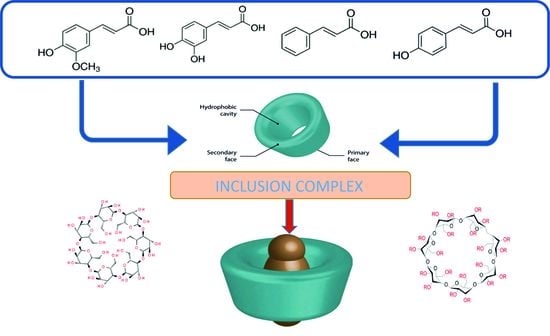
Conductometric Studies of Formation the Inclusion Complexes of Phenolic Acids with β-Cyclodextrin and 2-HP-β-Cyclodextrin in Aqueous Solutions
Abstract
1. Introduction
2. Results and Discussion
3. Materials and Methods
3.1. Materials
3.2. Characterization Methods
4. Conclusions
Supplementary Materials
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Szetjli, J. Introduction and general overview of cyclodextrin chemistry. Chem. Rev. 1998, 98, 1743–1753. [Google Scholar]
- Munzos-Botella, S.; del Castillo, B.; Martin, M.A. Cyclodextrin properties and applications of inclusion complex formation. ARS Pharm. 1995, 36, 187–198. [Google Scholar]
- Martin Del Valle, E.M. Cyclodextrin and their uses: A review. Process Biochem. 2004, 39, 1033–1046. [Google Scholar] [CrossRef]
- Fujishima, N.; Kusaka, K.; Umino, T.; Urushinata, T.; Terumi, K. Flour based foods containing highly branched cyclodextrins. Jpn. Pat. JP 2001, 136, 898. [Google Scholar]
- Bhardway, R.; Dorr, R.T.; Blanchard, J. Approaches to reducing toxicity of parenteral anticancer drug formulations using cyclodextrins. J. Pharm. Sci. Technol. 2000, 54, 233–239. [Google Scholar]
- Bibby, D.C.; Davies, N.M.; Tucker, I.G. Mechanism by which cyclodextrins modify drug release from polymeric drug delivery systems. Int. J. Pharm. 2000, 197, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Buschmann, H.J.; Scollmeyer, E. Applications of cyclodextrins in cosmetic products: A review. J. Cosmet. Sci. 2002, 53, 185–191. [Google Scholar]
- Foley, P.R.; Kaiser, C.E.; Sadler, J.D.; Burckhardt, E.E.; Liu, Z. Detergent Composition with Cyclodextrin Perfume Complexes to Mask Malodours. U.S. Patent WO2001023516A1, 29 September 2000. [Google Scholar]
- Hedges, R.A. Industrial applications of cyclodextrins. Chem. Rev. 1998, 98, 2035–2044. [Google Scholar] [CrossRef] [PubMed]
- Baudin, C.; Pean, C.; Perly, B.; Goselin, P. Inclusion of organic pollutants in cyclodextrin and derivatives. Int. J. Environ. Anal. Chem. 2000, 77, 233–242. [Google Scholar] [CrossRef]
- Lezcano, M.; Ai-Soufi, W.; Novo, M.; Rodriguez-Nunez, E.; Tato, J.V. Complexation of several benzimidazoletype fugicides with alpha and beta- cyclodextrins. J. Agric. Food Chem. 2002, 50, 108–112. [Google Scholar] [CrossRef]
- Koukiekolo, R.; Desseaux, V.; Moreau, Y.; Marchis, M.G.; Santimone, M. Mechanism of porcine pancreatic alphaamylase inhibition of amylose and maltopentaose hydrolysis by alpha, beta- and gamma-cyclodextrins. Eur. J. Biochem. 2001, 268, 841–848. [Google Scholar] [CrossRef] [PubMed]
- Schneiderman, E.; Stalcup, A.M. Cyclodextrins: A versatile tool in separation science. J. Chromatogr. B Biomed. Sci. Appl. 2000, 745, 83–102. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Chen, Y. Chiral separation of amino acids derivatives with fluoresceine-5-isothiocyanate by capillary electrophoresis and laser-induced fluorescene detection using mixed selectors of beta-cyclodextrin and sodium taurocholate. J. Chromatogr. A 2002, 955, 133–140. [Google Scholar] [CrossRef] [PubMed]
- Zarzycki, P.K.; Kulhanek, K.M.; Smith, R. Chromatographic behaviour of selected steroids and their inclusion complexes with beta-cyclodextrin on octadecylsilica stationary phases with different carbon loads. J. Chromatogr. A 2002, 955, 125–131. [Google Scholar] [CrossRef] [PubMed]
- Szejtli, J.; Szente, L. Elimination of bitter, disgusting tastes of drugs and foods by cyclodextrin. Eur. J. Pharm. Biopharm. 2005, 61, 115–125. [Google Scholar] [CrossRef] [PubMed]
- Siquet, C.; Paiva-Martins, F.; Lima, J.; Reis, S.; Borges, F. Antioxidant profile of dihydroxy- and trihydroxyphenolic acid—A structure—Activity relationship study. Free Radic. Res. 2006, 40, 433–442. [Google Scholar] [CrossRef]
- Cuvelier, M.E.; Richard, H.; Berset, C. Comparison of the antioxidative activity of some acid-phenols: Structure—Activity relationship. Biosci. Biotechnol. Biochem. 1992, 56, 324–325. [Google Scholar] [CrossRef]
- Chen, J.H.; Ho, C.-T. Antioxidant activities of caffeic acid and its related hydroxy- cinnamic acid compounds. J. Agric. Food Chem. 1997, 45, 2374–2378. [Google Scholar] [CrossRef]
- Medina, I.; Gallardo, J.M.; González, M.J.; Lois, A.S.; Hedges, N. Effect of molecular structure of phenolic families as hydroxycinnamic acids and catechins on their antioxidant effectiveness in minced fish muscle. J. Agric. Food Chem. 2007, 55, 3889–3895. [Google Scholar] [CrossRef]
- Sroka, Z.; Cisowski, W. Hydrogen peroxide scavenging, antioxidant and anti-radical activity of some phenolic acids. Food Chem. Toxicol. 2003, 41, 753–758. [Google Scholar] [CrossRef]
