2.1. Thermogravimetric Analysis (TGA)
Traditionally, amino acids are reported to melt or decompose at temperatures above 450 K [
6,
11]. However, literature data exhibits a significant scatter. Moreover, a recent paper by Weiss et al. [
13] suggests that all amino acids studied do not melt but rather decompose, which could damage calorimeters as a result. Weiss et al. [
13] reported an evolution of CO
2, NH
3, and H
2O during the decomposition of L-cysteine at the temperature of about 518 K. For the remaining four amino acids of this study, Weiss et al. [
13] reported the decomposition without specifying its temperature and mechanism. Therefore, reinvestigation of the melting/decomposition process for all five amino acids was undertaken to reconcile the literature data. All the samples were studied by TGA; results are shown in
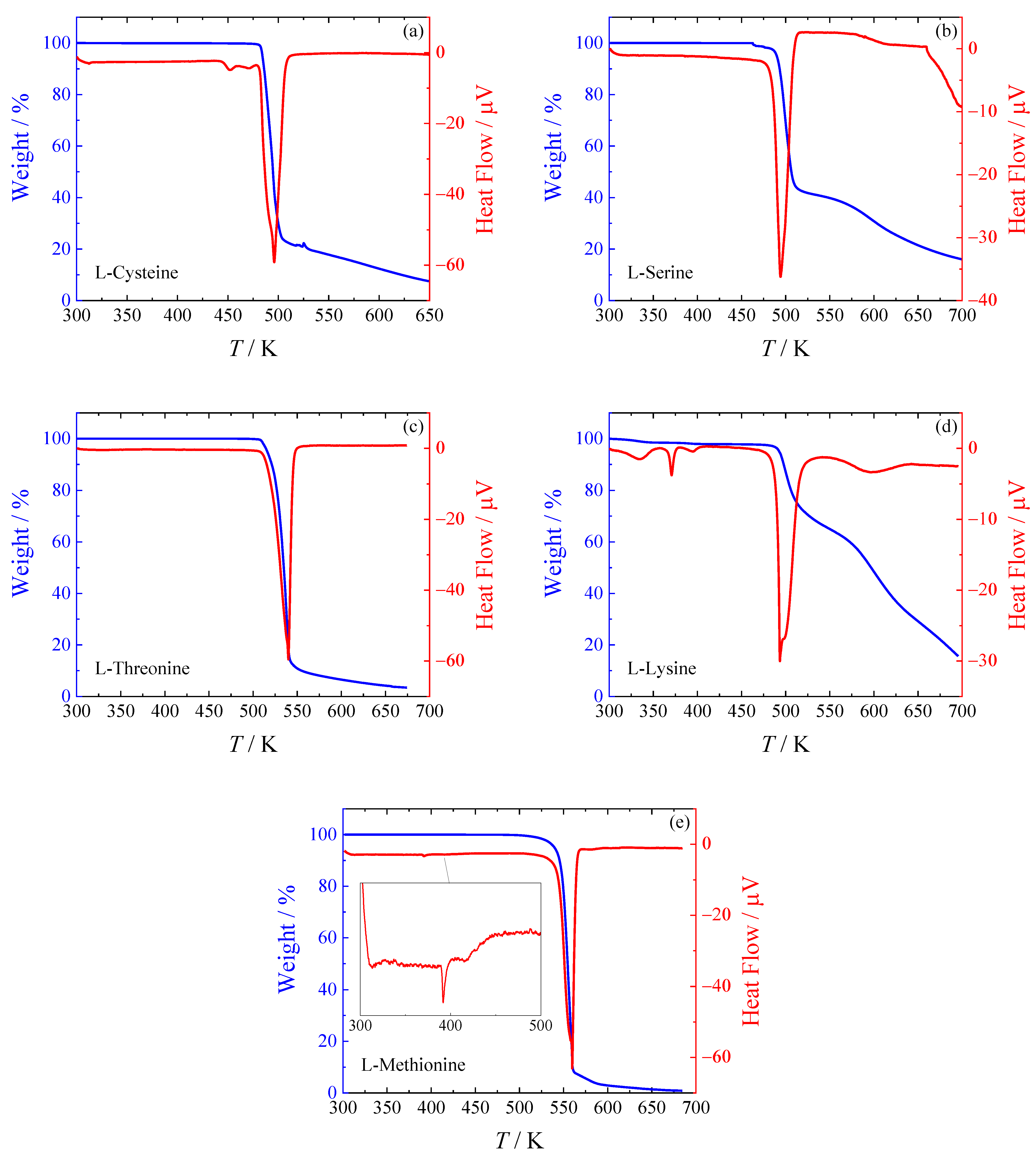
Figure 1.
