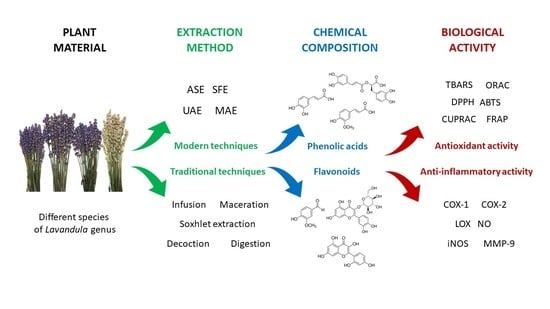
Phytochemical Profiling, Antioxidant and Anti-Inflammatory Activity of Plants Belonging to the Lavandula Genus
Abstract
1. Introduction
2. Extraction Process
2.1. Traditional Extraction Techniques
2.2. Modern Extraction Techniques
| Extraction Method | Extraction Procedure and Conditions | Yield [%] | Lavender Species | Part of Plant | Detection Method | Antioxidant Assays | Ref. | |
|---|---|---|---|---|---|---|---|---|
| Types of Assays | Activity | |||||||
| Refluxed extraction | 3 g were refluxed with 100 mL of MeOH in a water bath for 1 h. Plant material was re-extracted twice with the same solvent (2 × 100 mL). Then, the SPE procedure was used to obtain the phenolic acid fractions. | ND | L. angustifolia | flowers | SPE/RP-HPLC | ND | [53] | |
| Methanol extraction | 0.34 g was extracted with 30 mL MeOH. | ND | L. stoechas | flowers | Folin–Ciocalteu (760 nm) HPLC/ESI-MS | DPPH [mg/mL] | 7.05 | [51] |
| Ethanol extraction | 300 g were extracted with 90% EtOH. Then, the dried extract was suspended in water and fractionated with ethyl acetate. | 10 | L. coronopifolia | aerial parts | UPLC- ESI- MS/MS | DPPH [µg/mL] | 17.8 ± 0.8 | [54] |
| 2 g were extracted with 10 mL of 96% EtOH for 24 h in a water bath at 45 °C. | ND | L. angustifolia | flowers | RP-HPLC | ND | [1] | ||
| Aqueous-ethanol extraction | 2 g were extracted with 90 mL of 50% EtOH at 85 °C for 1 h. | ND | L. angustifolia ssp. angustifolia | flowers | Folin–Ciocalteu (765 nm) | DPPH [µg/mL] Fe2+ chelation assay [µg/mL] | 95.60 ± 1.70 54.46 ± 0.55 101.40 ± 0.90 50.60 ± 0.40 | [4] |
| L. angustifolia ssp. angustifolia ‘Munstead’ | ||||||||
| L. angustifolia. angustifolia ‘Hidicote Blue’ | 96.53 ± 1.45 49.93 ± 0.75 | |||||||
| L. angustifolia ssp. pyrenaica | 110.36 ± 1.40 81.90 ± 1.40 | |||||||
| L. hybrida | 73.53 ± 1.25 49.90 ± 0.90 | |||||||
| Aqueous extraction | 0.34 g was extracted with 30 mL of H2O | ND | L. stoechas | flowers | Folin–Ciocalteu (760 nm) | DPPH [mg/mL] | 1.78 | [51] |
| Infusion | 1 g was extracted with 200 mL of boiling water for 10 min. | ND | L. angustifolia | flowers | Folin–Ciocalteu (760 nm) | ABTS [mM] | 0.72 ± 0.07 | [50] |
| 2 g was extracted with 200 mL of boiling distilled water and left to stand at room temperature for 5 min. | ND | L. pedunculata | flowering stems with inflorescences | HPLC-DAD-ESI/MSn | DPPH [μg/mL] TBARS [μg/mL] reducing power [μg/mL] | 68 ± 0.5–191 ± 2 14 ± 1–39.1 ± 0.1 51 ± 1–167 ± 1 | [16] | |
| 1 g was homogenized in 20 mL of hot water (90 °C) for 5 min. | 22.5 | L. pedunculata ssp. lusitanica | aerial parts | HPLC-DAD | TEAC (μmol TE/g extract) ORAC (μmol TE/g extract) TBARS [%] Fe2+ chelation assay [%] | 866 ± 12.5 3018 ± 91.1 100 ± 0.0 48.0 ± 5.0 | [48] | |
| Infusion with stirring | 20 g was extracted with 400 mL of boiling water and stirred for 15 min. | 10.8 | L. stoechas | plant material from local market | Folin–Ciocalteu (760 nm) | DPPH [%] Fe2+ chelation assay superoxide anion | 45 ± 0.0 84 ± 0.0 78 ± 0.0 | [55] |
| [%] | ||||||||
| Stirring | 1 g was extracted with 25 mL of EtOH:H2O (80:20 v/v) and stirred for 1 h at 25 °C at 150 rpm. | ND | L. pedunculata | flowering stems with inflorescences | HPLC-DAD-ESI/MSn | DPPH [μg/mL] | 87 ± 2–257 ± 7 17 ± 1–63.5 ± 0.1 67 ± 1–216 ± 6 | [16] |
| TBARS [μg/mL] reducing power [μg/mL] | ||||||||
| 30 g was extracted with 1500 mL of deionized water, heated to a specific temperature (40, 60, 80 °C ± 0.5 °C) and stirred for 90 min at 500 rpm. | 0.24 | L. x hybrida | plant material from herbal store | Folin–Ciocalteu (760 nm) | ABTS [mol Trolox/g DM] | 0.216 ± 0.038 | [56] | |
| Shaking | Samples were extracted with 80% aqueous methanol and shaken at room temperature for 15 h. | ND | L. angustifolia ‘Lady’ L. angustifolia ‘Hidcote’ L. latifolia | leaves | HPLC-MS, Folin–Ciocalteu (735 nm) | DPPH [μmol TEAC/g DW] | 14.17 ± 9.09 9.00 ± 3.00 6.56 ± 1.13 | [49] |
| Shaking | Different protocols: | ND | ||||||
| ST1: SLE using H2O, shaking for 5 h | ||||||||
| ST2: H2O:EtOH (1:1; v/v), shaking for 2 h | ||||||||
| ST3: H2O:EtOH (1:1; v/v), shaking for 5 h | L. spica | plant material from local herbal market | SLE-SPE-UHPLC-MS/MS | ND | [57] | |||
| ST4: EtOH, shaking for 5 h | ||||||||
| ST5: H2O:MeOH (1:1; v/v), shaking for 2 h | ||||||||
| ST6: H2O:MeOH (1:1; v/v), shaking for 2 h twice | ||||||||
| ST7: H2O:MeOH (1:1; v/v), shaking for 5 h | ||||||||
| ST8: MeOH, shaking for 5 h | ||||||||
| 2 g was extracted with 20 mL of MilliQ water and shaken for 1 h at ambient temperature | ND | L. angustifolia | herb | UHPLC-DA | ABTS [mmol/100 g DW] | 22.00 ± 0.00 20.19 ± 2.55 | [58] | |
| Randall Extraction | 2 g was extracted with 20 mL of MilliQ water by Randall extraction for 1 h at 100 °C. | |||||||
| Plant material was extracted with hexane and then with ethanol at room temperature for 48 h with plant material: solvent ratio of 1:10 (w/w). | 12.2 | L. stoechas ssp. luisieri | herb | HPLC | DPPH [µg/mL] | 30.66 ± 1.9 | [59] | |
| Maceration Maceration | 10 g were soaked overnight at room temperature in 200 mL of each solvent: water (w), water: ethanol (1:1) (w/e), ethanol (e). | 22.1 21.3 12.8 | L. viridis L’Her | aerial parts | HPLC–DAD | ORAC (w, w/e, e) [μmol TE/g extract] | 1502.22 ±39.95 4030.26 ±02.40 1183.95 ±90.78 | [11] |
| TEAC (w, w/e, e) [μmol TE/g extract] | 670.95 ± 4.24 1149.82± 17.31 332.06 ± 2.52 | |||||||
| 10 g was soaked overnight at room temperature in 200 mL of: water (w), water: ethanol (1:1) (w/e), ethanol (e). | 22.4 19.4 19.6 | L. pedunculata ssp. lusitanica | aerial parts | HPLC-DAD | TEAC (w, w/e, e) [μmol TE/g extract] ORAC (w, w/e, e) [μmol TE/g extract] TBARS (w, w/e, e) [%] Fe2+ chelation assay (w, w/e, e) [%] | 569 ± 1.99 688 ± 10.59 224 ± 6.41 | [48] | |
