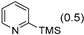
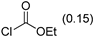
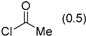
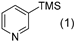
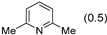
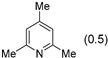
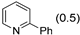
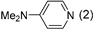
3.2.9. Specific Procedures and Characterization
3-(Benzo[d][1,3]dioxol-5-yl)-5-chloroisoxazole (2c). Compound 2c was prepared following the general procedure of GP-A from 3-(benzo[d][1,3]dioxol-5-yl)isoxazol-5(4H)-one (616 mg, 3 mmol) as colorless solid (443 mg, yield 66%). Mp: 82–83 °C (Et2O–hexane). 1H NMR (400 MHz, CDCl3) δ 7.28 (s, 1H), 7.25–7.20 (m, 1H), 6.93–6.85 (m, 1H), 6.41 (s, 1H), 6.05 (s, 2H). 13C{1H} NMR (100 MHz, CDCl3) δ 163.7, 154.9, 149.6, 148.3, 122.1, 121.2, 108.7, 106.6, 101.6, 99.4. HRMS-ESI [M + H]+ calcd for C10H735ClNO3+, 224.0109; found, 224.0112.
5-Chloro-3-(2,3-dihydrobenzo[b][1,4]dioxin-6-yl)isoxazole (2d). Compound 2d was prepared following the general procedure of GP-A from 3-(2,3-dihydrobenzo[b][1,4]dioxin-6-yl)isoxazol-5(4H)-one (658 mg, 3 mmol) as colorless solid (506 mg, yield 71%). Mp: 78–79 °C (Et2O–hexane). 1H NMR (400 MHz, CDCl3) δ 7.29 (s, 1H), 7.26–7.21 (m, 1H), 6.96–6.89 (m, 1H), 6.39 (s, 1H), 4.36–4.25 (s, 4H). 13C{1H} NMR (100 MHz, CDCl3) δ 163.6, 154.7, 145.6, 143.9, 121.4, 120.0, 117.8, 115.6, 99.4, 64.5, 64.2. HRMS-ESI [M + Na]+ calcd for C11H835ClNNaO3+, 260.0085; found, 260.0079.
5-Chloro-3-(2,3-dihydrobenzo[b][
1,
4]
dioxin-6-yl)isoxazole (
2e). Compound
2e was prepared following the general procedure of GP-A from 3-([1,1’-biphenyl]-4-yl)isoxazol-5(4
H)-one (712 mg, 3 mmol) as colorless solid (506 mg, yield 66%). Mp: 139–140 °C (Et
2O–hexane).
1H NMR (400 MHz, CDCl
3)
δ 7.89–7.83 (m, 2H), 7.75–7.71 (m, 2H), 7.68–7.63 (m, 2H), 7.53–7.47 (m, 2H), 7.46–7.39 (m, 1H), 6.54 (s, 1H).
13C{1H} NMR (100 MHz, CDCl
3)
δ 163.9, 155.1, 143.4, 140.0, 128.9, 127.9, 127.7 (2C), 127.1, 127.0, 99.6. HRMS-ESI [M + H]
+ calcd for C
15H
1135ClNO
+, 256.0524; found, 256.0527.
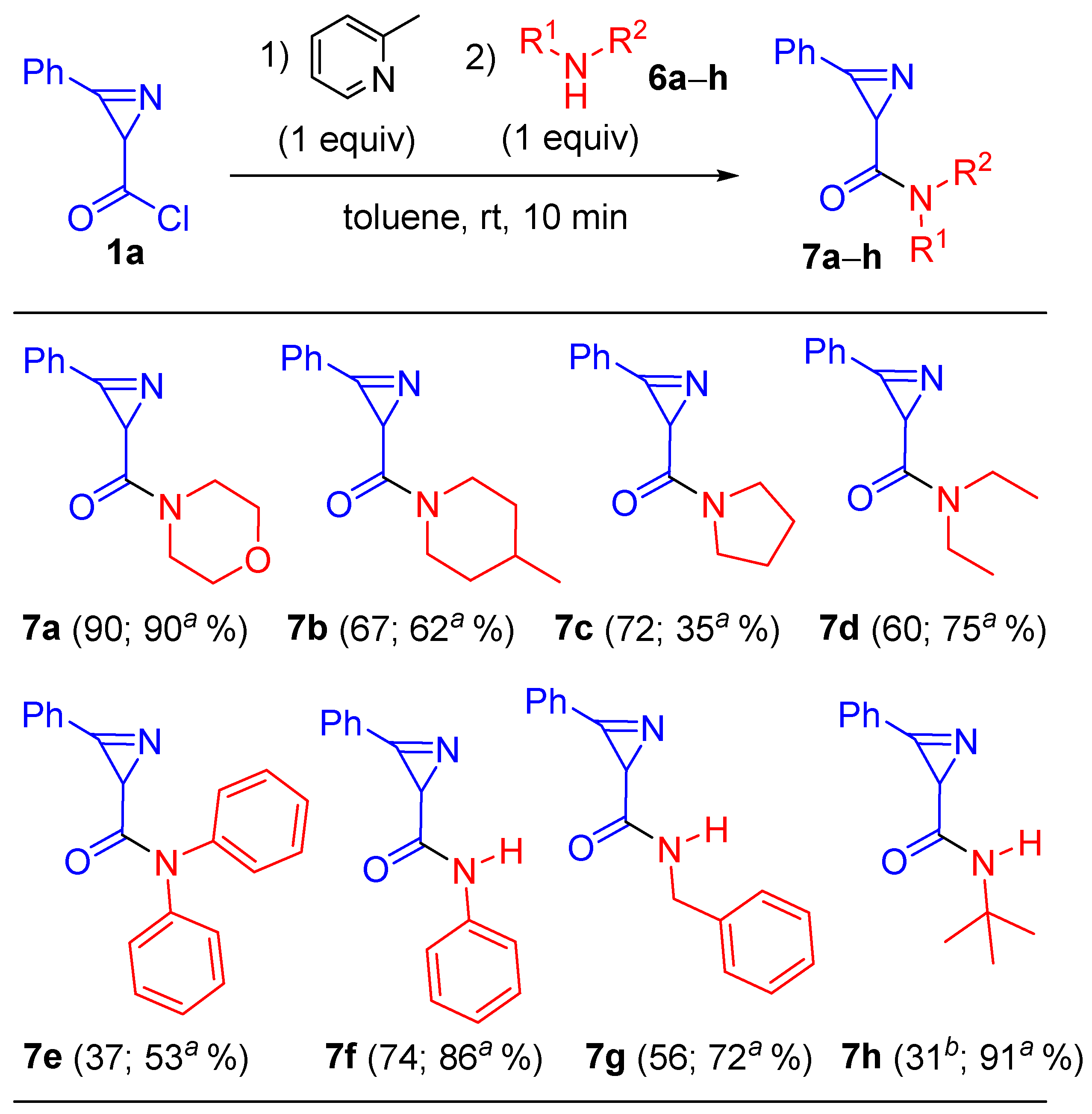
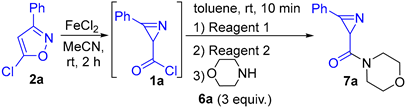
Morpholino(3-phenyl-2H-azirin-2-yl)methanone (7a). Compound 7a was prepared following GP-B–F procedure from 5-chloro-3-phenylisoxazole 2a (359 mg, 2 mmol, 1 equiv.) with morpholine 6a (GP-B and GP-E: 348 mg, 4 mmol, 2 equiv.; GP-C: 174 mg, 2 mmol, 1 equiv.; GP-D: 522 mg, 6 mmol, 3 equiv.; GP-F: 209 mg, 2.4 mmol, 1.2 equiv.) as a nucleophile. A mixture of PE–EtOAc (from 5:1 to 1:1) was used as an eluent for chromatography. Orange solid (GP-B: 322 mg, yield 70%; GP-C and GP-D: 506 mg, yield 90%; GP-E: 276 mg, yield 60%; GP-F: 355 mg, yield 77%). Mp: 73–75 °C (Et2O–hexane). 1H NMR (400 MHz, CDCl3) δ 7.96–7.82 (m, 2H), 7.65–7.51 (m, 3H), 3.97–3.57 (m, 8H), 3.04 (s, 1H). 13C{1H} NMR (100 MHz, CDCl3) δ 169.0, 159.3, 133.5, 130.2, 129.1, 122.9, 66.7 (2C), 45.9, 42.6, 28.5. HRMS-ESI [M + Na]+ calcd for C13H14N2NaO2+, 253.0948; found, 253.0949.
