Electrically Polarized Withaferin A and Alginate-Incorporated Biphasic Calcium Phosphate Microspheres Exhibit Osteogenicity and Antibacterial Activity In Vitro
Abstract
1. Introduction
2. Results
2.1. X-ray Diffraction Analysis
2.2. Fourier Transform Infrared Spectroscopy
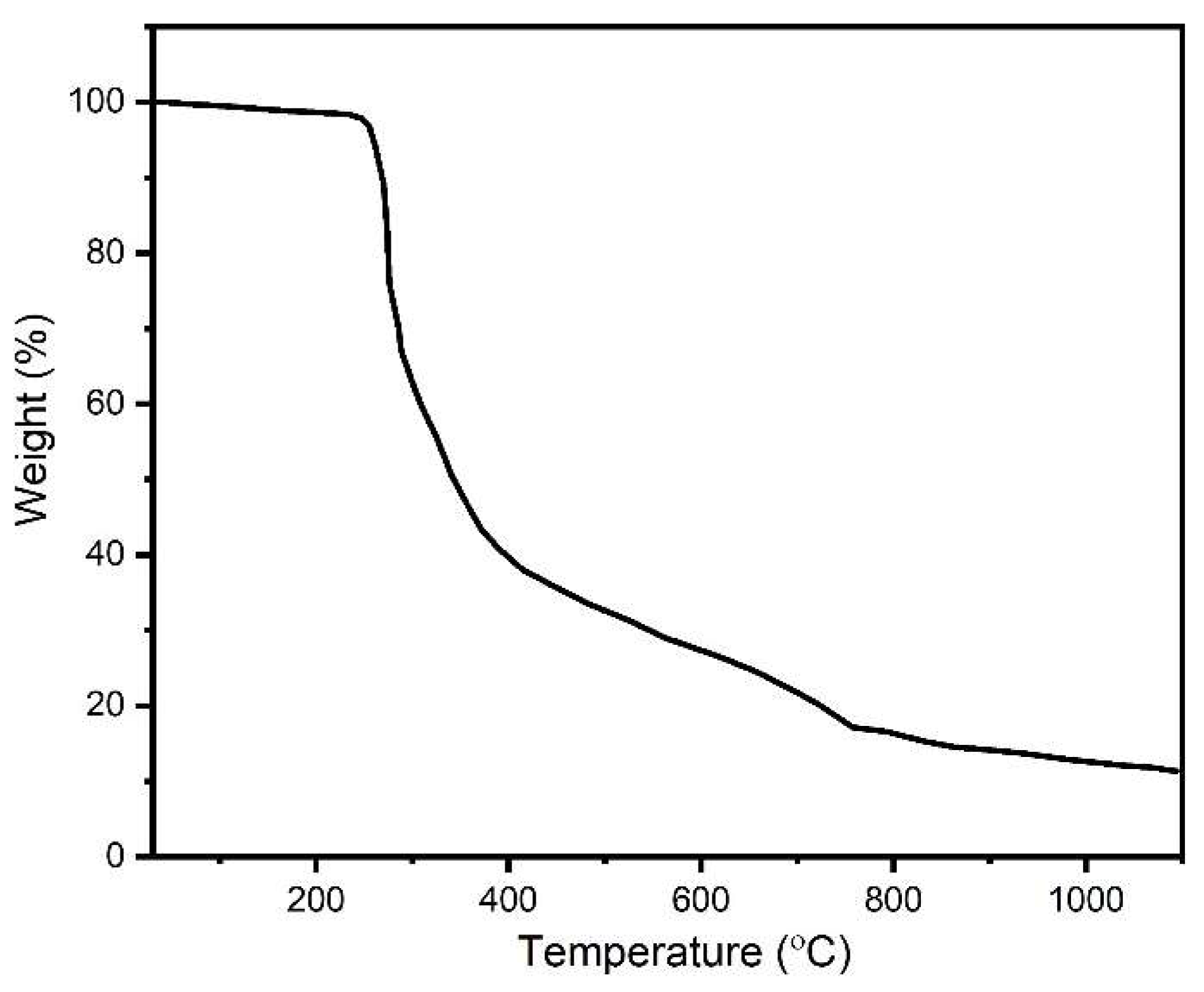
2.3. Thermogravimetric Analysis
2.4. Thermally Stimulated Depolarization Current
2.5. Contact Angle
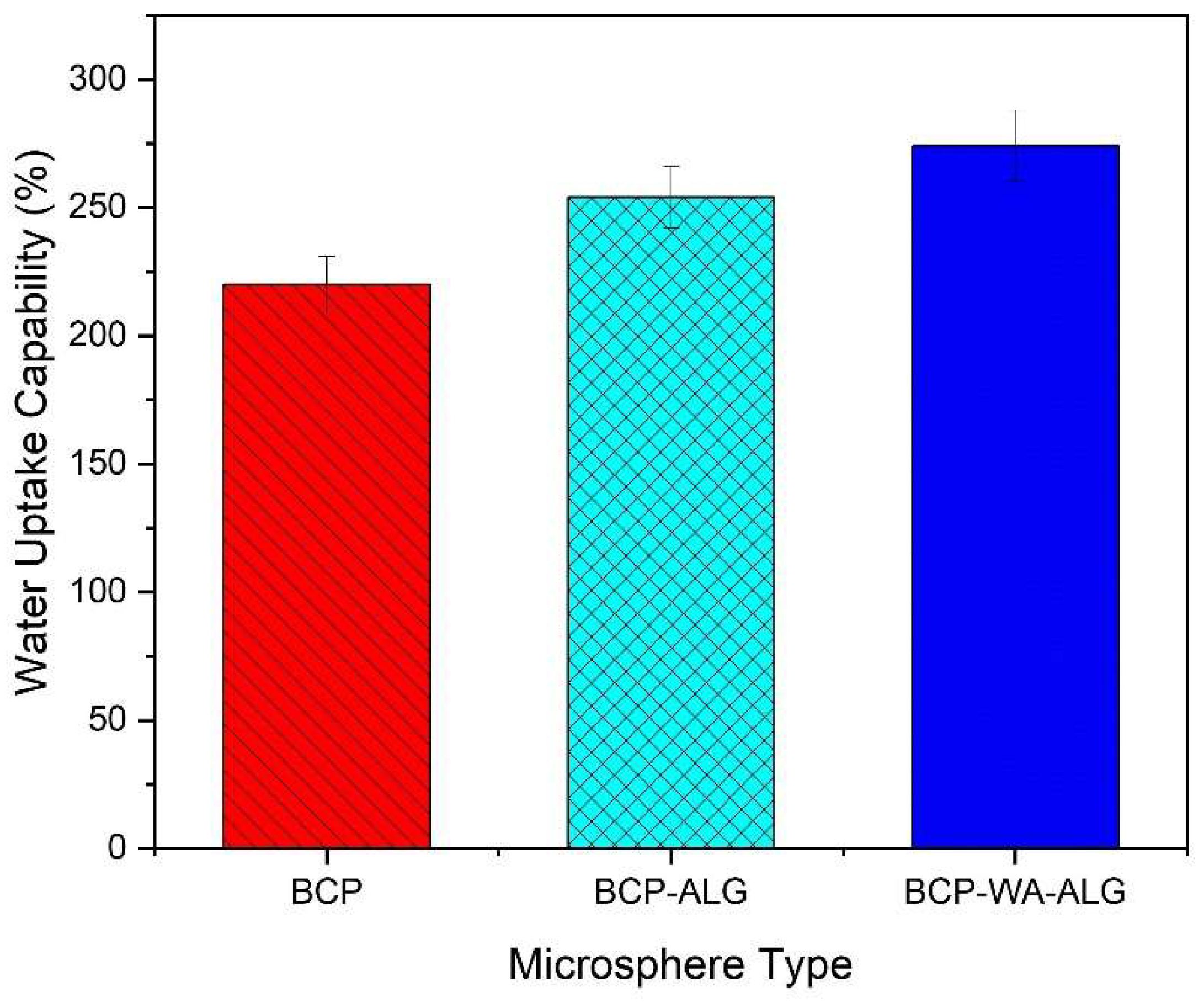
2.6. Swelling Ratio
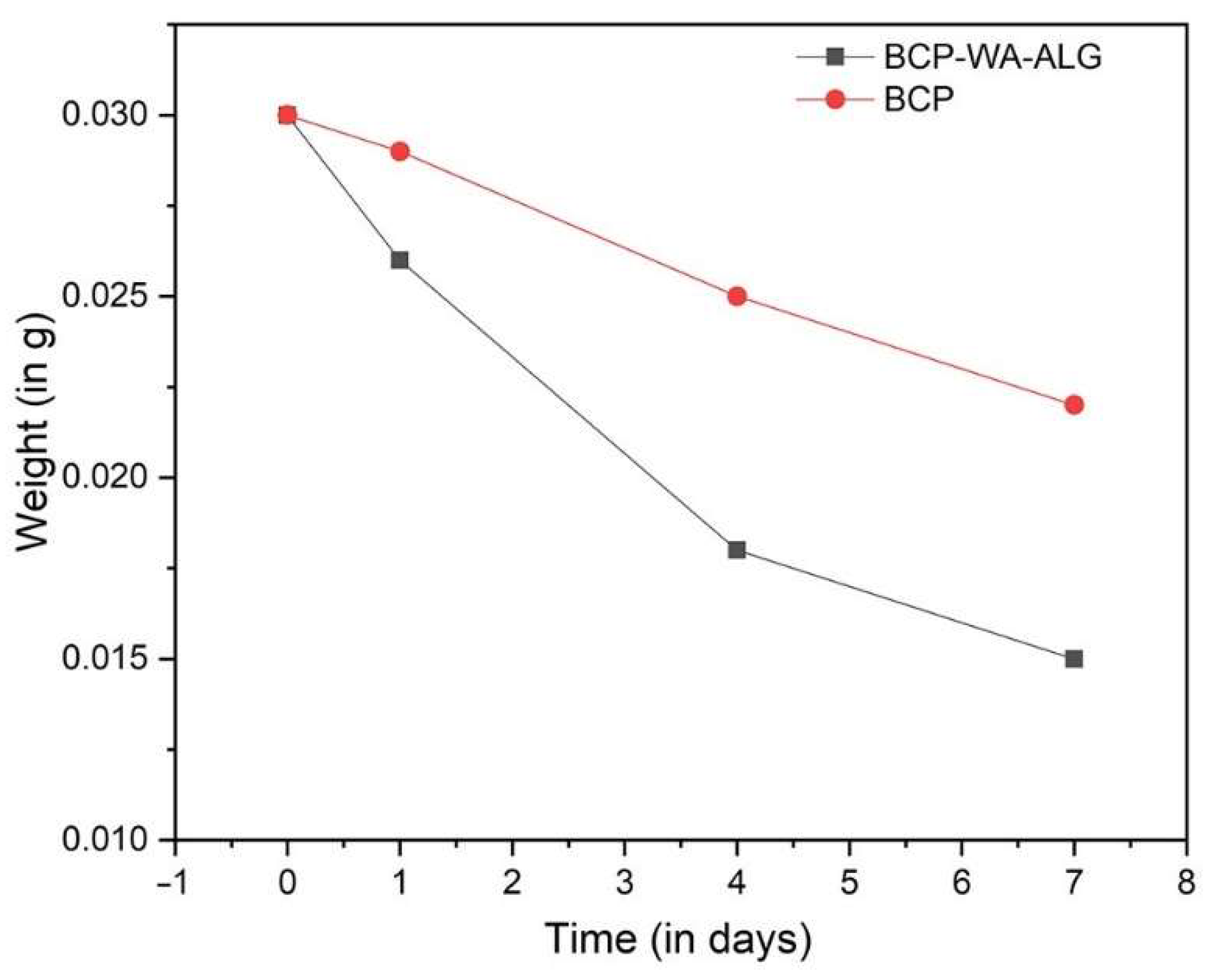
2.7. Degradation
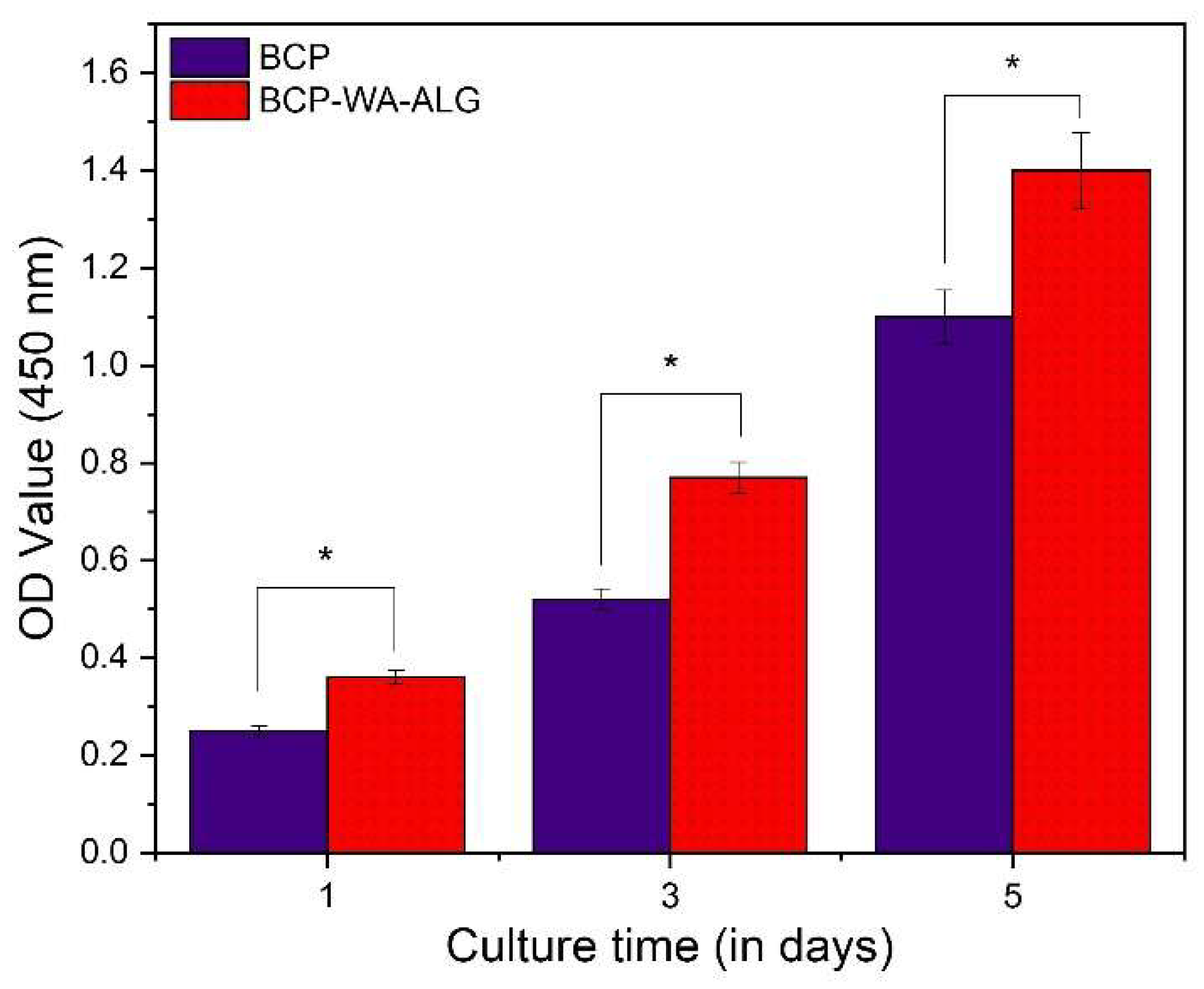
2.8. MTT Assay Study
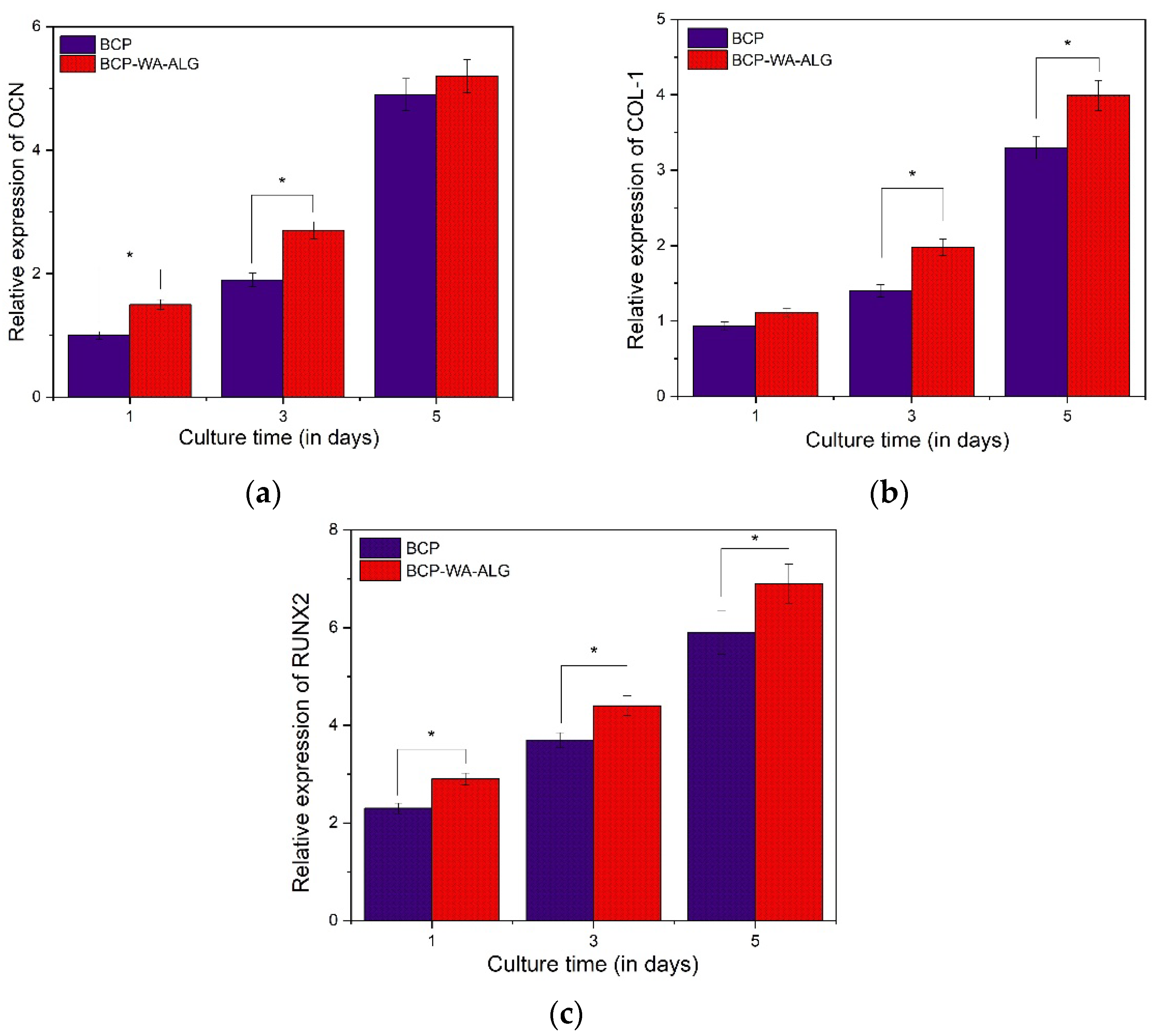
2.9. Osteogenic Expression
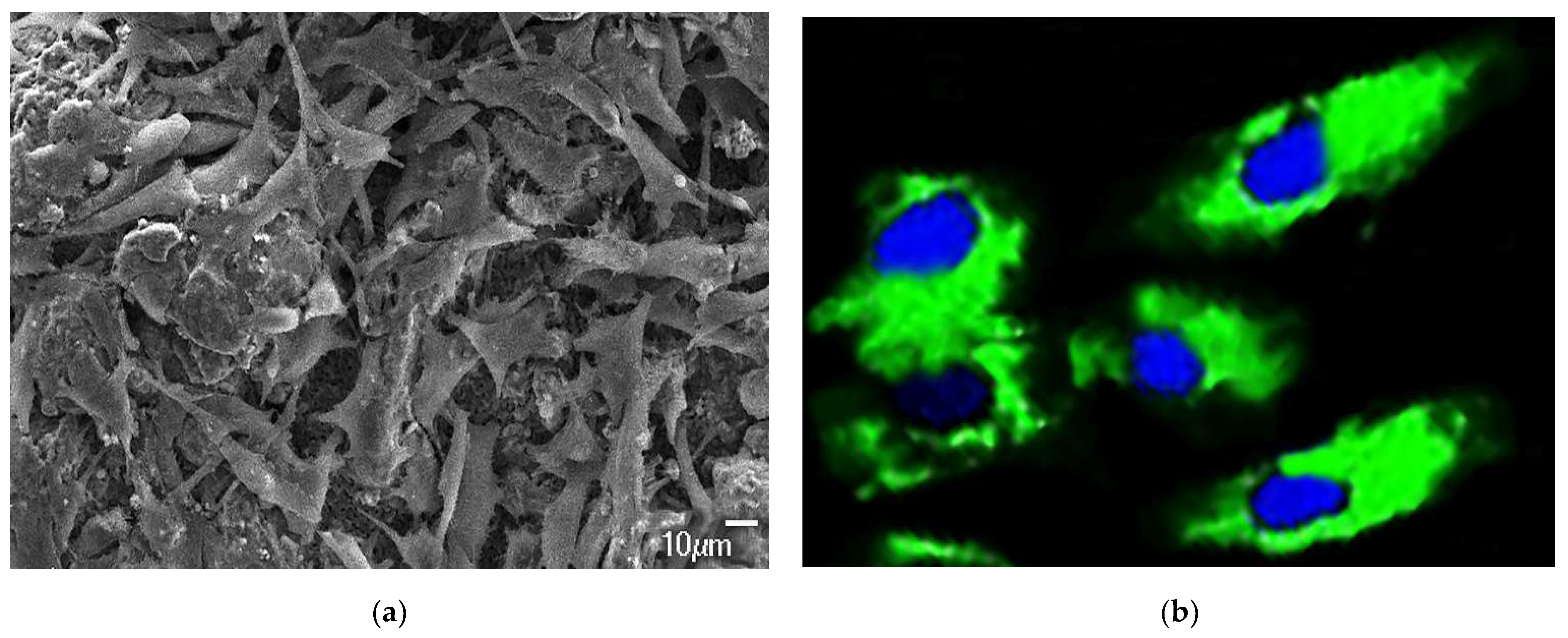
2.10. Cellular Response
3. Discussion
4. Materials and Methods
4.1. Formation of BCP Nanoparticles and Microspheres
4.2. Polarization of the Microspheres
4.3. Characterization of the Microspheres
4.3.1. X-ray Diffraction (XRD)
4.3.2. FTIR Analysis
4.3.3. TGA Study
4.3.4. Swelling Ratio and Degradation Analysis of BCP-WFA-ALG Microspheres
4.3.5. Release Rate
4.3.6. In Vitro Cell Proliferation Testing: MTT Assay
4.3.7. Osteogenesis-Related Gene Expression
4.3.8. Cell Proliferation and Cytoskeletal Response
