Antimicrobial Activities Evaluation and Phytochemical Screening of Some Selected Plant Materials Used in Traditional Medicine
Abstract
1. Introduction
2. Results and Discussion
3. Materials and Methods
3.1. Tested Material
- Rowan fruits (Sorbus aucuparia L.) were collected from natural habitats in October 2022.
- Guelder rose fruits (Viburnum opulus L.) were collected from natural habitats in October 2022.
- Sea buckthorn fruits (Hippophaë rhamnoides L.) were collected from natural habitats in September 2022.
- Bitter melon fruits (Momordica charantia L.) were collected from natural habitats in September 2021.
- Elderberry (Sambucus nigra L.) flowers were collected from natural habitats in June 2022.
- Beach rose (Rosa rugosa Thunb.) leaves were collected from natural habitats in July 2022.
3.2. Extraction Procedure
3.3. Total Phenolic Acids and Total Flavonoids
3.4. ABTS+ Antioxidant Activity
3.5. Determination of LMWOA (Low Molecular Weight Organic Acid)
3.6. Carotenoids Determination
3.7. Phenolic Compounds Determination
3.8. Sterol Analysis
3.9. MIC Measurement
3.10. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Bernhoft, A.; Siem, H.; Bjertness, E.; Meltzer, M.; Flaten, T.; Holmsen, E. Bioactive Compounds in Plants–Benefits and Risks for Man and Animals; The Norwegian Academy of Science and Letters: Oslo, Norway, 2010. [Google Scholar]
- Croteau, R.; Kutchan, T.M.; Lewis, N.G. Natural Products (Secondary Metabolites). Biochem. Mol. Biol. Plant 2000, 24, 1250–1319. [Google Scholar]
- Azmir, J.; Zaidul, I.S.M.; Rahman, M.M.; Sharif, K.M.; Mohamed, A.; Sahena, F.; Omar, A.K.M. Techniques for extraction of bioactive compounds from plant materials: A review. J. Food Eng. 2013, 117, 426–436. [Google Scholar] [CrossRef]
- Muzykiewicz, A.; Zielonka-Brzezicka, J.; Klimowicz, A. The antioxidant potential of flesh, albedo and flavedo extracts from different varieties of grapefruits. Acta Sci. Pol. Technol. Aliment. 2019, 18, 4. [Google Scholar] [CrossRef]
- Proestos, C.; Komaitis, M. Application of microwave-assisted extraction to the fast extraction of plant phenolic compounds. LWT-Food Sci. Technol. 2008, 41, 652–659. [Google Scholar] [CrossRef]
- Tan, S.P.; Kha, T.C.; Parks, S.E.; Roach, P.D. Bitter melon (Momordica charantia L.) bioactive composition and health benefits: A review. Food Rev. Int. 2016, 32, 181–202. [Google Scholar] [CrossRef]
- Daka, D. Antibacterial effect of garlic (Allium sativum) on Staphyloccus aureus: An in vitro study. Afr. J. Biotechnol. 2011, 10, 666–669. [Google Scholar]
- Fasseas, M.K.; Mountzouris, K.C.; Tarantilis, P.A.; Polissiou, M.; Zervas, G. Antioxidant activity in meat treated with oregano and sage essential oils. Food Chem. 2007, 106, 1188–1194. [Google Scholar] [CrossRef]
