Abstract
The advent of graphene opens up the research into two-dimensional (2D) materials, which are considered revolutionary materials. Due to its unique geometric structure, graphene exhibits a series of exotic physical and chemical properties. In addition, single-element-based 2D materials (Xenes) have garnered tremendous interest. At present, 16 kinds of Xenes (silicene, borophene, germanene, phosphorene, tellurene, etc.) have been explored, mainly distributed in the third, fourth, fifth, and sixth main groups. The current methods to prepare monolayers or few-layer 2D materials include epitaxy growth, mechanical exfoliation, and liquid phase exfoliation. Although two Xenes (aluminene and indiene) have not been synthesized due to the limitations of synthetic methods and the stability of Xenes, other Xenes have been successfully created via elaborate artificial design and synthesis. Focusing on elemental 2D materials, this review mainly summarizes the recently reported work about tuning the electronic, optical, mechanical, and chemical properties of Xenes via surface modifications, achieved using controllable approaches (doping, adsorption, strain, intercalation, phase transition, etc.) to broaden their applications in various fields, including spintronics, electronics, optoelectronics, superconducting, photovoltaics, sensors, catalysis, and biomedicines. These advances in the surface modification of Xenes have laid a theoretical and experimental foundation for the development of 2D materials and their practical applications in diverse fields.
1. Introduction
Graphene, initially isolated from graphite via mechanical exfoliation, possesses a layered honeycomb structure and exhibits fascinating electrical and thermal properties [1]. Subsequently, two-dimensional (2D) materials, including elemental monolayers (Xenes) [2], transition metal chalcogenides (TMCs) [3,4], oxides [5], halides [6,7], and carbides (MXenes) [8,9], have shown intriguing properties, such as high carrier mobility [10], layer-dependent band structures and magnetic properties [6,11,12], nontrivial topology [13,14,15,16], valleytronics [17], etc. Therefore, 2D materials have become promising candidates for various applications relating to next-generation technology, including spintronics, superconducting, nanoelectronics, nanosensing, etc.
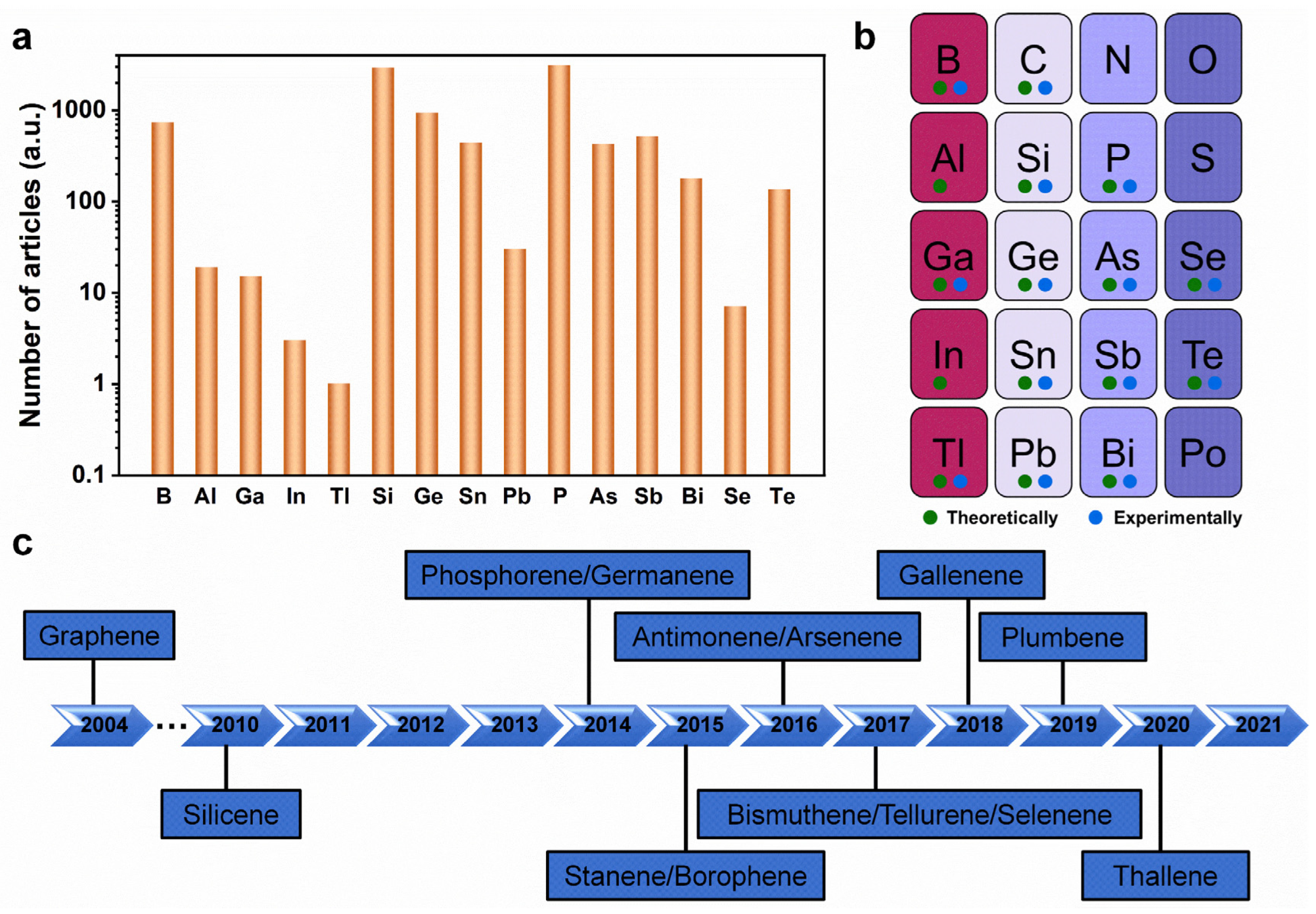
Over the past few years, many studies have focused on searching for other 2D Xenes with distinctive and exciting properties beyond graphene (Figure 1a) [18,19]. The successful experimental realization of non-graphene 2D analogs (silicene and phosphorene) sparked a continuous expansion of the list of elements with atomically thin forms [20,21]. Sixteen elemental-main-group 2D Xenes have been predicted theoretically or created experimentally to date (Figure 1b) [22,23,24,25,26,27,28,29,30,31,32]. To the best of our knowledge, except aluminene and indiene, the other 13 non-graphene Xenes have been experimentally obtained (Figure 1c). Currently, monolayer or few-layer Xenes can be created using mechanical exfoliation, liquid phase exfoliation, and epitaxial growth methods [1,12,22,33].

Figure 1.
Development of 2D Xenes. (a) Statistics diagram of the number of research articles on non-graphene 2D Xenes from 2010 to 2021. (b) Overview of 2D analogs of main-group elements, explored using either experimental or theoretical routes. The elements with no signs have not been explored to date. (c) The timeline of the experimental creation of 2D Xenes.
Even though 2D Xenes have demonstrated great potential in numerous applications, their intrinsic properties usually constrain the expansion of these applications. For instance, despite the high mobility, the gapless band structure in graphene restricts its applications in electronic devices. Therefore, finetuning the properties of 2D Xenes plays an essential role in overcoming their intrinsic constraints [34,35,36,37]. Surface modifications can effectively tailor the properties of 2D Xenes for practical applications. The common approaches for surface modifications include heteroatom-doping [38,39], adsorption [34,36,40], in-plane heterostructure formation [41,42,43], boundary formation [44], edge shape [32,45,46,47,48], phase transition [49], strain [50,51], twisted structure [52,53], etc. Motivated by recent advances in the surface modifications of 2D Xenes, herein, we review the reported work to date, connect the surface modifications of various 2D Xenes to their measured/predicted properties, and evaluate their advantages and disadvantages for various applications.
2. Classification of 2D Xenes
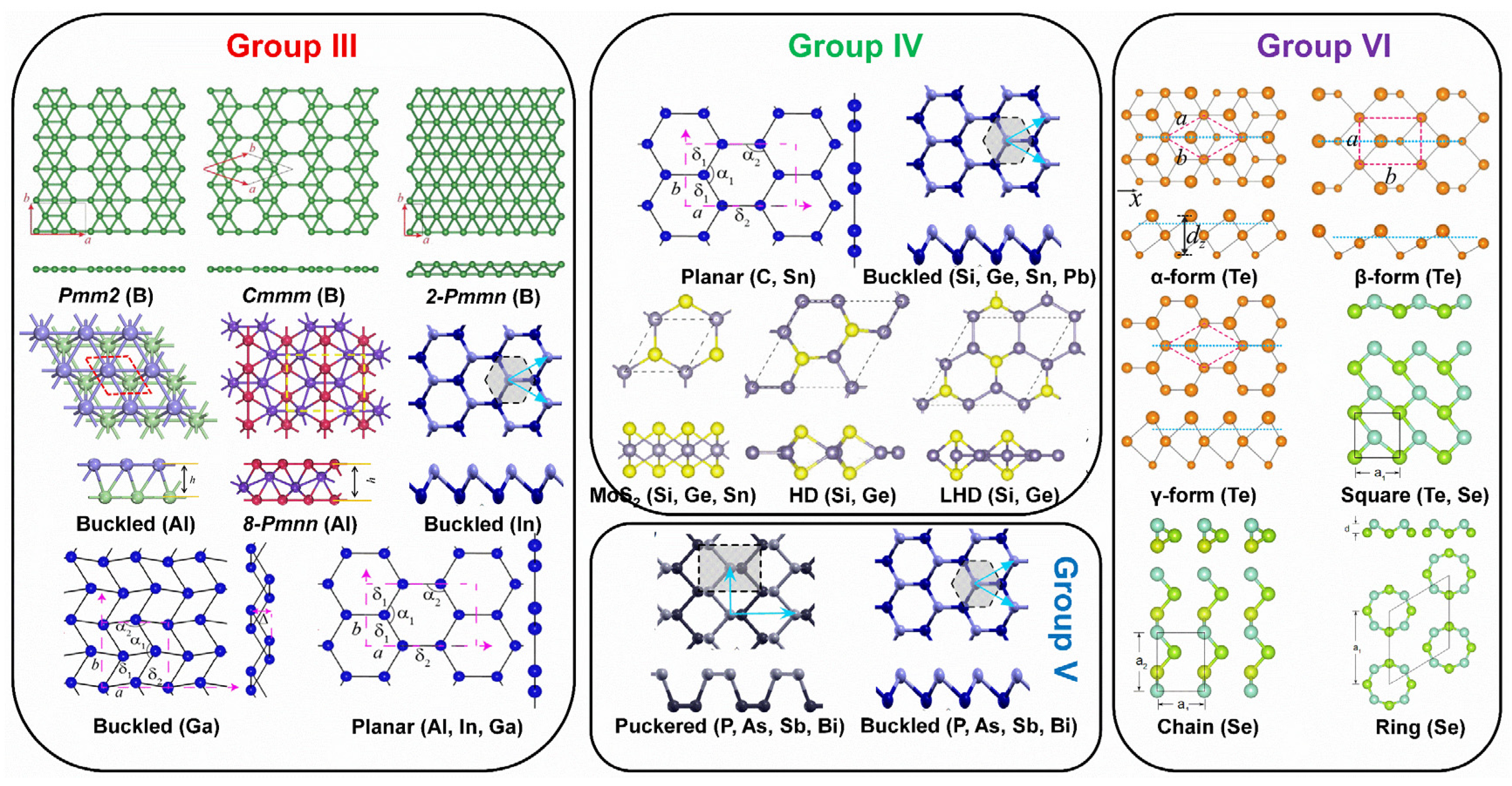
The material properties (electronic, optical, chemical, etc.) of 2D Xenes are not only determined by their chemical compositions but are also strongly associated with the atom arrangement in the lattice. Due to the preferred orbital hybridization of various elements in the main group, 2D Xenes have been theoretically predicted or experimentally verified to possess allotropes with diverse crystal lattices (Figure 2). The reported 16 Xenes are classified into main groups, including group III (borophene, aluminene, gallenene, indiene, and thallene); group IV (graphene, silicene, germanene, stanene, and plumbene); group V (phosphorene, arsenene, antimonene, and bismuthene); and group VI (selenene, and tellurene).
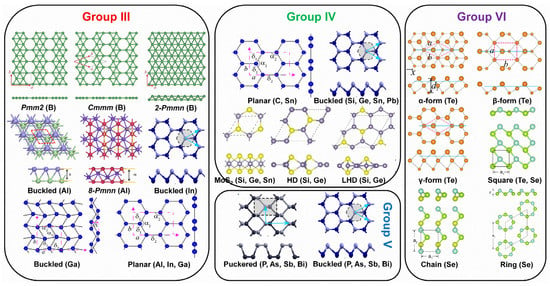
Figure 2.
Allotropes of various 2D Xenes in main groups predicted theoretically or created experimentally. The dotted boxes represent the unit cells. Allotropes of borophene: Reprinted with permission from Ref. [54]. Copyright 2017, Informa UK Limited, trading as Taylor & Francis Group. Allotropes of aluminene: Reprinted with permission from Ref. [55]. Copyright 2017, Elsevier Ltd. Allotropes of gallenene: Reprinted with permission from Ref. [29]. Copyright 2018, American Association for the Advancement of Science. Buckled and puckered structures: Reprinted with permission from Ref. [56]. Copyright 2016, American Physical Society. MoS2-like, HD, and LHD structures: Reprinted with permission from Ref. [57]. Copyright 2015, American Physical Society. α-form, β-form, and γ-form of tellurene: Reprinted with permission from Ref. [27]. Copyright 2017, American Physical Society. Square-, chain-, and ring-Se: Reprinted with permission from Ref. [58]. Copyright 2017, IOP Publishing.
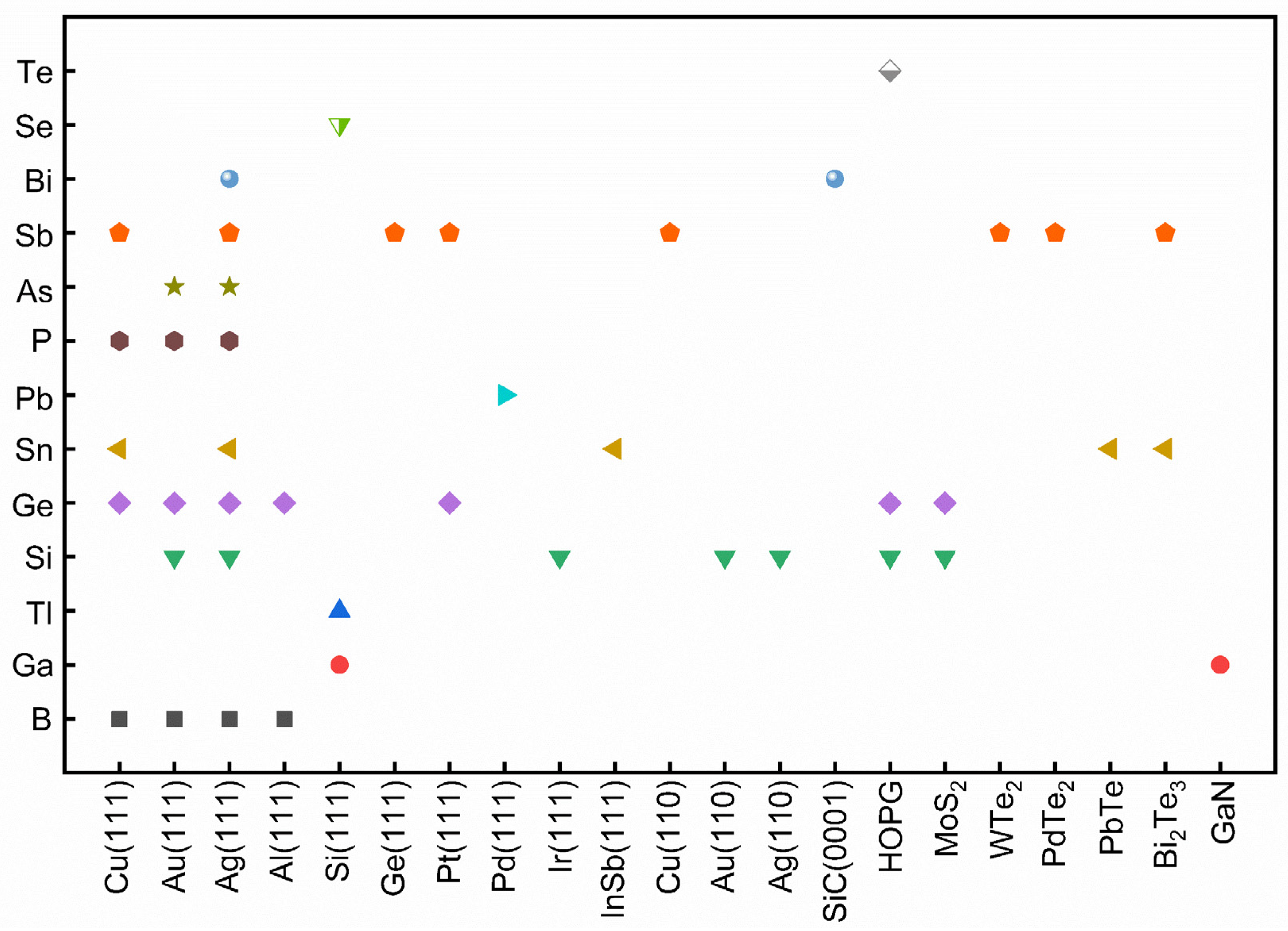
In group III elements, theoretical calculations have predicted many allotropes of 2D borophene (B1−ν☐ν; ν represents the vacancy concentration) with various vacancy concentrations and 2D aluminene with various forms [55,59,60,61]. However, only two allotropes of 2D gallenene (buckled and planar structures) have been successfully exfoliated [29]. Similarly, 2D indiene has been predicted to possess buckled, planar, and puckered geometry [62]. Among group IV elements, the favorable hybridization state is somewhere between sp2 and sp3, leading to various 2D allotropic structures, such as planar honeycomb graphene/stanene [63,64,65], buckled silicene/germanene/stanene/plumbene [25,30,66,67], pha-/penta-graphene [68,69], MoS2-like stanene [57], and honeycomb dumbbell (HD)/large honeycomb dumbbell (LHD) silicene/germanene [57]. Different from group III and group IV, all the elements in group V prefer the sp3 hybridization state to create buckled (α-form) or puckered (β-form) lattices [24,70,71,72,73,74,75]. Meanwhile, a γ-form and a δ-form of arsenene have also been proposed [56]. In group VI, four different allotropic forms of tellurene have been predicted [27,58], while Se can form a chain, a ring, or a square structure [58]. To verify the atomic structures of various elemental Xenes, epitaxial growth has been attempted on many substrates, analogous to the epitaxial growth of graphene. The suitable substrates for the epitaxial growth of various non-graphene Xenes (Figure 3) reveal that the substrate choices affect whether the synthesis can succeed in addition to an allotropic lattice structure.

