Advances in the Synthesis of Fused 1,2,3-Triazoles via a MCR-Intramolecular Azide-Alkyne Cycloaddition Approach
Abstract
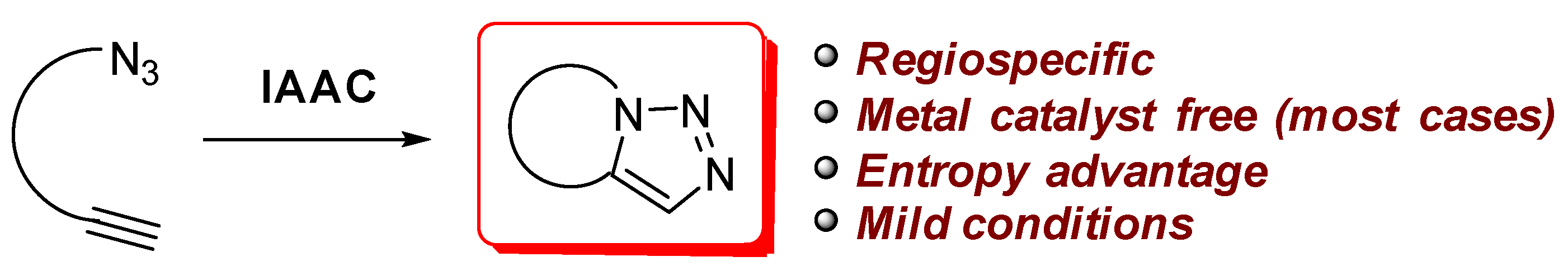
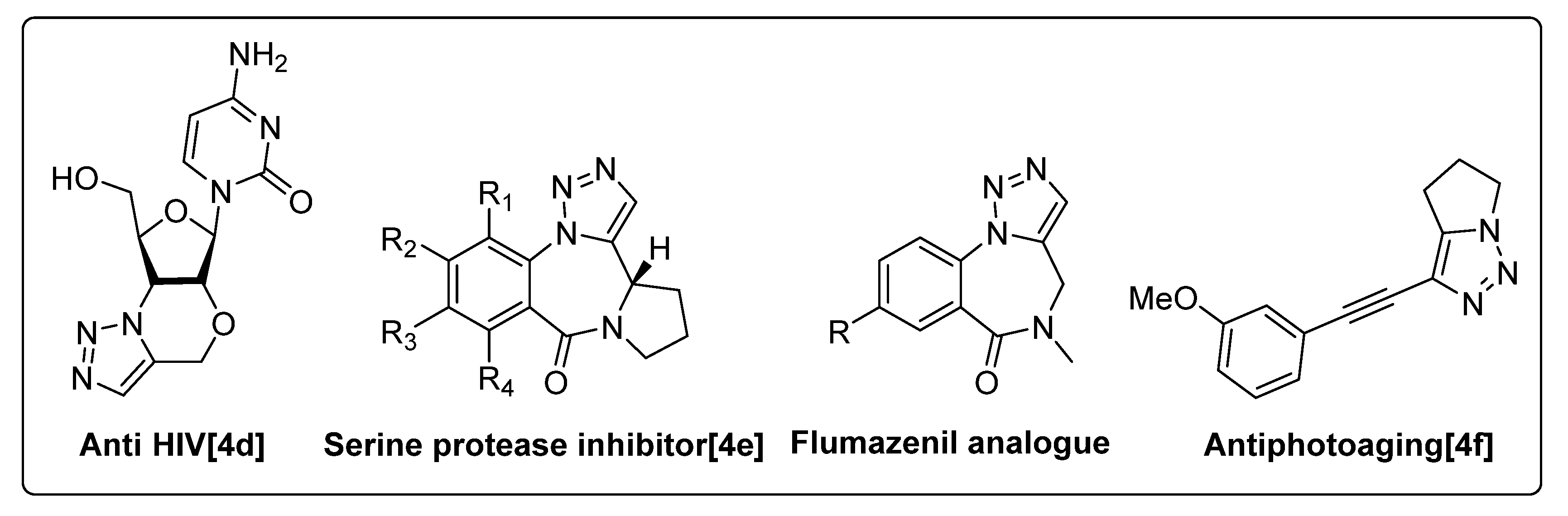
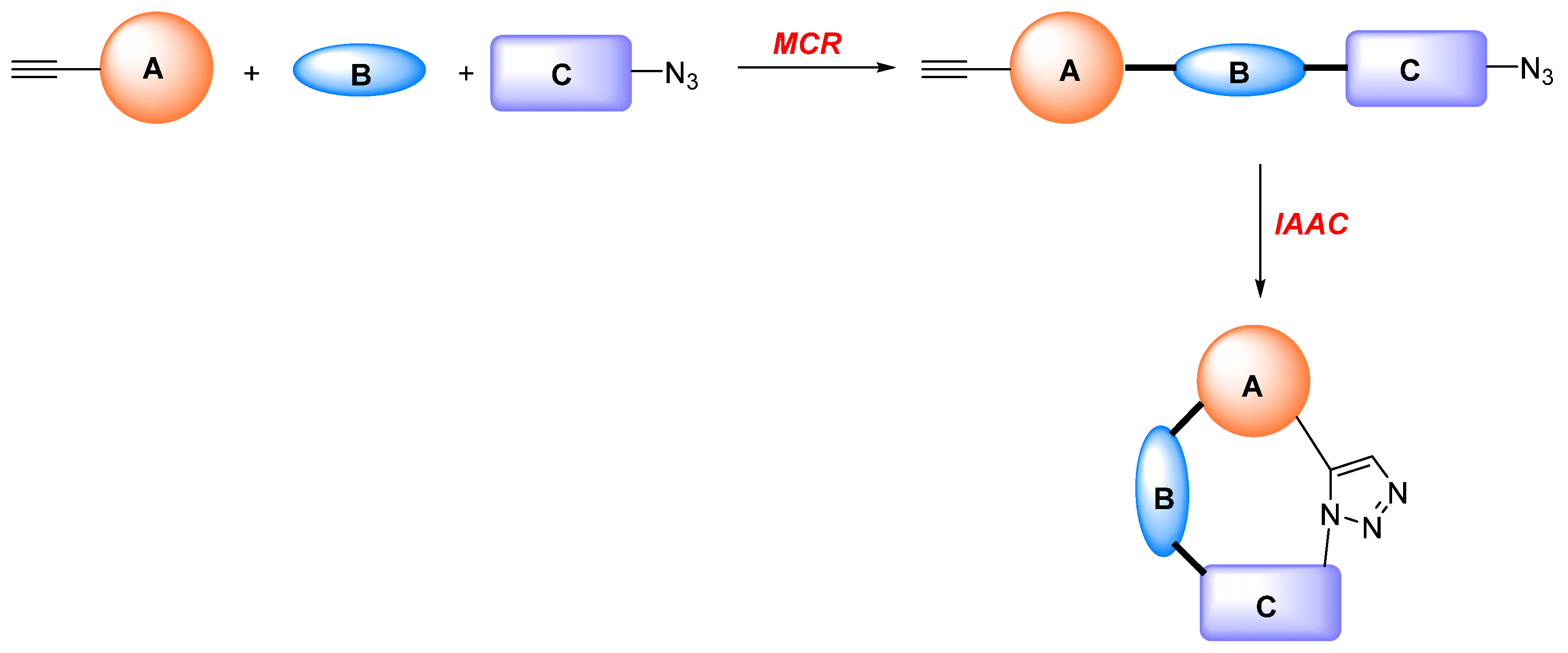
1. Introduction
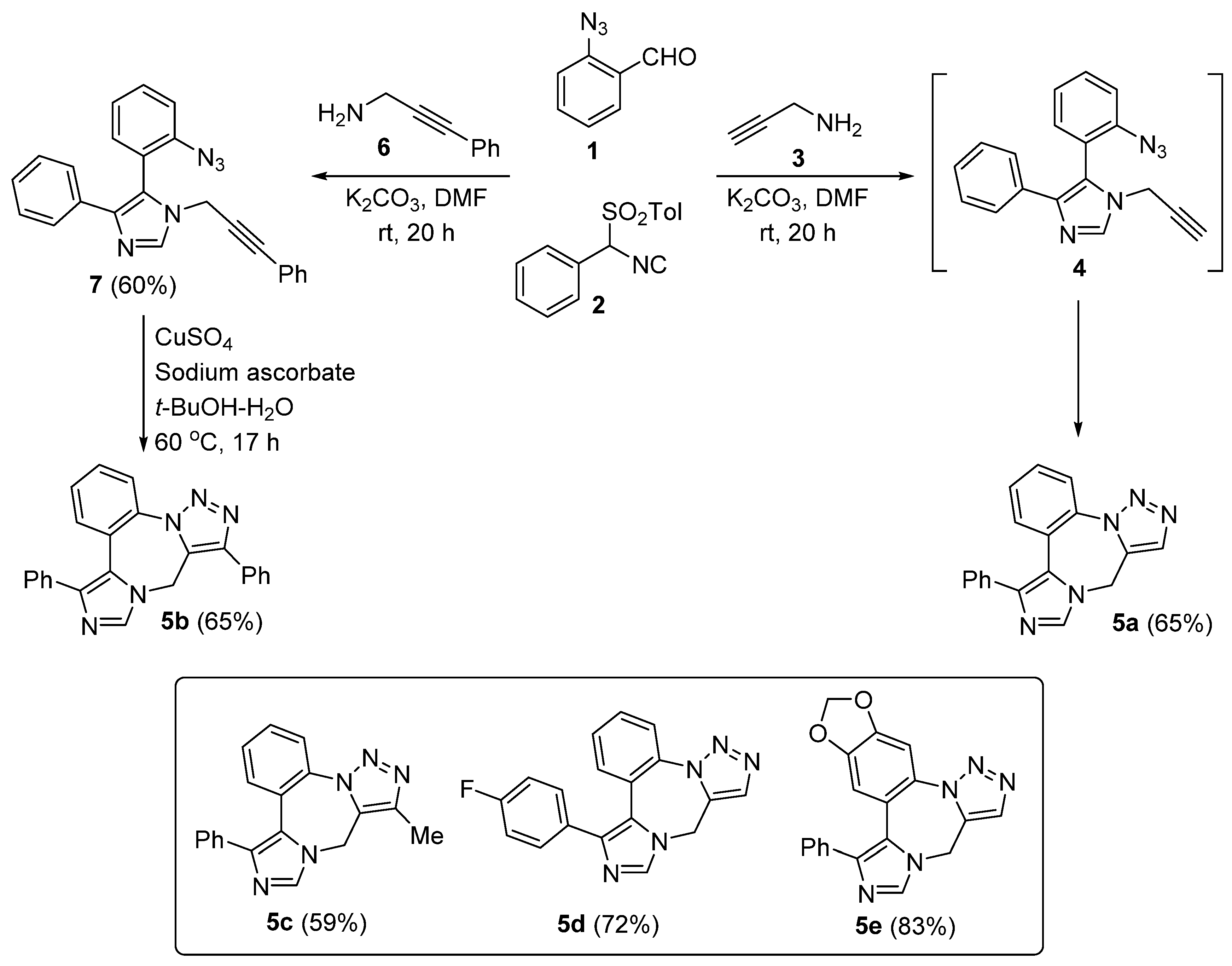
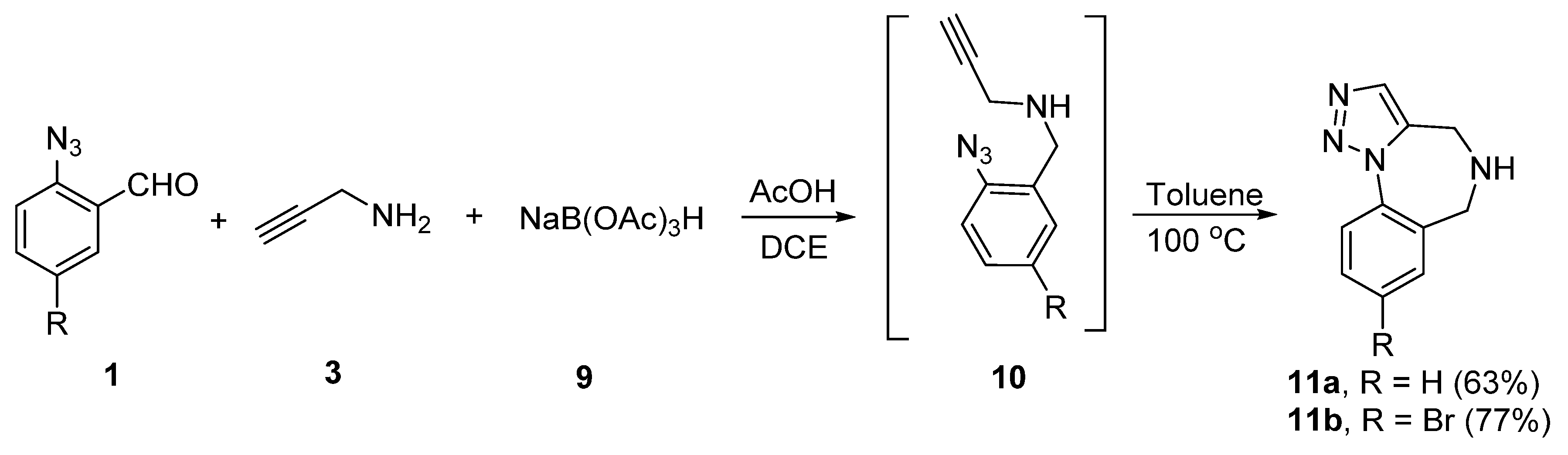
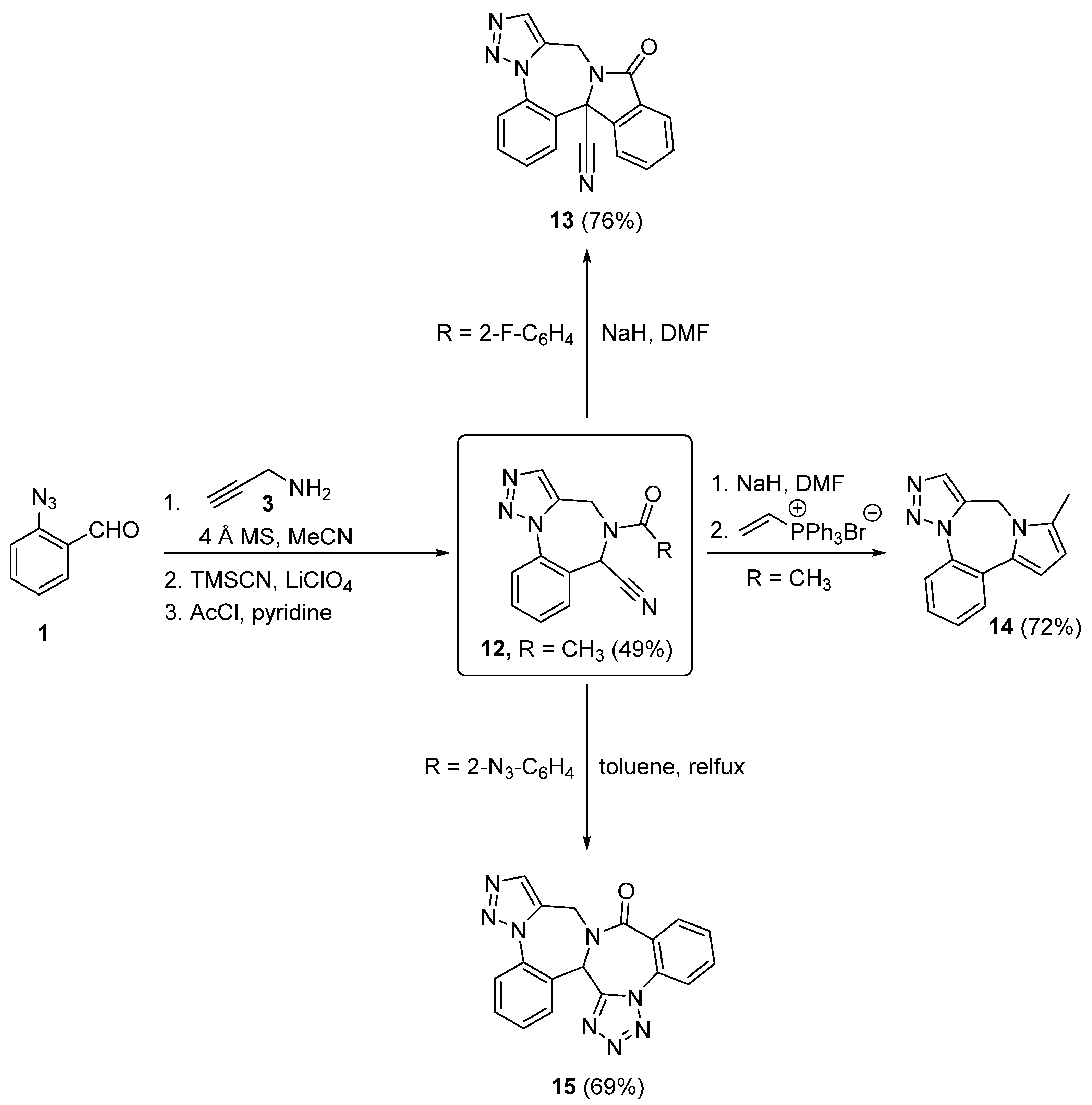
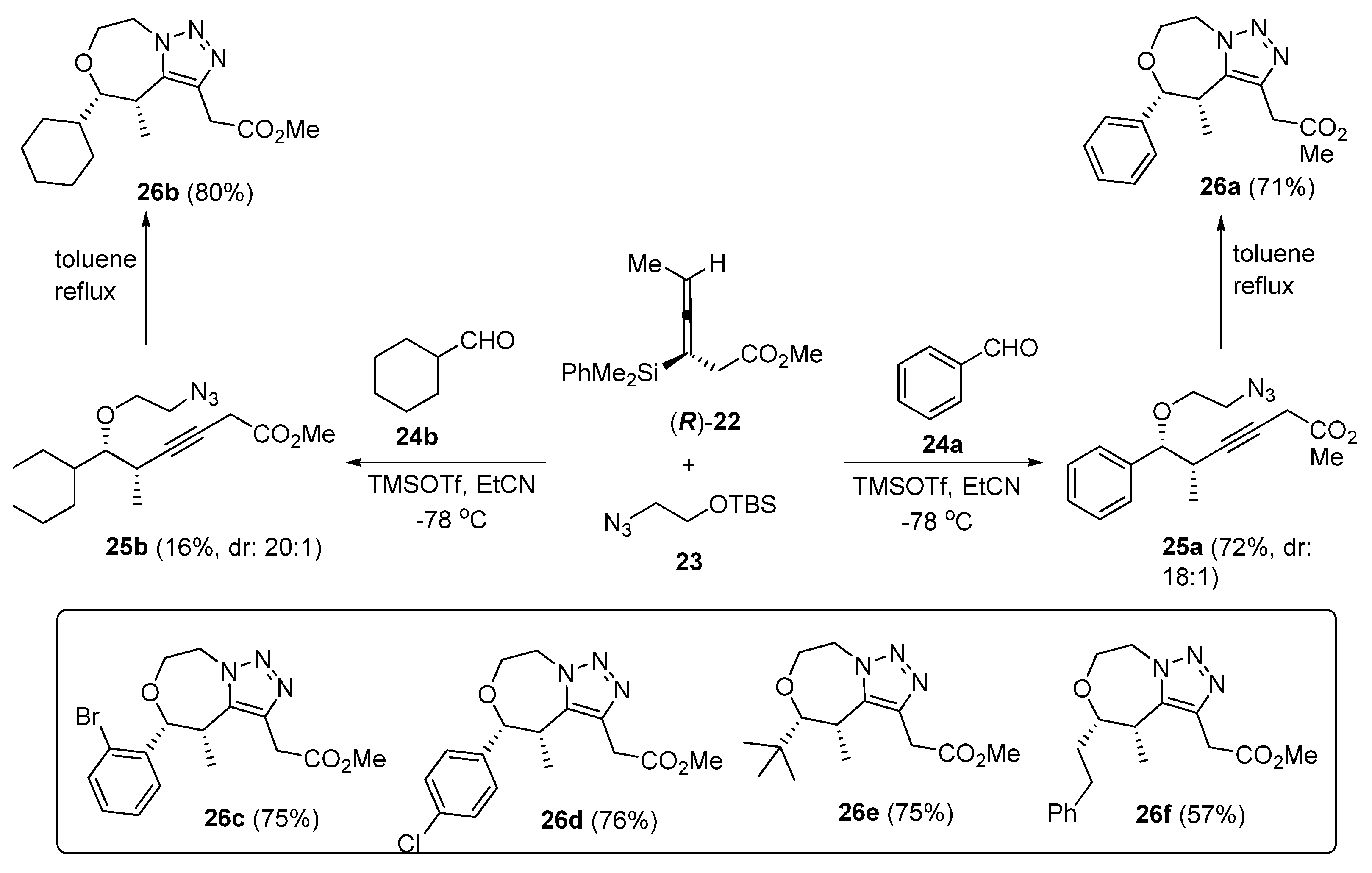
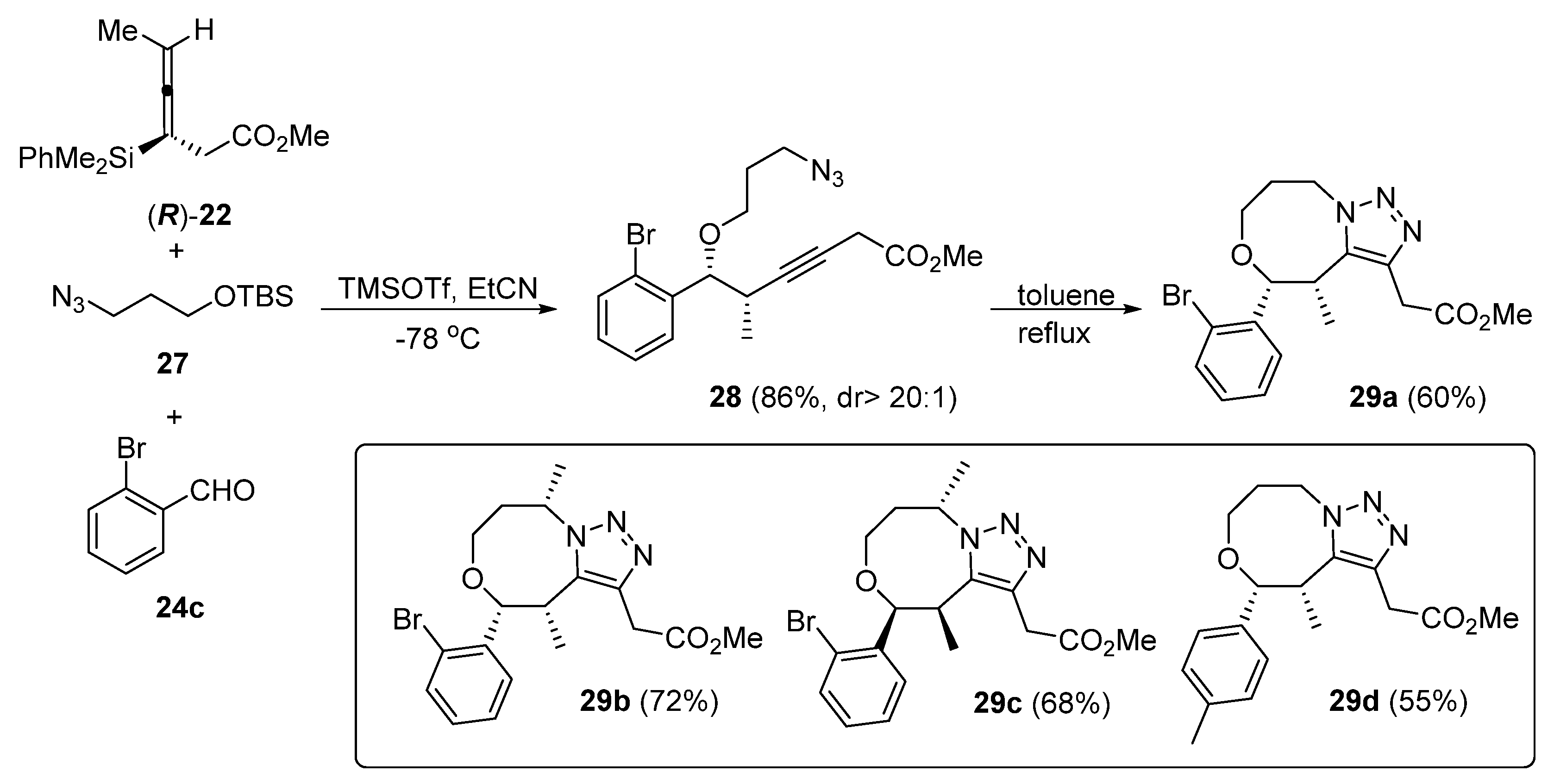
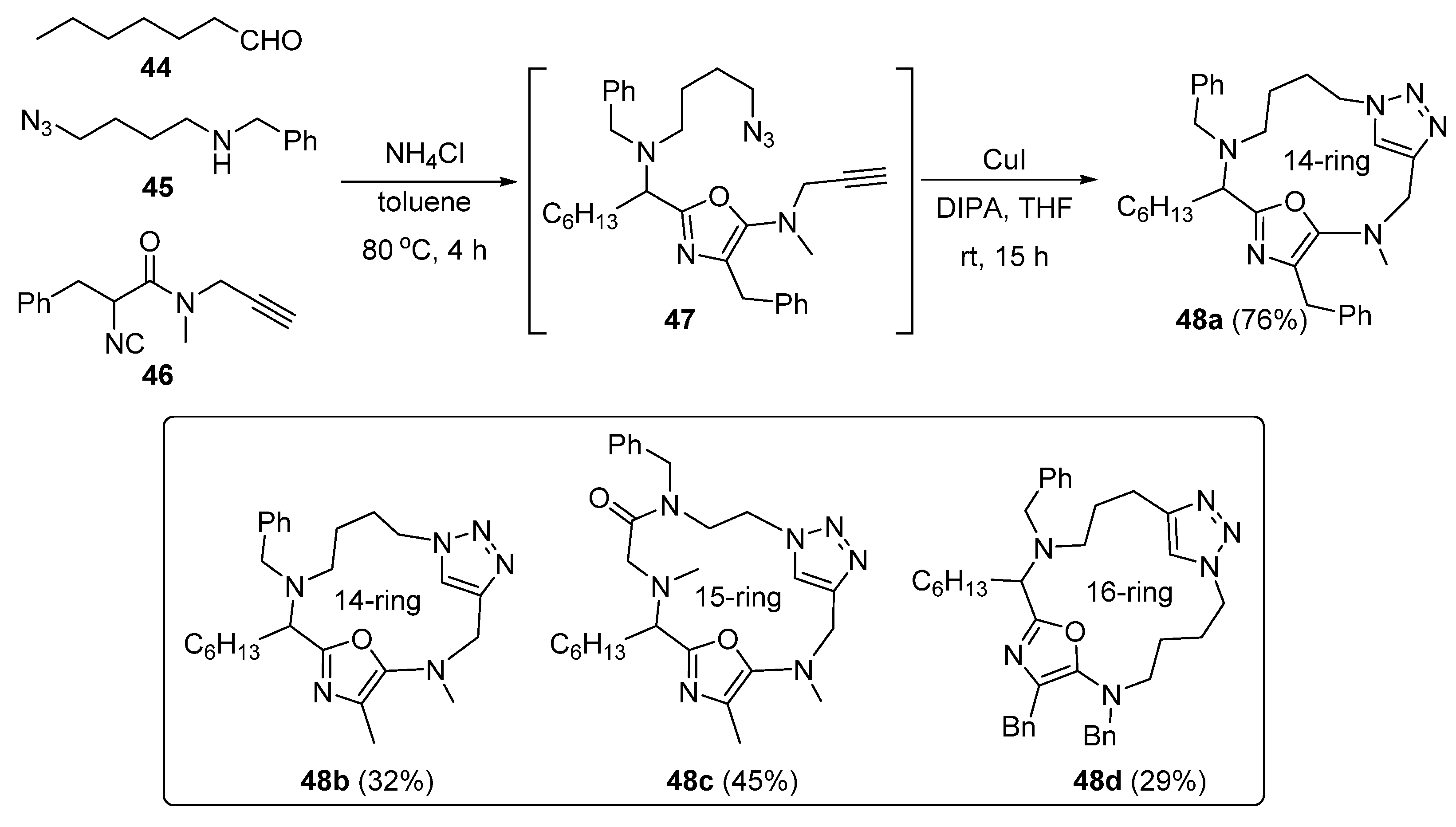
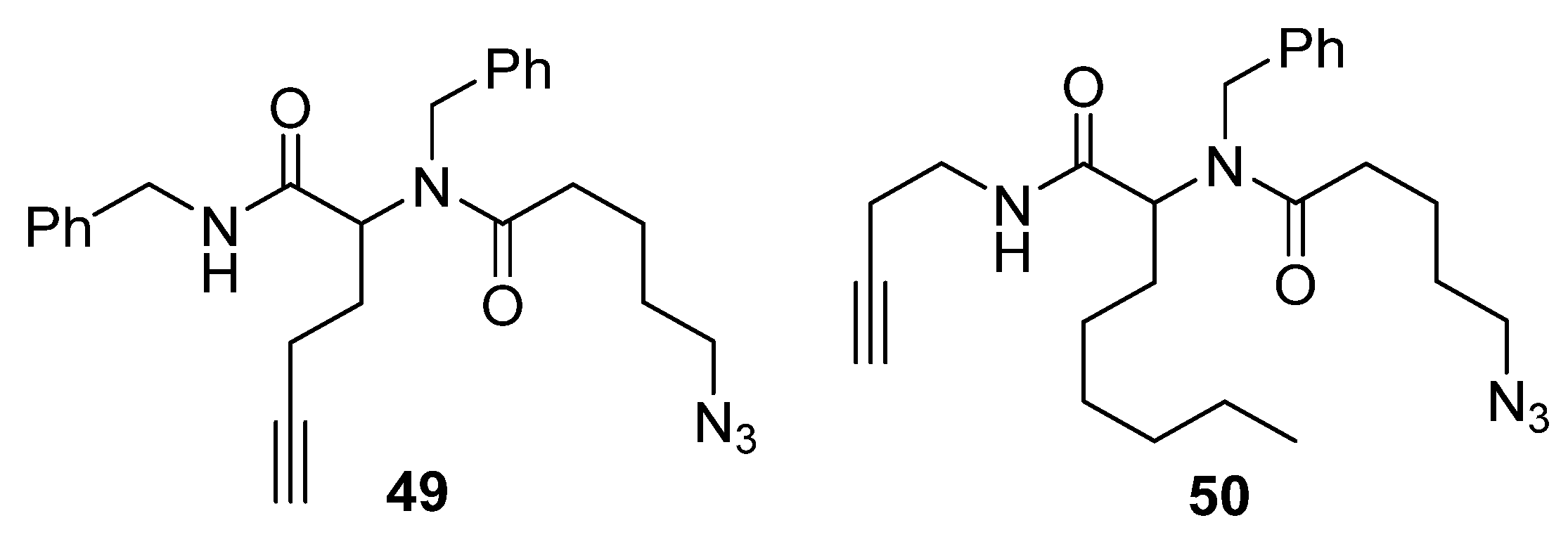
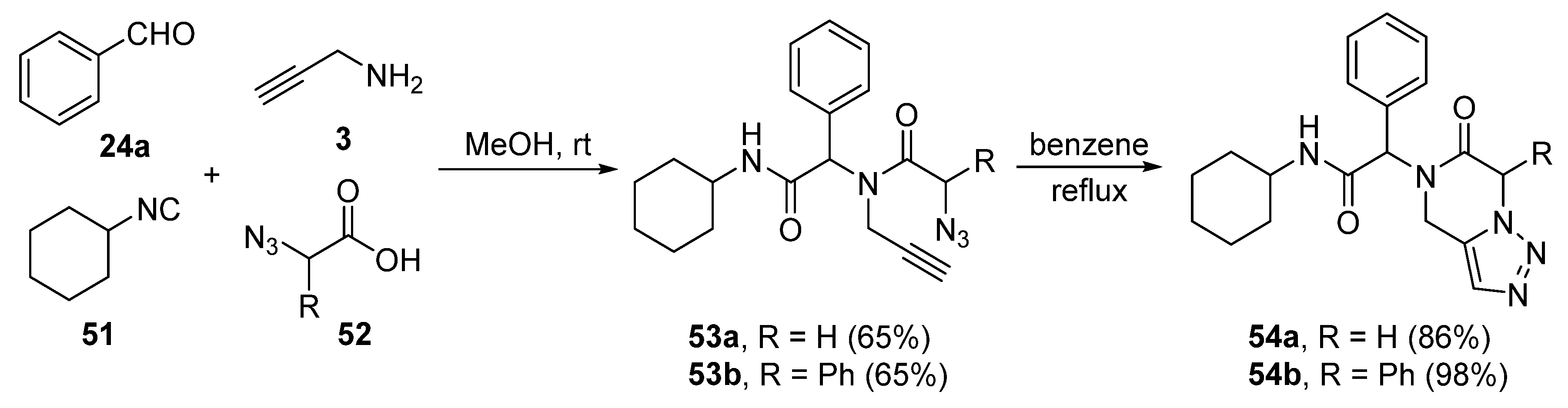
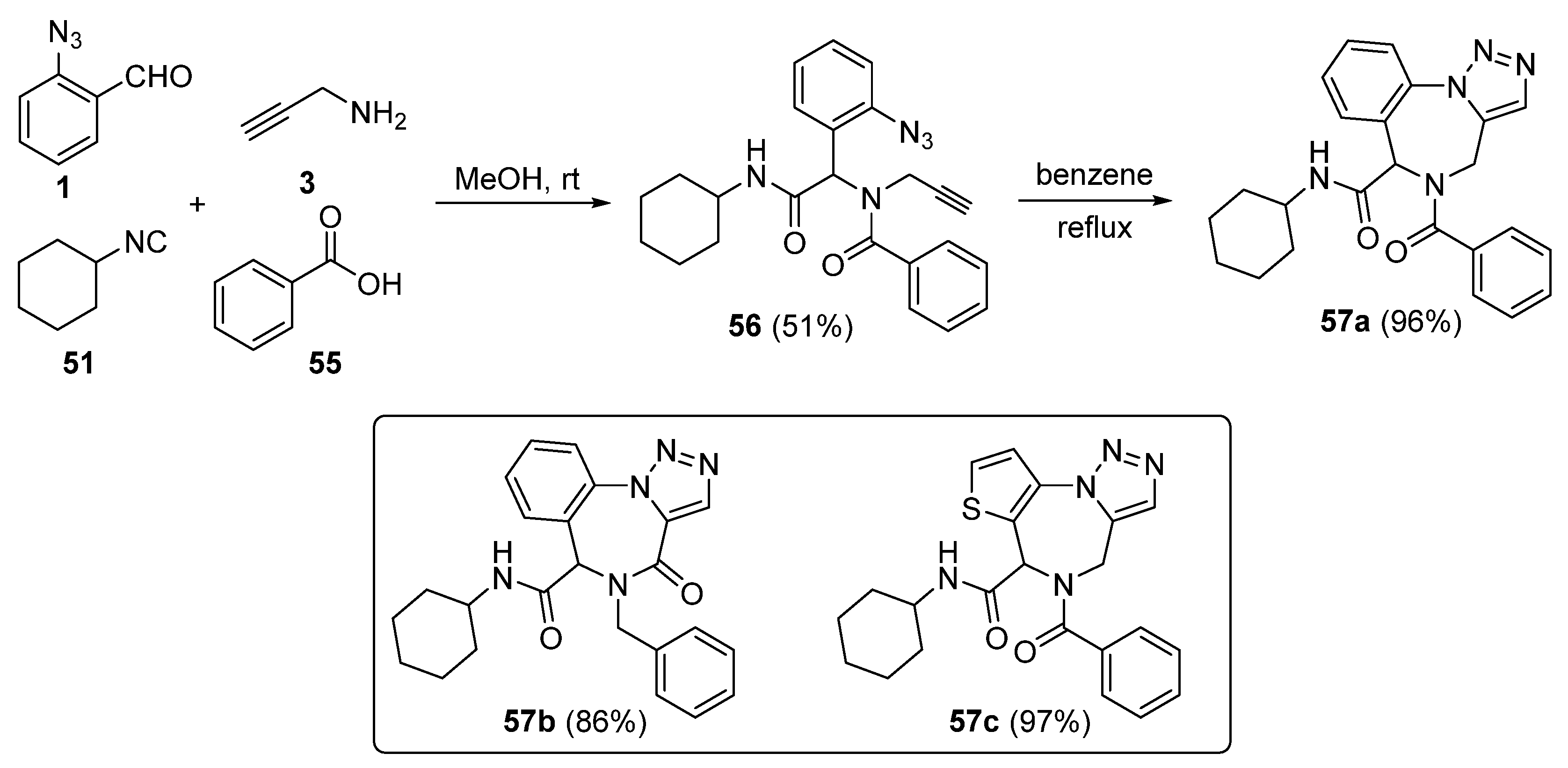
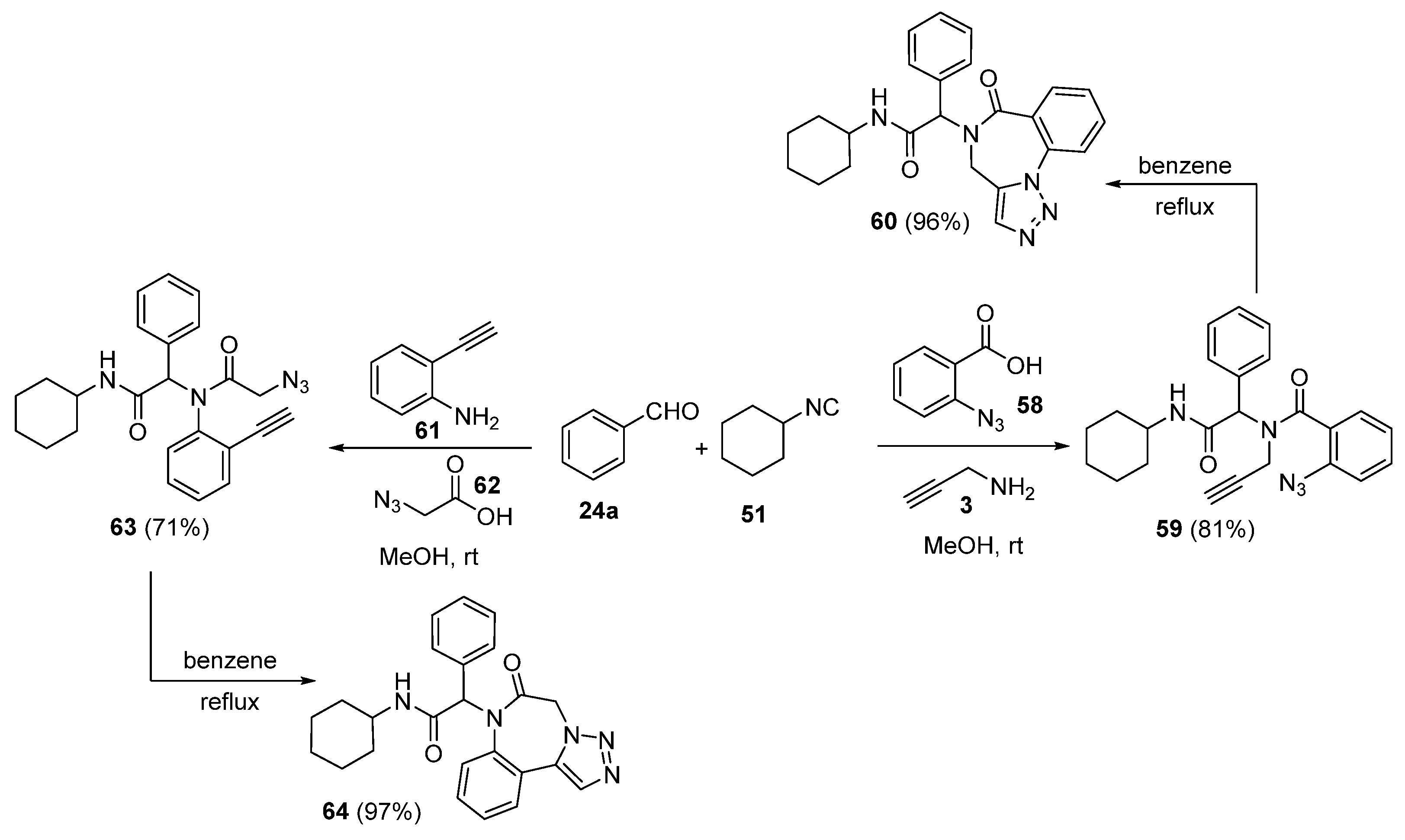
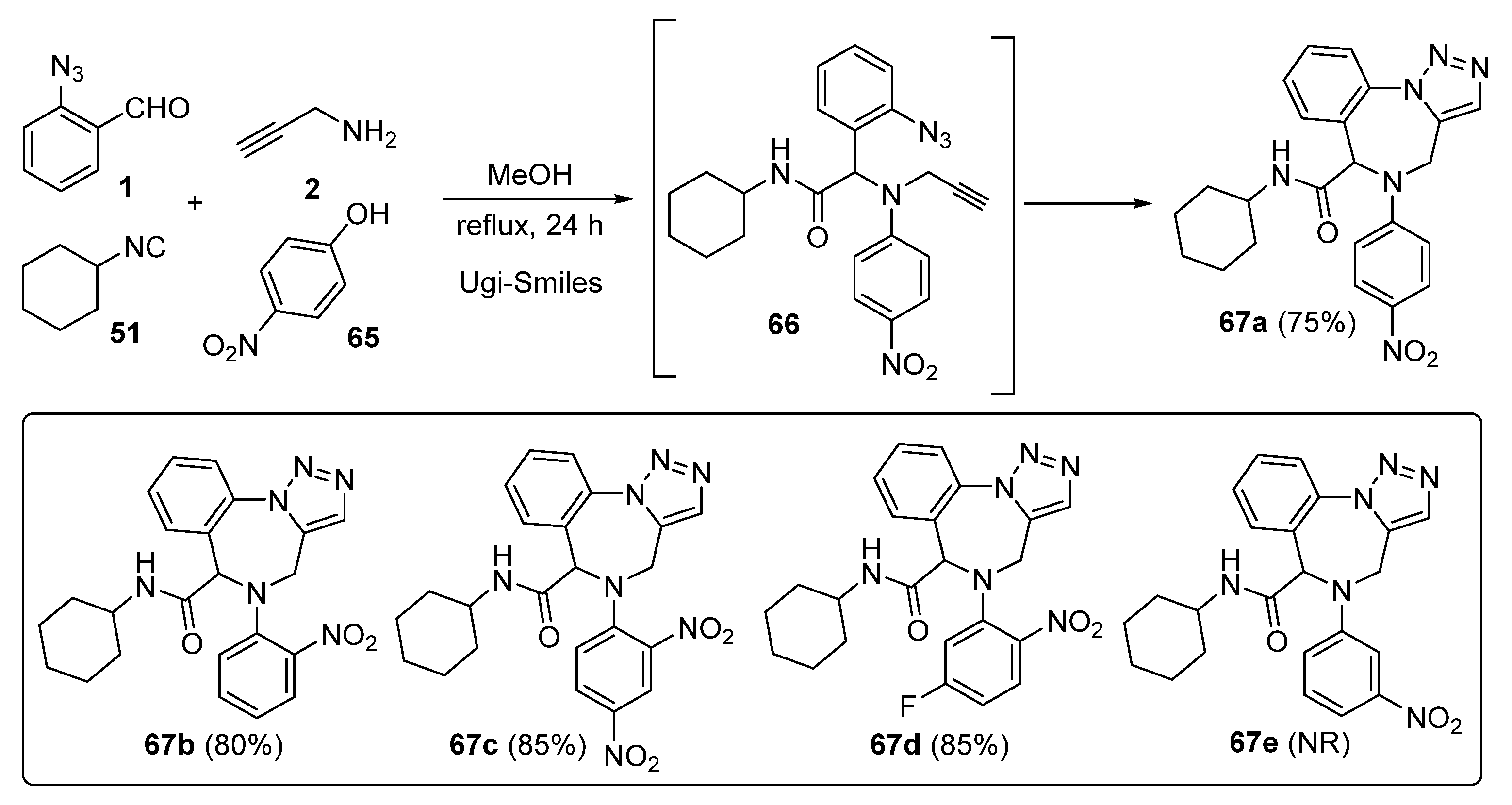
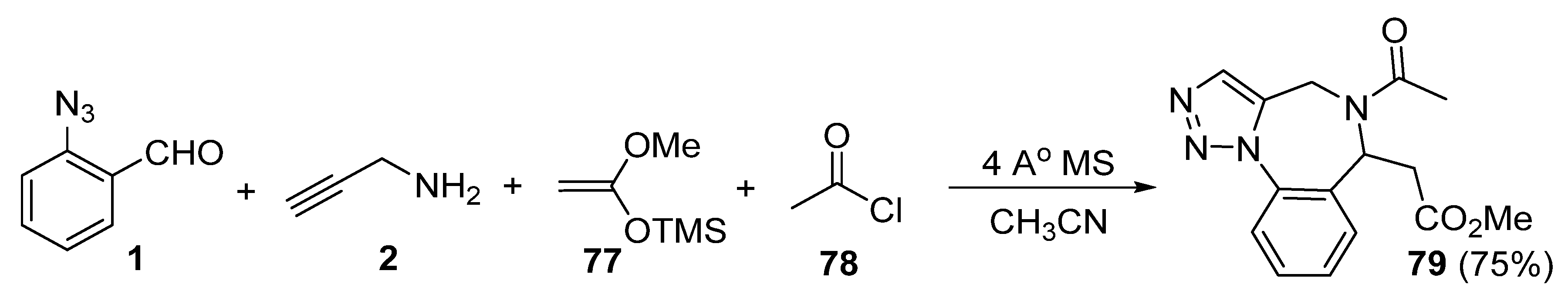
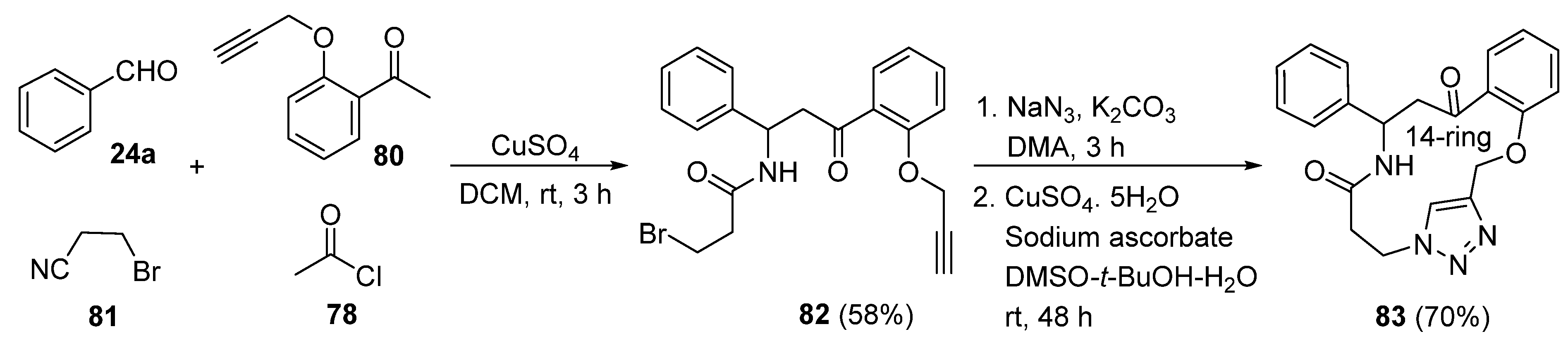
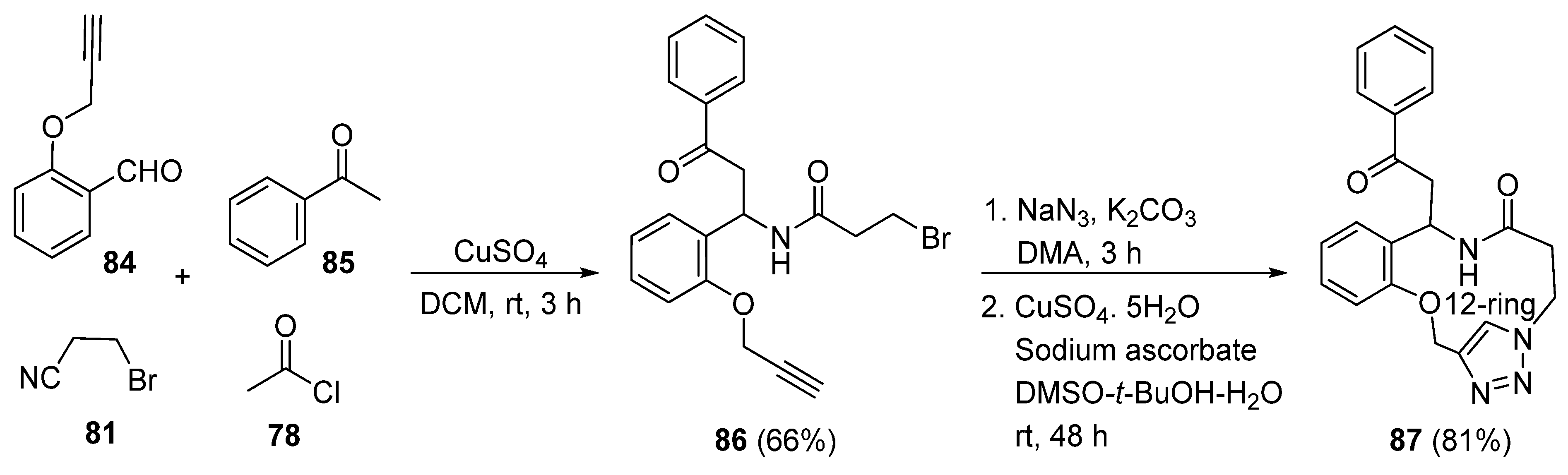
2. Synthesis of Fused 1,2,3-Triazole Moieties via Sequential 3-CR-IAAC Approach
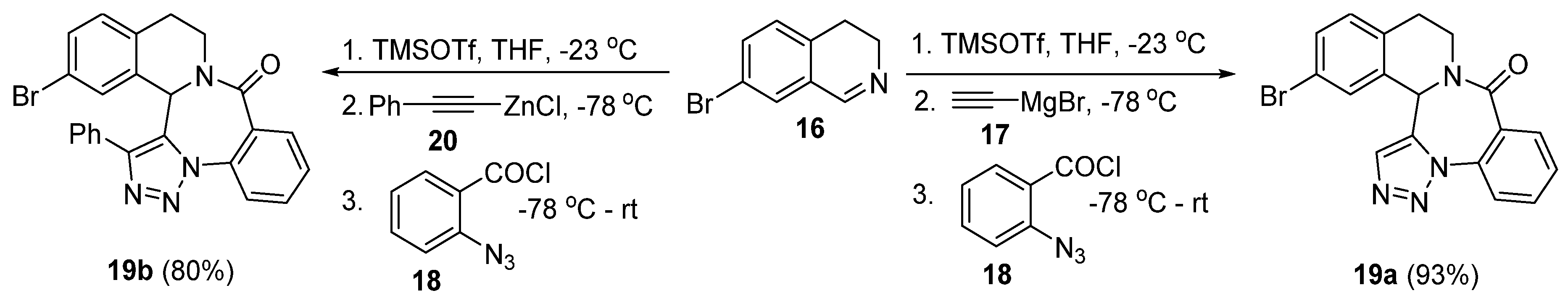
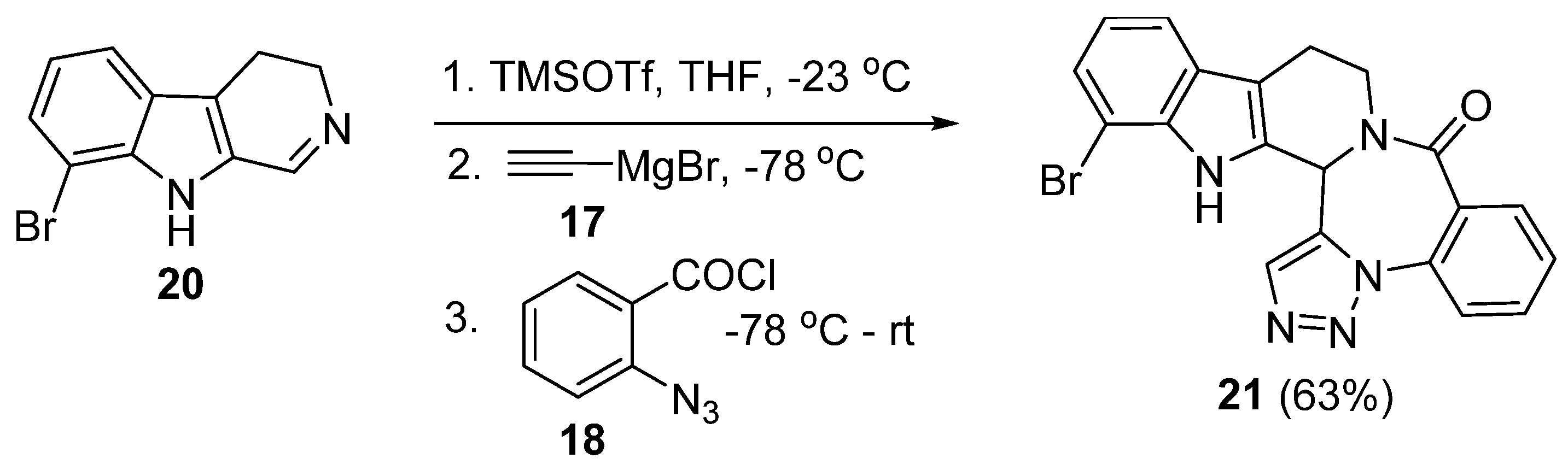
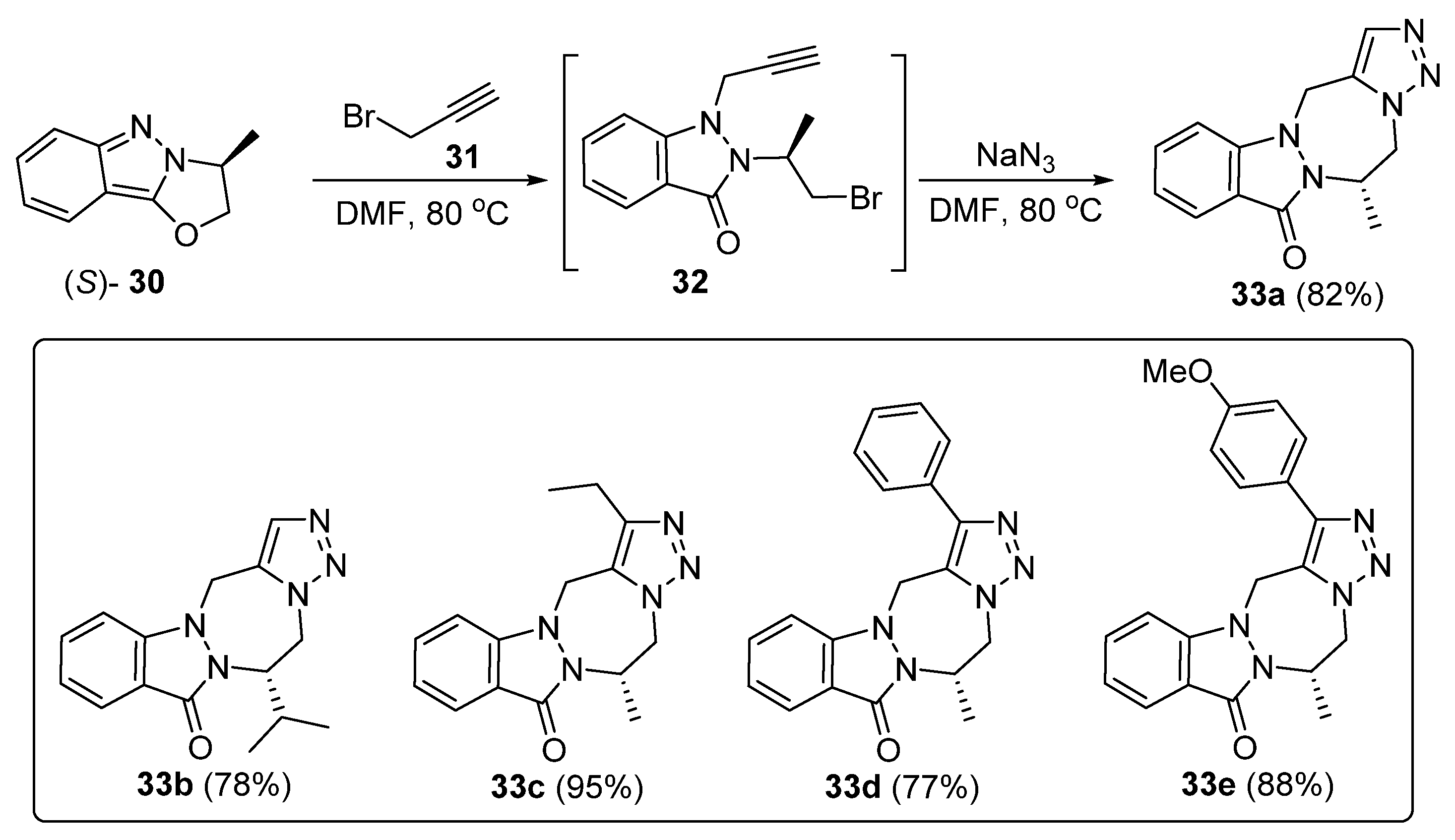
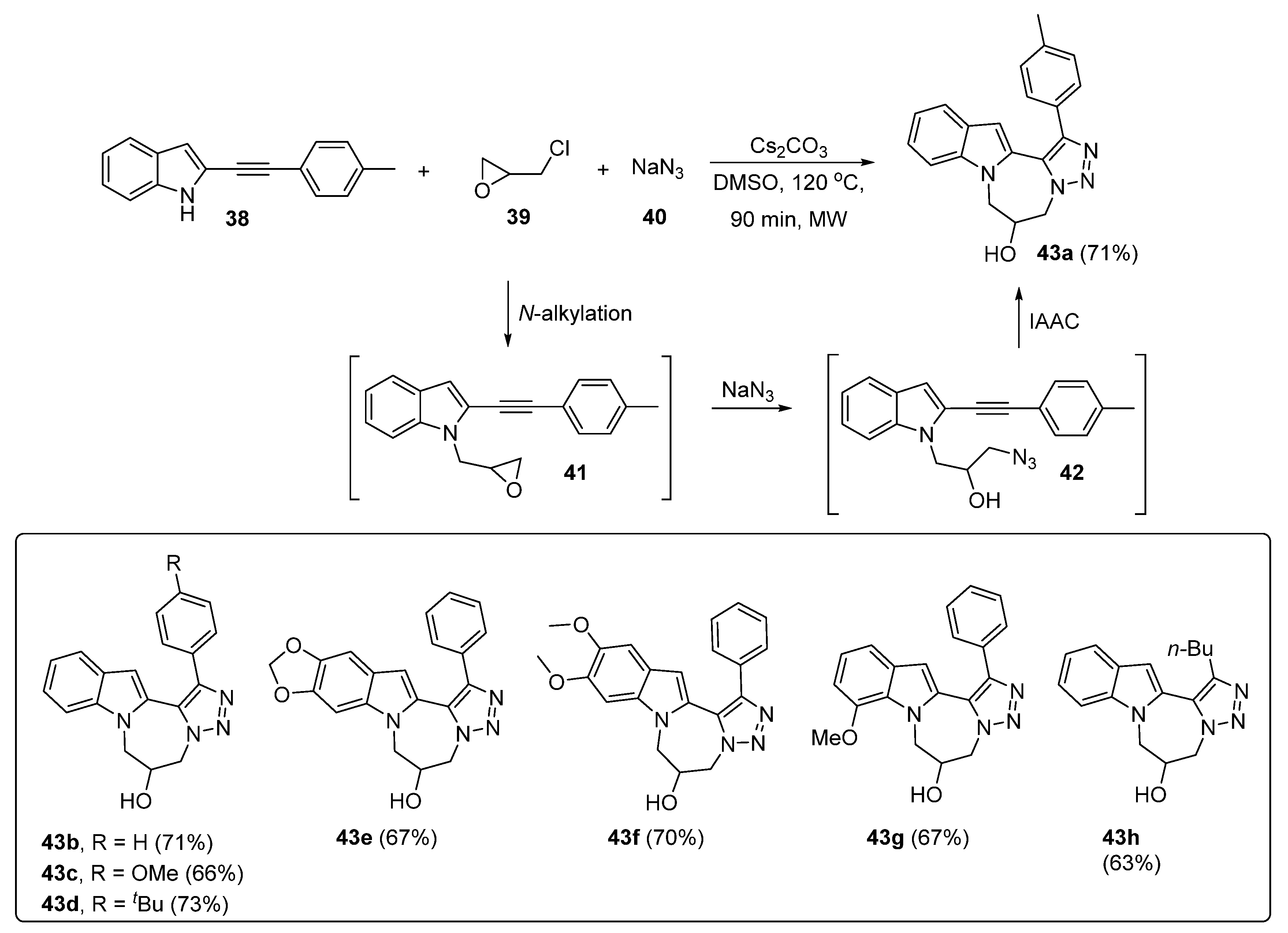
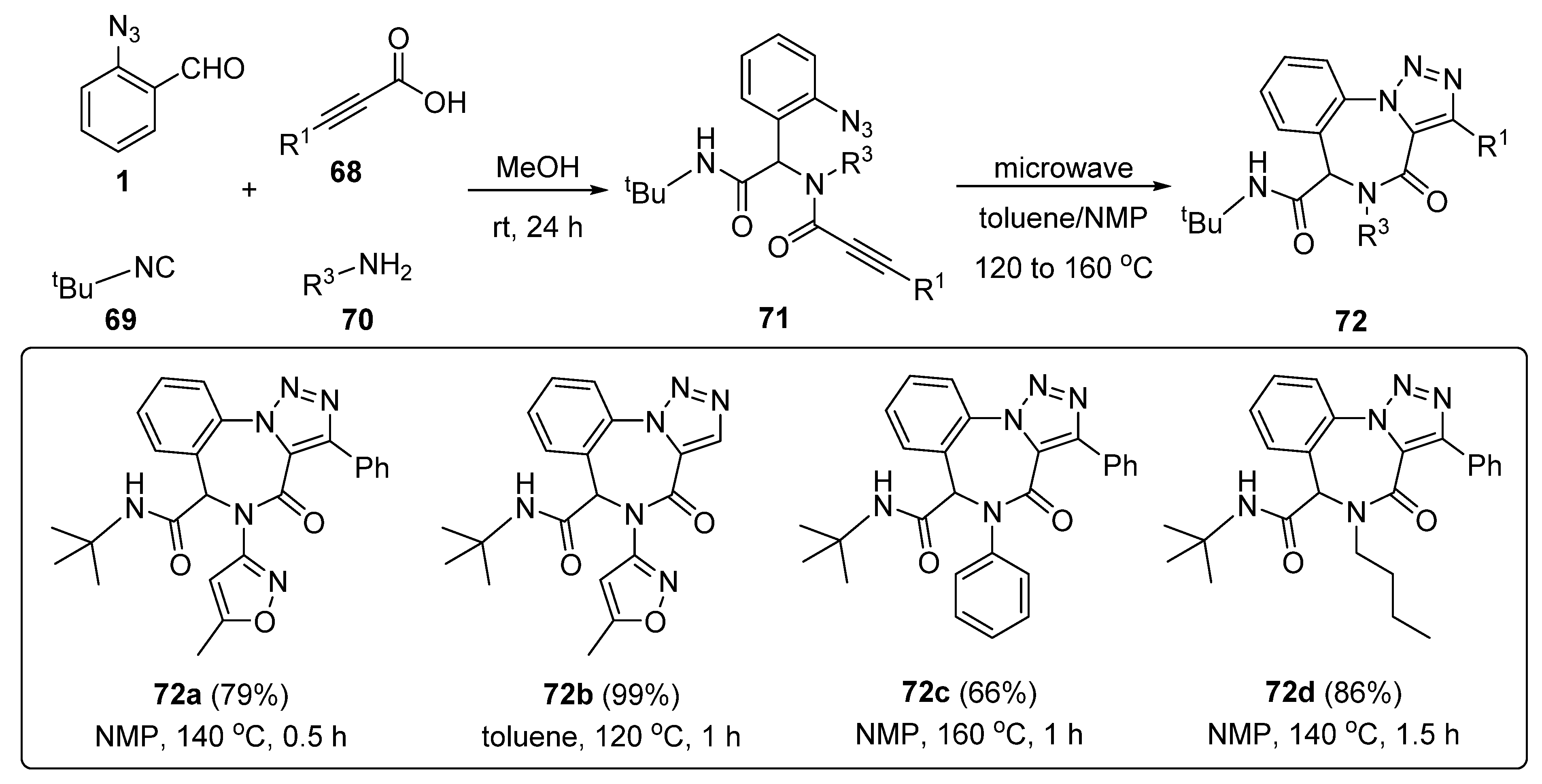
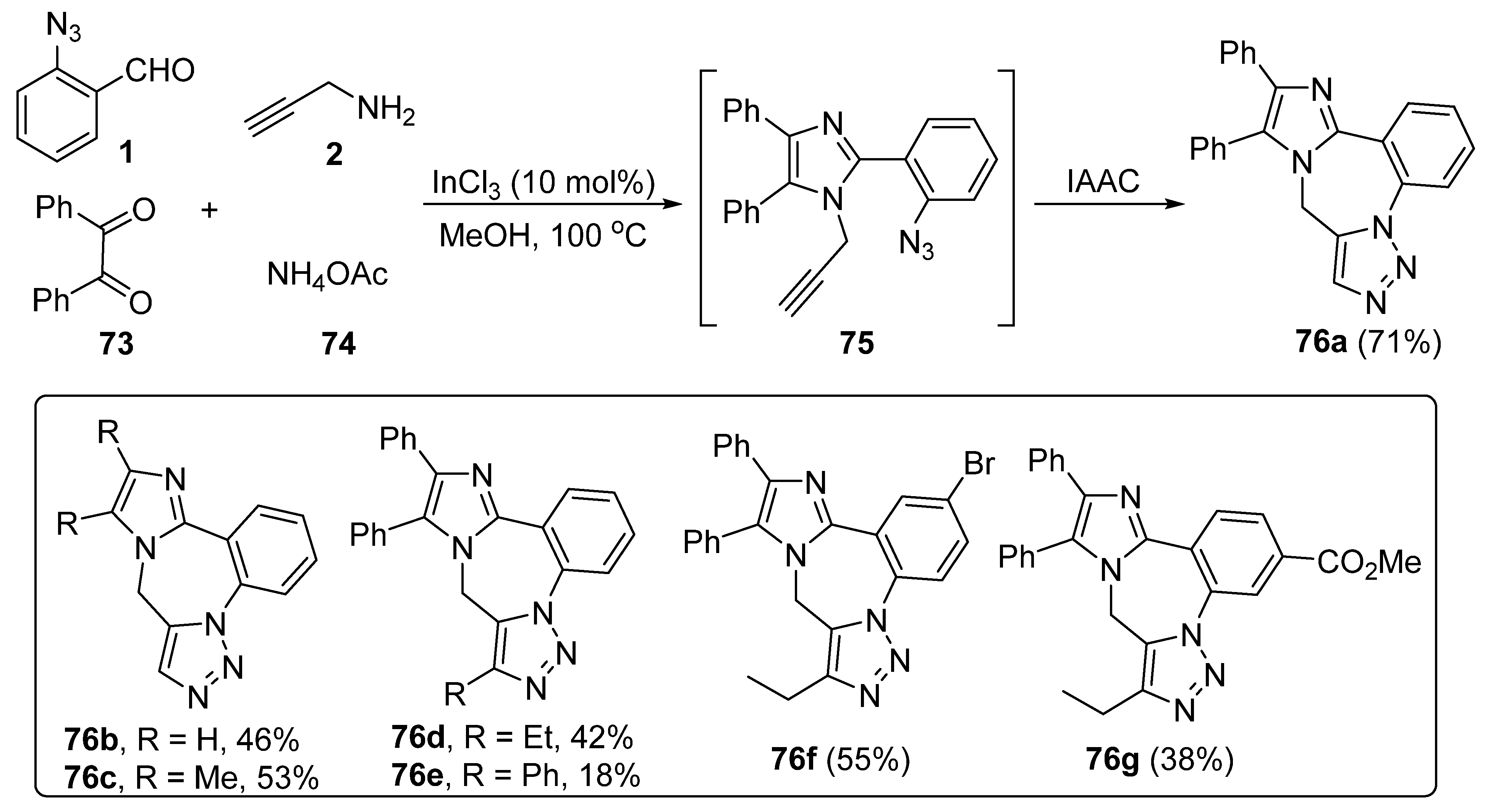
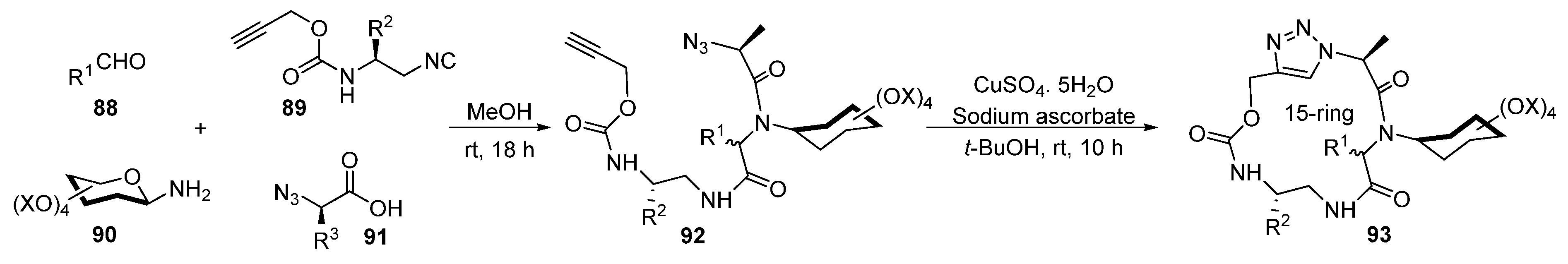
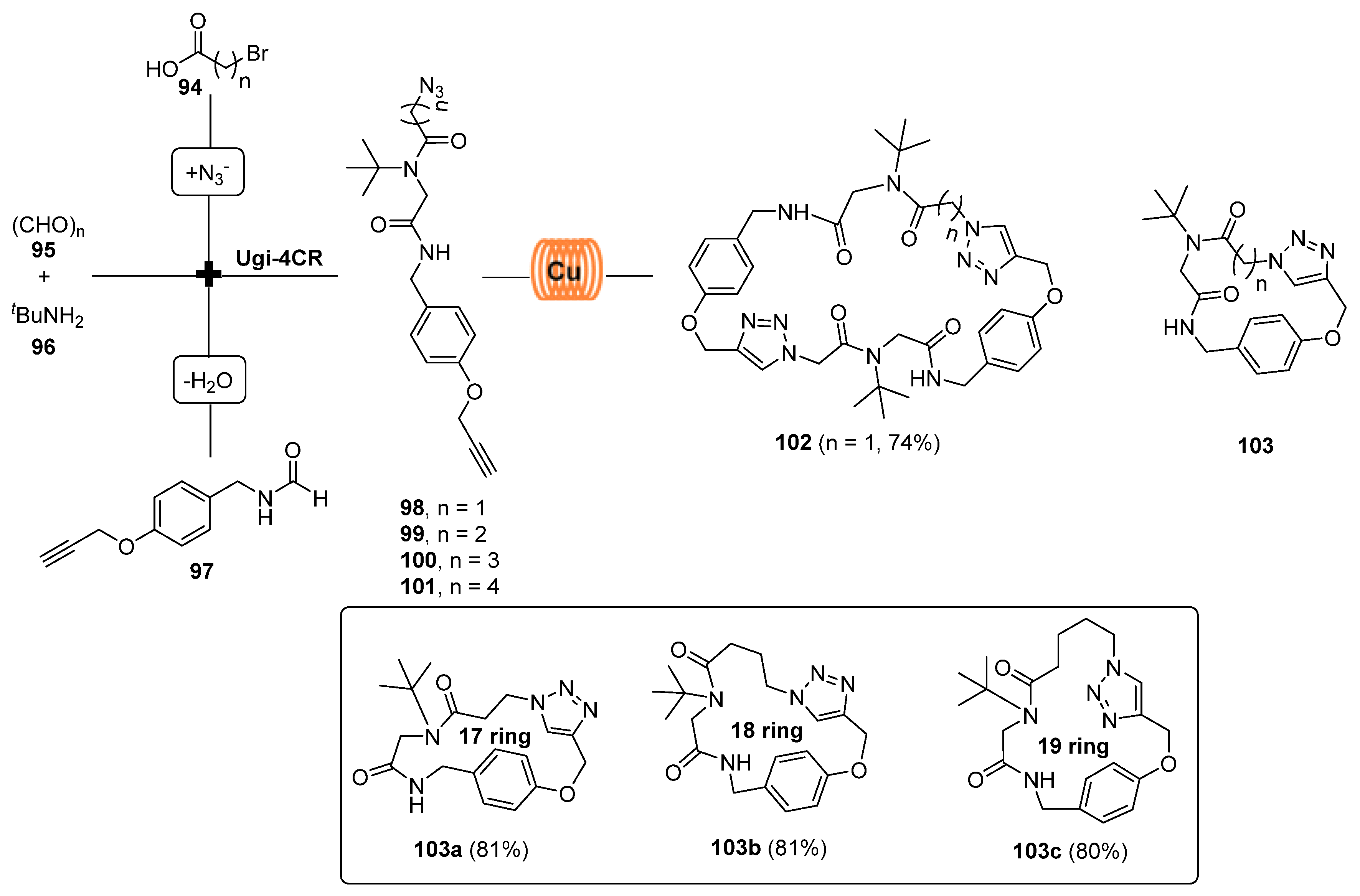
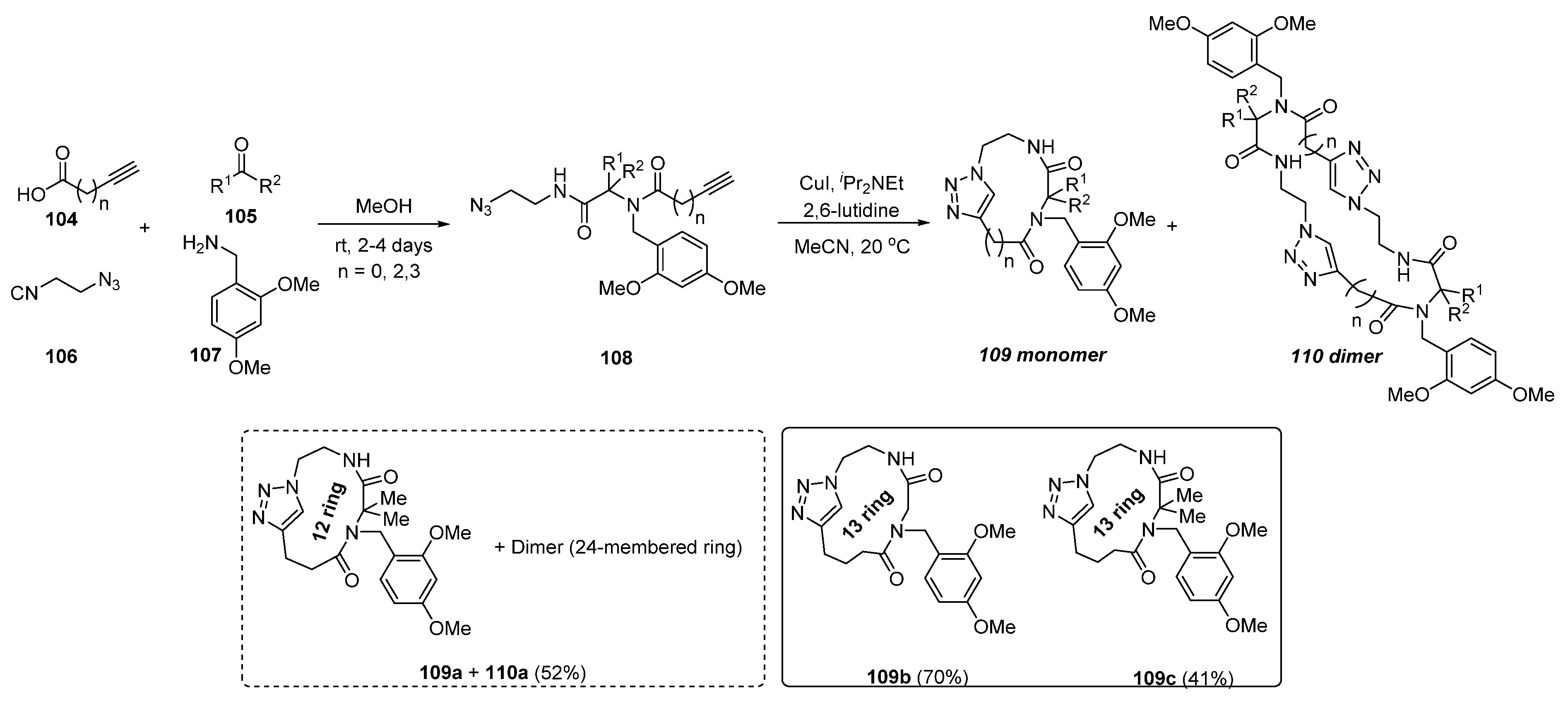
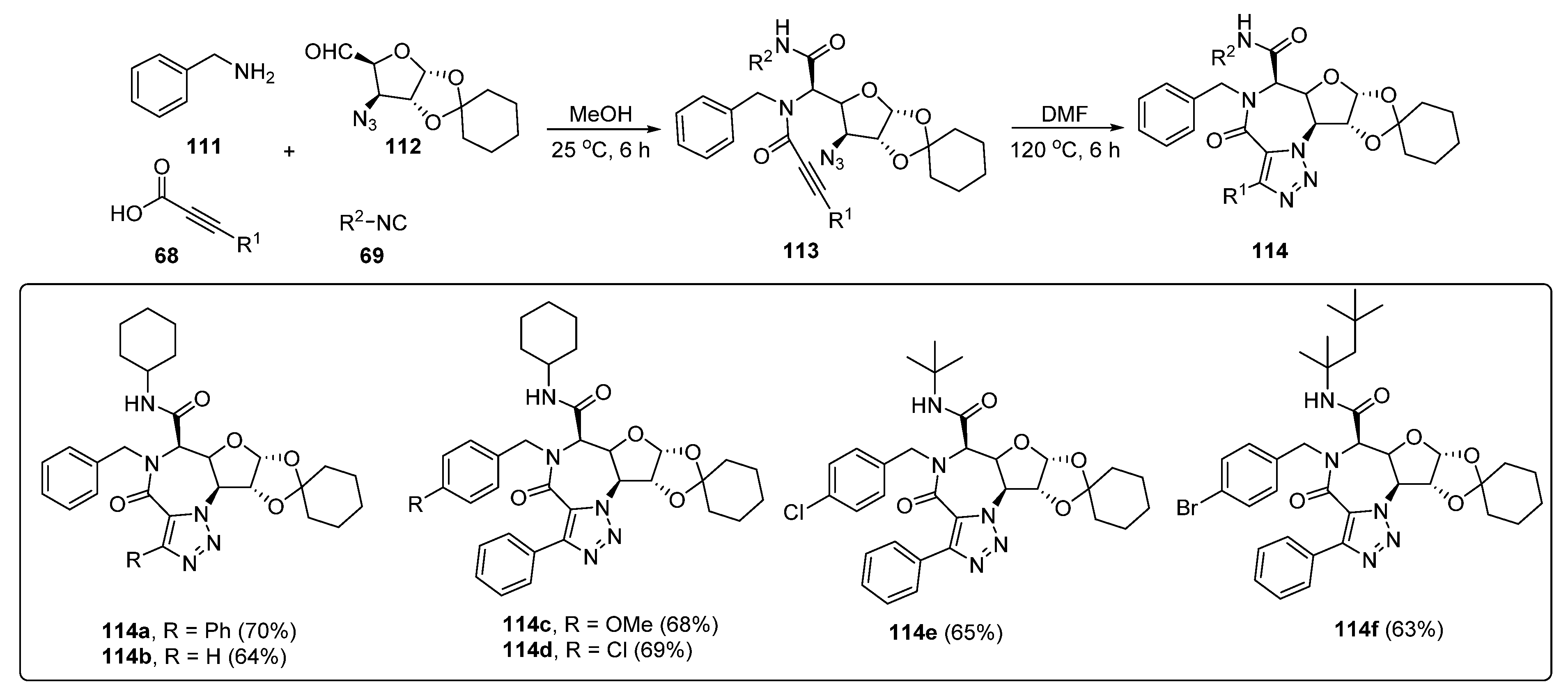
3. Synthesis of Fused 1,2,3-Triazole Moieties via Sequential 4-CR-IAAC Approach
4. Concluding Remarks
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Meldal, M.; Tomøe, C.W. Cu-Catalyzed Azide−Alkyne Cycloaddition. Chem. Rev. 2008, 108, 2952–3015. [Google Scholar] [CrossRef] [PubMed]
