Abstract
Matricaria chamomilla L. (MC) and Chamaemelum nobile (L.) All. (CN) are two varieties of Chamomile. These herbs have been used for thousands of years in Greece, Rome and ancient Egypt. Chamomile has been used for the treatment of stomach problems, cramps, dermatitis, and minor infections. The purpose of this study was to introduce the botanical characteristics and geographical distribution, traditional uses, chemical constituents, pharmacological activities, toxicity studies and quality control studies, and lay a theoretical foundation for the rational development and utilization of chamomile. This review powered that chemical constituents include flavonoids, coumarins, volatile oils, terpenes, organic acids, polysaccharides, and others. These compounds possess anticancer, anti-infective, anti-inflammatory, antithrombotic, antioxidant, hypolipidaemic, hypoglycaemic, antihypertensive, antidepressant, neuroprotective activities, among others. Chamomile is a widely used herb in traditional medicine. It brings great economic value due to its numerous pharmacological effects and traditional uses. However, more toxicity tests should be carried out to confirm its safety. There is need for further research to provide concrete scientific evidence and validate its medicinal properties.
1. Introduction
Traditional Chinese medicine (TCM) has been used for a long time in China and recorded for more than 5000 years. Numerous herbs have been used in TCM [1]. This field is becoming an integral part of various traditional medicine and modern medicine systems globally. In modern medicine, it is primarily used to prevent various diseases. Therefore, TCM is gaining popularity worldwide and supports human health greatly [2].
Chamomile is an annual or perennial plant belonging to the family Asteraceae. The plant improves the appetite and relieves painful swellings and sweating [3]. Chamomile is native to temperate regions of Asia and Europe, and cultivated worldwide for its high medicinal, cosmetics and food value [4]. It has been used for thousands of years in Greece, Rome and ancient Egypt. In China, the detailed use of this plant was first recorded in Uyghur medicine. Veteran doctors of TCM believe that preparations containing chamomile have a calming effect. In addition, the plant is also used in other traditional, homoeopathic and Unani preparations [5,6]. There are two main varieties of chamomile: Matricaria chamomilia L. (MC) and Anthemis nobilis (L.) All. (CN). Matricaria chamomilia L. belongs to the genus Matricaria. It is an annual plant, and the flowering period is from May to July in China. Chamaemelum nobile (L.) All. is a perennial plant of the genus Chamaemelum. The flowering period is from April to May in China [7,8]. Matricaria chamomilia L. is relatively common and has been researched and used widely. At present, 26 countries around the world have included this plant in their pharmacopoeia. Chamomile flower heads are commonly used for medicinal purposes [9]. Chamomile contains flavonoids, coumarins, volatile oils, terpenes, sterols, organic acids, and polysaccharides, among other compounds. Having a wide array of compounds, chamomile exhibits various pharmacological activities such as anticancer, anti-infective, anti-inflammatory, antioxidant, hypoglycaemic, hypotensive, hypolipidaemic, antiallergic, antidepressant, and neuroprotective effects, and others [3,4,5,6,7,8]. In general, this plant has outstanding research value. Still, there are very few reviews of it in the literature. This article provides a comprehensive review of the botanical characteristics and distribution, traditional uses, chemical constituents, pharmacological effects, and quality control methods of chamomile.
Pictures of two species of chamomile from the Global Biodiversity Information Facility “https://www.gbif.org (accessed on 16 November 2022)” are shown in Figure 1.

Figure 1.
Two species of Chamomilla: Matricaria chamomilia L. (a), Chamaemelum nobile (L.) All. (b).
2. Methodology
In this review, the information about the botanical characteristics and geographical distribution, traditional uses, chemical constituents, pharmacological activities, adverse reactions, toxicity studies and quality control studies of chamomile is collected. All information on chamomile and its two species were collected from textbooks, electronic databases and library materials, including PubMed, Web of Science, China National Knowledge Infrastructure, MDPI, Springer, Elsevier, Google Scholar, Yahoo search and Google Scholar. The keywords used to obtain the information. included ‘Yang Gan Jv’, ‘Chamomile’, ‘Matricaria chamomilia L.’, ‘Chamaemelum nobile (L.) All.’, ‘botany’, ‘traditional uses’, ‘pharmacological activities’, ‘chemical constituents’, ‘toxicity’, and ‘quality control studies.’ The scientific names and photos of two species of chamomile were confirmed from the World Flora Online database (www.worldfloraonline.org). The chemical constituents were verified using PubChem, and the structures were drawn using ChemDraw.
