Triazine: An Important Building Block of Organic Materials for Solar Cell Application
Abstract
1. Introduction
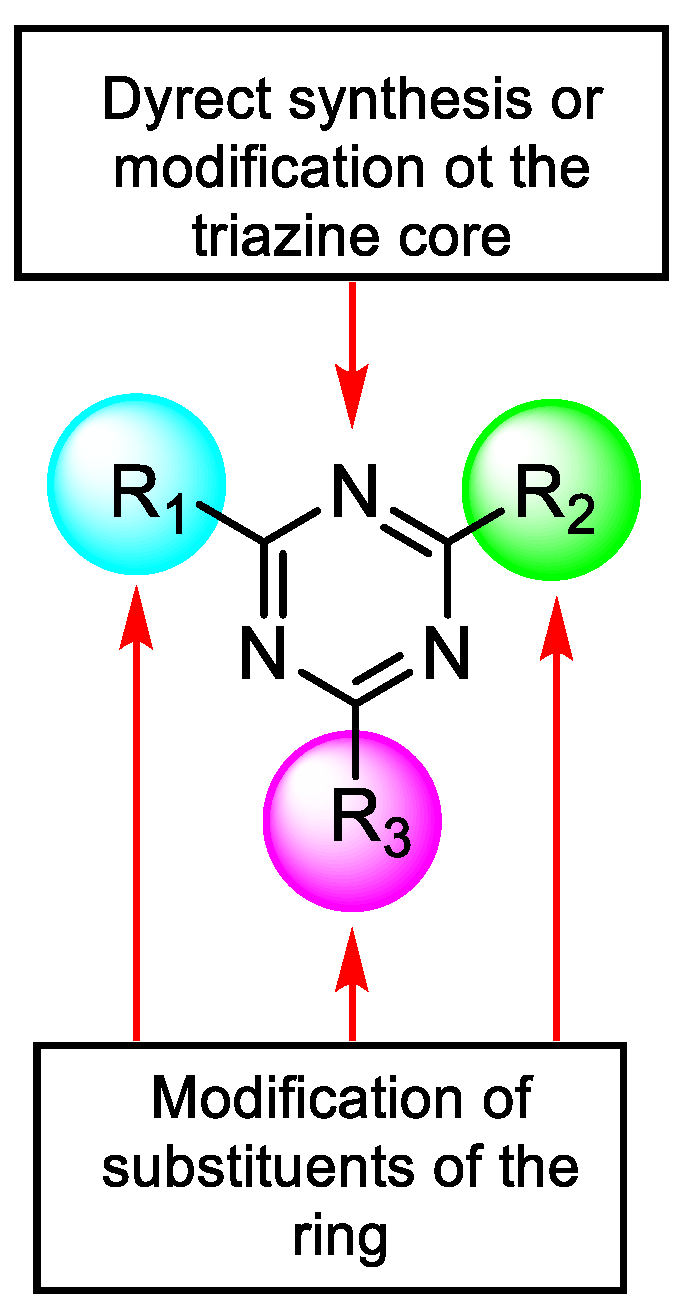
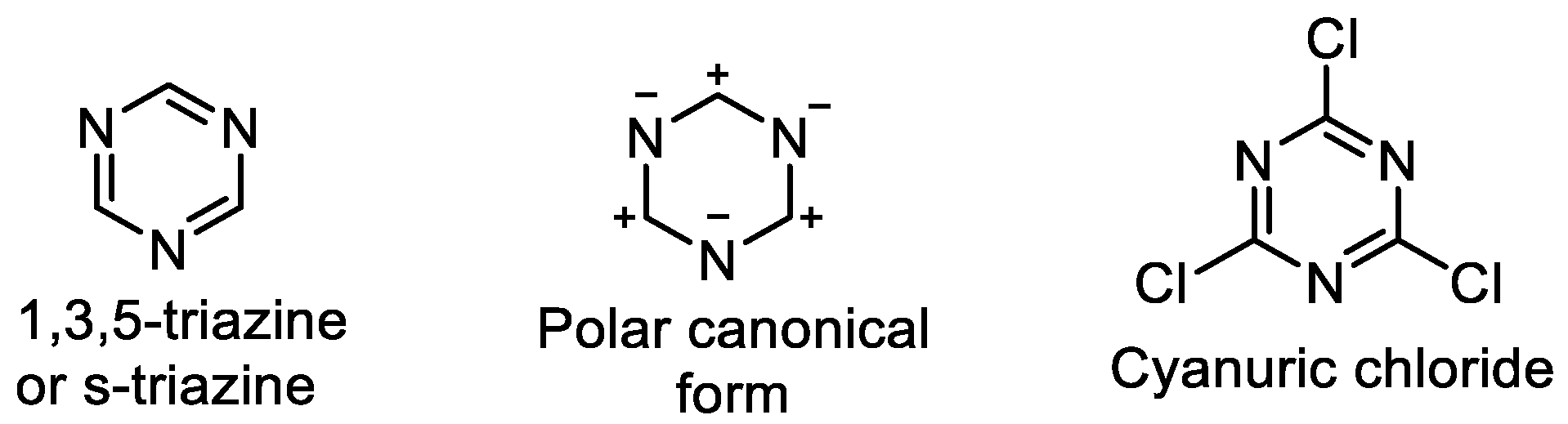
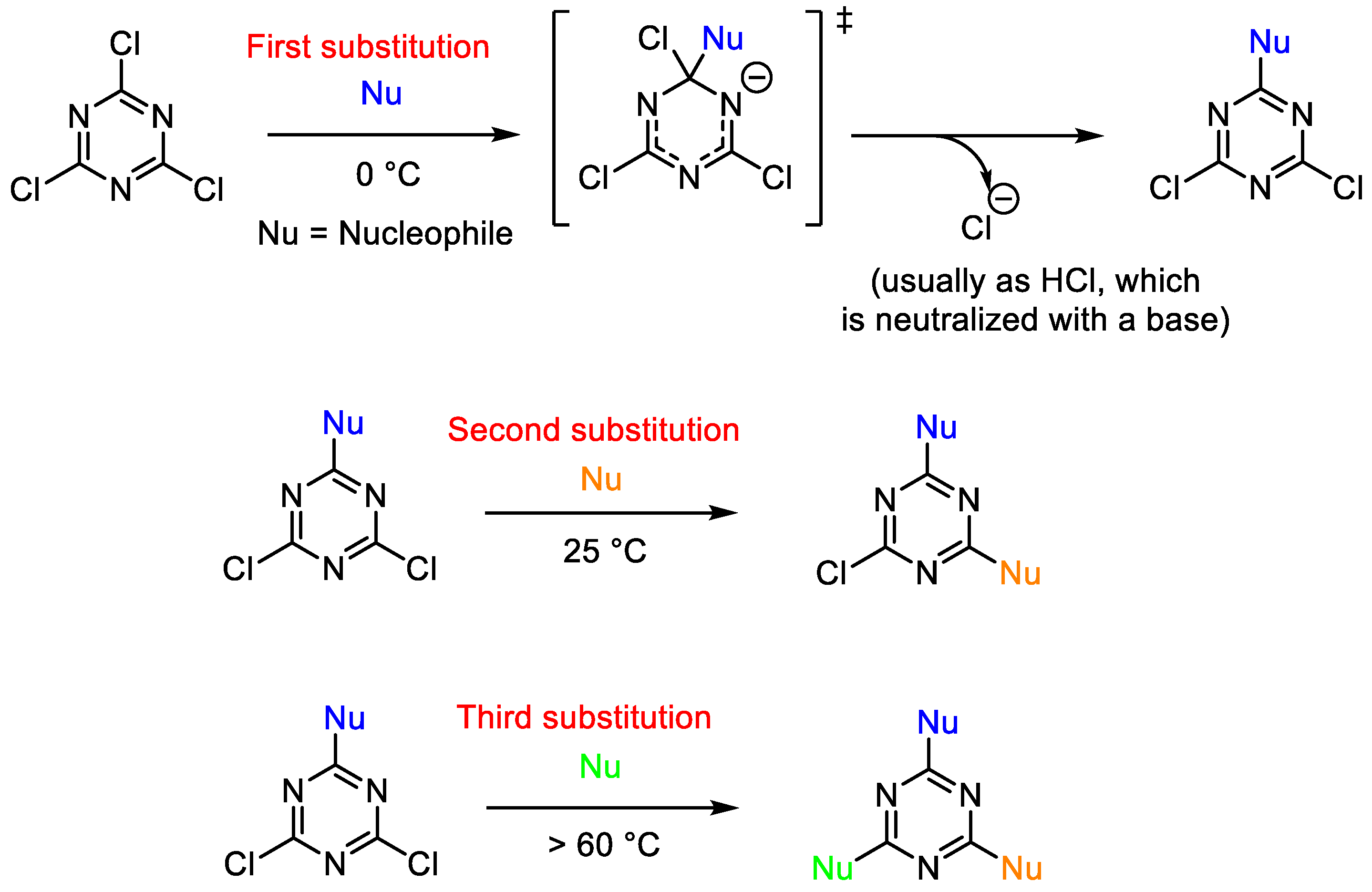
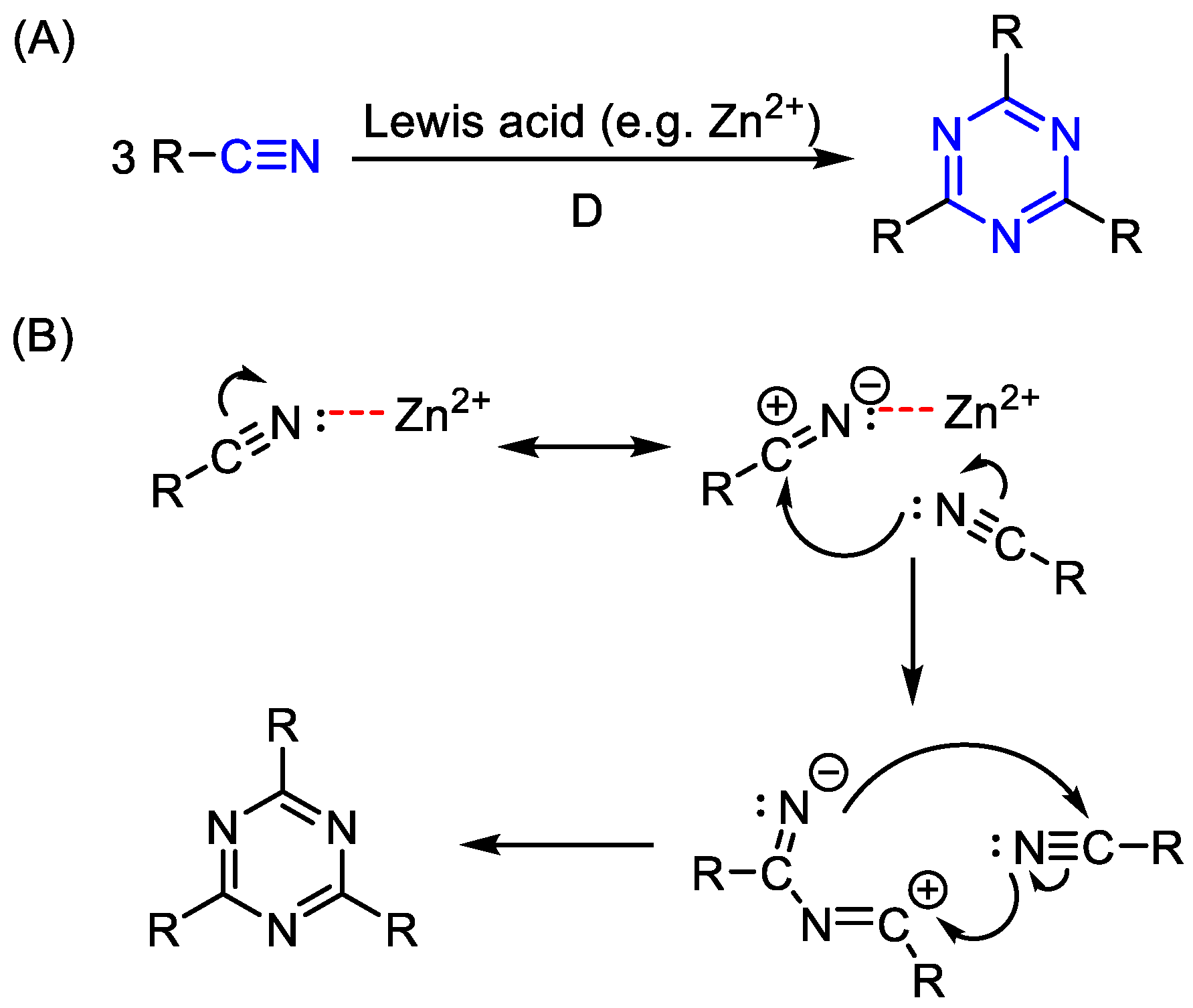
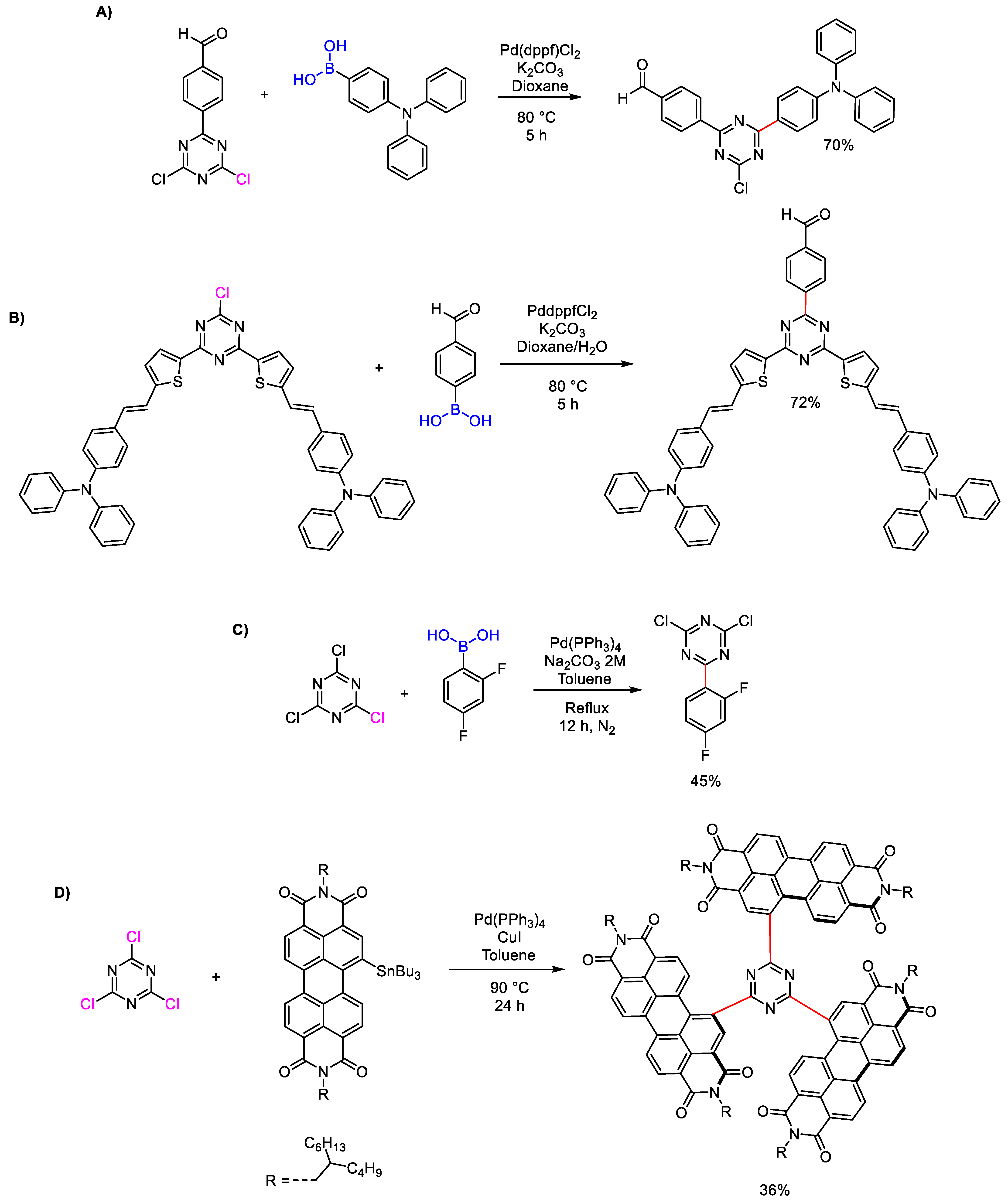
2. Common Synthetic Approaches to Obtain Substituted 1,3,5-Triazines
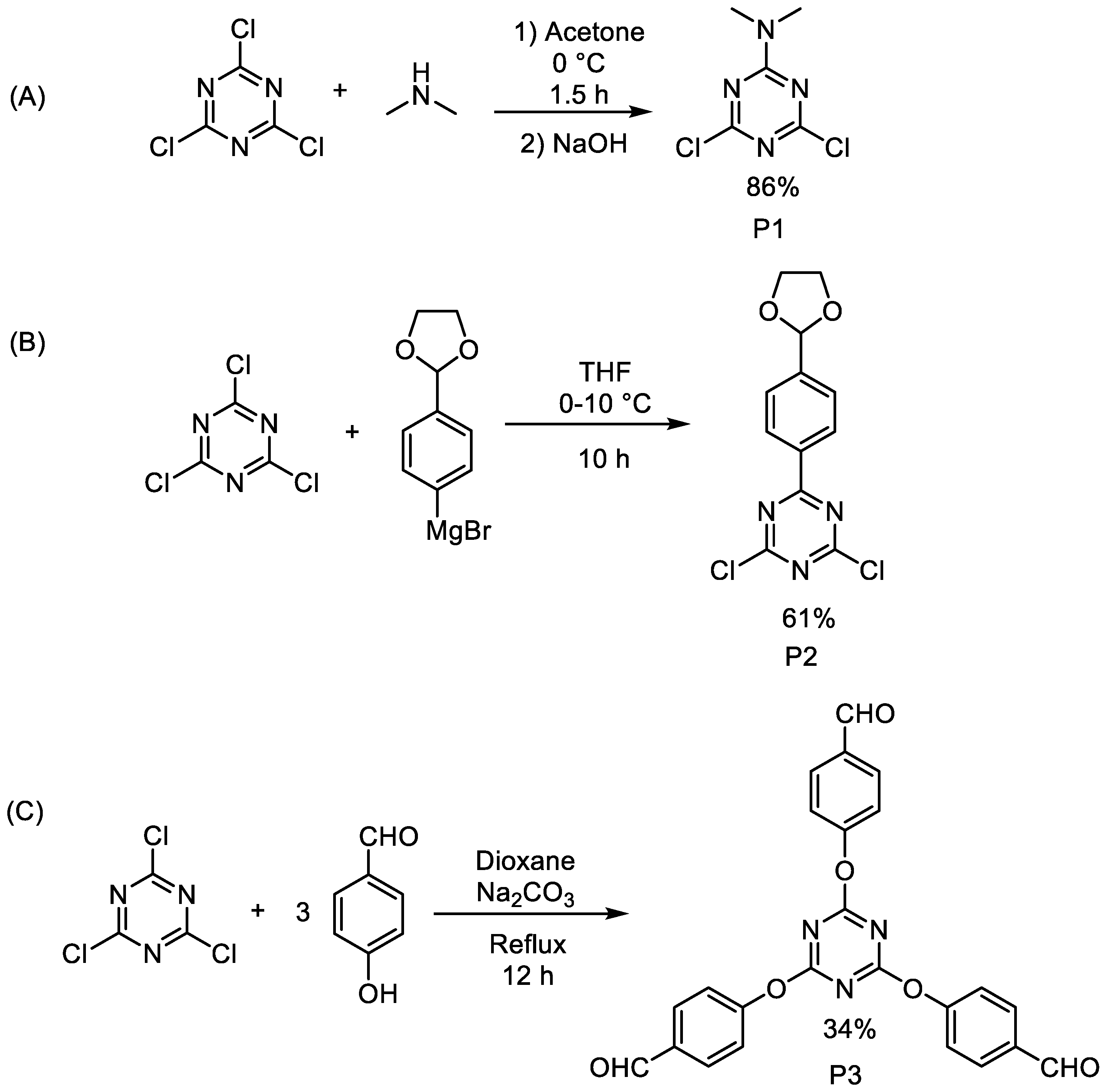
2.1. Direct Synthesis or Modification of a Triazine Core
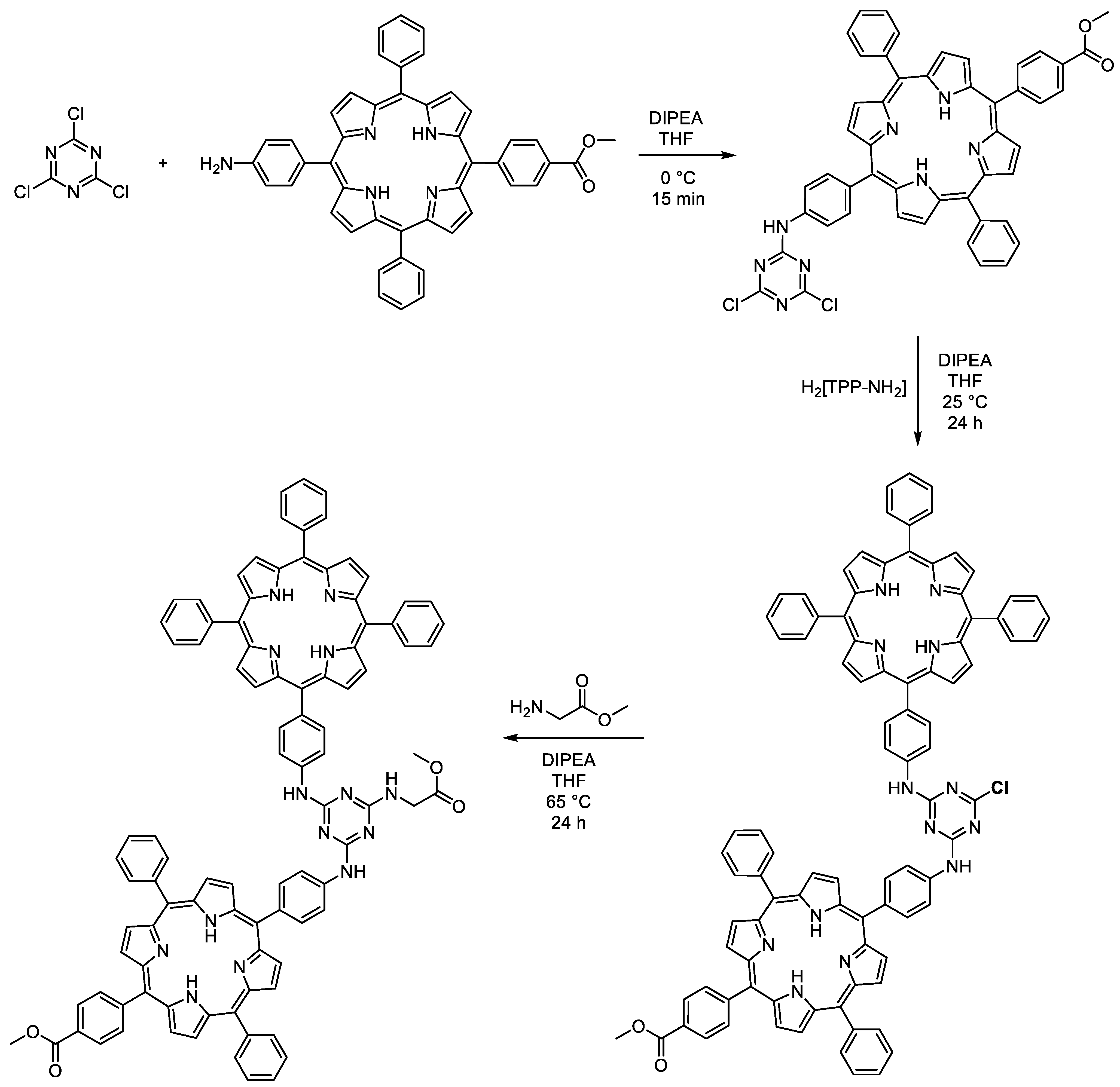
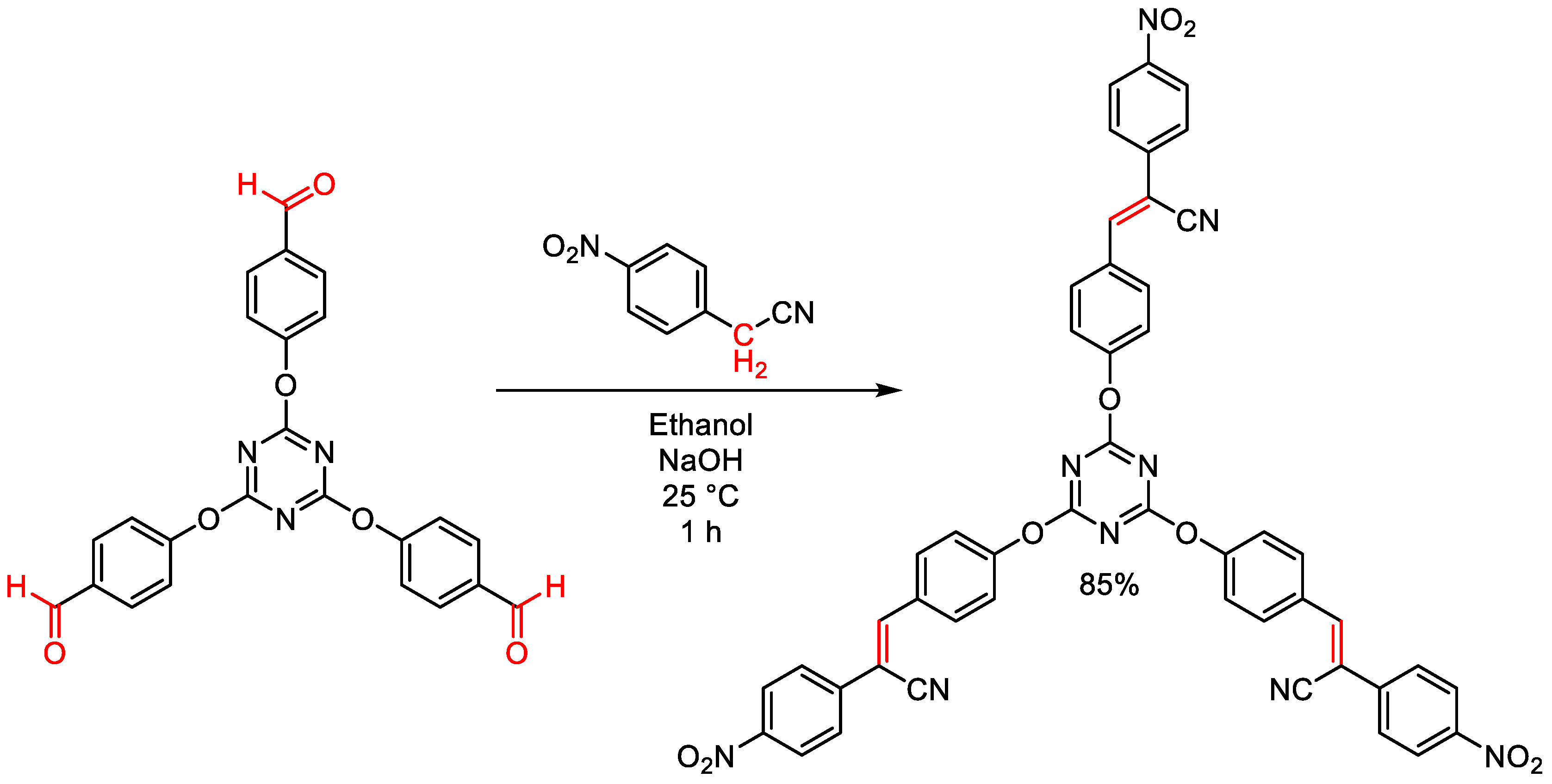
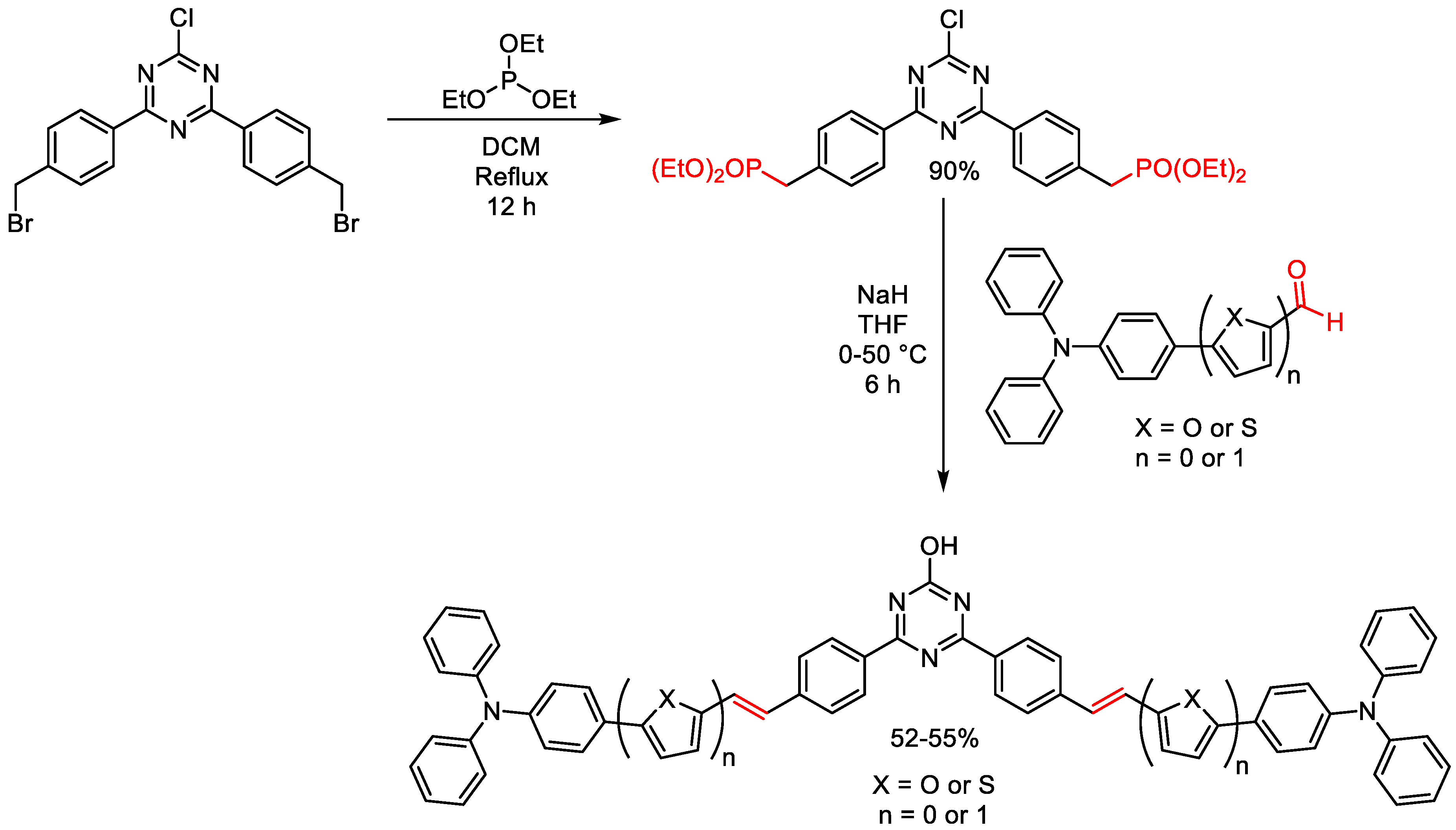
2.2. Structural Modifications of Substituents of Triazine Cores
3. Triazine and Its Derivatives in OSCs
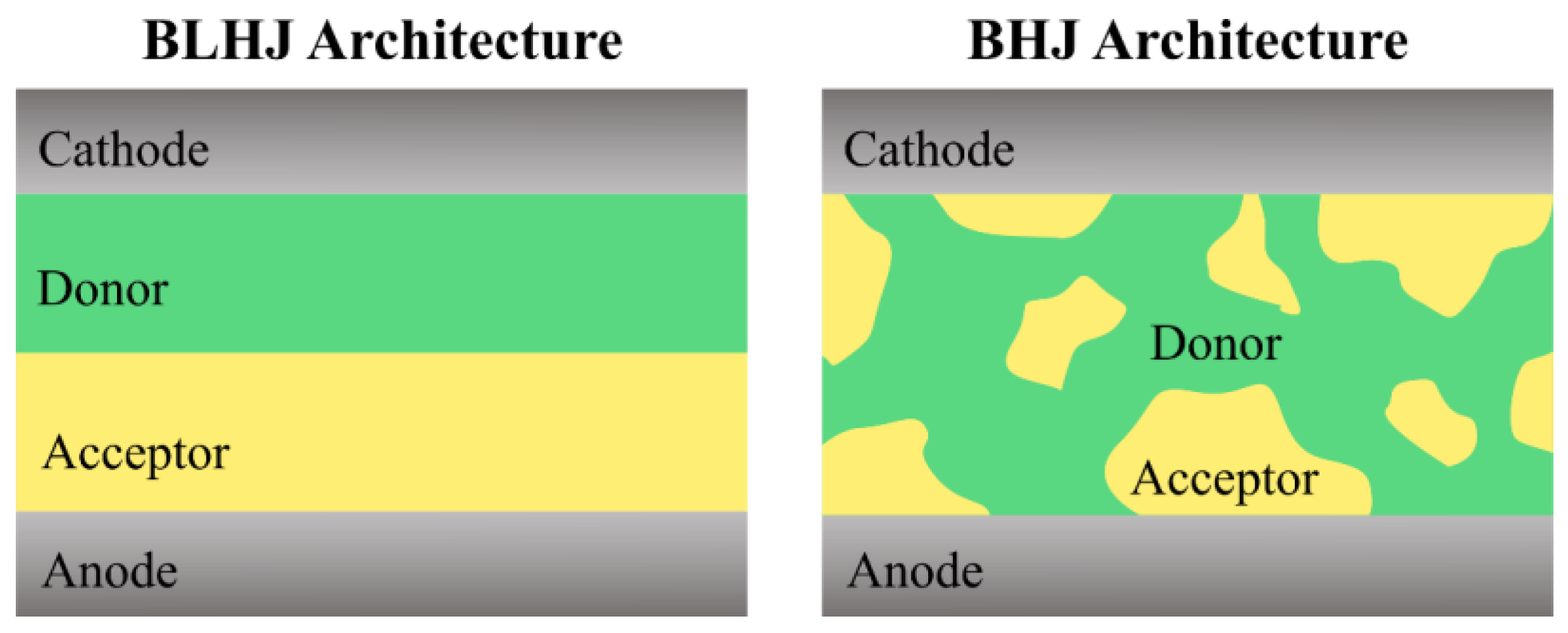
3.1. Brief Introduction to OSCs
3.2. Triazine Derivatives in OSCs
3.2.1. Donor Materials
3.2.2. Acceptor Materials
3.2.3. Triazine Derivatives as Interfacial Layers for OSCs
4. Triazine and Its Derivatives in DSSCs
4.1. Brief Introduction to DSSCs
4.2. Triazine-Based Photosensitizers in DSSCs
4.2.1. Metal-Free Triazine-Based Dyes
4.2.2. Zn-Metalated Porphyrin-Based Substituted Triazines
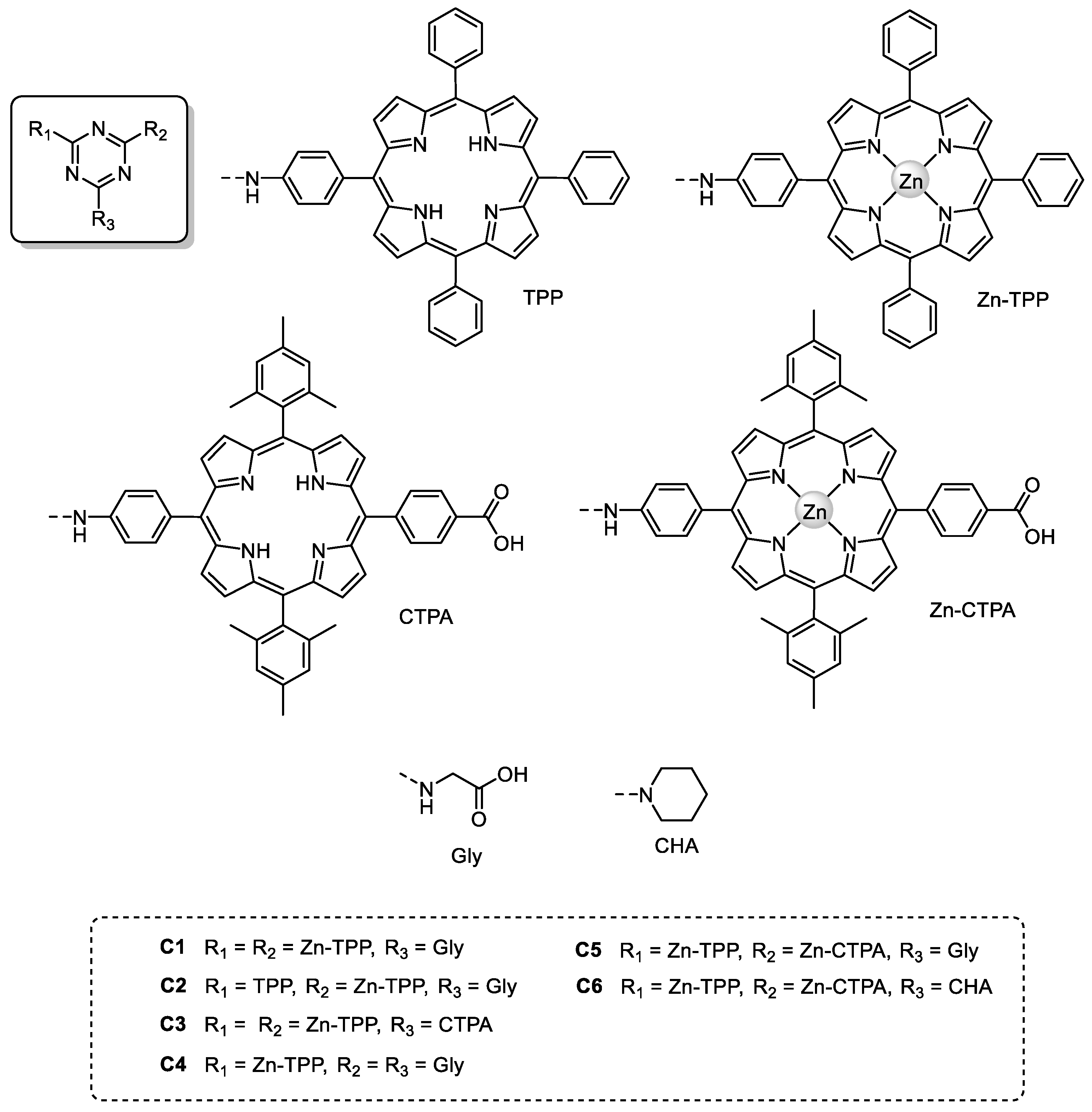
| Triazine-Based Molecule | λMAX a (nm) | ε b (×104 M−1 cm−1) | Eox c (V) | Ered d (V) | Redox Couple | VOC (V) | JSC (mA/cm2) | FF (%) | PCE (%) | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|
| C1 | 422 | - | 1.16 | −1.13 | I−/I3– | 0.63 | 3.33 | 65 | 3.61 | [84] |
| C2 | 422 | - | 1.16 | –0.89 | I–/I3– | 0.66 | 1.67 | 68 | 4.46 | [84] |
| C3 | 422 | 81.7 | 0.92 g | –1.16 g | I–/I3– | 0.63–0.66 | 9.43–10.94 | 64–68 | 3.80–4.91 | [85] |
| C3 (+CDCA) | - | - | - | - | I–/I3– | 0.64 | 12.42 | 70 | 5.56 | [85] |
| C4 | 420 | - | 0.83 | –1.33 | I–/I3– | 0.64 | 10.85 | 68 | 4.72 | [83] |
| C4 (+D) | - | - | - | - | I–/I3– | 0.70 | 14.78 | 71 | 7.34 | [83] |
| C5 | 425 | 66.7 | 1.04 | –1.08 | I–/I3– | 0.68 | 10.78 | 72 | 5.28 | [35] |
| C6 | 425 | 66.6 | 1.29 | –1.09 | I–/I3– | 0.62 | 8.56 | 63 | 3.50 | [35] |
5. Triazine and Its Derivatives in PSCs
5.1. A Brief Introduction to PSCs
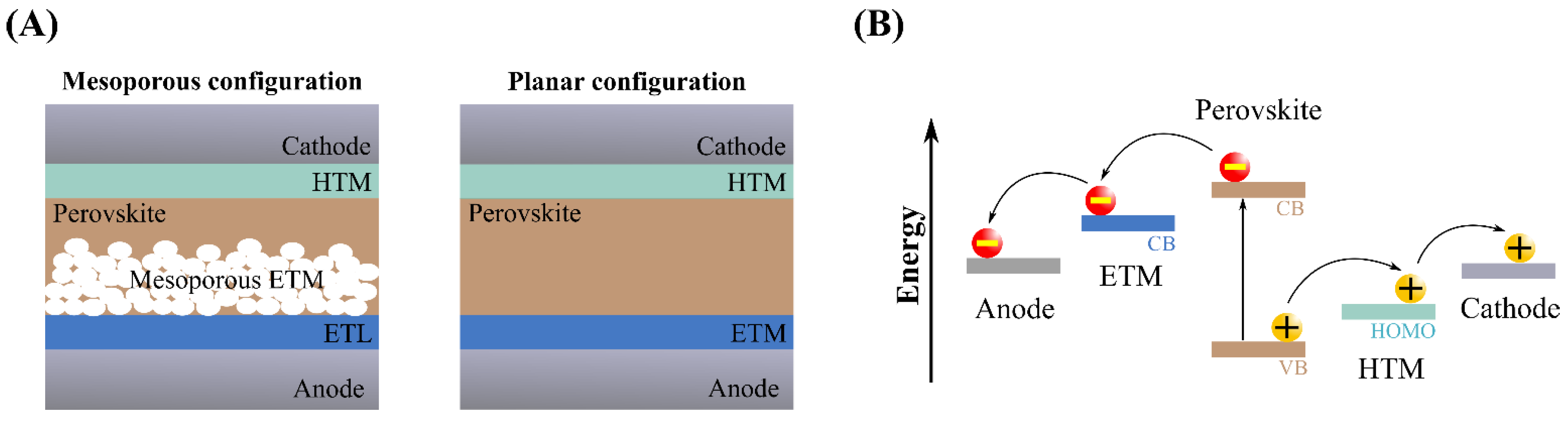
5.2. Triazine-Based Organic Materials in PSCs
5.2.1. Triazine Derivatives as HTMs
5.2.2. Triazine Derivatives as ILs for PSCs
5.2.3. Triazine Derivatives for Surface Passivation
6. Conclusions and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Correction Statement
Sample Availability
References
- Shahbaz, M.; Urano, S.; LeBreton, P.R.; Rossman, M.A.; Hosmane, R.S.; Leonard, N.J. Tri-s-Triazine: Synthesis, Chemical Behavior, and Spectroscopic and Theoretical Probes of Valence Orbital Structure. J. Am. Chem. Soc. 1984, 106, 2805–2811. [Google Scholar] [CrossRef]
- Cannon, J.G. Comprehensive Heterocyclic Chemistry II Edited by A. R. Katritzky, C.W. Rees, and E. F. V. Scriven. Pergamon Press, Elsevier Science, Ltd., Tarrytown, NY. 1996. 12 Volumes: Vol. 1A, Xxvii + 505 Pp; Vol. 1B, 860 Pp; Vol. 2, Xiii + 1102 Pp; Vol. 3, Xiii +. J. Med. Chem. 1997, 40, 4165–4166. [Google Scholar] [CrossRef]
- Grundmann, C.; Kreutzberger, A. Triazines. IX. 1,3,5-Triazine and Its Formation from Hydrocyanic Acid1,2. J. Am. Chem. Soc. 1954, 76, 5646–5650. [Google Scholar] [CrossRef]
- Smolin, E.M.; Rapoport, L. S-Triazines and Derivatives, Volume 13; John Wiley & Sons: Hoboken, NJ, USA, 2009; Volume 13, ISBN 047018812X. [Google Scholar]
- Murase, T.; Fujita, M. Highly Blue Luminescent Triazine−Amine Conjugated Oligomers. J. Org. Chem. 2005, 70, 9269–9278. [Google Scholar] [CrossRef] [PubMed]
- Xu, F.; Sun, J.-H.; Yan, H.-B.; Shen, Q. Cyclotrimerization of Nitriles Catalyzed by SmI2/Amines: Synthesis of 2,4,6-Trisubstituted-S-Triazines. Synth. Commun. 2000, 30, 1017–1022. [Google Scholar] [CrossRef]
- Hao, Z.; Xueding, W.; Weiqing, Y.; Yuanyuan, Z.; Hua, C.; Yuliang, W.; Menglin, M.; Quan, D. Study of the Friedel-Crafts Reaction of Cyanuric Chloride with Low-Boiling Aromatic Ring. Chinese J. Org. Chem. 2017, 37, 2697–2704. [Google Scholar]
- Audebert, P.; Clavier, G.; Allain, C. Chapter 6.3—Triazines, tetrazines, and fused ring polyaza systems. In Progress in Heterocyclic Chemistry; Gribble, G., Joule, J., Eds.; Elsevier: Amsterdam, The Netherlands, 2017; Volume 29, pp. 483–518. ISBN 0959-6380. [Google Scholar]
- Zhang, R.-F.; Hu, W.-J.; Liu, Y.A.; Zhao, X.-L.; Li, J.-S.; Jiang, B.; Wen, K. A Shape-Persistent Cryptand for Capturing Polycyclic Aromatic Hydrocarbons. J. Org. Chem. 2016, 81, 5649–5654. [Google Scholar] [CrossRef]
- Klinkebiel, A.; Beyer, O.; Malawko, B.; Lüning, U. Elongated and Substituted Triazine-Based Tricarboxylic Acid Linkers for MOFs. Beilstein J. Org. Chem. 2016, 12, 2267–2273. [Google Scholar] [CrossRef]