- Cheng, J.-C.; Dai, F.; Zhou, B.; Yang, L.; Liu, Z.L. Antioxidant activity of hydrocinnamic acid derivative in human low density lipoprotein: Mechanism and structure-activity relationship. Food Chem. 2007, 104, 132–139. [Google Scholar] [CrossRef]
- Marinova, E.M.; Toneva, A.; Yanishlieva, N. Comparison of the antioxidative properties of caffeic and chlorogenic acids. Food Chem. 2009, 114, 1498–1502. [Google Scholar] [CrossRef]
- Kinart, Z.; Tomaš, R. Studies of the Formation of Inclusion Complexes Derivatives of Cinnamon Acid with α-Cyclodextrin in a Wide Range of Temperatures Using Conductometric Methods. Molecules 2022, 27, 4420. [Google Scholar] [CrossRef] [PubMed]
- Salomon, M.; Hefter, G.T. Mobilities of cation-macrocyclic ligand complexes. Pure Appl. Chem. 1993, 65, 1533–1540. [Google Scholar] [CrossRef][Green Version]
- Salomon, M. Conductometric study of cationic and anionic complexes in propylene carbonate. J. Solut. Chem. 1990, 19, 1225–1236. [Google Scholar] [CrossRef]
- Hoglen, N.C.; Waller, S.C.; Sipes, I.G.; Liebler, D.C. Reactions of peroxynitrite with gam-ma-tocopherol. Chem. Res. Toxicol. 1997, 10, 401–407. [Google Scholar] [CrossRef]
- Conney, R.V.; Harwood, P.J.; Franke, A.A.; Narala, K.; Sundstrom, A.K.; Berggren, P.O.; Mordan, L.J. Products of γ-tocopherol reaction with NO2 and their formation in rat insu- linoma (RINm5F) cells. Free. Radic. Biol. Med. 1995, 19, 259–269. [Google Scholar] [CrossRef]
- Conney, R.V.; Franke, A.A.; Harwood, P.J.; Hatch-pigott, V.; Custer, L.J. γ-Tocopherol detoxification of nitrogen dioxide: Superiority to α-tocopherol. Proc. Natl. Acad. Sci. USA 1993, 90, 1771–1775. [Google Scholar] [CrossRef]
- Ohkatsu, Y.; Kajiyama, T.; Arai, Y. Antioxidant acivities of tocopherols. Polym. Degrad. Stab. 2001, 72, 303–311. [Google Scholar] [CrossRef]
- Williamson, K.S.; Gabbita, S.P.; Mou, S.; West, M.; Pye, Q.N.; Markesbery, W.R.; Cooney, R.V.; Grammas, P.; Reimann-Philipp, U.; Floyd, R.A.; et al. The nitration product 5-nitro-gamma-tocopherol is increased in the Alzheimer brain. Nitric Oxide 2002, 6, 221–227. [Google Scholar] [CrossRef]
- Mura, P. Analytical techniques for characterization of cyclodextrin complexes in aqueous solution: A review. J. Pharm. Biomed. Anal. 2014, 101, 238–250. [Google Scholar] [CrossRef] [PubMed]
- Górnas, P.; Neunert, G.; Baczyńśki, K.; Polewski, K. Beta-cyclodextrin complexes with chlorogenic and caffeic acids from coffeebrew: Spectroscopic, thermodynamic and molecular modelling study. Food Chem. 2009, 114, 190–196. [Google Scholar] [CrossRef]
- Aksamija, A.; Tomao, V.; Dangles, O.; Plasson, R. Encapsulation of phenolic acids into cyclodextrins: A globalstatistical analysis of the effects of pH, temperature and concentrations on binding constants measured by ACE methods. Electrophoresis 2022, 43, 2290–2301. [Google Scholar] [CrossRef] [PubMed]
- Matejczyk, M.; Swisłocka, R.; Golonko, A.; Lewandowski, W.; Hawrylik, E. Cytotoxic, genotoxic and antimicrobial activity of caffeic and rosmarinic acids and their lithium, sodium and potassium salts as potential anticancer compounds. Adv. Med. Sci. 2018, 63, 14–21. [Google Scholar] [CrossRef] [PubMed]
- Swisłocka, R. Spectroscopic (FT-IR, FT-Raman, UV absorption, 1H and 13C NMR) and theoretical (in B3LYP/6-311++G** level) studies on alkalimetal salts of caffeic acid. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2013, 100, 21–30. [Google Scholar] [CrossRef] [PubMed]
- Kinart, Z.; Tomaš, R. Conductivity properties of selected aliphatic monocarboxylic acid anions in water at 298.15 K. Int. J. Electrochem. Sci. 2020, 15, 10007–10027. [Google Scholar] [CrossRef]
- Bešter-Rogač, M.; Neueder, R.; Barthel, J.; Appeblat, A. Conductivity studies on aqueous solutions of stereoisomers of tartaricacids and tartrates. Part III. Acidic tartrates. J. Solut. Chem. 1998, 27, 299–307. [Google Scholar] [CrossRef]
- Barthel, J.; Feuerlein, F.; Neueder, R.; Wachter, R. Calibration of conductance cells at various temperatures. J. Solut. Chem. 1980, 9, 209–219. [Google Scholar] [CrossRef]
 -trans-cinnamic acid;
-trans-cinnamic acid;  -trans–p-coumaric acid;
-trans–p-coumaric acid;  -trans-caffeic acid;
-trans-caffeic acid;  -trans-ferulic acid and 2-HP-β-cyclodextrin with the studied salts.:
-trans-ferulic acid and 2-HP-β-cyclodextrin with the studied salts.:  -trans-cinnamic acid;
-trans-cinnamic acid;  -trans–p-coumaric acid;
-trans–p-coumaric acid;  -trans-caffeic acid;
-trans-caffeic acid;  -trans-ferulic acid.
