Novel MCM-41 Supported Dicationic Imidazolium Ionic Liquids Catalyzed Greener and Efficient Regioselective Synthesis of 2-Oxazolidinones from Aziridines and Carbon Dioxide
Abstract
:1. Introduction
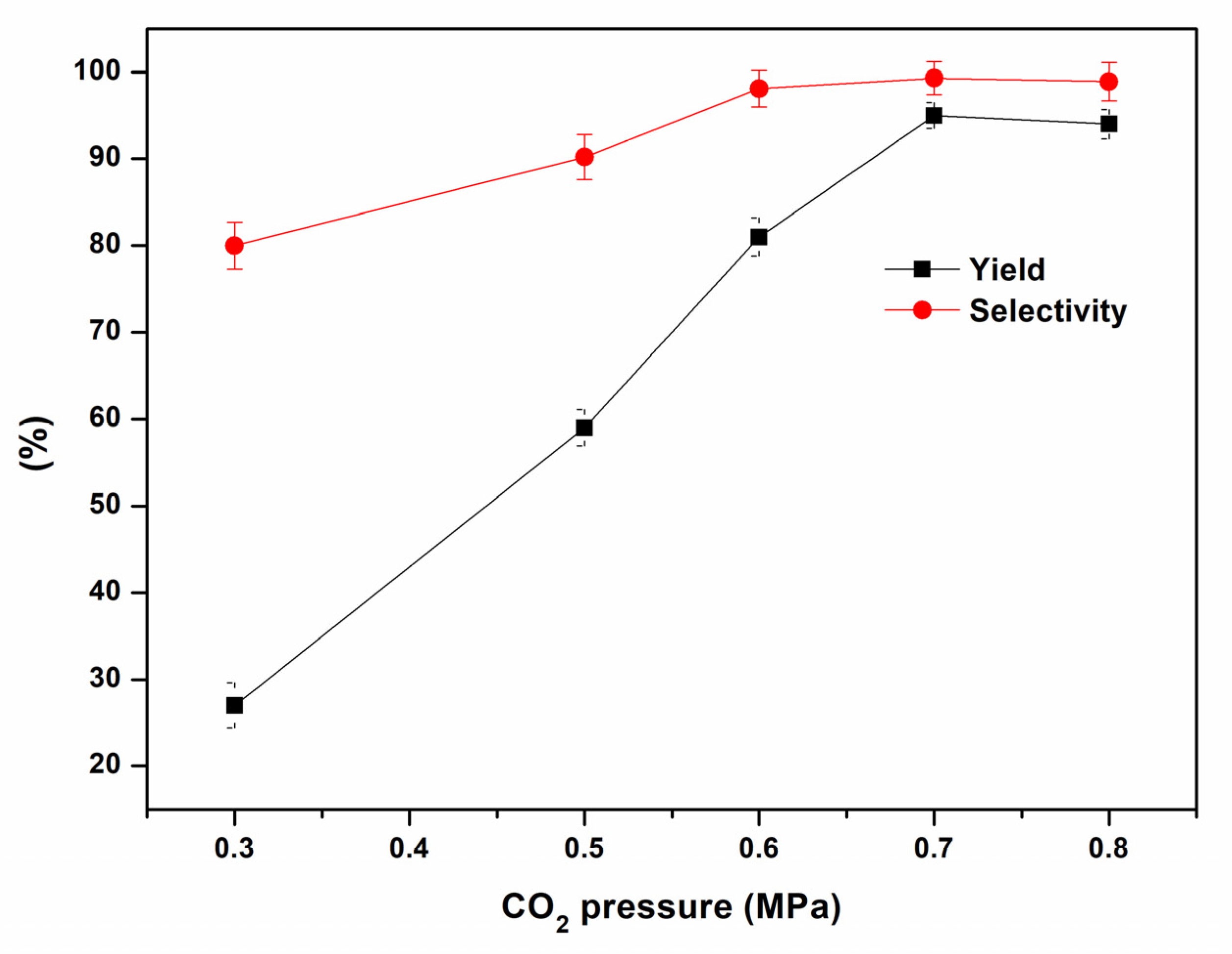
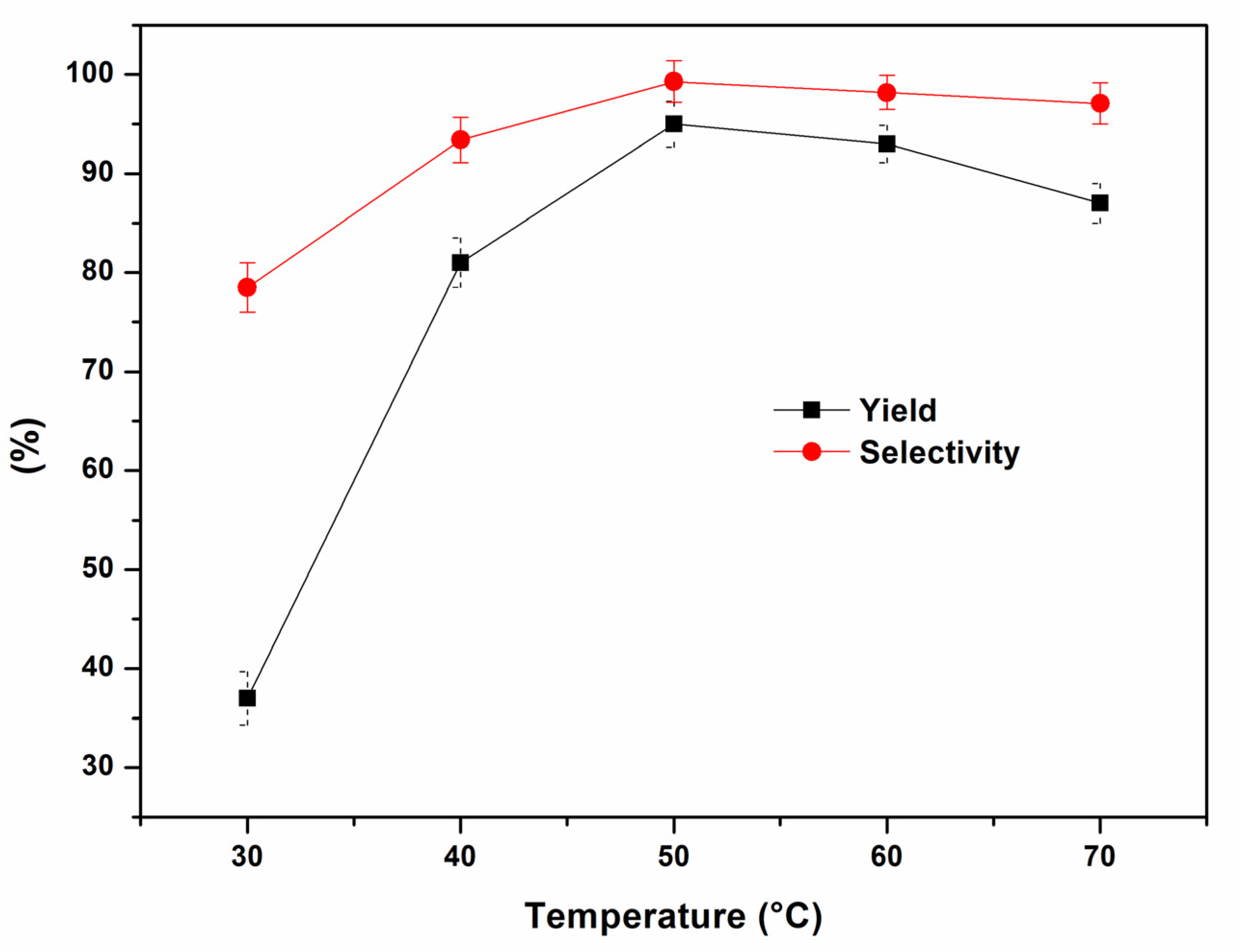
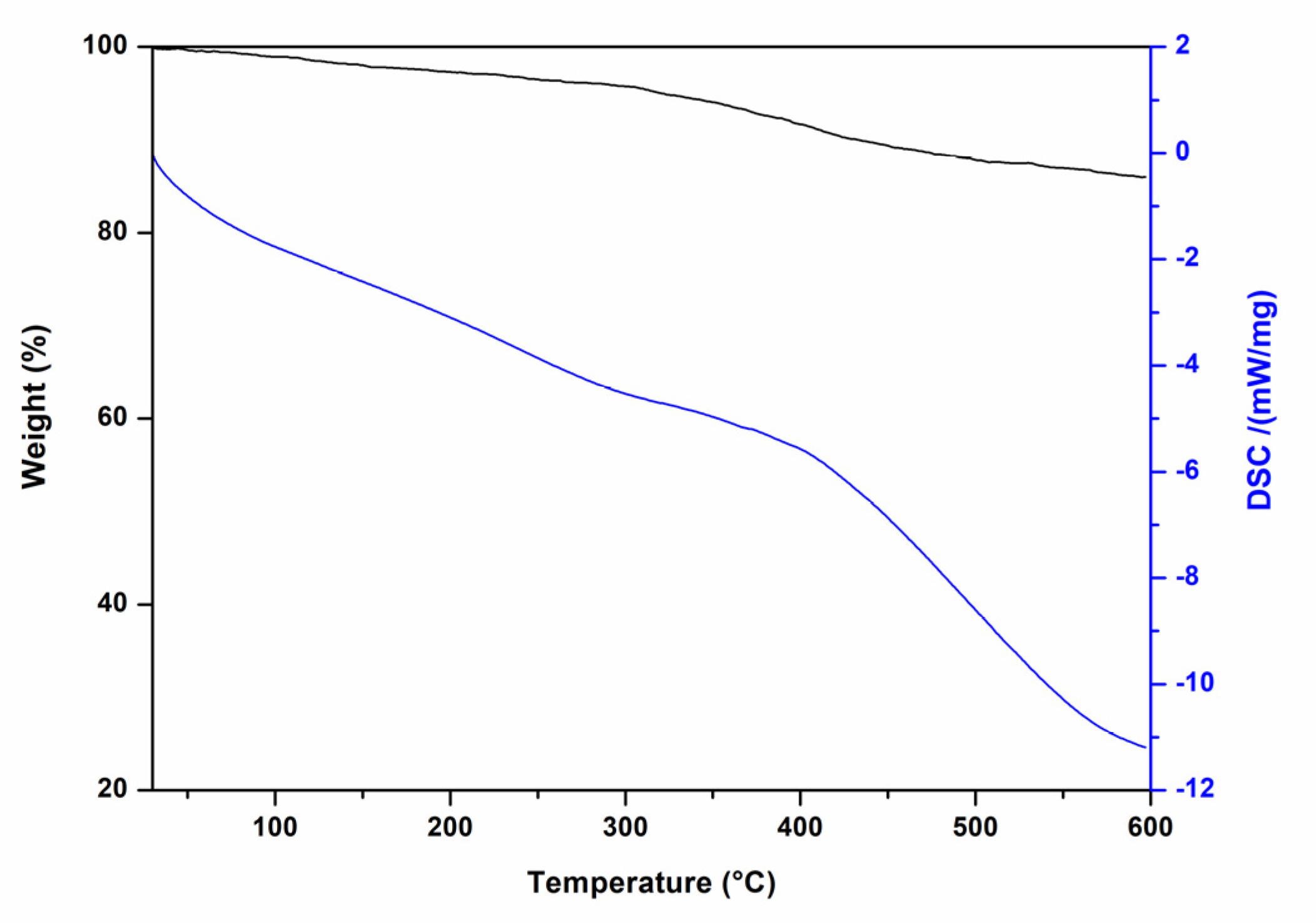
2. Results and Discussion
3. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Frogneux, X.; Blondiaux, E.; Thuéry, P.; Cantat, T. Bridging Amines with CO2: Organocatalyzed Reduction of CO2 to Aminals. ACS Catal. 2015, 5, 3983–3987. [Google Scholar] [CrossRef]
- Multilayer graphtriyne membranes for separation and storage of CO2: Molecular dynamics simulations of post-combustion model mixtures. Molecules 2022, 27, 5958. [CrossRef] [PubMed]
- Senila, L.; Scurtu, D.A.; Kovacs, E.; Levei, E.A.; Cadar, O.; Becze, A.; Varaticeanu, C. High-pressure supercritical CO2 pretreatment of apple orchard waste for carbohydrates production using response surface methodology and method uncertainty evaluation. Molecules 2022, 27, 7783. [Google Scholar] [CrossRef] [PubMed]
- Karapinar, D.; Creissen, C.E.; de la Cruz, J.G.R.; Schreiber, M.W.; Fontecave, M. Electrochemical CO2 reduction to ethanol with copper-based catalysts. ACS Energy Lett. 2021, 6, 694–706. [Google Scholar] [CrossRef]
- Pulla, S.; Felton, C.M.; Gartia, Y.; Ramidi, P.; Ghosh, A. Synthesis of 2-oxazolidinones by direct condensation of 2-aminoalcohols with carbon dioxide using chlorostannoxanes. ACS Sustain. Chem. Eng. 2013, 1, 309–312. [Google Scholar] [CrossRef]
