Abstract
The photo-induced denitrogenative annulations of a variety of 1-alkenylbenzotriazoles were investigated. By judiciously manipulating the structural variations of 1-alkenylbenzotriazoles, two characteristic polycyclic skeletons associated with monoterpene indole alkaloids were constructed through a diverted and controllable manner. The present work not only enriches the photochemistry of 1-alkenylbenzotriazoles, but also offers a unified approach to access skeletally diverse indole alkaloid scaffolds.
1. Introduction
Monoterpene indole alkaloids comprise a large class of natural products that exhibit diverse molecular architectures and broad biological activities [1,2]. Among them, an array of strychnos and akuammiline alkaloids featuring a 4a,9a-heterocycle-fused tetrahydrocarbazole skeleton (I, Figure 1), as shown in minfiensine (1), vincorine (2) and aspidophylline A (3), have spurred great interest of synthetic chemists in the past decades. Characterized by a unique [4.3.3]propellane core containing two adjacent quaternary stereocenters, tetracyclic skeleton I has been viewed as one of the major synthetic challenges associated with this group of natural products, and thus a plethora of synthetic approaches have been developed to access it and related scaffolds [3,4,5]. Besides the above-mentioned molecules, there also exist some monoterpene indole alkaloids that bear the same A/B/C tricyclic ring system to I but differ in the connectivity patterns between the A/B/C and D rings [1]. Taking alsmaphorazine D (4), melodinine E (5) and leuconoxine (6) as examples, all of them share a characteristic octahydropyrido[1,2-a]pyrrolo[2,3-b]indole core (II), in which the D ring is connected to A/B/C ring at N1 and C9a instead of C4a and C9a as shown in I. The structural similarity between I and II could be rationalized by their inherent biosynthetic relationship. It has been suggested that the latter should be biosynthetically derived from the former through the cleavage of the C4a–C4 bond followed by the formation of a N1–C4 bond [6,7]. Of note, although this biosynthetic hypothesis seems to be inspiring, it has remained yet to be validated in practice [8].
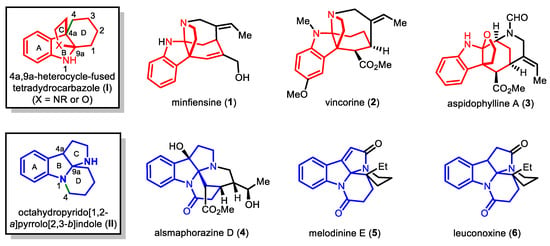
Figure 1.
Representative indole alkaloids containing the tetracyclic skeleton I and II.
1,2,3-Benzotriazoles are an important class of heterocycles that have found widespread applications in organic synthesis, medicinal chemistry, and material science [9,10,11,12,13]. Historically, it has been reported that some 1-substituted 1,2,3-benzotriazoles could undergo denitrogenative transformations to generate other valuable products. This unique reactivity has aroused considerable interest from the synthetic community in recent years, which leads to the development of a broad range of novel denitrogenative transformations of 1,2,3-benzotriazoles [14,15,16,17,18,19]. Among them, some photochemical reactions are particularly attractive due to their appealing synthetic potential and environmentally benign nature [20,21,22,23,24,25,26,27]. For example, it has been reported that 1-vinyl-1,2,3-benzotriazole 7 could undergo sequential photo-induced nitrogen extrusion, N-radical 1,3-shift, 1,5-diradical combination and tautomerization to yield the indole product 10 (Scheme 1A). From a synthetic point of view, this unique photo transformation represents a promising synthetic tool to access indole-derived natural products. However, such potential has rarely been explored, with only sporadic cases documented [22,23]. In 1980s, Wender and co-workers reported a seminal work on this subject, in which a series of natural product-relevant indole scaffolds were prepared by this method, as exemplified by the case leading to tetrahydrocarbazole 12 [22]. Another notable example was reported by Johnson and co-workers, in which a spiro-oxindole derivative 14 was obtained through the photo-induced denitrogenative annulation of 13 followed by hydrolysis of the in situ-generated indolenine intermediate [23].
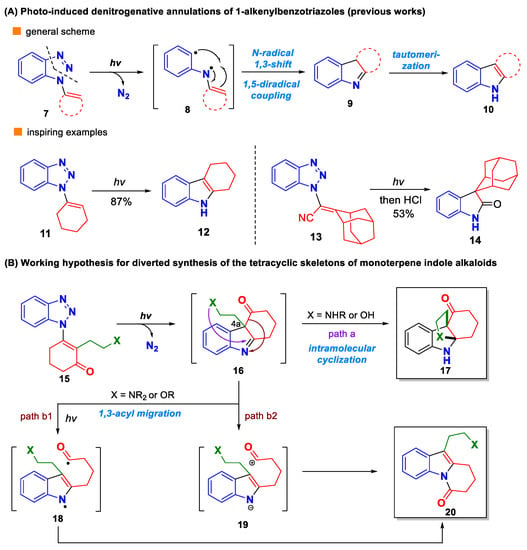
Scheme 1.
Research background and working hypothesis (A,B).
In recent years, our group has been striving to develop conceptually novel and practically useful denitrogenative transformations of 1,2,3-benzotriazoles [28,29,30]. During this course, we realized that the photo-induced denitrogenative annulation of 1-alkenylbenzotriazoles, if combined with rational design, might serve as an enabling tool for the synthesis of monoterpene indole alkaloids. As depicted in Scheme 1B, we envisioned that a functionalized 1-alkenylbenzotriazole like 15 could undergo the photo-induced denitrogenative annulation to give 2,3,3-trisubstituted indolenine intermediate 16. Once formed, the imine moiety of 16 could be captured by a nitrogen- or oxygen-derived nucleophile (e.g., NHR or OH) pre-installed on the side chain at C4a (path a), thus affording the 4a,9a-heterocycle-fused tetrahydrocarbazole derivative 17. On the other hand, if the nitrogen- or oxygen-derived nucleophile exists in a masked form (e.g., NR2, or OR), a different reaction pathway could be imagined for the intermediate 16. Based on some inspiring cases [8], 1,3-acyl migration could take place, giving rise to the dihydropyrido[1,2-a]indolone derivative 20, either through the diradical intermediate 18 (retro-photo-Fries rearrangement, path b1) [31,32,33,34,35] or zwitterion species 19 (path b2) [36,37,38,39]. Finally, 20 could be elaborated into the tetracyclic skeleton II through some additional operations. From a synthetic perspective, the above-mentioned synthetic blueprint appears to be attractive, since it enables the facile access of two different polycyclic skeletons associated with monoterpene indole alkaloids in a diverted and controllable manner.
2. Results and Discussion
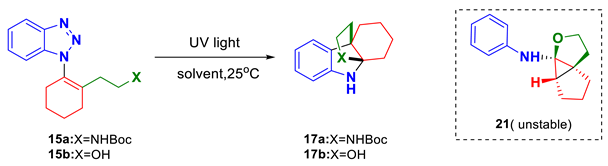
At the initial of our study, two 1-alkenylbenzotrizoles 15a and 15b, which bear a NHBoc and OH moiety on the side chain, respectively, were prepared through a couple of steps (for details, see Supplementary Materials). With these precursors in hand, we then focused our attention on exploring the designed photo-induced denitrogenative annulation reaction. Based on some relevant works [17,18,19,20,21,22], we first chose to conduct the photoreaction of 15a with MeCN as the solvent and 254 nm UV lamp as the light resource. To our disappointment, the reaction outcomes turned out to be complicated, and no desired product 17a could be identified in the reaction mixtures (Table 1, entry 1). In sharp comparison, the photoreaction of 15b appeared to be more encouraging, with the expected product 17b obtained in 23% yield (entry 2). Besides, we also identified another unstable product (ca. 30%) in the reaction, which was assigned to be the 5/3/5 tricyclic compound 21 based on the extensive spectroscopic studies. Furtherly, we conducted a condition screening using 15b as the substrate. However, only a slightly improved yield of 17b (30%) was obtained when the reaction was performed in THF (entry 3).

