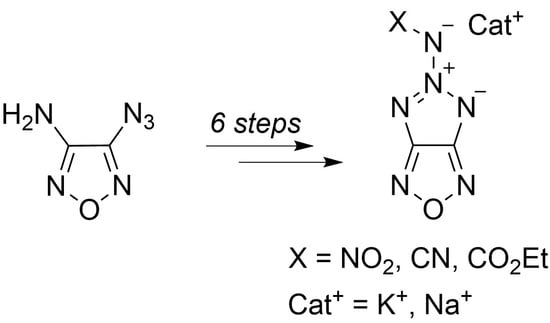
Anions Containing Tripoid Conjugated N4− System: Salts of 5-(Substituted Amino)-[1,2,3]triazolo[4,5-c][1,2,5]oxadiazol-5-ium-4-ides, as well as Their Synthesis, Structure, and Thermal Stability
Abstract
:1. Introduction
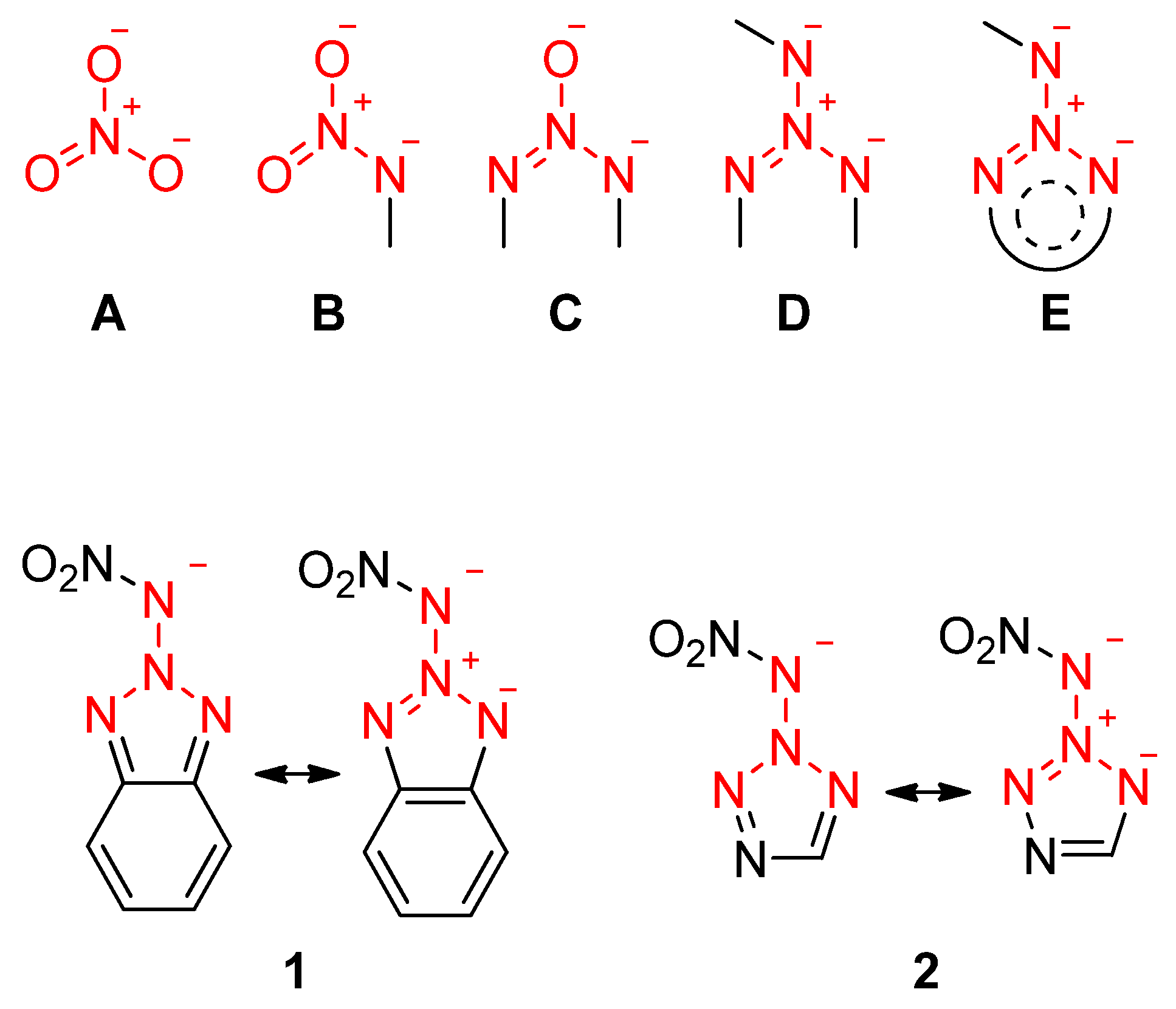
2. Results and Discussion
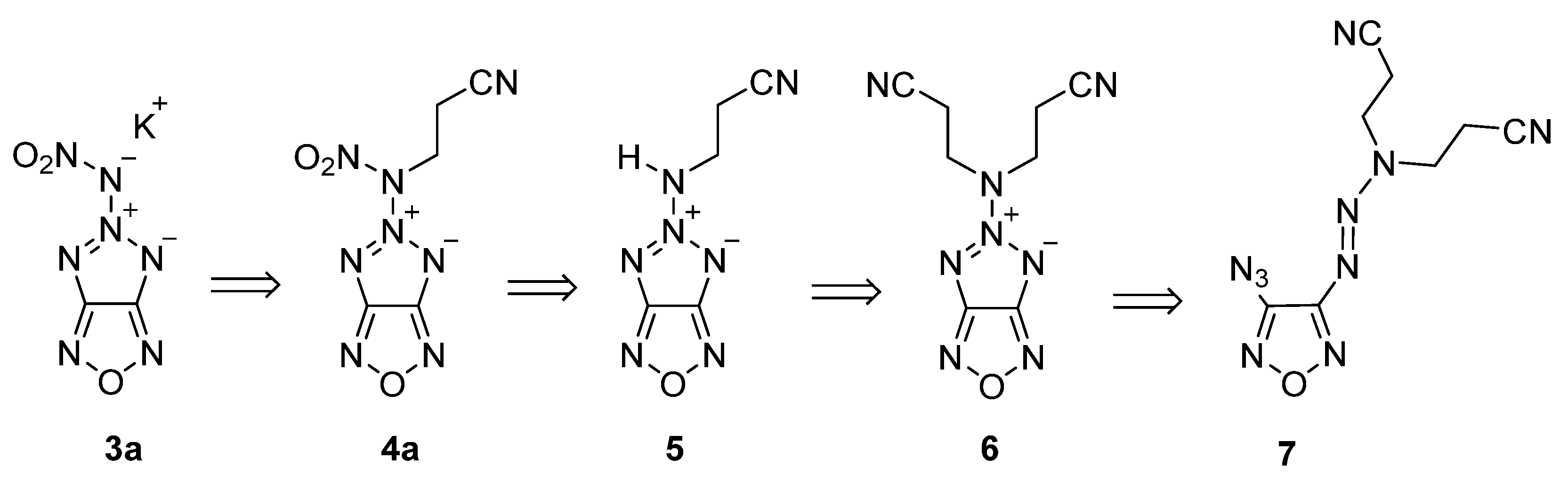
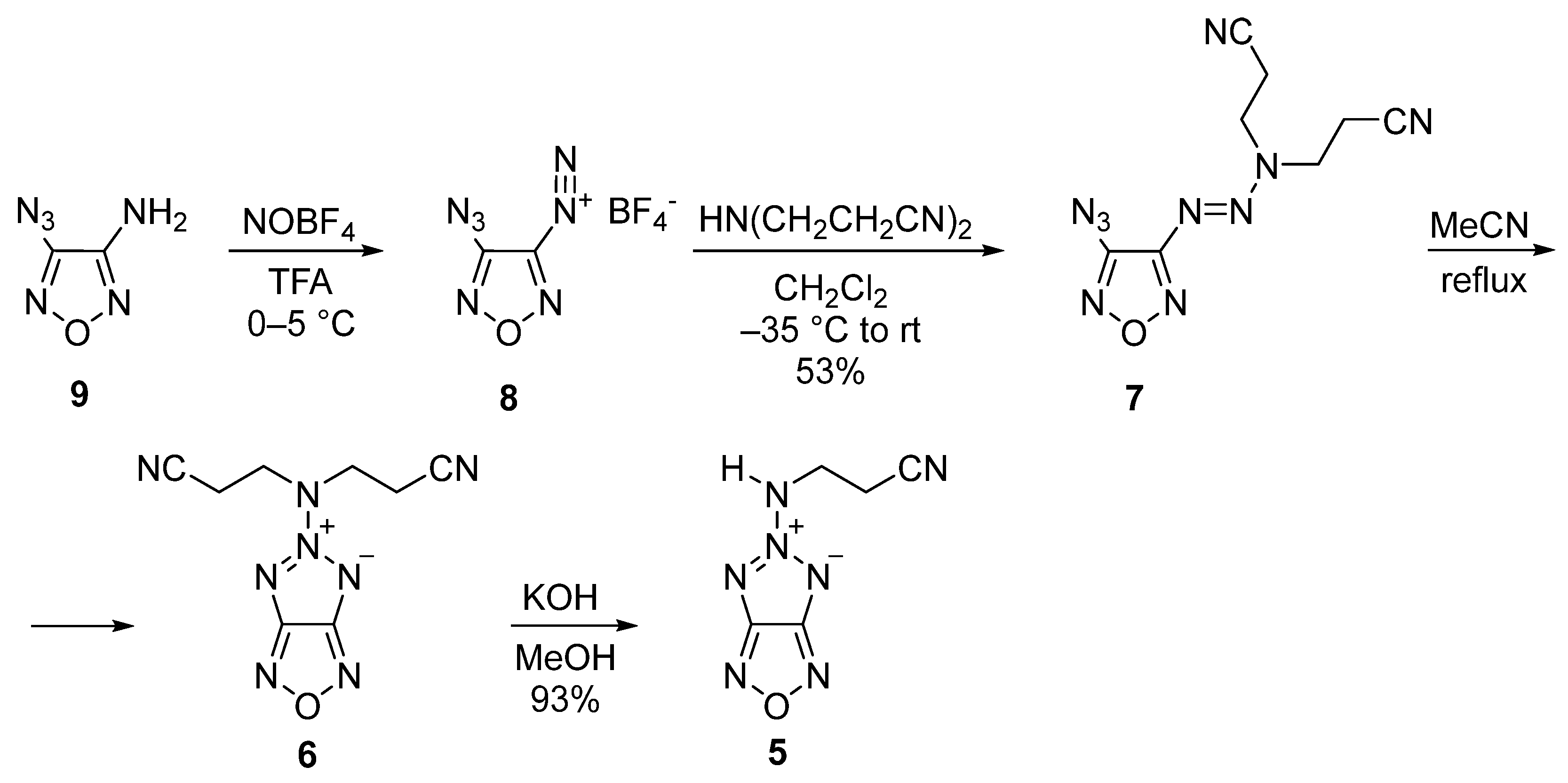
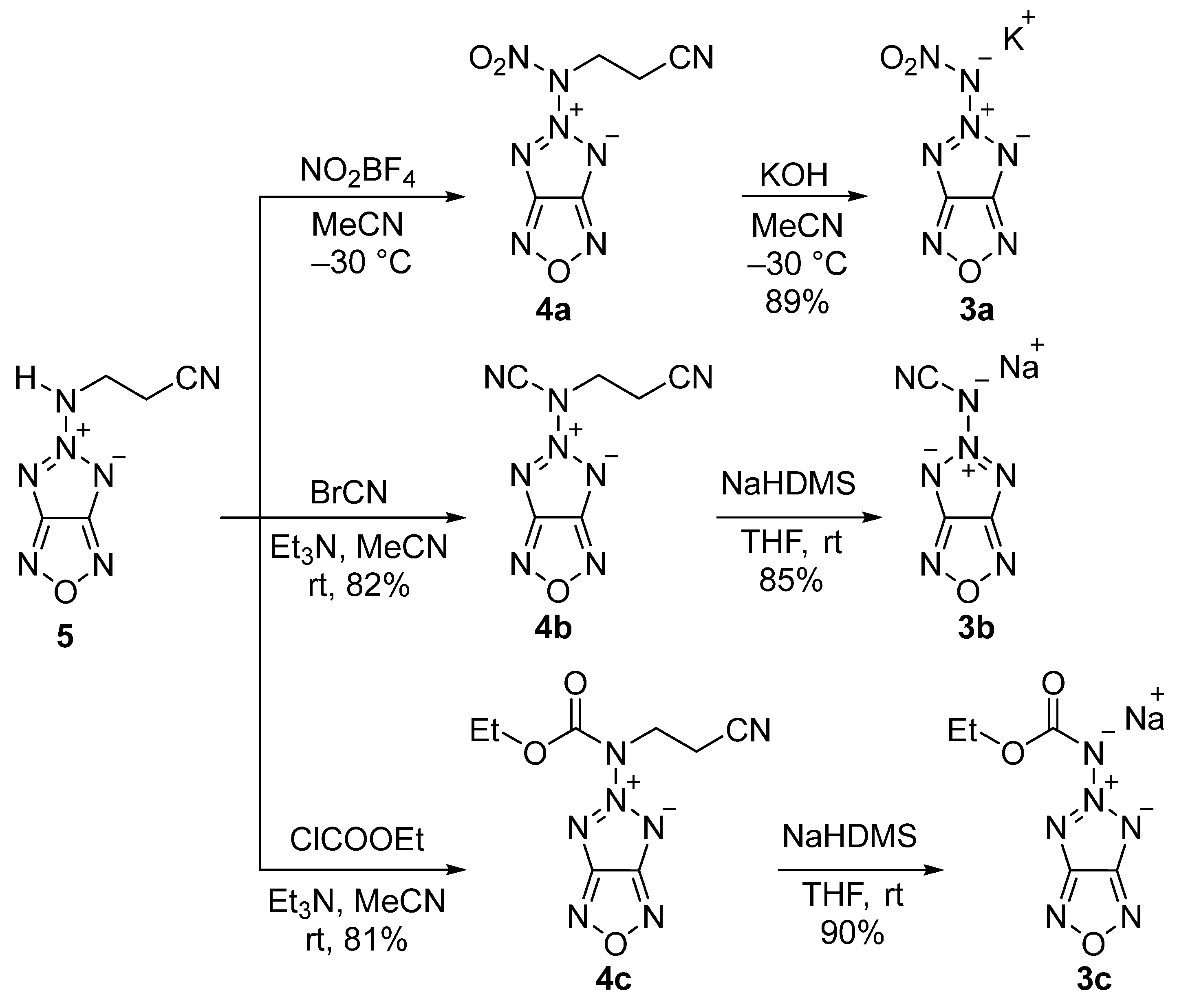
2.1. Synthesis
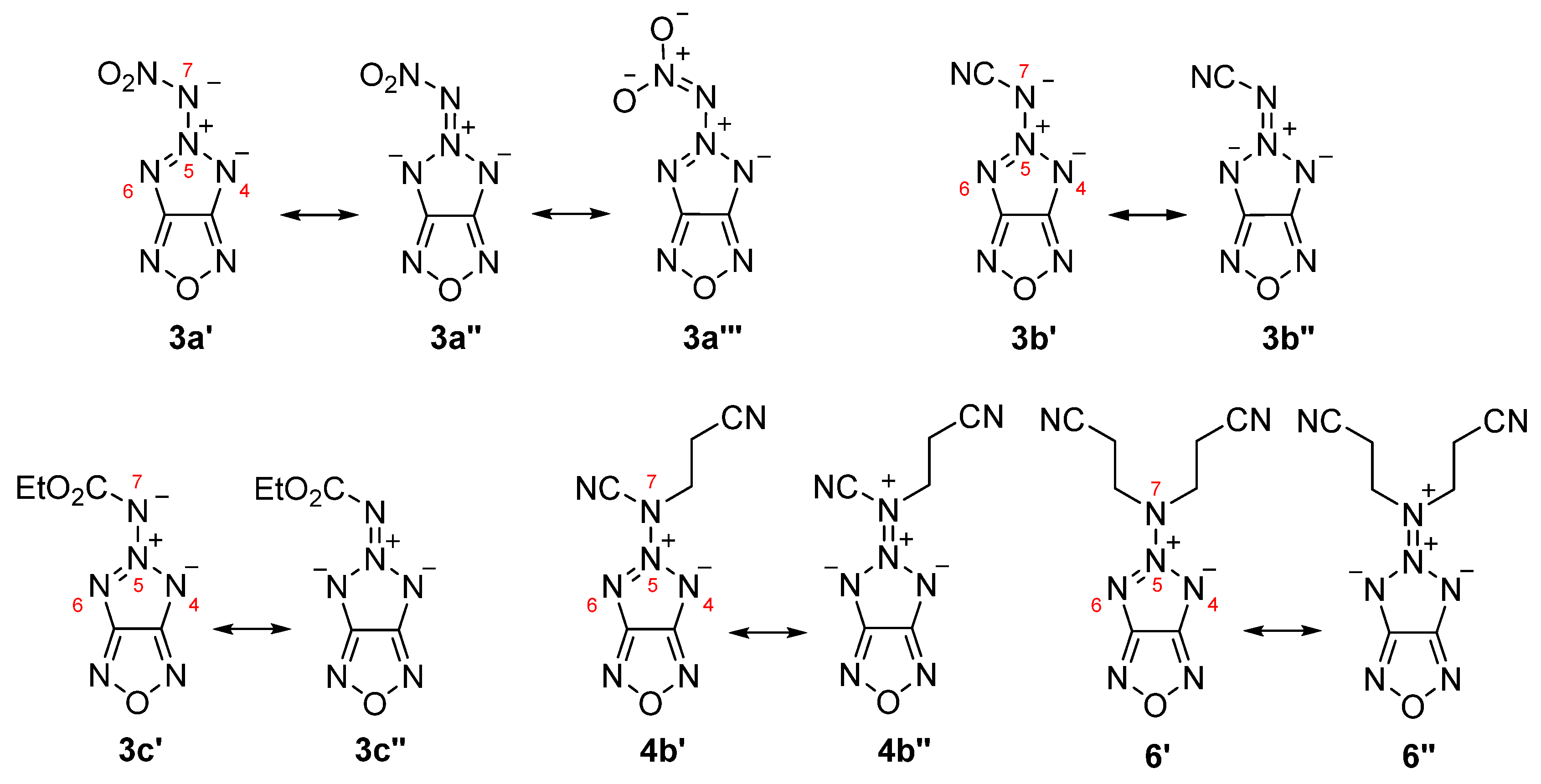
2.2. Spectroscopy
2.3. X-ray Structure Analysis
2.4. Thermal Behavior
3. Conclusions
4. Materials and Methods
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Dworkin, A.; Naumann, R.; Seigfred, C.; Karty, J.M.; Mo, Y. Y-aromaticity: Why is the trimethylenemethane dication more stable than the butadienyl dication? J. Org. Chem. 2005, 70, 7605–7616. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Zhu, H.; Zhang, Q.; Zhao, Q.; Zhao, H.; Suo, B.; Zhai, G.; Zou, W.; Han, H.; Song, Q.; et al. Theoretical study on the two novel planar-type all-nitrogen N4(4−) anions: Structures, stability, reaction rate and their stable mechanisms via protonation. Chem. Phys. Lett. 2021, 771, 138519. [Google Scholar] [CrossRef]
