Simultaneous Measurement of Cortisol, Cortisone, Dexamethasone and Additional Exogenous Corticosteroids by Rapid and Sensitive LC–MS/MS Analysis
Abstract
1. Introduction
2. Results and Discussion
2.1. Method Development
2.2. Method Validation
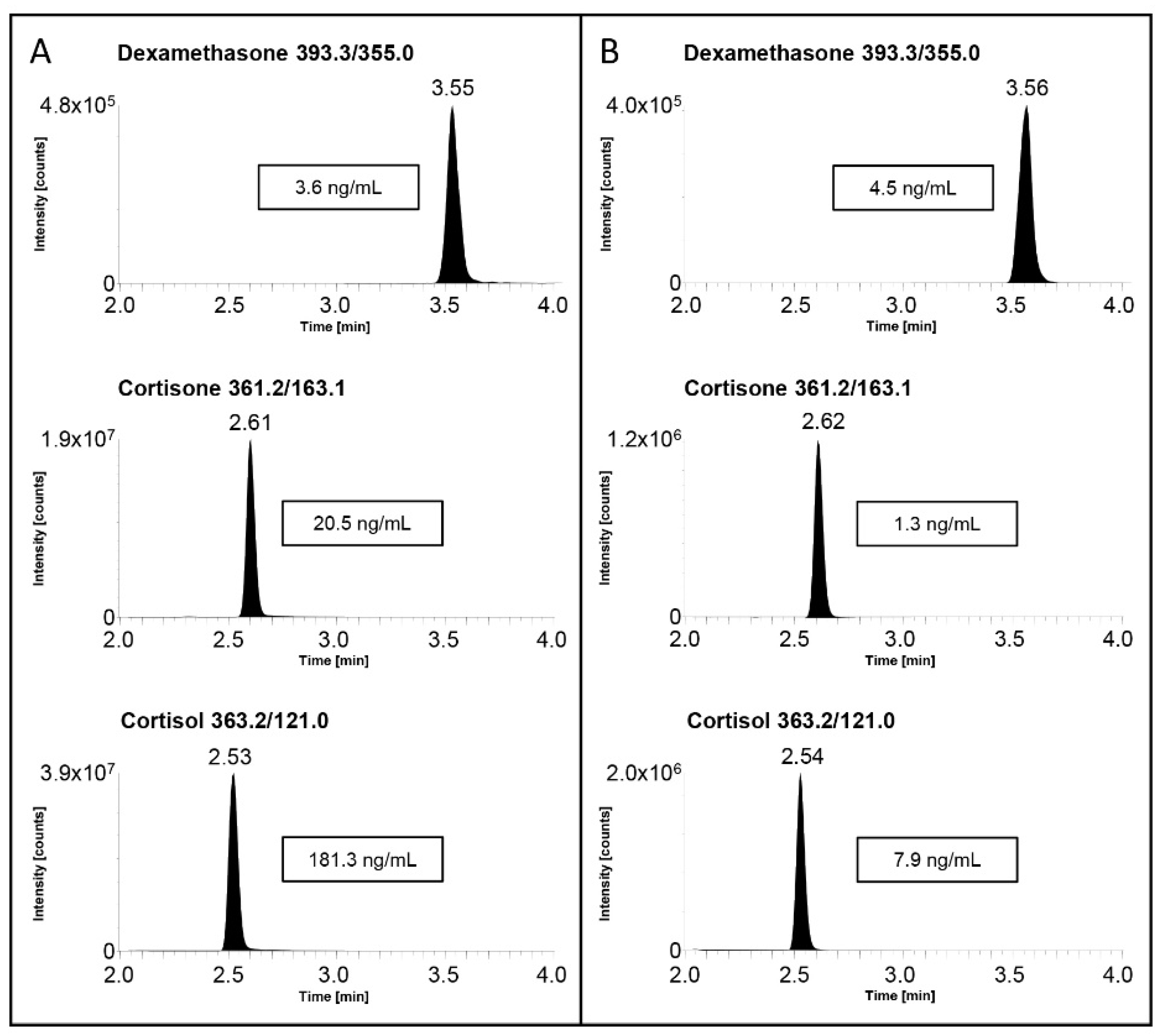
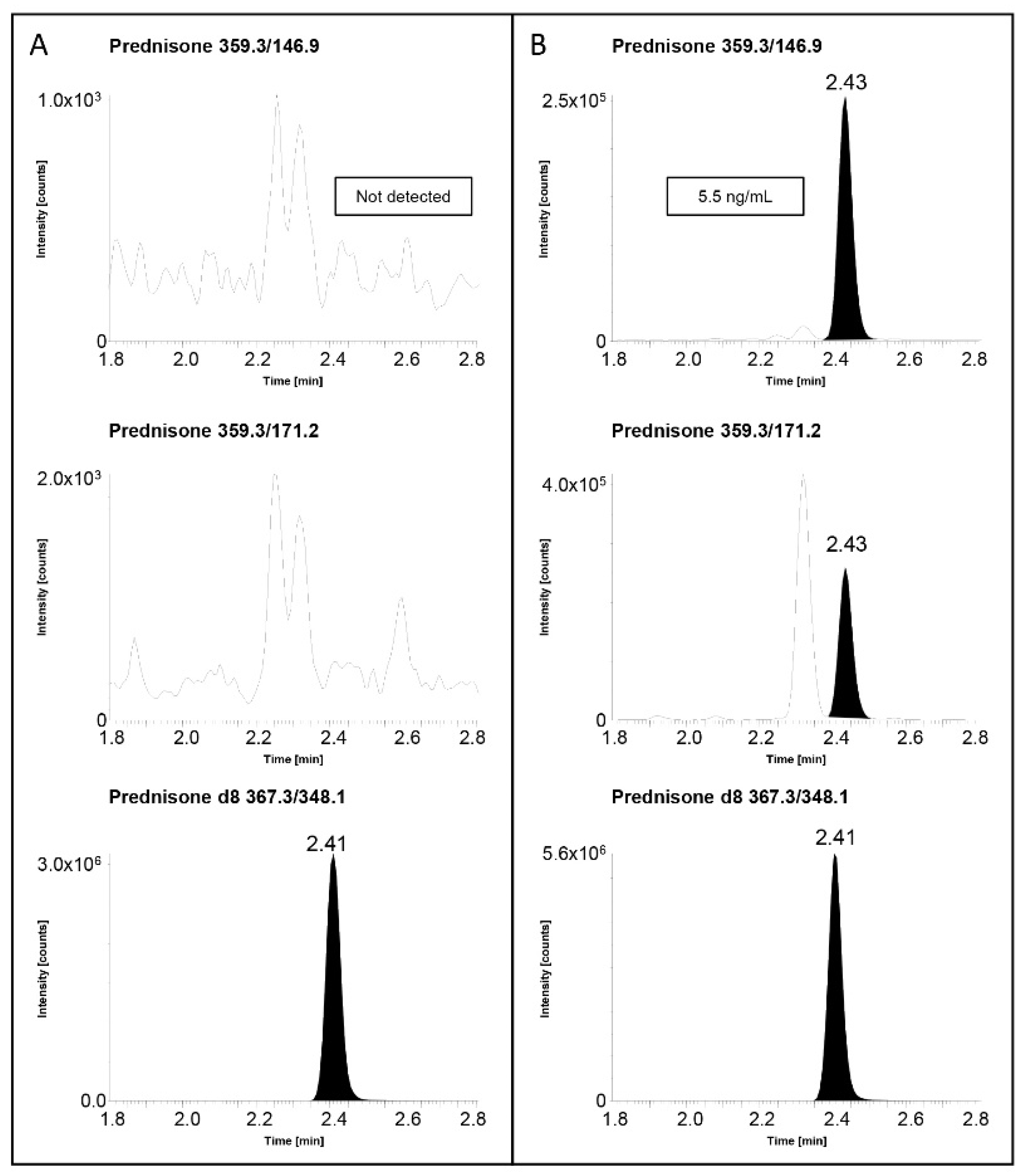
2.3. Real Samples’ Applications
3. Materials and Methods
3.1. Chemicals and Reagents
3.2. Sample Preparation
3.3. LC–MS/MS Analysis
3.4. Method Validation
3.5. Real Samples Applications
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Schleimer, R.P. An overview of glucocorticoid anti-inflammatory actions. Eur. J. Clin. Pharmacol. 1993, 45 (Suppl. 1), S3–S7. [Google Scholar] [CrossRef]
- De Keyser, J.; Zwanikken, C.; Zorgdrager, A.; Oenema, D.; Boon, M. Treatment of acute relapses in multiple sclerosis at home dexamethasone: A pilot study. J. Clin. Neurosci. 1999, 6, 382–384. [Google Scholar] [CrossRef]
- Parasiliti-Caprino, M.; Bioletto, F.; Frigerio, T.; D’Angelo, V.; Ceccato, F.; Ferraù, F.; Ferrigno, R.; Minnetti, M.; Scaroni, C.; Cannavò, S.; et al. A New Clinical Model to Estimate the Pre-Test Probability of Cushing’s Syndrome: The Cushing Score. Front. Endocrinol. 2021, 12, 747549. [Google Scholar] [CrossRef]
- Nieman, L.K.; Biller, B.M.K.; Findling, J.W.; Newell-Price, J.; Savage, M.O.; Stewart, P.M.; Montori, V.M. he diagnosis of Cushing’s syndrome: An Endocrine Society Clinical Practice Guideline. J. Clin. Endocrinol. Metab. 2008, 93, 1526–1540. [Google Scholar] [CrossRef]
- Fleseriu, M.; Auchus, R.; Bancos, I.; Ben-Shlomo, A.; Bertherat, J.; Biermasz, N.R.; Boguszewski, C.L.; Bronstein, M.D.; Buchfelder, M.; Carmichael, J.D.; et al. Consensus on diagnosis and management of Cushing’s disease: A guideline update. Lancet Diabetes Endocrinol. 2021, 9, 847–875. [Google Scholar] [CrossRef]
- Fassnacht, M.; Arlt, W.; Bancos, I.; Dralle, H.; Newell-Price, J.; Sahdev, A.; Tabarin, A.; Terzolo, M.; Tsagarakis, S.; Dekkers, O.M. Management of adrenal incidentalomas: European Society of Endocrinology Clinical Practice Guideline in collaboration with the European Network for the study of adrenal tumours. Eur. J. Endocrinol. 2016, 175, G1–G34. [Google Scholar] [CrossRef]