4.4. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- LeGeros, R.Z. Properties of Osteoconductive Biomaterials: Calcium Phosphates. Clin. Orthop. Relat. Res. 2002, 395, 81–98. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Fan, H.; Zhang, Z.Y.; Lou, A.J.; Pei, G.X.; Jiang, S.; Mu, T.W.; Qin, J.J.; Chen, S.Y.; Jin, D. Osteogenesis and angio-genesis of tissue-engineered bone constructed by prevascularized β-tricalcium phosphate scaffold and mesenchymal stem cells. Biomaterials 2010, 31, 9452–9461. [Google Scholar] [CrossRef] [PubMed]
- Swain, S.; Patra, A.; Kumari, S.; Praharaj, R.; Mishra, S.; Rautray, T. Corona poled gelatin—Magnesium hydroxyapatite composite demonstrates osteogenicity. Mater. Today Proc. 2022, 62, 6131–6135. [Google Scholar] [CrossRef]
- Mohapatra, B.; Rautray, T.R. Strontium-substituted biphasic calcium phosphate scaffold for orthopedic applications. J. Korean Ceram. Soc. 2020, 57, 392–400. [Google Scholar] [CrossRef]
- Roohani-Esfahani, S.; Nouri-Khorasani, S.; Lu, Z.; Appleyard, R.; Zreiqat, H. Effects of bioactive glass nanoparticles on the mechanical and biological behavior of composite coated scaffolds. Acta Biomater. 2011, 7, 1307–1318. [Google Scholar] [CrossRef]
- LeGeros, R.Z.; Lin, S.; Rohanizadeh, R.; Mijares, D.; LeGeros, J.P. Biphasic calcium phosphate bioceramics: Preparation, properties and applications. J. Mater. Sci. Mater. Med. 2003, 14, 201–209. [Google Scholar] [CrossRef]
