Abstract
Vitamin B12, also known as the anti-pernicious anemia factor, is an essential micronutrient totally dependent on dietary sources that is commonly integrated with food supplements. Four vitamin B12 forms—cyanocobalamin, hydroxocobalamin, 5′-deoxyadenosylcobalamin, and methylcobalamin—are currently used for supplementation and, here, we provide an overview of their biochemical role, bioavailability, and efficacy in different dosage forms. Since the effective quantity of vitamin B12 depends on the stability of the different forms, we further provide a review of their main reactivity and stability under exposure to various environmental factors (e.g., temperature, pH, light) and the presence of some typical interacting compounds (oxidants, reductants, and other water-soluble vitamins). Further, we explore how the manufacturing process and storage affect B12 stability in foods, food supplements, and medicines and provide a summary of the data published to date on the content-related quality of vitamin B12 products on the market. We also provide an overview of the approaches toward their stabilization, including minimization of the destabilizing factors, addition of proper stabilizers, or application of some (innovative) technological processes that could be implemented and contribute to the production of high-quality vitamin B12 products.
1. Introduction
Vitamin B12 is an essential micronutrient that belongs to the group of water-soluble B-complex vitamins. In a narrow sense, the term vitamin B12 implies cyanocobalamin, the anti-pernicious anemia factor isolated and crystallized in Glaxo laboratories and structurally characterized by the Nobel Prize winner Dorothy Hodgkin in the early 1950s. This is the primary vitamin B12 form used for food supplementation purposes, however, more broadly, the term includes a group of corrinoid compounds known as cobalamins (Cbls) [1,2,3]. These include the two Cbl forms with biological activity: 5′-deoxyadenosylcobalamin (AdoCbl) and methylcobalamin (MeCbl), as well as other forms, such as cyanocobalamin (CNCbl), hydroxocobalamin/aquocobalamin (OHCbl/H2OCbl+), and analogous Cbl derivates (glutathionyl-, nitrite-, nitroso-, sulfito-, etc.) [1,3,4,5].
The major dietary source of vitamin B12 is animal-derived food, i.e., meat, milk, eggs, and fish [6,7]. Plant foods typically do not contain substantial vitamin B12 amounts, with some exceptions (dried green and purple lavers) [6,8]. Therefore, vegetarians and vegans are especially prone to vitamin B12 deficiency; vitamin B12 supplementation through food supplements and fortified food is recommended to prevent deficiency in these populations [9,10]. On the other hand, vitamin B12 in the form of prescribed medicines is mainly used for the prevention and treatment of vitamin B12 deficiency [9]. Vitamin B12 medicines, food supplements, and fortified foods most commonly contain CNCbl, due to its better stability compared to other vitamin B12 forms [1,3,11]. Alternatively, OHCbl may also be found in medicines and foods. In accordance with the European Commission Regulation No 1170/2009 of 30 November 2009, CNCbl, OHCbl, AdoCbl, and MeCbl may be used in the manufacture of food supplements, whereas CNCbl and OHCbl may also be added to foods [12]. The stability of each form is thus an important factor to consider during the formulation and manufacture of quality medicines, food supplements, and fortified foods and is also associated with their efficient and safe use. Therefore, the aim of this manuscript is to provide a review of vitamin B12’s activity and use, with a special focus on its stability and approaches for the stabilization of its frequently used forms in foods, food supplements, and medicines. Aspects related to its absorption, distribution, metabolism, excretion (ADME), activation, and stability in complexes with its transport proteins in the human body, which all affect the clinical outcomes and efficiency of different cobalamines supplements, will also be reported.
2. Vitamin B12 ADME Properties, Activation, and Stability in the Human Body
Dietary vitamin B12 is usually bound to food proteins [13,14]. Its active absorption begins with its release from food, which is facilitated by the low pH in the gastric lumen (Figure 1) [13,15]. The liberated vitamin B12 is immediately bound to haptocorrin (transcobalamin-I) (HC), a vitamin B12-binding glycoprotein produced by salivary and oesophageal glands, which protects it from acid hydrolysis in the stomach [15,16,17]. The vitamin B12-HC complex is degraded by pancreatic proteases in the duodenum, thus releasing vitamin B12, which further binds to intrinsic factor (IF) produced by the parietal cells of the stomach [14,17,18]. The vitamin B12-IF complex then passes to the distal ileum, which is its primary absorption site [15,17]. This complex binds to its enterocyte membrane receptor and enters the enterocytes by endocytosis. A small portion of the ingested vitamin B12 (1–2%) is absorbed by the less efficient passive diffusion, which becomes more important in patients with a deficiency or lack of IF (e.g., gastrectomized patients), as well as in the event of exceeded capacity of the IF system [1,15]. The capacity of the IF system to absorb vitamin B12 is limited and it decreases with the application of larger vitamin B12 doses, ranging from 80% for 0.1 μg oral dose to 50% for 1 μg oral dose and <10% in doses near or above 20 μg. High vitamin B12 oral doses (≥500 μg) result in about 1% absorption [15,16,19]. Passive vitamin B12 absorption also occurs in the nasal mucous membranes after its nasal application [15]. The available literature indicates different vitamin B12 bioavailability after nasal application, depending on its applied form, and is estimated to be 2–5% for OHCbl [20], 2–6% for CNCbl [21], and ~20% for MeCbl [22].
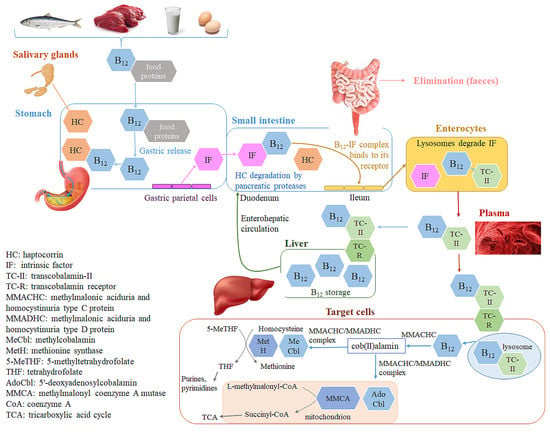
Figure 1.
Schematic representation of vitamin B12 absorption, distribution, metabolism, excretion (ADME), enterohepatic circulation, and cellular uptake and activity.
Within the enterocytes, lysosomes degrade IF and the free vitamin B12 binds to transcobalamin-II (TC-II), a nonglycosylated protein, which transports vitamin B12 in the blood stream [14,17,18]. The vitamin B12-TC-II complex has a half-life of 60–90 min and is quickly taken up by the target cells [23]. The cellular uptake of the vitamin B12-TC-II complex proceeds as receptor-mediated endocytosis, followed by its intracellular degradation in the lysosomes and the release of vitamin B12. Within the target cell, vitamin B12, regardless of its ingested form, is converted to its two active forms, AdoCbl and MeCbl, by a complex intracellular pathway in which different chaperones and transporters are involved [24,25,26,27]. The methylmalonic aciduria and homocystinuria type C protein (MMACHC) is the main chaperone, which binds the released vitamin B12 from the lysosomes in a base-off conformation (the ligand 5’,6´-dimethylbenzimidazole in vitamin B12 is replaced with a histidine residue of the protein). The MMACHC also catalyzes the conversion of any vitamin B12 form into the cob(II)alamin intermediate by the decyanation of CNCbl and dealkylation of alkylCbls, which also requires the activity of glutathione S-transferase. The following step of cob(II)alamin conversion into AdoCbl and MeCbl involves the activity of various enzymes, including MMACHC, methylmalonic aciduria and homocystinuria type D protein (MMADHC), and methionine synthase reductase [25,26,27,28]. AdoCbl and MeCbl exhibit their physiological effects upon binding to their target enzymes methionine synthase (MetH), and methylmalonyl coenzyme A mutase (MMCA) (Figure 1) [14,15,18]. Thus, MeCbl is the predominant vitamin B12 form in the plasma and AdoCbl in all tissues [1].
Excess vitamin B12 is accumulated in the liver and kidney. The average vitamin B12 content in heathy adults is estimated to be 2–3 mg, about half of which is stored in the liver [1,29]. Part of the vitamin B12, stored in the liver (0.5–5.0 μg vitamin B12/day), is secreted in the bile (Figure 1). A majority (50–80%) of it is reabsorbed, bound to IF, in the ileal enterocytes [1,15,17]. Enterohepatic circulation with vitamin B12 recycling is thus another important pathway to maintain adequate vitamin B12 levels in the body. This reabsorption is the reason for relatively low losses of B12 and the slow manifestation of vitamin B12 deficiency even in the absence of its intake [1]. The unabsorbed vitamin B12 from food or bile is predominantly excreted in the feces, with an estimated daily loss of approximately 0.1% of its body stores [1,30]. In situations where the circulating vitamin B12 in the blood exceeds its binding capacity (e.g., after injection), the excess amounts are excreted in the urine [1,30].
Vitamin B12 Stability in the Complex with Transport Proteins in the Human Body
Vitamin B12 forms a series of complexes with its transport proteins (HC, IF, TC-II, Figure 1). The structures of the transport proteins are very similar [31]. Each transport protein almost completely envelops and protects vitamin B12 (Figure 2). Cbls bind tightly to their transport proteins with Kd values in the subpicomolar range (TC-II-Cbl complex has a Kd of 5 × 10−15 M) [32]. The solvent-accessible area of the complexed Cbl is very low (7–17%, depending on the vitamin B12 form) and only the ribosyl CH2OH group and in some cases the β-ligand are exposed to solvent (Figure 2) [18]. Although many interactions between cobalamin and its binding site are conserved among the transporters, a significant difference of TC-II is the presence of a His residue, absent in HC and IF, which coordinates to the Co ion of Cbl in humans (after displacement of H2O at the upper axial position of H2O-Cbl) [31]. A protective and stabilizing role of TC-II during B12 transport in plasma was hypothesized. TC-II binding might be advantageous to impede the coordination of exogenous molecules to the Co ion, which causes the intracellular reductive conversion of Cbls to be more difficult [33]. Therefore, the most significant functional group unprotected by protein complexation is the 5′-hydroxyl group on the ribose of the nucleotide moiety of Cbl (Figure 2). This is the ideal site for the synthesis of vitamin B12 bioconjugates, which maintain a high affinity toward the transport protein in plasma [34].
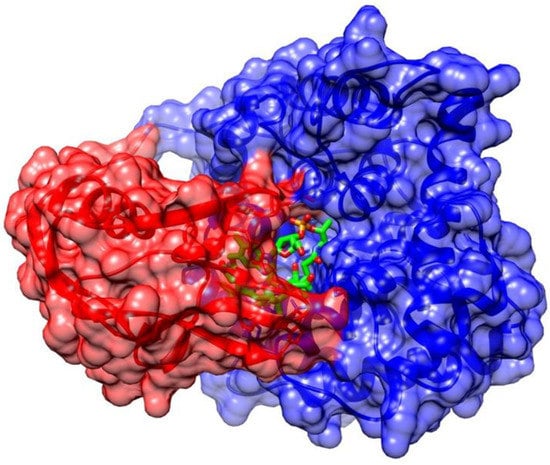
Figure 2.
Representation of TC-II protein structure [35]. Secondary structure cartoon showing the N-terminal α-domain (blue), the C-terminal β-domain (red), and the Cbl (green) in the central domain interface. The protected Cbl is tightly encapsulated between the two protein domains.
3. Mechanism of Action of Vitamin B12 in the Human Body
In the human body, vitamin B12 acts as a cofactor of several vitamin B12-dependent enzymes, including methyltransferases, such as MetH, and mutases, such as MMCA [18,36]. The latter is an AdoCbl-dependent enzyme, involved in the metabolism of branched-chain amino acids, odd-chain fatty acids, and cholesterol [36,37]. MMCA catalyzes the isomerization of L-methylmalonyl-coenzyme A (CoA) into succinyl-CoA for further metabolic reactions within the tricarboxylic acid cycle [36,38]. Therefore, vitamin B12 deficiency leads to the accumulation and increased serum and urine concentration of methylmalonic acid, the precursor of L-methylmalonyl-CoA, which is utilized in the early diagnosis of vitamin B12 deficiency [39,40]. Improper isomerization of L-methylmalonyl-CoA into succinyl-CoA and the accumulation of methylmalonic acid cause the formation of non-physiological fatty acids, which are incorporated into neuronal lipids in the myelin sheaths. This results in impaired signal transmission and the various neurological and neuropsychiatric consequences associated with vitamin B12 deficiency (nerve atrophy, neuropathy, myelopathy, depression, dementia, etc.) [36,37,39,41]. MetH is a MeCbl-dependent enzyme, involved in the methionine cycle, in which the active folate form 5-methyltetrahydrofolate transfers a methyl group to homocysteine, generating methionine and tetrahydrofolate [36,42,43]. This reaction is thus of particular importance not only for the formation of the essential amino acid methionine but also for its further conversion to S-adenosylmethionine (SAM). The latter is a universal methyl donor in a variety of vital physiological processes including the methylation of DNA, RNA, histones, and other proteins [36,43,44].
4. Vitamin B12 Deficiency
Vitamin B12 deficiency is an important public health issue, affecting an estimated 6% of the worldwide population and between 1.6% and 10% of the European populations [45]. The prevalence of vitamin B12 deficiency is generally higher among the elderly [46,47], vegetarians and vegans [48,49], pregnant women [50,51], infants breastfeeding from mothers with B12 deficiency [13,46], and, in general, in developing countries [45,51]. Due to its small losses, as a result of enterohepatic circulation, and its high amount of storage in the body (on average between 2 and 3 mg in healthy adults), vitamin B12 deficiency caused by its insufficient intake usually clinically manifests after a few years [1]. However, its deficiency becomes apparent more rapidly in the case of vitamin B12 malabsorption (e.g., due to pernicious anemia, food-bound vitamin B12 malabsorption, celiac disease, inflammatory bowel disease, Whipple disease), especially if combined with its low dietary intake [16]. Other common causes of vitamin B12 deficiency include inherited disorders (TC-II deficiency), the use of some medications (e.g., cholestyramine, metformin), and chronic alcoholism [9,13,39,46]. Considering the above-discussed actions of vitamin B12 in the human body, its deficiency affects important processes requiring intense cell replication, such as erythropoiesis. Therefore, vitamin B12 deficiency typically manifests clinically with hematological (megaloblastic anemia) and neurological symptoms (irritability, dementia, depression, visual disturbances, tingling or numbness of the extremities, paranoia, and psychosis) as well as some cellular and molecular consequences (cellular stress, apoptosis, and accumulation of homocysteine and methylmalonic acid) [9,39,46,52]. The diagnosis of severe vitamin B12 deficiency is typically based on hematological changes (elevated mean corpuscular volume of erythrocytes) and serum vitamin B12 concentration (<148 pmol/L (200 ng/L)). Other sensitive biomarkers of vitamin B12 deficiency include elevated plasma total homocysteine and methylmalonic acid levels and decreased serum vitamin B12-TC-II complex concentrations [1,46,53].
5. Treatment and Prevention of Vitamin B12 Deficiency
In healthy individuals, vitamin B12 daily intakes of 1–4 μg are considered sufficient to meet nutritional requirements [1]. Based on different biomarkers of vitamin B12 status and its mean intakes in the EU, the European Food Safety Authority (EFSA) Panel established adequate intakes for vitamin B12 at 4 μg/day for adults, 4.5 μg/day for pregnant women, and 5 μg/day for breastfeeding women. They also proposed estimated adequate intakes ranging between 1.5 μg/day for infants and 4 μg/day in children above 15 years [1]. The majority of the healthy population can achieve these values through the regular consumption of a mixed diet, including foods that are naturally rich in vitamin B12 (meat, milk and dairy products, fish, and eggs) [54]. However, dietary supplementation with vitamin B12 supplements is recommended in specific population groups, who are at higher risk for developing vitamin B12 deficiency (vegetarians, vegans, the elderly, and pregnant and lactating women) as the most efficient way of preventing its deficiency, even when vitamin B12 fortified foods are consumed [54,55,56,57]. A wide variety of vitamin B12 supplements may be found on the market in terms of the contained vitamin B12 form (mostly CNCbl due to its stability, but also MeCbl and OHCbl), its content (from a few μg up to a few mg), the presence of other micronutrients (supplements containing only vitamin B12 or in combinations with other B-complex vitamins and multivitamin/mineral supplements), and their dosage forms (tablets, effervescent tablets, drops, syrups, oral sprays, capsules, etc.) [54,58]. Contrary to dietary supplements, which are generally used for the prevention of vitamin B12 deficiency, high dose vitamin B12 treatments are usually indicated and used in conditions of its medically diagnosed deficiency. It should be noted that there is a different regulation of medicines, which are subjected to marketing authorization procedure to assure their quality, safety, and efficacy, and food supplements, which are concentrated sources of nutrients or other substances with a nutritional or physiological effect and are regulated as foods. Therefore, the FDA-approved indications of vitamin B12 medicines include the following conditions: pernicious anemia, vitamin B12 malabsorption or dietary deficiency, atrophic gastritis, chronic use of acid-reducing medication, total or partial gastrectomy, small bowel bacteria overgrowth, infection with diphyllobothrium latum or helicobacter pylori, and pancreatic insufficiency [59]. All these conditions are associated with vitamin B12 deficiency and usually respond well to various vitamin B12 treatment strategies [60]. Intramuscular, oral (including sublingual), or intranasal applications have all been reported as effective routes for the treatment of vitamin B12 deficiency [60,61,62,63]. Irrespective of the cause, vitamin B12 deficiency is most commonly treated by intramuscular administration [13,64,65,66]. For example, in Spain, vitamin B12 deficiency is conventionally treated with 1000 μg CNCbl intramuscular injections, administered daily during the first week, followed by weekly administration for one month, and then every month or two months for life [66]. The recommended vitamin B12 form in the intramuscular injections and their treatment schedules differ from country to country, but treatment typically involves similar loading of doses at the beginning of the treatment, followed by maintenance treatment [39]. For comparison, the standard treatment of vitamin B12 deficiency without neurological symptoms in the UK is the intramuscular application of 1000 μg OHCbl three times a week for the first two weeks, followed by its application every 3 months until improvement, or for life in the case of pernicious anemia. The patients with neurological symptoms are treated with 1000 μg OHCbl intramuscular injections every other day until improvement, followed by a maintenance treatment every 2 months [13,53].
The application of high vitamin B12 doses is considered safe, as no adverse effects have been identified in association with its high intakes in both healthy individuals and patients with compromised vitamin B12 absorption. Vitamin B12 has also not been found to be carcinogenic and genotoxic in vivo or in vitro nor teratogenic or with adverse effects on fertility and post-natal development. Therefore, the EFSA Panel has not derived a tolerable upper intake level for vitamin B12 [1].