- Chiodini, I.; Ramos-Rivera, A.; Marcus, A.O.; Yau, H. Adrenal hypercortisolism: A closer look at screening, diagnosis, and important considerations of different testing modalities. J. Endocr. Soc. 2019, 3, 1097–1109. [Google Scholar] [CrossRef]
- Arnaldi, G.; Angeli, A.; Atkinson, A.B.; Bertagna, X.; Cavagnini, F.; Chrousos, G.P.; Fava, G.A.; Findling, J.W.; Gaillard, R.C.; Grossman, A.B.; et al. Diagnosis and complications of Cushing’s syndrome: A consensus statement. J. Clin. Endocrinol. Metab. 2003, 88, 5593–5602. [Google Scholar] [CrossRef]
- Ceccato, F.; Marcelli, G.; Martino, M.; Concettoni, C.; Brugia, M.; Trementino, L.; Michetti, G.; Arnaldi, G. The diagnostic accuracy of increased late night salivary cortisol for Cushing’s syndrome: A real-life prospective study. J. Endocrinol. Investig. 2019, 42, 327–335. [Google Scholar] [CrossRef]
- Ponzetto, F.; Settanni, F.; Parasiliti-Caprino, M.; Rumbolo, F.; Nonnato, A.; Ricciardo, M.; Amante, E.; Priolo, G.; Vitali, S.; Anfossi, L.; et al. Reference Ranges of Late-Night Salivary Cortisol and Cortisone Measured by LC–MS/MS and Accuracy for the Diagnosis of Cushing’s Syndrome. J. Endocrinol. Investig. 2020, 43, 1797–1806. [Google Scholar] [CrossRef]
- Lavagnino, L.; Amiant, F.; Parasiliti Caprino, M.; Maccario, M.; Arvat, E.; Ghigo, E.; Abbate Daga, G.; Fassino, S. Urinary cortisol and psychopathology in obese binge eating subjects. Appetite 2014, 83, 112–116. [Google Scholar] [CrossRef]
- Elias, P.C.L.; Martinez, E.Z.; Barone, B.F.C.; Mermejo, L.M.; Castro, M.; Moreira, A.C. Late-night salivary cortisol has a better performance than urinary free cortisol in the diagnosis of Cushing’s syndrome. J. Clin. Endocrinol. Metab. 2014, 99, 2045–2051. [Google Scholar] [CrossRef]
- Meikle, A.W. Dexamethasone suppression tests: Usefulness of simultaneous measurement of plasma cortisol and dexamethasone. Clin. Endocrinol. 1982, 16, 401–408. [Google Scholar] [CrossRef] [PubMed]
- Morris, H.; Varr, V.; Gilliland, J.; Hooper, M. Dexamethasone concentrations and the dexamethasone suppression test in psychiatric disorders. Br. J. Psychiatry 1986, 148, 66–69. [Google Scholar] [CrossRef] [PubMed]
- O’Sullivan, B.T.; Cutler, D.J.; Hunt, G.E.; Walters, C.; Johnson, G.F.; Caterson, I.D. Pharmacokinetics of dexamethasone and its relationship to dexamethasone suppression test outcome in depressed patients and healthy control subjects. Biol. Psychiatry 1997, 41, 574–584. [Google Scholar] [CrossRef] [PubMed]