The decomposition behavior differs for particular amino acids. The simplest decomposition path can be seen in the case of L-cysteine (
Figure 1a), L-threonine (
Figure 1c), and L-methionine (
Figure 1e), which show a one-stage decomposition. For L-serine (
Figure 1b), two decomposition stages can be clearly distinguished. L-lysine (
Figure 1d) exhibits the most complicated decomposition behavior. We can see two distinct decomposition stages, but the first decrease in sample weight appears already around 300 K and also around 395 K, both accompanied by wide DSC peaks. They most likely correspond to the evaporation of water from the hygroscopic sample, as exposure to air cannot be avoided during the preparation of the sample for the TGA measurements. The weight fraction before the main decomposition peak is around 0.97, meaning water accounts for approximately 3 % of the total sample weight. It is known that L-lysine absorbs air moisture and forms hemihydrate easily [
9], which should contain 5 % of water in weight.
One thermal event was observed for L-cysteine around 445 K (i.e., shortly below the decomposition temperature) and two in L-methionine (at 393 K and 415 K). In the case of L-lysine, another sharp peak at 371 K was also observed. Any of these peaks are not accompanied by weight loss, and thus they correspond to phase transitions. A more detailed analysis of the phase behavior was performed using HF DSC, as described in
Section 2.2.
A summary of decomposition temperatures obtained in this work, as well as those reported in the literature, is presented in
Table 1. It should be noted that the decomposition kinetics is influenced by a number of factors (heating rate, sample size, purge gas, etc.); this is reflected by a relatively large range of reported decomposition temperatures. Recently reported temperatures of fusion obtained with fast scanning DSC [
14] (see
Table 1) are higher than those of decomposition by 50–140 K. To avoid the decomposition, Do et al. [
14] used scanning rates up to 20,000 K·s
−1.
The agreement of our TGA data with the literature is only partial. TGA data by Rodante et al. [
15] for L-cysteine, L-serine, and L-threonine lie within 6 K from our data. For L-lysine and L-methionine, the differences are very large (152 K and 38 K, respectively). In the case of L-lysine, the low “decomposition temperature” value reported by Rodante et al. [
15] likely corresponds to the evaporation of water from a wet sample, as also observed in our experiments. In the case of L-methionine, the reason for this discrepancy is unclear. When comparing to another set of TGA data by Rodriguez-Mendez et al. [
16], there is again a reasonable agreement for three of the amino acids (decomposition temperatures of L-cysteine, L-serine, and L-lysine fall within 20 K from our values); however, their data for L-threonine and L-methionine are significantly lower (by 38 K and 49 K, respectively). If we compare to data recommended by the Syracuse Research Corporation (retrieved from [
17]), there is an agreement within 10 K for four of the compounds (this time, the decomposition temperature for L-cysteine is in poor agreement). Agreement with other data obtained by DSC, differential thermal analysis (DTA), or differential thermal gravimetry (DTG) is similarly inconsistent.
Table 1.
Temperatures of Fusion and Decomposition of L-Cysteine, L-Serine, L-Threonine, L-Lysine, and L-Methionine a.
Table 1.
Temperatures of Fusion and Decomposition of L-Cysteine, L-Serine, L-Threonine, L-Lysine, and L-Methionine a.
