Spectroscopic Investigations of Porphyrin-TiO2 Nanoparticles Complexes
Abstract
1. Introduction
2. Results and Discussions
2.1. Morphological Characterization of the Porphyrin-TiO2 Complexes
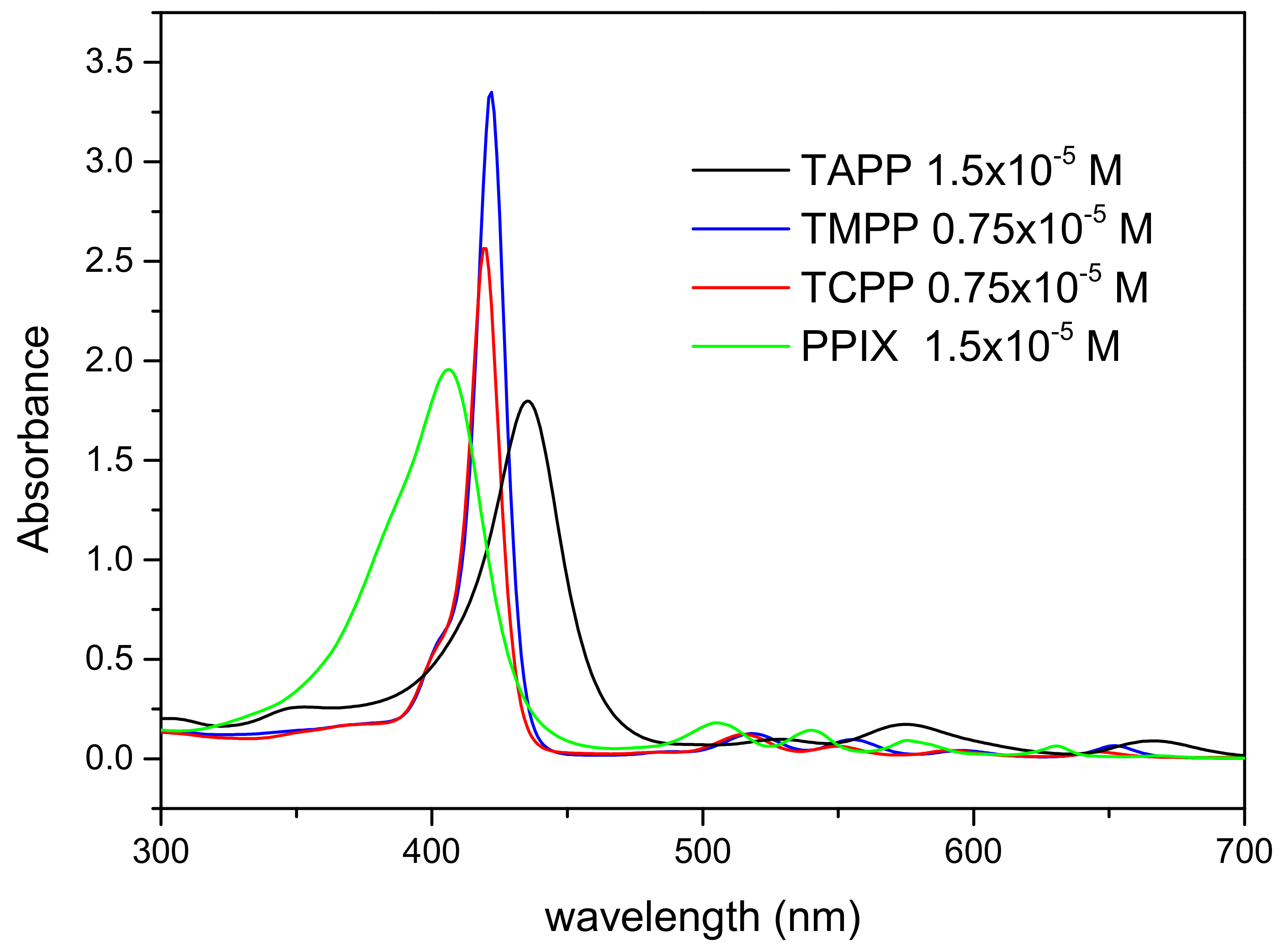
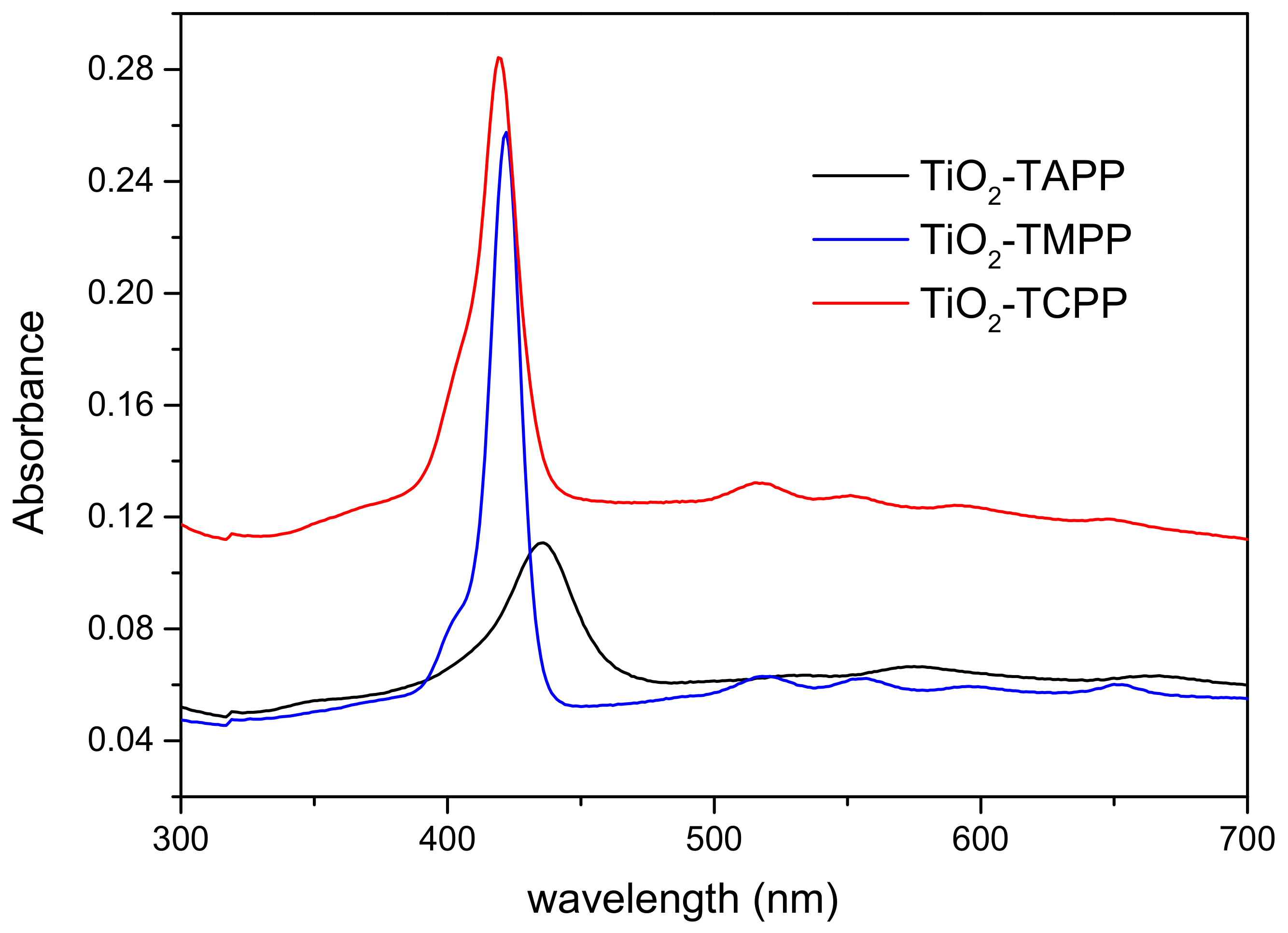
2.2. UV Vis Absorption Spectroscopy
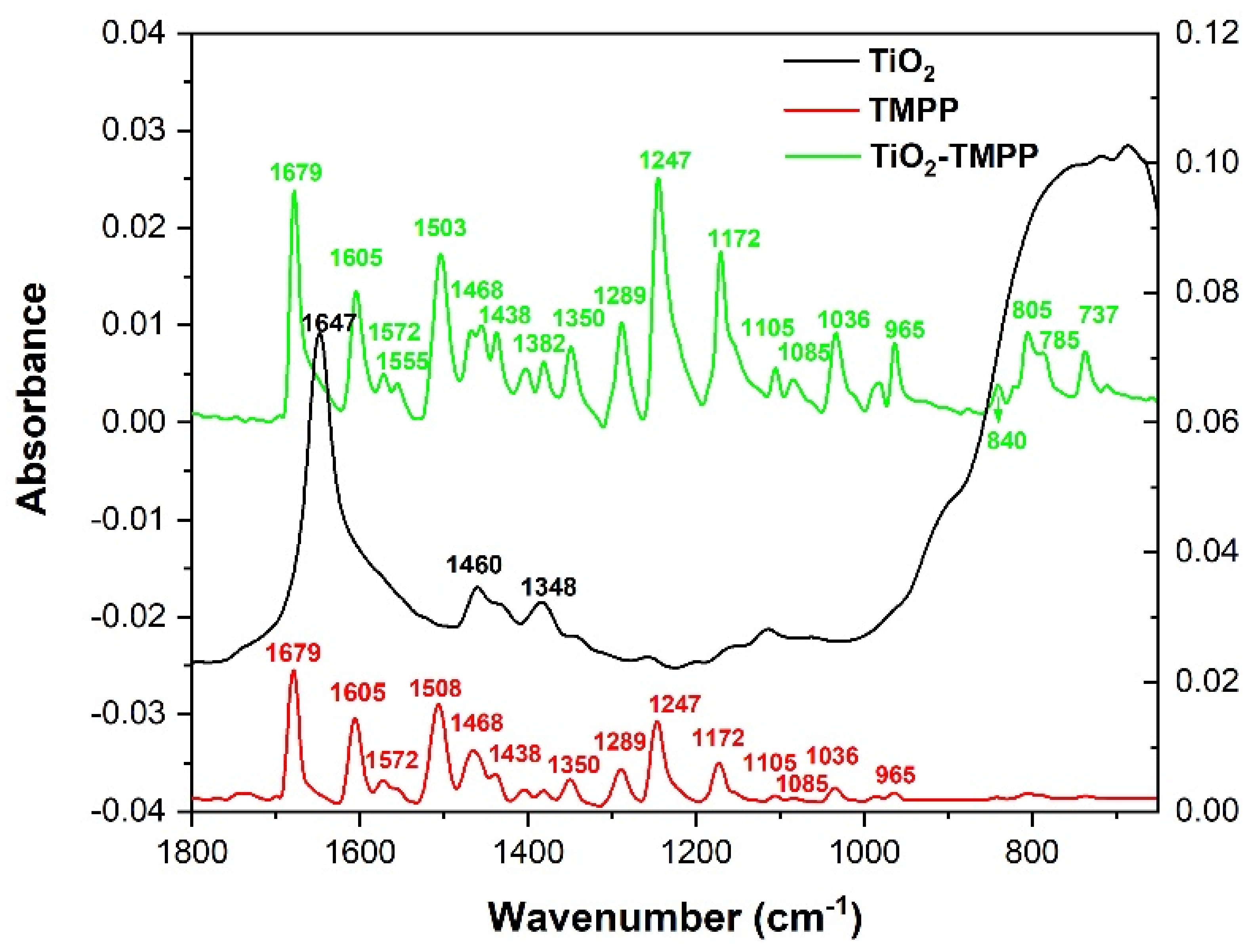
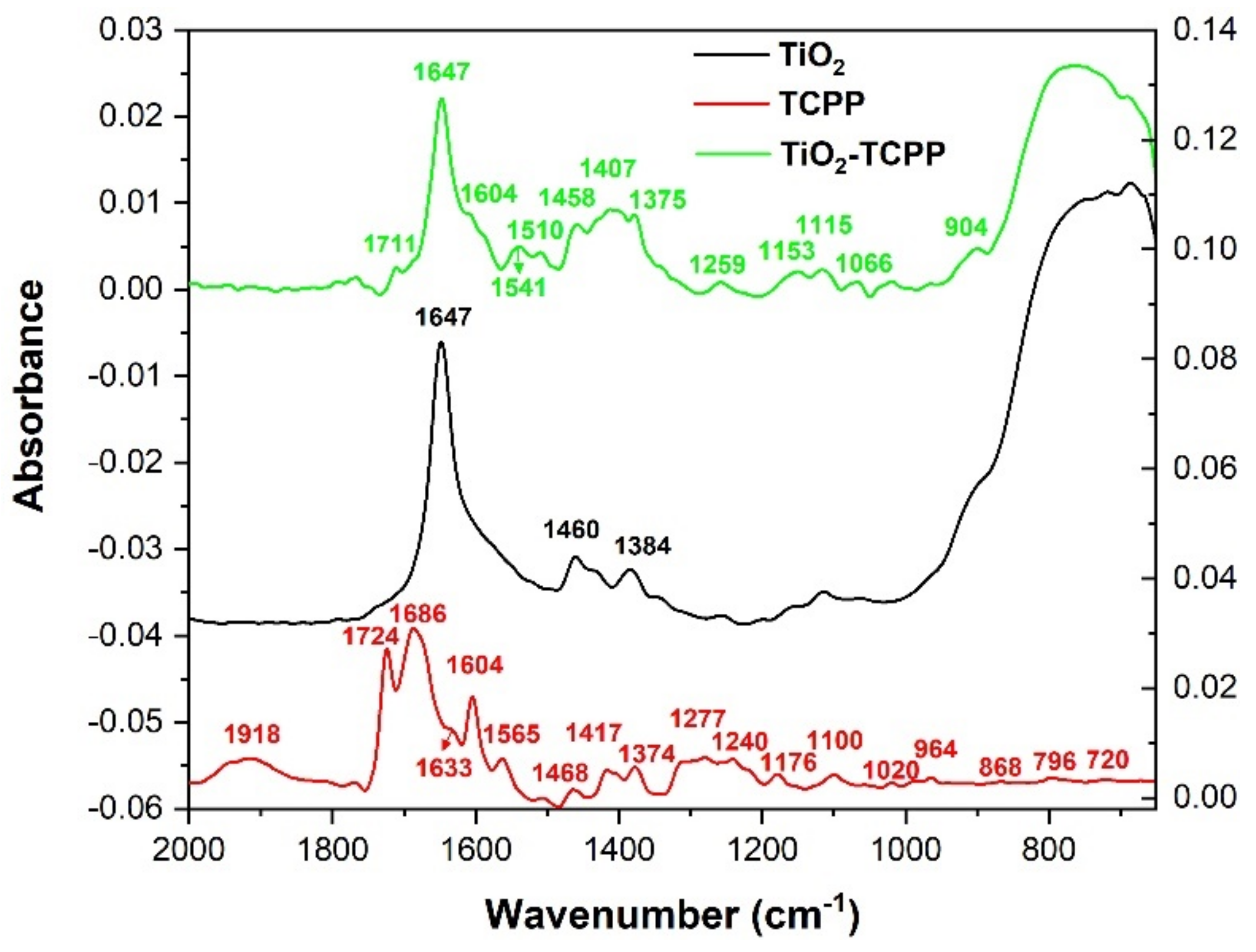
2.3. Fourier Transform Infrared Spectroscopy–Attenuated Total Reflection (FTIR-ATR)
2.4. Singlet Oxygen Generation
3. Materials and Methods
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Correia, J.H.; Rodrigues, J.A.; Pimenta, S.; Dong, T.; Yang, Z. Photodynamic Therapy Review: Principles, Photosensitizers, Applications, and Future Directions. Pharmaceutics 2021, 13, 1332. [Google Scholar] [CrossRef] [PubMed]
- Dolmans, D.E.J.G.J.; Fukumura, D.; Jain, R.K. Photodynamic Therapy for Cancer. Nat. Rev. Cancer 2003, 3, 380–387. [Google Scholar] [CrossRef] [PubMed]
- Wainwright, M. Photodynamic Antimicrobial Chemotherapy (PACT). J. Antimicrob. Chemother. 1998, 42, 13–28. [Google Scholar] [CrossRef]
- Kwiatkowski, S.; Knap, B.; Przystupski, D.; Saczko, J.; Kędzierska, E.; Knap-Czop, K.; Kotlińska, J.; Michel, O.; Kotowski, K.; Kulbacka, J. Photodynamic Therapy—Mechanisms, Photosensitizers and Combinations. Biomed. Pharm. 2018, 106, 1098–1107. [Google Scholar] [CrossRef] [PubMed]
- Solban, N.; Rizvi, I.; Hasan, T. Targeted Photodynamic Therapy. Lasers Surg. Med. 2006, 38, 522–531. [Google Scholar] [CrossRef] [PubMed]
- Staicu, A.; Pascu, A.; Nuta, A.; Ana-Alexandra, S.; Raditoiu, V.; Pascu, M.L. Studies about Phthalocyanine Photosensitizers to Be Used in Photodynamic Therapy. Rom. Rep. Phys. 2013, 65, 1032–1051. [Google Scholar]
- Montaseri, H.; Kruger, C.A.; Abrahamse, H. Inorganic Nanoparticles Applied for Active Targeted Photodynamic Therapy of Breast Cancer. Pharmaceutics 2021, 13, 296. [Google Scholar] [CrossRef]
- Kessel, D.; Oleinick, N.L. Photodynamic Therapy and Cell Death Pathways. In Photodynamic Therapy: Methods and Protocols; Gomer, C.J., Ed.; Methods in Molecular Biology; Humana Press: Totowa, NJ, USA, 2010; pp. 35–46. ISBN 978-1-60761-697-9. [Google Scholar]
- Fotinos, N.; Convert, M.; Piffaretti, J.-C.; Gurny, R.; Lange, N. Effects on Gram-Negative and Gram-Positive Bacteria Mediated by 5-Aminolevulinic Acid and 5-Aminolevulinic Acid Derivatives. Antimicrob Agents Chemother. 2008, 52, 1366–1373. [Google Scholar] [CrossRef]
- Nordmann, P.; Poirel, L.; Toleman, M.A.; Walsh, T.R. Does Broad-Spectrum Beta-Lactam Resistance Due to NDM-1 Herald the End of the Antibiotic Era for Treatment of Infections Caused by Gram-Negative Bacteria? J. Antimicrob. Chemother. 2011, 66, 689–692. [Google Scholar] [CrossRef]