- Bouler, J.M.; Pilet, P.; Gauthier, O.; Verron, E. Biphasic calcium phosphate ceramics for bone reconstruction: A review of bio-logical response. Acta. Biomater. 2017, 53, 1–12. [Google Scholar] [CrossRef]
- Swain, S.; Mishra, S.; Patra, A.; Praharaj, R.; Rautray, T. Dual action of polarised zinc hydroxyapatite—guar gum composite as a next generation bone filler material. Mater. Today Proc. 2022, 62, 6125–6130. [Google Scholar] [CrossRef]
- Mishra, S.; Rautray, T.R. Silver-incorporated hydroxyapatite–albumin microspheres with bactericidal effects. J. Korean Ceram. Soc. 2020, 57, 175–183. [Google Scholar] [CrossRef]
- Mishra, S.; Rautray, T.R. Fabrication of Xanthan gum-assisted hydroxyapatite microspheres for bone regeneration. Mater. Technol. 2019, 35, 364–371. [Google Scholar] [CrossRef]
- Chen, Y.; Liu, Z.; Jiang, T.; Zou, X.; Lei, L.; Yan, W.; Yang, J.; Li, B. Strontium-substituted biphasic calcium phosphate mi-crospheres promoted degradation performance and enhanced bone regeneration. J. Biomed. Mater. Res. A 2020, 108, 895–905. [Google Scholar] [CrossRef] [PubMed]
