Wet-Chemical Synthesis of TiO2/PVDF Membrane for Energy Applications
Abstract
1. Introduction
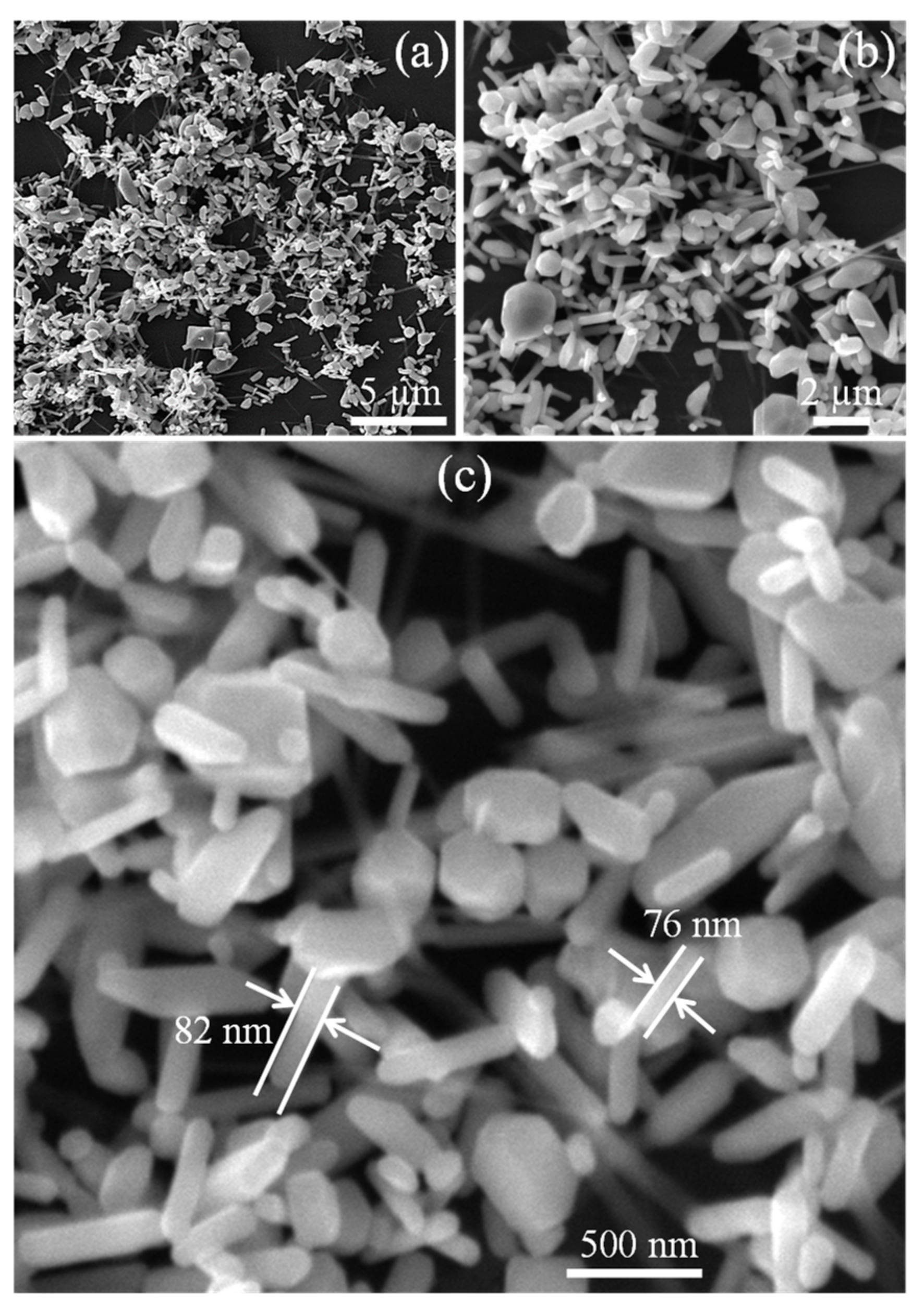
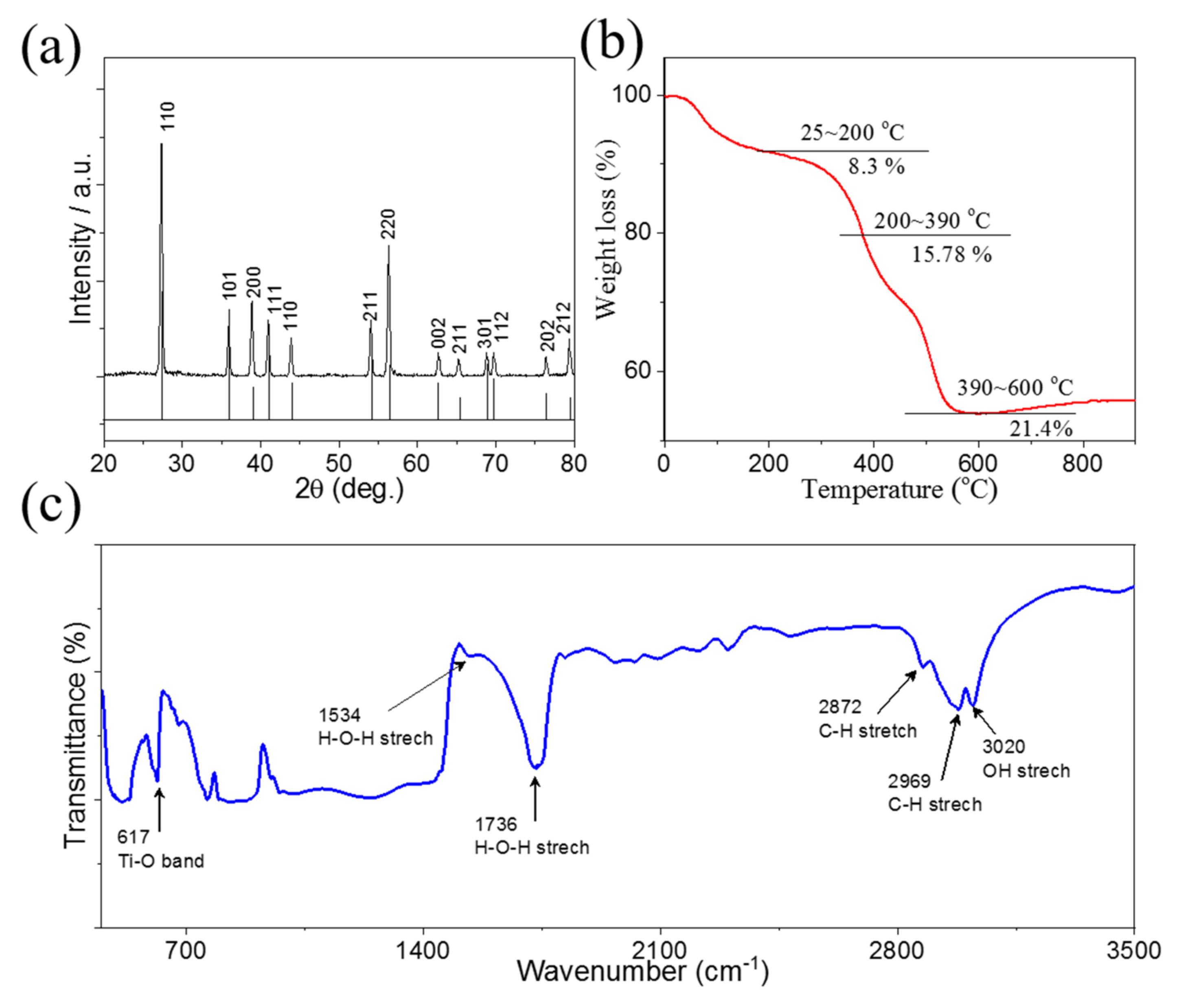
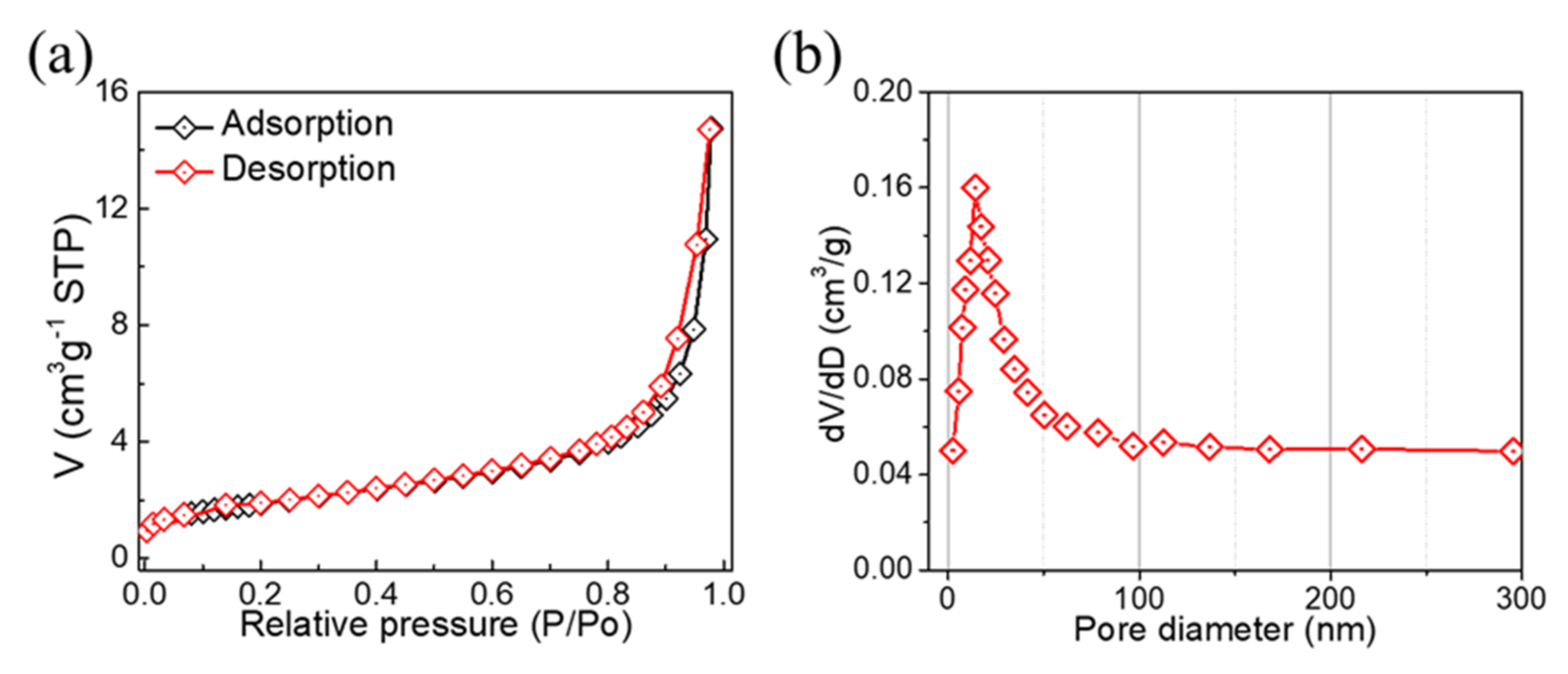
2. Results and Discussion
3. Experimental Section
3.1. Synthesis of TiO2 Nanoplates
3.2. Synthesis of TiO2/PVDF Membrane
3.3. Physical Characterization of TiO2/PVDF Membrane
3.4. Electrochemical Characterization of TiO2/PVDF Membrane
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Russell, J.C.; Posey, V.A.; Gray, J.; May, R.; Reed, D.A.; Zhang, H.; Marbella, L.E.; Steigerwald, M.L.; Yang, Y.; Roy, X.; et al. High-performance organic pseudocapacitors via molecular contortion. Nat. Mater. 2021, 20, 1136–1141. [Google Scholar] [CrossRef] [PubMed]
- He, C.; Bo, T.; Ke, Y.; Wang, B.-T.; Tao, J.; Shen, P.K. Black potassium titanate nanobelts: Ultrafast and durable aqueous redox electrolyte energy storage. J. Power Sources 2021, 483, 229140. [Google Scholar] [CrossRef]
- Javed, M.S.; Khan, A.J.; Ahmad, A.; Siyal, S.H.; Akram, S.; Zhao, G.; Bahajjaj, A.A.A.; Ouladsmane, M.; Alfakeer, M. Design and fabrication of bimetallic oxide nanonest-like structure/carbon cloth composite electrode for supercapacitors. Ceram. Int. 2021, 47, 30747–30755. [Google Scholar] [CrossRef]
- Shao, Y.; El-Kady, M.F.; Sun, J.; Li, Y.; Zhang, Q.; Zhu, M.; Wang, H.; Dunn, B.; Kaner, R.B. Design and Mechanisms of Asymmetric Supercapacitors. Chem. Rev. 2018, 118, 9233–9280. [Google Scholar] [CrossRef] [PubMed]
- Javed, M.S.; Lei, H.; Li, J.; Wang, Z.; Mai, W. Construction of highly dispersed mesoporous bimetallic-sulfide nanoparticles locked in N-doped graphitic carbon nanosheets for high energy density hybrid flexible pseudocapacitors. J. Mater. Chem. A 2019, 7, 17435–17445. [Google Scholar] [CrossRef]
- Liu, L.; Hu, X.; Zeng, H.-Y.; Yi, M.-Y.; Shen, S.-G.; Xu, S.; Cao, X.; Du, J.-Z. Preparation of NiCoFe-hydroxide/polyaniline composite for enhanced-performance supercapacitors. J. Mater. Sci. Technol. 2019, 35, 1691–1699. [Google Scholar]