- Youf, R.; Müller, M.; Balasini, A.; Thétiot, F.; Müller, M.; Hascoët, A.; Jonas, U.; Schönherr, H.; Lemercier, G.; Montier, T.; et al. Antimicrobial Photodynamic Therapy: Latest Developments with a Focus on Combinatory Strategies. Pharmaceutics 2021, 13, 1995. [Google Scholar] [CrossRef]
- Amos-Tautua, B.M.; Songca, S.P.; Oluwafemi, O.S. Application of Porphyrins in Antibacterial Photodynamic Therapy. Molecules 2019, 24, 2456. [Google Scholar] [CrossRef]
- Mesquita, M.Q.; Dias, C.J.; Neves, M.G.P.M.S.; Almeida, A.; Faustino, M.A.F. Revisiting Current Photoactive Materials for Antimicrobial Photodynamic Therapy. Molecules 2018, 23, 2424. [Google Scholar] [CrossRef] [PubMed]
- Almeida, A.; Cunha, A.; Faustino, M.A.F.; Tomé, A.C.; Neves, M.G.P.M.S. Chapter 5. Porphyrins as Antimicrobial Photosensitizing Agents. In Comprehensive Series in Photochemical & Photobiological Sciences; Hamblin, M.R., Jori, G., Eds.; Royal Society of Chemistry: Cambridge, UK, 2011; pp. 83–160. ISBN 978-1-84973-144-7. [Google Scholar]
- Huang, Y.-Y.; Sharma, S.K.; Yin, R.; Agrawal, T.; Chiang, L.Y.; Hamblin, M.R. Functionalized Fullerenes in Photodynamic Therapy. J. Biomed. Nanotechnol. 2014, 10, 1918–1936. [Google Scholar] [CrossRef] [PubMed]
- Marin, D.M.; Payerpaj, S.; Collier, G.S.; Ortiz, A.L.; Singh, G.; Jones, M.; Walter, M.G. Efficient Intersystem Crossing Using Singly Halogenated Carbomethoxyphenyl Porphyrins Measured Using Delayed Fluorescence, Chemical Quenching, and Singlet Oxygen Emission. Phys. Chem. Chem. Phys. 2015, 17, 29090–29096. [Google Scholar] [CrossRef] [PubMed]
- Abada, Z.; Cojean, S.; Pomel, S.; Ferrié, L.; Akagah, B.; Lormier, A.T.; Loiseau, P.M.; Figadère, B. Synthesis and Antiprotozoal Activity of Original Porphyrin Precursors and Derivatives. Eur. J. Med. Chem. 2013, 67, 158–165. [Google Scholar] [CrossRef]
- Zoltan, T.; Vargas, F.; López, V.; Chávez, V.; Rivas, C.; Ramírez, Á.H. Influence of Charge and Metal Coordination of Meso-Substituted Porphyrins on Bacterial Photoinactivation. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2015, 135, 747–756. [Google Scholar] [CrossRef]
- Kou, J.; Dou, D.; Yang, L. Porphyrin Photosensitizers in Photodynamic Therapy and Its Applications. Oncotarget 2017, 8, 81591–81603. [Google Scholar] [CrossRef]
- Jain, M.; Zellweger, M.; Wagnières, G.; van den Bergh, H.; Cook, S.; Giraud, M.-N. Photodynamic Therapy for the Treatment of Atherosclerotic Plaque: Lost in Translation? Cardiovasc. Ther. 2017, 35, e12238. [Google Scholar] [CrossRef]
- Habermeyer, B.; Guilard, R. Some Activities of PorphyChem Illustrated by the Applications of Porphyrinoids in PDT, PIT and PDI. Photochem. Photobiol. Sci. 2018, 17, 1675–1690. [Google Scholar] [CrossRef]
- Nasir, A.; Khan, A.; Li, J.; Naeem, M.; Khalil, A.A.K.; Khan, K.; Qasim, M. Nanotechnology, A Tool for Diagnostics and Treatment of Cancer. Curr. Top. Med. Chem. 2021, 21, 1360–1376. [Google Scholar] [CrossRef]