-trans-ferulic acid.
 -trans-cinnamic acid;
-trans-cinnamic acid;  -trans–p-coumaric acid;
-trans–p-coumaric acid;  -trans-caffeic acid;
-trans-caffeic acid;  -trans-ferulic acid and 2-HP-β-cyclodextrin with the studied salts.:
-trans-ferulic acid and 2-HP-β-cyclodextrin with the studied salts.:  -trans-cinnamic acid;
-trans-cinnamic acid;  -trans–p-coumaric acid;
-trans–p-coumaric acid;  -trans-caffeic acid;
-trans-caffeic acid;  -trans-ferulic acid.
-trans-ferulic acid.
 -trans-cinnamic acid;
-trans-cinnamic acid;  -trans–p-coumaric acid;
-trans–p-coumaric acid;  -trans-caffeic acid;
-trans-caffeic acid;  -trans-ferulic acid and 2-HP-β-cyclodextrin with the studied salts.:
-trans-ferulic acid and 2-HP-β-cyclodextrin with the studied salts.:  -trans-cinnamic acid;
-trans-cinnamic acid;  -trans–p-coumaric acid;
-trans–p-coumaric acid;  -trans-caffeic acid;
-trans-caffeic acid;  -trans-ferulic acid.
-trans-ferulic acid.
 -trans-cinnamic acid;
-trans-cinnamic acid;  -trans–p-coumaric acid;
-trans–p-coumaric acid;  -trans-caffeic acid;
-trans-caffeic acid;  -trans-ferulic acid and 2-HP-β-cyclodextrin with the studied salts.:
-trans-ferulic acid and 2-HP-β-cyclodextrin with the studied salts.:  -trans-cinnamic acid;
-trans-cinnamic acid;  -trans–p-coumaric acid;
-trans–p-coumaric acid;  -trans-caffeic acid;
-trans-caffeic acid;  -trans-ferulic acid.
-trans-ferulic acid.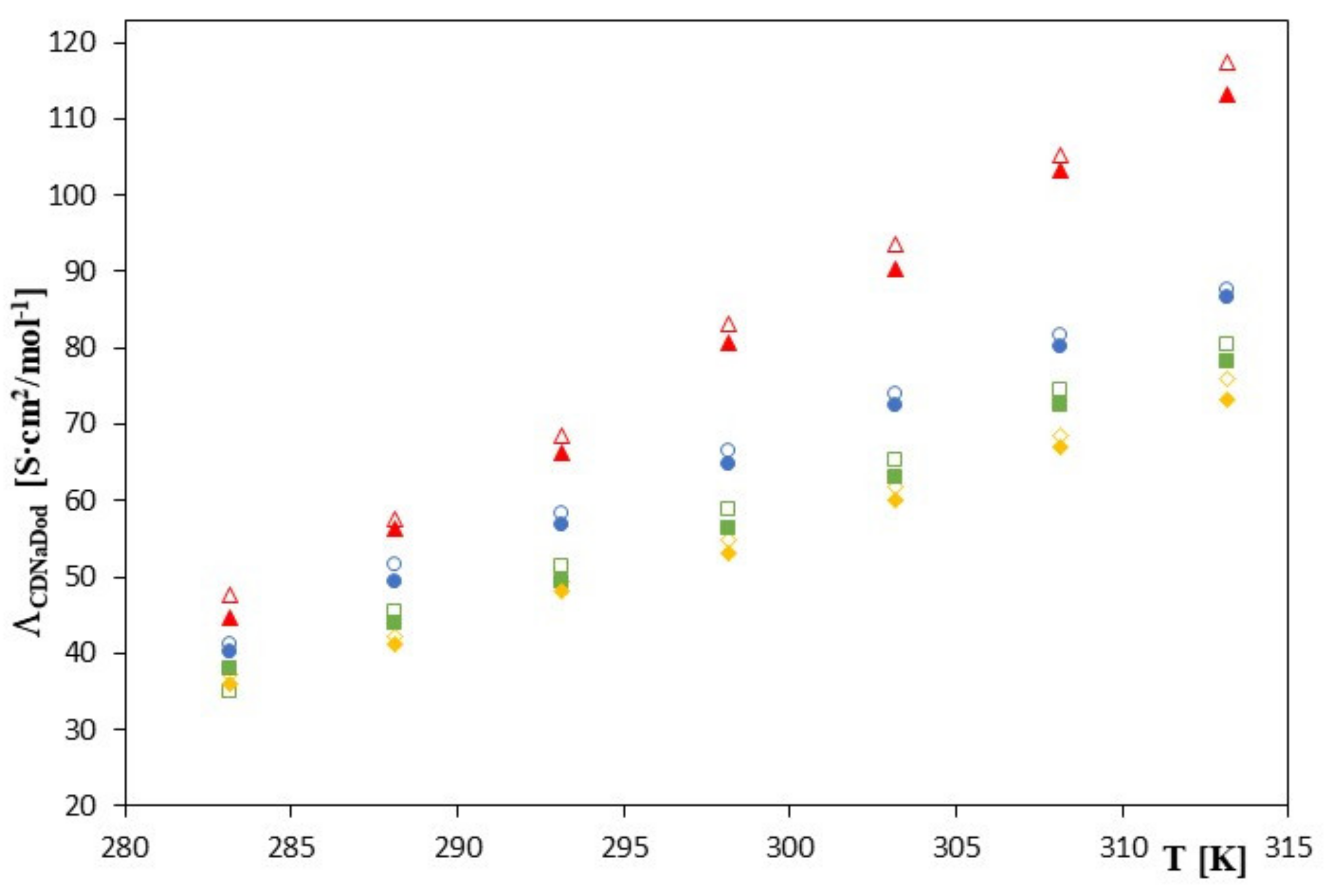
 -trans-cinnamic acid;
-trans-cinnamic acid;  -trans–p-coumaric acid;
-trans–p-coumaric acid;  -trans-caffeic acid;
-trans-caffeic acid;  -trans-ferulic acid and 2-HP-β-cyclodextrin with the studied salts.:
-trans-ferulic acid and 2-HP-β-cyclodextrin with the studied salts.:  -trans-cinnamic acid;
-trans-cinnamic acid;  -trans –p-coumaric acid;
-trans –p-coumaric acid;  -trans-caffeic acid;
-trans-caffeic acid;  -trans-ferulic acid.