Figure 3.
Summary of the successful substrate choices for the epitaxial growth of various non-graphene 2D Xenes. Each color represents one kind of Xenes.
3. Surface Modifications of 2D Xenes in Various Main Groups
3.1. Group III
Borophene. Borophene, a monolayer of boron atoms, has been synthesized on many metal substrates under ultrahigh vacuum conditions [22,76,77,78]. Several phases of borophenes, including 2-Pmmn, ν1/6 (β12), ν1/5 (χ3), ν1/12, and honeycomb, which are realized experimentally, exhibit metallic properties [22,76,77,78]. However, plenty of borophene allotropes with various vacancy concentrations have been reported with theoretical calculations [59,60,79,80,81]. Moreover, the relationship between the formation energy and vacancy concentration shows a V-shaped function [82], demonstrating that the vacancy concentration is directly correlated with the stability of borophene.
Borophene possesses some unique physical and chemical properties. Due to the strong B-B bonds and distinctive atomic structure, borophene exhibits an ultrahigh mechanical modulus [83,84]. Young’s modulus of the Pmmm and 2-Pmmn phases of borophene along the armchair direction can reach 574.61 and 398 N m−1, respectively, larger than that of graphene. In fact, several factors, including the vacancy concentration, chemical modification, layer numbers, and temperature, can affect the mechanical properties of borophene. Young’s modulus of borophene was found to decrease with an increasing vacancy concentration, layer numbers, and temperature, as well as hydrogenation or fluorination [83,84,85,86,87,88,89]. Because of its outstanding flexibility and excellent electronic conductivity, borophene has a wide range of intriguing applications in flexible electronic devices [79]. In addition, the thermal conductivity of the 2-Pmmn phase of borophene is different along the zigzag and armchair directions due to its highly anisotropic atomic structure [90,91]. The electronic band structures of borophene show 1D, nearly free electron states and metallic Dirac fermions [92,93,94,95]. However, the metallic-to-semiconducting transition can be achieved via fluorination and a uniaxial or biaxial strain [85,96,97]. Last but not least, the superconductivity of borophene is the most notable characteristic, sparking plenty of research interest [80,98,99,100,101,102,103,104,105,106]. The superconducting transition temperatures (Tc) can be tuned with vacancy concentration, doping, strain, and Mg intercalation. Tc exhibits a V-shaped function as the hexagon hole density [99], illustrating that Tc gradually decreases with a rising boron vacancy concentration of up to ν = 1/9; thereafter, Tc steadily increases with the vacancy concentration. Furthermore, tensile strain or hole-doping can increase Tc; in contrast, a compressive strain or electron-doping decreases Tc [106]. The suppression induced by electron-doping makes it difficult to experimentally probe superconductivity in substrate-supported borophene.
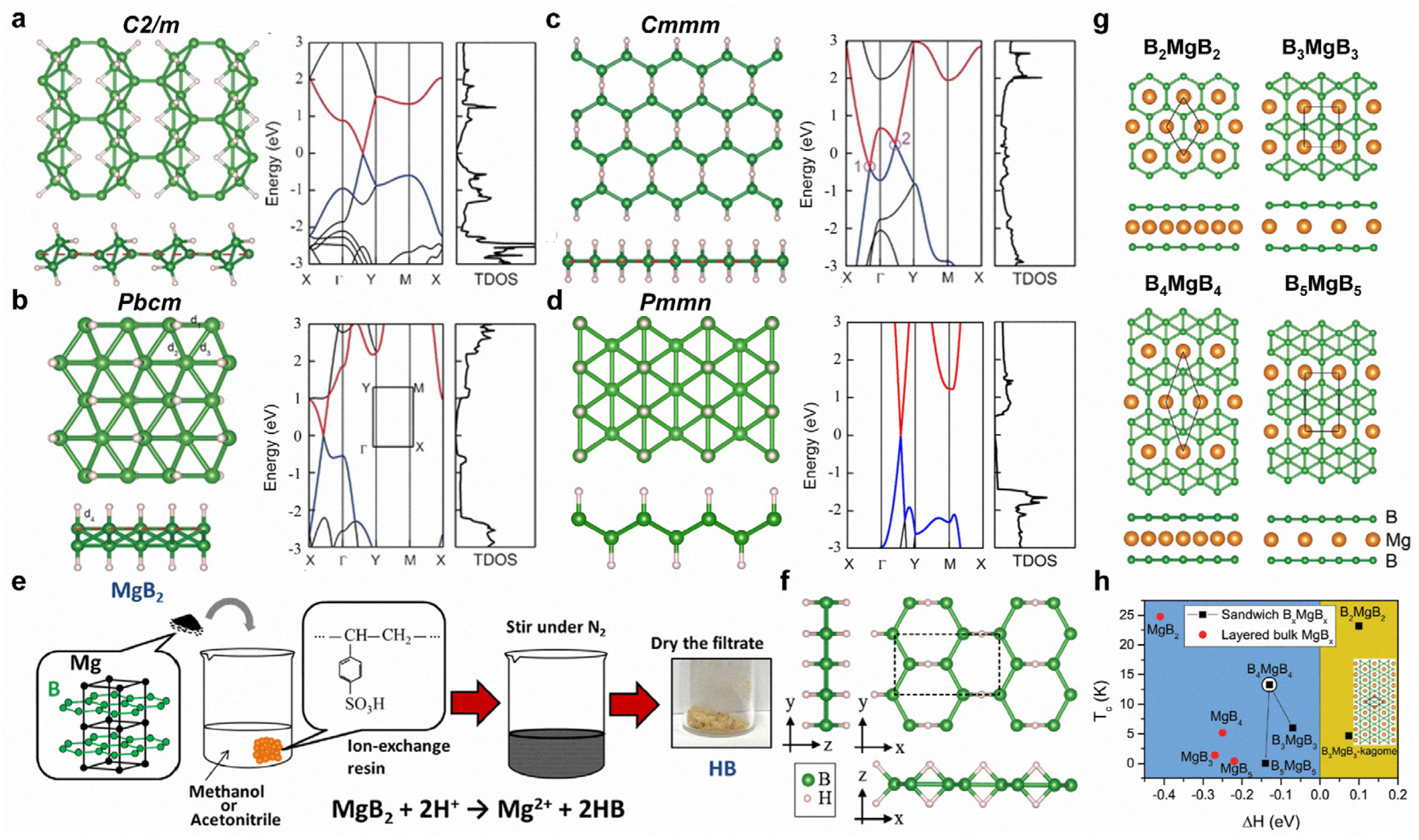
The physical and chemical properties of borophene can be tuned using surface modifications, such as hydrogenation, fluorination, doping, intercalation, strain, etc. Young’s modulus of borophene decreases after hydrogenation or fluorination. Furthermore, hydrogenation can lead to Dirac cones with massless Dirac fermions in C2/m, Pbcm, and Pmmn structures, while Cmmm structures exhibit Dirac ring features (Figure 4a–d) [107]. Interestingly, BH sheets have been successfully prepared from MgB2 by using the cation exchange method (Figure 4e,f) [108]. For Mg intercalation, the intercalated bilayer borophene (B2MgB2) can exhibit good phonon-mediated superconductivity with a high Tc of 23.2 K (Figure 4g,h) [102]. Moreover, tensile strain in borophene is beneficial for superconducting [106]. Li-doped borophene–graphene heterostructure shows gas-sensitive properties, and this is promising for borophene-based gas sensors [109].
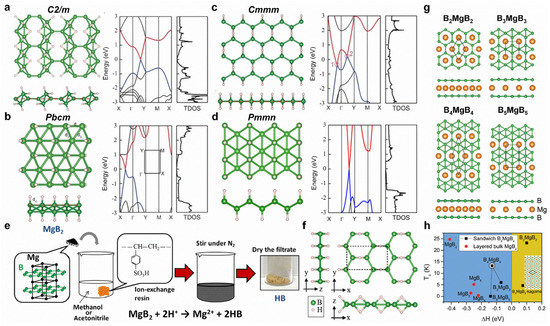
Figure 4.
Surface modifications of borophene. (a–d) Top and side views as well as calculated band structures of (a) C2/m, (b) Pbcm, (c) Cmmm, and (d) Pmmn BH structures. The red and blue lines represent the unoccupied and occupied band dispersions near Fermi level, respectively. (e) Synthesis process of BH sheets. (f) Proposed structure model of synthesized BH sheet. (g) Top and side views of 2D sandwich structures of B2MgB2, B3MgB3, B4MgB4, and B5MgB5. (h) Calculated Tc as a function of the formation enthalpy. (a–d) Reprinted with permission from Ref. [107]. Copyright 2016, Wiley. (e,f) Reprinted with permission from Ref. [108]. Copyright 2017, American Chemical Society. (g,h) Reprinted with permission from Ref. [102]. Copyright 2017, Royal Society of Chemistry.
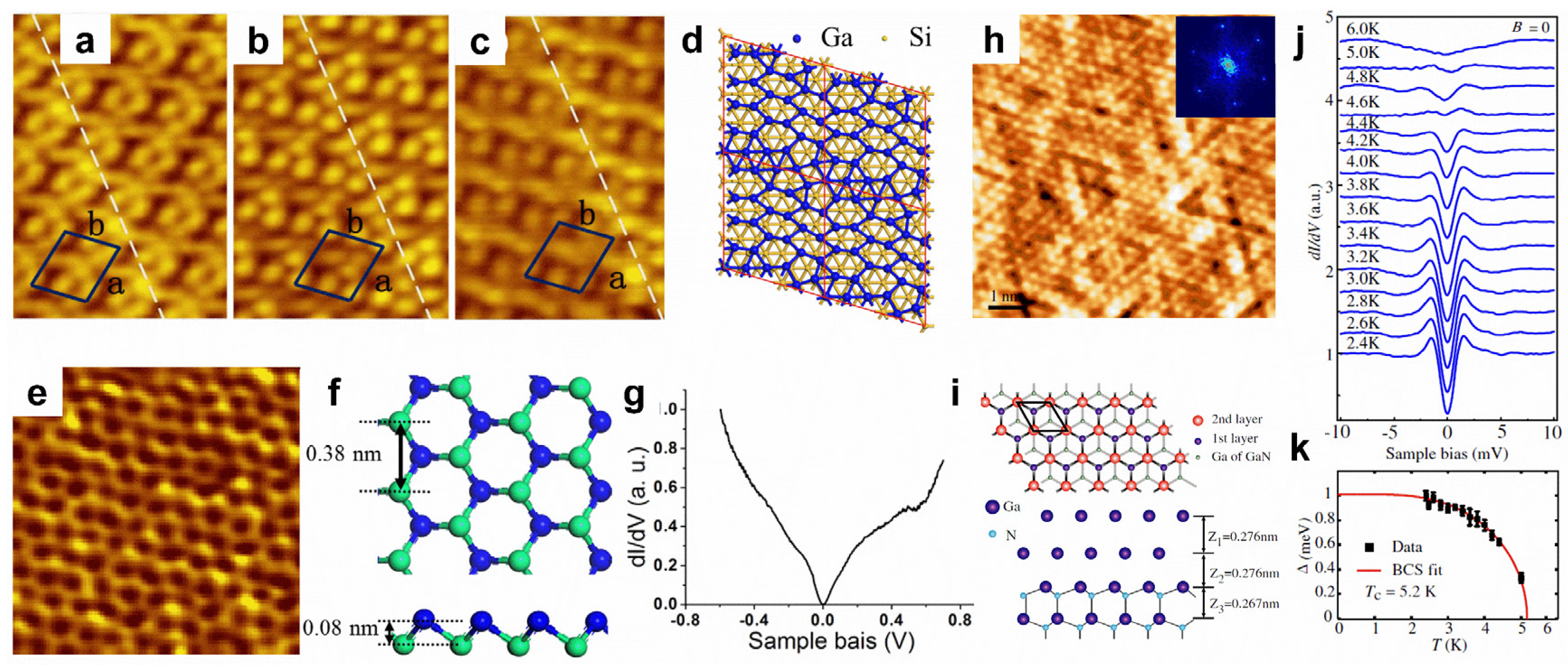
Gallenene and thallene. The surface modifications of aluminene and indiene have not been reported before, as studies on them are still in the theoretical research stage, without experimental realization. Therefore, this section will focus on gallenene and thallene. In 2018, few-layer gallenene was first obtained using the solid melt exfoliation technique [29]. Thereafter, the epitaxial growth method was used to prepare gallenene. The substrate and the loading amount of gallium can modify the atomic and electronic structures of gallenene. With a low loading amount of gallium, the monolayer gallenene grown on Si(111) displays a 4 × √13 superstructure (Figure 5a–d), while the second-layer gallenene exhibits a hexagonal honeycomb structure with a high loading amount (Figure 5e,f) [110]. The buckled honeycomb gallenene shows metallic properties (Figure 5g) [110]. Nevertheless, the growth behavior of gallenene on a GaN(0001) substrate is totally different, showing a bilayer flat gallenene (Figure 5h,i) [111]. Excitingly, the bilayer hexagonal gallenene exhibits superconducting with a Tc of 5.4 K (Figure 5j,k) [111]. However, thallene has rarely been reported. Recently, honeycomb thallene was successfully formed on a NiSi2/Si(111) substrate [112].
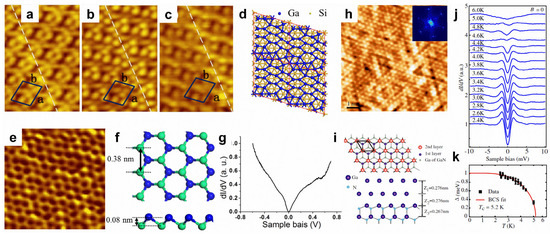
Figure 5.
Surface modifications of gallenene. (a–c) STM images with biases of 2.0, 1.6, and 1.0 V of monolayer gallenene on Si(111) substrate. (d) Proposed configuration of monolayer gallenene on Si(111). (e) STM image of second-layer gallenene on Si(111) substrate. (f) Top and side views of the proposed structure for second-layer gallenene. (g) dI/dV spectrum recorded on the second-layer gallenene. (h) High-resolution STM image of gallenene on GaN(0001) substrate. (i) Top and side views of proposed gallenene structure on GaN(0001) substrate. (j) A series of dI/dV spectra recorded on gallenene/GaN at various temperatures. (k) Temperature-dependent superconducting gap magnitude and fitting to BCS gap function for gallenene/GaN. (a–g) Reprinted with permission from Ref. [110]. Copyright 2018, IOP Publishing Ltd. (h–k) Reprinted with permission from Ref. [111]. Copyright 2015, American Physical Society.
3.2. Group IV
Graphene. The sp2 hybridization of carbon atoms leads to the formation of flat honeycomb graphene with a σ bond between neighboring carbon atoms [113]. The σ bond is the key to the high robustness of graphene, with a Young’s modulus of 1T Pa and a fracture strength of 130 GPa [114]. The unhybridized p orbit, perpendicular to the planar structure of graphene, binds covalently with neighboring carbon atoms to form a π band that is half-filled. Graphene is a semimetal, showing linear dispersion bands near the Fermi level with massless Dirac fermions [113]. As a result, graphene displays an ambipolar electric field effect and high carrier mobility [115]. In addition to its excellent transparent properties, graphene becomes a low-cost alternative to indium tin oxides [116]. Furthermore, graphene exhibits impressive thermal properties with a thermal conductivity ranging from 3000–5000 W m−1 K−1 [117].
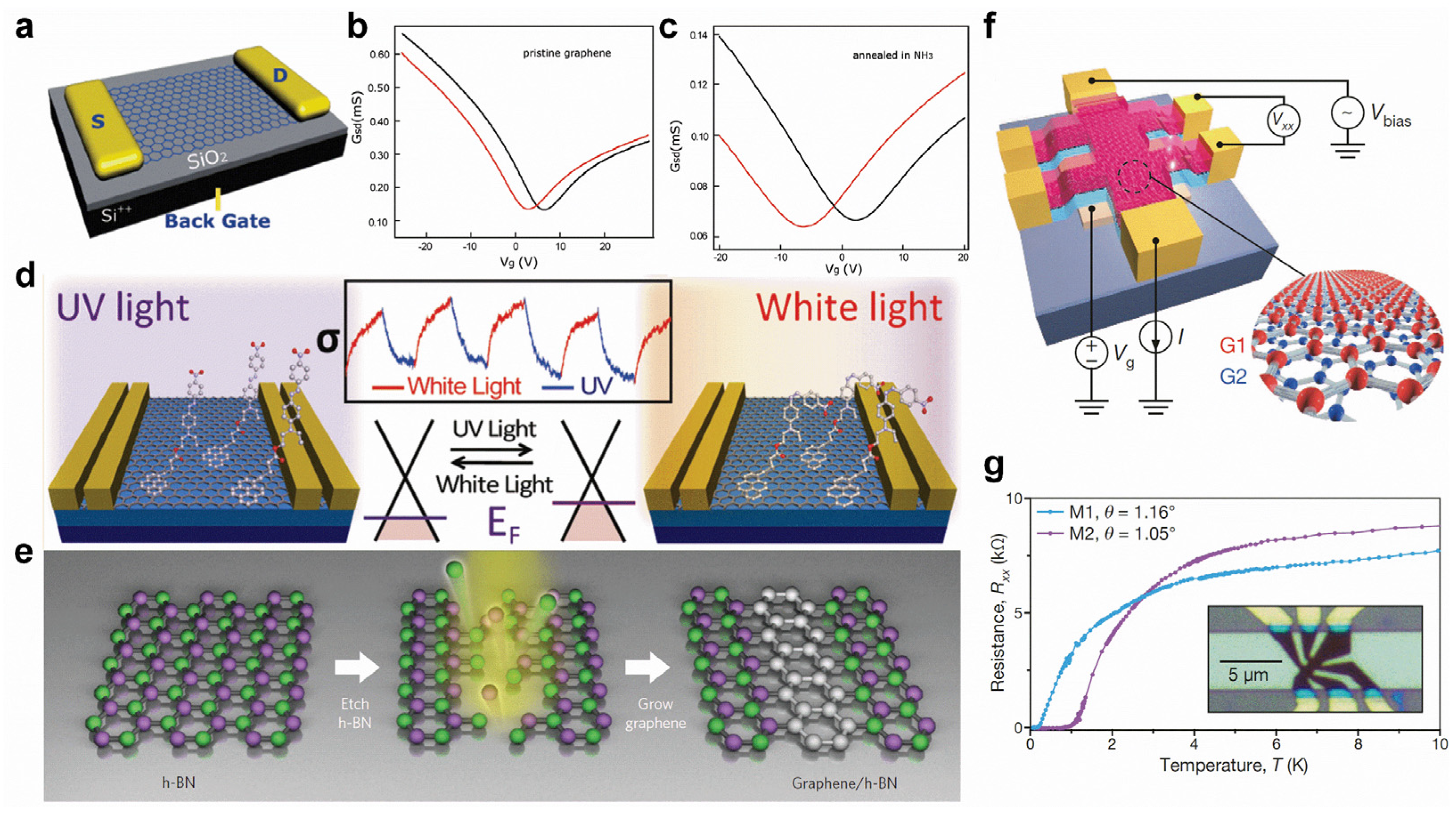
Graphene’s distinct physical characteristics make it potentially useful for field-effect transistors (FET), sensors, transparent conductive films, energy devices, etc., but its intrinsic gapless character still constrains its further applications. First, heteroatom-doping or chemical adsorption can effectively tune the electronic properties of graphene. N-doping can not only induce an n-type-doping effect (Figure 6a–c) [118] but can also give rise to p-type-doping with different configurations of N substitutions. For example, graphitic N induces n-type-doping, but pyridinic N induces p-type-doping [119]. In addition, B-doping can introduce p-type transfer characteristics [120]. Chemical functional groups can also produce various doping effects. For example, the adsorption of spiropyran and DR1P molecules introduce n-type and p-type-doping to graphene, respectively [36,121]. Moreover, light can reversibly switch the molecular transformations, resulting in the controllable shift of the Dirac point of graphene (Figure 6d) [36,121]. By seamlessly and precisely stitching the domains of graphene and h-BN (Figure 6e), the hybrid atomic layers of in-plane heterostructures can be applied for intriguing electronic applications [42,122]. Interestingly, in the proper twisted angles of bilayer graphene, the electronic band structure shows flat bands near Fermi energy, resulting in the correlated insulating states at half-filling and unconventional superconductivity with a Tc of 1.7 K (Figure 6f,g) [52].
