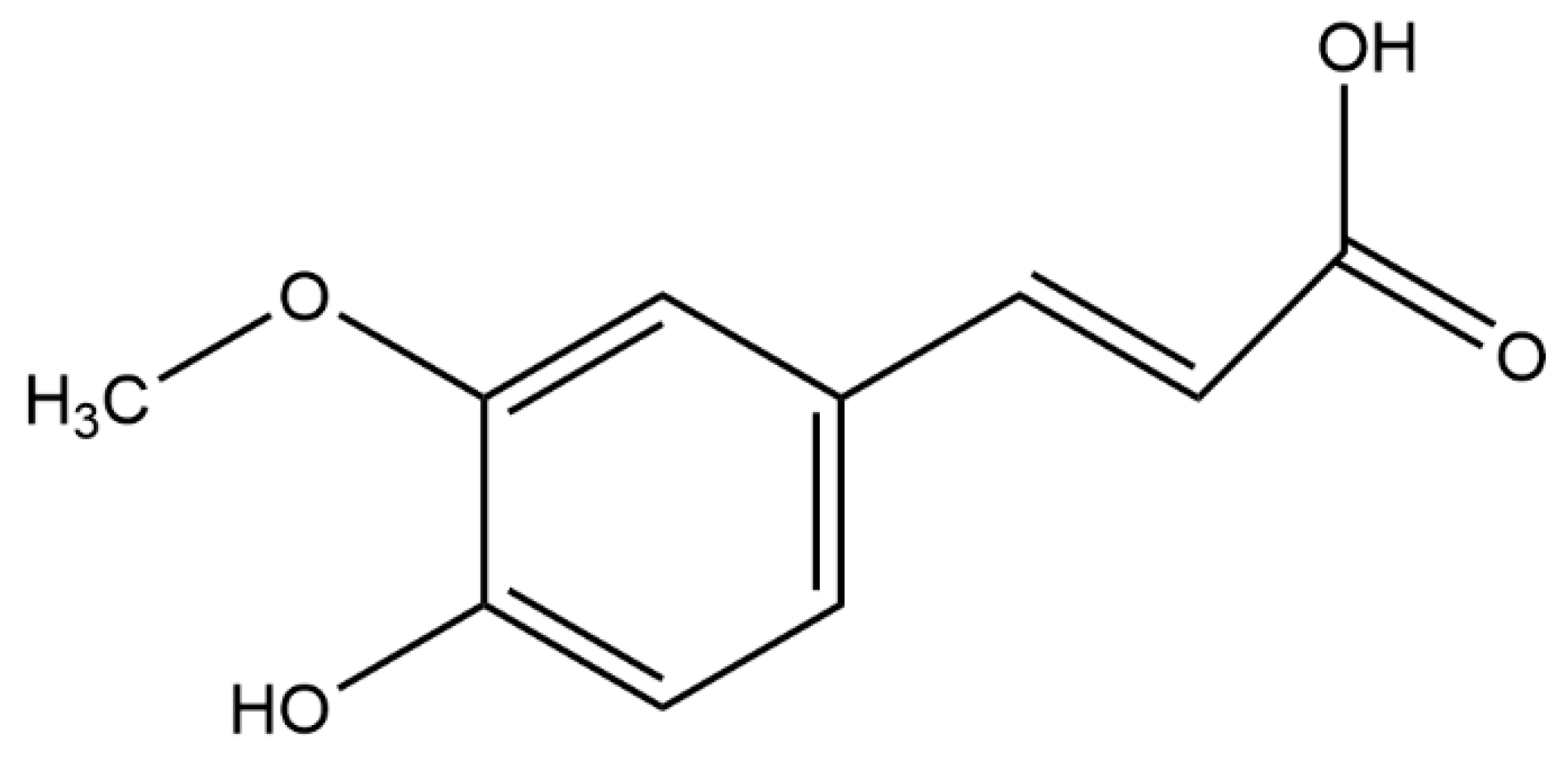
Protective Effects of Ferulic Acid on Metabolic Syndrome: A Comprehensive Review
Abstract
1. Introduction
2. Literature Search
3. Ferulic Acid and Diabetes
3.1. The Animal Studies
3.2. The Human Studies
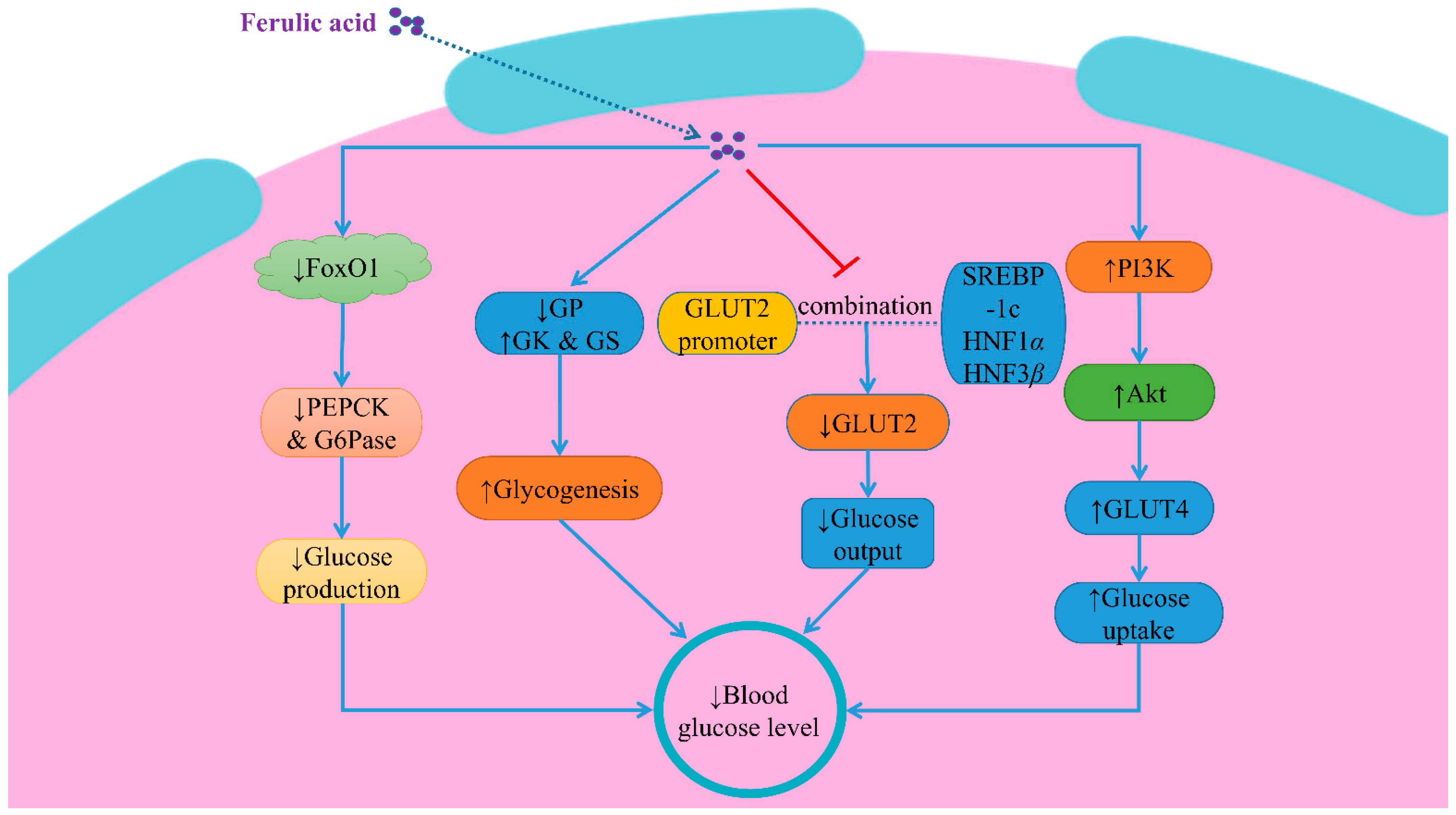
3.3. Possible Mechanisms
3.3.1. Inhibition of Expressions of Gluconeogenic Enzymes
3.3.2. Inhibition of GLUT2 Expression
3.3.3. Improvement of PI3K/Akt/GLUT4 Signaling Pathway
3.3.4. Others
4. Ferulic Acid and Hypertension
4.1. The Animal Studies
4.2. Possible Mechanisms
4.2.1. Improvement of Endothelial Function
4.2.2. Renin-Angiotensin-Aldosterone System
5. Ferulic Acid and Hyperlipidemia
5.1. The Animal Studies
5.2. The Human Studies
5.3. Possible Mechanisms
6. Ferulic Acid and Obesity
6.1. The Animal Studies
6.2. The In Vitro Studies
6.3. Possible Mechanisms
6.3.1. MAPK and MEK/ERK1/2 Signaling Pathways
6.3.2. AMPK Signaling Pathways
6.3.3. Inhibition of PPAR γ and C/EBP α/β Expression
6.3.4. Others
| Effects of FA | Experimental Models | Dose/Concentration of FA | Course of Treatment | Results | Mechanisms | References |
|---|---|---|---|---|---|---|
| Anti-hyperglycemia | HFD-induced obese mice | 25 and 50 mg/kg | 8 weeks | ↓ blood glucose level ↓ insulin resistance ↑ the serum adiponectin level | ↓ gluconeogenic genes | [36] |
| Isolated psoas muscle tissues of rat/α-glucosidase, α-amylase (in vitro) | 15, 30, 60, 120 and 240 μg/mL | 2 h | ↓ blood glucose level | ↑ muscle glucose uptake ↓ carbohydrate enzyme activities | [41] | |
| High fat and fructose-induced T2D rat | 50 mg/kg | 30 days | ↓ blood glucose and serum insulin levels ↑ glucose tolerance and insulin tolerance | ↓ gluconeogenesis ↓ negative regulators of insulin signaling ↑ hepatic glycogenesis | [59] | |
| HFD-induced obese male C57BL/6N mice | High-fat diet supplemented with 0.5% FA | 7 weeks | ↓ blood glucose level | ↓ gluconeogenesis ↑ glucokinase activity ↑ insulin secretion | [60] | |
| HFD-induced obese C57BL/6 mice | 10 mg/kg | 12 weeks | ↓ blood glucose level | Phosphorylation and inactivation of FoxO1 | [62] | |
| HFD and high fructose water-induced diabetic Wistar rats (in vitro) | 50 mg/kg | 30 days | ↓ hepatic GLUT2 expression | Impairing the interaction between these transcription factors (SREBP1c, HNF1α and HNF3β) and GLUT2 gene promoter. | [63] | |
| STZ-induced diabetic Wistar rats (in vitro and vivo) | 50 mg/kg | 8 weeks | ↓ blood glucose level ↑ plasma insulin level | ↑ phosphorylation of PI3K, Akt, AMPK | [66] | |
| Differentiated L6 myotubes (in vitro) | 25 μM | 3 h | ↑ uptake of 2-deoxyglucose | Regulation of P13K-dependent pathway | [68] | |
| 3T3-L1 adipocytes (in vitro) | 25 μM | 24 h | ↑ uptake of 2-deoxyglucose | ↑ PI3K expression | [69] | |
| Alloxan-induced diabetic mice | 10 mg/kg | 15 days | ↓ basic biochemical marker (glucose, urea and uric acid, etc.) | ↓ the proinflammatory factor, NF-κB | [72] | |
| HFD-gestational diabetic rats | 20 mg/kg | 12 weeks | ↓ β-cells apoptosis Improvement of insulin signaling | ↑ the expression of p-IRS1, p-IRS2, p-PI3K, GLUT1, GLUT3 and GLUT4 ↑ protein expression of visfatin | [76] | |
| Human amylin peptide (in vitro) | 10 μM and 40 μM | 6 h | ↓ β-cells apoptosis ↑ β-cells mass | ↓ islet amyloid cytotoxicity to β-cells | [77] | |
| Anti-hypertension and anti-hyperlipidemia | Thoracic aortic rings from male WKY rats and SHR (in vitro) | 10−5 to 10−3 mol/L | 30 min | ↑ endothelial function | ↑ bioavailability of basal and stimulated NO | [84] |
| 2K1C hypertensive rats | 10−5 to 10−3 mol/L | 30 min | ↑ endothelial function | ↑ bioavailability of NO | [87] | |
| Stroke-prone spontaneously hypertensive rats | 9.5 mg/kg | 6 h | ↓ blood pressure | ↓ ACE activity in the plasma | [98] | |
| Diet-induced hypercholesterolemia rats | high-cholesterol diet supplemented with 0.013% FA | 5 weeks | ↓ the plasma TG and TC concentrations | ↓ HMG-Co A reductase | [117] | |
| Diet-induced hypercholesterolemia weaned piglets | diet supplemented with 0.05% and 0.45% FA | 5 weeks | ↑ lipid metabolism | ↑ lipolysis and fatty acid oxidation | [118] | |
| Oleic-acid-treated HepG2 cells (in vitro) | 0, 12.5, 25 and 50 μg/ml | 24 h | ↓ cellular lipid accumulation | ↓ ERK1/2, JNK1/2/3 and HGMB1 expression | [119] | |
| Diet-induced hypercholesterolemia mice | 0.5%FA diet | 7 weeks | ↓ plasma and hepatic TC and TG concentrations ↓ lipid peroxidation rate ↓ high-density lipoprotein cholesterol level | ↑ fecal lipid excretion Regulation of lipogenic enzymes activities | [120] | |
| Eight-week-old male db/db diabetic mice | 25, 50 and 100 mg/kg | 7 days | ↑ lipid metabolism | Trigger of the mitochondrial membrane distribution of ACSL 1 | [121] | |
| HFD-induced ApoE−/− mice | 40 mg/kg | 12 weeks | ↑ lipid metabolism | ↑ AMPK α phosphorylation ↓ SREBP 1 and ACC 1 expression | [122] | |
| Anti-obesity | 3T3-L1 adipocytes (in vitro) | 10 μM | 24 h | ↑ release of glycerol content ↓ lipogenic activities | ↓ PPAR γ, C/EBP α and FAS expression ↑ lipolysis-related factors | [123] |
| 3T3-L1 adipocytes (in vitro)/HFD-induced obese mice | 0.2–2 mM/25 and 50 mg/kg | 10 days/90 days | ↓ cellular lipid accumulation ↓ adipogenesis and lipid accumulation ↓ body weight gain | ↓ key transcriptional factors expression ↑ p38MAPK and ERK1/2 signaling pathways Activation of pAMP-α to upregulate HSL | [143] | |
