Chemical Constituents from the Leaves of Ligustrum robustum and Their Bioactivities
Abstract
1. Introduction
2. Results and Discussion
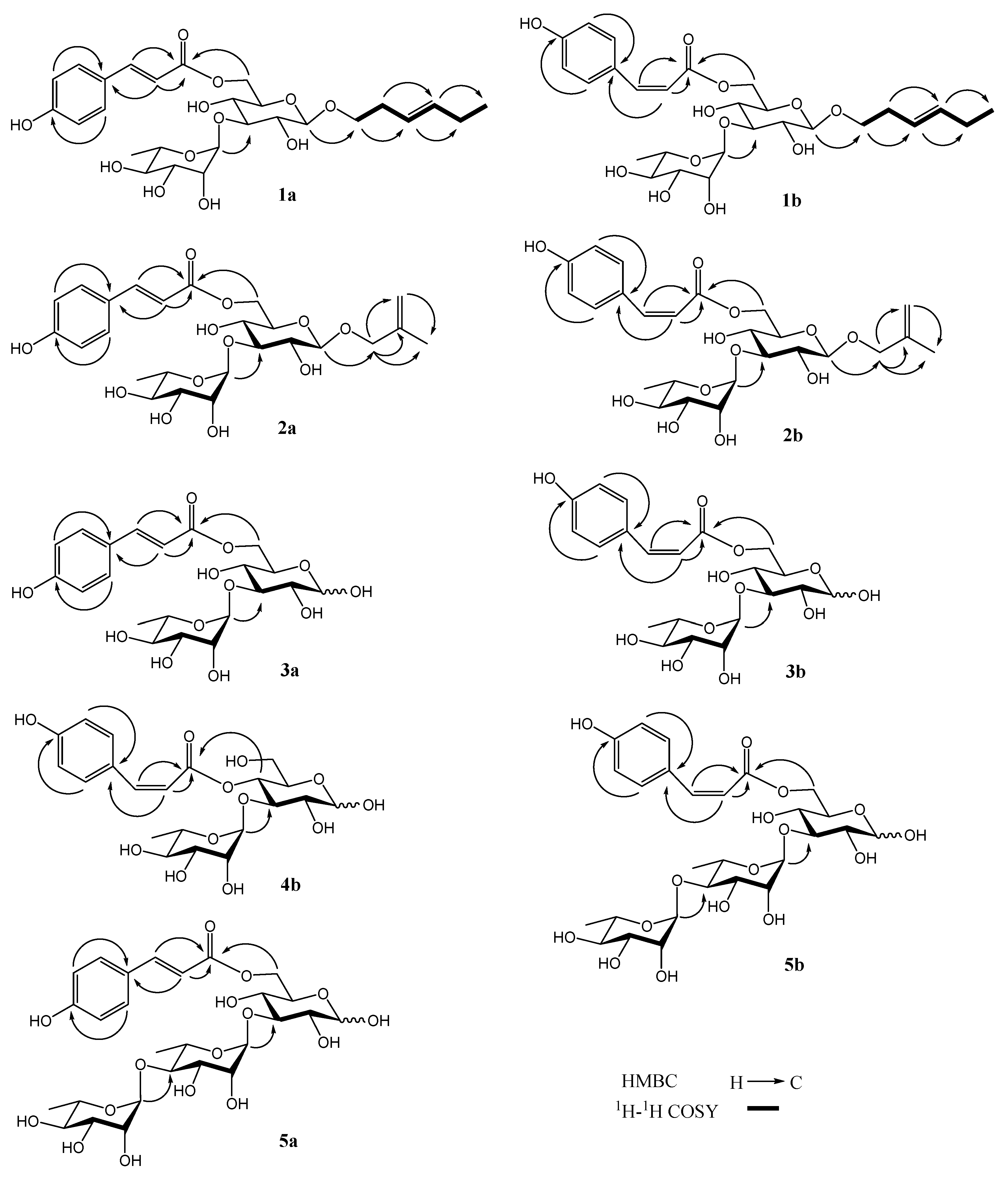
2.1. Identification of Compounds 1–10
2.2. The Bioactivities of Compounds 1–10
3. Materials and Methods
3.1. General Experimental Procedure
3.2. Plant Material
3.3. Extraction and Isolation
3.4. Acid Hydrolysis of Compounds 1–5
3.5. Determination of Bioactivities
3.6. Statistical Analyses
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
Abbreviation
| Abbreviation | Full Spelling |
| ABTS | 2,2′-azino-bis(3-ethylbenzthiazoline-6-sulphonic acid) ammonium salt |
| Ac-CoA | acetyl-coenzyme A |
| ANOVA | one-way analysis of variance |
| Caff | caffeoyl |
| CC | column chromatography |
| 1H-1H COSY | 1H-1H homonuclear chemical shift correlation spectroscopy |
| Cou | coumaroyl |
| DMSO | dimethyl sulfoxide |
| DPPH | 2,2-diphenyl-1-picrylhydrazyl |
| EtOAc | ethyl acetate |
| FAS | fatty acid synthase |
| Glc | glucosyl |
| HMBC | heteronuclear multiple bond coherence spectroscopy |
| HRESIMS | high-resolution electrospray ionization mass spectroscopy |
| HSQC | heteronuclear single quantum coherence spectroscopy |
| IC50 | half inhibitory concentration |
| IR | infrared absorption spectrum |
| Mal-CoA | methylmalonyl coenzyme A |
| NMR | nuclear magnetic resonance |
| HPLC | high-performance liquid chromatography |
| SD | standard deviation |
| Rha | rhamnosyl |
| TLC | thin-layer chromatography |
| UV | ultraviolet visible absorption spectrum |
References
- Ansari, P.; Akther, S.; Hannan, J.M.A.; Seidel, V.; Nujat, N.J.; Abdel-Wahab, Y.H.A. Pharmacologically active phytomolecules isolated from traditional antidiabetica plants and their therapeutic role for the management of diabetes mellitus. Molecules 2022, 27, 4278. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.-Q.; Chen, W.; Ma, K.; Liu, Z.-Z.; Gao, Y.; Zhang, J.-G.; Wang, T.; Yang, Y.-J. Akkermansia muciniphila suppresses high-fat diet-induced obesity and related metabolic disorders in beagles. Molecules 2022, 27, 6074. [Google Scholar] [CrossRef] [PubMed]
- Mika, K.; Szafarz, M.; Zadrozna, M.; Nowak, B.; Bednarski, M.; Szczepa´nska, K.; Pociecha, K.; Kubacka, M.; Nicosia, N.; Juda, I.; et al. KSK-74: Dual histamine H3 and sigma-2 receptor ligand with anti-obesity potential. Int. J. Mol. Sci. 2022, 23, 7011. [Google Scholar] [CrossRef] [PubMed]