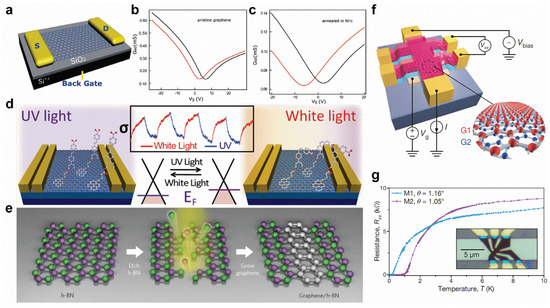
Figure 6.
Surface modifications of graphene. (a–c) Comparison between the transfer characteristics of different graphene-based FETs (black and red curves were measured in air and in vacuum respectively). (a) Scheme of a graphene-based FET device. (b,c) The transport properties of (b) pristine graphene and (c) graphene annealed in NH3 after irradiation measured at Vsd = 0.03 V. (d) Light-driven conductance modulation of graphene with adsorption of DR1P molecules. (e) Fabrication of in-plane graphene/h-BN heterostructures. (f) Schematic of a twisted bilayer graphene device and four-probe measurement system. (g) Superconductivity in twisted bilayer graphene. (a–c) Reprinted with permission from Ref. [118]. Copyright, 2010 American Chemical Society. (d) Reprinted with permission from Ref. [36]. Copyright 2012, American Chemical Society. (e) Reprinted with permission from Ref. [42]. Copyright 2013, Springer Nature Ltd. (f,g) Reprinted with permission from Ref. [52]. Copyright 2018, Springer Nature Ltd.
Silicene. Silicene, the silicon analog of graphene, was first predicted in a theoretical study [123] and first realized experimentally via epitaxial growth on Ag(110) [20]. Unlike the way the sp2 hybridization of carbon atoms induces a flat honeycomb structure in graphene, silicon prefers mixed sp2-sp3 hybridization to form low-buckled honeycomb silicene, retaining the existence of Dirac fermions [124,125]. Considering the spin-orbit coupling (SOC) effect, silicene is predicted to have a spin-orbit gap of 1.55 meV, much larger than that of graphene [16]. Owing to its topologically nontrivial electronic structures, silicene exhibits many unique physical properties, including the quantum spin Hall (QSH) effect [16,126], giant magnetoresistance [127,128], the field-tunable bandgap [129,130], and nonlinear electro-optic effects [131]. Hence, silicene shows great potential for device applications, especially for gate-controlled topological FET [132]. Although silicene has been prepared on many substrates, the poor air stability of silicene is the major challenge, requiring the proper encapsulation or passivation of the reactive surface for device fabrication [133,134].
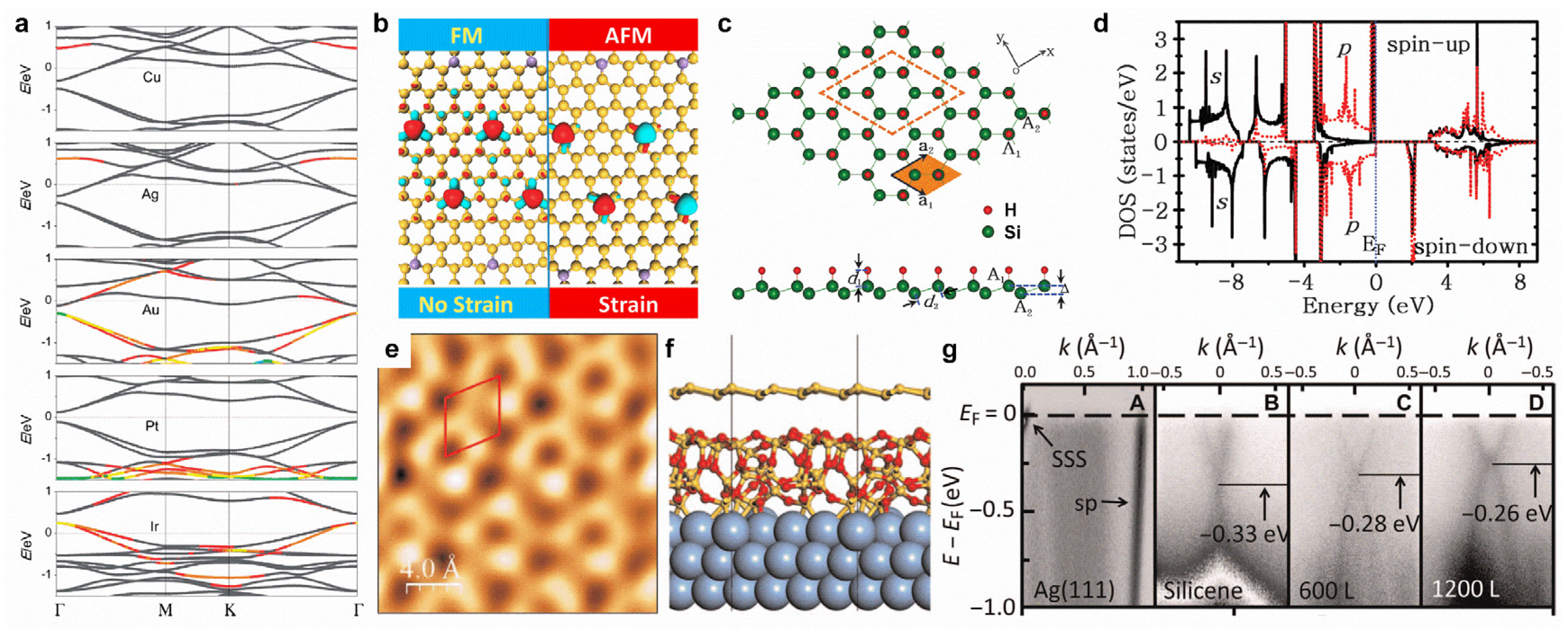
Due to the limitations of the poor air stability of silicene, it is difficult to experimentally perform surface modifications on silicene. Theoretical investigations on the surface modifications of silicene focus on doping, strain, hydrogenation, intercalation, and chemical adsorption. Transition metal adsorption can induce various doping effects in silicene (n-type via Cu, Ag, and Au adsorption; p-type via Ir adsorption; and neutral type via Pt adsorption in Figure 7a) [135], and so can applying strain [136]. Moreover, Mn-doping can induce a ferromagnetic state in silicene, which can be transformed into an antiferromagnetic state with the application of biaxial strain (Figure 7b) [137]. Under a certain level of pressure strain, the spin-orbit bandgap of silicene will increase from 1.55 to 2.9 meV [16]. One-side semi-hydrogenation can introduce ferromagnetism to silicene, as well as make it semiconducting with a direct bandgap of 1.74 eV (Figure 7c,d) [138]. Oxygen intercalation into underlying silicene on a Ag(111) surface can effectively reduce the orbital hybridization of the top-layer silicene and Ag substrate, leading to massless Dirac fermions (Figure 7e–g) [139]. However, in K-intercalated bilayer silicene, the Dirac cones are recovered with a small bandgap of 0.27 eV [140]. Chemical adsorption (gas and organic molecules) can tune the electronic properties of silicene, which could be a better candidate for detecting gas and organic molecules compared with graphene [109,141,142].
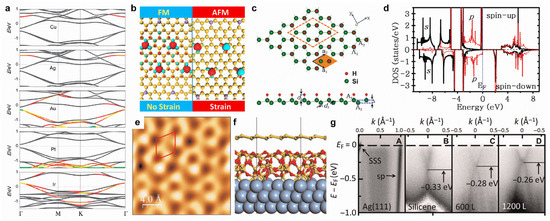
Figure 7.
Surface modifications of silicene. (a) Electronic band structures of Cu-, Ag-, Au-, Pt-, and Ir-covered silicene with a coverage of 5.6%. The Fermi level is set to zero. (b) Strain-controlled magnetic exchange coupling in Mn-doped silicene. (c) Top and side views of the atomic structure for one-side semi-hydrogenated silicene. (d) Partial density of states calculated by HSE06 for semi-hydrogenated silicene. (e) High-resolution STM image of oxygen-intercalated epitaxial silicene grown on Ag(111). (f) Atomic structure of silicene/SiOx/Ag(111) from ab initio molecular dynamics (AIMD) simulation. (g) Energy versus k dispersion measured using ARPES for clean Ag(111) surface (A), as-grown √3 × √3 silicene formed on buffer layer (B), oxygen-intercalated silicene with an oxygen dose of 600 L (C), and intercalated silicene with an oxygen dose of 1200 L (D). (a) Reprinted with permission from Ref. [135]. Copyright 2014, Royal Society of Chemistry. (b) Reprinted with permission from Ref. [137]. Copyright 2017, American Chemical Society. (c,d) Reprinted with permission from Ref. [138]. Copyright 2012, Royal Society of Chemistry. (e–g) Reprinted with permission from Ref. [139]. Copyright 2016, American Association for the Advancement of Science.
Germanene. Germanene, similar to silicene, shows a low-buckled honeycomb structure, leading to a topologically nontrivial electronic structure with a spin-orbit bandgap of 23.9 meV due to a greater SOC than that of graphene and silicene [16,143,144]. These characteristics make germanene a promising candidate for applications in high-speed and low-energy-consumption devices since it has high charge-carrier mobility and exhibits QSHE [144]. Germanene has also been successfully prepared via epitaxial growth on various substrates.
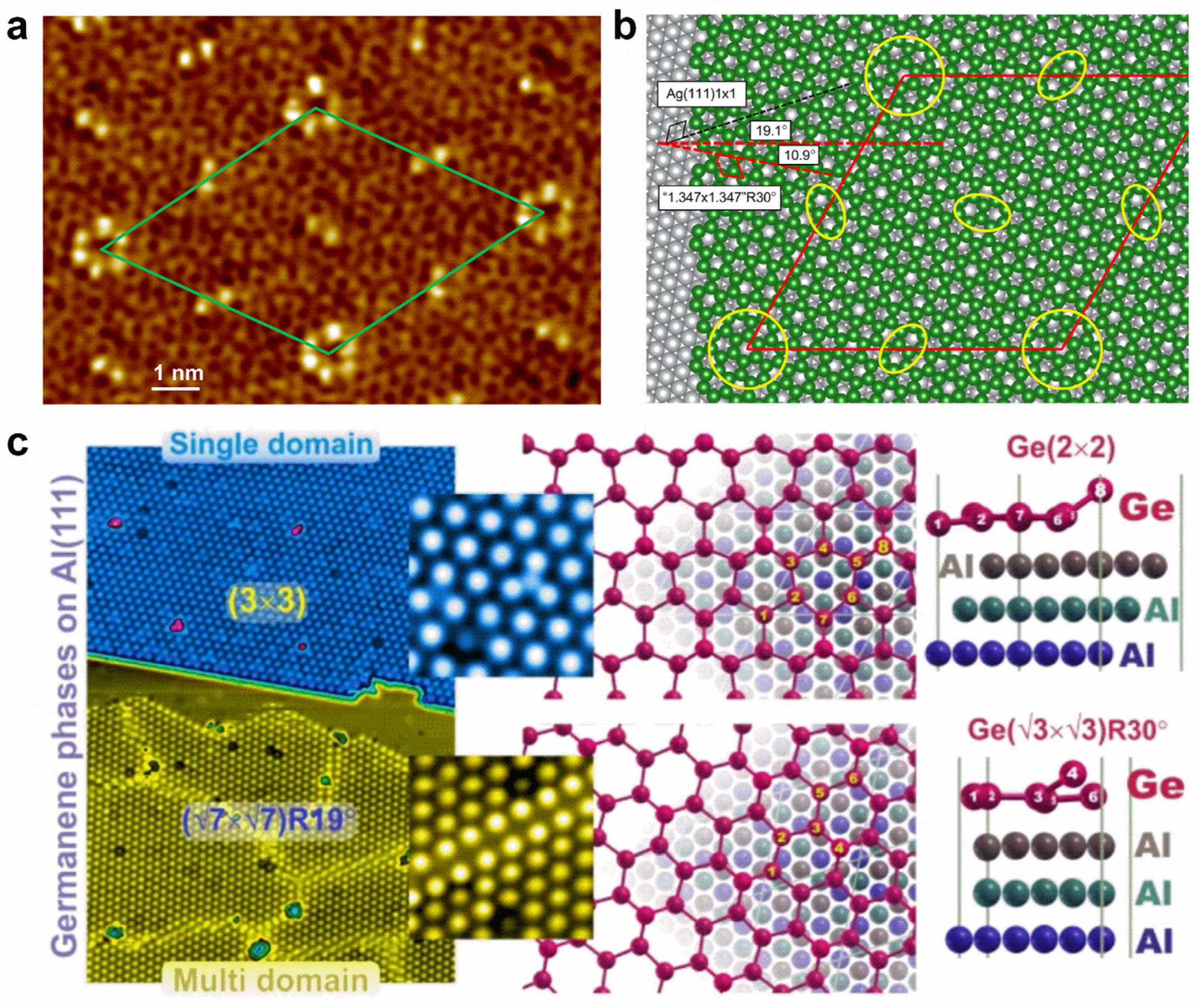
Doping various atoms can introduce totally different influences on the physical properties of germanene. For instance, the adsorption of alkali metal atoms makes semi-metallic germanene become metallic, with the Dirac point moving below the Fermi level and an opened small bandgap at the Dirac point, while the adsorption of halogen atoms could lead to relatively large bandgaps, ranging from 0.416 to 1.596 eV, which is promising for optoelectronic applications [145,146,147]. The adsorption of transition metal atoms (e.g., Ti, Sc, V, Cr, Mn, Fe, and Co) can induce magnetism, while nonmagnetic semiconducting states can be realized for Ni, Cu, and Zn adsorption [148,149]. The atomic structures of germanene can be controlled with supported substrate and growth conditions. Directly grown on a Ag(111) surface, two distinct phases of germanene can be observed: one shows a striped phase, a honeycomb lattice partially commensurate with the substrate; the other is a quasi-freestanding phase, a honeycomb lattice incommensurate with the substrate [150]. By epitaxially preparing it on Ag(111) thin-film grown on Ge(111) with a segregation method, the germanene shows a highly ordered long-range superstructure with two types of protrusions (hexagon and line), resulting in a (7√7 × 7√7)R19.1° supercell with respect to Ag(111) (Figure 8a,b) [151]. However, the single domain (3 × 3) and multidomain (√7 × √7)R(±19.1°) of germanene can exist simultaneously on an Al(111) surface (Figure 8c) [152]. On MoS2 substrate, germanene islands can be formed at high deposition rates, whereas Ge atoms prefer to intercalate between MoS2 layers to form Ge clusters at low deposition rates [147,153]. On a Au(111) surface, honeycomb (1 × 1) germanene with a buckled structure was identified in a (√7 × √7) superstructure, exhibiting distinctive vibrational phonon modes and enhancing electron–phonon coupling induced by the tensile strain [154].
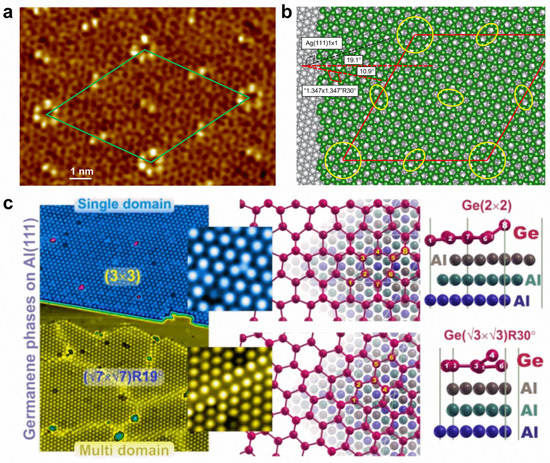
Figure 8.
Surface modifications of germanene. (a) Atomic-scale STM image of germanene grown on a Ag(111) thin film. (b) Structural model of germanene on Ag(111). (c) Two different phases of germanene grown on an Al(111) surface. (a,b) Reprinted with permission from Ref. [151]. Copyright 2018, American Chemical Society. (c) Reprinted with permission from Ref. [152]. Copyright 2019, Springer Nature Ltd.
