Regiospecific Photochemical Synthesis of Methylchrysenes
Abstract
1. Introduction
2. Results and Discussion
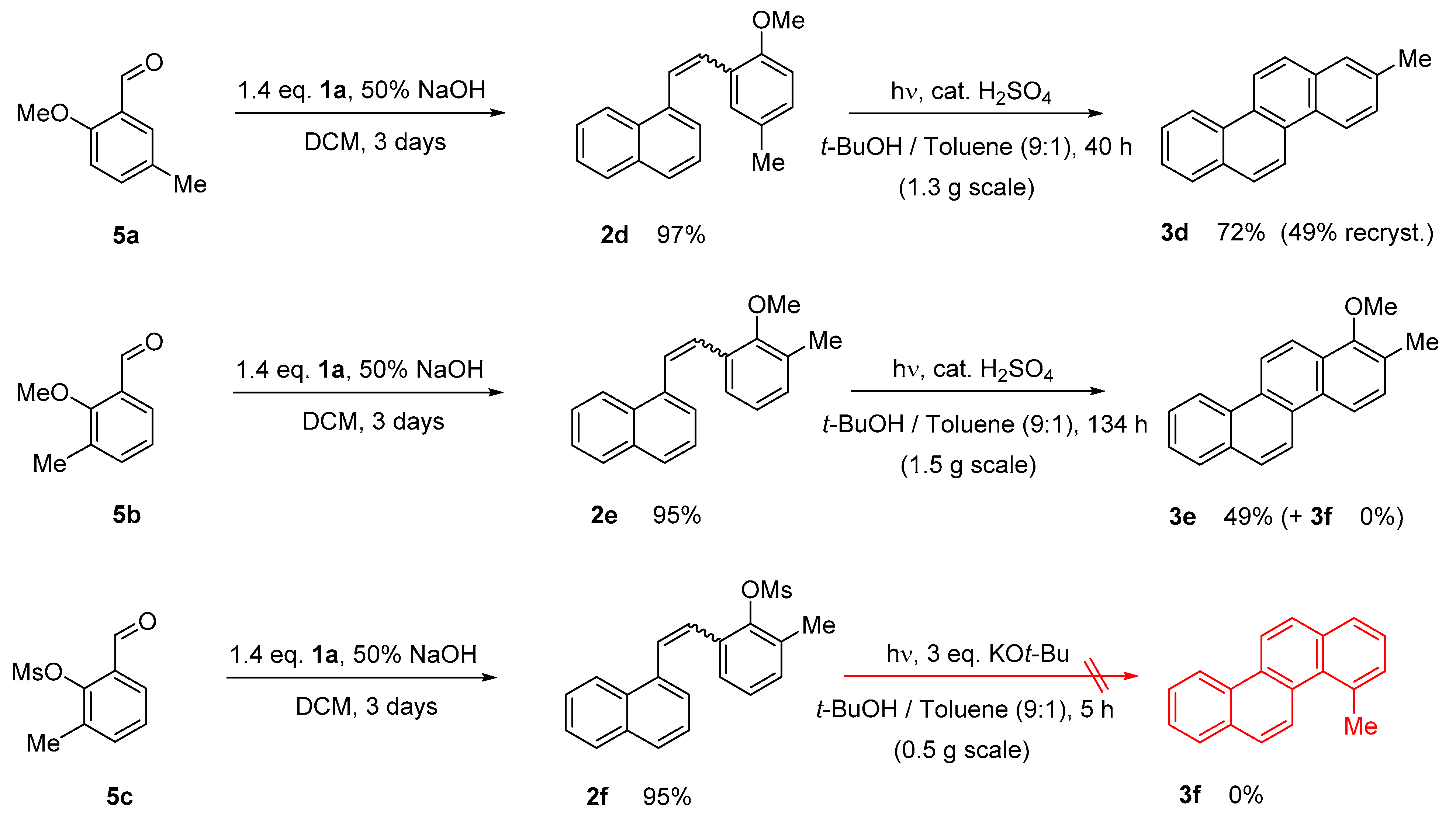
2.1. Photochemical Cyclization Using Stoichiometric Amount of I2
2.2. Photochemical Cyclization under Eliminative Conditions
2.3. Direct Oxidation of Methylchrysene 3b
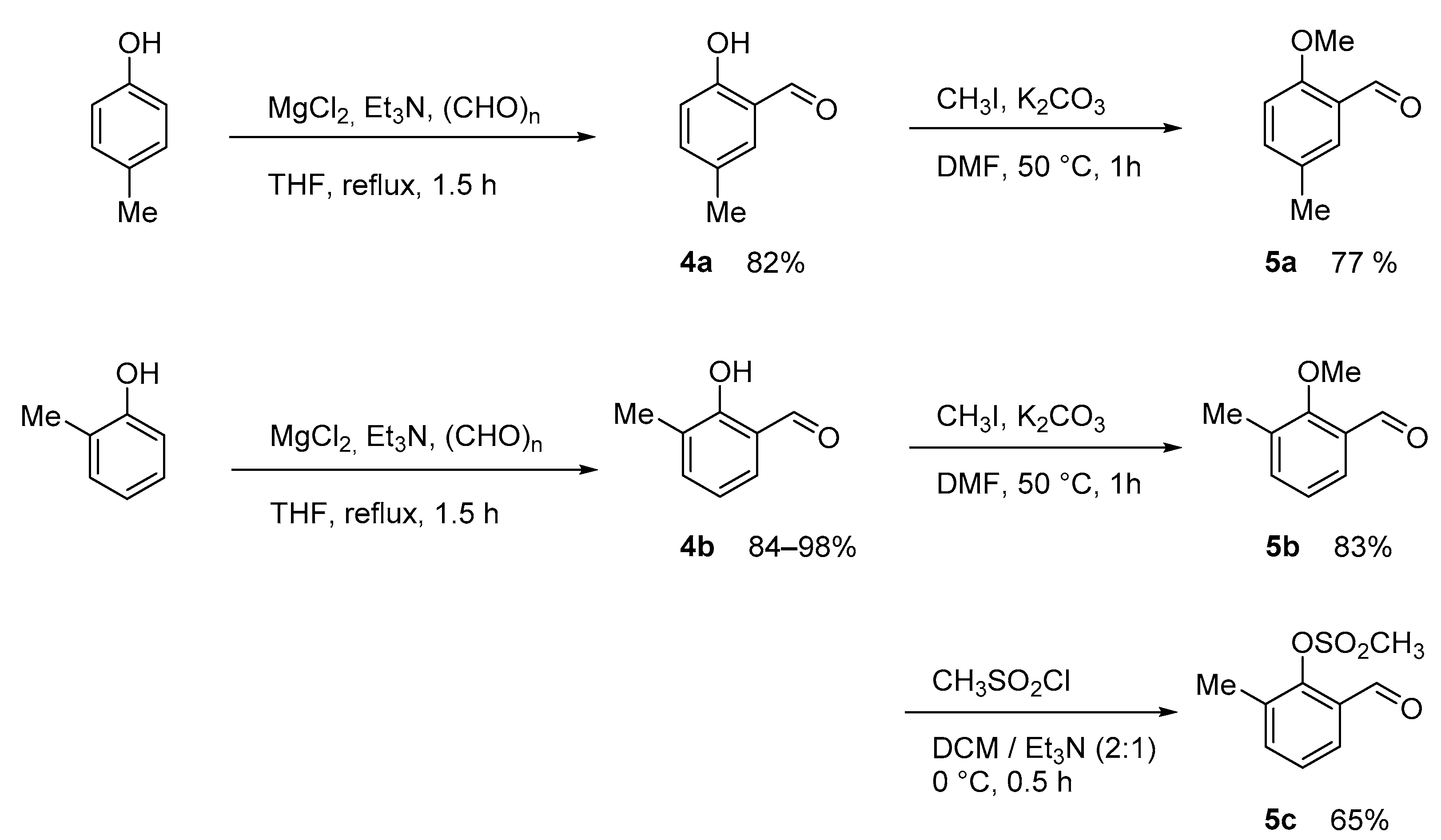
3. Materials and Methods
3.1. General Information
3.2. Synthesis
3.2.1. Synthesis of Wittig-Reagents
3.2.2. Synthesis of Stilbenes
3.2.3. Photochemical cyclization of stilbenes
3.2.4. Formylation of phenoles
3.2.5. Protection of Hydroxybenzaldehydes
3.2.6. Oxidation of Methylchrysene
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Pampanin, D.M.; Sydnes, M.O. Polycyclic aromatic hydrocarbons a constituent of petroleum: Presence and influence in the aquatic environment. Hydrocarbon 2013, 5, 83–118. [Google Scholar] [CrossRef][Green Version]
- Baird, S.J.S.; Bailey, E.A.; Vorhees, D.J. Evaluating human risk from exposure to alkylated PAHs in an aquatic system. Hum. Ecol. Risk Assess. 2007, 13, 322–338. [Google Scholar] [CrossRef]
- Yang, C.; Zhang, G.; Wang, Z.; Yang, Z.; Hollebone, B.; Landriault, M.; Shah, K.; Brown, C.E. Development of a methodology for accurate quantitation of alkylated polycyclic aromatic hydrocarbons in petroleum and oil contaminated environmental samples. Anal. Methods 2014, 6, 7760–7771. [Google Scholar] [CrossRef]
- Hawthorne, S.B.; Miller, D.J.; Kreitinger, J.P. Measurement of total polycyclic aromatic hydrocarbon concentrations in sediments and toxic units used for estimating risk to benthic invertebrates at manufactured gas plant sites. Environ. Toxicol. Chem. 2006, 25, 287–296. [Google Scholar] [CrossRef]
- Soerhus, E.; Donald, C.E.; da Silva, D.; Thorsen, A.; Karlsen, O.; Meier, S. Untangling mechanisms of crude oil toxicity: Linking gene expression, morphology and PAHs at two developmental stages in a cold-water fish. Sci. Total Environ. 2021, 757, 143896. [Google Scholar] [CrossRef]