(4-Methylpiperidin-1-yl)(3-phenyl-2H-azirin-2-yl)methanone (7b). Compound 7b was prepared following GP-C and GP-D procedures from 5-chloro-3-phenylisoxazole 2a (359 mg, 2 mmol, 1 equiv) with 4-methylpiperidine 6b (GP-C: 198 mg, 2 mmol, 1 equiv; GP-D: 595 mg, 6 mmol, 3 equiv) as a nucleophile. A mixture of PE–EtOAc (from 5:1 to 1:1) was used as an eluent for chromatography. Orange oil (GP-C: 325 mg, yield 67%; GP-D: 300 mg, yield 62%). 1H NMR (400 MHz, CDCl3) δ 7.99–7.86 (m, 2H), 7.66–7.52 (m, 3H), 4.64–4.39 (m, 2H), 3.38–3.20 (m, 1H), 3.10 (s, 1H), 2.78–2.60 (m, 1H), 1.95–1.79 (m, 1H), 1.77–1.65 (m, 2H), 1.41–1.13 (m, 2H), 1.02 (d, J = 6.2 Hz, 3H). 13C{1H} NMR (100 MHz, CDCl3) δ 168.5, 159.8 (d, J = 17.5 Hz), 133.3, 130.2, 129.1, 123.3, 45.9 (d, J = 11.6 Hz), 42.8 (d, J = 13.9 Hz), 34.9 (d, J = 13.3 Hz), 33.6, 31.1 (d, J = 13.1 Hz), 29.0, 21.7. HRMS-ESI [M + H]+ calcd for C15H19N2O+, 243.1492; found, 243.1494.
(3-Phenyl-2H-azirin-2-yl)(pyrrolidin-1-yl)methanone (7c). Compound 7c was prepared following GP-C and GP-D procedures from 5-chloro-3-phenylisoxazole 2a (359 mg, 2 mmol, 1 equiv.) with pyrrolidine 6c (GP-C: 142 mg, 2 mmol, 1 equiv.; GP-D: 427 mg, 6 mmol, 3 equiv.) as a nucleophile. A mixture of PE–EtOAc (from 5:1 to 1:1) was used as an eluent for chromatography. Orange solid (GP-C: 309 mg, yield 72%; GP-D: 150 mg, yield 35%). Mp: 58–59 °C (Et2O–hexane). 1H NMR (400 MHz, CDCl3) δ 7.94–7.84 (m, 2H), 7.64–7.50 (m, 3H), 3.93–3.74 (m, 2H), 3.61–3.49 (m, 2H), 2.97 (s, 1H), 2.13–1.83 (m, 4H). 13C{1H} NMR (100 MHz, CDCl3) δ 168.6, 159.4, 133.3, 130.2, 129.0, 123.1, 46.4, 46.3, 30.0, 26.2, 24.1. HRMS-ESI [M + H]+ calcd for C13H15N2O+, 215.1179; found, 215.1181.
N,N-Diethyl-3-phenyl-2H-azirine-2-carboxamide (7d). Compound 7d was prepared following GP-C and GP-D procedures from 5-chloro-3-phenylisoxazole 2a (359 mg, 2 mmol, 1 equiv.) with diethylamine 6d (GP-C: 146 mg, 2 mmol, 1 equiv.; GP-D: 439 mg, 6 mmol, 3 equiv.) as a nucleophile. A mixture of PE–EtOAc (from 5:1 to 1:1) was used as an eluent for chromatography. Orange solid (GP-C: 260 mg, yield 60%; GP-D: 324 mg, yield 75%). Mp: 53–54 °C (Et2O–hexane). 1H NMR (400 MHz, CDCl3) δ 7.92–7.86 (m, 2H), 7.62–7.51 (m, 3H), 3.80–3.70 (m, 1H), 3.70–3.59 (m, 1H), 3.58–3.48 (m, 1H), 3.44–3.34 (m, 1H), 3.04 (s, 1H), 1.40 (t, J = 7.1 Hz, 3H), 1.16 (t, J = 7.1 Hz, 3H). 13C{1H} NMR (100 MHz, CDCl3) δ 169.4, 159.4, 133.2, 130.1, 129.0, 123.3, 42.0, 41.1, 29.0, 15.1, 13.0. HRMS-ESI [M + H]+ calcd for C13H17N2O+, 217.1335; found, 217.1338.