| 1530 ± 121 2567 ± 151 861 ± 6.00 | ||||||||
| 96 ± 2 100 ± 0 4 ± 2 | ||||||||
| 65.9 ± 1.27 50.1 ± 0.14 32.0 ± 0.50 | ||||||||
| 10 g was extracted with 100 mL of 70% MeOH and shaken in a water bath at 40 °C for 5 min. | ND | L. pubescens | aerial parts | Folin–Ciocalteu (760 nm) | DPPH [μg/mL] | |||
| Ultrasonic- microwave-assisted extraction (UMAE) | 10 g were immersed in 100 mL of 70% MeOH. The mixture was exposed to acoustic waves at 40 °C for 5 min (ultrasonic power 50 W, frequency 40 kHz, microwave power 480 W). | ND | L. pubescens | aerial parts | Folin–Ciocalteu (760 nm) | DPPH [μg/mL] | 24.83 19.54 22.04 | [38] |
| Ultrasonic- homogenizer-assisted extraction | 10 g was extracted with 100 mL of 70% MeOH using magnetic stirring (ultrasonic power 150 W, frequency 20 kHz, 40 °C, 5 min). | |||||||
| Microwave- assisted extraction (MAE) | 1 g was extracted with 15 mL of 60% and 80% methanol, ethanol and acetone at 80 °C and 500 W. | ND | L. officinalis | flowers | UPLC-DAD-ESI-MS/MS Folin–Ciocalteu (750 nm) | CUPRAC [mmol TR/g] DPPH [µg/mL] | 0.39 ± 0.01 125 ± 4.6 | [37] |
| Ultrasonic- assisted extraction (UAE) | 30 g was extracted twice with 500 mL of 80% EtOH using an ultrasonic bath for 30 min. | 14.8 14.2 10.9 23.9 20.8 14.6 | L. angustifolia | flowers * leaves inflorescence stalks | HPTLC | DPPH * [µg/mL] TBARS * [µg/mL] Fe2+ chelation assay * reducing power * | 11.37 ± 0.69 89.36 ± 5.00 319.21 ± 21.96 25.17 ± 0.16 | [60] |
| L. intermedia ‘Budrovka’ | flowers * leaves inflorescence stalks | HPTLC | DPPH * [µg/mL] TBARS * [µg/mL] Fe2+ chelation assay * reducing power * | 17.17 ± 0.33 116.54 ± 9.96 397.71 ± 10.26 33.78 ± 2.34 | ||||
| 0.5 g was immersed in 40 mL of 62.5% MeOH. Then, 10 mL of 6 M HCl was added and the mixture was submitted to ultrasounds for 15 min and refluxed in a water bath at 90 °C for 2 h. | ND | L. vera (L. angustifolia) | leaves | RP-HPLC | ND | [52] | ||
| Ultrasonic- assisted extraction (UAE) | 2 g was sonicated with 20 mL of MilliQ water for 15 min at ambient temperature. | ND | L. angustifolia | herb | UHPLC-PDA | ABTS [mmol/100 g DW] | 10.00 ± 0.00 | [58] |
| Pulsed ultrasound-assisted extraction (PUAE) | 1 g of flower residues was extracted with 40 mL of 70% EtOH using ultrasound applied in pulsed modality (frequency 26 kHz, power 200 W, temperature < 60 °C, extraction time 10 min). | ND | L. angustifolia ‘Rosa’ | flower residues after the distillation of essential oil | RP-HPLC Folin–Ciocalteu (760 nm) | DPPH [mg TE/g of dry waste] | 107.29 ± 0.05 | [34] |
| Accelerated solvent extraction (ASE) | 5 g was mixed with washed sea sand and extracted with 30 mL of 50% MeOH at 1500 PSI and 80 °C for 10 min. | 20 14 | L. dentata L. stoechas | aerial parts | RP-HPLC-DAD-MS | DPPH [µg/mL] | 71.1 ± 8.7 67.0 ± 6.5 | [5] |
| Supercritical fluid extraction (SFE) | 100 g was extracted with CO2 at 200–300 bar and 40–60 °C for 15–45 min, CO2 flow rate 10 kg/h. | ND 0.53–7.28 | L. angustifolia | flowers | HPLC Folin–Ciocalteu (765 nm) RP-HPLC | DPPH [%] | 50.55 ± 0.7 78.83 ± 1.3 ND | [47,61] |
| 40 g was extracted at 100–300 bar and 40–60 °C for 90 min, CO2 flow rate 1–3 kg/h. | ||||||||
| Supercritical antisolvent fractionation (SAF) | The ethanolic maceration extract was dissolved in 3% EtOH and fractionated using SAF with CO2 at 130 bar, CO2 flow rate 30 g/min. | ND | L. stoechas ssp. luisieri | herb | HPLC | DPPH [µg/mL] | 16.17 ± 0.7 | [59] |
3. Chemical Composition
4. Antioxidant Activity
5. Anti-Inflammatory Activity
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Turgut, A.C.; Emen, F.M.; Canbay, H.S.; Demirdöğen, R.E.; Çam, N.; Kılıç, D.; Yeşilkaynak, T. Chemical characterization of Lavandula angustifolia Mill. which is a phytocosmetic species and investigation of its antimicrobial effect in cosmetic products. J. Turk. Chem. Soc. Sect. A Chem. 2016, 4, 283–298. [Google Scholar] [CrossRef]
- Polish Pharmacopoeia, Poland. In Polish Pharmacopoeia: Supplement 2021, 12th ed.; Office for Registration of Medicinal Products MDaBP: Warsaw, Poland, 2021.
- Danh, L.T.; Triet, N.D.A.; Han, L.T.N.; Zhao, J.; Mammucari, R.; Foster, N. Antioxidant activity, yield and chemical composition of lavender essential oil extracted by supercritical CO2. J. Supercrit. Fluids 2012, 70, 27–34. [Google Scholar] [CrossRef]
- Robu, S.; Aprotosoaie, A.C.; Miron, A.; Cioancǎ, O.; Stǎnescu, U.; Hǎncianu, M. In vitro antioxidant activity of ethanolic extracts from some Lavandula species cultivated in Romania. Farmacia 2012, 60, 394–401. [Google Scholar]
- Algieri, F.; Rodriguez-Nogales, A.; Vezza, T.; Garrido-Mesa, J.; Garrido-Mesa, N.; Utrilla, M.P.; González-Tejero, M.R.; Casares-Porcel, M.; Molero-Mesa, J.; Contreras, M.D.M.; et al. Anti-inflammatory activity of hydroalcoholic extracts of Lavandula dentata L. and Lavandula stoechas L. J. Ethnopharmacol. 2016, 190, 142–158. [Google Scholar] [CrossRef]
- Shaikh, R.; Pund, M.; Dawane, A.; Iliyas, S. Evaluation of Anticancer, Antioxidant, and Possible Anti-inflammatory Properties of Selected Medicinal Plants Used in Indian Traditional Medication. J. Tradit. Complement. Med. 2014, 4, 253–257. [Google Scholar] [CrossRef]
- Sosa, S.; Altinier, G.; Politi, M.; Braca, A.; Morelli, I.; Della Loggia, R. Extracts and constituents of Lavandula multifida with topical anti-inflammatory activity. Phytomedicine 2005, 12, 271–277. [Google Scholar] [CrossRef]
