Stabilization of Natural Pigments in Ethanolic Solutions for Food Applications: The Case Study of Chlorella vulgaris
Abstract
1. Introduction
2. Results and Discussion
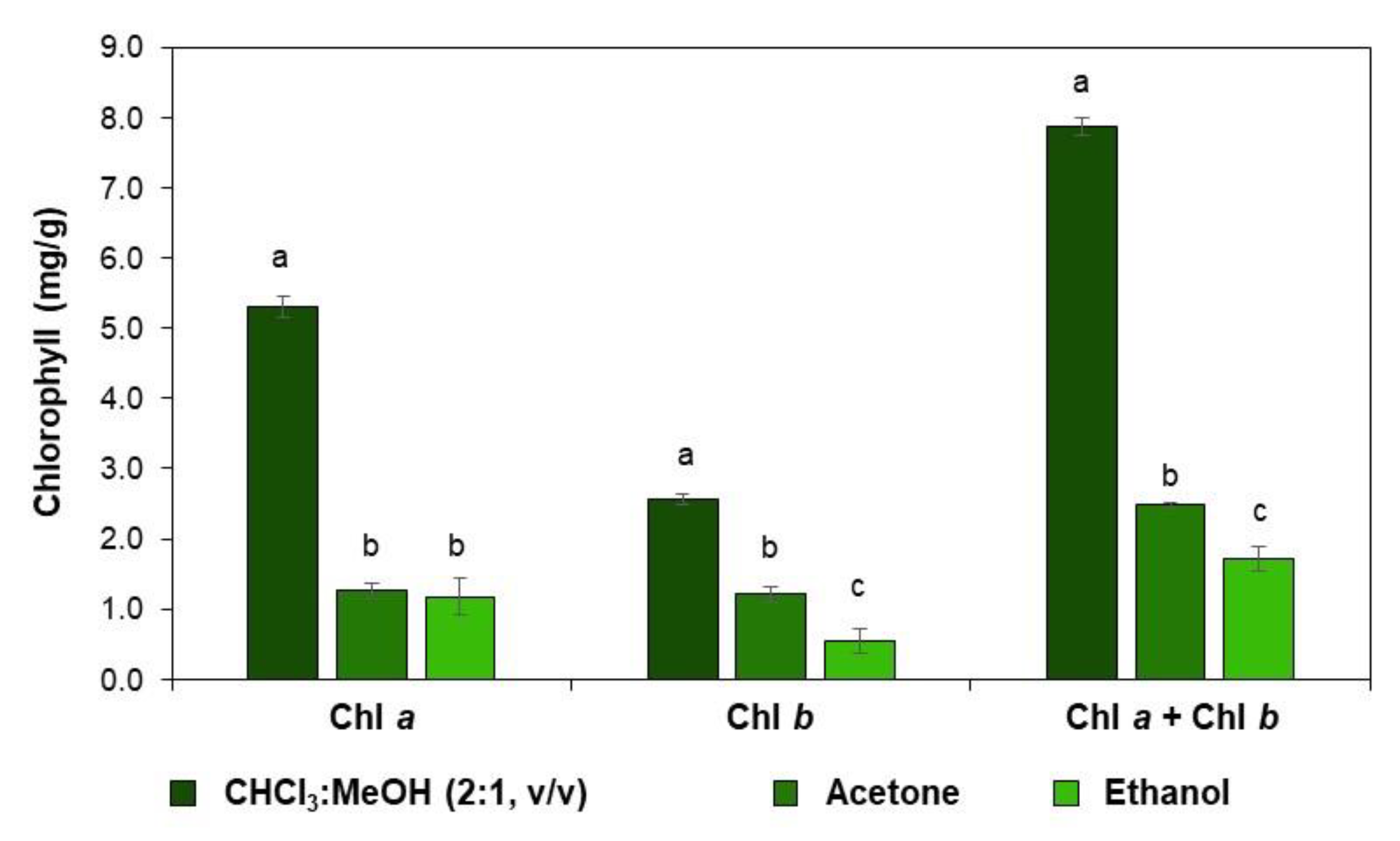
2.1. Evaluation of the Efficiency of Different Solvents to Extract C. vulgaris Pigments
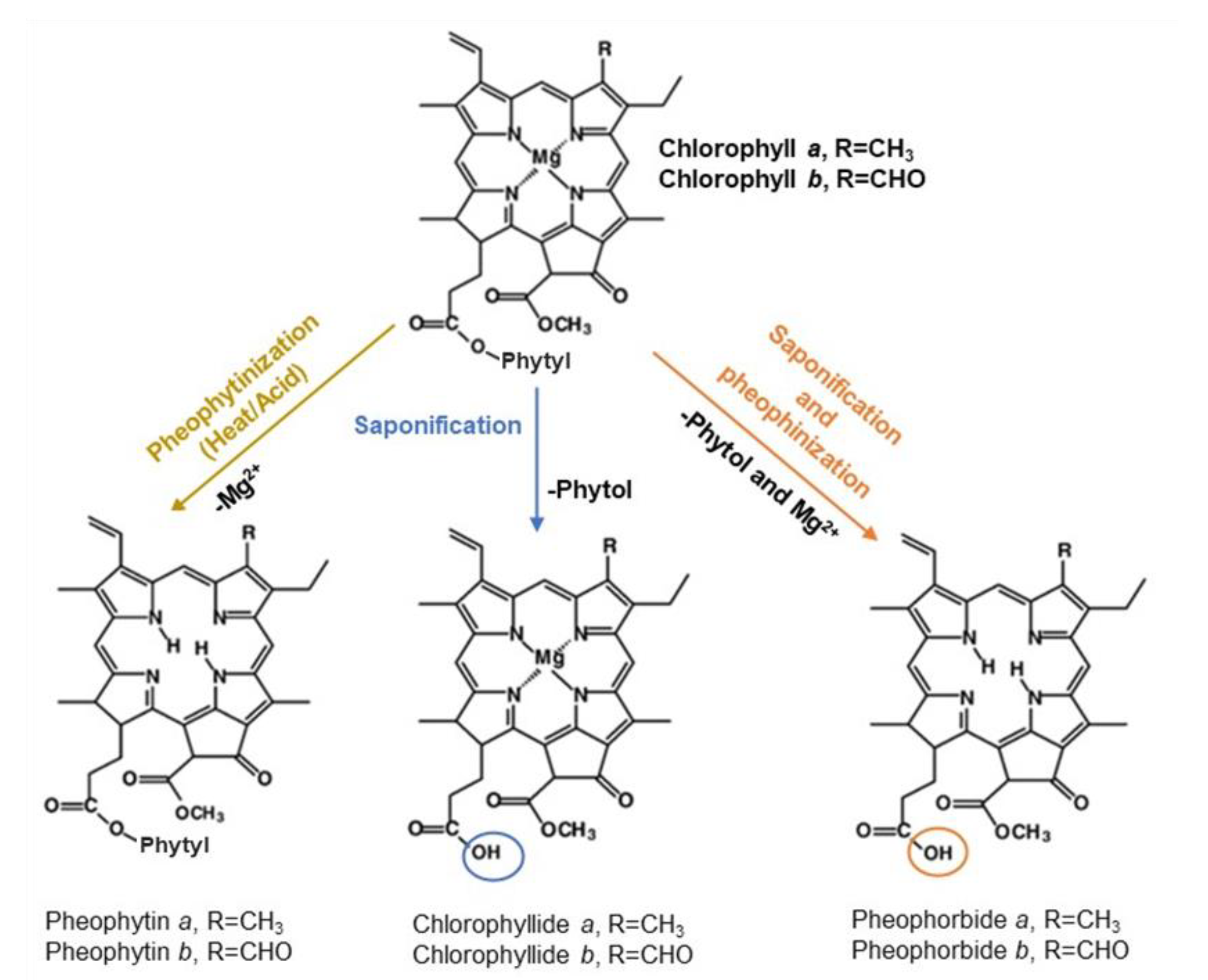
2.2. C. vulgaris Pigments Identification
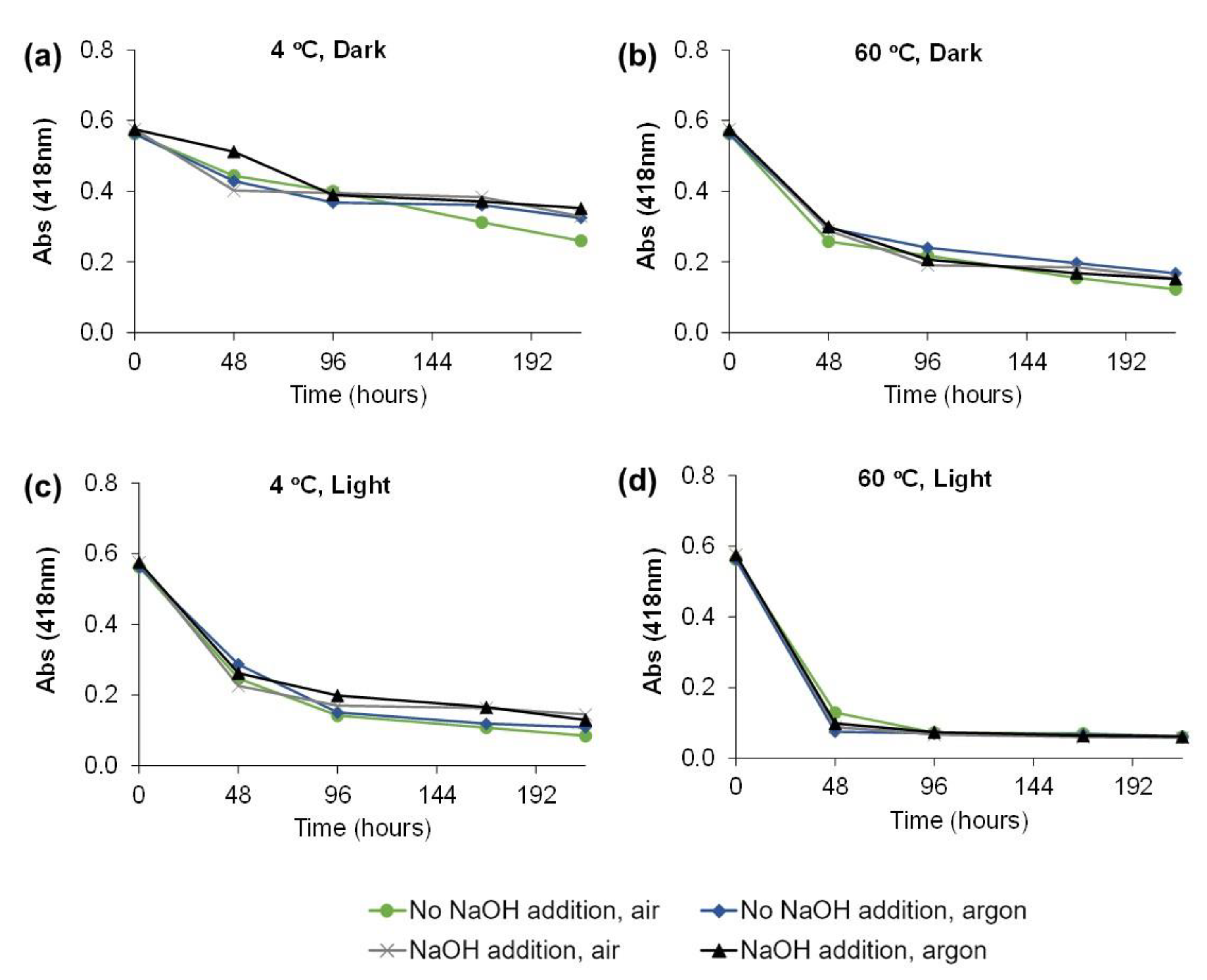
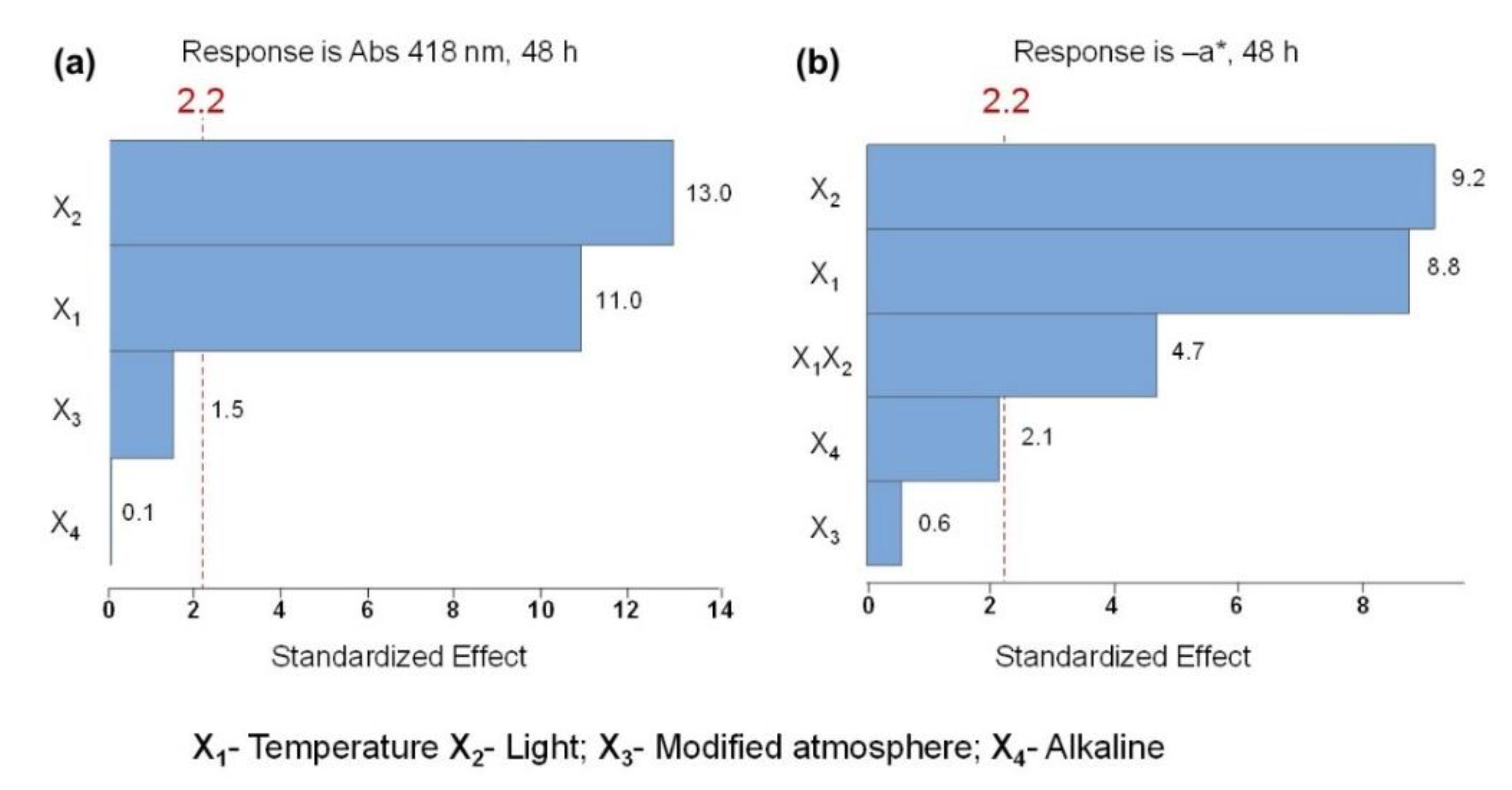
2.3. Evaluation of C. vulgaris Color Stability
2.4. Degradation Kinetic of Green Color at Different Temperatures
2.5. Food Application
3. Materials and Methods
3.1. Extraction
3.2. Total Chlorophylls Content Determination
3.3. Pigments Identification by Thin Layer Chromatography (TLC)
3.4. Evaluation of Pigments Stabilization and Color Loss
3.5. Degradation Kinetic of Green Color
3.6. Food Application of C. vulgaris Pigments
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Kong, W.; Liu, N.; Zhang, J.; Yang, Q.; Hua, S.; Song, H.; Xia, C. Optimization of Ultrasound-Assisted Extraction Parameters of Chlorophyll from Chlorella Vulgaris Residue after Lipid Separation Using Response Surface Methodology. J. Food Sci. Technol. 2014, 51, 2006–2013. [Google Scholar] [CrossRef]
