Isolation of Various Flavonoids with TRAIL Resistance-Overcoming Activity from Blumea lacera
Abstract
1. Introduction
2. Materials and Methods
2.1. General Experimental Procedures
2.2. Plant Material
2.3. Cell Cultures
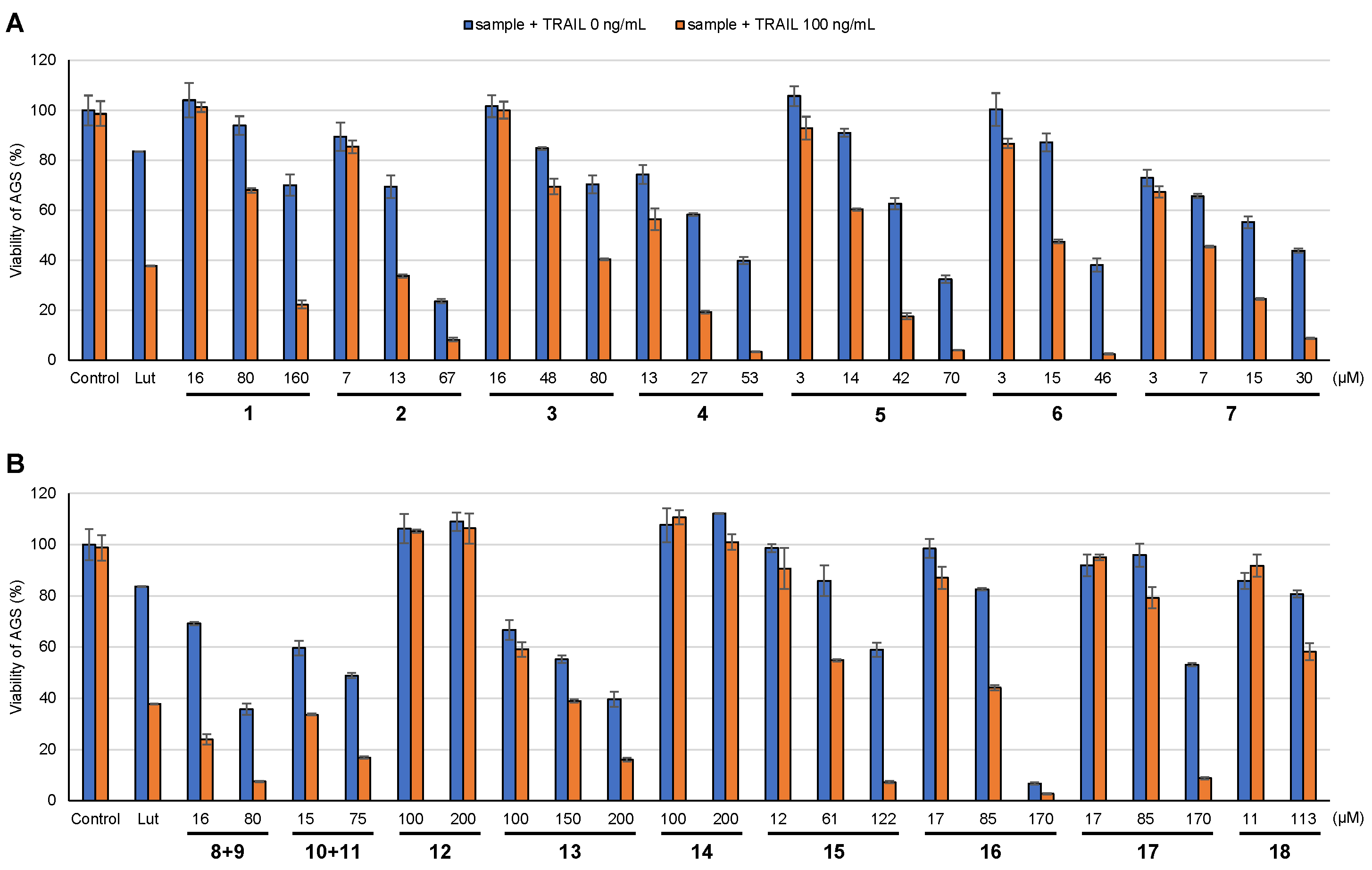
2.4. Assay of Cell Viability (TRAIL Resistance-Overcoming Activity Assay)
2.5. Extraction and Isolation
3. Results and Discussion
3.1. Isolation of Compounds from Blumea Lacera
3.2. TRAIL Resistance-Overcoming Activity of Isolated Compounds
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Von Karstedt, S.; Montinaro, A.; Walczak, H. Exploring the TRAILs less travelled: TRAIL in cancer biology and therapy. Nat. Rev. Cancer 2017, 17, 352–366. [Google Scholar] [CrossRef]
- Pham, X.P.; Nhung, T.T.T.; Trinh, H.N.; Trung, D.M.; Giang, D.T.; Vu, B.D.; Diep, N.T.; Van Long, N.; Nguyen, V.T.; Van Men, C. Isolation and structural characterization of compounds from Blumea lacera. Pharmacogn. J. 2021, 13, 999–1004. [Google Scholar] [CrossRef]
- Chiang, L.-C.; Cheng, H.-Y.; Chen, C.-C.; Lin, C.-C. In vitro Anti-leukemic and Antiviral Activities of Traditionally Used Medicinal Plants in Taiwan. Am. J. Chin. Med. 2004, 32, 695–704. [Google Scholar] [CrossRef]
- Akter, R.; Uddin, S.J.; Tiralongo, J.; Grice, I.D.; Tiralongo, E. A new cytotoxic steroidal glycoalkaloid from the methanol extract of Blumea lacera leaves. J. Pharm. Pharm. Sci. 2015, 18, 616–633. [Google Scholar] [CrossRef]
- Akter, R.; Uddin, S.J.; Tiralongo, J.; Grice, I.D.; Tiralongo, E. A new cytotoxic diterpenoid glycoside from the leaves of Blumea lacera and its effects on apoptosis and cell cycle. Nat. Prod. Res. 2016, 30, 2688–2693. [Google Scholar] [CrossRef]
- Agarwal, R.; Singh, R.; Siddiqui, I.R.; Singh, J. Triterpenoid and prenylated phenol glycosides from Blumea lacera. Phytochemistry 1995, 38, 935–938. [Google Scholar] [CrossRef]
- Jitrangsri, K.; Takaya, A.; Hara, Y.; Sadhu, S.K.; Ahmed, F.; Ishibashi, M. Bioactivity-guided isolation of TRAIL-resistance-overcoming activity compounds from the leaves of Murraya exotica. Nat. Prod. Commun. 2021, 16. [Google Scholar] [CrossRef]
- Manome, T.; Hara, Y.; Ahmed, F.; Sadhu, S.K.; Ishibashi, M. Thannilignan glucoside and 2-(β-glucopyranosyl)-3-isoxazolin-5-one derivative, two new compounds isolated from Terminalia bellirica. J. Nat. Med. 2022, 76, 482–489. [Google Scholar] [CrossRef]
- Lindhagen, E.; Nygren, P.; Larsson, R. The fluorometric microculture cytotoxicity assay. Nat. Protoc. 2008, 3, 1364–1369. [Google Scholar] [CrossRef]