- Chen, J.M.; Qi, L.; Zhang, L.; Li, L.J.; Hou, C.Y.; Li, W.; Wang, L.J. Copper/DTBP-promoted oxyselenation of propargylic amines with diselenides and CO2: Synthesis of selenyl 2-oxazolidinones. J. Org. Chem. 2020, 85, 10924–10933. [Google Scholar] [CrossRef]
- Brunel, P.; Monot, J.; Kefalidis, C.E.; Maron, L.; Martin-Vaca, B.; Bourissou, D. Valorization of CO2: Preparation of 2-oxazolidinones by metal / ligand cooperative catalysis with SCS indenediide Pd complexes. ACS Catal. 2017, 7, 2652–2660. [Google Scholar] [CrossRef]
- Sinast, M.; Zuccolo, M.; Wischnat, J.; Sube, T.; Hasnik, F.; Baro, A.; Dallavalle, S.; Laschat, S. Samarium iodide-promoted asymmetric reformatsky reaction of 3-(2-haloacyl)-2-oxazolidinones with enals. J. Org. Chem. 2019, 84, 10050–10064. [Google Scholar] [CrossRef]
- Wu, Y.; Liu, G. Organocatalyzed cycloaddition of carbon dioxide to aziridines. Tetrahedron Lett. 2011, 52, 6450–6452. [Google Scholar] [CrossRef]
- Liu, H.; Hua, R. Conversion of carbon dioxide into 2-oxazolidinones and 2(3H)-oxazolones catalyzed by 2,2′,2″-terpyridine. Tetrahedron 2016, 72, 1200–1204. [Google Scholar] [CrossRef]
- Sonzini, P.; Berthet, N.; Damiano, C.; Dufaud, V.; Gallo, E. A metal-free porphyrin heterogenised onto SBA-15 silica: A performant material for the CO2 cycloaddition to epoxides and aziridines. J. Catal. 2022, 414, 143–154. [Google Scholar] [CrossRef]
- Li, Y.N.; Xu, Q.N.; Wu, L.F.; Guo, Y.H.; Yue, H.; Zhou, J.; Ge, C.L.; Chang, H.R. Water-promoted selective cycloaddition of CO2 and aziridine in confined nanospaces of hierarchical porous silica: Synergetic effect of chemical function and physical microenvironment. J. Environ. Chem. Eng. 2021, 9, 105607. [Google Scholar] [CrossRef]
