Abstract
Lavender is a valuable medicinal plant belonging to the Lamiaceae family. Currently 39 species are known, but only Lavandula angustifolia is a pharmacopoeial raw material. Lavender has a long history of medicinal use and mainly exhibits antioxidant, anti-inflammatory, sedative, antidepressant, spasmolytic, anticholinesterases, antifungal and antibacterial properties. Used internally, it relieves symptoms of mental stress and insomnia and supports digestion. Topical use of lavender in aromatherapy, neuralgia and antiseptics is also known. The constant interest in lavender, and in particular in Lavandula angustifolia, in the field of medicine and pharmacy is evidenced by the growing number of publications. In view of so many studies, it seems important to review traditional and modern extraction techniques that determine the chemical composition responsible for the antioxidant and anti-inflammatory effects of various extracts from the species of the Lavandula genus.
1. Introduction
Lavender is a valuable medicinal plant belonging to the Lamiaceae family. It is native to the Mediterranean region and grows in natural sites of the lower parts of the mountains. Lavender is cultivated as an ornamental plant in many countries in Europe, north Africa, southwest Asia, western Iran, eastern India, China and Japan (Figure 1). The Lavandula genus includes 39 species, but only Lavandula angustifolia is considered a pharmacopoeial raw material [1,2]. Lavender has a long history of medicinal use. In traditional medicine it is a popular herb used to treat multiple diseases. Lavender has antioxidant [3,4], anti-inflammatory [5,6,7,8], sedative [9], antidepressant [10], spasmolytic, anticholinesterases [11], antifungal [8] and antibacterial [1] properties. Lavender is known as a medicinal product used internally to relieve symptoms of mental stress, insomnia and digestive disorders, and externally in aromatherapy, neuralgia and as an antiseptic. Lavender decoctions and hydrolates are applied as compresses that have a beneficial effect on the skin. Lavender infusions and lavender oil in the form of inhalation have sedative and anxiolytic effects that have been confirmed in both animal and human studies [9,10,12]. Lavender is a very popular aromatic plant and is commonly used in food and cosmetics thanks to its antibacterial, antifungal, antioxidant and anti-inflammatory properties. Lavender essential oil is present in eau de toilette, lotions, soaps, shampoos and household cleaners [13,14]. The essential oil of lavender can be added to cosmetics without the need for preservatives [1]. Because of its unique composition, pro-health benefits and attractive sensory attributes lavender can be used in the processing industry as a component of products with functional properties [3]. Owing to its medicinal activities, lavender, in particular Lavandula angustifolia, enjoys constant interest in the medical and pharmaceutical areas, as evidenced by an increasing number of publications in the last 25 years (Figure 2).
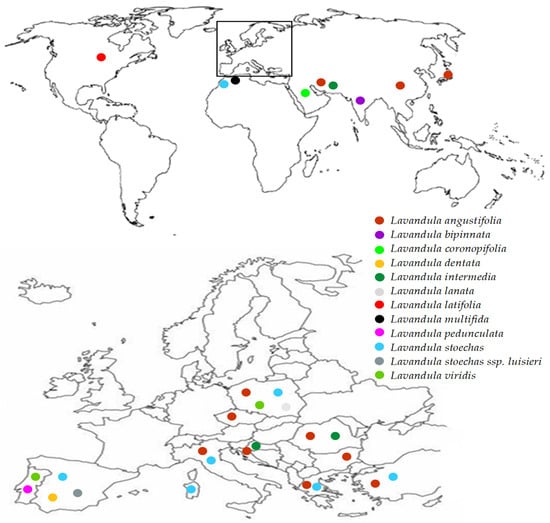
Figure 1.
Global distribution map of various species belonging to the Lavandula genus. Inset: distribution of Lavandula in Europe.
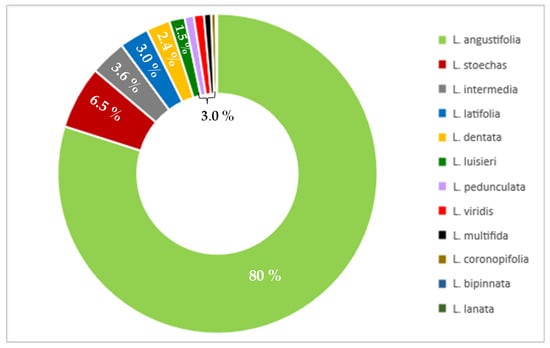
Figure 2.
Percentage of publications for various lavender species in the last 25 years (PubMed, November 2022).
In this review are presented the traditional and modern extraction techniques, chemical composition as well as antioxidant and anti-inflammatory activity of the extracts from different species of the Lavandula genus. The specific characteristics of each species are presented in Table 1 [15,16].

Table 1.
The specific characteristics of different species of Lavandula [15,16].
Recent reviews on lavender concerned mainly the anti-anxiety, antidepressant and wound-healing properties of essential oil [9,10,12,17,18]. However, to the best of our knowledge this work is the first attempt to review the data concerning the extraction and profile of other bioactive components of lavender, namely phenolic acids and flavonoids, as well as the antioxidant and anti-inflammatory properties of lavender phenolic-rich extracts.
The species of Lavandula presented in Table 1 differ in terms of the height of the shrub, color of leaves, flowers and flowering period. Lavandula coronopifolia, which occurs naturally in northern Africa, the Arabian Peninsula and Western Asia, starts flowering the earliest, in January, whereas Lavandula pubescens, which occurs naturally in Syria, Jordan, Israel, Egypt, Yemen and Saudi Arabia, starts flowering from August to September. In recent years, more and more species have been cultivated outside their natural habitat. The same species grown in different areas may show morphological differences in the color of leaves, flowers, plant density or seed weight. The morphological variability, and thus the quantitative composition of individual compounds, is due to climatic conditions such as temperature, insolation, rainfall, humidity and altitude as well as environmental conditions such as fertilization, soil type and its pH level [16,19].
2. Extraction Process
Extraction of plant material is a process of separating bioactive compounds from the sample by means of selective solvents and standard extraction procedures [20,21]. A high extraction yield results from the appropriate selection of the solvent which should be suited to the nature of the compounds to be extracted [21,22]. The polarity of the targeted compounds is especially important. For the extraction of non-polar compounds, hexane and chloroform are used [23]. Moreover, methanol, ethanol and acetone as well as hydroalcoholic mixtures are the most generally used solvents for the extraction of polar compounds [24]. Phenolic compounds are more stable at low pH, hence the acidified hydroalcoholic solvents are frequently used for their extraction [25]. The extraction process is influenced by the physicochemical parameters of the solvents (boiling point, viscosity, density, vapor pressure and solvent power), their cost, non-flammability and non-toxicity [21,24], as well as sample preparation (drying, grinding and sample particle size) and extraction parameters (extraction time, temperature, number of extraction steps, ratio of solvent to sample and use of co-solvent) [21,22,26,27].
2.1. Traditional Extraction Techniques
The conventional methods include solid-liquid extraction, such as maceration, digestion, percolation, infusion, decoction and Soxhlet extraction [28]. They are the general techniques used for the extraction of medicinal plants and are mostly applied for galenical preparations. One of these is tincture which is made as a result of maceration or percolation of plant material with ethanol of a suitable concentration [2]. Maceration is based on soaking plant material in a solvent at room temperature for several hours up to several days. The process of maceration that takes place at elevated temperature is called digestion [29]. The use of repeated maceration, grinding of plant material, high temperature and stirring during the extraction process increases its efficiency [29,30]. Moreover, percolation is a continuous extraction method in which after 24 h maceration a fresh solvent flows through the comminuted plant material and thus allows it to be completely etched. Percolation usually takes less time than maceration and requires percolators, that is, vessels with a conical shape that facilitate the removal of the extracted raw material [28,29]. Infusion is obtained by macerating the ground plant material with cold or boiling water for 5 to 15 min. Decoction differs from infusion in that the raw material is boiled in parallel with water from 15 to 30 min. In both cases the thus-obtained extract is cooled and filtered [28,29]. One of the most widely used traditional methods for the extraction of heat-stable compounds of medicinal plants is Soxhlet extraction. It is a form of continuous hot extraction in which the target compounds are extracted from solids with repeated washing with organic solvents such as ethyl acetate or hexane [24]. This technique is often used in the industry, but it should be remembered that it is not environmentally friendly because it uses large amounts of toxic solvents. During the extraction of lavender essential oil with hexane, other substances such as waxes, pigments and albuminous materials are extracted in addition to volatile compounds. The hexane extracts obtained in this way can be purified, but this is time-consuming and contributes to yield loss [3]. Additionally, long-term extraction time and high solvent temperature may result in the decomposition of valuable substances [22]. The disadvantages of traditional extraction techniques are that they require long analytical times and large quantities of solvents [21,25,27,30], and also they may contribute to the degradation of thermolabile compounds [22,27], thus resulting in a lower extraction yield [21,27]. Moreover, these methods are characterized by low selectivity and reproducibility [25]. Despite numerous drawbacks, these methods are still used because they are simple, easy to implement and do not require specialized equipment [31].
2.2. Modern Extraction Techniques
Several modern extraction methods which are environmentally friendly and thus called green techniques, such as ultrasound-assisted extraction (UAE), microwave-assisted extraction (MAE), accelerated solvent extraction (ASE) and supercritical fluid extraction (SFE), have recently been introduced for the extraction of compounds from plant materials [27,30]. These techniques are characterized by lower volumes of organic solvents (up to 100 mL), shorter extraction times (up to 1 h, as compared to even days for, e.g., maceration) and thus lower energy consumption [24]. By using these methods better reproducibility and selectivity as well as higher quality of the extract can be obtained than with traditional techniques [21,24,25].
Ultrasound is sound waves with frequencies from 20 kHz to 100 MHz [30]. The best extraction frequency for lavender is 20–40 kHz [17,32,33,34]. The propagation of ultrasound waves in the liquid is related to changes in the acoustic pressure causing the cavitation phenomenon. Furthermore, cavitation leads to the formation and collapse of gas bubbles, thus resulting in mechanical disruption of the cell walls thereby causing the release of target compounds from plant material [21,35]. Ultrasound extraction is a simple extraction technique requiring only a water bath or an ultrasonic probe applied directly to the sample [30,36]. The application of ultrasound can be an alternative technique for the extraction of phenolic compounds due to the reduction in the extraction time and the amount of the solvent [22]. This technique enables the extraction of several samples at the same time in water bath; however, after the extraction process, filtration or decantation is required. The extraction efficiency is influenced by many factors such as sample size, temperature, frequency and sonication time, as well as the kind of solvent and its pH [24]. For instance, polyphenolic compounds show higher stability at a lower pH of the solvent [25].