- Gayathry, K.S.; John, J.A. A comprehensive review on bitter gourd (Momordica charantia L.) as a gold mine of functional bioactive components for therapeutic foods. Food Prod Process Nutr. 2022, 4, 10. [Google Scholar] [CrossRef]
- Samadov, B.S. The chemical composition of the medicinal plant Momordica Charantia L used in folk medicine. Themat. J. Chem. 2022, 6, 34–50. [Google Scholar]
- Fascella, G.; D’Angiolillo, F.; Mammano, M.M.; Granata, G.; Napoli, E. Effect of petal color, water status, and extraction method on qualitative characteristics of Rosa rugosa Liqueur. Plants 2022, 11, 1859. [Google Scholar] [CrossRef]
- Olech, M.; Nowak, R.; Pecio, Ł.; Łoś, R.; Malm, A.; Rzymowska, J.; Oleszek, W. Multidirectional characterisation of chemical composition and health-promoting potential of Rosa rugosa hips. Nat. Prod. Res. 2017, 31, 667–671. [Google Scholar] [CrossRef] [PubMed]
- Güney, M.; Gündeşlİ, M.A. The Necessities of Cranberry bush (Viburnum opulus) Evaluation for Horticultural Cultivation. MAS J. Appl. Sci. 2022, 7, 1033–1041. [Google Scholar]
- Zadernowski, R.; Naczk, M.; Amarowicz, R. Tocopherols in sea buckthorn (Hippophae rhamnoides L.) berry oil. J. Am. Oil Chem. Soc. 2003, 80, 55–58. [Google Scholar] [CrossRef]
- Zadernowski, R.; Naczk, M.; Czaplicki, S.; Rubinskiene, M.; Szałkiewicz, M. Composition of phenolic acids in sea buckthorn (Hippophae rhamnoides L.) berries. J. Am. Oil Chem. Soc. 2005, 82, 175–179. [Google Scholar] [CrossRef]
- Yang, B.; Kallio, H. Composition and physiological effects of sea buckthorn (Hippophae) lipids. Trends Food Sci. Technol. 2002, 13, 160–167. [Google Scholar] [CrossRef]
- Papuc, C.; Diaconescu, C.; Nicorescu, V. Antioxidant activity of sea buckthorn (Hippophae Rhamnoides) extracts compared with common food additives. Rom. Biotechnol. Lett. 2008, 13, 4049–4053. [Google Scholar]
- Przybylska-Balcerek, A.; Szablewski, T.; Szwajkowska-Michałek, L.; Świerk, D.; Cegielska-Radziejewska, R.; Krejpcio, Z.; Stuper-Szablewska, K. Sambucus Nigra Extracts–Natural Antioxidants and Antimicrobial Compounds. Molecules 2021, 26, 2910. [Google Scholar] [CrossRef]
- Ou, S.; Kwok, K. Ferulic acid: Pharmaceutical functions, preparation and applications in foods. J. Sci. Food Agric. 2004, 84, 1261–1269. [Google Scholar] [CrossRef]
- Ozcan, T.A.; Akpinar-Bayizit, A.; Yilmaz-Ersan, L.; Delikanli, B. Phenolics in Human Health. Int. J. Chem. Eng. Appl. 2014, 5, 393–396. [Google Scholar] [CrossRef]
- Przybylska-Balcerek, A.; Góral, T.; Kurasiak-Popowska, D.; Stuper-Szablewska, K. Phenolic acids in various genotypes of cereals grown in Poland in the years 2017–2018. Acad. J. Med. Plant 2019, 7, 92–99. [Google Scholar]
- Parus, A. Antioxidant and pharmacological properties of phenolic acids. Post. Phytotherapist. 2013, 1, 48–53. [Google Scholar]