- Yang, M.; Li, J.; Gu, P.; Fan, X. The Application of Nanoparticles in Cancer Immunotherapy: Targeting Tumor Microenvironment. Bioact. Mater. 2020, 6, 1973–1987. [Google Scholar] [CrossRef] [PubMed]
- Ziental, D.; Czarczynska-Goslinska, B.; Mlynarczyk, D.T.; Glowacka-Sobotta, A.; Stanisz, B.; Goslinski, T.; Sobotta, L. Titanium Dioxide Nanoparticles: Prospects and Applications in Medicine. Nanomaterials 2020, 10, 387. [Google Scholar] [CrossRef] [PubMed]
- Horikoshi, S.; Serpone, N. Introduction to Nanoparticles. In Microwaves in Nanoparticle Synthesis; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2013; pp. 1–24. ISBN 978-3-527-64812-2. [Google Scholar]
- De Jong, W.H.; Borm, P.J.A. Drug Delivery and Nanoparticles:Applications and Hazards. Int. J. Nanomed. 2008, 3, 133–149. [Google Scholar] [CrossRef] [PubMed]
- Youssef, Z.; Vanderesse, R.; Colombeau, L.; Baros, F.; Roques-Carmes, T.; Frochot, C.; Wahab, H.; Toufaily, J.; Hamieh, T.; Acherar, S.; et al. The Application of Titanium Dioxide, Zinc Oxide, Fullerene, and Graphene Nanoparticles in Photodynamic Therapy. Cancer Nanotechnol. 2017, 8, 6. [Google Scholar] [CrossRef]
- Shanmugapriya, K.; Kang, H.W. Engineering Pharmaceutical Nanocarriers for Photodynamic Therapy on Wound Healing: Review. Mater. Sci. Eng. C 2019, 105, 110110. [Google Scholar] [CrossRef] [PubMed]
- Colombeau, L.; Acherar, S.; Baros, F.; Arnoux, P.; Gazzali, A.M.; Zaghdoudi, K.; Toussaint, M.; Vanderesse, R.; Frochot, C. Inorganic Nanoparticles for Photodynamic Therapy. Top Curr. Chem. 2016, 370, 113–134. [Google Scholar] [CrossRef]
- Kirar, S.; Chaudhari, D.; Thakur, N.S.; Jain, S.; Bhaumik, J.; Laha, J.K.; Banerjee, U.C. Light-Assisted Anticancer Photodynamic Therapy Using Porphyrin-Doped Nanoencapsulates. J. Photochem. Photobiol. B: Biol. 2021, 220, 112209. [Google Scholar] [CrossRef]
- Huang, C.; Lv, Y.; Zhou, Q.; Kang, S.; Li, X.; Mu, J. Visible Photocatalytic Activity and Photoelectrochemical Behavior of TiO2 Nanoparticles Modified with Metal Porphyrins Containing Hydroxyl Group. Ceram. Int. 2014, 40, 7093–7098. [Google Scholar] [CrossRef]
- Krakowiak, R.; Frankowski, R.; Mylkie, K.; Kotkowiak, M.; Mlynarczyk, D.T.; Dudkowiak, A.; Stanisz, B.J.; Zgoła-Grześkowiak, A.; Ziegler-Borowska, M.; Goslinski, T. Titanium(IV) Oxide Nanoparticles Functionalized with Various Meso-Porphyrins for Efficient Photocatalytic Degradation of Ibuprofen in UV and Visible Light. J. Environ. Chem. Eng. 2022, 10, 108432. [Google Scholar] [CrossRef]
- Cherian, S.; Wamser, C.C. Adsorption and Photoactivity of Tetra(4-Carboxyphenyl)Porphyrin (TCPP) on Nanoparticulate TiO2. J. Phys. Chem. B 2000, 104, 3624–3629. [Google Scholar] [CrossRef]