Microwave radiation is electromagnetic radiation in the range of 300 MHz to 300 GHz [30]. Microwave power from 230 to 500 W is most often used to extract lavender [33,37,38]. Microwave-assisted extraction (MAE) can be applied both for polar and non-polar solvents; however due to the high dielectric constant, polar solvents are more often used [21]. Solvents absorb microwave radiation and are heated to a temperature above the boiling point, which results in the rapid isolation of target compounds and high extraction efficiency [22]. Generally speaking, microwave-assisted extraction could reduce extraction time and solvent consumption and also increase the purity of the obtained extract in comparison with conventional methods [22,30,36]. Additionally, this technique allows processing of several samples at the same time [39]. Extraction conditions, such as temperature and extraction time, are very important but they should be chosen with caution to avoid thermal degradation of phenolic compounds [40].
Accelerated solvent extraction (ASE), also called pressurized liquid extraction (PLE), is performed at an elevated pressure, thus resulting in a higher extraction yield in comparison with conventional techniques. The extraction efficiency is influenced by parameters such as temperature, pressure, extraction time, the nature and volume of the solvent used as well as the solid-to-liquid ratio [35,41]. The extraction is carried out at temperatures between 40 °C and 200 °C and pressures between 3.3 and 20.3 MPa, and takes up to 20 min [22,24]. Increased pressure allows one to keep the solvent in the liquid phase above its boiling point whereas high temperature accelerates the kinetics of extraction [22,29]. This leads to a reduction in surface tension and solvent viscosity, and thus to an increase in the solubility of the compounds [27]. In accelerated solvent extraction ethanol or water are used to extract polyphenols [35]. Accelerated solvent extraction can be an alternative to other techniques because of the reduction in solvent quantities and extraction time, automation of the process and the possibility of extracting samples with high humidity [24,30]. On the other hand, the use of high temperatures may contribute to the decomposition of thermolabile compounds [29].
Supercritical fluid extraction (SFE) is an attractive technique for the extraction of bioactive compounds present in medicinal plants. Supercritical fluids exhibit the characters of both a liquid and a gas at their critical point [40]. Their densities are similar to the density of a liquid whereas low viscosity and surface tension make them similar to a gas [29,30]. Owing to their high diffusivity, supercritical fluids increase the extraction rate. The extraction procedure usually consists of static and dynamic phases. In the first phase the vessel with sample is filled with supercritical gas and thermostated for 10–15 min. During the dynamic phase the supercritical fluid is continuously passed through the sample for about an hour and then the extract is collected in a receiver [3,42]. Solvents such as carbon dioxide, water, ethane, propane and dimethyl ether could be used in supercritical extraction [43,44]. However, the most commonly used supercritical fluid is carbon dioxide, which is considered safe for humans and the environment due to its non-toxicity, non-flammability and non-explosiveness. It enables the recovery of thermolabile compounds owing to its low critical temperature (31.1 °C) and pressure (7.38 MPa) [43,45]. Carbon dioxide is mainly used to extract carotenoids, lipids and essential oils due to its non-polarity [22,30]. The addition of up to 15–20% of co-solvent (ethanol, methanol, water) to the supercritical fluid extraction process enables the extraction of polyphenolic compounds, which are polar [46]. The total use of carbon dioxide during extraction depends on its pressure, flow rate, temperature, sample size and extraction time. In the study of Woźniak et al., 2017 [42] the CO2 consumption was 120–130 g for a 10 g sample and a CO2 flow of 1.8 g/min. The major advantage of this method is the possibility to modify the solubility of individual compounds by changing the extraction parameters such as temperature and pressure [44]. Optimal extraction conditions to obtain the maximum yield and polyphenol content from lavender flowers were a temperature of 54.5 °C, a pressure of 29.79 MPa and an extraction time of 45 min [47]. Further advantages of supercritical fluid extraction are the lack of oxygen which prevents unfavorable oxidation processes [30] and the ease of separating the extractant from the product [22,45]. Various methods used for the extraction of secondary plant metabolites are presented in Table 2.
As can be seen, in the case of lavender the majority of studies used traditional methods, i.e., maceration, infusion or digestion with water, alcohol or hydroalcoholic mixtures (17 studies). Among them the best results in terms of total yield were obtained for maceration [11,48]. As for the modern techniques, the most often used (five studies) was ultrasound-assisted extraction. This is probably due to the relatively easy access to ultrasound devices in scientific laboratories. From the analysis of various approaches to the extraction of bioactive compounds other than the essential oil components from lavender, the best method of extraction seems to be ultrasound-assisted extraction with a hydroalcoholic solvent, which decreases the extraction time compared with traditional methods. As the phenolics in lavender are not very thermolabile, an elevated temperature (up to 60 °C) can be used, increasing the yield. Unfortunately, in many cases the yield of extraction could be determined because no dry extract was obtained [37,49,50,51,52], and in some of the papers the mass of the extract and the extraction yield were not given.

Table 2.
Extraction techniques for various lavender species.
Table 2.
Extraction techniques for various lavender species.
| Extraction Method | Extraction Procedure and Conditions | Yield [%] | Lavender Species | Part of Plant | Detection Method | Antioxidant Assays | Ref. | |
|---|---|---|---|---|---|---|---|---|
| Types of Assays | Activity | |||||||
| Refluxed extraction | 3 g were refluxed with 100 mL of MeOH in a water bath for 1 h. Plant material was re-extracted twice with the same solvent (2 × 100 mL). Then, the SPE procedure was used to obtain the phenolic acid fractions. | ND | L. angustifolia | flowers | SPE/RP-HPLC | ND | [53] | |
| Methanol extraction | 0.34 g was extracted with 30 mL MeOH. | ND | L. stoechas | flowers | Folin–Ciocalteu (760 nm) HPLC/ESI-MS | DPPH [mg/mL] | 7.05 | [51] |
| Ethanol extraction | 300 g were extracted with 90% EtOH. Then, the dried extract was suspended in water and fractionated with ethyl acetate. | 10 | L. coronopifolia | aerial parts | UPLC- ESI- MS/MS | DPPH [µg/mL] | 17.8 ± 0.8 | [54] |
| 2 g were extracted with 10 mL of 96% EtOH for 24 h in a water bath at 45 °C. | ND | L. angustifolia | flowers | RP-HPLC | ND | [1] | ||
| Aqueous-ethanol extraction | 2 g were extracted with 90 mL of 50% EtOH at 85 °C for 1 h. | ND | L. angustifolia ssp. angustifolia | flowers | Folin–Ciocalteu (765 nm) | DPPH [µg/mL] Fe2+ chelation assay [µg/mL] | 95.60 ± 1.70 54.46 ± 0.55 101.40 ± 0.90 50.60 ± 0.40 | [4] |
| L. angustifolia ssp. angustifolia ‘Munstead’ | ||||||||
| L. angustifolia. angustifolia ‘Hidicote Blue’ | 96.53 ± 1.45 49.93 ± 0.75 | |||||||
| L. angustifolia ssp. pyrenaica | 110.36 ± 1.40 81.90 ± 1.40 | |||||||
| L. hybrida | 73.53 ± 1.25 49.90 ± 0.90 | |||||||
| Aqueous extraction | 0.34 g was extracted with 30 mL of H2O | ND | L. stoechas | flowers | Folin–Ciocalteu (760 nm) | DPPH [mg/mL] | 1.78 | [51] |
| Infusion | 1 g was extracted with 200 mL of boiling water for 10 min. | ND | L. angustifolia | flowers | Folin–Ciocalteu (760 nm) | ABTS [mM] | 0.72 ± 0.07 | [50] |
| 2 g was extracted with 200 mL of boiling distilled water and left to stand at room temperature for 5 min. | ND | L. pedunculata | flowering stems with inflorescences | HPLC-DAD-ESI/MSn | DPPH [μg/mL] TBARS [μg/mL] reducing power [μg/mL] | 68 ± 0.5–191 ± 2 14 ± 1–39.1 ± 0.1 51 ± 1–167 ± 1 | [16] | |
| 1 g was homogenized in 20 mL of hot water (90 °C) for 5 min. | 22.5 | L. pedunculata ssp. lusitanica | aerial parts | HPLC-DAD | TEAC (μmol TE/g extract) ORAC (μmol TE/g extract) TBARS [%] Fe2+ chelation assay [%] | 866 ± 12.5 3018 ± 91.1 100 ± 0.0 48.0 ± 5.0 | [48] | |
| Infusion with stirring | 20 g was extracted with 400 mL of boiling water and stirred for 15 min. | 10.8 | L. stoechas | plant material from local market | Folin–Ciocalteu (760 nm) | DPPH [%] Fe2+ chelation assay superoxide anion | 45 ± 0.0 84 ± 0.0 78 ± 0.0 | [55] |
| [%] | ||||||||
| Stirring | 1 g was extracted with 25 mL of EtOH:H2O (80:20 v/v) and stirred for 1 h at 25 °C at 150 rpm. | ND | L. pedunculata | flowering stems with inflorescences | HPLC-DAD-ESI/MSn | DPPH [μg/mL] | 87 ± 2–257 ± 7 17 ± 1–63.5 ± 0.1 67 ± 1–216 ± 6 | [16] |