- Zuzarte, M.; Gonçalves, M.J.; Cavaleiro, C.; Cruz, M.T.; Benzarti, A.; Marongiu, B.; Maxia, A.; Piras, A.; Salgueiro, L. Antifungal and anti-inflammatory potential of Lavandula stoechas and Thymus herba-barona essential oils. Ind. Crops Prod. 2013, 44, 97–103. [Google Scholar] [CrossRef]
- De Moura Linck, V.; da Silva, A.L.; Figueiró, M.; Piato, A.L.; Herrmann, A.P.; Birck, F.D.; Elisabetsky, E. Inhaled linalool-induced sedation in mice. Phytomedicine 2009, 16, 303–307. [Google Scholar] [CrossRef]
- Kageyama, A.; Ueno, T.; Oshio, M.; Masuda, H.; Horiuchi, H.; Yokogoshi, H. Antidepressant-like Effects of an Aqueous Extract of Lavender (Lavandula angustifolia Mill.) in Rats. Food Sci. Technol. Res. 2012, 18, 473–479. [Google Scholar] [CrossRef]
- Costa, P.; Gonçalves, S.; Valentão, P.; Andrade, P.B.; Romano, A. Accumulation of phenolic compounds in in vitro cultures and wild plants of Lavandula viridis L’Hér and their antioxidant and anti-cholinesterase potential. Food Chem. Toxicol. 2013, 57, 69–74. [Google Scholar] [CrossRef]
- Kritsidima, M.; Newton, T.; Asimakopoulou, K.; Newton, J.T. The effects of lavender scent on dental patient anxiety levels: A cluster randomised-controlled trial. Community Dent. Oral Epidemiol. 2010, 38, 83–87. [Google Scholar] [CrossRef] [PubMed]
- Carrasco, A.; Ortiz-Ruiz, V.; Martinez-Gutierrez, R.; Tomas, V.; Tudela, J. Lavandula stoechas essential oil from Spain: Aromatic profile determined by gas chromatography–mass spectrometry, antioxidant and lipoxygenase inhibitory bioactivities. Ind. Crops Prod. 2015, 73, 16–27. [Google Scholar] [CrossRef]
- Prusinowska, R.; Śmigielski, K.B. Composition, biological properties and therapeutic effects of Lavender L). A review. Herba Pol. 2014, 60, 56–66. [Google Scholar] [CrossRef]
- Lis-Balchin, M. Lavender: The Genus Lavandula; CRC Press: Boca Raton, FL, USA, 2002. [Google Scholar]
- Lopes, C.L.; Pereira, E.; Soković, M.; Carvalho, A.M.; Barata, A.M.; Lopes, V.; Rocha, F.; Calhelha, R.C.; Barros, L.; Ferreira, I.C. Phenolic Composition and Bioactivity of Lavandula pedunculata (Mill.) Cav. Samples from Different Geographical Origin. Molecules 2018, 23, 1037. [Google Scholar] [CrossRef] [PubMed]
- Da Porto, C.; Decorti, D.; Kikic, I. Flavour compounds of Lavandula angustifolia L. to use in food manufacturing: Comparison of three different extraction methods. Food Chem. 2009, 112, 1072–1078. [Google Scholar] [CrossRef]
- Luo, W.; Du, Z.; Zheng, Y.; Liang, X.; Huang, G.; Zhang, Q.; Liu, Z.; Zhang, K.; Zheng, X.; Lin, L.; et al. Phytochemical composition and bioactivities of essential oils from six Lamiaceae species. Ind. Crops Prod. 2019, 133, 357–364. [Google Scholar] [CrossRef]
- Delgado, F.; Ribeiro, S.; Alves, Á.; Bettencourt, E.; Dias, S. Morphological, ecological and genetic variability of Lavandula luisieri (Rozeira) Rivas-Martínez in central eastern Portugal. Plant Genet. Resour. 2010, 8, 82–90. [Google Scholar] [CrossRef]
- Abubakar, A.R.; Haque, M. Preparation of medicinal plants: Basic extraction and fractionation procedures for experimental purposes. J. Pharm. Bioallied Sci. 2020, 12, 1–10. [Google Scholar] [CrossRef]
- Belwal, T.; Ezzat, S.M.; Rastrelli, L.; Bhatt, I.D.; Daglia, M.; Baldi, A.; Devkota, H.P.; Orhan, I.E.; Patra, J.K.; Das, G.; et al. A critical analysis of extraction techniques used for botanicals: Trends, priorities, industrial uses and optimization strategies. TrAC Trends Anal. Chem. 2018, 100, 82–102. [Google Scholar] [CrossRef]
- Lourenço, S.C.; Moldão-Martins, M.; Alves, V.D. Antioxidants of Natural Plant Origins: From Sources to Food Industry Applications. Molecules 2019, 24, 4132. [Google Scholar] [CrossRef]
- Stalikas, C.D. Extraction, separation, and detection methods for phenolic acids and flavonoids. J. Sep. Sci. 2007, 30, 3268–3295. [Google Scholar] [CrossRef] [PubMed]
- Arceusz, A.; Wesolowski, M.; Konieczynski, P. Methods for Extraction and Determination of Phenolic Acids in Medicinal Plants: A Review. Nat. Prod. Commun. 2013, 8, 1821–1829. [Google Scholar] [CrossRef] [PubMed]
- Domínguez-Rodríguez, G.; Marina, M.L.; Plaza, M. Strategies for the extraction and analysis of non-extractable polyphenols from plants. J. Chromatogr. A 2017, 1514, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Costa, D.C.; Costa, H.S.; Albuquerque, T.G.; Ramos, F.; Castilho, M.C.; Sanches-Silva, A. Advances in phenolic compounds analysis of aromatic plants and their potential applications. Trends Food Sci. Technol. 2015, 45, 2236–2254. [Google Scholar] [CrossRef]
- Tzima, K.; Brunton, N.; Rai, D. Qualitative and Quantitative Analysis of Polyphenols in Lamiaceae Plants—A Review. Plants 2018, 7, 25. [Google Scholar] [CrossRef]
- Saravanabavan, N.; Salwe, K.J.; Codi, R.S.; Kumarappan, M. Herbal extraction procedures: Need of the hour. Int. J. Basic Clin. Pharmacol. 2020, 9, 1135–1139. [Google Scholar] [CrossRef]
- United Nations Industrial Development Organization; Handa, S.S.; Khanuja, S.P.S.; Longo, G.; Rakesh, D.D. Extraction Technologies for Medicinal and Aromatic Plants: Earth, Environmental and Marine Sciences and Technologies; United Nations Industrial Development Organization: Trieste, Italy, 2008. [Google Scholar]
- Soquetta, M.B.; Terra, L.D.M.; Bastos, C.P. Green technologies for the extraction of bioactive compounds in fruits and vegetables. CyTA J. Food 2018, 16, 400–412. [Google Scholar] [CrossRef]
- Rasul, M.G. Conventional extraction methods use in medicinal plants, their advantages and disadvantages. Int. J. Basic Sci. Appl. Comput. 2018, 2, 10–14. [Google Scholar]