- Guthrie, S. The impact of dexamethasone pharmacokinetics on the DST: A review. Psychopharmacol. Bull. 1991, 27, 565–576. [Google Scholar]
- Vilar, L.; Freitas, M.D.C.; Faria, M.; Montenegro, R.; Casulari, L.A.; Naves, L.; Bruno, O.D. Pitfalls in the diagnosis of Cushing’s syndrome. Arq. Bras. Endocrinol. Metab. 2007, 51, 1207–1216. [Google Scholar] [CrossRef]
- Ueland, G.Å.; Methlie, P.; Kellmann, R.; Bjørgaas, M.; Åsvold, B.O.; Thorstensen, K.; Kelp, O.; Thordarson, H.B.; Mellgren, G.; Løvås, K.; et al. Simultaneous assay of cortisol and dexamethasone improved diagnostic accuracy of the dexamethasone suppression test. Eur. J. Endocrinol. 2017, 176, 705–713. [Google Scholar] [CrossRef]
- de Graaf, A.J.; Mulder, A.L.; Krabbe, J.G. Retrospective analysis of repeated dexamethasone suppression tests—The added value of measuring dexamethasone. Ann. Clin. Biochem. 2019, 56, 708–710. [Google Scholar] [CrossRef]
- Zou, J.J.; Dai, L.; Ding, L.; Xiao, D.W.; Bin, Z.Y.; Fan, H.W.; Liu, L.; Wang, G.J. Determination of betamethasone and betamethasone 17-monopropionate in human plasma by liquid chromatography-positive/negative electrospray ionization tandem mass spectrometry. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2008, 873, 159–164. [Google Scholar] [CrossRef]
- Li, X.; Tong, H.; Xu, B.; Deng, Y.; Li, Y.; Huang, J.; Mao, Y.; Liu, M.; Zhang, P.; Guo, S. A sensitive and high-throughput LC-ESI-MS/MS method to detect budesonide in human plasma: Application to an evaluation of pharmacokinetics of budesonide intranasal formulations with and without charcoal-block in healthy volunteers. Drug. Dev. Ind. Pharm. 2021, 47, 329–336. [Google Scholar] [CrossRef] [PubMed]
- Szeitz, A.; Manji, J.; Riggs, K.W.; Thamboo, A.; Javer, A.R. Validated assay for the simultaneous determination of cortisol and budesonide in human plasma using ultra high performance liquid chromatography-tandem mass spectrometry. J. Pharm. Biomed. Anal. 2014, 90, 198–206. [Google Scholar] [CrossRef] [PubMed]
- Ruiter, A.F.C.; Teeninga, N.; Nauta, J.; Endert, E.; Ackermans, M.T. Determination of unbound prednisolone, prednisone and cortisol in human serum and saliva by on-line solid-phase extraction liquid chromatography tandem mass spectrometry and potential implications for drug monitoring of prednisolone and prednisone in saliva. Biomed. Chromatogr. 2012, 26, 789–796. [Google Scholar] [PubMed]