| Reference | Tdecomp, onset/K | Tdecomp, peak top/K | Method | Scanning Rate, Purge Gas |
|---|
| L-cysteine (one-stage decomposition) (Tfus= 604 ± 7 K b) |
| SRC recommendation c | 513 | | | |
| Weiss et al. [13] | | 518 | TGA | 5 K min−1, argon |
| Olafsson and Bryan [18] | | 521 | DSC | 10 K min−1, nitrogen |
| Rodriguez-Mendez et al. [16] | 493 | | TGA | 10 K min−1, air |
| Rodriguez-Mendez et al. [16] | 496 | | DTA | 10 K min−1, air |
| Rodante et al. [15] | 393,441,490 d | | TGA | 10 K min−1, nitrogen |
| Rodante et al. [15] | | 415,453,521 | DSC | 10 K min−1, nitrogen |
| Wesolowski and Erecinska [19] | | 473 | DTA | 5 K min−1, air |
| Wesolowski and Erecinska [19] | | 473 | DTG | 5 K min−1, air |
| This work | 484 | | TGA | 5 K min−1, argon |
| L-serine (two-stage decomposition) (Tfus= 519 ± 7 K b) |
| SRC recommendation c | 501 | | | |
| Olafsson and Bryan [18] | | 505 | DSC | 10 K min−1, nitrogen |
| Rodriguez-Mendez et al. [16] | 503 | | TGA | 10 K min−1, air |
| Rodriguez-Mendez et al. [16] | 502 | | DTA | 10 K min−1, air |
| Rodante et al. [20] | 496 | | TGA | 10 K min−1, nitrogen |
| Rodante et al. [20,21] | | 508 | DSC | 10 K min−1, nitrogen |
| This work | 491 | | TGA | 5 K min−1, argon |
| L-threonine (one-stage decomposition) (Tfus= 587 ± 9 K b) |
| SRC recommendation c | 529 | | | |
| Olafsson and Bryan [18] | | 532 | DSC | 10 K min−1, nitrogen |
| Rodriguez-Mendez et al. [16] | 488 | | TGA | 10 K min−1, air |
| Rodriguez-Mendez et al. [16] | 471 | | DTA | 10 K min−1, air |
| Rodante et al. [20] | 520 | | TGA | 10 K min−1, nitrogen |
| Rodante et al. [20,22] | | 540 | DSC | 10 K min−1, nitrogen |
| Contineanu et al. [23] | 521 | 529 | DSC | 4 K min−1, nosp. |
| Wesolowski and Erecinska [19] | | 523 | DTA | 5 K min−1, air |
| Wesolowski and Erecinska [19] | | 518 | DTG | 5 K min−1, air |
| This work | 526 | | TGA | 5 K min−1, argon |
| L-lysine (two-stage decomposition) (Tfus= 529 ± 9 K b) |
| SRC recommendation c | 498 | | | |
| Olafsson and Bryan [18] | | 506 | DSC | 10 K min−1, nitrogen |
| Rodriguez-Mendez et al. [16] | 509 | | TGA | 10 K min−1, air |
| Rodriguez-Mendez et al. [16] | 531 | | DTA | 10 K min−1, air |
| Rodante et al. [20] | 336 | | TGA | 10 K min−1, nitrogen |
| Rodante et al. [20] | | 339,515,532 | DSC | 10 K min−1, nitrogen |
| This work | 492 | | TGA | 5 K min−1, argon |
| L-methionine (one-stage decomposition) (Tfus= n.a. e) |
| SRC recommendation c | 555 | | | |
| Olafsson and Bryan [18] | | 562 | DSC | 10 K min−1, nitrogen |
| Rodriguez-Mendez et al. [16] | 498 | | TGA | 10 K min−1, air |
| Rodriguez-Mendez et al. [16] | 467 | | DTA | 10 K min−1, air |
| Rodante et al. [22] | 509 d | | TGA | 10 K min−1, nitrogen |
| Rodante et al. [22] | | 568 | DSC | 10 K min−1, nitrogen |
| This work | 547 | | TGA | 5 K min−1, argon |
2.2. Phase Behavior
All amino acids studied are zwitterionic crystals at 298.15 K whose structures determined by XRPD are provided in
Table 2. Subsequently, the phase behavior was investigated in the temperature range from 183 K to the thermal decomposition temperature using heat-flux DSC to confirm/exclude the presence of phase transitions. The phase transitions observed in this work are listed and compared with literature values in
Table 3.