5.1. Different Routes of Vitamin B12 Application
The intramuscular route of vitamin B12 administration has gained wide acceptance due to its quite constant bioavailability (10% of the injected dose) in comparison with the variable bioavailability of the oral route, which depends on various transporters [39,60]. However, after the discovery of passive, transporter-independent vitamin B12 absorption, the effectiveness of its oral applications has been increasingly re-evaluated. Thus, high vitamin B12 doses (1000–2000 μg of CNCbl) are usually administered to ensure sufficient absorption to meet daily needs, even in the absence of transporter-mediated absorption [66,67,68,69,70,71]. The main conclusion of these studies, as well as of a Cochrane review on the efficacy of the oral versus the intramuscular route of vitamin B12 administration for the treatment of its deficiency, is that both application routes are similarly safe and effective in normalizing vitamin B12 serum concentrations [66,67,68,69,70,71,72]. These studies also highlight the main benefits of oral over the intramuscular route of vitamin B12 application, such as reduced patient injection-related discomfort and increased patient convenience, reduced risk of bleeding in anticoagulated patients, and reduced number of hospital visits [66,67,68,69,70,71,72]. In addition to these advantages, the use of orally administered vitamin B12 also results in a significant reduction in healthcare costs [64,65,73,74]. Therefore, some countries, such as Sweden and Canada, have implemented the routine use of oral vitamin B12 for deficiency treatment [75], and others, such as the UK, consider the use of oral vitamin B12 acceptable for the treatment of asymptomatic deficiencies, as a maintenance treatment, or for the prevention of vitamin B12 deficiency [53]. In addition to intramuscular and oral vitamin B12 administration, new routes, such as sublingual and nasal administration, have recently become available. In addition to the obvious advantages over the oral and intramuscular routes (patient convenience and good adherence, safety, cost-effectiveness), sublingual and nasal treatments also provide a promising alternative for specific populations (infants, children, and the elderly) and patients with some specific conditions (swallowing disorders or malabsorption due to intestinal surgery, inflammatory intestinal diseases, or short bowel syndrome), for which oral and intramuscular routes are less appropriate [60,63,76,77]. Their efficacy has been extensively evaluated in recent years and has been demonstrated to be comparable to those of oral or intramuscular vitamin B12 administration. Namely, the administration of sublingual vitamin B12 was shown to be comparable or even superior to the intramuscular route in infants [77,78], children [76,77], adults [60,79], and some specific population groups (patients with type 2 diabetes treated with metformin [80], and vegans and vegetarians) [81]. Similarly, nasal vitamin B12 has a demonstrated comparable efficacy to intramuscular vitamin B12 in children [61], adults [82], and the elderly [20,63,83].
5.2. Different Vitamin B12 Forms
CNCbl, the synthetic vitamin B12 form, has been traditionally used for the manufacture of vitamin B12 supplements and medicines, primarily due to its better stability compared to other vitamin B12 forms [3,84,85]. However, it is not a naturally present form of vitamin B12. It was first isolated from the liver, as an anti-pernicious anemia factor, due to an artefact of the purification method [84]. It is found only in trace amounts in human tissues as a result of cyanide binding produced by smoking or other sources [85]. More recently, the naturally occurring vitamin B12 forms—HCbl, MeCbl, and AdoCbl—have also become available for supplementation and therapy. These forms are often marketed and promoted with certain claims, including the superiority of supplemental AdoCbl in increasing B12 intramitochondrial levels or the superiority of supplemental MeCbl in intracellular methylation reactions, which are not necessarily scientifically based [85]. Some food supplements on the market contain edible blue-green algae (cyanobacteria), which typically contain pseudovitamin B12. However, this vitamin B12 form is inactive in humans [6].
All four authorized vitamin B12 forms are reported as being effective in improving its level in the human body [62,70,79,86,87,88,89]. As outlined above, before reaching their target cells, vitamin B12 is absorbed and transported by several vitamin B12 transporters, including HC, IF, and TC-II (Figure 1). Their affinity for different vitamin B12 forms is mainly reported as comparable [28,90,91,92,93]. Intracellularly, the free vitamin B12 form is released in the cytosol for its further activation (Figure 1). However, mutations in the genes encoding the lysosomal membrane protein LMBD1 result in an impaired vitamin B12 lysosomal release, its accumulation in the lysosomes, and reduced production of MeCbl and AdoCbl, resulting in methylmalonic aciduria and homocystinuria [94,95,96]. In this particular case, OHCbl was found to be superior to CNCbl in resolving the consequent neurological symptoms, suggesting its ability to bypass this classic lysosomal release mechanism [96,97]. As the free vitamin B12 form in the cytosol is further activated to its biologically active MeCbl and AdoCbl forms, these two forms seem to have a theoretical advantage over CNCbl and OHCbl, which require proper activation. However, numerous studies on vitamin B12 metabolism have shown that all four vitamin B12 forms are converted to the cob(II)alamin intermediate in the cytosol (Figure 1). During this process, which involves the activity of cytosolic MMACHC chaperon, assisted by methionine synthase reductase, flavins, NADPH, or reduced glutathione, the ligands of the specific supplemented vitamin B12 form—cyano, hydroxy, adenosyl, and methyl—are removed [28,85,98,99,100,101,102]. The cob(II)alamin intermediate is then converted into MeCbl in the cytosol or into AdoCbl in the mitochondrion (Figure 1). Thus, the amount of active vitamin B12 forms in the cells depends on the type of cell and on specific conditions, as well as on genetic polymorphisms, and not only on the supplemented vitamin B12 form [85,98]. The most common genetic disorder of intracellular vitamin B12 metabolism is Cobalamin C (CblC) deficiency, caused by mutations in the MMACHC genes [103,104]. It is characterized by impaired MeCbl and AdoCbl synthesis and impaired MMCA and MetH activity and clinically manifests as hyperhomocysteinemia, methylmalonic acidemia, and hypomethioninemia. The main treatment of CblC deficiency is OHCbl, which improves the biochemical parameters, hematological abnormalities, neuropsychiatric symptoms, and growth parameters [105,106,107,108]. The exact mechanism of CblC patients’ responses to OHCbl is still under investigation. Possible explanations include the non-specific, presumably glutathione-dependent, OHCbl reduction to the cob(II)alamin intermediate, causing OHCbl to be the only so-far reported vitamin B12 form that can bypass the MMACHC pathway [28]; and the higher binding affinity of OHCbl with respect to CNCbl for some MMACHC mutants related to CblC disorder [109].
Therefore, for the greater part of the population, the bioavailability of all four vitamin B12 forms—CNCbl, OHCbl, MeCbl, and AdoCbl—is considered comparable. However, the use of a specific vitamin B12 form, namely OHCbl, is advantageous in individuals with a genetic disorder of the intracellular vitamin B12 metabolism [28,85]. Moreover, there is a growing body of research demonstrating lower tissue retention, higher urinary excretion, and, consequently, lower overall bioavailability of vitamin B12, supplemented in the form of CNCbl, compared to OHCbl, MeCbl, and AdoCbl [85,110,111]. Additionally, some researchers suggest the use of natural vitamin B12 forms (OHCbl, MeCbl, or AdoCbl) instead of CNCbl for its long-term supplementations to avoid the accumulation of cyanide in human tissues, which is especially important for smokers, as tobacco smoke is one of the major sources of cyanide [85,112,113,114]. Consequently, there is an evident trend of the replacement of CNCbl supplements, which were previously almost exclusively found on the market, with its natural forms, especially MeCbl, presumably because of its cost-effectiveness [85].
6. Chemistry and Reactivity of Vitamin B12
Chemically, cobalamins consist of a corrin ring formed by four reduced pyrrole rings (A–D) and a central cobalt atom, chelated by the four pyrrole nitrogens and two additional ligands—nitrogen of the 5´,6´-dimethylbenzimidazole at the lower α-side and a ligand labeled R1 in Figure 3 at the upper β-side. Different vitamin B12 forms differ in the β-ligand and are listed in Figure 3. The fifth ligand-5´,6´-dimethylbenzimidazole- is linked to a ribose, which is connected to the corrin ring through a phosphate and propionamide group. Seven amide side chains (four propionamides and three acetamides) are also linked to the corrin ring [3,11,18,42,115,116,117]. Due to its complex structure comprising different functional groups, vitamin B12 is prone to various degradation reactions. The most typical degradation reactions in the corrin ring, its side chains, the nucleotide moiety, and the β-ligand are summarized below and are schematically presented in Figure 3.
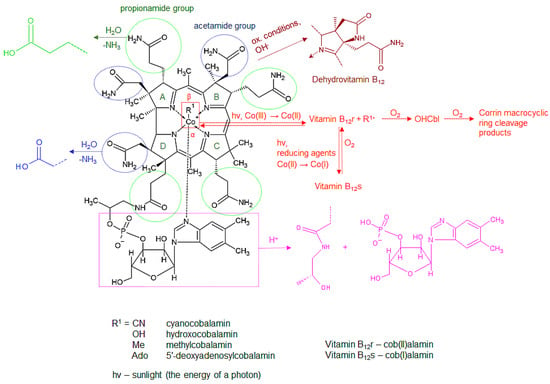
Figure 3.
Typical degradation reactions of vitamin B12.
- Hydrolytic deamidation of the side chains
The amide (propionamide, shown in green, and acetamide, shown in blue, in Figure 3) side chains are susceptible to acid and alkaline hydrolysis of their associated carboxylic acids. The extent of the hydrolysis depends on the strength of the acid/base, time of exposure, and other exposure conditions (temperature, concentrations) and may be partial or complete (from mono- to heptacarboxylic acid). These hydrolytic degradation products are not biologically active [3,19,118,119].
- Lactam formation
The acetamide side chain of the B ring in the structure of vitamin B12 is also susceptible to cyclization and the formation of a lactam in oxidative or alkaline conditions and elevated temperature. This reaction is indicated in purple in Figure 3. The formed compound, known as dehydrovitamin B12, has physical properties similar to CNCbl but without its biological activity [11,19,118,119].
- Phosphate bond hydrolysis in the nucleotide moiety
Specific conditions (high acidity or presence of chromium(III) hydroxide and elevated temperature) cause the hydrolysis of the phosphate bond in the nucleotide moiety, yielding cobinamide and the benzimidazole nucleotide (labeled in pink in Figure 3), which is susceptible to further degradation [11,19,118].
- Reduction in the cobalt atom
Chemical, electrochemical, or photochemical reduction in the central chelated cobalt atom (Co(III)) in the corrin ring of vitamin B12 may result in the formation of its Co(II) or Co(I) analogues, indicated as vitamin B12r and B12s, respectively (red color in Figure 3). Both of them are susceptible to oxidation and conversion into the Co(III) form [19,116,119,120]. As presented in Figure 3, the vitamin B12r form is easily oxidized into OHCbl in the presence of oxygen [11,19,121]. The formed OHCbl is in turn susceptible to further irreversible oxidative degradation [19,122].
- Cleavage the of β-ligand Co-C bond
The cleavage and subsequent reformation of the Co-C bond in the vitamin B12 β-ligand occur both in vivo and in vitro as a consequence of external factors (light or elevated temperature) or the activity of Cbl enzymes in the body. These reactions are the basis for the biological activity of vitamin B12 [19,116,123]. The Co-C bond cleavage may be either homolytic or heterolytic. The homolytic cleavage, presented in red in Figure 3, is typical for AdoCbl and results in the formation of vitamin B12r and an adenosyl radical. The heterolytic cleavage is mostly catalyzed by MeCbl-linked enzymes and is, therefore, characteristic of MeCbl. It yields vitamin B12s with a lone electron pair, also known as “supernucleophile”, which further reacts with electrophiles (e.g., methylating agents) [19,116,117,120,123,124,125].
7. Vitamin B12 Stability
7.1. Factors Affecting the Stability of Different Vitamin B12 Forms
The intrinsic stability of vitamin B12 and the effects of various factors on its stability have wide reaching practical implications in the storage and management of B12-containing samples and they have been discussed for more than seven decades in the scientific literature. Initially, the research was focused on CNCbl, especially in the presence of ascorbic acid [126,127,128,129,130]. CNCbl is recognized as the most stable form of vitamin B12 and, as such, is still its most commonly used form for supplementation purposes [3,85,118,131,132]. However, with progress in the food, supplements, and pharmaceutical sectors and advances in stabilization approaches, there is a growing use of the other approved vitamin B12 forms (OHCbl, AdoCbl, and MeCbl) [85,133,134], resulting also in an increased interest in their stability. In general, considering Cbls’ structure and reactivity (Figure 3), we can conclude that they are susceptible to photolytic, hydrolytic, oxidative, and thermal degradation. Although some of these degradation reactions result in the formation of a biologically active form of vitamin B12 (e.g., formation of OHCbl after reductive decyanation of the cobalt atom in CNCbl, Figure 3), such conversions between different vitamin B12 forms in finished products, which specify the content of a single vitamin B12 form, are not acceptable in terms of their regulation. Thus, in the following, we provide a review of the stability of different vitamin B12 forms under different conditions, which, depending on the degradation mechanisms, considerably affect their stability.
7.1.1. Temperature
Commercial vitamin B12 supplements (CNCbl, OHCbl, AdoCbl, and MeCbl) are typically supplied in dry solid forms (powders), with different recommended storage temperatures: 2–8 °C for CNCbl and H2OCbl+ as acetate, chloride, or sulfate and −20 °C for AdoCbl and MeCbl [135,136,137,138,139,140]. CNCbl is reported to be stable in its solid form even at elevated temperatures (up to 100 °C) for a few hours [3,117]. However, temperature is an important factor that strongly affects CNCbl stability in aqueous solutions. Temova et al. reported CNCbl degradation in aqueous solution after 24 h and 48 h of storage at 60 °C (5.3% and 8.6% degradation, respectively), which did not occur at room temperature (<1% degradation after 48 h) [141]. Bajaj and Singhal also showed that temperature significantly accelerates the CNCbl degradation rate in aqueous solutions with pH values between 2 and 10. The degradation follows first-order kinetics, with the half-life at pH 2 decreasing from 63 days at 4 °C to 8 days at 37 °C, while at pH 6 it decreases from 231 days to 116 days at the same temperatures. The maximum stability observed was at pH 6 and 4 °C with a halving of the lifetime at 37 °C. The high correlations between the degradation rate constants and the three evaluated temperatures (4, 25, and 37 °C) showed that CNCbl degradation followed the Arrhenius equation at tested solution aqueous media. The considerably different activation energy at pH 2 (41.9 kJ/mol) compared to the activation energies at pH 4–10 (between 10.3 and 15.4 kJ/mol) indicate different CNCbl degradation mechanisms at pH 2. The authors conclude that temperature has a greater effect on the stability of CNCbl in highly acidic conditions [142]. MeCbl is thermally stable in neutral aqueous solutions in the dark, as it persists after heating up to 100 °C for 20 min. However, the effect of temperature on MeCbl stability becomes evident in the presence of light, as it accelerates the photochemical transformation of MeCbl into OHCbl [3,86]. OHCbl is reported to be stable in a reconstituted solution for injection for up to 6 h at 40 °C [143]. However, the literature data regarding the comparative thermal stability of OHCbl are not fully consistent. Thus, Mander et al. report that the effect of temperature on the stability of OHCbl is comparable to that on CNCbl and MeCbl, leading to a similar extent of degradation under the same conditions [144], whereas Hadinata Lie et al. found that CNCbl is more heat stable than OHCbl and MeCbl [145]. Nonetheless, it can be concluded that the stability of CNCbl, MeCbl, and OHCbl in aqueous solutions is significantly affected by the temperature. The mechanism of thermolysis of AdoCbl is very well established. Homolysis dominated at high temperatures while heterolysis dominates at low temperatures [146]. The half-life at pH 7.5 changes from 690 days at 30 °C to 15 h at 85 °C [147]. The crystalline form is reported to decompose at temperatures >210 °C [3,86].
7.1.2. PH of the Solution
Considering the liability of vitamin B12 to both acidic and alkaline hydrolysis, the effect of pH is important for its stability. Different vitamin B12 forms are generally unstable in both strongly acidic or alkaline media [3,117,148]. Among the different vitamin B12 forms, the most detailed information on the effect of pH on stability is available for CNCbl. Although some of the literature sources only highlight CNCbl’s instability in acidic conditions (pH < 3) [148], recent forced degradation studies showed an even greater extent of CNCbl degradation in strongly alkaline solutions. Specifically, CNCbl completely degraded in 0.1 M NaOH after 48 h of storage at room temperature (85% degradation after 1 h at 80 °C) in comparison with 11.2% degradation in 0.1 M HCl under the same conditions of CNCbl concentration, storage time, and temperature (61% degradation after 1 h at 80 °C) [141,149]. As reported above, Bajaj and Singhal evaluated CNCbl degradation kinetics at less extreme pH values (2, 4, 6, 8, and 10) at three temperatures (4, 25, and 37 °C) with the highest degradation extent and rate at pH 2 and lowest at pH 6 [142]. Other papers report the highest CNCbl stability in different pH ranges: 4–7 [3,117], 4–6.5 [150], 4.5–5 [151], and 4–4.5 [131]. The official requirements for the pH of CNCbl solutions are 4.0–5.0 for CNCbl oral solution in the British Pharmacopeia [152], and 4.5–7.0 for CNCbl injections in the United States Pharmacopeia [153]. To the best of our knowledge, pH profiles of the remaining vitamin B12 forms have not yet been published. However, the comparative stability of MeCbl and OHCbl with respect to CNCbl have been evaluated at pH 3.0, 4.5, 8.0, and 9.0 within 24 h of storage at room temperature. MeCbl degradation was most pronounced (78% at pH 3.0 and 64% at pH 9.0), followed by OHCbl (20% at pH 3.0 and 24% at pH 9.0) and CNCbl (16% at pH 3.0 and 15% at pH 9.0) [145]. Similar to CNCbl in the pH range 4–7, OHCbl, MeCbl, and AdoCbl are also reported to have the highest stability [145]. However, it should be considered that OHCbl is in equilibrium with its conjugate acid H2OCbl+. The corresponding logK of the following equilibrium equation H2OCbl+ + OH− ⇄ OHCbl + H2O is 6.2 [154]. The official monographs specify a pH range for OHCbl preparations: between 3.8 and 5.5 for OHCbl injections in the British Pharmacopeia [152] and between 3.5 and 5.0 in the United States Pharmacopeia [153]. In this pH range, the H2OCbl+ is the prevalent form, however at neutral pH OHCbl is the main species.
