Abstract
In the context of the rapid development of the world’s nuclear power industry, it is vital to establish reliable and efficient radioanalytical methods to support sound environment and food radioactivity monitoring programs and a cost-effective waste management strategy. As one of the most import fission products generated during human nuclear activities, 90Sr has been widely determined based on different analytical techniques for routine radioactivity monitoring, emergency preparedness and radioactive waste management. Herein, we summarize and critically review analytical methods developed over the last few decades for the determination of 90Sr in environmental and biological samples. Approaches applied in different steps of the analysis including sample preparation, chemical separation and detection are systematically discussed. The recent development of modern materials for 90Sr concentration and advanced instruments for rapid 90Sr measurement are also addressed.
1. Introduction
90Sr (T½ = 28.79 y) is one of the most important hazardous radionuclides with respect to radiological safety to human and the environment due to its long half-life and high fission yield [1,2,3]. 90Sr is a fission product of 235U and 239Pu; its decay chain is shown in Figure 1 [4]. 90Sr emits beta particles with a maximum energy of 546 keV and decays to short-lived 90Y (t½ = 64 h). 90Y decays to a stable nuclide 90Zr by emitting beta particles with a maximum energy of 2280 keV [5]. There are three main sources of 90Sr in the environment: (1) Global fallout from the atmospheric nuclear weapons testing during the 1950s–1980s [6,7,8,9], with a total 90Sr inventory of 804 PBq [10,11,12]. (2) Nuclear accidents including the former Soviet Union Chernobyl nuclear power plant (SUCNPP) accident in 1986 and the Fukushima Daiichi nuclear power plant (FDNPP) accident in 2011 in Japan. The Chernobyl NPP accident had released up to 10 PBq of 90Sr into the surrounding environment, especially in the Black Sea [13,14,15,16], while the Fukushima accident was reported to release 1 PBq of 90Sr mostly into the Pacific Ocean [17,18,19,20,21]. (3) Regulated releases from the operation of nuclear facilities such as nuclear power plants and nuclear reprocessing plants [22].

Figure 1.
The decay chain of 90Sr.
Strontium is easily dissolved in water; thus, it is very mobile in the environment [23,24]. 90Sr can be transported by different pathways from the environment into the food chain and finally enter into human bodies [25]. Sr and Ca are both elements of group IIA, their chemical properties are similar. Therefore, the biological process of 90Sr in the human body is also very similar to that of Ca, which belongs to the typical bone-seeking nuclides. Once 90Sr enters the body, it will follow the uptake of Ca and be quickly accumulated on the surface layer of bone salt as Sr3(PO4)2 [26]. With time, 90Sr participates in the formation of bone salt and enters the crystals of bone inorganic salt; thus, it becomes immobilized in the bone with the physiological osteogenesis process. The release of high-energy beta particles from 90Y imposes severe damage to the human bone and bone marrow hematopoietic tissues, leading to bone cancer or leukemia [27,28].
With the rapid development of the global nuclear power industry and the widespread application of nuclear technology, it sets high demands in radiation protection and radiological risk assessment during routine operation and nuclear emergency situations. Therefore, it is of great significance to establish efficient analytical methods to be applied for the determination of radiological toxic radionuclides including 90Sr. In the past few decades, substantial efforts have been devoted to method development for 90Sr determination in various samples. Several researchers have made good reviews about the analytical methodologies of 90Sr, some focusing on the development of radiochemical separation procedures for 90Sr based on the use of radiometric measurement techniques [29], and some focusing on methods for 90Sr routine environmental monitoring [1,29] or determination in milk [30]. In the present work, we aim to summarize the analytical methods developed in the past few decades for low-level (e.g., in the range of 1–1000 mBq/L in water or 1–1000 mBq/kg in solid samples) 90Sr analysis in environmental and biological samples and critically review the pros and cons of different analytical approaches. Here, we focus on presenting the recent progresses in technical development for low-level 90Sr analysis, specifically on the development and application of advanced materials for Sr isolation/purification and modern instruments for Sr measurement.
In our literature search, keywords ‘strontium-90′, ‘determination’, ‘analysis’, ‘separation’, ‘environmental’, and ‘biological’ were used. Table 1 summarizes the analytical methods reported for 90Sr determination in environmental and biological samples since the 21st century. Generally, analytical procedures for 90Sr determination are divided into three stages including sample pretreatment, chemical purification and measurement as illustrated in Figure 2 and detailed in this review. It is noteworthy that the quantification of 90Sr can be performed by directly measuring 90Sr using a radiometric or mass spectrometric method or by an indirect measurement through its daughter 90Y using a radiometric method. In both cases, the isolation of 90Sr or 90Y from the sample matrix and interfering elements is necessary due to the relatively low penetrating abilities and continuous energy spectrum of beta particles. Thereby, the development of sample pretreatment and chemical purification protocol is always served for the measurement technique selected for 90Sr quantification.

Table 1.
Overview of analytical methods developed for determining 90Sr in environmental and biological samples since 2000.
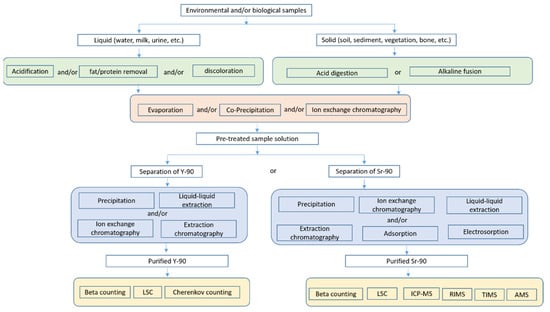
Figure 2.
Overview of analytical scheme for 90Sr in environmental and biological samples.
2. Sample Pretreatment
The purpose of sample pretreatment is to obtain a homogeneous sample, pre-concentrate the analyte and remove the majority of sample matrix such as organic matter and stable elements. The selection of sample pretreatment methods depends on the sample type.
2.1. Pretreatment of Environmental Samples
For environmental solid samples including soil, sediment, sludge, etc., the commonly used pretreatment method is acid digestion and, in some cases, the acid digestion is assisted with microwave [7,8,59,60,61,62,63,64]. Prior to the acid digestion, the sample needs to be dried and combusted to decompose organic matter. Acid digestion using mineral acids such as HNO3, HF, HCl, HClO4 or mixed acids (e.g., mixture of HF, HClO4 and HNO3, mixture of HF, HNO3 and HCl) has been widely used to extract 90Sr from solid samples [32,35,65,66,67]. The advantage of the acid digestion method is its easy operation and capacity of processing large sample amounts (e.g., up to 200 g); however, it requires a large amount of aggressive acids and long operation time [68].
With the use of microwave-assisted acid digestion, the sample processing time can be significantly reduced [4,33,35,69]. For instance, Feuerstein et al. [35] took only 25 min to digest a sample with HF and HClO4 in a microwave oven. Compared with traditional acid digestion operated in open vessels, microwave-assisted digestion is much faster. However, the sample size is usually limited to a few grams [37] as restricted by the volume of microwave ovens and digestion vessels. Therefore, the microwave-assisted acid digestion is not suitable to process low-level environmental samples, where tens or hundreds of grams of sample would be needed for 90Sr determination.
Another pretreatment method for solid environmental samples is alkaline fusion, in which fusion flux such as Na2CO3 and Na2O2, NaOH or LiBO3 is mixed with the solid sample and melted at a high temperature, e.g., 300–1000 °C [70,71,72,73,74,75]. Jurecic et al. [72] compared conventional acid digestion using a mixture of HNO3, HClO4 and HF, microwave-assisted digestion using HNO3 and HF, and alkali fusion with Na2CO3 and Na2O2. The complete decomposition of soil samples could only be achieved by alkaline fusion. The disadvantage of alkaline fusion is the high temperature and aggressive reaction required, which concerns the safety of the analysts. As restricted by the operating condition, alkaline fusion is mostly applicable to smaller sized samples, e.g., up to 5–10 g.
For environmental water samples including seawater, groundwater, lake water and river water, evaporation or co-precipitation is the commonly used pretreatment method for 90Sr determination [76]. For large volume of environmental samples, evaporation is seldom used due to its time-consuming feature. 90Sr in environmental water samples is usually co-precipitated with oxalates or carbonates, e.g., 90Sr in natural waters can be co-precipitated with calcium oxalate under lower pH (pH = 4–5) which is thereafter decomposed into carbonate [2] or co-precipitated directly with calcium carbonate under higher pH (pH > 10).
2.2. Pretreatment of Biological Samples
A number of biological samples including bones, teeth, blood, urine, plants, milk, etc., are often analyzed for 90Sr in radioactivity monitoring and radiological risk assessment. For biological samples, similar sample pretreatment approaches as for environmental samples can be applied [7,61,62,77,78,79] but with a focus on tackling challenges introduced by the complex biological matrices. For example, bones and teeth usually contain substantial amount of calcium. Milk contains large amount of fat and protein as well as antiseptic additives, e.g., formaldehyde or sodium azide [48,80]. Urine contains a large amount of organic compounds and inorganic salts [54,55,81,82]. Blood has a high concentration of iron and even more complex matrix composition.
Therefore, Gasa et al. [77] used a combination of HF, HNO3, HCl, and H3BO3 to digest mammalian skulls. Fuming nitric acid is also commonly used to digest biological samples (such as teeth and bones) enrich in calcium [19]. With the addition of oxidizing reagents such as H2O2, the acid digestion process for bones using mineral acids can be accelerated [53].
The typical protocol for the pretreatment of fresh milk includes drying, ashing and then acid digestion of the ashed sample. To ensure the complete decomposition of organic substance in milk, the ashing usually last for 3–5 days, which is somewhat time-consuming. The microwave-assisted acid digestion has been demonstrated successfully to increase the efficiency for organic matter decomposition by a factor of 2–5 [45] and has become popular for biological sample pretreatment [83]. Microwave-assisted enzymatic digestion has also been proven to substantially reduce digestion times to 20 min for proteins [84], which might also have the potential to be applied in milk pretreatment for 90Sr analysis. After digestion, co-precipitation is generally used to isolate Sr from the milk samples [49].