- Special issue on click chemistry. Chem. Soc. Rev. 2010, 39, 1221–1408.
- Pedersen, D.S.; Abell, A. 1,2,3-Triazoles in Peptidomimetic Chemistry. Eur. J. Org. Chem. 2011, 2011, 2399–2411. [Google Scholar] [CrossRef]
- Agalave, S.G.; Maujan, S.R.; Pore, V.S. Click Chemistry: 1,2,3-Triazoles as Pharmacophore. Chem. Asian J. 2011, 6, 2696–2718. [Google Scholar] [CrossRef]
- Thirumurugan, P.; Matosiuk, D.; Jozwiak, K. Click Chemistry for Drug Development and Diverse Chemical–Biology Applications. Chem. Rev. 2013, 113, 4905–4979. [Google Scholar] [CrossRef]
- Li, L.; Zhang, Z. Development and Applications of the Copper-Catalyzed Azide-Alkyne Cycloaddition (CuAAC) as a Bioorthogonal Reaction. Molecules 2016, 21, 1393. [Google Scholar] [CrossRef]
- Neumann, S.; Biewend, M.; Rana, S.; Binder, W.H. The CuAAC: Principles, Homogeneous and Heterogeneous Catalysts, and Novel Developments and Applications. Macromol. Rapid Commun. 2020, 41, 1900359. [Google Scholar] [CrossRef]
- John, J.; Thomas, J.; Dehaen, W. Organocatalytic Routes toward Substituted 1,2,3-Triazoles. Chem. Commun. 2015, 51, 10797–10806. [Google Scholar] [CrossRef]
- Dai, J.; Tian, S.; Yang, X.; Liu, Z. Synthesis methods of 1,2,3-/1,2,4-triazoles: A review. Front. Chem. 2022. [Google Scholar] [CrossRef]
- Alvarez, R.; Velazquez, S.; San-Felix, A.; Aquaro, S.; De Clercq, E.; Perno, C.-F.; Karlsson, A.; Balzarini, J.; Camarasa, M.J. 1,2,3-Triazole-[2,5-Bis-O-(tert-butyldimethylsilyl)-beta-D-ribofuranosyl}-3′-spiro-5″-(4″-amino-1″,2″-oxathiole 2″,2″-dioxide) (TSAO) Analogs: Synthesis and Anti-HIV-1 Activity. J. Med. Chem. 1994, 37, 4185–4194. [Google Scholar] [CrossRef]