- Deng, Y.-R.; Song, A.-X.; Wang, H.-Q. Chemical components of Seriphidium santolium Poijak. J. Chin. Chem. Soc. 2004, 51, 629–636. [Google Scholar] [CrossRef]
- Habib, E.S.; El-Bsoumy, E.; Ibrahim, A.K.; Helal, M.A.; El-Magd, M.A.; Ahmed, S.A. Anti-inflammatory effect of methoxyflavonoids from Chiliadenus montanus (Jasonia Montana) growing in Egypt. Nat. Prod. Res. 2021, 35, 5909–5913. [Google Scholar] [CrossRef]
- Ai, D.T.T.; Ngan, T.B.; Hien, N.T.; Linh, N.T.; Van Cuong, P.; Huyen, V.T.; Huong, D.T.M. Chemical constituents and cytotoxic activities of stem bark of Dialium cochinchinense. Chem. Nat. Compd. 2021, 57, 360–363. [Google Scholar] [CrossRef]
- Huong, D.T.; Luong, D.V.; Thao, T.T.P.; Sung, T.V. A new flavone and cytotoxic activity of flavonoid constituents isolated from Miliusa balansae (Annonaceae). Pharmazie 2005, 60, 627–629. [Google Scholar] [CrossRef]
- Pereira, C.R.P.; da Silva, Y.D.S.; Cechinel-Zanchett, C.C.; Mariano, L.N.B.; Boeing, T.; Cechinel Filho, V.; Monache, F.D.; de Souza, P.; Niero, R. A rare 6-O-glucoside flavonoid from Citharexylum myrianthum Cham. exhibit diuretic and potassium-sparing effect in rats. J. Mol. Struct. 2021, 1239, 130483. [Google Scholar] [CrossRef]
- Nagao, T.; Abe, F.; Kinjo, J.; Okabe, H. Antiproliferative constituents in plants 10. Flavones from the leaves of Lantana montevidensis Briq. and consideration of structure-activity relationship. Biol. Pharm. Bull. 2002, 25, 875–879. [Google Scholar] [CrossRef]
- Nakasugi, T.; Nakashima, M.; Komai, K. Antimutagens in Gaiyou (Artemisia argyi Levl. et Vant.). J. Agric. Food Chem. 2000, 48, 3256–3266. [Google Scholar] [CrossRef]
- Wang, Q.; Jin, J.; Dai, N.; Han, N.; Han, J.; Bao, B. Anti-inflammatory effects, nuclear magnetic resonance identification, and high-performance liquid chromatography isolation of the total flavonoids from Artemisia frigida. J. Food Drug Anal. 2016, 24, 385–391. [Google Scholar] [CrossRef]
- Williams, C.A.; Harborne, J.B.; Geiger, H.; Hoult, J.R.S. The flavonoids of Tanacetum parthenium and T. vulgare and their anti-inflammatory properties. Phytochemistry 1999, 51, 417–423. [Google Scholar] [CrossRef]
- Glasl, S.; Mucaji, P.; Werner, I.; Presser, A.; Jurenitsch, J. Sesquiterpenes and flavonoid aglycones from a Hungarian taxon of the Achillea millefolium group. Z. Nat. C 2002, 57, 976–982. [Google Scholar] [CrossRef]