- Lima, D.B.; de Souza, M.A.A.; de Lima, G.G.; FerreiraSouto, E.P.; Oliveira, H.M.L.; Fook, M.V.L.; de Sá, M.J.C. Injectable bone substitute based on chitosan with polyethylene glycol polymeric solution and biphasic calcium phosphate microspheres. Car-bohydr. Polym. 2020, 245, 116575. [Google Scholar] [CrossRef] [PubMed]
- Berghe, W.V.; Sabbe, L.; Kaileh, M.; Haegeman, G.; Heyninck, K. Molecular insight in the multifunctional activities of Withaferin A. Biochem. Pharmacol. 2012, 84, 1282–1291. [Google Scholar] [CrossRef] [PubMed]
- Behl, T.; Sharma, A.; Sharma, L.; Sehgal, A.; Zengin, G.; Brata, R.; Fratila, O.; Bungau, S. Exploring the Multifaceted Therapeutic Potential of Withaferin A and Its Derivatives. Biomedicines 2020, 8, 571. [Google Scholar] [CrossRef]
- Mohan, R.; Hammers, H.J.; Bargagna-Mohan, P.; Zhan, X.H.; Herbstritt, C.J.; Ruiz, A.; Zhang, L.; Hanson, A.D.; Conner, B.P.; Rougas, J.; et al. Withaferin A is a potent inhibitor of angiogenesis. Angiogenesis 2004, 7, 115–122. [Google Scholar] [CrossRef]
- Swain, S.; Bowen, C.; Rautray, T. Dual response of osteoblast activity and antibacterial properties of polarized strontium substituted hydroxyapatite—Barium strontium titanate composites with controlled strontium substitution. J. Biomed. Mater. Res. Part A 2021, 109, 2027–2035. [Google Scholar] [CrossRef]
