Abstract
The DYRK (Dual-specificity tyrosine phosphorylation-regulated kinase) family of protein kinases is involved in the pathogenesis of several neurodegenerative diseases. Among them, the DYRK1A protein kinase is thought to be implicated in Alzheimer’s disease (AD) and Down syndrome, and as such, has emerged as an appealing therapeutic target. DYRKs are a subset of the CMGC (CDK, MAPKK, GSK3 and CLK) group of kinases. Within this group of kinases, the CDC2-like kinases (CLKs), such as CLK1, are closely related to DYRKs and have also sparked great interest as potential therapeutic targets for AD. Based on inhibitors previously described in the literature (namely TG003 and INDY), we report in this work a new class of dihydroquinolines exhibiting inhibitory activities in the nanomolar range on hDYRK1A and hCLK1. Moreover, there is overwhelming evidence that oxidative stress plays an important role in AD. Pleasingly, the most potent dual kinase inhibitor 1p exhibited antioxidant and radical scavenging properties. Finally, drug-likeness and molecular docking studies of this new class of DYRK1A/CLK1 inhibitors are also discussed in this article.
1. Introduction
Alzheimer’ s disease (AD) is a neurodegenerative disease, clinically characterized by a progressive deterioration of cognitive functions, leading to an inexorable decline in functional abilities. In addition, AD patients often present neuropsychiatric and behavioral symptoms such as depression, restlessness and hallucinations leading to a deterioration of their quality of life [1]. The pathogenesis of AD is multifactorial, characterized by specific brain anatomical and pathological abnormalities: a significant extent of oxidative stress associated with the presence of extracellular deposits of amyloid-β(Aβ) peptide and intraneuronal neurofibrillary tangles [2,3,4].
Dual-specificity tyrosine phosphorylation-regulated kinase 1A (DYRK1A) is a protein kinase that is abnormally expressed in many diseases such as Down syndrome or AD [5,6,7]. In the literature, it was reported that DYRK1A is able to phosphorylate both APP on Thr668 [8] and presenilin-1 on Thr354 [9]. When the latter is hyper-phosphorylated, the activity of γ-secretase, one of the proteases that cleave APP, is increased. This leads to an abnormally high production of β-amyloid peptides, the main components of amyloid plaques, that are responsible for neurodegeneration [10,11].
DYRK1A was also found to be involved in the hyperphosphorylation of Tau protein (Tubulin-Associated Unit) [12,13,14,15,16], which triggers Tau to dissociate from neuronal microtubules and, shortly afterwards, causes microtubules to disassemble while leading to the formation of neurofibrillary tangles and final neurodegeneration.
Furthermore, the CDK-like kinase (CLK) family, composed of four isoforms: CLK1, CLK2, CLK3 and CLK4, is also an interesting target for treating AD [7,17,18,19,20]. As for DYRK1A, they are dual-specificity kinases. In particular, CLK1 is likely involved in AD by phosphorylating the serine residue in serine/arginine-rich (SR) proteins [19]. SR proteins are a family of splicing factors involved in the alternative splicing of Tau.
Although some natural compounds, such as epigallocatechin-3-gallate (EGCG) [21], harmine [22], and leucettamine B [23], have been identified as potent inhibitors of DYRK1A or CLK1, significant research effort has been devoted to developing synthetic DYRK1A and/or CLK1 inhibitors based on relatively sophisticated heterocyclic scaffolds [7,19,24,25,26,27,28]. A large majority of these kinase inhibitors are type I, that is, they bind to the active form of the kinase in the ATP pocket [29].
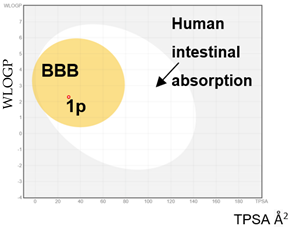
As part of a research program seeking new and readily accessible classes of DYRK1A and/or CLK1 kinase inhibitors, our attention was drawn to INDY [(Z)-1-(3-Ethyl-5-hydroxy-2(3H)-benzothiazolylidene)-2-propanone)] and its derivatives such as TG003 (its synthetic precursor) and ProINDY (its prodrug), reported by Ogawa [30] and Hagiwara [31], respectively (Figure 1). These DYRK1A/CLK1 inhibitors are all derived from 5-methoxy-2-methylbenzothiazole and show significant structural analogy to the 3-acetyl 7-substituted 1,4-dihydroquinoline derivatives. It is worth mentioning that this new class of dual DYRK1A and CLK1 inhibitors based on a biooxidizable 1,4-dihydroquinoline scaffold may also undergo oxidation into quinolinium salts. That could lead to antioxidant properties going hand in hand with the attenuation of oxidative stress in brain tissues. In the present article, we report the synthesis of these biooxidizable 1,4-dihydroquinoline derivatives, a preliminary in vitro biological evaluation toward DYRK1A and CLK1 kinases, and an examination of their redox properties in various biological media.
Figure 1.
INDY, TG003 and ProINDY. INDY and TG003: DYRK1A/CLK1 inhibitors and structural analogies with 3-acetyl 7-substituted 1,4-dihydroquinolines.
2. Results and Discussion
2.1. Synthesis of the Studied Dual hDYRK1A and hCLK1 Inhibitors
Apart from commercial quinoline 6a, 3-acetyl quinoline derivatives 6b–h were obtained by reacting the corresponding o-nitrobenzaldehyde derivatives 3b–h with 4,4-dimethoxybutan-2-one 4 in the presence of tin chloride as a reducing reagent (Scheme 1). This domino nitro reduction-Friedländer cyclisation, previously reported in the literature for the preparation of quinoline-3-carboxylic acid esters [32], provided access to the required 3-acetyl quinoline derivatives 6b–h in a one-step procedure from readily available and stable starting materials.
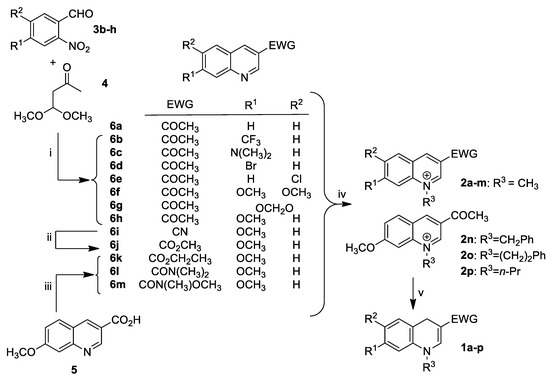
Scheme 1.
(i) SnCl2·H2O, EtOH, reflux, 4 h (16–96%); (ii) TMSCl, methanol, 50 °C, 4 h (65%); (iii) SOCl2, ethanol, 78 °C, 18 h (75%) for 6k, T3P®, (CH3)2N·HCl, DMF, 130 °C, 18 h for 6l, CDI, CH3ONHCH3·HCl, DMF, NEt3, 18 h (76%) for 6m; (iv) CH3I, CH3CN, sealed tube, 85 °C, 18 h (70–95%) for 2a–m, PhCH2Br, CH3CN, 85 °C, 3 days (91%) for 2n, PhCH2CH2Br, CH3CN, 85 °C, 4 days (80%) for 2o, n-PrBr, CH3CN, 85 °C, 2 days (70%) for 2p; (v) BNAH, CH2Cl2, 20 °C, 4 h (10–86%).
We then focused on the preparation of compounds 6i–m to investigate the influence of the electron withdrawing group (EWG) at C-3 position of the quinoline ring on the inhibitory activity of the DYRK1A and CLK1 kinases. To this end, the 3-cyano-7-methoxy quinoline 6i previously reported in our group [33] was reacted with chlorotrimethylsilane and anhydrous methanol [34] to provide methyl ester 6j. Quinoline ester 6k was obtained by reacting thionyl chloride on the reported quinoline 3-carboxylic acid 5 [35] in ethanol. Finally 6l,m were prepared from quinoline derivative 5 using T3P® and CDI amide coupling reagents, respectively. Subsequently, quinoline derivatives 6a–m were subjected to a classical quaternization reaction with alkyl halides to afford the desired quinolinium salts 2a–p, which were then successfully regioselectively reduced with BNAH to the corresponding 1,4-dihydroquinolines 1a–p. With the target compounds 1a–p in hand, readily obtained in a few steps, and in most cases, in a good overall yield, we embarked on an initial biological evaluation of these dihydroquinoline scaffolds as potential new DYRK1A and CLK1 inhibitors (Table 1).

Table 1.
Inhibitory activities of dihydroquinoline derivatives on kinases (hDYRK1A, hCLK1).
2.2. In Vitro Evaluation on hDYRK1A and hCLK1 Kinases
We initially focused on the influence of the substitution patterns at the benzene ring by comparing the hDYRK1A and hCLK1 inhibitory activities of a set of dihydroquinolines 1a–h differently substituted at C-6 and C-7 (EWG = COMe and R3 = Me). To our delight, compound 1h displayed interesting three-digit nanomolar hDYRK1A and hCLK1 inhibitory activities (Entry 8) comparable to those of INDY and TG003, whereas compounds 1a–d showed no inhibition on either of the two kinases (Entries 1−4). This first series revealed that a methoxy group at C-7 is essential while an additional substituent at C-6 appears to be deleterious, resulting in a complete loss of the inhibitory activity (Entries 6,7). The influence of the EWG at C-3 position, which is crucial to ensure good stability of the 1,4-dihydroquinoline, was then assessed by replacing the acetyl group in 1h by a variety of EWGs (nitrile, amides, esters). The resulting compounds 1i–m turned out to be inactive toward both hDYRK1A and hCLK1 kinases (Entries 9−13), with the sole exception of the ester derivatives 1j,k that exhibited micromolar hCLK1 inhibitory activities (Entries 10,11). After demonstrating that both an acetyl group at C-3 position and a methoxy group at C-7 position are required, we turned our attention to the role of the alkyl group on the nitrogen dihydroquinoline scaffold. Compared to N-methyl dihydroquinoline 1h, N-benzyl and N-phenethyl dihydroquinolines 1n,o exhibited lower hDYRK1A inhibitory activities, while displaying comparable three-digit nanomolar hCLK1 inhibitory activities (Entries 14,15). Gratifyingly, N-propyl dihydroquinoline 1p turned out to be 2.5-fold more potent than N-methyl dihydroquinoline 1h toward hDYRK1A and 8-fold more potent than N-methyl dihydroquinoline 1h toward hCLK1 (Entries 8,16). To complete this SAR analysis, we sought to determine whether or not the enamine function is a crucial element for this new class of dual DYRK1A/CLK1 inhibitors. To tackle this issue, quinolinium salts 2h and quinoline 6h were also evaluated for inhibition of hDYRK1A/hCLK1. None of these synthetic intermediates showed inhibitory activities on hDYRK1A/hCLK1 kinases (IC50 > 10 mM). These observations led us to conclude that, in addition to the acetyl group at C-3 and the methoxy group at C-7, the enamine-like nitrogen atom is also a prerequisite for inhibition of hDYRK1A/hCLK1.
2.3. Evaluation of the Antioxidant and Radical Scavenging Activities of 1p
Oxidative stress is also an important hallmark of AD [36,37,38,39,40] and is closely linked with the formation of both the neurofibrillary tangles and the amyloid plaques.
A further interesting aspect of this novel class of hDYRK1A/hCLK1 inhibitors is related to the potential of 1,4-dihydroquinolines 1 to prevent oxidative stress by exhibiting in vivo antioxidant and radical scavenging properties during their conversion to the corresponding quinolinium salts 2 in the Central Nervous System (CNS) [35,41].
To evaluate the ability of 1,4-dihydroquinolines 1 to act as antioxidants, we studied their behavior by incubating them in different oxidative media [42] (NAD+, mouse brain homogenate, H2O2, riboflavin) (Figure 2). First of all, in vitro stability of the most potent inhibitor 1p was evaluated in PBS and human plasma at 37 °C. After 3.5 h incubation in these non-oxidizing media, only 3% of the oxidation product 2p was observed in PBS and 4% in human plasma (8% after 24 h for both media). This finding led us to predict a good stability of 1p at the periphery. Then, in the presence of the oxidizing medium consisting of 0.2% NAD+ in PBS, the oxidation rate of dihydroquinoline 1p was relatively low (5% after 3.5 h and 16% after 24 h). However, the formation of the oxidation product 2p was shown to be much faster when using 2% NAD+ (24% after 3.5 h and 77% after 24 h). A similar result was observed when 1p was exposed to 20% fresh mouse brain homogenate (25% after 3.5 h and 72% after 24 h), while hydrogen peroxide 0.1% appeared to be slightly less effective (13% after 3.5 h and 53% after 24 h). Finally, in vitro oxidation with 0.1% riboflavin showed an extremely rapid oxidative conversion of 1p into the corresponding quaternary salt 2p (100% in less than 3.5 h).
