Synthesis and Evaluation of Reactive Oxygen Species Sensitive Prodrugs of a NAMPT Inhibitor FK866
Abstract
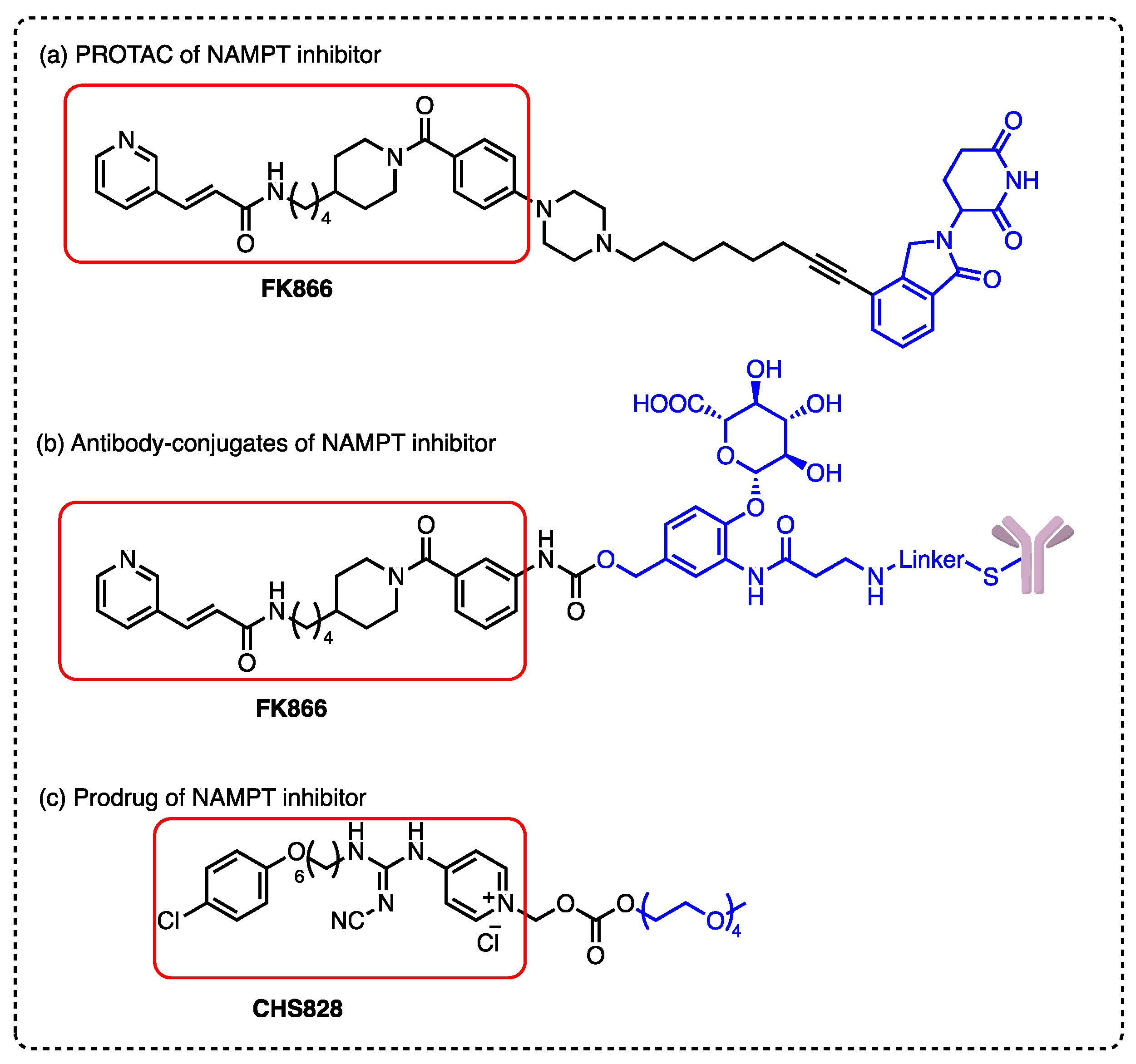
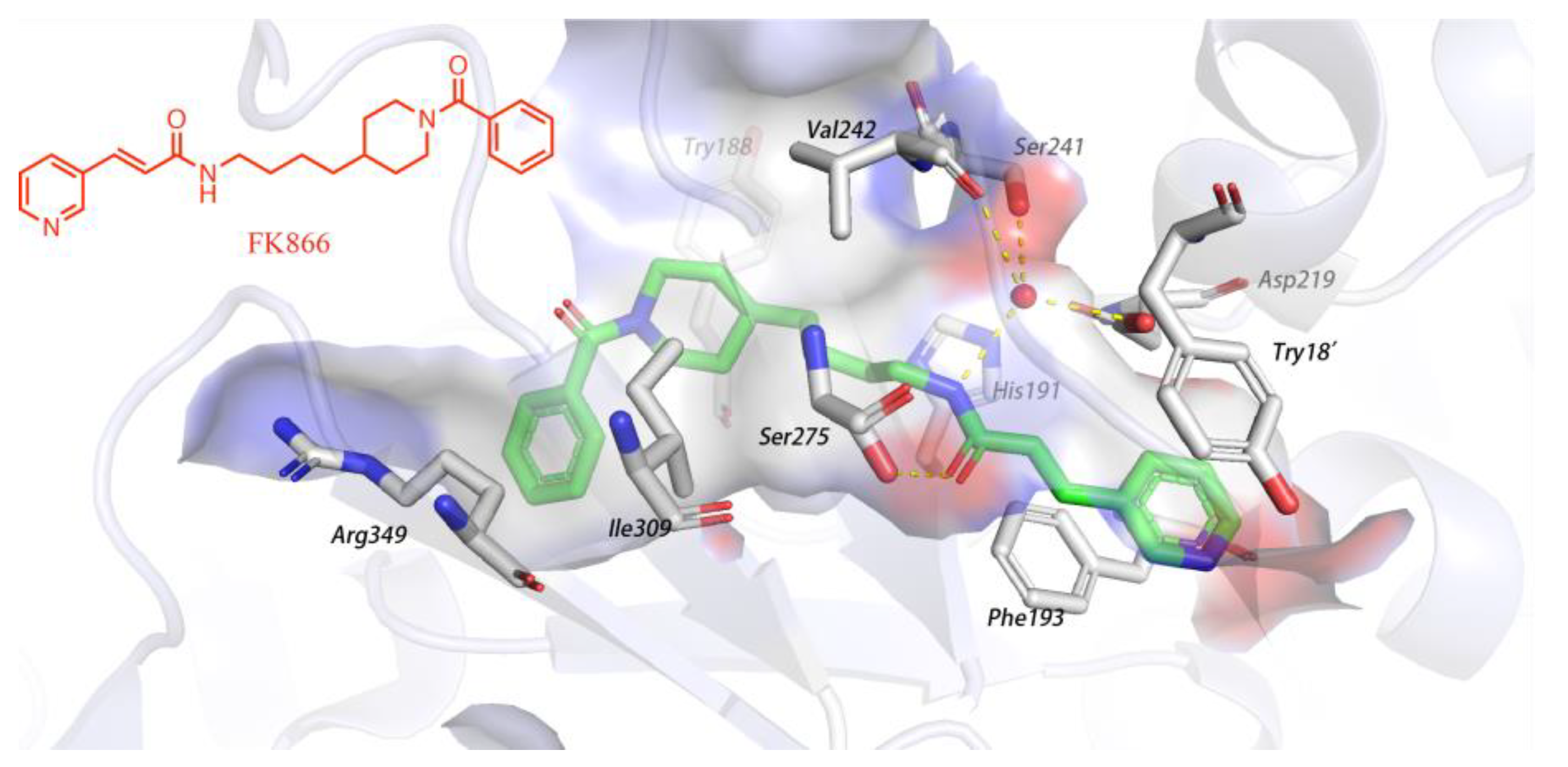
1. Introduction
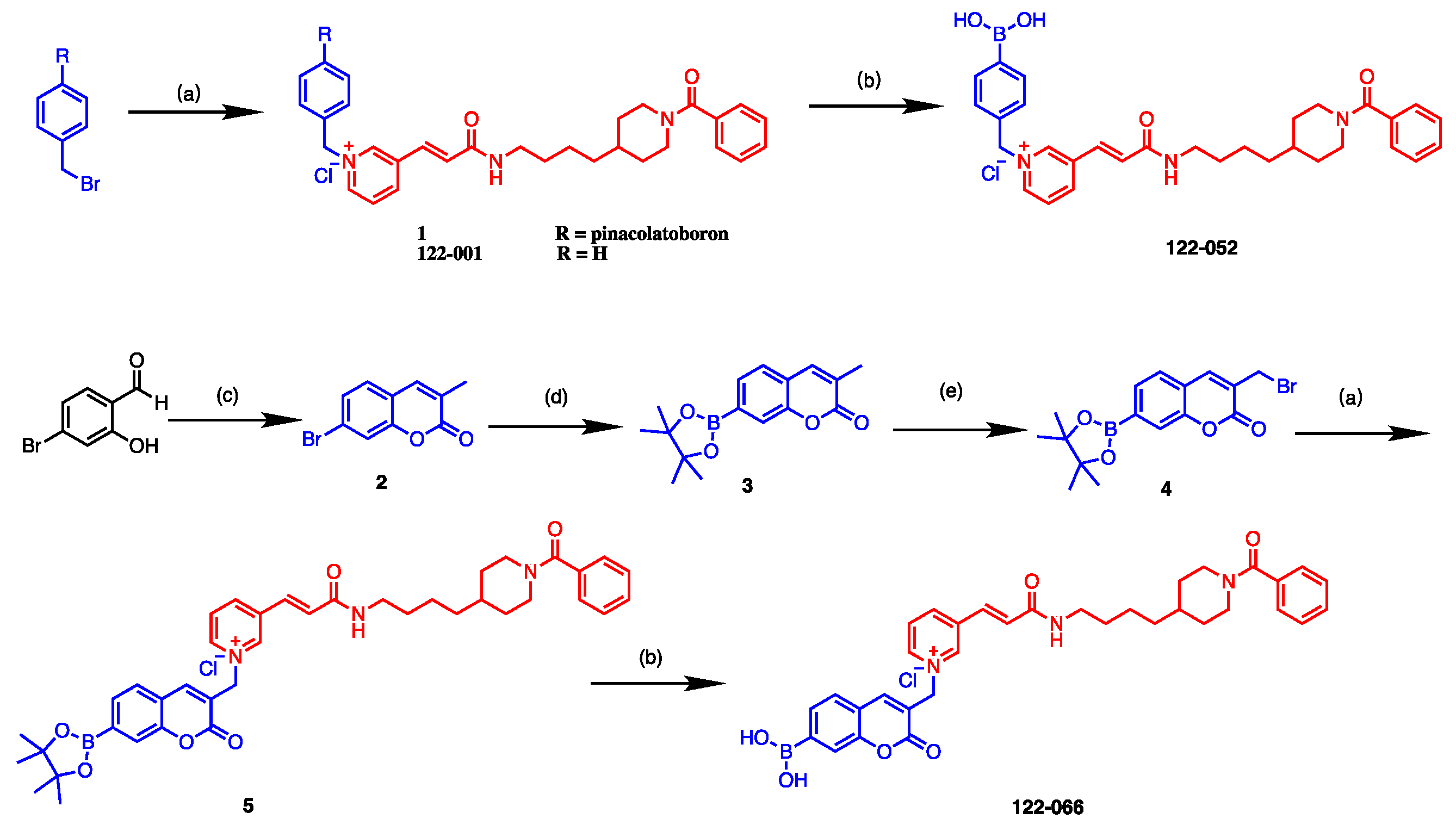
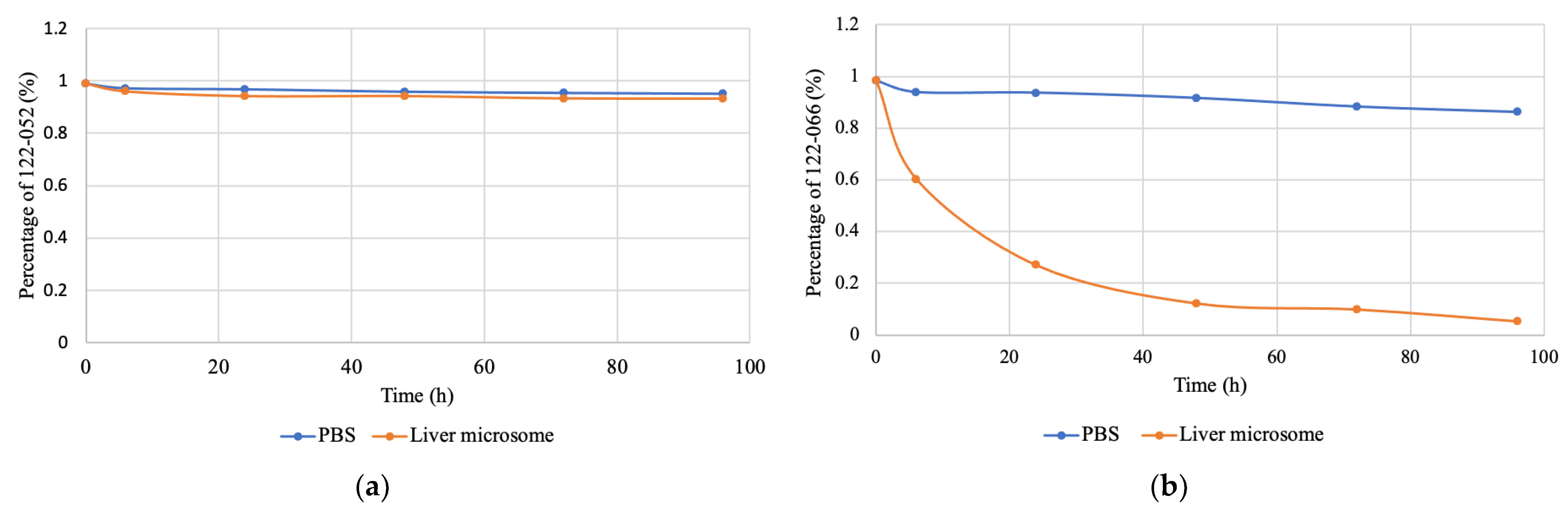
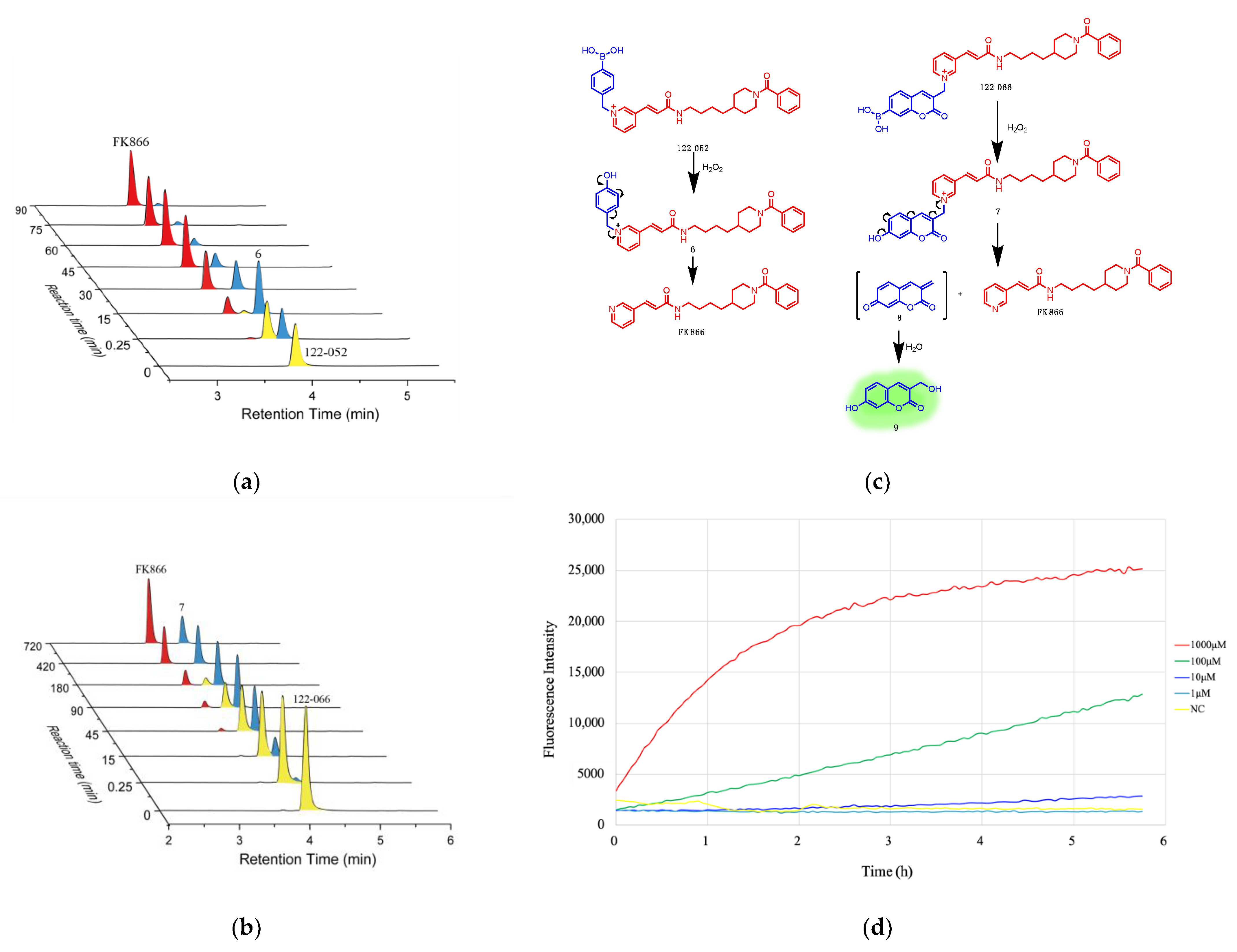
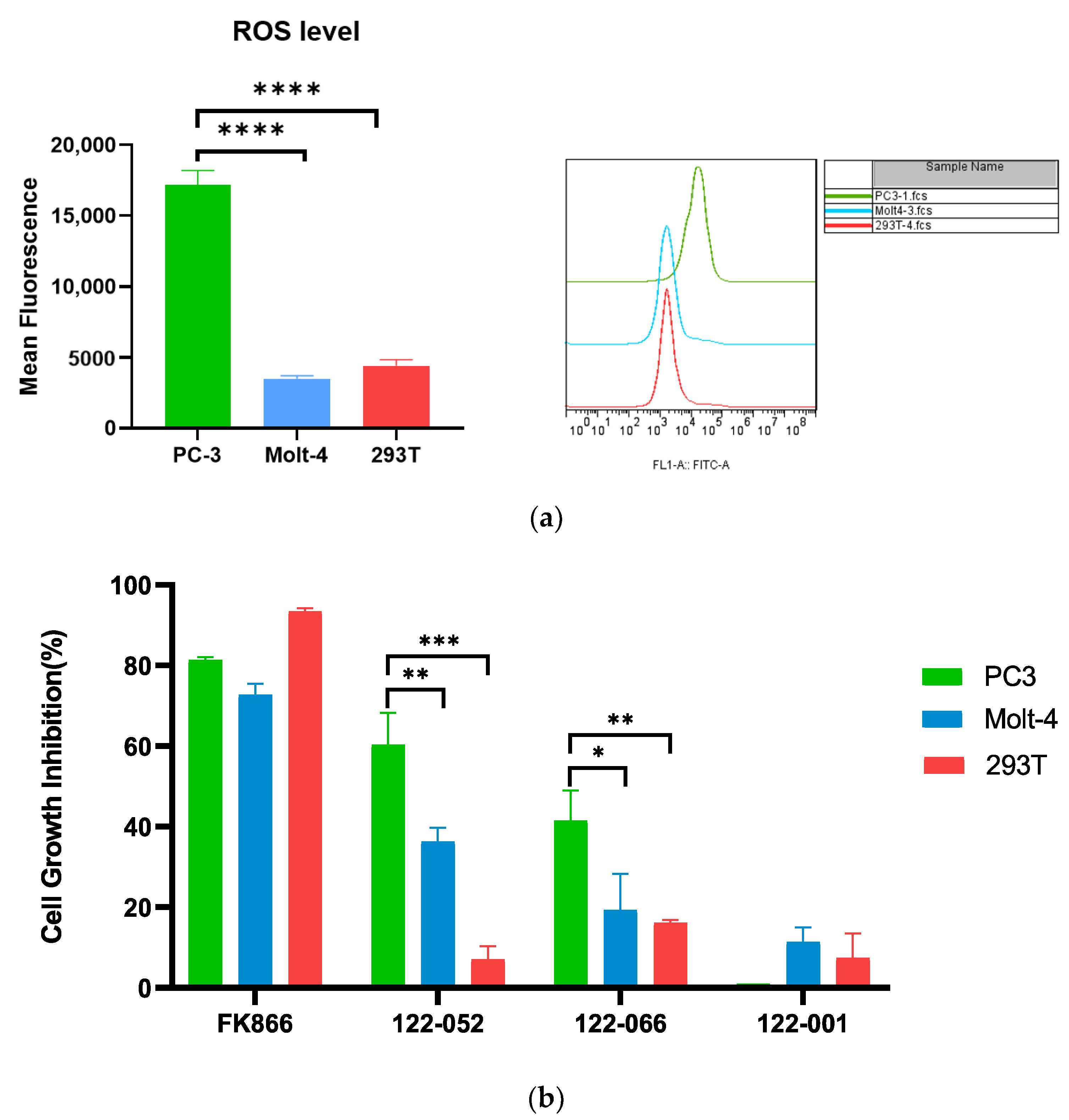
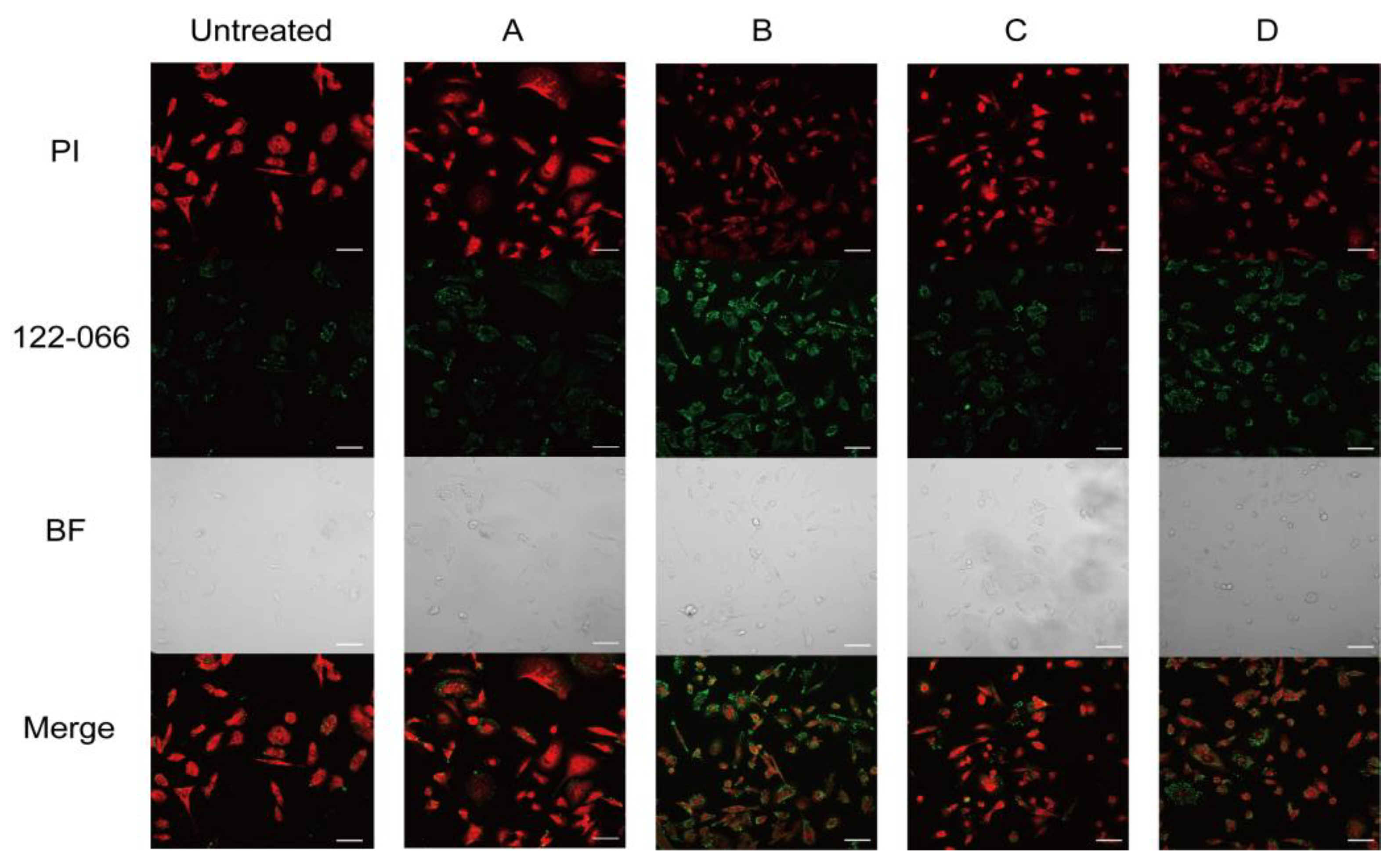
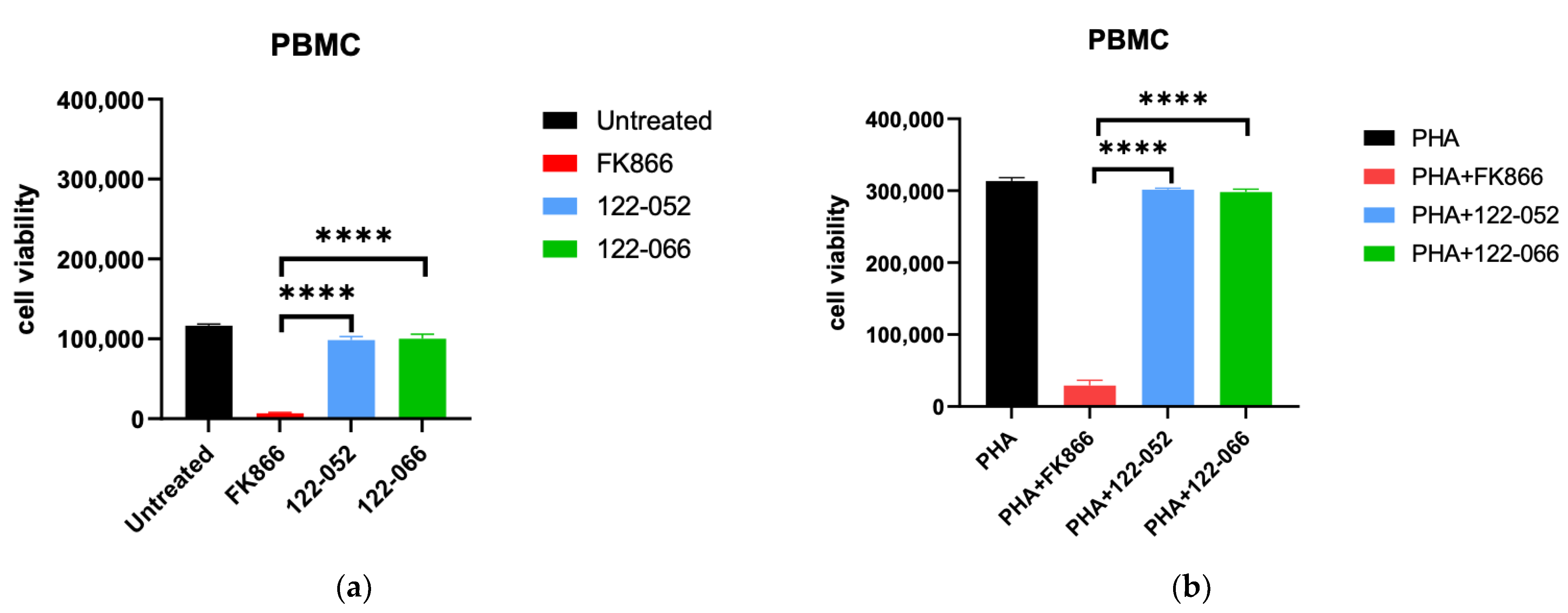
2. Results and Discussion
3. Materials and Methods
3.1. Chemistry
3.2. HPLC Analysis of Drug Release Triggered by H2O2
3.3. HPLC Analysis of Stability of Prodrugs
3.4. Fluorescence Assay
3.5. Cell lines and Culture
3.6. Reactive Oxygen Species ASSAY
3.7. In Vitro Cytotoxicity Assays
3.8. Viability Assay
3.9. Fluorescence Imaging
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Wei, Y.C.; Xiang, H.T.; Zhang, W.Q. Review of various NAMPT inhibitors for the treatment of cancer. Front. Pharmacol. 2022, 13, 970553. [Google Scholar] [CrossRef] [PubMed]