N,N,3-Triphenyl-2H-azirine-2-carboxamide (7e). Compound 7e was prepared following GP-C and GP-D procedures from 5-chloro-3-phenylisoxazole 2a (359 mg, 2 mmol, 1 equiv.) with diphenylamine 6e (GP-C: 334 mg, 2 mmol, 1 equiv.; GP-D: 1.02 g, 6 mmol, 3 equiv.) as a nucleophile. A mixture of PE–EtOAc (from 5:1 to 1:1) was used as an eluent for chromatography. Orange solid (GP-C: 231 mg, yield 37%; GP-D: 331 mg, yield 53%). Mp: 189–190 °C (Et2O–hexane). 1H NMR (400 MHz, CDCl3) δ 7.99–7.87 (m, 2H), 7.67–7.56 (m, 3H), 7.53–7.24 (m, 10H), 2.82 (s, 1H). 13C{1H} NMR (100 MHz, CDCl3) δ 170.4, 158.6, 142.4, 133.4, 130.2, 129.4, 129.1, 127.1, 123.0, 31.3. HRMS-ESI [M + H]+ calcd for C21H17N2O+, 313.1335; found, 313.1340.
N,3-Diphenyl-2H-azirine-2-carboxamide (7f). Compound 7f was prepared following GP-C and GP-D procedures from 5-chloro-3-phenylisoxazole 2a (359 mg, 2 mmol, 1 equiv.) with aniline 6f (GP-C: 186 mg, 2 mmol, 1 equiv; GP-D: 559 mg, 6 mmol, 3 equiv.) as a nucleophile. A mixture of PE–EtOAc (from 5:1 to 1:1) was used as an eluent for chromatography. Orange solid (GP-C: 350 mg, yield 74%; GP-D: 406 mg, yield 86%). Mp: 179–180 °C (Et2O–hexane). 1H NMR (400 MHz, CDCl3) δ 8.01–7.93 (m, 2H), 7.71–7.65 (m, 1H), 7.63–7.57 (m, 2H), 7.55–7.50 (m, 2H), 7.43 (s, 1H), 7.34–7.26 (m, 2H), 7.13–7.07 (m, 1H), 2.94 (s, 1H). 13C{1H} NMR (100 MHz, CDCl3) δ 168.6, 162.2, 137.4, 134.3, 130.6, 129.4, 128.9, 124.4, 122.3, 119.7, 32.0. HRMS-ESI [M + Na]+ calcd for C15H12N2NaO+, 259.0842; found, 259.0842.
N-Benzyl-3-phenyl-2H-azirine-2-carboxamide (
7g) [
16,
17]. Compound
7g was prepared following GP-C and GP-D procedures from 5-chloro-3-phenylisoxazole
2a (359 mg, 2 mmol, 1 equiv.) with phenylmethanamine
6g (GP-C: 214 mg, 2 mmol, 1 equiv.; GP-D: 643 mg, 6 mmol, 3 equiv.) as a nucleophile. A mixture of PE–EtOAc (from 5:1 to 1:1) was used as an eluent for chromatography. Light yellow solid (GP-C: 280 mg, yield 56%; GP-D: 360 mg, yield 72%). Mp: 145–146 °C (Et
2O–hexane).
1H NMR (400 MHz, CDCl
3)
δ 7.96–7.89 (m, 2H), 7.70–7.65 (m, 1H), 7.63–7.57 (m, 2H), 7.33–7.20 (m, 5H), 5.90 (s, 1H), 4.54–4.35 (m, 2H), 2.85 (s, 1H).
13C{1H} NMR (100 MHz, CDCl
3)
δ 170.5, 162.1, 137.9, 134.1, 130.5, 129.4, 128.6, 127.6, 127.4, 122.4, 43.4, 31.4. HRMS-ESI [M + Na]
+ calcd for C
16H
14N
2NaO
+, 273.0998; found, 273.1001.
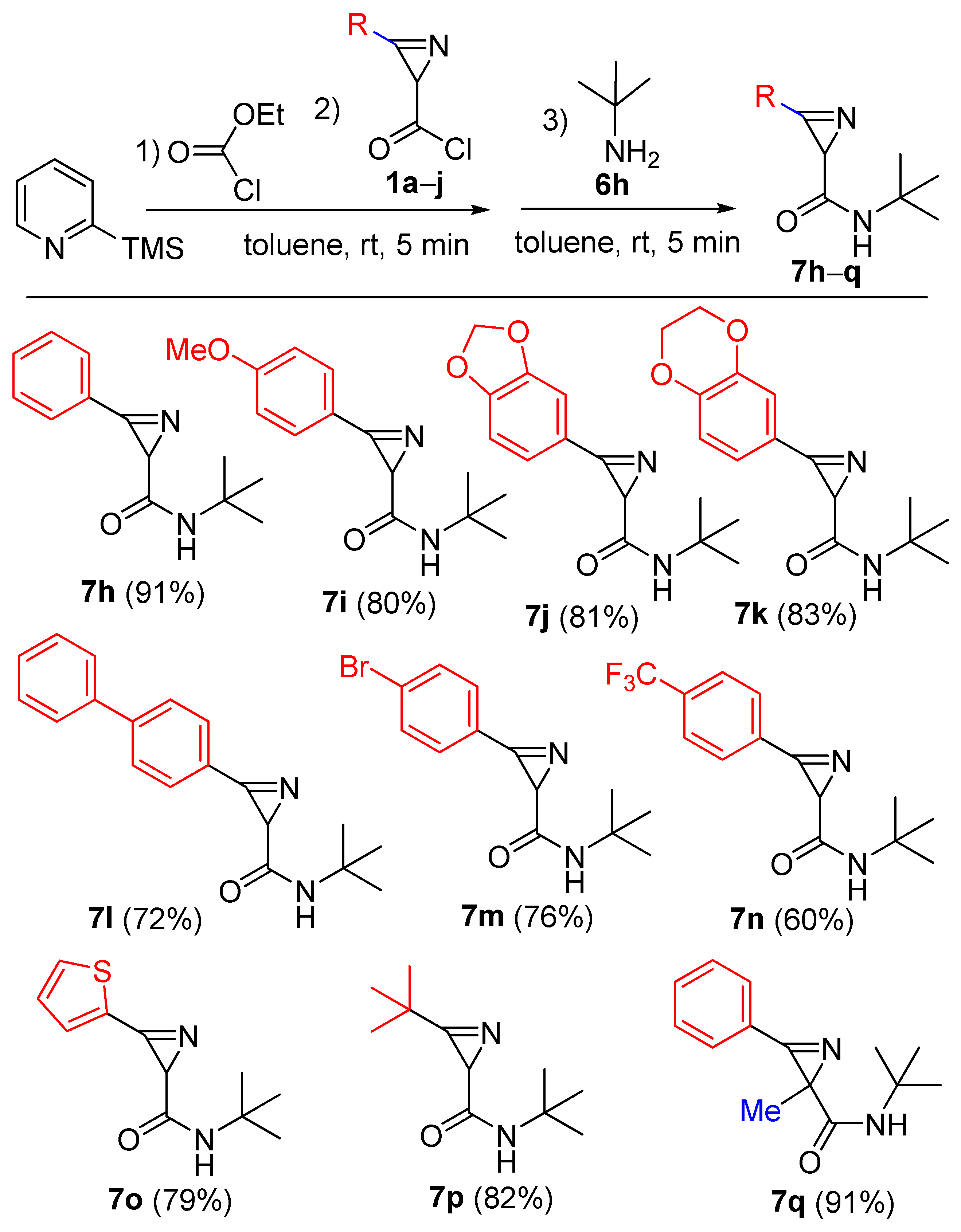