2.1. Botanical Characteristics and Geographical Distribution
Chamomile is an annual or perennial plant native to temperate regions of Asia and Europe. It is widely cultivated worldwide, such as in Germany, Hungary, France, Russia, Brazil and western Xinjiang of China. This plant is native to tropical conditions but it can be cultivated in cold climatic conditions [4]. The roots are thin, spindle-shaped and grow straight. The stems can grow to 10–80 cm. The leaves are long, narrow and pinnate, with fissures. The head is about 10–30 mm in diameter [5,10]. Matricaria chamomilia L. has small white flowers of the Compositae family and, at their center, a yellow tubular petal is present. Anthemis nobilis (L.) All. contains flowers with double petals, soft stems, and has a green apple fragrance. It is also called “the apple of the ground”. In addition, it is also known as the “Physician of Plants” due to its ability to heal sick plants around it. Although MC and CN are similar in appearance, they have distinct differences. The petals of MC are turned down and have a raised conical center, whereas the center of CN is flat [8]. Additionally, MC and CN have four and three germination holes in pollen grains, respectively. It is worth noting that CN has a non-glandular hair structure [6,11]. In some areas, such as Europe, Mexico, South America, Russia, the chamomile flower head is the main medicinal part and is used for anti-inflammation and sedation. However, in China, in the majority of cases the whole plant is collected to treat various diseases [7].
2.2. Traditional Uses
As early as the Eastern Han Dynasty in China, a monograph recorded the use of TCM by human beings to treat sundry diseases, namely “Shennong’s Classic of Materia Medica” (《神农本草经》) [12]. Several uses of chamomile are reported in the TCM literature, and it is one of the most commonly used herbal medicines to treat stomach problems, cramps, dermatitis, and minor infections [13]. Chamomile has been used for thousands of years in Greece, Rome, and ancient Egypt. In China, the plant was first recorded in detail in Uyghur medicine. In the “Zhu Medical Canon” (《注医典》), a Uyghur medical work written in the 10th century, chamomile is called “Bamu Nai”. Its taste and fragrance is slightly bitter. The plant nourishes the nerves and the stomach. It improves the appetite and relieves painful swellings and sweating, and is frequently used for chronic headaches, constipation, poor sweating, joint swelling, and urinary system disorders [3,5]. “Chinese Herbs. Uyghur Medicine Volume” (《中华草本.维药卷》) recorded that Chamomile can strengthen the brain and muscles, can be a diuretic and improves e menstrual flow, nourishes the stomach and can be used as an appetizer. It mainly treats symptoms of muscle relaxation, joint swelling and pain, stomach deficiency and anorexia, amenorrhea, and urine retention. “Chinese Ethnic Medicine” (《中国民族药志》) indicates that Chamomile can detoxify, clear heat, stop dysentery, dispel wind, and eliminate paralysis. Meanwhile, the Uyghur folk medicine book “Baidi Yi Medicine Book” (《拜地依药书》) recorded that the plant has a favourable effect on cold headache, black fat and mucous typhoid, and kidney stones [6,7]. It is worth mentioning that 26 countries worldwide have included Chamomile in pharmacopoeia, including German Pharmacopoeia, European Pharmacopoeia, United States Pharmacopeia, British Pharmacopoeia, and more [9]. It can be used as an ingredient in many traditional medicine preparations, such as Zukamu granules, Fufang Munizi granules, and Strong Madsiri Ayat honey ointment, and is also used in Homeopathy and Unani preparations [5,7]. Furthermore, chamomile is the main active ingredient in many mouthwash preparations [14]. The oral consumption of this plant relieves pain caused by functional digestive disorders and symptoms of gastrointestinal disorders. Topical application of chamomile essence (as a lotion or powder) can be used to repel mosquitoes, treat skin diseases, wounds, haemorrhoids and inflammation of the eyes, nose, and throat [7,15,16]. It has been used in herbal baths for thousands of years, and is commonly consumed as a decoction (in water) in TCM [17]. According to numerous records, chamomile is one of various TCM bath agents.
In fact, in addition to being used as a medicine, chamomile is also used in cosmetics, food, and more [18,19]. The essential oil in this plant serves as perfume, an in skincare products, massage oil, and toothpaste. [20]. A small amount of chamomile in bathing water results in emollient and anti-inflammatory effects [21]. Tea made from dried flowers is reported to induce good sleep, regulate the intestines and sweat, and prevent cold [18]. Honey chamomile tea brewed with 5 g of Chamomile, two slices of lemon, and one tablespoon of honey has been reported to lower blood pressure, detoxify, and relieve inflammation [22]. Gould et al. found that chamomile tea could influence hemodynamics in patients with heart disease [23]. Guo et al. used the essential oil of CN to prepare a clear and transparent flower tea product with a unique flavor [24]. A medicinal preparation containing chamomile and other herbs produces calming and tranquillizing effects. The traditional uses of chamomile are shown in Table 1.

Table 1.
Traditional uses of chamomile.