- Bohacek, R.S.; McMartin, C.; Guida, W.C. The art and practice of structure-based drug design: A molecular modeling perspective. Med. Res. Rev. 1996, 16, 3–50. [Google Scholar] [CrossRef]
- Soltis, M.J.; Yeh, H.J.; Cole, K.A.; Whittaker, N.; Wersto, R.P.; Kohn, E.C. Identification and characterization of human metabolites of CAI [5-amino-1-1(4′-chlorobenzoyl-3,5-dichlorobenzyl)-1,2,3-triazole-4-carboxamide). Drug Metab. Dispos. 1996, 24, 799–806. [Google Scholar]
- Kharb, R.; Sharma, P.C.; Yar, M.S. Pharmacological significance of triazole scaffold. J. Enzyme Inhib. Med. Chem. 2011, 26, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, I.; Kummetha, I.R.; Singh, G.; Sharova, N.; Lichinchi, G.; Dang, J.; Stevenson, M.; Rana, T.M. 1,2,3-Triazoles as Amide Bioisosteres: Discovery of a New Class of Potent HIV-1 Vif Antagonists. J. Med. Chem. 2016, 59, 7677–7682. [Google Scholar] [CrossRef] [PubMed]
- Shafran, E.A.; Bakulev, V.A.; Rozin, Y.A.; Shafran, Y.M. Condensed 1,2,3-triazoles (review). Chem. Heterocycl. Compd. 2008, 44, 1040–1069. [Google Scholar] [CrossRef]
- Majumdar, K.C.; Ray, K. Synthesis of 1,2,3-Triazole-Fused Heterocycles via Intramolecular Azide-Alkyne Cycloaddition Reactions. Synthesis 2011, 3767–3783. [Google Scholar] [CrossRef]
- Kumar, H.; Dhameja, M.; Rizvi, M.; Gupta, P. Progress in the Synthesis of Fused 1,2,3-Triazoles. ChemistrySelect 2021, 6, 4889–4947. [Google Scholar] [CrossRef]
- Dheer, D.; Singh, V.; Shankar, R. Medicinal attributes of 1,2,3-triazoles: Current developments. Bioorg. Chem. 2017, 71, 30–54. [Google Scholar] [CrossRef]