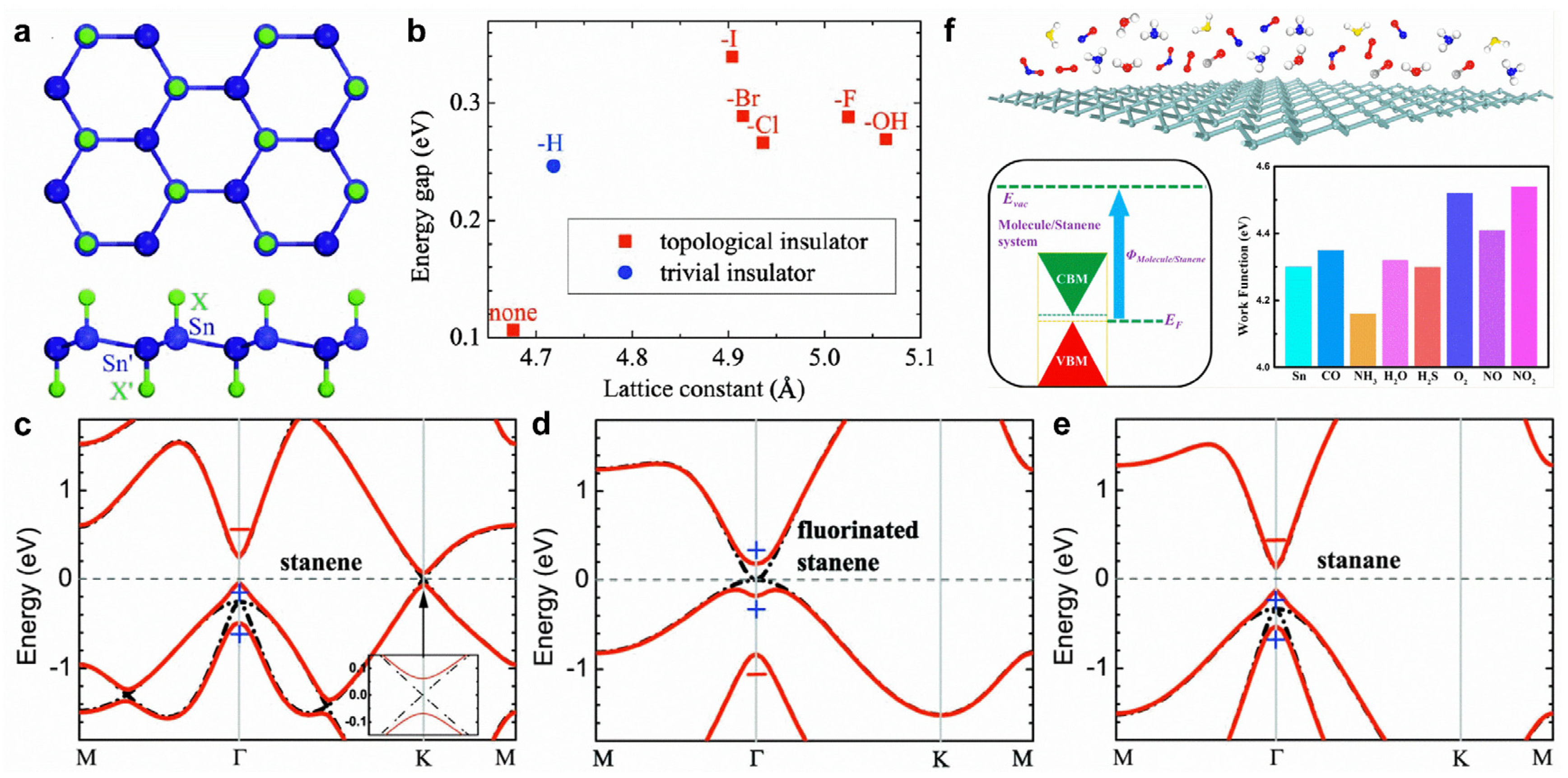
Stanene. Analogously, monolayer Sn prefers to form a low-buckled structure due to the mixed sp2-sp3 hybridization of Sn atoms [155]. Stanene has been predicted to have massless Dirac fermions and open a spin-obit bandgap of 0.1 eV at the K point with SOC [155]. The bandgap at the Dirac point can produce a conductive 1D helical edge state with opposite spin polarization, allowing for low-power spintronic applications [13,156].
Double-side-decorated stanene created by chemical functional groups appears in the most stable configuration (Figure 9a). For pristine stanene, SOC can open a bandgap of 0.1 eV at the K point; thus, stanene becomes a QSH insulator (Figure 9c). After hydorgenation or fluorination, the bandgap at the K point is substantially enlarged because of the saturation of the π orbital (Figure 9d,e). Fluorination induces a parity exchange between the occupied and unoccupied bands at the Γ point. In detail, a negative-parity Bloch state shifts downward into valence bands, leaving a positive-parity Bloch state as the conduction band minimum (Figure 9d), leading to a topologically nontrivial band structure. Nonetheless, the band inversion at the Γ point cannot be seen in hydrogenated stanene (Figure 9e). In fact, the band inversion exists for several chemical functional groups (halogen atoms and -OH) (Figure 9b).

Figure 9.
Surface modifications of stanene. (a) Crystal structure of decorated stanene by hydrogen or halogen atoms on both sides. (b) The calculated energy gaps of stanene and decorated stanene. (c–e) Band structures for (c) stanene, (d) fluorinated stanene, and (e) stanene with (red solid lines) and without (black dash-dotted lines) SOC. Parities of the Bloch states at the Γ point are denoted by +, −. (f) Structural model of stanene for the adsorption of various molecules and work function of stanene with various adsorbed molecules. (a–e) Reprinted with permission from Ref. [155]. Copyright 2013, American Physical Society. (f) Reprinted with permission from Ref. [157]. Copyright 2016, American Chemical Society.
Stanene is predicted to show superior sensing performance for small molecules. CO, O2, NO, NO2, and SO2 molecules act as charge acceptors, whereas H2O, NH3, and H2S molecules act as charge donors [157,158]. In addition, molecule adsorption can effectively tune the work function of stanene (Figure 9f) [157]. The edge shapes of stanene play a key role in the physical properties of stanene nanoribbons (NRs). Armchair stanene NRs are nonmagnetic semiconductors with tunable bandgaps by ribbon width, whereas zigzag stanene NRs present antiferromagnetic ground states with an opposite spin order between the two edges [159]. Generally, the energy gap (0.1 eV) of monolayer stanene rules out phonon-mediated superconductivity. Interestingly, doping with Ca (Li) can lead to superconductivity with a low Tc of ~1.3 K (~1.4 K), lower than the value (3.7 K) of bulk β-tin [160].
Plumbene. Unlike the other four Xenes in main group IV, no Dirac point crosses linearly from the Pb pz orbit at the K point without SOC. Although turning on SOC opens a large bandgap of ~400 meV, no Dirac edge state has been observed in the bandgap of plumbene [161]. In addition, resulting from the energy decrease in the s antibonding state from graphene to plumbene, the s antibonding state is lower than all p bonding and antibonding states at the Γ point in plumbene, totally different from graphene, silicene, germanene, and stanene [162]. Therefore, plumbene is a normal insulator with a topologically trivial character. However, through electron-doping, plumbene can become a topological insulator with a large bulk gap (~200 meV) [163]. Plumbene decorated by chemical function groups (hydrogen and halogen atoms) can transform from a normal insulator to a QSH insulator with giant bulk gaps from 1.03 to 1.34 eV [161]. Plumbene has been predicted to be magnetic with Ti-, V-, Cr-, Mn-, Fe-, and Co-doping, while Sc- and Ni-doped plumbene is nonmagnetic [164]. It is interesting that plumbene can be successfully grown using segregation on a Pd1−xPbx(111) alloy surface [30]. Furthermore, a c(2 × 4) structure of Pb forms on Ir(111) substrate, whereas a flat honeycomb plumbene can be formed on an Fe monolayer on Ir(111) [165].
3.3. Group V
Phosphorene. Puckered and buckled structures in phosphorene are the most common allotropic monolayer structures, corresponding to the individual atomic layers of black phosphorous and blue phosphorous crystals, respectively [166]. Puckered monolayer phosphorene is semiconducting with a direct bandgap of 1.83 eV, whereas buckled monolayer phosphorene is a semiconductor with an indirect bandgap of 2.0 eV. Phosphorene can be obtained through mechanical exfoliation, liquid phase exfoliation, electrochemical exfoliation, chemical vapor deposition, epitaxial growth, etc. [166]. To date, the semiconducting character of phosphorene has led to some experimental demonstrations in various applications, including electronics, optoelectronics, photovoltaics, supercapacitors, and catalysis [73,167].
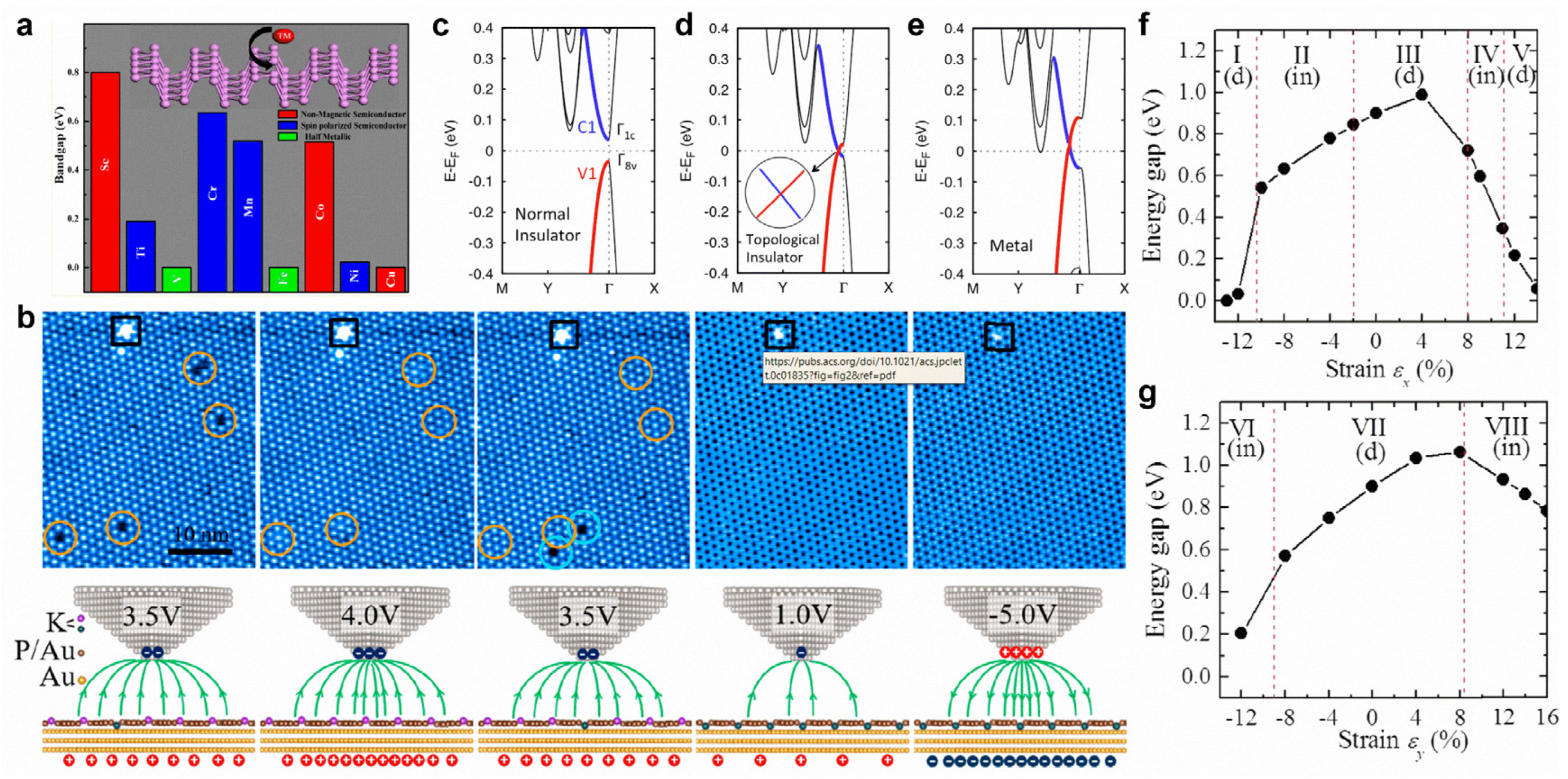
Transition metal-doped black phosphorene possesses dilute magnetic semiconducting properties. In particular, the substitutional doping of Ti, Cr, and Mn can create a spin-polarized semiconducting state, while a half-metallic state is realized via V- and Fe-doping (Figure 10a) [168]. Both Fe-doping and N-doping can significantly improve the electrocatalytic activity of black phosphorene for nitrogen reduction reactions [169,170]. For blue phosphorene, B-doping and C-doping can both improve the sensitivity of NH3 gas molecules, and the sensitivity of CO gas molecules can be enhanced by B-doping [171]. With a monotonic increase in an external electric field, black phosphorene can transition from a normal insulator to a topological insulator and, eventually, to a metal (Figure 10c–e) [172]. In addition, an external electric field can realize reversible potassium intercalation in a blue phosphorene–Au network (Figure 10b) [173]. When axial strain is applied in the zigzag or armchair direction, the bandgap of black phosphorene will exhibit a direct–indirect–direct transition (Figure 10f,g) [51]. Moreover, a topological phase transition of black phosphorene can be realized via the application of compressive biaxial in-plane strain and perpendicular tensile strain [174].
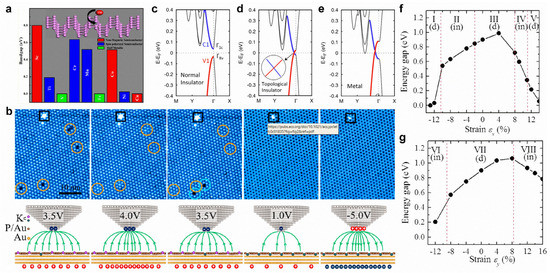
Figure 10.
Surface modifications of phosphorene. (a) Electronic and magnetic properties of transition-metal-doped phosphorene. (b) STM images of blue phosphorene–Au network with 1 ML K deposition scanned with different bias voltages. (c–e) Band structures of 4-layer phosphorene with an external electric field of (c) 0 V Å−1, (d) 0.45 V Å−1, and (e) 0.6 V Å−1. (f,g) Bandgap of phosphorene as a function of strain: (f) εx, applied in the zigzag and (g) εy, in the armchair directions. (a) Reprinted with permission from Ref. [168]. Copyright 2015, American Chemical Society. (b) Reprinted with permission from Ref. [173]. Copyright 2020, American Chemical Society. (c–e) Reprinted with permission from Ref. [172]. Copyright 2015, American Chemical Society. (f,g) Reprinted with permission from Ref. [51]. Copyright 2014, American Physical Society.
Arsenene. Arsenene mainly has two kinds of allotropic structures, including buckled and puckered [166]. Buckled honeycomb monolayer arsenene, derived from semi-metallic gray arsenic, has an indirect bandgap of 2.49 eV [24], while puckered monolayer arsenene, exfoliated from black semiconducting arsenic, possesses an indirect bandgap of 0.831 eV [75]. Both buckled arsenene and puckered arsenene have thickness-dependent bandgaps. The methods to obtain arsenene include top-down (mechanical exfoliation and liquid phase exfoliation) and bottom-up strategies (molecular beam epitaxy, chemical vapor deposition, physical vapor deposition, etc.) [175]. For epitaxial growth, monolayer buckled arsenene was successfully grown on a Ag(111) substrate [176], whereas monolayer armchair arsenene nanochains have been formed on a Au(111) surface [32]. Two-dimensional arsenene has been theoretically predicted to exhibit various physical properties, such as an indirect-to-direct bandgap transition, a semimetal-to-semiconductor transition, superconductivity, and a QSH effect, deserving many research efforts [24,177,178]. Recently, few-layer black arsenene was proved to exhibit a particle–hole asymmetric Rashba valley and exotic quantum Hall states due to synergetic effects between the spin-orbit interaction and the Stark effect [179].
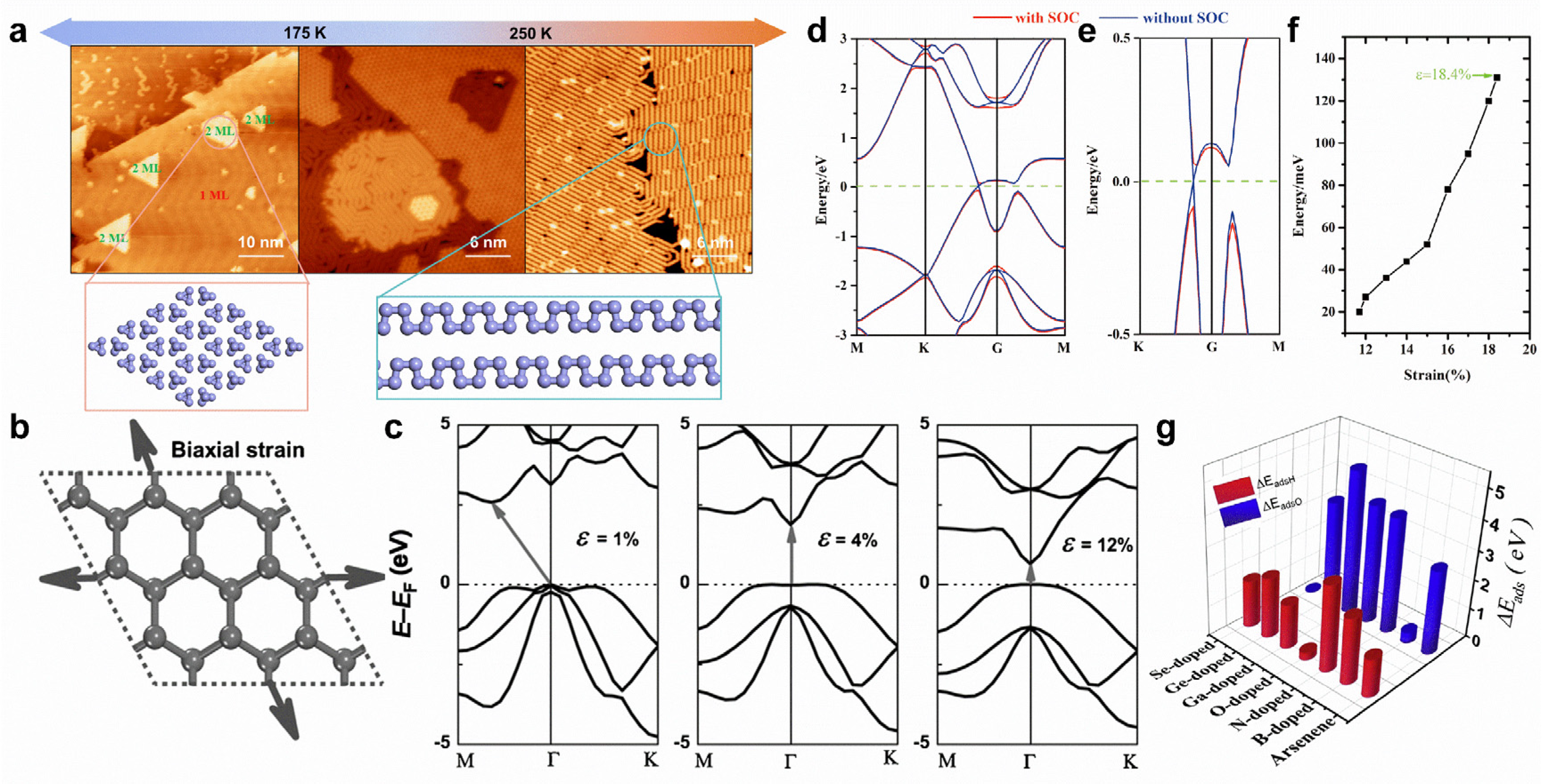
Substrate temperatures can modify the formation of arsenic nanostructures. On Au(111) substrate, the arsenic monolayer formed by 0D tetrahedral As4 clusters can transform into a monolayer formed by 1D armchair arsenene nanochains (Figure 11a) [32]. The application of biaxial tensile strain can effectively tune the band structures of arsenene. Under low tensile strain, arsenene can transform from an indirect to a direct bandgap (Figure 11b,c) [24]. By further enlarging the tensile strain, the direct bandgap will gradually disappear, causing band inversion at the Γ point (Figure 11d,e) [177]. The consideration of SOC can open a spin-orbit bandgap (~131 meV) under a tensile strain of 18.4% (Figure 11f), indicating a QSH effect in arsenene [177]. Intriguingly, under proper biaxial tensile strain and electron-doping, arsenene can be superconducting, with a Tc of 30.8 K [178]. Indeed, 3D-transition-metal-doping can strongly tailor the electronic and magnetic properties of arsenene. Ti-, V-, Cr-, Mn-, and Fe-doping can induce magnetic states for arsenene [180]. Meanwhile, Ti- and Mn-doping leads to a half-metallic state, while V-, Cr-, and Fe-doping results in a spin-polarized semiconducting state [180]. In addition, doping can further modify chemical properties, making arsenene potentially useful for hydrogen evolution and oxygen evolution reactions (Figure 11g) [181].