Table 1.
Condition optimization of the photoreaction.
The above-mentioned results suggest that although the designed photo-induced denitrogenative annulation is feasible, the reaction outcomes largely rely on the judicious manipulation of the substrate structures. The distinct results associated with 15a and 15b indicate that a nucleophile with favorable steric and electronic nature should be employed to capture the in situ-generated indolenine intermediate. Apparently, the OH group appears to be a better choice than the NHBoc for the current reaction. On the other hand, the identification of byproduct 21 indicated that there exist some unexpected reaction pathways besides the desired one. The plausible mechanism of the photoreaction of 15b is outlined in Scheme 2. Simply, upon photo irradiation, 15b will undergo nitrogen extrusion to generate diradical species I-15b. Subsequently, I-15b will go through radical 1,3-shift to give 1,5-diradicals II-15b and III-15b as a pair of cis/trans isomers. Between them, the cis-isomer II-15b could advance to 17b though 1,5-diradical combination followed by imine capture by the hydroxyl group. Comparably, for trans-isomer III-15b, a 1,5-hydrogen abstraction will take place preferentially, leading to a new 1,3-diradical species (V-15b) [40]. Finally, a 1,3-diradical combination followed by the cyclization of the hydroxyl group onto the imine moiety will give rise to the observed byproduct 21.
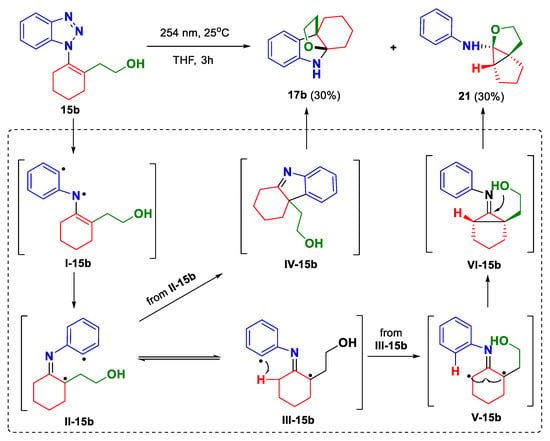
Scheme 2.
Initial results of the photo-induced denitrogenative annulation.
Having the mechanistic rationalization in mind, we sought to improve the reaction by judiciously manipulating the substrate structure. As we envisioned, introducing two substitutions on the C1 position of the substrate (e.g., 15c and 15d in Scheme 3) will facilitate the reaction from two aspects. First, the competitive 1,5-hydrogen abstraction would be precluded in this scenario due to the absence of suitable C–H bonds on the C1 position. Second, the equilibrium between the cis- and trans-imine intermediates will shift towards the desired direction. Taking 15c as an example, II-15c should be thermodynamically more favorable than III-15c, and thus the desired reaction pathway would take place preferentially. To our delight, this speculation was validated quickly in practice. As shown in Scheme 3, when 1-alkenylbenzotrizoles 15c and 15d were submitted to the photoreaction, the corresponding annulation products 17c (56%) and 17d (54%) were obtained in notably improved yields.
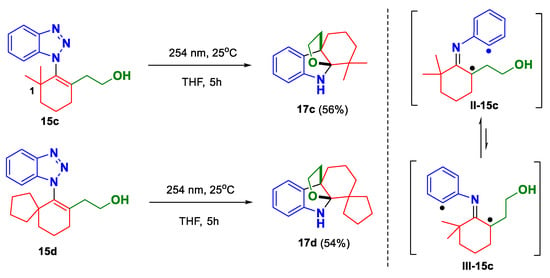
Scheme 3.
Photo-induced denitrogenative annulation of 1-alkenylbenzotrizoles 15c and 15d.
Besides the C1 substitute effect, we found that the structural variation on the side chain also exerted a profound effect on the reaction outcome. Indeed, when 1-alkenylbenzotrizole 15e bearing a carboxylic acid on side chain was attempted in the photoreaction, a different result was obtained. In this case, two products were isolated, between which the minor component turned out to be the desired product 17e (38%) and the major one was assigned as tetrahydrocarbazole 22 (Scheme 4A). The structure of 22 was confirmed by the comparison of its spectroscopic data with those reported in literature [41]. Although the exact mechanism for the formation of 22 remains unclear at this stage, a plausible one is suggested in Scheme 4B. Naturally, the reaction also starts from the denitrogenation of 15e. However, different from the above-mentioned cases, in the current scenario the resulting I-15e more likely advances to the zwitterion species II-15e and III-15e through N-radical 1,3-shift and intramolecular proton transfer from the carboxylic acid to the imine moiety. Between them, II-15e could convert to 17e following the same pathway as suggested above. Comparably, III-15e might go through an intramolecular 1,5-hydrogen transfer followed by radical 1,3-shift to yield N-radical cation species IV-15e. Subsequently, a Nazarov-type cyclization would take place, and the resulting product V-15e could undergo sequential tautomerization and decarboxylation to yield byproduct 22.
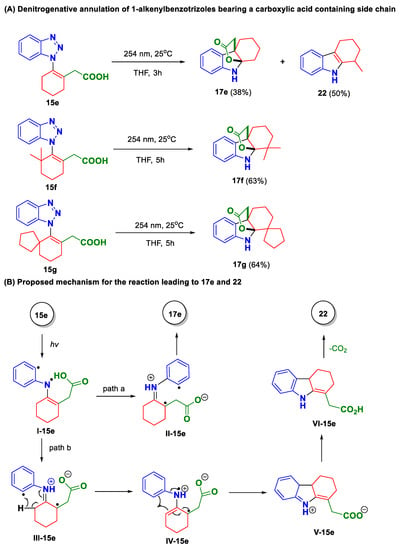
Scheme 4.
Photo-induced denitrogenative annulation of 1-alkenylbenzotrizole bearing a carboxylic acid-containing side chain (A,B).
Taking the above outcomes into consideration, we decided to integrate the structural modifications on the C-1 position and C-4a side chain into a single molecule. To this end, 1-alkenylbenzotrizoles 15f and 15g were synthetized. As we expected, both 15f and 15g proved to be excellent substrates for the photoreaction by providing the corresponding products in good yields (17f: 63%; 17g: 64%).
Having the 4a,9a-heterocycle-fused tetrahydrocarbazole skeleton (I) secured in an acceptable efficiency, we then moved to explore the proposed chemistry leading to the tetracyclic skeleton II (Figure 1). According to our design, two structural modifications should be implemented on the 1-alkenylbenzotrizoles. First, the nucleophile on the side chain should be masked, and thus the in situ-generated indolenine intermediate (e.g., 16, Scheme 1) could not be captured. Secondly, a carbonyl group should be introduced onto the C-4 position of the cyclohexene unit, which allows the proposed 1,3-acyl migration to take place. Taking these rationalizations in mind, 1-alkenylbenzotrizoles 15h and 15i were prepared and evaluated in the photoreactions. To our delight, the designed chemistry worked well under the conventional photo conditions (254 nm UV, CH3CN, 25 ℃), with the desired dihydropyrido[1,2-a]indolone derivatives 20h and 20i obtained in 42% and 35% yields, respectively. Although the yields of the above reactions appeared to be moderated, their overall efficiency is appreciable, since they actually integrate several transformations into a single operation.