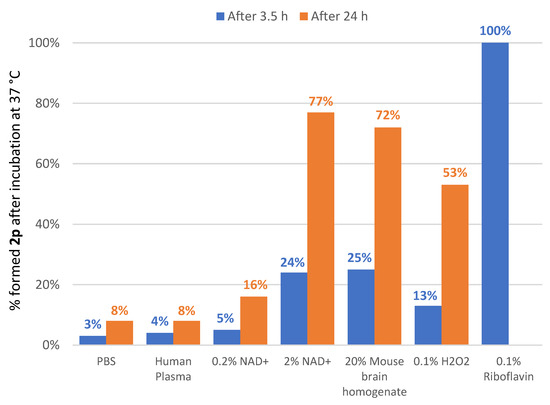
Figure 2.
In vitro stability of 1p in various non-oxidizing and oxidizing media.
Finally, the aptitude of 1p to act as a free radical scavenger was assessed using the rapid, inexpensive and widely used DPPH• (2,2-diphenyl-1-picrylhydrazyl) scavenging method [43]. Dark purple DPPH• is a stable free radical that can accept an electron or hydrogen to become a stable diamagnetic molecule with the loss of violet color. Interestingly, we were able to determine that dihydroquinoline 1p exhibited a noticeable radical scavenging activity (EC50 = 128 μM). Moreover, 1H NMR experiments conducted in DMSO-d6 showed that dihydroquinoline 1p reacts with DPPH• almost instantly to furnish the corresponding inactive quinolinium salt 2p (see Supplementary Materials). Thus, this novel class of hDYRK1A/hCLK1 inhibitors are all the more interesting that they may also potentially display an additional antioxidant activity to combat oxidative stress in AD.
2.4. Drug-likeness Evaluation
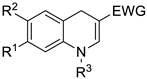
Drug candidates should possess favorable ADME (Absorption, Distribution, Metabolism, and Excretion) properties. In the literature, Lipinski [44], based on a study of orally active drugs, established a set of simple rules for estimating permeability. These rules were later adjusted and others were added to design successful CNS drugs [45]. To predict the CNS druggability of 1p, its ability to cross the blood-brain barrier (BBB) by passive diffusion was examined using the free online server SwissADME [46] (Table 2). According to the results obtained (see Supplementary Materials) and as illustrated by the “BOILED-egg” (Brain Or IntestinaL EstimateD permeation) [47], 1,4-dihydroquinoline 1p may be viewed as a promising CNS drug candidate. The “BOILED-egg” method is based on a descriptive delineation of well-absorbed drugs. This robust and accurate predictive model of passive absorption is based on the calculation of physicochemical descriptors.

Table 2.
Calculation with 1p and comparison of parameters required for drugs targeting the CNS.
To confirm these encouraging results, the ability of 1p to cross the BBB by passive diffusion was assessed through the parallel artificial membrane permeability assay of blood-brain barrier (PAMPA-BBB) [48]. The obtained permeability value suggests that 1p easily diffuses across the BBB (Pe = 20.0 0.9 × 10−6 cm·s−1).
2.5. Docking Studies
Visualization of the hDYRK1A structure co-crystallized with INDY (PDB ID: 3ANQ [30]), a DYRK1A benzothiazole derivative ATP-competitive inhibitor (IC50 = 0.24 µM and Ki = 0.18 µM), and hCLK1 co-crystallized with the INDY derivative inhibitor TG003 (PDB ID: 6YTE [49], Kd = 75 nM) showed that the two ligands bind in both kinases’ active sites through a very similar interaction network (Figure 3A,B). In both structures, the aromatic ring of the ligands was placed parallel to the beta sheet delimiting the ATP binding site and the ligands established two hydrogen bonds: (i) a first one with a backbone NH of Leu241-hDYRK1A or Leu244-hCLK1, and (ii) a second one with NH3+ of the Lys188-hDYRK1A or Lys191-hCLK1 side chain. Even though the lysine side chain is long and flexible, in both kinases its orientation is fixed through salt bridges with Glu203-hDYRK1A and Glu206-hCLK1. Therefore, for a ligand binding in the “INDY way” to the two kinases, it is necessary to have two hydrogen bond acceptors at a distance of about 8.5 Å. Among the synthetized ligands, only ligands with the EWG = COCH3 and R1 substituent = OCH3 presented interesting activities. Indeed, the distance between their oxygen atoms is about 8.7 Å. To gain further insight into the binding mode of the dihydroquinoline derivatives, compound 1h docking studies using the GOLD program applying the ChemPLP scoring function were carried out. GOLD is an automated ligand docking program that uses a genetic algorithm. GOLD’s evolutionary algorithm modifies the position, orientation and conformation of a ligand to fit into one or more low energy states of the protein’s active site. Our docking studies confirmed that compound 1h reproduces well the INDY binding mode in hDYRK1A and in hCLK1. For both kinases, the proposed poses by GOLD converged to the same ligand orientation and the best scoring ones are represented on Figure 3C,D. The compound 1h ChemPLP fit score was 63.59 in hDYRK1A and 67.99 in hCLK1. As can be seen on Figure 3, compound 1h is able to establish the two crucial hydrogen bonds with both kinases, the first one with the Leu backbone NH and the second one with NH3+ of Lys.
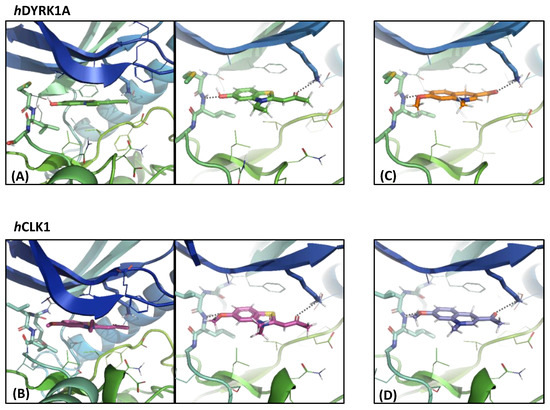
Figure 3.
Two perpendicular views of (A) INDY inhibitor position from the hDYRK1 X-ray structure, and (B) INDY derivative TG003 position from the hCLK1 X-ray structure compared to the compound 1h positioned using the docking studies in the (C) hDYRK1A binding site and (D) hCLK1 binding site. The INDY, INDY derivative TG003, compound 1h and the selected residue or residue side chains of the binding site are in stick form and the protein in ribbon representation. This figure was made with PYMOL (DeLano Scientific, 2002, San Carlo, CA, USA).
3. Materials and Methods
3.1. Docking Studies
The initial model of compound 1h was built using BIOVIA Discovery Studio v19.1 (BIOVIA, San Diego, CA, USA) and its preferential protonation state at pH 7.4 was checked using standard tools of the ChemAxon Package (ChemAxon Ltd., Budapest, Hungary) [50]; the majority nonprotonated microspecies was used for the docking studies.
The crystallographic coordinates of human CLK1 used in this study were obtained from the X-ray structure of CLK1 bound with the benzothiazole TG003 inhibitor (PDB ID 6YTE [49], a structure refined to 2.30 Å with an R factor of 18.0%) and those of human DYRK1A from the X-ray structure of DYRK1A co-crystallized with the INDY inhibitor (PDB ID 3ANQ [30], a structure refined to 2.60 Å with an R factor of 23.6%).
The docking of compound 1h into the hCLK1 and hDYRK1 was carried out with the GOLD program v5.3 (The Cambridge Crystallographic Data Centre CCDC, Cambridge, United Kingdom) using the default parameters [51,52]. This program applies a genetic algorithm to explore conformational spaces and ligand binding modes. To evaluate the proposed ligand positions, the ChemPLP fitness function was applied. The binding site in both kinases was defined as a 6 Å sphere from the co-crystallized ligand.
3.2. Chemistry
All commercial reagents were used without further purification. The solvents were dried with appropriate desiccants and distilled prior to use or were obtained anhydrous from commercial suppliers. Silica gel (60, 230–400 mesh or 70–230 mesh) was used for column chromatography. Reactions were monitored by thin layer chromatography on silica gel precoated aluminum plates. UV light at 254 nm or KMnO4 stains were used to visualize TLC plates. 1H, 13C, 19F NMR spectra were recorded using a spectrometer operating at 300, 75 and 282 MHz, respectively. Abbreviations used for peak multiplicities are s: singlet, d: doublet, t: triplet, q: quadruplet dd = doublet of doublet, br = broad and m: multiplet. Coupling constants J are in Hz and chemical shifts are given in ppm and calibrated with DMSO-d6 or CDCl3 (residual solvent signals). 1H NMR spectra obtained in CDCl3 were referenced to 7.26 ppm. 13C NMR spectra obtained in CDCl3 were referenced to 77.16 ppm and in DMSO-d6 were referenced to 39.52 ppm. 19F NMR chemical shifts (δ) were determined relative to CFCl3 as an internal standard (19F, δ = 0.0 ppm). Nitrobenzaldehyde derivatives 3b–e, g, h and 4,4-dimethoxybutan-2-one 4 were obtained from Sigma-Aldrich, while 3f was commercially available at Fisher Scientific. 7-methoxy quinoline carboxylic acid 5 was prepared as previously reported in the literature [33,35,41]. 3-acetylquinoline 6a was purchased from Fisher Scientific. 7-methoxyquinoline-3-carbonitrile 6i was prepared using a synthesis previously described [33]. Elemental analyses were performed by the microanalysis service of the University of Rouen and were recorded with a Thermo Scientific™ FLASH 2000 analyzer (Thermo Fisher Scientific, Waltham, MA, USA).
General procedure A for the synthesis of compounds1a–p
The corresponding quinolinium salt 2a–p and BNAH were placed in dry and degassed dichloromethane in a round-bottomed flask and under inert atmosphere. The resulting suspension was stirred in the dark at 20 °C for 4 h. Thereafter, degassed dichloromethane (20 mL) was added to the reaction mixture and the solution was washed with degassed water (2 × 20 mL) and brine. The organic phase was dried on MgSO4 and evaporated to dryness to give the corresponding dihydroquinoline.
1-(1-Methyl-1,4-dihydroquinolin-3-yl)ethanone (1a)
According to procedure A, from quinolinium salt 2a (150 mg, 0.48 mmol), BNAH (103 mg, 0.48 mmol), dry dichloromethane (2.0 mL). Compound 1a was obtained (63 mg, 70%) as a brown amorphous solid. 1H NMR (300 MHz, CDCl3) δ 7.19–7.07 (m, 3H), 6.99 (dd, J = 7.4, 1.2 Hz, 1H), 6.78 (dd, J = 8.0, 1.1 Hz, 1H), 3.77 (s, 2H), 3.29 (s, 3H), 2.24 (s, 3H); 13C NMR (75 MHz, CDCl3) δ 194.26, 144.92, 138.45, 129.84, 127.13, 123.82, 123.59, 112.77, 109.45, 39.34, 25.65, 24.12; HRMS (ESI): m/z calcd. for C12H14NO: 188.1075 [M + H]+, found 188.4068. Anal. Calcd. for C12H13NO: C, 76.98; H, 7.00; N, 7.48. Found: C, 77.09; H, 7.03; N, 7.53.
1-(1-Methyl-7-(trifluoromethyl)-1,4-dihydroquinolin-3-yl)ethanone (1b)
According to procedure A, from quinolinium salt 2b (150 mg, 0.39 mmol), BNAH (84 mg, 0.39 mmol), dry dichloromethane (2.0 mL). Compound 1b was obtained (66 mg, 66%) as an orange solid. m.p. 141–142 °C; 1H NMR (300 MHz, CDCl3) δ 7.22–7.12 (m, 3H), 6.93 (d, J = 1.6 Hz, 1H), 3.82–3.70 (m, 2H), 3.30 (s, 3H), 2.24 (s, 3H); 19F NMR (282 MHz, CDCl3) δ −62.7; 13C NMR (75 MHz, CDCl3) δ 194.30, 144.47, 139.22, 130.22, 129.67 (q, J = 32.5 Hz), 127.80 (d, J = 1.4 Hz), 124.08 (q, J = 1080 Hz) 120.07 (q, J = 3.9 Hz), 109.88, 109.31 (q, J = 3.9 Hz), 39.38, 25.69, 24.23; HRMS (ESI): m/z calcd. for C13H13F3NO: 256.0949 [M + H]+, found 256.0955. Anal. Calcd. for C13H12F3NO: C, 61.17; H, 4.74; N, 5.49. Found: C, 61.09; H, 4.83; N, 5.53.