- Pawłowska, K.A.; Baracz, T.; Skowrońska, W.; Piwowarski, J.P.; Majdan, M.; Malarz, J.; Stojakowska, A.; Zidorn, C.; Granica, S. The contribution of phenolics to the anti-inflammatory potential of the extract from Bolivian coriander (Porophyllum ruderale subsp. ruderale). Food Chem. 2022, 371, 131116. [Google Scholar] [CrossRef]
- Ulubelen, A.; Kerr, K.M.; Mabry, T.J. New 6-hydroxyflavonoids and their methyl ethers and glycosides from Neurolaena oaxacana. Phytochemistry 1980, 19, 1761–1766. [Google Scholar] [CrossRef]
- Wei, X.; Huang, H.; Wu, P.; Cao, H.; Ye, W. Phenolic constituents from Mikania micrantha. Biochem. Syst. Ecol. 2004, 32, 1091–1096. [Google Scholar] [CrossRef]
- Wang, X.-L.; Hay, A.-E.; Matheeussen, A.; Gupta, M.P.; Hostettmann, K. Structure elucidation and NMR assignments of two new triterpenoids from the stems of Paragonia pyramidata (Bignoniaceae). Magn. Reson. Chem. 2011, 49, 184–189. [Google Scholar] [CrossRef]
- Iacazio, G. Easy access to various natural keto polyunsaturated fatty acids and their corresponding racemic alcohols. Chem. Phys. Lipids 2003, 125, 115–121. [Google Scholar] [CrossRef]
- Stavri, M.; Mathew, K.T.; Gibbons, S. Antimicrobial constituents of Scrophularia deserti. Phytochemistry 2006, 67, 1530–1533. [Google Scholar] [CrossRef]
- Tang, J.; Tewtrakul, S.; Wang, Z.T.; Tu, Z.B. Aurantiamide acetate from stems of Zanthoxylum dissitum Hemsley. J. Chin. Pharm. Sci. 2003, 12, 231–233. [Google Scholar]
- Hasegawa, H.; Yamada, Y.; Komiyama, K.; Hayashi, M.; Ishibashi, M.; Yoshida, T.; Sakai, T.; Koyano, T.; Kam, T.-S.; Murata, K.; et al. Dihydroflavonol BB-1, an extract of natural plant Blumea balsamifera, abrogates TRAIL resistance in leukemia cells. Blood 2006, 107, 679–688. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Manome, T.; Hara, Y.; Ishibashi, M. Isolation of Various Flavonoids with TRAIL Resistance-Overcoming Activity from Blumea lacera. Molecules 2023, 28, 264. https://doi.org/10.3390/molecules28010264
Manome T, Hara Y, Ishibashi M. Isolation of Various Flavonoids with TRAIL Resistance-Overcoming Activity from Blumea lacera. Molecules. 2023; 28(1):264. https://doi.org/10.3390/molecules28010264
Chicago/Turabian StyleManome, Teruhisa, Yasumasa Hara, and Masami Ishibashi. 2023. "Isolation of Various Flavonoids with TRAIL Resistance-Overcoming Activity from Blumea lacera" Molecules 28, no. 1: 264. https://doi.org/10.3390/molecules28010264
APA StyleManome, T., Hara, Y., & Ishibashi, M. (2023). Isolation of Various Flavonoids with TRAIL Resistance-Overcoming Activity from Blumea lacera. Molecules, 28(1), 264. https://doi.org/10.3390/molecules28010264



