 | 3.6 | 2.2 |
| 24 | 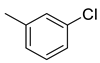 | 398.5 | 55.4 | 36.1 | 17.3 | 7.2 | 0.7 | 0.3 |
| 25 | 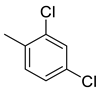 | 336 | 6.65 | 48.7 | 37.2 | 50.5 | 7.3 | 5.6 |
| 26 | 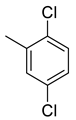 | 800 | 3.64 | 57.7 | 41.9 | 219.8 | 15.8 | 11.5 |
| 27 | 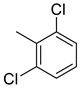 | 24.6 | 3.57 | 54.2 | 68.7 | 6.9 | 15.2 | 19.2 |
| 28 | 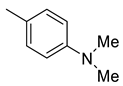 | 1060 | 5.39 | 159 | 22.9 | 196.7 | 29.5 | 4.2 |
| 29 | 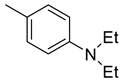 | 45.0 | 4.27 | 64.1 | 42.1 | 10.5 | 15.0 | 9.9 |
| AAZ | 250.0 | 12.0 | 25.0 | 5.7 | 20.8 | 2.1 | 0.5 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moi, D.; Vittorio, S.; Angeli, A.; Balboni, G.; Supuran, C.T.; Onnis, V. Investigation on Hydrazonobenzenesulfonamides as Human Carbonic Anhydrase I, II, IX and XII Inhibitors. Molecules 2023, 28, 91. https://doi.org/10.3390/molecules28010091
Moi D, Vittorio S, Angeli A, Balboni G, Supuran CT, Onnis V. Investigation on Hydrazonobenzenesulfonamides as Human Carbonic Anhydrase I, II, IX and XII Inhibitors. Molecules. 2023; 28(1):91. https://doi.org/10.3390/molecules28010091
Chicago/Turabian StyleMoi, Davide, Serena Vittorio, Andrea Angeli, Gianfranco Balboni, Claudiu T. Supuran, and Valentina Onnis. 2023. "Investigation on Hydrazonobenzenesulfonamides as Human Carbonic Anhydrase I, II, IX and XII Inhibitors" Molecules 28, no. 1: 91. https://doi.org/10.3390/molecules28010091
APA StyleMoi, D., Vittorio, S., Angeli, A., Balboni, G., Supuran, C. T., & Onnis, V. (2023). Investigation on Hydrazonobenzenesulfonamides as Human Carbonic Anhydrase I, II, IX and XII Inhibitors. Molecules, 28(1), 91. https://doi.org/10.3390/molecules28010091