7.1.3. Exposure to Light
All four vitamin B12 forms, especially MeCbl and AdoCbl, are susceptible to photodegradation. The research in the field of vitamin B12 photostability goes back to 1956, with DeMerre and Wilson’s publication on the photolysis of CNCbl after exposure to different types of radiation. They reported that each hour of exposure to sunlight or UV light causes approximately 20% CNCbl degradation [155]. Since then, this field has been updated by numerous publications and is still being researched [156,157,158,159,160,161,162]. Vitamin B12 instability upon exposure to light is also recognized by the responsible authorities and manufacturers of vitamin B12 preparations, which recommend that vitamin B12 active ingredients (CNCbl, OHCbl, and MeCbl) and their preparations (injections and tablets) should be stored protected from light [140,152,153].
Different vitamin B12 forms undergo different photodegradation mechanisms. Upon light exposure, the cyanide ligand in CNCbl is replaced with an OH− group to form OHCbl (in neutral or alkaline solutions) or with water to form H2OCbl+ (in acidic solutions) [122,163,164]. MeCbl and AdoCbl undergo photoreduction to vitamin B12r, which is quickly oxidized to OHCbl or H2OCbl+, depending on the pH [159,165,166]. Under the influence of light, OHCbl is irreversibly degraded to unknown corrin ring oxidation products [122,163].
In the literature, there are inconstancies regarding the comparative photostability of different vitamin B12 forms. Hadinata Lie et al. reported that CNCbl is significantly less susceptible to photolysis compared to OHCbl and MeCbl (4% CNCbl degradation after 60 min UV exposure compared to 28% for OHCbl and 39% for MeCbl in their aqueous solutions under the same experimental conditions) [145]. Juzeniene and Nizauskaite studied the photodegradation of different vitamin B12 forms in aqueous solutions at physiological pH (7.4) under UVA exposure. Under these conditions, OHCbl was the most stable vitamin B12 form, followed by CNCbl. The latter had around a five-fold higher photodegradation rate. MeCbl and AdoCbl were both particularly unstable and photodegraded within seconds of the light exposure. MeCbl was found to be three-fold more sensitive to UVA exposure than AdoCbl [164]. One of the possible reasons for the inconsistent results, apart from the different light sources, is the difference in the pH of the evaluated vitamin B12 aqueous solutions, which has been shown to significantly affect the rate of photodegradation. For example, Ahmad and Fareedi evaluated zero-order CNCbl photolysis in an aqueous solution across the pH range 1–12 and determined that the protonated CNCbl, present at lower pH, is more liable for photolysis (k = 1.316 × 10−7 M min−1 at pH = 1) than the neutral CNCbl form with the maximum photostability at pH = 8.5 (k = 0.039 × 10−7 M min−1) [161]. On the contrary, Vaid et al. showed that the protonated MeCbl was less susceptible to photolysis and that the first-order reaction rate increases with the pH up to pH 5.0 (kobs = 0.134 min−1 at pH 1 and kobs = 0.273 min−1 at pH 5) [165].
7.1.4. Presence of Oxidizing and Reducing Agents
Vitamin B12 is unstable in the presence of both oxidizing and reducing agents [118,131,167,168]. Therefore, the instability of vitamin B12 in the presence of ascorbic acid, which was first discussed by Gakenheimer and Feller in 1949 [129], is the most known vitamin B12 incompatibility. Some reducing agents with the thiol functional group, including cysteine, homocysteine, N–acetyl–cysteine, cysteamine, pentafluorophenylthiolate, glutathione, and dithiothreitol form molecular complexes with vitamin B12 [163,169,170,171,172,173,174]. However, more in general, reducing agents, such as ascorbic acid, reducing sugars, thiols, formaldehyde, mercaptide ion, NaHSO3 or FeSO4, typically cause a reduction in the cobalt ion from Co(III) to Co(II), yielding vitamin B12r by the cleavage of the β-ligand [122,163,175,176,177]. In the presence of air, the vitamin B12r thus formed is oxidized to OHCbl [122,130]. Similar to the oxidation of other vitamin B12 forms, in some conditions (e.g., depleted oxygen concentrations and presence of hydrogen peroxide), vitamin B12r oxidation may also lead to irreversible cleavage of the corrin ring [118,122,178]. To ensure the oxidative stability of vitamin B12 active ingredients (CNCbl, OHCbl, and MeCbl) and preparations, the authorities recommend their storage in airtight containers [140,152,153].
Ascorbic acid is the most typical representative of reducing agents that react with vitamin B12 [122,126,127,129,130,179,180]. Its presence in solution is reported to cause up to 65% CNCbl degradation after 24 h of storage at 25 °C in the dark [122]. The destabilizing effect of ascorbic acid increases with the temperature, ascorbic acid concentration, and exposure of the solutions to light [122,179,181]. It also depends on the pH of the solution (faster reaction up to pH around 5.0 for both CNCbl and OHCbl) and on the vitamin B12 form (MeCbl is the least stable vitamin B12 form, followed by OHCbl and finally CNCbl) [122,145,179].
On the other hand, H2O2 is widely used as an oxidant, especially in forced degradation studies. Two published CNCbl forced degradation studies confirmed its oxidative instability following exposure to H2O2 [141,149]. However, the extent of CNCbl degradation in 3% H2O2 solution significantly differs between the two studies (~2% degradation after 24 h versus 55% degradation after 1 h at room temperature). Higher temperatures led to a significant increase in the rate and extent of CNCbl degradation in the presence of H2O2 [149].
7.2. Characteristic Interactions in Vitamin B12 Products
In addition to the above-described well-known interaction with ascorbic acid, the stability of vitamin B12 is also affected by the presence of some other compounds which are common ingredients of vitamin B12 preparations (foods or pharmaceutical preparations), such as other B-complex vitamins and some frequently used excipients. The most characteristic interactions are summarized below.
7.2.1. Vitamin B12—Reducing Sugars (Dextrose and Sucrose)
Dextrose and sucrose are commonly used as excipients in both solid and liquid dosage forms, including parenteral preparations [182,183]. However, similar to the case of ascorbic acid, they have a reported incompatibility with CNCbl due to their reducing properties [183,184]. Barr et al. reported that, compared to sucrose, dextrose had a more pronounced effect on CNCbl degradation. There was a loss of about 40% in vitamin B12 content in the dextrose solutions and of nearly 30% in the sucrose solutions after 112 days at 45 °C. Dextrose was the only substance that produced an observable loss in vitamin B12 content at 25 °C. The deterioration at this temperature amounted to about a 25% loss in vitamin B12 content after 140 days [184].
7.2.2. Vitamin B12—Riboflavin
Riboflavin is an effective photosensitizer that catalyzes the photochemical changes of other compounds, including also vitamin B12 [185,186,187]. Ahmad et al. evaluated the effect of riboflavin on CNCbl photostability in aqueous solutions in the pH range of 2–12. They concluded that riboflavin promoted CNCbl photolysis to OHCbl and further products, with a more than two-fold higher first-order reaction rate constant at pH 7 compared to the same solution in its absence. CNCbl photolysis in the presence of riboflavin showed the highest stability around pH 7–8 [188]. Similarly, Juzeniene and Nizauskaite evaluated OHCbl photodegradation in the presence of riboflavin at physiological pH (7.4) and concluded it was up to three-fold faster than its photodegradation in the absence of riboflavin [164].
7.2.3. Vitamin B12—Thiamine
Vitamin B12 stability in aqueous solutions is also affected by the presence of thiamine or, more precisely, its degradation products. Feller and Macek as well as Mukherjee and Sen reported that CNCbl is stable in thiamine aqueous solutions at pH between 3.5 and 4.5 during prolonged storage at ambient temperature, but unstable under conditions that cause thiamine degradation (elevated temperature, high pH). They found the extent of CNCbl degradation related to thiamine degradation [189,190]. Doerge et al. identified the thiol-containing thiamine degradation product, cysteine, as responsible for CNCbl degradation [191]. This destabilization effect is enhanced by the presence of high thiamine concentrations and the presence of pyridoxine or nicotinamide [150,177,192].
7.2.4. Vitamin B12—Nicotinamide
The adverse effect of nicotinamide on the stability of CNCbl in aqueous solutions has also been recognized by researchers for quite some time, with reports on their incompatibility since 1958–9 [190,193]. Ahmad et al. have more recently reported that nicotinamide accelerates CNCbl photolysis in an aqueous solution. They also evaluated the effect of pH in the range 1–7 and concluded that nicotinamide accelerates CNCbl photolysis across this pH range. In fact, pH significantly affects the rate of CNCbl photolysis in the presence of nicotinamide, which occurred as a second-order reaction (first-order in both species) at the highest rate at pH 1 (k2 = 0.57 M−1 min−1) and gradually decreased up to pH 7 (k2 = 0.075 M−1 min−1) [194].
7.2.5. Vitamin B12—Mixtures of Water-Soluble Vitamins
Individual water-soluble vitamin deficiency is often associated with insufficient intake of other water-soluble vitamins. Therefore, they are commonly found all together in solid or liquid pharmaceutical preparation [141,195]. As described above, the presence of individual water-soluble vitamins (ascorbic acid, riboflavin, thiamine, and nicotinamide) affects vitamin B12 stability. Hadinata Lie et al. evaluated the stability of CNCbl, MeCbl, and OHCbl in the presence of both thiamine and nicotinic acid and showed that their presence causes the degradation of all three vitamin B12 forms, among which they identified MeCbl as the least stable form [145]. Temova et al. recently comparatively evaluated the stability of CNCbl in aqueous solutions alone and in multivitamin preparations (ascorbic acid, thiamine, riboflavin, riboflavin sodium 5′phosphate, nicotinamide, calcium-pantothenate, dexpanthenol, pyridoxine, biotin, and folic acid). They concluded that the presence of other water-soluble vitamins significantly decreased CNCbl stability under oxidative, thermal, and photolytic conditions, but did not affect its hydrolytic stability (in 0.1 M HCl and 0.1 M NaOH) [141].
8. Vitamin B12 in Foods, Food Supplements, and Medicines
8.1. Vitamin B12 Stability in Foods
Vitamin B12 is a natural component of certain foods (e.g., meat, milk, eggs, and fish) [6,7]. It is also commonly added to specific fortified foods (e.g., breakfast cereals, plant milk, and bread) in the food industry or formulated as medicines or food supplements in the pharmaceutical and food supplement industries [196,197]. In association with the general vitamin B12 instability, which, as described above, is affected by several factors, there are also various reports on its stability in foods and pharmaceutical preparations.
The effect of some typical food production processes (e.g., pasteurization, sterilization, and extrusion) on vitamin B12 content and stability has been examined primarily to evaluate possible food fortification with vitamin B12. Thus, Ottaway reported that vitamin B12 is stable during milk pasteurization. However, its stability in milk depends on the severity of the heat processing of the milk, with up to 20% and 35% loss during milk sterilization and spray drying, respectively [198]. Vitamin B12 (CNCbl and OHCbl) stability has also been evaluated throughout the processes of baking bread. Thus, Edelmann et al. concluded that the proofing step did not significantly affect CNCbl and OHCbl stability. Straight- and sponge-dough baking did not affect CNCbl content but caused an average of 21% and 31% OHCbl loss, respectively. The sourdough baking process caused both OHCbl (44%) and CNCbl degradation (23%) [199]. Extrusion has become a popular food processing method used for the production of foods, also vitamin-fortified, in various shapes and colors. Riaz et al., Killeit and Bajaj, and Singhal have evaluated how different extrusion variables affect CNCbl stability. Based on their research, we may conclude that CNCbl is susceptible to degradation during extrusion. Thus, the extent of CNCbl mostly depended on the die temperature (from 23% CNCbl loss at 140 °C to almost complete CNCbl degradation at 194 °C), whereas the screw speed and feed rate had a lesser effect on CNCbl stability [200,201,202]. In addition to the processes that vitamin B12 is exposed to during food production, the packaging of the foods is also reported to affect its stability. Hemery et al. determined CNCbl stability in fortified wheat flour packed in multilayer PET/aluminum bags or paper bags. The multilayer PET/aluminum bags, which are non-permeable to humidity and oxygen, provided sufficient CNCbl stabilization as no significant CNCbl loss was observed after 6 months of storage at 25 °C and 65% or 85% relative humidity (RH) or 40 °C and 65% or 85% RH. In contrast, the storage of the flour in paper bags at 65% RH resulted in a 30–48% and 49–63% loss of CNCbl after 1.5 and 6 months, respectively. Thus, packaging choice is of critical importance in CNCbl degradation in the flour samples [203].
Food processing has also been reported to significantly affect vitamin B12 stability, with a typical maximum vitamin B12 loss of up to 45% after cooking, 50% after cooking and draining, and 45% after reheating, as reported by Devi [204]. The effect of microwaving on vitamin B12 stability in foods was initially evaluated by Watanabe et al., who reported up to 40% total vitamin B12 loss after 6 min of microwave heating of beef, pork, and cow milk [205]. Similar conclusions were reached by Czerwonka et al. [206] and Bennink and Ono [207]. Nishioka et al. evaluated in greater detail how different cooking methods affect the stability of naturally present vitamin B12 in fish (round herring) meats. They reported a total vitamin B12 degradation of 59% after 7.5 min of grilling and 1.0 min of microwaving, 47% after 5.0 min of boiling, 43% after 4.0 min of frying, 41% after 9.0 min of steaming, and no vitamin B12 loss after 30 min of vacuum-packed pouch cooking. Thus, they concluded that vitamin B12 stability in food depends on the cooking temperature and time as well as the presence of other food ingredients, which was supported by a thermal stability study of OHCbl solutions [208]. Some specific food ingredients affecting vitamin B12 stability were identified by Johns et al. Specifically, they discovered that cocoa polyphenols (gallic acid, caffeic acid, epigallocatechin gallate, catechin, and its oligomers) accelerate CNCbl degradation, causing a three-fold greater vitamin B12 loss in heated, chocolate-flavored milk compared to unflavored milk [209]. The effect of the food matrix on vitamin B12 stability was also demonstrated by Bajaj and Singhal, who determined significantly higher CNCbl degradation rates in two model juices (lime and carrot) at 25 °C and 37 °C than in aqueous solutions with similar pH values. For instance, CNCbl degradation rate in lime juice (pH 2.16) was almost four-fold higher than in aqueous solutions with pH 2, which they presumed was a consequence of the high ascorbic acid concentration in lime juice. The role of the different complex matrices on CNCbl stability was evidenced by the different rate and extent of CNCbl degradation between the two juices evaluated (15% CNCbl loss after 28 days of storage at 25 °C in carrot juice compared to 95% loss in lime juice under the same experimental conditions) [142].
8.2. Vitamin B12 Stability in Food Supplements and Medicines
8.2.1. Vitamin B12 Stability in Liquid Dosage Forms
Research on vitamin B12 stability in pharmaceutical preparations goes back to the 1950s, with Rosenblum and Woodbury’s publication on its stability in four different multivitamin combinations [210] and the report from Blitz et al. on its stability in commercial B-complex parenteral solutions [211]. The published results reveal incorrect vitamin B12 contents (between 5 and 80% of the labeled claim) in four of the six evaluated B-complex injectable solutions. After 3 months of storage at room temperature, an additional significant vitamin B12 loss was observed in all the tested preparations, resulting in its total degradation in half of the evaluated products and contents between 12 and 58% of the labeled claim in the remaining three solutions. Further storage at room temperature caused additional vitamin B12 degradation in all the evaluated preparations, except one. The authors observed a correlation between enhanced vitamin B12 degradation and higher thiamine and niacinamide concentrations [211]. Similar conclusions were drawn in a more recent study, performed by Ahmad and Hussain, evaluating CNCbl stability in seven commercial parenteral preparations. The initial CNCbl contents were in accordance with the labeled claims (101–110%) in three evaluated preparations that contained CNCbl only, in contrast to its low determined content (71–87%) in four tested injections that also contained thiamine and pyridoxine. A considerable difference in CNCbl stability between the single- and multi-ingredient preparations was generally observed. Thus, CNCbl contents remained at >95% of the labeled claim after 12 months of storage at room temperature in all three single-ingredient preparations, whereas its contents decreased to 28–37% of the labeled claim in the four multi-ingredient preparations [151]. CNCbl degradation in commercial parenteral preparations containing thiamine and pyridoxine, after two months of their light-protected storage at 40 °C, was additionally confirmed by Monajjemzadeh et al. By evaluating the CNCbl stability after 5 days at 55 °C in experimentally prepared parenteral formulations containing different CNCbl, thiamine, and pyridoxine combinations, they confirmed the CNCbl stability in a single-ingredient CNCbl solution as well as in combination with pyridoxine (97%) and its degradation in solutions with thiamine (79%) and especially in combination with thiamine and pyridoxine (41%) [150]. Recently, there is an increasing number of studies that highlight the photolability of MeCbl in liquid dosage forms and the need for its light-protected storage and proper stabilization [212,213,214].
8.2.2. Vitamin B12 Stability in Solid Dosage Forms
In comparison with liquid dosage forms, the research on vitamin B12 stability in food supplements and medicines in solid dosage forms is less common. Jacob et al. first pointed out an unexpected vitamin B12 loss in film-coated multivitamin tablets and identified the use of methanol as a solvent for the film coating as a plausible cause for vitamin B12 degradation [215]. A more recent study, performed by Ohmoria et al., evaluated CNCbl stability in powders, granules, plain tablets, and sugar-coated tablets. They focused on the effect of humidity and the presence of other B-complex vitamins: fursultiamine (the disulfide derivative of thiamine), riboflavin, and pyridoxine. This study showed that moisture affects the CNCbl stability in powders, as the CNCbl powder remained stable after 4 months of storage at 40 °C/6% RH and 40 °C/57% RH but was unstable at 40 °C/75% RH. Compared to powders, CNCbl was more susceptible to moisture in granules that also contained fursultiamine, riboflavin, and pyridoxine. They also showed a negative correlation between water activity, expressed as equilibrium relative humidity (ERH), and CNCbl stability in plain tablets and sugar-coated tablets. Moreover, by comparison with uncoated tablets with the same ingredients and ERH levels, they demonstrated that the sugar-coating layer decreases the CNCbl stability. Evaluating the CNCbl stability in sugar-coated tablets with different compositions, they also showed that the presence of both riboflavin and pyridoxine reduced its stability, whereas fursultiamine, the tetrahydrofurfuryl thiamine derivative, improved its stability [216]. In addition to humidity and the presence of other interacting ingredients, light is also an important factor affecting the vitamin B12 stability in solid dosage forms. Its effects, as well as the importance of MeCbl light protection in solid dosage forms, were demonstrated by Saeki et al. Their six evaluated commercial MeCbl preparations in solid dosage forms (tablets and capsules) showed a different extent of MeCbl degradation (between 5 and 31% in the primary packaging and between 8 and 44% in the opened samples) after 20 days of light exposure, which depended on the packaging material and also the light-resistant properties of the products coating [217].