- Damiano, C.; Sonzini, P.; Cavalleri, M.; Manca, G.; Gallo, E. The CO2 cycloaddition to epoxides and aziridines promoted by porphyrin-based catalysts. Inorg. Chim. Acta 2022, 540, 121065. [Google Scholar] [CrossRef]
- Miller, A.W.; Nguyen, S.T. (Salen)chromium(III)/DMAP: An efficient catalyst system for the selective synthesis of 5-substituted oxazolidinones from carbon dioxide and aziridines. Org. Lett. 2004, 6, 2301–2304. [Google Scholar] [CrossRef]
- Bresciani, G.; Zacchini, S.; Marchetti, F.; Pampaloni, G. Non-precious metal carbamates as catalysts for the aziridine/CO2 coupling reaction under mild conditions. Dalton Trans. 2021, 50, 5351–5359. [Google Scholar] [CrossRef]
- Saptal, V.; Shinde, D.B.; Banerjee, R.; Bhanage, B.M. State-of-the-Art catechol porphyrin COF catalyst for chemical fixation of carbon dioxide via cyclic carbonates and oxazolidinones. Catal. Sci. Technol. 2016, 6, 6152–6158. [Google Scholar] [CrossRef]
- Tian, X.R.; Shi, Y.; Hou, S.L.; Ma, Y.; Zhao, B. Efficient Cycloaddition of CO2 and Aziridines Activated by a Quadruple-Interpenetrated Indium–Organic Framework as a Recyclable Catalyst. Inorg. Chem. 2021, 60, 15383–15389. [Google Scholar] [CrossRef]
- Cao, C.S.; Shi, Y.; Xu, H.; Zhao, B. A multifunctional MOFs as recyclable catalyst for fixation of CO2 with aziridines or epoxides and luminescent probe of Cr(VI). Dalton Trans. 2018, 47, 4545–4553. [Google Scholar] [CrossRef]
- Hu, T.; Ding, Y. Mechanism for CO2 fixation with aziridines synergistically catalyzed by HKUST-1 and TBAB: A DFT study. Organometallics 2020, 39, 505–515. [Google Scholar] [CrossRef]