| Embryo stem cells (ESCs) and adipose-derived mesenchymal stem cells (ADMSCs) (in vitro) | Diet with ferulic acid (5 g/kg diet) | 8 weeks | ↑ body weight loss ↑ glucose homeostasis, lipid profiling and hepatic steatosis | ↑ ADMSCs self-renewal | [149] | |
| 3T3-L1 adipocytes (in vitro) | 25, 50 and 100 μM | 8 days | ↓ adipogenesis | ↑ HO-1 expression | [150] |
7. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Samson, S.L.; Garber, A.J. Metabolic syndrome. Endocrin. Metab. Clin. 2014, 43, 1–23. [Google Scholar] [CrossRef] [PubMed]
- Huang, P.L. A comprehensive definition for metabolic syndrome. Dis. Model. Mech. 2009, 2, 231–237. [Google Scholar] [CrossRef] [PubMed]
- O’Neill, S.; O’Driscoll, L. Metabolic syndrome: A closer look at the growing epidemic and its associated pathologies. Obes. Rev. 2015, 16, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Malarvizhi, R.; Mani, S.; Sali, V.K.; Bhardwaj, M.; Vasanthi, H.R. Macrotyloma uniflorum a plant food alleviates the metabolic syndrome through modulation of adipokines and ppars. J. Food Biochem. 2021, 45, e13595. [Google Scholar] [CrossRef]
- Prasun, P. Mitochondrial dysfunction in metabolic syndrome. Biochim. Biophys. Acta Mol. Basis Dis. 2020, 1866, 165838. [Google Scholar] [CrossRef]
- Shewry, P.R.; Ward, J.L. Exploiting genetic variation to improve wheat composition for the prevention of chronic diseases. Food Energy Secur. 2012, 1, 47–60. [Google Scholar] [CrossRef]
- Roche, A.; Ross, E.; Walsh, N.; O’Donnell, K.; Williams, A.; Klapp, M.; Fullard, N.; Edelstein, S. Representative literature on the phytonutrients category: Phenolic acids. Crit. Rev. Food Sci. Nutr. 2017, 57, 1089–1096. [Google Scholar] [CrossRef]
- Heleno, S.A.; Martins, A.; Queiroz, M.J.R.P.; Ferreira, I.C.F.R. Bioactivity of phenolic acids: Metabolites versus parent compounds: A review. Food Chem. 2015, 173, 501–513. [Google Scholar] [CrossRef]
- Ibitoye, O.B.; Ajiboye, T.O. Dietary phenolic acids reverse insulin resistance, hyperglycaemia, dyslipidaemia, inflammation and oxidative stress in high-fructose diet-induced metabolic syndrome rats. Arch. Physiol. Biochem. 2018, 124, 410–417. [Google Scholar] [CrossRef]
- Guo, X.; Wang, O.; Wang, Y.; Wang, K.; Ji, B.; Zhou, F. Phenolic acids alleviate high-fat and high-fructose diet-induced metabolic disorders in rats. J. Food Biochem. 2017, 41, e12419. [Google Scholar] [CrossRef]
- Adisakwattana, S. Cinnamic acid and its derivatives: Mechanisms for prevention and management of diabetes and its complications. Nutrients 2017, 9, 163. [Google Scholar] [CrossRef] [PubMed]
- Srinivasan, M.; Sudheer, A.R.; Menon, V.P. Ferulic acid: Therapeutic potential through its antioxidant property. J. Clin. Biochem. Nutr. 2007, 40, 92–100. [Google Scholar] [CrossRef]
- Boz, H. Ferulic acid in cereals—A review. Czech J. Food Sci. 2016, 33, 1–7. [Google Scholar] [CrossRef]
- Zhang, X.; Han, B.; Feng, Z.; Yang, Y.; Jiang, J.; Zhang, P. Ferulic acid derivatives from ligusticum chuanxiong. Fitoterapia 2018, 125, 147–154. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Tang, Y.; Liu, X.; Zhu, M.; Tao, W.; Li, W.; Duan, J. Effects of ferulic acid on antioxidant activity in angelicae sinensis radix, chuanxiong rhizoma, and their combination. Chin. J. Nat. Med. 2015, 13, 401–408. [Google Scholar] [CrossRef] [PubMed]
- Zheng, K.Y.Z.; Zhang, Z.X.; Du, C.Y.Q.; Zhang, W.L.; Bi, C.W.C.; Choi, R.C.Y.; Dong, T.T.X.; Tsim, K.W.K. Ferulic acid enhances the chemical and biological properties of astragali radix: A stimulator for danggui buxue tang, an ancient chinese herbal decoction. Planta Med. 2014, 80, 159–164. [Google Scholar] [CrossRef]
- Al-Mutairi, A.; Rahman, A.; Rao, M.S. Low doses of thymoquinone and ferulic acid in combination effectively inhibit proliferation of cultured mda-mb 231 breast adenocarcinoma cells. Nutr. Cancer 2021, 73, 282–289. [Google Scholar] [CrossRef]
- Wang, J.; Yuan, Z.; Zhao, H.; Ju, D.; Chen, Y.; Chen, X.; Zhang, J. Ferulic acid promotes endothelial cells proliferation through up-regulating cyclin d1 and vegf. J. Ethnopharmacol. 2011, 137, 992–997. [Google Scholar] [CrossRef]
- Lampiasi, N.; Montana, G. An in vitro inflammation model to study the nrf2 and nf-κb crosstalk in presence of ferulic acid as modulator. Immunobiology 2018, 223, 349–355. [Google Scholar] [CrossRef]
- Xu, L.; Wu, J.; Xu, F.; Chu, C.; Li, X.; Shi, X.; Zheng, W.; Wang, Z.; Jia, Y.; Xiao, W. Use of ferulic acid in the management of diabetes mellitus and its complications. Molecules 2022, 27, 6010. [Google Scholar] [CrossRef]
- Baek, S.E.; Kim, J.Y.; Song, W.T.; Lee, S.H.; Hong, J.H.; Lee, C.K.; Kang, S.G. Neuroprotective effect of rice bran extract supplemented with ferulic acid in the rat model of ischemic brain injury. Anim. Cells Syst. 2014, 18, 93–100. [Google Scholar] [CrossRef][Green Version]
- Chen, S.; Lin, Y.; Miao, L.; Pan, W.; Jiang, W.; Qian, L.; Hao, J.; Xi, B.; Liu, B.; Ge, X. Ferulic acid alleviates lipopolysaccharide-induced acute liver injury in megalobrama amblycephala. Aquaculture 2021, 532, 735972. [Google Scholar] [CrossRef]
- Wu, X.; Lin, L.; Wu, H. Ferulic acid alleviates lipopolysaccharide-induced acute lung injury through inhibiting tlr4/nf-κb signaling pathway. J. Biochem. Mol. Toxic. 2021, 35, e22664. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.; Jiang, J.; Lu, W. Ferulic acid exerts anti-angiogenic and anti-tumor activity by targeting fibroblast growth factor receptor 1-mediated angiogenesis. Int. J. Mol. Sci. 2015, 16, 24011–24031. [Google Scholar] [CrossRef] [PubMed]