Similar to milk, urine samples are usually digested by concentrated acids and pre-concentrated by co-precipitation [58,85]. In recent years, adsorption and chromatographic methods have been applied directly in biological sample pretreatment to remove the matrix. For example, Hawkins et al. [55] developed a rapid method for detecting 90Sr in human urine, wherein the organic matrix was removed by activated carbon. Sadi et al. [54] applied Sr resin in an HPLC system to remove the urine matrix, while some other researchers used cation exchange resin [54,55]. It is noteworthy that direct pretreatment with adsorption and chromatographic methods are mostly applicable to small-size samples (e.g., up to 250 mL). For large volumes (e.g., >1 L) of biological samples, the co-precipitation approach is more practical for the sample pre-concentration.
3. Chemical Purification
Chemical purification is a method by precipitation, calcination, acid and alkali treatment, leaching and so on. The main purpose of chemical purification is to concentrate the target radionuclide and to remove interferences, thus obtaining a purified fraction. Interferences for 90Sr or 90Y measurement with radiometric methods include all other beta emitters which will directly affect the detection of 90Sr and any alpha/gamma emitters which will increase the background. When using mass spectrometric methods for 90Sr measurement, focus should be given to remove isobaric and polyatomic interferences formed by stable isotopes, such as 90Zr and 89Y1H. In any case, 90Sr or 90Y must be isolated in the chemical purification step, wherein different techniques have been applied including precipitation, liquid–liquid extraction, ion exchange chromatography, extraction chromatography, electrosorption and adsorption.
3.1. Precipitation
The nitrate precipitation using fuming nitric acid is a classical method and widely applied for 90Sr purification [1,29,60,64,86]. The method is based on the low solubility of Sr(NO3)2 in high concentration (>14 M) of nitric acid to achieve the selective isolation of Sr. The method using fuming nitric acid treatment is recommended by the International Atomic Energy Agency (IAEA, ISO 18589-5: 2019 [87], ISO 13160: 2012 [88]), the United States Department of Energy and other agencies (e.g., HJ815-2016 GB [89], GB 14883.3-2016 [90]). In many cases, nitrate precipitation is combined with other precipitation approaches including chromates, hydroxides, carbonates, etc. to achieve a complete removal of interferences and concentration of Sr as reviewed by Shao et al. [1]. The precipitation method is robust and provides reliable results. However, it is very tedious and labor intensive, as it often requires repeated precipitation for 3–5 times. In addition, the method involves the use of offensive fuming nitric acid, which could potentially impose health risks to the operator.
3.2. Liquid–Liquid Extraction
Liquid–liquid extraction is a separation process based on the different solubility of a solute in two partially miscible liquid solvents. In 90Sr purification, Sr is typically extracted from an aqueous phase into an organic solvent containing hydrophobic ligand (so-called extractant) which forms a stable electrically neutral complex with Sr. Traditional extractants such as di- (2-ethylhexyl) phosphoric acid (HDEHP), tributyl phosphate (TBP), trioctylphosphine oxide (TOPO), and thiopheneyl trifluoroacetone (TTA) have been used to separate 90Sr and 90Y. For example, HDEHP is a phosphorous extractant and maintains a good extraction rate of high-valent metal ions when the aqueous phase has high acidity. Borcherding et al. firstly adopted HDEHP as an extractant for 90Sr purification in 1968 [91]. Reddy et al. [39] studied the effect of HDEHP concentration and pH on the extraction efficiency and observed that about 90% of 90Sr can be extracted into the organic phase with a 10% (v/v) mixture of HDEHP and toluene at pH 4–4.5. In 1969, Pedersen proposed that crown ethers can form stable coordination compounds with alkaline earth metals [92]. The crown ether molecules modified with hydrophobic groups, such as benzo crown ether and dibenzo crown ether, make the crown ether complex easily enter the organic phase and improve the extraction rate of metal ions. Cyclohexyl and substituted cyclohexyl-modified crown ethers, such as dicyclohexyl-18-crown-6 (DCH18C6), di-tert-butyl dicyclohexyl-18-crown-6 (DtBuCH18C6), etc., have high extraction efficiency on Sr [93]. Tormos et al. [94] used the DCH18C6-Cl2CHCHCl2 system as a rapid separation method for 90Sr in soil and plant samples. The chemical yield of strontium was 70–85%. The advantage of liquid–liquid extraction is its simplicity and high efficiency. However, organic solvents are usually volatile and toxic. Therefore, liquid–liquid extraction for 90Sr analysis is gradually fading out in radioanalytical laboratories for human and environmental safety reasons.
3.3. Ion Exchange Chromatography
Ion exchange chromatography is based on the different affinities of ions and polar molecules onto ion exchangers to separate the target analyte from interfering elements. Ion exchange resins commonly used for 90Sr (or 90Y) purification include Dowex-50, Amberlite IR-120, Zeokarb 225, AG 1-X8 and AG 50W-X8 [47,49,59,93]. Castrillejo et al. [95] combined anion (AG1-X8) and cation (AG50W-X8) exchange chromatography to purify 90Y, and 63–93% of chemical yield was achieved. The Ministry of Environmental Protection of China has recommended a national standard method for 90Sr determination in water and biological samples, where Sr is separated based on the selective adsorption of EDTA-Sr complexes on a cation exchanger [89]. Nguyen et al. [96] used cation exchange resin to analyze radioactive strontium in water and the result was compared with current methods from the U.S. Environmental Protection Agency (EPA) and Food Emergency Response Network (FERN). The comparison indicated that the method using cation exchange resin to separate radiostrontium was more environmentally friendly and easier to perform. In short, the advantages of ion exchange chromatography include that it can handle large volumes of samples and is highly applicable to different sample matrixes. However, the selectivity of ion exchangers is not very high; thus, repeated chromatographic separation is usually needed, making the analytical processes tedious and less effective.
3.4. Extraction Chromatography
Extraction chromatography is also called solid phase extraction, which employs the same basic principle as liquid–liquid extraction, but the extractant is immobilized on the surface of an inert solid support material. HDEHP and Crown ether have been widely used in extraction chromatographic separation for 90Sr. Horwitz et al. [97] developed the Sr resin consisting of DtBuCH18C6-n-butanol system on an inert support [51], based on the research of crown ether liquid–liquid extraction. Nowadays, the Sr resin has been widely used in many international, e.g., ISO18589-5: 2019 and national standards, e.g., the American Society for Testing and Materials (US-ASTM), the French Association for Standardization (AFNOR), and the British Standards Institute (GB-BSI). Chu et al. [86] compared the performance of strontium nitrate precipitation, ion exchange chromatography, and extraction chromatography for the determination of 90Sr in tea, brown rice, milk powder and soil samples. The results showed that the extraction chromatographic method is more effective and can reduce the analytical time to 2 h. Compared with ion exchange chromatography, extraction chromatography has a higher selectivity. However, Grahek et al. [98] noticed that the separation efficiency on the Sr resin decreased with the increase in Sr, Ca and Na concentrations in the sample. By pre-separating matrix elements (e.g., Na and Ca) on an anion exchange column, and further isolating Sr from Ca on a Sr resin column, the Sr chemical yield can be improved from 50% to 75%. Therefore, it is a good strategy to combine ion exchange chromatography with extraction chromatography to achieve high selectivity for 90Sr and meanwhile ensure high-capacity sample processing. For example, Taylor et al. [37] directly loaded 1 L of water onto a 50 mL cation exchange column followed by purification of 90Sr using a Sr resin, and they achieved satisfactory results with 84–98% of chemical yields.
3.5. Adsorption
Adsorption is a process of uptaking target analytes from aqueous phase onto the surface of an adsorbent. The adsorption method has the advantages of cost-effective, high capacity and flexibility in operation and no generation of secondary pollutants [99,100]. Thus, adsorption is considered to be the most effective method for removing environmental pollutants [101]. Various adsorbents, including metal sulfides, metal–organic frameworks (MOFs) and graphene oxide (GO), have been developed to remove strontium through different mechanisms. Soft S2− ligands in their frameworks have an innate strong affinity for soft metal ions (Sr2+) rather than coexisting hard ions (H+, Na+, K+) [102]; thus, they can effectively remove Sr from wastewater. Zhang et al. [103] synthesized a metal sulfide (NaTS), which can reach adsorption equilibrium within 5 min for Sr2+, and a maximum adsorption capacity of 80 mg/g. However, when multiple ions coexist in the system, the strongly bound ions dominate the surface since adsorption depends on the binding strength of the ions onto the adsorbent surface [104]. Thus, the selectivity enhancement of adsorbents to target nuclides remains a great challenge. To address the challenges, an ion imprinting technique has been developed to effectively improve the selectivity of materials by which a functional monomer and a crosslinker were polymerized in the presence of a template ion [105,106]. It can create specific cavities containing a ligand for the template ion that possesses the right size and charge [107,108,109]. Yin et al. [110] employed biogene-derived aerogels for the selective adsorption of strontium (II) by the imprinting method from a high-salt environment.