Two ambient-pressure polymorphs of L-cysteine are usually considered in the literature: I (orthorhombic) [
37], which exhibits a phase transition at 70 K [
24] and should be thus considered two phases (Ia and Ib), and II (monoclinic) [
38]. We are not aware that their thermodynamic relationship was resolved. Additionally, two high-pressure polymorphs, III and IV, were described [
39]. The thermal event observed with TGA near 450 K (see
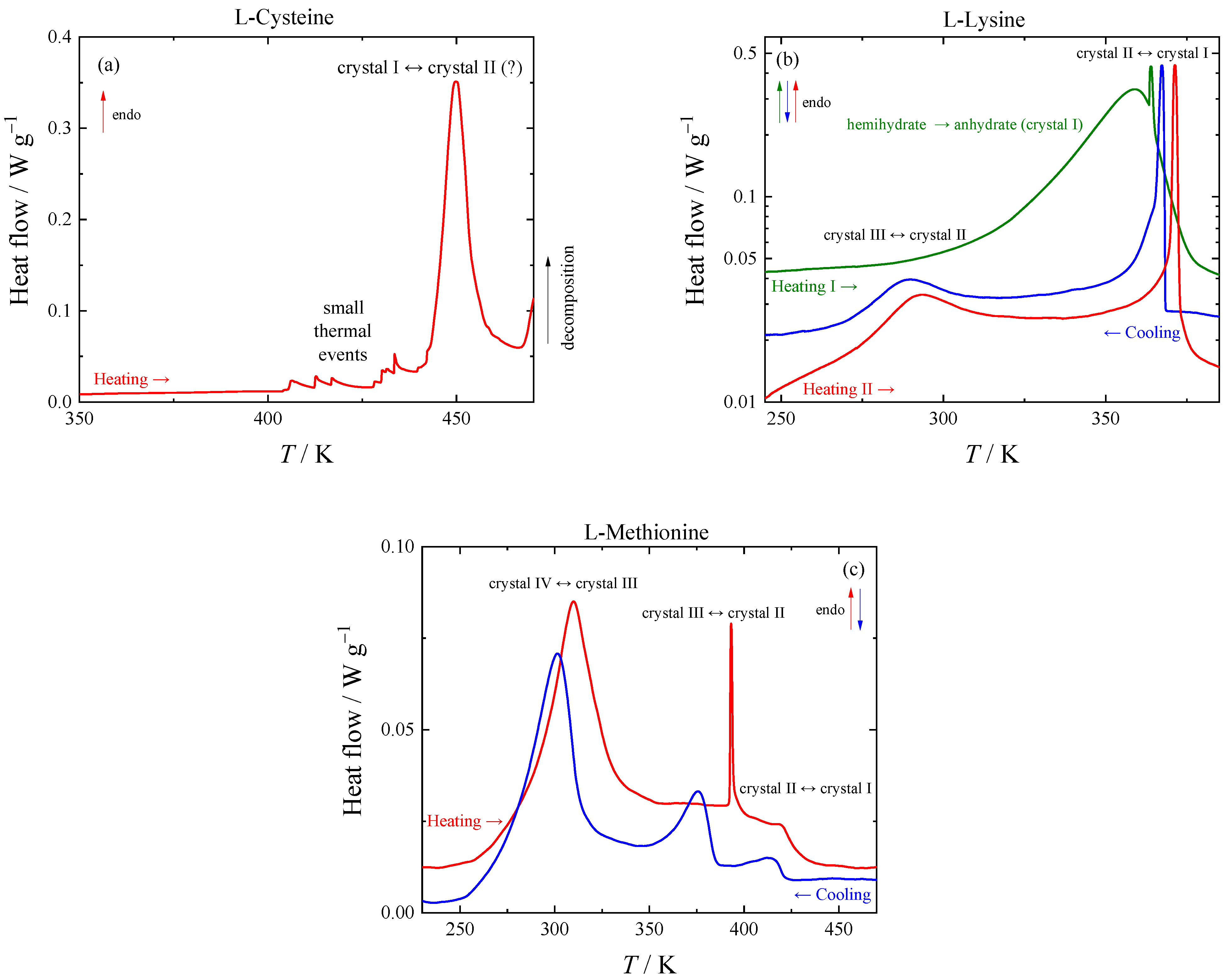
Figure 1a) was confirmed using HF DSC, as shown in
Figure 2a. This phase transition could represent a transition from I
b to II; in that case, the phases would be enantiotropically related with form II stable above approximately 400 K. Detailed study of the phase transition is complicated because of thermal decomposition and identity of high-temperature phase with crystal structure II could not be confirmed.