2.3. Chemical Constituents
2.3.1. Organic Acids
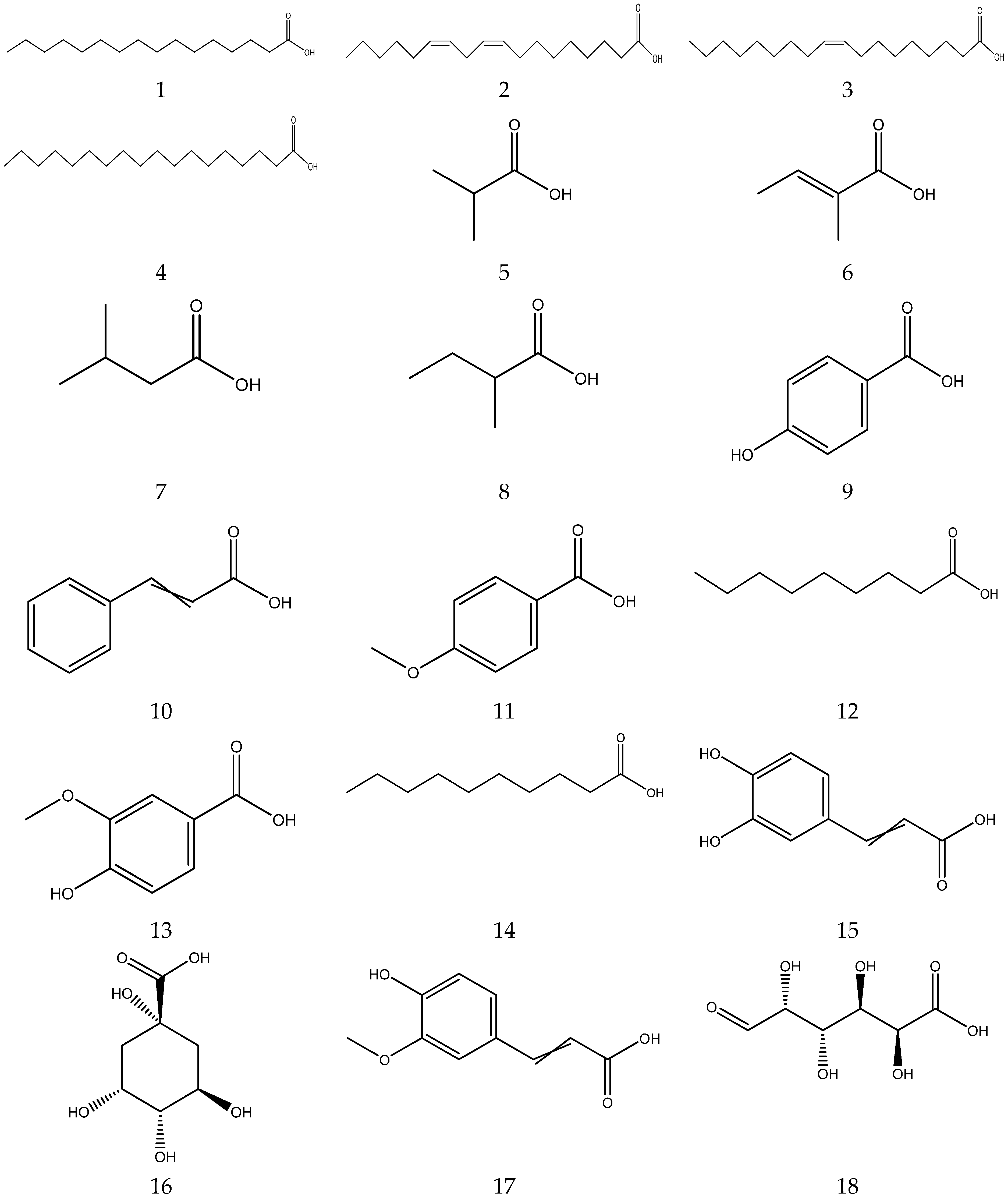
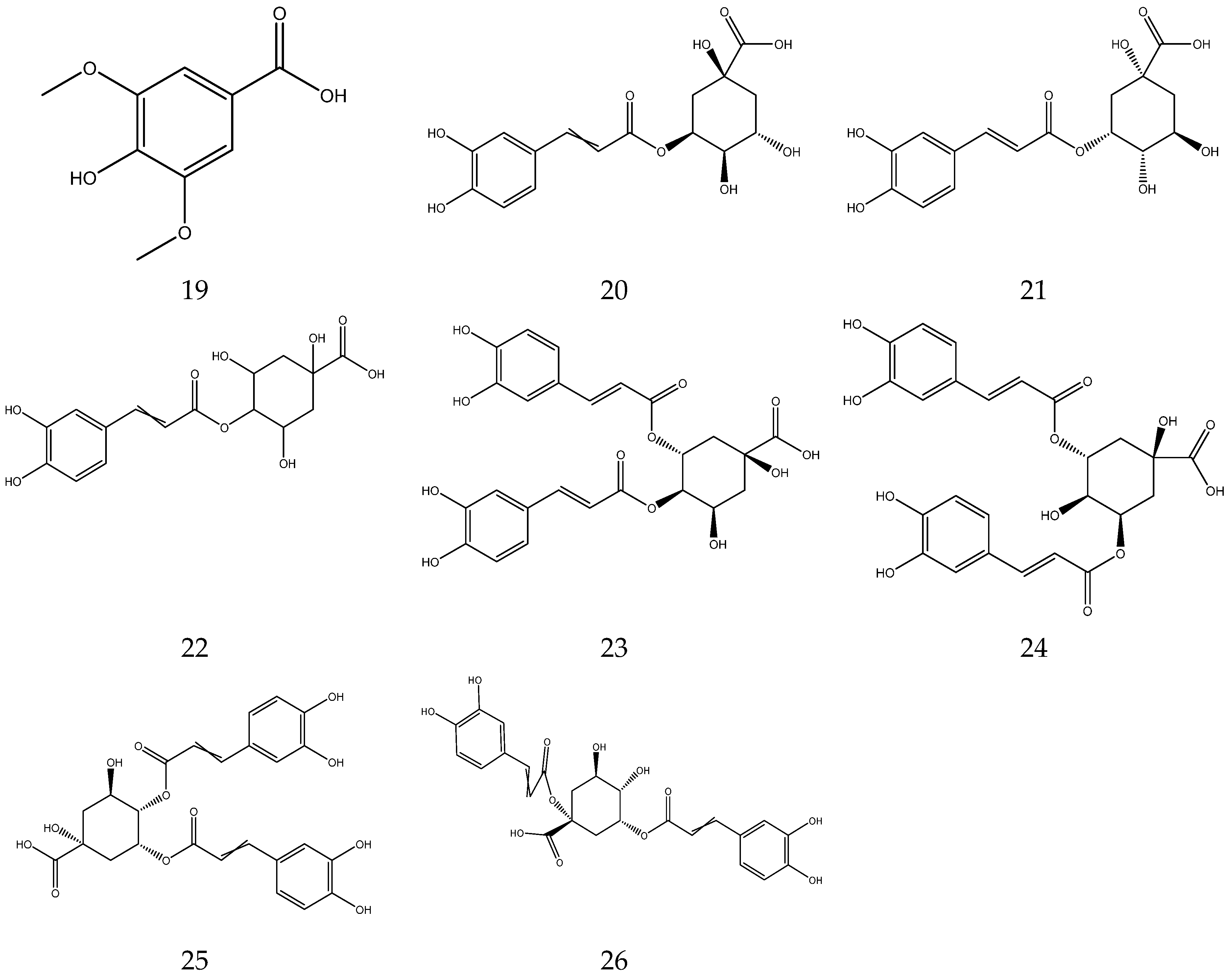
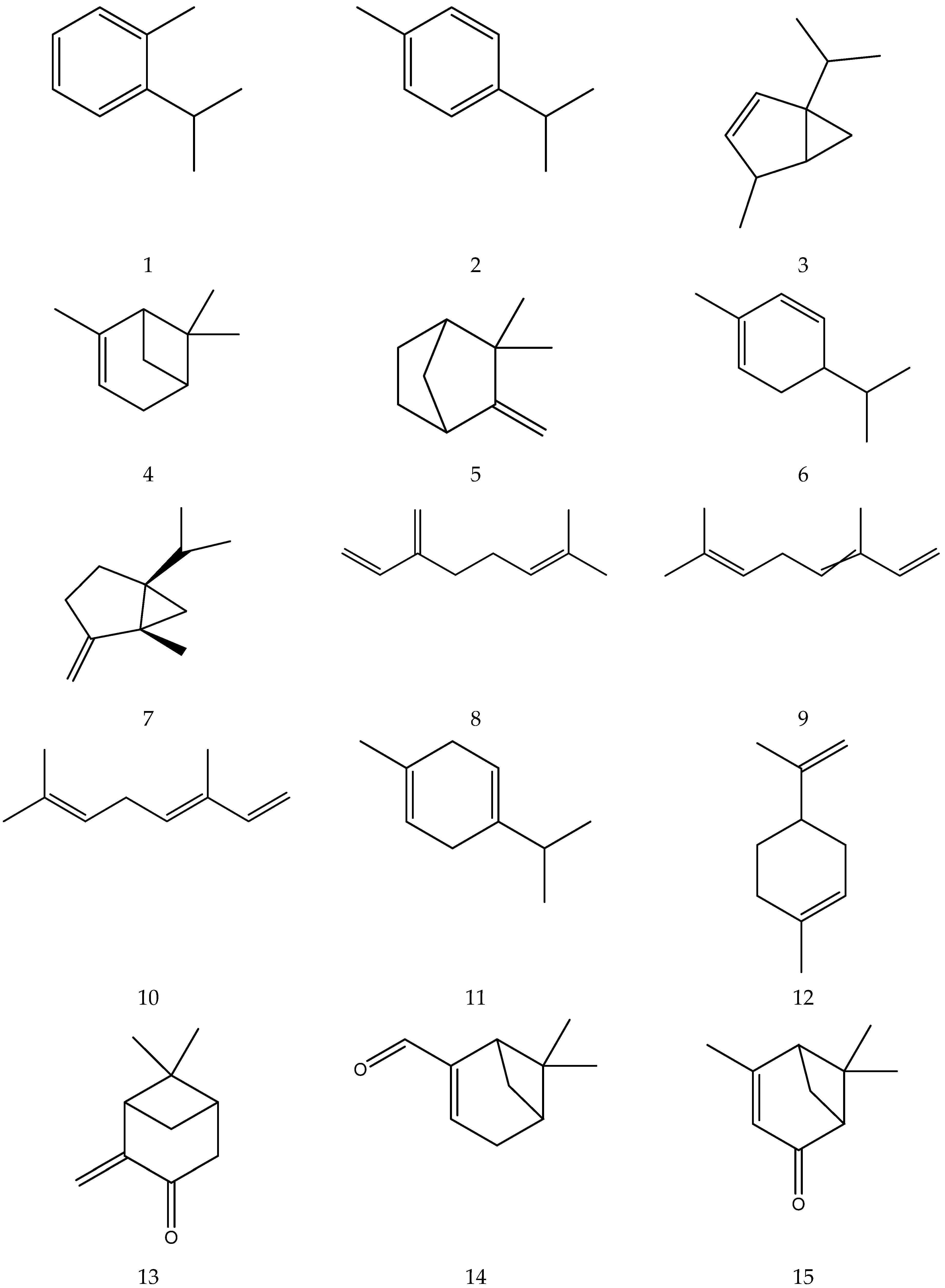
Organic acids contain carboxylic acid, and sulfonic acid functional groups. A total of 26 organic acids have been isolated from chamomile, among which four acids are primary metabolites and are essential compounds for the growth and development of living organisms [33,34,35,36,37,38]. The compounds also have great potential in the treatment of cardiovascular diseases, immune system diseases and cancer [39]. In addition, the remaining 22 acids are secondary metabolites.