- Song, M.-X.; Deng, X.-Q. Recent developments on triazole nucleus in anticonvulsant compounds: A review. J. Enzyme Inhib. Med. Chem. 2018, 33, 453–478. [Google Scholar] [CrossRef]
- Bozorova, K.; Zhao, J.; Aisa, H.A. 1,2,3-Triazole-containing hybrids as leads in medicinal chemistry: A recent overview. Bioorg. Med. Chem. 2019, 27, 3511–3531. [Google Scholar] [CrossRef]
- Sun, J.; Duan, R.; Li, H.; Wu, J. Synthesis and Anti-HIV Activity of Triazolo-Fused 2′,3′-Cyclic Nucleoside Analogs Prepared by an Intramolecular Huisgen 1,3-Dipolar Cycloaddition. Helv. Chim. Acta 2013, 96, 59–68. [Google Scholar] [CrossRef]
- Mohapatra, D.K.; Maity, P.K.; Shabab, M.; Khan, M.I. Click chemistry based rapid one-pot synthesis and evaluation for protease inhibition of new tetracyclic triazole fused benzodiazepine derivatives. Bioorg. Med. Chem. Lett. 2009, 19, 5241–5245. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, H.-Y.; Lee, W.-C.; Senadi, G.C.; Hu, W.-P.; Liang, J.-J.; Tsai, T.-R.; Chou, Y.-W.; Kuo, K.-K.; Chen, C.-Y.; Wang, J.-J. Discovery, Synthetic Methodology, and Biological Evaluation for Antiphotoaging Activity of Bicyclic[1,2,3]triazoles: In Vitro and in Vivo Studies. J. Med. Chem. 2013, 56, 5422–5435. [Google Scholar] [CrossRef] [PubMed]
- Ugi, I.; Dömling, A.; Hörl, W. Multicomponent reactions in organic chemistry. Endeavour 1994, 18, 115–122. [Google Scholar] [CrossRef]
- Zhu, J.; Bienaymé, H. (Eds.) Multicomponent Reactions; Wiley-VCH: Weinheim, Germany, 2005. [Google Scholar]
- Dömling, A. Recent Developments in Isocyanide Based Multicomponent Reactions in Applied Chemistry. Chem. Rev. 2006, 106, 17–89. [Google Scholar] [CrossRef] [PubMed]