- Taylor, R.L.; Grebe, S.K.; Singh, R.J. Quantitative, highly sensitive liquid chromatography-tandem mass spectrometry method for detection of synthetic corticosteroids. Clin. Chem. 2004, 50, 2345–2352. [Google Scholar] [CrossRef] [PubMed]
- Methlie, P.; Hustad, S.S.; Kellmann, R.; Almås, B.; Moter Erichsen, M.; Husebye, E.; Løvås, K. Multisteroid LC-MS/MS assay for glucocorticoids and androgens, and its application in Addison’s disease. Endocr. Connect. 2013, 2, 125–136. [Google Scholar] [CrossRef]
- Ceccato, F.; Artusi, C.; Barbot, M.; Lizzul, L.; Pinelli, S.; Costantini, G.; Niero, S.; Antonelli, G.; Plebani, M.; Scaroni, C. Dexamethasone measurement during low-dose suppression test for suspected hypercortisolism: Threshold development with and validation. J. Endocrinol. Investig. 2020, 43, 1105–1113. [Google Scholar] [CrossRef]
- Vogg, N.; Kurlbaum, M.; Deutschbein, T.; Gra, B.; Fassnacht, M.; Kroiss, M. Method-Specific Cortisol and Dexamethasone Thresholds Increase Clinical Specificity of the Dexamethasone Suppression Test for Cushing Syndrome. Clin. Chem. 2021, 67, 998–1007. [Google Scholar] [CrossRef]
- Hawley, J.M.; Owen, L.J.; Debono, M.; Newell-Price, J.; Keevil, B.G. Development of a rapid liquid chromatography tandem mass spectrometry method for the quantitation of serum dexamethasone and its clinical verification. Ann. Clin. Biochem. 2018, 55, 665–672. [Google Scholar] [CrossRef]
- Meikle, A.W.; Lagerquist, L.G.; Tyler, F.H. Apparently normal pituitary-adrenal suppressibility in Cushing’s syndrome: Dexamethasone metabolism and plasma levels. J. Lab. Clin. Med. 1975, 86, 472–478. [Google Scholar]
- Djedovic, N.K.; Rainbow, S.J. Detection of synthetic glucocorticoids by liquid chromatography-tandem mass spectrometry in patients being investigated for Cushing’s syndrome. Ann. Clin. Biochem. 2011, 48 Pt 6, 542–549. [Google Scholar] [CrossRef]
- Kuz’mina, N.E.; Moiseev, S.V.; Severinova, E.Y.; Stepanov, E.A.; Bunyatyan, N.D. Identification and Quantification by NMR Spectroscopy of the 22 R and 22 S Epimers in Budesonide Pharmaceutical Forms. Molecules 2022, 27, 2262. [Google Scholar] [CrossRef] [PubMed]