- Zheng, X.; Li, P.; Zhu, H.; Zhao, G.; Rui, K.; Shu, J.; Xu, X.; Wang, X.; Sun, W.; Dou, S.X. Understanding the structural and chemical evolution of layered potassium titanates for sodium ion batteries. Energy Storage Mater. 2020, 25, 502–509. [Google Scholar] [CrossRef]
- Javed, M.S.; Mateen, A.; Ali, S.; Zhang, X.; Hussain, I.; Imran, M.; Shah, S.S.A.; Han, W.J.S. The Emergence of 2D MXenes Based Zn-Ion Batteries: Recent Development and Prospects. Small 2022, 18, 2201989. [Google Scholar] [CrossRef]
- Javed, M.S.; Khan, A.J.; Asim, S.; Shah, S.S.A.; Najam, T.; Siyalg, S.H.; Tahir, M.F.; Zhao, Z.; Mai, W. Insights to pseudocapacitive charge storage of binary metal-oxide nanobelts decorated activated carbon cloth for highly-flexible hybrid-supercapacitors. J. Energy Storage 2020, 31, 101602. [Google Scholar] [CrossRef]
- Wang, S.; Liang, Y.; Zhuo, W.; Lei, H.; Javed, M.S.; Liu, B.; Wang, Z.; Mai, W. Freestanding polypyrrole/carbon nanotube electrodes with high mass loading for robust flexible supercapacitors. Mater. Chem. Front. 2021, 5, 1324–1329. [Google Scholar]
- Javed, M.S.; Shah, S.S.A.; Najam, T.; Aslam, M.K.; Li, J.; Hussain, S.; Ahmad, M.A.; Ashfaq, M.; Raza, R.; Mai, W. Synthesis of mesoporous defective graphene-nanosheets in a space-confined self-assembled nanoreactor: Highly efficient capacitive energy storage. Electrochim. Acta 2019, 305, 517–527. [Google Scholar] [CrossRef]
- Mateen, A.; Javed, M.S.; Khan, S.; Saleem, A.; Majeed, M.K.; Khan, A.J.; Tahir, M.F.; Ahmad, M.A.; Assiri, M.A.; Peng, K.-Q. Metal-organic framework-derived walnut-like hierarchical Co-O-nanosheets as an advanced binder-free electrode material for flexible supercapacitor. J. Energy Storage 2022, 49, 104150. [Google Scholar] [CrossRef]
- Abbas, Q.; Siyal, S.H.; Mateen, A.; Hassan, N.U.; Idrees, A.; Rehman, Z.U.; Din, E.M.T.E.; Bajaber, M.A.; Javed, M.S.J.M. Hydrothermal Synthesis of Binder-Free Metallic NiCo2O4 Nano-Needles Supported on Carbon Cloth as an Advanced Electrode for Supercapacitor Applications. Materials 2022, 15, 4499. [Google Scholar] [CrossRef]