- Senaphan, K.; Kukongviriyapan, U.; Sangartit, W.; Pakdeechote, P.; Pannangpetch, P.; Prachaney, P.; Greenwald, S.; Kukongviriyapan, V. Ferulic acid alleviates changes in a rat model of metabolic syndrome induced by high-carbohydrate, high-fat diet. Nutrients 2015, 7, 6446–6464. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wang, T.; Hu, X.; Chen, G. Vitamin a and diabetes. J. Med. Food 2021, 24, 775–785. [Google Scholar] [CrossRef]
- Salin Raj, P.; Swapna, S.U.S.; Raghu, K.G. High glucose induced calcium overload via impairment of serca/pln pathway and mitochondrial dysfunction leads to oxidative stress in h9c2 cells and amelioration with ferulic acid. Fund. Clin. Pharmacol. 2019, 33, 412–425. [Google Scholar] [CrossRef]
- Elhessy, H.M.; Eltahry, H.; Erfan, O.S.; Mahdi, M.R.; Hazem, N.M.; El-Shahat, M.A. Evaluation of the modulation of nitric oxide synthase expression in the cerebellum of diabetic albino rats and the possible protective effect of ferulic acid. Acta Histochem. 2020, 122, 151633. [Google Scholar] [CrossRef]
- Ghosh, S.; Chowdhury, S.; Sarkar, P.; Sil, P.C. Ameliorative role of ferulic acid against diabetes associated oxidative stress induced spleen damage. Food Chem. Toxicol. 2018, 118, 272–286. [Google Scholar] [CrossRef]
- Reis, J.S.; Veloso, C.A.; Mattos, R.T.; Purish, S.; Nogueira-Machado, J.A. Oxidative stress: A review on metabolic signaling in type 1 diabetes. Arq. Bras. Endocrinol. Metabol. 2008, 52, 1096–1105. [Google Scholar] [CrossRef]
- West, I.C. Radicals and oxidative stress in diabetes. Diabet. Med. 2000, 17, 171–180. [Google Scholar] [CrossRef] [PubMed]
- Vinayagam, R.; Jayachandran, M.; Xu, B. Antidiabetic effects of simple phenolic acids: A comprehensive review. Phytother. Res. 2016, 30, 184–199. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Rui, Y.; Guo, S.; Luan, F.; Liu, R.; Zeng, N. Ferulic acid: A review of its pharmacology, pharmacokinetics and derivatives. Life Sci. 2021, 284, 119921. [Google Scholar] [CrossRef]
- Ohnishi, M.; Matuo, T.; Tsuno, T.; Hosoda, A.; Nomura, E.; Taniguchi, H.; Sasaki, H.; Morishita, H. Antioxidant activity and hypoglycemic effect of ferulic acid in stz-induced diabetic mice and kk-ay mice. Biofactors 2004, 21, 315–319. [Google Scholar] [CrossRef]
- Roy, S.; Metya, S.K.; Sannigrahi, S.; Rahaman, N.; Ahmed, F. Treatment with ferulic acid to rats with streptozotocin-induced diabetes: Effects on oxidative stress, pro-inflammatory cytokines, and apoptosis in the pancreatic β cell. Endocrine 2013, 44, 369–379. [Google Scholar] [CrossRef]
- Naowaboot, J.; Piyabhan, P.; Munkong, N.; Parklak, W.; Pannangpetch, P. Ferulic acid improves lipid and glucose homeostasis in high-fat diet-induced obese mice. Clin. Exp. Pharmacol. Physiol. 2016, 43, 242–250. [Google Scholar] [CrossRef] [PubMed]
- Jung, E.H.; Ran Kim, S.; Hwang, I.K.; Youl Ha, T. Hypoglycemic effects of a phenolic acid fraction of rice bran and ferulic acid in c57bl/ksj-db/db mice. J. Agric. Food Chem. 2007, 55, 9800–9804. [Google Scholar] [CrossRef]
- Song, Y.; Wu, T.; Yang, Q.; Chen, X.; Wang, M.; Wang, Y.; Peng, X.; Ou, S. Ferulic acid alleviates the symptoms of diabetes in obese rats. J. Funct. Foods 2014, 9, 141–147. [Google Scholar] [CrossRef]
- Huang, J.; Wang, X.; Tao, G.; Song, Y.; Ho, C.; Zheng, J.; Ou, S. Feruloylated oligosaccharides from maize bran alleviate the symptoms of diabetes in streptozotocin-induced type 2 diabetic rats. Food Funct. 2018, 9, 1779–1789. [Google Scholar] [CrossRef] [PubMed]
- Matowane, G.R.; Ramorobi, L.M.; Mashele, S.S.; Bonnet, S.L.; Noreljaleel, A.E.M.; Swain, S.S.; Makhafola, T.J.; Chukwuma, C.I. Complexation potentiated promising anti-diabetic and anti-oxidative synergism between zn(ii) and ferulic acid: A multimode study. Diabet. Med. 2022, 39, e14905. [Google Scholar] [CrossRef]
- Salau, V.F.; Erukainure, O.L.; Koorbanally, N.A.; Islam, M.S. Ferulic acid promotes muscle glucose uptake and modulate dysregulated redox balance and metabolic pathways in ferric-induced pancreatic oxidative injury. J. Food Biochem. 2022, 46, e13641. [Google Scholar] [CrossRef] [PubMed]
- Gogoi, B.; Chatterjee, P.; Mukherjee, S.; Buragohain, A.K.; Bhattacharya, S.; Dasgupta, S. A polyphenol rescues lipid induced insulin resistance in skeletal muscle cells and adipocytes. Biochem. Biophys. Res. Commun. 2014, 452, 382–388. [Google Scholar] [CrossRef] [PubMed]
- Naowaboot, J.; Piyabhan, P.; Tingpej, P.; Munkong, N.; Parklak, W.; Pannangpetch, P. Anti-insulin resistant effect of ferulic acid on high fat diet-induced obese mice. Asian Pac. J. Trop. Biomed. 2018, 8, 604. [Google Scholar] [CrossRef]
- Kang, B.B.; Chiang, B.H. A novel phenolic formulation for treating hepatic and peripheral insulin resistance by regulating glut4-mediated glucose uptake. J. Tradit. Complement. Med. 2022, 12, 195–205. [Google Scholar] [CrossRef] [PubMed]
- Nankar, R.; Prabhakar, P.K.; Doble, M. Hybrid drug combination: Combination of ferulic acid and metformin as anti-diabetic therapy. Phytomedicine 2017, 37, 10–13. [Google Scholar] [CrossRef]
- Prabhakar, P.K.; Prasad, R.; Ali, S.; Doble, M. Synergistic interaction of ferulic acid with commercial hypoglycemic drugs in streptozotocin induced diabetic rats. Phytomedicine 2013, 20, 488–494. [Google Scholar] [CrossRef]