-trans-ferulic acid.
 -trans-cinnamic acid;
-trans-cinnamic acid;  -trans–p-coumaric acid;
-trans–p-coumaric acid;  -trans-caffeic acid;
-trans-caffeic acid;  -trans-ferulic acid and 2-HP-β-cyclodextrin with the studied salts.:
-trans-ferulic acid and 2-HP-β-cyclodextrin with the studied salts.:  -trans-cinnamic acid;
-trans-cinnamic acid;  -trans –p-coumaric acid;
-trans –p-coumaric acid;  -trans-caffeic acid;
-trans-caffeic acid;  -trans-ferulic acid.
-trans-ferulic acid.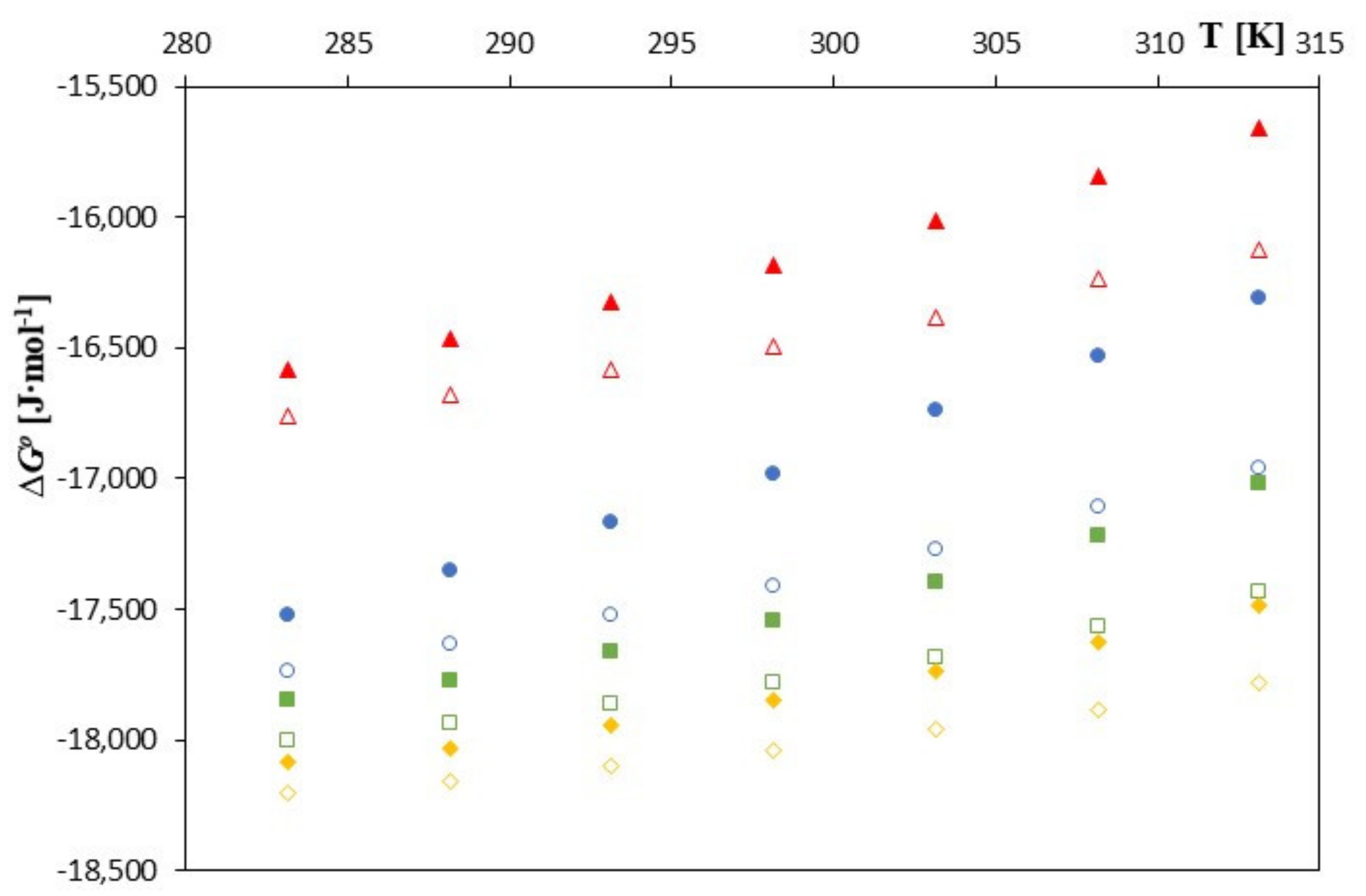
 -trans-cinnamic acid;
-trans-cinnamic acid;  -trans–p-coumaric acid;
-trans–p-coumaric acid;  -trans-caffeic acid;
-trans-caffeic acid;  -trans-ferulic acid and 2-HP-β-cyclodextrin with the studied salts.:
-trans-ferulic acid and 2-HP-β-cyclodextrin with the studied salts.:  -trans-cinnamic acid;
-trans-cinnamic acid;  -trans–p-coumaric acid;
-trans–p-coumaric acid;  -trans-caffeic acid;
-trans-caffeic acid;  -trans-ferulic acid.
-trans-ferulic acid.
 -trans-cinnamic acid;
-trans-cinnamic acid;  -trans–p-coumaric acid;
-trans–p-coumaric acid;  -trans-caffeic acid;
-trans-caffeic acid;  -trans-ferulic acid and 2-HP-β-cyclodextrin with the studied salts.:
-trans-ferulic acid and 2-HP-β-cyclodextrin with the studied salts.:  -trans-cinnamic acid;
-trans-cinnamic acid;  -trans–p-coumaric acid;
-trans–p-coumaric acid;  -trans-caffeic acid;
-trans-caffeic acid;  -trans-ferulic acid.