- Lu, S.-H.; Huang, J.; Zuo, H.-J.; Zhou, Z.-B.; Yang, C.-Y.; Huang, Z.-L. Monoterpenoid glycosides from the leaves of Ligustrum robustum and their bioactivities. Molecules 2022, 27, 3709. [Google Scholar] [CrossRef] [PubMed]
- Martiz, R.M.; Patil, S.M.; Thirumalapura Hombegowda, D.; Shbeer, A.M.; Alqadi, T.; Al-Ghorbani, M.; Ramu, R.; Prasad, A. Phyto-computational intervention of diabetes mellitus at multiple stages using isoeugenol from Ocimum tenuiflorum: A combination of pharmacokinetics and molecular modelling approaches. Molecules 2022, 27, 6222. [Google Scholar] [CrossRef]
- Akinyede, K.A.; Oyewusi, H.A.; Hughes, G.D.; Ekpo, O.E.; Oguntibeju, O.O. In vitro evaluation of the anti-diabetic potential of aqueous acetone helichrysum petiolare extract (AAHPE) with molecular docking relevance in diabetes mellitus. Molecules 2022, 27, 155. [Google Scholar] [CrossRef]
- He, Z.D.; Lau, K.M.; But, P.P.-H.; Jiang, R.W.; Dong, H.; Ma, S.C.; Fung, K.P.; Ye, W.C.; Sun, H.D. Antioxidative glycosides from the leaves of Ligustrum robustum. J. Nat. Prod. 2003, 66, 851–854. [Google Scholar] [CrossRef]
- Zhu, F.; Cai, Y.Z.; Sun, M.; Ke, J.X.; Lu, D.Y.; Corke, H. Comparison of major phenolic constituents and in vitro antioxidant activity of diverse kudingcha genotypes from Ilex kudingcha, Ilex cornuta, and Ligustrum robustum. J. Agric. Food Chem. 2009, 57, 6082–6089. [Google Scholar] [CrossRef]
- Yang, R.M.; Liu, F.; He, Z.D.; Ji, M.; Chu, X.X.; Kang, Z.Y.; Cai, D.Y.; Gao, N.N. Anti-obesity effect of total phenylpropanoid glycosides from Ligustrum robustum Blume in fatty diet-fed mice via up-regulating leptin. J. Ethnopharmacol. 2015, 169, 459–465. [Google Scholar] [CrossRef]
- Li, L.; Peng, Y.; Xu, L.J.; Wu-Lan, T.N.; Shi, R.B.; Xiao, P.G. Chemical constituents from Ligustrum robustum Bl. Biochem. Syst. Ecol. 2010, 38, 398–401. [Google Scholar] [CrossRef]
- Li, L.; Peng, Y.; Liu, Y.; Xu, L.J.; Guo, N.; Shi, R.B.; Xiao, P.G. Two new phenethanol glycosides from Ligustrum robustum. Chin. Chem. Lett. 2011, 22, 326–329. [Google Scholar] [CrossRef]
- Tian, J.; Zhang, H.J.; Sun, H.D.; Pan, L.T.; Yao, P.; Chen, D.Y. Monoterpenoid glycosides from Ligustrum robustum. Phytochemistry 1998, 48, 1013–1018. [Google Scholar] [CrossRef]
- Tian, J.; Sun, H.D. New monoterpenoid glycosides from Ligustrum robustum. Chin. J. Appl. Environ. Biol. 1999, 5, 501–506. [Google Scholar]