- Chen, D.; Yang, D.; Geng, J.; Zhu, J.; Jiang, Z. Improving Visible-Light Photocatalytic Activity of N-Doped TiO2 Nanoparticles via Sensitization by Zn Porphyrin. Appl. Surf. Sci. 2008, 255, 2879–2884. [Google Scholar] [CrossRef]
- Gaeta, M.; Sanfilippo, G.; Fraix, A.; Sortino, G.; Barcellona, M.; Oliveri Conti, G.; Fragalà, M.E.; Ferrante, M.; Purrello, R.; D’Urso, A. Photodegradation of Antibiotics by Noncovalent Porphyrin-Functionalized TiO2 in Water for the Bacterial Antibiotic Resistance Risk Management. IJMS 2020, 21, 3775. [Google Scholar] [CrossRef] [PubMed]
- Sułek, A.; Pucelik, B.; Kobielusz, M.; Łabuz, P.; Dubin, G.; Dąbrowski, J.M. Surface Modification of Nanocrystalline TiO2 Materials with Sulfonated Porphyrins for Visible Light Antimicrobial Therapy. Catalysts 2019, 9, 821. [Google Scholar] [CrossRef]
- Castro, K.A.D.F.; Moura, N.M.M.; Figueira, F.; Ferreira, R.I.; Simões, M.M.Q.; Cavaleiro, J.A.S.; Faustino, M.A.F.; Silvestre, A.J.D.; Freire, C.S.R.; Tomé, J.P.C.; et al. New Materials Based on Cationic Porphyrins Conjugated to Chitosan or Titanium Dioxide: Synthesis, Characterization and Antimicrobial Efficacy. IJMS 2019, 20, 2522. [Google Scholar] [CrossRef]
- Tomaszewska, E.; Soliwoda, K.; Kadziola, K.; Tkacz-Szczesna, B.; Celichowski, G.; Cichomski, M.; Szmaja, W.; Grobelny, J. Detection Limits of DLS and UV-Vis Spectroscopy in Characterization of Polydisperse Nanoparticles Colloids. J. Nanomater. 2013, 2013, 313081. [Google Scholar] [CrossRef]
- Nistorescu, S.; Udrea, A.-M.; Badea, M.A.; Lungu, I.; Boni, M.; Tozar, T.; Dumitrache, F.; Maraloiu, V.-A.; Popescu, R.G.; Fleaca, C.; et al. Low Blue Dose Photodynamic Therapy with Porphyrin-Iron Oxide Nanoparticles Complexes: In Vitro Study on Human Melanoma Cells. Pharmaceutics 2021, 13, 2130. [Google Scholar] [CrossRef]
- Mudalige, T.; Qu, H.; Van Haute, D.; Ansar, S.M.; Paredes, A.; Ingle, T. Characterization of Nanomaterials. In Nanomaterials for Food Applications; Elsevier: Amsterdam, The Netherlands, 2019; pp. 313–353. ISBN 978-0-12-814130-4. [Google Scholar]
- Ishii, F.; Nii, T. Lipid Emulsions and Lipid Vesicles Prepared from Various Phospholipids as Drug Carriers. In Colloid and Interface Science in Pharmaceutical Research and Development; Elsevier: Amsterdam, The Netherlands, 2014; pp. 469–501. ISBN 978-0-444-62614-1. [Google Scholar]
- Giovanelli, L.; Lee, H.-L.; Lacaze-Dufaure, C.; Koudia, M.; Clair, S.; Lin, Y.-P.; Ksari, Y.; Themlin, J.-M.; Abel, M.; Cafolla, A.A. Electronic Structure of Tetra(4-Aminophenyl)Porphyrin Studied by Photoemission, UV–Vis Spectroscopy and Density Functional Theory. J. Electron Spectrosc. Relat. Phenom. 2017, 218, 40–45. [Google Scholar] [CrossRef]
- Frisch, M.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.; et al. Gaussian 09; Gaussian, Inc.: Wallingford, CT, USA, 2009. [Google Scholar]