For L-serine, four polymorphs are known to date. The only polymorph stable at normal pressure is I (sometimes also labeled 1). The other polymorphs were reported to exist at high pressures: II [
40], III [
41], and IV [
42]. In addition to a single known ambient-pressure polymorph I of L-threonine, three high-pressure polymorphs were reported: I’, II, and III [
43]. No phase transition for L-serine and L-threonine has been observed in this work in the studied temperature range.
Since L-lysine forms hemihydrate and monohydrate in a moist atmosphere, no studies of the phase behavior of anhydrate were reported to our knowledge. Multiple phase transitions have been detected for L-lysine in this work, which can be seen on the respective DSC curves in
Figure 2b. During the first heating of L-lysine hemihydrate in a pierced DSC pan, a wide peak corresponding to the evaporation of water is observed. In a subsequent run, L-lysine anhydrate exhibits a wide peak with a maximum of 292 K, which corresponds to a second-order transition, and a sharp peak of a first-order phase transition at 370.3 ± 0.5 K.
Three structures of L-methionine (II, III, and IV), all with disordered side chains, were recently solved [
29]. On the other hand, previous crystal structure reports at lower [
44,
45] and room [
28] temperatures did not reveal any disorder. Solving the structure of high-temperature phase I was not attempted because of the complicated disorder of phase II [
29]. The structures resolved at 120 K [
44], 293 K [
29], and 320 K [
29] are all monoclinic; they have very close unit-cell parameters and differ only in occupancies of the disordered side chain (e.g., molecule B has occupancy 1:0:0:0 [
44], 0.69:0.12:0.08:0.11 [
29], and 0.41:0.15:0.20:0.24 [
29], respectively). However, the previous calorimetric studies [
34,
36], as well as this work, revealed only one phase transition in the temperature region 120– 320 K manifested by a very broad peak with a start around 250 K and a maximum at 307. K. Lima Jr. et al. [
31] reported another anomaly in the intensities of some Raman bands in the interval 160–140 K and around 220 K during heat capacity measurements “by relaxation calorimetry (note that the paper does not tabulate any heat capacity data) but not in their DSC experiments. The anomalies scattered over wide temperature ranges are said to be related to conformational changes of at least one of the independent molecules in the unit cell [
31]. Based on an unsubstantiated criterion Δ
trS > 1 J mol
−1 K
−1, the phase transition with a maximum at 307 K is then established as a first-order transition by authors [
31].
The tangled story of the L-methionine phase behavior below 350 K has a consistent and logical solution, although somewhat uncommon. The shape and negligible hysteresis of the peak corresponding to the phase transition suggests that it might be a lambda transition, i.e., a second-order transition, where the continuous structural changes intensify while increasing the temperature and suddenly decelerate or stop after reaching a certain point. From the calorimetric point of view, the intensification of the structural changes manifests through a “divergence” of heat capacity followed by a fast drop to a value following the trend well below the temperature region of the structural changes (see
Figure 2c). At cooling, this lambda transition shows almost no hysteresis—the heat capacity rises very fast, reaches the maximum at the same temperature, and then slowly decays. The temperature of the maximum at the heat capacity curve (about 308 K) would be considered as the phase transition temperature (here between phases IV and III) since any other characteristic temperature cannot be evaluated for the transition with similar accuracy.
From the crystallographic point of view, the situation is somewhat surprising. Polymorph IV exhibits a continuous (and equilibrium) change of the occupancies of conformations, starting from a fully ordered structure at low temperatures all the way until reaching occupancies corresponding to polymorph III. Initially, the occupancies of alternative conformations are below the detection limit, but with rising temperature, the rise of their occupancies intensifies, and once it reaches values for polymorph III, they remain stable. In other words, disorder occupancies of polymorph IV may match any values between ordered structure and values for polymorph III, depending on the temperature. A variable-temperature x-ray study should confirm this hypothesis, but its execution is very demanding because of the large number of occurring conformations.
Although the explained behavior differs from the common idea of polymorphism, it is consistent and logical. One might argue that the moment where the disorder starts to appear should be considered a phase transition instead of the moment when the occupancies stabilize. However, because of the diverging nature of the process, it is not possible to determine its beginning while its end is well-defined. It is vital to watch the behavior of the first derivatives of the thermodynamic and structural properties in the case of second-order phase transitions. While the thermodynamic properties and structural parameters are continuous, their first derivatives are discontinued—and despite the limited amount of structural data, calorimetric experiments clearly place this discontinuity at 308 K.