- Katritzky, A.R.; Mitchell, J.W. N-oxides and related compounds. Part XLIII. Formation of N-nitroimides by base-catalysed nitration. J. Chem. Soc. Perkin Trans. 1 1973, 2624–2626. [Google Scholar] [CrossRef]
- Ilyushin, M.A.; Terpigorev, A.N.; Tselinskii, I.V. Effect of isomeric structure of (N-nitramino) tetrazoles on the properties of their salts. Russ. J. Gen. Chem. 1999, 69, 1654–1657. [Google Scholar]
- Cliff, M.D.; Smith, M.W. Thermal characteristics of alkali metal dinitramide salts. J. Energ. Mater. 1999, 17, 69–86. [Google Scholar] [CrossRef]
- Rahm, M.; Brinck, T. On the anomalous decomposition and reactivity of ammonium and potassium dinitramide. J. Phys. Chem. A 2010, 114, 2845–2854. [Google Scholar] [CrossRef]
- Muravyev, N.V.; Koga, N.; Meerov, D.B.; Pivkina, A.N. Kinetic analysis of overlapping multistep thermal decomposition comprising exothermic and endothermic processes: Thermolysis of ammonium dinitramide. Phys. Chem. Chem. Phys. 2017, 19, 3254–3264. [Google Scholar] [CrossRef]