- Socrates, G. Infrared and Raman Characteristic Group Frequencies: Tables and Charts, 3rd ed.; Reprint as Paperback; Wiley: Chichester, UK, 2010; ISBN 978-0-470-09307-8. [Google Scholar]
- Coates, J. Interpretation of Infrared Spectra, A Practical Approach. In Encyclopedia of Analytical Chemistry; Meyers, R.A., Ed.; John Wiley & Sons, Ltd: Chichester, UK, 2006; p. a5606. ISBN 978-0-470-02731-8. [Google Scholar]
- Aydin, M. Comparative Study of the Structural and Vibroelectronic Properties of Porphyrin and Its Derivatives. Molecules 2014, 19, 20988–21021. [Google Scholar] [CrossRef]
- León, A.; Reuquen, P.; Garín, C.; Segura, R.; Vargas, P.; Zapata, P.; Orihuela, P. FTIR and Raman Characterization of TiO2 Nanoparticles Coated with Polyethylene Glycol as Carrier for 2-Methoxyestradiol. Appl. Sci. 2017, 7, 49. [Google Scholar] [CrossRef]
- García-Serrano, J.; Gómez-Hernández, E.; Ocampo-Fernández, M.; Pal, U. Effect of Ag Doping on the Crystallization and Phase Transition of TiO2 Nanoparticles. Curr. Appl. Phys. 2009, 9, 1097–1105. [Google Scholar] [CrossRef]
- Duan, M.; Li, J.; Mele, G.; Wang, C.; Lü, X.; Vasapollo, G.; Zhang, F. Photocatalytic Activity of Novel Tin Porphyrin/TiO 2 Based Composites. J. Phys. Chem. C 2010, 114, 7857–7862. [Google Scholar] [CrossRef]
- Min, K.S.; Kumar, R.S.; Lee, J.H.; Kim, K.S.; Lee, S.G.; Son, Y.-A. Synthesis of New TiO2/Porphyrin-Based Composites and Photocatalytic Studies on Methylene Blue Degradation. Dye. Pigment. 2019, 160, 37–47. [Google Scholar] [CrossRef]
- Zoltan, T.; Rosales, M.C.; Yadarola, C. Reactive Oxygen Species Quantification and Their Correlation with the Photocatalytic Activity of TiO2 (Anatase and Rutile) Sensitized with Asymmetric Porphyrins. J. Environ. Chem. Eng. 2016, 4, 3967–3980. [Google Scholar] [CrossRef]
- Wang, H.; Zhou, D.; Wu, Z.; Wan, J.; Zheng, X.; Yu, L.; Phillips, D.L. The Visible Light Degradation Activity and the Photocatalytic Mechanism of Tetra(4-Carboxyphenyl) Porphyrin Sensitized TiO2. Mater. Res. Bull. 2014, 57, 311–319. [Google Scholar] [CrossRef]
- Ahmed, M.A.; Abou-Gamra, Z.M.; Medien, H.A.A.; Hamza, M.A. Effect of Porphyrin on Photocatalytic Activity of TiO 2 Nanoparticles toward Rhodamine B Photodegradation. J. Photochem. Photobiol. B Biol. 2017, 176, 25–35. [Google Scholar] [CrossRef]
- Kollhoff, F.; Schneider, J.; Li, G.; Barkaoui, S.; Shen, W.; Berger, T.; Diwald, O.; Libuda, J. Anchoring of Carboxyl-Functionalized Porphyrins on MgO, TiO2, and Co3O4 Nanoparticles. Phys. Chem. Chem. Phys. 2018, 20, 24858–24868. [Google Scholar] [CrossRef]
- Myrzakhmetov, B.; Arnoux, P.; Mordon, S.; Acherar, S.; Tsoy, I.; Frochot, C. Photophysical Properties of Protoporphyrin IX, Pyropheophorbide-a, and Photofrin® in Different Conditions. Pharmaceuticals 2021, 14, 138. [Google Scholar] [CrossRef]