An interesting link between the crystallographic and calorimetric observations can be found that supports the hypothesis above. If the total entropy change Δ
IV-IIIS is evaluated from the heat capacity description (
Section 2.3), a value of 11.4 J mol
−1 K
−1 is obtained. Although a somewhat arbitrary baseline needs to be selected, this value is in good agreement with a mixing entropy corresponding to the disorder of molecule B in the crystal structure of form III calculated from occupancies reported in [
29], Δ
mixS = 10.9 J mol
−1 K
−1. Disorder of molecule A would correspond to additional Δ
mixS = 5.2 J mol
−1 K
−1, but since the two reported conformations [
29] are noticeably similar, the mixing entropy might be an irrational construct in this case. Agreement between calorimetric Δ
IV-IIIS and crystallographic Δ
mixS supports the hypothesis that the wide calorimetric peak corresponds to a continuous transformation from the fully ordered form IV to form III. The Raman spectroscopic experiment [
31] seems to be in agreement with this hypothesis, while the anomaly in relaxation calorimetry heat capacity measurements [
31] might be related to the metastable occurrence of fully ordered structure at elevated temperatures and relaxation to equilibrium disordered form IV.
The discussion of the other phase transitions of L-methionine visible in
Figure 2c is simpler. Phase transition III-II is sharp, exhibits hysteresis, and its shape differs on heating and on cooling. Structures are determined at a single temperature for both polymorphs [
29] and show that new conformations are introduced during the phase transition. This description agrees with typical features of a first-order phase transition. Phase transition II-I is associated with the smallest entropy change, and it is difficult to reveal the shape of its calorimetric peak without ambiguity. Resolving the structure of form I was not attempted in [
29] because of expecting a complex disorder. The choice of considering the phase transition to be of the first or second order brings negligible difference to the description of thermodynamic quantities.
2.3. Heat Capacities
Experimental heat capacities obtained in this work with two Tian-Calvet calorimeters (SETARAM μDSC IIIa, SETARAM MicroCalvet), PerkinElmer power compensation DSC 8500 (PC DSC), and Quantum Design PPMS relaxation calorimeter are listed in the
Supplementary Materials (SM) in Tables S1–S7 including correction scaling factors applied for PerkinElmer DSC 8500 and Quantum Design PPMS results. Available literature data on crystal heat capacities are summarized in
Table 4. Experimental data from
Table 4 were fitted with Equations (1) and (2) parameters which are given in
Table 5.
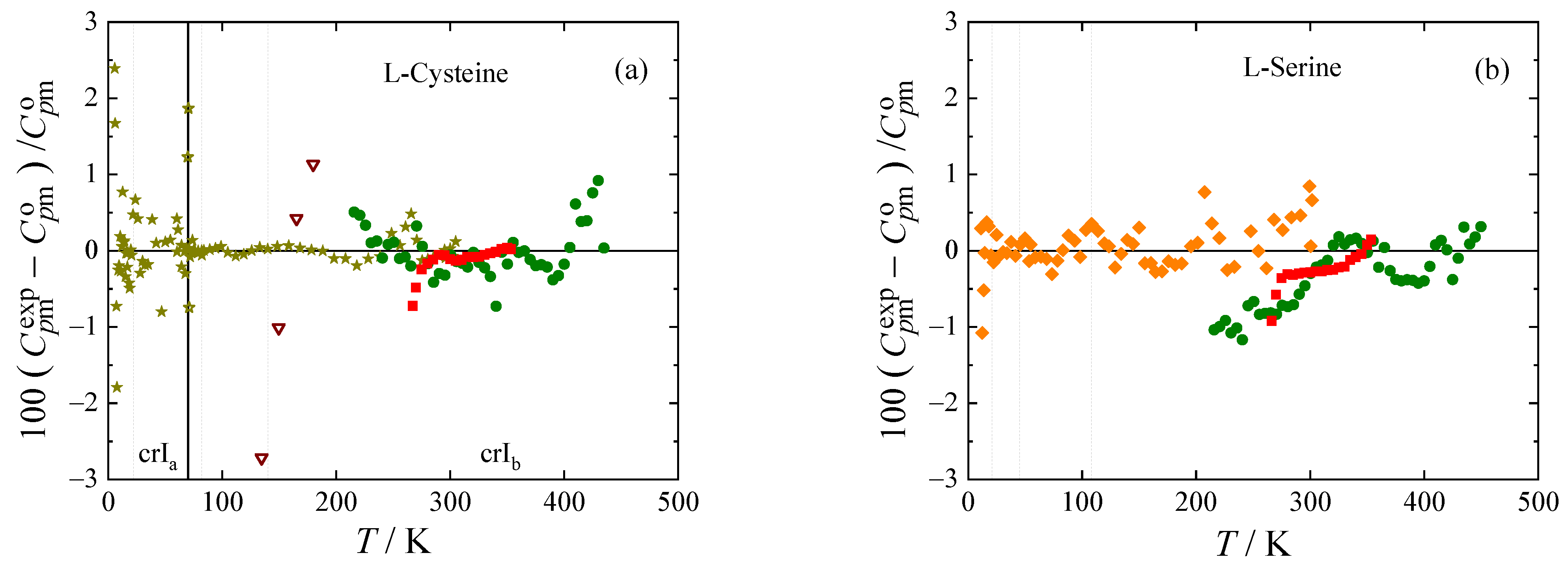
The experimental heat capacities for all amino acids studied are compared with the smoothed values obtained using Equations (1) and (2) in
Figure 3. The deviations of the selected experimental data (marked bold in
Table 4) from the smoothed values do not exceed 1 % with the exception of lowest temperatures, where all experiments have higher uncertainty, and vicinity of phase transitions, where the data points were excluded from the fit.