- Du, Y.; Wu, Y.; Liu, A.H.; He, L.N. Quaternary ammonium bromide functionalized polyethylene glycol: A highly efficient and recyclable catalyst for selective synthesis of 5-aryl-2-oxazolidinones from carbon dioxide and aziridines under solvent-free conditions. J. Org. Chem. 2008, 73, 4709–4712. [Google Scholar] [CrossRef]
- Morán-Ramallal, R.; Liz, R.; Gotor, V. Regioselective and stereospecific synthesis of enantiopure 1,3-oxazolidin-2-ones by intramolecular ring opening of 2-(boc-aminomethyl)aziridines. Preparation of the antibiotic linezolid. Org. Lett. 2008, 10, 1935–1938. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Lu, C.; Zhao, B.; Wang, Q.; Yao, Y. Cycloaddition of aziridine with CO2/CS2 catalyzed by amidato divalent lanthanide complexes. J. Org. Chem. 2019, 84, 1951–1958. [Google Scholar] [CrossRef] [PubMed]
- Bresciani, G.; Bortoluzzi, M.; Pampaloni, G.; Marchetti, F. Diethylammonium iodide as catalyst for the metal-free synthesis of 5-aryl-2-oxazolidinones from aziridines and carbon dioxide. Org. Biomol. Chem. 2021, 19, 4152–4161. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.J.; Lu, X.M. Ionic Liquids: From Fundamental Research to Industrial Applications; Science Press: Beijing, China, 2006. [Google Scholar]
- Lozano, P. Sustainable Catalysis in Ionic Liquids; CRC Press: Boca Raton, FL, USA, 2018. [Google Scholar]
- Goossens, K.; Lava, K.; Bielawski, C.W.; Binnemans, K. Ionic liquid crystals: Versatile materials. Chem. Rev. 2016, 116, 4643–4807. [Google Scholar] [CrossRef] [PubMed]