- Salau, V.F.; Erukainure, O.L.; Olofinsan, K.A.; Msomi, N.Z.; Ijomone, O.K.; Islam, M.S. Ferulic acid mitigates diabetic cardiomyopathy via modulation of metabolic abnormalities in cardiac tissues of diabetic rats. Fund. Clin. Pharmacol. 2022. [Google Scholar] [CrossRef]
- Dhaliwal, J.; Dhaliwal, N.; Akhtar, A.; Kuhad, A.; Chopra, K. Beneficial effects of ferulic acid alone and in combination with insulin in streptozotocin induced diabetic neuropathy in sprague dawley rats. Life Sci. 2020, 255, 117856. [Google Scholar] [CrossRef]
- Park, S.; Moon, N.R.; Kang, S.; Kim, D.S. Ferulic acid and vinpocetine intake improves memory function by enhancing insulin sensitivity and reducing neuroinflammation and oxidative stress in type 2 diabetic animals with induced alzheimer’s disease. J. Funct. Foods 2022, 95, 105180. [Google Scholar] [CrossRef]
- Anand, S.; Pandey, P.; Begum, M.Y.; Chidambaram, K.; Arya, D.K.; Gupta, R.K.; Sankhwar, R.; Jaiswal, S.; Thakur, S.; Rajinikanth, P.S. Electrospun biomimetic multifunctional nanofibers loaded with ferulic acid for enhanced antimicrobial and wound-healing activities in stz-induced diabetic rats. Pharmaceuticals 2022, 15, 302. [Google Scholar] [CrossRef]
- Bairagi, U.; Mittal, P.; Singh, J.; Mishra, B. Preparation, characterization, and in vivo evaluation of nano formulations of ferulic acid in diabetic wound healing. Drug Dev. Ind. Pharm. 2018, 44, 1783–1796. [Google Scholar] [CrossRef] [PubMed]
- Yin, Y.; Qi, F.; Song, Z.; Zhang, B.; Teng, J. Ferulic acid combined with astragaloside iv protects against vascular endothelial dysfunction in diabetic rats. Biosci. Trends 2014, 8, 217–226. [Google Scholar] [CrossRef] [PubMed]
- Choi, R.; Kim, B.H.; Naowaboot, J.; Lee, M.Y.; Hyun, M.R.; Cho, E.J.; Lee, E.S.; Lee, E.Y.; Yang, Y.C.; Chung, C.H. Effects of ferulic acid on diabetic nephropathy in a rat model of type 2 diabetes. Exp. Mol. Med. 2011, 43, 676–683. [Google Scholar] [CrossRef] [PubMed]
- Ma, R.; He, Y.; Fang, Q.; Xie, G.; Qi, M. Ferulic acid ameliorates renal injury via improving autophagy to inhibit inflammation in diabetic nephropathy mice. Biomed. Pharmacother. 2022, 153, 113424. [Google Scholar] [CrossRef]
- Zhu, D.; Zou, W.; Cao, X.; Xu, W.; Lu, Z.; Zhu, Y.; Hu, X.; Hu, J.; Zhu, Q. Ferulic acid attenuates high glucose-induced apoptosis in retinal pigment epithelium cells and protects retina in db/db mice. PeerJ 2022, 10, e13375. [Google Scholar] [CrossRef]
- Sompong, W.; Cheng, H.; Adisakwattana, S. Protective effects of ferulic acid on high glucose-induced protein glycation, lipid peroxidation, and membrane ion pump activity in human erythrocytes. PLoS ONE 2015, 10, e129495. [Google Scholar] [CrossRef]
- Costabile, G.; Vitale, M.; Della Pepa, G.; Cipriano, P.; Vetrani, C.; Testa, R.; Mena, P.; Bresciani, L.; Tassotti, M.; Calani, L.; et al. A wheat aleurone-rich diet improves oxidative stress but does not influence glucose metabolism in overweight/obese individuals: Results from a randomized controlled trial. Nutr. Metab. Cardiovasc. Dis. 2022, 32, 715–726. [Google Scholar] [CrossRef]
- Cao, R.; Cronk, Z.X.; Zha, W.; Sun, L.; Wang, X.; Fang, Y.; Studer, E.; Zhou, H.; Pandak, W.M.; Dent, P.; et al. Bile acids regulate hepatic gluconeogenic genes and farnesoid x receptor via gαi-protein-coupled receptors and the akt pathway. J. Lipid Res. 2010, 51, 2234–2244. [Google Scholar] [CrossRef]
- Narasimhan, A.; Chinnaiyan, M.; Karundevi, B. Ferulic acid exerts its antidiabetic effect by modulating insulin-signalling molecules in the liver of high-fat diet and fructose-induced type-2 diabetic adult male rat. Appl. Physiol. Nutr. Metab. 2015, 40, 769–781. [Google Scholar] [CrossRef]
- Son, M.J.; Rico, C.W.; Nam, S.H.; Kang, M.Y. Effect of oryzanol and ferulic acid on the glucose metabolism of mice fed with a high-fat diet. J. Food Sci. 2011, 76, H7–H10. [Google Scholar] [CrossRef]
- Calnan, D.R.; Brunet, A. The foxo code. Oncogene 2008, 27, 2276–2288. [Google Scholar] [CrossRef] [PubMed]
- Kinyua, A.W.; Ko, C.M.; Doan, K.V.; Yang, D.J.; Huynh, M.K.Q.; Moh, S.H.; Choi, Y.; Kim, K.W. 4-hydroxy-3-methoxycinnamic acid regulates orexigenic peptides and hepatic glucose homeostasis through phosphorylation of foxo1. Exp. Mol. Med. 2018, 50, e437. [Google Scholar] [CrossRef] [PubMed]
- Narasimhan, A.; Chinnaiyan, M.; Karundevi, B. Ferulic acid regulates hepatic glut2 gene expression in high fat and fructose-induced type 2 diabetic adult male rat. Eur. J. Pharmacol. 2015, 761, 391–397. [Google Scholar] [CrossRef] [PubMed]
- Matsuzaka, T.; Shimano, H.; Yahagi, N.; Amemiya-Kudo, M.; Okazaki, H.; Tamura, Y.; Iizuka, Y.; Ohashi, K.; Tomita, S.; Sekiya, M.; et al. Insulin-independent induction of sterol regulatory element-binding protein-1c expression in the livers of streptozotocin-treated mice. Diabetes 2004, 53, 560–569. [Google Scholar] [CrossRef] [PubMed]
- Brownsey, R.W.; Boone, A.N.; Allard, M.F. Actions of insulin on the mammalian heart: Metabolism, pathology and biochemical mechanisms. Cardiovasc. Res. 1997, 34, 3–24. [Google Scholar] [CrossRef]
- Chowdhury, S.; Ghosh, S.; Rashid, K.; Sil, P.C. Deciphering the role of ferulic acid against streptozotocin-induced cellular stress in the cardiac tissue of diabetic rats. Food Chem. Toxicol. 2016, 97, 187–198. [Google Scholar] [CrossRef]