- Brauch, S.; van Berkel, S.S.; Westermann, B. Higher-order multicomponent reactions: Beyond four reactants. Chem. Soc. Rev. 2013, 42, 4948–4962. [Google Scholar] [CrossRef]
- Gracias, V.; Darczak, D.; Gasiecki, A.F.; Djuric, S.W. Synthesis of fused triazolo-imidazole derivatives by sequential van Leusen/alkyne–azide cycloaddition reactions. Tetrahedron Lett. 2005, 46, 9053–9056. [Google Scholar] [CrossRef]
- Donald, J.R.; Martin, S.F. Synthesis and Diversification of 1,2,3-Triazole-Fused 1,4-Benzodiazepine Scaffolds. Org. Lett. 2011, 13, 852–855. [Google Scholar] [CrossRef]
- Granger, B.A.; Kaneda, K.; Martin, S.F. Multicomponent Assembly Strategies for the Synthesis of Diverse Tetrahydroisoquinoline Scaffolds. Org. Lett. 2011, 13, 4542–4545. [Google Scholar] [CrossRef]
- Granger, B.A.; Wang, Z.; Kaneda, K.; Fang, Z.; Martin, S.F. Multicomponent Assembly Processes for the Synthesis of Diverse Yohimbine and Corynanthe Alkaloid Analogues. ACS Comb. Sci. 2013, 15, 379–386. [Google Scholar] [CrossRef][Green Version]
- Brawn, R.A.; Welzel, M.; Lowe, J.T.; Panek, J.S. Regioselective Intramolecular Dipolar Cycloaddition of Azides and Unsymmetrical Alkynes. Org. Lett. 2010, 12, 336–339. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Conrad, W.E.; Rodriguez, K.X.; Nguyen, H.H.; Fettinger, J.C.; Haddadin, M.J.; Kurth M., J. A One-Pot-Three-Step Route to Triazolotriazepinoindazolones from Oxazolino-2H-indazoles. Org. Lett. 2012, 14, 3870–3873. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Dhongde, A.P.; Afraj, S.N.; Nuzlia, C.; Chen, C.; Lee, G.-H. A Facile Synthesis of 4,6,7,8,8a,9-Hexahydropyrrolo[1,2-a][1,2,3]triazole [1,5-d]pyrazines by a Three-Component Coupling Reaction Followed by Intramolecular 1,3-Dipolar Cycloaddition. Eur. J. Org. Chem. 2013, 4119–4130. [Google Scholar]