- Garten, A.; Petzold, S.; Korner, A.; Imai, S.; Kiess, W. Nampt: Linking NAD biology, metabolism and cancer. Trends Endocrin. Met. 2009, 20, 130–138. [Google Scholar] [CrossRef] [PubMed]
- Lin, T.C. Updated Functional Roles of NAMPT in Carcinogenesis and Therapeutic Niches. Cancers 2022, 14, 2059. [Google Scholar] [CrossRef] [PubMed]
- Travelli, C.; Colombo, G.; Mola, S.; Genazzani, A.A.; Porta, C. NAMPT: A pleiotropic modulator of monocytes and macrophages. Pharmacol. Res. 2018, 135, 25–36. [Google Scholar] [CrossRef]
- Sampath, D.; Zabka, T.S.; Misner, D.L.; O’Brien, T.; Dragovich, P.S. Inhibition of nicotinamide phosphoribosyltransferase(NAMPT) as a therapeutic strategy in cancer. Pharmacol. Therapeut. 2015, 151, 16–31. [Google Scholar] [CrossRef]
- Khan, J.A.; Tao, X.; Tong, L.A. Molecular basis for the inhibition of human NMPRTase, a novel target for anticancer agents. Nat. Struct. Mol. Biol. 2006, 13, 582–588. [Google Scholar] [CrossRef]