- Vattikuti, S.V.P.; Devarayapalli, K.C.; Dang, N.N.; Shim, J. 1D/1D Na2Ti3O7/SWCNTs electrode for split-cell-type asymmetric supercapacitor device. Ceram. Int. 2021, 47, 11602–11610. [Google Scholar] [CrossRef]
- Cao, K.; Liu, H.; Li, W.; Xu, C.; Han, Q.; Zhang, Z.; Jiao, L. K2Ti6O13 nanorods for potassium-ion battery anodes. J. Electroanal. Chem. 2019, 841, 51–55. [Google Scholar] [CrossRef]
- Pant, B.; Park, M.; Park, S.-J. TiO2 NPs Assembled into a Carbon Nanofiber Composite Electrode by a One-Step Electrospinning Process for Supercapacitor Applications. Polymers 2019, 11, 899. [Google Scholar] [CrossRef]
- Yu, X.; Lin, D.; Li, P.; Su, Z. Recent advances in the synthesis and energy applications of TiO2-graphene nanohybrids. Sol. Energy Mater. Sol. Cells 2017, 172, 252–269. [Google Scholar] [CrossRef]
- Lee, H.; Jin, S.; Yim, S. Titanium oxide nanoparticle-embedded mesoporous manganese oxide microparticles for supercapacitor electrodes. J. Phys. Chem. Solids 2020, 138, 109264. [Google Scholar] [CrossRef]
- Elmouwahidi, A.; Bailón-García, E.; Castelo-Quibén, J.; Pérez-Cadenas, A.; Maldonado-Hódar, F.; Carrasco-Marín, F. Carbon–TiO2 composites as high-performance supercapacitor electrodes: Synergistic effect between carbon and metal oxide phases. J. Mater. Chem. A 2018, 6, 633–644. [Google Scholar] [CrossRef]
- Zhang, F.; Ma, X.; Cao, C.; Li, J.; Zhu, Y.J. Poly (vinylidene fluoride)/SiO2 composite membranes prepared by electrospinning and their excellent properties for nonwoven separators for lithium-ion batteries. J. Power Sources 2014, 251, 423–431. [Google Scholar] [CrossRef]
- Xu, Y.; Lin, Y.; Lee, M.; Malde, C.; Wang, R. Development of low mass-transfer-resistance fluorinated TiO2-SiO2/PVDF composite hollow fiber membrane used for biogas upgrading in gas-liquid membrane contactor. J. Membr. Sci. 2018, 552, 253–264. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, Y.; Dong, S.; Ye, Z.; Guo, Y. One-step hydrothermal synthesis of a TiO2-Ti3C2Tx nanocomposite with small sized TiO2 nanoparticles. Ceram. Int. 2017, 43, 11065–11070. [Google Scholar] [CrossRef]