3.6. Electrosorption
Electrosorption, integrating adsorption and electrochemistry, is a separation technique through a non-Faradic process independent of electron gain and loss [111]. Electrosorption such as the half-wave rectified alternating current electrochemical (HW-ACE) method [112] and asymmetrical alternating or direct (DC)/alternating current (AC) electrochemical method [104] have been extensively developed for the removal of heavy metal ions from aqueous solutions. Different from the traditional adsorption method, which is intrinsically constrained by the limited utilization of the surface adsorption sites, electric field-induced ion migration fully utilizes the active sites on the adsorbent surface. Xiang et al. [113] achieved a significant enhancement of the Sr2+ adsorption capacity by using the modified porous carbon material as a capacitive deionization electrode. Furthermore, the ions are released from the electrical double layers (EDL) through reverse potential or power cut, and then, the electrode material is regenerated without generating waste liquid, avoiding the secondary pollution [111]. Wang et al. [114] grafted 4′-aminobenzene-18-crown-6 ether onto the surface of carbon felt to obtain a composite (CE@CF), which was used as an electrode to extract Sr2+ from aqueous solutions and simulated seawater by an asymmetric pulsed current-assisted electrochemical (APCE) method. The Sr2+ ions adsorbed on the surface of the CE@CF electrode could be desorbed by applying a positive voltage without an eluent, and the CE@CF electrode displayed good recyclability. Currently, electrochemical adsorption is a rapidly developing method for the removal of Sr2+ from industrial wastes.
4. Measurement
As mentioned earlier, the measurement of 90Sr can be performed via directly measuring the isolated 90Sr or by an indirect measurement through its daughter 90Y. The techniques for 90Sr measurement include radiometric method to detect the beta decay of 90Sr using a beta counter or liquid scintillation counting (LSC) and mass spectrometry to detect 90Sr ions. The measurement of 90Y is typically performed by detecting the higher energy (2280 Kev) beta emission of 90Y by LSC/beta counter or Cerenkov counting.
4.1. Beta Counter
Beta counters commonly used for 90Sr measurement include proportional counters and Geiger–Müller counter (GM counters). The proportional counter has a satisfactorily high efficiency (about 50%) and a relatively low background, which can be used as a sensitive detection tool for 90Sr. The GM counter can also be used to detect relatively high-energy beta radiation. However, none of them can distinguish between 89Sr, 90Sr, and 90Y. In such case, the sample is repeatedly counted for the in-growth of 90Y; thereby, the activity of 90Sr can be calculated. Sato et al. [115] have developed a technique to visualize the locations of 90Sr source in 3D by combining a directional GM counters with Structure from Motion (SfM) and a method to estimate the radioactivity of the visualized source. Additionally, by combining a beta-ray detector with a Compton camera, they demonstrated that 90Sr and 137Cs can be visualized separately.
4.2. Electrosorption Liquid Scintillation Counting
Liquid scintillation technology is widely used to measure the activity of radionuclides, especially low energy β emitters. LSC is favored for 90Sr determination because of the high resolution which can distinguish 89Sr, 90Sr and 90Y [116] and very high counting efficiencies (close to 100%) for 89Sr, 90Sr and 90Y. The samples prepared for LSC generally have high transparency and low self-absorption, but they could have a quenching effect [117,118]. LSC has the advantages of high detection efficiency and fast measurement, which is suitable for the rapid analysis of 90Sr in emergency situations. The samples can be measured immediately after 90Sr is separated, without waiting for 90Sr–90Y equilibrium or 90Y separation. The measurement can also wait until the activities of 90Sr and 90Y reach equilibrium. In the latter case, the analytical turnover time is longer but with high precision and high counting efficiency.
4.3. Cherenkov Counting
Cherenkov radiation is a bluish light that occurs when an electrically charged particle travels faster than the local speed of light in an optical medium. As long as the beta particle energy is greater than 0.26 MeV, Cherenkov radiation can be generated in the water, and this radiation can be detected by a low background LSC. The energy (0.546 MeV) of β particles of 90Sr is relatively low, which is not suitable for direct measurement by Cherenkov counting. Both 89Sr (β energy is 1.46 MeV) and 90Y (β energy is 2.29 MeV) have high Cherenkov counting efficiencies (>40%); therefore, 90Y needs to be separated from 89Sr before measuring by Cherenkov counting. Cherenkov counting can also be combined with LSC to obtain a fast quantification for 90Sr. After the chemical separation of Sr, Cherenkov counting can be performed immediately (ignoring the in-growth of 90Y). The sample is measured again by LSC after mixing with cocktail. Cherenkov counting provides a count rate of 89Sr, and the LSC measurement provides the sum of 90Sr and 89Sr; thereby, the 90Sr count rate can be calculated. Compared with LSC, the Cherenkov counting has the advantages of simple sample preparation, no addition of organic scintillator and thus no chemical quenching, and a recyclable sample for other uses. However, it also has disadvantages such as lower counting efficiency and severe color quenching effect compared with LSC. Other studies [69] showed that when 90Sr and 137Cs coexist, the beta rays emitted by 137Cs could affect the Cherenkov measurement of 90Sr. The contribution of 137Cs could be mathematically corrected by multiple linear regression calibration to avoid the chemical separation step.
Grahek et al. [98] observed that the 90Sr measured on the proportional counter by counting 90Sr–90Y in the radioactive equilibrium can significantly reduce the detection limit compared to the measurement on the low-level scintillation counter by Cherenkov counting. Rondahl et al. [119] compared three measurement strategies for 89Sr and 90Sr, wherein Method A was based on a two-stage chemical separation and measures 89Sr and 90Sr (via 90Y) sequentially; B was a spectral subtraction method, and C was a spectral convolution method (Figure 3). The results indicate that Method A is suitable for the determination of 90Sr over the entire range of the 89Sr/90Sr ratio. The simultaneous determination of 89Sr and 90Sr in Methods B and C has great uncertainty, especially for 90Sr. In an emergency, the most appropriate method is to measure 89Sr after the first chemical separation and then measure 90Sr and 90Y after the second chemical separation.
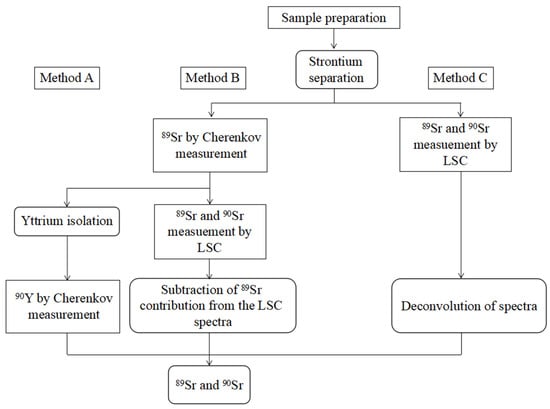
Figure 3.
Comparison of different strategies for measurement of 89Sr and 90Sr. Second chemical separation (Method A), spectrum subtraction (Method B) and spectrum deconvolution (Method C). Reprinted with permission from Ref. [119]. 2018, Stina Holmgren Rondahl.
4.4. Mass Spectrometry
Mass spectrometric techniques are highly sensitive for the measurement of radionuclides, especially for the long-lived radionuclides. Compared with radiometric methods, 90Sr measurement with mass spectrometric methods may not be advantageous in terms of detection limit due to the relatively short half-life of 90Sr (28.79 y), but it provides high analytical throughput and multi-isotope capability. In recent years, efforts were increasing devoted to develop modern mass spectrometric setups to push the detection limit for 90Sr to a lower end.
4.4.1. Inductively Coupled Plasma Mass Spectrometry (ICP-MS)
For the measurement of radionuclides, inductively coupled plasma mass spectrometry (ICP-MS) is the most widely used mass spectrometry technique due to its relatively low cost, easy operation, and high sensitivity. Therefore, ICP-MS has been adopted for the rapid determination of radionuclides in environmental samples since the 1990s [32,36,37,82,120]. For 90Sr measurement, a variety of ICP-MS setups including single-quadrupole ICP-MS (ICP-QMS), triple-quadrupole ICP-MS (ICP-MS/MS), sector filed ICP-MS (SF-ICP-MS), dynamic reaction cell ICP-MS (DRC-ICP-MS), and electrothermal vaporization ICP-MS (ETV-ICP-MS) have been applied [4,33,35,40,71,74,121,122]. Among different mass spectrometric methods, ICP-MS is the mainstream in the measurement of 90Sr.
Nevertheless, 90Sr measurement by ICP-MS is challenged by spectroscopic and non-spectroscopic interferences, matrix and carry-over effects. Table 2 shows the interferences affecting 90Sr in the mass spectrometric measurement, including both isobaric/polyatomic interfaces, primarily 90Zr due to its high content in sample matrix and chemical reagents, and peak tailing of 88Sr. Therefore, besides the thorough chemical purification of 90Sr prior to the detection, sufficient suppression of the signals from interferences during the ICP-MS measurement is important to ensure the analytical accuracy.

Table 2.
Interferences affecting the determination of 90Sr by mass spectrometry.
In an ICP-QMS with a reaction cell, the reaction gas reacts with the interfering substance, e.g., 90Zr thus suppresses the 90Zr signal but still keeps high sensitivity for 90Sr. The reaction gas can be selected based on thermodynamic and kinetic data [1,3,4,33,71,74,123,124]. The most commonly used reaction gas for the suppression of 90Zr from 90Sr is Q2 [125]. An ultrasonic nebulizer (USN) can be adopted to enhance the sensitivity of ICP-MS for the determination of 90Sr, e.g., 22-fold sensitivity enrichment was reported by Takagai et al. [33]. Al-Meer et al. [36] established a new method for the determination of 90Sr in soil samples by ICP-MS/MS, introducing oxygen into the reaction cell to suppress the interference of 90Zr. The detection limit was found to be 0.1 pg/g, using a microflow nebulizer.