- Braveenth, R.; Chai, K.Y. Triazine-Acceptor-Based Green Thermally Activated Delayed Fluorescence Materials for Organic Light-Emitting Diodes. Materials 2019, 12, 2646. [Google Scholar] [CrossRef]
- Furue, R.; Nishimoto, T.; Park, I.S.; Lee, J.; Yasuda, T. Aggregation-Induced Delayed Fluorescence Based on Donor/Acceptor-Tethered Janus Carborane Triads: Unique Photophysical Properties of Nondoped OLEDs. Angew. Chemie Int. Ed. 2016, 55, 7171–7175. [Google Scholar] [CrossRef]
- Suh, M.C.; Park, S.-R.; Cho, Y.R.; Shin, D.H.; Kang, P.-G.; Ahn, D.A.; Kim, H.S.; Kim, C.-B. Synthesis of Soluble Host Materials for Highly Efficient Red Phosphorescent Organic Light-Emitting Diodes. ACS Appl. Mater. Interfaces 2016, 8, 18256–18265. [Google Scholar] [CrossRef] [PubMed]
- Zhong, H.; Lai, H.; Fang, Q. New Conjugated Triazine Based Molecular Materials for Application in Optoelectronic Devices: Design, Synthesis, and Properties. J. Phys. Chem. C 2011, 115, 2423–2427. [Google Scholar] [CrossRef]
- Green, M.A.; Ho-Baillie, A.; Snaith, H.J. The Emergence of Perovskite Solar Cells. Nat. Photonics 2014, 8, 506–514. [Google Scholar] [CrossRef]
- Urbani, M.; Grätzel, M.; Nazeeruddin, M.K.; Torres, T. Meso-Substituted Porphyrins for Dye-Sensitized Solar Cells. Chem. Rev. 2014, 114, 12330–12396. [Google Scholar] [CrossRef]
- Lin, Y.; Li, Y.; Zhan, X. Small Molecule Semiconductors for High-Efficiency Organic Photovoltaics. Chem. Soc. Rev. 2012, 41, 4245–4272. [Google Scholar] [CrossRef] [PubMed]
- Günes, S.; Neugebauer, H.; Sariciftci, N.S. Conjugated Polymer-Based Organic Solar Cells. Chem. Rev. 2007, 107, 1324–1338. [Google Scholar] [CrossRef]
- Do, K.; Choi, H.; Lim, K.; Jo, H.; Cho, J.W.; Nazeeruddin, M.K.; Ko, J. Star-Shaped Hole Transporting Materials with a Triazine Unit for Efficient Perovskite Solar Cells. Chem. Commun. 2014, 50, 10971–10974. [Google Scholar] [CrossRef]
- Cherioux, F.; Audebert, P.; Hapiot, P. New Star-Shaped Molecules with Extended Electronic Delocalization. Chem. Mater. 1998, 10, 1984–1989. [Google Scholar] [CrossRef]
- Thomas, K.R.J.; Venkateswararao, A.; Balasaravanan, R.; Li, C.-T.; Ho, K.-C. Triazine-Branched Mono- and Dianchoring Organic Dyes: Effect of Acceptor Arms on Optical and Photovoltaic Properties. Dye. Pigment. 2019, 165, 182–192. [Google Scholar] [CrossRef]
- Aryal, U.K.; Reddy, S.S.; Choi, J.; Woo, C.Y.; Jang, S.; Lee, Y.; Kim, B.S.; Lee, H.W.; Jin, S.-H. Efficient Cathode Interfacial Materials Based on Triazine/Phosphine Oxide for Conventional and Inverted Organic Solar Cells. Macromol. Res. 2020, 28, 727–732. [Google Scholar] [CrossRef]
- Berger, R.; Hauser, J.; Labat, G.; Weber, E.; Hulliger, J. New Symmetrically Substituted 1,3,5-Triazines as Host Compounds for Channel-Type Inclusion Formation. CrystEngComm 2012, 14, 768–770. [Google Scholar] [CrossRef]
- El-Faham, A.; Farooq, M.; Almarhoon, Z.; Alhameed, R.A.; Wadaan, M.A.M.; de la Torre, B.G.; Albericio, F. Di- and Tri-Substituted s-Triazine Derivatives: Synthesis, Characterization, Anticancer Activity in Human Breast-Cancer Cell Lines, and Developmental Toxicity in Zebrafish Embryos. Bioorg. Chem. 2020, 94, 103397. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.-Y.; Lee, W.-H.; Lee, R.-H. Solution Processable Star-Shaped Molecules with a Triazine Core and Branching Thienylenevinylenes for Bulk Heterojunction Solar Cells. RSC Adv. 2014, 4, 48150–48162. [Google Scholar] [CrossRef]
- Grundmann, C.; Kreutzberger, A. Triazines. XIII. The Ring Cleavage of s-Triazine by Primary Amines. A New Method for the Synthesis of Heterocycles1,2. J. Am. Chem. Soc. 1955, 77, 6559–6562. [Google Scholar] [CrossRef]
- Blotny, G. Recent Applications of 2,4,6-Trichloro-1,3,5-Triazine and Its Derivatives in Organic Synthesis. Tetrahedron 2006, 62, 9507–9522. [Google Scholar] [CrossRef]
- Mibu, N.; Yokomizo, K.; Takemura, S.; Ueki, N.; Itohara, S.; Zhou, J.; Miyata, T.; Sumoto, K. Synthesis and Biological Evaluation of Symmetrical 2,4,6-Trisubstituted 1,3,5-Triazine Derivatives. Chem. Pharm. Bull. 2013, 61, 823–833. [Google Scholar] [CrossRef][Green Version]
- Al-Zaydi, K.M.; Khalil, H.H.; El-Faham, A.; Khattab, S.N. Synthesis, Characterization and Evaluation of 1,3,5-Triazine Aminobenzoic Acid Derivatives for Their Antimicrobial Activity. Chem. Cent. J. 2017, 11, 39. [Google Scholar] [CrossRef]
- Mikroyannidis, J.A.; Sharma, S.S.; Vijay, Y.K.; Sharma, G.D. Novel Low Band Gap Small Molecule and Phenylenevinylene Copolymer with Cyanovinylene 4-Nitrophenyl Segments: Synthesis and Application for Efficient Bulk Heterojunction Solar Cells. ACS Appl. Mater. Interfaces 2010, 2, 270–278. [Google Scholar] [CrossRef]