- Mangos, T.J.; Berger, R.G. Determination of Major Chlorophyll Degradation Products. Zeitschrift Leb. Forsch. A 1997, 204, 345–350. [Google Scholar] [CrossRef]
- Cuaresma, M.; Janssen, M.; Vílchez, C.; Wijffels, R.H. Horizontal or Vertical Photobioreactors? How to Improve Microalgae Photosynthetic Efficiency. Bioresour. Technol. 2011, 102, 5129–5137. [Google Scholar] [CrossRef]
- Fradique, M.; Batista, A.P.; Nunes, M.C.; Gouveia, L.; Bandarra, N.M.; Raymundo, A. Isochrysis Galbana and Diacronema Vlkianum Biomass Incorporation in Pasta Products as PUFA’s Source. LWT-Food Sci. Technol. 2013, 50, 312–319. [Google Scholar] [CrossRef]
- Ventura, S.P.M.; Nobre, B.P.; Ertekin, F.; Hayes, M.; Garciá-Vaquero, M.; Vieira, F.; Koc, M.; Gouveia, L.; Aires-Barros, M.R.; Palavra, A.M.F. Extraction of Value-Added Compounds from Microalgae. In Microalgae-Based Biofuels and Bioproducts; Elsevier: Amsterdam, The Netherlands, 2017; pp. 461–483. [Google Scholar]
- Pangestuti, R.; Kim, S.-K. Biological Activities and Health Benefit Effects of Natural Pigments Derived from Marine Algae. J. Funct. Foods 2011, 3, 255–266. [Google Scholar] [CrossRef]
- Hynstova, V.; Sterbova, D.; Klejdus, B.; Hedbavny, J.; Huska, D.; Adam, V. Separation, Identification and Quantification of Carotenoids and Chlorophylls in Dietary Supplements Containing Chlorella Vulgaris and Spirulina Platensis Using High Performance Thin Layer Chromatography. J. Pharm. Biomed. Anal. 2018, 148, 108–118. [Google Scholar] [CrossRef]