| TBARS [μg/mL] reducing power [μg/mL] | ||||||||
| 30 g was extracted with 1500 mL of deionized water, heated to a specific temperature (40, 60, 80 °C ± 0.5 °C) and stirred for 90 min at 500 rpm. | 0.24 | L. x hybrida | plant material from herbal store | Folin–Ciocalteu (760 nm) | ABTS [mol Trolox/g DM] | 0.216 ± 0.038 | [56] | |
| Shaking | Samples were extracted with 80% aqueous methanol and shaken at room temperature for 15 h. | ND | L. angustifolia ‘Lady’ L. angustifolia ‘Hidcote’ L. latifolia | leaves | HPLC-MS, Folin–Ciocalteu (735 nm) | DPPH [μmol TEAC/g DW] | 14.17 ± 9.09 9.00 ± 3.00 6.56 ± 1.13 | [49] |
| Shaking | Different protocols: | ND | ||||||
| ST1: SLE using H2O, shaking for 5 h | ||||||||
| ST2: H2O:EtOH (1:1; v/v), shaking for 2 h | ||||||||
| ST3: H2O:EtOH (1:1; v/v), shaking for 5 h | L. spica | plant material from local herbal market | SLE-SPE-UHPLC-MS/MS | ND | [57] | |||
| ST4: EtOH, shaking for 5 h | ||||||||
| ST5: H2O:MeOH (1:1; v/v), shaking for 2 h | ||||||||
| ST6: H2O:MeOH (1:1; v/v), shaking for 2 h twice | ||||||||
| ST7: H2O:MeOH (1:1; v/v), shaking for 5 h | ||||||||
| ST8: MeOH, shaking for 5 h | ||||||||
| 2 g was extracted with 20 mL of MilliQ water and shaken for 1 h at ambient temperature | ND | L. angustifolia | herb | UHPLC-DA | ABTS [mmol/100 g DW] | 22.00 ± 0.00 20.19 ± 2.55 | [58] | |
| Randall Extraction | 2 g was extracted with 20 mL of MilliQ water by Randall extraction for 1 h at 100 °C. | |||||||
| Plant material was extracted with hexane and then with ethanol at room temperature for 48 h with plant material: solvent ratio of 1:10 (w/w). | 12.2 | L. stoechas ssp. luisieri | herb | HPLC | DPPH [µg/mL] | 30.66 ± 1.9 | [59] | |
| Maceration Maceration | 10 g were soaked overnight at room temperature in 200 mL of each solvent: water (w), water: ethanol (1:1) (w/e), ethanol (e). | 22.1 21.3 12.8 | L. viridis L’Her | aerial parts | HPLC–DAD | ORAC (w, w/e, e) [μmol TE/g extract] | 1502.22 ±39.95 4030.26 ±02.40 1183.95 ±90.78 | [11] |
| TEAC (w, w/e, e) [μmol TE/g extract] | 670.95 ± 4.24 1149.82± 17.31 332.06 ± 2.52 | |||||||
| 10 g was soaked overnight at room temperature in 200 mL of: water (w), water: ethanol (1:1) (w/e), ethanol (e). | 22.4 19.4 19.6 | L. pedunculata ssp. lusitanica | aerial parts | HPLC-DAD | TEAC (w, w/e, e) [μmol TE/g extract] ORAC (w, w/e, e) [μmol TE/g extract] TBARS (w, w/e, e) [%] Fe2+ chelation assay (w, w/e, e) [%] | 569 ± 1.99 688 ± 10.59 224 ± 6.41 | [48] | |
| 1530 ± 121 2567 ± 151 861 ± 6.00 | ||||||||
| 96 ± 2 100 ± 0 4 ± 2 | ||||||||
| 65.9 ± 1.27 50.1 ± 0.14 32.0 ± 0.50 | ||||||||
| 10 g was extracted with 100 mL of 70% MeOH and shaken in a water bath at 40 °C for 5 min. | ND | L. pubescens | aerial parts | Folin–Ciocalteu (760 nm) | DPPH [μg/mL] | |||
| Ultrasonic- microwave-assisted extraction (UMAE) | 10 g were immersed in 100 mL of 70% MeOH. The mixture was exposed to acoustic waves at 40 °C for 5 min (ultrasonic power 50 W, frequency 40 kHz, microwave power 480 W). | ND | L. pubescens | aerial parts | Folin–Ciocalteu (760 nm) | DPPH [μg/mL] | 24.83 19.54 22.04 | [38] |
| Ultrasonic- homogenizer-assisted extraction | 10 g was extracted with 100 mL of 70% MeOH using magnetic stirring (ultrasonic power 150 W, frequency 20 kHz, 40 °C, 5 min). | |||||||
| Microwave- assisted extraction (MAE) | 1 g was extracted with 15 mL of 60% and 80% methanol, ethanol and acetone at 80 °C and 500 W. | ND | L. officinalis | flowers | UPLC-DAD-ESI-MS/MS Folin–Ciocalteu (750 nm) | CUPRAC [mmol TR/g] DPPH [µg/mL] | 0.39 ± 0.01 125 ± 4.6 | [37] |
| Ultrasonic- assisted extraction (UAE) | 30 g was extracted twice with 500 mL of 80% EtOH using an ultrasonic bath for 30 min. | 14.8 14.2 10.9 23.9 20.8 14.6 | L. angustifolia | flowers * leaves inflorescence stalks | HPTLC | DPPH * [µg/mL] TBARS * [µg/mL] Fe2+ chelation assay * reducing power * | 11.37 ± 0.69 89.36 ± 5.00 319.21 ± 21.96 25.17 ± 0.16 | [60] |
| L. intermedia ‘Budrovka’ | flowers * leaves inflorescence stalks | HPTLC | DPPH * [µg/mL] TBARS * [µg/mL] Fe2+ chelation assay * reducing power * | 17.17 ± 0.33 116.54 ± 9.96 397.71 ± 10.26 33.78 ± 2.34 | ||||
| 0.5 g was immersed in 40 mL of 62.5% MeOH. Then, 10 mL of 6 M HCl was added and the mixture was submitted to ultrasounds for 15 min and refluxed in a water bath at 90 °C for 2 h. | ND | L. vera (L. angustifolia) | leaves | RP-HPLC | ND | [52] | ||
| Ultrasonic- assisted extraction (UAE) | 2 g was sonicated with 20 mL of MilliQ water for 15 min at ambient temperature. | ND | L. angustifolia | herb | UHPLC-PDA | ABTS [mmol/100 g DW] | 10.00 ± 0.00 | [58] |
| Pulsed ultrasound-assisted extraction (PUAE) | 1 g of flower residues was extracted with 40 mL of 70% EtOH using ultrasound applied in pulsed modality (frequency 26 kHz, power 200 W, temperature < 60 °C, extraction time 10 min). | ND | L. angustifolia ‘Rosa’ | flower residues after the distillation of essential oil | RP-HPLC Folin–Ciocalteu (760 nm) | DPPH [mg TE/g of dry waste] | 107.29 ± 0.05 | [34] |
| Accelerated solvent extraction (ASE) | 5 g was mixed with washed sea sand and extracted with 30 mL of 50% MeOH at 1500 PSI and 80 °C for 10 min. | 20 14 | L. dentata L. stoechas | aerial parts | RP-HPLC-DAD-MS | DPPH [µg/mL] | 71.1 ± 8.7 67.0 ± 6.5 | [5] |
| Supercritical fluid extraction (SFE) | 100 g was extracted with CO2 at 200–300 bar and 40–60 °C for 15–45 min, CO2 flow rate 10 kg/h. | ND 0.53–7.28 | L. angustifolia | flowers | HPLC Folin–Ciocalteu (765 nm) RP-HPLC | DPPH [%] | 50.55 ± 0.7 78.83 ± 1.3 ND | [47,61] |
| 40 g was extracted at 100–300 bar and 40–60 °C for 90 min, CO2 flow rate 1–3 kg/h. | ||||||||
| Supercritical antisolvent fractionation (SAF) | The ethanolic maceration extract was dissolved in 3% EtOH and fractionated using SAF with CO2 at 130 bar, CO2 flow rate 30 g/min. | ND | L. stoechas ssp. luisieri | herb | HPLC | DPPH [µg/mL] | 16.17 ± 0.7 | [59] |
ND—no data. * denotes analyzes performed for flowers.
3. Chemical Composition
Lavender flowers (Lavandulae flos), harvested before the flowering period, are the medicinal raw material. The main biologically active compounds of lavender are components of essential oil, phenolic compounds, triterpenes and sterols [62]. Essential oil, for which lavender is mainly known, is present in amounts from 2% to 3%. It is obtained from the flowers by hydrodistillation or steam distillation. The essential oil consists of more than a hundred components, the main of which are linalool (from 9.3% to 68.8%) and linalyl acetate (from 1.2% to 59.4%). The quality of essential oil of lavender depends both on the high content of linalool and linalyl acetate, and on their mutual proportions [3,14]. The predominant compounds include terpenes: borneol, limonene, camphene, eucalyptol, β-ocimene, 1,8-cineol, camphor, fenchone, lavandulol acetate, lavandulol, α-terpineol, β-caryophyllene, geraniol and α-pinen as well as non-terpenoid aliphatic components: octanon, octenol, octenylacetate and octanol [13,14,17,48,62].
An equally important group of compounds present in lavender flowers are polyphenols. They are secondary plant metabolites with various biological properties. They are found in various parts of plants: flowers, leaves, stems, fruits and seeds [63,64,65]. So far, more than 8000 polyphenolic compounds have been identified. In terms of chemical structure, they are characterized by the presence of one or more aromatic rings in a molecule and different numbers of hydroxyl groups [63]. Polyphenols are biosynthesized through the shikimic acid pathway [66]. They can be divided into several different groups: phenolic acids, flavonoids, coumarins, stilbenes and lignans [65,66]. Most phenolic compounds are found in combination with sugars, organic acids and esters [63,64,67].
There are two groups of phenolic acids: derivatives of hydroxybenzoic acid and hydroxycinnamic acid [67]. The phenolic acids most common in lavender are presented in Table 3. Rosmarinic acid is the most dominantly present popular hydroxycinnamic acid of the Lavandula genus [1,5,11,16,48,51,54]. The other representatives of this group include cinnamic acid, hydroxyhydrocinnamic acid glucoside, caffeic acid, caffeic acid 3-glucoside, chlorogenic acid, cryptochlorogenic acid, neochlorogenic acid, caftaric acid derivative, chicoric acid, p-coumaric acid, hydro-p-coumaric acid, coumaric acid hexoside, ferulic acid, ferulic acid-4-O-glucoside, lithospermic acid A, rosmarinic acid, salvianolic acid B (lithospermic acid B), salvianolic acid C and G, sinapic acid and yunnaneic acid F [1,5,11,16,48,49,51,52,54,57,58,68]. The hydroxybenzoic acid derivatives are less abundant in plants than the hydroxycinnamic acid derivatives. The main representatives of this group are benzoic acid, 3-hydroxybenzoic acid, 4-hydroxybenzoic acid, vanillic acid, syringic acid, protocatechuic acid, gallic acid, homoprotocatechuic acid and homovanillic acid [1,52,57,58] (Table 3).