The organic acids from chamomile are shown in Table 2, and their chemical structures are shown in Figure 2.

Table 2.
Organic acids from Chamomile.
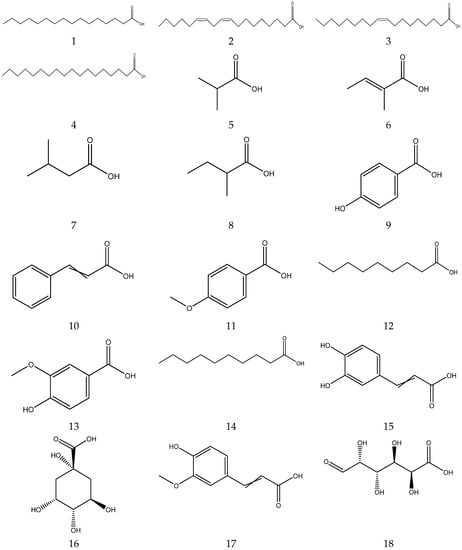
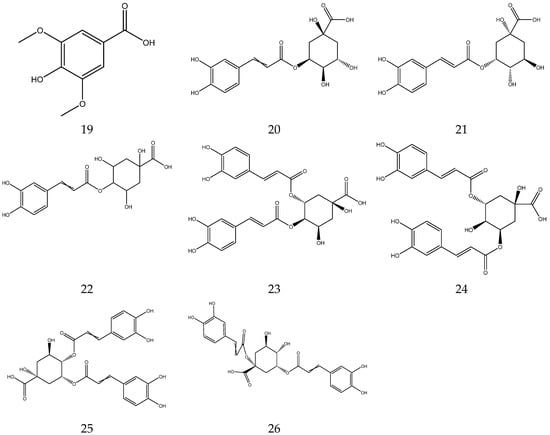
Figure 2.
The chemical structures of organic acid from Chamomile.
2.3.2. Flavonoids
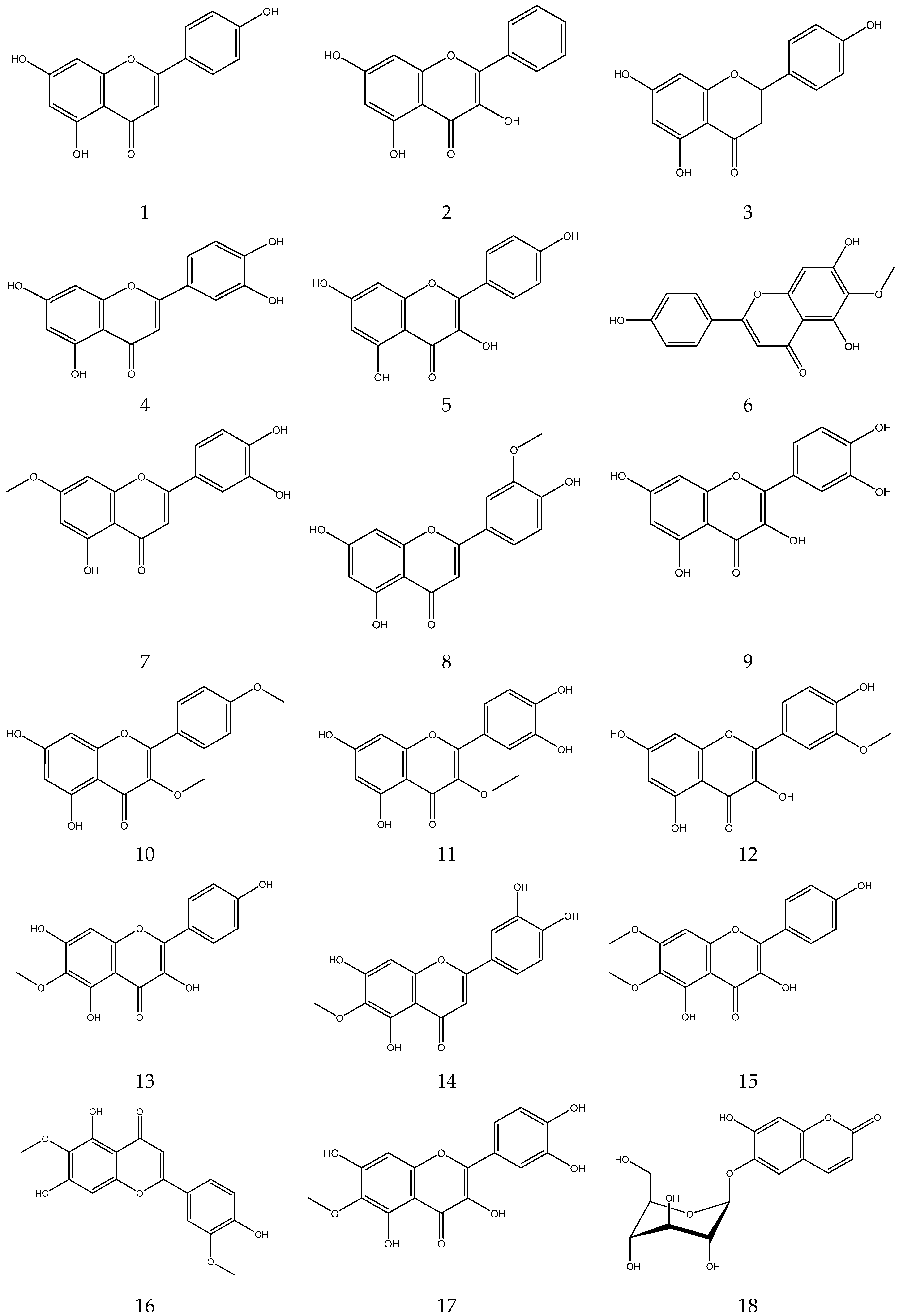
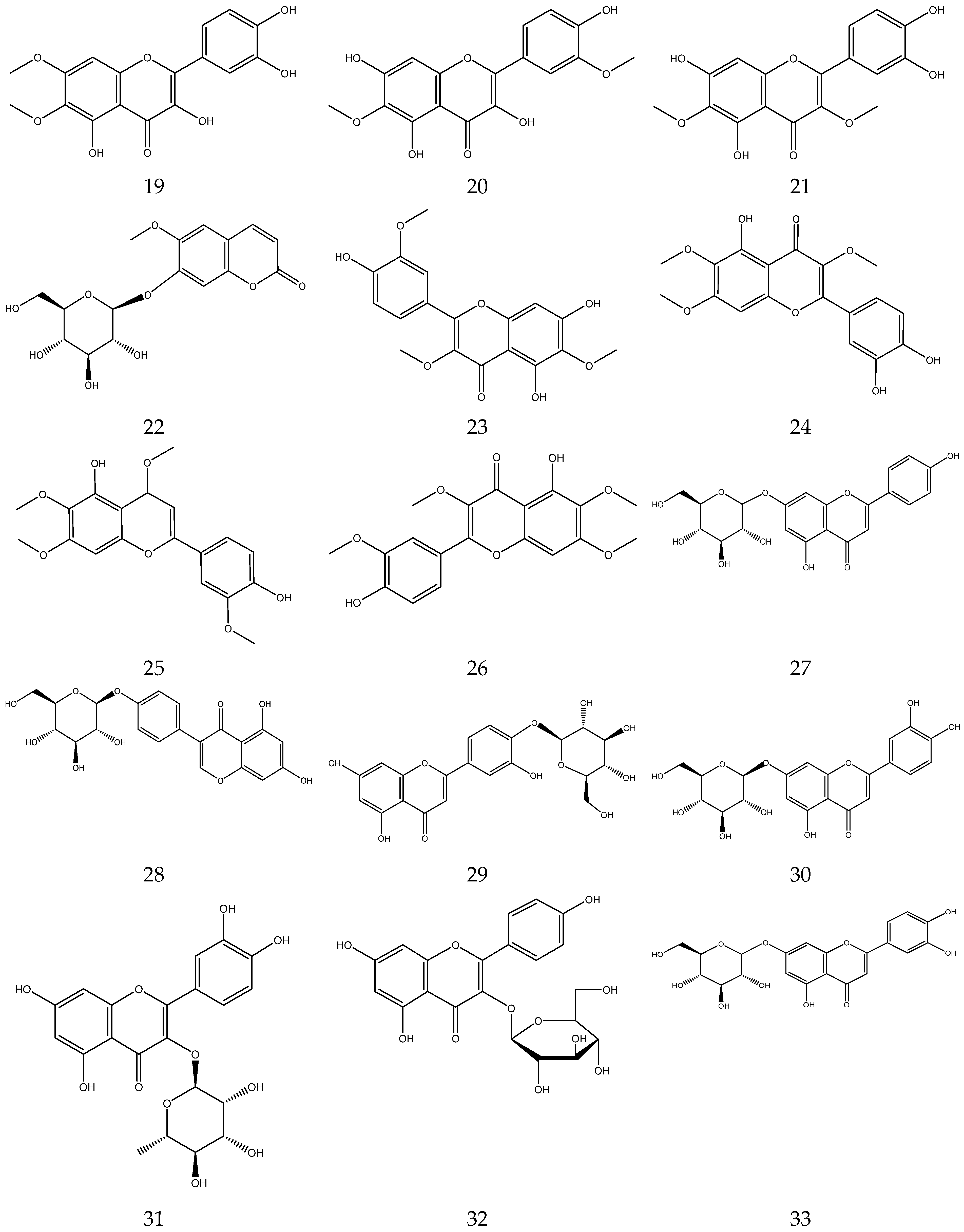