- Hodson, P.V.; Qureshi, K.; Noble, C.A.J.; Akhtar, P.; Brown, R.S. Inhibition of CYP1A enzymes by α-naphthoflavone causes both synergism and antagonism of retene toxicity to rainbow trout (Oncorhynchus mykiss). Aquat. Toxicol. 2007, 81, 275–285. [Google Scholar] [CrossRef]
- Lin, H.; Morandi, G.D.; Brown, R.S.; Snieckus, V.; Rantanen, T.; Joergensen, K.B.; Hodson, P.V. Quantitative structure-activity relationships for chronic toxicity of alkyl-chrysenes and alkyl-benz[a]anthracenes to Japanese medaka embryos (Oryzias latipes). Aquat. Toxicol. 2015, 159, 109–118. [Google Scholar] [CrossRef]
- Lille-Langoey, R.; Joergensen, K.B.; Goksoeyr, A.; Pampanin, D.M.; Sydnes, M.O.; Karlsen, O.A. Substituted Two- to Five-Ring Polycyclic Aromatic Compounds Are Potent Agonists of Atlantic Cod (Gadus morhua) Aryl Hydrocarbon Receptors Ahr1a and Ahr2a. Environ. Sci. Technol. 2021, 55, 15123–15135. [Google Scholar] [CrossRef]
- Malmquist, L.M.V.; Selck, H.; Joergensen, K.B.; Christensen, J.H. Polycyclic Aromatic Acids Are Primary Metabolites of Alkyl-PAHs-A Case Study with Nereis diversicolor. Environ. Sci. Technol. 2015, 49, 5713–5721. [Google Scholar] [CrossRef]
- Davies, W.; Wilmshurst, J.R. Synthesis of polycyclic aromatic hydrocarbons. IV. Convenient synthesis of chrysene and three methylcrysenes. J. Chem. Soc. 1961, 4079–4082. [Google Scholar] [CrossRef]
- Paul, S.; Jana, R.; Ray, J.K. Palladium-catalyzed intramolecular C-H activation: A synthetic approach towards polycyclic aromatic hydrocarbons. Synlett 2010, 10, 1463–1468. [Google Scholar] [CrossRef]
- Mallory, F.B.; Mallory, C.W. Photocyclization of stilbenes and related molecules. Org. React. 1984, 30, 1–456. [Google Scholar] [CrossRef]
- Joergensen, K.B. Photochemical oxidative cyclisation of stilbenes and Stilbenoids—The Mallory-reaction. Molecules 2010, 15, 4334–4358. [Google Scholar] [CrossRef] [PubMed]