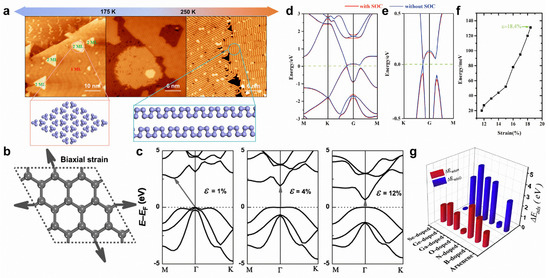
Figure 11.
Surface modifications of arsenene. (a) Schematic diagram of substrate-temperature-dependent arsenic nanostructures on Au(111) substrate. (b) Schematic representation of arsenene under biaxial tensile strain. (c) Variations in electronic band structures of arsenene under various biaxial strains. The arrows represent the transitions of the bandgaps. (d,e) Band structure of arsenene under a biaxial tensile strain of 18.4% and an enlarged view. (f) Bandgap variation in arsenene with the tensile strain. (g) Adsorption energy of hydrogen and oxygen on pristine and B-, N-, O-, Ga-, Ge-, and Se-doped arsenene. (a) Reprinted with permission from Ref. [32]. Copyright 2022, American Chemical Society. (b,c) Reprinted with permission from Ref. [24]. Copyright 2015, Wiley. (d–f) Reprinted with permission from Ref. [177]. Copyright 2015, Royal Society of Chemistry. (g) Reprinted with permission from Ref. [181]. Copyright 2018, Elsevier Ltd.
Antimonene and bismuthene. The most stable structures of antimonene and bismuthene are α-form (puckered) and β-form (buckled), which are the monolayers of black and gray bulk allotropes, respectively. Monolayer β-Sb and β-Bi possess indirect bandgaps of 2.28 and 0.99 eV, respectively, whereas the direct bandgaps of monolayer α-Sb and α-Bi are 1.18 and 0.36 eV, respectively [74,182,183]. Notably, the calculated bandgaps may vary depending on the methods used. Both antimonene and bismuthene exhibit tunable bandgaps, high carrier mobility, high stability, and in-plane anisotropy, providing a basis for multifunctional applications in electronics, optoelectronics, sensors, batteries, etc. [184,185,186].
Doping with 3d transition metal atoms for antimonene can lead to significant changes in the bandgap and the magnetic moment [187]. For Cr-doped β-antimonene, a spin-polarized semiconducting state was predicted. For Ti-, Mn-, and V-doped β-antimonene, half-metallic behavior was calculated. Similarly, a Cr-doped bismuthene structure leads to a spin-polarized semiconducting state, while V-doped bismuthene can produce a magnetic metal character, and Mn- and Fe-replacing systems result in half-metal features [188]. Additionally, V-doped systems exhibit ferromagnetism (FM) when two V atoms are far apart, but Cr-, Mn-, and Fe-doped bismuthene exhibits anti-ferromagnetism (AFM) when two impurity atoms are close together or far apart [188]. Bivacancy-doping in β-antimonene can reduce the bandgap of pristine β-antimonene, but monovacancy-doped β-antimonene exhibits a metallic character [189]. Electron-doping and Ca-intercalation can transform bilayer β-antimonene from a semimetal into a superconductor [190]. Moreover, the physisorption of the organic molecules tetrathiafulvalene and tetracyanoquinodimethane can induce n-type- and p-type-doping for antimonene, respectively [191]. Under a monotonic increase in biaxial tensile strain, β-antimonene and β-bismuthene undergo indirect-to-direct bandgap and semiconducting to semi-metallic transitions and even topological phase transitions [24,192,193].
3.4. Group VI
Selenene and tellurene. Selenene has been predicted to have three allotropic structures, including a 1T-MoS2-like structure (t-Se or α-Se), a tiled helical-chain structure (c-Se), and s square structure (s-Se) [194]. Both t-Se and c-Se are semiconductors with indirect bandgaps of 0.71 and 1.74 eV, respectively, while s-Se is semi-metallic. The formation energy of c-Se is the lowest, indicating that c-Se is the most stable phase. In addition, a ring structure of selenene (r-Se) is proposed [58]. However, theoretical investigations predict the existence of three phases of tellurene, including α-, β-, and γ-phases, possessing 1T-MoS2-like, rectangle, and 2H-MoS2-like structures, respectively [27]. It was found that α- and β-Te show semiconducting characteristics with indirect bandgaps of 0.76 and 1.17 eV, respectively, whereas γ-Te is a metal. It is interesting that the band structures of square selenene and tellurene (s-Se and s-Te) show Dirac-cone-like dispersions. A large bandgap (~0.1 eV) opened by SOC makes them become topological insulators and host nontrivial edge states [58]. Therefore, square selenene and tellurene become promising candidates for spintronic applications. The tensile strain can monotonically decrease the bandgaps of α-Se and α-Te [195,196]. Meanwhile, for 2D square tellurene under a strain effect, the system displays three structural phases, buckled square, buckled rectangle, and planar square phases, which exhibit extraordinary topological properties [196]. In particular, the buckled rectangle tellurene can act as a QSH insulator with a bandgap of 0.24 eV.
4. Applications of 2D Xenes
As mentioned above, 2D Xenes possess various physical and chemical properties, such as flexibility, layer-dependent semiconducting, high carrier mobility, molecule and light sensitivity, topologically nontrivial band structures, etc.
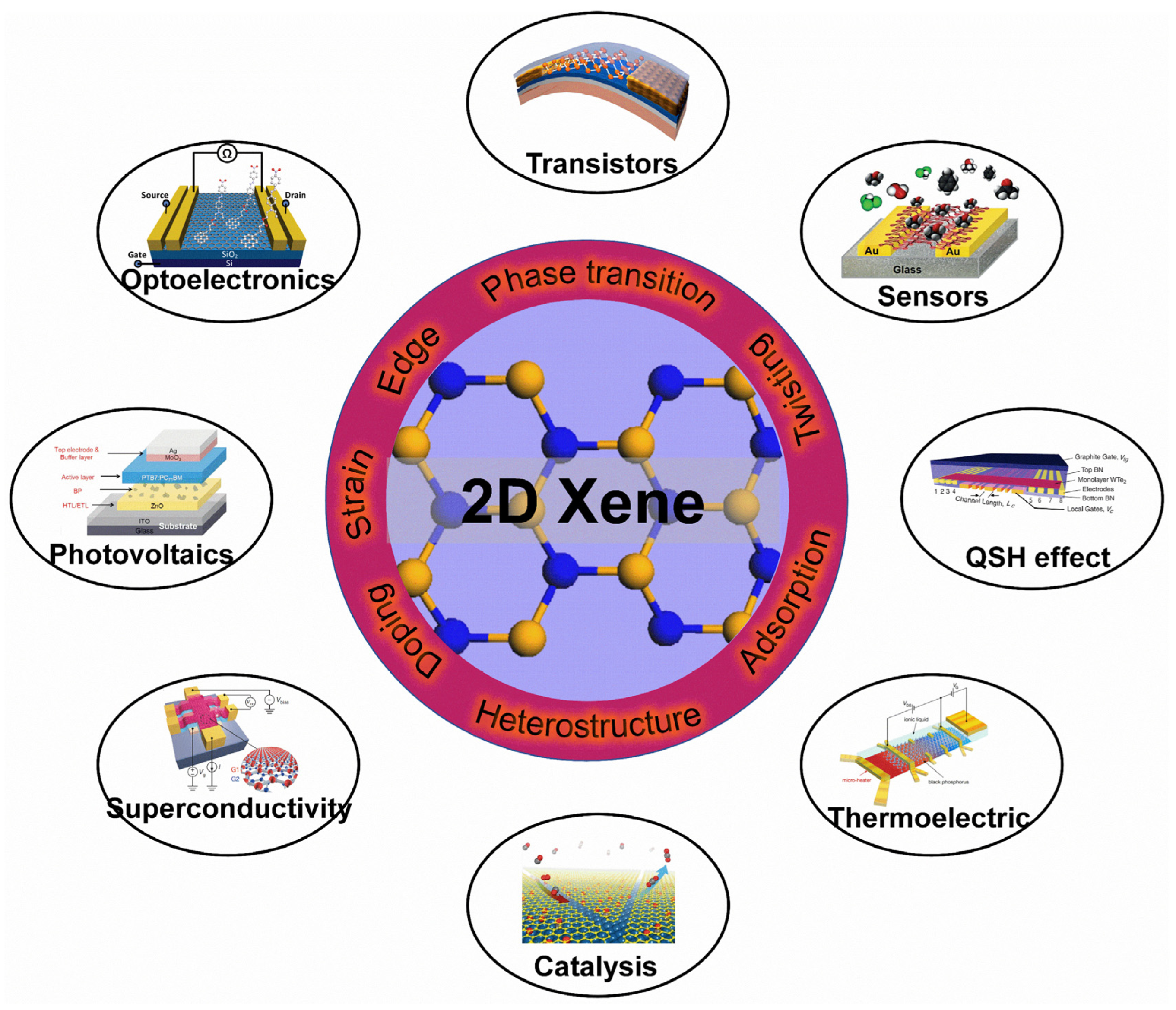
In order to utilize 2D Xenes more effectively, surface modifications become particularly important (Figure 12) to tune the properties of 2D Xenes. For instance, the FET of acene-type graphene nanoribbons exhibits excellent semiconductor characteristics with an on/off ratio of 88 [197]. To enhance the mid-infrared (MIR) absorption of graphene, the localized surface plasmon resonance of B-doped Si quantum dots (QDs) results in a QD/graphene hybrid photodetector with ultrahigh responsivity, gain, and specific detectivity in the UV-to-MIR region [34]. A 2D bismuthene/Si(111) heterostructure exhibits excellent photodetection performance in the Vis-MIR region due to the promoted generation and transportation of charge carriers in the heterojunction [198]. Solution-exfoliated black phosphorene flakes, as an electron transport layer, can enhance the performance of organic solar cells [199]. In addition, layered black phosphorene exhibits the selective detection of methanol [200]. The thermoelectric power (S) in black phosphorene can be effectively controlled with ion-gating. In the hole-depleted state, the S of black phosphorene can reach +510 μV/K at 210 K, much higher than the bulk single crystal value of +340 μV/K at 300 K [201]. Under the proper electron-doping and biaxial tensile strain, buckled arsenene shows superconductivity with a high Tc of 30.8 K [178]. Iodine-decorated stanene exhibits a topologically nontrivial band structure with a larger gap of ~320 meV than that of pristine stanene (~100 meV) [155]. Graphene/Pt(111) surfaces can cause CO adsorption/desorption and CO oxidation surface reactions [202]. MoS2/graphene hybrids decorated by CdS nanocrystals can act as high-performance photocatalysts for H2 evolution under visible light irradiation [203]. Moreover, 2D Xenes are also regarded as promising agents for biomedical applications [204]. For example, an ultrathin bismuthene can act as a sensing platform to detect microRNA with a detection limit of 60 PM [205]. Polyethylene-coated antimonene quantum dots can be used as photothermal agents with a high photothermal conversion efficacy of 45.5% for photothermal therapy in cancer theranostics [206]. Overall, thanks to surface modifications, 2D Xenes show great potential for applications in plenty of fields.
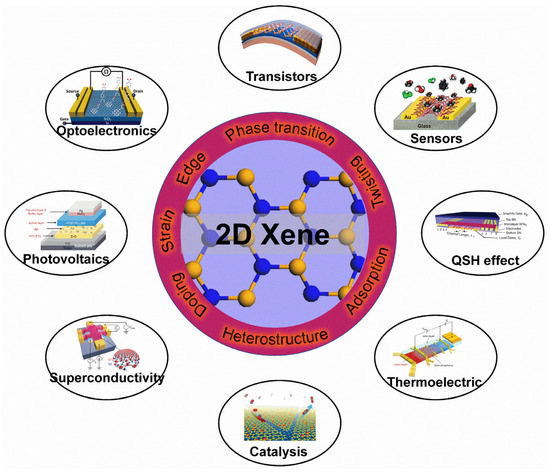
Figure 12.
Applications of 2D Xenes with surface modifications. Transistors: Reprinted with permission from Ref. [207]. Copyright 2015, American Chemical Society. Optoelectronics: Reprinted with permission from Ref. [36]. Copyright 2012, American Chemical Society. Sensors: Reprinted with permission from Ref. [200]. Copyright 2015, American Chemical Society. Photovoltaics: Reprinted with permission from Ref. [199]. Copyright 2016, Wiley. QSH effect: Reprinted with permission from Ref. [208]. Copyright 2018, American Association for the Advancement of Science. Superconductivity: Reprinted with permission from Ref. [52]. Copyright 2018, Springer Nature Ltd. Thermoelectric: Reprinted with permission from Ref. [201]. Copyright 2016, American Chemical Society. Catalysis: Reprinted with permission from Ref. [202]. Copyright 2014, National Academy of Science.
5. Conclusions
In total, except graphene, 15 different elemental 2D materials of the main group elements have been experimentally created or theoretically predicted to date. In fact, 14 Xenes, including borophene, graphene, silicene, phosphorene, gallenene, germanene, arsenene, selenene, stanene, antimonene, tellurene, thallene, plumbene, and bismuthene, have been successfully grown on proper substrates using epitaxial methods. Although a lot of research, engineering, and development related to 2D Xenes—most of which were investigated theoretically—has been reported in recent years, experimental studies are still desperately required for the development of synthesis strategies and novel applications. Before realizing the surface modifications of 2D Xenes for the applications of interest, three significant challenges need to be overcome:
(i) The synthesis strategies of 2D Xenes must not only ensure reliably large-scale production but also be tailored for the requirements of the application. For example, the applications of electronics and batteries demand low costs and scalable techniques, while plasmonics and spintronics require high fidelity and reproducible techniques. Hence, reliable synthesis approaches play a crucial role in functional design and practical applications.
(ii) The strategies to enhance the environmental stability and mitigate the degradation of 2D Xenes must be taken into consideration for certain applications. For instance, an optoelectronic device based on black phosphorene must be concerned with ambient stability, requiring appropriate encapsulation or the passivation of the surface.
(iii) Many of the predicted and fascinating properties of 2D Xenes require further efforts to find strategies for their implementation, which may offer opportunities to discover revolutionary technologies. In addition, the exciting and novel properties necessitate not just “one-off” research, but also statistical evaluation to ascertain their viability and accessibility on a commercial scale.
Despite the challenges ahead, the exceptional properties of 2D Xenes will significantly impact future applications in various fields. It is hoped that this review will inspire more exhilarating discoveries and applications in the growing family of 2D Xenes.
Author Contributions
Conceptualization, Y.M.; validation, J.C., C.W., X.X. (Xudong Xiao), W.Z. and Y.M.; investigation, J.C., C.W., H.L., X.X. (Xin Xu), J.Y., Z.H., L.W. and Y.M.; resources, J.C., C.W. and Y.M.; data curation, J.C., C.W. and Y.M.; writing—original draft preparation, J.C., C.W. and Y.M.; writing—review and editing, X.X. (Xudong Xiao), W.Z. and Y.M.; visualization, Y.M.; project administration, Y.M.; funding acquisition, Y.M., J.C. and C.W. contributed equally to this work. All authors have read and agreed to the published version of the manuscript.
Funding
This work is financially supported by the Start-Up Foundation of Henan University (Grant No.: CX3050A0970531).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are contained within the article.