Naturally, we also attempted the carboxylate-derived 1-alkenylbenzotrizoles 15j and 15k in the photoreaction. Again, these two substrates showed slightly different reactivity from 15h and 15i. As shown, besides the expected products 20j and 20k, we also identified another product in these reactions, which were assigned to be the tricyclic compounds 23 and 24, respectively. Interestingly, compared with 20j and 20k, 23 and 24 not only showed different tricyclic ring systems, but also lost a unit of -CH2CO2Me. A plausible mechanism for the above-mentioned transformations is suggested in Scheme 5B. Taking 15j as example, a photo-induced denitrogenative annulation will take place first, leading to indolenine intermediate 16j. Without a suitable nucleophile to trap the imine moiety, 16j would go through two different pathways upon further photo irradiation. On one hand, the proposed retro-photo-Fries rearrangement will take place through the cleavage of C4a-C4 bond (path a), and the resulting diradical species 18j readily converts to the product 20j through 1,6-radical combination. On the other hand, the cleavage of C4a-C5 bond might also occur as a competitive pathway (path b), with the release of an α-carbonyl radical species. Subsequently, the resulting 18j’ will abstract a hydrogen form the reaction system to yield the byproduct 23. Interestingly, we did not observe similar byproducts in the reactions with 15h and 15i, indicating that the generation of a stabilized α-carbonyl radical species should be the driving force of path b.
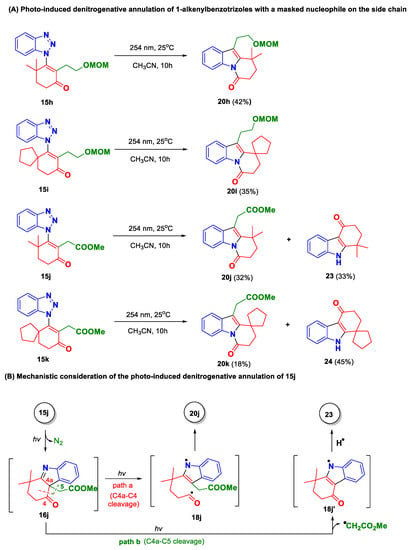
Scheme 5.
Photo-induced denitrogenative annulation of 1-alkenylbenzotrizoles with a masked nucleophile on the side chain (A,B).
3. Materials and Methods
3.1. General Information
Commercially available reagents were purchased from commercial sources and used as received without further purification. If no further details are given, the reaction was performed under ambient atmosphere and temperature. Analytical thin layer chromatography (TLC) was performed on silica gel-coated plates (Merck, 60 F254) with the indicated solvent mixture, and visualization was performed using ultraviolet (UV) irradiation (λ = 254 nm) and/or staining with aqueous KMnO4. If not specially mentioned, flash column chromatography used silica gel (200–300 mesh) supplied by Tsingtao Haiyang Chemicals (Qingdao, China).
1H NMR spectra were recorded on a Bruker Avance III 400 (400 MHz) spectrometer. TMS (δH 0.00) were used as the internal reference. 13C NMR spectra were recorded on a Bruker Advance III 400 (100 MHz) spectrometer in CDCl3 (δC 77.16) using their central resonance as the internal reference. All 13C NMR spectra were proton decoupled. High-resolution mass spectra (HRMS) were recorded on a Waters Xevo G2 QTOF MS. A commercially available UV lamp (model: Philips TUV 25W/G25 T8, emission wave-length range: 200–280 nm; λmax: 254 nm) was used as light resource.
3.2. General Procedure
A quartz tube was charged with 1-alkenylbenzotriazoles (40 mg) at N2 atmosphere. The freshly distilled THF or MeCN (5.0 mL) was added and the reaction mixture was stirred at room temperature for 3–10 h upon photolysis (254 nm). The resulting solution was concentrated in vacuo. The residue was directly purified by flash chromatography (silica gel, 200–300 mesh, hexanes/EtOAc or CH2Cl2/MeOH) to yield the corresponding product (Irradiation system, see Supplementary Materials).
2-(2-(1H-Benzo[d][1,2,3]triazol-1-yl)cyclohex-1-en-1-yl) ethanol (15b): colorless oil; Yield: 86%; 1H NMR (400 MHz, CDCl3) δ 1.78–1.93 (m, 4H), 1.99 (t, J = 6.5 Hz, 2H), 2.33–2.39 (m, 2H), 2.41–2.47 (m, 2H), 2.53 (t, J = 5.3 Hz, 1H), 3.59–3.65 (m, 2H), 7.35 (ddd, J = 8.1, 5.9, 1.9 Hz, 1H), 7.42–7.51 (m, 2H), 8.02 (dd, J = 8.4, 1.0 Hz, 1H). 13C NMR (100 MHz, CDCl3) δ 145.6, 136.6, 132.9, 130.0, 127.8, 124.1, 120.1, 110.3, 60.0, 35.5, 29.6, 28.8, 22.9, 22.2. HRMS m/z calcd for C14H18N3O [M+H]+: 244.1450; found: 244.1449.
(4bR,8aR)-5,6,7,8-Tetrahydro-9H-8a,4b-(epoxyethano)carbazole (17b): white solid; Yield: 30%; 1H NMR (400 MHz, CDCl3) δ 1.35–1.47 (m, 3H), 1.60–1.68 (m, 2H), 1.70–1.80 (m, 1H), 1.92–1.99 (m, 2H), 2.17–2.25 (m, 2H), 3.57–3.67 (m, 1H), 3.89–3.96 (m, 1H), 4.30 (s, 1H), 6.61 (m, 1H), 6.76 (t, J = 7.4 Hz, 1H), 7.02–7.08 (m, 2H).13C NMR (100 MHz, CDCl3) δ 148.3, 135.6, 127.8, 122.8, 119.2, 109.2, 102.4, 66.2, 53.5, 38.0, 33.1, 32.4, 20.8, 19.7. HRMS m/z calcd for C14H18NO [M+H]+: 216.1388; found: 216.1387.
N-Phenylhexahydro-3aH-cyclopenta[2,3]cyclopropa[1,2-b]furan-3a-amine (21): white solid; Yield: ca. 30%; 1H NMR (400 MHz, CDCl3) δ 1.49–1.64 (m, 2H), 1.70–1.99 (m, 4H), 1.97–2.07 (m, 1H), 2.05–2.16 (m, 1H), 2.46 (q, J = 10.2 Hz, 1H), 3.53 (ddd, J = 10.1, 9.0, 7.7 Hz, 1H), 4.12 (td, J = 9.0, 2.2 Hz, 1H), 4.62 (s, 1H), 6.72–6.81 (m, 3H), 7.18 (td, J = 8.5, 7.2 Hz, 2H). 13C NMR (100 MHz, CDCl3) δ 145.8, 129.3, 118.7, 113.8, 79.4, 65.7, 39.8, 31.9, 30.8, 28.6, 26.3, 23.2. HRMS m/z calcd for C14H18NO [M+H]+: 216.1388; found: 216.1387.
2-(2-(1H-Benzo[d][1,2,3]triazol-1-yl)-3,3-dimethylcyclohex-1-en-1-yl) ethanol (15c): colorless oil; Yield: 92%; 1H NMR (400 MHz, CDCl3) δ 0.89 (s, 3H), 1.15 (s, 3H), 1.50 (s, 1H), 1.56–1.74 (m, 2H), 1.75–1.83 (m, 2H), 1.83–1.94 (m, 2H), 2.26–2.46 (m, 2H), 3.50 (m, 2H), 7.36 (ddd, J = 8.1, 6.6, 1.4 Hz, 1H), 7.40–7.51 (m, 2H), 8.06 (dt, J = 8.3, 1.1 Hz, 1H). 13C NMR (100 MHz, CDCl3) δ 145.1, 138.2, 137.3, 134.8, 127.7, 123.9, 119.9, 110.9, 60.2, 39.2, 36.5, 35.8, 30.0, 28.5, 27.9, 19.0. HRMS m/z calcd for C16H22N3O [M+H]+: 272.1763; found: 272.1761.