1-(7-(Dimethylamino)-1-methyl-1,4-dihydroquinolin-3-yl)ethanone (1c)
According to procedure A, from quinolinium salt 2c (150 mg, 0.42 mmol), BNAH (90 mg, 0.42 mmol), dry dichloromethane (2.0 mL). Compound 1c was obtained (65 mg, 67%) as an orange solid. m.p. 126–127 °C; 1H NMR (300 MHz, CDCl3) δ 7.14 (d, J = 0.8 Hz, 1H), 6.94 (d, J = 8.4 Hz, 1H), 6.38 (dd, J = 8.3, 2.5 Hz, 1H), 6.12 (d, J = 2.4 Hz, 1H), 3.65 (s, 2H), 3.28 (s, 3H), 2.91 (s, 6H), 2.21 (s, 3H); 13C NMR (75 MHz, CDCl3) δ 194.45, 150.19, 145.12, 138.88, 130.23, 111.92, 110.02, 108.19, 97.92, 40.88, 39.41, 24.77, 24.16; HRMS (ESI): m/z calcd. for C14H19N2O: 231.1497 [M + H]+, found 231.1494. Anal. Calcd. for C14H18N2O: C, 73.01; H, 7.88; N, 12.16. Found: C, 72.89; H, 7.82; N, 12.23.
1-(7-Bromo-1-methyl-1,4-dihydroquinolin-3-yl)ethanone (1d)
According to procedure A, from quinolinium salt 2d (150 mg, 0.38 mmol), BNAH (82 mg, 0.38 mmol), dry dichloromethane (2.0 mL). Compound 1d was obtained (75 mg, 74%) as a beige solid. m.p. 179–180 °C; 1H NMR (300 MHz, CDCl3) δ 7.13–7.05 (m, 2H), 6.95 (dt, J = 8.1, 1.1 Hz, 1H), 6.89 (d, J = 1.9 Hz, 1H), 3.69 (s, 2H), 3.26 (s, 3H), 2.24 (s, 3H); 13C NMR (75 MHz, CDCl3) δ 194.33, 144.39, 139.93, 131.10, 126.16, 122.72, 120.46, 115.77, 110.09, 39.40, 25.27, 24.25; HRMS (ESI): m/z calcd. for C12H13NO79Br: 266.0181 [M + H]+, found 266.0182. Anal. Calcd. for C12H12NOBr: C, 54.16; H, 4.54; N, 5.26. Found: C, 54.06; H, 4.67; N, 5.23.
1-(6-Chloro-1-methyl-1,4-dihydroquinolin-3-yl)ethanone (1e)
According to procedure A, from quinolinium salt 2e (150 mg, 0.43 mmol), BNAH (93 mg, 0.43 mmol), dry dichloromethane (2.0 mL). Compound 1e was obtained (74 mg, 77%) as a beige solid. m.p. 124–125 °C; 1H NMR (300 MHz, CDCl3) δ 7.14–7.05 (m, 3H), 6.72–6.65 (m, 1H), 3.73 (d, J = 1.1 Hz, 2H), 3.27 (s, 3H), 2.23 (s, 3H); 13C NMR (75 MHz, CDCl3) δ 194.10, 144.52, 137.17, 129.45, 128.34, 126.92, 125.63, 113.91, 109.22, 39.41, 25.60, 24.13; HRMS (ESI): m/z calcd. for C12H13NO35Cl: 222.0686 [M + H]+, found 222.0692. Anal. Calcd. for C12H12NOCl: C, 65.02; H, 5.46; N, 6.32. Found: C, 64.97; H, 5.53; N, 6.22.
1-(6,7-Dimethoxy-1-methyl-1,4-dihydroquinolin-3-yl)ethanone (1f)
According to procedure A, from quinolinium salt 2f (160 mg, 0.43 mmol), BNAH (92 mg, 0.43 mmol), dry dichloromethane (2.0 mL). Compound 1f was obtained (10 mg, 10%) as a yellow solid. m.p. 235–236 °C; 1H NMR (300 MHz, CDCl3) δ 7.14 (s, 1H), 6.62 (t, J = 0.9 Hz, 1H), 6.38 (s, 1H), 3.87 (s, 3H), 3.83 (s, 3H), 3.70 (s, 2H), 3.30 (s, 3H), 2.22 (s, 3H); 13C NMR (75 MHz, CDCl3) δ 194.29, 147.87, 145.58, 144.72, 131.86, 115.79, 113.28, 108.77, 98.74, 56.49, 56.36, 39.63, 25.62, 24.21; HRMS (ESI): m/z calcd. for C14H18NO3: 248.1287 [M + H]+, found 248.1282. Anal. Calcd. for C14H17NO3: C, 68.00; H, 6.93; N, 5.66. Found: C, 67.91; H, 6.83; N, 5.73.
1-(5-Methyl-5,8-dihydro-[1,3]dioxolo[4,5-g]quinolin-7-yl)ethanone (1g)
According to procedure A, from quinolinium salt 2g (150 mg, 0.42 mmol), BNAH (90 mg, 0.42 mmol), dry dichloromethane (2.0 mL). Compound 1g was obtained (65 mg, 67%) as an orange solid. m.p. 152–153 °C; 1H NMR (300 MHz, CDCl3) δ 7.09 (s, 1H), 6.55 (t, J = 0.9 Hz, 1H), 6.36 (s, 1H), 5.87 (s, 2H), 3.64 (s, 2H), 3.22 (s, 3H), 2.19 (s, 3H); 13C NMR (75 MHz, CDCl3) δ 194.16, 146.71, 144.66, 143.60, 132.73, 116.50, 109.61, 108.49, 101.23, 95.40, 39.88, 25.97, 24.12; HRMS (ESI): m/z calcd. for C13H14NO3: 232.0974 [M + H]+, found 232.0975. Anal. Calcd. for C13H13NO3: C, 67.52; H, 5.67; N, 6.06. Found: C, 67.72; H, 5.63; N, 6.11.
1-(7-Methoxy-1-methyl-1,4-dihydroquinolin-3-yl)ethanone (1h)
According to procedure A, from quinolinium salt 2h (138 mg, 0.47 mmol), BNAH (100 mg, 0.47 mmol), dry dichloromethane (2.0 mL). Compound 1h was obtained (83 mg, 82%) as an orange solid. m.p. 99–100 °C; 1H NMR (300 MHz, CDCl3) δ 7.14 (d, J = 0.8 Hz, 1H), 7.02 (dt, J = 8.3, 1.1 Hz, 1H), 6.53 (dd, J = 8.3, 2.5 Hz, 1H), 6.35 (d, J = 2.4 Hz, 1H), 3.79 (s, 3H), 3.69 (s, 2H), 3.27 (s, 3H), 2.24 (s, 3H); 13C NMR (75 MHz, CDCl3) δ 194.54, 158.97, 144.79, 139.42, 130.46, 116.12, 110.25, 107.36, 100.48, 55.54, 39.52, 24.93, 24.29; HRMS (ESI): m/z calcd. for C13H16NO2: 218.1181 [M + H]+, found 218.1191. Anal. Calcd. for C13H15NO2: C, 71.87; H, 6.96; N, 6.45. Found: C, 71.91; H, 6.93; N, 6.53.
7-Methoxy-1-methyl-1,4-dihydroquinoline-3-carbonitrile (1i)
According to procedure A, from quinolinium salt 2i (100 mg, 0.31 mmol), BNAH (66 mg, 0.31 mmol), dry dichloromethane (2.0 mL). Compound 1i was obtained (45 mg, 73%) as a yellow solid. m.p. 127–128 °C; 1H NMR (300 MHz, CDCl3) δ 6.88 (dt, J = 8.3, 1.0 Hz, 1H), 6.66 (t, J = 1.0 Hz, 1H), 6.51 (dd, J = 8.3, 2.5 Hz, 1H), 6.29 (d, J = 2.4 Hz, 1H), 3.78 (s, 3H), 3.64 (d, J = 1.1 Hz, 2H), 3.15 (s, 3H); 13C NMR (75 MHz, CDCl3) δ 159.33, 144.92, 139.15, 129.87, 121.41, 112.54, 107.50, 100.36, 77.51, 55.47, 38.95, 26.68; HRMS (ESI): m/z calcd. for C12H13N2O: 201.1028 [M + H]+, found 201.1035. Anal. Calcd. for C12H12N2O: C, 71.98; H, 6.04; N, 13.99. Found: C, 72.05; H, 5.98; N, 14.08.
Methyl 7-methoxy-1-methyl-1,4-dihydroquinoline-3-carboxylate (1j)
According to procedure A, from quinolinium salt 2j (100 mg, 0.28 mmol), BNAH (60 mg, 0.28 mmol), dry dichloromethane (2.0 mL). Compound 1j was obtained (51 mg, 79%) as a yellow solid. m.p. 95–96 °C; 1H NMR (300 MHz, CDCl3) δ 7.22 (d, J = 0.9 Hz, 1H), 6.97 (dt, J = 8.3, 1.1 Hz, 1H), 6.50 (dd, J = 8.3, 2.5 Hz, 1H), 6.31 (d, J = 2.4 Hz, 1H), 3.79 (s, 3H), 3.72 (d, J = 6.7 Hz, 5H), 3.20 (s, 3H); 13C NMR (75 MHz, CDCl3) δ 168.37, 159.00, 143.10, 139.91, 130.24, 115.39, 106.95, 100.20, 97.69, 55.46, 51.13, 39.05, 25.56; HRMS (ESI): m/z calcd. for C13H16NO3: 234.1130 [M + H]+, found 234.1135. Anal. Calcd. for C13H15NO3: C, 66.94; H, 6.48; N, 6.00. Found: C, 67.03; H, 6.53; N, 6.09.
Ethyl 7-methoxy-1-methyl-1,4-dihydroquinoline-3-carboxylate (1k)
According to procedure A, from quinolinium salt 2k (100 mg, 0.27 mmol), BNAH (58 mg, 0.27 mmol), dry dichloromethane (2.0 mL). Compound 1k was obtained (57 mg, 86%) as a yellow solid. m.p. 93–94 °C; 1H NMR (300 MHz, CDCl3) δ 7.20 (d, J = 0.9 Hz, 1H), 6.96 (dt, J = 8.3, 1.1 Hz, 1H), 6.49 (dd, J = 8.3, 2.5 Hz, 1H), 6.29 (d, J = 2.5 Hz, 1H), 4.18 (q, J = 7.1 Hz, 2H), 3.78 (s, 3H), 3.69 (d, J = 1.1 Hz, 2H), 3.19 (s, 3H), 1.29 (t, J = 7.1 Hz, 3H); 13C NMR (75 MHz, CDCl3) δ 167.96, 158.99, 142.88, 139.99, 130.22, 115.45, 106.87, 100.14, 98.03, 59.66, 55.46, 39.03, 25.56, 14.69; HRMS (ESI): m/z calcd. for C14H18NO3: 248.1287 [M + H]+, found 248.1279. Anal. Calcd. for C14H17NO3: C, 68.00; H, 6.93; N, 5.66. Found: C, 68.05; H, 6.99; N, 5.59.
7-Methoxy-N,N,1-trimethyl-1,4-dihydroquinoline-3-carboxamide (1l)
According to procedure A, from quinolinium salt 2l (150 mg, 0.40 mmol), BNAH (86 mg, 0.40 mmol), dry dichloromethane (2.0 mL). Compound 1l was obtained (59.7 mg, 60%) as a yellow solid. m.p. 219–220 °C; 1H NMR (300 MHz, CDCl3) δ 6.92 (dt, J = 8.3, 1.0 Hz, 1H), 6.48–6.39 (m, 2H), 6.25 (d, J = 2.5 Hz, 1H), 3.76 (s, 3H), 3.65 (d, J = 1.1 Hz, 2H), 3.11 (s, 3H), 3.01 (s, 6H); 13C NMR (75 MHz, CDCl3) δ 171.95, 159.04, 141.19, 138.15, 129.58, 114.53, 105.96, 102.37, 99.17, 55.39, 38.49, 37.79, 27.75; HRMS (ESI): m/z calcd. for C14H19N2O2: 247.1447 [M + H]+, found 247.1457. Anal. Calcd. For C14H18N2O2: C, 68.27; H, 7.37; N, 11.37. Found: C, 68.07; H, 7.18; N, 11.29.