- Sharma, G.D.; Zervaki, G.E.; Angaridis, P.A.; Kitsopoulos, T.N.; Coutsolelos, A.G. Triazine-Bridged Porphyrin Triad as Electron Donor for Solution-Processed Bulk Hetero-Junction Organic Solar Cells. J. Phys. Chem. C 2014, 118, 5968–5977. [Google Scholar] [CrossRef]
- Chakravarthi, N.; Gunasekar, K.; Cho, W.; Long, D.X.; Kim, Y.-H.; Song, C.E.; Lee, J.-C.; Facchetti, A.; Song, M.; Noh, Y.-Y.; et al. A Simple Structured and Efficient Triazine-Based Molecule as an Interfacial Layer for High Performance Organic Electronics. Energy Environ. Sci. 2016, 9, 2595–2602. [Google Scholar] [CrossRef]
- Liu, J.; Wang, K.; Zhang, X.; Li, C.; You, X. Triazine Dyes as Photosensitizers for Dye-Sensitized Solar Cells. Tetrahedron 2013, 69, 190–200. [Google Scholar] [CrossRef]
- Liu, J.; Wang, K.; Xu, F.; Tang, Z.; Zheng, W.; Zhang, J.; Li, C.; Yu, T.; You, X. Synthesis and Photovoltaic Performances of Donor–π–Acceptor Dyes Utilizing 1,3,5-Triazine as π Spacers. Tetrahedron Lett. 2011, 52, 6492–6496. [Google Scholar] [CrossRef]
- Zervaki, G.E.; Angaridis, P.A.; Koukaras, E.N.; Sharma, G.D.; Coutsolelos, A.G. Dye-Sensitized Solar Cells Based on Triazine-Linked Porphyrin Dyads Containing One or Two Carboxylic Acid Anchoring Groups. Inorg. Chem. Front. 2014, 1, 256–270. [Google Scholar] [CrossRef]
- Sharma, G.D.; Zervaki, G.E.; Angaridis, P.; Coutsolelos, A.G. New Solution Processed Bulk-Heterojunction Organic Solar Cells Based on a Triazine-Bridged Porphyrin Dyad as Electron Donor. RSC Adv. 2014, 4, 50819–50827. [Google Scholar] [CrossRef]
- Sharma, A.; El-Faham, A.; de la Torre, B.G.; Albericio, F. Exploring the Orthogonal Chemoselectivity of 2,4,6-Trichloro-1,3,5-Triazine (TCT) as a Trifunctional Linker With Different Nucleophiles: Rules of the Game. Front. Chem. 2018, 6, 516. [Google Scholar] [CrossRef]
- Herrera, A.; Riaño, A.; Moreno, R.; Caso, B.; Pardo, Z.D.; Fernández, I.; Sáez, E.; Molero, D.; Sánchez-Vázquez, A.; Martínez-Alvarez, R. One-Pot Synthesis of 1,3,5-Triazine Derivatives via Controlled Cross-Cyclotrimerization of Nitriles: A Mechanism Approach. J. Org. Chem. 2014, 79, 7012–7024. [Google Scholar] [CrossRef]
- de la Hoz, A.; Díaz-Ortiz, A.; Elguero, J.; Martínez, L.J.; Moreno, A.; Sánchez-Migallón, A. Solvent-Free Preparation of Tris-Pyrazolyl-1,3,5-Triazines. Tetrahedron 2001, 57, 4397–4403. [Google Scholar] [CrossRef]
- Díaz-Ortiz, A.; de la Hoz, A.; Moreno, A.; Sánchez-Migallón, A.; Valiente, G. Synthesis of 1,3,5-Triazines in Solvent-Free Conditions Catalysed by Silica-Supported Lewis Acids. Green Chem. 2002, 4, 339–343. [Google Scholar] [CrossRef]
- Díaz-Ortiz, Á.; Elguero, J.; Foces-Foces, C.; de la Hoz, A.; Moreno, A.; del Carmen Mateo, M.; Sánchez-Migallón, A.; Valiente, G. Green Synthesis and Self-Association of 2,4-Diamino-1,3,5-Triazine Derivatives. New J. Chem. 2004, 28, 952–958. [Google Scholar] [CrossRef]
- Chalermnon, M.; Cherdchom, S.; Sereemaspun, A.; Rojanathanes, R.; Khotavivattana, T. Biguanide-Based Synthesis of 1,3,5-Triazine Derivatives with Anticancer Activity and 1,3,5-Triazine Incorporated Calcium Citrate Nanoparticles. Molecules 2021, 26, 1028. [Google Scholar] [CrossRef]
- Linder, T.; Schnürch, M.; Mihovilovic, M.D. One-Pot Synthesis of Triazines as Potential Agents Affecting Cell Differentiation. Mon. für Chemie-Chem. Mon. 2018, 149, 1257–1284. [Google Scholar] [CrossRef]
- Throckmorton, J.; Palmese, G. Acceleration of Cyanate Ester Trimerization by Dicyanamide RTILs. Polymer 2016, 91, 7–13. [Google Scholar] [CrossRef]
- You, Q.; Wang, F.; Wu, C.; Shi, T.; Min, D.; Chen, H.; Zhang, W. Synthesis of 1,3,5-Triazines via Cu(OAc)2-Catalyzed Aerobic Oxidative Coupling of Alcohols and Amidine Hydrochlorides. Org. Biomol. Chem. 2015, 13, 6723–6727. [Google Scholar] [CrossRef] [PubMed]
- Shiau, S.-Y.; Chang, C.-H.; Chen, W.-J.; Wang, H.-J.; Jeng, R.-J.; Lee, R.-H. Star-Shaped Organic Semiconductors with Planar Triazine Core and Diketopyrrolopyrrole Branches for Solution-Processed Small-Molecule Organic Solar Cells. Dye. Pigment. 2015, 115, 35–49. [Google Scholar] [CrossRef]
- Duan, Y.; Xu, X.; Yan, H.; Wu, W.; Li, Z.; Peng, Q. Pronounced Effects of a Triazine Core on Photovoltaic Performance–Efficient Organic Solar Cells Enabled by a PDI Trimer-Based Small Molecular Acceptor. Adv. Mater. 2017, 29, 1605115. [Google Scholar] [CrossRef] [PubMed]
- Johansson Seechurn, C.C.C.; Kitching, M.O.; Colacot, T.J.; Snieckus, V. Palladium-Catalyzed Cross-Coupling: A Historical Contextual Perspective to the 2010 Nobel Prize. Angew. Chemie Int. Ed. 2012, 51, 5062–5085. [Google Scholar] [CrossRef]