- Yu, Z.L.; Gao, H.X.; Zhang, Z.; He, Z.; He, Q.; Jia, L.R.; Zeng, W.C. Inhibitory effects of Ligustrum robustum (Roxb.) Blume extract on α-amylase and α-glucosidase. J. Funct. Foods 2015, 19, 204–213. [Google Scholar] [CrossRef]
- Lu, S.-H.; Zuo, H.-J.; Shi, J.-X.; Li, C.-R.; Li, Y.-H.; Wang, X.; Li, L.-R.; Huang, J. Two new glycosides from the leaves of Ligustrum robustum and their antioxidant activities and inhibitory effects on α-glucosidase and α-amylase. S. Afr. J. Bot. 2019, 125, 521–526. [Google Scholar] [CrossRef]
- Lu, S.-H.; Zuo, H.-J.; Huang, J.; Chen, R.; Pan, J.-P.; Li, X.-X. Phenylethanoid and phenylmethanoid glycosides from the leaves of Ligustrum robustum and their bioactivities. Molecules 2022, 27, 7390. [Google Scholar] [CrossRef]
- Ito, H.; Otsuki, A.; Mori, H.; Li, P.; Kinoshita, M.; Kawakami, Y.; Tsuji, H.; Fang, D.Z.; Takahashi, Y. Two new monoterpene glycosides from Qing Shan Lu Shui tea with inhibitory effects on leukocyte-type 12-lipoxygenase activity. Molecules 2013, 18, 4257–4266. [Google Scholar] [CrossRef]
- Kawakami, Y.; Otsuki, A.; Mori, Y.; Kanzaki, K.; Suzuki-Yamamoto, T.; Fang, D.Z.; Ito, H.; Takahashi, Y. Involvement of the hydroperoxy group in the irreversible inhibition of leukocyte-type 12-lipoxygenase by monoterpene glycosides contained in the Qing Shan Lu Shui tea. Molecules 2019, 24, 304. [Google Scholar] [CrossRef]
- Wu, Y.; Yang, J.; Liu, X.J.; Zhang, Y.; Lei, A.L.; Yi, R.K.; Tan, F.; Zhao, X. Preventive effect of small-leaved Kuding tea (Ligustrum robustum) on high-diet-induced obesity in C57BL/6J mice. Food Sci. Nutr. 2020, 8, 4512–4522. [Google Scholar] [CrossRef]
- Karasawa, H.; Kobayashi, H.; Takizawa, N.; Miyase, T.; Fukushima, S. Studies on the constituents of Cistanchis herba. VII. Isolation and structures of citanoside H and I. Yakugaku Zasshi 1986, 106, 562–566. [Google Scholar] [CrossRef]
- Kobayashi, H.; Karasawa, H.; Miyase, T.; Fukushima, S. Studies on the constituents of Cistanchis herba. V. Isolation and structures of two phenylpropanoid glycosides, citanoside E and F. Chem. Pharm. Bull. 1985, 33, 1452–1457. [Google Scholar] [CrossRef]
- Zheng, Z.-P.; Liang, J.-Y.; Hu, L.-H. Water-soluble constituents of Cudrania tricuspidata (Carr.) Bur. J. Integr. Plant Biol. 2006, 48, 996–1000. [Google Scholar] [CrossRef]