- Luk’yanov, O.A.; Gorelik, V.P.; Tartakovsky, V.A. Dinitramide and its Salts 1. Synthesis of dinitramide salts by decyanoethylation of N,N-dinitro-β-aminopropionitrile. Russ. Chem. Bull. 1994, 43, 89–92. [Google Scholar]
- Chen, F.Y.; Xuan, C.L.; Lu, Q.Q.; Xiao, L.; Yang, J.Q.; Hu, Y.B.; Zhang, G.P.; Wang, Y.L.; Zhao, F.Q.; Hao, G.Z.; et al. A review on the high energy oxidizer ammonium dinitramide: Its synthesis, thermal decomposition, hygroscopicity, and application in energetic materials. Def. Technol. 2022, in press. [Google Scholar] [CrossRef]
- Voronin, A.A.; Fedyanin, I.V.; Churakov, A.M.; Pivkina, A.N.; Muravyev, N.V.; Strelenko, Y.A.; Klenov, M.S.; Lempert, D.B.; Tartakovsky, V.A. 4H-[1,2,3]Triazolo [4,5-c][1,2,5]oxadiazole 5-oxide and Its Salts: Promising Multipurpose Energetic Materials. ACS Appl. Energy Mater. 2020, 3, 9401–9407. [Google Scholar] [CrossRef]
- Pei, L.; Xie, C.; Yin, P.; Pang, S. N-amination of nitrogen-rich scaffolds: From single N–N bond formation to diverse energetic functionalization strategies. Energ. Mater. Front. 2021, 2, 306–316. [Google Scholar] [CrossRef]
- Tselinskii, I.V.; Mel’nikova, S.F.; Vergizov, S.N. Azidofurazans in the synthesis of condensed systems. J. Org. Chem. USSR 1981, 17, 994–995. [Google Scholar]