(4bR,8aR)-8,8-Dimethyl-5,6,7,8-tetrahydro-9H-8a,4b-(epoxyethano)carbazole (17c): white solid; Yield: 56%; M.p. 106–107 ℃; 1H NMR (400 MHz, CDCl3) δ 1.08 (s, 3H), 1.09 (s, 3H), 1.36–1.45 (m, 3H), 1.51–1.66 (m, 2H), 2.05–2.15 (m, 1H), 2.20–2.37 (m, 2H), 3.59 (ddd, J = 10.5, 8.1, 6.5 Hz, 1H), 3.91 (td, J = 8.1, 1.8 Hz, 1H), 4.41 (s, 1H), 6.63 (dt, J = 7.4, 1.0 Hz, 1H), 6.75 (td, J = 7.4, 1.0 Hz, 1H), 7.04 (t, J = 7.5 Hz, 2H). 13C NMR (100 MHz, CDCl3) δ 147.8, 136.9, 127.7, 122.3, 119.0, 109.3, 105.9, 67.4, 54.3, 38.5, 37.4, 36.3, 33.6, 26.5, 25.2, 17.5. HRMS m/z calcd for C16H22NO [M+H]+: 244.1701; found: 244.1702.
2-(6-(1H-Benzo[d][1,2,3]triazol-1-yl)spiro[4.5]dec-6-en-7-yl) ethanol (15d): colorless oil; Yield: 91%; 1H NMR (400 MHz, CDCl3) δ 1.03–1.13 (m, 1H), 1.13–1.19 (m, 1H), 1.20–1.31 (m, 1H), 1.35–1.75 (m, 7H), 1.78–1.88 (m, 4H), 2.03–2.15 (m, 1H), 2.26–2.38 (m, 2H), 3.39–3.52 (m, 2H), 7.33 (ddd, J = 8.1, 6.6, 1.3 Hz, 1H), 7.38–7.49 (m, 2H), 7.97–8.04 (m, 1H). 13C NMR (100 MHz, CDCl3) δ 145.0, 138.9, 135.9, 135.1, 127.8, 123.9, 119.9, 110.7, 60.3, 47.5, 37.6, 36.5, 36.0, 36.0, 29.7, 24.2, 23.9, 19.7. HRMS m/z calcd for C18H24N3O [M+H]+: 298.1919; found: 298.1921.
(4b′R,8a′R)-6′,7′-Dihydro-5′H,9′H-spiro[cyclopentane-1,8′-[8a,4b](epoxyethano)carbazole] (17d): white solid; Yield: 54%; M.p. 84–85 ℃; 1H NMR (400 MHz, CDCl3) δ 1.34–1.50 (m, 4H), 1.52–1.75 (m, 7H), 1.80–1.90 (m, 2H), 1.92–2.01 (m, 1H), 2.10–2.25 (m, 2H), 3.54 (ddd, J = 11.3, 8.3, 5.8 Hz, 1H), 3.91 (ddd, J = 8.7, 7.7, 1.3 Hz, 1H), 4.38 (s, 1H), 6.57 (dt, J = 7.6, 0.8 Hz, 1H), 6.72 (td, J = 7.4, 1.0 Hz, 1H), 6.99–7.06 (m, 2H). 13C NMR (100 MHz, CDCl3) δ 148.9, 135.7, 127.8, 122.8, 118.6, 108.3, 106.2, 66.4, 54.7, 49.0, 39.7, 35.8, 34.3, 32.5, 32.2, 26.2, 25.0, 17.1. HRMS m/z calcd for C18H24NO [M+H]+: 270.1858; found: 270.1857.
2-(2-(1H-Benzo[d][1,2,3]triazol-1-yl) cyclohex-1-en-1-yl) acetic acid (15e): white solid; Yield: 93%; 1H NMR (400 MHz, CDCl3) δ 1.79–2.00 (m, 4H), 2.38–2.58 (m, 4H), 2.86 (s, 2H), 7.39 (m, 1H), 7.44–7.56 (m, 2H), 8.07 (d, J = 8.3 Hz, 1H), 11.34 (s, 1H). 13C NMR (100 MHz, CDCl3) δ 174.9, 145.2, 133.0, 132.3, 131.7, 128.1, 124.4, 119.9, 110.3, 37.8, 29.8, 29.4, 22.7, 22.0. HRMS m/z calcd for C14H16N3O2 [M+H]+: 258.1243; found: 258.1244.
(4bR,8aR)-5,6,7,8-Tetrahydro-9H-8a,4b-(epoxyethano)carbazol-11-one (17e): yellow solid; Yield: 38%; 1H NMR (400 MHz, CDCl3) δ 1.24–1.46 (m, 3H), 1.60–1.83 (m, 3H), 2.06–2.12 (m, 1H), 2.36–2.44 (m, 1H), 2.84–2.99 (m, 2H), 4.82 (s, 1H), 6.72 (dt, J = 7.8, 0.8 Hz, 1H), 6.85 (td, J = 7.5, 1.0 Hz, 1H), 7.06–7.16 (m, 2H). 13C NMR (100 MHz, CDCl3) δ 174.1, 145.8, 134.9, 128.8, 123.1, 120.7, 110.4, 50.3, 38.4, 33.4, 33.0, 22.1, 20.2. HRMS m/z calcd for C14H16NO2 [M+H]+: 230.1181; found: 230.1183.
1-Methyl-2,3,4,9-tetrahydro-1H-carbazole (22): light yellow oil; Yield: 50%; 1H NMR (400 MHz, CDCl3) δ 1.31 (d, J = 7.0 Hz, 3H), 1.49–1.58 (m, 1H), 1.71–1.85 (m, 1H), 1.96–2.09 (m, 2H), 2.66–2.78 (m, 2H), 2.93–3.05 (m, 1H), 7.04–7.16 (m, 2H), 7.27–7.33 (m, 1H), 7.44–7.50 (m, 1H), 7.76 (s, 1H). 13C NMR (100 MHz, CDCl3) δ 138.7, 135.8, 127.8, 121.2, 119.3, 118.1, 110.5, 109.9, 32.5, 28.8, 22.1, 21.3, 20.4. HRMS m/z calcd for C13H16N [M+H]+: 186.1283; found: 186.1278.
2-(2-(1H-Benzo[d][1,2,3]triazol-1-yl)-3,3-dimethylcyclohex-1-en-1-yl)acetic acid (15f): white solid; Yield: 94%; M.p. 190–191 °C; 1H NMR (400 MHz, CDCl3) δ 0.86 (s, 3H), 1.18 (s, 3H), 1.77–1.83 (m, 2H), 1.85–1.96 (m, 2H), 2.30 (dt, J = 17.9, 5.8 Hz, 1H), 2.40 (d, J = 16.7 Hz, 1H), 2.48 (dt, J = 18.1, 6.4 Hz, 1H), 2.58 (d, J = 16.7 Hz, 1H), 7.33 (ddd, J = 7.9, 6.5, 1.2 Hz, 1H), 7.38–7.47 (m, 2H), 8.04 (d, J = 8.2 Hz, 1H), 11.31 (s, 1H). 13C NMR (100 MHz, CDCl3) δ 175.4, 144.8, 138.9, 134.8, 134.1, 127.9, 124.2, 119.7, 111.1, 39.0, 37.7, 36.6, 30.4, 28.3, 27.6, 18.8. HRMS m/z calcd for C16H20N3O2 [M+H]+: 286.1556; found: 286.1556.