- Sugiyama, M.; Nagayama, E.; Kikuchi, M. Lignan and phenylpropanoid glycosides from Osmanthus asiaticus. Phytochemistry 1993, 33, 1215–1219. [Google Scholar] [CrossRef]
- Leng, L.-F.; Yi, C.-D.; Zhao, W.-K.; Yin, J.-L.; Zeng, G.-Z. A new lupane-type triterpenoid from Dichroa hirsuta. Zhongguo Zhong Yao Za Zhi 2019, 44, 1829–1835. [Google Scholar] [PubMed]
- Kuang, T.-D.; Chen, H.-Q.; Li, W.; Yang, J.-L.; Zhou, L.-M.; Cai, C.-H.; Dong, W.-H.; Mei, W.-L.; Dai, H.-F. A new sesquiterpene from Chinese agarwood induced by artificial holing. Zhongguo Zhong Yao Za Zhi 2017, 42, 4618–4623. [Google Scholar]
- Liu, N.-Z.; Zhao, B.-Q.; Qian, Q.-G.; Chen, N.-H.; Zhou, X.-J. Chemical constituents from Scropularia ningpoensis. Chin. Trad. Pat. Med. 2019, 41, 576–579. [Google Scholar]
- Fan, H.J.; Wu, D.; Tian, W.X.; Ma, X.F. Inhibitory effects of tannic acid on fatty acid synthase and 3T3-L1 preadipocyte. Biochim. Biophys. Acta 2013, 1831, 1260–1266. [Google Scholar] [CrossRef]
- Wu, D.; Ma, X.F.; Tian, W.X. Pomegranate husk extract, punicalagin and ellagic acid inhibit fatty acid synthase and adipogenesis of 3T3-L1 adipocyte. J. Funct. Foods 2013, 5, 633–641. [Google Scholar] [CrossRef]
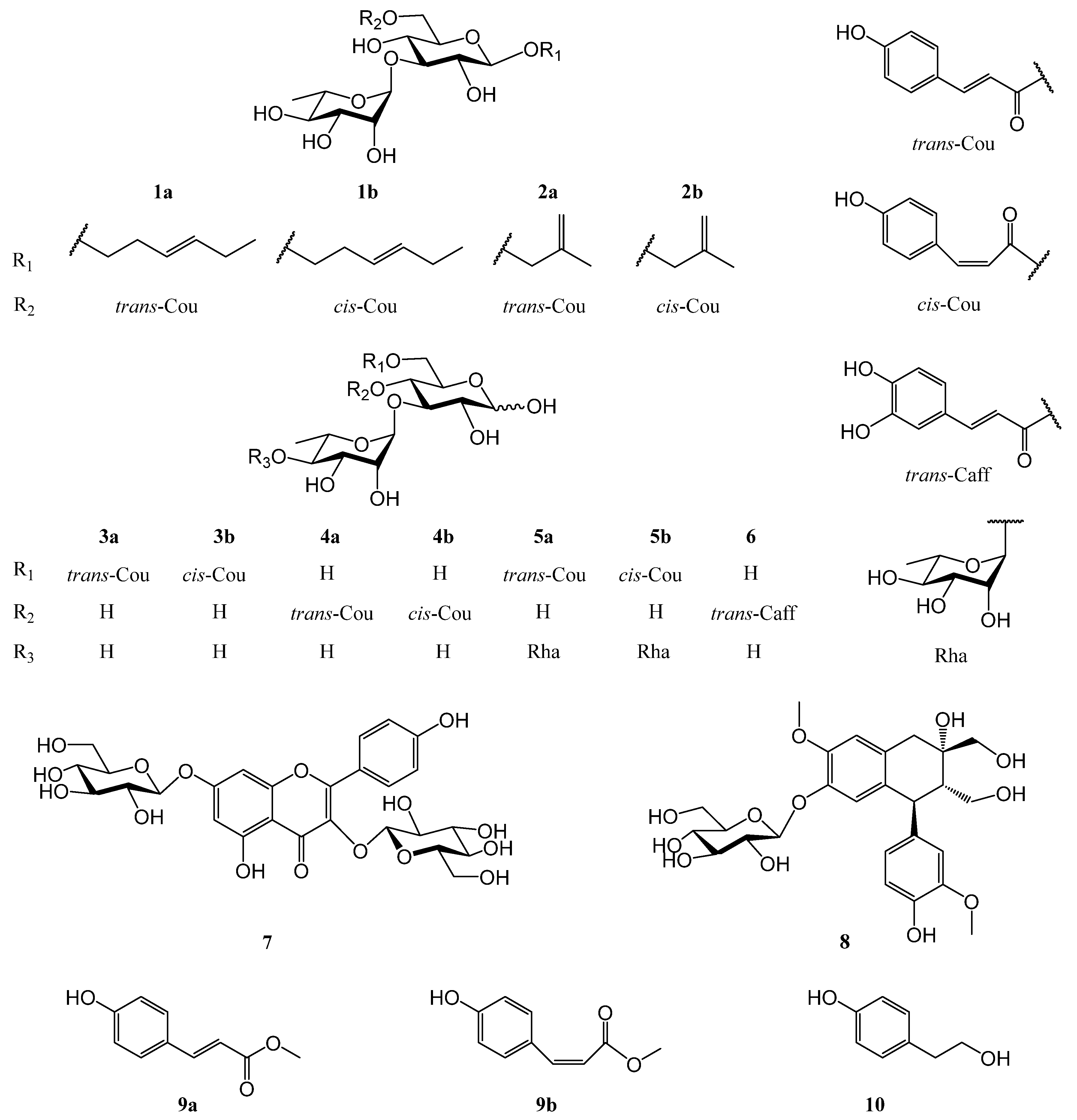
| No. | 1a | 1b | 2a | 2b |
|---|---|---|---|---|
| 1a | 3.55 m | 3.55 m | 4.07 d (12.6) | 4.10 d (12.6) |
| 1b | 3.80 m | 3.80 m | 4.20 d (12.6) | 4.15 d (12.6) |
| 2 | 2.37 m | 2.37 m | ||
| 3a | 5.36 dt (17.4, 6.6) | 5.36 dt (17.4, 6.6) | 4.88 br. s | 4.88 br. s |
| 3b | 5.02 br. s | 5.02 br. s | ||
| 4 | 5.42 dt (17.4, 6.6) | 5.42 dt (17.4, 6.6) | 1.75 s | 1.73 s |
| 5 | 2.05 m | 2.05 m | ||
| 6a | 0.93 t (7.2) | 0.93 t (7.2) | ||
| 6b | 0.97 t (7.2) | 0.97 t (7.2) | ||
| Glc | ||||
| 1′ | 4.31 d (8.4) | 4.27 d (7.8) | 4.30 d (7.2) | 4.26 d (7.8) |
| 2′ | 3.30 m | 3.30 m | 3.34 m | 3.34 m |
| 3′ | 3.51 m | 3.51 m | 3.52 m | 3.52 m |
| 4′ | 3.40 t (9.6) | 3.40 t (9.6) | 3.42 br. d (9.0) | 3.42 br. d (9.0) |
| 5′ | 3.54 m | 3.54 m | 3.52 m | 3.52 m |
| 6′a | 4.35 dd (12.0, 6.0) | 4.34 dd (12.0, 6.0) | 4.36 dd (12.0, 6.0) | 4.36 dd (12.0, 6.0) |
| 6′b | 4.48 dd (12.0, 2.4) | 4.46 dd (12.0, 2.4) | 4.48 dd (12.0, 1.8) | 4.46 dd (12.0, 1.8) |
| Rha | ||||
| 1″ | 5.18 d (1.8) | 5.16 d (1.8) | 5.18 d (1.8) | 5.16 d (1.8) |
| 2″ | 3.94 m | 3.94 m | 3.94 dd (3.6, 1.8) | 3.94 dd (3.6, 1.8) |
| 3″ | 3.71 dd (9.6, 3.6) | 3.71 dd (9.6, 3.6) | 3.70 dd (9.6, 3.6) | 3.70 dd (9.6, 3.6) |