- Von Heideman, A.; Berglund, A.; Larsson, R.; Nygren, P. Safety and efficacy of NAD depleting cancer drugs: Results of a phase I clinical trial of CHS 828 and overview of published data. Cancer Chemoth. Pharm. 2010, 65, 1165–1172. [Google Scholar] [CrossRef] [PubMed]
- Holen, K.; Saltz, L.B.; Hollywood, E.; Burk, K.; Hanauske, A.R. The pharmacokinetics, toxicities, and biologic effects of FK866, a nicotinamide adenine dinucleotide biosynthesis inhibitor. Investig. New Drug. 2008, 26, 45–51. [Google Scholar] [CrossRef]
- Ghanem, M.S.; Monacelli, F.; Nencioni, A. Advances in NAD-Lowering Agents for Cancer Treatment. Nutrients 2021, 13, 1665. [Google Scholar] [CrossRef]
- Hasmann, M.; Schemainda, I. FK866, a highly specific noncompetitive inhibitor of nicotinamide phosphoribosyltransferase, represents a novel mechanism for induction of tumor cell apoptosis. Cancer Res. 2003, 63, 7436–7442. [Google Scholar]
- Deng, Y.X.; Hu, B.Y.; Miao, Y.Z.; Wang, J.; Zhang, S.D.; Wan, H.; Wu, Z.; Lv, Y.F.; Feng, J.; Ji, N.; et al. A Nicotinamide Phosphoribosyltransferase Inhibitor, FK866, Suppresses the Growth of Anaplastic Meningiomas and Inhibits Immune Checkpoint Expression by Regulating STAT1. Front. Oncol. 