- Mohapatra, B.; Rautray, T.R. Facile fabrication of Luffa cylindrica-assisted 3D hydroxyapatite scaffolds. Bioinspired Biomim. Nanobiomaterials 2021, 10, 37–44. [Google Scholar] [CrossRef]
- Detsch, R.; Mayr, H.; Ziegler, G. Formation of osteoclast-like cells on HA and TCP ceramics. Acta Biomater. 2008, 4, 139–148. [Google Scholar] [CrossRef]
- Kwon, S.-H.; Jun, Y.-K.; Hong, S.-H.; Kim, H.-E. Synthesis and dissolution behavior of β-TCP and HA/β-TCP composite powders. J. Eur. Ceram. Soc. 2003, 23, 1039–1045. [Google Scholar] [CrossRef]
- Victor, S.; Kumar, T.S.S. BCP ceramic microspheres as drug delivery carriers: Synthesis, characterisation and doxycycline release. J. Mater. Sci. Mater. Med. 2007, 19, 283–290. [Google Scholar] [CrossRef]
- Habraken, W.; Habibovic, P.; Epple, M.; Bohner, M. Calcium phosphates in biomedical applications: Materials for the future? Mater. Today 2016, 19, 69–87. [Google Scholar] [CrossRef]
- Khedgikar, V.; Kushwaha, P.; Gautam, J.; Verma, A.; Changkija, B.; Kumar, A.; Sharma, S.; Nagar, G.K.; Singh, D.; Trivedi, P.K.; et al. Withaferin A: A proteasomal inhibitor promotes healing after injury and exerts anabolic effect on osteoporotic bone. Cell Death Dis. 2013, 4, e778. [Google Scholar] [CrossRef] [PubMed]