N-(tert-Butyl)-3-phenyl-2H-azirine-2-carboxamide (7h). Compound 7h was prepared following methods GP-B, GP-C and GP-D from 5-chloro-3-phenylisoxazole 2a (359 mg, 2 mmol, 1 equiv.) with 2-methylpropan-2-amine 6h (GP-B: 293 mg, 4 mmol, 2 equiv.; GP-C: 146 mg, 2 mmol, 1 equiv.; GP-D: 439 mg, 6 mmol, 3 equiv.) as a nucleophile. A mixture of PE–EtOAc (from 5:1 to 1:1) was used as an eluent for chromatography. Colorless solid (GP-B: 182 mg, yield 42%; GP-C: 134 mg, yield 31%; GP-D: 394 mg, yield 91%). Mp: 171–172 °C (Et2O–hexane). 1H NMR (400 MHz, CDCl3) δ 7.95–7.89 (m, 2H), 7.69–7.63 (m, 1H), 7.63–7.56 (m, 2H), 5.32 (s, 1H), 2.69 (s, 1H), 1.32 (s, 9H). 13C{1H} NMR (100 MHz, CDCl3) δ 169.6, 162.6, 133.9, 130.3, 129.3, 122.7, 51.3, 32.1, 28.7. HRMS-ESI [M + H]+ calcd for C13H17N2O+, 217.1335; found, 217.1338.
N-(tert-Butyl)-3-(4-methoxyphenyl)-2H-azirine-2-carboxamide (7i). Compound 7i was prepared following GP-D procedure from 5-chloro-3-(4-methoxyphenyl)isoxazole 2b (419 mg, 2 mmol, 1 equiv.) with 2-methylpropan-2-amine 6h (439 mg, 6 mmol, 3 equiv.) as a nucleophile. A mixture of PE–EtOAc (from 5:1 to 1:1) was used as an eluent for chromatography. Colorless solid (394 mg, yield 80%). Mp: 157–158 °C (Et2O–hexane). 1H NMR (400 MHz, CDCl3) δ 7.88–7.82 (m, 2H), 7.10–7.04 (m, 2H), 5.26 (s, 1H), 3.91 (s, 3H), 2.63 (s, 1H), 1.30 (s, 9H). 13C{1H} NMR (100 MHz, CDCl3) δ 170.0, 164.1, 161.3, 132.5, 115.0, 114.9, 55.6, 51.1, 31.8, 28.6. HRMS-ESI [M + Na]+ calcd for C14H18N2NaO2+, 269.1260; found, 269.1262.
3-(Benzo[d][1,3]dioxol-5-yl)-N-(tert-butyl)-2H-azirine-2-carboxamide (7j). Compound 7j was prepared following GP-D procedure from 3-(benzo[d][1,3]dioxol-5-yl)-5-chloroisoxazole 2c (447 mg, 2 mmol, 1 equiv.) with 2-methylpropan-2-amine 6h (439 mg, 6 mmol, 3 equiv.) as a nucleophile. A mixture of PE–EtOAc (from 5:1 to 1:1) was used as an eluent for chromatography. Light yellow solid (422 mg, yield 81%). Mp: 150–151 °C (Et2O–hexane). 1H NMR (400 MHz, CDCl3) δ 7.45–7.41 (m, 1H), 7.38–7.35 (m, 1H), 7.01–6.96 (m, 1H), 6.11 (s, 2H), 5.27 (s, 1H), 2.63 (s, 1H), 1.31 (s, 9H). 13C{1H} NMR (100 MHz, CDCl3) δ 169.7, 161.6, 152.6, 148.6, 127.2, 116.5, 109.1, 109.0, 102.2, 51.2, 32.3, 28.6. HRMS-ESI [M + H]+ calcd for C14H17N2O3+, 261.1234; found, 261.1237.
N-(tert-Butyl)-3-(2,3-dihydrobenzo[b][1,4]dioxin-6-yl)-2H-azirine-2-carboxamide (7k). Compound 7k was prepared following GP-D procedure from 5-chloro-3-(2,3-dihydrobenzo[b][1,4]dioxin-6-yl)isoxazole 2d (475 mg, 2 mmol, 1 equiv.) with 2-methylpropan-2-amine 6h (439 mg, 6 mmol, 3 equiv.) as a nucleophile. A mixture of PE–EtOAc (from 5:1 to 1:1) was used as an eluent for chromatography. Colorless solid (455 mg, yield 83%). Mp: 123–124 °C (Et2O–hexane). 1H NMR (400 MHz, CDCl3) δ 7.46–7.39 (m, 2H), 7.08–7.00 (m, 1H), 5.24 (s, 1H), 4.38–4.29 (m, 4H), 2.61 (s, 1H), 1.31 (s, 9H). 13C{1H} NMR (100 MHz, CDCl3) δ 169.9, 161.5, 148.7, 144.1, 124.4, 119.3, 118.4, 115.7, 64.7, 64.0, 51.2, 32.0, 28.6. HRMS-ESI [M + Na]+ calcd for C15H18N2NaO3+, 297.1210; found, 297.1212.