N,7-Dimethoxy-N,1-dimethyl-1,4-dihydroquinoline-3-carboxamide (1m)
According to procedure A, from quinolinium salt 2m (150 mg, 0.39 mmol), BNAH (83 mg, 0.39 mmol), dry dichloromethane (3.0 mL). Compound 1m was obtained (68 mg, 67%) as a yellow solid. 1H NMR (300 MHz, CDCl3) δ 7.23 (d, J = 0.9 Hz, 1H), 6.95 (dt, J = 8.2, 1.0 Hz, 1H), 6.47 (dd, J = 8.3, 2.5 Hz, 1H), 6.28 (d, J = 2.4 Hz, 1H), 3.77 (d, J = 3.1 Hz, 5H), 3.64 (s, 3H), 3.21 (s, 3H), 3.17 (s, 3H); 13C NMR (75 MHz, CDCl3) δ 169.90, 158.96, 142.60, 140.41, 129.78, 115.72, 106.51, 100.47, 99.61, 60.38, 55.43, 38.95, 34.17, 27.13; HRMS (ESI): m/z calcd. For C14H19NO3: 263.1396 [M + H]+, found 263.1397. Anal. Calcd. for C14H18N2O3: C, 64.10; H, 6.92; N, 10.68. Found: C, 64.19; H, 6.90; N, 10.73.
1-(1-Benzyl-7-methoxy-1,4-dihydroquinolin-3-yl)ethanone (1n)
According to procedure A, from quinolinium salt 2n (160 mg, 0.43 mmol), BNAH (92 mg, 0.43 mmol), dry dichloromethane (2.0 mL). Compound 1n was obtained (86 mg, 68%) as an orange solid. m.p. 129–130 °C; 1H NMR (300 MHz, CDCl3) δ 7.39–7.25 (m, 6H), 7.01 (dt, J = 8.3, 1.1 Hz, 1H), 6.47 (dd, J = 8.3, 2.4 Hz, 1H), 6.24 (d, J = 2.4 Hz, 1H), 4.80 (s, 2H), 3.75 (s, 2H), 3.63 (s, 3H), 2.23 (s, 3H); 13C NMR (75 MHz, CDCl3) δ 194.75, 158.74, 144.41, 138.41, 136.25, 130.59, 129.11, 127.83, 126.18, 116.02, 110.79, 107.70, 101.31, 55.32, 55.27, 25.00, 24.28; HRMS (ESI): m/z calcd. for C19H20NO2: 294.1494 [M + H]+, found 294.1490. Anal. Calcd. for C19H19NO2: C, 77.79; H, 6.53; N, 4.77. Found: C, 77.86; H, 6.50; N, 4.70.
1-(7-Methoxy-1-phenethyl-1,4-dihydroquinolin-3-yl)ethanone (1o)
According to procedure A, from quinolinium salt 2o (150 mg, 0.39 mmol), BNAH (83 mg, 0.39 mmol), dry dichloromethane (2.0 mL). Compound 1o was obtained (90 mg, 75%) as an orange solid. m.p. 92–93 °C; 1H NMR (300 MHz, CDCl3) δ 7.29 (dd, J = 6.9, 1.3 Hz, 2H), 7.26–7.13 (m, 3H), 7.02 (dt, J = 8.3, 1.1 Hz, 1H), 6.72 (s, 1H), 6.53 (dd, J = 8.3, 2.4 Hz, 1H), 6.47 (d, J = 2.4 Hz, 1H), 3.80 (s, 5H), 3.62 (s, 2H), 2.99 (t, J = 6.9 Hz, 2H), 1.99 (s, 3H); 13C NMR (75 MHz, CDCl3) δ 194.63, 158.94, 144.23, 138.13, 137.87, 130.95, 129.05, 128.86, 126.97, 116.40, 109.69, 107.23, 100.53, 55.47, 53.25, 34.24, 24.76, 23.97; HRMS (ESI): m/z calcd. for C20H22NO2: 308.1651 [M + H]+, found 308.1647. Anal. Calcd. for C20H21NO2: C, 78.15; H, 6.89; N, 4.56. Found: C, 78.25; H, 6.91; N, 4.49.
1-(7-Methoxy-1-propyl-1,4-dihydroquinolin-3-yl)ethanone (1p)
According to procedure A, from quinolinium salt 2p (130 mg, 0.40 mmol), BNAH (86 mg, 0.40 mmol), dry dichloromethane (2.0 mL). Compound 1p was obtained (60 mg, 61%) as an orange solid. m.p. 98–99 °C; 1H NMR (300 MHz, CDCl3) δ 7.15 (d, J = 0.8 Hz, 1H), 7.01 (dt, J = 8.4, 1.1 Hz, 1H), 6.50 (dd, J = 8.3, 2.4 Hz, 1H), 6.35 (d, J = 2.4 Hz, 1H), 3.78 (s, 3H), 3.68 (s, 2H), 3.59–3.47 (m, 2H), 2.23 (s, 3H), 1.82–1.69 (m, 2H), 0.99 (t, J = 7.4 Hz, 3H); 13C NMR (75 MHz, CDCl3) δ 194.59, 158.92, 144.22, 138.25, 130.82, 116.46, 110.00, 107.10, 100.82, 55.53, 53.60, 25.07, 24.29, 21.38, 11.33; HRMS (ESI): m/z calcd. for C15H20NO2: 246.1494 [M + H]+, found 246.1507. Anal. Calcd. for C15H19NO2: C, 73.44; H, 7.81; N, 5.71. Found: C, 73.46; H, 7.80; N, 5.80.
General procedure B for the synthesis of compounds2a–p
In a glass tube, to a solution of the corresponding quinoline 6a–m in dry acetonitrile was added a large excess of alkyl halide at 20 °C. The tube was sealed and the resulting mixture was heated at 85 °C until reaction completion (18 h for MeI and up to 4 days for the rest of the alkylating agents). After cooling at room temperature, dichloromethane (5 mL) and diethyl ether (15 mL) were added to the suspension and the mixture was stirred for 10 min. The formed precipitate was filtered, rinsed twice with diethyl ether, and dried under vacuum to afford the corresponding quinolinium salt.
3-Acetyl-1-methylquinolin-1-ium iodide (2a)
According to procedure B, from commercially available 3-acetylquinoline (200 mg, 1.17 mmol), MeI (1.0 mL), dry acetonitrile (2.0 mL). Quinolinium salt 2a (340 mg, 93%) was obtained as an orange powder. m.p. 235–236 °C; 1H NMR (300 MHz, DMSO) δ 9.97 (s, 1H), 9.90 (s, 1H), 8.79–8.53 (m, 2H), 8.42 (td, J = 7.2, 3.5 Hz, 1H), 8.16 (t, J = 7.6 Hz, 1H), 4.73 (s, 3H), 2.84 (s, 3H); 13C NMR (75 MHz, DMSO) δ 194.42, 149.89, 146.97, 139.08, 137.56, 132.05, 130.78, 129.54, 128.43, 119.50, 45.64, 27.26; HRMS (ESI): m/z calcd. for C12H12NO: 186.0919 [M-I]+, found 186.0916.
3-Acetyl-1-methyl-7-(trifluoromethyl)quinolin-1-ium iodide (2b)
According to procedure B, from quinoline 6b (200 mg, 0.43 mmol), MeI (1.0 mL), dry acetonitrile (4.0 mL). Quinolinium salt 2b (261 mg, 82%) was obtained as a reddish-orange powder. m.p. 227–228 °C; 1H NMR (300 MHz, DMSO) δ 10.10 (dd, J = 1.9, 0.8 Hz, 1H), 9.99 (s, 1H), 8.93 (s, 1H), 8.86 (d, J = 8.6 Hz, 1H), 8.48 (dd, J = 8.5, 1.5 Hz, 1H), 4.83 (s, 3H), 2.86 (s, 3H); 13C NMR (75 MHz, CDCl3) δ 196.34, 150.43, 148.74, 136.92, 133.26 (q, J = 32.9 Hz), 130.55, 129.01, 128.34, 127.19 (q, J = 4.4 Hz), 125.40, 123.20 (q, J = 3.1 Hz), 121.78, 26.86; 19F NMR (282 MHz, CDCl3) δ −61.35; HRMS (ESI): m/z calcd. for C13H11NOF3: 254.0793 [M-I]+, found 254.0798. Anal. Calcd. for C13H11NOF3I: C, 40.97; H, 2.91; N, 3.68. Found: C, 40.82; H, 2.98; N, 3.75.
3-Acetyl-7-dimethylamino-1-methylquinolin-1-ium iodide (2c)
According to procedure B, from quinoline 6c (200 mg, 0.93 mmol), MeI (1.0 mL), dry acetonitrile (4.0 mL). Quinolinium salt 2c (304 mg, 91%) was obtained as a brick orange powder. m.p. 279–280 °C; 1H NMR (300 MHz, DMSO) δ 9.48 (d, J = 1.9 Hz, 1H), 9.23 (d, J = 1.5 Hz, 1H), 8.26 (d, J = 9.5 Hz, 1H), 7.66 (dd, J = 9.4, 2.3 Hz, 1H), 6.93–6.78 (m, 1H), 4.38 (s, 3H), 3.33 (s, 6H), 2.69 (s, 3H); 13C NMR (75 MHz, DMSO) δ 193.93, 155.70, 148.17, 142.46, 142.04, 133.36, 123.36, 121.26, 119.32, 94.15, 44.21, 40.53, 26.77; HRMS (ESI): m/z calcd. for C14H17N2O: 229.1341 [M-I]+, found 229.1337. Anal. Calcd. for C14H17N2OI: C, 47.21; H, 4.81; N, 7.86. Found: C, 47.11; H, 4.78; N, 7.77.
3-Acetyl-7-bromo-1-methylquinolin-1-ium iodide (2d)
According to procedure B, from quinoline 6d (170 mg, 0.680 mmol), methyl iodide (1.0 mL), dry acetonitrile (2.0 mL). Quinolinium salt 2d (240 mg, 90%) was obtained as a deep yellow powder. m.p. 248–249 °C; 1H NMR (300 MHz, DMSO) δ 9.97 (dd, J = 1.9, 0.8 Hz, 1H), 9.87 (d, J = 1.8 Hz, 1H), 8.89 (t, J = 1.2 Hz, 1H), 8.56 (d, J = 8.7 Hz, 1H), 8.35 (dd, J = 8.7, 1.7 Hz, 1H), 4.70 (s, 3H), 2.82 (s, 3H); 13C NMR (75 MHz, DMSO) δ 194.19, 150.65, 146.82, 139.56, 134.20, 133.38, 132.10, 129.76, 127.40, 122.40, 45.77, 27.18; HRMS (ESI): m/z calcd. for C12H11NO79Br: 264.0024 [M-I]+, found 264.0023. Anal. Calcd. for C12H11NOBrI: C, 36.76; H, 2.83; N, 3.57. Found: C, 36.70; H, 2.78; N, 3.49.
3-Acetyl-6-chloro-1-methylquinolin-1-ium iodide (2e)
According to procedure B, from quinoline 6e (200 mg, 0.973 mmol), methyl iodide (1.0 mL), dry acetonitrile (2.0 mL). Quinolinium salt 2e (321 mg, 95%) was obtained as a bright orange powder. m.p. 259–260 °C; 1H NMR (300 MHz, DMSO) δ 10.00 (dd, J = 1.9, 0.8 Hz, 1H), 9.84–9.77 (m, 1H), 8.79 (d, J = 2.4 Hz, 1H), 8.62 (d, J = 9.3 Hz, 1H), 8.45 (dd, J = 9.4, 2.4 Hz, 1H), 4.73 (s, 3H); 13C NMR (75 MHz, DMSO) δ 194.05, 150.34, 145.92, 137.88, 137.22, 135.21, 130.53, 130.24, 129.41, 121.89, 45.85, 27.17; HRMS (ESI): m/z calcd. for C12H11NOCl: 220.0529 [M-I]+, found 220.0536. Anal. Calcd. for C12H11NOClI: C, 41.47; H, 3.19; N, 4.03. Found: C, 41.50; H, 3.28; N, 3.97.
3-Acetyl-6,7-dimethoxy-1-methylquinolin-1-ium iodide (2f)
According to procedure B, from quinoline 6f (200 mg, 0.87 mmol), MeI (1.0 mL), dry acetonitrile (4.0 mL). Quinolinium salt 2f (290 mg, 90%) was obtained as a yellow powder. m.p. 235–236°C; 1H NMR (300 MHz, DMSO) δ 9.71–9.64 (m, 1H), 9.53 (d, J = 1.8 Hz, 1H), 8.04 (s, 1H), 7.71 (s, 1H), 4.66 (s, 3H), 4.20 (s, 3H), 4.03 (s, 3H), 2.79 (s, 3H); 13C NMR (75 MHz, DMSO) δ 194.21, 158.23, 151.48, 145.88, 142.81, 137.42, 127.55, 125.03, 108.95, 99.19, 57.66, 56.64, 45.56, 27.08; HRMS (ESI): m/z calcd. for C14H16NO3: 246.1130 [M-I]+, found 246.1138. Anal. Calcd. for C14H16NO3I: C, 45.06; H, 4.32; N, 3.75. Found: C, 45.11; H, 4.46; N, 3.78.