- Kędzia, B.; Hołderna-Kędzia, E. Antimicrobial activity of plant phenol derivatives. Borgis-Adv. Phytother. 2012, 3, 151–155. [Google Scholar]
- Vieira, V.; Pires, T.C.S.P.; Calhelha, R.C.; Alves, M.J.; Ferreira, O.; Barros, L.; Ferreira, I.C.F.R. Phenolic profile, antioxidant and antibacterial properties of Juglans regia L. (walnut) leaves from the Northeast of Portugal. Ind. Crops Prod. 2019, 134, 347–355. [Google Scholar] [CrossRef]
- Pernin, A.; Dubois-Brissonnet, F.; Roux, S.; Masson, M.; Bosc, V.; Noëlle Maillard, M. Phenolic compounds can delaythe oxidation of polyunsaturated fatty acids and the growth of Listeria monocytogenes: Structure-activity relationships. J. Sci. Food Agric. 2018, 98, 5401–5408. [Google Scholar] [CrossRef] [PubMed]
- Camargo, A.C.; Bismara Regitano-d’Arce, M.A.; Rasera, G.B.; Canniatti-Brazaca, S.G.; Prado-Silva, L.; Alvarenga, V.O.; Sant’Ana, A.S.; Shahidi, F. Phenolic acids and flavonoids of peanut by-products: Antioxidant capacity andantimicrobial effects. Food Chem. 2017, 237, 538–544. [Google Scholar] [CrossRef] [PubMed]
- Ali, R.M.; Houghton, P.J.; Raman, A.; Hoult, J.R.S. Antimicrobial and anti-inflammatory activities of extracts and constituents of Oroxylum indicum. Phytomedicine 1998, 5, 375–381. [Google Scholar]
- Iwagawa, T.; Kawasaki, J.; Hase, T.; Sako, S.; Ōkubo, T.; Ishida, M.; Kim, M. An acetylated flavonol glycoside from Lasiobema japonica. Phytochemistry 1990, 29, 1013–1014. [Google Scholar] [CrossRef]
- Basile, A.; Giordano, S.; López-Sáez, J.A.; Cobianchi, R.C. Antibacterial activity of pure flavonoids isolated from mosses. Phytochemistry 1999, 52, 1479–1482. [Google Scholar] [CrossRef]
- Waage, S.K.; Hedin, P.A. Quercetin 3-O-galactosyl-(1Æ6)-glucoside, a compound from narrowleaf vetch with antibacterial activity. Phytochemistry 1985, 24, 243–245. [Google Scholar] [CrossRef]
- Liu, H.; Orjala, J.; Sticher, O.; Rali, T. Acylated flavonol glycosides from leaves of Stenochlaena palustris. J. Nat. Prod. 1999, 62, 70–75. [Google Scholar] [CrossRef]
- Mitrokotsa, D.; Demetzos, C.; Harvala, C.; Mentis, A.; Perez, S.; Kokkinopoulo, D. Bioactive compounds from the buds of Plantanus orientalis and isolation of a new kaempferol glycoside. Planta Med. 1993, 59, 517–520. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Hamburger, M.; Gueho, J.; Hostettmann, K. Antimicrobial flavonoids from Psiadia trinervia and their methylated and acetylated derivatives. Phytochemistry 1989, 28, 2323–2327. [Google Scholar] [CrossRef]
- Kilcar, A.Y.; Yildiz, O.; Dogan, T.; Sulu, E.; Takan, G.; Muftuler, F.Z. The effect of bitter melon (Momordica charantia) extract on the uptake of 99mtc labeled paclitaxel: In vitro monitoring in breast cancer cells. Curr. Med. Chem. Anticancer Agents 2020, 20, 1497–1503. [Google Scholar] [CrossRef] [PubMed]