- Arigela, R.K.; Sharma, S.K.; Kumar, B.; Kundu, B. Microwave-assisted three-component domino reaction: Synthesis of indolodiazepinotriazoles. Beilstein J. Org. Chem. 2013, 9, 401–405. [Google Scholar] [CrossRef] [PubMed]
- Pirali, T.; Tron, G.C.; Zhu, J. One-Pot Synthesis of Macrocycles by a Tandem Three-Component Reaction and Intramolecular [3+2] Cycloaddition. Org. Lett. 2006, 8, 4145–4148. [Google Scholar] [CrossRef] [PubMed]
- Akritopoulou-Zanze, I.; Gracias, V.; Djuric, S.W. A versatile synthesis of fused triazolo derivatives by sequential Ugi/alkyne–azide cycloaddition reactions. Tetrahedron Lett. 2004, 45, 8439–8441. [Google Scholar] [CrossRef]
- Mina Saeedi, Mohammad Mahdavi, Alireza Foroumadi, Abbas Shafiee, Synthesis of novel fused 4,5-dihydro-1,2,3-triazolo[1,5-a][1,4]benzodiazepine derivatives via four-component Ugi-Smiles-type reaction. Tetrahedron 2013, 69, 3506–3510. [CrossRef]
- Mazur, M.O.; Zhelavskyi, O.S.; Zviagin, E.M.; Shishkina, S.V.; Musatov, V.I.; Kolosov, M.A.; Shvets, E.H.; Andryushchenko, A.Y.; Chebanov, V.A. Effective microwave-assisted approach to 1,2,3-triazolobenzodiazepinones via tandem Ugi reaction/catalyst-free intramolecular azide–alkyne cycloaddition. Org. Biomol. Chem. 2021, 17, 678–687. [Google Scholar] [CrossRef]
- Nguyen, H.H.; Palazzo, T.A.; Kurth, M.J. Facile One-Pot Assembly of Imidazotriazolobenzodiazepines via Indium(III)-Catalyzed Multicomponent Reactions. Org. Lett. 2013, 15, 4492–4495. [Google Scholar] [CrossRef]