- Mezzullo, M.; Pelusi, C.; Fazzini, A.; Repaci, A.; Di Dalmazi, G.; Gambineri, A.; Pagotto, U.; Fanelli, F. Female and male serum reference intervals for challenging sex and precursor steroids by liquid chromatography—Tandem mass spectrometry. J. Steroid Biochem. Mol. Biol. 2020, 197, 105538. [Google Scholar] [CrossRef] [PubMed]
- Schiffer, L.; Shaheen, F.; Gilligan, L.C.; Storbeck, K.H.; Hawley, J.M.; Keevil, B.G.; Arlt, W.; Taylor, A.E. Multi-steroid profiling by UHPLC-MS/MS with post-column infusion of ammonium fluoride. J. Chromatogr. B Analyt. Technol. Biomed. Life. Sci. 2022, 1209, 123413. [Google Scholar] [CrossRef] [PubMed]
- Takkis, K.; Aro, R.; Kõrgvee, L.T.; Varendi, H.; Lass, J.; Herodes, K.; Kipper, K. Signal Enhancement in the HPLC-ESI-MS/MS analysis of spironolactone and its metabolites using HFIP and NH4F as eluent additives. Anal. Bioanal. Chem. 2017, 409, 3145–3151. [Google Scholar] [CrossRef]
- Ponzetto, F.; Mehl, F.; Boccard, J.; Baume, N.; Rudaz, S.; Saugy, M.; Nicoli, R. Longitudinal monitoring of endogenous steroids in human serum by UHPLC-MS/MS as a tool to detect testosterone abuse in sports. Anal. Bioanal. Chem. 2016, 408, 705–719. [Google Scholar] [CrossRef]
- van der Gugten, J.G.; Holmes, D.T. Quantitation of Aldosterone in Serum or Plasma Using Liquid Chromatography-Tandem Mass Spectrometry (LC-MS/MS). Methods Mol. Biol. 2022, 2546, 45–54. [Google Scholar]
- Hawley, J.M.; Adaway, J.E.; Owen, L.J.; Keevil, B.G. Development of a total serum testosterone, androstenedione, 17-hydroxyprogesterone, 11β-hydroxyandrostenedione and 11-ketotestosterone LC-MS/MS assay and its application to evaluate pre-analytical sample stability. Clin. Chem. Lab. Med. 2020, 58, 741–752. [Google Scholar] [CrossRef]
- Matuszewski, B.K.; Costanzer, M.L.; Chavez-Eng, C.M. Strategies for the assessment of matrix effect in quantitative bioanalytical methods based on HPLC-MS/MS. Anal. Chem. 2003, 75, 3019–3030. [Google Scholar] [CrossRef]
- ISO 5725-1:1994; Accuracy (Trueness and Precision) of Measurement Methods and Results—Part 1: General Principles and Definitions. International Organization for Standardization (ISO): Geneva, Switzerland, 1994. Available online: https://www.iso.org/obp/ui/#iso:std:iso:5725:-1:ed-1:v1:en (accessed on 2 November 2022).