Table 3.
Hydroxycinnamic and hydroxybenzoic acids in different species of lavender.
The presence of phenolic compounds in plant tissues protects them against adverse environmental conditions, such as high and low temperature, ultraviolet radiation, drought and salinity, and also against attack by herbivores, insects and microorganisms [64,66,69]. Besides biotic and abiotic stresses, the geographical area [16] and environmental factors such as soil composition, mineral fertilization, rainfall or temperature exert a notable effect on the content of polyphenolic compounds [49,58,64]. On the other hand, a study by Costa et al. [11] showed that the cultivation method affects the level of phenolic compounds. In vitro cultures of L. viridis were characterized by a higher content of phenolic compounds than wild plants, which was caused by differences between the growing conditions. The content of polyphenolic compounds also depends on the species, cultivars and selection of parts of the plant material. In the study of Blažeković et al. [60] the extracts prepared from the inflorescence stalk were characterized by a lower content of total polyphenols (3.09% and 4.54% for Lavandula x intermedia ‘Budrovka’ and Lavandula angustifolia, respectively) than lavender flower extracts (6.65% and 8.46%). However, the highest content of polyphenols was found in leaf extracts (7.05% and 9.20%). Likewise, Adaszyńska-Skwirzyńska and Dzięcioł [70] obtained the highest total polyphenol content for leafy stalk extracts (4.06 and 3.71 mg GAE/g d.m. for Lavandula angustifolia ‘Blue River’ and Lavandula angustifolia ‘Ellagance Purple’ extracts, respectively), and much lower for flower extracts (1.13 and 1.12 mg GAE/g d.m.). Moreover, the study by Bajalan et al. [71] showed that population variability has a significant effect on the variation in the content of phenolic compounds. Additionally, a positive correlation was found between the content of those compounds and the content of phosphorus in the soil.
Flavonoids, occurring mainly as glycosides, are the most abundant group of polyphenols [72]. They are composed of two aromatic rings connected by a three-carbon heterocyclic ring. Depending on the differences in the structure of the heterocyclic ring, these compounds are divided into several subgroups: flavones, isoflavones, flavonols, flavanols, flavanones, anthocyanins, coumarins and chalcones [63,67]. In this review flavonoids from different subclasses present in lavender flowers are listed in Table 4. Flavones are represented by apigenin, apigenin-O-glucoside, apigenin-O-glucuronide, apigenin hexoside, genkwanin (7-methylapigenin), isoscutellarein-O-glucuronide, luteolin, luteolin-O-glucoside, luteolin-O-glucuronide, luteolin-O-hexosyl-O-glucuronide and methylluteolin-O-glucuronide [5,11,16,48,51,68]. Among the isoflavones and flavanols present in lavender formononetin and catechin occur most often, respectively [52,57]. As concerns the flavonols, quercetin, quercetin 3-O-glucoside, rutin, myricetin, taxifolin (dihydroquercetin) and fisetin (5-desoxyquercetin) are the main representatives of this group of compounds [51,57,68]. On the other hand, the representatives of flavanones include hesperetin, hesperidin, neohesperidin, naringenin, narirutin, naringin, eriodictyol, eriodictyol-O-glucuronide, eriocitrin, pinocembrin, liquiritigenin, liquiritin and vanillin [11,16,52,57,58]. More recently, new phenolic compounds such as lavandunat, lavandufurandiol, lavandufluoren, lavandupyrones A and B and lavandudiphenyls A and B have been isolated from Lavandula angustifolia [73]. Lavender flowers also contain coumarin derivatives (umbelliferon, herniarine), triterpenes (ursolic acid, oleanolic acid and mictomeric acid) and sterols (cholesterol, campesterol, stigamsterol and β-sitosterol) [7,62].

Table 4.
Flavonoids in different species of lavender.
4. Antioxidant Activity
Free radicals are formed as a result of endogenous processes, i.e., enzymatic and nonenzymatic reactions in the cells, as well as due to exogenous factors such as environmental pollution, cigarette smoke, ionizing radiation, ultraviolet radiation, industrial solvents and pesticides. Free radicals contain at least one unpaired electron on the valence shell and react readily with the molecules in their vicinity, acting as prooxidants.
According to their structure, prooxidants can be classified into radical reactive species (superoxide anion radical, hydroxyl radical, peroxyl radical, nitric oxide radical) and non-free radical reactive species (peroxynitrite, singlet oxygen, hydrogen peroxide). Their overproduction leads to imbalance in the organism and damage to lipids, proteins and DNA due to their high reactivity. The ROS and RNS thus contribute to premature skin aging and the development of multiple diseases such as diabetes mellitus, hypertension, atherosclerosis, cardiovascular disease, liver diseases, renal failure, arthritis, cancer, as well as Alzheimer’s and Parkinson’s disease [74,75,76].
The antioxidant activity of polyphenols results from their ability to prevent the formation of free radicals or scavenge the reactive oxygen species. They can donate a hydrogen atom or an electron showing reducing properties [74]. These compounds can prevent oxidation processes by inhibition of xanthine oxidase, induction of antioxidant enzymes such as superoxide dismutase, glutathione dismutase, glutathione peroxidase and catalase, and chelating capacity due to metal ions [67,75]. Besides binding ferrous and copper ions, phenolic compounds also absorb UV radiation [77].
The antioxidant activity of phenolic compounds is associated with the presence and position of hydroxyl groups in their molecule. Stronger antioxidant properties were found for hydroxycinnamic acids than for hydroxybenzoic acids. Reports indicate that the highest antioxidant activity is demonstrated by rosmarinic acid, followed by chicoric acid and caffeic acid [78,79]. In addition, the presence of a methoxy group increases the antioxidant activity of phenolic acids. Ferulic acid having one group attached to the benzene ring is a less effective antioxidant than synapic acid which has two methoxy groups [80]. On the other hand, the antioxidant properties of flavonols are the consequence of the presence of a hydroxyl group at the C3 position of the flavonoid skeleton [81].
The methods of measuring antioxidant capacity can be described according to the reaction mechanism, namely SET (single electron transfer) or HAT (hydrogen atom transfer) mechanism, or both. The SET mechanism is based on donating one electron, whereas the HAT mechanism is based on the hydrogen atom transfer by the antioxidant [77,82].
The SET methods based on the reduction of ions include the FRAP and CUPRAC assays. The FRAP (Ferric Reducing Antioxidant Power) assay measures the reduction of ferric ion (Fe3+) to ferrous ion (Fe2+) through the donation of an electron. This reaction is carried out in an acidic medium (pH 3.6) to maintain the solubility of iron and leads to the formation of an intensely blue ferrous-tripyridyl-S-triazine (TPTZ) complex with an absorption maximum at 593 nm. TPTZ is the most popular iron-binding ligand used in the FRAP assay. The absorbance value of the sample is directly proportional to the concentration of the antioxidant. Moreover, in the CUPRAC (Cupric Ion Reducing Antioxidant Capacity) method, the reduction of Cu(II) ions to Cu(I) ions is used to measure the antioxidant capacity. The Cu(I) ions form an orange-yellow complex with neocuproin with an absorption maximum at 450 nm [37,77].
The ORAC assay (Oxygen Radical Absorbance Capacity) is one of the methods based on the HAT mechanism. It uses the process of deactivating peroxide radicals. It is based on measuring the decrease in the fluorescence intensity of fluorescein—a molecular probe that is oxidized by peroxide radicals. In this assay, AAPH (2,2′-azobis (2-amidinopropane) dihydrochloride) is mostly used as a source of free radicals. The degradation of fluorescein is slower when there are more antioxidants in the sample [48,82].
The most commonly used methods based on both mechanisms are the DPPH and the ABTS assays. DPPH (2,2-diphenyl-1-picrylhydrazyl) is a stable radical that can accept an electron or a hydrogen atom [83]. The DPPH alcoholic solution is dark purple in color with an absorption maximum at 517 nm. As a result of the reaction with phenolic compounds the ethanolic solution of the DPPH radical changes its color to light yellow [77]. The decrease in absorbance of the solution or signal intensity of electron paramagnetic resonance spectroscopy (EPR) is proportional to the amount of the reduced DPPH form that was formed during the reaction [74,84]. On the other hand, the ABTS method uses the ABTS radical cation (2,2′-azobis (3-ethylbenzothiazoline-6-sulfonate) which is formed as a result of chemical or enzymatic reactions. The ABTS radicals produced during the reaction with potassium persulfate are blue-green in color and have an absorption maximum at 734 nm. In the presence of antioxidants, the radical cation is reduced, resulting in discoloration of the solution [58,79], proportional to the antioxidant content in the sample [50]. This assay enables the measurement of the antioxidant activity of hydrophilic and lipophilic compounds due to the solubility of the ABTS radical in both aqueous and organic solvents [58]. Antioxidant activity of the extracts is usually expressed as Trolox equivalents, the synthetic vitamin E derivative [48,58,79]. In addition, Relative Antioxidant Capacity Index (RACI) can be used to comprehensively determine the total antioxidant capacity of the samples as determined using various methods, including ABTS, DPPH and ORAC [85].