(4bR,8aR)-8,8-Dimethyl-5,6,7,8-tetrahydro-9H-8a,4b-(epoxyethano)carbazol-11-one (17f): white solid; Yield: 63%; M.p. 117–118 °C; 1H NMR (400 MHz, CDCl3) δ 1.12 (s, 3H), 1.23 (s, 3H), 1.31–1.41 (m, 1H), 1.45–1.54 (m, 3H), 1.55–1.67 (m, 1H), 2.08–2.17 (m, 1H), 2.84–3.01 (m, 2H), 4.81 (s, 1H), 6.72 (dt, J = 7.8, 0.7 Hz, 1H), 6.84 (td, J = 7.5, 1.0 Hz, 1H), 7.04 (dd, J = 7.5, 1.2 Hz, 1H), 7.11 (td, J = 7.7, 1.3 Hz, 1H). 13C NMR (100 MHz, CDCl3) δ 174.8, 145.2, 136.1, 128.7, 122.7, 120.7, 110.4, 51.4, 40.7, 37.3, 37.2, 33.9, 26.4, 23.8, 17.3. HRMS m/z calcd for C16H20NO2 [M+H]+: 258.1494; found: 258.1491.
2-(6-(1H-Benzo[d][1,2,3]triazol-1-yl)spiro[4.5]dec-6-en-7-yl)acetic acid (15g): white solid; Yield: 84%; M.p. 204–205 °C; 1H NMR (400 MHz, CDCl3) δ 1.05–1.21 (m, 2H), 1.22–1.32 (m, 1H), 1.39–1.59 (m, 3H), 1.60–1.70 (m, 1H), 1.72–1.91 (m, 4H), 2.11–2.22 (m, 1H), 2.23–2.32 (m, 1H), 2.35 (d, J = 16.7 Hz, 1H), 2.43–2.51 (m, 1H), 2.56 (d, J = 16.7 Hz, 1H), 7.35 (ddd, J = 8.1, 6.2, 1.7 Hz, 1H), 7.38–7.47 (m, 2H), 8.04 (d, J = 8.3 Hz, 1H), 10.40 (s, 1H). 13C NMR (100 MHz, CDCl3) δ 175.4, 144.8, 137.7, 135.0, 134.7, 127.9, 124.2, 119.8, 110.9, 47.6, 37.9, 37.7, 36.5, 36.0, 30.2, 24.3, 24.1, 19.6. HRMS m/z calcd for C18H22N3O2 [M+H]+: 312.1712; found: 312.1708.
(4b′R,8a′R)-6′,7′-Dihydro-5′H,9′H-spiro[cyclopentane-1,8′-[8a,4b](epoxyethano)carbazol]-11′-one (17g): white solid; Yield: 64%; 1H NMR (400 MHz, CDCl3) δ 1.37–1.79 (m, 12H), 1.90–2.13 (m, 2H), 2.88 (d, J = 2.9 Hz, 2H), 4.83 (s, 1H), 6.71 (d, J = 7.8 Hz, 1H), 6.83 (t, J = 7.4 Hz, 1H), 7.05 (d, J = 7.4 Hz, 1H), 7.11 (t, J = 7.7 Hz, 1H). 13C NMR (100 MHz, CDCl3) δ 175.0, 145.6, 135.8, 128.7, 122.8, 120.5, 110.2, 51.6, 48.9, 40.1, 36.1, 33.5, 32.4, 25.3, 23.1, 17.3. HRMS m/z calcd for C18H22NO2 [M+H]+: 284.1651; found: 284.1656.
3-(1H-Benzo[d][1,2,3]triazol-1-yl)-2-(2-(methoxymethoxy)ethyl)-4,4-dimethylcyclohex-2-en-1-one (15h): light yellow oil; Yield: 72%; 1H NMR (400 MHz, CDCl3) δ 0.95 (s, 3H), 1.39 (s, 3H), 1.92 (ddd, J = 13.3, 7.6, 5.8 Hz, 1H), 2.09–2.25 (m, 3H), 2.67–2.87 (m, 2H), 3.09 (s, 3H), 3.21–3.37 (m, 2H), 4.28–4.35 (m, 2H), 7.36–7.44 (m, 2H), 7.52 (ddd, J = 8.1, 7.0, 1.1 Hz, 1H), 8.11 (dt, J = 8.4, 1.0 Hz, 1H). 13C NMR (100 MHz, CDCl3) δ 198.1, 156.8, 145.2, 136.0, 134.0, 128.4, 124.3, 120.3, 110.4, 95.9, 65.3, 55.1, 38.0, 37.1, 34.7, 27.0, 26.3, 26.1. HRMS m/z calcd for C18H24N3O3 [M+H]+: 330.1818; found: 330.1805.
10-(2-(Methoxymethoxy)ethyl)-9,9-dimethyl-8,9-dihydropyrido[1,2-a]indol-6(7H)-one (20h): colorless oil; Yield: 42%; 1H NMR (400 MHz, CDCl3) δ 1.53 (s, 6H), 1.95 (t, J = 6.6 Hz, 2H), 2.81 (dd, J = 7.1, 6.2 Hz, 2H), 3.16 (dd, J = 8.2, 7.2 Hz, 2H), 3.36 (s, 3H), 3.78 (dd, J = 8.3, 7.2 Hz, 2H), 4.65 (s, 2H), 7.26–7.34 (m, 2H), 7.46–7.52 (m, 1H), 8.47–8.53 (m, 1H). 13C NMR (100 MHz, CDCl3) δ 169.5, 141.3, 134.5, 131.1, 124.8, 123.9, 118.0, 116.8, 113.6, 96.6, 67.3, 55.4, 37.0, 32.7, 30.9, 28.6, 25.7. HRMS m/z calcd for C18H24NO3 [M+H]+: 302.1756; found: 302.1751.
6-(1H-Benzo[d][1,2,3]triazol-1-yl)-7-(2-(methoxymethoxy)ethyl)spiro[4.5]dec-6-en-8-one (15i): light yellow oil; Yield: 74%; 1H NMR (400 MHz, CDCl3) δ 1.20–1.28 (m, 2H), 1.39–1.63 (m, 4H), 1.81–1.99 (m, 2H), 2.10–2.22 (m, 3H), 2.31–2.42 (m, 1H), 2.65–2.81 (m, 2H), 3.08 (s, 3H), 3.22–3.34 (m, 2H), 4.26–4.34 (m, 2H), 7.35–7.44 (m, 2H), 7.53 (ddd, J = 8.1, 7.0, 1.0 Hz, 1H), 8.11 (dt, J = 8.3, 1.0 Hz, 1H). 13C NMR (100 MHz, CDCl3) δ 198.3, 155.9, 145.2, 136.5, 134.3, 128.5, 124.4, 120.3, 110.3, 95.8, 65.4, 55.1, 48.7, 36.9, 35.2, 34.6, 33.9, 26.5, 24.5, 24.3. HRMS m/z calcd for C20H26N3O3 [M+H]+: 356.1974; found: 356.1961.