- Sunderhaus, J.D.; Dockendorff, C.; Martin, S.F. Applications of Multicomponent Reactions for the Synthesis of Diverse Heterocyclic Scaffolds. Org. Lett. 2007, 9, 4223–4226. [Google Scholar] [CrossRef]
- Bahulayan, D.; Arun, S. An easy two step synthesis of macrocyclic peptidotriazoles via a four-component reaction and copper catalyzed intramolecular azide–alkyne [3+ 2] click cycloaddition. Tetrahedron Lett. 2012, 53, 2850–2855. [Google Scholar] [CrossRef]
- Samarasimhareddy, M.; Hemantha, H.P.; Sureshbabu, V.V. A simple protocol for the synthesis of triazole-linked cyclic glycopeptidomimetics: A sequential Ugi-MCR and azide–alkyne cycloaddition approach. Tetrahedron Lett. 2012, 53, 3104–3107. [Google Scholar] [CrossRef][Green Version]
- Salvador, C.E.M.; Pieber, B.; Neu, P.M.; Torvisco, A.; Andrade, C.K.Z.; Kappe, C.O. A Sequential Ugi Multicomponent/Cu-Catalyzed Azide–Alkyne Cycloaddition Approach for the Continuous Flow Generation of Cyclic Peptoids. J. Org. Chem. 2015, 80, 4590–4602. [Google Scholar] [CrossRef] [PubMed]
- Zakharova, E.A.; Shmatova, O.I.; Kutovaya, I.V.; Khrustalev, V.N.; Nenajdenko, V.G. Synthesis of macrocyclic peptidomimetics via the Ugi-click-strategy. Org. Biomol. Chem. 2019, 17, 3433–3445. [Google Scholar] [CrossRef] [PubMed]
- Reddy, B.V.S.; Majumder, N.; Rao, T.P. Four-Component, One-Pot Synthesis of N-Alkyl-4-oxo-3-phenylhexahydro-4H spiro{[1,3]dioxolo[4′,5′:4,5]furo[2,3-f}[1,2,3]triazolo[1,5-a][1,4]diazepine-9,1′-cyclohexane}-6-carboxamide Derivatives. Synthesis 2014, 46, 3408–3414. [Google Scholar] [CrossRef]