3-([1,1’-Biphenyl]-4-yl)-N-(tert-butyl)-2H-azirine-2-carboxamide (7l). Compound 7l was prepared following GP-D procedure from 3-([1,1’-biphenyl]-4-yl)-5-chloroisoxazole 2e (511 mg, 2 mmol, 1 equiv.) with 2-methylpropan-2-amine 6h (439 mg, 6 mmol, 3 equiv.) as a nucleophile. A mixture of PE–EtOAc (from 5:1 to 1:1) was used as an eluent for chromatography. Light yellow solid (421 mg, yield 72%). Mp: 161–162 °C (Et2O–hexane). 1H NMR (400 MHz, CDCl3) δ 8.02–7.98 (m, 2H), 7.84–7.80 (m, 2H), 7.68–7.64 (m, 2H), 7.54–7.42 (m, 3H), 5.33 (s, 1H), 2.72 (s, 1H), 1.35 (s, 9H). 13C{1H} NMR (100 MHz, CDCl3) δ 169.7, 162.3, 146.8, 139.5, 130.8, 129.0, 128.6, 128.0, 127.3, 121.3, 51.3, 32.1, 28.7. HRMS-ESI [M + H]+ calcd for C19H21N2O+, 293.1648; found, 293.1652.
3-(4-Bromophenyl)-N-(tert-butyl)-2H-azirine-2-carboxamide (7m). Compound 7m was prepared following GP-D procedure from 3-(4-bromophenyl)-5-chloroisoxazole 2f (517 mg, 2 mmol, 1 equiv.) with 2-methylpropan-2-amine 6h (439 mg, 6 mmol, 3 equiv.) as a nucleophile. A mixture of PE–EtOAc (from 5:1 to 1:1) was used as an eluent for chromatography. Colorless solid (449 mg, yield 76%). Mp: 171–172 °C (Et2O–hexane). 1H NMR (400 MHz, CDCl3) δ 7.81–7.72 (m, 4H), 5.34 (s, 1H), 2.69 (s, 1H), 1.33 (s, 9H). 13C{1H} NMR (100 MHz, CDCl3) δ 169.2, 162.1, 132.8, 131.5, 129.1, 121.6, 51.4, 32.3, 28.7. HRMS-ESI [M + H]+ calcd for C13H1679BrN2O+, 295.0441; found, 295.0443.
N-(tert-butyl)-3-(4-(trifluoromethyl)phenyl)-2H-azirine-2-carboxamide (7n). Compound 7n was prepared following GP-D procedure from 5-chloro-3-(4-(trifluoromethyl)phenyl)isoxazole 2g (495 mg, 2 mmol, 1 equiv.) with 2-methylpropan-2-amine 6h (439 mg, 6 mmol, 3 equiv.) as a nucleophile. A mixture of PE–EtOAc (from 5:1 to 1:1) was used as an eluent for chromatography. Colorless solid (341 mg, yield 60%). Mp: 142–143 °C (Et2O–hexane). 1H NMR (400 MHz, CDCl3) δ 8.07–8.01 (m, 2H), 7.88–7.83 (m, 2H), 5.47 (s, 1H), 2.75 (s, 1H), 1.34 (s, 9H). 13C{1H} NMR (100 MHz, CDCl3) δ 168.9, 162.2, 135.3 (q, J = 33.0 Hz), 130.6, 126.4 (q, J = 3.7 Hz), 126.2, 123.3 (q, J = 272.9 Hz), 51.6, 32.6, 28.7. HRMS-ESI [M + H]+ calcd for C14H16F3N2O+, 285.1209; found, 285.1213.
N-(tert-butyl)-3-(thiophen-2-yl)-2H-azirine-2-carboxamide (7o). Compound 7o was prepared following GP-D procedure from 5-chloro-3-(thiophen-2-yl)isoxazole 2h (371 mg, 2 mmol, 1 equiv.) with 2-methylpropan-2-amine 6h (439 mg, 6 mmol, 3 equiv.) as a nucleophile. A mixture of PE–EtOAc (from 5:1 to 1:1) was used as an eluent for chromatography. Colorless solid (351 mg, yield 79%). Mp: 135–136 °C (Et2O–hexane). 1H NMR (400 MHz, CDCl3) δ 7.89 (d, J = 3.9 Hz, 1H), 7.74 (d, J = 2.7 Hz, 1H), 7.28 (dd, J = 5.0, 3.7 Hz, 1H), 5.35 (s, 1H), 2.70 (s, 1H), 1.32 (s, 9H). 13C{1H} NMR (100 MHz, CDCl3) δ 169.2, 155.8, 135.6, 135.5, 128.6, 125.1, 51.3, 32.8, 28.6. HRMS-ESI [M + H]+ calcd for C11H15N2Os+, 223.0900; found, 223.0903.
N,3-Di-tert-butyl-2H-azirine-2-carboxamide (7p). Compound 7p was prepared following GP-D procedure from 3-(tert-butyl)-5-chloroisoxazole 2i (319 mg, 2 mmol, 1 equiv.) with 2-methylpropan-2-amine 6h (439 mg, 6 mmol, 3 equiv.) as a nucleophile. A mixture of PE–EtOAc (from 5:1 to 1:1) was used as an eluent for chromatography. Colorless solid (322 mg, yield 82%). Mp: 129–130 °C (Et2O–hexane). 1H NMR (400 MHz, CDCl3) δ 5.15 (s, 1H), 2.34 (s, 1H), 1.31 (s, 9H), 1.30 (s, 9H). 13C{1H} NMR (100 MHz, CDCl3) δ 13C NMR (101 MHz, CDCl3) δ 171.9, 170.0, 51.1, 33.3, 32.3, 28.7, 25.7. HRMS-ESI [M + H]+ calcd for C11H21N2O+, 197.1648; found, 197.1651.
N-(tert-Butyl)-2-methyl-3-phenyl-2H-azirine-2-carboxamide (7q). Compound 7q was prepared following GP-D procedure from 5-chloro-4-methyl-3-phenylisoxazole 2j (387 mg, 2 mmol, 1 equiv.) with 2-methylpropan-2-amine 6h (439 mg, 6 mmol, 3 equiv.) as a nucleophile. A mixture of PE–EtOAc (from 10:1 to 5:1) was used as an eluent for chromatography. Colorless solid (419 mg, yield 91%). Mp: 84–85 °C (Et2O–hexane).1H NMR (400 MHz, CDCl3) δ 7.91–7.85 (m, 2H), 7.68–7.63 (m, 1H), 7.62–7.56 (m, 2H), 5.22 (s, 1H), 1.60 (s, 3H), 1.27 (s, 9H). 13C{1H} NMR (100 MHz, CDCl3) δ 171.5, 168.0, 133.8, 130.1, 129.4, 122.7, 51.0, 37.1, 28.5, 17.4. HRMS-ESI [M + H]+ calcd for C14H19N2O+, 231.1492; found, 231.1495.