2022, 12, 836257. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Liu, H.; Chen, L.; Wu, C.; Liu, X.; Cang, Y.; Jiang, B.; Yang, X.; Fan, G. Addressing the Enzyme-independent tumor-promoting function of NAMPT via PROTAC-mediated degradation. Cell Chem. Biol. 2022, 29, 1616–1629.e12. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Pu, C.Y.; Fu, Y.X.; Dong, G.Q.; Huang, M.; Sheng, C.Q. NAMPT-targeting PROTAC promotes antitumor immunity via suppressing myeloid-derived suppressor cell expansion. Acta Pharm. Sin. B 2022, 12, 2859–2868. [Google Scholar] [CrossRef] [PubMed]
- Su, Z.; Xie, F.; Xu, X.; Liu, L.Q.; Xiao, D.; Zhou, X.B.; Li, S. Development of a nitroreductase-dependent theranostic payload for antibody-drug conjugate. Bioorg. Chem. 2022, 129, 106190. [Google Scholar] [CrossRef] [PubMed]
- Karpov, A.S.; Abrams, T.; Clark, S.; Raikar, A.; D’Alessio, J.A.; Dillon, M.P.; Gesner, T.G.; Jones, D.; Lacaud, M.; Mallet, W.; et al. Nicotinamide Phosphoribosyltransferase Inhibitor as a Novel Payload for Antibody-Drug Conjugates. ACS Med. Chem. Lett. 2018, 9, 838–842. [Google Scholar] [CrossRef]