- Ferreira, A.S.; Ferreira, S.S.; Correia, A.; Vilanova, M.; Silva, T.H.; Coimbra, M.A.; Nunes, C. Reserve, Structural and Extracellular Polysaccharides of Chlorella Vulgaris: A Holistic Approach. Algal Res. 2020, 45, 101757. [Google Scholar] [CrossRef]
- Koca, N.; Karadeniz, F.; Burdurlu, H.S. Effect of PH on Chlorophyll Degradation and Colour Loss in Blanched Green Peas. Food Chem. 2007, 100, 609–615. [Google Scholar] [CrossRef]
- Ryan, A.A.; Senge, M.O. How Green Is Green Chemistry? Chlorophylls as a Bioresource from Biorefineries and Their Commercial Potential in Medicine and Photovoltaics. Photochem. Photobiol. Sci. 2015, 14, 638–660. [Google Scholar] [CrossRef]
- Zheng, Y.; Shi, J.; Pan, Z.; Cheng, Y.; Zhang, Y.; Li, N. Effect of Heat Treatment, PH, Sugar Concentration, and Metal Ion Addition on Green Color Retention in Homogenized Puree of Thompson Seedless Grape. LWT-Food Sci. Technol. 2014, 55, 595–603. [Google Scholar] [CrossRef]
- Nisha, P.; Singhal, R.S.; Pandit, A.B. A Study on the Degradation Kinetics of Visual Green Colour in Spinach (Spinacea oleracea L.) and the Effect of Salt Therein. J. Food Eng. 2004, 64, 135–142. [Google Scholar] [CrossRef]