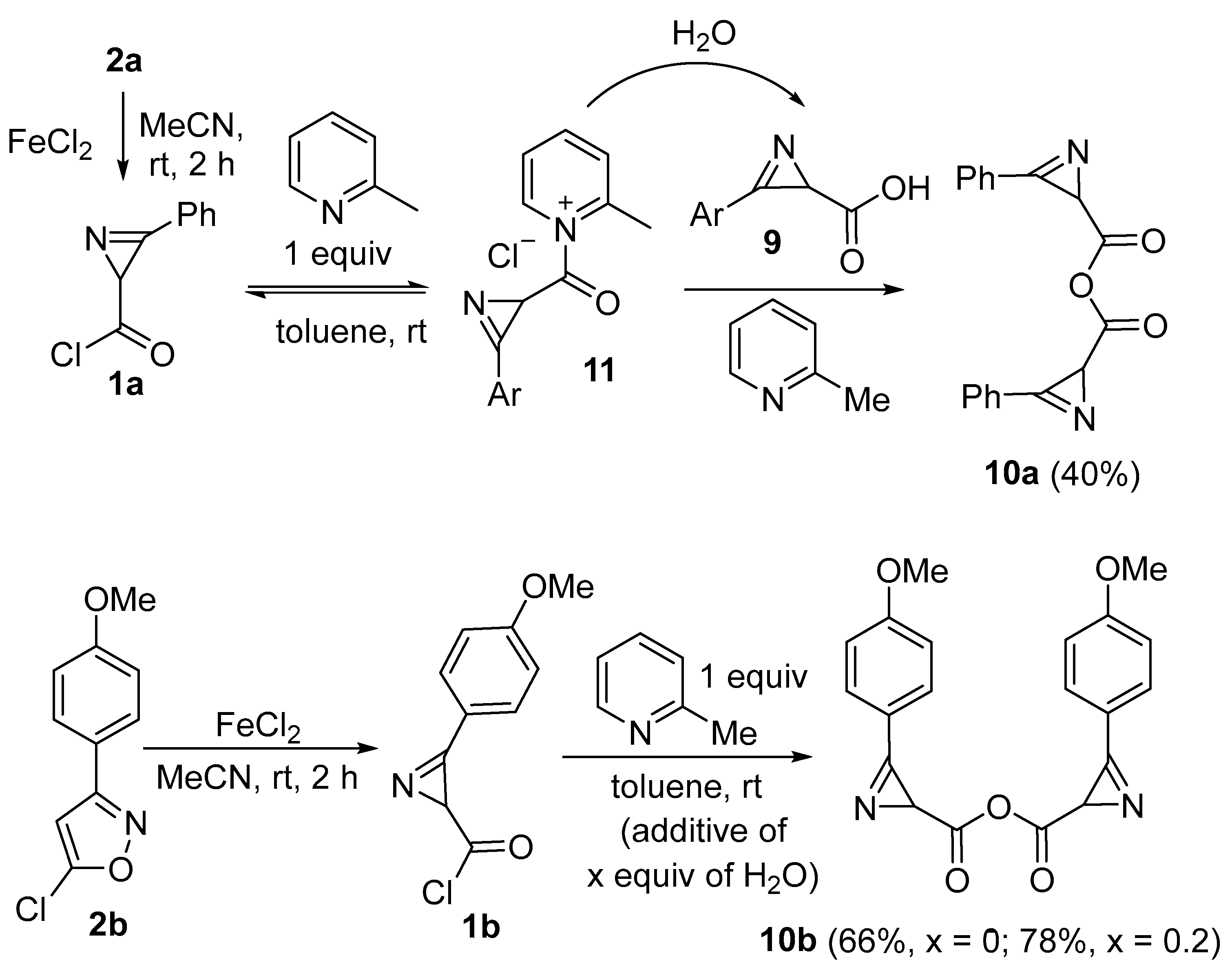
3-Phenyl-2H-azirine-2-carboxylic anhydride (10a). Compound 10a was prepared following GP-G procedure from 5-chloro-3-phenylisoxazole 2a (359 mg, 2 mmol, 1 equiv.). A mixture of PE–EtOAc (from 10:1 to 5:1) was used as an eluent for chromatography. Colorless solid (122 mg, yield 40%). Mp: 92–93 °C (Et2O–hexane). 1H NMR (400 MHz, C6D6) δ 7.32–7.23 (m, 4H), 7.03–6.95 (m, 2H), 6.92–6.83 (m, 4H), 2.80–2.74 (m, 2H). 13C{1H} NMR (100 MHz, C6D6) δ 174.21, 174.18, 158.0 (2C), 134.2, 131.1, 130.7 (2C), 129.44, 129.41, 121.10, 121.07, 37.7 (2C). HRMS-ESI [M + Na]+ calcd for C18H12N2NaO3+, 327.0740; found, 327.0733.
3-(4-Methoxyphenyl)-2H-azirine-2-carboxylic anhydride (10b). Compound 10b was prepared following GP-G procedure from 2b (419 mg, 2 mmol, 1 equiv.). A mixture of PE–EtOAc (from 10:1 to 2:1) was used as an eluent for chromatography. Colorless solid (242 mg, yield 66%). Mp: 96–98 °C (Et2O–hexane). 1H NMR (400 MHz, C6D6) δ 7.49–7.37 (m, 4H), 6.53–6.43 (m, 4H), 3.12 (s, 6H), 2.78–2.60 (m, 2H). 13C{1H} NMR (100 MHz, CDCl3) δ 177.3 (2C), 168.1, 167.9, 164.4, 164.3, 155.8, 155.7, 132.82, 132.79, 115.0, 114.9, 113.4, 113.3, 55.62, 55.60, 29.7, 28.7. HRMS-ESI [M + Na]+ calcd for C20H16N2NaO5+, 387.0951; found, 387.0955.