- Gunasekaran, A.; Boyer, J.H. Dense energetic compounds of C, H, N, and O atoms. III. 5-[4-nitro-(1,2,5) oxadiazolyl]-5H-[1,2,3] triazolo [4,5-c][1,2,5] oxadiazole. Heteroat. Chem. 1993, 4, 521–524. [Google Scholar] [CrossRef]
- Khisamutdinov, G.K.; Mrakhutzina, T.A.; Gabdullin, R.M.; Abdrakhmanov, I.S.; Smirnov, S.P.; Ugrak, B.I. Synthesis, properties, and structures of 5-nitroalkyl-5H-(1,2,3)triazolo[4,5-c] furazans. Russ. Chem. Bull. 1995, 44, 1269–1271. [Google Scholar] [CrossRef]
- Zelenov, V.P.; Lobanova, A.A.; Lyukshenko, N.I.; Sysolyatin, S.V.; Kalashnikov, A.I. Behavior of [1,2,5]oxadiazolo[3,4-e][1,2,3,4]tetrazine 4,6-dioxide in various media. Russ. Chem. Bull. 2008, 57, 1384–1389. [Google Scholar] [CrossRef]
- Xiaobo, S.; Zhao, S.; Chen, W.; Chen, C.; Qiu, H. Copper-Catalyzed Cascade Cyclization Reaction of 2-Haloaryltriazenes and Sodium Azide: Selective Synthesis of 2H-Benzotriazoles in Water. Chem. Eur. J. 2014, 20, 1825–1828. [Google Scholar]
- Hall, J.H. Thermal decomposition of o-azidoazobenzenes. II. Synthesis of 2-substituted benzotriazoles. J. Org. Chem. 1968, 33, 2954–2956. [Google Scholar] [CrossRef]