- Neumann, C.S.; Olivas, K.C.; Anderson, M.E.; Cochran, J.H.; Jin, S.; Li, F.; Loftus, L.V.; Meyer, D.W.; Neale, J.; Nix, J.C.; et al. Targeted Delivery of Cytotoxic NAMPT Inhibitors Using Antibody-Drug Conjugates. Mol. Cancer Ther. 2018, 17, 2633–2642. [Google Scholar] [CrossRef]
- Rautio, J.; Kumpulainen, H.; Heimbach, T.; Oliyai, R.; Oh, D.; Jarvinen, T.; Savolainen, J. Prodrugs: Design and clinical applications. Nat. Rev. Drug Discov. 2008, 7, 255–270. [Google Scholar] [CrossRef]
- Wang, P.F.; Gong, Q.J.; Hu, J.B.; Li, X.; Zhang, X.J. Reactive Oxygen Species(ROS)-Responsive Prodrugs, Probes, and Theranostic Prodrugs: Applications in the ROS-Related Diseases. J. Med. Chem. 2021, 64, 298–325. [Google Scholar] [CrossRef]
- Cheung, E.C.; Vousden, K.H. The role of ROS in tumour development and progression. Nat. Rev. Cancer 2022, 22, 280–297. [Google Scholar] [CrossRef]