-trans-ferulic acid.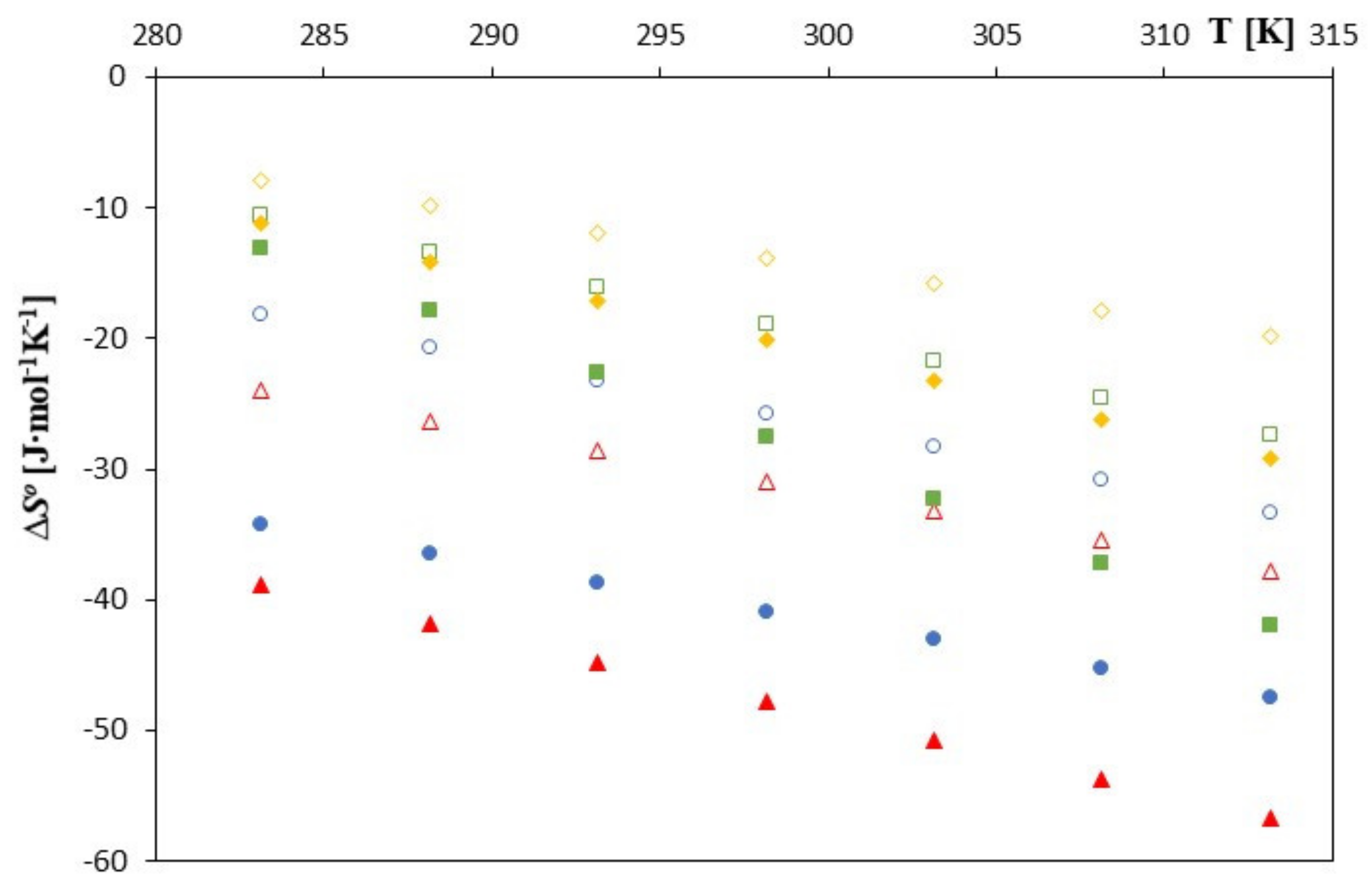
 -trans-cinnamic acid;
-trans-cinnamic acid;  -trans–p-coumaric acid;
-trans–p-coumaric acid;  -trans-caffeic acid;
-trans-caffeic acid;  -trans-ferulic acid and 2-HP-β-cyclodextrin with the studied salts.:
-trans-ferulic acid and 2-HP-β-cyclodextrin with the studied salts.:  -trans-cinnamic acid;
-trans-cinnamic acid;  -trans–p-coumaric acid;
-trans–p-coumaric acid;  -trans-caffeic acid;
-trans-caffeic acid;  -trans-ferulic acid.
-trans-ferulic acid.
 -trans-cinnamic acid;
-trans-cinnamic acid;  -trans–p-coumaric acid;
-trans–p-coumaric acid;  -trans-caffeic acid;
-trans-caffeic acid;  -trans-ferulic acid and 2-HP-β-cyclodextrin with the studied salts.:
-trans-ferulic acid and 2-HP-β-cyclodextrin with the studied salts.:  -trans-cinnamic acid;
-trans-cinnamic acid;  -trans–p-coumaric acid;
-trans–p-coumaric acid;  -trans-caffeic acid;
-trans-caffeic acid;  -trans-ferulic acid.