- Wang, Y.; Li, L.; Huang, X.; Li, Q.; Li, G. New insights into fluorinated TiO2 (brookite, anatase and rutile) nanoparticles as efficient photocatalytic redox catalysts. RSC Adv. 2015, 5, 34302–34313. [Google Scholar] [CrossRef]
- Li, X.W.; Song, R.G.; Jiang, Y.; Wang, C.; Jiang, D. Surface modification of TiO2 nanoparticles and its effect on the properties of fluoropolymer/TiO2 nanocomposite coatings. Appl. Surf. Sci. 2013, 276, 761–768. [Google Scholar] [CrossRef]
- Gabbott, P. Principles and Applications of Thermal Analysis; John Wiley & Sons: Hoboken, NJ, USA, 2008. [Google Scholar]
- Menczel, J.D.; Prime, R.B. Thermal Analysis of Polymers: Fundamentals and Applications; John Wiley & Sons: Hoboken, NJ, USA, 2009. [Google Scholar]
- Zheng, X.; Liu, Y.; Liu, X.; Li, Q.; Zheng, Y. A novel PVDF-TiO2@g-C3N4 composite electrospun fiber for efficient photocatalytic degradation of tetracycline under visible light irradiation. Ecotoxicol. Environ. Saf. 2021, 210, 111866. [Google Scholar] [CrossRef]
- Sun, F.; Ren, H.-T.; Huang, S.-Y.; Li, T.-T.; Peng, H.-K.; Lin, Q.; Lou, C.-W.; Lin, J.-H. Polyvinylidene Fluoride Electrospun Fibers Loaded TiO2 for Photocatalytic Degradation and Oil/Water Separation. Fibers Polym. 2020, 21, 1475–1487. [Google Scholar] [CrossRef]
- Wang, H.; Ding, K. Effect of Self-Made TiO2 Nanoparticle Size on the Performance of the PVDF Composite Membrane in MBR for Landfill Leachate Treatment. Membranes 2022, 12, 216. [Google Scholar] [CrossRef]
- Javed, M.S.; Shah, S.S.A.; Hussain, S.; Tan, S.; Mai, W. Mesoporous manganese-selenide microflowers with enhanced electrochemical performance as a flexible symmetric 1.8 V supercapacitor. Chem. Eng. J. 2020, 382, 122814. [Google Scholar] [CrossRef]
- Lukatskaya, M.R.; Kota, S.; Lin, Z.; Zhao, M.-Q.; Shpigel, N.; Levi, M.D.; Halim, J.; Taberna, P.-L.; Barsoum, M.W.; Simon, P.; et al. Ultra-high-rate pseudocapacitive energy storage in two-dimensional transition metal carbides. Nat. Energy 2017, 2, 17105. [Google Scholar] [CrossRef]