- Mahmood, A.; Raja, G.K.; Mahmood, T.; Gulfraz, M.; Khanum, A. Isolation and characterization of antimicrobial activity conferring component (s) from seeds of bitter gourd (Momordica charantia). J. Med. Plants Res. 2012, 6, 566–573. [Google Scholar] [CrossRef]
- Kubola, J.; Siriamornpun, S. Phenolic contents and antioxidant activities of bitter gourd (Momordica charantia L.) leaf, stem and fruit fraction extracts in vitro. Food Chem. 2008, 110, 881–890. [Google Scholar] [CrossRef]
- Ozusaglam, M.A.; Karakoca, K. Antimicrobial and antioxidant activities of Momordica charantia from Turkey. Afr. J. Biotechnol. 2013, 12, 1548–1558. [Google Scholar]
- Wu, S.J.; Ng, L.T. Antioxidant and free radical scavenging activities of wild bitter melon (Momordica charantia Linn. var. abbreviata Ser.) in Taiwan. Food Sci. Technol. 2008, 41, 323–330. [Google Scholar] [CrossRef]
- Krawitz, C.; Mraheil, M.A.; Stein, M.; Imirzalioglu, C.; Domann, E.; Pleschka, S.; Hain, T. Inhibitory activity of a standardized elderberry liquid extract against clini-cally-relevant human respiratory bacterial pathogens and influenza A and B viruses. BMC Complementary Altern. Med. 2011, 11, 16. [Google Scholar] [CrossRef]
- Hearst, C.; McCollum, G.; Nelson, D.; Ballard, L.M.; Millar, B.C.; Goldsmith, C.E.; Rooney, P.J.; Loughrey, A.; Moore, J.E.; Rao, J.R. Antibacterial activity of elder (Sambucus nigra L.) flower or berry against hospital pathogens. J. Med. Plant Res. 2010, 4, 1805–1809. [Google Scholar]
- Sadeghi-Kiakhani, M.; Arami, M.; Gharanjig, K. Application of a biopolymer chitosan-poly (propylene) imine dendrimer hybrid as an antimicrobial agent on the wool fabrics. Iran. Polym. J. 2013, 22, 931–940. [Google Scholar]
- Liepiņa, I.; Nikolajeva, V.; Jākobsone, I. Antimicrobial activity of extracts from fruits of Aronia melanocarpa and Sorbus aucuparia. Environ. Exp. Biol. 2013, 11, 195–199. [Google Scholar]
- Krisch, J.; Galgóczy, L.; Tölgyesi, M.; Papp, T.; Vágvölgyi, C. Effect of fruit juices and pomace extracts on the growth of Gram-positive and Gram-negative bacteria. Acta Biol. Szeged. 2008, 52, 267–270. [Google Scholar]
- Kylli, P.; Nohynek, L.; Puopponen-Pimiä, R.; Westerlund-Wikstrom, B.; McDougall, G.; Stewart, D.; Heinonen, M. Rowanberry phenolics: Compositional analysis and bioactivities. J. Agric. Food Chem. 2010, 58, 11985–11992. [Google Scholar] [CrossRef]
- Sagdic, O.; Aksoy, A.; Ozkan, G. Evaluation of the antibacterial and antioxidant potentials of cranberry (gilaburu, Viburnum opulus L.) fruit extract. Acta Aliment. 2006, 35, 487–492. [Google Scholar] [CrossRef]
- Özkan, G.; Sağdiç, O.; Özcan, M. Inhibition of pathogenic bacteria by essential oils at different concentrations. Food Sci. Technol. Int. 2003, 9, 85–88. [Google Scholar] [CrossRef]