8.3. Vitamin B12 Content in Fortified Foods, Food Supplements, and Medicines and Their Regulation
The intrinsic instability of vitamin B12 in finished products is an important issue in ensuring their proper content-related quality. More specifically, its insufficient stabilization within formulations leads to its degradation during the production and storage of the finished products, which results in decreased content. Aware of this issue, manufacturers of products with such issues frequently adopt the strategy of the addition of sensitive active ingredients in higher contents than declared to compensate for degradation and therefore to guarantee the content and achieve a commercially acceptable shelf life [141,218,219,220]. Thus, this concept of overages is consistent with current Good Manufacturing Practices and is well recognized among the manufacturers of vitamin products [218,221,222]. The amount of used overage depends on the intrinsic stability of the active ingredient and its anticipated loss and should be reduced to a minimum [222]. To ensure food safety and the protection of consumers’ interests, the European Commission has published a guidance document for competent authorities for the control of compliance with EU legislation on the setting of tolerances for nutrient values declared on a label. The tolerances for vitamins, including vitamin B12 content, are between 65 and 150% in foods and between 80% and 150% in food supplements including measurement uncertainty. In the case of vitamin C in liquids, higher upper tolerances may be accepted [223]. In the USA, the U.S. Food and Drug Administration (FDA) regulates the lowest but not the highest vitamin content in fortified foods and food supplements. Thus, the FDA requires that Class I nutrients, which are nutrients (vitamins, minerals, protein, dietary fiber, or potassium) added in fortified or fabricated foods and food supplements, must be present at 100% or more of the value declared on the label; whereas Class II nutrients (vitamins, minerals, protein, total carbohydrate, dietary fiber, other carbohydrate, polyunsaturated and monounsaturated fat, or potassium) that occur naturally in food products must be present at 80% or more of the value declared on the label [224]. Tolerances for vitamin B12 content in food supplements are also provided in the United States Pharmacopeia-National Formulary (USP–NF) monographs, and are between 90 and 150% of its declared content on the label in capsules and tablets, whereas 90–450% of the labeled content is considered acceptable for oral solutions [153]. Vitamin B12 contents in medicines and the explanation of the acceptable tolerance are a part of the registration documentation and are directly regulated by the responsible regulation agency.
With the increase in health awareness, the aging population, lifestyle changes, and the global increasing cost of healthcare, the availability, and consumption of food supplements and fortified foods have been steadily increasing over the past decade worldwide [221,225]. Unfortunately, their growing use has not been accompanied by an increase in their quality. Due to variations in both manufacturing methods and regulatory controls, the actual contents of the active ingredients are unknown to researchers and consumers, who depend on product quality to ensure a sufficient intake to achieve the desired effects on one hand but avoid excessive consumption on the other [221,226,227]. Therefore, in line with the growing concern about their quality, there is also an increasing number of studies evaluating the contents of the active ingredients in fortified foods, food supplements, and medicines, including vitamin B12. To date, the most extensive study in this field was performed by Andrews et al., as a part of the analysis of multivitamin/mineral products in the United States market for the Dietary Supplement Ingredient Database. They analyzed a nationally representative range of multivitamin/mineral products, in which vitamin B12 was most often labeled in contents above its recommended Dietary Allowance (RDA), published by the US Institute of Medicine [228]. The determined vitamin B12 contents in the 98 tested food supplements had a labeled vitamin B12 content above its RDA between 50.6 and 164.3% of its labeled content, with an overall 8.4% mean percentage difference from the labeled content. The determined vitamin B12 content in the one additional food supplement with its labeled content below its RDA was 65.1% of the labeled content. The complete range of the final laboratory data, including 337 vitamin B12 food supplements, confirmed the wide distribution of its analytically determined contents, with the vitamin B12 content between 95% and 125% of the labeled contents in most of the tested supplements (25th–75th percentiles) [221]. The results of the remaining published studies evaluating the analytically determined content of vitamin B12 in relation to the labeled contents in fortified foods, food supplements, and medicines from around the globe were reviewed and are summarized in Table 1.

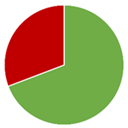
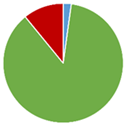
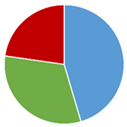
Table 1.
The compliance of the determined vitamin B12 contents in the tested fortified foods, food supplements, and medicines with the label claims along with the lowest (Min), highest (Max), and average (AV) determined contents and the number of tested products in each category (No.). The pie chart represents the shares of products with determined vitamin B12 content below (blue), within (green), and above (red) the acceptable tolerances for each product category (65–150% in fortified foods, 80–150% in food supplements, and 90–110% for medicines).
The summarized results (Table 1) provide an overview of the overall quality of vitamin B12 products. The published results of the vitamin B12 contents in fortified foods and food supplements are mostly spread between 100 and 150% of its labeled content, thus confirming the assumption that vitamin B12 overages are often added to commercial products. Nevertheless, the vitamin B12 contents were within the acceptable tolerances in the majority of the evaluated fortified foods (69%) and food supplements (87%). However, the available published data discloses vitamin B12 contents above the highest acceptable tolerance (150%) in a significant share of evaluated fortified foods (31%) and food supplements (11%), which reveal the need for more strict quality control of such products. The results on vitamin B12 contents in the evaluated medicines are also very concerning, especially in view of the significantly lower determined vitamin B12 content (<10% of the labeled content in three medicines and <80% of the labeled content in five additional medicines), which may be critical in the treatment of its deficiency. However, as the majority of these results on vitamin B12 content in medicines are outdated and published between 1956 and 2000, this field should be updated with some more recent data to draw firmer conclusions.
9. Strategies for Vitamin B12 Stabilization
Proper vitamin B12 stabilization and content in relation to the values declared on a label is very important in ensuring the quality, efficacy, and safety of vitamin B12 preparations. Therefore, a part of the scientific community has recently focused on researching possibilities for vitamin B12 stabilization, most of which are based on the minimization of the destabilizing factors, the addition of proper stabilizers, or the application of some technological processes to enhance its stability. Considering the aforementioned hydrolytic, thermal, oxido-reductive, and photolytic instability, vitamin B12 can be significantly stabilized by adjusting the pH of the solution to 4–7, storing it at a lower temperature, protecting it from light, oxidants, and reductants, and formulating it into solid dosage forms [145,150,161,164].
The most recognized additives that stabilize vitamin B12 include sorbitol, ferric salts (ferric chloride and saccharated iron oxide), whey proteins (alpha-lactalbumin, beta-lactoglobulin, and lactoferrin), and halide salts (potassium, magnesium, and calcium halides). The protective effect of sorbitol on the stability of vitamin B12 was first mentioned by Barr et al. (1957), which was explained by its ability to decrease the quantity of available water that promotes its hydrolysis [184]. After a research intermission, the stabilizing effect of sorbitol on CNCbl, MeCbl, and OHCbl in solutions and medical food has been recently evaluated in more detail. Hadinata Lie et al. showed that sorbitol significantly reduced the extent of photodegradation of CNCbl (from 3.8% to 0% degradation loss after 60 min of exposure to UV light), OHCbl (from 28% to 10% loss), and especially MeCbl (from 39% to 1% loss) in solutions. Similarly, they showed that the addition of sorbitol to their solutions significantly improved the thermal stability of all three evaluated vitamin B12 forms (from 38% to 1% degradation loss of CNCbl, from 34% to 10% for OHCbl, and from 38% to 20% for MeCbl after 60 min at 100 °C). The stability of CNCbl, OHCbl, and MeCbl in the presence of ascorbic acid or both thiamine and nicotinic acid also significantly improved in the presence of sorbitol. Moreover, sorbitol effectively protected CNCbl and MeCbl against acidic and alkaline hydrolysis but did not have a sufficient stabilizing effect on the hydrolysis of OHCbl [145]. Sorbitol, being a polyol sweetener with an antihyperglycemic effect, is a common ingredient in the food and pharmaceutical industry, including medical food for diabetic patients. Therefore, Lee et al. evaluated its adequacy as a CNCbl stabilizing agent during the production process of medical food. In addition to its protective effect on the thermal and ascorbic acid-induced degradation of CNCbl in solutions, Lee et al. also demonstrated that sorbitol efficiently protected CNCbl against degradation in medical foods, caused by heat (two-step sterilization) and the food matrix, which also contained ascorbic acid [181].
Similar to the case of sorbitol, the stabilizing effect of ferric chloride on vitamin B12 stability in the presence of reducing agents, first mentioned by Mukherjee in 1959 [190], was recently explored in more detail by Lee et al. [181]. The protective effect of ferric chloride is based on its oxidizing ability, which protects the B12 from reducing agents such as ascorbic acid [142,181,190]. Lee et al. showed that ferric chloride reduced CNCbl degradation, caused by the combination of ascorbic acid and heat, to a minimum. However, they concluded that despite its protective effects, ferric chloride is less appropriate than sorbitol for CNCbl stabilization in commercial products because of its catalyzing effect on the oxidation of ascorbic acid, which is a common ingredient of multivitamin products. In addition, there are limitations on its added amounts due to the recommended daily intake of chlorine and iron [181]. Ichikawa et al. showed that the incompatibility of vitamin B12 and ascorbic acid can be also overcome by the addition of various halide salts. They demonstrated that the presence of potassium, magnesium, and calcium halides improves the stability of both CNCbl and ascorbic acid in the pH range of 3.5–5.3 and that their stabilization effect increases with an increase in the concentrations and the atomic number of the halide anion Cl− < Br− < I−) [242].
Vitamin B12 complexation with whey proteins (alpha-lactalbumin, beta-lactoglobulin, and lactoferrin) is also an effective approach for its stabilization. Wang et al. evaluated the effect of alpha-lactalbumin and beta-lactoglobulin on the thermal and photostability of CNCbl and AdoCbl and showed an increase in their stability by 10–30%. Thus, they showed that these whey proteins improve the stability of CNCbl and AdoCbl upon exposure to heat and light during food processing and also during in vitro stomach digestion, leading to their improved bioavailability [243]. Similarly, Uchida et al. patented the production of the CNCbl-lactoferrin complex, in which lactoferrin provided a considerable increase in CNCbl stability against acidic hydrolysis. Due to the high photostability of the thus prepared complex, the innovators claim its suitability for widespread use in foods and drinks fortification and the production of medicines [244].
Researchers have also responded to the high demand for effective vitamin B12 stabilization for fortification and supplementation purposes by exploring technological approaches for improving its stability and compatibility with other, commonly present ingredients, such as other vitamins and, particularly, ascorbic acid or minerals. Such strategies include the use of viscosigens, co-crystallization and microencapsulation techniques, vitamin B12 incorporation in bilayer tablets, liposomal preparations, and nanosystems [245,246,247].
Grissom et al. reported that CNCbl can be significantly stabilized against photolysis in anaerobic conditions (aqueous solutions purged with nitrogen or argon for more than 30 min and sealed with a rubber septum) by the use of viscosigens such as glycerol (≥25%) or Ficoll®, which is a hydrophilic copolymer of saccharose and epichlorohydrin (≥10%). They propose the decrease in diffusion and enhancement of radical-pair recombination as the likely mechanism for CNCbl stabilization by viscosigens [245].
Bajaj and Singhal approached the stabilization of CNCbl using co-crystallization, which is a previously reported and widely used technique for the stabilization of different sensitive compounds in the food industry, including flavor compounds, antioxidants, and some vitamins (K3 and D3) [246,248,249]. They reported that the CNCbl co-crystallization with sucrose and gum acacia, as a hydrocolloid (2.5 g/100 g sucrose), results in substantial CNCbl stabilization, which is additionally enhanced by storage at lower temperature and RH (25 °C/33% RH). At these storage conditions, the authors report a low first-order degradation rate constant (0.001 day−1) and a half-life of 693 days. The authors conclude that co-crystallization is a simple, easily adoptable, and cost-effective approach for CNCbl stabilization and its food fortification [246].
The development of a stable food supplement formulation, containing CNCbl in combination with all other B-complex vitamins (thiamine mononitrate, riboflavin, nicotinamide, calcium pantothenate, pyridoxine hydrochloride, folic acid, and biotin), as well as vitamins C (ascorbic acid), A (retinyl acetate), D (cholecalciferol), and E (tocopherol) and the following minerals: copper, iodine, magnesium, manganese, molybdenum, silicon, and zinc, was addressed by Rajakumari et al. [247]. In this recent research, they pointed out the disadvantages of conventional food supplement formulations (film-coated and sugar-coated tablets), including decreased stability of the active ingredients and their interactions with other ingredients and highlighted the need for the development of contemporary food supplement formulations with improved properties. They described the formulation of a film-coated bilayer tablet using a direct compression method in which vitamins are incorporated in the first and minerals in the second layers. By optimizing the composition of the bilayer tablet, through the inclusion of microcrystalline cellulose, carmellose calcium, sodium starch glycolate, croscarmellose sodium, and pregelatinised starch as excipients, they achieved appropriate physicochemical stability for all the incorporated vitamins and minerals. The authors claim the determined shelf-life (≥ 90% of the initial content of all the active ingredients) of 24 months increased production and time- and cost-effectiveness as the main benefits of the proposed approach for its applicability in the food and pharmaceutical industry [247].
Another of the explored approaches for vitamin B12 stabilization is based on the particle size reduction and includes microencapsulation, nanoencapsulation, and the production of liposomes. Microencapsulation is an advanced and emerging technology in the food and pharmaceutic industry for the protection of unstable food components or active ingredients by their incorporation into particles of micrometer size [250,251]. Several techniques, including spray-drying [252,253], spray-chilling [254], and emulsion technique [255] have been reported for CNCbl microencapsulation. All these authors report that by optimization of the microencapsulation process and composition, high CNCbl encapsulation efficiency and stability can be achieved, causing this technique to be a promising solution for the development of quality and stable vitamin B12 products. For instance, Carlan et al. incorporated CNCbl into modified chitosan microparticles and achieved a ≤ 10% CNCbl loss after 6 months of storage at 25 °C [253], while Mazzocato et al. report a ≤ 5% loss of CNCbl incorporated into solid lipid microparticles after 4 months of storage at 25 °C, compared to the ≈25% loss of its free form under the same conditions [254]. Further reduction in the particle size to the nano-scale and the use of nanocarrier-based delivery systems is another promising approach for the improvement of vitamin B12 stability [256]. In that sense, Britto et al. describe the encapsulation of CNCbl into polymeric nanoparticles, formed by chitosan and tripolyphosphate. Under the tested conditions, the encapsulated and non-encapsulated control (aqueous CNCbl solution (pH 6.8) at 25 °C, in the dark for three weeks) remained stable [257]. Unfortunately, the conditions used did not allow for the observation of the stabilizing effect of B12 nanoencapsulation, while the stabilization effect of nanoencapsulation was observed for vitamin C. Maiorova et al. emphasized the applicability of nanosystems as carriers for CNCbl by the development and evaluation of nanoengineered polymer capsules and lyotropic liquid-crystalline nanosystems [258]. The incorporation of vitamin B12 into liposomes has also been shown as an effective approach for its protection from the effect of destabilizing factors [259,260,261]. Liposomes are closed spherical vesicles with a unique structure, composed of a phospholipid bilayer, enabling vitamin B12 incorporation inside the center aqueous phase, which protects it from degradation. This approach was utilized by Arsalan et al., who investigated the effect of CNCbl entrapment in liposomal preparations on its photostability. They determined a two-six-fold lower photolysis rate of CNCbl in liposomes, compared to the unentrapped CNCbl under the same conditions. This result can be further improved by alteration in the used phospholipid (phosphatidylcholine) and the size of the liposomes [259]. Similarly, Lee et at. demonstrated that CNCbl incorporation into ordinary or chitosan-coated liposomes enhances its stabilization in the presence of ascorbic acid. The stabilization effect was more evident at higher storage temperatures and can be exploited for the production of quality food supplements containing both CNCbl and ascorbic acid [261].
10. Conclusions
Vitamin B12 has been recognized as an essential micronutrient for almost one century. In the form of medicines, it is mainly used for the treatment of vitamin B12 deficiency, whereas vitamin B12 supplements (including fortified foods) are commonly used for the prevention of its deficiency, especially in high-risk population groups (vegetarians, vegans, the elderly, pregnant, and lactating women). Four vitamin B12 forms—CNCbl, OHCbl, AdoCbl, and MeCbl are authorized for supplementation purposes. The overview of the chemical, biochemical, and pharmacological aspects of these vitamin B12 forms, reported in this review, provides the necessary background to understand the factors affecting the stability of this micronutrient in simple and complex matrices. CNCbl has been traditionally used in vitamin B12 supplements and medicines, primarily due to its superior stability compared to other vitamin B12 forms. Thus, it is also the most researched form both in terms of efficacy and stability, but its use is recently on the decline, due to concerns about the potential accumulation of cyanide in human tissues. In that sense, MeCbl, OHCbl, and AdoCbl could be more appropriate and promising vitamin B12 forms. However, their intrinsic lower stability is an obstacle that needs to be overcome for their wide use for vitamin B12 supplementation in clinical practice. Stability is one of the main aspects in ensuring the quality, efficacy, and safety of the vitamins. Based on the present review of the literature, we conclude that research on strategies for vitamin B12 stabilization is principally limited to CNCbl. However, considering the inferior stability of AdoCbl, OHCbl, and MeCbl compared to CNCbl, such research would be more valuable for these three vitamin B12 forms. In any case, we believe that this extensive review of the approaches for stabilization of different vitamin B12s will provide researchers, manufacturers of fortified foods, food supplements, and medicines, and interested professionals with an overview of this issue and contribute toward an increase in the quality of vitamin B12-containing products.
Author Contributions
Conceptualization, Ž.T.R., R.R., N.H. and S.G.; Formal Analysis, Ž.T.R.; Investigation, Ž.T.R., R.R., N.H. and S.G.; Writing—Original Draft Preparation, Ž.T.R.; Writing—Review and Editing, Ž.T.R., R.R., N.H. and S.G.; Project Administration, S.G.; Funding Acquisition, R.R. and S.G. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Slovenian Research Agency (ARRS) [grant number P1-0189] and by the University of Trieste [grant number FRA-2022].
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Sample Availability
Not applicable.