| 4″ | 3.39 t (9.6) | 3.39 t (9.6) | 3.40 br. d (9.6) | 3.40 br. d (9.6) |
| 5″ | 4.00 m | 4.00 m | 4.00 m | 4.00 m |
| 6″ | 1.25 d (6.0) | 1.24 d (6.0) | 1.25 d( 6.6) | 1.25 d( 6.6) |
| Cou | ||||
| 2′″ | 7.43 d (8.4) | 7.65 d (8.4) | 7.47 d (8.4) | 7.65 d (8.4) |
| 3′″ | 6.77 d (8.4) | 6.75 d (8.4) | 6.80 d (8.4) | 6.76 d (8.4) |
| 5′″ | 6.77 d (8.4) | 6.75 d (8.4) | 6.80 d (8.4) | 6.76 d (8.4) |
| 6′″ | 7.43 d (8.4) | 7.65 d (8.4) | 7.47 d (8.4) | 7.65 d (8.4) |
| 7′″ | 7.63 d (15.6) | 6.88 d (13.2) | 7.65 d (16.2) | 6.89 d (12.6) |
| 8′″ | 6.33 d (15.6) | 5.79 d (13.2) | 6.37 d (16.2) | 5.80 d (12.6) |
| No. | 1a | 1b | 2a | 2b |
|---|---|---|---|---|
| 1 | 70.8 | 70.7 | 74.0 | 73.8 |
| 2 | 28.9 | 28.9 | 143.1 | 143.1 |
| 3 | 125.8 | 125.8 | 113.4 | 113.4 |
| 4 | 134.6 | 134.6 | 19.7 | 19.7 |
| 5 | 21.5 | 21.5 | ||
| 6 | 14.6 | 14.6 | ||
| Glc | ||||
| 1′ | 104.4 | 104.2 | 103.0 | 103.0 |
| 2′ | 75.6 | 75.6 | 75.7 | 75.7 |
| 3′ | 84.0 | 84.0 | 84.0 | 84.0 |
| 4′ | 70.5 | 70.4 | 70.4 | 70.4 |
| 5′ | 75.6 | 75.3 | 75.4 | 75.4 |
| 6′ | 64.6 | 64.5 | 64.6 | 64.6 |
| Rha | ||||
| 1″ | 102.7 | 102.8 | 102.8 | 102.8 |
| 2″ | 72.4 | 72.4 | 72.4 | 72.4 |
| 3″ | 72.3 | 72.3 | 72.3 | 72.3 |
| 4″ | 74.0 | 74.0 | 74.0 | 74.0 |
| 5″ | 70.0 | 70.0 | 70.0 | 70.0 |
| 6″ | 17.9 | 17.9 | 17.9 | 17.9 |
| Cou | ||||
| 1′″ | 126.3 | 127.5 | 126.9 | 127.5 |
| 2′″ | 131.3 | 133.8 | 131.2 | 133.8 |
| 3′″ | 117.4 | 116.0 | 116.9 | 115.9 |
| 4′″ | 163.0 | 160.4 | 161.6 | 160.2 |
| 5′″ | 117.4 | 116.0 | 116.9 | 115.9 |
| 6′″ | 131.3 | 133.8 | 131.2 | 133.8 |
| 7′″ | 147.1 | 145.3 | 146.9 | 145.3 |
| 8′″ | 114.1 | 116.2 | 114.8 | 116.2 |
| CO | 169.2 | 168.1 | 169.1 | 168.1 |
| No. | 3a b | 3b b | 4b c | ||
|---|---|---|---|---|---|
| β | α | β | α | β | |
| Glc | |||||
| 1 | 4.52 d (7.8) | 5.08 d (3.6) | 4.49 d (7.8) | 5.06 d (4.2) | 4.52 d (7.6) |
| 2 | 3.27 m | 3.49 dd (9.6, 3.6) | 3.26 m | 3.48 dd (9.6, 4.2) | 3.33 m |
| 3 | 3.53 t (9.6) | 3.81 t (9.6) | 3.52 t (9.0) | 3.77 t (9.6) | 3.75 t (9.2) |
| 4 | 3.40 m | 3.41 m | 3.39 m | 3.40 m | 4.85 t (9.2) |
| 5 | 3.58 m | 4.08 dd (9.6, 3.6) | 3.57 m | 4.07 dd (9.6, 3.6) | 3.55 m |
| 6a | 4.36 dd (12.0, 6.0) | 4.32 dd (12.0, 3.6) | 4.26 dd (12.0, 5.4) | 4.26 dd (12.0, 3.6) | 3.52 m |
| 6b | 4.45 dd (12.0, 1.8) | 4.49 dd (12.0, 1.8) | 4.39 dd (12.0, 1.8) | 4.45 dd (12.0, 1.8) | 3.58 m |
| Rha | |||||