- Sułek, A.; Pucelik, B.; Kuncewicz, J.; Dubin, G.; Dąbrowski, J.M. Sensitization of TiO2 by Halogenated Porphyrin Derivatives for Visible Light Biomedical and Environmental Photocatalysis. Catal. Today 2019, 335, 538–549. [Google Scholar] [CrossRef]
- Li, Z.; Pan, X.; Wang, T.; Wang, P.-N.; Chen, J.-Y.; Mi, L. Comparison of the Killing Effects between Nitrogen-Doped and Pure TiO2 on HeLa Cells with Visible Light Irradiation. Nanoscale Res. Lett. 2013, 8, 96. [Google Scholar] [CrossRef]
- Pan, X.; Liang, X.; Yao, L.; Wang, X.; Jing, Y.; Ma, J.; Fei, Y.; Chen, L.; Mi, L. Study of the Photodynamic Activity of N-Doped TiO2 Nanoparticles Conjugated with Aluminum Phthalocyanine. Nanomaterials 2017, 7, 338. [Google Scholar] [CrossRef]
- Kathiravan, A.; Renganathan, R. Effect of Anchoring Group on the Photosensitization of Colloidal TiO2 Nanoparticles with Porphyrins. J. Colloid Interface Sci. 2009, 331, 401–407. [Google Scholar] [CrossRef] [PubMed]
- Lü, X.; Li, J.; Wang, C.; Duan, M.; Luo, Y.; Yao, G.; Wang, J.-L. Enhanced Photoactivity of CuPp-TiO2 Photocatalysts under Visible Light Irradiation. Appl. Surf. Sci. 2010, 257, 795–801. [Google Scholar] [CrossRef]
- Staicu, A.; Smarandache, A.; Pascu, A.; Pascu, M.L. Photophysics of Covalently Functionalized Single Wall Carbon Nanotubes with Verteporfin. Appl. Surf. Sci. 2017, 417, 170–174. [Google Scholar] [CrossRef]
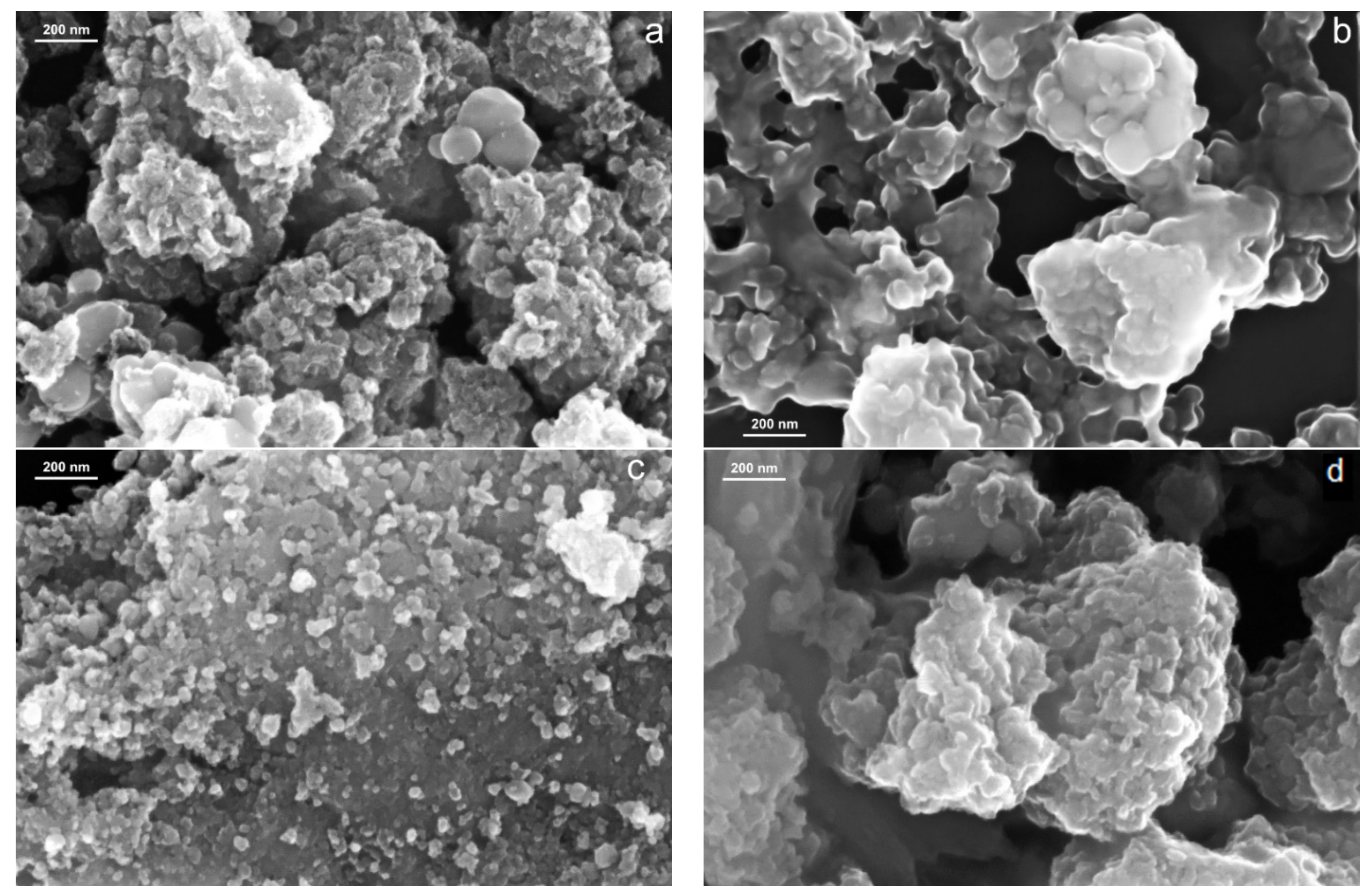


| Sample | Mean Size/nm SEM | Element | Weight % | Atomic % |
|---|---|---|---|---|
| TiO2 | 20 | C K | 5.02 | 6.48 |
| O K | 28.01 | 46.37 | ||
| Ti K | 66.97 | 47.15 | ||
| TiO2-TAPP | 43 | C K | 12.31 | 21.62 |
| N K | 2.68 | 4.03 | ||
| O K | 42.06 | 55.26 | ||
| Ti K | 42.95 | 19.09 | ||
| TiO2-TCPP | 21 | C K | 3.19 | 7.54 |
| N K | 1.63 | 3.31 | ||
| O K | 27.73 | 49.17 | ||
| Ti K | 67.45 | 39.98 | ||
| TiO2-TMPP | 31 | C K | 3.34 | 8.02 |
| N K | 2.14 | 4.40 | ||
| O K | 29.49 | 43.59 | ||
| Ti K | 65.03 | 43.99 |
| Sample | Mean Size (nm) | Standard Deviation (nm) | Polydispersity Index | Zeta Potential (mV) |
|---|---|---|---|---|
| TiO2 | 559.1 | 126.9 | 0.403 | −50.3 |
| TiO2-TAPP | 1213.7 | 233.0 | 0.733 | −46.8 |
| TiO2-TMPP | 1148.4 | 322.5 | 0.954 | −44.1 |
| TiO2-TCPP | 1225.8 | 274.1 | 0.956 | −44.0 |
| Observed Frequency (cm−1) | Calculated Frequency (cm−1) | Assigned Vibrations |
|---|---|---|
| 741 | 702 | NH2 bending and CH bending from aminophenyl radicals |
| 801 | 749 | NH bending; CH bending |
| 842 | 858 | NH bending; CH bending |
| 964 | 986 | CC bending; CN stretching; CH bending |
| 1095 | 1017 | CH bending; CN stretching; CC bending |
| 1126 | 1158 | NH2 bending; CH bending |
| 1158 | 1183 | CN stretching; CH bending |
| 1178 | 1208 | CH bending; NH bending |
| 1196 | 1209 | CH bending |
| 1216 | 1217 | NH bending; CH bending |