- Keevil, B.G. Improving the Dexamethasone Suppression Test. Clin. Chem. 2021, 67, 929–931. [Google Scholar] [CrossRef]



| Reference | Matrix | Sample Volume (uL) | Sample Preparation | LC Column | Analysis Time (min) | Analytes | LLOQ (ng/mL) | Additional Analytes |
|---|---|---|---|---|---|---|---|---|
| Taylor RL et al. [24] | Serum Urine Table extracts | 500 μL | LLE | Synergi Max_RP (50 × 4.6 mm) Phenomenex | 15.00 | F E Dex | 0.3–0.7 0.3–0.7 0.3–0.7 | 13 synthetic glucocorticoids |
| Methlie P et al. [25] | Serum | 85 μL | LLE | Zorbax RRHD C18 (50 × 2.1 mm, 1.8 μm) Agilent | 6.10 | F E Dex | 0.7 0.6 0.03 | 2 synthetic glucocorticoids + 5 steroid hormones |
| Ceccato F et al. [26] | Serum | 100 μL | PPE | Acquity UPLC HSS C18 (150 × 2.1 mm, 1.8 µm) Waters | - | Dex | 0.4 | - |
| Vogg N et al. [27] | Serum | 200 μL | PPE | XBridge BEH C18 (75 × 3.0 mm, 2.5 µm) Waters | 5.35 | F Dex | 1 1 | - |
| Hawley JM et al. [28] | Serum | 100 μL | SLE | Kinetex C8 (30 × 2.1 mm, 2.6 µm) Phenomenex | 2.20 | Dex | 0.1 | - |
| Compound | Ret. Time (min) | Trueness (%) | Repeatability (%) | Intermediate Precision (%) | Combined Uncertainty (%) | Linearity Range (ng/mL) | LLOQ (pg/mL) | Extraction Recovery (CV) (%) | Matrix Effect (CV) (%) |
|---|---|---|---|---|---|---|---|---|---|
| Beclomethasone | 3.93 | 94.2–106.3 | 6.3–9.5 | 6.1–10.1 | 8.4–12.5 | 0.5–75 | 500 | 89.9 (6.3) | 93.9 (0.9) |
| Betamethasone | 3.43 | 98.8–105.4 | 5.8–8.9 | 6.2–9.5 | 9.1–13.4 | 0.5–60 | 500 | 85.4 (7.7) | 95.5 (9.5) |
| Budesonide | 5.77 | 93.6–104.9 | 7.1–10.3 | 8.3–11.1 | 9.9–14.0 | 0.5–60 | 500 | 79.5 (6.9) | 87.3 (3.4) |
| Cortisol | 2.53 | 97.8–105.5 | 5.5–8.5 | 5.3–9.2 | 8.4–12.1 | 1–500 | 1000 | 83.2 (6.3) | 92.6 (1.5) |
| Cortisone | 2.61 | 95.6–105.6 | 4.6–8.6 | 5.2–8.8 | 7.4–12.2 | 0.1–50 | 100 | 80.4 (6.5) | 94.9 (0.5) |
| Dexamethasone | 3.55 | 98.6–105.9 | 6.7–9.2 | 6.1–9.6 | 11.4–13.6 | 0.5–60 | 500 | 97.8 (6.2) | 94.7 (9.5) |
| Flumethasone | 3.65 | 93.2–108.4 | 6.6–9.9 | 7.2–10.8 | 8.5–13.6 | 1–100 | 1000 | 79.2 (4.2) | 106.6 (7.8) |
| Prednisone | 2.44 | 96.6–106.3 | 5.7–8.8 | 6.2–9.9 | 8.4–14.1 | 0.25–250 | 250 | 84.9 (9.7) | 94.7 (0.8) |
| Triamcinolone | 1.70 | 94.8–105.0 | 7.4–10.1 | 7.6–10.4 | 8.8–12.8 | 1–100 | 1000 | 67.0 (10.8) | 96.6 (5.9) |
| Compound | Ionization Mode | Q1 Mass (Da) | Q3 Mass (Da) | DP (V) | EP (V) | CE (V) | CXP (V) |
|---|---|---|---|---|---|---|---|
| Beclomethasone | Pos | 409.3 * | 391.1 | 41 | 10 | 15 | 16 |
| 409.3 | 147.1 | 41 | 10 | 23 | 16 | ||