10′-(2-(Methoxymethoxy)ethyl)-7′,8′-dihydro-6′H-spiro[cyclo-pentane-1,9′-pyrido[1,2-a]indol]-6′-one (20i): light yellow oil; Yield: 35%; 1H NMR (400 MHz, CDCl3) δ 1.79–1.96 (m, 6H), 1.99 (dd, J = 7.1, 6.0 Hz, 2H), 2.15–2.29 (m, 2H), 2.76 (dd, J = 7.1, 5.9 Hz, 2H), 3.10 (dd, J = 8.3, 7.2 Hz, 2H), 3.36 (s, 3H), 3.81 (dd, J = 8.3, 7.2 Hz, 2H), 4.65 (s, 2H), 7.23–7.32 (m, 2H), 7.46–7.51 (m, 1H), 8.46–8.52 (m, 1H). 13C NMR (100 MHz, CDCl3) δ 169.7, 141.9, 134.5, 131.1, 124.6, 123.8, 118.0, 116.8, 112.8, 96.5, 67.0, 55.3, 43.4, 39.3, 34.5, 31.7, 25.7, 25.6. HRMS m/z calcd for C20H26NO3 [M+H]+: 328.1913; found: 328.1909.
Methyl 2-(2-(1H-benzo[d][1,2,3]triazol-1-yl)-3,3-dimethyl-6-oxocyclohex-1-en-1-yl) acetate (15j): light yellow oil; Yield: 73%; 1H NMR (400 MHz, CDCl3) δ 0.95 (s, 3H), 1.45 (s, 3H), 2.11–2.27 (m, 2H), 2.48 (d, J = 16.9 Hz, 1H), 2.77–2.86 (m, 2H), 3.03 (d, J = 16.9 Hz, 1H), 3.50 (s, 3H), 7.37–7.47 (m, 2H), 7.51 (ddd, J = 8.0, 6.8, 1.0 Hz, 1H), 8.11 (d, J = 8.4 Hz, 1H). 13C NMR (100 MHz, CDCl3) δ 197.3, 170.4, 157.4, 145.2, 134.0, 132.8, 128.7, 124.6, 120.3, 110.3, 52.1, 38.0, 36.9, 34.2, 31.2, 26.7, 25.9. HRMS m/z calcd for C17H20N3O3 [M+H]+: 314.1505; found: 314.1498.
Methyl 2-(9,9-dimethyl-6-oxo-6,7,8,9-tetrahydropyrido[1,2-a]indol -10-yl) acetate (20j): colorless oil; Yield: 32%; 1H NMR (400 MHz, CDCl3) δ 1.52 (s, 6H), 1.97 (t, J = 6.7 Hz, 2H), 2.83 (dd, J = 7.1, 6.2 Hz, 2H), 3.70 (s, 3H), 3.86 (s, 2H), 7.26–7.35 (m, 2H), 7.44–7.49 (m, 1H), 8.47–8.53 (m, 1H). 13C NMR (100 MHz, CDCl3) δ 171.6, 169.4, 142.1, 134.4, 130.8, 125.0, 124.1, 117.9, 116.8, 109.9, 52.3, 36.9, 32.6, 30.9, 30.7, 28.2. HRMS m/z calcd for C17H20NO3 [M+H]+: 286.1443; found: 286.1449.
1,1-Dimethyl-1,2,3,9-tetrahydro-4H-carbazol-4-one (23): white solid; Yield: 33%; M.p. 240–241 ℃; 1H NMR (400 MHz, CDCl3) δ 1.48 (s, 6H), 2.10 (dd, J = 7.0, 6.0 Hz, 2H), 2.68 (dd, J = 7.0, 6.0 Hz, 2H), 7.21–7.26 (m, 2H), 7.34–7.40 (m, 1H), 8.21–8.29 (m, 1H), 8.65 (s, 1H). 13C NMR (100 MHz, CDCl3) δ 194.2, 158.1, 135.7, 125.1, 123.5, 122.8, 121.9, 111.6, 111.1, 38.7, 35.6, 32.2, 27.5. HRMS m/z calcd for C14H16NO [M+H]+: 214.1232; found: 214.1227.
Methyl 2-(6-(1H-benzo[d][1,2,3]triazol-1-yl)-8-oxospiro[4.5]dec-6-en-7-yl)acetate (15k): light yellow oil; Yield: 66%; 1H NMR (400 MHz, CDCl3) δ 1.19–1.29 (m, 2H), 1.37–1.58 (m, 2H), 1.60–1.70 (m, 2H), 1.96 (ddd, J = 12.9, 7.7, 4.3 Hz, 1H), 2.20 (t, J = 6.3 Hz, 2H), 2.38–2.51 (m, 2H), 2.77 (td, J = 6.9, 5.9, 4.1 Hz, 2H), 3.00 (d, J = 16.8 Hz, 1H), 3.49 (s, 3H), 7.38–7.48 (m, 2H), 7.49–7.56 (m, 1H), 8.11 (d, J = 8.3 Hz, 1H). 13C NMR (100 MHz, CDCl3) δ 197.6, 170.4, 156.6, 145.1, 134.2, 133.3, 128.7, 124.6, 120.3, 110.3, 52.1, 48.7, 36.8, 34.8, 34.8, 33.9, 31.2, 24.6, 24.5. HRMS m/z calcd for C19H22N3O3 [M+H]+: 340.1661; found: 340.1653.
Methyl 2-(6′-oxo-7′,8′-dihydro-6′H-spiro[cyclopentane-1,9′-pyrido[1,2-a]indol]-10′- yl)acetate (20k): light yellow oil; Yield: 18%; 1H NMR (400 MHz, CDCl3) δ 1.81–1.96 (m, 6H), 2.02 (t, J = 6.5 Hz, 2H), 2.15–2.24 (m, 2H), 2.78 (dd, J = 7.0, 6.0 Hz, 2H), 3.71 (s, 3H), 3.80 (s, 2H), 7.27–7.35 (m, 2H), 7.40–7.47 (m, 1H), 8.43–8.53 (m, 1H). 13C NMR (100 MHz, CDCl3) δ 171.6, 169.7, 142.9, 134.4, 130.8, 124.9, 124.0, 118.0, 116.8, 109.4, 52.4, 43.2, 39.3, 34.7, 31.8, 30.7, 25.9. HRMS m/z calcd for C19H22NO3 [M+H]+: 312.16; found: 312.1588.
2,3-Dihydrospiro[carbazole-1,1′-cyclopentan]-4(9H)-one (24): white solid; Yield: 45%; M.p. 229–230 °C; 1H NMR (400 MHz, CDCl3) δ 1.84–1.93 (m, 4H), 1.93–2.08 (m, 4H), 2.14 (dd, J = 7.0, 5.9 Hz, 2H), 2.65 (dd, J = 7.0, 5.9 Hz, 2H), 7.20–7.26 (m, 2H), 7.32–7.41 (m, 1H), 8.21–8.29 (m, 1H), 8.89 (s, 1H). 13C NMR (100 MHz, CDCl3) δ 194.5, 158.3, 135.8, 125.1, 123.4, 122.7, 121.7, 112.3, 111.1, 43.3, 38.2, 36.6, 36.2, 25.5. HRMS m/z calcd for C16H18NO [M+H]+: 240.1388; found: 240.1386.