- Bellomo, M.G.; Fallico, B.; Muratore, G. Stability of Pigments and Oil in Pistachio Kernels during Storage. Int. J. Food Sci. Technol. 2009, 44, 2358–2364. [Google Scholar] [CrossRef]
- Clydesdale, F.M.; Francis, F.J. Chlorophyll Changes in Thermally Processed Spinach as Influenced by Enzyme Conversion and PH Adjustment. Food Technol. 1968, 22, 135. [Google Scholar]
- Salama, M.F.; Moharram, H.A. Relationship between Colour Improvement and Metallo-Chlorophyll Complexes during Blanching of Peas and Broccoli. Alex. J. Food Sci. Technol. 2007, 4, 11–18. [Google Scholar]
- Rudra, S.G.; Sarkar, B.C.; Shivhare, U.S. Thermal Degradation Kinetics of Chlorophyll in Pureed Coriander Leaves. Food Bioprocess Technol. 2008, 1, 91–99. [Google Scholar] [CrossRef]
- Araujo, P.W.; Brereton, R.G. Stability Studies of Chlorophyll a Using Different Storage Systems. In Proceedings of the Analytical Proceedings including Analytical Communications; The Royal Society of Chemistry: London, UK, 1995; Volume 32, pp. 415–417. [Google Scholar]
- Shen, S.-C.; Hsu, H.-Y.; Huang, C.-N.; Wu, J.S.-B. Color Loss in Ethanolic Solutions of Chlorophyll A. J. Agric. Food Chem. 2010, 58, 8056–8060. [Google Scholar] [CrossRef] [PubMed]