- Mansinhos, I.; Gonçalves, S.; Rodríguez-Solana, R.; Ordóñez-Díaz, J.L.; Moreno-Rojas, J.M.; Romano, A. Ultrasonic-Assisted Extraction and Natural Deep Eutectic Solvents Combination: A Green Strategy to Improve the Recovery of Phenolic Compounds from Lavandula pedunculata subsp. lusitanica (Chaytor) Franco. Antioxidants 2021, 10, 582. [Google Scholar] [CrossRef]
- Pande, J.; Chanda, S. Determination of phytochemical profile and antioxidant efficacy of Lavendula bipinnata leaves collected during Magha Nakshatra days and Normal days using LC-QTOF-MS technique. J. Pharm. Biomed. Anal. 2020, 186, 113347. [Google Scholar] [CrossRef]
- Turrini, F.; Beruto, M.; Mela, L.; Curir, P.; Triglia, G.; Boggia, R.; Zunin, P.; Monroy, F. Ultrasound-Assisted Extraction of Lavender (Lavandula angustifolia Miller, Cultivar Rosa) Solid By-Products Remaining after the Distillation of the Essential Oil. Appl. Sci. 2021, 11, 5495. [Google Scholar] [CrossRef]
- Giacometti, J.; Kovačević, D.B.; Putnik, P.; Gabrić, D.; Bilušić, T.; Krešić, G.; Jambrak, A.R. Extraction of bioactive compounds and essential oils from mediterranean herbs by conventional and green innovative techniques: A review. Food Res. Int. 2018, 113, 245–262. [Google Scholar] [CrossRef] [PubMed]
- Altemimi, A.; Lakhssassi, N.; Baharlouei, A.; Watson, D.G.; Lightfoot, D.A. Phytochemicals: Extraction, Isolation, and Identification of Bioactive Compounds from Plant Extracts. Plants 2017, 6, 42. [Google Scholar] [CrossRef] [PubMed]
- Çelik, S.E.; Tufan, A.N.; Bekdeşer, B.; Özyürek, M.; Güçlü, K.; Apak, R. Identification and Determination of Phenolics in Lamiaceae Species by UPLC-DAD-ESI-MS/MS. J. Chromatogr. Sci. 2016, 55, 291–300. [Google Scholar] [CrossRef] [PubMed]
- Rashed, M.M.; Tong, Q.; Abdelhai, M.H.; Gasmalla, M.A.; Ndayishimiye, J.B.; Chen, L.; Ren, F. Effect of ultrasonic treatment on total phenolic extraction from Lavandula pubescens and its application in palm olein oil industry. Ultrason. Sonochemistry 2016, 29, 39–47. [Google Scholar] [CrossRef] [PubMed]
- Llompart, M.; Celeiro, M.; Dagnac, T. Microwave-assisted extraction of pharmaceuticals, personal care products and industrial contaminants in the environment. TrAC Trends Anal. Chem. 2019, 116, 136–150. [Google Scholar] [CrossRef]
- Azwanida, N.N. A Review on the Extraction Methods Use in Medicinal Plants, Principle, Strength and Limitation. Med. Aromat. Plants 2015, 4, 1–6. [Google Scholar]
- Ong, E.S. Extraction methods and chemical standardization of botanicals and herbal preparations. J Chromatogr B Anal. Technol Biomed Life Sci. 2004, 812, 23–33. [Google Scholar] [CrossRef]
- Woźniak, Ł.; Marszałek, K.; Skąpska, S.; Jędrzejczak, R. The Application of Supercritical Carbon Dioxide and Ethanol for the Extraction of Phenolic Compounds from Chokeberry Pomace. Appl. Sci. 2017, 7, 322. [Google Scholar] [CrossRef]
- Cvjetko Bubalo, M.; Vidović, S.; Radojčić Redovniković, I.; Jokić, S. New perspective in extraction of plant biologically active compounds by green solvents. Food Bioprod. Process. 2018, 109, 52–73. [Google Scholar] [CrossRef]
- Da Silva, R.P.F.F.; Rocha-Santos, T.A.P.; Duarte, A.C. Supercritical fluid extraction of bioactive compounds. TrAC Trends Anal. Chem. 2016, 76, 40–51. [Google Scholar] [CrossRef]
- Chemat, F.; Abert-Vian, M.; Fabiano-Tixier, A.S.; Strube, J.; Uhlenbrock, L.; Gunjevic, V.; Cravotto, G. Green extraction of natural products. Origins, current status, and future challenges. TrAC Trends Anal. Chem. 2019, 118, 248–263. [Google Scholar] [CrossRef]
- De Melo, M.M.R.; Silvestre, A.J.D.; Silva, C.M. Supercritical fluid extraction of vegetable matrices: Applications, trends and future perspectives of a convincing green technology. J. Supercrit. Fluids 2014, 92, 115–176. [Google Scholar] [CrossRef]
- Tyśkiewicz, K.; Konkol, M.; Rój, E. Supercritical Carbon Dioxide (scCO2) Extraction of Phenolic Compounds from Lavender (Lavandula angustifolia) Flowers: A Box-Behnken Experimental Optimization. Molecules 2019, 24, 3354. [Google Scholar] [CrossRef] [PubMed]
- Costa, P.; Gonçalves, S.; Valentão, P.; Andrade, P.B.; Almeida, C.; Nogueira, J.M.; Romano, A. Metabolic profile and biological activities of Lavandula pedunculata subsp. lusitanica (Chaytor) Franco: Studies on the essential oil and polar extracts. Food Chem. 2013, 141, 2501–2506. [Google Scholar] [CrossRef] [PubMed]
- Ahn, J.; Alford, A.R.; Niemeyer, E.D. Variation in phenolic profiles and antioxidant properties among medicinal and culinary herbs of the Lamiaceae family. J. Food Meas. Charact. 2020, 14, 1720–1732. [Google Scholar] [CrossRef]
- Ivanova, D.; Gerova, D.; Chervenkov, T.; Yankova, T. Polyphenols and antioxidant capacity of Bulgarian medicinal plants. J. Ethnopharmacol. 2005, 96, 145–150. [Google Scholar] [CrossRef]
- Karabagias, I.K.; Karabagias, V.K.; Riganakos, K.A. Physico-Chemical Parameters, Phenolic Profile, In Vitro Antioxidant Activity and Volatile Compounds of Ladastacho (Lavandula stoechas) from the Region of Saidona. Antioxidants 2019, 8, 80. [Google Scholar] [CrossRef]
- Proestos, C.; Sereli, D.; Komaitis, M. Determination of phenolic compounds in aromatic plants by RP-HPLC and GC-MS. Food Chem. 2006, 95, 44–52. [Google Scholar] [CrossRef]
- Zgórka, G.; Głowniak, K. Variation of free phenolic acids in medicinal plants belonging to the Lamiaceae family. J. Pharm. Biomed. Anal. 2001, 26, 79–87. [Google Scholar] [CrossRef]
- Sahar, A.; Wafaa, H.B.H.; Ahmed, E.M.E.; Hanan, M.A.-Y.; May, A.E.; Rasha, A. Ultra performance liquid chromatography-tandem mass spectrometeric analysis of ethyl acetate fraction from saudi Lavandula coronopifolia Poir and evaluation of its cytotoxic and antioxidant activities. J. Herbmed Pharmacol. 2020, 9, 268–276. [Google Scholar]