4. Conclusions
In summary, we systematically explored the photo-induced denitrogenative transformations of various 1-alkenylbenzotrizoles. Through rationally manipulating the structures of 1-alkenylbenzotrizole precursors, we could access two different types of polycyclic skeletons associated with monoterpene indole alkaloids. Specifically, starting from 1-alkenylbenzotrizoles bearing a suitable nucleophile (e.g., OH and COOH) on their side chains, the 4a,9a-heterocycle-fused tetrahydrocarbazole skeleton could be assembled through a photo-induced denitrogenative annulation followed by the cyclization of the nucleophilic side chain onto the indolenine intermediate. Comparably, for 1-alkenylbenzotrizoles with a masked side chain, the resulting indolenine intermediate will divert to the other tricyclic skeleton, namely dihydropyrido[1,2-a]indolone, through further skeletal rearrangement reaction. Taken together, the results of the present study clearly showcase the appealing pottnial of photo-induced denitrogenative transformations of 1-alkenylbenzotrizoles, which, as we anticipated, may find considerable application in the total synthesis of complex natural products.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/molecules28010363/s1, Scheme S1: The general procedure for the preparation of 1-alkenylbenzotriazoles; Figure S1: Setup of the photoreactor. Furthermore, all 1H and C NMR spectra of synthesized compounds are shown [42,43,44,45,46,47,48,49,50].
Author Contributions
Conceptualization, Y.T. and Z.W.; methodology, Z.W., Y.C. and Z.D.; investigation, Z.W., Y.C. and Z.D.; writing—original draft preparation, Z.W. and Y.C.; writing—review and editing, Y.T., Z.W. and Y.C.; supervision, Y.T.; project administration, Y.T.; funding acquisition, Y.T. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by National Natural Science Foundation of China (grant number: 21971140; 22271172), Tsinghua University Spring Breeze Fund (grant number: 2021Z81CFY006), Tsinghua-Toyota Joint Research Fund (grant number: 20213930008; 20213930027), and Beijing Natural Science Foundation (grant number: M21011).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Sample Availability
Samples of the compounds are available from the authors.
References
- Mohammed, A.E.; Abdul-Hameed, Z.H.; Alotaibi, M.O.; Bawakid, N.O.; Sobahi, T.R.; Abdel-Lateff, A.; Alarif, W.M. Chemical diversity and bioactivities of monoterpene indole alkaloids (mias) from six apocynaceae genera. Molecules 2021, 26, 488–554. [Google Scholar] [CrossRef] [PubMed]
- Rosales, P.F.; Bordin, G.S.; Gower, A.E.; Moura, S. Indole alkaloids: 2012 until now, highlighting the new chemical structures and biological activities. Fitoterapia 2020, 143, 104558–104583. [Google Scholar] [CrossRef] [PubMed]
- Adams, G.L.; Smith, A.B. Chapter three—The chemistry of the akuammiline alkaloids. In The Alkaloids: Chemistry and Biology; Knölker, H.-J., Ed.; Academic Press: Cambridge, MA, USA, 2016; Volume 76, pp. 171–257. [Google Scholar]
- Eckermann, R.; Gaich, T. The akuammiline alkaloids; origin and synthesis. Synthesis 2013, 45, 2813–2823. [Google Scholar]
- Hosoya, K.; Iida, K.; Odagi, M.; Nagasawa, K. Recent advances in synthetic strategies for the c4a,c9a-fused tetracyclic hydrocarbazole core structure of minfiensine and related akuammiline alkaloids. Heterocycles 2021, 103, 110–128. [Google Scholar]
- Koyama, K.; Hirasawa, Y.; Nugroho, A.E.; Kaneda, T.; Hoe, T.C.; Chan, K.-L.; Morita, H. Alsmaphorazines c–e, indole alkaloids from alstonia pneumatophora. Tetrahedron 2012, 68, 1502–1506. [Google Scholar] [CrossRef]
- Pfaffenbach, M.; Gaich, T. The diaza[5.5.6.6]fenestrane skeleton—Synthesis of leuconoxine alkaloids. Chem. Eur. J. 2016, 22, 3600–3610. [Google Scholar] [CrossRef]
- Gao, B.; Yao, F.; Zhang, Z.; Ding, H. Total synthesis of (+)-alsmaphorazine c and formal synthesis of (+)-strictamine: A photo-fries approach. Angew. Chem. Int. Ed. 2021, 60, 10603–10607. [Google Scholar] [CrossRef]
- Katritzky, A.R.; Rachwal, S. Synthesis of heterocycles mediated by benzotriazole. 1. Monocyclic systems. Chem. Rev. 2010, 110, 1564–1610. [Google Scholar] [CrossRef]
- Katritzky, A.R.; Rachwal, S. Synthesis of heterocycles mediated by benzotriazole. 2. Bicyclic systems. Chem. Rev. 2011, 111, 7063–7120. [Google Scholar] [CrossRef]
- Bollikolla, H.B.; Boddapati, S.N.M.; Thangamani, S.; Mutchu, B.R.; Alam, M.M.; Hussien, M.; Jonnalagadda, S.B. Advances in synthesis and biological activities of benzotriazole analogues: A micro review. J. Heterocycl. Chem. 2022. [Google Scholar] [CrossRef]
- Briguglio, I.; Piras, S.; Corona, P.; Gavini, E.; Nieddu, M.; Boatto, G.; Carta, A. Benzotriazole: An overview on its versatile biological behavior. Eur. J. Med. Chem. 2015, 97, 612–648. [Google Scholar] [CrossRef] [PubMed]
- Akter, M.; Rupa, K.; Anbarasan, P. 1,2,3-triazole and its analogues: New surrogates for diazo compounds. Chem. Rev. 2022, 122, 13108–13205. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, Z.; Tang, Y. Renaissance of ring-opening chemistry of benzotriazoles: New wine in an old bottle. Chem. Rec. 2020, 20, 693–709. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Singh, A.S.; Yan, G.; Yu, J.; Tiwari, V.K. Recent developments on denitrogenative functionalization of benzotriazoles. Synthesis 2020, 52, 3781–3800. [Google Scholar]
- Li, W.; Zhang, J. Synthesis of heterocycles through denitrogenative cyclization of triazoles and benzotriazoles. Chem. Eur. J. 2020, 26, 11931–11945. [Google Scholar] [CrossRef] [PubMed]
- An, T.; Liu, C.; Yin, Y.; Wu, X.-F.; Yin, Z. Palladium-catalyzed denitrogenative carbonylation of benzotriazoles with cr(co)6 as the carbonyl source. Organometallics 2022, 41, 1731–1737. [Google Scholar] [CrossRef]
- Li, Y.-L.; Zhang, P.-C.; Wu, H.-H.; Zhang, J. Palladium-catalyzed asymmetric tandem denitrogenative heck/tsuji–trost of benzotriazoles with 1,3-dienes. J. Am. Chem. Soc. 2021, 143, 13010–13015. [Google Scholar] [CrossRef]
- Hari Balakrishnan, M.; Mannathan, S. Palladium/copper-catalyzed denitrogenative alkylidenation and ortho-alkynylation reaction of 1,2,3-benzotriazin-4(3h)-ones. Org. Lett. 2020, 22, 542–546. [Google Scholar] [CrossRef]
- Alekseyev, R.S.; Kurkin, A.V.; Yurovskaya, M.A. Γ-carbolines and their hydrogenated derivatives. 1. Aromatic γ-carbolines: Methods of synthesis, chemical and biological properties (review). Chem. Heterocycl. Compd. 2009, 45, 889–925. [Google Scholar] [CrossRef]