As a different approach, the TBARS method (Thiobarbituric Acid Reactive Substances Assay) is used to measure lipid peroxidation products. It is based on the spectrophotometric measurement of malondialdehyde (MDA) produced during lipid peroxidation. As a result of the reaction of thiobarbituric acid (TBA) with MDA, a pink complex is formed that absorbs at a wavelength of 532–535 nm. The absorbance value is proportional to the concentration of MDA, a compound that is commonly used as an oxidative stress marker. In the presence of antioxidants the formation of MDA is inhibited [82]. The results of the antioxidant activity assessment of lavender extracts are reported in Table 2. Significant differences in antioxidant activity can result from the sample origin, the extraction method used, as well as the differences between species. Unfortunately, the way in which the results of antioxidant activity are presented by different scientific teams is difficult to compare due to different conversions for extracts, different units and substances as equivalents. In addition, the extraction conditions such as different concentrations of reagents, solvents and temperature are responsible for different obtained values even for the same substance marked with the same test. We have made an effort to standardize these values; however, the publications lack many details required for such conversion. Robu et al. [4] performed a comparative study of antioxidant activity for various cultivars of Lavandula angustifolia with Lavandula hybrida. The highest result was obtained for Lavandula hybrida (IC50 = 49.90 and 73.53 μg/mL for ferrous ion chelating and DPPH assay, respectively), followed by Lavandula angustifolia ‘Hidcote Blue’ (IC50 = 49.93 and 96.53 μg/mL), and Lavandula angustifolia ‘Munstead’ (IC50 = 50.60 and 101.40 μg/mL). Whereas, in the study by Blazeković et al. [60] in most of the tests a slightly higher antioxidant activity was observed for Lavandula angustifolia extracts as compared with Lavandula x intermedia ‘Budrovka’ extracts, which could be due to their higher polyphenolic contents. However, in both studies all Lavandula extracts showed a concentration-dependent antioxidant activity, wherein the strongest DPPH-radical-scavenging and iron-chelating activity were observed at a higher concentration of the extracts. On the other hand, in the study by Ahn et al. [49] the aqueous-methanolic leaf extract of Lavandula angustifolia ‘Lady’ afforded an over twice as high DPPH value (14.17 μmol TEAC/g DW) than the Lavandula latifolia extract (6.56 μmol TEAC/g DW). In another study, the antioxidant activity of methanolic extracts of different species and cultivars of lavender was analyzed [68]. The ability to scavenge free radicals of plant extracts decreased in the following order: Lavandula viridis (99.47% of inhibition), Lavandula stoechas (95.18%), Lavandula angustifolia ‘Rosea’ (93.92%), Lavandula lanata (92.78%), Lavandula angustifolia ‘Afropurpurea’ (92.09%) and Lavandula angustifolia (91.51%). The highest inhibition of the Lavandula viridis extract can be due to the presence of ferulic acid which was found only in this sample.
The above studies showed a significant antiradical activity of lavender extracts. On the other hand, a study by Celik et al. [37] showed that the antioxidant activity of the microwave-assisted lavender extract is significantly lower than that of other plants of the Lamiaceae family. The highest TAC values evaluated by the CUPRAC assay were obtained for Origanum majorana (0.66 mmol TR/g) and Mentha pulegium (0.58 mmol TR/g) extracts whereas the lowest ones were obtained for the Lavandula officinalis extracts (0.39 mmol TR/g). Likewise, Nicolai et al. [86] observed that ultrasound-assisted ethanolic extracts of Melissa officinalis, Salvia officinalis, Hypericum perforatum and Rosmarinum officinalis afforded the strongest DPPH-radical-scavenging activity of 95.2%, 94.7%, 92.7% and 72.5%, respectively. In contrast, the Lavandula angustifolia extract had a significantly lower value equal to 17.7%.
5. Anti-Inflammatory Activity
Inflammation is a protective response elicited by numerous biological (bacteria, fungi, viruses, endo- and exotoxins), chemical (acids, bases, carrageenan) and physical (mechanical factors, ultrasonic waves, ionizing radiation, magnetic field) agents. Each of these factors triggers a body defensive reaction [87]. Inflammation can be acute or chronic, and each type is related to different effects. In the acute phase, lasting from several minutes to a few days, neutrophils migrate from dilated blood vessels to the site of infection, causing redness, swelling and pain. Moreover, the state of chronic inflammation can lead to the development of multiple diseases such as rheumatoid arthritis, gout, cardiovascular diseases, diabetes, bowel diseases, Alzheimer’s disease, cancer and depression.
Inflammation is associated with excessive activity of the immune system by releasing inflammatory cells such as macrophages, neutrophils and lymphocytes [88,89]. The key role of the immune system is also related to the expression of inflammatory mediators, the most important of which are vasoactive amines (histamine, serotonin) and peptide (bradykinin), arachidonic acid metabolites (eicosanoids—prostaglandins, thromboxanes, leukotrienes and lipoxins), proinflammatory cytokines (IL-1β, IL-6, IL-8, IL-12, TNF-α), chemokines and proteolytic enzymes (elastin, cathepsins, matrix metalloproteinases) [88,90]. Molecules such as nuclear factor kappa-light-chain-enhancer of activated B cells (NF-ĸB), transforming growth factor β (TGF-β), reactive oxygen species (ROS), reactive nitrogen species (RNS), inducible nitric oxide synthase (iNOS) and cyclooxygenases (COX) are also released during the inflammatory process [88,89,91].
For testing anti-inflammatory activity several animal experimental models are used (Table 5) such as the carrageenan-induced paw edema in mice [5] and in rats [92], the formalin test in mice [93], the croton-oil-induced ear edema in mice [7], the TPA-induced ear edema in mice [18], and the cell line stimulated with LPS [16]. In these studies anti-inflammatory drugs such as dexamethasone [16,93] or the nonsteroidal anti-inflammatory drugs (NSAIDs) such as indomethacin [7,93], aspirin [6], ibuprofen [18] and diclofenac [5] are used as a positive control. NSAIDs work by inhibiting cyclooxygenase, the enzyme responsible for eicosanoid synthesis [94]. Cyclooxygenase (COX) converts arachidonic acid to prostaglandin G2, then into prostaglandin H2, and finally to prostaglandins (PGF2α and PGE2), prostacyclin (PGI2) and thromboxane A2 (TXA2) [91]. There are three cyclooxygenase isoforms. COX-1 is a constitutive form that plays a role in normal physiological processes. COX-2 is an inducible form involved in inflammation, whereas the least known COX-3 is associated with the central nervous system. The majority of NSAIDs inhibit both COX-1 and COX-2 [91,95]. On the other hand, lipoxygenase (5-LOX) metabolizes arachidonic acid to 5-hydroxyeicosatetraenoic acids and leukotrienes (LTs) [88,96].

Table 5.
Anti-inflammatory activity of Lavandula extracts.
Research has confirmed that the anti-inflammatory activity of lavender is due to the presence of essential oil components, non-volatile terpenoids and polyphenols. In the study by Carrascoet al. [13], thymol, fenchone and camphor from Lavandula stoechas essential oil showed an inhibitory effect on lipoxygenase (LOX). Luo et al. [18] observed that essential oil of Lavandula angustifolia reduced the expression of inflammatory mediators such as IL-6, NF-κB and TNF-α in the TPA-induced ear edema model in mice. Furthermore, in the study by Sosa et al. [7] the higher inhibition of croton-oil-induced ear edema in mice was observed for ethanolic extracts of Lavandula multifida than for aqueous extracts, but in both cases the antiphlogistic activity was dose-dependent. The terpenoids ursolic acid, oleanolic acid and maslinic acid and phenolic monoterpene carvacrol were obtained as a result of fractionation of the ethanolic extract. Numerous scientific reports [5,6,16,92] indicate that plants of the Lavandula genus are a source of polyphenolic compounds with anti-inflammatory activity. One of many antiphlogistic mechanisms of polyphenols is their ability to inhibit enzymes including iNOS, LOX and COX [92,96]. The anti-inflammatory activity of the Lavandula extracts was assessed using a fluorimetric test based on the detection of prostaglandin G2 generated by the COX enzyme. Cyclooxygenase COX-2 was more strongly inhibited by lavender extracts than cyclooxygenase COX-1. Shaikh et al. [6] observed that the ethanolic fraction of Lavandula bipinnata obtained by Soxhlet extraction inhibited COX-2 by 50%, compared to COX-1 by 19%. A high-performance thin-layer chromatography (HPTLC) analysis showed that the extract was rich in flavonoids. Likewise, Husseini et al. [93] demonstrated that the anti-inflammatory activity of hydroalcoholic macerate of Lavandula officinalis was associated with the inhibition of COX-2 by 45% and COX-1 by 33%. In addition, the anti-inflammatory effect of COX-2 increased with the increasing concentration of the extract. Researchers also showed that the extract inhibited the chronic (inflammatory) phase of formalin-induced pain in mice, whereas it had no effect on the acute (neurogenic) phase. Moreover, in the study of Algieri et al. [5], Lavandula stoechas and Lavandula dentata hydromethanolic extracts, obtained using accelerated solvent extraction (ASE), showed anti-inflammatory activity against carrageenan-induced paw edema in mice. The hydromethanolic extract of Lavandula stoechas at a dose of 100 mg/kg significantly decreased the expression of the pro-inflammatory cytokines IL-1β, IL-6 and TNF-α and the enzymes iNOS, COX-2 and MMP-9, whereas the extract of Lavandula dentata decreased the expression of only iNOS, COX-2 and IL-1β. The qualitative analysis of the extracts performed using RP-HPLC-DAD-MS showed that they contained the phenolic acids hydroxybenzoic acid, hydroxycinnamic acid and their derivatives as well as flavones which are a subclass of flavonoids. Similarly, Yassine et al. [92] observed the anti-inflammatory activity of the extracts of Lavandula stoechas on carrageenan-induced paw edema in rats. A significant inhibition of edema was found in the case of hydroethanolic extract obtained using ultrasound-assisted extraction and two fractions rich in flavonoids and mucilages, whereas that effect was not observed for the tannin fraction. Furthermore, Lopes et al. [16] used the mouse macrophage-like cell line RAW 264.7 stimulated with LPS to study the anti-inflammatory activity of the extracts of Lavandula pedunculata. The hydroethanolic extracts displayed a higher anti-inflammatory potential through inhibition of NO production than the aqueous extracts (infusions).
6. Conclusions
According to Pharmacopoeia XI, only Lavandula angustifolia is currently recognized as a medicinal raw material. However, in recent years other species such as Lavandula stoechas, Lavandula intermedia, Lavandula latifolia, Lavandula dentata and Lavandula luisieri have also gained increasing interest in the medical and pharmaceutical areas. Many of these species are a rich source of phenolic compounds such as phenolic acids and flavonoids, thus resulting in antioxidant and anti-inflammatory properties. However, in order to obtain a high-quality extract, it is crucial to select the appropriate extraction method.