- Szatrowski, T.P.; Nathan, C.F. Production of Large Amounts of Hydrogen-Peroxide by Human Tumor-Cells. Cancer Res. 1991, 51, 794–798. [Google Scholar]
- Gorrini, C.; Harris, I.S.; Mak, T.W. Modulation of oxidative stress as an anticancer strategy. Nat. Rev. Drug Discov. 2013, 12, 931–947. [Google Scholar] [CrossRef] [PubMed]
- Antonio, J.P.M.; Carvalho, J.I.; Andre, A.S.; Dias, J.N.R.; Aguiar, S.I.; Faustino, H.; Lopes, R.M.R.M.; Veiros, L.F.; Bernardes, G.J.L.; Silva, F.A.; et al. Diazaborines Are a Versatile Platform to Develop ROS-Responsive Antibody Drug Conjugates**. Angew. Chem. Int. Edit. 2021, 60, 25914–25921. [Google Scholar] [CrossRef] [PubMed]
- Binderup, E.; Bjorkling, F.; Hjarnaa, P.V.; Latini, S.; Baltzer, B.; Carlsen, M.; Binderup, L. EB1627: A soluble prodrug of the potent anticancer cyanoguanidine CHS828. Bioorg. Med. Chem. Lett. 2005, 15, 2491–2494. [Google Scholar] [CrossRef] [PubMed]
- Maslah, H.; Skarbek, C.; Pethe, S.; Labruere, R. Anticancer boron-containing prodrugs responsive to oxidative stress from the tumor microenvironment. Eur. J. Med. Chem. 2020, 207, 112670. [Google Scholar] [CrossRef] [PubMed]