- Wood, L.W. Optimization of Ultrasound-Assisted Extraction Parameters of Chlorophyll from Chlorella Vulgaris Residue after Lipid Separation Using Response Surface Methodology. Can. J. Fish. Aquat. Sci. 1985, 42, 38–43. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K.; Buschmann, C. Extraction of Phtosynthetic Tissues: Chlorophylls and Carotenoids. Curr. Protoc. Food Anal. Chem. 2001, 1, F4.2.1–F4.2.6. [Google Scholar] [CrossRef]
- Safafar, H.; Uldall Nørregaard, P.; Ljubic, A.; Møller, P.; Løvstad Holdt, S.; Jacobsen, C. Enhancement of Protein and Pigment Content in Two Chlorella Species Cultivated on Industrial Process Water. J. Mar. Sci. Eng. 2016, 4, 84. [Google Scholar] [CrossRef]
- Sathya, S. Separation of Algal Pigments by Thin Layer Chromatography (TLC) and High Performance Liquid Chromatography (HPLC). World J. Pharm. Res. 2017, 6, 1275–1284. [Google Scholar]
- Gong, M.; Bassi, A. Investigation of Chlorella Vulgaris UTEX 265 Cultivation under Light and Low Temperature Stressed Conditions for Lutein Production in Flasks and the Coiled Tree Photo-Bioreactor (CTPBR). Appl. Biochem. Biotechnol. 2017, 183, 652–671. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Kim, M.; Lee, S.; Jin, E. Development of a Chlorella Vulgaris Mutant by Chemical Mutagenesis as a Producer for Natural Violaxanthin. Algal Res. 2020, 46, 101790. [Google Scholar] [CrossRef]