- Pflieger, R.; Lejeune, M.; Draye, M. Sonoluminescence spectra in the first tens of seconds of sonolysis of [BEPip][NTf2], at 20 kHz under Ar. Molecules 2022, 27, 6050. [Google Scholar] [CrossRef]
- Pham-Truong, T.N.; Ghilane, J. Investigating localized electrochemical of ferrocenyl-imidazolium in ionic liquid usingcanning electrochemical microscopy configuration. Molecules 2022, 27, 6004. [Google Scholar] [CrossRef]
- Yang, Z.Z.; Li, Y.N.; Wei, Y.Y.; He, L.N. Protic onium salts-catalyzed synthesis of 5-aryl-2-oxazolidinones from aziridines and CO2 under mild conditions. Green Chem. 2011, 13, 2351–2353. [Google Scholar] [CrossRef]
- Chen, Y.; Luo, R.; Yang, Z.; Zhou, X.; Ji, H. Imidazolium-based ionic liquids decorated zinc porphyrin catalyst for converting CO2 into five-membered heterocyclic molecules. Sustain. Energy Fuels 2018, 2, 125–132. [Google Scholar] [CrossRef]
- Virtanen, P.; Salminen, E.; Mikkola, J.P. Modeling of supported ionic liquid catalysts systems—From idea to applications. Ind. Eng. Chem. Res. 2017, 56, 12852–12862. [Google Scholar] [CrossRef]
- Sharma, J.; Kumar, P.; Sillanpaa, M.; Kumar, D.; Nemiwal, M. Immobilized ionic liquids on Fe3O4 nanoparticles: A potential catalyst for organic synthesis. Inorg. Chem. Commun. 2022, 145, 110055. [Google Scholar] [CrossRef]
- Gholinejad, M.; Zareh, F.; Sheibani, H.; Nájera, C.; Yus, M. Magnetic ionic liquids as catalysts in organic reactions. J. Mol. Liq. 2022, 367, 120395. [Google Scholar] [CrossRef]
- Baimoldina, A.; Yang, F.; Kolla, K.; Altemose, P.; Wang, B.; Clifford, C.; Kowall, C.; Li, L. Separating miscible liquid–liquid mixtures using supported ionic liquid membranes. Ind. Eng. Chem. Res. 2022, 61, 747–753. [Google Scholar] [CrossRef]