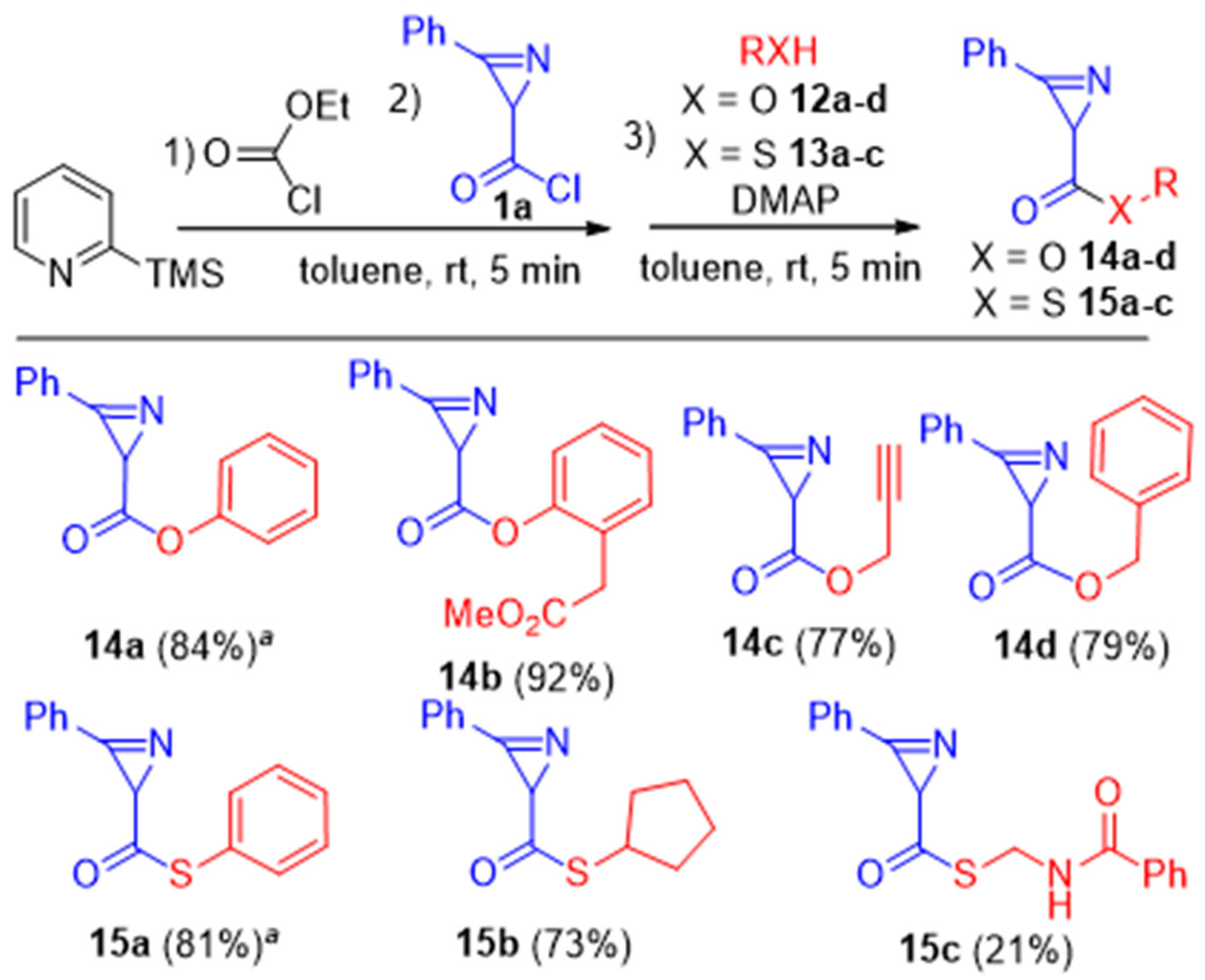
Phenyl 3-phenyl-2H-azirine-2-carboxylate (14a). Compound 14a was prepared following GP-H procedure from 5-chloro-3-phenylisoxazole 2a (359 mg, 2 mmol, 1 equiv.) with phenol 12a (282 mg, 3 mmol, 1.5 equiv.) as a nucleophile and N,N-dimethylpyridin-4-amine (733 mg, 6 mmol, 3 equiv.) as a base. A mixture of PE–EtOAc (from 20:1 to 5:1) was used as an eluent for chromatography. Colorless solid (399 mg, yield 84%). Mp: 82–83 °C (Et2O–hexane). 1H NMR (400 MHz, CDCl3) δ 8.04–7.95 (m, 2H), 7.74–7.59 (m, 3H), 7.43–7.34 (m, 2H), 7.28–7.21 (m, 1H), 7.18–7.08 (m, 2H), 3.09 (s, 1H). 13C{1H} NMR (100 MHz, CDCl3) δ 170.1, 158.2, 150.6, 134.1, 130.5, 129.4, 129.3, 125.9, 122.0, 121.3, 29.6. HRMS-ESI [M + Na]+ calcd for C15H11NNaO2+, 260.0682; found, 260.0686.
2-(2-Methoxy-2-oxoethyl)phenyl 3-phenyl-2H-azirine-2-carboxylate (14b). Compound 14b was prepared following GP-H procedure from 5-chloro-3-phenylisoxazole 2a (359 mg, 2 mmol, 1 equiv.) with methyl 2-(2-hydroxyphenyl)acetate 12b (499 mg, 3 mmol, 1.5 equiv.) as a nucleophile and N,N-dimethylpyridin-4-amine (733 mg, 6 mmol, 3 equiv.) as a base. A mixture of PE–EtOAc (from 20:1 to 5:1) was used as an eluent for chromatography. Colorless solid (835 mg, yield 92%). Mp: 52–53 °C (Et2O–hexane). 1H NMR (400 MHz, CDCl3) δ 8.08–7.99 (m, 2H), 7.74–7.61 (m, 3H), 7.35–7.29 (m, 2H), 7.25–7.15 (m, 2H), 3.64 (s, 3H), 3.58 (AB-q, J = 15.6 Hz, 2H), 3.09 (s, 1H). 13C{1H} NMR (100 MHz, CDCl3) δ 170.9, 169.8, 158.1, 148.9, 134.1, 131.3, 130.6, 129.4, 128.5, 126.3, 126.2, 122.4, 122.0, 52.0, 36.1, 29.5. HRMS-ESI [M + H]+ calcd for C18H16NO4+, 310.1074; found, 310.1077.