- Mallory, F.B.; Wood, C.S.; Gordon, J.T. Photochemistry of Stilbenes. III. Some Aspects of the Mechanism of Photocyclization to Phenanthrenes. J. Am. Chem. Soc. 1964, 86, 3094–3102. [Google Scholar] [CrossRef]
- Laarhoven, W.H.; Cuppen, T.J.H.M.; Nivard, R.J.F. Photodehydrocyclizations in stilbene-like compounds. III. Effect of steric factors. Tetrahedron 1970, 26, 4865–4881. [Google Scholar] [CrossRef]
- McAtee, C.C.; Riehl, P.S.; Schindler, C.S. Polycyclic Aromatic Hydrocarbons via Iron(III)-Catalyzed Carbonyl-Olefin Metathesis. J. Am. Chem. Soc. 2017, 139, 2960–2963. [Google Scholar] [CrossRef] [PubMed]
- Browne, C.E.; Dobbs, T.K.; Hecht, S.S.; Eisenbraun, E.J. Stereochemical assignment of (E)- and (Z)-2-(1-naphthyl)-1-phenylpropene and their photocyclization to 5-methylchrysene. J. Org. Chem. 1978, 43, 1656–1660. [Google Scholar] [CrossRef]
- Nagel, D.L.; Kupper, R.; Antonson, K.; Wallcave, L. Synthesis of alkyl-substituted benzo[c]phenanthrenes and chrysenes by photocyclization. J. Org. Chem. 1977, 42, 3626–3628. [Google Scholar] [CrossRef]
- Leznoff, C.C.; Hayward, R.J. Photocyclization Reactions of Aryl polyenes. V. Photochemical Synthesis of Substituted Chrysenes. Can. J. Chem. 1972, 50, 528–533. [Google Scholar] [CrossRef]
- Karatsu, T.; Hiresaki, T.; Arai, T.; Sakuragi, H.; Tokumaru, K.; Wirz, J. Triplet intermediates in cis-trans photoisomerization of 3-chrysenylethylenes. Bull. Chem. Soc. Jpn. 1991, 64, 3355–3362. [Google Scholar] [CrossRef]
- Okamoto, H.; Takahashi, H.; Takane, T.; Nishiyama, Y.; Kakiuchi, K.; Gohda, S.; Yamaji, M. Convenient Phenacene Synthesis by Sequentially Performed Wittig Reaction and Mallory Photocyclization Using Continuous-Flow Techniques. Synth. Stuttg. 2017, 49, 2949–2957. [Google Scholar] [CrossRef]
- Okamoto, H.; Takane, T.; Gohda, S.; Kubozono, Y.; Sato, K.; Yamaji, M.; Satake, K. Efficient Synthetic Photocyclization for Phenacenes Using a Continuous Flow Reactor. Chem. Lett. 2014, 43, 994–996. [Google Scholar] [CrossRef]