- Chong, S.Y.; Wang, T.T.; Cheng, L.C.; Lv, H.Y.; Ji, M. Metal-organic framework MIL-101-NH2 supported acetatebased butylimidazolium ionic liquid as a highly efficient heterogeneous catalyst for the synthesis of 3-aryl-2-oxazolidinones. Langmuir 2019, 35, 495–503. [Google Scholar] [CrossRef] [PubMed]
- Tamanoi, F. Mesoporous Silica-Based Nanomaterials and Biomedical Applications—Part A. The Enzymes; Elsevier: Amsterdam, The Netherlands, 2018. [Google Scholar]
- Sun, L.B.; Liu, X.Q.; Zhou, H.C. Design and fabrication of mesoporous heterogeneous basic catalysts. Chem. Soc. Rev. 2015, 44, 5092–5147. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Williams, C.T. Recent advances in the applications of mesoporous silica in heterogeneous catalysis. Catal. Sci. Technol. 2022, 12, 5765–5794. [Google Scholar] [CrossRef]
- Pal, N.; Bhaumik, A. Mesoporous material: A versatile support in heterogeneous catalysis for the liquid phase catalytic transformations. RSC Adv. 2015, 5, 24363–24391. [Google Scholar] [CrossRef]
- la Torre, C.; Gavara, R.; García-Fernández, A.; Mikhaylov, M.; Sokolov, M.N.; Miravet, J.F.; Sancenón, F.; Martínez-Máñez, R.; Galindo, F. Enhancement of photoactivity and cellular uptake of (Bu4N)2[Mo6I8(CH3COO)6] complex by loading on porous MCM-41 support. Photodynamic studies as an anticancer agent. Biomater. Adv. 2022, 140, 213057. [Google Scholar] [CrossRef]