- Baydar, N.; Ozkan, G.; Yaşar, S. Evaluation of the antiradical and antioxidant potential of grape extracts. Food Control 2007, 18, 1131–1136. [Google Scholar] [CrossRef]
- Yue, X.F.; Shang, X.; Zhang, Z.J.; Zhang, Y.N. Phytochemical composition and antibacterial activity of the essential oils from different parts of sea buckthorn (Hippophae rhamnoides L.). J. Food Drug Anal. 2017, 25, 327–332. [Google Scholar] [CrossRef]
- Garza-Reyes, J.A.; Kumar, V.; Chaikittisilp, S.; Tan, K.H. The effect of lean methods and tools on the environmental performance of manufacturing organisations. Int. J. Prod. Econ. 2018, 200, 170–180. [Google Scholar] [CrossRef]
- Tajkarimi, M.; Ibrahim, S.A. Antimicrobial activity of ascorbic acid alone or in combination with lactic acid on Escherichia coli O157: H7 in laboratory medium and carrot juice. Food Control 2011, 22, 801–804. [Google Scholar] [CrossRef]
- Giannuzzi, L.; Zaritzky, N.E. Effect of Ascorbic Acid in Comparison to Citric and Lactic Acid on Listeria monocytogenes Inhibition at Refrigeration Temperatures. LWT-Food Sci. Technol. 1996, 29, 278–285. [Google Scholar] [CrossRef]
- Wijngaard, H.H.; Brunton, N. The optimisation of solid–liquid extraction of antioxidants from apple pomace by response surface methodology. J. Food Eng. 2010, 96, 134–140. [Google Scholar] [CrossRef]
- Palafox-Carlos, H.; Yahia, E.M.; González-Aguilar, G.A. Identification and quantification of major phenolic compounds from mango (Mangifera indica, cv. Ataulfo) fruit by HPLC–DAD–MS/MS-ESI and their individual contribution to the antioxidant activity during ripening. Food Chem. 2012, 135, 105–111. [Google Scholar] [CrossRef]
- Przybylska, A.; Stuper-Szablewska, K.; Matysiak, A.; Perkowski, J. Optimization of extraction conditions total phenolic compounds and antioxidant activity from wheat grain. ABiD 2018, 23, 4–12. [Google Scholar]
- Gąsecka, M.; Siwulski, M.; Magdziak, Z.; Budzyńska, S.; Stuper-Szablewska, K.; Niedzielski, P.; Mleczek, M. The effect of drying temperature on bioactive compounds and antioxidant activity of Leccinum scabrum (Bull.) Gray and Hericium erinaceus (Bull.) Pers. J. Food Sci. Technol. 2020, 57, 513–525. [Google Scholar] [CrossRef]
- Suchowilska, E.; Bieńkowska, T.; Stuper-Szablewska, K.; Wiwart, M. Concentrations of Phenolic Acids, Flavonoids and Carotenoids and the Antioxidant Activity of the Grain, Flour and Bran of Triticum polonicum as Compared with Three Cultivated Wheat Species. Agriculture 2020, 10, 591. [Google Scholar] [CrossRef]
- Bilska, K.; Stuper-Szablewska, K.; Kulik, T.; Buśko, M.; Załuski, D.; Jurczak, S.; Perkowski, J. Changes in Phenylpropanoid and Trichothecene Production by Fusarium culmorum and F. graminearum Sensu Stricto via Exposure to Flavonoids. Toxins 2018, 10, 110. [Google Scholar] [CrossRef]