In this review are presented both the traditional and modern extraction methods, also called green techniques. These techniques are characterized by lower consumption of the solvents and shorter extraction times, which means lower energy consumption. By using these methods better reproducibility and selectivity as well as higher quality of the extracts can be obtained than by traditional techniques. Each of the presented methods has its advantages and disadvantages. Which method a given laboratory chooses depends on many factors, such as the availability of equipment, solvents and trained staff, as well as maintenance costs.
However, since in the analyzed studies there was a great variation in both extraction protocols and plant material, there is a need for a systematic study comparing various extraction techniques of the same plant material to reliably recommend the optimal approach. Further studies are needed with special attention paid to the optimization extraction and activity-guided extraction. Additionally, a key issue is to standardize the units and substances as equivalents in which the obtained results of activity determination are presented. There is also a gap concerning the extraction and analysis of lavender flower residues left after the distillation of essential oil—currently the main product of lavender—which could be a valuable source of phenolics exhibiting antioxidant and anti-inflammatory properties.
Author Contributions
Conceptualization, K.D.Z. and K.P.; methodology, N.D.; formal analysis, N.D.; investigation, N.D.; resources, N.D.; data curation, N.D.; writing—original draft preparation, N.D.; writing—review and editing, K.D.Z. and K.P.; visualization, N.D.; supervision, K.D.Z. and K.P. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Turgut, A.C.; Emen, F.M.; Canbay, H.S.; Demirdöğen, R.E.; Çam, N.; Kılıç, D.; Yeşilkaynak, T. Chemical characterization of Lavandula angustifolia Mill. which is a phytocosmetic species and investigation of its antimicrobial effect in cosmetic products. J. Turk. Chem. Soc. Sect. A Chem. 2016, 4, 283–298. [Google Scholar] [CrossRef]
- Polish Pharmacopoeia, Poland. In Polish Pharmacopoeia: Supplement 2021, 12th ed.; Office for Registration of Medicinal Products MDaBP: Warsaw, Poland, 2021.
- Danh, L.T.; Triet, N.D.A.; Han, L.T.N.; Zhao, J.; Mammucari, R.; Foster, N. Antioxidant activity, yield and chemical composition of lavender essential oil extracted by supercritical CO2. J. Supercrit. Fluids 2012, 70, 27–34. [Google Scholar] [CrossRef]
- Robu, S.; Aprotosoaie, A.C.; Miron, A.; Cioancǎ, O.; Stǎnescu, U.; Hǎncianu, M. In vitro antioxidant activity of ethanolic extracts from some Lavandula species cultivated in Romania. Farmacia 2012, 60, 394–401. [Google Scholar]
- Algieri, F.; Rodriguez-Nogales, A.; Vezza, T.; Garrido-Mesa, J.; Garrido-Mesa, N.; Utrilla, M.P.; González-Tejero, M.R.; Casares-Porcel, M.; Molero-Mesa, J.; Contreras, M.D.M.; et al. Anti-inflammatory activity of hydroalcoholic extracts of Lavandula dentata L. and Lavandula stoechas L. J. Ethnopharmacol. 2016, 190, 142–158. [Google Scholar] [CrossRef]
- Shaikh, R.; Pund, M.; Dawane, A.; Iliyas, S. Evaluation of Anticancer, Antioxidant, and Possible Anti-inflammatory Properties of Selected Medicinal Plants Used in Indian Traditional Medication. J. Tradit. Complement. Med. 2014, 4, 253–257. [Google Scholar] [CrossRef]
- Sosa, S.; Altinier, G.; Politi, M.; Braca, A.; Morelli, I.; Della Loggia, R. Extracts and constituents of Lavandula multifida with topical anti-inflammatory activity. Phytomedicine 2005, 12, 271–277. [Google Scholar] [CrossRef]
- Zuzarte, M.; Gonçalves, M.J.; Cavaleiro, C.; Cruz, M.T.; Benzarti, A.; Marongiu, B.; Maxia, A.; Piras, A.; Salgueiro, L. Antifungal and anti-inflammatory potential of Lavandula stoechas and Thymus herba-barona essential oils. Ind. Crops Prod. 2013, 44, 97–103. [Google Scholar] [CrossRef]
- De Moura Linck, V.; da Silva, A.L.; Figueiró, M.; Piato, A.L.; Herrmann, A.P.; Birck, F.D.; Elisabetsky, E. Inhaled linalool-induced sedation in mice. Phytomedicine 2009, 16, 303–307. [Google Scholar] [CrossRef]
- Kageyama, A.; Ueno, T.; Oshio, M.; Masuda, H.; Horiuchi, H.; Yokogoshi, H. Antidepressant-like Effects of an Aqueous Extract of Lavender (Lavandula angustifolia Mill.) in Rats. Food Sci. Technol. Res. 2012, 18, 473–479. [Google Scholar] [CrossRef]
- Costa, P.; Gonçalves, S.; Valentão, P.; Andrade, P.B.; Romano, A. Accumulation of phenolic compounds in in vitro cultures and wild plants of Lavandula viridis L’Hér and their antioxidant and anti-cholinesterase potential. Food Chem. Toxicol. 2013, 57, 69–74. [Google Scholar] [CrossRef]
- Kritsidima, M.; Newton, T.; Asimakopoulou, K.; Newton, J.T. The effects of lavender scent on dental patient anxiety levels: A cluster randomised-controlled trial. Community Dent. Oral Epidemiol. 2010, 38, 83–87. [Google Scholar] [CrossRef] [PubMed]
- Carrasco, A.; Ortiz-Ruiz, V.; Martinez-Gutierrez, R.; Tomas, V.; Tudela, J. Lavandula stoechas essential oil from Spain: Aromatic profile determined by gas chromatography–mass spectrometry, antioxidant and lipoxygenase inhibitory bioactivities. Ind. Crops Prod. 2015, 73, 16–27. [Google Scholar] [CrossRef]
- Prusinowska, R.; Śmigielski, K.B. Composition, biological properties and therapeutic effects of Lavender L). A review. Herba Pol. 2014, 60, 56–66. [Google Scholar] [CrossRef]
- Lis-Balchin, M. Lavender: The Genus Lavandula; CRC Press: Boca Raton, FL, USA, 2002. [Google Scholar]
- Lopes, C.L.; Pereira, E.; Soković, M.; Carvalho, A.M.; Barata, A.M.; Lopes, V.; Rocha, F.; Calhelha, R.C.; Barros, L.; Ferreira, I.C. Phenolic Composition and Bioactivity of Lavandula pedunculata (Mill.) Cav. Samples from Different Geographical Origin. Molecules 2018, 23, 1037. [Google Scholar] [CrossRef] [PubMed]
- Da Porto, C.; Decorti, D.; Kikic, I. Flavour compounds of Lavandula angustifolia L. to use in food manufacturing: Comparison of three different extraction methods. Food Chem. 2009, 112, 1072–1078. [Google Scholar] [CrossRef]
- Luo, W.; Du, Z.; Zheng, Y.; Liang, X.; Huang, G.; Zhang, Q.; Liu, Z.; Zhang, K.; Zheng, X.; Lin, L.; et al. Phytochemical composition and bioactivities of essential oils from six Lamiaceae species. Ind. Crops Prod. 2019, 133, 357–364. [Google Scholar] [CrossRef]
- Delgado, F.; Ribeiro, S.; Alves, Á.; Bettencourt, E.; Dias, S. Morphological, ecological and genetic variability of Lavandula luisieri (Rozeira) Rivas-Martínez in central eastern Portugal. Plant Genet. Resour. 2010, 8, 82–90. [Google Scholar] [CrossRef]
- Abubakar, A.R.; Haque, M. Preparation of medicinal plants: Basic extraction and fractionation procedures for experimental purposes. J. Pharm. Bioallied Sci. 2020, 12, 1–10. [Google Scholar] [CrossRef]
- Belwal, T.; Ezzat, S.M.; Rastrelli, L.; Bhatt, I.D.; Daglia, M.; Baldi, A.; Devkota, H.P.; Orhan, I.E.; Patra, J.K.; Das, G.; et al. A critical analysis of extraction techniques used for botanicals: Trends, priorities, industrial uses and optimization strategies. TrAC Trends Anal. Chem. 2018, 100, 82–102. [Google Scholar] [CrossRef]
- Lourenço, S.C.; Moldão-Martins, M.; Alves, V.D. Antioxidants of Natural Plant Origins: From Sources to Food Industry Applications. Molecules 2019, 24, 4132. [Google Scholar] [CrossRef]
- Stalikas, C.D. Extraction, separation, and detection methods for phenolic acids and flavonoids. J. Sep. Sci. 2007, 30, 3268–3295. [Google Scholar] [CrossRef] [PubMed]
- Arceusz, A.; Wesolowski, M.; Konieczynski, P. Methods for Extraction and Determination of Phenolic Acids in Medicinal Plants: A Review. Nat. Prod. Commun. 2013, 8, 1821–1829. [Google Scholar] [CrossRef] [PubMed]
- Domínguez-Rodríguez, G.; Marina, M.L.; Plaza, M. Strategies for the extraction and analysis of non-extractable polyphenols from plants. J. Chromatogr. A 2017, 1514, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Costa, D.C.; Costa, H.S.; Albuquerque, T.G.; Ramos, F.; Castilho, M.C.; Sanches-Silva, A. Advances in phenolic compounds analysis of aromatic plants and their potential applications. Trends Food Sci. Technol. 2015, 45, 2236–2254. [Google Scholar] [CrossRef]