- Pumilia, G.; Cichon, M.J.; Cooperstone, J.L.; Giuffrida, D.; Dugo, G.; Schwartz, S.J. Changes in Chlorophylls, Chlorophyll Degradation Products and Lutein in Pistachio Kernels (Pistacia vera L.) during Roasting. Food Res. Int. 2014, 65, 193–198. [Google Scholar] [CrossRef]
- Wellburn, A.R. The Spectral Determination of Chlorophylls a and b, as Well as Total Carotenoids, Using Various Solvents with Spectrophotometers of Different Resolution. J. Plant Physiol. 1994, 144, 307–313. [Google Scholar] [CrossRef]
- Lopes, G.R.; Passos, C.P.; Rodrigues, C.; Teixeira, J.A.; Coimbra, M.A. Modulation of Infusion Processes to Obtain Coffee-Derived Food Ingredients with Distinct Composition. Eur. Food Res. Technol. 2019, 245, 2133–2146. [Google Scholar] [CrossRef]
- Aronoff, S.; Mackinney, G. The Photo-Oxidation of Chlorophyll. J. Am. Chem. Soc. 1943, 65, 956–958. [Google Scholar] [CrossRef]
- Tijskens, L.M.M.; Schijvens, E.; Biekman, E.S.A. Modelling the Change in Colour of Broccoli and Green Beans during Blanching. Innov. Food Sci. Emerg. Technol. 2001, 2, 303–313. [Google Scholar] [CrossRef]
- Mínguez-Mosquera, M.I.; Gandul-Rojas, B. High-Performance Liquid Chromatographic Study of Alkaline Treatment of Chlorophyll. J. Chromatogr. A 1995, 690, 161–176. [Google Scholar] [CrossRef]