- Stuper-Szablewska, K.; Kurasiak-Popowska, D.; Nawracała, J.; Perkowski, J. Response of non-enzymatic antioxidative mechanisms to stress caused by infection with Fusarium fungi and chemical protection in different wheat genotypes. Chem. Ecol. 2017, 33, 949–962. [Google Scholar] [CrossRef]
- Kurasiak-Popowska, D.; Ryńska, B.; Stuper-Szablewska, K. Analysis of Distribution of Selected Bioactive Compoundsin Camelina sativa from Seeds to Pomace and Oil. Agronomy 2019, 9, 168. [Google Scholar] [CrossRef]

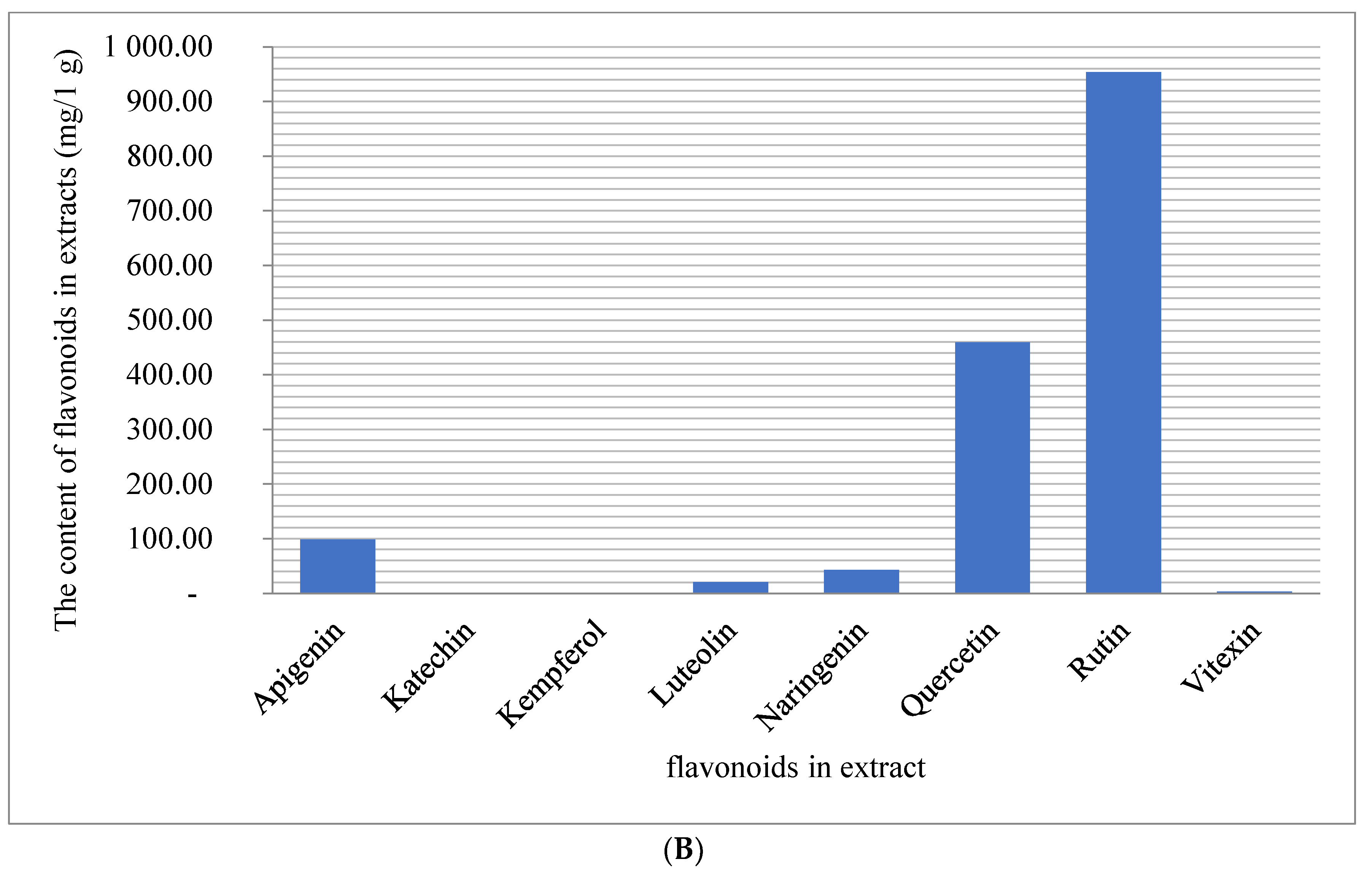

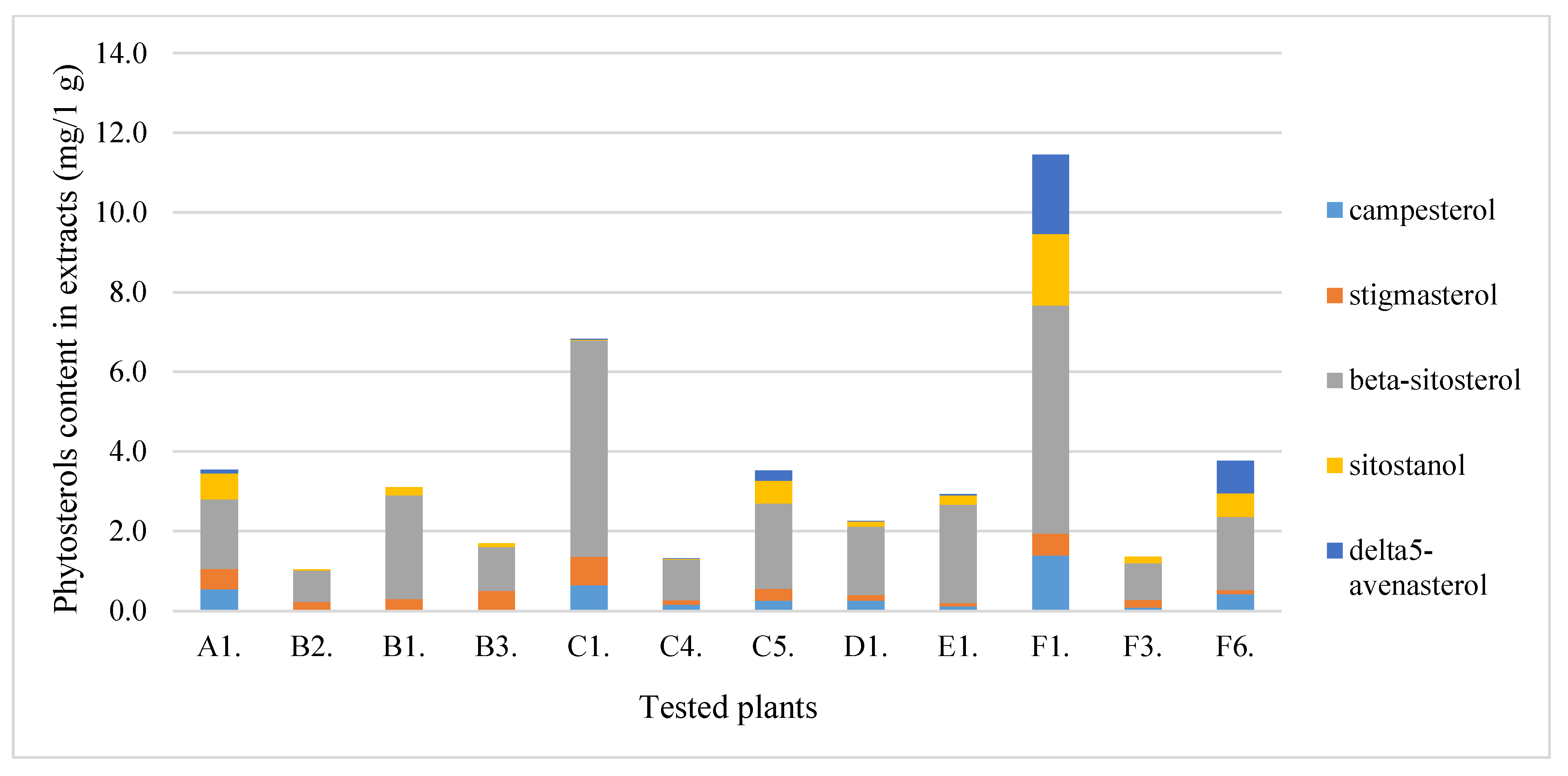




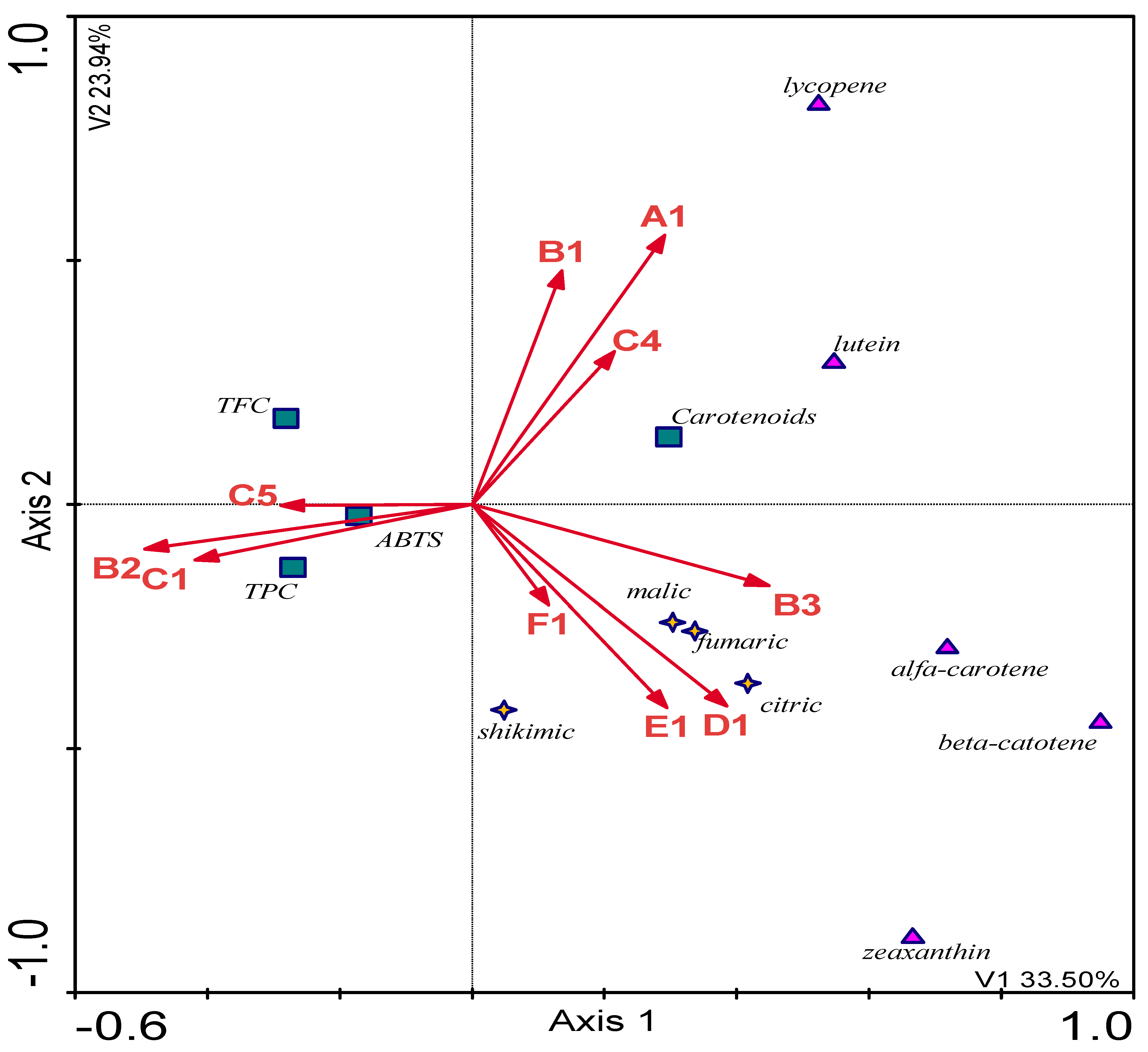
| Nr. | Plants | TPC (mg GAE/1 g Extract) | ABTS (µmoLTROLOX/1 g Extract s.m.) | TFC (mg RUTE/1 g Extract) |
|---|---|---|---|---|
| A1. | Bitter melon fruits (Momordica charantia L.) | 11.61 | 11.81 | 3.74 |
| B2. | Elderberry flower (Sambucus nigra L.) | 34.98 | 50.28 | 21.52 |
| B1. | Elderberry fruit (Sambucus nigra L.) | 9.64 | 33.15 | 18.09 |
| B3. | Elderberry leaves (Sambucus nigra L.) | 1.35 | 1.66 | 0.21 |
| C1. | Beach rose fruit (Rosa rugosa Thunb.) | 60.96 | 47.10 | 38.30 |
| C4. | Beach rose pulp (Rosa rugosa Thunb.) | 2.01 | 9.39 | 17.95 |
| C5. | Beach rose pips (Rosa rugosa Thunb.) | 31.06 | 23.54 | 15.09 |
| D1. | Rowan fruits (Sorbus aucuparia L.) | 7.59 | 10.18 | 0.23 |
| E1. | Guelder rose fruits (Viburnum opulus L.) | 12.03 | 14.07 | 0.42 |
| F1. | Sea-buckthorn fruits (Hippophaë rhamnoides L.) | 15.59 | 16.22 | 4.18 |
| F3. | Sea-buckthorn leaves dried in the sun (Hippophaë rhamnoides L.) | 5.03 | 5.34 | 2.51 |
| F6. | Sea-buckthorn pomace after juice-thermal treatment (Hippophaë rhamnoides L.) | 1.77 | 3.61 | 1.05 |
| min | 1.31 | 1.6 | 0.19 | |
| max | 61.04 | 50.86 | 38.5 | |
| mean | 16.3 | 18.86 | 10.27 | |
| SD | 17.29 | 15.95 | 11.64 |
| Tested Bacteria | ||||||||
|---|---|---|---|---|---|---|---|---|
| Escherichia coli | Salmonella enteritidis | Proteus mirabilis | Pseudomonas fluorescens | Pesudomonas fragii | Listeria innocua | Micrococcus luteus | ||
| MIC | MIC | MIC | MIC | MIC | MIC | MIC | ||
| Momordica charantia L. | SD | 0.02 | 0.02 | 0.02 | 0.02 | 0.02 | 0.01 | 0.00 |
| Min | 0.03 | 0.05 | 0.1 | 0.05 | 0.05 | 0.05 | 0.05 | |
| Max | 0.08 | 0.025 | 0.075 | 0.075 | 0.025 | 0.05 | 0.05 | |
| Mean Value | 0.05 | 0.05 | 0.10 | 0.05 | 0.05 | 0.06 | 0.05 | |
| Sambucus nigra L. (Sambuci flos) | SD | 0.04 | 0.05 | 0.01 | 0.01 | 0.03 | 0.02 | 0.00 |
| Min | 0.13 | 0.1 | 0.175 | 0.15 | 0.05 | 0.11 | 0.05 | |
| Max | 0.20 | 0.2 | 0.2 | 0.175 | 0.1 | 0.15 | 0.05 | |
| Mean Value | 0.16 | 0.16 | 0.18 | 0.15 | 0.08 | 0.13 | 0.05 | |
| Sambucus nigra L. (Sambuci fructus) | SD | 0.01 | 0.06 | 0.03 | 0.05 | - | 0.03 | 0.00 |
| Min | 0.10 | 0.1 | 0.1 | 0.1 | 0.15 | 0.1 | 0.05 | |