7-Acetyl-5-methyl-[1,3]dioxolo[4,5-g]quinolin-5-ium iodide (2g)
According to procedure B, from quinoline 6g (150 mg, 0.745 mmol), methyl iodide (1.0 mL), dry acetonitrile (4.0 mL). Quinolinium salt 2g (222 mg, 89%) was obtained as a beige powder. m.p. 235–236 °C; 1H NMR (300 MHz, DMSO) δ 9.70–9.63 (m, 1H), 9.47 (d, J = 1.8 Hz, 1H), 8.06 (d, J = 0.8 Hz, 1H), 7.94 (s, 1H), 6.54 (s, 2H), 4.56 (s, 3H), 2.77 (s, 3H); 13C NMR (75 MHz, DMSO) δ 194.23, 157.51, 150.50, 146.24, 143.30, 139.54, 127.89, 126.93, 105.65, 105.15, 97.21, 45.98, 27.11; HRMS (ESI): m/z calcd. for C13H12NO3: 230.0817 [M-I]+, found 230.0823. Anal. Calcd. for C13H12NO3I: C, 43.72; H, 3.39; N, 3.92. Found: C, 43.77; H, 3.29; N, 3.93.
3-Acetyl-7-methoxy-1-methylquinolin-1-ium iodide (2h)
According to procedure B, from quinoline 6h (58 mg, 0.29 mmol), MeI (0.5 mL), dry acetonitrile (1.0 mL). Quinolinium salt 2h (70 mg, 70%) was obtained as an orange powder. m.p. 223–224 °C; 1H NMR (300 MHz, DMSO) δ 9.83 (d, J = 1.8 Hz, 1H), 9.70 (d, J = 1.8 Hz, 1H), 8.55 (d, J = 9.1 Hz, 1H), 7.84–7.71 (m, 2H), 4.64 (s, 3H), 4.17 (s, 3H), 2.79 (s, 3H); 13C NMR (75 MHz, DMSO) δ 194.19, 166.59, 149.27, 145.50, 141.93, 133.88, 127.15, 123.90, 122.76, 99.62, 57.32, 45.37, 27.05; HRMS (ESI): m/z calcd. for C13H14NO2: 216.1025 [M-I]+, found 216.1015. Anal. Calcd. for C13H14NO2I: C, 45.50; H, 4.11; N, 4.08. Found: C, 45.50; H, 4.17; N, 4.13.
3-Cyano-7-methoxy-1-methylquinolin-1-ium (2i)
According to procedure B, from quinoline 6i (100 mg, 0.54 mmol), MeI (0.5 mL), dry acetonitrile (2.0 mL). Quinolinium salt 2i (133 mg, 75%) was obtained as an orange powder. m.p. 223–224 °C; 1H NMR (300 MHz, DMSO) δ 9.95 (s, 1H), 9.68 (s, 1H), 8.42 (d, J = 9.2 Hz, 1H), 7.91–7.65 (m, 2H), 4.55 (s, 3H), 4.18 (s, 3H); 13C NMR (75 MHz, DMSO) δ 167.38, 151.67, 149.64, 141.92, 133.29, 123.83, 123.48, 114.89, 102.97, 99.79, 57.62, 45.74; HRMS (ESI): m/z calcd. for C12H11N2O: 199.0875 [M-I]+, found 199.0871.
7-Methoxy-3-(methoxycarbonyl)-1-methylquinolin-1-ium (2j)
According to procedure B, from quinoline 6j (100 mg, 0.46 mmol), MeI (0.5 mL), dry acetonitrile (2.0 mL). Quinolinium salt 2j (141 mg, 85%) was obtained as a deep yellow powder. m.p. 240−241 °C; 1H NMR (300 MHz, DMSO) δ 9.85 (d, J = 1.8 Hz, 1H), 9.67 (d, J = 1.9 Hz, 1H), 8.59 (d, J = 9.1 Hz, 1H), 7.90–7.64 (m, 2H), 4.63 (s, 3H), 4.16 (s, 3H), 4.02 (s, 3H); 13C NMR (75 MHz, DMSO) δ 166.79, 162.88, 149.66, 146.27, 142.35, 133.81, 124.06, 122.89, 120.77, 99.55, 57.40, 53.31, 45.36; HRMS (ESI): m/z calcd. for C13H14NO3: 232.0974 [M-I]+, found 232.0979. Anal. Calcd. for C13H14NO3I: C, 43.47; H, 3.93; N, 3.90. Found: C, 43.60; H, 3.90; N, 3.90.
3-(Ethoxycarbonyl)- 7-methoxy-1-methylquinolin-1-ium (2k)
According to procedure B, from quinoline 6k (100 mg, 0.43 mmol), MeI (0.5 mL), dry acetonitrile (2.0 mL). Quinolinium salt 2k (161 mg, 84%) was obtained as a deep yellow powder. m.p. 218−219 °C; 1H NMR (300 MHz, DMSO) δ 9.83 (d, J = 1.9 Hz, 1H), 9.66 (d, J = 2.2 Hz, 1H), 8.59 (d, J = 9.1 Hz, 1H), 7.79 (dd, J = 9.0, 2.2 Hz, 1H), 7.74 (d, J = 2.3 Hz, 1H), 4.63 (s, 3H), 4.49 (q, J = 7.1 Hz, 2H), 4.16 (s, 3H), 1.41 (t, J = 7.1 Hz, 3H);13C NMR (75 MHz, DMSO) δ 166.76, 162.38, 149.65, 146.20, 142.35, 133.81, 124.07, 122.87, 120.99, 99.54, 62.34, 57.38, 45.38, 14.21; HRMS (ESI): m/z calcd. for C14H16NO3: 246.1130 [M-I]+, found 246.1235. Anal. Calcd. For C14H16NO3I: C, 45.06; H, 4.32; N, 3.75. Found: C, 45.11; H, 4.46; N, 3.78.
3-(Dimethylcarbamoyl)-7-methoxy-1-methylquinolin-1-ium iodide (2l)
According to procedure B, from quinoline 6l (200 mg, 0.87 mmol), MeI (1.0 mL), dry acetonitrile (4.0 mL). Quinolinium salt 2l (278 mg, 91%) was obtained as a khaki powder. M.p. 219–220 °C; 1H NMR (300 MHz, DMSO) δ 9.56 (d, J = 1.8 Hz, 1H), 9.28 (d, J = 1.8 Hz, 1H), 8.42 (d, J = 9.0 Hz, 1H), 7.81–7.65 (m, 2H), 4.59 (s, 3H), 4.15 (s, 3H), 3.10 (s, 6H). 13C NMR (75 MHz, DMSO) δ 165.48, 164.57, 148.25, 143.75, 140.93, 132.60, 126.91, 124.00, 122.64, 98.98, 57.23, 45.38, 35.34; HRMS (ESI): m/z calcd. For C14H17N2O2: 245.1290 [M-I]+, found 245.1282. Anal. Calcd. for C14H17N2O2I: C, 45.18; H, 4.60; N, 7.53. Found: C, 45.03; H, 4.66; N, 7.48.
7-Methoxy-3-(methoxy(methyl)carbamoyl)-1-methylquinolin-1-ium iodide (2m)
According to procedure B, from quinoline 6m (350 mg, 0.745 mmol), MeI (3.0 mL), no solvent, 24 h. Quinolinium salt 2m (471 mg, 85%) was obtained as a khaki powder. m.p. 206–207 °C; 1H NMR (300 MHz, DMSO) δ 9.63 (d, J = 1.8 Hz, 1H), 9.43 (d, J = 1.8 Hz, 1H), 8.51 (d, J = 9.1 Hz, 1H), 7.84–7.65 (m, 2H), 4.61 (s, 3H), 4.15 (s, 3H), 3.65 (s, 3H), 3.41 (s, 3H), 3.34 (s, 3H); 13C NMR (75 MHz, DMSO) δ 166.00, 162.95, 149.04, 145.11, 141.35, 133.22, 124.38, 123.83, 122.69, 99.21, 61.59, 57.28, 45.50; HRMS (ESI): m/z calcd. for C14H17N2O3: 261.1239 [M-I]+, found 261.1243. Anal. Calcd. for C14H17N2O3I: C, 43.32; H, 4.41; N, 7.22. Found: C, 43.47; H, 4.46; N, 7.17.
3-Acetyl-1-benzyl-7-methoxyquinolin-1-ium bromide (2n)
According to procedure B, from quinoline 6h (150 mg, 0.745 mmol), Benzyl bromide (1.0 mL), dry acetonitrile (4.0 mL), 3 days. Quinolinium salt 2n (252 mg, 91%) was obtained as a beige powder. m.p. 218–219 °C; 1H NMR (300 MHz, DMSO) δ 10.10 (d, J = 1.9 Hz, 1H), 9.79 (d, J = 1.8 Hz, 1H), 8.57 (d, J = 8.9 Hz, 1H), 7.80–7.65 (m, 2H), 7.54–7.29 (m, 5H), 6.44 (s, 2H), 4.00 (s, 3H), 2.84 (s, 3H); 13C NMR (75 MHz, DMSO) δ 194.25, 166.49, 149.56, 146.28, 140.94, 134.41, 133.63, 129.10, 128.86, 127.76, 127.65, 124.68, 122.56, 100.21, 59.97, 57.19, 27.16; HRMS (ESI): m/z calcd. for C19H18NO2: 292.1338 [M-Br]+, found 292.1336. Anal. Calcd. for C19H18NO2Br: C, 61.30; H, 4.87; N, 3.76. Found: C, 61.41; H, 4.95; N, 3.79.
3-Acetyl-7-methoxy-1-phenethylquinolin-1-ium bromide (2o)
According to procedure B, from quinoline 6h (150 mg, 0.745 mmol), phenethyl bromide (1.0 mL), dry acetonitrile (4.0 mL), 4 days. Quinolinium salt 2o (230 mg, 80%) was obtained as a greenish-brown powder. m.p. 183–184 °C; 1H NMR (300 MHz, DMSO) δ 9.68 (d, J = 1.8 Hz, 1H), 9.60 (d, J = 1.9 Hz, 1H), 8.54 (d, J = 9.8 Hz, 1H), 7.76 (d, J = 7.9 Hz, 2H), 7.31–7.16 (m, 5H), 5.41 (t, J = 7.4 Hz, 2H), 4.17 (s, 3H), 3.33 (t, J = 7.3 Hz, 2H), 2.71 (s, 3H); 13C NMR (75 MHz, DMSO) δ 193.96, 166.85, 148.67, 145.78, 141.08, 136.49, 134.18, 129.16, 128.57, 127.10, 127.01, 124.40, 122.96, 99.38, 57.29, 34.71, 26.99; HRMS (ESI): m/z calcd. for C20H20NO2: 306.1494 [M-Br]+, found 306.1489. Anal. Calcd. for C20H20NO2Br: C, 62.19; H, 5.22; N, 3.63. Found: C, 62.07; H, 5.27; N, 3.59.
3-Acetyl-7-methoxy-1-propylquinolin-1-ium bromide (2p)
According to procedure B, from quinoline 6h (150 mg, 0.745 mmol), propyl bromide (1.0 mL), dry acetonitrile (4.0 mL), 2 days. Quinolinium salt 2p (170 mg, 70%) was obtained as a beige powder. m.p. 183–184 °C; 1H NMR (300 MHz, DMSO) δ 9.84 (d, J = 1.9 Hz, 1H), 9.72 (d, J = 1.8 Hz, 1H), 8.57 (d, J = 9.7 Hz, 1H), 7.79 (d, J = 7.8 Hz, 2H), 5.10 (t, J = 7.5 Hz, 2H), 4.17 (s, 3H), 2.80 (s, 3H), 2.07–1.94 (m, 2H), 0.99 (t, J = 7.3 Hz, 3H); 13C NMR (75 MHz, DMSO) δ 194.26, 166.83, 148.75, 145.74, 141.03, 134.29, 127.40, 124.51, 122.69, 99.49, 58.44, 57.40, 27.11, 22.29, 10.57; HRMS (ESI): m/z calcd. for C15H18NO2: 244.1338 [M-Br]+, found 244.1333. Anal. Calcd. for C15H18NO2Br: C, 55.57; H, 5.60; N, 4.32. Found: C, 55.67; H, 5.47; N, 4.25.