- Gülçin, Ì.; Şat, İ.G.; Beydemir, Ş.; Elmastaş, M.; Küfrevioǧlu, Ö.İ. Comparison of antioxidant activity of clove (Eugenia caryophylata Thunb) buds and lavender (Lavandula stoechas L.). Food Chem. 2004, 87, 393–400. [Google Scholar] [CrossRef]
- Tušek, A.J.; Jurina, T.; Benković, M.; Valinger, D.; Belščak-Cvitanović, A.; Kljusurić, J.G. Application of multivariate regression and artificial neural network modelling for prediction of physical and chemical properties of medicinal plants aqueous extracts. J. Appl. Res. Med. Aromat. Plants. 2020, 16, 100229. [Google Scholar] [CrossRef]
- Bajkacz, S.; Baranowska, I.; Buszewski, B.; Kowalski, B.; Ligor, M. Determination of Flavonoids and Phenolic Acids in Plant Materials Using SLE-SPE-UHPLC-MS/MS Method. Food Anal. Methods 2018, 11, 3563–3575. [Google Scholar] [CrossRef]
- Dvorackova, E.; Snóblová, M.; Hrdlicka, P. Content of phenolic compounds in herbs used in the Czech Republic. Int. Food Res. J. 2014, 21, 1495. [Google Scholar]
- Giménez-Rota, C.; Lorán, S.; Mainar, A.M.; Hernáiz, M.J.; Rota, C. Supercritical Carbon Dioxide Antisolvent Fractionation for the Sustainable Concentration of Lavandula luisieri (Rozeira) Riv.-Mart Antimicrobial and Antioxidant Compounds and Comparison with Its Conventional Extracts. Plants 2019, 8, 455. [Google Scholar] [CrossRef]
- Blažeković, B.; Vladimir-Knezević, S.; Brantner, A.; Štefan, M.B. Evaluation of Antioxidant Potential of Lavandula x intermedia Emeric ex Loisel. ‘Budrovka’: A Comparative Study with L. angustifolia Mill. Molecules 2010, 15, 5971–5987. [Google Scholar] [CrossRef]
- Jerković, I.; Molnar, M.; Vidović, S.; Vladić, J.; Jokić, S. Supercritical CO2 Extraction of Lavandula angustifolia Mill. Flowers: Optimisation of Oxygenated Monoterpenes, Coumarin and Herniarin Content. Phytochem. Anal. 2017, 28, 558–566. [Google Scholar] [CrossRef]
- EMA. Assessment Report on Lavandula angustifolia Miller, Aetheroleum and Lavandula angustifolia Miller; Flos; Monograph European Medicines Agency: Amsterdam, The Netherlands, 27 March 2012. [Google Scholar]
- Crozier, A.; Jaganath, I.B.; Clifford, M.N. Dietary phenolics: Chemistry, bioavailability and effects on health. Nat. Prod. Rep. 2009, 26, 1001–1043. [Google Scholar] [CrossRef]
- Hussain, T.; Tan, B.; Yin, Y.; Blachier, F.; Tossou, M.C.B.; Rahu, N. Oxidative Stress and Inflammation: What Polyphenols Can Do for Us? Oxid. Med. Cell Longev. 2016, 2016, 7432797–7432799. [Google Scholar] [CrossRef]
- Myburgh, K.H. Polyphenol Supplementation: Benefits for Exercise Performance or Oxidative Stress? Sports Med. 2014, 44 (Suppl. S1), 57–70. [Google Scholar] [CrossRef] [PubMed]
- Santos-Sánchez, N.F.; Salas-Coronado, R.; Hernández-Carlos, B.; Villanueva-Cañongo, C. Shikimic Acid Pathway in Biosynthesis of Phenolic Compounds. In Plant Physiological Aspects of Phenolic Compounds; IntechOpen: London, UK, 2019. [Google Scholar]
- Tsao, R. Chemistry and Biochemistry of Dietary Polyphenols. Nutrients 2010, 2, 1231–1246. [Google Scholar] [CrossRef] [PubMed]
- Hawrył, A.; Hawrył, M.; Waksmundzka-Hajnos, M. Liquid chromatography fingerprint analysis and antioxidant activity of selected lavender species with chemometric calculations. PLoS ONE 2019, 14, e0218974. [Google Scholar] [CrossRef] [PubMed]
- Marranzano, M.; Rosa, R.L.; Malaguarnera, M.; Palmeri, R.; Tessitori, M.; Barbera, A.C. Polyphenols: Plant Sources and Food Industry Applications. Curr. Pharm. Des. 2018, 24, 4125–4130. [Google Scholar] [CrossRef]
- Adaszyńska-Skwirzyńska, M.; Dzięcioł, M. Comparison of phenolic acids and flavonoids contents in various cultivars and parts of common lavender (Lavandula angustifolia) derived from Poland. Nat. Prod. Res. 2017, 31, 2575–2580. [Google Scholar] [CrossRef]
- Bajalan, I.; Mohammadi, M.; Alaei, M.; Pirbalouti, A.G. Total phenolic and flavonoid contents and antioxidant activity of extracts from different populations of lavandin. Ind. Crops Prod. 2016, 87, 255–260. [Google Scholar] [CrossRef]
- Rahman, M.; Rahaman, S.; Islam, R.; Rahman, F.; Mithi, F.M.; Alqahtani, T.; Almikhlafi, M.A.; Alghamdi, S.Q.; Alruwaili, A.S.; Hossain, S.; et al. Role of Phenolic Compounds in Human Disease: Current Knowledge and Future Prospects. Molecules 2021, 27, 233. [Google Scholar] [CrossRef]
- Yadikar, N.; Bobakulov, K.; Li, G.; Aisa, H.A. Seven new phenolic compounds from Lavandula angustifolia. Phytochem. Lett. 2018, 23, 149–154. [Google Scholar] [CrossRef]
- Boligon, A.A.; Machado, M.M.; Athayde, M.L. Technical evaluation of antioxidant activity. Med Chem. 2014, 4, 517–522. [Google Scholar] [CrossRef]
- De Lima Cherubim, D.J.; Buzanello Martins, C.V.; Oliveira Fariña, L.; da Silva de Lucca, R.A. Polyphenols as natural antioxidants in cosmetics applications. J. Cosmet. Dermatol. 2020, 19, 33–37. [Google Scholar] [CrossRef] [PubMed]
- Lobo, V.; Patil, A.; Phatak, A.; Chandra, N. Free radicals, antioxidants and functional foods: Impact on human health. Pharmacogn. Rev. 2010, 4, 118–126. [Google Scholar] [CrossRef] [PubMed]
- Munteanu, I.G.; Apetrei, C. Analytical Methods Used in Determining Antioxidant Activity: A Review. Int. J. Mol. Sci. 2021, 22, 3380–3410. [Google Scholar] [CrossRef] [PubMed]
- Godlewska-Żyłkiewicz, B.; Świsłocka, R.; Kalinowska, M.; Golonko, A.; Świderski, G.; Arciszewska, Ż.; Nalewajko-Sieliwoniuk, E.; Naumowicz, M.; Lewandowski, W. Biologically Active Compounds of Plants: Structure-Related Antioxidant, Microbiological and Cytotoxic Activity of Selected Carboxylic Acids. Materials 2020, 13, 4454. [Google Scholar] [CrossRef] [PubMed]
- Prommajak, T.; Kim, S.M.; Pan, C.-H.; Kim, S.M.; Surawang, S.; Rattanapanone, N. Identification of Antioxidants in Lamiaceae Vegetables by HPLC-ABTS and HPLC-MS. Chiang Mai Univ. J. Nat. Sci. 2016, 15, 21–38. [Google Scholar] [CrossRef]