- Guo, X.; Zeng, Z.; Qian, Y.; Qiu, J.; Wang, K.; Wang, Y.; Ji, B.; Zhou, F. Wheat flour, enriched with γ-oryzanol, phytosterol, and ferulic acid, alleviates lipid and glucose metabolism in high-fat-fructose-fed rats. Nutrients 2019, 11, 1697. [Google Scholar] [CrossRef] [PubMed]
- Prabhakar, P.K.; Doble, M. Synergistic effect of phytochemicals in combination with hypoglycemic drugs on glucose uptake in myotubes. Phytomedicine 2009, 16, 1119–1126. [Google Scholar] [CrossRef]
- Prabhakar, P.K.; Doble, M. Interaction of cinnamic acid derivatives with commercial hypoglycemic drugs on 2-deoxyglucose uptake in 3t3-l1 adipocytes. J. Agric. Food Chem. 2011, 59, 9835–9844. [Google Scholar] [CrossRef]
- Kang, B.B.; Chiang, B.H. Amelioration of insulin resistance using the additive effect of ferulic acid and resveratrol on vesicle trafficking for skeletal muscle glucose metabolism. Phytother. Res. 2020, 34, 808–816. [Google Scholar] [CrossRef]
- Kumar, A.; Kaundal, R.K.; Iyer, S.; Sharma, S.S. Effects of resveratrol on nerve functions, oxidative stress and dna fragmentation in experimental diabetic neuropathy. Life Sci. 2007, 80, 1236–1244. [Google Scholar] [CrossRef] [PubMed]
- Ramar, M.; Manikandan, B.; Raman, T.; Priyadarsini, A.; Palanisamy, S.; Velayudam, M.; Munusamy, A.; Marimuthu Prabhu, N.; Vaseeharan, B. Protective effect of ferulic acid and resveratrol against alloxan-induced diabetes in mice. Eur. J. Pharmacol. 2012, 690, 226–235. [Google Scholar] [CrossRef] [PubMed]
- Adisakwattana, S.; Chantarasinlapin, P.; Thammarat, H.; Yibchok-Anun, S. A series of cinnamic acid derivatives and their inhibitory activity on intestinal α-glucosidase. J. Enzym. Inhib. Med. Chem. 2009, 24, 1194–1200. [Google Scholar] [CrossRef] [PubMed]
- Azay-Milhau, J.; Ferrare, K.; Leroy, J.; Aubaterre, J.; Tournier, M.; Lajoix, A.; Tousch, D. Antihyperglycemic effect of a natural chicoric acid extract of chicory (Cichorium intybus L.): A comparative in vitro study with the effects of caffeic and ferulic acids. J. Ethnopharmacol. 2013, 150, 755–760. [Google Scholar] [CrossRef] [PubMed]
- Wagman, A.S.; Nuss, J.M. Current therapies and emerging targets for the treatment of diabetes. Curr. Pharm. Des. 2001, 7, 417–450. [Google Scholar] [CrossRef]
- Zhao, J.; Gao, J.; Li, H. Ferulic acid confers protection on islet beta cells and placental tissues of rats with gestational diabetes mellitus. Cell. Mol. Biol. 2020, 66, 37–41. [Google Scholar] [CrossRef]
- Mirhashemi, S.M. To evaluate likely antiamyloidogenic property of ferulic acid and baicalein against human islet amyloid polypeptide aggregation, in vitro study. Afr. J. Pharm. Pharmcol. 2012, 6, 671–676. [Google Scholar] [CrossRef]
- Fuchs, F.D.; Whelton, P.K. High blood pressure and cardiovascular disease. Hypertension 2020, 75, 285–292. [Google Scholar] [CrossRef]
- Laurent, S. Antihypertensive drugs. Pharmacol. Res. 2017, 124, 116–125. [Google Scholar] [CrossRef]
- Fuchs, F.D.; Fuchs, S.C. Blood pressure targets in the treatment of high blood pressure: A reappraisal of the j-shaped phenomenon. J. Hum. Hypertens. 2014, 28, 80–84. [Google Scholar] [CrossRef]
- Suzuki, A.; Kagawa, D.; Fujii, A.; Ochiai, R.; Tokimitsu, I.; Saito, I. Short- and long-term effects of ferulic acid on blood pressure in spontaneously hypertensive rats. Am. J. Hypertens. 2002, 15, 351–357. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Peng, Y.; Ma, Q.; Li, Z.; Zhang, X. Study on the formation of antihypertensive t win drugs by caffeic acid and ferulic acid with t elmisartan. Drug Des. Dev. Ther. 2020, 14, 977–992. [Google Scholar] [CrossRef] [PubMed]
- Badawy, D.; El-Bassossy, H.M.; Fahmy, A.; Azhar, A. Aldose reductase inhibitors zopolrestat and ferulic acid alleviate hypertension associated with diabetes: Effect on vascular reactivity. Can. J. Physiol. Pharm. 2013, 91, 101–107. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, A.; Yamamoto, M.; Jokura, H.; Fujii, A.; Tokimitsu, I.; Hase, T.; Saito, I. Ferulic acid restores endothelium-dependent vasodilation in aortas of spontaneously hypertensive rats. Am. J. Hypertens. 2007, 20, 508–513. [Google Scholar] [CrossRef] [PubMed]
- Shirakawa, H.; Koseki, T.; Hashizume, K.; Komai, M. The driselase-treated fraction of rice bran is a more effective dietary factor to improve hypertension, glucose and lipid metabolism in stroke-prone spontaneously hypertensive rats compared to ferulic acid. Br. J. Nutr. 2007, 97, 67–76. [Google Scholar] [CrossRef]
- Van Rymenant, E.; Van Camp, J.; Pauwels, B.; Boydens, C.; Vanden Daele, L.; Beerens, K.; Brouckaert, P.; Smagghe, G.; Kerimi, A.; Williamson, G.; et al. Ferulic acid-4-o-sulfate rather than ferulic acid relaxes arteries and lowers blood pressure in mice. J Nutr. Biochem. 2017, 44, 44–51. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.; Kim, H.I.; Park, S.H.; Lee, M.J.; Jun, J.Y.; Kim, H.L.; Chung, J.H.; Yeum, C.H. Endothelium-dependent vasodilation by ferulic acid in aorta from chronic renal hypertensive rats. Kidney Res. Clin. Pract. 2012, 31, 227–233. [Google Scholar] [CrossRef]
- Huang, Y.; Xu, M.; Li, J.; Chen, K.; Xia, L.; Wang, W.; Ren, P.; Huang, X. Ex vivo to in vivo extrapolation of syringic acid and ferulic acid as grape juice proxies for endothelium-dependent vasodilation: Redefining vasoprotective resveratrol of the french paradox. Food Chem. 2021, 363, 130323. [Google Scholar] [CrossRef]