- Ulbrich, M.; Schwurack, B.; Flöter, E. Alkaline Dissolution of Native Potato Starch—Impact of the Preparation Conditions on the Solution Properties Determined by Means of SEC-MALS. Starch Stärke 2017, 69, 1600256. [Google Scholar] [CrossRef]
- Weemaes, C.A.; Ooms, V.; Van Loey, A.M.; Hendrickx, M.E. Green Chilli Puree. J. Agric. Food Chem. 1999, 47, 2404–2409. [Google Scholar] [CrossRef]
- Ahmed, J.; Kaur, A.; Shivhare, U. Color Degradation Kinetics of Spinach, Mustard Leaves, and Mixed Puree. J. Food Sci. 2002, 67, 1088–1091. [Google Scholar] [CrossRef]
- Ahmed, J.; Shivhare, U.S.; Raghavan, G.S. V Rheological Characteristics and Kinetics of Colour Degradation of Green Chilli Puree. J. Food Eng. 2000, 44, 239–244. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K.; Buschmann, C. Chlorophylls and Carotenoids: Measurement and Characterization by UV-VIS Spectroscopy. Curr. Protoc. Food Anal. Chem. 2001, 1, F4.3.1–F4.3.8. [Google Scholar] [CrossRef]

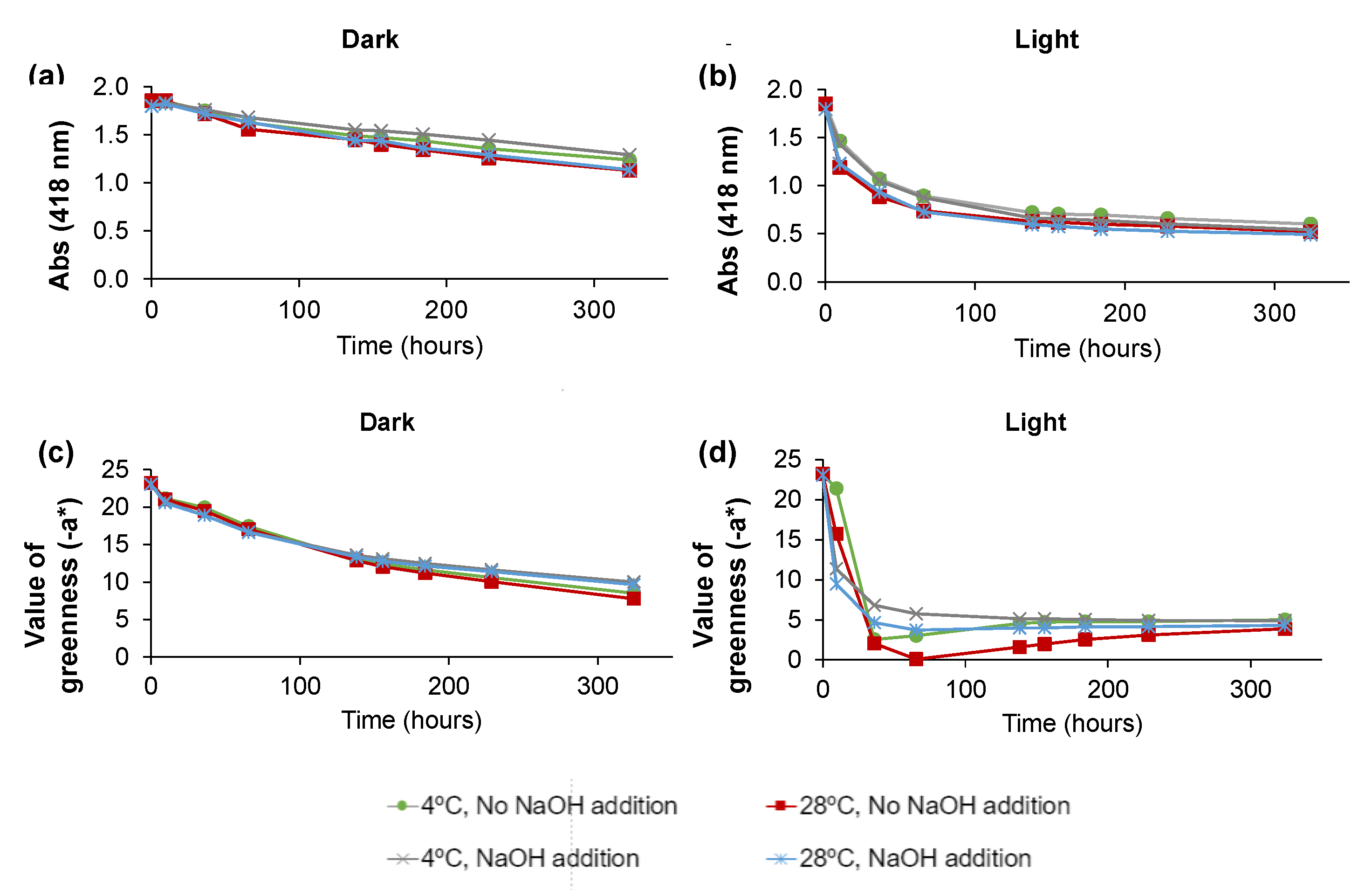
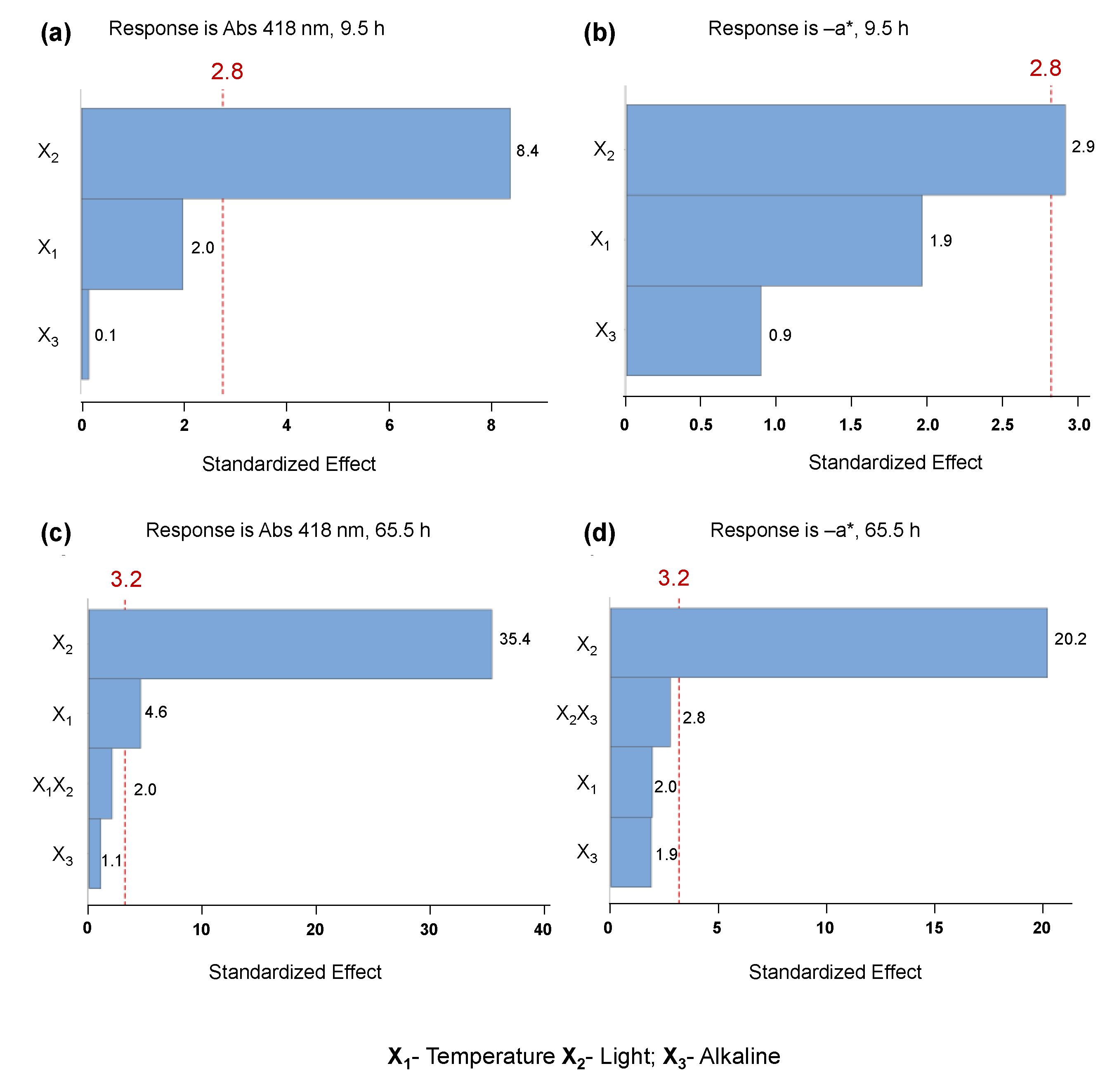
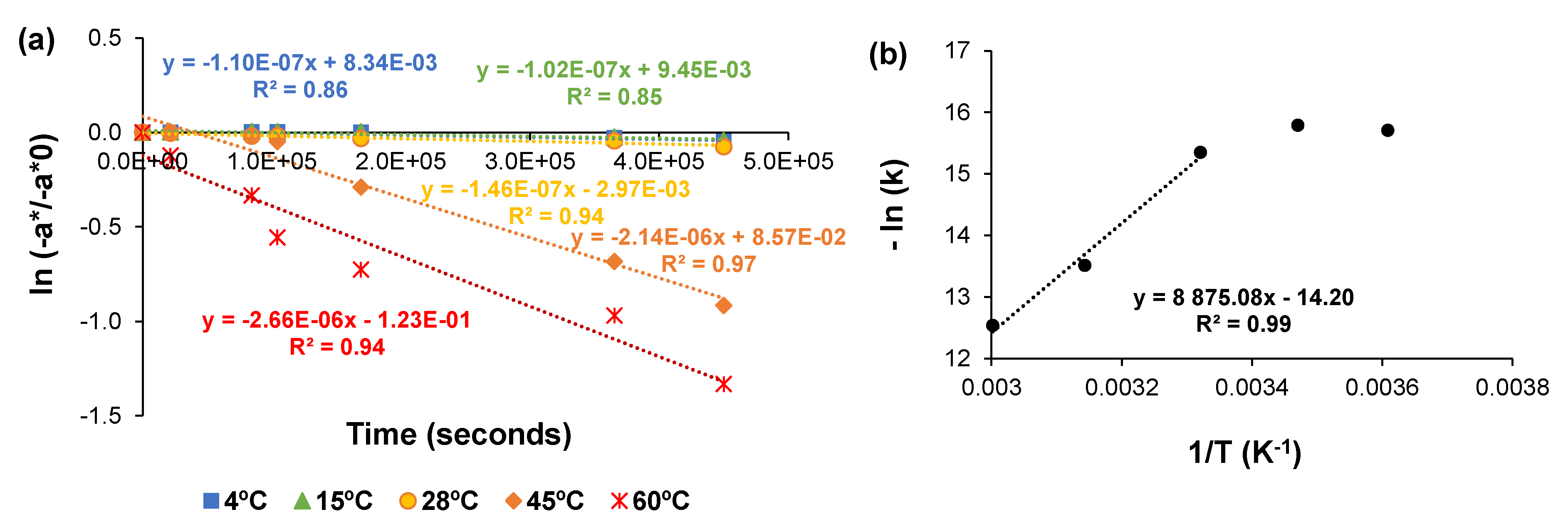

| Conditions | Runs | A418 | −a* | ||||
|---|---|---|---|---|---|---|---|
| Set 1 | Set 2 | %dif | Set 1 | Set 2 | %dif | ||
| No NaOH addition, dark | 1 | −0.0025 | −0.0029 | 13.8 | −0.0667 | −0.0892 | 25.2 |
| No NaOH addition, light | 2 | −0.0066 | −0.0163 | 59.5 | −0.1931 | −0.4264 | 54.7 |