- Parus, A. Antioxidant and pharmacological properties of phenolic acids. Postępy Fitoterapii. 2012, 2013, 48–53. [Google Scholar]
- Burda, S.; Oleszek, W. Antioxidant and Antiradical Activities of Flavonoids. J. Agric. Food Chem. 2001, 49, 2774–2779. [Google Scholar] [CrossRef]
- Dasgupta, A.; Klein, K. Antioxidants in Food, Vitamins and Supplements: Prevention and Treatment of Disease; Elsevier: Saint Louis, MO, USA, 2014. [Google Scholar]
- Ionita, P. The chemistry of dpph∙free radical and congeners. Int. J. Mol. Sci. 2021, 22, 1–15. [Google Scholar] [CrossRef]
- Sanna, D.; Delogu, G.; Mulas, M.; Schirra, M.; Fadda, A. Determination of Free Radical Scavenging Activity of Plant Extracts Through DPPH Assay: An EPR and UV–Vis Study. Food Anal. Methods 2012, 5, 759–766. [Google Scholar] [CrossRef]
- Brahmi, N.; Scognamiglio, M.; Pacifico, S.; Mekhoukhe, A.; Madani, K.; Fiorentino, A.; Monaco, P. 1 H NMR based metabolic profiling of eleven Algerian aromatic plants and evaluation of their antioxidant and cytotoxic properties. Food Res. Int. 2015, 76, 334–341. [Google Scholar] [CrossRef]
- Nicolai, M.; Pereira, P.; Vitor, R.F.; Reis, C.P.; Roberto, A.; Rijo, P. Antioxidant activity and rosmarinic acid content of ultrasound-assisted ethanolic extracts of medicinal plants. Measurement 2016, 89, 328–332. [Google Scholar] [CrossRef]
- Całkosiński, I.; Dobrzyński, M.; Całkosińska, M.; Seweryn, E.; Bronowicka-Szydełko, A.; Dzierzba, K.; Ceremuga, I.; Gamian, A. Characterization of an inflammatory response. Postepy Hig. Med. Dosw. 2009, 63, 395–408. [Google Scholar]
- Abdulkhaleq, L.A.; Assi, M.A.; Abdullah, R.; Zamri-Saad, M.; Taufiq-Yap, Y.H.; Hezmee, M.N.M. The crucial roles of inflammatory mediators in inflammation: A review. Veter. World 2018, 11, 627–635. [Google Scholar] [CrossRef]
- Arulselvan, P.; Fard, M.T.; Tan, W.S.; Gothai, S.; Fakurazi, S.; Norhaizan, M.E.; Kumar, S.S. Role of Antioxidants and Natural Products in Inflammation. Oxid. Med. Cell. Longev. 2016, 2016, 5276130. [Google Scholar] [CrossRef]
- Medzhitov, R. Origin and physiological roles of inflammation. Nature 2008, 454, 428–435. [Google Scholar] [CrossRef]
- Wong, R.S.Y. Role of Nonsteroidal Anti-Inflammatory Drugs (NSAIDs) in Cancer Prevention and Cancer Promotion. Adv. Pharmacol. Sci. 2019, 2019, 1–10. [Google Scholar] [CrossRef]
- Yassine, E.Z.; Dalila, B.; Latifa, E.M.; Smahan, B.; Lebtar, S.; Sanae, A.; Abdellah, F. Phytochemical screening, anti-inflammatory activity and acute toxicity of hydro-ethanolic, flavonoid, tannin and mucilage extracts of Lavandula stoechas L. from Morocco. Int. J. Pharmacogn. Phytochem. Res. 2016, 8, 31–37. [Google Scholar]
- Husseini, Y.; Sahraei, H.; Meftahi, G.H.; Dargahian, M.; Mohammadi, A.; Hatef, B.; Zardooz, H.; Ranjbaran, M.; Hosseini, S.B.; Alibeig, H.; et al. Analgesic and anti-inflammatory activities of hydro-alcoholic extract of Lavandula officinalis in mice: Possible involvement of the cyclooxygenase type 1 and 2 enzymes. Rev. Bras. Farm. 2016, 26, 102–108. [Google Scholar] [CrossRef]
- Bozimowski, G. A Review of Nonsteroidal Anti-inflammatory Drugs. AANA J. 2015, 83, 425–433. [Google Scholar]
- Duda, M.; Olczyk, P.; Fenig, A.; Komosińska-Vassev, K. Cyklooksygenaza–znaczenie w biotechnologii, medycynie i farmacji. Farm. Dyplomie 2014, 70, 619–628. [Google Scholar]
- Yahfoufi, N.; Alsadi, N.; Jambi, M.; Matar, C. The Immunomodulatory and Anti-Inflammatory Role of Polyphenols. Nutrients 2018, 10, 1618. [Google Scholar] [CrossRef]
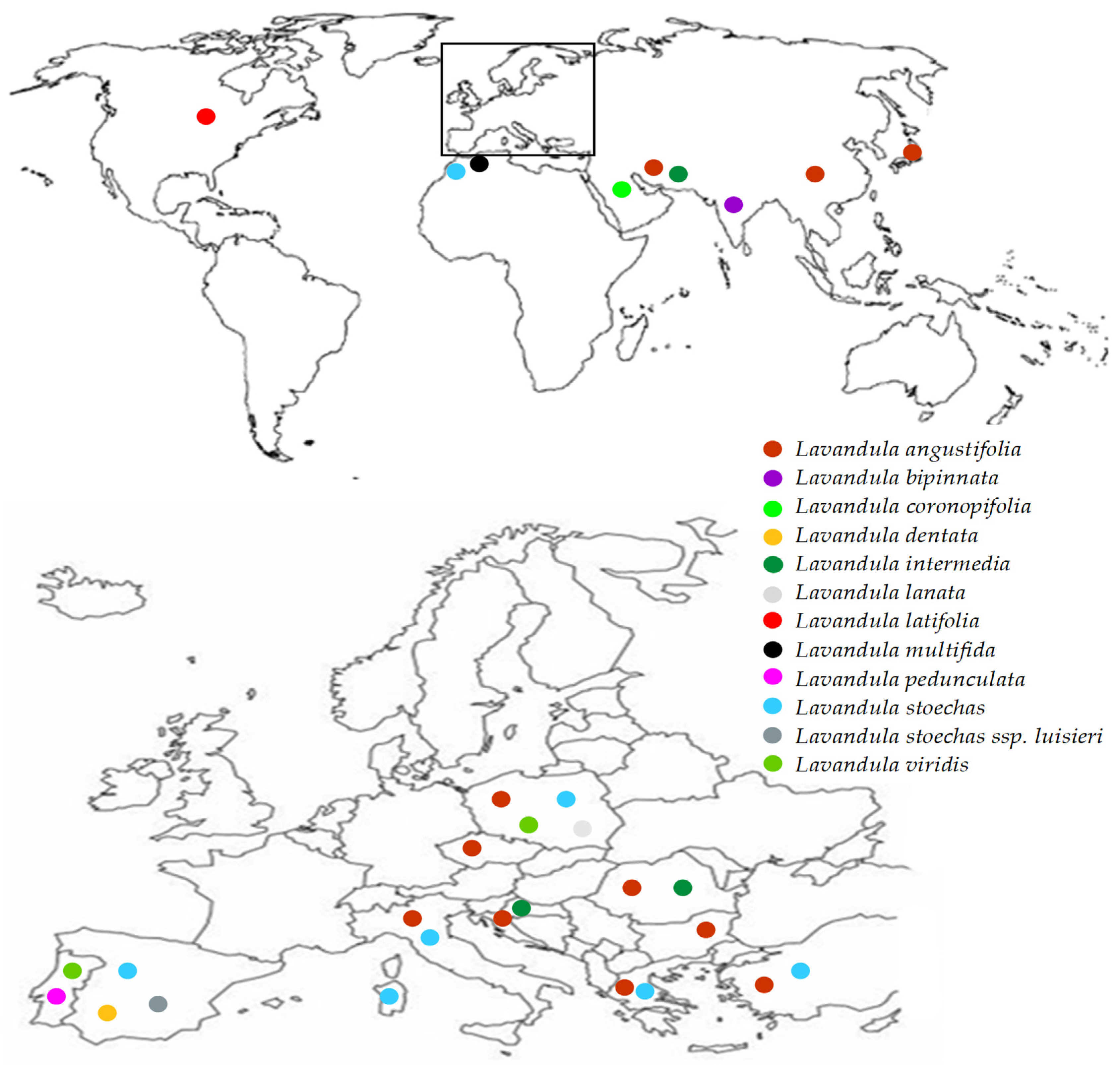
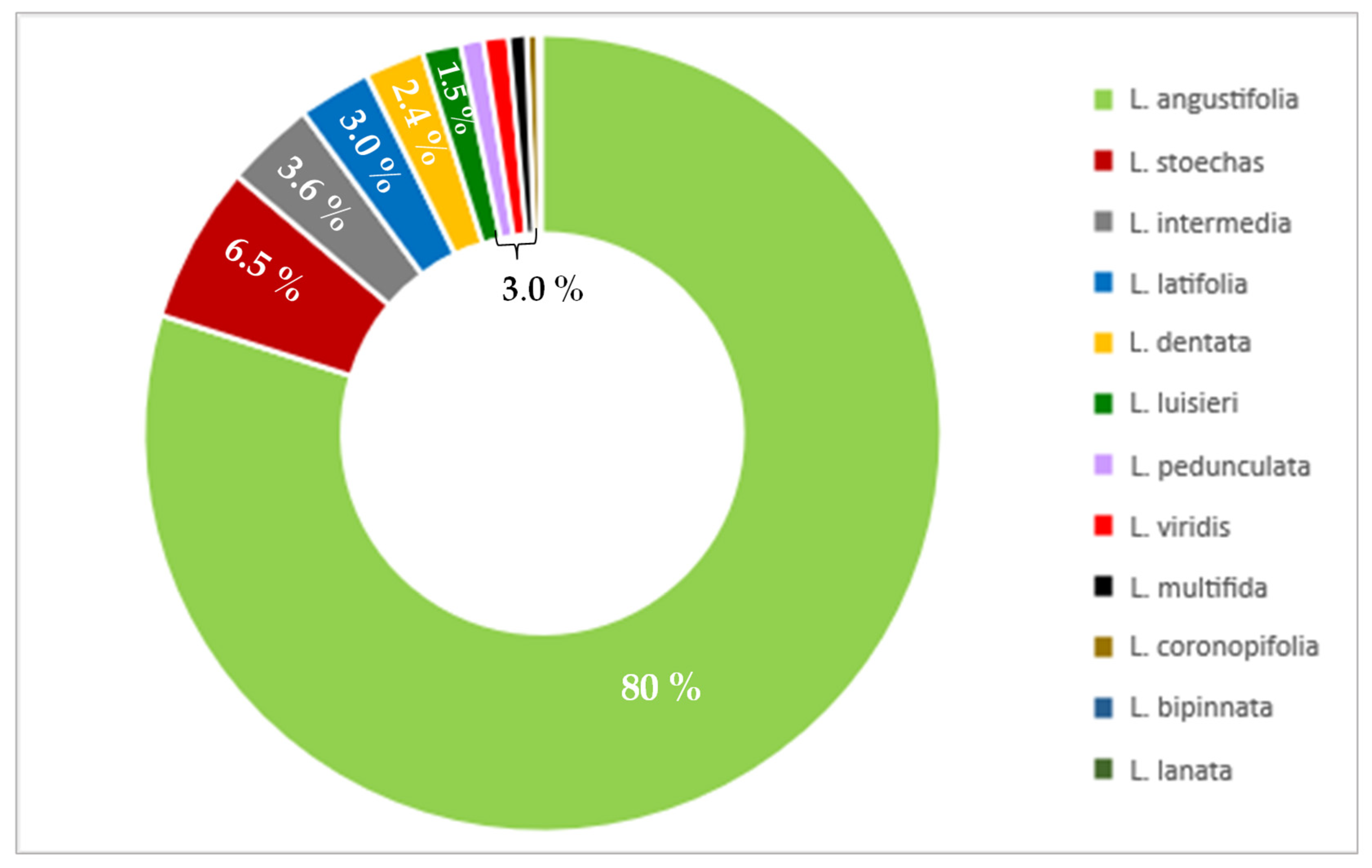
| Systematic Name | Common Name | Height of Shrub [cm] | Color of Leaves | Color of Flowers | Flowering Period | Place of Native Origin |
|---|---|---|---|---|---|---|
| Lavandula angustifolia (L. officinalis, L. vera) | British lavender | to 50 | grey leaves becoming green as they mature | shades of blue or mauve, white | mid-June to July | Southwest and South Central Europe in mountainous areas over 1500 m |
| Lavandula bipinnata | - | 15–100 | green | pale blue | August | Central and South India |
| Lavandula coronopifolia | - | to 80 | green to grey-green | sky blue to lilac | January to April | Cape Verde Islands, North Africa, Western Asia, Arabian Peninsula |
| Lavandula dentata | - | 50–100 | green to grey-green | shades of violet-blue to mauve | June to August | South Spain, Balearic Islands, North Africa, South West Arabian Peninsula, Ethiopia |
| Lavandula intermedia (L. hybrida) | lavandin | 60–150 | grey tomentose | shades of lilac-purple to white | late June to July | France, Spain, Italy |
| Lavandula lanata | woolly lavender | 50–80 | leaves covered with dense white woolly hairs | dark purple | mid-to late July | mountainous areas in South Spain over 2000 m |
| Lavandula latifolia (L. spica) | spike lavender | 50–70 (100) | grey | blue to mauve | From mid-July | Southwest and South Central Europe to 1000 m |
| Lavandula multifida | Egyptian lavender | to 40 | grey-green | violet to blue-violet | June to September | South Spain and Italy, North Africa |
| Lavandula pedunculata | butterfly lavender | to 60 | grey-green | mauve | June to July | Iberian Peninsula, North Africa and Turkey |
| Lavandula pubescens | - | 30–60 | green | violet-blue | August to September | Syria, Jordan, Israel, Egypt, Saudi Arabia, Yemen |
| Lavandula stoechas | French lavender | 40–70 | grey tomentose | black-purple to mauve | May to September | Mediterranean basin |