- Fukuda, T.; Kuroda, T.; Kono, M.; Hyoguchi, M.; Tanaka, M.; Matsui, T. Augmentation of ferulic acid-induced vasorelaxation with aging and its structure importance in thoracic aorta of spontaneously hypertensive rats. Naunyn Schmiedebergs Arch. Pharmacol. 2015, 388, 1113–1117. [Google Scholar] [CrossRef]
- El-Bassossy, H.; Badawy, D.; Neamatallah, T.; Fahmy, A. Ferulic acid, a natural polyphenol, alleviates insulin resistance and hypertension in fructose fed rats: Effect on endothelial-dependent relaxation. Chem. Biol. Interact. 2016, 254, 191–197. [Google Scholar] [CrossRef]
- Alam, M.A.; Sernia, C.; Brown, L. Ferulic acid improves cardiovascular and kidney structure and function in hypertensive rats. J. Cardiovasc. Pharm. 2013, 61, 240–249. [Google Scholar] [CrossRef] [PubMed]
- Cooke, M.J.P.; Dzau, M.V.J. Nitric oxide synthase: Role in the genesis of vascular disease. Annu. Rev. Med. 1997, 48, 489–509. [Google Scholar] [CrossRef] [PubMed]
- Behrendt, D.; Ganz, P. Endothelial function: From vascular biology to clinical applications. Am. J. Cardiol. 2002, 90, L40–L48. [Google Scholar] [CrossRef] [PubMed]
- Steinberg, H.O.; Chaker, H.; Leaming, R.; Johnson, A.; Brechtel, G.; Baron, A.D. Obesity/insulin resistance is associated with endothelial dysfunction. Implications for the syndrome of insulin resistance. J. Clin. Investig. 1996, 97, 2601–2610. [Google Scholar] [CrossRef] [PubMed]
- Akbari, C.M.; Logerfo, F.W. Diabetes and peripheral vascular disease. J. Vasc. Surg. 1999, 30, 373–384. [Google Scholar] [CrossRef] [PubMed]
- Te Riet, L.; van Esch, J.H.M.; Roks, A.J.M.; van den Meiracker, A.H.; Danser, A.H.J. Hypertension. Circ. Res. 2015, 116, 960–975. [Google Scholar] [CrossRef] [PubMed]
- Lever, A.F.; Lyall, F.; Morton, J.J.; Folkow, B. Angiotensin ii, vascular structure and blood pressure. Kidney Int. Suppl. 1992, 37, S51–S55. [Google Scholar]
- Ohsaki, Y.; Shirakawa, H.; Koseki, T.; Komai, M. Novel effects of a single administration of ferulic acid on the regulation of blood pressure and the hepatic lipid metabolic profile in stroke-prone spontaneously hypertensive rats. J. Agric. Food Chem. 2008, 56, 2825–2830. [Google Scholar] [CrossRef]
- Nelson, R.H. Hyperlipidemia as a risk factor for cardiovascular disease. Prim. Care 2013, 40, 195–211. [Google Scholar] [CrossRef]
- Xenoulis, P.G.; Steiner, J.M. Canine hyperlipidaemia. J. Small Anim. Pract. 2015, 56, 595–605. [Google Scholar] [CrossRef]
- Bilen, O.; Pokharel, Y.; Ballantyne, C.M. Genetic testing in hyperlipidemia. Endocrin. Metab. Clin. 2016, 45, 129–140. [Google Scholar] [CrossRef] [PubMed]
- Malloy, M.J.; Kane, J.P. Hyperlipidemia and cardiovascular disease. Curr. Opin. Lipidol. 2012, 23, 591–592. [Google Scholar] [CrossRef] [PubMed]
- Wierzbicki, A.S.; Mikhailidis, D.P. Editorial: The year in hyperlipidaemia. Curr. Opin. Cardiol. 2021, 36, 461. [Google Scholar] [CrossRef]
- Sharma, R.D. Effect of hydroxy acids on hypercholesterolaemia in rats. Atherosclerosis 1980, 37, 463. [Google Scholar] [CrossRef] [PubMed]
- Yeh, Y.H.; Lee, Y.T.; Hsieh, H.S.; Hwang, D.F. Dietary caffeic acid, ferulic acid and coumaric acid supplements on cholesterol metabolism and antioxidant activity in rats. J. Food Drug Anal. 2009, 17, 123–132. [Google Scholar] [CrossRef]
- Sri Balasubashini, M.; Rukkumani, R.; Menon, V.P. Protective effects of ferulic acid on hyperlipidemic diabetic rats. Acta Diabetol. 2003, 40, 118–122. [Google Scholar] [CrossRef] [PubMed]
- Yogeeta, S.K.; Hanumantra, R.B.; Gnanapragasam, A.; Senthilkumar, S.; Subhashini, R.; Devaki, T. Attenuation of abnormalities in the lipid metabolism during experimental myocardial infarction induced by isoproterenol in rats: Beneficial effect of ferulic acid and ascorbic acid. Basic Clin. Pharmacol. Toxicol. 2006, 98, 467–472. [Google Scholar] [CrossRef]
- Bocco, B.M.; Fernandes, G.W.; Lorena, F.B.; Cysneiros, R.M.; Christoffolete, M.A.; Grecco, S.S.; Lancellotti, C.L.; Romoff, P.; Lago, J.H.G.; Bianco, A.C.; et al. Combined treatment with caffeic and ferulic acid from Baccharis uncinella c. Dc. (Asteraceae) protects against metabolic syndrome in mice. Braz. J. Med. Biol. Res. 2016, 49. [Google Scholar] [CrossRef]
- Sudheer, A.R.; Chandran, K.; Marimuthu, S.; Menon, V.P. Ferulic acid modulates altered lipid profiles and prooxidant/antioxidant status in circulation during nicotine-induced toxicity: A dose-dependent study. Toxicol. Mech. Method. 2008, 15, 375–381. [Google Scholar] [CrossRef]
- Lambruschini, C.; Demori, I.; El Rashed, Z.; Rovegno, L.; Canessa, E.; Cortese, K.; Grasselli, E.; Moni, L. Synthesis, photoisomerization, antioxidant activity, and lipid-lowering effect of ferulic acid and feruloyl amides. Molecules 2021, 26, 89. [Google Scholar] [CrossRef]
- Luo, Z.; Li, M.; Yang, Q.; Zhang, Y.; Liu, F.; Gong, L.; Han, L.; Wang, M. Ferulic acid prevents nonalcoholic fatty liver disease by promoting fatty acid oxidation and energy expenditure in c57bl/6 mice fed a high-fat diet. Nutrients 2022, 14, 2530. [Google Scholar] [CrossRef] [PubMed]
- Bhaskaragoud, G.; Rajath, S.; Mahendra, V.P.; Kumar, G.S.; Gopala, K.A.; Kumar, G.S. Hypolipidemic mechanism of oryzanol components- ferulic acid and phytosterols. Biochem. Biophys. Res. Commun. 2016, 476, 82–89. [Google Scholar] [CrossRef] [PubMed]
- Jain, P.G.; Surana, S.J. Isolation, characterization and hypolipidemic activity of ferulic acid in high-fat-diet-induced hyperlipidemia in laboratory rats. EXCLI J. 2016, 15, 599–613. [Google Scholar] [CrossRef] [PubMed]