-trans-ferulic acid.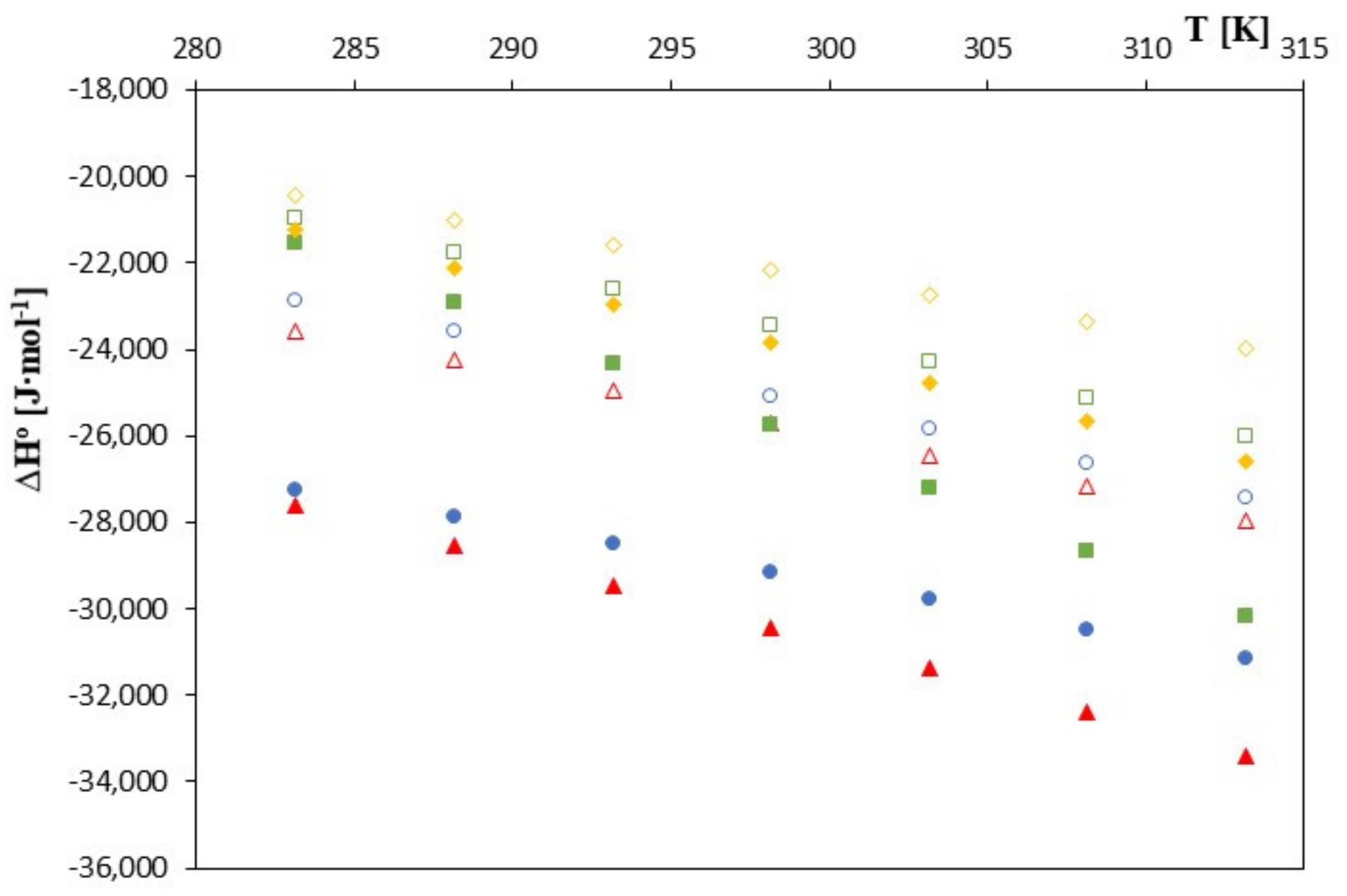
| β-Cyclodextrin | 2-HP-β-Cyclodextrin | |||||||
|---|---|---|---|---|---|---|---|---|
| T [K] | Kf [dm3/mol] | lnKf [dm3/mol] | ΛCDNaDod [S∙cm2/mol−1] | σ(Λ) | Kf [dm3/mol] | lnKf [dm3/mol] | ΛCDNaDod [S∙cm2/mol−1] | σ(Λ) |
| 283.15 | 1235 ± 8 | 7.1188 | 47.56 ± 0.02 | 0.01 | 1145 ± 8 | 7.0432 | 44.75 ± 0.01 | 0.01 |
| 288.15 | 1055 ± 4 | 6.9613 | 57.55 ± 0.01 | 0.01 | 965 ± 6 | 6.8721 | 56.23 ± 0.01 | 0.02 |
| 293.15 | 900 ± 2 | 6.8024 | 68.52 ± 0.01 | 0.02 | 810 ± 4 | 6.6970 | 66.32 ± 0.02 | 0.02 |
| 298.15 | 775 ± 2 | 6.6529 | 83.235 ± 0.02 | 0.02 | 685 ± 3 | 6.5294 | 80.75 ± 0.01 | 0.01 |
| 303.15 | 665 ± 3 | 6.4998 | 93.62 ± 0.01 | 0.01 | 575 ± 3 | 6.3544 | 90.45 ± 0.01 | 0.01 |
| 308.15 | 565 ± 2 | 6.3368 | 105.24 ± 0.01 | 0.01 | 485 ± 2 | 6.1841 | 103.23 ± 0.01 | 0.01 |
| 313.15 | 489 ± 0.9 | 6.1924 | 117.32 ± 0.01 | 0.01 | 409 ± 1 | 6.0137 | 113.15 ± 0.01 | 0.02 |
| β-Cyclodextrin | 2-HP-β-Cyclodextrin | |||||||
|---|---|---|---|---|---|---|---|---|
| T [K] | Kf [dm3/mol] | lnKf [dm3/mol] | ΛCDNaDod [S∙cm2/mol−1] | σ(Λ) | Kf [dm3/mol] | lnKf [dm3/mol] | ΛCDNaDod [S∙cm2/mol−1] | σ(Λ) |
| 283.15 | 1870 ± 7 | 7.5337 | 41.24 ± 0.01 | 0.01 | 1710 ± 6 | 7.4442 | 40.11 ± 0.01 | 0.02 |
| 288.15 | 1570 ± 4 | 7.3588 | 51.73 ± 0.01 | 0.01 | 1400 ± 5 | 7.2442 | 49.52 ± 0.02 | 0.02 |
| 293.15 | 1324 ± 3 | 7.1884 | 58.23 ± 0.02 | 0.02 | 1144 ± 4 | 7.0423 | 56.85 ± 0.01 | 0.02 |
| 298.15 | 1124 ± 3 | 7.0246 | 66.56 ± 0.01 | 0.02 | 944 ± 2 | 6.8501 | 64.78 ± 0.02 | 0.01 |
| 303.15 | 945 ± 2 | 6.8512 | 73.87 ± 0.02 | 0.01 | 765 ± 1 | 6.6399 | 72.54 ± 0.01 | 0.01 |
| 308.15 | 795 ± 1 | 6.6783 | 81.56 ± 0.01 | 0.01 | 635 ± 0.9 | 6.4536 | 80.12 ± 0.01 | 0.01 |
| 313.15 | 675 ± 0.8 | 6.5147 | 87.52 ±0.01 | 0.02 | 525 ± 0.9 | 6.2634 | 86.65 ± 0.02 | 0.02 |
| β-Cyclodextrin | 2-HP-β-Cyclodextrin | |||||||
|---|---|---|---|---|---|---|---|---|
| T [K] | Kf [dm3/mol] | lnKf [dm3/mol] | ΛCDNaDod [S∙cm2/mol−1] | σ(Λ) | Kf [dm3/mol] | lnKf [dm3/mol] | ΛCDNaDod [S∙cm2/mol−1] | σ(Λ) |
| 283.15 | 2092 ± 6 | 7.6459 | 35.12 ± 0.01 | 0.01 | 1962 ± 6 | 7.5817 | 38.05 ± 0.01 | 0.01 |
| 288.15 | 1785 ± 4 | 7.4872 | 45.55 ± 0.01 | 0.01 | 1665 ± 5 | 7.4176 | 43.89 ± 0.01 | 0.02 |
| 293.15 | 1522 ± 4 | 7.3278 | 51.42 ± 0.02 | 0.02 | 1402 ± 3 | 7.2457 | 49.52 ± 0.01 | 0.02 |
| 298.15 | 1305 ± 2 | 7.1740 | 58.74 ± 0.01 | 0.02 | 1185 ± 2 | 7.0775 | 56.32 ± 0.02 | 0.01 |
| 303.15 | 1115 ± 1 | 7.0166 | 65.32 ± 0.01 | 0.01 | 995 ± 1 | 6.9027 | 63.12 ± 0.02 | 0.01 |
| 308.15 | 950 ± 0.9 | 6.8565 | 74.59 ± 0.01 | 0.01 | 830 ± 1 | 6.7214 | 72.56 ± 0.01 | 0.01 |
| 313.15 | 810 ± 0.8 | 6.6970 | 80.45 ± 0.01 | 0.01 | 690 ± 0.9 | 6.5367 | 78.15 ± 0.01 | 0.01 |