- Tzima, K.; Brunton, N.; Rai, D. Qualitative and Quantitative Analysis of Polyphenols in Lamiaceae Plants—A Review. Plants 2018, 7, 25. [Google Scholar] [CrossRef]
- Saravanabavan, N.; Salwe, K.J.; Codi, R.S.; Kumarappan, M. Herbal extraction procedures: Need of the hour. Int. J. Basic Clin. Pharmacol. 2020, 9, 1135–1139. [Google Scholar] [CrossRef]
- United Nations Industrial Development Organization; Handa, S.S.; Khanuja, S.P.S.; Longo, G.; Rakesh, D.D. Extraction Technologies for Medicinal and Aromatic Plants: Earth, Environmental and Marine Sciences and Technologies; United Nations Industrial Development Organization: Trieste, Italy, 2008. [Google Scholar]
- Soquetta, M.B.; Terra, L.D.M.; Bastos, C.P. Green technologies for the extraction of bioactive compounds in fruits and vegetables. CyTA J. Food 2018, 16, 400–412. [Google Scholar] [CrossRef]
- Rasul, M.G. Conventional extraction methods use in medicinal plants, their advantages and disadvantages. Int. J. Basic Sci. Appl. Comput. 2018, 2, 10–14. [Google Scholar]
- Mansinhos, I.; Gonçalves, S.; Rodríguez-Solana, R.; Ordóñez-Díaz, J.L.; Moreno-Rojas, J.M.; Romano, A. Ultrasonic-Assisted Extraction and Natural Deep Eutectic Solvents Combination: A Green Strategy to Improve the Recovery of Phenolic Compounds from Lavandula pedunculata subsp. lusitanica (Chaytor) Franco. Antioxidants 2021, 10, 582. [Google Scholar] [CrossRef]
- Pande, J.; Chanda, S. Determination of phytochemical profile and antioxidant efficacy of Lavendula bipinnata leaves collected during Magha Nakshatra days and Normal days using LC-QTOF-MS technique. J. Pharm. Biomed. Anal. 2020, 186, 113347. [Google Scholar] [CrossRef]
- Turrini, F.; Beruto, M.; Mela, L.; Curir, P.; Triglia, G.; Boggia, R.; Zunin, P.; Monroy, F. Ultrasound-Assisted Extraction of Lavender (Lavandula angustifolia Miller, Cultivar Rosa) Solid By-Products Remaining after the Distillation of the Essential Oil. Appl. Sci. 2021, 11, 5495. [Google Scholar] [CrossRef]
- Giacometti, J.; Kovačević, D.B.; Putnik, P.; Gabrić, D.; Bilušić, T.; Krešić, G.; Jambrak, A.R. Extraction of bioactive compounds and essential oils from mediterranean herbs by conventional and green innovative techniques: A review. Food Res. Int. 2018, 113, 245–262. [Google Scholar] [CrossRef] [PubMed]
- Altemimi, A.; Lakhssassi, N.; Baharlouei, A.; Watson, D.G.; Lightfoot, D.A. Phytochemicals: Extraction, Isolation, and Identification of Bioactive Compounds from Plant Extracts. Plants 2017, 6, 42. [Google Scholar] [CrossRef] [PubMed]
- Çelik, S.E.; Tufan, A.N.; Bekdeşer, B.; Özyürek, M.; Güçlü, K.; Apak, R. Identification and Determination of Phenolics in Lamiaceae Species by UPLC-DAD-ESI-MS/MS. J. Chromatogr. Sci. 2016, 55, 291–300. [Google Scholar] [CrossRef] [PubMed]
- Rashed, M.M.; Tong, Q.; Abdelhai, M.H.; Gasmalla, M.A.; Ndayishimiye, J.B.; Chen, L.; Ren, F. Effect of ultrasonic treatment on total phenolic extraction from Lavandula pubescens and its application in palm olein oil industry. Ultrason. Sonochemistry 2016, 29, 39–47. [Google Scholar] [CrossRef] [PubMed]
- Llompart, M.; Celeiro, M.; Dagnac, T. Microwave-assisted extraction of pharmaceuticals, personal care products and industrial contaminants in the environment. TrAC Trends Anal. Chem. 2019, 116, 136–150. [Google Scholar] [CrossRef]
- Azwanida, N.N. A Review on the Extraction Methods Use in Medicinal Plants, Principle, Strength and Limitation. Med. Aromat. Plants 2015, 4, 1–6. [Google Scholar]
- Ong, E.S. Extraction methods and chemical standardization of botanicals and herbal preparations. J Chromatogr B Anal. Technol Biomed Life Sci. 2004, 812, 23–33. [Google Scholar] [CrossRef]
- Woźniak, Ł.; Marszałek, K.; Skąpska, S.; Jędrzejczak, R. The Application of Supercritical Carbon Dioxide and Ethanol for the Extraction of Phenolic Compounds from Chokeberry Pomace. Appl. Sci. 2017, 7, 322. [Google Scholar] [CrossRef]
- Cvjetko Bubalo, M.; Vidović, S.; Radojčić Redovniković, I.; Jokić, S. New perspective in extraction of plant biologically active compounds by green solvents. Food Bioprod. Process. 2018, 109, 52–73. [Google Scholar] [CrossRef]
- Da Silva, R.P.F.F.; Rocha-Santos, T.A.P.; Duarte, A.C. Supercritical fluid extraction of bioactive compounds. TrAC Trends Anal. Chem. 2016, 76, 40–51. [Google Scholar] [CrossRef]
- Chemat, F.; Abert-Vian, M.; Fabiano-Tixier, A.S.; Strube, J.; Uhlenbrock, L.; Gunjevic, V.; Cravotto, G. Green extraction of natural products. Origins, current status, and future challenges. TrAC Trends Anal. Chem. 2019, 118, 248–263. [Google Scholar] [CrossRef]
- De Melo, M.M.R.; Silvestre, A.J.D.; Silva, C.M. Supercritical fluid extraction of vegetable matrices: Applications, trends and future perspectives of a convincing green technology. J. Supercrit. Fluids 2014, 92, 115–176. [Google Scholar] [CrossRef]
- Tyśkiewicz, K.; Konkol, M.; Rój, E. Supercritical Carbon Dioxide (scCO2) Extraction of Phenolic Compounds from Lavender (Lavandula angustifolia) Flowers: A Box-Behnken Experimental Optimization. Molecules 2019, 24, 3354. [Google Scholar] [CrossRef] [PubMed]
- Costa, P.; Gonçalves, S.; Valentão, P.; Andrade, P.B.; Almeida, C.; Nogueira, J.M.; Romano, A. Metabolic profile and biological activities of Lavandula pedunculata subsp. lusitanica (Chaytor) Franco: Studies on the essential oil and polar extracts. Food Chem. 2013, 141, 2501–2506. [Google Scholar] [CrossRef] [PubMed]
- Ahn, J.; Alford, A.R.; Niemeyer, E.D. Variation in phenolic profiles and antioxidant properties among medicinal and culinary herbs of the Lamiaceae family. J. Food Meas. Charact. 2020, 14, 1720–1732. [Google Scholar] [CrossRef]
- Ivanova, D.; Gerova, D.; Chervenkov, T.; Yankova, T. Polyphenols and antioxidant capacity of Bulgarian medicinal plants. J. Ethnopharmacol. 2005, 96, 145–150. [Google Scholar] [CrossRef]
- Karabagias, I.K.; Karabagias, V.K.; Riganakos, K.A. Physico-Chemical Parameters, Phenolic Profile, In Vitro Antioxidant Activity and Volatile Compounds of Ladastacho (Lavandula stoechas) from the Region of Saidona. Antioxidants 2019, 8, 80. [Google Scholar] [CrossRef]
- Proestos, C.; Sereli, D.; Komaitis, M. Determination of phenolic compounds in aromatic plants by RP-HPLC and GC-MS. Food Chem. 2006, 95, 44–52. [Google Scholar] [CrossRef]
- Zgórka, G.; Głowniak, K. Variation of free phenolic acids in medicinal plants belonging to the Lamiaceae family. J. Pharm. Biomed. Anal. 2001, 26, 79–87. [Google Scholar] [CrossRef]
- Sahar, A.; Wafaa, H.B.H.; Ahmed, E.M.E.; Hanan, M.A.-Y.; May, A.E.; Rasha, A. Ultra performance liquid chromatography-tandem mass spectrometeric analysis of ethyl acetate fraction from saudi Lavandula coronopifolia Poir and evaluation of its cytotoxic and antioxidant activities. J. Herbmed Pharmacol. 2020, 9, 268–276. [Google Scholar]
- Gülçin, Ì.; Şat, İ.G.; Beydemir, Ş.; Elmastaş, M.; Küfrevioǧlu, Ö.İ. Comparison of antioxidant activity of clove (Eugenia caryophylata Thunb) buds and lavender (Lavandula stoechas L.). Food Chem. 2004, 87, 393–400. [Google Scholar] [CrossRef]
- Tušek, A.J.; Jurina, T.; Benković, M.; Valinger, D.; Belščak-Cvitanović, A.; Kljusurić, J.G. Application of multivariate regression and artificial neural network modelling for prediction of physical and chemical properties of medicinal plants aqueous extracts. J. Appl. Res. Med. Aromat. Plants. 2020, 16, 100229. [Google Scholar] [CrossRef]
- Bajkacz, S.; Baranowska, I.; Buszewski, B.; Kowalski, B.; Ligor, M. Determination of Flavonoids and Phenolic Acids in Plant Materials Using SLE-SPE-UHPLC-MS/MS Method. Food Anal. Methods 2018, 11, 3563–3575. [Google Scholar] [CrossRef]
- Dvorackova, E.; Snóblová, M.; Hrdlicka, P. Content of phenolic compounds in herbs used in the Czech Republic. Int. Food Res. J. 2014, 21, 1495. [Google Scholar]
- Giménez-Rota, C.; Lorán, S.; Mainar, A.M.; Hernáiz, M.J.; Rota, C. Supercritical Carbon Dioxide Antisolvent Fractionation for the Sustainable Concentration of Lavandula luisieri (Rozeira) Riv.-Mart Antimicrobial and Antioxidant Compounds and Comparison with Its Conventional Extracts. Plants 2019, 8, 455. [Google Scholar] [CrossRef]