4.4.2. Resonance Ionization Mass Spectrometry (RIMS)
The Resonance Ionization Mass Spectrometry (RIMS) can avoid some isobaric interference and improve the selectivity of traditional mass spectrometry toward isotopes of interest. The system is mainly composed of three parts: an ion source sample loading system, atomic ionization continuous wave laser system and mass spectrometer [126]. In RIMS, a laser beam is used to selectively/resonantly excite and ionize analyte atoms, thereby eliminating the isobaric interference caused by other elements. In a recent work, Cheon et al. [127] developed an interference filtered external cavity diode laser system, which significantly improved the selectivity of 90Sr. In the study of Bushaw et.al [128], a diode laser-based scheme for the isotopically selective excitation and ionization of strontium was presented. With the use of graphite crucible atomization, the detection limit was demonstrated as low as 0.8 fg, and the overall 90Sr selectivity was >1010 against stable Sr. Due to the limited accessibility, the application of RIMS in 90Sr measurement for environmental and biological samples is still scarce.
4.4.3. Thermal Ionization Mass Spectrometry (TIMS)
The thermal ionization mass spectrometry (TIMS) is equipped with a sample changer, a sector magnetic field mass analyzer, and a Faraday cup detector [4,25,70,124,129]. By setting the magnetic field, TIMS works in a peak-hopping mode, in which the specified ion beam is directed to the detector in sequence, and voltage data are acquired in a digital format as a measure of peak intensity and used to calculate the isotope ratio. TIMS is suitable for measuring the Sr isotope ratio, such as 90Sr/86Sr, and then calculating the concentration of 90Sr by multiplying the concentration of 86Sr. Compared to ICP-MS, TIMS has higher precision and a lower detection limit for 90Sr measurement [129]. In addition, TIMS measurement is not affected by isobaric interferences and any carry-over effect. However, the target preparation for TIMS is very time-consuming; therefore, TIMS has not gained as high popularity as ICP-MS for 90Sr detection.
4.4.4. Accelerator Mass Spectrometry (AMS)
Accelerator mass spectrometry (AMS) can also measure trace amounts of 90Sr, which has the characteristics of high sensitivity for radionuclides with long half-lives [123,130]. Environmental 90Sr/Sr atomic ratios are estimated to be 10−7–10−14; therefore, AMS may extend capability in research into environmental 90Sr owing to superior abundance sensitivity of 10−15 [131]. However, 90Sr-AMS is affected by two major difficulties, which are related to the Sr beam current and interference by 90Zr. Removal of the interference by using a chemical process would drastically improve the AMS detection limit. Satou et al. have developed a simple chemical procedure for rapid AMS-based 90Sr-level determination and confirmed the validity of the chemical procedure [132]. Very recently, Ion-Laser Interaction Mass Spectrometry (ILIAMS), a novel technique for the efficient suppression of a stable isobaric background, has been developed and achieved isobar suppression factors of >105 for the fission products including 90Sr [133]. This new approach has already been validated for 90Sr in selected reference materials (e.g., IAEA-A-12) and is ready for application in environmental studies. In addition, the high cost and high technical demands in AMS instrument maintenance and operation still restrict the application of AMS in the measurement of 90Sr.
5. Automation in 90Sr Analytical Methods
In recent years, the application of automation techniques in 90Sr analytical methods has become popular. Flow-based techniques such as flow injection, sequential injection, and lab-on-valve were the main approaches reported for the automated chemical separation of 90Sr [41]. These developed flow systems were mostly designed and assembled in-house of research laboratories with the use of commercial available components (such as pumps, valves, fittings, etc.); some of them were directly hyphatened to ICP-MS instruments for the rapid on-line detection of 90Sr. Takagai et al. [33] integrated Sr resin (50 mg) into a lab-on-valve platform which was coupled with an DRC-ICP-MS for the on-line separation and detection of 90Sr from microwave digested soil samples. 90Sr can be detected at levels of femtograms per gram of soil. The analytical turnover time for on-line separation and detection was only 14.6 min. Habibi et al. [34] developed a protocol for the rapid determination of actinides and 90Sr in soil samples (0.5 g/sample), wherein automated chemical separation and on-line ICP-MS measurement were performed after alkaline fusion for the sample pretreatment. The protocol provided satisfactory chemical yields (>80%) and sample throughput (10 samples in 24 h).
6. Quality Control
Quality control is important to ensure the reliability of the obtained analytical methods. This includes laboratory background control, the calibration of instruments, evaluation of trueness of the method, and uncertainty calculations. For each batch of samples, a procedure blank should be analyzed following the sample procedure for the samples. Under normal circumstances, the number of blank samples should not be less than 5% of the total number of samples analyzed.
In order to evaluate the impact of various operational processes on data quality, IAEA [134] conducted an inter-comparison exercise for 90Sr determination in ore samples. It was found that the main sources of deviations were from ineffective purification procedures, high background values and lack of statistical control over background values. The main source of bias that led to the underestimation was the overestimated chemical yield due to failure to correct for stable strontium in the sample and quenching correction in LSC. Cheng’s report [135] analyzed the uncertainty composition of the 90Sr measurement results of environmental soil samples. For environmental soil samples, the relative uncertainty was 22% (k = 1). The results show that the factors that contribute greatly to the uncertainty of the measurement results are the uncertainties in β radioactivity measurement (counting statistics), instrument detection efficiency, chemical yield, and sample mass. The relative uncertainties are 22%, 4.4%, 1.1%, 0.83% and 0.69% (k = 1), respectively. In Jiang’s report [136], the fishbone diagram method was used to analyze the uncertainty of 90Sr measurement for water samples. The uncertainty caused by the chemical yield was the largest, which was 6.65%, because the sample preparation of the method was complicated.
7. Conclusions and Perspectives
We herein review the analytical methods of 90Sr in environmental and biological samples, discuss in detail the advantages and disadvantages of different pretreatments, chemical purification methods and measurement techniques developed for 90Sr. At present, the most widely used pretreatment method is acid digestion. Crown ether extraction chromatography is gradually replacing the precipitation method because of its superior separation efficiency. Among the different measurement methods of 90Sr, LSC is widely accepted as the most practical analytical technique for quantifying radioactive strontium isotopes. Cerenkov counter can also quickly measure 90Sr by 90Y. In terms of detection limit and instrument cost, radiometric measurement methods are superior to mass spectrometry.
Traditional methods often take two weeks or more, and the standard processes established by most countries still continue the routine procedure developed in the last century. To improve this, it is necessary to increase the efficiency of pretreatment, chemical separation and detection methods. In terms of pretreatment, the rapid processing of large volume samples still needs to be explored. In terms of chemical separation, crown ether extraction chromatography has become a new trend, but synthesis of new modern materials (such as nanomaterials) and technologies (such as electrosorption) with high absorption capacity, high selectivity toward 90Sr and low cost is still desired. In terms of detection methods, the advantages of mass spectrometry are obvious and attractive. The promises of modern ICP-MS/MS and AMS setups for low-level 90Sr environmental and biological assay are foreseen yet requiring dedicated efforts in instrumental development and optimization. In addition, rapid analytical methods based on automated techniques for 90Sr still need further exploration, especially in the direct hyphenation with mass spectrometry for online measurement.