General procedure C for the synthesis of compounds6b–h
Tin(II) chloride dihydrate was added to a solution of the corresponding 2-nitrobenzaldehyde derivative 3b–h in EtOH. Thereafter, 4,4-dimethoxybutan-2-one 4 was introduced. The reaction mixture was heated to reflux for 4 h. After cooling to room temperature, the reaction mixture was concentrated and the residue was dissolved in EtOAc and hydrolyzed with saturated aqueous potassium sodium tartrate. The resulting emulsion was stirred overnight and the liquid was decanted. The organic phase was separated and the remaining aqueous layer was extracted with EtOAc. The combined organic layers were washed with brine, dried on MgSO4, and evaporated. The residue was purified by flash chromatography on silica gel using a mixture of petroleum ether/Et2O/EtOAc (ratio 5:3:2 to 4:3:3).
3-Acetyl-7-trifluoromethylquinoline (6b)
According to procedure C, 4-trifluoromethyl-2-nitrobenzaldehyde 3b (1.0 g, 4.6 mmol), 4,4-dimethoxybutan-2-one 4 (1.51 g, 11.4 mmol), SnCl2·2H2O (4.12 g, 18.25 mmol) in ethanol (30 mL). Quinoline 6b (0.425 g, 39%) was obtained as a beige powder. m.p. 102–103 °C; 1H NMR (300 MHz, CDCl3) δ 9.53 (d, J = 2.2 Hz, 1H), 8.77 (dd, J = 2.2, 0.8 Hz, 1H), 8.48 (dd, J = 1.8, 0.9 Hz, 1H), 8.10 (d, J = 8.6 Hz, 1H), 7.82 (dd, J = 8.6, 1.8 Hz, 1H), 2.79 (s, 3H); 13C NMR (75 MHz, CDCl3) δ 196.34, 150.43, 148.74, 136.92, 133.26 (q, J = 32.9 Hz), 130.55, 129.01, 128.34, 127.19 (q, J = 4.4 Hz), 125.40, 123.20 (q, J = 3.1 Hz), 26.86; 19F NMR (282 MHz, CDCl3) δ −62.94; HRMS (ESI): m/z calcd. for C12H9NOF3: 240.0636 [M + H]+, found 240.0634. Anal. Calcd. for C12H8NOF3: C, 60.26; H, 3.37; N, 5.86. Found: C, 60.16; H, 3.47; N, 5.77.
3-Acetyl-7-dimethylaminoquinoline (6c)
According to procedure C, 4-dimethylamino-2-nitrobenzaldehyde 3c (1.0 g, 5.15 mmol), 4,4-dimethoxybutan-2-one 4 (1.70 g, 12.9 mmol), SnCl2·2H2O (4.65 g, 20.6 mmol) in ethanol (30 mL). Quinoline 6c (0.480 g, 44%) was obtained as a beige powder. m.p. 150–151 °C; 1H NMR (300 MHz, CDCl3) δ 9.24 (d, J = 2.3 Hz, 1H), 8.48 (dd, J = 2.3, 0.8 Hz, 1H), 7.71 (d, J = 9.0 Hz, 1H), 7.22–7.07 (m, 2H), 3.14 (s, 6H), 2.65 (s, 3H); 13C NMR (75 MHz, CDCl3) δ 196.63, 152.94, 151.92, 150.27, 136.92, 130.48, 125.77, 118.97, 116.63, 106.22, 40.38, 26.62; HRMS (ESI): m/z calcd. for C13H15N2O: 215.1184 [M + H]+, found 215.1183. Anal. Calcd. for C13H14N2O: C, 72.87; H, 6.59; N, 13.07. Found: C, 72.99; H, 6.60; N, 13.23.
3-Acetyl-7-bromoquinoline (6d)
According to procedure C, 4-bromo-2-nitrobenzaldehyde 3d (1.0 g, 4.35 mmol), 4,4-dimethoxybutan-2-one 4 (1.44 g, 10.9 mmol), SnCl2·2H2O (3.92 g, 17.4 mmol) in ethanol (30 mL). Quinoline 6d (0.780 g, 72%) was obtained as a beige powder. m.p. 126–127 °C; 1H NMR (300 MHz, CDCl3) δ 9.43 (d, J = 2.2 Hz, 1H), 8.69 (dd, J = 2.3, 0.9 Hz, 1H), 8.36 (dd, J = 1.8, 0.9 Hz, 1H), 7.83 (dt, J = 8.7, 0.5 Hz, 1H), 7.73 (dd, J = 8.7, 1.9 Hz, 1H), 2.75 (s, 3H); 13C NMR (75 MHz, CDCl3) δ 196.44, 150.28, 150.24, 137.23, 132.01, 131.38, 130.54, 129.52, 126.69, 125.56, 26.95; HRMS (ESI): m/z calcd. for C11H9NO79Br: 249.9868 [M + H]+, found 249.9873. Anal. Calcd. For C11H8NOBr: C, 52.83; H, 3.22; N, 5.60. Found: C, 52.86; H, 3.32; N, 5.75.
3-Acetyl-6-chloroquinoline (6e)
According to procedure C, 5-chloro-2-nitrobenzaldehyde 3e (3.0 g, 16.2 mmol), 4,4-dimethoxybutan-2-one 4 (5.34 g, 40.4 mmol), SnCl2·2H2O (14.59 g, 64.7 mmol) in ethanol (90 mL). Quinoline 6e (2 g, 60%) was obtained as a beige powder. M.p. 146–147 °C; 1H NMR (300 MHz, CDCl3) δ 9.40 (d, J = 2.2 Hz, 1H), 8.61 (dd, J = 2.2, 0.8 Hz, 1H), 8.09 (dt, J = 9.0, 0.7 Hz, 1H), 7.92 (d, J = 2.3 Hz, 1H), 7.76 (dd, J = 9.0, 2.3 Hz, 1H), 2.74 (s, 3H);13C NMR (75 MHz, CDCl3) δ 196.56, 149.52, 148.27, 136.42, 133.57, 133.00, 131.19, 129.98, 127.92, 127.65, 27.04; HRMS (ESI): m/z calcd. For C11H9NO35Cl: 206.0373 [M + H]+, found 206.0368. Anal. Calcd. for C11H8NOCl: C, 64.25; H, 3.92; N, 6.81. Found: C, 64.55; H, 3.82; N, 6.90.
3-Acetyl-6,7-dimethoxyquinoline (6f)
According to procedure C, 6-nitroveratraldehyde 3f (2.0 g, 9.5 mmol), 4,4-dimethoxybutan-2-one 4 (3.13 g, 23.7 mmol), SnCl2·2H2O (8.55 g, 37.9 mmol) in ethanol (60 mL). Quinoline 6f (2.1 g, 96%) was obtained as a yellow powder. m.p. 218–219 °C; 1H NMR (300 MHz, CDCl3) δ 9.23 (d, J = 2.2 Hz, 1H), 8.56 (d, J = 2.2 Hz, 1H), 7.46 (s, 1H), 7.14 (s, 1H), 4.06 (s, 3H), 4.03 (s, 3H), 2.71 (s, 3H). 13C NMR (75 MHz, CDCl3) δ 196.90, 154.73, 150.67, 147.62, 147.52, 135.27, 128.20, 122.70, 108.05, 106.31, 56.48, 56.31, 26.90. HRMS (ESI): m/z calcd. for C13H14NO3: 232.0970 [M + H]+, found 232.0974. Anal. Calcd. for C13H13NO3: C, 67.52; H, 5.67; N, 6.06. Found: C, 67.56; H, 5.52; N, 6.00.
7-Acetyl-[1,3]dioxolo[4,5-g]quinoline (6g)
According to procedure C, 6-nitropiperonal 3g (1.0 g, 5.1 mmol), 4,4-dimethoxybutan-2-one 4 (1.69 g, 12.8 mmol), SnCl2·2H2O (4.63 g, 20.5 mmol) in ethanol (30 mL). Quinoline 6g (0.178 g, 16%) was obtained as a beige powder. m.p. 165–166 °C; 1H NMR (300 MHz, CDCl3) δ 9.22 (d, J = 2.2 Hz, 1H), 8.52 (dt, J = 2.2, 0.6 Hz, 1H), 7.43 (d, J = 0.6 Hz, 1H), 7.16 (s, 1H), 6.17 (s, 2H), 2.71 (s, 3H); 13C NMR (75 MHz, CDCl3) δ 196.82, 152.97, 148.89, 148.75, 147.47, 135.68, 128.12, 124.16, 105.94, 103.91, 102.38, 26.89; HRMS (ESI): m/z calcd. for C12H10NO3: 216.0661 [M + H]+, found 216.0653. Anal. Calcd. for C12H9NO3: C, 66.97; H, 4.22; N, 6.51. Found: C, 67.13; H, 4.42; N, 6.40.
3-Acetyl-7-methoxyquinoline (6h)
According to procedure C, 4-methoxy-2-nitrobenzaldehyde 3h (1.0 g, 5.5 mmol), 4,4-dimethoxybutan-2-one 4 (1.82 g, 13.8 mmol), SnCl2·2H2O (4.98 g, 22.1 mmol) in ethanol (30 mL). Quinoline 6h (0.71 g, 64%) was obtained as a beige powder. m.p. 129–130 °C; 1H NMR (300 MHz, CDCl3) δ 9.32 (d, J = 2.2 Hz, 1H), 8.58 (dd, J = 2.2, 0.8 Hz, 1H), 7.77 (d, J = 9.0 Hz, 1H), 7.41 (d, J = 2.6 Hz, 1H), 7.22 (dd, J = 9.0, 2.5 Hz, 1H), 3.95 (s, 3H), 2.68 (s, 3H); 13C NMR (75 MHz, CDCl3) δ 196.69, 162.91, 151.97, 149.80, 136.93, 130.51, 127.64, 122.00, 121.02, 107.48, 55.80, 26.79; HRMS (ESI): m/z calcd. for C12H12NO2: 202.0868 [M + H]+, found 202.0863. Anal. Calcd. for C12H11NO2: C, 71.63; H, 5.51; N, 6.96. Found: C, 71.76; H, 5.63; N, 7.07.
Methyl 7-methoxyquinoline-3-carboxylate (6j)
Methanol (0.9 mL, 22.26 mmol), TMSCl (1.18 g, 10.86 mmol), and quinoline 6i (1.0 g, 5.43 mmol) were sequentially added to a dry flask under inert atmosphere at rt. The reaction mixture was heated at 50 °C for 4 h. After being cooled to rt, water (0.2 mL) was added to the mixture and followed by the addition of Na2CO3 (0.58 g) and CH2Cl2 (30 mL). The organic layer was dried on MgSO4 and concentrated under vacuum to afford 770 mg of ester 6j (65%). 1H NMR (300 MHz, CDCl3) δ 8.94 (d, J = 2.1 Hz, 1H), 8.40 (dd, J = 2.1, 0.8 Hz, 1H), 7.75 (d, J = 9.0 Hz, 1H), 7.44 (d, J = 2.5 Hz, 1H), 7.30 (dd, J = 9.0, 2.5 Hz, 1H), 3.98 (s, 3H); 13C NMR (75 MHz, CDCl3) δ 163.41, 151.11, 150.51, 140.70, 129.44, 122.06, 121.67, 117.63, 107.80, 104.14, 55.97. Anal. Calcd. for C12H11NO3: C, 66.35; H, 5.10; N, 6.45. Found: C, 66.37; H, 5.05; N, 6.51.
Ethyl 7-methoxyquinoline-3-carboxylate (6k)
To a suspension of 5 (1.10 g, 5.41 mmol) in ethanol was added, dropwise, SOCl2 (2.76 mL, 38 mmol). The resulting mixture was stirred under reflux for 18 h. After concentration under reduced pressure, water (50 mL) was added and the crude mixture was neutralized to pH 7 with 20% solution Na2CO3. The aqueous solution was extracted with CH2Cl2 (3 × 50 mL), the combined organic layers were dried on MgSO4 and concentrated under vacuum to afford 940 mg (75%) of the desired product 6k.1H NMR (300 MHz, CDCl3) δ 9.32 (d, J = 2.1 Hz, 1H), 8.67 (d, J = 2.1 Hz, 1H), 7.73 (d, J = 9.0 Hz, 1H), 7.40 (d, J = 2.4 Hz, 1H), 7.19 (dd, J = 9.0, 2.5 Hz, 1H), 4.41 (q, J = 14.1, 6.9 Hz, 2H), 3.93 (s, 3H), 1.41 (t, J = 7.2 Hz, 3H); 13C NMR (75 MHz, CDCl3) δ 165.57, 162.58, 151.84, 150.49, 138.03, 130.08, 121.98, 121.23, 120.71, 107.40, 61.25, 55.64, 14.35. Anal. Calcd. for C13H13NO3: C, 67.52; H, 5.67; N, 6.06. Found: C, 67.46; H, 5.72; N, 6.18.