- Luk’yanov, O.A.; Anikin, O.V.; Gorelik, V.P.; Tartakovsky, V.A. Dinitramide and its Salts 2. Dinitramide in Michael- and retro-Michael-type reactions. Russ. Chem. Bull. 1994, 43, 1200–1202. [Google Scholar]
- Zhilin, E.S.; Fershtat, L.L.; Bystrov, D.M.; Kulikov, A.S.; Dmitrienko, A.O.; Ananyev, I.V.; Makhova, N.N. Renaissance of 1,2,5-Oxadiazolyl Diazonium Salts: Synthesis and Reactivity. Eur. J. Org. Chem. 2019, 26, 4248–4259. [Google Scholar] [CrossRef]
- Rakitin, O.A.; Zalesova, O.A.; Kulikov, A.S.; Makhova, N.N.; Godovikova, T.I.; Khmel’nitskii, L.I. Synthesis and reactivity of furazanyl-and furoxanyldiazonium salts. Russ. Chem. Bull. 1993, 42, 1865–1870. [Google Scholar] [CrossRef]
- Akhtar, M.H.; McDaniel, R.S.; Feser, M.; Oehlschlager, A.C. NMR study of hindered rotation in 1-aryl-3,3-dimethyltriazenes. Tetrahedron 1968, 24, 3899–3906. [Google Scholar] [CrossRef]
- Churakov, A.M.; Ioffe, S.L.; Strelenko, Y.A.; Tartakovsky, V.A. Synthesis of 4H-[1,2,3]triazolo[4,5-c][1,2,5]oxadiazole 5-oxide and its N-and O-alkyl derivatives. Tetrahedron Lett. 1996, 37, 8577–8580. [Google Scholar] [CrossRef]