- Alimi, I.; Remy, R.; Bochet, C.G. Photochemical c–h activation: Generation of indole and carbazole libraries, and first total synthesis of clausenawalline d. Eur. J. Org. Chem. 2017, 2017, 3197–3210. [Google Scholar] [CrossRef]
- Wender, P.A.; Cooper, C.B. The photochemistry of 1-alkenylbenzotriazoles: Methodology for the synthesis of indoles. Tetrahedron 1986, 42, 2985–2991. [Google Scholar] [CrossRef]
- Dutton, J.K.; Pleynet, D.P.M.; Johnson, A.P. Synthesis of hindered spiro-oxindoles by photolysis of 1-(1-alkenyl)benzotriazoles. Tetrahedron 1999, 55, 11927–11942. [Google Scholar] [CrossRef]
- Kazuo, T.; Mamoru, O.; Teijiro, Y. The photochemical decomposition of benzotriazoles. Bull. Chem. Soc. Jpn. 1972, 45, 515–519. [Google Scholar]
- Al-Jalal, N.A.; Ibrahim, M.R.; Al-Awadi, N.A.; Elnagdi, M.H.; Ibrahim, Y.A. Photochemistry of benzotriazoles: Generation of 1,3-diradicals and intermolecular cycloaddition as a new route toward indoles and dihydropyrrolo[3,4-b]indoles. Molecules 2014, 19, 20695–20708. [Google Scholar] [CrossRef] [PubMed]
- Teders, M.; Gómez-Suárez, A.; Pitzer, L.; Hopkinson, M.N.; Glorius, F. Diverse visible-light-promoted functionalizations of benzotriazoles inspired by mechanism-based luminescence screening. Angew. Chem. Int. Ed. 2017, 56, 902–906. [Google Scholar] [CrossRef]
- Teders, M.; Pitzer, L.; Buss, S.; Glorius, F. Regioselective synthesis of 2-substituted indoles from benzotriazoles and alkynes by photoinitiated denitrogenation. ACS Catal. 2017, 7, 4053–4056. [Google Scholar] [CrossRef]
- Wang, Y.; Wu, Y.; Li, Y.; Tang, Y. Denitrogenative suzuki and carbonylative suzuki coupling reactions of benzotriazoles with boronic acids. Chem. Sci. 2017, 8, 3852–3857. [Google Scholar] [CrossRef]
- Wang, Y.; Li, Y.; Fan, Y.; Wang, Z.; Tang, Y. Palladium-catalyzed denitrogenative functionalizations of benzotriazoles with alkenes and 1,3-dienes. Chem. Commun. 2017, 53, 11873–11876. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, Z.; Chen, X.; Tang, Y. Palladium-catalyzed denitrogenative cycloadditions and alkenylations of benzotriazoles with alkynes. Org. Chem. Front. 2018, 5, 2815–2819. [Google Scholar] [CrossRef]
- Teuber, H.-J.; Cornelius, D.; Worbs, E. Indole aus cyclischen β-dicarbonyl-verbindungen, i. Tetrahedron Lett. 1964, 5, 331–333. [Google Scholar] [CrossRef]
- Ban, Y.; Yoshida, K.; Goto, J.; Oishi, T.; Takeda, E. Ishigamori A synthetic road to the forest of strychnos, aspidosperma, schizozygane and eburnamine alkaloids by way of the novel photoisomerization. Tetrahedron 1983, 39, 3657–3668. [Google Scholar] [CrossRef]
- Ban, Y.; Yoshida, K.; Goto, J.; Oishi, T. Novel photoisomerization of 1-acylindoles to 3-acylindolenines. General entry to the total synthesis of strychnos and aspidosperma alkaloids. J. Am. Chem. Soc. 1981, 103, 6990–6992. [Google Scholar] [CrossRef]
- Somei, M.; Natsume, M. Photochemical rearrangements for the syntheses of 3-, 4-, and 6-substituted indoles. Tetrahedron Lett. 1973, 14, 2451–2454. [Google Scholar] [CrossRef]
- Takechi, H.; Machida, M.; Kanaoka, Y. Intramolecular photoreactions of phthalimide-alkene systems. Oxetane formation of n-(ω-indol-3-ylalkyl)phthalimides. Chem. Pharm. Bull. 1988, 36, 2853–2863. [Google Scholar]
- Teuber, H.-J.; Worbs, E.; Cornelius, D. Indole aus cyclischen 1.3-dicarbonylverbindungen mit substituierter 2-stellung. Arch. Pharm. 1982, 315, 388–396. [Google Scholar] [CrossRef]
- Edmondson, S.D.; Mastracchio, A.; Parmee, E.R. Palladium-catalyzed coupling of vinylogous amides with aryl halides: Applications to the synthesis of heterocycles. Org. Lett. 2000, 2, 1109–1112. [Google Scholar] [CrossRef]
- Li, W.; Dong, Z.; Zhang, Y.; Zeng, Z.; Usman, M.; Liu, W.-B. Cu-catalyzed arylation/acyl migration cascade reaction of enaminones: Access to n-fused polycyclic and 2,3-disubstituted indoles. J. Org. Chem. 2019, 84, 7995–8005. [Google Scholar] [CrossRef]
- Dong, Z.; Zhang, X.-W.; Li, W.; Li, Z.-M.; Wang, W.-Y.; Zhang, Y.; Liu, W.; Liu, W.-B. Synthesis of n-fused polycyclic indoles via ligand-free palladium-catalyzed annulation/acyl migration reaction. Org. Lett. 2019, 21, 1082–1086. [Google Scholar] [CrossRef]
- Barker, S.J.; Storr, R.C. Flash-pyrolysis of 1-vinylbenzotriazoles. J. Chem. Soc. Perkin Trans. 1990, 1, 485–488. [Google Scholar] [CrossRef]
- Naruse, Y.; Ito, Y.; Inagaki, S. Highly regioselective functionalization of 2,3-dialkyl substituents on indoles. J. Org. Chem. 1991, 56, 2256–2258. [Google Scholar] [CrossRef]
- Bhunia, S.; Ghorpade, S.; Huple, D.B.; Liu, R.S. Gold-catalyzed oxidative cyclizations of cis-3-en-1-ynes to form cyclopentenone derivatives. Angew. Chem. Int. Ed. 2012, 51, 2939–2942. [Google Scholar] [CrossRef] [PubMed]
- Das, J.; Sarkar, A.; Ghosh, P. Friedelane triterpenoids: Transformations toward A-ring modifications including 2-homoderivatives. N. J. Chem. 2018, 42, 6673–6688. [Google Scholar] [CrossRef]
- Lee, E.; Shin, I.J.; Kim, T.S. Enantiocontrolled synthesis of quaternary carbon centers via anionic oxy-cope rearrangement: An efficient synthesis of (+)-Dihydromayurone. J. Am. Chem. Soc. 1990, 112, 260–264. [Google Scholar] [CrossRef]
- Wong, L.S.-M.; Sherburn, M.S. IMDA-radical cyclization approach to (+)-Himbacine. Org. Lett. 2003, 5, 3603–3606. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.G.; Zhang, J.S.; Ma, S.M. Iron catalysis for room temperature aerobic oxidation of alcohols to carboxylic acids. J. Am. Chem. Soc. 2016, 138, 8344–8347. [Google Scholar] [CrossRef]
- Guo, Y.L.; Quan, T.F.; Lu, Y.D.; Luo, T.P. Enantioselective total synthesis of (+)-Wortmannin. J. Am. Chem. Soc. 2017, 139, 6815–6818. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Yamamoto, H. Tungsten-catalyzed asymmetric epoxidation of allylic and homoallylic alcohols with hydrogen peroxide. J. Am. Chem. Soc. 2014, 136, 1222–1225. [Google Scholar] [CrossRef]
- Li, Z.; Watkins, E.B.; Liu, H.; Chittiboyina, A.G.; Carvalho, P.B.; Avery, M.A. 1,3-Diaxially substituted trans-decalins: Potential nonsteroidal human progesterone receptor inhibitors. J. Org. Chem. 2008, 73, 7764–7767. [Google Scholar] [CrossRef]
- Daniel, D.; Andrew, A.S. Reductive N-alkylation of amides, carbamates and ureas. Tetrahedron Lett. 1999, 40, 2295–2298. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).