| 1252 | 1272 | CN stretching; NH bending; CH bending; CC stretching |
| 1285 | 1301 | CN stretching; NH bending; CH bending |
| 1348 | 1372 | CH bending; NH bending; CC stretching; CN stretching |
| 1386 | 1388 | CH bending; NH bending; CC stretching; CN stretching |
| 1437 | 1428 | CH bending; CC stretching |
| 1468 | 1499 | CC and CN stretching from porphyrin ring; CH bending |
| 1500 | 1548 | CH bending; NH bending; CC stretching |
| 1512 | 1554 | CH bending; CC stretching |
| 1555 | 1594 | CC stretching; CH bending; NH bending; CN stretching |
| 1617 | 1656 | CC stretching; NH2 bending; CH bending |
| 1668 | 1657 | CC stretching; NH2 bending; CH bending |
| 1715 | 1691 | NH2 bending |
| 1730 | 1692 | NH2 bending |
| 3029 | 3131 | CH stretching |
| 3118 | 3158 | CH stretching from aminophenyl radicals |
| 3213 | 3160 | CH stretching from aminophenyl radicals |
| 3337 | 3237 | CH stretching from porphyrin ring |
| 3421 | 3519 | NH2 symmetrical stretching |
| 3440 | 3543 | NH stretching from porphyrin ring |
| Observed Frequency (cm−1) | Calculated Frequency (cm−1) | Assigned Vibrations |
|---|---|---|
| 965 | 986 | CC and CN stretching from porphyrin ring; CH bending |
| 1036 | 1003 | CC and CN stretching from porphyrin ring; CH bending |
| 1085 | 1064 | CO stretching; CC stretching; CH bending |
| 1105 | 1130 | CH bending |
| 1172 | 1196 | CH bending; CN stretching; CC stretching |
| 1247 | 1273 | CC and CO stretching; CH bending from methoxyphenyl radicals; CC and CN stretching, CH and NH bending from porphyrin ring |
| 1289 | 1277 | CH bending; NH bending; CN stretching; CC stretching |
| 1350 | 1323, 1331 | CC stretching; CH bending |
| 1382 | 1374 | CC stretching, CN stretching, CH bending from porphyrin ring |
| 1403 | 1392 | NH bending; CH bending; C=C and CN stretching from porphyrin ring |
| 1438 | 1478 | CH3 wagging; CH bending |
| 1468 | 1503, 1505 | CC stretching; CH3 bending |
| 1508 | 1540 | CH bending; CC stretching |
| 1605 | 1596 | CC stretching from porphyrin ring; CH bending; NH bending |
| 1679 | 1650 | CC in-ring stretching from methoxyphenyl radicals; CH bending |
| 3002 | 3000 | CH3 symmetrical stretching |
| 3032 | 3057 | CH3 asymmetrical stretching |
| 3069 | 3132 | CH stretching from CH3 |
| 3105 | 3174 | CH stretching from methoxyphenyl radicals |
| 3118 | 3204 | CH stretching from methoxyphenyl radicals |
| 3320 | 3259 | CH stretching from porphyrin ring |
| - | 3548 | NH stretching |
| Observed Frequency (cm−1) | Calculated Frequency (cm−1) | Assigned Vibrations |
|---|---|---|
| 673 | 658 | NH, CH, OH, OC=O and CC bending |
| 720 | 760 | CH, NH and CC bending |
| 765 | 780 | CC, CO, OH, CH and NH bending |
| 781 | 787 | CC, CO, OH, CH and NH bending |
| 796 | 789 | CC, CO, OH, CH and NH bending |
| 868 | 827 | CH and NH bending |