- Deng, H.L.; Luo, X.S.; Lin, Z.; Niu, J.; Huang, M.H. 14N NMR as a General Tool to Characterize the Nitrogen-Containing Species and Monitor the Nitration Process. J. Org. Chem. 2021, 86, 16699–16706. [Google Scholar] [CrossRef] [PubMed]
- Arriau, J.; Deschamps, J.; Reginald, J.; Duke, C.; Epsztajn, J.; Katritzky, A.R.; Lunt, E.; Mitchell, J.W.; Rizvi, S.A.; Roch, G. Pyridine 1-nitroimide: Crystal structure, conformation, electronic structure, site of protonation, and reactivity. Tetrahedron Lett. 1974, 44, 3865–3868. [Google Scholar] [CrossRef]
- Muravyev, N.V.; Monogarov, K.A.; Asachenko, A.F.; Nechaev, M.S.; Ananyev, I.V.; Fomenkov, I.V.; Kiselev, V.G.; Pivkina, A.N. Pursuing reliable thermal analysis techniques for energetic materials: Decomposition kinetics and thermal stability of dihydroxylammonium 5,5′-bistetrazole-1,1′-diolate (TKX-50). Phys. Chem. Chem. Phys. 2017, 19, 436–449. [Google Scholar] [CrossRef]
- Krause, L.; Herbst-Irmer, R.; Sheldrick, G.M.; Stalke, D. Comparison of Silver and Molybdenum Microfocus X-ray Sources for Single-Crystal Structure Determination. J. Appl. Crystallogr. 2015, 48, 3. [Google Scholar] [CrossRef]