| β-Cyclodextrin | 2-HP-β-Cyclodextrin | |||||||
|---|---|---|---|---|---|---|---|---|
| T [K] | Kf [dm3/mol] | lnKf [dm3/mol] | ΛCDNaDod [S∙cm2/mol−1] | σ(Λ) | Kf [dm3/mol] | lnKf [dm3/mol] | ΛCDNaDod [S∙cm2/mol−1] | σ(Λ) |
| 283.15 | 2280 ± 7 | 7.7319 | 37.28 ± 0.01 | 0.02 | 2165 ± 5 | 7.6802 | 36.11 ± 0.01 | 0.02 |
| 288.15 | 1955 ± 6 | 7.5781 | 42.31 ± 0.01 | 0.01 | 1860 ± 5 | 7.5283 | 41.10 ± 0.01 | 0.02 |
| 293.15 | 1680 ± 5 | 7.4265 | 49.47 ± 0.02 | 0.02 | 1575 ± 4 | 7.3620 | 48.11 ± 0.01 | 0.02 |
| 298.15 | 1446 ± 3 | 7.2766 | 54.95 ± 0.01 | 0.02 | 1341 ± 4 | 7.2012 | 53.05 ± 0.01 | 0.01 |
| 303.15 | 1244 ± 2 | 7.1261 | 61.73 ± 0.02 | 0.01 | 1139 ± 3 | 7.0379 | 60.1 ± 0.01 | 0.01 |
| 308.15 | 1076 ± 2 | 6.9810 | 68.41 ± 0.01 | 0.01 | 971 ± 2 | 6.8783 | 67.14 ± 0.01 | 0.01 |
| 313.15 | 925 ± 1 | 6.8298 | 75.95 ± 0.01 | 0.02 | 825 ± 1 | 6.7154 | 73.12 ± 0.02 | 0.01 |
| β-Cyclodextrin | 2-HP-β-Cyclodextrin | |||||
|---|---|---|---|---|---|---|
| T [K] | ∆G0 [J∙mol−1] | ∆S0 [J∙mol−1] | ∆H0 [J∙mol−1] | ∆G0 [J∙mol−1] | ∆S0 [J∙mol−1‧K−1] | ∆H0 [J∙mol−1] |
| 283.15 | −16,758 | −24.036 | −23,565 | −16,580 | −38.888 | −27,592 |
| 288.15 | −16,677 | −26.319 | −24,261 | −16,463 | −41.844 | −28,521 |
| 293.15 | −16,579 | −28.602 | −24,964 | −16,322 | −44.800 | −29,456 |
| 298.15 | −16,491 | −30.885 | −25,700 | −16,185 | −47.756 | −30,424 |
| 303.15 | −16,382 | −33.168 | −26,437 | −16,016 | −50.712 | −31,389 |
| 308.15 | −16,235 | −35.451 | −27,159 | −15,844 | −53.668 | −32,382 |
| 313.15 | −16,122 | −37.734 | −27,939 | −15,657 | −56.624 | −33,389 |
| β-Cyclodextrin | 2-HP-β-Cyclodextrin | |||||
|---|---|---|---|---|---|---|
| T [K] | ∆G0 [J∙mol−1] | ∆S0 [J∙mol−1] | ∆H0 [J∙mol−1] | ∆G0 [J∙mol−1] | ∆S0 [J∙mol−1‧K−1] | ∆H0 [J∙mol−1] |
| 283.15 | −17,735 | −18.180 | −22,883 | −17,525 | −34.296 | −27,235 |
| 288.15 | −17,629 | −20.714 | −23,598 | −17,355 | −36.486 | −27,868 |
| 293.15 | −17,520 | −23.248 | −24,335 | −17,164 | −38.676 | −28,502 |
| 298.15 | −17,413 | −25.782 | −25,100 | −16,980 | −40.866 | −29,164 |
| 303.15 | −17,268 | −28.316 | −25,852 | −16,735 | −43.056 | −29,787 |
| 308.15 | −17,110 | −30.850 | −26,616 | −16,534 | −45.246 | −30,476 |
| 313.15 | −16,961 | −33.384 | −27,416 | −16,307 | −47.436 | −31,161 |
| β-Cyclodextrin | 2-HP-β-Cyclodextrin | |||||
|---|---|---|---|---|---|---|
| T [K] | ∆G0 [J∙mol−1] | ∆S0 [J∙mol−1] | ∆H0 [J∙mol−1] | ∆G0 [J∙mol−1] | ∆S0 [J∙mol−1‧K−1] | ∆H0 [J∙mol−1] |
| 283.15 | −17,999 | −10.534 | −20,982 | −17,848 | −13.040 | −21,540 |
| 288.15 | −17,937 | −13.343 | −21,782 | −17,770 | −17.865 | −22,918 |
| 293.15 | −17,860 | −16.152 | −22,594 | −17,660 | −22.690 | −24,311 |