- Carrera, M.; de la Viuda, M.; Guijarro, A. 3,3′,5,5′-Tetra-tert-butyl-4,4′-diphenoquinone (DPQ)-Air as a New Organic Photocatalytic System: Use in the Oxidative Photocyclization of Stilbenes to Phenacenes. Synlett 2016, 27, 2783–2787. [Google Scholar] [CrossRef]
- Liu, L.; Yang, B.; Katz, T.J.; Poindexter, M.K. Improved methodology for photocyclization reactions. J. Org. Chem. 1991, 56, 3769–3775. [Google Scholar] [CrossRef]
- Jorgensen, K.B.; Joensen, M. Photochemical synthesis of chrysenols. Polycycl. Aromat. Compd. 2008, 28, 362–372. [Google Scholar] [CrossRef]
- Zhang, F.J.; Harvey, R.G. Efficient synthesis of the carcinogenic anti-diol epoxide metabolite of 5-methylchrysene. J. Org. Chem. 1998, 63, 2771–2773. [Google Scholar] [CrossRef] [PubMed]
- Alacid, E.; Najera, C. Palladium-Catalyzed Cross-Coupling Reactions of Potassium Alkenyltrifluoroborates with Organic Halides in Aqueous Media. J. Org. Chem. 2009, 74, 2321–2327. [Google Scholar] [CrossRef]
- Amin, S.; Hecht, S.S.; Di Raddo, P.; Harvey, R.G. Comparative tumor initiating activities of cyclopentano and methyl derivatives of 5-methylchrysene and chrysene. Cancer Lett. 1990, 51, 17–20. [Google Scholar] [CrossRef]
- Olsen, R.J.; Pruett, S.R. Photocyclization of o-Halostilbenes. J. Org. Chem. 1985, 50, 5457–5460. [Google Scholar] [CrossRef]
- Mallory, F.B.; Rudolph, M.J.; Oh, S.M. Photochemistry of stilbenes. 8. Eliminative photocyclization of o-methoxystilbenes. J. Org. Chem. 1989, 54, 4619–4626. [Google Scholar] [CrossRef]
- Hansen, T.V.; Skatteboel, L. Ortho-formylation of phenols: Preparation of 3-bromosalicylaldehyde. Org. Synth. 2005, 82, 64–68. [Google Scholar] [CrossRef]
- Hofslokken, N.U.; Skattebol, L. Convenient method for the ortho-formylation of phenols. Acta Chem. Scand. 1999, 53, 258–262. [Google Scholar] [CrossRef]
- Vogel, A.I.; Furniss, B.S. Vogel’s Textbook of Practical Organic Chemistry, 5th ed.; Furniss, B.S., Ed.; Longman: Harlow, UK, 1989; p. 1514. [Google Scholar]