Acknowledgments
We thank J. Li from Beihang University for helpful discussions.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Novoselov, K.S.; Geim, A.K.; Morozov, S.V.; Jiang, D.-e.; Zhang, Y.; Dubonos, S.V.; Grigorieva, I.V.; Firsov, A.A. Electric field effect in atomically thin carbon films. Science 2004, 306, 666–669. [Google Scholar] [CrossRef] [PubMed]
- Mannix, A.J.; Kiraly, B.; Hersam, M.C.; Guisinger, N.P. Synthesis and chemistry of elemental 2D materials. Nat. Rev. Chem. 2017, 1, 0014. [Google Scholar] [CrossRef]
- Kis, A. Graphene is not alone. Nat. Nanotechnol. 2012, 7, 683. [Google Scholar]
- Butler, S.Z.; Hollen, S.M.; Cao, L.; Cui, Y.; Gupta, J.A.; Gutiérrez, H.R.; Heinz, T.F.; Hong, S.S.; Huang, J.; Ismach, A.F. Progress, challenges, and opportunities in two-dimensional materials beyond graphene. ACS Nano 2013, 7, 2898–2926. [Google Scholar] [CrossRef] [PubMed]
- Xiao, X.; Song, H.; Lin, S.; Zhou, Y.; Zhan, X.; Hu, Z.; Zhang, Q.; Sun, J.; Yang, B.; Li, T. Scalable salt-templated synthesis of two-dimensional transition metal oxides. Nat. Commun. 2016, 7, 11296. [Google Scholar] [CrossRef] [PubMed]
- Huang, B.; Clark, G.; Navarro-Moratalla, E.; Klein, D.R.; Cheng, R.; Seyler, K.L.; Zhong, D.; Schmidgall, E.; McGuire, M.A.; Cobden, D.H. Layer-dependent ferromagnetism in a van der Waals crystal down to the monolayer limit. Nature 2017, 546, 270–273. [Google Scholar] [CrossRef] [PubMed]
- Burch, K.S.; Mandrus, D.; Park, J.-G. Magnetism in two-dimensional van der Waals materials. Nature 2018, 563, 47–52. [Google Scholar] [CrossRef]
- Zhang, Y.-Z.; El-Demellawi, J.K.; Jiang, Q.; Ge, G.; Liang, H.; Lee, K.; Dong, X.; Alshareef, H.N. MXene hydrogels: Fundamentals and applications. Chem. Soc. Rev. 2020, 49, 7229–7251. [Google Scholar] [CrossRef]
- Zhang, C.J.; McKeon, L.; Kremer, M.P.; Park, S.-H.; Ronan, O.; Seral-Ascaso, A.; Barwich, S.; Coileáin, C.Ó.; McEvoy, N.; Nerl, H.C. Additive-free MXene inks and direct printing of micro-supercapacitors. Nat. Commun. 2019, 10, 1795. [Google Scholar] [CrossRef]
- Novoselov, K.; Mishchenko, O.A.; Carvalho, O.A.; Castro Neto, A. 2D materials and van der Waals heterostructures. Science 2016, 353, aac9439. [Google Scholar] [CrossRef]
- Li, L.; Kim, J.; Jin, C.; Ye, G.J.; Qiu, D.Y.; Da Jornada, F.H.; Shi, Z.; Chen, L.; Zhang, Z.; Yang, F. Direct observation of the layer-dependent electronic structure in phosphorene. Nat. Nanotechnol. 2017, 12, 21–25. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Shao, X.; Li, J.; Dong, B.; Hu, Z.; Zhou, Q.; Xu, H.; Zhao, X.; Fang, H.; Li, X. Electrochemically exfoliated platinum dichalcogenide atomic layers for high-performance air-stable infrared photodetectors. ACS Appl. Mater. Interfaces 2021, 13, 8518–8527. [Google Scholar] [CrossRef] [PubMed]
- Hasan, M.Z.; Kane, C.L. Colloquium: Topological insulators. Rev. Mod. Phys. 2010, 82, 3045. [Google Scholar] [CrossRef]
- Konig, M.; Wiedmann, S.; Brune, C.; Roth, A.; Buhmann, H.; Molenkamp, L.W.; Qi, X.-L.; Zhang, S.-C. Quantum spin Hall insulator state in HgTe quantum wells. Science 2007, 318, 766–770. [Google Scholar] [CrossRef] [PubMed]
- Kane, C.L.; Mele, E.J. Quantum spin Hall effect in graphene. Phys. Rev. Lett. 2005, 95, 226801. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.-C.; Feng, W.; Yao, Y. Quantum spin Hall effect in silicene and two-dimensional germanium. Phys. Rev. Lett. 2011, 107, 076802. [Google Scholar] [CrossRef]
- Schaibley, J.R.; Yu, H.; Clark, G.; Rivera, P.; Ross, J.S.; Seyler, K.L.; Yao, W.; Xu, X. Valleytronics in 2D materials. Nat. Rev. Mater. 2016, 1, 16055. [Google Scholar] [CrossRef]
- Castellanos-Gomez, A. Why all the fuss about 2D semiconductors? Nat. Photonics 2016, 10, 202–204. [Google Scholar] [CrossRef]
- Xu, M.; Liang, T.; Shi, M.; Chen, H. Graphene-like two-dimensional materials. Chem. Rev. 2013, 113, 3766–3798. [Google Scholar] [CrossRef]
- Aufray, B.; Kara, A.; Vizzini, S.; Oughaddou, H.; Léandri, C.; Ealet, B.; Le Lay, G. Graphene-like silicon nanoribbons on Ag (110): A possible formation of silicene. Appl. Phys. Lett. 2010, 96, 183102. [Google Scholar] [CrossRef]
- Dávila, M.; Xian, L.; Cahangirov, S.; Rubio, A.; Le Lay, G. Germanene: A novel two-dimensional germanium allotrope akin to graphene and silicene. New J. Phys. 2014, 16, 095002. [Google Scholar] [CrossRef]
- Mannix, A.J.; Zhou, X.-F.; Kiraly, B.; Wood, J.D.; Alducin, D.; Myers, B.D.; Liu, X.; Fisher, B.L.; Santiago, U.; Guest, J.R. Synthesis of borophenes: Anisotropic, two-dimensional boron polymorphs. Science 2015, 350, 1513–1516. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Yu, Y.; Ye, G.J.; Ge, Q.; Ou, X.; Wu, H.; Feng, D.; Chen, X.H.; Zhang, Y. Black phosphorus field-effect transistors. Nat. Nanotechnol. 2014, 9, 372–377. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Yan, Z.; Li, Y.; Chen, Z.; Zeng, H. Atomically thin arsenene and antimonene: Semimetal–semiconductor and indirect–direct band-gap transitions. Angew. Chem. 2015, 127, 3155–3158. [Google Scholar] [CrossRef]
- Zhu, F.-F.; Chen, W.-J.; Xu, Y.; Gao, C.-L.; Guan, D.-D.; Liu, C.-H.; Qian, D.; Zhang, S.-C.; Jia, J.-F. Epitaxial growth of two-dimensional stanene. Nat. Mater. 2015, 14, 1020–1025. [Google Scholar] [CrossRef]
- Reis, F.; Li, G.; Dudy, L.; Bauernfeind, M.; Glass, S.; Hanke, W.; Thomale, R.; Schäfer, J.; Claessen, R. Bismuthene on a SiC substrate: A candidate for a high-temperature quantum spin Hall material. Science 2017, 357, 287–290. [Google Scholar] [CrossRef]
- Zhu, Z.; Cai, X.; Yi, S.; Chen, J.; Dai, Y.; Niu, C.; Guo, Z.; Xie, M.; Liu, F.; Cho, J.-H. Multivalency-driven formation of Te-based monolayer materials: A combined first-principles and experimental study. Phys. Rev. Lett. 2017, 119, 106101. [Google Scholar] [CrossRef]
- Qin, J.; Qiu, G.; Jian, J.; Zhou, H.; Yang, L.; Charnas, A.; Zemlyanov, D.Y.; Xu, C.-Y.; Xu, X.; Wu, W. Controlled growth of a large-size 2D selenium nanosheet and its electronic and optoelectronic applications. ACS Nano 2017, 11, 10222–10229. [Google Scholar] [CrossRef]
- Kochat, V.; Samanta, A.; Zhang, Y.; Bhowmick, S.; Manimunda, P.; Asif, S.A.S.; Stender, A.S.; Vajtai, R.; Singh, A.K.; Tiwary, C.S. Atomically thin gallium layers from solid-melt exfoliation. Sci. Adv. 2018, 4, e1701373. [Google Scholar] [CrossRef]
- Yuhara, J.; He, B.; Matsunami, N.; Nakatake, M.; Le Lay, G. Graphene’s latest cousin: Plumbene epitaxial growth on a “nano WaterCube”. Adv. Mater. 2019, 31, 1901017. [Google Scholar] [CrossRef]
- Yeoh, K.H.; Yoon, T.L.; Ong, D.S.; Lim, T.L. First-principles studies on the superconductivity of aluminene. Appl. Surf. Sci. 2018, 445, 161–166. [Google Scholar] [CrossRef]
- Liu, G.; Xu, S.-G.; Ma, Y.; Shao, X.; Xiong, W.; Wu, X.; Zhang, S.; Liao, C.; Chen, C.; Wang, X. Arsenic Monolayers Formed by Zero-Dimensional Tetrahedral Clusters and One-Dimensional Armchair Nanochains. ACS Nano 2022, 16, 17087–17096. [Google Scholar] [CrossRef] [PubMed]
- Hernandez, Y.; Nicolosi, V.; Lotya, M.; Blighe, F.M.; Sun, Z.; De, S.; McGovern, I.T.; Holland, B.; Byrne, M.; Gun’Ko, Y.K. High-yield production of graphene by liquid-phase exfoliation of graphite. Nat. Nanotechnol. 2008, 3, 563–568. [Google Scholar] [CrossRef] [PubMed]
- Ni, Z.; Ma, L.; Du, S.; Xu, Y.; Yuan, M.; Fang, H.; Wang, Z.; Xu, M.; Li, D.; Yang, J. Plasmonic silicon quantum dots enabled high-sensitivity ultrabroadband photodetection of graphene-based hybrid phototransistors. ACS Nano 2017, 11, 9854–9862. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Liu, X.; Wu, B.; Nan, H.; Guo, H.; Ni, Z.; Wang, F.; Wang, X.; Shi, Y.; Wang, X. Improving the performance of graphene phototransistors using a heterostructure as the light-absorbing layer. Nano Lett. 2017, 17, 6391–6396. [Google Scholar] [CrossRef]
- Kim, M.; Safron, N.S.; Huang, C.; Arnold, M.S.; Gopalan, P. Light-driven reversible modulation of doping in graphene. Nano Lett. 2012, 12, 182–187. [Google Scholar] [CrossRef]
- Cai, J.; Pignedoli, C.A.; Talirz, L.; Ruffieux, P.; Söde, H.; Liang, L.; Meunier, V.; Berger, R.; Li, R.; Feng, X. Graphene nanoribbon heterojunctions. Nat. Nanotechnol. 2014, 9, 896–900. [Google Scholar] [CrossRef]
- Zhu, H.; Gan, X.; McCreary, A.; Lv, R.; Lin, Z.; Terrones, M. Heteroatom doping of two-dimensional materials: From graphene to chalcogenides. Nano Today 2020, 30, 100829. [Google Scholar] [CrossRef]
- de la Torre, B.; Švec, M.; Hapala, P.; Redondo, J.; Krejčí, O.; Lo, R.; Manna, D.; Sarmah, A.; Nachtigallová, D.; Tuček, J. Non-covalent control of spin-state in metal-organic complex by positioning on N-doped graphene. Nat. Commun. 2018, 9, 2831. [Google Scholar] [CrossRef]
- Wehling, T.; Novoselov, K.; Morozov, S.; Vdovin, E.; Katsnelson, M.; Geim, A.; Lichtenstein, A. Molecular doping of graphene. Nano Lett. 2008, 8, 173–177. [Google Scholar] [CrossRef]
- Gong, Y.; Lin, J.; Wang, X.; Shi, G.; Lei, S.; Lin, Z.; Zou, X.; Ye, G.; Vajtai, R.; Yakobson, B.I. Vertical and in-plane heterostructures from WS2/MoS2 monolayers. Nat. Mater. 2014, 13, 1135–1142. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Ma, L.; Shi, G.; Zhou, W.; Gong, Y.; Lei, S.; Yang, X.; Zhang, J.; Yu, J.; Hackenberg, K.P. In-plane heterostructures of graphene and hexagonal boron nitride with controlled domain sizes. Nat. Nanotechnol. 2013, 8, 119–124. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Wan, X.; Xie, W.; Wen, J.; Kang, Z.; Zeng, X.; Chen, H.; Xu, J. Lateral built-in potential of monolayer MoS2–WS2 in-plane heterostructures by a shortcut growth strategy. Adv. Mater. 2015, 27, 6431–6437. [Google Scholar] [CrossRef] [PubMed]
- Sangwan, V.K.; Jariwala, D.; Kim, I.S.; Chen, K.-S.; Marks, T.J.; Lauhon, L.J.; Hersam, M.C. Gate-tunable memristive phenomena mediated by grain boundaries in single-layer MoS2. Nat. Nanotechnol. 2015, 10, 403–406. [Google Scholar] [CrossRef] [PubMed]
- Ruffieux, P.; Wang, S.; Yang, B.; Sánchez-Sánchez, C.; Liu, J.; Dienel, T.; Talirz, L.; Shinde, P.; Pignedoli, C.A.; Passerone, D. On-surface synthesis of graphene nanoribbons with zigzag edge topology. Nature 2016, 531, 489–492. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Cohen, M.L.; Louie, S.G. Magnetic edge-state excitons in zigzag graphene nanoribbons. Phys. Rev. Lett. 2008, 101, 186401. [Google Scholar] [CrossRef] [PubMed]
- Jacobberger, R.M.; Kiraly, B.; Fortin-Deschenes, M.; Levesque, P.L.; McElhinny, K.M.; Brady, G.J.; Rojas Delgado, R.; Singha Roy, S.; Mannix, A.; Lagally, M.G. Direct oriented growth of armchair graphene nanoribbons on germanium. Nat. Commun. 2015, 6, 8006. [Google Scholar] [CrossRef]
- Li, G.; Yoon, K.-Y.; Zhong, X.; Wang, J.; Zhang, R.; Guest, J.R.; Wen, J.; Zhu, X.-Y.; Dong, G. A modular synthetic approach for band-gap engineering of armchair graphene nanoribbons. Nat. Commun. 2018, 9, 1687. [Google Scholar] [CrossRef]
- Cho, S.; Kim, S.; Kim, J.H.; Zhao, J.; Seok, J.; Keum, D.H.; Baik, J.; Choe, D.-H.; Chang, K.J.; Suenaga, K. Phase patterning for ohmic homojunction contact in MoTe2. Science 2015, 349, 625–628. [Google Scholar] [CrossRef]
- Yang, S.; Chen, Y.; Jiang, C. Strain engineering of two-dimensional materials: Methods, properties, and applications. InfoMat 2021, 3, 397–420. [Google Scholar] [CrossRef]
- Peng, X.; Wei, Q.; Copple, A. Strain-engineered direct-indirect band gap transition and its mechanism in two-dimensional phosphorene. Phys. Rev. B 2014, 90, 085402. [Google Scholar] [CrossRef]
- Cao, Y.; Fatemi, V.; Fang, S.; Watanabe, K.; Taniguchi, T.; Kaxiras, E.; Jarillo-Herrero, P. Unconventional superconductivity in magic-angle graphene superlattices. Nature 2018, 556, 43–50. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Fatemi, V.; Demir, A.; Fang, S.; Tomarken, S.L.; Luo, J.Y.; Sanchez-Yamagishi, J.D.; Watanabe, K.; Taniguchi, T.; Kaxiras, E. Correlated insulator behaviour at half-filling in magic-angle graphene superlattices. Nature 2018, 556, 80–84. [Google Scholar] [CrossRef] [PubMed]
- Peng, B.; Zhang, H.; Shao, H.; Ning, Z.; Xu, Y.; Ni, G.; Lu, H.; Zhang, D.W.; Zhu, H. Stability and strength of atomically thin borophene from first principles calculations. Mater. Res. Lett. 2017, 5, 399–407. [Google Scholar] [CrossRef]
- Yuan, J.; Yu, N.; Xue, K.; Miao, X. Stability, electronic and thermodynamic properties of aluminene from first-principles calculations. Appl. Surf. Sci. 2017, 409, 85–90. [Google Scholar] [CrossRef]
- Mardanya, S.; Thakur, V.K.; Bhowmick, S.; Agarwal, A. Four allotropes of semiconducting layered arsenic that switch into a topological insulator via an electric field: Computational study. Phys. Rev. B 2016, 94, 035423. [Google Scholar] [CrossRef]
- Matusalem, F.; Marques, M.; Teles, L.K.; Bechstedt, F. Stability and electronic structure of two-dimensional allotropes of group-IV materials. Phys. Rev. B 2015, 92, 045436. [Google Scholar] [CrossRef]
- Xian, L.; Paz, A.P.; Bianco, E.; Ajayan, P.M.; Rubio, A. Square selenene and tellurene: Novel group VI elemental 2D materials with nontrivial topological properties. 2D Mater. 2017, 4, 041003. [Google Scholar] [CrossRef]
- Zhang, Z.; Yang, Y.; Gao, G.; Yakobson, B.I. Two-dimensional boron monolayers mediated by metal substrates. Angew. Chem. 2015, 127, 13214–13218. [Google Scholar] [CrossRef]
- Liu, Y.; Penev, E.S.; Yakobson, B.I. Probing the synthesis of two-dimensional boron by first-principles computations. Angew. Chem. Int. Ed. 2013, 52, 3156–3159. [Google Scholar] [CrossRef]
- Huang, Y.; Shirodkar, S.N.; Yakobson, B.I. Two-dimensional boron polymorphs for visible range plasmonics: A first-principles exploration. J. Am. Chem. Soc. 2017, 139, 17181–17185. [Google Scholar] [CrossRef] [PubMed]
- Singh, D.; Gupta, S.K.; Lukačević, I.; Sonvane, Y. Indiene 2D monolayer: A new nanoelectronic material. RSC Adv. 2016, 6, 8006–8014. [Google Scholar] [CrossRef]
- Deng, J.; Xia, B.; Ma, X.; Chen, H.; Shan, H.; Zhai, X.; Li, B.; Zhao, A.; Xu, Y.; Duan, W. Epitaxial growth of ultraflat stanene with topological band inversion. Nat. Mater. 2018, 17, 1081–1086. [Google Scholar] [CrossRef]
- Ren, W.; Cheng, H.-M. The global growth of graphene. Nat. Nanotechnol. 2014, 9, 726–730. [Google Scholar] [CrossRef] [PubMed]
- Sprinkle, M.; Ruan, M.; Hu, Y.; Hankinson, J.; Rubio-Roy, M.; Zhang, B.; Wu, X.; Berger, C.; De Heer, W.A. Scalable templated growth of graphene nanoribbons on SiC. Nat. Nanotechnol. 2010, 5, 727–731. [Google Scholar] [CrossRef]