- Giuliani, N.; Morandi, F.; Tagliaferri, S.; Lazzaretti, M.; Bonomini, S.; Crugnola, M.; Mancini, C.; Martella, E.; Ferrari, L.; Tabilio, A.; et al. The proteasome inhibitor bortezomib affects osteoblast differentiation in vitro and in vivo in multiple myeloma patients. Blood 2007, 110, 334–338. [Google Scholar] [CrossRef] [PubMed]
- Rautray, T.R.; Narayanan, R.; Kim, K.H. Ion implantation of titaniumbased biomaterials. Prog. Mater. Sci. 2011, 56, 1137–1177. [Google Scholar] [CrossRef]
- Behera, D.R.; Nayak, P.; Rautray, T.R. Phosphatidylethanolamine impregnated Zn-HA coated on titanium for enhanced bone growth with antibacterial properties. J. King Saud Univ. Sci. 2019, 32, 848–852. [Google Scholar] [CrossRef]
- Swain, S.; Ong, J.L.; Narayanan, R.; Rautray, T.R. Ti-9Mn β-type alloy exhibits better osteogenicity than Ti-15Mn alloy in vitro. J. Biomed. Mater. Res. Part B Appl. Biomater. 2021, 109, 2154–2161. [Google Scholar] [CrossRef]
- Rautray, T.R.; Mohapatra, B.; Kim, K.-H. Fabrication of Strontium–Hydroxyapatite Scaffolds for Biomedical Applications. Adv. Sci. Lett. 2014, 20, 879–881. [Google Scholar] [CrossRef]
- Swain, S.; Rautray, T.R. Estimation of Trace Elements, Antioxidants, and Antibacterial Agents of Regularly Consumed Indian Medicinal Plants. Biol. Trace Element Res. 2020, 199, 1185–1193. [Google Scholar] [CrossRef]