| 1′ | 5.18 d (1.8) | 5.13 d (1.8) | 5.15 d (1.8) | 5.10 d (1.8) | 5.12 d (2.0) |
| 2′ | 3.97 m | 3.97 m | 3.96 m | 3.96 m | 3.93 m |
| 3′ | 3.72 m | 3.72 m | 3.71 m | 3.71 m | 3.58 m |
| 4′ | 3.41 m | 3.41 m | 3.40 m | 3.40 m | 3.32 m |
| 5′ | 4.02 dd (9.6, 6.0) | 4.02 dd (9.6, 6.0) | 4.01 dd (9.6, 6.0) | 4.01 dd (9.6, 6.0) | 3.63 m |
| 6′ | 1.26 d (6.0) | 1.26 d (6.0) | 1.25 d (6.0) | 1.25 d (6.0) | 1.17 d (6.0) |
| Cou | |||||
| 2″ | 7.45 d (8.4) | 7.45 d (8.4) | 7.66 d (7.8) | 7.66 d (7.8) | 7.72 d (8.8) |
| 3″ | 6.80 d (8.4) | 6.80 d (8.4) | 6.75 d (7.8) | 6.75 d (7.8) | 6.76 d (8.8) |
| 5″ | 6.80 d (8.4) | 6.80 d (8.4) | 6.75 d (7.8) | 6.75 d (7.8) | 6.76 d (8.8) |
| 6″ | 7.45 d (8.4) | 7.45 d (8.4) | 7.66 d (7.8) | 7.66 d (7.8) | 7.72 d (8.8) |
| 7″ | 7.63 d (16.2) | 7.63 d (16.2) | 6.86 d (13.2) | 6.86 d (13.2) | 6.94 d (12.8) |
| 8″ | 6.33 d (16.2) | 6.33 d (16.2) | 5.76 d (13.2) | 5.76 d (13.2) | 5.81 d (12.8) |
| No. | 4b c | 5a c | 5b c | ||
| α | β | α | β | α | |
| Glc | |||||
| 1 | 5.11 d (3.6) | 4.51 d (8.0) | 5.07 d (3.6) | 4.51 d (8.0) | 5.06 d (3.6) |
| 2 | 3.56 m | 3.26 m | 3.48 m | 3.26 m | 3.48 m |
| 3 | 4.06 t (9.2) | 3.53 m | 3.81 t (9.2) | 3.53 m | 3.81 t (9.2) |
| 4 | 4.88 t (9.2) | 3.40 m | 3.40 m | 3.40 m | 3.40 m |
| 5 | 4.01 m | 3.56 m | 4.07 m | 3.56 m | 4.07 m |
| 6a | 3.52 m | 4.33 dd (12.0, 5.6) | 4.30 dd (12.0, 6.0) | 4.33 dd (12.0, 5.6) | 4.30 dd (12.0, 6.0) |
| 6b | 3.58 m | 4.45 dd (12.0, 2.0) | 4.50 dd (12.0, 2.0) | 4.45 dd (12.0, 2.0) | 4.50 dd (12.0, 2.0) |
| Inner-Rha | |||||
| 1′ | 5.17 d (2.0) | 5.19 d (1.6) | 5.13 d (1.6) | 5.17 d (1.6) | 5.11 d (1.6) |
| 2′ | 3.93 m | 3.91 m | 3.91 m | 3.91 m | 3.91 m |
| 3′ | 3.58 m | 3.61 dd (9.6, 3.2) | 3.85 dd (9.2, 3.2) | 3.61 dd (9.6, 3.2) | 3.85 dd (9.2, 3.2) |
| 4′ | 3.32 m | 3.54 m | 3.54 m | 3.54 m | 3.54 m |
| 5′ | 3.63 m | 4.12 dd (9.6, 6.0) | 4.12 dd (9.6, 6.0) | 4.12 dd (9.6, 6.0) | 4.12 dd (9.6, 6.0) |
| 6′ | 1.16 d (6.0) | 1.29 d (6.0) | 1.29 d (6.0) | 1.29 d (6.0) | 1.29 d (6.0) |
| Outer-Rha | |||||
| 1″ | 5.20 d (1.6) | 5.20 d (1.6) | 5.20 d (1.6) | 5.20 d (1.6) | |
| 2″ | 3.95 dd (3.2, 1.6) | 3.95 dd (3.2, 1.6) | 3.95 dd (3.2, 1.6) | 3.95 dd (3.2, 1.6) | |
| 3″ | 3.61 dd (9.6, 3.2) | 3.61 dd (9.6, 3.2) | 3.61 dd (9.6, 3.2) | 3.61 dd (9.6, 3.2) | |
| 4″ | 3.40 m | 3.40 m | 3.40 m | 3.40 m | |
| 5″ | 3.72 dd (9.2, 6.0) | 3.72 dd (9.2, 6.0) | 3.72 dd (9.2, 6.0) | 3.72 dd (9.2, 6.0) | |