- Roy, D.; Uozumi, Y. Recent Advances in Palladium-Catalyzed Cross-Coupling Reactions at Ppm to Ppb Molar Catalyst Loadings. Adv. Synth. Catal. 2018, 360, 602–625. [Google Scholar] [CrossRef]
- Christoffel, F.; Ward, T.R. Palladium-Catalyzed Heck Cross-Coupling Reactions in Water: A Comprehensive Review. Catal. Lett. 2018, 148, 489–511. [Google Scholar] [CrossRef]
- Lattanzi, A.; Orelli, L.R.; Barone, P.; Massa, A.; Iannece, P.; Scettri, A. Convenient Procedure of Horner–Wadsworth–Emmons Olefination for the Synthesis of Simple and Functionalized α,β-Unsaturated Nitriles. Tetrahedron Lett. 2003, 44, 1333–1337. [Google Scholar] [CrossRef]
- Pastoetter, D.L.; Xu, S.; Borrelli, M.; Addicoat, M.; Biswal, B.P.; Paasch, S.; Dianat, A.; Thomas, H.; Berger, R.; Reineke, S.; et al. Synthesis of Vinylene-Linked Two-Dimensional Conjugated Polymers via the Horner–Wadsworth–Emmons Reaction. Angew. Chemie Int. Ed. 2020, 59, 23620–23625. [Google Scholar] [CrossRef]
- Naka, A.; Fujishima, K.; Okada, E.; Noguchi, M.; Ohshita, J.; Adachi, Y.; Ooyama, Y.; Ishikawa, M. Synthesis of Pentamethyldisilanyl-Substituted Starlike Molecule with Triazine Core and Its Application to Dye-Sensitized Solar Cells. J. Organomet. Chem. 2016, 825–826, 63–68. [Google Scholar] [CrossRef]
- Yeh, N.; Yeh, P. Organic Solar Cells: Their Developments and Potentials. Renew. Sustain. Energy Rev. 2013, 21, 421–431. [Google Scholar] [CrossRef]
- Qing, L.; Qian, K.; Yi, Y.; Zhong, Z.; Jinzhao, Q.; Bowei, X.; Jianhui, H. Highly Stable Organic Solar Cells Based on an Ultraviolet-Resistant Cathode Interfacial Layer. CCS Chem. 2021, 4, 938–948. [Google Scholar] [CrossRef]
- Silvestri, F.; Marrocchi, A.; Seri, M.; Kim, C.; Marks, T.J.; Facchetti, A.; Taticchi, A. Solution-Processable Low-Molecular Weight Extended Arylacetylenes: Versatile p-Type Semiconductors for Field-Effect Transistors and Bulk Heterojunction Solar Cells. J. Am. Chem. Soc. 2010, 132, 6108–6123. [Google Scholar] [CrossRef] [PubMed]
- Kumavat, P.P.; Sonar, P.; Dalal, D.S. An Overview on Basics of Organic and Dye Sensitized Solar Cells, Their Mechanism and Recent Improvements. Renew. Sustain. Energy Rev. 2017, 78, 1262–1287. [Google Scholar] [CrossRef]
- Marinova, N.; Valero, S.; Delgado, J.L. Organic and Perovskite Solar Cells: Working Principles, Materials and Interfaces. J. Colloid Interface Sci. 2017, 488, 373–389. [Google Scholar] [CrossRef]
- Wang, D.; Geng, Z. Design and Characteration of Planar Star-Shaped Oligomer Electron Donors for Organic Solar Cells: A DFT Study. Can. J. Chem. 2015, 93, 1181–1190. [Google Scholar] [CrossRef]
- Zhang, X.; Jin, R. Rational Design of Low-Band Gap Star-Shaped Molecules With 2,4,6-Triphenyl-1,3,5-Triazine as Core and Diketopyrrolopyrrole Derivatives as Arms for Organic Solar Cells Applications. Front. Chem. 2019, 7, 122. [Google Scholar] [CrossRef]
- Wan, X.; Li, C.; Zhang, M.; Chen, Y. Acceptor–Donor–Acceptor Type Molecules for High Performance Organic Photovoltaics—Chemistry and Mechanism. Chem. Soc. Rev. 2020, 49, 2828–2842. [Google Scholar] [CrossRef]
- Kan, B.; Kan, Y.; Zuo, L.; Shi, X.; Gao, K. Recent Progress on All-Small Molecule Organic Solar Cells Using Small-Molecule Nonfullerene Acceptors. InfoMat 2021, 3, 175–200. [Google Scholar] [CrossRef]
- Piradi, V.; Yan, F.; Zhu, X.; Wong, W.-Y. A Recent Overview of Porphyrin-Based π-Extended Small Molecules as Donors and Acceptors for High-Performance Organic Solar Cells. Mater. Chem. Front. 2021, 5, 7119–7133. [Google Scholar] [CrossRef]
- Li, X.; Zhang, W.; Usman, K.; Fang, J. Small Molecule Interlayers in Organic Solar Cells. Adv. Energy Mater. 2018, 8, 1702730. [Google Scholar] [CrossRef]
- Gao, X.; Wu, Y.; Song, X.; Tao, X.; He, Y.; Yang, T.; Yu, R.; Li, Z.; Tao, Y. Star-Shaped Small Molecular Donors Based on a Zn-Porphyrin Core and DPP Arms via Different Linkers for Organic Solar Cells. Dye. Pigment. 2021, 188, 109216. [Google Scholar] [CrossRef]
- Mone, M.; Tang, S.; Murto, P.; Abdulahi, B.A.; Larsen, C.; Wang, J.; Mammo, W.; Edman, L.; Wang, E. Star-Shaped Diketopyrrolopyrrole–Zinc Porphyrin That Delivers 900 Nm Emission in Light-Emitting Electrochemical Cells. Chem. Mater. 2019, 31, 9721–9728. [Google Scholar] [CrossRef]
- Cao, B.; He, X.; Fetterly, C.R.; Olsen, B.C.; Luber, E.J.; Buriak, J.M. Role of Interfacial Layers in Organic Solar Cells: Energy Level Pinning versus Phase Segregation. ACS Appl. Mater. Interfaces 2016, 8, 18238–18248. [Google Scholar] [CrossRef]
- Zhang, H.; Li, Y.; Zhang, X.; Zhang, Y.; Zhou, H. Role of Interface Properties in Organic Solar Cells: From Substrate Engineering to Bulk-Heterojunction Interfacial Morphology. Mater. Chem. Front. 2020, 4, 2863–2880. [Google Scholar] [CrossRef]