| Lavandula stoechas subsp. luisieri (Lavandula luisieri) | Spanish lavender | 40–60 | green | mauve | spring | Southwest Spain, Central and South Portugal |
| Lavandula viridis | white lavender | 30–50 | green | white | spring | Southwest Spain, South Portugal, Madeira |
| Hydroxycinnamic Acids | Species | Part of Plant | Contents [μg/g] | Ref. |
|---|---|---|---|---|
| cinnamic acid | Lavandula angustifolia | herb | 0.028–0.050 | [58] |
| Lavandula angustifolia | flowers | 0.001 | [1] | |
| hydroxycinnamic acid glucoside | Lavandula dentata | aerial parts | n.q. | [5] |
| Lavandula stoechas | n.q. | |||
| caffeic acid (3,4-dihydroxycinnamic acid) | Lavandula angustifolia | herb | 0.018–0.062 | [58] |
| Lavandula angustifolia | flowers | 0.015 | [1] | |
| Lavandula angustifolia | herb | n.q. | [68] | |
| Lavandula angustifolia ‘Rosea’ | n.q. | |||
| Lavandula angustifolia ‘Afropurpurea’ | n.q. | |||
| Lavandula lanata | n.q. | |||
| Lavandula stoechas | n.q. | |||
| Lavandula viridis | n.q. | |||
| Lavandula coronopifolia | aerial parts | n.q. | [54] | |
| Lavandula pedunculata | flowering stems | n.q. | [16] | |
| Lavandula spica | herb | 0.585 | [57] | |
| Lavandula stoechas | flowers | n.q. | [51] | |
| Lavandula vera | leaves | 0.001 | [52] | |
| caffeic acid 3-glucoside | Lavandula angustifolia ‘Lady’ | leaves | n.q. | [49] |
| chlorogenic acid (3-O-caffeoylquinic acid) | Lavandula angustifolia | flowers | 0.007 | [1] |
| Lavandula angustifolia | herb | n.q. | [68] | |
| Lavandula angustifolia ‘Rosea’ | n.q. | |||
| Lavandula angustifolia ‘Afropurpurea’ | n.q. | |||
| Lavandula lanata | n.q. | |||
| Lavandula stoechas | n.q. | |||
| Lavandula viridis | n.q. | |||
| Lavandula pedunculata subsp. lusitanica | aerial parts | 0.012 | [48] | |
| Lavandula viridis L’Her | aerial parts | 0.096 | [11] | |
| cryptochlorogenic acid (4-O-caffeoylquinic acid) | Lavandula pedunculata subsp. lusitanica | aerial parts | 0.053–0.692 | [48] |
| Lavandula viridis L’Her | aerial parts | 1.335–1.825 | [11] | |
| neochlorogenic acid (5-O-caffeoylquinic acid) | Lavandula pedunculata subsp. lusitanica | aerial parts | 0.130–1.232 | [48] |
| Lavandula viridis L’Her | aerial parts | 0.605–2.332 | [11] | |
| caftaric acid derivative | Lavandula coronopifolia | aerial parts | n.q. | [54] |
| chicoric acid (dicaffeoyltartaric acid) | Lavandula coronopifolia | aerial parts | n.q. | [54] |
| p-coumaric acid (4-hydroxycinnamic acid) | Lavandula angustifolia | herb | 0.365–0.422 | [58] |
| Lavandula angustifolia | flowers | 0.005 | [1] | |
| Lavandula spica | herb | 0.520 | [57] | |
| hydro-p-coumaric acid | Lavandula spica | herb | 0.558 | [57] |
| coumaric acid hexoside | Lavandula dentata | aerial parts | n.q | [5] |
| Lavandula stoechas | n.q. | |||
| ferulic acid (4-hydroxy-3-methoxycinnamic acid) | Lavandula angustifolia | herb | 0.053–0.542 | [58] |
| Lavandula angustifolia | flowers | 0.0002 | [1] | |
| Lavandula angustifolia ‘Lady’ | leaves | n.q. | [49] | |
| Lavandula spica | herb | 0.380 | [57] | |
| Lavandula vera | leaves | 0.005 | [52] | |
| Lavandula viridis | herb | n.q. | [68] | |
| ferulic acid-4-O-glucoside | Lavandula angustifolia ‘Lady’ | leaves | n.q. | [49] |
| lithospermic acid A | Lavandula pedunculata | flowering stems | n.q. | [16] |
| rosmarinic acid | Lavandula angustifolia | flowers | 0.010 | [1] |
| Lavandula coronopifolia | aerial parts | n.q. | [54] | |
| Lavandula pedunculata subsp. lusitanica | aerial parts | 0.011–6.246 | [48] | |
| Lavandula pedunculata | flowering stems | n.q. | [16] | |
| Lavandula dentata | aerial parts | n.q. | [5] | |
| Lavandula stoechas | flowers | n.q. | [51] | |
| Lavandula viridis L’Her | aerial parts | 1.346–20.714 | [11] | |
| salvianolic acid B (lithospermic acid B) | Lavandula pedunculata | flowering stems | n.q. | [16] |
| Lavandula stoechas | aerial parts | n.q. | [5] | |
| Lavandula stoechas | flowers | n.q. | [51] | |
| salvianolic acid C and G | Lavandula coronopifolia | aerial parts | n.q. | [54] |
| sinapic acid (4-hydroxy-3,5-dimethoxycinnamic acid) | Lavandula angustifolia | herb | 0.362–2.352 | [58] |
| yunnaneic acid F | Lavandula dentata | aerial parts | n.q. | [5] |
| Lavandula stoechas | n.q. | |||
| benzoic acid | Lavandula spica | herb | 0.687 | [57] |
| 3-hydroxybenzoic acid | Lavandula spica | herb | 0.018 | [57] |
| 4-hydroxybenzoic acid | Lavandula angustifolia | herb | 0.002 | [58] |
| Lavandula angustifolia | flowers | 0.011 | [1] | |
| Lavandula spica | herb | 1.578 | [57] | |
| Lavandula vera | leaves | 0.002 | [52] | |
| vanillic acid (4-hydroxy-3-methoxybenzoic acid) | Lavandula angustifolia Lavandula angustifolia Lavandula vera | herb flowers leaves | 0.003–0.010 0.0007 0.001 | [58] [1] [52] |
| syringic acid (4-hydroxy-3,5-dimethoxybenzoic acid) | Lavandula angustifolia | herb | 0.017–0.025 | [58] |
| protocatechuic acid (3,4-dihydroxybenzoic acid) | Lavandula angustifolia | flowers | 0.003 | [1] |
| Lavandula angustifolia | herb | 0.007–0.047 | [58] | |
| Lavandula spica | herb | 0.301 × 10−3 | [57] | |
| gallic acid (3,4,5-trihydroxybenzoic acid) | Lavandula angustifolia | herb | 0.005–0.017 | [58] |
| Lavandula angustifolia | flowers | 0.0001 | [1] | |
| homoprotocatechuic acid (3,4-dihydroxyphenylacetic acid) | Lavandula spica | herb | 0.007 | [57] |
| homovanillic acid (4-hydroxy-3-methoxyphenylacetic acid) | Lavandula spica | herb | 0.065 | [57] |
| Flavonoids | Species | Part of Plant | Contents [μg/g] | Ref. |
|---|---|---|---|---|
| Flavones | ||||
| apigenin (4’,5,7-trihydroxyflavone) | Lavandula angustifolia | herb | n.q. | [68] |
| Lavandula angustifolia ‘Rosea’ | n.q. | |||
| Lavandula stoechas | n.q. | |||