| NaOH addition, dark | 3 | −0.0036 | −0.0012 | −66.7 | −0.0313 | −0.1048 | 70.1 |
| NaOH addition, light | 4 | −0.0073 | −0.0170 | 57.1 | −0.1981 | −0.3470 | 42.9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ferreira, A.S.; Pereira, L.; Canfora, F.; Silva, T.H.; Coimbra, M.A.; Nunes, C. Stabilization of Natural Pigments in Ethanolic Solutions for Food Applications: The Case Study of Chlorella vulgaris. Molecules 2023, 28, 408. https://doi.org/10.3390/molecules28010408
Ferreira AS, Pereira L, Canfora F, Silva TH, Coimbra MA, Nunes C. Stabilization of Natural Pigments in Ethanolic Solutions for Food Applications: The Case Study of Chlorella vulgaris. Molecules. 2023; 28(1):408. https://doi.org/10.3390/molecules28010408
Chicago/Turabian StyleFerreira, Andreia S., Liliana Pereira, Feliciana Canfora, Tiago H. Silva, Manuel A. Coimbra, and Cláudia Nunes. 2023. "Stabilization of Natural Pigments in Ethanolic Solutions for Food Applications: The Case Study of Chlorella vulgaris" Molecules 28, no. 1: 408. https://doi.org/10.3390/molecules28010408
APA StyleFerreira, A. S., Pereira, L., Canfora, F., Silva, T. H., Coimbra, M. A., & Nunes, C. (2023). Stabilization of Natural Pigments in Ethanolic Solutions for Food Applications: The Case Study of Chlorella vulgaris. Molecules, 28(1), 408. https://doi.org/10.3390/molecules28010408