- Vangeli, O.C.; Romanos, G.E.; Beltsios, K.G.; Fokas, D.; Kouvelos, E.P.; Stefanopoulos, K.L.; Kanellopoulos, N.K. Grafting of imidazolium based ionic liquid on the pore surface of nanoporous materialss study of physicochemical and thermodynamic properties. J. Phys. Chem. B 2010, 114, 6480–6491. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Zhang, J.; Zou, X.; Dong, H.; Yao, P. Selective oxidation of styrene to 1,2-epoxyethylbenzene by hydrogen peroxide over heterogeneous phosphomolybdic acid supported on ionic liquid modified MCM-41. Chem. Eng. J. 2015, 260, 172–177. [Google Scholar] [CrossRef]
- Fehrmann, R.; Riisager, A.; Haumann, M. Supported Ionic Liquids: Fundamentals and Applications; Wiley-VCH: Weinheim, Germany, 2014. [Google Scholar]
- Kang, M.; Jin, F.; Li, Z.; Song, H.; Chen, J. Research and application of supported ionic liquids. Prog. Chem. 2020, 32, 1274–1293. [Google Scholar] [CrossRef]
- Muniandy, L.; Adam, F.; Rahman, N.R.A.; Ng, E.P. Highly selective synthesis of cyclic carbonates via solvent free cycloaddition of CO2 and epoxides using ionic liquid grafted on rice husk derived MCM-41 Inorg. Chem. Commun. 2019, 104, 1–7. [Google Scholar] [CrossRef]
- Li, X.; Zhang, L.; Zheng, Y.; Zheng, C. SO2 absorption performance enhancement by ionic liquid supported on mesoporous molecular sieve. Energy Fuel 2015, 29, 942–953. [Google Scholar] [CrossRef]
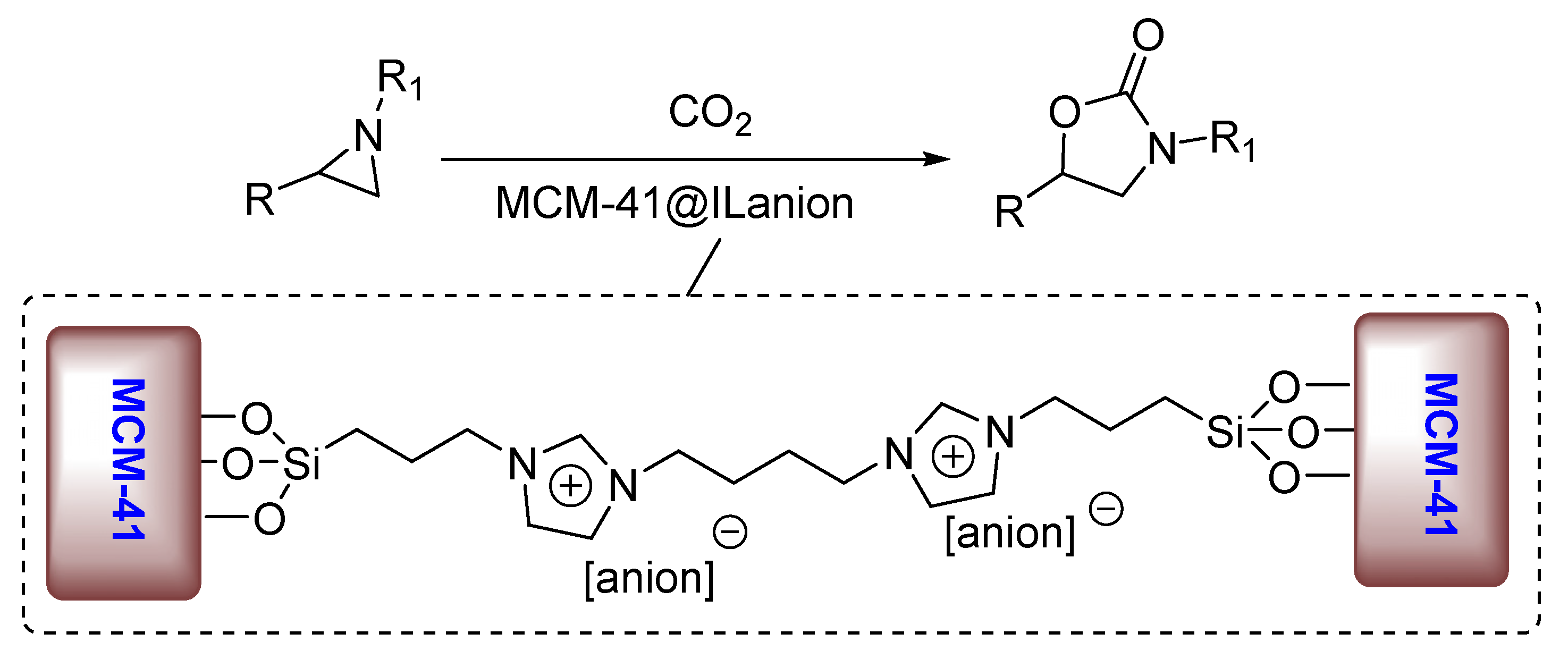

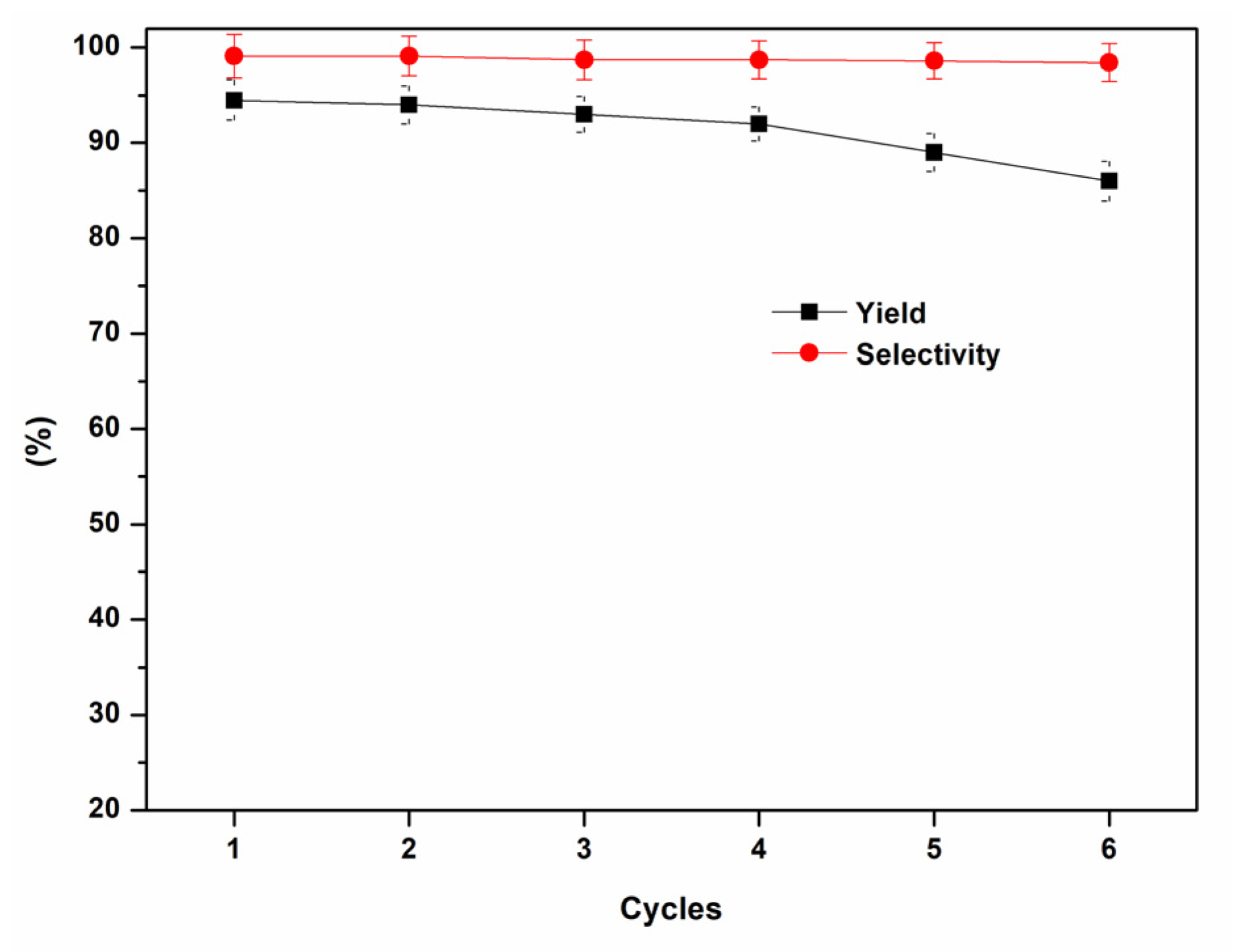
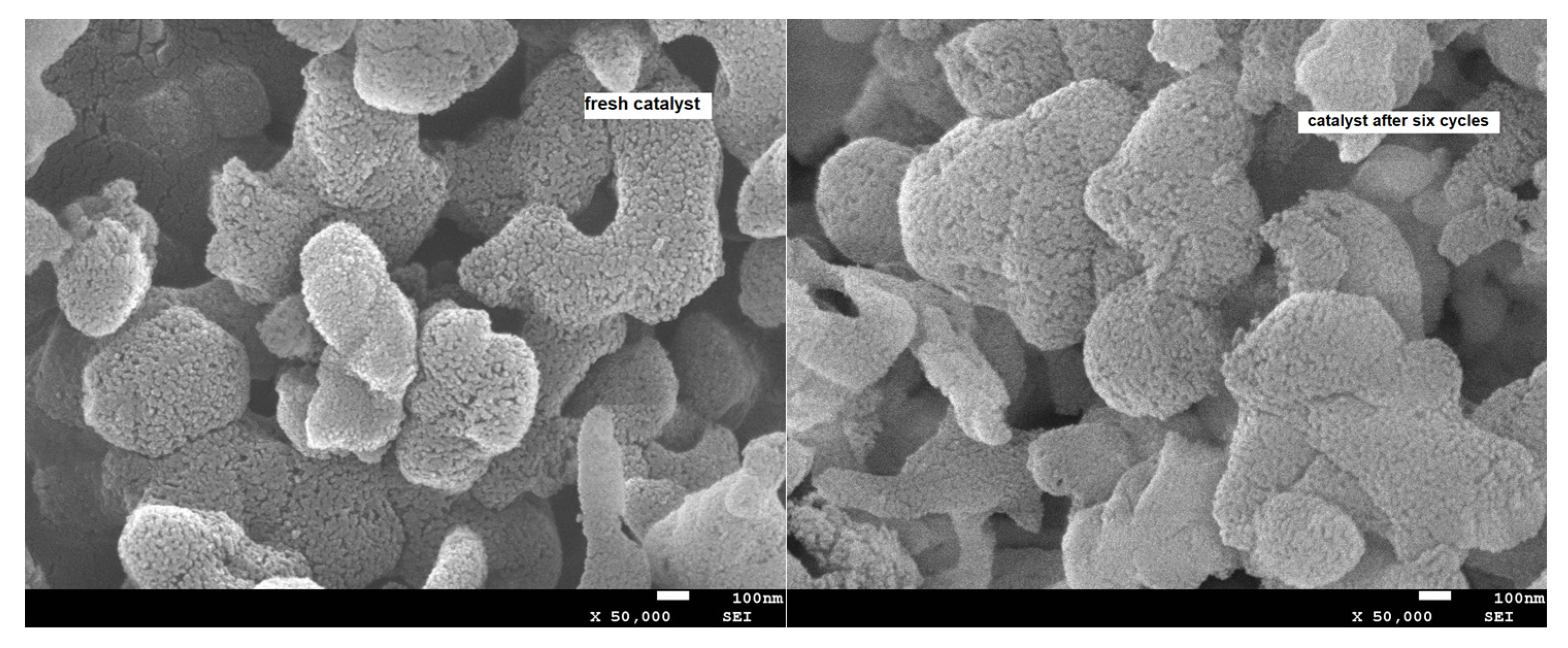

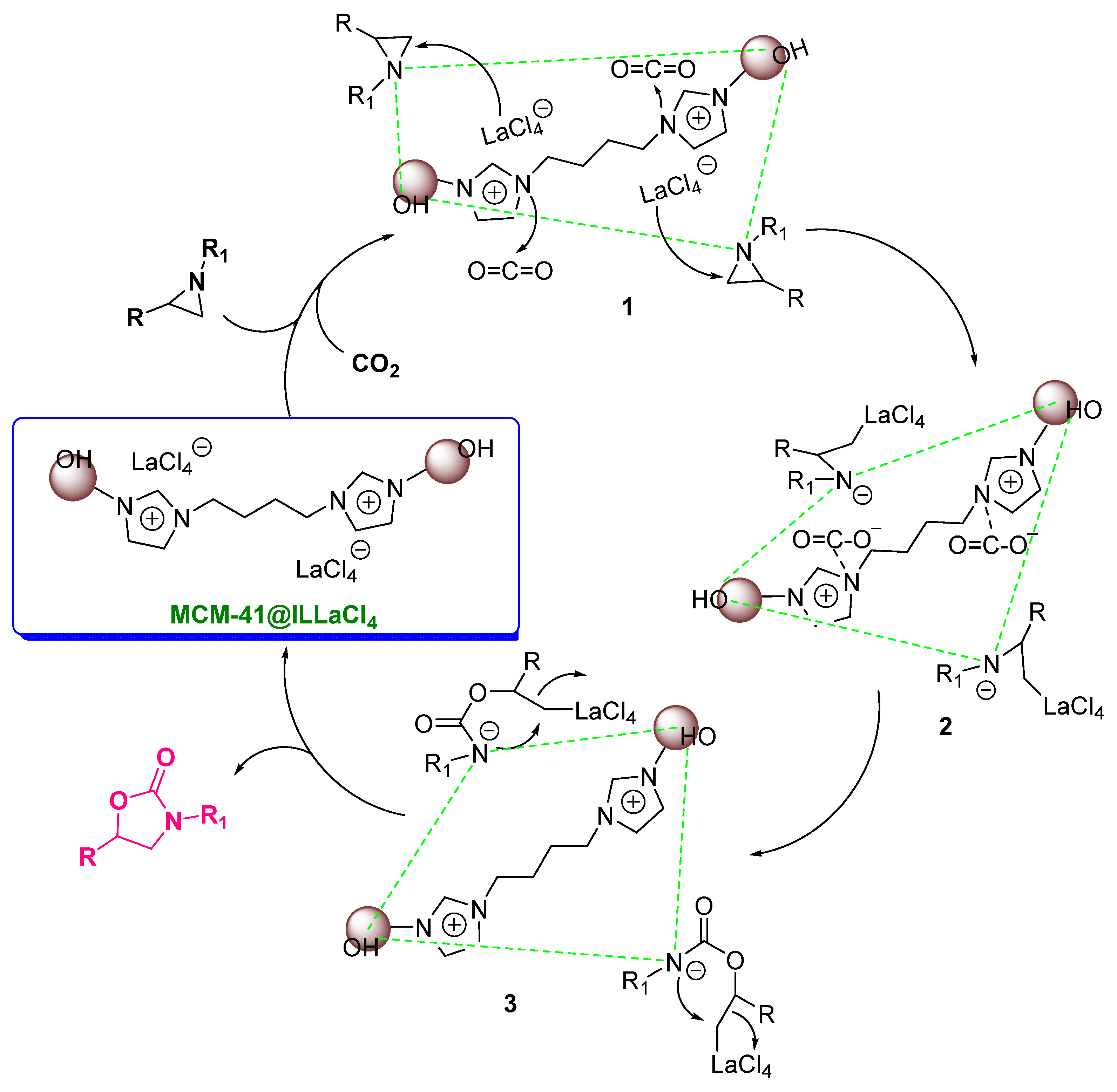
| Entry | Aziridine | Product | Time (h) | Yield (%) b | Selectivity (%) c |
|---|---|---|---|---|---|
| 1 | 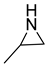 | 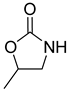 | 3 | 95 | 99.3 |
| 2 |  | 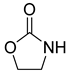 | 3 | 97 | 99.8 |