- Du, H.; Jiao, L.; Wang, Q.; Yang, J.; Guo, L.; Si, Y.; Wang, Y.; Yuan, H. Facile carbonaceous microsphere templated synthesis of Co3O4 hollow spheres and their electrochemical performance in supercapacitors. Nano Res. 2013, 6, 87–98. [Google Scholar] [CrossRef]
- Javed, M.S.; Mateen, A.; Hussain, I.; Ahmad, A.; Mubashir, M.; Khan, S.; Assiri, M.A.; Eldin, S.M.; Shah, S.S.A.; Han, W. Recent progress in the design of advanced MXene/metal oxides-hybrid materials for energy storage devices. Energy Storage Mater. 2022, 53, 827–872. [Google Scholar] [CrossRef]
- Javed, M.S.; Mateen, A.; Hussain, I.; Ali, S.; Asim, S.; Ahmad, A.; Eldin, E.t.; Bajaber, M.A.; Najam, T.; Han, W. The quest for negative electrode materials for Supercapacitors: 2D materials as a promising family. Chem. Eng. J. 2023, 452, 139455. [Google Scholar] [CrossRef]
- Javed, M.S.; Dai, S.; Wang, M.; Guo, D.; Chen, L.; Wang, X.; Hu, C.; Xi, Y. High performance solid state flexible supercapacitor based on molybdenum sulfide hierarchical nanospheres. J. Power Sources 2015, 285, 63–69. [Google Scholar] [CrossRef]
- Lal, M.S.; Lavanya, T.; Ramaprabhu, S. An efficient electrode material for high performance solid-state hybrid supercapacitors based on a Cu/CuO/porous carbon nanofiber/TiO2 hybrid composite. Beilstein J. Nanotechnol. 2019, 10, 781–793. [Google Scholar]
- Zhang, M.; Li, Q.; Fang, D.; Ayhan, I.A.; Zhou, Y.; Dong, L.; Xiong, C.; Wang, Q. NiO hierarchical hollow nanofibers as high-performance supercapacitor electrodes. RSC Adv. 2015, 5, 96205–96212. [Google Scholar] [CrossRef]
- Pazhamalai, P.; Krishnamoorthy, K.; Mariappan, V.K.; Kim, S.-J. Blue TiO2 nanosheets as a high-performance electrode material for supercapacitors. J. Colloid Interface Sci. 2019, 536, 62–70. [Google Scholar] [CrossRef]
- Kumar, R.; Singh, B.K.; Soam, A.; Parida, S.; Sahajwalla, V.; Bhargava, P. In situ carbon-supported titanium dioxide (ICS-TiO2) as an electrode material for high performance supercapacitors. Nanoscale Adv. 2020, 2, 2376–2386. [Google Scholar] [CrossRef]