- O’Regan, B.; Grätzel, M. A Low-Cost, High-Efficiency Solar Cell Based on Dye-Sensitized Colloidal TiO2 Films. Nature 1991, 353, 737–740. [Google Scholar] [CrossRef]
- Boschloo, G. Improving the Performance of Dye-Sensitized Solar Cells. Front. Chem. 2019, 7, 77. [Google Scholar] [CrossRef]
- Kokkonen, M.; Talebi, P.; Zhou, J.; Asgari, S.; Soomro, S.A.; Elsehrawy, F.; Halme, J.; Ahmad, S.; Hagfeldt, A.; Hashmi, S.G. Advanced Research Trends in Dye-Sensitized Solar Cells. J. Mater. Chem. A 2021, 9, 10527–10545. [Google Scholar] [CrossRef]
- Rondán-Gómez, V.; Montoya De Los Santos, I.; Seuret-Jiménez, D.; Ayala-Mató, F.; Zamudio-Lara, A.; Robles-Bonilla, T.; Courel, M. Recent Advances in Dye-Sensitized Solar Cells. Appl. Phys. A 2019, 125, 836. [Google Scholar] [CrossRef]
- Hagfeldt, A.; Boschloo, G.; Sun, L.; Kloo, L.; Pettersson, H. Dye-Sensitized Solar Cells. Chem. Rev. 2010, 110, 6595–6663. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Lan, Z.; Lin, J.; Huang, M.; Huang, Y.; Fan, L.; Luo, G. Electrolytes in Dye-Sensitized Solar Cells. Chem. Rev. 2015, 115, 2136–2173. [Google Scholar] [CrossRef] [PubMed]
- Bousrez, G.; Renier, O.; Adranno, B.; Smetana, V.; Mudring, A.-V. Ionic Liquid-Based Dye-Sensitized Solar Cells—Insights into Electrolyte and Redox Mediator Design. ACS Sustain. Chem. Eng. 2021, 9, 8107–8114. [Google Scholar] [CrossRef]
- Denizalti, S.; Ali, A.K.; Ela, Ç.; Ekmekci, M.; Erten-Ela, S. Dye-Sensitized Solar Cells Using Ionic Liquids as Redox Mediator. Chem. Phys. Lett. 2018, 691, 373–378. [Google Scholar] [CrossRef]
- Jang, Y.J.; Thogiti, S.; Lee, K.; Kim, J.H. Long-Term Stable Solid-State Dye-Sensitized Solar Cells Assembled with Solid-State Polymerized Hole-Transporting Material. Crystals 2019, 9, 452. [Google Scholar] [CrossRef]
- Mehmood, U.; Rahman, S.; Harrabi, K.; Hussein, I.A.; Reddy, B.V.S. Recent Advances in Dye Sensitized Solar Cells. Adv. Mater. Sci. Eng. 2014, 2014, 974782. [Google Scholar] [CrossRef]
- Shalini, S.; Balasundaraprabhu, R.; Kumar, T.S.; Prabavathy, N.; Senthilarasu, S.; Prasanna, S. Status and Outlook of Sensitizers/Dyes Used in Dye Sensitized Solar Cells (DSSC): A Review. Int. J. Energy Res. 2016, 40, 1303–1320. [Google Scholar] [CrossRef]
- Ooyama, Y.; Harima, Y. Photophysical and Electrochemical Properties, and Molecular Structures of Organic Dyes for Dye-Sensitized Solar Cells. ChemPhysChem 2012, 13, 4032–4080. [Google Scholar] [CrossRef]
- Sharma, K.; Sharma, V.; Sharma, S.S. Dye-Sensitized Solar Cells: Fundamentals and Current Status. Nanoscale Res. Lett. 2018, 13, 381. [Google Scholar] [CrossRef]
- Zhang, H.; Zang, X.-F.; Hong, Y.-P.; Chen, Z.-E. Theoretical and Photovoltaic Investigations of 1,3,5-Triazine-Based Photosensitizers Achieving Highly Efficient DSSCs. Synth. Met. 2021, 280, 116882. [Google Scholar] [CrossRef]
- Sharma, G.D.; Angaridis, P.A.; Pipou, S.; Zervaki, G.E.; Nikolaou, V.; Misra, R.; Coutsolelos, A.G. Efficient Co-Sensitization of Dye-Sensitized Solar Cells by Novel Porphyrin/Triazine Dye and Tertiary Aryl-Amine Organic Dye. Org. Electron. 2015, 25, 295–307. [Google Scholar] [CrossRef]
- Zervaki, G.E.; Roy, M.S.; Panda, M.K.; Angaridis, P.A.; Chrissos, E.; Sharma, G.D.; Coutsolelos, A.G. Efficient Sensitization of Dye-Sensitized Solar Cells by Novel Triazine-Bridged Porphyrin–Porphyrin Dyads. Inorg. Chem. 2013, 52, 9813–9825. [Google Scholar] [CrossRef] [PubMed]
- Zervaki, G.E.; Papastamatakis, E.; Angaridis, P.A.; Nikolaou, V.; Singh, M.; Kurchania, R.; Kitsopoulos, T.N.; Sharma, G.D.; Coutsolelos, A.G. A Propeller-Shaped, Triazine-Linked Porphyrin Triad as Efficient Sensitizer for Dye-Sensitized Solar Cells. Eur. J. Inorg. Chem. 2014, 2014, 1020–1033. [Google Scholar] [CrossRef]
- Kojima, A.; Teshima, K.; Shirai, Y.; Miyasaka, T. Organometal Halide Perovskites as Visible-Light Sensitizers for Photovoltaic Cells. J. Am. Chem. Soc. 2009, 131, 6050–6051. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, K.; Takahashi, T.; Ban, T.; Kondo, T.; Uchida, K.; Miura, N. Comparative Study on the Excitons in Lead-Halide-Based Perovskite-Type Crystals CH3NH3PbBr3 CH3NH3PbI3. Solid State Commun. 2003, 127, 619–623. [Google Scholar] [CrossRef]
- Kim, H.G.; Borse, P.H.; Jang, J.S.; Jeong, E.D.; Lee, J.S. Enhanced Photochemical Properties of Electron Rich W-Doped PbBi2Nb2O9 Layered Perovskite Material under Visible-Light Irradiation. Mater. Lett. 2008, 62, 1427–1430. [Google Scholar] [CrossRef]
- Im, J.-H.; Lee, C.-R.; Lee, J.-W.; Park, S.-W.; Park, N.-G. 6.5% Efficient Perovskite Quantum-Dot-Sensitized Solar Cell. Nanoscale 2011, 3, 4088–4093. [Google Scholar] [CrossRef]