| Max | 0.13 | 0.2 | 0.15 | 0.15 | 0.152 | 0.15 | 0.05 | |
| Mean Value | 0.11 | 0.13 | 0.13 | 0.15 | 0.15 | 0.12 | 0.05 | |
| Sambucus nigra L. (Sambuci folium) | SD | 0.03 | 0.03 | 0.01 | 0.03 | 0.02 | 0.03 | 0.03 |
| Min | 2.35 | 2.65 | 2.4 | 1.7 | 1.7 | 2.55 | 1.4 | |
| Max | 2.40 | 2.7 | 2.425 | 1.75 | 1.73 | 2.6 | 1.45 | |
| Mean Value | 2.38 | 2.67 | 2.43 | 1.73 | 1.72 | 2.57 | 1.43 | |
| Rosa canina L. (Fructus Rosae) | SD | 0.03 | 0.01 | 0.01 | 0 | 0.01 | 0.03 | 0.01 |
| Min | 0.10 | 0.1 | 0.15 | 0.05 | 0.05 | 0.1 | 0.05 | |
| Max | 0.15 | 0.125 | 0.175 | 0.051 | 0.075 | 0.15 | 0.075 | |
| Mean Value | 0.12 | 0.12 | 0.15 | 0.05 | 0.07 | 0.12 | 0.06 | |
| Rosa canina L. (Rosa impetus) | SD | 0.05 | 0.05 | 0.03 | 0.05 | 0.05 | 0.03 | 0.00 |
| Min | 0.60 | 0.8 | 0.8 | 0.55 | 0.4 | 0.075 | 0.05 | |
| Max | 0.70 | 0.9 | 0.85 | 0.6 | 0.5 | 0.125 | 0.05 | |
| Mean Value | 0.65 | 0.85 | 0.82 | 0.55 | 0.45 | 0.10 | 0.05 | |
| Rosa canina L. (Rosa ravageur) | SD | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.00 |
| Min | 0.05 | 0.075 | 0.1 | 0.05 | 0.05 | 0.05 | 0.05 | |
| Max | 0.08 | 0.1 | 0.125 | 0.075 | 0.075 | 0.075 | 0.05 | |
| Mean Value | 0.06 | 0.09 | 0.11 | 0.06 | 0.06 | 0.07 | 0.05 | |
| Sorbus aucuparia L. (Fructus) | SD | 0.03 | 0.02 | 0.03 | 0.05 | 0.03 | 0.03 | 0.01 |
| Min | 0.50 | 0.25 | 0.3 | 0.2 | 0.225 | 0.25 | 0.1 | |
| Max | 0.55 | 0.3 | 0.35 | 0.3 | 0.275 | 0.3 | 0.075 | |
| Mean Value | 0.53 | 0.28 | 0.32 | 0.26 | 0.25 | 0.28 | 0.08 | |
| Viburnum opulus L. (Fructus) | SD | 0.01 | 0.01 | 0.02 | 0.03 | 0.03 | 0.01 | 0.01 |
| Min | 0.20 | 0.3 | 0.3 | 0.15 | 0.15 | 0.2 | 0.1 | |
| Max | 0.18 | 0.31 | 0.25 | 0.175 | 0.15 | 0.225 | 0.075 | |
| Mean Value | 0.20 | 0.3 | 0.275 | 0.2 | 0.2 | 0.2 | 0.075 | |
| Hippophae rhamnoides L. (Fructus) | SD | 0.03 | 0.10 | 0.03 | 0.03 | 0.04 | 0.03 | 0.03 |
| Min | 0.50 | 1.75 | 1.2 | 0.85 | 0.8 | 1 | 0.45 | |
| Max | 0.50 | 1.8 | 1.25 | 0.9 | 0.725 | 1 | 0.4 | |
| Mean Value | 0.52 | 1.72 | 1.23 | 0.87 | 0.77 | 0.98 | 0.42 | |
| Hippophae rhamnoides L. (Folium) | SD | 0.03 | 0.05 | 0.03 | 0.03 | 0.03 | 0.05 | 0.06 |
| Min | 2.95 | 3.1 | 2.9 | 2.25 | 2.2 | 2.8 | 2 | |
| Max | 3.00 | 3.2 | 2.95 | 2.3 | 2.25 | 2.9 | 2.1 | |
| Mean Value | 2.97 | 3.15 | 2.92 | 2.27 | 2.23 | 2.85 | 2.07 | |
| Hippophae rhamnoides L. (pomace) | SD | 1.47 | 1.55 | 1.44 | 1.12 | 1.10 | 1.40 | 1.01 |
| Min | 2.90 | 3.1 | 2.9 | 2.2 | 2.25 | 2.85 | 2.15 | |
| Max | 3.00 | 3.2 | 2.95 | 2.25 | 2.3 | 2.85 | 2.25 | |
| Mean Value | 2.95 | 3.15 | 2.90 | 2.23 | 2.27 | 2.83 | 2.18 | |
| Variable | p-Value | F-Value | [%] Expl. |
|---|---|---|---|
| Listeria innocua | 46.848 | 0.001 | 41.74 |
| Escherichia coli | 4.243 | 0.002 | 12.41 |
| Proteus mirabilis | 3.315 | 0.020 | 5.56 |
| Pseudomonas fluorescens | 3.487 | 0.011 | 6.53 |