- Shen, D.; Rao, A.M.; Zhou, J.; Lu, B. High-Potential Cathodes with Nitrogen Active Centres for Quasi-Solid Proton-Ion Batteries. Angew. Chem. 2022, 134, e202201972. [Google Scholar] [CrossRef]
- Tanwar, S.; Arya, A.; Singh, N.; Yadav, B.C.; Kumar, V.; Rai, A.; Sharma, A.L. High efficient carbon coated TiO2 electrode for ultra-capacitor applications. J. Phys. D Appl. Phys. 2021, 55, 055501. [Google Scholar]
- Tolba, G.; Motlak, M.; Bastaweesy, A.; Ashour, E.; Abdelmoez, W.; El-Newehy, M.; Barakat, N. Synthesis of novel Fe-doped amorphous TiO2/C nanofibers for supercapacitors applications. Int. J. Electrochem. Sci. 2015, 10, 3117–3123. [Google Scholar]
- Sun, X.; Xie, M.; Wang, G.; Sun, H.; Cavanagh, A.S.; Travis, J.J.; George, S.M.; Lian, J. Atomic Layer Deposition of TiO2 on Graphene for Supercapacitors. J. Electrochem. Soc. 2012, 159, A364–A369. [Google Scholar]
- Sharavath, V.; Sarkar, S.; Ghosh, S. One-pot hydrothermal synthesis of TiO2/graphene nanocomposite with simultaneous nitrogen-doping for energy storage application. J. Electroanal. Chem. 2018, 829, 208–216. [Google Scholar] [CrossRef]
- Jiang, L.; Ren, Z.; Chen, S.; Zhang, Q.; Lu, X.; Zhang, H.; Wan, G. Bio-derived three-dimensional hierarchical carbon-graphene-TiO2 as electrode for supercapacitors. Sci. Rep. 2018, 8, 4412. [Google Scholar] [CrossRef] [PubMed]
- Selvakumar, M.; Bhat, D.K. Microwave synthesized nanostructured TiO2-activated carbon composite electrodes for supercapacitor. Appl. Surf. Sci. 2012, 263, 236–241. [Google Scholar] [CrossRef]
- Hsieh, C.-T.; Chang, C.-C.; Chen, W.-Y.; Hung, W.-M. Electrochemical capacitance from carbon nanotubes decorated with titanium dioxide nanoparticles in acid electrolyte. J. Phys. Chem. Solids 2009, 70, 916–921. [Google Scholar] [CrossRef]
- Ramadoss, A.; Kim, G.-S.; Kim, S.J. Fabrication of reduced graphene oxide/TiO2 nanorod/reduced graphene oxide hybrid nanostructures as electrode materials for supercapacitor applications. CrystEngComm 2013, 15, 10222–10229. [Google Scholar] [CrossRef]