- Kim, J.Y.; Lee, J.-W.; Jung, H.S.; Shin, H.; Park, N.-G. High-Efficiency Perovskite Solar Cells. Chem. Rev. 2020, 120, 7867–7918. [Google Scholar] [CrossRef]
- Im, J.-H.; Chung, J.; Kim, S.-J.; Park, N.-G. Synthesis, Structure, and Photovoltaic Property of a Nanocrystalline 2H Perovskite-Type Novel Sensitizer (CH3CH2NH3)PbI3. Nanoscale Res. Lett. 2012, 7, 353. [Google Scholar] [CrossRef]
- Li, W.; Li, J.; Wang, L.; Niu, G.; Gao, R.; Qiu, Y. Post Modification of Perovskite Sensitized Solar Cells by Aluminum Oxide for Enhanced Performance. J. Mater. Chem. A 2013, 1, 11735–11740. [Google Scholar] [CrossRef]
- Urieta-Mora, J.; García-Benito, I.; Molina-Ontoria, A.; Martín, N. Hole Transporting Materials for Perovskite Solar Cells: A Chemical Approach. Chem. Soc. Rev. 2018, 47, 8541–8571. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.-S.; Lee, C.-R.; Im, J.-H.; Lee, K.-B.; Moehl, T.; Marchioro, A.; Moon, S.-J.; Humphry-Baker, R.; Yum, J.-H.; Moser, J.E.; et al. Lead Iodide Perovskite Sensitized All-Solid-State Submicron Thin Film Mesoscopic Solar Cell with Efficiency Exceeding 9%. Sci. Rep. 2012, 2, 591. [Google Scholar] [CrossRef]
- Lee, M.M.; Teuscher, J.; Miyasaka, T.; Murakami, T.N.; Snaith, H.J. Efficient Hybrid Solar Cells Based on Meso-Superstructured Organometal Halide Perovskites. Science 2012, 338, 643–647. [Google Scholar] [CrossRef] [PubMed]
- Noh, J.H.; Jeon, N.J.; Choi, Y.C.; Nazeeruddin, M.K.; Grätzel, M.; Seok, S. Il Nanostructured TiO2/CH3NH3PbI3 Heterojunction Solar Cells Employing Spiro-OMeTAD/Co-Complex as Hole-Transporting Material. J. Mater. Chem. A 2013, 1, 11842–11847. [Google Scholar] [CrossRef]
- Etgar, L.; Gao, P.; Xue, Z.; Peng, Q.; Chandiran, A.K.; Liu, B.; Nazeeruddin, M.K.; Grätzel, M. Mesoscopic CH3NH3PbI3/TiO2 Heterojunction Solar Cells. J. Am. Chem. Soc. 2012, 134, 17396–17399. [Google Scholar] [CrossRef] [PubMed]
- Zu, F.; Shin, D.; Koch, N. Electronic Properties of Metal Halide Perovskites and Their Interfaces: The Basics. Mater. Horizons 2022, 9, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Sharma, R.; Sharma, A.; Agarwal, S.; Dhaka, M.S. Stability and Efficiency Issues, Solutions and Advancements in Perovskite Solar Cells: A Review. Sol. Energy 2022, 244, 516–535. [Google Scholar] [CrossRef]
- Hussain, I.; Tran, H.P.; Jaksik, J.; Moore, J.; Islam, N.; Uddin, M.J. Functional Materials, Device Architecture, and Flexibility of Perovskite Solar Cell. Emergent Mater. 2018, 1, 133–154. [Google Scholar] [CrossRef]
- Vivo, P.; Salunke, J.K.; Priimagi, A. Hole-Transporting Materials for Printable Perovskite Solar Cells. Materials 2017, 10, 1087. [Google Scholar] [CrossRef]
- Calió, L.; Kazim, S.; Grätzel, M.; Ahmad, S. Hole-Transport Materials for Perovskite Solar Cells. Angew. Chemie Int. Ed. 2016, 55, 14522–14545. [Google Scholar] [CrossRef]
- Kazim, S.; Nazeeruddin, M.K.; Grätzel, M.; Ahmad, S. Perovskite as Light Harvester: A Game Changer in Photovoltaics. Angew. Chemie Int. Ed. 2014, 53, 2812–2824. [Google Scholar] [CrossRef] [PubMed]
- Lim, K.; Kang, M.-S.; Myung, Y.; Seo, J.-H.; Banerjee, P.; Marks, T.J.; Ko, J. Star-Shaped Hole Transport Materials with Indeno [1,2-b] Thiophene or Fluorene on a Triazine Core for Efficient Perovskite Solar Cells. J. Mater. Chem. A 2016, 4, 1186–1190. [Google Scholar] [CrossRef]
- Shi, L.; Sun, X.; Yuan, H.; Zhang, K.; Li, X.; Zhang, Y.; Ban, X.; Huang, Z.; Zhang, D. Para-Halogenated Triphenyltriazine Induced Surface Passivation toward Efficient and Stable Perovskite Solar Cells. Appl. Surf. Sci. 2022, 590, 153051. [Google Scholar] [CrossRef]
- Chen, S.; Pan, Q.; Li, J.; Zhao, C.; Guo, X.; Zhao, Y.; Jiu, T. Grain Boundary Passivation with Triazine-Graphdiyne to Improve Perovskite Solar Cell Performance. Sci. China Mater. 2020, 63, 2465–2476. [Google Scholar] [CrossRef]
- Kim, S.-G.; Chen, J.; Seo, J.-Y.; Kang, D.-H.; Park, N.-G. Rear-Surface Passivation by Melaminium Iodide Additive for Stable and Hysteresis-Less Perovskite Solar Cells. ACS Appl. Mater. Interfaces 2018, 10, 25372–25383. [Google Scholar] [CrossRef]
- Kuo, D.-W.; Liu, G.-Z.; Lee, R.-H. Star-Shaped Molecule with Planar Triazine Core and Perylene Diimide Branches as an n-Type Additive for Bulk-Heterojunction Perovskite Solar Cells. Dye. Pigment. 2019, 170, 107562. [Google Scholar] [CrossRef]
- Gkini, K.; Verykios, A.; Balis, N.; Kaltzoglou, A.; Papadakis, M.; Adamis, K.S.; Armadorou, K.-K.; Soultati, A.; Drivas, C.; Gardelis, S.; et al. Enhanced Organic and Perovskite Solar Cell Performance through Modification of the Electron-Selective Contact with a Bodipy–Porphyrin Dyad. ACS Appl. Mater. Interfaces 2020, 12, 1120–1131. [Google Scholar] [CrossRef]