- Weinstain, R.; Segal, E.; Satchi-Fainaro, R.; Shabat, D. Real-time monitoring of drug release. Chem. Commun. 2010, 46, 553–555. [Google Scholar] [CrossRef] [PubMed]
- Behara, K.K.; Rajesh, Y.; Venkatesh, Y.; Pinninti, B.R.; Mandal, M.; Singh, N.D.P. Cascade photocaging of diazeniumdiolate: A novel strategy for one and two photon triggered uncaging with real time reporting. Chem. Commun. 2017, 53, 9470–9473. [Google Scholar] [CrossRef]
- Galli, U.; Ercolano, E.; Carraro, L.; Roman, C.R.B.; Sorba, G.; Canonico, P.L.; Genazzani, A.A.; Tron, G.C.; Billington, R.A. Synthesis and biological evaluation of isosteric analogues of FK866, an inhibitor of NAD salvage. ChemMedChem 2008, 3, 771–779. [Google Scholar] [CrossRef] [PubMed]
- Fentem, J.H.; Fry, J.R. Metabolism of Coumarin by Rat, Gerbil and Human Liver-Microsomes. Xenobiotica 1992, 22, 357–367. [Google Scholar] [CrossRef]
- Born, S.L.; Api, A.M.; Ford, R.A.; Lefever, F.R.; Hawkins, D.R. Comparative metabolism and kinetics of coumarin in mice and rats. Food Chem. Toxicol. 2003, 41, 247–258. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, Z.; Wang, H.; Liu, H.; Chen, H.; Jiang, B. Synthesis and Evaluation of Reactive Oxygen Species Sensitive Prodrugs of a NAMPT Inhibitor FK866. Molecules 2023, 28, 169. https://doi.org/10.3390/molecules28010169
Xu Z, Wang H, Liu H, Chen H, Jiang B. Synthesis and Evaluation of Reactive Oxygen Species Sensitive Prodrugs of a NAMPT Inhibitor FK866. Molecules. 2023; 28(1):169. https://doi.org/10.3390/molecules28010169
Chicago/Turabian StyleXu, Zili, Huihui Wang, Haixia Liu, Hongli Chen, and Biao Jiang. 2023. "Synthesis and Evaluation of Reactive Oxygen Species Sensitive Prodrugs of a NAMPT Inhibitor FK866" Molecules 28, no. 1: 169. https://doi.org/10.3390/molecules28010169
APA StyleXu, Z., Wang, H., Liu, H., Chen, H., & Jiang, B. (2023). Synthesis and Evaluation of Reactive Oxygen Species Sensitive Prodrugs of a NAMPT Inhibitor FK866. Molecules, 28(1), 169. https://doi.org/10.3390/molecules28010169