- Duchnowicz, P.; Broncel, M.; Podsędek, A.; Koter-Michalak, M. Hypolipidemic and antioxidant effects of hydroxycinnamic acids, quercetin, and cyanidin 3-glucoside in hypercholesterolemic erythrocytes (in vitro study). Eur. J. Nutr. 2012, 51, 435–443. [Google Scholar] [CrossRef]
- Bumrungpert, A.; Lilitchan, S.; Tuntipopipat, S.; Tirawanchai, N.; Komindr, S. Ferulic acid supplementation improves lipid profiles, oxidative stress, and inflammatory status in hyperlipidemic subjects: A randomized, double-blind, placebo-controlled clinical trial. Nutrients 2018, 10, 713. [Google Scholar] [CrossRef]
- Little, R.; Houghton, M.J.; Carr, I.M.; Wabitsch, M.; Kerimi, A.; Williamson, G. The ability of quercetin and ferulic acid to lower stored fat is dependent on the metabolic background of human adipocytes. Mol. Nutr. Food Res. 2020, 64, 2000034. [Google Scholar] [CrossRef]
- Kim, H.K.; Jeong, T.S.; Lee, M.K.; Park, Y.B.; Choi, M.S. Lipid-lowering efficacy of hesperetin metabolites in high-cholesterol fed rats. Clin. Chim. Acta 2003, 327, 129–137. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, X.; Huang, Z.; Chen, D.; Yu, B.; Yu, J.; Chen, H.; He, J.; Luo, Y.; Zheng, P. Dietary ferulic acid supplementation improves antioxidant capacity and lipid metabolism in weaned piglets. Nutrients 2020, 12, 3811. [Google Scholar] [CrossRef]
- Wang, Z.; Yang, Y.; Zhang, J.; Hu, J.; Yan, X.; Zeng, S.; Huang, X.; Lin, S. Ferulic acid ameliorates intrahepatic triglyceride accumulation in vitro but not in high fat diet-fed c57bl/6 mice. Food Chem. Toxicol. 2021, 149, 111978. [Google Scholar] [CrossRef]
- Son, M.J.; Rico, C.W.; Nam, S.H.; Kang, M.Y. Influence of oryzanol and ferulic acid on the lipid metabolism and antioxidative status in high fat-fed mice. J. Clin. Biochem. Nutr. 2010, 46, 150–156. [Google Scholar] [CrossRef]
- Gao, J.; Gu, X.; Zhang, M.; Zu, X.; Shen, F.; Hou, X.; Hao, E.; Bai, G. Ferulic acid targets acsl1 to ameliorate lipid metabolic disorders in db/db mice. J. Funct. Foods 2022, 91, 105009. [Google Scholar] [CrossRef]
- Gu, Y.; Zhang, Y.; Li, M.; Huang, Z.; Jiang, J.; Chen, Y.; Chen, J.; Jia, Y.; Zhang, L.; Zhou, F. Ferulic acid ameliorates atherosclerotic injury by modulating gut microbiota and lipid metabolism. Front Pharmacol. 2021, 12, 621339. [Google Scholar] [CrossRef] [PubMed]
- Kuppusamy, P.; Ilavenil, S.; Hwang, I.H.; Kim, D.; Choi, K.C. Ferulic acid stimulates adipocyte-specific secretory proteins to regulate adipose homeostasis in 3t3-l1 adipocytes. Molecules 2021, 26, 1984. [Google Scholar] [CrossRef] [PubMed]
- Ben-Shlomo, A.; Fleseriu, M. Obesity. Endocrin. Metab. Clin. 2016, 45, xiii. [Google Scholar] [CrossRef] [PubMed]
- Gadde, K.M.; Martin, C.K.; Berthoud, H.; Heymsfield, S.B. Obesity: Pathophysiology and management. J. Am. Coll. Cardiol. 2018, 71, 69–84. [Google Scholar] [CrossRef] [PubMed]
- Sarma, S.; Sockalingam, S.; Dash, S. Obesity as a multisystem disease: Trends in obesity rates and obesity-related complications. Diabetes Obes. Metab. 2021, 23, 3–16. [Google Scholar] [CrossRef]
- Esser, N.; Legrand-Poels, S.; Piette, J.; Scheen, A.J.; Paquot, N. Inflammation as a link between obesity, metabolic syndrome and type 2 diabetes. Diabetes Res. Clin. Pract. 2014, 105, 141–150. [Google Scholar] [CrossRef]
- Alam, M.A.; Subhan, N.; Hossain, H.; Hossain, M.; Reza, H.M.; Rahman, M.M.; Ullah, M.O. Hydroxycinnamic acid derivatives: A potential class of natural compounds for the management of lipid metabolism and obesity. Nutr. Metab. 2016, 13, 27. [Google Scholar] [CrossRef]
- Wang, O.; Zhang, N.; Han, C.; Huang, J. Regular exercise combined with ferulic acid exhibits antiobesity effect and regulates metabolic profiles in high-fat diet-induced mice. Front Nutr. 2022, 9, 957321. [Google Scholar] [CrossRef]
- Pérez-Torres, I.; Castrejón-Téllez, V.; Soto, M.E.; Rubio-Ruiz, M.E.; Manzano-Pech, L.; Guarner-Lans, V. Oxidative stress, plant natural antioxidants, and obesity. Int. J. Mol. Sci. 2021, 22, 1786. [Google Scholar] [CrossRef]
- Halter, B.; Ildari, N.; Cline, M.A.; Gilbert, E.R. Ferulic acid, a phytochemical with transient anorexigenic effects in birds. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2021, 259, 111015. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Pan, Y.; Wang, L.; Zhou, H.; Song, G.; Wang, Y.; Liu, J.; Li, A. Optimal dietary ferulic acid for suppressing the obesity-related disorders in leptin-deficient obese c57bl/6j -ob/ob mice. J. Agric. Food Chem. 2019, 67, 4250–4258. [Google Scholar] [CrossRef] [PubMed]
- Cheng, H.; Liu, J.; Tan, Y.; Feng, W.; Peng, C. Interactions between gut microbiota and berberine, a necessary procedure to understand the mechanisms of berberine. J. Pharm. Anal. 2022, 12, 541–555. [Google Scholar] [CrossRef] [PubMed]
- Tian, B.; Geng, Y.; Wang, P.; Cai, M.; Neng, J.; Hu, J.; Xia, D.; Cao, W.; Yang, K.; Sun, P. Ferulic acid improves intestinal barrier function through altering gut microbiota composition in high-fat diet-induced mice. Eur. J. Nutr. 2022, 61, 3767–3783. [Google Scholar] [CrossRef]
- Justo, M.L.; Claro, C.; Zeyda, M.; Stulnig, T.M.; Herrera, M.D.; Rodríguez-Rodríguez, R. Rice bran prevents high-fat diet-induced inflammation and macrophage content in adipose tissue. Eur. J. Nutr. 2016, 55, 2011–2019. [Google Scholar] [CrossRef]