- Balis, N.; Verykios, A.; Soultati, A.; Constantoudis, V.; Papadakis, M.; Kournoutas, F.; Drivas, C.; Skoulikidou, M.-C.; Gardelis, S.; Fakis, M.; et al. Triazine-Substituted Zinc Porphyrin as an Electron Transport Interfacial Material for Efficiency Enhancement and Degradation Retardation in Planar Perovskite Solar Cells. ACS Appl. Energy Mater. 2018, 1, 3216–3229. [Google Scholar] [CrossRef]
- Chakravarthi, N.; Park, H.-Y.; Kranthiraja, K.; Kim, H.; Shin, J.; Song, M.; Jin, S.-H. Substituent Position Engineering of Phosphine Oxide Functionalized Triazine-Based Cathode Interfacial Materials for Flexible Organic and Perovskite Solar Cells. Org. Electron. 2018, 54, 54–63. [Google Scholar] [CrossRef]
- Xiang, W.; Liu, S.; Tress, W. Interfaces and Interfacial Layers in Inorganic Perovskite Solar Cells. Angew. Chemie Int. Ed. 2021, 60, 26440–26453. [Google Scholar] [CrossRef]
- Vasilopoulou, M.; Fakharuddin, A.; Coutsolelos, A.G.; Falaras, P.; Argitis, P.; Yusoff, A.R. bin M.; Nazeeruddin, M.K. Molecular Materials as Interfacial Layers and Additives in Perovskite Solar Cells. Chem. Soc. Rev. 2020, 49, 4496–4526. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Q.; Zhao, Y.; Zhang, X.; Yang, X.; Chen, Y.; Chu, Z.; Ye, Q.; Li, X.; Yin, Z.; You, J. Surface Passivation of Perovskite Film for Efficient Solar Cells. Nat. Photonics 2019, 13, 460–466. [Google Scholar] [CrossRef]
- Girish, K.H. Advances in Surface Passivation of Perovskites Using Organic Halide Salts for Efficient and Stable Solar Cells. Surfaces Interfaces 2021, 26, 101420. [Google Scholar] [CrossRef]
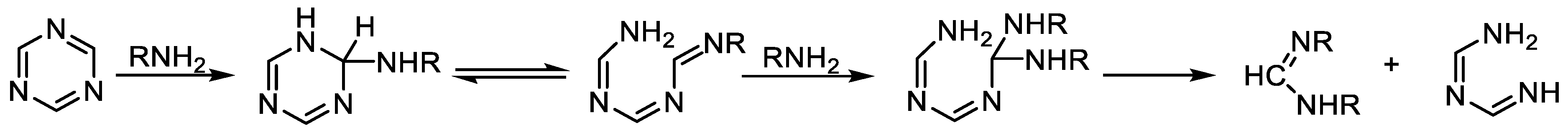
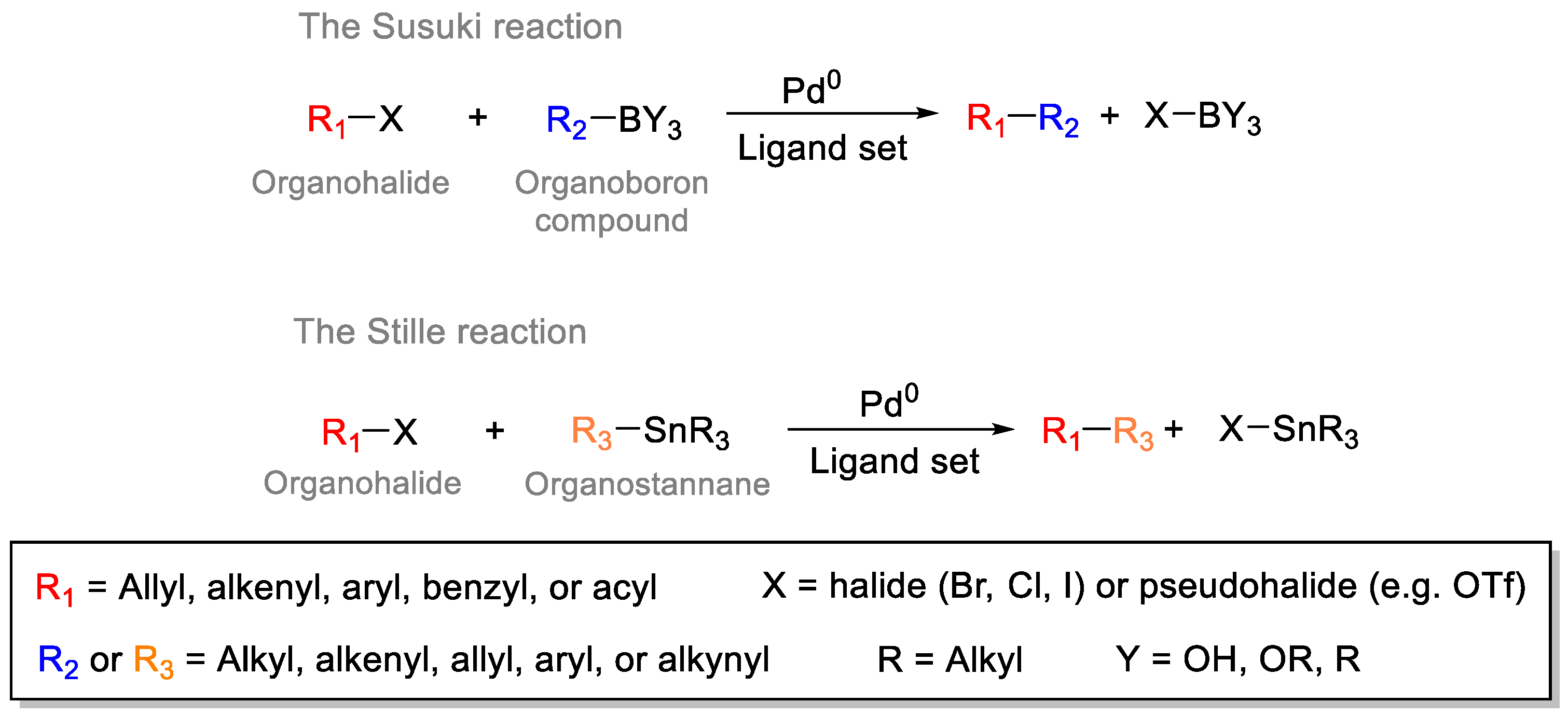

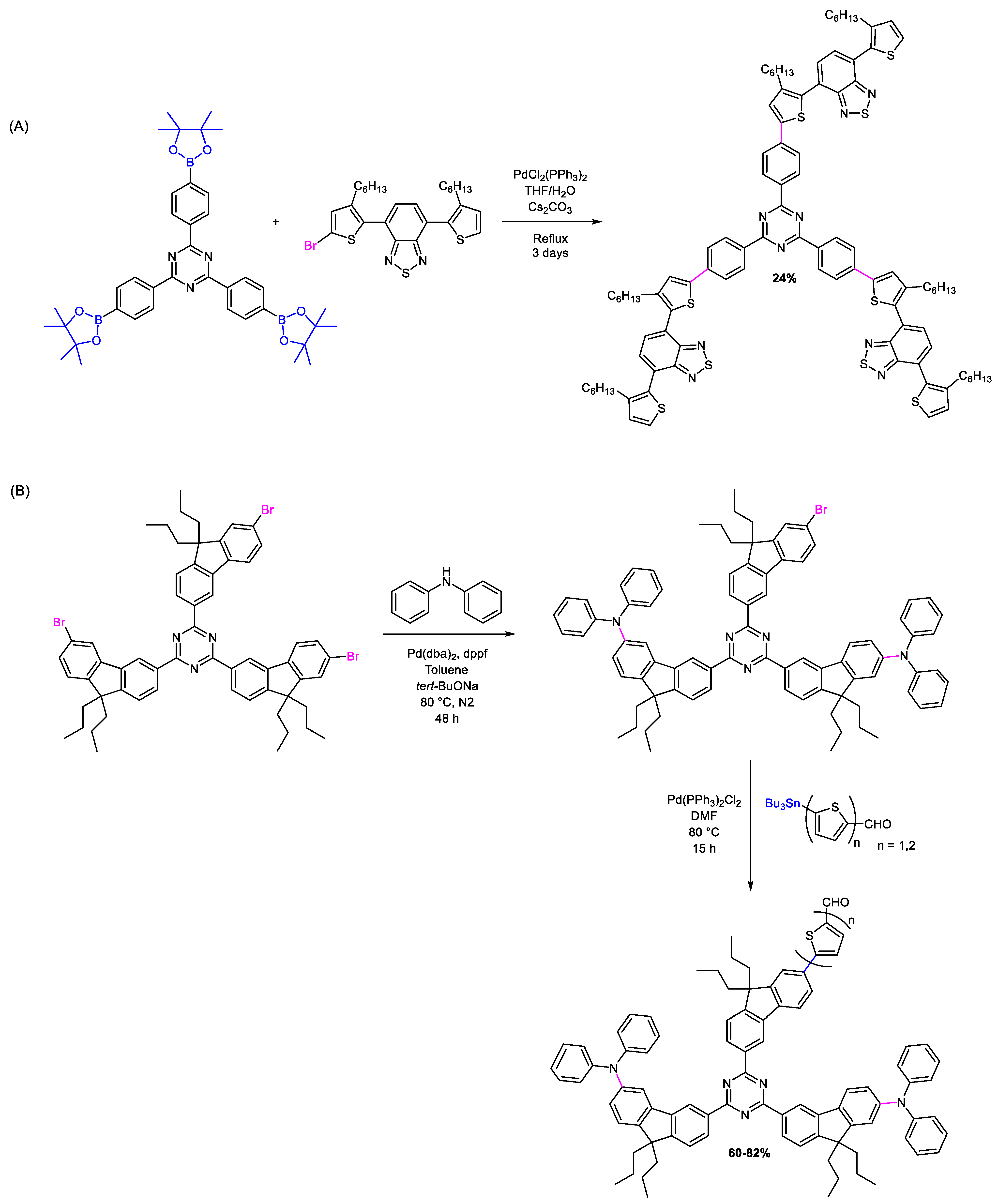
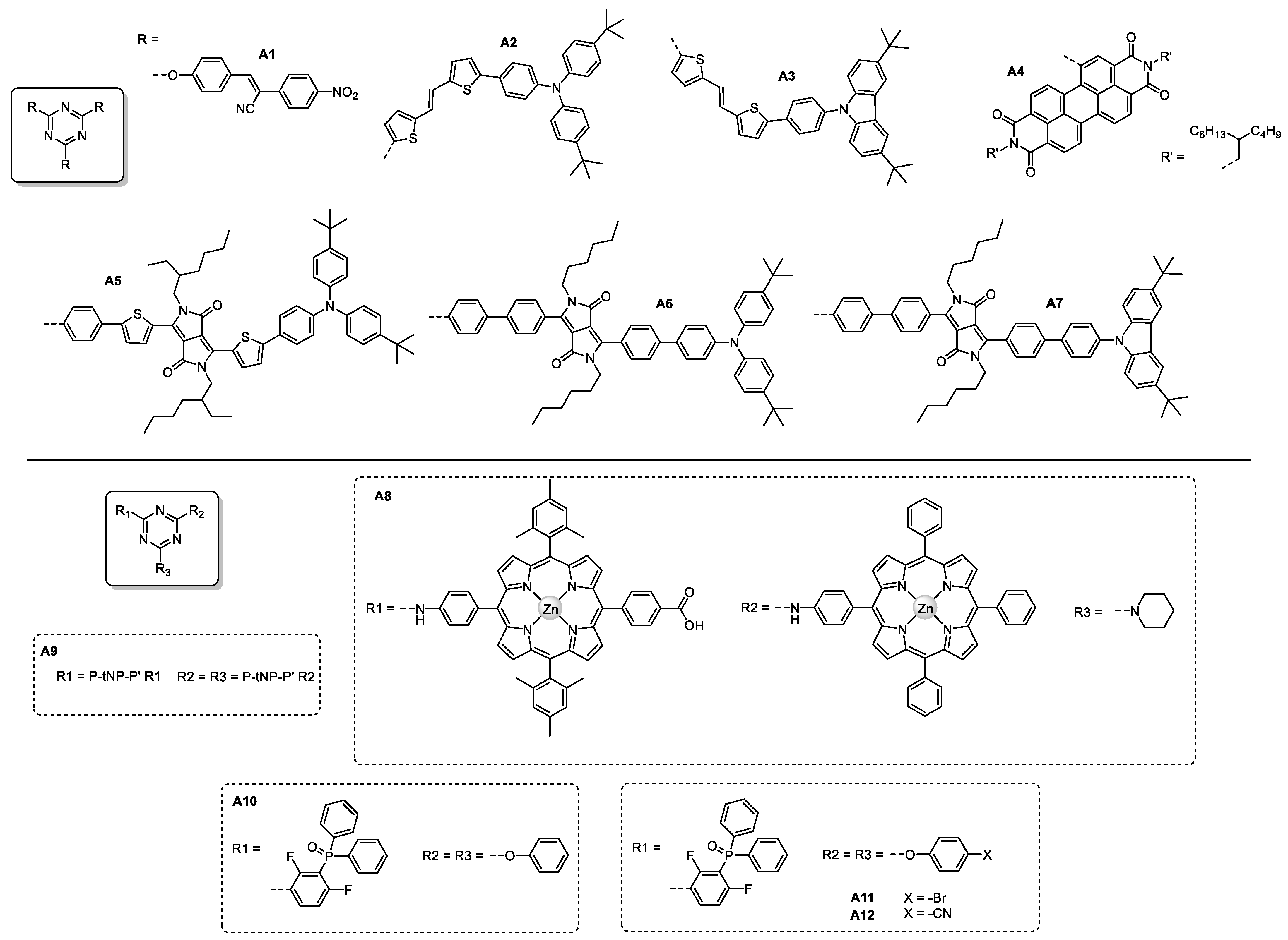
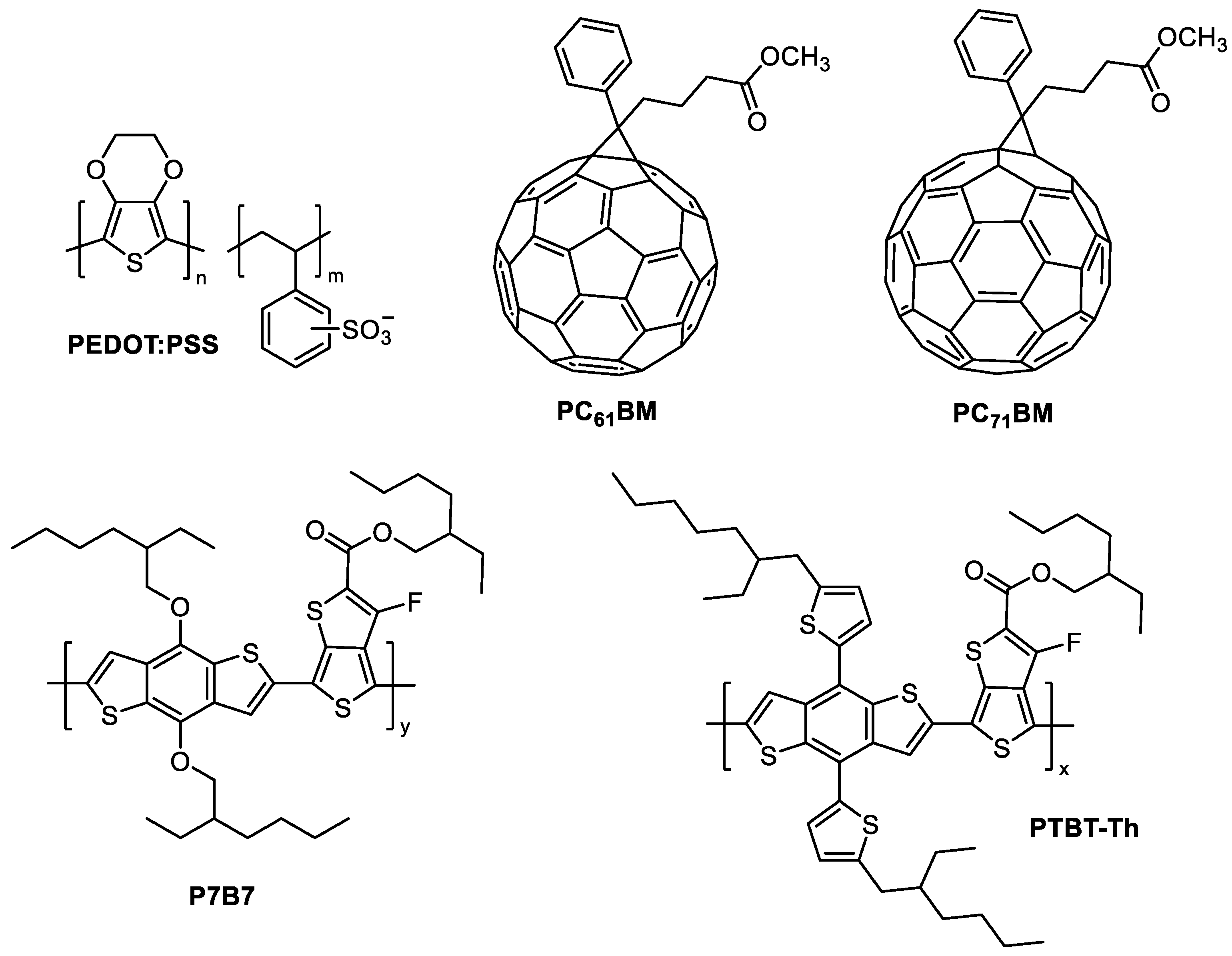

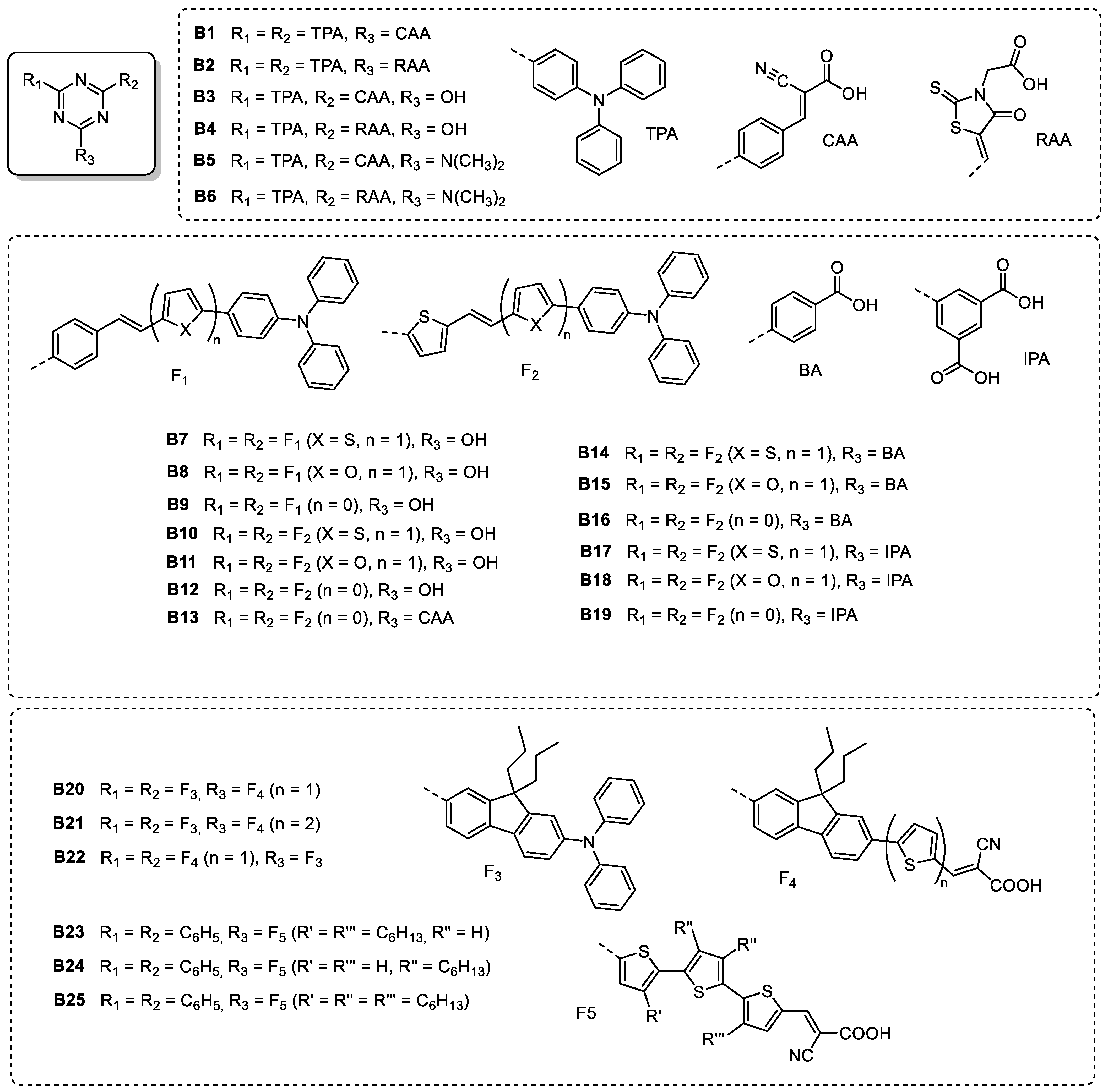


| Triazine-Based Molecule | λMAX (nm) | Egopt (eV) | HOMO/ LUMO (eV) | Photoactive Layer | VOC (V) | JSC (mA/cm2) | FF | PCE (%) | Ref. |
|---|---|---|---|---|---|---|---|---|---|
| A1 | 648(sol/tf) | 1.57 | –5.2/–3.6 | a1:PC61BM | 0.89–0.92 | 5.1–7.4 | 0.54–0.58 | 2.53–3.82 | [30] |
| A2 | 495(sol)/488(tf) | 2.05 | –5.47/–3.42 | a2:PC61BM | 0.56–0.69 | 1.06–4.80 | 0.33–0.36 | 0.20–1.18 | [25] |
| a2:PC71BM | 0.69 | 10.57 | 0.34 | 2.48 | [25] | ||||
| A3 | 442(sol)/446(tf) | 2.09 | –5.58/–3.49 | a3:PC61BM | 0.70–0.78 | 1.19–3.82 | 0.26–0.32 | 0.22–0.93 | [25] |
| a3:PC71BM | 0.78 | 6.00 | 0.31 | 1.45 | [25] | ||||
| A4 | 515(sol)/530(tf) | 2.05 | –6.03/–3.81 | a4:PTB7-Th | 0.78 | 17.10 | 0.68 | 8.91 | [47] |
| A5 | 623(sol)/640(tf) | 1.58 | –5.26/–3.68 | a5:PC61BM | 0.63–0.70 | 2.10–4.81 | 0.30–0.32 | 0.40–1.08 | [46] |
| a5:PC71BM | 0.73 | 6.34 | 0.34 | 1.57 | [46] | ||||
| A6 | 515(sol)/523(tf) | 2.06 | –5.26/–3.41 | a6:PC61BM | 0.48–0.52 | 1.40–4.63 | 0.30–0.33 | 0.25–0.79 | [46] |
| a6:PC71BM | 0.61 | 5.85 | 0.32 | 1.14 | [46] | ||||
| A7 | 505(sol)/514(tf) | 2.09 | –5.51/–3.42 | a7:PC61BM | 0.43–0.47 | 0.47–1.80 | 0.25–0.30 | 0.06–0.24 | [46] |
| a7:PC71BM | 0.50 | 2.75 | 0.28 | 0.39 | [46] | ||||
| A8 | 425(sol)/~435(tf) | 1.94 | –5.69/–3.31 | a8:PC71BM | 0.94–0.98 | 6.75–8.52 | 0.44–0.52 | 2.91–4.16 | [36] |
| A9 | 425(sol)/~435(tf) | 1.94 | –5.63/–3.55 | a9:PC71BM | 0.92–0.92 | 6.45–8.06 | 0.46–0.53 | 2.85–3.93 | [31] |
| A10 | ~270(sol/tf) | 3.99 | –6.96/–2.97 | PTB7:PC71BM | 0.74 | 15.04–18.85 | 0.68 | 7.64–9.66 | [32] |
| PTB7-Th: PC71BM | 0.81 | 17.69 | 0.70 | 9.77 | [32] | ||||
| A11 | <300 | 4.0 | –5.98/–1.98 | PTB7:PC71BM | 0.76–0.77 | 14.80–15.34 | 0.70 | 8.15–8.23 | [22] |
| A12 | <300 | 4.0 | –6.04/–2.04 | PTB7:PC71BM | 0.76 | 15.31–15.87 | 0.69 | 8.19–8.33 | [22] |
| Triazine-Based Molecule | λMAX a (nm) | ε b (×104 M−1 cm−1) | Eox c (V) | Ered d (V) | Redox Couple | VOC (V) | JSC (mA/cm2) | FF (%) | PCE | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|
| B1 | 396 | 5.44 | –1.49 e | 0.99 f | I–/I3– | 0.757 | 3.33 | 71.8 | 1.81 | [34] |
| B2 | 403 | 7.89 | –1.37 e | 1.02 f | I–/I3– | 0.648 | 1.67 | 75.9 | 0.82 | [34] |
| B3 | 397 | 2.31 | –1.36 e | 1.08 f | I–/I3– | 0.550 | 2.81 | 78.5 | 1.21 | [34] |
| B4 | 396 | 5.16 | –1.38 e | 1.00 f | I–/I3– | 0.510 | 0.96 | 70.8 | 0.35 | [34] |
| B5 | 374 | 2.75 | –1.52 e | 1.13 f | I–/I3– | 0.688 | 1.45 | 77.7 | 0.78 | [34] |
| B6 | 395 | 6.00 | –1.52 e | 0.97 f | I–/I3– | 0.543 | 1.37 | 64.1 | 0.48 | [34] |
| B7 | 448 | 7.70 | 1.26 | –0.76 | I–/I3– | 0.602 | 3.65 | 62.1 | 1.37 | [33] |
| B8 | 455 | 6.82 | 1.30 | –0.71 | I–/I3– | 0.615 | 3.72 | 59.5 | 1.36 | [33] |