- Sheldrick, G.M. XS. version 2013/1, Georg-August-Universität Göttingen, Germany, 2013; b) GM Sheldrick. Acta Crystallogr. Sect. A 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Crystal Structure Refinement with SHELXL. Acta Crystallogr. C 2015, 71, 3–8. [Google Scholar] [CrossRef]


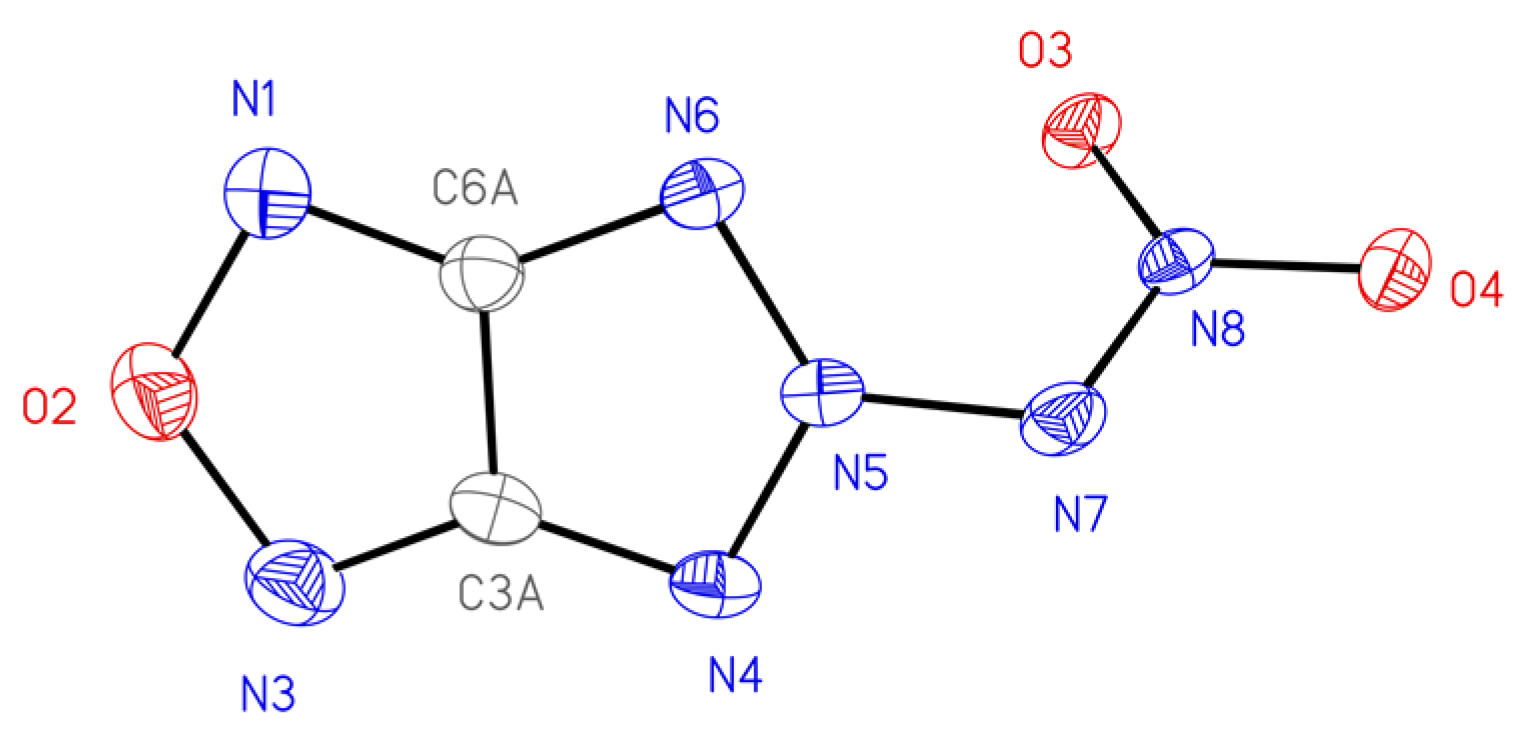
| Compound | 3a | 3b | 3c | 4b | 6 | 12 1 |
|---|---|---|---|---|---|---|
| bond lengths, Å | ||||||
| N4–N5 | 1.337(2) | 1.3420(18) | 1.360(2) | 1.328(2) | 1.3465(8) | 1.3545(16) |
| N5–N6 | 1.339(2) | 1.3535(17) | 1.347(2) | 1.324(2) | 1.3465(8) | 1.3443(16) |
| N5–N7 | 1.382(2) | 1.3368(18) | 1.338(2) | 1.379(2) | 1.3320(14) | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Voronin, A.A.; Balabanova, S.P.; Fedyanin, I.V.; Churakov, A.M.; Pivkina, A.N.; Strelenko, Y.A.; Klenov, M.S.; Tartakovsky, V.A. Anions Containing Tripoid Conjugated N4− System: Salts of 5-(Substituted Amino)-[1,2,3]triazolo[4,5-c][1,2,5]oxadiazol-5-ium-4-ides, as well as Their Synthesis, Structure, and Thermal Stability. Molecules 2022, 27, 6287. https://doi.org/10.3390/molecules27196287