References
- EFSA Panel on Dietetic Products, Nutrition, and Allergies (NDA) Scientific Opinion on Dietary Reference Values for Cobalamin (Vitamin B12). EFSA J. 2015, 13, 4150. [CrossRef]
- Kumar, N. Chapter 60-Neurologic Aspects of Cobalamin (B12) Deficiency. In Handbook of Clinical Neurology; Neurologic Aspects of Systemic Disease Part II; Biller, J., Ferro, J.M., Eds.; Elsevier: Amsterdam, The Netherlands, 2014; Volume 120, pp. 915–926. [Google Scholar]
- Jägerstad, M.; Arkbåge, K. Cobalamins | Properties and Determination. In Encyclopedia of Food Sciences and Nutrition, 2nd ed.; Caballero, B., Ed.; Academic Press: Oxford, UK, 2003; pp. 1419–1427. ISBN 978-0-12-227055-0. [Google Scholar]
- Anes, J.M.; Beck, R.A.; Brink, J.J.; Goldberg, R.J. Nitritocobalamin and Nitrosocobalamin May Be Confused with Sulfitocobalamin Using Cation-Exchange Chromatography. J. Chromatogr. B Biomed. Appl. 1994, 660, 180–185. [Google Scholar] [CrossRef]
- Wiley, T.E.; Miller, N.A.; Miller, W.R.; Sofferman, D.L.; Lodowski, P.; Toda, M.J.; Jaworska, M.; Kozlowski, P.M.; Sension, R.J. Off to the Races: Comparison of Excited State Dynamics in Vitamin B12 Derivatives Hydroxocobalamin and Aquocobalamin. J. Phys. Chem. A 2018, 122, 6693–6703. [Google Scholar] [CrossRef]
- Watanabe, F. Vitamin B12 Sources and Bioavailability. Exp. Biol. Med. 2007, 232, 1266–1274. [Google Scholar] [CrossRef]
- Watanabe, F.; Bito, T. Vitamin B12 Sources and Microbial Interaction. Exp. Biol. Med. 2018, 243, 148–158. [Google Scholar] [CrossRef]
- Watanabe, F.; Yabuta, Y.; Bito, T.; Teng, F. Vitamin B₁₂-Containing Plant Food Sources for Vegetarians. Nutrients 2014, 6, 1861–1873. [Google Scholar] [CrossRef]
- Langan, R.C.; Goodbred, A.J. Vitamin B12 Deficiency: Recognition and Management. AFP 2017, 96, 384–389. [Google Scholar]
- Ryan-Harshman, M.; Aldoori, W. Vitamin B12 and Health. Can. Fam. Physician 2008, 54, 536–541. [Google Scholar]
- Schnellbaecher, A.; Binder, D.; Bellmaine, S.; Zimmer, A. Vitamins in Cell Culture Media: Stability and Stabilization Strategies. Biotechnol. Bioeng. 2019, 116, 1537–1555. [Google Scholar] [CrossRef]
- Commission Regulation (EC) No 1170/2009 of 30 November 2009 Amending Directive 2002/46/EC of the European Parliament and of Council and Regulation (EC) No 1925/2006 of the European Parliament and of the Council as Regards the Lists of Vitamin and Minerals and Their Forms That Can Be Added to Foods, Including Food Supplements (Text with EEA Relevance). Available online: https://www.legislation.gov.uk/eur/2009/1170/contents (accessed on 18 January 2022).
- Shipton, M.J.; Thachil, J. Vitamin B12 Deficiency—A 21st Century Perspective. Clin. Med. 2015, 15, 145–150. [Google Scholar] [CrossRef]
- Romain, M.; Sviri, S.; Linton, D.M.; Stav, I.; van Heerden, P.V. The Role of Vitamin B12 in the Critically Ill—A Review. Anaesth. Intensive Care 2016, 44, 447–452. [Google Scholar] [CrossRef] [PubMed]
- Brito, A.; Habeych, E.; Silva-Zolezzi, I.; Galaffu, N.; Allen, L.H. Methods to Assess Vitamin B12 Bioavailability and Technologies to Enhance Its Absorption. Nutr. Rev. 2018, 76, 778–792. [Google Scholar] [CrossRef] [PubMed]
- Allen, L.H. Causes of Vitamin B12 and Folate Deficiency. Food Nutr. Bull. 2008, 29, S20–S34. [Google Scholar] [CrossRef] [PubMed]
- Kozyraki, R.; Cases, O. Vitamin B12 Absorption: Mammalian Physiology and Acquired and Inherited Disorders. Biochimie 2013, 95, 1002–1007. [Google Scholar] [CrossRef]
- Randaccio, L.; Geremia, S.; Demitri, N.; Wuerges, J. Vitamin B12: Unique Metalorganic Compounds and the Most Complex Vitamins. Molecules 2010, 15, 3228–3259. [Google Scholar] [CrossRef]
- Schneider, Z.; Stroinski, A. Comprehensive B12: Chemistry, Biochemistry, Nutrition, Ecology, Medicine; Walter de Gruyter: Berlin, Germany; Boston, MA, USA, 2011; ISBN 978-3-11-084479-5. [Google Scholar]
- van Asselt, D.Z.B.; Merkus, F.W.H.M.; Russel, F.G.M.; Hoefnagels, W.H.L. Nasal Absorption of Hydroxocobalamin in Healthy Elderly Adults. Br. J. Clin. Pharmacol. 1998, 45, 83–86. [Google Scholar] [CrossRef]
- Nascobal® Nasal Spray (Cyanocobalamin, USP) 500 Mcg/Spray Pack Insert. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2005/021642lbl.pdf (accessed on 18 January 2022).
- Dave, H.; Agarwal, V.; Maseeh, A.; Patel, K. Novel Methylcobalamin Nasal Spray versus Intramuscular Injection: A Randomized, Crossover Comparative Bioavailability Study. Int. J. Endocrinol. Metabol. Dis. 2021, 7, 1–6. [Google Scholar] [CrossRef]
- Quadros, E.V.; Sequeira, J.M. Cellular Uptake of Cobalamin: Transcobalamin and the TCblR/CD320 Receptor. Biochimie 2013, 95, 1008–1018. [Google Scholar] [CrossRef]
- Mascarenhas, R.; Li, Z.; Gherasim, C.; Ruetz, M.; Banerjee, R. The Human B12 Trafficking Protein CblC Processes Nitrocobalamin. J. Biol. Chem. 2020, 295, 9630–9640. [Google Scholar] [CrossRef]
- Moreno-Garcia, M.A.; Rosenblatt, D.S.; Jerome-Majewska, L.A. Vitamin B12 Metabolism during Pregnancy and in Embryonic Mouse Models. Nutrients 2013, 5, 3531–3550. [Google Scholar] [CrossRef]
- Kim, J.; Hannibal, L.; Gherasim, C.; Jacobsen, D.W.; Banerjee, R. A Human Vitamin B12 Trafficking Protein Uses Glutathione Transferase Activity for Processing Alkylcobalamins. J. Biol. Chem. 2009, 284, 33418–33424. [Google Scholar] [CrossRef] [PubMed]
- Yamada, K.; Gherasim, C.; Banerjee, R.; Koutmos, M. Structure of Human B12 Trafficking Protein CblD Reveals Molecular Mimicry and Identifies a New Subfamily of Nitro-FMN Reductases. J. Biol. Chem. 2015, 290, 29155–29166. [Google Scholar] [CrossRef] [PubMed]
- Obeid, R.; Fedosov, S.N.; Nexo, E. Cobalamin Coenzyme Forms Are Not Likely to Be Superior to Cyano- and Hydroxyl-Cobalamin in Prevention or Treatment of Cobalamin Deficiency. Mol. Nutr. Food Res. 2015, 59, 1364–1372. [Google Scholar] [CrossRef] [PubMed]
- Reizenstein, P.; Ek, G.; Matthews, C.M. Vitamin B12 Kinetics in Man. Implications on Total-Body-B12-Determinations, Human Requirements, and Normal and Pathological Cellular B12 Uptake. Phys. Med. Biol. 1966, 11, 295–306. [Google Scholar] [CrossRef] [PubMed]
- O’Leary, F.; Samman, S. Vitamin B12 in Health and Disease. Nutrients 2010, 2, 299–316. [Google Scholar] [CrossRef]
- Wuerges, J.; Geremia, S.; Randaccio, L. Structural Study on Ligand Specificity of Human Vitamin B12 Transporters. Biochem. J. 2007, 403, 431–440. [Google Scholar] [CrossRef]
- Fedosov, S.N.; Grissom, C.B.; Fedosova, N.U.; Moestrup, S.K.; Nexø, E.; Petersen, T.E. Application of a fluorescent cobalamin analogue for analysis of the binding kinetics. A study employing recombinant human transcobalamin and intrinsic factor. FEBS J. 2006, 273, 4742–4753. [Google Scholar] [CrossRef]
- Wuerges, J.; Geremia, S.; Fedosov, S.N.; Randaccio, L. Vitamin B12 Transport Proteins: Crystallographic Analysis of Beta-Axial Ligand Substitutions in Cobalamin Bound to Transcobalamin. IUBMB Life 2007, 59, 722–729. [Google Scholar] [CrossRef]
- Siega, P.; Wuerges, J.; Arena, F.; Gianolio, E.; Fedosov, S.N.; Dreos, R.; Geremia, S.; Aime, S.; Randaccio, L. Release of Toxic Gd3+Ions to Tumour Cells by Vitamin B12 Bioconjugates. Chemistry 2009, 15, 7980–7989. [Google Scholar] [CrossRef]
- Wuerges, J.; Garau, G.; Geremia, S.; Fedosov, S.N.; Petersen, T.E.; Randaccio, L. Structural Basis for Mammalian Vitamin B12 Transport by Transcobalamin. Proc. Natl. Acad. Sci. USA 2006, 103, 4386–4391. [Google Scholar] [CrossRef]
- Lyon, P.; Strippoli, V.; Fang, B.; Cimmino, L. B Vitamins and One-Carbon Metabolism: Implications in Human Health and Disease. Nutrients 2020, 12, 2867. [Google Scholar] [CrossRef] [PubMed]
- Rizzo, G.; Laganà, A.S.; Rapisarda, A.M.C.; La Ferrera, G.M.G.; Buscema, M.; Rossetti, P.; Nigro, A.; Muscia, V.; Valenti, G.; Sapia, F.; et al. Vitamin B12 among Vegetarians: Status, Assessment and Supplementation. Nutrients 2016, 8, 767. [Google Scholar] [CrossRef] [PubMed]
- Takahashi-Iñiguez, T.; García-Hernandez, E.; Arreguín-Espinosa, R.; Flores, M.E. Role of Vitamin B12 on Methylmalonyl-CoA Mutase Activity. J. Zhejiang. Univ. Sci. B 2012, 13, 423–437. [Google Scholar] [CrossRef] [PubMed]
- Oh, R.C.; Brown, D.L. Vitamin B12 Deficiency. AFP 2003, 67, 979–986. [Google Scholar]
- Herrmann, W.; Obeid, R. Cobalamin Deficiency. Subcell. Biochem. 2012, 56, 301–322. [Google Scholar] [CrossRef]
- Miller, A.; Korem, M.; Almog, R.; Galboiz, Y. Vitamin B12, Demyelination, Remyelination and Repair in Multiple Sclerosis. J. Neurol. Sci. 2005, 233, 93–97. [Google Scholar] [CrossRef]
- Zelder, F. Recent Trends in the Development of Vitamin B12 Derivatives for Medicinal Applications. Chem. Commun. 2015, 51, 14004–14017. [Google Scholar] [CrossRef]
- Froese, D.S.; Fowler, B.; Baumgartner, M.R. Vitamin B12, Folate, and the Methionine Remethylation Cycle—Biochemistry, Pathways, and Regulation. J. Inherit. Metab. Dis. 2019, 42, 673–685. [Google Scholar] [CrossRef]
- Selhub, J. Folate, Vitamin B12 and Vitamin B6 and One Carbon Metabolism. J. Nutr. Health Aging 2002, 6, 39–42. [Google Scholar]
- Azzini, E.; Raguzzini, A.; Polito, A. A Brief Review on Vitamin B12 Deficiency Looking at Some Case Study Reports in Adults. Int. J. Mol. Sci. 2021, 22, 9694. [Google Scholar] [CrossRef]
- Green, R.; Allen, L.H.; Bjørke-Monsen, A.-L.; Brito, A.; Guéant, J.-L.; Miller, J.W.; Molloy, A.M.; Nexo, E.; Stabler, S.; Toh, B.-H.; et al. Vitamin B12 Deficiency. Nat. Rev. Dis. Primers 2017, 3, 17040. [Google Scholar] [CrossRef] [PubMed]
- Nalder, L.; Zheng, B.; Chiandet, G.; Middleton, L.T.; de Jager, C.A. Vitamin B12 and Folate Status in Cognitively Healthy Older Adults and Associations with Cognitive Performance. J. Nutr. Health Aging 2021, 25, 287–294. [Google Scholar] [CrossRef]
- Pawlak, R. Vitamin B12 Status Is a Risk Factor for Bone Fractures among Vegans. Med. Hypotheses 2021, 153, 110625. [Google Scholar] [CrossRef] [PubMed]
- Desmond, M.A.; Sobiecki, J.G.; Jaworski, M.; Płudowski, P.; Antoniewicz, J.; Shirley, M.K.; Eaton, S.; Książyk, J.; Cortina-Borja, M.; De Stavola, B.; et al. Growth, Body Composition, and Cardiovascular and Nutritional Risk of 5- to 10-y-Old Children Consuming Vegetarian, Vegan, or Omnivore Diets. Am. J. Clin. Nutr. 2021, 113, 1565–1577. [Google Scholar] [CrossRef] [PubMed]
- Saravanan, P.; Sukumar, N.; Adaikalakoteswari, A.; Goljan, I.; Venkataraman, H.; Gopinath, A.; Bagias, C.; Yajnik, C.S.; Stallard, N.; Ghebremichael-Weldeselassie, Y.; et al. Association of Maternal Vitamin B12 and Folate Levels in Early Pregnancy with Gestational Diabetes: A Prospective UK Cohort Study (PRiDE Study). Diabetologia 2021, 64, 2170–2182. [Google Scholar] [CrossRef]
- Behere, R.V.; Deshmukh, A.S.; Otiv, S.; Gupte, M.D.; Yajnik, C.S. Maternal Vitamin B12 Status During Pregnancy and Its Association with Outcomes of Pregnancy and Health of the Offspring: A Systematic Review and Implications for Policy in India. Front. Endocrinol. 2021, 12, 619176. [Google Scholar] [CrossRef]
- Rashid, S.; Meier, V.; Patrick, H. Review of Vitamin B12 Deficiency in Pregnancy: A Diagnosis Not to Miss as Veganism and Vegetarianism Become More Prevalent. Eur. J. Haematol. 2021, 106, 450–455. [Google Scholar] [CrossRef]
- Devalia, V.; Hamilton, M.S.; Molloy, A.M. British Committee for Standards in Haematology Guidelines for the Diagnosis and Treatment of Cobalamin and Folate Disorders. Br. J. Haematol. 2014, 166, 496–513. [Google Scholar] [CrossRef]
- Ströhle, A.; Richter, M.; González-Gross, M.; Neuhäuser-Berthold, M.; Wagner, K.-H.; Leschik-Bonnet, E.; Egert, S.; for the German Nutrition Society (DGE). The Revised D-A-CH-Reference Values for the Intake of Vitamin B12: Prevention of Deficiency and Beyond. Mol. Nutr. Food Res. 2019, 63, 1801178. [Google Scholar] [CrossRef]
- de Benoist, B. Conclusions of a WHO Technical Consultation on Folate and Vitamin B12 Deficiencies. Food Nutr. Bull. 2008, 29, S238–S244. [Google Scholar] [CrossRef]
- Pawlak, R.; Lester, S.E.; Babatunde, T. The Prevalence of Cobalamin Deficiency among Vegetarians Assessed by Serum Vitamin B12: A Review of Literature. Eur. J. Clin. Nutr. 2014, 68, 541–548. [Google Scholar] [CrossRef] [PubMed]
- Mądry, E.; Lisowska, A.; Grebowiec, P.; Walkowiak, J. The Impact of Vegan Diet on B12 Status in Healthy Omnivores: Five-Year Prospective Study. Acta. Sci. Pol. Technol. Aliment. 2012, 11, 209–212. [Google Scholar] [PubMed]
- Office of Dietary Supplements-Vitamin B12. Available online: https://ods.od.nih.gov/factsheets/VitaminB12-HealthProfessional/ (accessed on 6 October 2022).