Prop-2-yn-1-yl 3-phenyl-2H-azirine-2-carboxylate (14c). Compound 14c was prepared following GP-H procedure from 5-chloro-3-phenylisoxazole 2a (359 mg, 2 mmol, 1 equiv.) with prop-2-yn-1-ol 12c (168 mg, 3 mmol, 1.5 equiv.) as a nucleophile and N,N-dimethylpyridin-4-amine (733 mg, 6 mmol, 3 equiv.) as a base. A mixture of PE–EtOAc (from 20:1 to 5:1) was used as an eluent for chromatography. Colorless solid (307 mg, yield 77%). Mp: 42–43 °C (Et2O–hexane). 1H NMR (400 MHz, CDCl3) δ 7.93–7.87 (m, 2H), 7.71–7.64 (m, 1H), 7.63–7.56 (m, 2H), 4.77 (dd, J = 2.4, 1.0 Hz, 2H), 2.91 (s, 1H), 2.50 (t, J = 2.4 Hz, 1H). 13C{1H} NMR (100 MHz, CDCl3) δ 170.9, 158.0, 134.0, 130.5, 129.3, 121.9, 77.2, 75.2, 52.6, 29.3. HRMS-ESI [M + Na]+ calcd for C12H9NNaO2+, 222.0526; found, 222.0527.
Benzyl 3-phenyl-2H-azirine-2-carboxylate (
14d) [
26]. Compound
14d was prepared following GP-H procedure from 5-chloro-3-phenylisoxazole
2a (359 mg, 2 mmol, 1 equiv.) with phenylmethanol
12d (324 mg, 3 mmol, 1.5 equiv.) as a nucleophile and
N,
N-dimethylpyridin-4-amine (733 mg, 6 mmol, 3 equiv.) as a base. A mixture of PE–EtOAc (from 20:1 to 5:1) was used as an eluent for chromatography. Colorless solid (636 mg, yield 79%). Mp: 65–66 °C (Et
2O–hexane).
1H NMR (400 MHz, CDCl
3)
δ 7.92–7.88 (m, 2H), 7.69–7.64 (m, 1H), 7.62–7.57 (m, 2H), 7.39–7.34 (m, 5H), 5.23 (AB-q,
J = 12.4 Hz, 2H), 2.93 (s, 1H).
13C{1H} NMR (100 MHz, CDCl
3)
δ 171.5, 158.4, 135.6, 133.9, 130.4, 129.3, 128.5, 128.2, 128.1, 122.2, 66.9, 29.6. HRMS-ESI [M + Na]
+ calcd for C
16H
13NNaO
2+, 274.0839; found, 274.0841.
S-Phenyl 3-phenyl-2H-azirine-2-carbothioate (15a). Compound 15a was prepared following GP-H procedure from 5-chloro-3-phenylisoxazole 2a (359 mg, 2 mmol, 1 equiv.) with benzenethiol 13a (331 mg, 3 mmol, 1.5 equiv.) as a nucleophile and N,N-dimethylpyridin-4-amine (733 mg, 6 mmol, 3 equiv.) as a base. A mixture of PE–EtOAc (from 20:1 to 5:1) was used as an eluent for chromatography. Colorless solid (410 mg, yield 81%). Mp: 54–55 °C (Et2O–hexane). 1H NMR (400 MHz, CDCl3) δ 8.01–7.96 (m, 2H), 7.74–7.69 (m, 1H), 7.67–7.61 (m, 2H), 7.45–7.39 (m, 5H), 3.22 (s, 1H). 13C{1H} NMR (100 MHz, CDCl3) δ 196.8, 158.8, 134.6, 134.3, 130.7, 129.5, 129.4, 129.1, 126.9, 121.7, 38.0. HRMS-ESI [M + Na]+ calcd for C15H11NNaOS+, 276.0454; found, 276.0456.
S-Cyclopentyl 3-phenyl-2H-azirine-2-carbothioate (15b). Compound 15b was prepared following GP-H procedure from 5-chloro-3-phenylisoxazole 2a (359 mg, 2 mmol, 1 equiv.) with cyclopentanethiol 13b (306 mg, 3 mmol, 1.5 equiv.) as a nucleophile and N,N-dimethylpyridin-4-amine (733 mg, 6 mmol, 3 equiv.) as a base. A mixture of PE–EtOAc (from 20:1 to 5:1) was used as an eluent for chromatography. Yellow oil (358 mg, yield 73%). 1H NMR (400 MHz, CDCl3) δ 7.97–7.86 (m, 2H), 7.70–7.57 (m, 3H), 3.83–3.71 (m, 1H), 3.11 (s, 1H), 2.19–2.01 (m, 2H), 1.70–1.45 (m, 6H). 13C{1H} NMR (100 MHz, CDCl3) δ 199.3, 158.7, 134.0, 130.6, 129.4, 121.9, 42.4, 38.1, 33.3, 33.1, 24.7, 24.7. HRMS-ESI [M + H]+ calcd for C14H16NOS+, 246.0947; found, 246.0951.
S-(Benzamidomethyl) 3-phenyl-2H-azirine-2-carbothioate (15c). Compound 15c was prepared following GP-H procedure from 5-chloro-3-phenylisoxazole 2a (359 mg, 2 mmol, 1 equiv.) with N-(mercaptomethyl)benzamide 13c (502 mg, 3 mmol, 1.5 equiv.) as a nucleophile and N,N-dimethylpyridin-4-amine (733 mg, 6 mmol, 3 equiv.) as a base. A mixture of PE–EtOAc (from 20:1 to 5:1) was used as an eluent for chromatography. Light yellow solid (130 mg, yield 21%). Mp: 112–113 °C (Et2O–hexane). 1H NMR (400 MHz, CDCl3) δ 8.15 (s, 1H), 7.95–7.88 (m, 2H), 7.75–7.86 (m, 1H), 7.65–7.58 (m, 2H), 7.51–7.44 (m, 2H), 7.37–7.30 (m, 2H), 7.18–7.08 (m, 1H), 3.70 (s, 2H), 3.24 (s, 1H). 13C{1H} NMR (100 MHz, CDCl3) δ 199.8, 166.0, 158.2, 137.5, 134.6, 130.8, 129.6, 128.9, 124.5, 121.1, 119.9, 37.9, 33.6. HRMS-ESI [M + H]+ calcd for C17H14N2OS+, 311.0849; found, 311.0852.