Adiabatic data for L-cysteine by Huffman and Ellis [
46] have a very different slope from data by Paukov et al. [
30] and data obtained in this work by both Tian-Calvet and power compensation DSC (deviations from −8 to +13%). This was commented on by Paukov et al. [
30], who believe that this discrepancy is caused by impurities (possibly DL form or monoclinic L form of cysteine) in the sample of Huffman and Ellis [
46]. In the high-temperature region, heat capacities of L-cysteine were only correlated below 435 K because of discrepancies in experimental data above this temperature, which probably correspond with the initiation of the suggested I
b-II phase transition observed during the phase behavior study. Adiabatic heat capacity data by Hutchens et al. [
47] is available for L-serine. Our Tian-Calvet and PC DSC data agree with theirs within 1%. Data by Lukyanova et al. [
12] for L-threonine is also obtained by adiabatic calorimetry [
49]. The stated uncertainty of 0.2 % [
12] is, however, questionable, when comparing data from the same laboratory [
50,
51] with previously published data [
4,
52,
53] (for more details see
Figure S1 and
Section 1 of the
Supporting Materials). Although not included in the correlation, most of the data points from [
12] deviate from our fit (based on our measurements using Tian-Calvet, PC DSC, and relaxation calorimetry) by less than 2%. Heat capacities of L-lysine obtained from the three calorimeters are in good mutual agreement, and all exhibit a wide peak with a maximum of around 291 K, which was found to be characteristic of anhydrous L-lysine during the phase-behavior study (see
Figure 2). This transition, as well as the one exhibited by L-methionine around 310 K, is treated as a lambda transition in the correlation. I.e., heat capacities of both phases in the vicinity of the phase transition are correlated separately, and the phase transition temperatures in
Table 3 are evaluated as the intersection of the extrapolated heat capacity curves. Consequently, heat capacity, as well as entropy and enthalpy, are continuous at these phase transitions. For the other phase transitions of L-methionine (at 393 K and 421 K) and L-lysine (at 370.3 K), a step change of enthalpy equal to the value obtained by DSC (
Table 3) is considered in the correlation. Heat capacities of the given phases are correlated separately except for the crII and crI phases of L-methionine, which were treated together since only two experimental data points were available for crII. From the heat capacity data for L-methionine by Hutchens et al. [
34], only those from the low-temperature calorimeter were used, which are in reasonable agreement with our Tian-Calvet and PC DSC data (within 1.5%), although they show some scatter when approaching the crIV-crIII phase transition. Heat capacity data from the high-temperature adiabatic calorimeter are related with a higher uncertainty according to the authors [
34], their scatter is noticeably high and agreement with our measurements for crIII is poor.