- Al Amin, A.S.M.; Gupta, V. Vitamin B12 (Cobalamin). In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar]
- Bensky, M.J.; Ayalon-Dangur, I.; Ayalon-Dangur, R.; Naamany, E.; Gafter-Gvili, A.; Koren, G.; Shiber, S. Comparison of Sublingual vs. Intramuscular Administration of Vitamin B12 for the Treatment of Patients with Vitamin B12 Deficiency. Drug Deliv. Transl. Res. 2019, 9, 625–630. [Google Scholar] [CrossRef] [PubMed]
- Estourgie-van Burk, G.F.; van der Kuy, P.H.M.; de Meij, T.G.; Benninga, M.A.; Kneepkens, C.M.F. Intranasal Treatment of Vitamin B12 Deficiency in Children. Eur. J. Pediatr. 2020, 179, 349–352. [Google Scholar] [CrossRef]
- Verma, D.; Chandra, J.; Kumar, P.; Shukla, S.; Sengupta, S. Efficacy of Oral Methylcobalamin in Treatment of Vitamin B12 Deficiency Anemia in Children. Pediatr. Blood. Cancer. 2017, 64, e26698. [Google Scholar] [CrossRef]
- Andrès, E.; Zulfiqar, A.-A.; Vogel, T. State of the Art Review: Oral and Nasal Vitamin B12 Therapy in the Elderly. QJM 2020, 113, 5–15. [Google Scholar] [CrossRef]
- Houle, S.K.D.; Kolber, M.R.; Chuck, A.W. Should Vitamin B12 Tablets Be Included in More Canadian Drug Formularies? An Economic Model of the Cost-Saving Potential from Increased Utilisation of Oral versus Intramuscular Vitamin B12 Maintenance Therapy for Alberta Seniors. BMJ Open 2014, 4, e004501. [Google Scholar] [CrossRef]
- Vidal-Alaball, J.; Butler, C.C.; Potter, C.C. Comparing Costs of Intramuscular and Oral Vitamin B12 Administration in Primary Care: A Cost-Minimization Analysis. Eur. J. Gen. Pr. 2006, 12, 169–173. [Google Scholar] [CrossRef]
- Sanz-Cuesta, T.; González-Escobar, P.; Riesgo-Fuertes, R.; Garrido-Elustondo, S.; del Cura-González, I.; Martín-Fernández, J.; Escortell-Mayor, E.; Rodríguez-Salvanés, F.; García-Solano, M.; González-González, R.; et al. Oral versus Intramuscular Administration of Vitamin B12 for the Treatment of Patients with Vitamin B12 Deficiency: A Pragmatic, Randomised, Multicentre, Non-Inferiority Clinical Trial Undertaken in the Primary Healthcare Setting (Project OB12). BMC Public Health 2012, 12, 394. [Google Scholar] [CrossRef]
- Bolaman, Z.; Kadikoylu, G.; Yukselen, V.; Yavasoglu, I.; Barutca, S.; Senturk, T. Oral versus Intramuscular Cobalamin Treatment in Megaloblastic Anemia: A Single-Center, Prospective, Randomized, Open-Label Study. Clin. Ther. 2003, 25, 3124–3134. [Google Scholar] [CrossRef]
- Kuzminski, A.M.; Del Giacco, E.J.; Allen, R.H.; Stabler, S.P.; Lindenbaum, J. Effective Treatment of Cobalamin Deficiency with Oral Cobalamin. Blood 1998, 92, 1191–1198. [Google Scholar] [CrossRef] [PubMed]
- Castelli, M.C.; Friedman, K.; Sherry, J.; Brazzillo, K.; Genoble, L.; Bhargava, P.; Riley, M.G.I. Comparing the Efficacy and Tolerability of a New Daily Oral Vitamin B12 Formulation and Intermittent Intramuscular Vitamin B12 in Normalizing Low Cobalamin Levels: A Randomized, Open-Label, Parallel-Group Study. Clin. Ther. 2011, 33, 358–371.e2. [Google Scholar] [CrossRef] [PubMed]
- Saraswathy, A.; Dutta, A.; Simon, E.; Chacko, A. Randomized Open Label Trial Comparing Efficacy of Oral versus Intramuscular Vitamin B12 Supplementation for Treatment of Vitamin B12 Deficiency. Gastroenterology 2012, 142, S-216. [Google Scholar] [CrossRef]
- Sanz-Cuesta, T.; Escortell-Mayor, E.; Cura-Gonzalez, I.; Martin-Fernandez, J.; Riesgo-Fuertes, R.; Garrido-Elustondo, S.; Mariño-Suárez, J.E.; Álvarez-Villalba, M.; Gómez-Gascón, T.; González-García, I.; et al. Oral versus Intramuscular Administration of Vitamin B12 for Vitamin B12 Deficiency in Primary Care: A Pragmatic, Randomised, Non-Inferiority Clinical Trial (OB12). BMJ Open 2020, 10, e033687. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Li, L.; Qin, L.L.; Song, Y.; Vidal-Alaball, J.; Liu, T.H. Oral Vitamin B12 versus Intramuscular Vitamin B12 for Vitamin B12 Deficiency. Cochrane Database Syst. Rev. 2018, 2018, CD004655. [Google Scholar] [CrossRef]
- Masucci, L.; Goeree, R. Vitamin B12 Intramuscular Injections Versus Oral Supplements. Ont. Health Technol. Assess. Ser. 2013, 13, 1–24. [Google Scholar]
- van Walraven, C.; Austin, P.; Naylor, C.D. Vitamin B12 Injections versus Oral Supplements. How Much Money Could Be Saved by Switching from Injections to Pills? Can. Fam. Physician 2001, 47, 79–86. [Google Scholar]
- Vidal-Alaball, J.; Butler, C.C.; Cannings-John, R.; Goringe, A.; Hood, K.; McCaddon, A.; McDowell, I.; Papaioannou, A. Oral Vitamin B12 versus Intramuscular Vitamin B12 for Vitamin B12 Deficiency. Cochrane Database Syst. Rev. 2005, 23, CD004655. [Google Scholar] [CrossRef]
- Tugba-Kartal, A.; Cagla-Mutlu, Z. Comparison of Sublingual and Intramuscular Administration of Vitamin B12 for the Treatment of Vitamin B12 Deficiency in Children. RIC 2021, 72, 4648. [Google Scholar] [CrossRef]
- Orhan Kiliç, B.; Kiliç, S.; Şahin Eroğlu, E.; Gül, E.; Belen Apak, F.B. Sublingual Methylcobalamin Treatment Is as Effective as Intramuscular and Peroral Cyanocobalamin in Children Age 0–3 Years. Hematology 2021, 26, 1013–1017. [Google Scholar] [CrossRef]
- Varkal, M.A.; Karabocuoglu, M. Efficiency of the Sublingual Route in Treating B12 Deficiency in Infants. Int. J. Vitam. Nutr. Res. 2021. [Google Scholar] [CrossRef] [PubMed]
- Sharabi, A.; Cohen, E.; Sulkes, J.; Garty, M. Replacement Therapy for Vitamin B12 Deficiency: Comparison between the Sublingual and Oral Route. Br. J. Clin. Pharmacol. 2003, 56, 635–638. [Google Scholar] [CrossRef] [PubMed]
- Parry-Strong, A.; Langdana, F.; Haeusler, S.; Weatherall, M.; Krebs, J. Sublingual Vitamin B12 Compared to Intramuscular Injection in Patients with Type 2 Diabetes Treated with Metformin: A Randomised Trial. N. Z. Med. J. 2016, 129, 67–75. [Google Scholar] [PubMed]
- Del Bo’, C.; Riso, P.; Gardana, C.; Brusamolino, A.; Battezzati, A.; Ciappellano, S. Effect of Two Different Sublingual Dosages of Vitamin B12 on Cobalamin Nutritional Status in Vegans and Vegetarians with a Marginal Deficiency: A Randomized Controlled Trial. Clin. Nutr. 2019, 38, 575–583. [Google Scholar] [CrossRef]
- van Campen, C.L.M.; Riepma, K.; Visser, F.C. Open Trial of Vitamin B12 Nasal Drops in Adults with Myalgic Encephalomyelitis/Chronic Fatigue Syndrome: Comparison of Responders and Non-Responders. Front. Pharmacol. 2019, 10, 1102. [Google Scholar] [CrossRef]
- Tillemans, M.P.H.; Donders, E.M.V.J.; Verweij, S.L.; Van der Hoeven, R.T.M.; Kalisvaart, K.J. Effect of Administration Route on the Pharmacokinetics of Cobalamin in Elderly Patients: A Randomized Controlled Trial. Curr. Ther. Res. Clin. Exp. 2014, 76, 21–25. [Google Scholar] [CrossRef]
- Randaccio, L.; Geremia, S.; Wuerges, J. Crystallography of Vitamin B12 Proteins. J. Organomet. Chem. 2007, 692, 1198–1215. [Google Scholar] [CrossRef]
- Paul, C.; Brady, D.M. Comparative Bioavailability and Utilization of Particular Forms of B12 Supplements with Potential to Mitigate B12-Related Genetic Polymorphisms. Integr. Med. 2017, 16, 42–49. [Google Scholar]
- European Food Safety Authority (EFSA). 5′-Deoxyadenosylcobalamin and Methylcobalamin as Sources for Vitamin B12 Added as a Nutritional Substance in Food Supplements-Scientific Opinion of the Scientific Panel on Food Additives and Nutrient Sources Added to Food. EFSA J. 2008, 6, 815. [Google Scholar] [CrossRef]
- Gräsbeck, R. Hooked to Vitamin B12 since 1955: A Historical Perspective. Biochimie 2013, 95, 970–975. [Google Scholar] [CrossRef]
- Yazaki, Y.; Chow, G.; Mattie, M. A Single-Center, Double-Blinded, Randomized Controlled Study to Evaluate the Relative Efficacy of Sublingual and Oral Vitamin B-Complex Administration in Reducing Total Serum Homocysteine Levels. J. Altern. Complement. Med. 2006, 12, 881–885. [Google Scholar] [CrossRef]
- Stabler, S.P. Clinical Practice. Vitamin B12 Deficiency. N. Engl. J. Med. 2013, 368, 149–160. [Google Scholar] [CrossRef]
- Seetharam, B.; Yammani, R.R. Cobalamin Transport Proteins and Their Cell-Surface Receptors. Expert Rev. Mol. Med. 2003, 5, 1–18. [Google Scholar] [CrossRef]
- Fedosov, S.N.; Berglund, L.; Fedosova, N.U.; Nexø, E.; Petersen, T.E. Comparative Analysis of Cobalamin Binding Kinetics and Ligand Protection for Intrinsic Factor, Transcobalamin, and Haptocorrin. J. Biol. Chem. 2002, 277, 9989–9996. [Google Scholar] [CrossRef]
- Hippe, E. Changes in Stokes Radius on Binding of Vitamin B12 to Human Intrinsic Factor and Transcobalamins. Biochim. BioPhys. Acta 1970, 208, 337–339. [Google Scholar] [CrossRef]
- Gruber, K.; Puffer, B.; Kräutler, B. Vitamin B12-Derivatives—Enzyme Cofactors and Ligands of Proteins and Nucleic Acids. Chem. Soc. Rev. 2011, 40, 4346–4363. [Google Scholar] [CrossRef]
- Gailus, S.; Höhne, W.; Gasnier, B.; Nürnberg, P.; Fowler, B.; Rutsch, F. Insights into Lysosomal Cobalamin Trafficking: Lessons Learned from CblF Disease. J. Mol. Med. 2010, 88, 459–466. [Google Scholar] [CrossRef]
- Rutsch, F.; Gailus, S.; Miousse, I.R.; Suormala, T.; Sagné, C.; Toliat, M.R.; Nürnberg, G.; Wittkampf, T.; Buers, I.; Sharifi, A.; et al. Identification of a Putative Lysosomal Cobalamin Exporter Altered in the CblF Defect of Vitamin B12 Metabolism. Nat. Genet. 2009, 41, 234–239. [Google Scholar] [CrossRef]
- Oladipo, O.; Rosenblatt, D.S.; Watkins, D.; Miousse, I.R.; Sprietsma, L.; Dietzen, D.J.; Shinawi, M. Cobalamin F Disease Detected by Newborn Screening and Follow-up on a 14-Year-Old Patient. Pediatrics 2011, 128, e1636–e1640. [Google Scholar] [CrossRef]
- Waggoner, D.J.; Ueda, K.; Mantia, C.; Dowton, S.B. Methylmalonic Aciduria (CblF): Case Report and Response to Therapy. Am. J. Med. Genet. 1998, 79, 373–375. [Google Scholar] [CrossRef]
- Chu, R.C.; Begley, J.A.; Colligan, P.D.; Hall, C.A. The Methylcobalamin Metabolism of Cultured Human Fibroblasts. Metabolism 1993, 42, 315–319. [Google Scholar] [CrossRef] [PubMed]
- Gherasim, C.; Lofgren, M.; Banerjee, R. Navigating the B12 Road: Assimilation, Delivery, and Disorders of Cobalamin. J. Biol. Chem. 2013, 288, 13186–13193. [Google Scholar] [CrossRef] [PubMed]
- Quadros, E.V.; Jackson, B.; Hoffbrand, A.V.; Linnell, J.C. Interconversion of Cobalamins in Human Lymphocytes in Vitro and the Influence of Nitrous Oxide on Synthesis of Cobalamin Coenzymes. In Interconversion of Cobalamins in Human Lymphocytes In Vitro and the Influence of Nitrous Oxide on Synthesis of Cobalamin Coenzymes; De Gruyter: Berlin, Germany, 2019; pp. 1045–1054. ISBN 978-3-11-151082-8. [Google Scholar]
- Banerjee, R. B12 Trafficking in Mammals: A for Coenzyme Escort Service. ACS Chem. Biol. 2006, 1, 149–159. [Google Scholar] [CrossRef] [PubMed]
- Quadros, E.V. Advances in the Understanding of Cobalamin Assimilation and Metabolism. Br. J. Haematol. 2010, 148, 195–204. [Google Scholar] [CrossRef]
- Kacpura, A.; Frigeni, M.; Gunther, K.; Farach, L. Clinical and Biochemical Outcomes in Cobalamin C Deficiency with Use of High-Dose Hydroxocobalamin in the Early Neonatal Period. Am. J. Med. Genet. A 2022, 188, 1831–1835. [Google Scholar] [CrossRef]
- Andersson, H.C.; Shapira, E. Biochemical and Clinical Response to Hydroxocobalamin versus Cyanocobalamin Treatment in Patients with Methylmalonic Acidemia and Homocystinuria (CblC). J. Pediatr. 1998, 132, 121–124. [Google Scholar] [CrossRef]
- Higashimoto, T.; Kim, A.Y.; Ogawa, J.T.; Sloan, J.L.; Almuqbil, M.A.; Carlson, J.M.; Manoli, I.; Venditti, C.P.; Gunay-Aygun, M.; Wang, T. High-dose Hydroxocobalamin Achieves Biochemical Correction and Improvement of Neuropsychiatric Deficits in Adults with Late Onset Cobalamin C Deficiency. JIMD Rep. 2019, 51, 17–24. [Google Scholar] [CrossRef]
- Carrillo-Carrasco, N.; Chandler, R.J.; Venditti, C.P. Combined Methylmalonic Acidemia and Homocystinuria, CblC Type. I. Clinical Presentations, Diagnosis and Management. J. Inherit. Metab. Dis. 2012, 35, 91–102. [Google Scholar] [CrossRef]
- Carrillo-Carrasco, N.; Sloan, J.; Valle, D.; Hamosh, A.; Venditti, C.P. Hydroxocobalamin Dose Escalation Improves Metabolic Control in CblC. J. Inherit. Metab. Dis. 2009, 32, 728–731. [Google Scholar] [CrossRef]
- Carrillo-Carrasco, N.; Venditti, C.P. Combined Methylmalonic Acidemia and Homocystinuria, CblC Type. II. Complications, Pathophysiology, and Outcomes. J. Inherit. Metab. Dis. 2012, 35, 103–114. [Google Scholar] [CrossRef]
- Froese, D.S.; Zhang, J.; Healy, S.; Gravel, R.A. Mechanism of Vitamin B12-Responsiveness in CblC Methylmalonic Aciduria with Homocystinuria. Mol. Genet. Metab. 2009, 98, 338–343. [Google Scholar] [CrossRef] [PubMed]
- Chalmers, J.N.; Shinton, N.K. Comparison of Hydroxocobalamin and Cyanocobalamin in the Treatment of Pernicious Anaemia. Lancet 1965, 2, 1305–1308. [Google Scholar] [CrossRef]
- Okuda, K.; Yashima, K.; Kitazaki, T.; Takara, I. Intestinal Absorption and Concurrent Chemical Changes of Methylcobalamin. J. Lab. Clin. Med. 1973, 81, 557–567. [Google Scholar] [PubMed]
- Freeman, A.G. Hydroxocobalamin versus Cyanocobalamin. J. R. Soc. Med. 1996, 89, 659. [Google Scholar] [CrossRef] [PubMed]
- Foulds, W.S.; Freeman, A.G.; Phillips, C.I.; Wilson, J. Cyanocobalamin: A Case for Withdrawal. Lancet 1970, 1, 35. [Google Scholar] [CrossRef]
- Carmel, R. Efficacy and Safety of Fortification and Supplementation with Vitamin B12: Biochemical and Physiological Effects. Food Nutr. Bull. 2008, 29, S177–S187. [Google Scholar] [CrossRef]
- Green, R. Cobalamins. In Encyclopedia of Human Nutrition, 2nd ed.; Caballero, B., Ed.; Elsevier: Oxford, UK, 2005; pp. 401–407. ISBN 978-0-12-226694-2. [Google Scholar]
- Kräutler, B. Biochemistry of B12-Cofactors in Human Metabolism. In Water Soluble Vitamins: Clinical Research and Future Application; Stanger, O., Ed.; Subcellular Biochemistry; Springer: Dordrecht, The Netherlands, 2012; pp. 323–346. ISBN 978-94-007-2199-9. [Google Scholar]
- Rucker, R.B.; Zempleni, J.; Suttie, J.W.; McCormick, D.B. Handbook of Vitamins, 4th ed.; Taylor & Francis: Oxford, UK, 2007; ISBN 978-0-8493-4022-2. [Google Scholar]
- Bonnett, R. The Chemistry of the Vitamin B12 Group. Chem. Rev. 1963, 63, 573–605. [Google Scholar] [CrossRef]
- ó Proinsias, K.; Giedyk, M.; Gryko, D. Vitamin B12: Chemical Modifications. Chem. Soc. Rev. 2013, 42, 6605–6619. [Google Scholar] [CrossRef]
- Giedyk, M.; Goliszewska, K.; Gryko, D. Vitamin B12 Catalysed Reactions. Chem. Soc. Rev. 2015, 44, 3391–3404. [Google Scholar] [CrossRef]
- Hogenkamp, H.P.C.; Barker, H.A.; Mason, H.S. An Electron-Spin Resonance Study of Coenzyme B12. Arch. Biochem. BioPhys. 1963, 100, 353–359. [Google Scholar] [CrossRef]
- Ahmad, I.; Qadeer, K.; Zahid, S.; Sheraz, M.A.; Ismail, T.; Hussain, W.; Ansari, I.A. Effect of Ascorbic Acid on the Degradation of Cyanocobalamin and Hydroxocobalamin in Aqueous Solution: A Kinetic Study. AAPS PharmSciTech 2014, 15, 1324–1333. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Banerjee, R. The Yin-Yang of Cobalamin Biochemistry. Chem. Biol. 1997, 4, 175–186. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Mamun, A.A.; Toda, M.J.; Lodowski, P.; Kozlowski, P.M. Photolytic Cleavage of Co–C Bond in Coenzyme B12-Dependent Glutamate Mutase. J. Phys. Chem. B 2019, 123, 2585–2598. [Google Scholar] [CrossRef] [PubMed]
- Jensen, K.P.; Ryde, U. How the Co−C Bond Is Cleaved in Coenzyme B12 Enzymes: A Theoretical Study. J. Am. Chem. Soc. 2005, 127, 9117–9128. [Google Scholar] [CrossRef]
- Trenner, N.R.; Buhs, R.P.; Bacher, F.A.; Gakenheimer, W.C. A Note Concerning the Incompatibility of Vitamin B12 and Ascorbic Acid. J. Am. Pharm. Assoc. 1950, 39, 361. [Google Scholar] [CrossRef]
- Bartilucci, A.J.; Digirolamo, R.; Eisen, H. Cyanocobalamin (Vitamin B12). II. Further Studies of the Effect of Ascorbic Acid Degradation Products on Cyanocobalamin. J. Am. Pharm. Assoc. 1958, 47, 42–43. [Google Scholar] [CrossRef]
- Rosenberg, A.J. The Copper-Promoted Decomposition of Vitamin B12 in Solutions of Ascorbic Acid. J. Biol. Chem. 1956, 219, 951–956. [Google Scholar] [CrossRef]
- Gakenheimer, W.C.; Feller, B.A. A Note on a Preliminary Observation of the Incompatibility of Vitamin B12 and Ascorbic Acid. J. Am. Pharm. Assoc. 1949, 38, 660. [Google Scholar] [CrossRef]
- Bartilucci, A.; Foss, N.E. Cyanocobalamin (Vitamin B12). I. A Study of the Stability of Cyanocobalamin and Ascorbic Acid in Liquid Formulations. J. Am. Pharm. Assoc. 1954, 43, 159–162. [Google Scholar] [CrossRef]
- Vitamin B12: A Review of Analytical Methods for Use in Food. Available online: https://www.gov.uk/government/publications/vitamin-b12-a-review-of-analytical-methods-for-use-in-food (accessed on 25 April 2022).