- Blažeković, B.; Vladimir-Knezević, S.; Brantner, A.; Štefan, M.B. Evaluation of Antioxidant Potential of Lavandula x intermedia Emeric ex Loisel. ‘Budrovka’: A Comparative Study with L. angustifolia Mill. Molecules 2010, 15, 5971–5987. [Google Scholar] [CrossRef]
- Jerković, I.; Molnar, M.; Vidović, S.; Vladić, J.; Jokić, S. Supercritical CO2 Extraction of Lavandula angustifolia Mill. Flowers: Optimisation of Oxygenated Monoterpenes, Coumarin and Herniarin Content. Phytochem. Anal. 2017, 28, 558–566. [Google Scholar] [CrossRef]
- EMA. Assessment Report on Lavandula angustifolia Miller, Aetheroleum and Lavandula angustifolia Miller; Flos; Monograph European Medicines Agency: Amsterdam, The Netherlands, 27 March 2012. [Google Scholar]
- Crozier, A.; Jaganath, I.B.; Clifford, M.N. Dietary phenolics: Chemistry, bioavailability and effects on health. Nat. Prod. Rep. 2009, 26, 1001–1043. [Google Scholar] [CrossRef]
- Hussain, T.; Tan, B.; Yin, Y.; Blachier, F.; Tossou, M.C.B.; Rahu, N. Oxidative Stress and Inflammation: What Polyphenols Can Do for Us? Oxid. Med. Cell Longev. 2016, 2016, 7432797–7432799. [Google Scholar] [CrossRef]
- Myburgh, K.H. Polyphenol Supplementation: Benefits for Exercise Performance or Oxidative Stress? Sports Med. 2014, 44 (Suppl. S1), 57–70. [Google Scholar] [CrossRef] [PubMed]
- Santos-Sánchez, N.F.; Salas-Coronado, R.; Hernández-Carlos, B.; Villanueva-Cañongo, C. Shikimic Acid Pathway in Biosynthesis of Phenolic Compounds. In Plant Physiological Aspects of Phenolic Compounds; IntechOpen: London, UK, 2019. [Google Scholar]
- Tsao, R. Chemistry and Biochemistry of Dietary Polyphenols. Nutrients 2010, 2, 1231–1246. [Google Scholar] [CrossRef] [PubMed]
- Hawrył, A.; Hawrył, M.; Waksmundzka-Hajnos, M. Liquid chromatography fingerprint analysis and antioxidant activity of selected lavender species with chemometric calculations. PLoS ONE 2019, 14, e0218974. [Google Scholar] [CrossRef] [PubMed]
- Marranzano, M.; Rosa, R.L.; Malaguarnera, M.; Palmeri, R.; Tessitori, M.; Barbera, A.C. Polyphenols: Plant Sources and Food Industry Applications. Curr. Pharm. Des. 2018, 24, 4125–4130. [Google Scholar] [CrossRef]
- Adaszyńska-Skwirzyńska, M.; Dzięcioł, M. Comparison of phenolic acids and flavonoids contents in various cultivars and parts of common lavender (Lavandula angustifolia) derived from Poland. Nat. Prod. Res. 2017, 31, 2575–2580. [Google Scholar] [CrossRef]
- Bajalan, I.; Mohammadi, M.; Alaei, M.; Pirbalouti, A.G. Total phenolic and flavonoid contents and antioxidant activity of extracts from different populations of lavandin. Ind. Crops Prod. 2016, 87, 255–260. [Google Scholar] [CrossRef]
- Rahman, M.; Rahaman, S.; Islam, R.; Rahman, F.; Mithi, F.M.; Alqahtani, T.; Almikhlafi, M.A.; Alghamdi, S.Q.; Alruwaili, A.S.; Hossain, S.; et al. Role of Phenolic Compounds in Human Disease: Current Knowledge and Future Prospects. Molecules 2021, 27, 233. [Google Scholar] [CrossRef]
- Yadikar, N.; Bobakulov, K.; Li, G.; Aisa, H.A. Seven new phenolic compounds from Lavandula angustifolia. Phytochem. Lett. 2018, 23, 149–154. [Google Scholar] [CrossRef]
- Boligon, A.A.; Machado, M.M.; Athayde, M.L. Technical evaluation of antioxidant activity. Med Chem. 2014, 4, 517–522. [Google Scholar] [CrossRef]
- De Lima Cherubim, D.J.; Buzanello Martins, C.V.; Oliveira Fariña, L.; da Silva de Lucca, R.A. Polyphenols as natural antioxidants in cosmetics applications. J. Cosmet. Dermatol. 2020, 19, 33–37. [Google Scholar] [CrossRef] [PubMed]
- Lobo, V.; Patil, A.; Phatak, A.; Chandra, N. Free radicals, antioxidants and functional foods: Impact on human health. Pharmacogn. Rev. 2010, 4, 118–126. [Google Scholar] [CrossRef] [PubMed]
- Munteanu, I.G.; Apetrei, C. Analytical Methods Used in Determining Antioxidant Activity: A Review. Int. J. Mol. Sci. 2021, 22, 3380–3410. [Google Scholar] [CrossRef] [PubMed]
- Godlewska-Żyłkiewicz, B.; Świsłocka, R.; Kalinowska, M.; Golonko, A.; Świderski, G.; Arciszewska, Ż.; Nalewajko-Sieliwoniuk, E.; Naumowicz, M.; Lewandowski, W. Biologically Active Compounds of Plants: Structure-Related Antioxidant, Microbiological and Cytotoxic Activity of Selected Carboxylic Acids. Materials 2020, 13, 4454. [Google Scholar] [CrossRef] [PubMed]
- Prommajak, T.; Kim, S.M.; Pan, C.-H.; Kim, S.M.; Surawang, S.; Rattanapanone, N. Identification of Antioxidants in Lamiaceae Vegetables by HPLC-ABTS and HPLC-MS. Chiang Mai Univ. J. Nat. Sci. 2016, 15, 21–38. [Google Scholar] [CrossRef]
- Parus, A. Antioxidant and pharmacological properties of phenolic acids. Postępy Fitoterapii. 2012, 2013, 48–53. [Google Scholar]
- Burda, S.; Oleszek, W. Antioxidant and Antiradical Activities of Flavonoids. J. Agric. Food Chem. 2001, 49, 2774–2779. [Google Scholar] [CrossRef]
- Dasgupta, A.; Klein, K. Antioxidants in Food, Vitamins and Supplements: Prevention and Treatment of Disease; Elsevier: Saint Louis, MO, USA, 2014. [Google Scholar]
- Ionita, P. The chemistry of dpph∙free radical and congeners. Int. J. Mol. Sci. 2021, 22, 1–15. [Google Scholar] [CrossRef]
- Sanna, D.; Delogu, G.; Mulas, M.; Schirra, M.; Fadda, A. Determination of Free Radical Scavenging Activity of Plant Extracts Through DPPH Assay: An EPR and UV–Vis Study. Food Anal. Methods 2012, 5, 759–766. [Google Scholar] [CrossRef]
- Brahmi, N.; Scognamiglio, M.; Pacifico, S.; Mekhoukhe, A.; Madani, K.; Fiorentino, A.; Monaco, P. 1 H NMR based metabolic profiling of eleven Algerian aromatic plants and evaluation of their antioxidant and cytotoxic properties. Food Res. Int. 2015, 76, 334–341. [Google Scholar] [CrossRef]
- Nicolai, M.; Pereira, P.; Vitor, R.F.; Reis, C.P.; Roberto, A.; Rijo, P. Antioxidant activity and rosmarinic acid content of ultrasound-assisted ethanolic extracts of medicinal plants. Measurement 2016, 89, 328–332. [Google Scholar] [CrossRef]
- Całkosiński, I.; Dobrzyński, M.; Całkosińska, M.; Seweryn, E.; Bronowicka-Szydełko, A.; Dzierzba, K.; Ceremuga, I.; Gamian, A. Characterization of an inflammatory response. Postepy Hig. Med. Dosw. 2009, 63, 395–408. [Google Scholar]
- Abdulkhaleq, L.A.; Assi, M.A.; Abdullah, R.; Zamri-Saad, M.; Taufiq-Yap, Y.H.; Hezmee, M.N.M. The crucial roles of inflammatory mediators in inflammation: A review. Veter. World 2018, 11, 627–635. [Google Scholar] [CrossRef]
- Arulselvan, P.; Fard, M.T.; Tan, W.S.; Gothai, S.; Fakurazi, S.; Norhaizan, M.E.; Kumar, S.S. Role of Antioxidants and Natural Products in Inflammation. Oxid. Med. Cell. Longev. 2016, 2016, 5276130. [Google Scholar] [CrossRef]
- Medzhitov, R. Origin and physiological roles of inflammation. Nature 2008, 454, 428–435. [Google Scholar] [CrossRef]
- Wong, R.S.Y. Role of Nonsteroidal Anti-Inflammatory Drugs (NSAIDs) in Cancer Prevention and Cancer Promotion. Adv. Pharmacol. Sci. 2019, 2019, 1–10. [Google Scholar] [CrossRef]
- Yassine, E.Z.; Dalila, B.; Latifa, E.M.; Smahan, B.; Lebtar, S.; Sanae, A.; Abdellah, F. Phytochemical screening, anti-inflammatory activity and acute toxicity of hydro-ethanolic, flavonoid, tannin and mucilage extracts of Lavandula stoechas L. from Morocco. Int. J. Pharmacogn. Phytochem. Res. 2016, 8, 31–37. [Google Scholar]
- Husseini, Y.; Sahraei, H.; Meftahi, G.H.; Dargahian, M.; Mohammadi, A.; Hatef, B.; Zardooz, H.; Ranjbaran, M.; Hosseini, S.B.; Alibeig, H.; et al. Analgesic and anti-inflammatory activities of hydro-alcoholic extract of Lavandula officinalis in mice: Possible involvement of the cyclooxygenase type 1 and 2 enzymes. Rev. Bras. Farm. 2016, 26, 102–108. [Google Scholar] [CrossRef]
- Bozimowski, G. A Review of Nonsteroidal Anti-inflammatory Drugs. AANA J. 2015, 83, 425–433. [Google Scholar]
- Duda, M.; Olczyk, P.; Fenig, A.; Komosińska-Vassev, K. Cyklooksygenaza–znaczenie w biotechnologii, medycynie i farmacji. Farm. Dyplomie 2014, 70, 619–628. [Google Scholar]
- Yahfoufi, N.; Alsadi, N.; Jambi, M.; Matar, C. The Immunomodulatory and Anti-Inflammatory Role of Polyphenols. Nutrients 2018, 10, 1618. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).