- Brown, G.S.; Barton, L.L.; Thomson, B.M. Permanganate oxidation of sorbed polycyclic aromatic hydrocarbons. Waste Manag. 2003, 23, 737–740. [Google Scholar] [CrossRef]
- Sala, T.; Sargent, M.V. Tetrabutylammonium permanganate; an efficient oxidant for organic substrates. J. Chem. Soc. Chem. Commun. 1978, 6, 253–254. [Google Scholar] [CrossRef]
- Chen, L.; Chen, Y.; Yao, K.; Zhou, W.; Li, F.; Chen, L.; Hu, R.; Tang, B.Z. A novel type of optically active helical liquid crystalline polymers: Synthesis and characterization of poly(p-phenylene)s containing terphenyl mesogen with different terminal groups. J. Polym. Sci. Part A Polym. Chem. 2009, 47, 4723–4735. [Google Scholar] [CrossRef]
- Al Mousawi, A.; Garra, P.; Dumur, F.; Bui, T.-T.; Goubard, F.; Toufaily, J.; Hamieh, T.; Graff, B.; Gigmes, D.; Fouassier, J.P.; et al. Novel carbazole skeleton-based photoinitiators for LED polymerization and LED projector 3D printing. Molecules 2017, 22, 2143. [Google Scholar] [CrossRef] [PubMed]
- Schwender, C.F.; Beers, S.A.; Malloy, E.; Demarest, K.; Minor, L.; Lau, K.H.W. 1-Naphthylmethylphosphonic acid derivatives as osteoclastic acid phosphatase inhibitors. Bioorg. Med. Chem. Lett. 1995, 5, 1801–1806. [Google Scholar] [CrossRef]
- McGeary, R.P.; Vella, P.; Mak, J.Y.W.; Guddat, L.W.; Schenk, G. Inhibition of purple acid phosphatase with α-alkoxynaphthylmethylphosphonic acids. Bioorg. Med. Chem. Lett. 2009, 19, 163–166. [Google Scholar] [CrossRef]
- Wang, Z.; Pitteloud, J.-P.; Montes, L.; Rapp, M.; Derane, D.; Wnuk, S.F. Vinyl tris(trimethylsilyl)silanes: Substrates for Hiyama coupling. Tetrahedron 2008, 64, 5322–5327. [Google Scholar] [CrossRef]
- Miura, M.; Hashimoto, H.; Itoh, K.; Nomura, M. Palladium-catalyzed desulfonylative vinylation of arenesulfonyl chlorides under solid-liquid phase-transfer conditions. J. Chem. Soc. Perkin Trans. 1 1990, 8, 2207–2211. [Google Scholar] [CrossRef]
- Hoffelner, K.; Libert, H.; Schmid, L. Aromatic cracking products from steroids. III. Z. Ernaehrungswiss. 1964, 5, 16–21. [Google Scholar] [CrossRef] [PubMed]