- Zironi, E.; Gazzotti, T.; Barbarossa, A.; Devicienti, C.; Scardilli, M.; Pagliuca, G. Technical Note: Development and Validation of a Method Using Ultra Performance Liquid Chromatography Coupled with Tandem Mass Spectrometry for Determination of Vitamin B12 Concentrations in Milk and Dairy Products. J. Dairy Sci. 2013, 96, 2832–2836. [Google Scholar] [CrossRef]
- Qiu, X.; Zhang, H.; Yin, Y.; Brandes, H.; Marsala, T.; Stenerson, K.; Cramer, H.; You, H. Determination of Active Vitamin B12 (Cobalamin) in Dietary Supplements and Ingredients by Reversed-Phase Liquid Chromatography: Single-Laboratory Validation. Food Chem. 2019, 298, 125010. [Google Scholar] [CrossRef]
- Kamath, A.; Pemminati, S. Methylcobalamin in Vitamin B12 Deficiency: To Give or Not to Give? J. Pharmacol. Pharmacother. 2017, 8, 33–34. [Google Scholar] [CrossRef]
- Cyanocobalamin Safety Data Sheet; Product No.: PHR1234; CAS-No.: 68-19-9; Merck Ife Science S.r.l.: Milano, Italy, 2021.
- Coenzyme B12 Safety Data Sheet; Product No.: C0884; CAS-No.: 13870-90-1; Merck Life Science S.r.l.: Milano, Italy, 2022.
- Hydroxocobalamin Acetate Safety Data Sheet; Product No.: H8017; CAS-No.: 22465-48-1; Merck Life Science S.r.l.: Milano, Italy, 2022.
- Methylcobalamin Safety Data Sheet; Product No.: M9756; CAS-No.: 13422-55-4; Merck Life Science S.r.l.: Milano, Italy, 2022.
- Cyanocobalamin Certified Reference Material Certificate of Analysis; Catalog No.: PHR1234; LOT No.: P500234; Fluca Analytical, Sigma-Aldrich RTC, Inc.: Laramie, WY, USA, 2022.
- Council of Europe. European Pharmacopoeia, 10th ed.; Council of Europe: Strasbourg, France, 2020. [Google Scholar]
- Temova Rakuša, Ž.; Grobin, A.; Roškar, R. A Comprehensive Approach for the Simultaneous Analysis of All Main Water-Soluble Vitamins in Multivitamin Preparations by a Stability-Indicating HPLC-DAD Method. Food Chem. 2021, 337, 127768. [Google Scholar] [CrossRef]
- Bajaj, S.R.; Singhal, R.S. Degradation Kinetics of Vitamin B12 in Model Systems of Different PH and Extrapolation to Carrot and Lime Juices. J. Food Eng. 2020, 272, 109800. [Google Scholar] [CrossRef]
- CYANOKIT® (Hydroxocobalamin for Injection) for Intravenous Infusion. Highlights of Prescribing Information; Merck Santé s.a.s.: Semoy, France, 2018.
- Mander, L.; Liu, H.W. Comprehensive Natural Products II: Chemistry and Biology, Volumes 1−10. J. Am. Chem. Soc. 2010, 132, 9929. [Google Scholar] [CrossRef]
- Hadinata Lie, A.; Chandra-Hioe, M.V.; Arcot, J. Sorbitol Enhances the Physicochemical Stability of B12 Vitamers. Int. J. Vitam. Nutr. Res. 2020, 90, 439–447. [Google Scholar] [CrossRef]
- Brown, K.L. Chemistry and Enzymology of Vitamin B12. Chem. Rev. 2005, 105, 2075–2150. [Google Scholar] [CrossRef]
- Brown, K.L.; Zou, X. Thermolysis of Coenzymes B12 at Physiological Temperatures: Activation Parameters for Cobalt-Carbon Bond Homolysis and a Quantitative Analysis of the Perturbation of the Homolysis Equilibrium by the Ribonucleoside Triphosphate Reductase from Lactobacillus Leichmannii. J. Inorg. Biochem. 1999, 77, 185–195. [Google Scholar] [CrossRef]
- Combs, G.F. Poglavje 1: Chemical and Physiological Properties of Vitamins. V. In The Vitamins: Fundamental Aspects in Nutrition and Health, 3rd ed.; Elsevier Academic Press: Amsterdam, The Netherlands, 2008; pp. 503–514. [Google Scholar]
- Yessaad, M.; Bernard, L.; Bourdeaux, D.; Chennell, P.; Sautou, V. Development of a Stability Indicating Method for Simultaneous Analysis of Five Water-Soluble Vitamins by Liquid Chromatography. Pharm. Technol. Hosp. Pharm. 2018, 3, 207–218. [Google Scholar] [CrossRef][Green Version]
- Monajjemzadeh, F.; Ebrahimi, F.; Zakeri-Milani, P.; Valizadeh, H. Effects of Formulation Variables and Storage Conditions on Light Protected Vitamin B12 Mixed Parenteral Formulations. Adv. Pharm. Bull. 2014, 4, 329–338. [Google Scholar] [CrossRef]
- Ahmad, I.; Hussain, W. Stability of Cyanocobalamin in Parenteral Preparations. Pak. J. Pharm. Sci. 1993, 6, 53–59. [Google Scholar] [PubMed]
- British Pharmacopoeia Commission Office: Medicines and Healthcare products Regulatory Agency. British Pharmacopoeia 2020; Crown Copyright 2019: London, UK, 2020. [Google Scholar]
- Convention, U.S.P. The United States Pharmacopeia: USP 44-NF 39; United States Pharmacopeial Convention Inc.: Washington, DC, USA, 2021; ISBN 978-1-889788-39-5. [Google Scholar]
- Randaccio, L.; Geremia, S.; Nardin, G.; Wuerges, J. X-Ray Structural Chemistry of Cobalamins. Coord. Chem. Rev. 2006, 11–12, 1332–1350. [Google Scholar] [CrossRef]
- Demerre, L.J.; Wilson, C. Photolysis of Vitamin B12. J. Am. Pharm. Assoc. 1956, 45, 129–134. [Google Scholar] [CrossRef] [PubMed]
- Andruniów, T.; Lodowski, P.; Garabato, B.D.; Jaworska, M.; Kozlowski, P.M. The Role of Spin-Orbit Coupling in the Photolysis of Methylcobalamin. J. Chem. Phys. 2016, 144, 124305. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, A.P.; Lodowski, P.; Kozlowski, P.M. Aerobic Photolysis of Methylcobalamin: Unraveling the Photoreaction Mechanism. Phys. Chem. Chem. Phys. 2022, 24, 6093–6106. [Google Scholar] [CrossRef] [PubMed]
- Kozlowski, P.M.; Garabato, B.D.; Lodowski, P.; Jaworska, M. Photolytic Properties of Cobalamins: A Theoretical Perspective. Dalton Trans. 2016, 45, 4457–4470. [Google Scholar] [CrossRef]
- Hogenkamp, H.P.C. The Photolysis of Methylcobalamin. Biochemistry 1966, 5, 417–422. [Google Scholar] [CrossRef]
- Yamada, R.; Shimizu, S.; Fukui, S. Formation of Vitamin B12s by Anaerobic Photolysis of Cobalt-Alkylcobalamins. Biochim. BioPhys. Acta 1966, 124, 197–200. [Google Scholar] [CrossRef]
- Ahmad, I.; Hussain, W.; Fareedi, A.A. Photolysis of Cyanocobalamin in Aqueous Solution. J. Pharm. Biomed. Anal. 1992, 10, 9–15. [Google Scholar] [CrossRef]
- Lodowski, P.; Jaworska, M.; Garabato, B.D.; Kozlowski, P.M. Mechanism of Co-C Bond Photolysis in Methylcobalamin: Influence of Axial Base. J. Phys. Chem. A 2015, 119, 3913–3928. [Google Scholar] [CrossRef]
- Qadeer, K.; Arsalan, A.; Ahmad, I.; Fatima, K.; Anwar, Z.; Ahmed, S.; Khattak, S.u.R.; Mahmud, S. Photochemical Interaction of Cyanocobalamin and Hydroxocobalamin with Cysteine. J. Mol. Struct. 2021, 1228, 129441. [Google Scholar] [CrossRef]
- Juzeniene, A.; Nizauskaite, Z. Photodegradation of Cobalamins in Aqueous Solutions and in Human Blood. J. Photochem. PhotoBiol. B Biol. 2013, 122, 7–14. [Google Scholar] [CrossRef]
- Vaid, F.H.M.; Zahid, S.; Faiyaz, A.; Qadeer, K.; Gul, W.; Anwar, Z.; Ahmad, I. Photolysis of Methylcobalamin in Aqueous Solution: A Kinetic Study. J. Photochem. PhotoBiol. A Chem. 2018, 362, 40–48. [Google Scholar] [CrossRef]
- Jones, A.R. The Photochemistry and Photobiology of Vitamin B12. Photochem. PhotoBiol. Sci. 2017, 16, 820–834. [Google Scholar] [CrossRef]
- Ahmed, L.M.; Saaed, S.I.; Marhoon, A.A. Effect of Oxidation Agents on Photo-Decolorization of Vitamin B12 in the Presence of ZnO/UV-A System. Indones. J. Chem. 2018, 18, 272–278. [Google Scholar] [CrossRef]
- Hunt, A.P. Kinetic and Mechanistic Studies on the Reaction of the Reduced Vitamin B12 Complex Cob(II)Alamin with Hydrogen Peroxide. Undergraduate Thesis, Kent State University, Kent, OH, USA, 2013. OhioLINK Electronic Theses and Dissertations Center. Available online: https://rave.ohiolink.edu/etdc/view?acc_num=ksuhonors1367864401 (accessed on 6 June 2022). OhioLINK Electronic Theses and Dissertations Center.
- Dubnoff, J.W. A Cobalamin Glutathione Complex. Biochem. BioPhys. Res. Commun. 1964, 16, 484–488. [Google Scholar] [CrossRef]
- Jacobsen, D.W.; Troxell, L.S.; Brown, K.L. Catalysis of Thiol Oxidation by Cobalamins and Cobinamides: Reaction Products and Kinetics. Biochemistry 1984, 23, 2017–2025. [Google Scholar] [CrossRef]
- Chiu, P.-C.; Reinhard, M. Transformation of Carbon Tetrachloride by Reduced Vitamin B12 in Aqueous Cysteine Solution. Environ. Sci. Technol. 1996, 30, 1882–1889. [Google Scholar] [CrossRef]
- Rodgers, Z.L.; Shell, T.A.; Brugh, A.M.; Nowotarski, H.L.; Forbes, M.D.E.; Lawrence, D.S. Fluorophore Assisted Photolysis of Thiolato-Cob(III)Alamins. Inorg. Chem. 2016, 55, 1962–1969. [Google Scholar] [CrossRef]
- Cavallini, D.; Scandurra, R.; Barboni, E.; Marcucci, M. The Inability of Thiols to Reduce Cobalamins in the Absence of a Metalion. FEBS Lett. 1968, 1, 272–274. [Google Scholar] [CrossRef]
- Randaccio, L.; Geremia, S.; Nardin, G.; Šlouf, M.; Srnova, I. Crystal Chemistry of Cobalamins. Structural Characterization of the Co−S Bond in Cobalamins. Inorg. Chem. 1999, 38, 4087–4092. [Google Scholar] [CrossRef]
- Hogenkamp, H.P.; Bratt, G.T.; Kotchevar, A.T. Reaction of Alkylcobalamins with Thiols. Biochemistry 1987, 26, 4723–4727. [Google Scholar] [CrossRef]
- Hogenkamp, H.P.C. The Interaction between Vitamin B12 and Vitamin C. Am. J. Clin. Nutr. 1980, 33, 1–3. [Google Scholar] [CrossRef]
- Macek, T.J. Stability Problems with Some Vitamins in Pharmaceuticals. Am. J. Pharm. Sci. Support. Public Health 1960, 132, 433–455. [Google Scholar]
- Nazhat, N.B.; Golding, B.T.; Johnson, G.R.A.; Jones, P. Destruction of Vitamin B12 by Reaction with Ascorbate: The Role of Hydrogen Peroxide and the Oxidation State of Cobalt. J. Inorg. Biochem. 1989, 36, 75–81. [Google Scholar] [CrossRef]
- Ahmad, I.; Qadeer, K.; Hafeez, A.; Zahid, S.; Sheraz, M.A.; ur Rehman Khattak, S. Effect of Ascorbic Acid on the Photolysis of Cyanocobalamin and Aquocobalamin/Hydroxocobalamin in Aqueous Solution: A Kinetic Study. J. Photochem. PhotoBiol. A Chem. 2017, 332, 92–100. [Google Scholar] [CrossRef]
- Hutchins, H.H.; Cravioto, P.J.; Macek, T.J. A Comparison of the Stability of Cyanocobalamin and Its Analogs in Ascorbate Solution. J. Am. Pharm. Assoc. 1956, 45, 806–808. [Google Scholar] [CrossRef]
- Lee, W.; Lee, Y.-B.; Huh, M.H.; Choi, J.K. Determination of the Chemical Stability of Cyanocobalamin in Medical Food by a Validated Immunoaffinity Column-Linked HPLC Method. J. Food. Qual. 2022, 2022, e1619936. [Google Scholar] [CrossRef]
- van der Merwe, J.; Steenekamp, J.; Steyn, D.; Hamman, J. The Role of Functional Excipients in Solid Oral Dosage Forms to Overcome Poor Drug Dissolution and Bioavailability. Pharmaceutics 2020, 12, 393. [Google Scholar] [CrossRef]
- Akers, M.J. Excipient-Drug Interactions in Parenteral Formulations. J. Pharm. Sci. 2002, 91, 2283–2300. [Google Scholar] [CrossRef]
- Barr, M.; Robert Kohn, S.; Tice, L.F. The Effect of Various Sugars and Polyols on the Stability of Vitamin B12. J. Am. Pharm. Assoc. 1957, 46, 650–652. [Google Scholar] [CrossRef] [PubMed]
- Cardoso, D.R.; Libardi, S.H.; Skibsted, L.H. Riboflavin as a Photosensitizer. Effects on Human Health and Food Quality. Food Funct. 2012, 3, 487–502. [Google Scholar] [CrossRef] [PubMed]
- Ansari, I.A.; Vaid, F.H.M.; Ahmad, I. Spectral Study of Photolysis of Aqueous Cyanocobalamin Solutions in Presence of Vitamins B and C. Pak. J. Pharm. Sci. 2004, 17, 93–99. [Google Scholar] [PubMed]
- Ahmad, I.; Hussain, W. Multicomponent Spectrophotometric Assay of Cyanocobalamin, Hydroxocobalamin and Riboflavin. Pak. J. Pharm. Sci. 1992, 5, 121–127. [Google Scholar]
- Ahmad, I.; Hafeez, A.; Akhter, N.; HM Vaid, F.; Qadeer, K. Effect of Riboflavin on the Photolysis of Cyanocobolamin in Aqueous Solution. Open Anal. Chem. J. 2012, 6, 22–27. [Google Scholar] [CrossRef]
- Feller, B.A.; Macek, T.J. Effect of Thiamine Hydrochloride on the Stability of Solutions of Crystalline Vitamin B12. J. Am. Pharm. Assoc. 1955, 44, 662–665. [Google Scholar] [CrossRef]
- Mukherjee, S.L.; Sen, S.P. Stability of Vitamin B12. Part II. Protection by an Iron Salt Against Destruction by Aneurine and Nicotinamide. J. Pharm. Pharmacol. 1959, 11, 26–31. [Google Scholar] [CrossRef]
- Doerge, R.F.; Ravin, L.J.; Caldwell, H.C. Effect of the Thiazole Moiety of Thiamine Hydrochloride and Selected Model Compounds on Cyanocobalamin Stability. J. Pharm. Sci. 1965, 54, 1038–1040. [Google Scholar] [CrossRef]
- Blitz, M.; Eigen, E.; Gunsberg, E. Vitamin B12 Studies-The Instability of Vitamin B12 in the Presence of Thiamine and Niacinamide. J. Am. Pharm. Assoc. 1954, 43, 651–653. [Google Scholar] [CrossRef]
- Gambier, A.S.; Rahn, E.P.G. Vitamin B12 in the Presence of Vitamin B1 and Niacinamide in Aqueous Combinations. J. Am. Pharm. Assoc. 1958, 47, 356–359. [Google Scholar] [CrossRef]
- Ahmad, I.; Ansari, I.A.; Ismail, T. Effect of Nicotinamide on the Photolysis of Cyanocobalamin in Aqueous Solution. J. Pharm. Biomed. Anal. 2003, 31, 369–374. [Google Scholar] [CrossRef]
- Chawla, J.; Kvarnberg, D. Hydrosoluble Vitamins. Handb. Clin. Neurol. 2014, 120, 891–914. [Google Scholar] [CrossRef]
- Russell, R.M.; Baik, H.; Kehayias, J.J. Older Men and Women Efficiently Absorb Vitamin B12 from Milk and Fortified Bread. J. Nutr. 2001, 131, 291–293. [Google Scholar] [CrossRef]
- Zeuschner, C.L.; Hokin, B.D.; Marsh, K.A.; Saunders, A.V.; Reid, M.A.; Ramsay, M.R. Vitamin B12 and Vegetarian Diets. Med. J. Aust. 2013, 199, S27-32. [Google Scholar] [CrossRef]
- Ottaway, P.B. Stability of Vitamins in Food. In The Technology of Vitamins in Food; Ottaway, P.B., Ed.; Springer: Boston, MA, USA, 1993; pp. 90–113. ISBN 978-1-4615-2131-0. [Google Scholar]
- Edelmann, M.; Chamlagain, B.; Santin, M.; Kariluoto, S.; Piironen, V. Stability of Added and in Situ-Produced Vitamin B12 in Breadmaking. Food Chem. 2016, 204, 21–28. [Google Scholar] [CrossRef]
- Riaz, M.N.; Asif, M.; Ali, R. Stability of Vitamins during Extrusion. Crit. Rev. Food Sci. Nutr. 2009, 49, 361–368. [Google Scholar] [CrossRef]
- Killeit, U. Vitamin Retention in Extrusion Cooking. Food Chem. 1994, 49, 149–155. [Google Scholar] [CrossRef]