Author Contributions
Manuscript writing and final approval of the manuscript, Z.Z. and Y.C.; manuscript writing, L.Z., H.R. and P.W.; final approval of the manuscript, H.Z. and X.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Zhejiang Provincial Foundation Public Welfare Research Project (No. LGC21H260001), Zhejiang Health Science and Technology Plan (No. 2021KY613, 2022RC120, 2022KY130, 2022KY132, 2023KY643), Project of South Zhejiang Institute of Radiation Medicine and Nuclear Technology (No. ZFY-2021-K-003, ZFY-2022-K-001, ZFY-2022-K-006).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Shao, Y.; Yang, G.; Tazoe, H.; Ma, L.; Yamada, M.; Xu, D. A review of measurement methodologies and their applications to environmental 90Sr. J. Environ. Radioact. 2018, 192, 321–333. [Google Scholar] [CrossRef] [PubMed]
- Tazoe, H.; Yamagata, T.; Tsujita, K.; Nagai, H.; Obata, H.; Tsumune, D.; Kanda, J.; Yamada, M. Observation of Dispersion in the Japanese Coastal Area of Released 90Sr, 134Cs, and 137Cs from the Fukushima Daiichi Nuclear Power Plant to the Sea in 2013. Int. J. Environ. Res. Public Health 2019, 16, 4094. [Google Scholar] [CrossRef] [PubMed]
- Russell, B.C.; Croudace, I.W.; Warwick, P.E. Determination of 135Cs and 137Cs in environmental samples: A review. Anal. Chim. Acta 2015, 890, 7–20. [Google Scholar] [CrossRef] [PubMed]
- Ješkovský, M.; Kaizer, J.; Kontuĺ, I.; Lujaniené, G.; Müllerová, M.; Povinec, P.P. Analysis of environmental radionuclides. In Handbook of Radioactivity Analysis; Academic Press: Cambridge, MA, USA, 2019; Volume 2, pp. 137–261. [Google Scholar]
- L’Annunziata, M.F. Table of Radioactive Isotopes. In Handbook of Radioactivity Analysis, 4th ed.; Academic Press: Cambridge, MA, USA, 2019; Volume 2, pp. 953–1012. [Google Scholar]
- Liu, W.-S.; Chu, T.C.; Weng, P.S. Measurement of Strontium-90 and Caesium-137 in Milk. Jpn. J. Health Phys. 1970, 5, 195–198. [Google Scholar] [CrossRef]
- Lu, J.G.; Huang, Y.; Li, F.; Wang, L.; Li, S.; Hsia, Y. The investigation of 137Cs and 90Sr background radiation levels in soil and plant around Tianwan NPP, China. J. Environ. Radioact. 2006, 90, 89–99. [Google Scholar] [CrossRef]
- Desideri, C.G.D.; Monte, L. Migration processes of 137Cs and 90Sr in compartments of a lake ecosystem. J. Radioanal. Nucl. Chem. 2005, 266, 31–37. [Google Scholar] [CrossRef]
- Hirose, K.; Igarashi, Y.; Aoyama, M. Analysis of the 50-year records of the atmospheric deposition of long-lived radionuclides in Japan. Appl. Radiat. Isot. 2008, 66, 1675–1678. [Google Scholar] [CrossRef]
- Igarashi, Y.; Aoyama, M.; Hirose, K.; Miyao, T.; Yabuki, S. Is it possible to use 90Sr and 137Cs as tracers for the aeolian dust transport? Water Air Soil Pollut. 2001, 130, 349–354. [Google Scholar] [CrossRef]
- Igarashi, Y.; Aoyama, M.; Hirose, K.; Povinec, P.; Yabuki, S. What anthropogenic radionuclides (90Sr and 137Cs) in atmospheric deposition, surface soils and aeolian dusts suggest for dust transport over Japan. Water Air Soil Pollut. Focus 2005, 5, 51–69. [Google Scholar] [CrossRef]
- Aoyama, M.; Hirose, K.; Igarashi, Y. Re-construction and updating our understanding on the global weapons tests 137Cs fallout. J. Environ. Monit. 2006, 8, 431–438. [Google Scholar] [CrossRef]
- Egorov, V.N.; Povinec, P.P.; Polikarpov, G.G.; Stokozov, N.A.; Gulin, S.B.; Kulebakina, L.G.; Osvath, I. 90Sr and 137Cs in the Black Sea after the Chernobyl NPP accident: Inventories, balance and tracer applications. J. Environ. Radioact. 1999, 43, 137–155. [Google Scholar] [CrossRef]
- Voitsekhovitch, O.V.; Kanivets, V.V.; Kristhuk, B.F. Project RER/2/003 Status Report of the Ukrainian Research Hydrometeorological Institute for 2000–2001. In Working Material of Regional Co-Operation Project RER/2/003 “Marine Environmental Assessment of the Black Sea”; IAEA: Vienna, Austria, 2004. [Google Scholar]
- Buesseler, K.O.; Livingston, H.D. Natural and man-made radionuclides in the Black Sea. In Radionuclides in the Ocean: Inputs and Inventories; IPSN: Paris, France, 1996; pp. 199–217. [Google Scholar]
- Mirzoyeva, N.Y.; Egorov, V.N.; Polikarpov, G.G. Distribution and migration of 90Sr in components of the Dnieper River basin and the Black Sea ecosystems after the Chernobyl NPP accident. J. Environ. Radioact. 2013, 125, 27–35. [Google Scholar] [CrossRef] [PubMed]
- Sahoo, S.K.; Kavasi, N.; Sorimachi, A.; Arae, H.; Tokonami, S.; Mietelski, J.W.; Lokas, E.; Yoshida, S. Strontium-90 activity concentration in soil samples from the exclusion zone of the Fukushima daiichi nuclear power plant. Sci. Rep. 2016, 6, 23925. [Google Scholar] [CrossRef]
- Hirose, K.; Povinec, P.P. 90Sr and 137Cs as tracers of oceanic eddies in the sea of Japan/East sea. J. Environ. Radioact. 2020, 216, 106179. [Google Scholar] [CrossRef] [PubMed]
- Koarai, K.; Kino, Y.; Takahashi, A.; Suzuki, T.; Shimizu, Y.; Chiba, M.; Osaka, K.; Sasaki, K.; Urushihara, Y.; Fukuda, T.; et al. 90Sr specific activity of teeth of abandoned cattle after the Fukushima accident—Teeth as an indicator of environmental pollution. J. Environ. Radioact. 2018, 183, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Hirose, K.; Povinec, P.P. 137Cs and 90Sr in surface waters of the Sea of Japan: Variations and the Fukushima Dai-ichi Nuclear Power Plant accident impact. Mar. Pollut. Bull. 2019, 146, 645–652. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Ninomiya, K.; Takahashi, N.; Saito, T.; Kita, K. Rapid isolation method for radioactive strontium using Empore Strontium Rad Disk. J. Nucl. Radiochem. Sci. 2016, 16, 15–21. [Google Scholar] [CrossRef]
- Aarkrog, A. Input of anthropogenic radionuclides into the World Ocean. Deep Sea Res. Part II Top. Stud. Oceanogr. 2003, 50, 2597–2606. [Google Scholar] [CrossRef]
- Ganzha, C.; Gudkov, D.; Ganzha, D.; Klenus, V.; Nazarov, A. Physicochemical forms of 90Sr and 137Cs in components of Glyboke Lake ecosystem in the Chornobyl exclusion zone. J. Environ. Radioact. 2014, 127, 176–181. [Google Scholar] [CrossRef]
- Povinec, P.P.; Aarkrog, A.; Buesseler, K.O.; Delfanti, R.; Hirose, K.; Hong, G.H.; Ito, T.; Livingston, H.D.; Nies, H.; Noshkin, V.E.; et al. 90Sr, 137Cs and 239,240Pu concentration surface water time series in the Pacific and Indian Oceans—WOMARS results. J. Environ. Radioact. 2005, 81, 63–87. [Google Scholar] [CrossRef]
- Li, W.B.; Hollriegl, V.; Roth, P.; Oeh, U. Influence of human biokinetics of strontium on internal ingestion dose of 90Sr and absorbed dose of 89Sr to organs and metastases. Radiat. Environ. Biophys. 2008, 47, 225–239. [Google Scholar] [CrossRef] [PubMed]
- Agency for Toxic Substances and Disease Registry; National Center for Environmental Health. Public Health Statement for Strontium. 2008. Available online: http://editors.eol.org/eoearth/wiki/Public_Health_Statement_for_Strontium (accessed on 10 June 2022).
- United Nations Scientific Committee on the Effects of Atomic Radiation. Ionizing Radiation: Sources and Effects of Ionizing Radiation; United Nations: New York, NY, USA, 1977. [Google Scholar]
- Vennart, M.C.T.J. The toxicity of 90Sr, 226Ra and 239Pu. Nature 1976, 263, 555–558. [Google Scholar]
- Vajda, N.; Kim, C.K. Determination of radiostrontium isotopes: A review of analytical methodology. Appl. Radiat. Isot. 2010, 68, 2306–2326. [Google Scholar] [CrossRef] [PubMed]
- Brun, S.; Bessac, S.; Uridat, D.; Boursier, B. Rapid method for the determination of radiostrontium in milk. J. Radioanal. Nucl. Chem. 2002, 253, 191–197. [Google Scholar] [CrossRef]
- Jabbar, T.; Khan, K.; Subhani, M.S.; Akhter, P. Determination of 90Sr in environment of district Swat, Pakistan. J. Radioanal. Nucl. Chem. 2009, 279, 377–384. [Google Scholar] [CrossRef]
- Amr, M.A.; Helal, A.I.; Al-Kinani, A.T.; Balakrishnan, P. Ultra-trace determination of 90Sr, 137Cs, 238Pu, 239Pu, and 240Pu by triple quadruple collision/reaction cell-ICP-MS/MS: Establishing a baseline for global fallout in Qatar soil and sediments. J. Environ. Radioact. 2016, 153, 73–87. [Google Scholar] [CrossRef]
- Takagai, Y.; Furukawa, M.; Kameo, Y.; Suzuki, K. Sequential inductively coupled plasma quadrupole mass-spectrometric quantification of radioactive strontium-90 incorporating cascade separation steps for radioactive contamination rapid survey. Anal. Methods 2014, 6, 355–362. [Google Scholar] [CrossRef]