7-Methoxy-N,N-dimethylquinoline-3-carboxamide (6l)
A 20 mL glass tube was charged with 7-methoxyquinoline-3-carboxylic acid 5 (0.5 g, 2.46 mmol), DMF (2 mL), T3P® in DMF 50% (1.74 g, 2.71 mmol) and (CH3)2N·HCl (2 M in Et2O, 0.62 mL) at 20°C. The tube was sealed, heated to 130 °C and stirred for 18 h. The solution was carefully hydrolyzed at 10 °C with aqueous saturated Na2CO3 (5 mL) and extracted with ethyl acetate (4 × 20 mL). Combined organic phases were dried on MgSO4 and concentrated under reduced pressure. The crude product (light brown oil) was used without further purification. m.p. 110–111 °C; 1H NMR (300 MHz, CDCl3) δ 8.76 (d, J = 2.2 Hz, 1H), 8.03 (dd, J = 2.2, 0.8 Hz, 1H), 7.56 (d, J = 9.0 Hz, 1H), 7.30–7.24 (m, 1H), 7.07 (dd, J = 8.9, 2.5 Hz, 1H), 3.79 (s, 3H), 2.99 (sbr, 3H), 2.93 (sbr, 3H). 13C NMR (75 MHz, CDCl3) δ 169.10, 161.47, 149.77, 148.59, 134.73, 129.10, 126.70, 121.87, 120.46, 106.92, 55.37, 39.53, 35.37. HRMS (ESI): m/z calcd. for C13H15N2O2: 231.1134 [M + H]+, found 231.1132.
N,7-Dimethoxy-N-methylquinoline-3-carboxamide (6m)
To a suspension of 7-methoxyquinoline-3-carboxylic acid 5 (635 mg, 3.1 mmol) in dry DMF (4 mL), CDI (597 mg, 3.4 mmol) was added and the resulting mixture was stirred at 20 °C under a stream of argon for 1 h. Thereafter, DMF (2.0 mL), N,O-dimethylhydroxylamine hydrochloride (396 mg, 4.1 mmol) and triethylamine (0.44 mL, 3.1 mmol) were successively added and the mixture was stirred for 18 h. The brown suspension was diluted with ethyl acetate (50 mL) and washed with water. The aqueous layer was extracted twice with ethyl acetate and the organic layers were combined. The organic phase obtained was washed with a saturated solution of NaHCO3 (2 × 50 mL), water (2 × 50 mL), saturated solution of NH4Cl (2 × 50 mL), brine. The organic layer was dried on MgSO4 and concentrated under vacuum to afford 594 mg of 6m as a pale orange oil (76%). 1H NMR (300 MHz, CDCl3) δ 9.18 (d, J = 2.1 Hz, 1H), 8.55 (d, J = 2.1 Hz, 1H), 7.79 (d, J = 8.9 Hz, 1H), 7.49 (d, J = 2.5 Hz, 1H), 7.26 (dd, J = 9.0, 2.5 Hz, 1H), 3.99 (s, 3H), 3.58 (s, 3H), 3.44 (s, 3H); 13C NMR (75 MHz, CDCl3) δ 167.63, 162.14, 150.63, 149.88, 136.94, 129.86, 124.73, 122.12, 120.62, 107.24, 61.32, 55.71, 33.39; HRMS (ESI): m/z calcd. for C13H15N2O3: 247.1083 [M + H]+, found 247.1082. Anal. Calcd. for C13H14N2O3: C, 63.40; H, 5.73; N, 11.38. Found: C, 63.61; H, 5.84; N, 11.48.
4. Conclusions
The search of new and readily accessible dual inhibitors of DYRK1A and CLK1, two kinases involved at different levels in the formation of amyloid plaques and neurofibrillary tangles in AD patients, remains a challenge in the development of new drug candidates in AD treatment. To address this issue, we designed herein a new class of dual inhibitors of DYRK1/CLK1 made up of a simple 1,4-dihydroquinoline structure, straightforwardly accessible in only three steps with good overall yields from commercial compounds. A preliminary SAR investigation highlighted that both an acetyl group at C-3 and a methoxy group at C-7 were required within the 1,4-dihydroquinoline scaffold to display the inhibition of hDYRK1A/hCLK1 kinases. Gratifyingly, compound 1p proved to be the most potent, exhibiting three- to two-digit nanomolar IC50 values (153 nM and 47 nM toward hDYRK1A and hCLK1, respectively). Interestingly, dihydroquinoline 1p exhibited antioxidant and radical scavenging properties (scavenging activity EC50 = 128 μM). This is a major asset over most existing inhibitors since oxidative stress is involved in the pathogenesis of AD. The ADME profile obtained for 1p is highly encouraging (predicting inter alia good BBB permeability). Finally, molecular docking studies showed that N-methyl-3-acetyl-7-methoxy 1,4-dihydroquinoline 1h reproduces the INDY binding mode in both kinases hDYRK1A and hCLK1. Overall, this work paves the way for further research efforts aiming at developing this novel class of dual-inhibitors of hDYRK1A and hCLK1 with in vitro antioxidant properties as promising drug candidates to treat AD.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/molecules28010036/s1, procedures for in vitro studies on hDYRK1A and hCLK1, PAMPA-BBB test of 1p, solubility assay with 1p, stability/oxidation and DPPH radical scavenging assays with 1p, 1H NMR oxidation of 1p to 2p in the presence of DPPH radical, ADME results for 1p, copies of 1H and 13C-NMR spectra for all synthesized compounds.
Author Contributions
Conceptualization, V.G., C.P. and V.L.; validation, V.G., C.P. and V.L.; formal analysis, J.S.-d.O.S.; investigation, M.-L.Ţ., L.P., F.A. and J.S.-d.O.S.; writing—original draft preparation, C.P., V.L., T.B. and J.S.-d.O.S.; writing—review and editing, C.P., T.B. and V.L.; supervision, V.G., C.P. and V.L. All authors have read and agreed to the published version of the manuscript.
Funding
This work was partially supported by the University of Rouen Normandy, INSA Rouen Normandy, the Centre National de la Recherche Scientifique (CNRS), European Regional Development Fund (ERDF), Labex SynOrg (ANR-11-LABX-0029), Carnot Institute I2C, the graduate school for research Xl-Chem (ANR-18-EURE-0020 XL CHEM), and by Région Normandie.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no competing financial interest.
Sample Availability
Samples of the compounds 1a–p are available from the authors.
Abbreviations
| Aβ | Amyloid-β |
| AD | Alzheimer’s disease |
| ADME | Absorption Distribution Metabolism Excretion |
| APP | Amyloid precursor protein |
| ATP | Adenosine triphosphate |
| BBB | Blood-brain barrier |
| BNAH | N-Benzyl-1,4-dihydronicotinamide |
| BOILED-egg | Brain or intestinal estimated permeation |
| CDC2 | Cell division control 2 kinase |
| CDI | 1,1′-carbonyldiimidazole |
| CDK | Cyclin-dependent kinase |
| ChemPLP | Piecewise linear potential |
| CLK | CDC-like kinase |
| CLK1 | CDC-like kinase 1 |
| CLK2 | CDC-like kinase 2 |
| CLK3 | CDC-like kinase 3 |
| CLK4 | CDC-like kinase 4 |
| cLogP | Calculated LogP |
| CMGC | CDK, MAPKK, GSK3, CLK group of kinases |
| CNS | Central nervous system |
| DMF | Dimethylformamide |
| DMSO | Dimethylsulfoxide |
| DPPH• | 2,2-diphenyl-1-picrylhydrazyl |
| DYRK | Dual-specificity tyrosine phosphorylation kinase |
| DYRK1A | Dual-specificity tyrosine phosphorylation kinase 1A |
| EC50 | Half maximal effective concentration |
| EGCG | Epigallocatechin-3-gallate |
| ESI | Electrospray ionization |
| EWG | Electron withdrawing group |
| Glu | Glutamic acid |
| GOLD | Genetic optimization for ligand docking |
| GSK3 | Glycogen synthase kinase 3 |
| hCLK1 | Human CDC-like kinase 1 |
| hDYRK1A | Human dual-specificity tyrosine phosphorylation kinase 1A |
| HRMS | High resolution mass spectroscopy |
| IC50 | Half maximal inhibitory concentration |
| ID | Identification code |
| INDY | Inhibitor of DYRK kinases |
| Leu | Leucine |
| Lys | Lysine |
| MAPK | Mitogen-activated protein kinase |
| NAD+ | Nicotinamide adenine dinucleotide |
| NMR | Nuclear magnetic resonance |
| PAMPA-BBB | Parallel artificial membrane permeability assay of blood-brain barrier |
| PBS | Phosphate-buffered saline |
| PDB | Protein data bank |
| proINDY | Proinhibitor of DYRK kinases |
| PyMOL | Python Molecular modelling |
| rt | Room temperature |
| SAR | Structure-activity relationship |
| SR | Serine/Arginine rich proteins |
| T3P | Propylphosphonic anhydride |
| Tau | Tubulin-associated unit |
| Thr | Threonine |
| TLC | Thin layer chromatography |
| TMSCl | Chlorotrimethylsilane |
| TPSA | Topological polar surface area |
| UV | Ultraviolet |
| WLOGP | Wildman-Crippen LogP |
References
- Alzheimer’s association report, 2022 Alzheimer’s disease facts and figures. Alzheimers Dement. 2022, 18, 700–789. [CrossRef] [PubMed]
- O’Brien, R.J.; Wong, P.C. Amyloid precursor protein processing and Alzheimer’s disease. Annu. Rev. Neurosci. 2011, 34, 185–204. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.-F.; Xu, T.-H.; Yan, Y.; Zhou, Y.-R.; Jiang, Y.; Melcher, K.; Xu, H.E. Amyloid beta: Structure, biology and structure-based therapeutic development. Acta Pharm. Sinic. 2017, 38, 1205–1235. [Google Scholar] [CrossRef] [PubMed]
- DeTure, M.A.; Dickson, D.W. The neuropathological diagnosis of Alzheimer’s disease. Mol. Neurodegener. 2019, 14, 32. [Google Scholar] [CrossRef] [PubMed]
- Wegiel, J.; Gong, C.-X.; Hwang, Y.-W. The role of DYRK1A in neurodegenerative diseases. FEBS J. 2011, 278, 236–245. [Google Scholar] [CrossRef]
- Becker, W.; Sippl, W. Activation, regulation, and inhibition of DYRK1A. FEBS J. 2011, 278, 246–256. [Google Scholar] [CrossRef]
- Lindberg, M.F.; Meijer, L. Dual-specificity, tyrosine phosphorylation-regulated kinases (DYRKs) and cdc2-like kinases (CLKs) in human disease, an overview. Int. J. Mol. Sci. 2021, 22, 6047. [Google Scholar] [CrossRef]
- Ryoo, S.-R.; Cho, H.-J.; Lee, H.-W.; Jeong, H.K.; Radnaabazar, C.; Kim, Y.-S.; Kim, M.-J.; Son, M.-Y.; Seo, H.; Chung, S.-H.; et al. Dual-specificity tyrosine(Y)-phosphorylation regulated kinase 1A-mediated phosphorylation of amyloid precursor protein: Evidence for a functional link between Down syndrome and Alzheimer’s disease. J. Neurochem. 2008, 104, 1333–1344. [Google Scholar] [CrossRef]
- Ryu, Y.S.; Park, S.Y.; Jung, M.-S.; Yoon, S.-H.; Kwen, M.-Y.; Lee, S.-Y.; Choi, S.-H.; Radnaabazar, C.; Kim, M.-K.; Kim, H.; et al. DYRK1A-mediated phosphorylation of Presenilin 1: A functional link between Down syndrome and Alzheimer’s disease. J. Neurochem. 2010, 115, 574–584. [Google Scholar] [CrossRef]
- Lee, M.-S.; Kao, S.-C.; Lemere, C.A.; Xia, W.; Tseng, H.-C.; Zhou, Y.; Neve, R.; Ahlijanian, M.K.; Tsai, L.-H. APP processing is regulated by cytoplasmic phosphorylation. J. Cell Biol. 2003, 163, 83–95. [Google Scholar] [CrossRef]
- Vingtdeux, V.; Hamdane, M.; Gompel, M.; Bégard, S.; Drobecq, H.; Ghestem, A.; Grosjean, M.-E.; Kostanjevecki, V.; Grognet, P.; Vanmechelen, E.; et al. Phosphorylation of amyloid precursor carboxy-terminal fragments enhances their processing by a gamma-secretase-dependent mechanism. Neurobiol. Dis. 2005, 20, 625–637. [Google Scholar] [CrossRef] [PubMed]