| 6″ | 1.25 d (6.0) | 1.25 d (6.0) | 1.25 d (6.0) | 1.25 d (6.0) | |
| Cou | |||||
| 2′″ | 7.72 d (8.8) | 7.46 d (8.4) | 7.46 d (8.4) | 7.64 d (8.4) | 7.63 d (8.4) |
| 3′″ | 6.76 d (8.8) | 6.81 d (8.4) | 6.81 d (8.4) | 6.76 d (8.4) | 6.75 d (8.4) |
| 5′″ | 6.76 d (8.8) | 6.81 d (8.4) | 6.81 d (8.4) | 6.76 d (8.4) | 6.75 d (8.4) |
| 6′″ | 7.72 d (8.8) | 7.46 d (8.4) | 7.46 d (8.4) | 7.64 d (8.4) | 7.63 d (8.4) |
| 7′″ | 6.95 d (12.8) | 7.64 d (16.0) | 7.64 d (16.0) | 6.87 d (12.8) | 6.87 d (12.8) |
| 8′″ | 5.80 d (12.8) | 6.35 d (16.0) | 6.34 d (16.0) | 5.79 d (12.8) | 5.78 d (12.8) |
| No. | 3a | 3b | 4b | 5a | 5b | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| β | α | β | α | β | α | β | α | β | α | |
| Glc | ||||||||||
| 1 | 98.1 | 94.0 | 98.1 | 94.1 | 98.2 | 94.0 | 98.1 | 94.1 | 98.1 | 94.1 |
| 2 | 76.8 | 74.2 | 76.7 | 74.2 | 77.3 | 74.6 | 77.0 | 74.4 | 77.0 | 74.4 |
| 3 | 84.1 | 81.7 | 84.2 | 81.8 | 81.9 | 79.4 | 83.6 | 81.3 | 83.6 | 81.3 |
| 4 | 70.6 | 70.4 | 70.7 | 70.5 | 70.6 | 70.5 | 70.6 | 70.4 | 70.6 | 70.4 |
| 5 | 75.4 | 70.8 | 75.3 | 70.8 | 76.1 | 71.2 | 75.5 | 70.9 | 75.5 | 70.9 |
| 6 | 64.8 | 64.8 | 64.6 | 64.6 | 62.4 | 62.5 | 64.9 | 64.9 | 64.9 | 64.9 |
| Inner-Rha | ||||||||||
| 1′ | 102.7 | 102.8 | 102.9 | 102.9 | 103.1 | 103.2 | 102.4 | 102.6 | 102.4 | 102.6 |
| 2′ | 72.3 | 72.3 | 72.3 | 72.3 | 72.3 | 72.3 | 72.9 | 72.9 | 72.9 | 72.9 |
| 3′ | 72.2 | 72.2 | 72.2 | 72.2 | 72.1 | 72.0 | 72.9 | 73.1 | 72.9 | 73.1 |
| 4′ | 74.0 | 74.0 | 74.1 | 74.0 | 73.8 | 73.8 | 81.2 | 81.1 | 81.2 | 81.1 |
| 5′ | 70.0 | 70.0 | 70.0 | 70.0 | 70.4 | 70.4 | 68.4 | 68.4 | 68.4 | 68.4 |
| 6′ | 17.9 | 17.9 | 17.9 | 17.9 | 18.2 | 18.2 | 18.6 | 18.6 | 18.6 | 18.6 |
| Outer-Rha | ||||||||||
| 1″ | 103.2 | 103.2 | 103.2 | 103.2 | ||||||
| 2″ | 72.4 | 72.4 | 72.4 | 72.4 | ||||||
| 3″ | 72.4 | 72.4 | 72.4 | 72.4 | ||||||
| 4″ | 73.9 | 73.9 | 73.9 | 73.9 | ||||||
| 5″ | 70.4 | 70.4 | 70.4 | 70.4 | ||||||
| 6″ | 17.8 | 17.8 | 17.8 | 17.8 | ||||||
| Cou | ||||||||||
| 1′″ | 126.9 | 126.9 | 127.5 | 127.5 | 127.5 | 127.5 | 127.2 | 127.1 | 127.5 | 127.5 |
| 2′″ | 131.1 | 131.1 | 133.7 | 133.7 | 134.3 | 134.3 | 131.2 | 131.2 | 133.8 | 133.8 |
| 3′″ | 116.9 | 116.9 | 115.9 | 115.9 | 115.8 | 115.9 | 116.8 | 116.8 | 115.9 | 115.9 |
| 4′″ | 161.6 | 161.6 | 160.2 | 160.2 | 160.4 | 160.5 | 161.3 | 161.3 | 160.4 | 160.4 |
| 5′″ | 116.9 | 116.9 | 115.9 | 115.9 | 115.8 | 115.9 | 116.8 | 116.8 | 115.9 | 115.9 |