- Logie, E.; Berghe, W.V. Tackling Chronic Inflammation with Withanolide Phytochemicals—A Withaferin a Perspective. Antioxidants 2020, 9, 1107. [Google Scholar] [CrossRef]
- Swain, S.; Padhy, R.N.; Rautray, T.R. Electrically stimulated hydroxyapatite–barium titanate composites demonstrate im-munocompatibility in vitro. J. Korean. Ceram. Soc. 2020, 57, 495–502. [Google Scholar] [CrossRef]
- Swain, S.; Padhy, R.N.; Rautray, T.R. Polarized piezoelectric bioceramic composites exhibit antibacterial activity. Mater. Chem. Phys. 2019, 239, 122002. [Google Scholar] [CrossRef]
- Swain, S.; Kumari, S.; Swain, P.; Rautray, T.R. Polarised strontium hydroxyapatite—xanthan gum composite exhibits osteo-genicity in vitro. Mater. Today Proc. 2022, 62, 6143–6147. [Google Scholar] [CrossRef]
- Swain, S.; Misra, R.D.K.; You, C.K.; Rautray, T.R. TiO2 nanotubes synthesised on Ti-6Al-4V ELI exhibits enhanced osteogenic activity: A potential next-generation material to be used as medical implants. Mater. Technol. 2020, 36, 393–399. [Google Scholar] [CrossRef]



| Gene | Forward | Reverse |
|---|---|---|
| COL1 | CCACCTTCTGCCCTAACACA | GACAAGAGGCTCAGGGTCAG |
| OCN | GGCGCTACCTGTATCAATGG | CCTCCTCTCCCTACACATGG |