| 964 | 986 | CC and CN stretching from porphyrin ring; CH bending |
| 1020 | 1096 | CC stretching; CH and OH bending from carboxyphenyl radicals |
| 1059 | 1107 | CC stretching; CH bending; NH bending |
| 1100 | 1191 | CH bending; OH bending |
| 1176 | 1215 | CC bending; CH bending; OH bending |
| 1222 | 1303 | CC bending; CH bending; OH bending |
| 1240 | 1312 | CC stretching; CH bending; OH bending |
| 1277 | 1318 | CC stretching; CH bending; OH bending |
| 1314 | 1336 | CH bending; OH bending |
| 1374 | 1376 | CC, CO, OH and CH bending from carboxyphenyl radicals; CC and CN stretching, and CH bending from porphyrin ring |
| 1403 | 1427 | CC stretching; CN bending; CH bending |
| 1417 | 1430 | CC stretching from carboxyphenyl radicals; CH bending |
| 1468 | 1431 | CC stretching from carboxyphenyl radicals; CH bending |
| 1506 | 1508 | CC stretching; CH bending; CN stretching from porphyrin ring |
| 1565 | 1595 | CC stretching; CH bending; NH bending |
| 1604 | 1601 | CC ring stretching; CH bending; NH bending |
| 1633 | 1646 | CC stretching; CH bending from carboxyphenyl radicals |
| 1686 | 1648 | CC stretching; CH bending from carboxyphenyl radicals |
| 1724 | 1803 | C=O stretching; OH bending; CC stretching |
| 1918 | 1834 | C=O stretching; OH bending |
| 3079 | 3154 | CH stretching from carboxyphenyl radicals |
| 3123 | 3180 | CH stretching from carboxyphenyl radicals |
| 3275 | 3187 | CH stretching from carboxyphenyl radicals |
| 3319 | 3260 | CH stretching from porphyrin ring |
| - | 3547 | NH stretching |
| Compound/Solvent DMF | 1O2 Lifetime (µs) | 1O2 Yield |
|---|---|---|
| TAPP | 24 | 0.54 |
| TCPP | 21.2 | 0.74 |
| TMPP | 24 | 0.92 |
| TiO2-TAPP | 19.5 | 0.65 |
| TiO2-TCPP | 19.5 | 0.62 |
| TiO2-TMPP | 20.53 | 0.96 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dinache, A.; Nistorescu, S.; Tozar, T.; Smarandache, A.; Boni, M.; Prepelita, P.; Staicu, A. Spectroscopic Investigations of Porphyrin-TiO2 Nanoparticles Complexes. Molecules 2023, 28, 318. https://doi.org/10.3390/molecules28010318
Dinache A, Nistorescu S, Tozar T, Smarandache A, Boni M, Prepelita P, Staicu A. Spectroscopic Investigations of Porphyrin-TiO2 Nanoparticles Complexes. Molecules. 2023; 28(1):318. https://doi.org/10.3390/molecules28010318
Chicago/Turabian StyleDinache, Andra, Simona Nistorescu, Tatiana Tozar, Adriana Smarandache, Mihai Boni, Petronela Prepelita, and Angela Staicu. 2023. "Spectroscopic Investigations of Porphyrin-TiO2 Nanoparticles Complexes" Molecules 28, no. 1: 318. https://doi.org/10.3390/molecules28010318
APA StyleDinache, A., Nistorescu, S., Tozar, T., Smarandache, A., Boni, M., Prepelita, P., & Staicu, A. (2023). Spectroscopic Investigations of Porphyrin-TiO2 Nanoparticles Complexes. Molecules, 28(1), 318. https://doi.org/10.3390/molecules28010318