| 6′″ | 131.1 | 131.1 | 133.7 | 133.7 | 134.3 | 134.3 | 131.2 | 131.2 | 133.8 | 133.8 |
| 7′″ | 146.8 | 146.8 | 145.3 | 145.3 | 147.1 | 147.3 | 146.7 | 146.8 | 145.2 | 145.2 |
| 8′″ | 114.7 | 114.7 | 116.2 | 116.2 | 116.1 | 116.1 | 115.0 | 114.9 | 116.3 | 116.3 |
| CO | 169.2 | 169.1 | 168.2 | 168.1 | 167.0 | 166.9 | 169.2 | 169.1 | 168.2 | 168.2 |
| Compound | FAS IC50 (μM) b | α-Glucosidase Inhibition at 0.1 mM (% ) | α-Amylase Inhibition at 0.1 mM (%) | DPPH IC50 (μM) b | ABTS•+ IC50 (μM) b |
|---|---|---|---|---|---|
| 1 | NA c | NA | 27.9 ± 6.4 bc | NA | 5.65 ± 0.19 b |
| 2 | 4.10 ± 0.12 a | NA | 24.0 ± 1.5 bc | NA | 103.4 ± 4.00 g |
| 3 | 6.25 ± 0.20 b | NA | 29.8 ± 1.8 bc | >250 | 12.04 ± 0.08 d |
| 4 | 10.49 ± 0.32 e | NA | 25.6 ± 1.0 bc | NA | 11.21 ± 0.40 cd |
| 5 | 9.75 ± 0.24 d | NA | 26.5 ± 4.0 bc | >250 | 15.54 ± 0.36 e |
| 6 | NA | NA | 23.0 ± 0.7 c | 46.66 ± 1.58 b | 17.01 ± 0.45 e |
| 7 | 8.10 ± 0.37 c | 15.6 ± 0.9 c | 31.8 ± 0.5 b | NA | 9.34 ± 0.04 cd |
| 8 | 8.01 ± 0.26 c | NA | 28.5 ± 2.7 bc | >250 | 29.13 ± 1.11 f |
| 9 | 15.41 ± 0.42 f | 33.8 ± 2.9 b | 29.5 ± 0.6 bc | >250 | 8.78 ± 0.09 c |
| 10 | NA | NA | 16.2 ± 5.0 d | NA | 3.41 ± 0.08 a |
| Orlistat d | 4.46 ± 0.13 a | ||||
| Acarbose d | 93.2 ± 0.1 a | 51.8 ± 2.5 a | |||
| l-(+)-ascorbic acid d | 13.66 ± 0.13 a | 10.06 ± 0.19 cd |
Disclaimer/Publisher′s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lu, S.-H.; Zuo, H.-J.; Huang, J.; Li, W.-N.; Huang, J.-L.; Li, X.-X. Chemical Constituents from the Leaves of Ligustrum robustum and Their Bioactivities. Molecules 2023, 28, 362. https://doi.org/10.3390/molecules28010362
Lu S-H, Zuo H-J, Huang J, Li W-N, Huang J-L, Li X-X. Chemical Constituents from the Leaves of Ligustrum robustum and Their Bioactivities. Molecules. 2023; 28(1):362. https://doi.org/10.3390/molecules28010362
Chicago/Turabian StyleLu, Shi-Hui, Hao-Jiang Zuo, Jing Huang, Wei-Neng Li, Jie-Lian Huang, and Xiu-Xia Li. 2023. "Chemical Constituents from the Leaves of Ligustrum robustum and Their Bioactivities" Molecules 28, no. 1: 362. https://doi.org/10.3390/molecules28010362
APA StyleLu, S.-H., Zuo, H.-J., Huang, J., Li, W.-N., Huang, J.-L., & Li, X.-X. (2023). Chemical Constituents from the Leaves of Ligustrum robustum and Their Bioactivities. Molecules, 28(1), 362. https://doi.org/10.3390/molecules28010362