- Meng, L.; Wang, Y.; Zhang, L.; Du, S.; Wu, R.; Li, L.; Zhang, Y.; Li, G.; Zhou, H.; Hofer, W.A. Buckled silicene formation on Ir (111). Nano Lett. 2013, 13, 685–690. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Lu, S.Z.; Pan, J.; Qin, Z.; Wang, Y.q.; Wang, Y.; Cao, G.y.; Du, S.; Gao, H.J. Buckled germanene formation on Pt (111). Adv. Mater. 2014, 26, 4820–4824. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Zhou, J.; Wang, Q.; Chen, X.; Kawazoe, Y.; Jena, P. Penta-graphene: A new carbon allotrope. Proc. Natl. Acad. Sci. USA 2015, 112, 2372–2377. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Zhou, X.-F.; Zhang, X.; Zhu, Q.; Dong, H.; Zhao, M.; Oganov, A.R. Phagraphene: A low-energy graphene allotrope composed of 5–6–7 carbon rings with distorted dirac cones. Nano Lett. 2015, 15, 6182–6186. [Google Scholar] [CrossRef]
- Guan, J.; Zhu, Z.; Tománek, D. Tiling phosphorene. ACS Nano 2014, 8, 12763–12768. [Google Scholar] [CrossRef]
- Wu, X.; Shao, Y.; Liu, H.; Feng, Z.; Wang, Y.L.; Sun, J.T.; Liu, C.; Wang, J.O.; Liu, Z.L.; Zhu, S.Y. Epitaxial growth and air-stability of monolayer antimonene on PdTe2. Adv. Mater. 2017, 29, 1605407. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Xu, W.; Zeng, M.; Yao, G.; Shen, L.; Yang, M.; Luo, Z.; Pan, F.; Wu, K.; Das, T. Topological properties determined by atomic buckling in self-assembled ultrathin Bi (110). Nano Lett. 2015, 15, 80–87. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, A.; Wang, M.; Zhu, X.; Rodin, A.S.; Su, H.; Castro Neto, A.H. Phosphorene: From theory to applications. Nat. Rev. Mater. 2016, 1, 16061. [Google Scholar] [CrossRef]
- Aktürk, E.; Aktürk, O.Ü.; Ciraci, S. Single and bilayer bismuthene: Stability at high temperature and mechanical and electronic properties. Phys. Rev. B 2016, 94, 014115. [Google Scholar] [CrossRef]
- Kamal, C.; Ezawa, M. Arsenene: Two-dimensional buckled and puckered honeycomb arsenic systems. Phys. Rev. B 2015, 91, 085423. [Google Scholar] [CrossRef]
- Feng, B.; Zhang, J.; Zhong, Q.; Li, W.; Li, S.; Li, H.; Cheng, P.; Meng, S.; Chen, L.; Wu, K. Experimental realization of two-dimensional boron sheets. Nat. Chem. 2016, 8, 563–568. [Google Scholar] [CrossRef]
- Li, W.; Kong, L.; Chen, C.; Gou, J.; Sheng, S.; Zhang, W.; Li, H.; Chen, L.; Cheng, P.; Wu, K. Experimental realization of honeycomb borophene. Sci. Bull. 2018, 63, 282–286. [Google Scholar] [CrossRef]
- Kiraly, B.; Liu, X.; Wang, L.; Zhang, Z.; Mannix, A.J.; Fisher, B.L.; Yakobson, B.I.; Hersam, M.C.; Guisinger, N.P. Borophene synthesis on Au (111). ACS Nano 2019, 13, 3816–3822. [Google Scholar] [CrossRef]
- Zhang, Z.; Yang, Y.; Penev, E.S.; Yakobson, B.I. Elasticity, flexibility, and ideal strength of borophenes. Adv. Funct. Mater. 2017, 27, 1605059. [Google Scholar] [CrossRef]
- Zhao, Y.; Zeng, S.; Ni, J. Superconductivity in two-dimensional boron allotropes. Phys. Rev. B 2016, 93, 014502. [Google Scholar] [CrossRef]
- Zhang, Z.; Penev, E.S.; Yakobson, B.I. Polyphony in B flat. Nat. Chem. 2016, 8, 525–527. [Google Scholar] [CrossRef] [PubMed]
- Penev, E.S.; Bhowmick, S.; Sadrzadeh, A.; Yakobson, B.I. Polymorphism of two-dimensional boron. Nano Lett. 2012, 12, 2441–2445. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Lü, T.-Y.; Wang, H.-Q.; Feng, Y.P.; Zheng, J.-C. High anisotropy of fully hydrogenated borophene. Phys. Chem. Chem. Phys. 2016, 18, 31424–31430. [Google Scholar] [CrossRef]
- Wang, H.; Li, Q.; Gao, Y.; Miao, F.; Zhou, X.-F.; Wan, X. Strain effects on borophene: Ideal strength, negative Possion’s ratio and phonon instability. New J. Phys. 2016, 18, 073016. [Google Scholar] [CrossRef]
- Peköz, R.; Konuk, M.; Kilic, M.E.; Durgun, E. Two-dimensional fluorinated boron sheets: Mechanical, electronic, and thermal properties. ACS Omega 2018, 3, 1815–1822. [Google Scholar] [CrossRef]
- Zhou, Y.-P.; Jiang, J.-W. Molecular dynamics simulations for mechanical properties of borophene: Parameterization of valence force field model and Stillinger-Weber potential. Sci. Rep. 2017, 7, 45516. [Google Scholar] [CrossRef]
- Le, M.Q.; Mortazavi, B.; Rabczuk, T. Mechanical properties of borophene films: A reactive molecular dynamics investigation. Nanotechnology 2016, 27, 445709. [Google Scholar] [CrossRef] [PubMed]
- Zhong, H.; Huang, K.; Yu, G.; Yuan, S. Electronic and mechanical properties of few-layer borophene. Phys. Rev. B 2018, 98, 054104. [Google Scholar] [CrossRef]
- Mortazavi, B.; Rahaman, O.; Dianat, A.; Rabczuk, T. Mechanical responses of borophene sheets: A first-principles study. Phys. Chem. Chem. Phys. 2016, 18, 27405–27413. [Google Scholar] [CrossRef]
- Zhou, H.; Cai, Y.; Zhang, G.; Zhang, Y.-W. Superior lattice thermal conductance of single-layer borophene. NPJ 2D Mater. Appl. 2017, 1, 14. [Google Scholar] [CrossRef]
- Li, D.; He, J.; Ding, G.; Tang, Q.; Ying, Y.; He, J.; Zhong, C.; Liu, Y.; Feng, C.; Sun, Q. Stretch-driven increase in ultrahigh thermal conductance of hydrogenated borophene and dimensionality crossover in phonon transmission. Adv. Funct. Mater. 2018, 28, 1801685. [Google Scholar] [CrossRef]
- Kong, L.; Liu, L.; Chen, L.; Zhong, Q.; Cheng, P.; Li, H.; Zhang, Z.; Wu, K. One-dimensional nearly free electron states in borophene. Nanoscale 2019, 11, 15605–15611. [Google Scholar] [CrossRef] [PubMed]
- Feng, B.; Zhang, J.; Liu, R.-Y.; Iimori, T.; Lian, C.; Li, H.; Chen, L.; Wu, K.; Meng, S.; Komori, F. Direct evidence of metallic bands in a monolayer boron sheet. Phys. Rev. B 2016, 94, 041408. [Google Scholar] [CrossRef]
- Zhou, X.-F.; Dong, X.; Oganov, A.R.; Zhu, Q.; Tian, Y.; Wang, H.-T. Semimetallic two-dimensional boron allotrope with massless Dirac fermions. Phys. Rev. Lett. 2014, 112, 085502. [Google Scholar] [CrossRef]
- Feng, B.; Sugino, O.; Liu, R.-Y.; Zhang, J.; Yukawa, R.; Kawamura, M.; Iimori, T.; Kim, H.; Hasegawa, Y.; Li, H. Dirac fermions in borophene. Phys. Rev. Lett. 2017, 118, 096401. [Google Scholar] [CrossRef]
- Shukla, V.; Grigoriev, A.; Jena, N.K.; Ahuja, R. Strain controlled electronic and transport anisotropies in two-dimensional borophene sheets. Phys. Chem. Chem. Phys. 2018, 20, 22952–22960. [Google Scholar] [CrossRef]
- Wang, Z.-Q.; Lü, T.-Y.; Wang, H.-Q.; Feng, Y.P.; Zheng, J.-C. Band structure engineering of borophane by first principles calculations. RSC Adv. 2017, 7, 47746–47752. [Google Scholar] [CrossRef]
- Gao, M.; Li, Q.-Z.; Yan, X.-W.; Wang, J. Prediction of phonon-mediated superconductivity in borophene. Phys. Rev. B 2017, 95, 024505. [Google Scholar] [CrossRef]
- Zhao, Y.; Zeng, S.; Ni, J. Phonon-mediated superconductivity in borophenes. Appl. Phys. Lett. 2016, 108, 242601. [Google Scholar] [CrossRef]
- Zhao, Y.; Zeng, S.; Lian, C.; Dai, Z.; Meng, S.; Ni, J. Multigap anisotropic superconductivity in borophenes. Phys. Rev. B 2018, 98, 134514. [Google Scholar] [CrossRef]
- Penev, E.S.; Kutana, A.; Yakobson, B.I. Can two-dimensional boron superconduct? Nano Lett. 2016, 16, 2522–2526. [Google Scholar] [CrossRef] [PubMed]
- Liao, J.-H.; Zhao, Y.-C.; Zhao, Y.-J.; Xu, H.; Yang, X.-B. Phonon-mediated superconductivity in Mg intercalated bilayer borophenes. Phys. Chem. Chem. Phys. 2017, 19, 29237–29243. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Zhao, Y.; Zeng, S.; Zulfiqar, M.; Ni, J. Strain effect on the superconductivity in borophenes. J. Phys. Chem. C 2018, 122, 16916–16924. [Google Scholar] [CrossRef]
- Cheng, C.; Sun, J.-T.; Liu, H.; Fu, H.-X.; Zhang, J.; Chen, X.-R.; Meng, S. Suppressed superconductivity in substrate-supported β12 borophene by tensile strain and electron doping. 2D Mater. 2017, 4, 025032. [Google Scholar] [CrossRef]
- Zheng, J.-C.; Zhu, Y. Searching for a higher superconducting transition temperature in strained Mg B 2. Phys. Rev. B 2006, 73, 024509. [Google Scholar] [CrossRef]
- Xiao, R.; Shao, D.; Lu, W.; Lv, H.; Li, J.; Sun, Y. Enhanced superconductivity by strain and carrier-doping in borophene: A first principles prediction. Appl. Phys. Lett. 2016, 109, 122604. [Google Scholar] [CrossRef]
- Jiao, Y.; Ma, F.; Bell, J.; Bilic, A.; Du, A. Two-Dimensional Boron Hydride Sheets: High Stability, Massless Dirac Fermions, and Excellent Mechanical Properties. Angew. Chem. 2016, 128, 10448–10451. [Google Scholar] [CrossRef]
- Nishino, H.; Fujita, T.; Cuong, N.T.; Tominaka, S.; Miyauchi, M.; Iimura, S.; Hirata, A.; Umezawa, N.; Okada, S.; Nishibori, E. Formation and characterization of hydrogen boride sheets derived from MgB2 by cation exchange. J. Am. Chem. Soc. 2017, 139, 13761–13769. [Google Scholar] [CrossRef]
- Wang, C.-B.; Lu, Q.; Zhang, L.-L.; Xu, T.-T.; Gong, W.-J. Li-decorated borophene–graphene heterostructure under gas adsorption. J. Phys. Chem. Solids 2022, 171, 111033. [Google Scholar] [CrossRef]
- Tao, M.-L.; Tu, Y.-B.; Sun, K.; Wang, Y.-L.; Xie, Z.-B.; Liu, L.; Shi, M.-X.; Wang, J.-Z. Gallenene epitaxially grown on Si (111). 2D Mater. 2018, 5, 035009. [Google Scholar] [CrossRef]
- Zhang, H.-M.; Sun, Y.; Li, W.; Peng, J.-P.; Song, C.-L.; Xing, Y.; Zhang, Q.; Guan, J.; Li, Z.; Zhao, Y. Detection of a superconducting phase in a two-atom layer of hexagonal Ga film grown on semiconducting GaN (0001). Phys. Rev. Lett. 2015, 114, 107003. [Google Scholar] [CrossRef] [PubMed]
- Gruznev, D.; Bondarenko, L.; Tupchaya, A.; Mihalyuk, A.; Eremeev, S.; Zotov, A.; Saranin, A. Thallene: Graphene-like honeycomb lattice of Tl atoms frozen on single-layer NiSi2. 2D Mater. 2020, 7, 045026. [Google Scholar] [CrossRef]
- Neto, A.C.; Guinea, F.; Peres, N.M.; Novoselov, K.S.; Geim, A.K. The electronic properties of graphene. Rev. Mod. Phys. 2009, 81, 109. [Google Scholar] [CrossRef]
- Lonkar, S.P.; Deshmukh, Y.S.; Abdala, A.A. Recent advances in chemical modifications of graphene. Nano Res. 2015, 8, 1039–1074. [Google Scholar] [CrossRef]
- Geim, A.K.; Novoselov, K.S. The rise of graphene. Nat. Mater. 2007, 6, 183–191. [Google Scholar] [CrossRef] [PubMed]
- Nair, R.R.; Blake, P.; Grigorenko, A.N.; Novoselov, K.S.; Booth, T.J.; Stauber, T.; Peres, N.M.; Geim, A.K. Fine structure constant defines visual transparency of graphene. Science 2008, 320, 1308. [Google Scholar] [CrossRef]
- Balandin, A.A.; Ghosh, S.; Bao, W.; Calizo, I.; Teweldebrhan, D.; Miao, F.; Lau, C.N. Superior thermal conductivity of single-layer graphene. Nano Lett. 2008, 8, 902–907. [Google Scholar] [CrossRef]
- Guo, B.; Liu, Q.; Chen, E.; Zhu, H.; Fang, L.; Gong, J.R. Controllable N-doping of graphene. Nano Lett. 2010, 10, 4975–4980. [Google Scholar] [CrossRef]
- Ma, C.; Liao, Q.; Sun, H.; Lei, S.; Zheng, Y.; Yin, R.; Zhao, A.; Li, Q.; Wang, B. Tuning the doping types in graphene sheets by N monoelement. Nano Lett. 2018, 18, 386–394. [Google Scholar] [CrossRef]
- Wang, H.; Zhou, Y.; Wu, D.; Liao, L.; Zhao, S.; Peng, H.; Liu, Z. Synthesis of boron-doped graphene monolayers using the sole solid feedstock by chemical vapor deposition. Small 2013, 9, 1316–1320. [Google Scholar] [CrossRef]
- Jang, A.-R.; Jeon, E.K.; Kang, D.; Kim, G.; Kim, B.-S.; Kang, D.J.; Shin, H.S. Reversibly light-modulated dirac point of graphene functionalized with spiropyran. ACS Nano 2012, 6, 9207–9213. [Google Scholar] [CrossRef] [PubMed]
- Levendorf, M.P.; Kim, C.-J.; Brown, L.; Huang, P.Y.; Havener, R.W.; Muller, D.A.; Park, J. Graphene and boron nitride lateral heterostructures for atomically thin circuitry. Nature 2012, 488, 627–632. [Google Scholar] [CrossRef] [PubMed]
- Takeda, K.; Shiraishi, K. Theoretical possibility of stage corrugation in Si and Ge analogs of graphite. Phys. Rev. B 1994, 50, 14916. [Google Scholar] [CrossRef] [PubMed]
- Cahangirov, S.; Topsakal, M.; Aktürk, E.; Şahin, H.; Ciraci, S. Two-and one-dimensional honeycomb structures of silicon and germanium. Phys. Rev. Lett. 2009, 102, 236804. [Google Scholar] [CrossRef]
- Ryu, S.; Hatsugai, Y. Topological origin of zero-energy edge states in particle-hole symmetric systems. Phys. Rev. Lett. 2002, 89, 077002. [Google Scholar] [CrossRef]
- Liu, C.-C.; Jiang, H.; Yao, Y. Low-energy effective Hamiltonian involving spin-orbit coupling in silicene and two-dimensional germanium and tin. Phys. Rev. B 2011, 84, 195430. [Google Scholar] [CrossRef]
- Xu, C.; Luo, G.; Liu, Q.; Zheng, J.; Zhang, Z.; Nagase, S.; Gao, Z.; Lu, J. Giant magnetoresistance in silicene nanoribbons. Nanoscale 2012, 4, 3111–3117. [Google Scholar] [CrossRef]
- Rachel, S.; Ezawa, M. Giant magnetoresistance and perfect spin filter in silicene, germanene, and stanene. Phys. Rev. B 2014, 89, 195303. [Google Scholar] [CrossRef]
- Ni, Z.; Liu, Q.; Tang, K.; Zheng, J.; Zhou, J.; Qin, R.; Gao, Z.; Yu, D.; Lu, J. Tunable bandgap in silicene and germanene. Nano Lett. 2012, 12, 113–118. [Google Scholar] [CrossRef]
- Drummond, N.; Zolyomi, V.; Fal’Ko, V. Electrically tunable band gap in silicene. Phys. Rev. B 2012, 85, 075423. [Google Scholar] [CrossRef]
- Bao, H.; Liao, W.; Guo, J.; Zhao, H.; Zhou, G. Terahertz electromagnetic response and its electric field manipulation of bulked silicene. Laser Phys. Lett. 2015, 12, 095902. [Google Scholar] [CrossRef]
- Molle, A.; Goldberger, J.; Houssa, M.; Xu, Y.; Zhang, S.-C.; Akinwande, D. Buckled two-dimensional Xene sheets. Nat. Mater. 2017, 16, 163–169. [Google Scholar] [CrossRef] [PubMed]
- Brumfiel, G. Sticky problem snares wonder material. Nature 2013, 495, 152–153. [Google Scholar] [CrossRef] [PubMed]
- De Padova, P.; Ottaviani, C.; Quaresima, C.; Olivieri, B.; Imperatori, P.; Salomon, E.; Angot, T.; Quagliano, L.; Romano, C.; Vona, A. 24 h stability of thick multilayer silicene in air. 2D Mater. 2014, 1, 021003. [Google Scholar] [CrossRef]
- Ni, Z.; Zhong, H.; Jiang, X.; Quhe, R.; Luo, G.; Wang, Y.; Ye, M.; Yang, J.; Shi, J.; Lu, J. Tunable band gap and doping type in silicene by surface adsorption: Towards tunneling transistors. Nanoscale 2014, 6, 7609–7618. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Ding, Y. Strain-induced self-doping in silicene and germanene from first-principles. Solid State Commun. 2013, 155, 6–11. [Google Scholar] [CrossRef]
- Li, S.; Ao, Z.; Zhu, J.; Ren, J.; Yi, J.; Wang, G.; Liu, W. Strain controlled ferromagnetic-antiferromagnetic transformation in Mn-doped silicene for information transformation devices. J. Phys. Chem. Lett. 2017, 8, 1484–1488. [Google Scholar] [CrossRef]
- Wang, X.-Q.; Li, H.-D.; Wang, J.-T. Induced ferromagnetism in one-side semihydrogenated silicene and germanene. Phys. Chem. Chem. Phys. 2012, 14, 3031–3036. [Google Scholar] [CrossRef]
- Du, Y.; Zhuang, J.; Wang, J.; Li, Z.; Liu, H.; Zhao, J.; Xu, X.; Feng, H.; Chen, L.; Wu, K. Quasi-freestanding epitaxial silicene on Ag (111) by oxygen intercalation. Sci. Adv. 2016, 2, e1600067. [Google Scholar] [CrossRef]