| 3 |  | 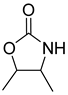 | 3 | 92 | 99.5 |
| 4 |  | 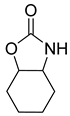 | 3 | 90 | 99.6 |
| 5 | 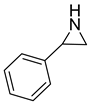 | 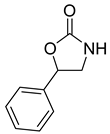 | 3 | 94 | 99.2 |
| 6 | 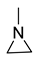 | 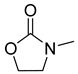 | 5 | 87 | 99 |
| 7 | 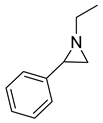 | 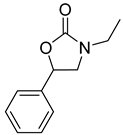 | 5 | 85 | 99.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, Y.; Yang, L.; Liu, X. Novel MCM-41 Supported Dicationic Imidazolium Ionic Liquids Catalyzed Greener and Efficient Regioselective Synthesis of 2-Oxazolidinones from Aziridines and Carbon Dioxide. Molecules 2023, 28, 242. https://doi.org/10.3390/molecules28010242
Hu Y, Yang L, Liu X. Novel MCM-41 Supported Dicationic Imidazolium Ionic Liquids Catalyzed Greener and Efficient Regioselective Synthesis of 2-Oxazolidinones from Aziridines and Carbon Dioxide. Molecules. 2023; 28(1):242. https://doi.org/10.3390/molecules28010242
Chicago/Turabian StyleHu, Yulin, Lili Yang, and Xiaobing Liu. 2023. "Novel MCM-41 Supported Dicationic Imidazolium Ionic Liquids Catalyzed Greener and Efficient Regioselective Synthesis of 2-Oxazolidinones from Aziridines and Carbon Dioxide" Molecules 28, no. 1: 242. https://doi.org/10.3390/molecules28010242
APA StyleHu, Y., Yang, L., & Liu, X. (2023). Novel MCM-41 Supported Dicationic Imidazolium Ionic Liquids Catalyzed Greener and Efficient Regioselective Synthesis of 2-Oxazolidinones from Aziridines and Carbon Dioxide. Molecules, 28(1), 242. https://doi.org/10.3390/molecules28010242