- Habibi, A.; Cariou, N.; Boulet, B.; Cossonnet, C.; Gurriaran, R.; Gleizes, M.; Cote, G.; Larivière, D. Automated chromatographic separation coupled on-line to ICP-MS measurements for the quantification of actinides and radiostrontium in soil samples. J. Radioanal. Nucl. Chem. 2017, 314, 127–139. [Google Scholar] [CrossRef]
- Feuerstein, J.; Boulyga, S.F.; Galler, P.; Stingeder, G.; Prohaska, T. Determination of 90Sr in soil samples using inductively coupled plasma mass spectrometry equipped with dynamic reaction cell (ICP-DRC-MS). J. Environ. Radioact. 2008, 99, 1764–1769. [Google Scholar] [CrossRef]
- Amr, S.H.A.-M.M.A. Ultratrace Determination of Strontium-90 in Environmental Soil Samples from Qatar by Collision/Reaction Cell-Inductively Coupled Plasma Mass Spectrometry (CRC-ICP-MS/MS). In Proceedings of the ASME 2013 15th International Conference on Environmental Remediation and Radioactive Waste Management, Facility Decontamination and Decommissioning; Environmental Remediation; Environmental Management/Public Involvement/Crosscutting Issues/Global Partnering, V002T04A010, Brussels, Belgium, 8–12 September 2013; Volume 2. [Google Scholar] [CrossRef]
- Taylor, V.F.; Evans, R.D.; Cornett, R.J. Determination of 90Sr in contaminated environmental samples by tuneable bandpass dynamic reaction cell ICP-MS. Anal. Bioanal. Chem. 2007, 387, 343–350. [Google Scholar] [CrossRef]
- Dai, X.; Kramer-Tremblay, S. Five-column chromatography separation for simultaneous determination of hard-to-detect radionuclides in water and swipe samples. Anal. Chem. 2014, 86, 5441–5447. [Google Scholar] [CrossRef] [PubMed]
- Reddy, P.J.; Pulhani, V.; Dhole, S.D.; Dahiwale, S.S.; Bhade, S.P.D.; Anilkumar, S.; Singh, R. Development of rapid solvent extraction based radioanalytical technique for estimation of 90Sr in the presence of natural and anthropogenic radionuclides. J. Radioanal. Nucl. Chem. 2017, 314, 359–370. [Google Scholar] [CrossRef]
- Zoriy, M.V.; Ostapczuk, P.; Halicz, L.; Hille, R.; Becker, J.S. Determination of 90Sr and Pu isotopes in contaminated groundwater samples by inductively coupled plasma mass spectrometry. Int. J. Mass Spectrom. 2005, 242, 203–209. [Google Scholar] [CrossRef]
- Kolacinska, K.; Chajduk, E.; Dudek, J.; Samczynski, Z.; Lokas, E.; Bojanowska-Czajka, A.; Trojanowicz, M. Automation of sample processing for ICP-MS determination of 90Sr radionuclide at ppq level for nuclear technology and environmental purposes. Talanta 2017, 169, 216–226. [Google Scholar] [CrossRef] [PubMed]
- Maxwell, S.L.; Culligan, B.K.; Utsey, R.C. Rapid determination of radiostrontium in seawater samples. J. Radioanal. Nucl. Chem. 2013, 298, 867–875. [Google Scholar] [CrossRef][Green Version]
- Sakama, M.; Nagano, Y.; Saze, T.; Higaki, S.; Kitade, T.; Izawa, N.; Shikino, O.; Nakayama, S. Application of ICP-DRC-MS to screening test of strontium and plutonium in environmental samples at Fukushima. Appl. Radiat. Isot. 2013, 81, 201–207. [Google Scholar] [CrossRef]
- Guerin, N.; Riopel, R.; Rao, R.; Kramer-Tremblay, S.; Dai, X. An improved method for the rapid determination of 90Sr in cow’s milk. J. Environ. Radioact. 2017, 175–176, 115–119. [Google Scholar] [CrossRef]
- Tenjović, B.; Stojković, I.; Nikolov, J.; Todorović, N.; Spasojević, J.; Agbaba, J.; Pajić, M.; Krmar, M. 90Sr/90Y determination in milk by Cherenkov radiation after microwave digestion. J. Radioanal. Nucl. Chem. 2019, 320, 679–687. [Google Scholar] [CrossRef]
- Stamoulis, K.C.; Ioannides, K.G.; Karamanis, D.T.; Patiris, D.C. Rapid screening of 90Sr activity in water and milk samples using Cherenkov radiation. J. Environ. Radioact. 2007, 93, 144–156. [Google Scholar] [CrossRef]
- Kim, C.K.; Al-Hamwi, A.; Torvenyi, A.; Kis-Benedek, G.; Sansone, U. Validation of rapid methods for the determination of radiostrontium in milk. Appl. Radiat. Isot. 2009, 67, 786–793. [Google Scholar] [CrossRef]
- Kabai, E.; Hornung, L.; Savkin, B.T.; Poppitz-Spuhler, A.; Hiersche, L. Fast method and ultra fast screening for determination of 90Sr in milk and dairy products. Sci. Total Environ. 2011, 410–411, 235–240. [Google Scholar] [CrossRef]
- Kabai, E.; Savkin, B.; Mehlsam, I.; Poppitz-Spuhler, A. Combined method for the fast determination of pure beta emitting radioisotopes in food samples. J. Radioanal. Nucl. Chem. 2016, 311, 1401–1408. [Google Scholar] [CrossRef]
- Amano, H.; Sakamoto, H.; Shiga, N.; Suzuki, K. Method for rapid screening analysis of Sr-90 in edible plant samples collected near Fukushima, Japan. Appl. Radiat. Isot. 2016, 112, 131–135. [Google Scholar] [CrossRef] [PubMed]
- Kong, X.; Dang, L.; Shao, X.; Yin, L.; Ji, Y. Rapid method for determination of 90Sr in biological samples by liquid scintillation counting after separation on synthesized column. J. Environ. Radioact. 2018, 193–194, 15–19. [Google Scholar] [CrossRef] [PubMed]
- Dulanská, S.; Remenec, B.; Mátel, Ľ.; Darážová, Ľ.; Galanda, D. Determination of 90Sr in bone samples using molecular recognition technology product AnaLig®Sr-01. J. Radioanal. Nucl. Chem. 2016, 311, 29–33. [Google Scholar] [CrossRef]
- Altzitzoglou, T.; Larosa, J.J.; Nicholl, C. Measurement of 90Sr in Bone Ash. Appl. Radiat. Isot. 1998, 49, 1313–1317. [Google Scholar] [CrossRef]
- Sadi, B.B.; Fontaine, A.; McAlister, D.; Li, C. Emergency Radiobioassay Method for Determination of 90Sr and 226Ra in a Spot Urine Sample. Anal. Chem. 2015, 87, 7931–7937. [Google Scholar] [CrossRef] [PubMed]
- Hawkins, C.A.; Shkrob, I.A.; Mertz, C.J.; Dietz, M.L.; Kaminski, M.D. Novel tandem column method for the rapid isolation of radiostrontium from human urine. Anal. Chim. Acta 2012, 746, 114–122. [Google Scholar] [CrossRef]
- Vonderheide, A.P.; Zoriy, M.V.; Izmer, A.V.; Pickhardt, C.; Caruso, J.A.; Ostapczuk, P.; Hille, R.; Becker, J.S. Determination of 90Sr at ultratrace levels in urine by ICP-MS. J. Anal. At. Spectrom. 2004, 19, 675–680. [Google Scholar] [CrossRef][Green Version]
- Dulanská, S.; Remenec, B.; Bilohuštin, J.; Labaška, M.; Galanda, D. Rapid determination of 90Sr in urine samples using AnaLig® Sr-01. J. Radioanal. Nucl. Chem. 2012, 295, 2189–2192. [Google Scholar] [CrossRef]
- Tomita, J.; Takeuchi, E. Rapid analytical method of 90Sr in urine sample: Rapid separation of Sr by phosphate co-precipitation and extraction chromatography, followed by determination by triple quadrupole inductively coupled plasma mass spectrometry (ICP-MS/MS). Appl. Radiat. Isot. 2019, 150, 103–109. [Google Scholar] [CrossRef] [PubMed]
- Qiao, J.; Nielsen, S. Radionuclide Monitoring. In Reference Module in Chemistry, Molecular Sciences and Chemical Engineering; Elsevier: Amsterdam, The Netherlands, 2018. [Google Scholar] [CrossRef]
- Sajeniouk, A.D. Routine radiochemical method for the determination of 90Sr, 238Pu, 239+240Pu, 241Am and 244Cm in environmental samples. J. Radioanal. Nucl. Chem. 2005, 264, 337–342. [Google Scholar] [CrossRef]
- Wang, J.J.; Wang, C.J.; Huang, C.C.; Lin, Y.M. Transfer Factors of 90Sr and 137Cs from Paddy Soil to the Rice Plant in Taiwan. J. Environ. Radioact. 1998, 39, 23–34. [Google Scholar] [CrossRef]
- Amano, H.; Arkhipov, T.U.A.; Paskevich, S.; Onuma, Y. Transfer of Long Lived Radionuclides in Chernobyl Soils to Edible Plants. IRPA-10. 2000, pp. 1–6. Available online: https://www.irpa.net/irpa10/cdrom/01059.pdf (accessed on 10 April 2022).
- Maxwell, S.L.; Culligan, B.K.; Shaw, P.J. Rapid determination of radiostrontium in large soil samples. J. Radioanal. Nucl. Chem. 2012, 295, 965–971. [Google Scholar] [CrossRef]
- Grahek, Ž.; Košutić, K.; Rožmarić-Mačefat, M. Strontium isolation from natural samples with Sr resin and subsequent determination of 90Sr. J. Radioanal. Nucl. Chem. 2006, 268, 179–190. [Google Scholar] [CrossRef]
- Bossew, P.; Lettner, H.; Hubmer, A.; Erlinger, C.; Gastberger, M. Activity ratios of 137Cs, 90Sr and 239+240Pu in environmental samples. J. Environ. Radioact. 2007, 97, 5–19. [Google Scholar] [CrossRef] [PubMed]
- Roane, J.E.; DeVol, T.A.; Leyba, J.D.; Fjeld, R.A. The use of extraction chromatography resins to concentrate actinides and strontium from soil for radiochromatographic analyses. J. Environ. Radioact. 2003, 66, 227–245. [Google Scholar] [CrossRef]
- Kavasi, N.; Sahoo, S.K.; Sorimachi, A.; Tokonami, S.; Aono, T.; Yoshida, S. Measurement of 90Sr in soil samples affected by the Fukushima Daiichi Nuclear Power Plant accident. J. Radioanal. Nucl. Chem. 2015, 303, 2565–2570. [Google Scholar] [CrossRef]