- Bajaj, S.R.; Singhal, R.S. Effect of Extrusion Processing and Hydrocolloids on the Stability of Added Vitamin B12 and Physico-Functional Properties of the Fortified Puffed Extrudates. LWT 2019, 101, 32–39. [Google Scholar] [CrossRef]
- Hemery, Y.M.; Fontan, L.; Laillou, A.; Jallier, V.; Moench-Pfanner, R.; Avallone, S.; Berger, J. Influence of Storage Conditions and Packaging of Fortified Wheat Flour on Microbial Load and Stability of Folate and Vitamin B12. Food Chem. X 2020, 5, 100076. [Google Scholar] [CrossRef]
- Devi, R. Food Processing and Impact on Nutrition. Sch. J. Agric. Vet. Sci. 2015, 2, 304–311. [Google Scholar]
- Watanabe, F.; Abe, K.; Fujita, T.; Goto, M.; Hiemori, M.; Nakano, Y. Effects of Microwave Heating on the Loss of Vitamin B12 in Foods. J. Agric. Food Chem. 1998, 46, 206–210. [Google Scholar] [CrossRef]
- Czerwonka, M.; Szterk, A.; Waszkiewicz-Robak, B. Vitamin B12 Content in Raw and Cooked Beef. Meat Sci. 2014, 96, 1371–1375. [Google Scholar] [CrossRef]
- Bennink, M.R.; Ono, K. Vitamin B12, E and D Content of Raw and Cooked Beef. J. Food Sci. 1982, 47, 1786–1792. [Google Scholar] [CrossRef]
- Nishioka, M.; Kanosue, F.; Yabuta, Y.; Watanabe, F. Loss of Vitamin B12 in Fish (Round Herring) Meats during Various Cooking Treatments. J. Nutr. Sci. Vitaminol. 2011, 57, 432–436. [Google Scholar] [CrossRef]
- Johns, P.W.; Das, A.; Kuil, E.M.; Jacobs, W.A.; Schimpf, K.J.; Schmitz, D.J. Cocoa Polyphenols Accelerate Vitamin B12 Degradation in Heated Chocolate Milk. Int. J. Food Sci. Technol. 2015, 50, 421–430. [Google Scholar] [CrossRef]
- Rosenblum, C.; Woodbury, D.T. The Determination of the Stability of Vitamin B12 in Multivitamin Mixture by a Radioactive Indicator Method. J. Am. Pharm. Assoc. 1952, 41, 368–371. [Google Scholar] [CrossRef]
- Blitz, M.; Eigen, E.; Gunsberg, E. Studies Relating to The Stability of Vitamin B12 in B-Complex Injectable Solutions. J. Am. Pharm. Assoc. 1956, 45, 803–806. [Google Scholar] [CrossRef]
- Chamle, A.H.; Shane, N.L.J.; Pai, A.; Muddukrishna, B.S. Photodegradation of Methylcobalamin and Its Determination in a Commercial Formulation. Indian J. Pharm. Sci. 2019, 81, 57–62. [Google Scholar] [CrossRef]
- Ip, K.; Banov, D.; Bassani, G.; Morgan, L. Physicochemical Stability of Extemporaneously Prepared Methylcobalamin Injections in the Presence and Absence of Preservative and the Impact of Light Exposure. Int. J. Pharm. Compd. 2019, 23, 167–175. [Google Scholar]
- Kusadome, C.; Shibayama, Y.; Nishi, Y.; Takeda, Y.; Yamada, K. Stability of High-Dose Methylcobalamin Injection. J. Appl. Ther. Res. 2008, 6, 15–18. [Google Scholar]
- Jacob, J.T.; Nessel, R.J.; Blodinger, J. Stability of Cyanocobalamin in Film-Coated Multivitamin Tablets. J Pharm. Sci. 1968, 57, 1854–1857. [Google Scholar] [CrossRef] [PubMed]
- Ohmori, S.; Kataoka, M.; Koyama, H. Stability of Cyanocobalamin in Sugar-Coated Tablets. Int. J. Pharm. 2007, 337, 161–168. [Google Scholar] [CrossRef] [PubMed]
- Saeki, T.; Katagiri, Y.; Hirano, H.; Naora, K. Photostability of Mecobalamin in Tablet and Capsule at the Dispensing Level. Shimane J. Med. Sci. 1984, 8, 1–8. [Google Scholar]
- LeDoux, M.A.; Appelhans, K.R.; Braun, L.A.; Dziedziczak, D.; Jennings, S.; Liu, L.; Osiecki, H.; Wyszumiala, E.; Griffiths, J.C. A Quality Dietary Supplement: Before You Start and after It’s Marketed—A Conference Report. Eur. J. Nutr. 2015, 54, 1–8. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Temova Rakuša, Ž.; Srečnik, E.; Roškar, R. Novel HPLC-UV Method for Simultaneous Determination of Fat-Soluble Vitamins and Coenzyme Q10 in Medicines and Supplements. Acta Chim. Slov. 2017, 64, 523–529. [Google Scholar] [CrossRef][Green Version]
- Flynn, A.; Kehoe, L.; Hennessy, Á.; Walton, J. Estimating Safe Maximum Levels of Vitamins and Minerals in Fortified Foods and Food Supplements. Eur. J. Nutr. 2017, 56, 2529–2539. [Google Scholar] [CrossRef]
- Andrews, K.W.; Roseland, J.M.; Gusev, P.A.; Palachuvattil, J.; Dang, P.T.; Savarala, S.; Han, F.; Pehrsson, P.R.; Douglass, L.W.; Dwyer, J.T.; et al. Analytical Ingredient Content and Variability of Adult Multivitamin/Mineral Products: National Estimates for the Dietary Supplement Ingredient Database12. Am. J. Clin. Nutr. 2017, 105, 526–539. [Google Scholar] [CrossRef]
- Yoo, S.J.; Walfish, S.L.; Atwater, J.B.; Giancaspro, G.; Sarma, N. Factors to Consider in Setting Adequate Overages of Vitamins and Minerals in Dietary Supplements. Pharm. Forum 2016, 42, 111–114. [Google Scholar]
- European Commission (2012) Guidance Document for Competent Authorities for the Control of Compliance with EU Legislation with Regard to the Setting of Tolerances for Nutrient Values Declared on a Label. 2012. Available online: https://www.fsai.ie/uploadedfiles/guidance_tolerances_december_2012.pdf (accessed on 19 May 2022).
- Center for Food Safety and Applied Nutrition (CFSAN). Guidance for Industry: Guide for Developing and Using Data Bases for Nutrition Labeling. Available online: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/guidance-industry-guide-developing-and-using-data-bases-nutrition-labeling (accessed on 19 June 2022).
- Chopra, A.S.; Lordan, R.; Horbańczuk, O.K.; Atanasov, A.G.; Chopra, I.; Horbańczuk, J.O.; Jóźwik, A.; Huang, L.; Pirgozliev, V.; Banach, M.; et al. The Current Use and Evolving Landscape of Nutraceuticals. Pharmacol. Res. 2022, 175, 106001. [Google Scholar] [CrossRef]
- Droz, N.; Marques-Vidal, P. Multivitamins/Multiminerals in Switzerland: Not as Good as It Seems. Nutr. J. 2014, 13, 24. [Google Scholar] [CrossRef]
- Dwyer, J.T.; Coates, P.M.; Smith, M.J. Dietary Supplements: Regulatory Challenges and Research Resources. Nutrients 2018, 10, 41. [Google Scholar] [CrossRef] [PubMed]
- Institute of Medicine (US) Standing Committee on the Scientific Evaluation of Dietary Reference Intakes and its Panel on Folate, Other B Vitamins, and Choline. Dietary Reference Intakes for Thiamin, Riboflavin, Niacin, Vitamin B6, Folate, Vitamin B12, Pantothenic Acid, Biotin, and Choline; National Academies Press (US): Washington, DC, USA, 1998; ISBN 978-0-309-06411-8. [Google Scholar]
- Klejdus, B.; Petrlová, J.; Potěšil, D.; Adam, V.; Mikelová, R.; Vacek, J.; Kizek, R.; Kubáň, V. Simultaneous Determination of Water- and Fat-Soluble Vitamins in Pharmaceutical Preparations by High-Performance Liquid Chromatography Coupled with Diode Array Detection. Anal. Chim. Acta 2004, 520, 57–67. [Google Scholar] [CrossRef]
- Fan, D.; Zhang, Y.; Wu, H. Development of a Simple and Sensitive HPLC-DAD Method for Quantification of Vitamin B12 Fortified in Infant Food. Anal. Methods 2021, 13, 4920–4925. [Google Scholar] [CrossRef]
- Luo, X.; Chen, B.; Ding, L.; Tang, F.; Yao, S. HPLC-ESI-MS Analysis of Vitamin B12 in Food Products and in Multivitamins-Multimineral Tablets. Anal. Chim. Acta 2006, 562, 185–189. [Google Scholar] [CrossRef]
- Raju, C.S.K.; Yu, L.L.; Schiel, J.E.; Long, S.E. A Simple and Sensitive LC-ICP-MS Method for the Accurate Determination of Vitamin B12 in Fortified Breakfast Cereals and Multivitamin Tablets. J. Anal. At. Spectrom. 2013, 28, 901–907. [Google Scholar] [CrossRef]
- Li, H.B.; Chen, F.; Jiang, Y. Determination of Vitamin B12 in Multivitamin Tablets and Fermentation Medium by High-Performance Liquid Chromatography with Fluorescence Detection. J. Chromatogr. A 2000, 891, 243–247. [Google Scholar] [CrossRef]
- Chatzimichalakis, P.F.; Samanidou, V.F.; Verpoorte, R.; Papadoyannis, I.N. Development of a Validated HPLC Method for the Determination of B-Complex Vitamins in Pharmaceuticals and Biological Fluids after Solid Phase Extraction. J. Sep. Sci. 2004, 27, 1181–1188. [Google Scholar] [CrossRef]
- Marszałł, M.L.; Lebiedzińska, A.; Czarnowski, W.; Szefer, P. High-Performance Liquid Chromatography Method for the Simultaneous Determination of Thiamine Hydrochloride, Pyridoxine Hydrochloride and Cyanocobalamin in Pharmaceutical Formulations Using Coulometric Electrochemical and Ultraviolet Detection. J. Chromatogr. A 2005, 1094, 91–98. [Google Scholar] [CrossRef]
- Chen, P.; Wolf, W.R.; Castanheira, I.; Sanches-Silva, A. A LC/UV/Vis Method for Determination of Cyanocobalamin (VB12) in Multivitamin Dietary Supplements with on-Line Sample Clean-Up. Anal. Methods 2010, 2, 1171–1175. [Google Scholar] [CrossRef]
- Li, H.B.; Chen, F. Determination of Vitamin B12 in Pharmaceutical Preparations by a Highly Sensitive Fluorimetric Method. Fresenius. J. Anal. Chem. 2000, 368, 836–838. [Google Scholar] [CrossRef]
- Patil, S.S.; Srivastava, A.K. Development and Validation of a Liquid Chromatography Method for the Simultaneous Determination of Eight Water-Soluble Vitamins in Multivitamin Formulations and Human Urine. J. AOAC Int. 2013, 96, 1273–1280. [Google Scholar] [CrossRef] [PubMed]
- D’Ulivo, L.; Yang, L.; Ding, J.; Pagliano, E.; Leek, D.M.; Thibeault, M.-P.; Mester, Z. Determination of Cyanocobalamin by Isotope Dilution LC-MS/MS. Anal. Chim. Acta 2017, 990, 103–109. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Liu, X.; Schnute, W. Simultaneous Analysis of Water-Soluble Vitamins in Vitamin-Enriched Beverages and Multivitamin Dietary Supplements by UHPLC-MS/MS; Application Note 294; Thermo Scientific: Waltham, MA, USA, 2016. [Google Scholar]
- Moreno, P.; Salvadó, V. Determination of Eight Water- and Fat-Soluble Vitamins in Multi-Vitamin Pharmaceutical Formulations by High-Performance Liquid Chromatography. J. Chromatogr. A 2000, 870, 207–215. [Google Scholar] [CrossRef] [PubMed]
- Ichikawa, M.; Ide, N.; Shiraishi, S.; Ono, K. Effect of Various Halide Salts on the Incompatibility of Cyanocobalamin and Ascorbic Acid in Aqueous Solution. Chem. Pharm. Bull. 2005, 53, 688–690. [Google Scholar] [CrossRef]
- Wang, H.; Shou, Y.; Zhu, X.; Xu, Y.; Shi, L.; Xiang, S.; Feng, X.; Han, J. Stability of Vitamin B12 with the Protection of Whey Proteins and Their Effects on the Gut Microbiome. Food Chem. 2019, 276, 298–306. [Google Scholar] [CrossRef]
- Uchida, T.; Suguri, T.; Harjinder, S. Folic Acid and/or Vitamin B12-Lactoferrin Complex. U.S. Patent US6500472B2, 31 December 2002. [Google Scholar]
- Grissom, C.B.; Chagovetz, A.M.; Wang, Z. Use of Viscosigens to Stabilize Vitamin B12 Solutions against Photolysis. J. Pharm. Sci. 1993, 82, 641–643. [Google Scholar] [CrossRef]
- Bajaj, S.R.; Singhal, R.S. Enhancement of Stability of Vitamin B12 by Co-Crystallization: A Convenient and Palatable Form of Fortification. J. Food Eng. 2021, 291, 110231. [Google Scholar] [CrossRef]
- Rajakumari, R.; Oluwafemi, O.S.; Thomas, S.; Kalarikkal, N. Dietary Supplements Containing Vitamins and Minerals: Formulation, Optimization and Evaluation. Powder Technol. 2018, 336, 481–492. [Google Scholar] [CrossRef]
- Wang, J.-R.; Zhou, C.; Yu, X.; Mei, X. Stabilizing Vitamin D3 by Conformationally Selective Co-Crystallization. Chem. Commun. 2014, 50, 855–858. [Google Scholar] [CrossRef]
- Zhu, B.; Wang, J.-R.; Zhang, Q.; Mei, X. Improving Dissolution and Photostability of Vitamin K3 via Cocrystallization with Naphthoic Acids and Sulfamerazine. Cryst. Growth Des. 2016, 16, 483–492. [Google Scholar] [CrossRef]
- Choudhury, N.; Meghwal, M.; Das, K. Microencapsulation: An Overview on Concepts, Methods, Properties and Applications in Foods. Food Front. 2021, 2, 426–442. [Google Scholar] [CrossRef]
- Singh, M.N.; Hemant, K.S.Y.; Ram, M.; Shivakumar, H.G. Microencapsulation: A Promising Technique for Controlled Drug Delivery. Res. Pharm. Sci. 2010, 5, 65–77. [Google Scholar] [PubMed]
- Carlan, I.C.; Estevinho, B.N.; Rocha, F. Study of Different Encapsulating Agents for the Microencapsulation of Vitamin B12. Environ. Eng. Manag. J. 2018, 17, 855–864. [Google Scholar] [CrossRef]
- Carlan, I.C.; Estevinho, B.N.; Rocha, F. Study of Microencapsulation and Controlled Release of Modified Chitosan Microparticles Containing Vitamin B12. Powder Technol. 2017, 318, 162–169. [Google Scholar] [CrossRef]
- Chalella Mazzocato, M.; Thomazini, M.; Favaro-Trindade, C.S. Improving Stability of Vitamin B12 (Cyanocobalamin) Using Microencapsulation by Spray Chilling Technique. Food Res. Int. 2019, 126, 108663. [Google Scholar] [CrossRef]
- Gupta, R.G.; Rao, B.C. Microencapsulation of Vitamin B12 by Emulsion Technique. Drug Dev. Ind. Pharm. 1985, 11, 41–53. [Google Scholar] [CrossRef]
- Oh, S.; Cave, G.; Lu, C. Vitamin B12 (Cobalamin) and Micronutrient Fortification in Food Crops Using Nanoparticle Technology. Front. Plant Sci. 2021, 12, 668819. [Google Scholar] [CrossRef]
- de Britto, D.; Pinola, F.G.; Mattoso, L.H.C.; Assis, O.B.G. Analysis of Thermal and Aqueous Suspension Stabilities of Chitosan Based Nanoencapsulated Vitamins. Química Nova 2016, 39, 1126–1130. [Google Scholar] [CrossRef]
- Maiorova, L.A.; Erokhina, S.I.; Pisani, M.; Barucca, G.; Marcaccio, M.; Koifman, O.I.; Salnikov, D.S.; Gromova, O.A.; Astolfi, P.; Ricci, V.; et al. Encapsulation of Vitamin B12 into Nanoengineered Capsules and Soft Matter Nanosystems for Targeted Delivery. Colloids Surf. B Biointerfaces 2019, 182, 110366. [Google Scholar] [CrossRef]
- Arsalan, A.; Ahmad, I.; Ali, S.A.; Qadeer, K.; Mahmud, S.; Humayun, F.; Beg, A.E. The Kinetics of Photostabilization of Cyanocobalamin in Liposomal Preparations. Int. J. Chem. Kinet. 2020, 52, 207–217. [Google Scholar] [CrossRef]
- Gorantla, S.; Wadhwa, G.; Jain, S.; Sankar, S.; Nuwal, K.; Mahmood, A.; Dubey, S.K.; Taliyan, R.; Kesharwani, P.; Singhvi, G. Recent Advances in Nanocarriers for Nutrient Delivery. Drug Deliv. Transl. Res. 2021, 12, 2359–2384. [Google Scholar] [CrossRef]
- Lee, J.; Yang, J.H.; Kim, K.S.; Park, G.D. Effect of Liposome Storage of Cyanocobalamin on Its Degradation by Ascorbic Acid. Food Suppl. Biomater. Health 2021, 1, e7. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).