| Betamethasone | Pos | 393.3 * | 373.2 | 44 | 10 | 13 | 10 |
| 393.3 | 355.2 | 44 | 10 | 16 | 10 | ||
| Budesonide | Pos | 413.3 + | 147.1 | 126 | 10 | 43 | 12 |
| 413.3 + | 173.1 | 126 | 10 | 43 | 12 | ||
| Budesonide d8 | Pos | 439.4 | 323.1 | 81 | 10 | 19 | 14 |
| Cortisol | Pos | 363.2 * | 121.0 | 101 | 10 | 23 | 16 |
| 363.2 | 90.9 | 106 | 10 | 83 | 16 | ||
| Cortisol d4 | Pos | 367.2 | 121.0 | 106 | 10 | 31 | 12 |
| Cortisone | Pos | 361.2 * | 163.1 | 121 | 10 | 28 | 14 |
| 361.2 | 91.0 | 126 | 10 | 85 | 10 | ||
| Cortisone d8 | Pos | 369.2 | 168.0 | 126 | 10 | 33 | 14 |
| Dexamethasone | Pos | 393.3 * | 355.2 | 44 | 10 | 16 | 10 |
| 393.3 | 373.2 | 44 | 10 | 13 | 10 | ||
| Dexamethasone d3 | Pos | 396.3 | 358.2 | 46 | 10 | 17 | 10 |
| Flumethasone | Pos | 411.3 * | 253.2 | 41 | 10 | 21 | 12 |
| 411.3 | 121.2 | 41 | 10 | 63 | 12 | ||
| Prednisone | Pos | 359.3 * | 171.3 | 135 | 10 | 46 | 10 |
| 359.3 | 146.9 | 135 | 10 | 46 | 10 | ||
| Prednisone d8 | Pos | 367.3 | 348.1 | 66 | 10 | 17 | 10 |
| Triamcinolone | Pos | 395.3 * | 375.2 | 51 | 10 | 13 | 14 |
| 395.3 | 165.1 | 51 | 10 | 95 | 14 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ponzetto, F.; Parasiliti-Caprino, M.; Settanni, F.; Nonnato, A.; Mengozzi, G.; Ghigo, E.; Giordano, R. Simultaneous Measurement of Cortisol, Cortisone, Dexamethasone and Additional Exogenous Corticosteroids by Rapid and Sensitive LC–MS/MS Analysis. Molecules 2023, 28, 248. https://doi.org/10.3390/molecules28010248
Ponzetto F, Parasiliti-Caprino M, Settanni F, Nonnato A, Mengozzi G, Ghigo E, Giordano R. Simultaneous Measurement of Cortisol, Cortisone, Dexamethasone and Additional Exogenous Corticosteroids by Rapid and Sensitive LC–MS/MS Analysis. Molecules. 2023; 28(1):248. https://doi.org/10.3390/molecules28010248
Chicago/Turabian StylePonzetto, Federico, Mirko Parasiliti-Caprino, Fabio Settanni, Antonello Nonnato, Giulio Mengozzi, Ezio Ghigo, and Roberta Giordano. 2023. "Simultaneous Measurement of Cortisol, Cortisone, Dexamethasone and Additional Exogenous Corticosteroids by Rapid and Sensitive LC–MS/MS Analysis" Molecules 28, no. 1: 248. https://doi.org/10.3390/molecules28010248
APA StylePonzetto, F., Parasiliti-Caprino, M., Settanni, F., Nonnato, A., Mengozzi, G., Ghigo, E., & Giordano, R. (2023). Simultaneous Measurement of Cortisol, Cortisone, Dexamethasone and Additional Exogenous Corticosteroids by Rapid and Sensitive LC–MS/MS Analysis. Molecules, 28(1), 248. https://doi.org/10.3390/molecules28010248