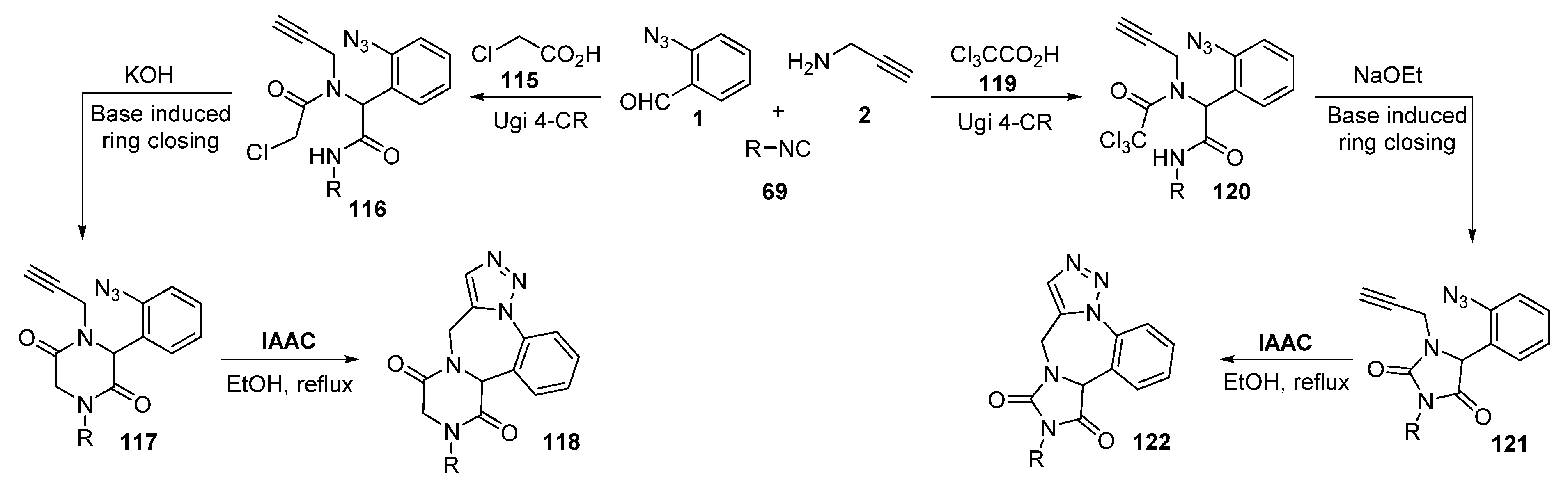
- Vroemans, R.; Bamba, F.; Winters, J.; Thomas, J.; Jacobs, J.; Van Meervelt, L.; John, J.; Dehaen, W. Sequential Ugi reaction/base-induced ring closing/IAAC protocol toward triazolobenzodiazepine-fused diketopiperazines and hydantoins. Beilstein J. Org. Chem. 2018, 14, 626–633. [Google Scholar] [CrossRef]
- Yan, Y.-M.; Li, H.-Y.; Zhang, M.; Wang, R.-X.; Zhou, C.-G.; Ren, Z.-X.; Ding, M.-W. One-Pot Synthesis of [1,2,3]Triazolo[1,5-a]quinoxalin-4(5H)-ones by a Metal-Free Sequential Ugi-4CR/Alkyne–Azide Cycloaddition Reaction. Synlett 2020, 31, 73–76. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
P, R.; Thomas, J.; Dehaen, W.; John, J. Advances in the Synthesis of Fused 1,2,3-Triazoles via a MCR-Intramolecular Azide-Alkyne Cycloaddition Approach. Molecules 2023, 28, 308. https://doi.org/10.3390/molecules28010308
P R, Thomas J, Dehaen W, John J. Advances in the Synthesis of Fused 1,2,3-Triazoles via a MCR-Intramolecular Azide-Alkyne Cycloaddition Approach. Molecules. 2023; 28(1):308. https://doi.org/10.3390/molecules28010308
Chicago/Turabian StyleP, Rahul, Joice Thomas, Wim Dehaen, and Jubi John. 2023. "Advances in the Synthesis of Fused 1,2,3-Triazoles via a MCR-Intramolecular Azide-Alkyne Cycloaddition Approach" Molecules 28, no. 1: 308. https://doi.org/10.3390/molecules28010308
APA StyleP, R., Thomas, J., Dehaen, W., & John, J. (2023). Advances in the Synthesis of Fused 1,2,3-Triazoles via a MCR-Intramolecular Azide-Alkyne Cycloaddition Approach. Molecules, 28(1), 308. https://doi.org/10.3390/molecules28010308