| B9 | 436 | 6.66 | 1.45 | –0.71 | I–/I3– | 0.607 | 3.07 | 64.4 | 1.20 | [33] |
| B10 | 493 | 6.12 | 1.27 | –0.68 | I–/I3– | 0.591 | 3.97 | 66.1 | 1.55 | [33] |
| B11 | 498 | 6.37 | 1.31 | –0.63 | I–/I3– | 0.611 | 4.16 | 59.5 | 1.51 | [33] |
| B12 | 477 | 5.97 | 1.42 | –0.65 | I–/I3– | 0.608 | 3.49 | 56.3 | 1.20 | [33] |
| B13 | 459 | 7.58 | 1.32 | –0.89 | I–/I3– | 0.691 | 7.76 | 68.8 | 3.69 | [33] |
| B14 | 465 | 8.13 | 1.23 | –0.88 | I–/I3– | 0.631 | 5.65 | 61.1 | 2.17 | [33] |
| B15 | 479 | 7.92 | 1.24 | –0.86 | I–/I3– | 0.614 | 6.18 | 61.1 | 2.32 | [33] |
| B16 | 451 | 7.00 | 1.34 | –0.90 | I–/I3– | 0.619 | 5.21 | 60.9 | 1.97 | [33] |
| B17 | 463 | 7.98 | 1.24 | –0.85 | I–/I3– | 0.639 | 6.29 | 67.0 | 2.70 | [33] |
| B18 | 473 | 7.58 | 1.26 | –0.83 | I–/I3– | 0.583 | 6.58 | 72.2 | 2.77 | [33] |
| B19 | 451 | 7.25 | 1.35 | –0.86 | I–/I3– | 0.601 | 5.60 | 64.1 | 2.15 | [33] |
| B20 | 423 | 9.65 | 1.24 | –1.27 | I–/I3– | 0.702 | 7.04 | 58.0 | 2.88 | [21] |
| B21 | 490 | 8.80 | 1.24 | –1.29 | I–/I3– | 0.679 | 10.67 | 59.0 | 4.29 | [21] |
| B22 | 429 | 5.70 | 1.25 | –1.27 | I–/I3– | 0.665 | 6.36 | 66.0 | 2.80 | [21] |
| B23 | 472 | 3.38 | –5.33 e | –2.92 f | I–/I3– | 0.708 | 14.31 | 70.2 | 7.11 | [82] |
| B23 (+CDCA) | – | - | - | - | I–/I3– | 0.689 | 13.91 | 70.1 | 6.71 | [82] |
| B24 | 478 | 3.45 | –5.46 e | –2.89 f | I–/I3– | 0.714 | 14.09 | 68.4 | 6.89 | [82] |
| B24 (+CDCA) | - | - | - | - | I–/I3– | 0.723 | 13.29 | 71.8 | 7.06 | [82] |
| B25 | 373 | 4.41 | –5.92 e | –2.73 f | I–/I3– | 0.649 | 6.38 | 74.7 | 3.09 | [82] |
| B25 (+CDCA) | - | - | - | - | I–/I3– | 0.593 | 3.65 | 77.8 | 1.69 | [82] |
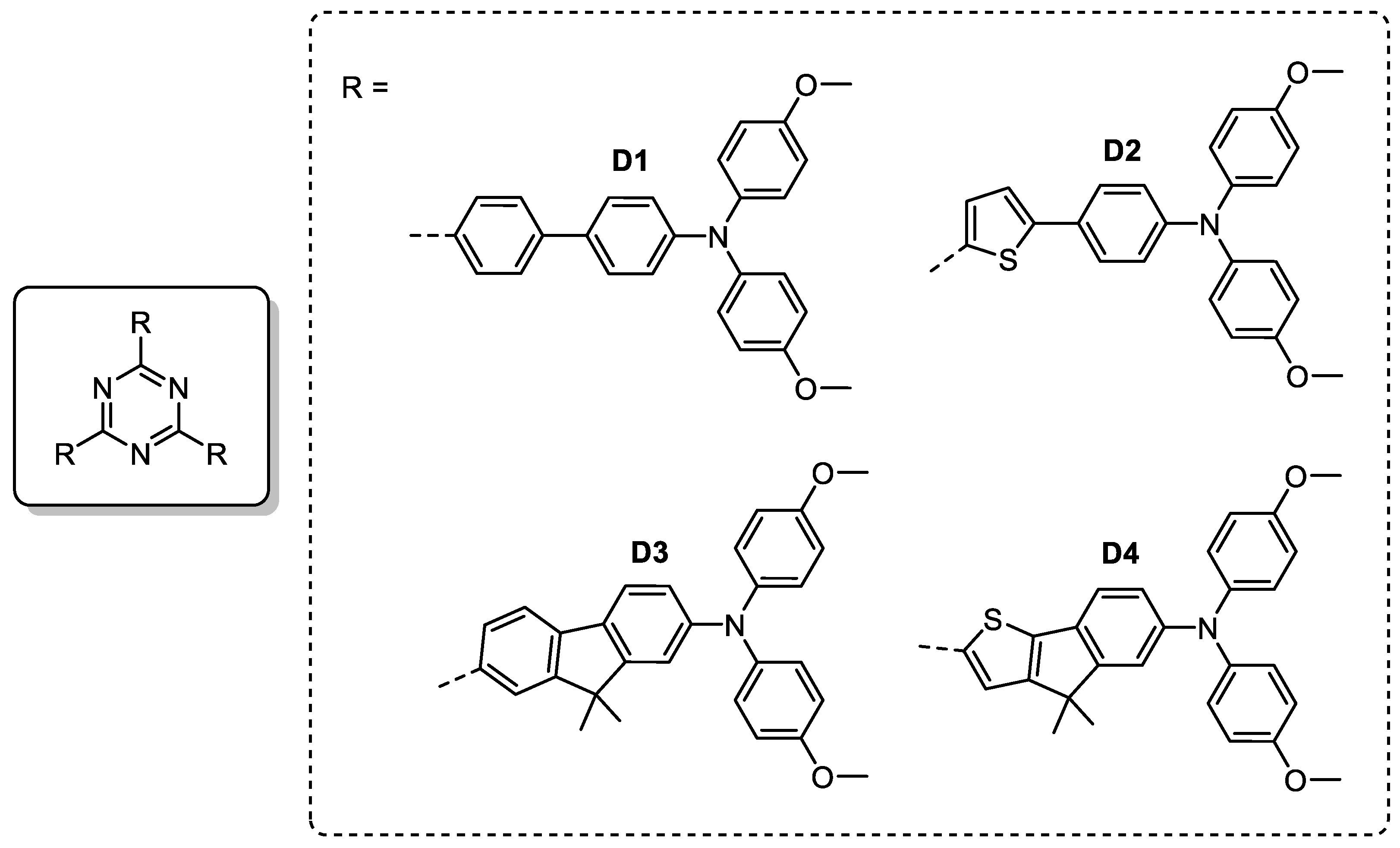
| Triazine-Based Molecule | HOMO (eV) | μh (×10−4 cm2 V−1 s−1) | Perovskite Material | ETM a | VOC (V) | JSC (mA/cm2) | FF (%) | PCE (%) | Ref. |
|---|---|---|---|---|---|---|---|---|---|
| D1 | –5.11 | 1.50 | MAPbI3 | TiO2 | 0.93 | 19.1 | 61 | 10.9 | [19] |
| D2 | –5.04 | 1.74 | MAPbI3 | TiO2 | 0.92 | 20.7 | 66 | 12.5 | [19] |
| D3 | –5.29 | 3.40 b | MAPbI3 | TiO2 | 0.95 | 20.2 | 64 | 12.3 | [104] |
| D4 | –5.26 | 4.35 b | MAPbI3 | TiO2 | 0.96 | 20.5 | 66 | 12.9 | [104] |
| Triazine-Based Molecule | HOMO (eV) | LUMO (eV) | Perovskite Material | ETM a | μe (×10−4 cm2 V−1 s−1) | VOC (V) | JSC (mA/cm2) | FF (%) | PCE (%) | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|
| E1 | –6.96 | –2.97 | MAPbI3 | ZnO | 1.32 | 1.06 | 20.5 | 76 | 16.2 | [32] |
| E2 | –6.72 | –2.73 | MAPbI3 | ZnO | 4.93 | 1.02 | 19.9 | 69 | 14.5 | [111] |
| E3 | –1.70 | - | MAPbI3 | TiO2 | - | 1.01 | 23.8 | 70 | 16.9 | [109] |
| E4 | –5.96 | –1.86 | MAPbI3 | ZnO | - | 1.11 | 21.1 | 74 | 17.3 | [110] |
| Triazine-Based Molecule | Additive Concentration/Content a | Perovskite Material | ETM | nt (×1016 cm−3) c | VOC (V) | JSC (mA cm−2) | FF (%) | PCE (%) | HI(%) | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|
| F1 | 0.0025–0.015 b | (FAPbI3)0.875(CsPbBr3)0.125 | Planar TiO2 | 0.708–0.749 (1.02) | 1.063–1.095 | 21.36–21.82 | 70.2–72.5 | 16.27–17.32 | 6.7–12.8 | [107] |
| F2 | 0.01–0.5 mM | MAPbI3 | Mesoporous TiO2 | 0.54 (1.00) | 1.05–1.06 | 23.1–23.6 | 77.7–79.2 | 18.94–19.81 | 1.2 | [105] |
| F3 | 0.01–0.5 mM | MAPbI3 | Mesoporous TiO2 | 0.59 (1.00) | 1.04–1.05 | 23.4 | 77.6–78.4 | 18.87–19.25 | 1.4 | [105] |
| F4 | 0.01–0.5 mM | MAPbI3 | Mesoporous TiO2 | 0.83 (1.00) | 1.03–1.04 | 23.3–23.4 | 77.1–78.7 | 18.81–19.22 | 1.8 | [105] |
| F5 | 0.6–3.6 mg | MAPbI3 | Planar PC61BM | - | 0.80–0.85 | 12.8–17.0 | 60.0–70.0 | 6.15–10.84 | - | [108] |
| F6 | 2 mg mL−1 | MAPbI3 | Planar SnO2 | 1.02 (1.83) | 1.09 | 23.3 | 79.7 | 20.33 | 0.5 | [106] |
| F6 | 2 mg mL−1 | FAPbI3 | Planar SnO2 | - | 1.13 | 24.4 | 76.7 | 21.2 | - | [106] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dávila Cerón, V.; Illicachi, L.A.; Insuasty, B. Triazine: An Important Building Block of Organic Materials for Solar Cell Application. Molecules 2023, 28, 257. https://doi.org/10.3390/molecules28010257
Dávila Cerón V, Illicachi LA, Insuasty B. Triazine: An Important Building Block of Organic Materials for Solar Cell Application. Molecules. 2023; 28(1):257. https://doi.org/10.3390/molecules28010257
Chicago/Turabian StyleDávila Cerón, Valeria, Luis Alberto Illicachi, and Braulio Insuasty. 2023. "Triazine: An Important Building Block of Organic Materials for Solar Cell Application" Molecules 28, no. 1: 257. https://doi.org/10.3390/molecules28010257
APA StyleDávila Cerón, V., Illicachi, L. A., & Insuasty, B. (2023). Triazine: An Important Building Block of Organic Materials for Solar Cell Application. Molecules, 28(1), 257. https://doi.org/10.3390/molecules28010257