- Bermejo-Barrera, P.; Moreda-Piñeiro, A.; Bermejo-Barrera, A. Sample pre-treatment methods for the trace elements determination in seafood products by atomic absorption spectrometry. Talanta 2001, 57, 969–984. [Google Scholar] [CrossRef]
- Torres, J.M.; Tent, J.; Llaurado, M.; Rauret, G. A rapid method for 90Sr determination in the presence of 137Cs in environmental samples. J. Environ. Radioact. 2002, 59, 113–125. [Google Scholar] [CrossRef]
- Dion, M.P.; Springer, K.W.E.; Sumner, R.I.; Thomas, M.-L.P.; Eiden, G.C. Analytical determination of radioactive strontium and cesium by Thermal Ionization Mass Spectrometry. Int. J. Mass Spectrom. 2020, 449, 116273. [Google Scholar] [CrossRef]
- Russell, B.C.; Croudace, I.W.; Warwick, P.E.; Milton, J.A. Determination of precise 135Cs/137Cs ratio in environmental samples using sector field inductively coupled plasma mass spectrometry. Anal. Chem. 2014, 86, 8719–8726. [Google Scholar] [CrossRef] [PubMed]
- Jurecic, S.; Benedik, L.; Planinsek, P.; Necemer, M.; Kump, P.; Pihlar, B. Analysis of uranium in the insoluble residues after decomposition of soil samples by various techniques. Appl. Radiat. Isot. 2014, 87, 61–65. [Google Scholar] [CrossRef] [PubMed]
- Maxwell, S.L.; Culligan, B.; Hutchison, J.B.; Utsey, R.C.; Sudowe, R.; McAlister, D.R. Rapid method to determine 89Sr/90Sr in large concrete samples. J. Radioanal. Nucl. Chem. 2016, 310, 399–411. [Google Scholar] [CrossRef]
- Taylor, V.F.; Evans, R.D.; Cornett, R.J. Preliminary evaluation of 135Cs/137Cs as a forensic tool for identifying source of radioactive contamination. J. Environ. Radioact. 2008, 99, 109–118. [Google Scholar] [CrossRef]
- Zhang, Z.; Igarashi, J.; Satou, Y.; Ninomiya, K.; Sueki, K.; Shinohara, A. Activity of 90Sr in Fallout Particles Collected in the Difficult-to-Return Zone around the Fukushima Daiichi Nuclear Power Plant. Environ. Sci. Technol. 2019, 53, 5868–5876. [Google Scholar] [CrossRef]
- Povinec, P.P.; Froehlich, K.; Gastaud, J.; Oregioni, B.; Pagava, S.V.; Pham, M.K.; Rusetski, V. Distribution of 90Sr, 137Cs and 239,240Pu in Caspian Sea water and biota. Deep Sea Res. Part II Top. Stud. Oceanogr. 2003, 50, 2835–2846. [Google Scholar] [CrossRef]
- Gaca, P.; Mietelski, J.W.; Kitowski, I.; Grabowska, S.; Tomankiewicz, E. 40K, 137Cs, 90Sr, 238,239+240Pu and 241Am in mammals’ skulls from owls’ pellets and owl skeletons in Poland. J. Environ. Radioact. 2005, 78, 93–103. [Google Scholar] [CrossRef]
- Lee, S.H.; Oh, J.S.; Lee, J.M.; Lee, K.B.; Park, T.S.; Lee, M.K.; Kim, S.H.; Choi, J.K. A preliminary study for the development of reference material using oyster for determination of 137Cs, 90Sr and plutonium isotopes. Appl. Radiat. Isot. 2016, 109, 109–113. [Google Scholar] [CrossRef]
- Sugiyama, H.; Terada, H.; Takahashi, M.; Iijima, I.; Isomura, K. Contents and Daily Intakes of Gamma-Ray Emitting Nuclides, 90Sr, and 238U using Market-Basket Studies in Japan. J. Health Sci. 2007, 53, 107–118. [Google Scholar] [CrossRef][Green Version]
- Brun, S.P.; Kergadallan, Y.; Boursier, B.; Fremy, J.-M.; Janin, F.O. Methodology for determination of radiostrontium in milk: A review. Lait 2003, 83, 1–15. [Google Scholar] [CrossRef]
- Puhakainen, M.; Heikkinen, T.; Rahola, T. Levels of 90Sr and 137Cs in the urine of Finnish people. Radiat. Prot. Dosim. 2003, 103, 255–262. [Google Scholar] [CrossRef] [PubMed]
- Navaneethan, U.; Parsi, M.A.; Lourdusamy, D.; Grove, D.; Sanaka, M.R.; Hammel, J.P.; Vargo, J.J.; Dweik, R.A. Volatile Organic Compounds in Urine for Noninvasive Diagnosis of Malignant Biliary Strictures: A Pilot Study. Dig. Dis. Sci. 2015, 60, 2150–2157. [Google Scholar] [CrossRef] [PubMed]
- Balarama Krishna, M.V.; Chandrasekaran, K.; Venkateswarlu, G.; Karunasagar, D. A cost-effective and rapid microwave-assisted acid extraction method for the multi-elemental analysis of sediments by ICP-AES and ICP-MS. Anal. Methods 2012, 4, 3290–3299. [Google Scholar] [CrossRef]
- Vesper, H.W.; Mi, L.; Enada, A.; Myers, G.L. Assessment of microwave-assisted enzymatic digestion by measuring glycated hemoglobin A1c by mass spectrometry. Rapid Commun. Mass Spectrom. 2005, 19, 2865–2870. [Google Scholar] [CrossRef]
- Alvarez, A.; Navarro, N. Method for actinides and Sr-90 determination in urine samples. Appl. Radiat. Isot. 1996, 47, 869–873. [Google Scholar] [CrossRef]
- Chu, T.C.; Wang, J.J.; Lin, Y.M. Radiostrontium Analytical Method using Crown-ether Compound and Cerenkov Counting and Its Applications in Environmental Monitoring. Appl. Radiat. Isot. 1998, 49, 1313–1317. [Google Scholar] [CrossRef]
- ISO 18589-5:2019; Measurement of Radioactivity in the Environment-Soil—Part 5: Strontium 90—Test Method Using Proportional Counting or Liquid Scintillation Counting. International Standard Organization: Geneva, Switzerland, 2019.
- ISO 13160:2012; Water Quality—Strontium 90 and Strontium 89—Test Methods Using Liquid Scintillation Counting or Proportional Counting. International Standard Organization: Geneva, Switzerland, 2012.
- HJ 815-2016; Radiochemical Analysis of Strontium-90 in Water and Ash of Biological Samples, Chinese Standard. Standard Press of China: Beijing, China, 2016.
- GB 14883.3-2016; Determination of Radioactive Substances Strontium-89 and Strontium-90 in Food, Chinese Standard. Standard Press of China: Beijing, China, 2016.
- Borcherding, J.; Nies, H. An improved method fo the determination of 90Sr in large samples of seawater. J. Radioanal. Nucl. Chem. 1986, 98, 127–131. [Google Scholar] [CrossRef]
- Pedersen, C.J. Crystalline Salt Complexes of Macrocyclic Polyethers. J. Am. Chem. Soc. 1969, 92, 386–391. [Google Scholar] [CrossRef]
- Vaney, B.; Friedli, C.; Geering, J.J.; Lerch, P. Rapid trace determination of radiostrontium in milk and drinking water. J. Radioanal. Nucl. Chem. 1989, 134, 87–95. [Google Scholar] [CrossRef]
- Tormos, J.; Jouve, A.; Revy, A.D.; Millan-Gomez, H.R.; Erarioa, M.J. Rapid Method for Determining Strontium-90 Contaminated Samples of Soil and Plant. J. Environ. Radioact. 1995, 27, 193–206. [Google Scholar] [CrossRef]
- Castrillejo, M.; Casacuberta, N.; Breier, C.F.; Pike, S.M.; Masque, P.; Buesseler, K.O. Reassessment of 90Sr, 137Csand 134Cs in the Coast off Japan Derived from the Fukushima Dai-ichi Nuclear Accident. Environ. Sci. Technol. 2016, 50, 173–180. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, D.M.; Moody, W.A.; Williamson, J.A. A Simple Method to Screen for Radiostrontium in Water by Ion Exchange Chromatography. Health Phys. 2019, 116, 771–775. [Google Scholar] [CrossRef] [PubMed]
- Horwitz, E.P.; Dietz, M.L.; Fisher, D.E. Correlation of the Extraction of Strontium Nitrate by a Crown Ether with the Water Content of the Organic Phase. Solvent Extr. Ion Exch. 1990, 8, 199–208. [Google Scholar] [CrossRef]
- Grahek, Z.; Dulanska, S.; Karanovic, G.; Coha, I.; Tucakovic, I.; Nodilo, M.; Matel, L. Comparison of different methodologies for the 90Sr determination in environmental samples. J. Environ. Radioact. 2018, 181, 18–31. [Google Scholar] [CrossRef] [PubMed]
- Pan, B.; Pan, B.; Zhang, W.; Lv, L.; Zhang, Q.; Zheng, S. Development of polymeric and polymer-based hybrid adsorbents for pollutants removal from waters. Chem. Eng. J. 2009, 151, 19–29. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, Y. Concurrent removal of Cu(II), Co(II) and Ni(II) from wastewater by nanostructured layered sodium vanadosilicate: Competitive adsorption kinetics and mechanisms. J. Environ. Chem. Eng. 2021, 9, 105945. [Google Scholar] [CrossRef]
- Zhang, N.; Ishag, A.; Li, Y.; Wang, H.; Guo, H.; Mei, P.; Meng, Q.; Sun, Y. Recent investigations and progress in environmental remediation by using covalent organic framework-based adsorption method: A review. J. Clean. Prod. 2020, 277, 123360. [Google Scholar] [CrossRef]
- Manos, M.J.; Kanatzidis, M.G. Metal sulfide ion exchangers: Superior sorbents for the capture of toxic and nuclear waste-related metal ions. Chem. Sci. 2016, 7, 4804–4824. [Google Scholar] [CrossRef]
- Zhang, Z.; Gu, P.; Zhang, M.; Yan, S.; Dong, L.; Zhang, G. Synthesis of a robust layered metal sulfide for rapid and effective removal of Sr2+ from aqueous solutions. Chem. Eng. J. 2019, 372, 1205–1215. [Google Scholar] [CrossRef]