- Salazar-López, N.; Astiazarán-García, H.; González-Aguilar, G.; Loarca-Piña, G.; Ezquerra-Brauer, J.; Domínguez Avila, J.; Robles-Sánchez, M. Ferulic acid on glucose dysregulation, dyslipidemia, and inflammation in diet-induced obese rats: An integrated study. Nutrients 2017, 9, 675. [Google Scholar] [CrossRef]
- Wang, W.; Pan, Y.; Zhou, H.; Wang, L.; Chen, X.; Song, G.; Liu, J.; Li, A. Ferulic acid suppresses obesity and obesity-related metabolic syndromes in high fat diet-induced obese c57bl/6j mice. Food Agric. Immunol. 2018, 29, 1116–1125. [Google Scholar] [CrossRef]
- De Melo, T.S.; Lima, P.R.; Carvalho, K.M.M.B.; Fontenele, T.M.; Solon, F.R.N.; Tomé, A.R.; de Lemos, T.L.G.; Da Cruz Fonseca, S.G.; Santos, F.A.; Rao, V.S.; et al. Ferulic acid lowers body weight and visceral fat accumulation via modulation of enzymatic, hormonal and inflammatory changes in a mouse model of high-fat diet-induced obesity. Braz. J. Med. Biol. Res. 2017, 50. [Google Scholar] [CrossRef]
- Chaiittianan, R.; Chayopas, P.; Rattanathongkom, A.; Tippayawat, P.; Sutthanut, K. Anti-obesity potential of corn silks: Relationships of phytochemicals and antioxidation, anti-pre-adipocyte proliferation, anti-adipogenesis, and lipolysis induction. J. Funct. Foods 2016, 23, 497–510. [Google Scholar] [CrossRef]
- Seo, C.; Yi, B.; Oh, S.; Kwon, S.; Kim, S.; Song, N.; Cho, J.Y.; Park, K.; Ahn, J.; Hong, J.; et al. Aqueous extracts of hulled barley containing coumaric acid and ferulic acid inhibit adipogenesis in vitro and obesity in vivo. J. Funct. Foods 2015, 12, 208–218. [Google Scholar] [CrossRef]
- Nagao, T.; Sayuri, T.; Tatsuya, T.; Risa, S.; Hideaki, S. Ferulic acid esters and weight-loss promoting effects in rats. J. Oleo Sci. 2012, 61, 331–336. [Google Scholar] [CrossRef][Green Version]
- Prusty, D.; Park, B.; Davis, K.E.; Farmer, S.R. Activation of mek/erk signaling promotes adipogenesis by enhancing peroxisome proliferator-activated receptor γ (pparγ) and c/ebpα gene expression during the differentiation of 3t3-l1 preadipocytes. J. Biol. Chem. 2002, 277, 46226–46232. [Google Scholar] [CrossRef] [PubMed]
- Ilavenil, S.; Kim, D.H.; Srigopalram, S.; Kuppusamy, P.; Valan Arasu, M.; Lee, K.D.; Lee, J.C.; Song, Y.H.; Jeong, Y.; Choi, K.C. Ferulic acid in lolium multiflorum inhibits adipogenesis in 3t3-l1 cells and reduced high-fat-diet-induced obesity in swiss albino mice via regulating p38mapk and p44/42 signal pathways. J. Funct. Foods 2017, 37, 293–302. [Google Scholar] [CrossRef]
- Li, C.; Chen, K.; Jia, M.; Ding, X.; Jiang, Z.; Li, L.; Zhang, D. Ampk promotes survival and adipogenesis of ischemia-challenged adscs in an autophagy-dependent manner. Biochim. Biophys. Acta Mol. Cell. Biol. Lipids 2018, 1863, 1498–1510. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.C.; Brooks, R.; Houskeeper, J.; Bremner, S.K.; Dunlop, J.; Viollet, B.; Logan, P.J.; Salt, I.P.; Ahmed, S.F.; Yarwood, S.J. Metformin suppresses adipogenesis through both amp-activated protein kinase (ampk)-dependent and ampk-independent mechanisms. Mol. Cell. Endocrinol. 2017, 440, 57–68. [Google Scholar] [CrossRef]
- Luna-Vital, D.; Luzardo-Ocampo, I.; Cuellar-Nuñez, M.L.; Loarca-Piña, G.; Gonzalez De Mejia, E. Maize extract rich in ferulic acid and anthocyanins prevents high-fat-induced obesity in mice by modulating sirt1, ampk and il-6 associated metabolic and inflammatory pathways. J. Nutr. Biochem. 2020, 79, 108343. [Google Scholar] [CrossRef]
- Park, T.J.; Park, A.; Kim, J.; Kim, J.Y.; Han, B.S.; Oh, K.J.; Lee, E.W.; Lee, S.C.; Bae, K.H.; Kim, W.K. Myonectin inhibits adipogenesis in 3t3-l1 preadipocytes by regulating p38 mapk pathway. BMB Rep. 2021, 54, 124–129. [Google Scholar] [CrossRef]
- Hu, E.; Kim, J.B.; Sarraf, P.; Spiegelman, B.M. Inhibition of adipogenesis through map kinase-mediated phosphorylation of pparγ. Science 1996, 274, 2100–2103. [Google Scholar] [CrossRef]
- Cho, J.; Park, E. Ferulic acid maintains the self-renewal capacity of embryo stem cells and adipose-derived mesenchymal stem cells in high fat diet-induced obese mice. J. Nutr. Biochem. 2020, 77, 108327. [Google Scholar] [CrossRef]
- Koh, E.-J.; Kim, K.-J.; Seo, Y.-J.; Choi, J.; Lee, B.-Y. Modulation of ho-1 by ferulic acid attenuates adipocyte differentiation in 3t3-l1 cells. Molecules 2017, 22, 745. [Google Scholar] [CrossRef]
- Koh, E.J.; Choi, J.; Lee, Y.J.; Hwang, J.H.; Seo, Y.J.; Song, J.H.; Lee, B.Y. Ferulic acid suppresses adipogenesis via activation of ho-1 in 3t3-l1 cells. FASEB J. 2016, 30, lb254. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ye, L.; Hu, P.; Feng, L.-P.; Huang, L.-L.; Wang, Y.; Yan, X.; Xiong, J.; Xia, H.-L. Protective Effects of Ferulic Acid on Metabolic Syndrome: A Comprehensive Review. Molecules 2023, 28, 281. https://doi.org/10.3390/molecules28010281
Ye L, Hu P, Feng L-P, Huang L-L, Wang Y, Yan X, Xiong J, Xia H-L. Protective Effects of Ferulic Acid on Metabolic Syndrome: A Comprehensive Review. Molecules. 2023; 28(1):281. https://doi.org/10.3390/molecules28010281
Chicago/Turabian StyleYe, Lei, Pan Hu, Li-Ping Feng, Li-Lu Huang, Yi Wang, Xin Yan, Jing Xiong, and Hou-Lin Xia. 2023. "Protective Effects of Ferulic Acid on Metabolic Syndrome: A Comprehensive Review" Molecules 28, no. 1: 281. https://doi.org/10.3390/molecules28010281
APA StyleYe, L., Hu, P., Feng, L.-P., Huang, L.-L., Wang, Y., Yan, X., Xiong, J., & Xia, H.-L. (2023). Protective Effects of Ferulic Acid on Metabolic Syndrome: A Comprehensive Review. Molecules, 28(1), 281. https://doi.org/10.3390/molecules28010281