| RUNX2 | CCCATTCATTAAAGTCCTCAAGA | TGGAAATTTGTTTTCTGAAATGC |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Priyadarshini, I.; Swain, S.; Koduru, J.R.; Rautray, T.R. Electrically Polarized Withaferin A and Alginate-Incorporated Biphasic Calcium Phosphate Microspheres Exhibit Osteogenicity and Antibacterial Activity In Vitro. Molecules 2023, 28, 86. https://doi.org/10.3390/molecules28010086
Priyadarshini I, Swain S, Koduru JR, Rautray TR. Electrically Polarized Withaferin A and Alginate-Incorporated Biphasic Calcium Phosphate Microspheres Exhibit Osteogenicity and Antibacterial Activity In Vitro. Molecules. 2023; 28(1):86. https://doi.org/10.3390/molecules28010086
Chicago/Turabian StylePriyadarshini, Itishree, Subhasmita Swain, Janardhan Reddy Koduru, and Tapash Ranjan Rautray. 2023. "Electrically Polarized Withaferin A and Alginate-Incorporated Biphasic Calcium Phosphate Microspheres Exhibit Osteogenicity and Antibacterial Activity In Vitro" Molecules 28, no. 1: 86. https://doi.org/10.3390/molecules28010086
APA StylePriyadarshini, I., Swain, S., Koduru, J. R., & Rautray, T. R. (2023). Electrically Polarized Withaferin A and Alginate-Incorporated Biphasic Calcium Phosphate Microspheres Exhibit Osteogenicity and Antibacterial Activity In Vitro. Molecules, 28(1), 86. https://doi.org/10.3390/molecules28010086