- Pei, J.-J.; Gong, C.-X.; Iqbal, K.; Grundke-Iqbal, I.; Wu, Q.L.; Winblad, B.; Cowburn, R.F. Subcellular distribution of protein phosphatases and abnormally phosphorylated τ in the temporal cortex from Alzheimer’s disease and control brains. J. Neural. Transm. 1998, 105, 69–83. [Google Scholar] [CrossRef]
- Jin, N.; Yin, X.; Yu, D.; Cao, M.; Gong, C.-X.; Iqbal, K.; Ding, F.; Gu, X.; Liu, F. Truncation and activation of GSK-3β by calpain I: A molecular mechanism links to tau hyperphosphorylation in Alzheimer’s disease. Sci. Rep. 2015, 5, 8187. [Google Scholar] [CrossRef] [PubMed]
- Ryoo, S.-R.; Jeong, H.K.; Radnaabazar, C.; Yoo, J.-J.; Cho, H.-J.; Lee, H.-W.; Kim, I.-S.; Cheon, Y.-H.; Ahn, Y.S.; Chung, S.-H.; et al. DYRK1A-mediated hyperphosphorylation of Tau. A functional link between Down syndrome and Alzheimer disease. J. Biol. Chem. 2007, 282, 34850–34857. [Google Scholar] [CrossRef] [PubMed]
- Azorsa, D.O.; Robeson, R.H.; Frost, D.; Hoovet, B.M.; Brautigam, G.R.; Dickey, C.; Beaudry, C.; Basu, G.D.; Holz, D.R.; Hernandez, J.A.; et al. High-content siRNA screening of the kinome identifies kinases involved in Alzheimer’s disease-related tau hyperphosphorylation. BMC Genom. 2010, 11, 25. [Google Scholar] [CrossRef] [PubMed]
- Woods, Y.L.; Cohen, P.; Becker, W.; Jakes, R.; Goedert, M.; Wang, X.; Proud, C.G. The kinase DYRK phosphorylates protein-synthesis initiation factor eIF2Bepsilon at Ser539 and the microtubule-associated protein tau at Thr212: Potential role for DYRK as a glycogen synthase kinase 3-priming kinase. Biochem. J. 2001, 355, 609–615. [Google Scholar] [CrossRef]
- Ledesma, M.D.; Correas, I.; Avila, J.; Díaz-Nido, J. Implication of brain cdc2 and MAP2 kinases in the phosphorylation of tau protein in Alzheimer’s disease. FEBS Lett. 1992, 308, 218–224. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.-Y.; Clark, A.W.; Rosales, J.L.; Chapman, K.; Fung, T.; Johnston, R.N. Elevated neuronal Cdc2-like kinase activity in the Alzheimer disease brain. Neurosci. Res. 1999, 34, 21–29. [Google Scholar] [CrossRef]
- Jain, P.; Karthikeyan, C.; Moorthy, N.S.; Waiker, D.K.; Jain, A.K.; Trivedi, P. Human CDC2-like kinase 1 (CLK1): A novel target for Alzheimer’s disease. Curr. Drug Targets 2014, 15, 539–550. [Google Scholar] [CrossRef]
- Martín Moyano, P.; Němec, V.; Paruch, K. Cdc-like kinases (CLKs): Biology, chemical probes, and therapeutic potential. Int. J. Mol. Sci. 2020, 21, 7549. [Google Scholar] [CrossRef]
- Bain, J.; McLauchlan, H.; Elliott, M.; Cohen, P. The specificities of protein kinase inhibitors: An update. Biochem. J. 2003, 371, 199–204. [Google Scholar] [CrossRef] [PubMed]
- Göckler, N.; Jofre, G.; Papadopoulos, C.; Soppa, U.; Tejedor, F.J.; Becker, W. Harmine specifically inhibits protein kinase DYRK1A and interferes with neurite formation. FEBS J. 2009, 276, 6324–6337. [Google Scholar] [CrossRef]
- Tahtouh, T.; Elkins, J.M.; Filippakopoulos, P.; Soundararajan, M.; Burgy, G.; Durieu, E.; Cochet, C.; Schmid, R.S.; Lo, D.C.; Delhommel, F.; et al. Selectivity, cocrystal structures, and neuroprotective properties of Leucettines, a family of protein kinase inhibitors derived from the marine sponge alkaloid Leucettamine, B. J. Med. Chem. 2012, 55, 9312–9330. [Google Scholar] [CrossRef] [PubMed]
- Stotani, S.; Giordanetto, F.; Medda, F. DYRK1A inhibition as potential treatment for Alzheimer’s disease. Future Med. Chem. 2016, 8, 681–696. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.L.; Fruit, C.; Hérault, Y.; Meijer, L.; Besson, T. Dual-specificity tyrosine phosphorylation-regulated kinase 1A (DYRK1A) inhibitors: A survey of recent patent literature. Expert Opin. Ther. Pat. 2017, 27, 1183–1199. [Google Scholar] [CrossRef]
- Pathak, A.; Rohilla, A.; Gupta, T.; Akhtar, M.J.; Haider, M.R.; Sharma, K.; Haider, K.; Yar, M.S. DYRK1A kinase inhibition with emphasis on neurodegeneration: A comprehensive evolution story-cum-perspective. Eur. J. Med. Chem. 2018, 158, 559–592. [Google Scholar] [CrossRef]
- Jarhad, D.B.; Mashelkar, K.K.; Kim, H.-R.; Noh, M.; Jeong, L.S. Dual-specificity tyrosine phosphorylation-regulated kinase 1A (DYRK1A) inhibitors as potential therapeutics. J. Med. Chem. 2018, 61, 9791–9810. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Xiong, X.; Liu, L.; Liang, Q.; Tong, R.; Feng, X.; Bai, L.; Shi, J. Recent research and development of DYRK1A inhibitors. Chin. Chem. Lett. 2022, 33, 1841–1849. [Google Scholar] [CrossRef]
- Zuccotto, F.; Ardini, E.; Casale, E.; Angiolini, M. Through the “gatekeeper door”: Exploiting the active kinase conformation. J. Med. Chem. 2010, 53, 2681–2694. [Google Scholar] [CrossRef]
- Ogawa, Y.; Nonaka, Y.; Goto, T.; Ohnishi, E.; Hiramatsu, T.; Kii, I.; Yoshida, M.; Ikura, T.; Onogi, H.; Shibuya, H.; et al. Development of a novel selective inhibitor of the Down syndrome-related kinase Dyrk1A. Nat. Commun. 2010, 1, 86. [Google Scholar] [CrossRef]
- Muraki, M.; Ohkawara, B.; Hosoya, T.; Onogi, H.; Koizumi, J.; Koizumi, T.; Sumi, K.; Yomoda, J.-I.; Murray, M.V.; Kimura, H.; et al. Manipulation of alternative splicing by a newly developed inhibitor of Clks. J. Biol. Chem. 2004, 279, 24246–24254. [Google Scholar] [CrossRef] [PubMed]
- Venkatesan, H.; Hocutt, F.M.; Jones, T.K.; Rabinowitz, M.H. A one-step synthesis of 2,4-unsubstituted quinoline-3-carboxylic acid esters from o-nitrobenzaldehydes. J. Org. Chem. 2010, 75, 3488–3491. [Google Scholar] [CrossRef] [PubMed]
- Charpentier, P.; Lobrégat, V.; Levacher, V.; Dupas, G.; Quéguiner, G.; Bourguignon, J. An efficient synthesis of 3-cyanoquinoline derivatives. Tetrahedron Lett. 1998, 39, 4013–4016. [Google Scholar] [CrossRef]
- Luo, F.-T.; Jeevanandam, A. Simple transformation of nitrile into ester by the use of chlorotrimethylsilane. Tetrahedron Lett. 1998, 39, 9455–9456. [Google Scholar] [CrossRef]
- Gourand, F.; Patin, D.; Henry, A.; Ibazizène, M.; Dhilly, M.; Fillesoye, F.; Tirel, O.; Tintas, M.-L.; Papamicaël, C.; Levacher, V.; et al. Chemical delivery system of MIBG to the central nervous system: Synthesis, 11C-radiosynthesis, and in vivo evaluation. ACS Med. Chem. Lett. 2019, 10, 352–357. [Google Scholar] [CrossRef] [PubMed]
- Gella, A.; Durany, N. Oxidative stress in Alzheimer disease. Cell. Adhes. Migr. 2009, 3, 88–93. [Google Scholar] [CrossRef] [PubMed]
- Manoharan, S.; Guillemin, G.J.; Abiramasundari, R.S.; Essa, M.M.; Akbar, M.; Akbar, M.D. The role of reactive oxygen species in the pathogenesis of Alzheimer’s disease, Parkinson’s disease, and Hutington’s disease: A mini review. Oxid. Med. Cell. Longev. 2016, 2016, 8590578. [Google Scholar] [CrossRef]
- Velena, A.; Zarkovic, N.; Troselj, K.G.; Bisenieks, E.; Krauze, A.; Poikans, J.; Duburs, G. 1,4-Dihydropyridine derivatives: Dihydronicotinamide analogues-model compounds targeting oxidative stress. Oxid. Med. Cell. Longev. 2016, 2016, 1892412. [Google Scholar] [CrossRef]
- Wojsiat, J.; Zoltowska, K.M.; Laskowska-Kaszub, K.; Wojda, U. Oxidant/antioxidant imbalance in Alzheimer’s disease: Therapeutic and diagnostic prospects. Oxid. Med. Cell. Long. 2018, 2018, 6435861. [Google Scholar] [CrossRef]
- Sinyor, B.; Mineo, J.; Ochner, C. Alzheimer’s disease, inflammation, and the role of antioxidants. J. Alzheimers Dis. Rep. 2020, 4, 175–183. [Google Scholar] [CrossRef]
- Gourand, F.; Ţînţaş, M.-L.; Henry, A.; Ibazizène, M.; Dhilly, M.; Fillesoye, F.; Papamicaël, C.; Levacher, V.; Barré, L. Delivering FLT to the central nervous system by means of a promising targeting system: Synthesis, [11C]radiosynthesis, and in vivo evaluation. ACS. Chem. Neurosci. 2017, 8, 2457–2467. [Google Scholar] [CrossRef] [PubMed]
- Peauger, L.; Azzouz, R.; Gembus, V.; Ţînţaş, M.-L.; Sopková-de Oliveira Santos, J.; Bohn, P.; Papamicaël, C.; Levacher, V. Donepezil-based central acetylcholinesterase inhibitors by means of a “bio-oxidizable” prodrug strategy: Design, synthesis, and in vitro biological evaluation. J. Med. Chem. 2017, 60, 5909–5926. [Google Scholar] [CrossRef] [PubMed]
- Blois, M.S. Antioxidant determinations by the use of a stable free radical. Nature 1958, 181, 1199–1200. [Google Scholar] [CrossRef]
- Lipinski, C.A.; Lombardo, F.; Dominy, B.W.; Feeney, P.J. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv. Drug. Deliver. Rev. 2001, 46, 3–26. [Google Scholar] [CrossRef] [PubMed]
- Pajouhesh, H.; Lenz, G.R. Medicinal chemical properties of successful central nervous system drugs. Neurotherapeutics 2005, 2, 541–553. [Google Scholar] [CrossRef] [PubMed]
- Daina, A.; Michielin, O.; Zoete, V. SwissADME: A free web tool to evaluate pharmacokinetics, drug-likeness and medicinal chemistry friendliness of small molecules. Sci. Rep. 2017, 7, 42717. [Google Scholar] [CrossRef]
- Daina, A.; Zoete, V. A BOILED-Egg to predict gastrointestinal absorption and brain penetration of small molecules. ChemMedChem 2016, 11, 1117–1121. [Google Scholar] [CrossRef]
- Kansy, M.; Senner, F.; Gubernator, K. Physicochemical high throughput screening: Parallel artificial membrane permeation assay in the description of passive absorption processes. J. Med. Chem. 1998, 41, 1007–1010. [Google Scholar] [CrossRef]
- Schröder, M.; Bullock, A.N.; Fedorov, O.; Bracher, F.; Chaikuad, A.; Knapp, S. DFG-1 Residue controls inhibitor binding mode and affinity, providing a basis for rational design of kinase inhibitor selectivity. J. Med. Chem. 2020, 63, 10224–10234. [Google Scholar] [CrossRef]
- ChemAxon-Software Solutions and Services for Chemistry & Biology, (n.d.). Available online: https://chemaxon.com/ (accessed on 6 February 2020).
- Jones, G.; Willet, P.; Glen, R.C. Molecular recognition of receptor sites using a genetic algorithm with a description of desolvation. J. Mol. Biol. 1995, 245, 43–53. [Google Scholar] [CrossRef]
- Jones, G.; Willet, P.; Glen, R.C.; Leach, A.R.; Taylor, R. Development and validation of a genetic algorithm for flexible docking. J. Mol. Biol. 1997, 267, 727–748. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).