| Salmonella enteritidis | 4.313 | 0.014 | 10.41 |
| Analyzed Phenolic Compounds | Retention Times [min] | Recovery Rates [%] |
|---|---|---|
| aempferol | 6.11 | 86 ± 5.3 |
| gallic acid | 8.85 | 92 ± 4.4 |
| vanillic | 9.71 | 79 ± 8.5 |
| luteolin | 11.89 | 96 ± 2.7 |
| protocatechuic acid | 12.23 | 90 ± 4.8 |
| vanillin acid | 14.19 | 88 ± 5.1 |
| apigenin | 16.43 | 93 ± 3.8 |
| catechin | 18.09 | 89 ± 5.7 |
| 4-hydroxybenzoic acid | 19.46 | 96 ± 3.78 |
| chlorogenic acid | 21.56 | 92 ± 2.8 |
| caffeic acid | 26.19 | 86 ± 6.7 |
| syringic acid | 28.05 | 94 ± 3.9 |
| naringenin | 31.22 | 88 ± 4.8 |
| vitexin | 35.41 | 95 ± 3.8 |
| rutin | 38.11 | 93 ± 4.9 |
| quercetin | 39.58 | 97 ± 1.9 |
| p-coumaric acid | 40.20 | 89 ± 3.6 |
| ferulic acid | 46.20 | 91 ± 4.9 |
| sinapic acid | 48.00 | 94 ± 5.1 |
| t-cinnamic acid | 52.40 | 97 ± 2.9 |
| Species | Designation | Plant Material | Designation |
|---|---|---|---|
| Bitter melon fruits (Momordica charantia L.) | A | Fruit | 1 |
| Elderberry (Sambucus nigra L.) | B | Flower | 2 |
| Beach rose (Rosa rugosa Thunb.) | C | Leaves | 3 |
| Rowan fruits (Sorbus aucuparia L.) | D | Pulp | 4 |
| Guelder rose fruits (Viburnum opulus L.) | E | Pips | 5 |
| Sea buckthorn fruits (Hippophaë rhamnoides L.) | F | Pomace | 6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stuper-Szablewska, K.; Szablewski, T.; Przybylska-Balcerek, A.; Szwajkowska-Michałek, L.; Krzyżaniak, M.; Świerk, D.; Cegielska-Radziejewska, R.; Krejpcio, Z. Antimicrobial Activities Evaluation and Phytochemical Screening of Some Selected Plant Materials Used in Traditional Medicine. Molecules 2023, 28, 244. https://doi.org/10.3390/molecules28010244
Stuper-Szablewska K, Szablewski T, Przybylska-Balcerek A, Szwajkowska-Michałek L, Krzyżaniak M, Świerk D, Cegielska-Radziejewska R, Krejpcio Z. Antimicrobial Activities Evaluation and Phytochemical Screening of Some Selected Plant Materials Used in Traditional Medicine. Molecules. 2023; 28(1):244. https://doi.org/10.3390/molecules28010244
Chicago/Turabian StyleStuper-Szablewska, Kinga, Tomasz Szablewski, Anna Przybylska-Balcerek, Lidia Szwajkowska-Michałek, Michał Krzyżaniak, Dariusz Świerk, Renata Cegielska-Radziejewska, and Zbigniew Krejpcio. 2023. "Antimicrobial Activities Evaluation and Phytochemical Screening of Some Selected Plant Materials Used in Traditional Medicine" Molecules 28, no. 1: 244. https://doi.org/10.3390/molecules28010244
APA StyleStuper-Szablewska, K., Szablewski, T., Przybylska-Balcerek, A., Szwajkowska-Michałek, L., Krzyżaniak, M., Świerk, D., Cegielska-Radziejewska, R., & Krejpcio, Z. (2023). Antimicrobial Activities Evaluation and Phytochemical Screening of Some Selected Plant Materials Used in Traditional Medicine. Molecules, 28(1), 244. https://doi.org/10.3390/molecules28010244