- Lutnaes, B.F.; Johansen, J.E. Characterization of all six mono-methylchrysenes by NMR and MS. Polycycl. Aromat. Compd. 2002, 22, 401–413. [Google Scholar] [CrossRef]
- Fieser, L.F.; Joshel, L.M.; Seligman, A.M. Synthetic experiments in the chrysene series. J. Am. Chem. Soc. 1939, 61, 2134–2139. [Google Scholar] [CrossRef]
- Bachmann, W.E.; Struve, W.S. The synthesis of derivatives of chrysene. J. Org. Chem. 1939, 4, 456–463. [Google Scholar] [CrossRef]
- Bhatt, S.; Nayak, S.K. Reductive deoxygenation of ortho-hydroxyaromatic aldehydes to 1,2-bis(hydroxyaryl)ethanes: Application to the synthesis of ethylene bridged calixarene-analogous metacyclophanes. Tetrahedron Lett. 2009, 50, 5823–5826. [Google Scholar] [CrossRef]
- Aspinall, H.C.; Beckingham, O.; Farrar, M.D.; Greeves, N.; Thomas, C.D. A general and convenient route to oxazolyl ligands. Tetrahedron Lett. 2011, 52, 5120–5123. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Böhme, T.; Egeland, M.; Lorentzen, M.; Mady, M.F.; Solbakk, M.F.; Sæbø, K.S.; Jørgensen, K.B. Regiospecific Photochemical Synthesis of Methylchrysenes. Molecules 2023, 28, 237. https://doi.org/10.3390/molecules28010237
Böhme T, Egeland M, Lorentzen M, Mady MF, Solbakk MF, Sæbø KS, Jørgensen KB. Regiospecific Photochemical Synthesis of Methylchrysenes. Molecules. 2023; 28(1):237. https://doi.org/10.3390/molecules28010237
Chicago/Turabian StyleBöhme, Thomas, Mari Egeland, Marianne Lorentzen, Mohamed F. Mady, Michelle F. Solbakk, Krister S. Sæbø, and Kåre B. Jørgensen. 2023. "Regiospecific Photochemical Synthesis of Methylchrysenes" Molecules 28, no. 1: 237. https://doi.org/10.3390/molecules28010237
APA StyleBöhme, T., Egeland, M., Lorentzen, M., Mady, M. F., Solbakk, M. F., Sæbø, K. S., & Jørgensen, K. B. (2023). Regiospecific Photochemical Synthesis of Methylchrysenes. Molecules, 28(1), 237. https://doi.org/10.3390/molecules28010237