| 298.15 | −17,783 | −18.961 | −23,436 | −17,544 | −27.515 | −25,747 |
| 303.15 | −17,685 | −21.770 | −24,284 | −17,398 | −32.340 | −27,201 |
| 308.15 | −17,566 | −24.579 | −25,140 | −17,220 | −37.165 | −28,672 |
| 313.15 | −17,436 | −27.388 | −26,012 | −17,018 | −41.990 | −30,168 |
| β-Cyclodextrin | 2-HP-β-Cyclodextrin | |||||
|---|---|---|---|---|---|---|
| T [K] | ∆G0 [J∙mol−1] | ∆S0 [J∙mol−1] | ∆H0 [J∙mol−1] | ∆G0 [J∙mol−1] | ∆S0 [J∙mol−1‧K−1] | ∆H0 [J∙mol−1] |
| 283.15 | −18,202 | −7.9240 | −20,445 | −18,080 | −11.204 | −21,252 |
| 288.15 | −18,155 | −9.8990 | −21,007 | −18,035 | −14.191 | −22,125 |
| 293.15 | −18,101 | −11.874 | −21,581 | −17,943 | −17.178 | −22,979 |
| 298.15 | −18,037 | −13.849 | −22,166 | −17,850 | −20.165 | −23,863 |
| 303.15 | −17,960 | −15.824 | −22,758 | −17,738. | −23.152 | −24,757 |
| 308.15 | −17,885 | −17.799 | −23,370 | −17,622 | −26.139 | −25,677 |
| 313.15 | −17,782 | −19.774 | −23,974 | −17,484 | −29.126 | −26,604 |
| Chemical Name | Chemical Formula | Chemical Formula | Source | CAS No | Mass Fraction Purity |
|---|---|---|---|---|---|
| Trans-ferulic acid | C10H10O4 | 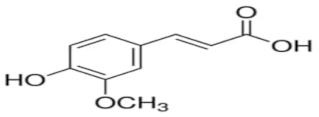 | TCI * | 537-98-4 | ≥0.998 |
| Trans-caffeic acid | C9H8O4 | 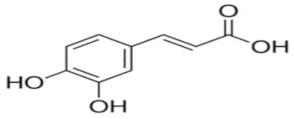 | TCI * | 331-39-5 | ≥0.998 |
| Trans –p-coumaric acid | C9H8O3 | 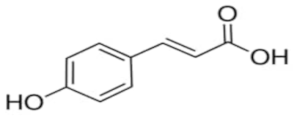 | TCI * | 501-98-4 | ≥0.998 |
| Trans-cinnamic acid | C9H8O2 | 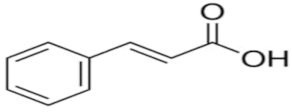 | TCI * | 140-10-3 | ≥0.998 |
| β -cyclodextrin | C42H70O35 |  | TCI * | 7585-39-9 | ≥0.998 |
| 2-HP-β-cyclodextrin Sodium hydroxide micropills | C66H112O42 NaOH | 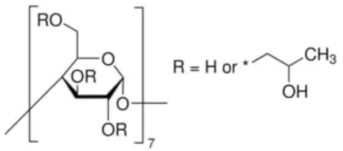 | TCI * Avantor | 128446-35-5 1310-73-2 | ≥0.998 ≥0.998 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kinart, Z. Conductometric Studies of Formation the Inclusion Complexes of Phenolic Acids with β-Cyclodextrin and 2-HP-β-Cyclodextrin in Aqueous Solutions. Molecules 2023, 28, 292. https://doi.org/10.3390/molecules28010292
Kinart Z. Conductometric Studies of Formation the Inclusion Complexes of Phenolic Acids with β-Cyclodextrin and 2-HP-β-Cyclodextrin in Aqueous Solutions. Molecules. 2023; 28(1):292. https://doi.org/10.3390/molecules28010292
Chicago/Turabian StyleKinart, Zdzisław. 2023. "Conductometric Studies of Formation the Inclusion Complexes of Phenolic Acids with β-Cyclodextrin and 2-HP-β-Cyclodextrin in Aqueous Solutions" Molecules 28, no. 1: 292. https://doi.org/10.3390/molecules28010292
APA StyleKinart, Z. (2023). Conductometric Studies of Formation the Inclusion Complexes of Phenolic Acids with β-Cyclodextrin and 2-HP-β-Cyclodextrin in Aqueous Solutions. Molecules, 28(1), 292. https://doi.org/10.3390/molecules28010292