Voronin AA, Balabanova SP, Fedyanin IV, Churakov AM, Pivkina AN, Strelenko YA, Klenov MS, Tartakovsky VA. Anions Containing Tripoid Conjugated N4− System: Salts of 5-(Substituted Amino)-[1,2,3]triazolo[4,5-c][1,2,5]oxadiazol-5-ium-4-ides, as well as Their Synthesis, Structure, and Thermal Stability. Molecules. 2022; 27(19):6287. https://doi.org/10.3390/molecules27196287
Chicago/Turabian StyleVoronin, Alexey A., Sofya P. Balabanova, Ivan V. Fedyanin, Aleksandr M. Churakov, Alla N. Pivkina, Yurii A. Strelenko, Michael S. Klenov, and Vladimir A. Tartakovsky. 2022. "Anions Containing Tripoid Conjugated N4− System: Salts of 5-(Substituted Amino)-[1,2,3]triazolo[4,5-c][1,2,5]oxadiazol-5-ium-4-ides, as well as Their Synthesis, Structure, and Thermal Stability" Molecules 27, no. 19: 6287. https://doi.org/10.3390/molecules27196287
APA StyleVoronin, A. A., Balabanova, S. P., Fedyanin, I. V., Churakov, A. M., Pivkina, A. N., Strelenko, Y. A., Klenov, M. S., & Tartakovsky, V. A. (2022). Anions Containing Tripoid Conjugated N4− System: Salts of 5-(Substituted Amino)-[1,2,3]triazolo[4,5-c][1,2,5]oxadiazol-5-ium-4-ides, as well as Their Synthesis, Structure, and Thermal Stability. Molecules, 27(19), 6287. https://doi.org/10.3390/molecules27196287