- Liu, H.; Han, N.; Zhao, J. Band gap opening in bilayer silicene by alkali metal intercalation. J. Phys. Condens. Matter 2014, 26, 475303. [Google Scholar] [CrossRef]
- Feng, J.-W.; Liu, Y.-J.; Wang, H.-X.; Zhao, J.-X.; Cai, Q.-H.; Wang, X.-Z. Gas adsorption on silicene: A theoretical study. Comp. Mater. Sci. 2014, 87, 218–226. [Google Scholar] [CrossRef]
- Osborne, D.A.; Morishita, T.; Tawfik, S.A.; Yayama, T.; Spencer, M.J. Adsorption of toxic gases on silicene/Ag (111). Phys. Chem. Chem. Phys. 2019, 21, 17521–17537. [Google Scholar] [CrossRef] [PubMed]
- Ye, X.-S.; Shao, Z.-G.; Zhao, H.; Yang, L.; Wang, C.-L. Intrinsic carrier mobility of germanene is larger than graphene’s: First-principle calculations. RSC Adv. 2014, 4, 21216–21220. [Google Scholar] [CrossRef]
- Dávila, M.E.; Le Lay, G. Few layer epitaxial germanene: A novel two-dimensional Dirac material. Sci. Rep. 2016, 6, 20714. [Google Scholar] [CrossRef]
- Hoat, D.; Nguyen, D.K.; Ponce-Perez, R.; Guerrero-Sanchez, J.; Van On, V.; Rivas-Silva, J.; Cocoletzi, G.H. Opening the germanene monolayer band gap using halogen atoms: An efficient approach studied by first-principles calculations. Appl. Surf. Sci. 2021, 551, 149318. [Google Scholar] [CrossRef]
- Pang, Q.; Zhang, C.-l.; Li, L.; Fu, Z.-q.; Wei, X.-m.; Song, Y.-l. Adsorption of alkali metal atoms on germanene: A first-principles study. Appl. Surf. Sci. 2014, 314, 15–20. [Google Scholar] [CrossRef]
- Jiao, Z.; Yao, Q.; Rudenko, A.; Zhang, L.; Zandvliet, H. Germanium/MoS2: Competition between the growth of germanene and intercalation. Phys. Rev. B 2020, 102, 205419. [Google Scholar] [CrossRef]
- Kaloni, T.P. Tuning the structural, electronic, and magnetic properties of germanene by the adsorption of 3d transition metal atoms. J. Phys. Chem. C 2014, 118, 25200–25208. [Google Scholar] [CrossRef]
- Sun, M.; Ren, Q.; Wang, S.; Zhang, Y.; Du, Y.; Yu, J.; Tang, W. Magnetism in transition-metal-doped germanene: A first-principles study. Comp. Mater. Sci. 2016, 118, 112–116. [Google Scholar] [CrossRef]
- Lin, C.-H.; Huang, A.; Pai, W.W.; Chen, W.-C.; Chen, T.-Y.; Chang, T.-R.; Yukawa, R.; Cheng, C.-M.; Mou, C.-Y.; Matsuda, I. Single-layer dual germanene phases on Ag (111). Phys. Rev. Mater. 2018, 2, 024003. [Google Scholar] [CrossRef]
- Yuhara, J.; Shimazu, H.; Ito, K.; Ohta, A.; Araidai, M.; Kurosawa, M.; Nakatake, M.; Le Lay, G. Germanene epitaxial growth by segregation through Ag (111) thin films on Ge (111). ACS Nano 2018, 12, 11632–11637. [Google Scholar] [CrossRef] [PubMed]
- Muzychenko, D.A.; Oreshkin, S.I.; Panov, V.I.; Van Haesendonck, C.; Oreshkin, A.I. Single and multi domain buckled germanene phases on Al (111) surface. Nano Res. 2019, 12, 2988–2996. [Google Scholar] [CrossRef]
- Zhang, L.; Bampoulis, P.; Rudenko, A.; Yao, Q.V.; Van Houselt, A.; Poelsema, B.; Katsnelson, M.; Zandvliet, H. Structural and electronic properties of germanene on MoS2. Phys. Rev. Lett. 2016, 116, 256804. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, J.; Gao, N.; Li, Z.; Xu, X.; Wang, J.; Zhao, J.; Dou, S.X.; Du, Y. Cooperative electron–phonon coupling and buckled structure in germanene on Au (111). ACS Nano 2017, 11, 3553–3559. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Yan, B.; Zhang, H.-J.; Wang, J.; Xu, G.; Tang, P.; Duan, W.; Zhang, S.-C. Large-gap quantum spin Hall insulators in tin films. Phys. Rev. Lett. 2013, 111, 136804. [Google Scholar] [CrossRef] [PubMed]
- Fu, L.; Kane, C.L.; Mele, E.J. Topological insulators in three dimensions. Phys. Rev. Lett. 2007, 98, 106803. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Tan, C.; Yang, Q.; Meng, R.; Liang, Q.; Cai, M.; Zhang, S.; Jiang, J. Ab initio study of the adsorption of small molecules on stanene. J. Phys. Chem. C 2016, 120, 13987–13994. [Google Scholar] [CrossRef]
- Monshi, M.M.; Aghaei, S.M.; Calizo, I. Doping and defect-induced germanene: A superior media for sensing H2S, SO2, and CO2 gas molecules. Surf. Sci. 2017, 665, 96–102. [Google Scholar] [CrossRef]
- Xiong, W.; Xia, C.; Peng, Y.; Du, J.; Wang, T.; Zhang, J.; Jia, Y. Spin–orbit coupling effects on electronic structures in stanene nanoribbons. Phys. Chem. Chem. Phys. 2016, 18, 6534–6540. [Google Scholar] [CrossRef]
- Shaidu, Y.; Akin-Ojo, O. First principles predictions of superconductivity in doped stanene. Comp. Mater. Sci. 2016, 118, 11–15. [Google Scholar] [CrossRef]
- Zhao, H.; Zhang, C.-W.; Ji, W.-X.; Zhang, R.-W.; Li, S.-S.; Yan, S.-S.; Zhang, B.-M.; Li, P.; Wang, P.-J. Unexpected giant-gap quantum spin Hall insulator in chemically decorated plumbene monolayer. Sci. Rep. 2016, 6, 20152. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.-L.; Wu, J. Evolution of the topological properties of two-dimensional group IVA materials and device design. Phys. Chem. Chem. Phys. 2018, 20, 2296–2307. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.-L.; Huang, L.; Wu, J. From a normal insulator to a topological insulator in plumbene. Phys. Rev. B 2017, 95, 125113. [Google Scholar] [CrossRef]
- Hashemi, D.; Iizuka, H. Magnetic properties of 3d transition metal (Sc–Ni) doped plumbene. RSC Adv. 2020, 10, 6884–6892. [Google Scholar] [CrossRef]
- Bihlmayer, G.; Sassmannshausen, J.; Kubetzka, A.; Blügel, S.; von Bergmann, K.; Wiesendanger, R. Plumbene on a magnetic substrate: A combined scanning tunneling microscopy and density functional theory study. Phys. Rev. Lett. 2020, 124, 126401. [Google Scholar] [CrossRef]
- Zhang, S.; Guo, S.; Chen, Z.; Wang, Y.; Gao, H.; Gómez-Herrero, J.; Ares, P.; Zamora, F.; Zhu, Z.; Zeng, H. Recent progress in 2D group-VA semiconductors: From theory to experiment. Chem. Soc. Rev. 2018, 47, 982–1021. [Google Scholar] [CrossRef]
- Batmunkh, M.; Bat-Erdene, M.; Shapter, J.G. Phosphorene and phosphorene-based materials–prospects for future applications. Adv. Mater. 2016, 28, 8586–8617. [Google Scholar] [CrossRef]
- Hashmi, A.; Hong, J. Transition metal doped phosphorene: First-principles study. J. Phys. Chem. C 2015, 119, 9198–9204. [Google Scholar] [CrossRef]
- Wei, Z.; Zhang, Y.; Wang, S.; Wang, C.; Ma, J. Fe-doped phosphorene for the nitrogen reduction reaction. J. Mater. Chem. A 2018, 6, 13790–13796. [Google Scholar] [CrossRef]
- Xu, G.; Li, H.; Bati, A.S.; Bat-Erdene, M.; Nine, M.J.; Losic, D.; Chen, Y.; Shapter, J.G.; Batmunkh, M.; Ma, T. Nitrogen-doped phosphorene for electrocatalytic ammonia synthesis. J. Mater. Chem. A 2020, 8, 15875–15883. [Google Scholar] [CrossRef]
- Safari, F.; Moradinasab, M.; Fathipour, M.; Kosina, H. Adsorption of the NH3, NO, NO2, CO2, and CO gas molecules on blue phosphorene: A first-principles study. Appl. Surf. Sci. 2019, 464, 153–161. [Google Scholar] [CrossRef]
- Liu, Q.; Zhang, X.; Abdalla, L.; Fazzio, A.; Zunger, A. Switching a normal insulator into a topological insulator via electric field with application to phosphorene. Nano Lett. 2015, 15, 1222–1228. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Liu, C.; Wang, J.; Gao, Q.; Hu, Z.; Hao, W.; Xu, X.; Du, Y.; Zhuang, J. Reversible Potassium Intercalation in Blue Phosphorene–Au Network Driven by an Electric Field. J. Phys. Chem. Lett. 2020, 11, 5584–5590. [Google Scholar] [CrossRef]
- Sisakht, E.T.; Fazileh, F.; Zare, M.; Zarenia, M.; Peeters, F. Strain-induced topological phase transition in phosphorene and in phosphorene nanoribbons. Phys. Rev. B 2016, 94, 085417. [Google Scholar] [CrossRef]
- Hu, Y.; Liang, J.; Xia, Y.; Zhao, C.; Jiang, M.; Ma, J.; Tie, Z.; Jin, Z. 2D arsenene and arsenic materials: Fundamental properties, preparation, and applications. Small 2022, 18, 2104556. [Google Scholar] [CrossRef] [PubMed]
- Shah, J.; Wang, W.; Sohail, H.M.; Uhrberg, R. Experimental evidence of monolayer arsenene: An exotic 2D semiconducting material. 2D Mater. 2020, 7, 025013. [Google Scholar] [CrossRef]
- Zhang, H.; Ma, Y.; Chen, Z. Quantum spin hall insulators in strain-modified arsenene. Nanoscale 2015, 7, 19152–19159. [Google Scholar] [CrossRef]
- Kong, X.; Gao, M.; Yan, X.-W.; Lu, Z.-Y.; Xiang, T. Superconductivity in electron-doped arsenene. Chin. Phys. B 2018, 27, 046301. [Google Scholar] [CrossRef]
- Sheng, F.; Hua, C.; Cheng, M.; Hu, J.; Sun, X.; Tao, Q.; Lu, H.; Lu, Y.; Zhong, M.; Watanabe, K. Rashba valleys and quantum Hall states in few-layer black arsenic. Nature 2021, 593, 56–60. [Google Scholar] [CrossRef]
- Sun, M.; Wang, S.; Du, Y.; Yu, J.; Tang, W. Transition metal doped arsenene: A first-principles study. Appl. Surf. Sci. 2016, 389, 594–600. [Google Scholar] [CrossRef]
- Som, N.N.; Mankad, V.; Jha, P.K. Hydrogen evolution reaction: The role of arsenene nanosheet and dopant. Int. J. Hydrog. Energy 2018, 43, 21634–21641. [Google Scholar] [CrossRef]
- Xu, Y.; Peng, B.; Zhang, H.; Shao, H.; Zhang, R.; Zhu, H. First-principle calculations of optical properties of monolayer arsenene and antimonene allotropes. Ann. Phys. 2017, 529, 1600152. [Google Scholar] [CrossRef]
- Zhang, S.; Xie, M.; Li, F.; Yan, Z.; Li, Y.; Kan, E.; Liu, W.; Chen, Z.; Zeng, H. Semiconducting group 15 monolayers: A broad range of band gaps and high carrier mobilities. Angew. Chem. 2016, 128, 1698–1701. [Google Scholar] [CrossRef]
- Batool, S.; Idrees, M.; Han, S.T.; Zhou, Y. 2D Layers of Group VA Semiconductors: Fundamental Properties and Potential Applications. Adv. Sci. 2022, 2022, 2203956. [Google Scholar] [CrossRef] [PubMed]
- Pumera, M.; Sofer, Z. 2D monoelemental arsenene, antimonene, and bismuthene: Beyond black phosphorus. Adv. Mater. 2017, 29, 1605299. [Google Scholar] [CrossRef] [PubMed]
- Ares, P.; Palacios, J.J.; Abellán, G.; Gómez-Herrero, J.; Zamora, F. Recent progress on antimonene: A new bidimensional material. Adv. Mater. 2018, 30, 1703771. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Song, Y.; Mi, W.; Wang, X. Prediction of spin-dependent electronic structure in 3d-transition-metal doped antimonene. Appl. Phys. Lett. 2016, 109, 022103. [Google Scholar] [CrossRef]
- Qi, M.; Dai, S.; Wu, P. Prediction of electronic and magnetic properties in 3d-transition-metal X-doped bismuthene (X = V, Cr, Mn and Fe). Appl. Surf. Sci. 2019, 486, 58–64. [Google Scholar] [CrossRef]
- Yang, L.; Song, Y.; Mi, W.; Wang, X. The electronic structure and spin–orbit-induced spin splitting in antimonene with vacancy defects. RSC Adv. 2016, 6, 66140–66146. [Google Scholar] [CrossRef]
- Zhang, J.-J.; Zhang, Y.; Dong, S. Protective layer enhanced the stability and superconductivity of tailored antimonene bilayer. Phys. Rev. Mater. 2018, 2, 126004. [Google Scholar] [CrossRef]
- Xie, M.; Zhang, S.; Cai, B.; Zou, Y.; Zeng, H. N-and p-type doping of antimonene. RSC Adv. 2016, 6, 14620–14625. [Google Scholar] [CrossRef]
- Zhao, M.; Zhang, X.; Li, L. Strain-driven band inversion and topological aspects in Antimonene. Sci. Rep. 2015, 5, 16108. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.-Y.; Huang, Y.; Chen, Q.-Y.; Li, Z.-Y.; Cao, C.; He, Y. Strain and electric field tunable electronic structure of buckled bismuthene. RSC Adv. 2017, 7, 39546–39555. [Google Scholar] [CrossRef]
- Liu, C.; Hu, T.; Wu, Y.; Gao, H.; Yang, Y.; Ren, W. 2D selenium allotropes from first principles and swarm intelligence. J. Phys. Condens. Matter 2019, 31, 235702. [Google Scholar] [CrossRef] [PubMed]
- Singh, J.; Jamdagni, P.; Jakhar, M.; Kumar, A. Stability, electronic and mechanical properties of chalcogen (Se and Te) monolayers. Phys. Chem. Chem. Phys. 2020, 22, 5749–5755. [Google Scholar] [CrossRef]
- Zhang, W.; Wu, Q.; Yazyev, O.V.; Weng, H.; Guo, Z.; Cheng, W.-D.; Chai, G.-L. Topological phase transitions driven by strain in monolayer tellurium. Phys. Rev. B 2018, 98, 115411. [Google Scholar] [CrossRef]
- Sakaguchi, H.; Song, S.; Kojima, T.; Nakae, T. Homochiral polymerization-driven selective growth of graphene nanoribbons. Nat. Chem. 2017, 9, 57–63. [Google Scholar] [CrossRef]
- Dang, Z.; Wang, W.; Chen, J.; Walker, E.S.; Bank, S.R.; Akinwande, D.; Ni, Z.; Tao, L. Vis-NIR photodetector with microsecond response enabled by 2D bismuth/Si (111) heterojunction. 2D Mater. 2021, 8, 035002. [Google Scholar] [CrossRef]
- Lin, S.; Liu, S.; Yang, Z.; Li, Y.; Ng, T.W.; Xu, Z.; Bao, Q.; Hao, J.; Lee, C.S.; Surya, C. Solution-processable ultrathin black phosphorus as an effective electron transport layer in organic photovoltaics. Adv. Funct. Mater. 2016, 26, 864–871. [Google Scholar] [CrossRef]
- Mayorga-Martinez, C.C.; Sofer, Z.; Pumera, M. Layered black phosphorus as a selective vapor sensor. Angew. Chem. Int. Ed. 2015, 54, 14317–14320. [Google Scholar] [CrossRef]
- Saito, Y.; Iizuka, T.; Koretsune, T.; Arita, R.; Shimizu, S.; Iwasa, Y. Gate-tuned thermoelectric power in black phosphorus. Nano Lett. 2016, 16, 4819–4824. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.; Fu, Q.; Zhang, Y.; Weng, X.; Li, H.; Chen, M.; Jin, L.; Dong, A.; Mu, R.; Jiang, P. Graphene cover-promoted metal-catalyzed reactions. Proc. Natl. Acad. Sci. USA 2014, 111, 17023–17028. [Google Scholar] [CrossRef] [PubMed]
- Chang, K.; Mei, Z.; Wang, T.; Kang, Q.; Ouyang, S.; Ye, J. MoS2/graphene cocatalyst for efficient photocatalytic H2 evolution under visible light irradiation. ACS Nano 2014, 8, 7078–7087. [Google Scholar] [CrossRef] [PubMed]
- Tao, W.; Kong, N.; Ji, X.; Zhang, Y.; Sharma, A.; Ouyang, J.; Qi, B.; Wang, J.; Xie, N.; Kang, C.; et al. Emerging two-dimensional monoelemental materials (Xenes) for biomedical applications. Chem. Soc. Rev. 2019, 48, 2891–2912. [Google Scholar] [CrossRef]
- Xue, T.; Bongu, S.R.; Huang, H.; Liang, W.; Wang, Y.; Zhang, F.; Liu, Z.; Zhang, Y.; Zhang, H.; Cui, X. Ultrasensitive detection of microRNA using a bismuthene-enabled fluorescence quenching biosensor. Chem. Commun. 2020, 56, 7041–7044. [Google Scholar] [CrossRef]
- Tao, W.; Ji, X.; Xu, X.; Islam, M.A.; Li, Z.; Chen, S.; Saw, P.E.; Zhang, H.; Bharwani, Z.; Guo, Z. Antimonene quantum dots: Synthesis and application as near-infrared photothermal agents for effective cancer therapy. Angew. Chem. 2017, 129, 12058–12062. [Google Scholar] [CrossRef]
- Zhu, W.; Yogeesh, M.N.; Yang, S.; Aldave, S.H.; Kim, J.-S.; Sonde, S.; Tao, L.; Lu, N.; Akinwande, D. Flexible black phosphorus ambipolar transistors, circuits and AM demodulator. Nano Lett. 2015, 15, 1883–1890. [Google Scholar] [CrossRef]
- Wu, S.; Fatemi, V.; Gibson, Q.D.; Watanabe, K.; Taniguchi, T.; Cava, R.J.; Jarillo-Herrero, P. Observation of the quantum spin Hall effect up to 100 kelvin in a monolayer crystal. Science 2018, 359, 76–79. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).