| Sr. No. | Electrode Material | Electrolyte | Specific Capacitance (GCD/CV) | No. of Cycles (n) | Capacitance Retention (%) | Ref. |
|---|---|---|---|---|---|---|
| 1 | TiO2/PVDF membrane | KOH | 283.74 F/g (1 A/g) | 8000 | 91 | This work |
| 2 | CB)/TiO2 | H2SO4 | 236 F/g (10 mV/s) | 10,000 | 90.3 | [41] |
| 3 | Fe-TiO2/C nanofibers | KOH | 137 F/g (5 mV/s) | - | - | [42] |
| 4 | TiO2-graphene composites | KOH | 84 F/g (10 mV/s) | 1000 | 87.5 | [43] |
| 5 | N-TiO2/NG | Na2SO4 | 205.1 F/g (1 mV/s) | 5000 | 78.8 | [44] |
| 6 | BC-G-TiO2 | H2SO4 | 250.8 F/g (2 A/g) | 100 | 84.4 | [45] |
| 7 | TiO2-activated carbon | Na2SO4 | 92 F/g (5 mV/s) | 5000 | 89.9 | [46] |
| 8 | TiO2-CNT | H2SO4 | 110 F/g (0.05 mA/cm2) | - | - | [47] |
| 9 | rGO/TiO2 NR/rGO | Na2SO4 | 114.5 F/g (5 mV/s) | 4000 | 85 | [48] |
| 10 | TiO2-carbon NFs | KOH | 106.57 F/g (1A/g) | 2000 | 84 | [16] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saleem, M.; Albaqami, M.D.; Bahajjaj, A.A.A.; Ahmed, F.; Din, E.; Arifeen, W.U.; Ali, S. Wet-Chemical Synthesis of TiO2/PVDF Membrane for Energy Applications. Molecules 2023, 28, 285. https://doi.org/10.3390/molecules28010285
Saleem M, Albaqami MD, Bahajjaj AAA, Ahmed F, Din E, Arifeen WU, Ali S. Wet-Chemical Synthesis of TiO2/PVDF Membrane for Energy Applications. Molecules. 2023; 28(1):285. https://doi.org/10.3390/molecules28010285
Chicago/Turabian StyleSaleem, Muhammad, Munirah D. Albaqami, Aboud Ahmed Awadh Bahajjaj, Fahim Ahmed, ElSayed Din, Waqas Ul Arifeen, and Shafaqat Ali. 2023. "Wet-Chemical Synthesis of TiO2/PVDF Membrane for Energy Applications" Molecules 28, no. 1: 285. https://doi.org/10.3390/molecules28010285
APA StyleSaleem, M., Albaqami, M. D., Bahajjaj, A. A. A., Ahmed, F., Din, E., Arifeen, W. U., & Ali, S. (2023). Wet-Chemical Synthesis of TiO2/PVDF Membrane for Energy Applications. Molecules, 28(1), 285. https://doi.org/10.3390/molecules28010285