| Lavandula pedunculata subsp. lusitanica | aerial parts | 0.768–2.736 | [48] | |
| apigenin-O-glucoside | Lavandula dentata | aerial parts | n.q. | [5] |
| Lavandula stoechas | n.q. | |||
| apigenin-O-glucuronide | Lavandula stoechas | flowers | n.q. | [51] |
| apigenin hexoside | Lavandula dentata | aerial parts | n.q. | [5] |
| Lavandula stoechas | n.q. | |||
| genkwanin (7-methylapigenin) | Lavandula dentata | aerial parts | n.q. | [5] |
| Lavandula stoechas | n.q. | |||
| isoscutellarein-O-glucuronide | Lavandula dentata | aerial parts | n.q. | [5] |
| luteolin (3’,4’,5,7-terahydroxyflavone) | Lavandula pedunculata subsp. lusitanica | aerial parts | 0.013–4.975 | [48] |
| Lavandula viridis | herb | n.q. | [68] | |
| Lavandula viridis L’Her | aerial parts | 0.175–7.086 | [11] | |
| luteolin-O-glucoside | Lavandula angustifolia | herb | n.q. | [68] |
| Lavandula angustifolia ‘Rosea’ | n.q. | |||
| Lavandula angustifolia ‘Afropurpurea’ | n.q. | |||
| Lavandula lanata | n.q. | |||
| Lavandula stoechas | n.q. | |||
| Lavandula viridis | n.q. | |||
| Lavandula dentata | aerial parts | n.q. | [5] | |
| Lavandula stoechas | n.q. | |||
| Lavandula stoechas | flowers | n.q. | [51] | |
| luteolin-O-glucuronide | Lavandula dentata | aerial parts | n.q. | [5] |
| Lavandula stoechas | n.q. | |||
| Lavandula pedunculata | flowering stems | n.q. | [16] | |
| Lavandula stoechas | flowers | n.q. | [51] | |
| luteolin-O-hexosyl-O-glucuronide | Lavandula pedunculata | flowering stems | n.q. | [16] |
| methylluteolin-O-glucuronide | Lavandula pedunculata | n.q. | [16] | |
| Isoflavones | ||||
| formononetin (7-hydroxy-4’-methoxyisoflavone) | Lavandula spica | herb | 0.007 | [57] |
| Flavonols | ||||
| quercetin (3,3’,4’,5,7-pentahydroxyflavone) | Lavandula spica | herb | 0.016 | [57] |
| quercetin 3-O-glucoside | Lavandula stoechas | flowers | n.q. | [51] |
| rutin (quercetin 3-rutinoside) | Lavandula spica | herb | 0.283 | [57] |
| taxifolin (dihydroquercetin) | Lavandula spica | herb | 0.004 | [57] |
| fisetin (5-desoxyquercetin) | Lavandula spica | herb | <0.001 | [57] |
| myricetin (3,5,7,3’,4’,5’-hexahydroxyflavone) | Lavandula angustifolia “Rosea” | herb | n.q. | [68] |
| Lavandula lanata | n.q. | |||
| Lavandula viridis | n.q. | |||
| Flavanol | ||||
| (+)-catechin | Lavandula vera | leaves | 0.004 | [52] |
| Flavanones | ||||
| hesperetin (3’,5,7,-trihydroxy-4’-methoxyflavanone) | Lavandula spica | herb | 0.001 | [57] |
| hesperidin (hesperetin-7- rutinoside) | Lavandula spica | herb | 0.023 | [57] |
| neohesperidin (hesperetin 7-O-neohesperidoside) | Lavandula spica | herb | 0.032 | [57] |
| naringenin (4’,5,7-trihydroxyflavanone) | Lavandula spica | herb | 0.398 | [57] |
| Lavandula vera | leaves | 0.003 | [52] | |
| narirutin (naringenin 7-O-rutinoside) | Lavandula spica | herb | 0.014 | [57] |
| naringin (naringenin-7-neohesperidoside) | Lavandula spica | herb | 0.001 | [57] |
| eriodictyol (tetrahydroxyflavanone) | Lavandula spica | whole plant | 0.007 | [57] |
| eriodictyol-O-glucuronide | Lavandula pedunculata | flowering stems | n.q. | [16] |
| eriocitrin (eriodictyol 7-O-rutinoside) | Lavandula spica | herb | 0.004 | [57] |
| pinocembrin (dihydrochrysin) | Lavandula spica | herb | 0.001 | [57] |
| Lavandula viridis L’Her | aerial parts | 4.934–12.745 | [11] | |
| liquiritigenin (4’,7-dihydroxyflavanone) | Lavandula spica | herb | <0.001 | [57] |
| liquiritin (7-hydroxyflavanone 4’-O-glucoside) | Lavandula spica | herb | 0.002 | [57] |
| vanillin (4-hydroxy-3-methoxybenzaldehyde) | Lavandula angustifolia | herb | 0.100–0.193 | [58] |
| Lavandula Species | Type of Extract | Animal Model of Inflammation | Anti-Inflammation Effect | Detection Method | Ref. |
|---|---|---|---|---|---|
| Lavandula multifida | ethanolic macerate, aqueous macerate | Croton-oil-induced ear edema in mice | edema reduction | HPLC | [7] |
| Lavandula bipinnata | Soxhlet extraction | - | inhibition of COX enzymes | HPTLC | [6] |
| Lavandula officinalis | hydroethanolic macerate | formalin test in mice | inhibition of COX enzymes | - | [93] |
| Lavandula dentata | hydromethanolic extracts (ASE) | carrageenan-induced paw edema in mice | decrease expression of iNOS, COX-2, IL-1β | RP-HPLC-DAD-MS | [5] |
| Lavandula stoechas | decrease expression of IL-1β, IL-6, TNF-α, iNOS, COX-2, MMP-9 | ||||
| Lavandula stoechas | hydroethanolic extract (UAE) | carrageenan-induced paw edema in rats | edema reduction | - | [92] |
| Lavandula pedunculata | hydroalcoholic extracts | mouse macrophage-like cell line RAW 264.7 stimulated with LPS | inhibition of NO production | HPLC-DAD-ESI/MSn | [16] |
| aqueous extracts (infusions) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dobros, N.; Zawada, K.D.; Paradowska, K. Phytochemical Profiling, Antioxidant and Anti-Inflammatory Activity of Plants Belonging to the Lavandula Genus. Molecules 2023, 28, 256. https://doi.org/10.3390/molecules28010256
Dobros N, Zawada KD, Paradowska K. Phytochemical Profiling, Antioxidant and Anti-Inflammatory Activity of Plants Belonging to the Lavandula Genus. Molecules. 2023; 28(1):256. https://doi.org/10.3390/molecules28010256
Chicago/Turabian StyleDobros, Natalia, Katarzyna Dorota Zawada, and Katarzyna Paradowska. 2023. "Phytochemical Profiling, Antioxidant and Anti-Inflammatory Activity of Plants Belonging to the Lavandula Genus" Molecules 28, no. 1: 256. https://doi.org/10.3390/molecules28010256
APA StyleDobros, N., Zawada, K. D., & Paradowska, K. (2023). Phytochemical Profiling, Antioxidant and Anti-Inflammatory Activity of Plants Belonging to the Lavandula Genus. Molecules, 28(1), 256. https://doi.org/10.3390/molecules28010256