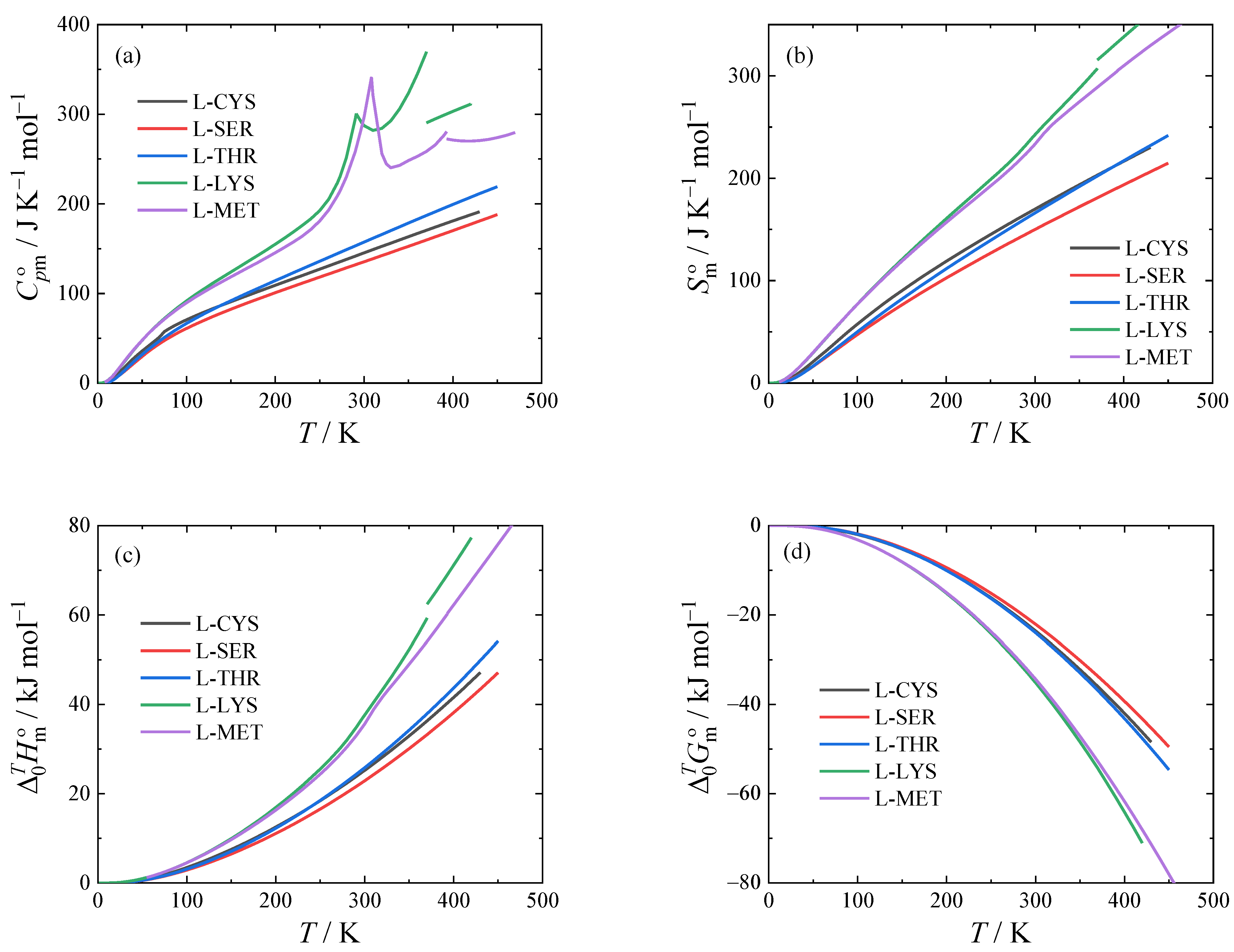
The thermodynamic functions of L-cysteine, L-serine, L-threonine, L-lysine, and L-methionine obtained using Equations (1) and (2) are tabulated in
Tables S8–S12 in the Supporting Materials and shown graphically in
Figure 4. Their values at
T = 298.15 K are shown in
Table 6 for convenience. Note that L-lysine and L-methionine are in close proximity to a second-order phase transition at
T = 298.15 K, leading to an increased heat capacity.