- Liu, C.; Wu, T.; Hsu, P.C.; Xie, J.; Zhao, J.; Liu, K.; Sun, J.; Xu, J.; Tang, J.; Ye, Z.; et al. Direct/Alternating Current Electrochemical Method for Removing and Recovering Heavy Metal from Water Using Graphene Oxide Electrode. ACS Nano 2019, 13, 6431–6437. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Zhou, L.; Zhang, L.; Zhang, S.; Chen, F.; Zhou, R.; Hua, D. Two-Dimensional Imprinting Strategy to Create Specific Nanotrap for Selective Uranium Adsorption with Ultrahigh Capacity. ACS Appl. Mater. Interfaces 2022, 14, 9408–9417. [Google Scholar] [CrossRef] [PubMed]
- Hojatpanah, M.R.; Khanmohammadi, A.; Khoshsafar, H.; Hajian, A.; Bagheri, H. Construction and application of a novel electrochemical sensor for trace determination of uranium based on ion-imprinted polymers modified glassy carbon electrode. Chemosphere 2022, 292, 133435. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Xu, M.; Yin, J.; Shui, R.; Yang, S.; Hua, D. Dual Ion-Imprinted Mesoporous Silica for Selective Adsorption of U(VI) and Cs(I) through Multiple Interactions. ACS Appl. Mater. Interfaces 2021, 13, 6322–6330. [Google Scholar] [CrossRef]
- Lee, H.-K.; Choi, J.-W.; Choi, S.-J. Magnetic ion-imprinted polymer based on mesoporous silica for selective removal of Co(II) from radioactive wastewater. Sep. Sci. Technol. 2020, 56, 1842–1852. [Google Scholar] [CrossRef]
- Fattahi, M.; Ezzatzadeh, E.; Jalilian, R.; Taheri, A. Micro solid phase extraction of cadmium and lead on a new ion-imprinted hierarchical mesoporous polymer via dual-template method in river water and fish muscles: Optimization by experimental design. J. Hazard. Mater. 2021, 403, 123716. [Google Scholar] [CrossRef]
- Yin, J.; Yang, S.; He, W.; Zhao, T.; Li, C.; Hua, D. Biogene-derived aerogels for simultaneously selective adsorption of uranium(VI) and strontium(II) by co-imprinting method. Sep. Purif. Technol. 2021, 271, 118849. [Google Scholar] [CrossRef]
- Huang, C.-C.; Siao, S.-F. Removal of copper ions from an aqueous solution containing a chelating agent by electrosorption on mesoporous carbon electrodes. J. Taiwan Inst. Chem. Eng. 2018, 85, 29–39. [Google Scholar] [CrossRef]
- Liu, C.; Hsu, P.-C.; Xie, J.; Zhao, J.; Wu, T.; Wang, H.; Liu, W.; Zhang, J.; Chu, S.; Cui, Y. A half-wave rectified alternating current electrochemical method for uranium extraction from seawater. Nat. Energy 2017, 2, 17007. [Google Scholar] [CrossRef]
- Xiang, S.; Mao, H.; Geng, W.; Xu, Y.; Zhou, H. Selective removal of Sr(II) from saliferous radioactive wastewater by capacitive deionization. J. Hazard. Mater. 2022, 431, 128591. [Google Scholar] [CrossRef]
- Wang, W.; Liu, S.; Zhou, Y.; Luo, J.; Shi, J.; Zhou, Z.; Ma, J. Extraction of Sr2+ from aqueous solutions using an asymmetric pulsed current-assisted electrochemical method. Sep. Purif. Technol. 2021, 276, 119235. [Google Scholar] [CrossRef]
- Sato, Y.; Minemoto, K.; Nemoto, M. Three-dimensional visualization of a beta-emitting nuclide by combining a directional Geiger-Mueller counter and structure from motion. J. Instrum. 2021, 16, 10008. [Google Scholar] [CrossRef]
- Kashirin, I.A.; Ermakov, A.I.; Malinovskiy, S.V.; Belanov, S.V.; Sapozhnikov, Y.A.; Efimov, K.M.; Tikhomirov, V.A.; Sobolev, A.I. Liquid scintillation determination of low level components in complex mixtures of radionuclides. Appl. Radiat. Isot. 2000, 53, 303–308. [Google Scholar] [CrossRef] [PubMed]
- Uesugi, M.; Watanabe, R.; Sakai, H.; Yokoyama, A. Rapid method for determination of 90Sr in seawater by liquid scintillation counting with an extractive scintillator. Talanta 2018, 178, 339–347. [Google Scholar] [CrossRef]
- Todorovic, N.; Stojkovic, I.; Nikolov, J.; Tenjovic, B. 90Sr determination in water samples using Cerenkov radiation. J. Environ. Radioact. 2017, 169–170, 197–202. [Google Scholar] [CrossRef]
- Rondahl, S.H.; Rameback, H. Evaluation of different methods for measuring 89Sr and 90Sr: Measurement uncertainty for the different methods as a function of the activity ratio. Appl. Radiat. Isot. 2018, 140, 87–95. [Google Scholar] [CrossRef]
- Aldave de las Heras, L.; Sandow, M.; Olszewski, G.; Serran-Purroy, M.; van Winckel, S.; Glatz, J.-P. Determination of traces of radionuclides by hyphenated techniques coupled to inductively coupled plasma mass spectrometry (ICP-MS). Rev. Soc. Catalana Quím. 2013, 12, 61–67. [Google Scholar]
- Zheng, J.; Bu, W.; Tagami, K.; Shikamori, Y.; Nakano, K.; Uchida, S.; Ishii, N. Determination of 135Cs and 135Cs/137Cs atomic ratio in environmental samples by combining ammonium molybdophosphate (AMP)-selective Cs adsorption and ion-exchange chromatographic separation to triple-quadrupole inductively coupled plasma-mass spectrometry. Anal. Chem. 2014, 86, 7103–7110. [Google Scholar] [CrossRef]
- Liu, Z.; Zheng, J.; Pan, S.; Dong, W.; Yamada, M.; Aono, T.; Guo, Q. Pu and 137Cs in the Yangtze River estuary sediments: Distribution and source identification. Environ. Sci. Technol. 2011, 45, 1805–1811. [Google Scholar] [CrossRef]
- Bu, W.; Zheng, J.; Liu, X.; Long, K.; Hu, S.; Uchida, S. Mass spectrometry for the determination of fission products 135Cs, 137Cs and 90Sr: A review of methodology and applications. Spectrochim. Acta Part B At. Spectrosc. 2016, 119, 65–75. [Google Scholar] [CrossRef]
- Hou, X.; Dai, X. Environmental liquid scintillation analysis. In Handbook of Radioactivity Analysis; Academic Press: Cambridge, MA, USA, 2020; Volume 2, pp. 41–136. [Google Scholar]
- Amr, M.A.; Abdel-Lateef, A.M. Comparing the capability of collision/reaction cell quadrupole and sector field inductively coupled plasma mass spectrometers for interference removal from 90Sr, 137Cs, and 226Ra. Int. J. Mass Spectrom. 2011, 299, 184–190. [Google Scholar] [CrossRef]
- Kluge, H.-J. Resonance ionization mass spectroscopy for nuclear research and trace analysis. Hyperfine Interact. 1987, 37, 347–364. [Google Scholar] [CrossRef][Green Version]
- Cheon, D.; Iwata, Y.; Miyabe, M.; Hasegawa, S. Development of Bandpass Filtered External Cavity Diode Laser System for RIMS of Radioactive Strontium Isotopes. In Proceedings of the Second International Symposium on Radiation Detectors and Their Uses (ISRD2018), Tsukuba, Japan, 23–26 January 2018. [Google Scholar] [CrossRef][Green Version]
- Bushaw, B.A.; Cannon, B.D. Diode laser based resonance ionization mass spectrometric measurement of strontium-90. Spectrochim. Acta Part B At. Spectrosc. 1997, 52, 1839–1854. [Google Scholar] [CrossRef]
- Steeb, J.L.; Graczyk, D.G.; Tsai, Y.; Mertz, C.J.; Essling, A.M.; Sullivan, V.S.; Carney, K.P.; Finck, M.R.; Giglio, J.J.; Chamberlain, D.B. Application of mass spectrometric isotope dilution methodology for 90Sr age-dating with measurements by thermal-ionization and inductively coupled-plasma mass spectrometry. J. Anal. At. Spectrom. 2013, 28, 1493–1507. [Google Scholar] [CrossRef]
- Paul, M.; Berkovits, D.; Cecil, L.D.; Feldstein, H.; Hershkowitz, A.; Kashiv, Y.; Vogt, S. Environmental 90Sr measurements. Nucl. Instrum. Methods Phys. Res. Sect. B Beam Interact. Mater. At. 1997, 123, 394–399. [Google Scholar] [CrossRef]
- Sasa, K.; Honda, M.; Hosoya, S.; Takahashi, T.; Takano, K.; Ochiai, Y.; Sakaguchi, A.; Kurita, S.; Satou, Y.; Sueki, K. A sensitive method for Sr-90 analysis by accelerator mass spectrometry. J. Nucl. Sci. Technol. 2020, 58, 72–79. [Google Scholar] [CrossRef]
- Satou, Y.; Sueki, K.; Sasa, K.; Matsunaka, T.; Takahashi, T.; Shibayama, N.; Izumi, D.; Kinoshita, N.; Matsuzaki, H. Technological developments for strontium-90 determination using AMS. Nucl. Instrum. Methods Phys. Res. Sect. B Beam Interact. Mater. At. 2015, 361, 233–236. [Google Scholar] [CrossRef]
- Hain, K.; Martschini, M.; Gülce, F.; Honda, M.; Lachner, J.; Kern, M.; Pitters, J.; Quinto, F.; Sakaguchi, A.; Steier, F.; et al. Developing Accelerator Mass Spectrometry Capabilities for Anthropogenic Radionuclide Analysis to Extend the Set of Oceanographic Tracers. Front. Mar. Sci. 2022, 9, 837515. [Google Scholar] [CrossRef]
- De Regge, P.; Radecki, Z.; Moreno, J.; Burns, K.; Kis-Benedek, G.; Bojanowski, R. The IAEA proficiency test on evaluation of methods for 90Sr measurement in a mineral matrix. J. Radioanal. Nucl. Chem. 2000, 246, 511–519. [Google Scholar] [CrossRef]
- Cheng, Q.; Jiang, S.; Wen, D.; Ma, J. Evaluation of Uncertainty for Measurement Result of 90Sr in Environmental Soil Samples. Sichuan Environ. 2009, 28, 22–25. [Google Scholar]
- Jiang, Y. Evaluation of Detection Uncertainty of Sr-90 in Water. Adm. Tech. Environ. Monit. 2004, 16, 15–18. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).