In general, flavonoids have a core structure of 2-phenyl chromone. A total of fifty flavonoids have been isolated from Chamomile and are its main active components [33,34,35,36]. They include quercetin, apigenin, luteolin, and rutin. These compounds exhibit antibacterial, antioxidant, anticancer, and other pharmacological effects. Yang et al. reported the presence of apigenin-7-O-β-D-glucoside and luteolin-7-O-β-D-glucose glycosides in an alcohol extract, and these are assumed to be the main bioactive flavonoids of chamomile [40]. Mire Ayi et al. evaluated the inhibitory effect of a total flavonoid extract on pancreatic lipase using 4-methylumbelliferone oleate (4-MUO) as a substrate [34]. Therefore, chamomile could be an alternative medicine to prevent and treat obesity. According to reports, apigenin has anti-inflammatory properties. Apigenin reduces inflammation in lipopolysaccharide (LPS)-stimulated BV2 microglia via the Glycogen Synthase Kinase 3β/Nuclear factor E2 related factor 2 (GSK-3β/Nrf2) signaling pathway [41]. In addition, it affects the levels of interferon-γ and interleukin-10 in lymphocytes [42].
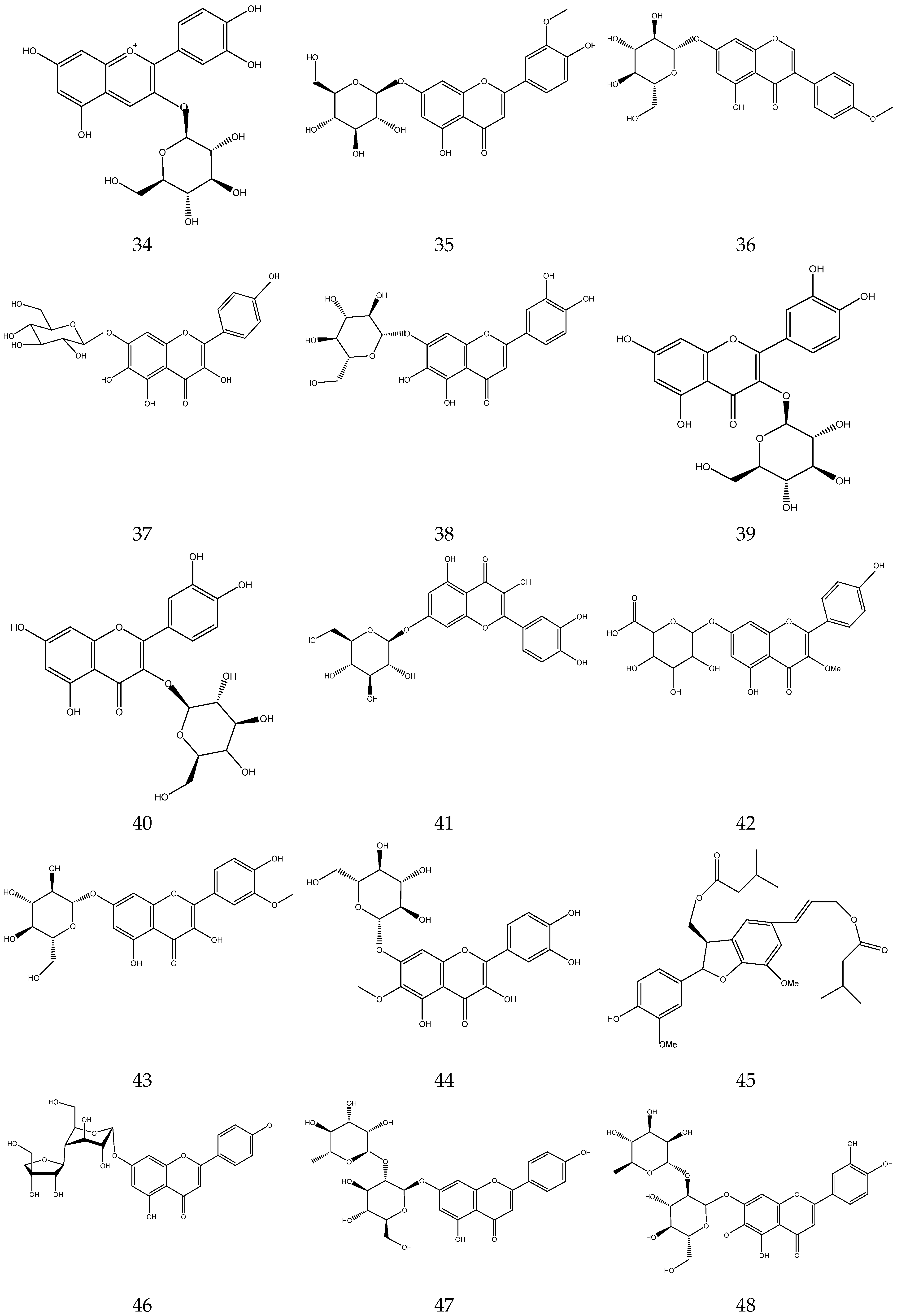
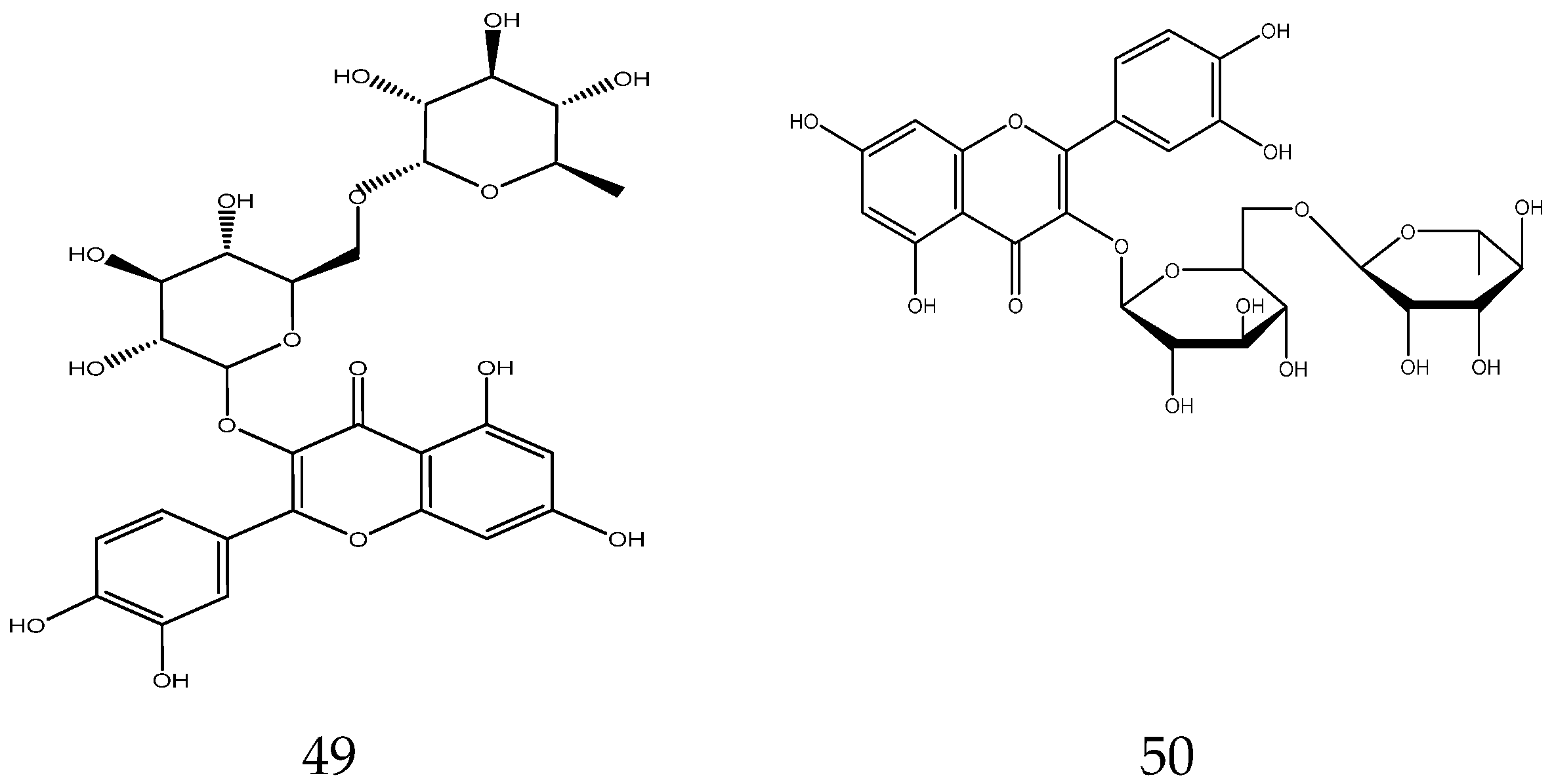
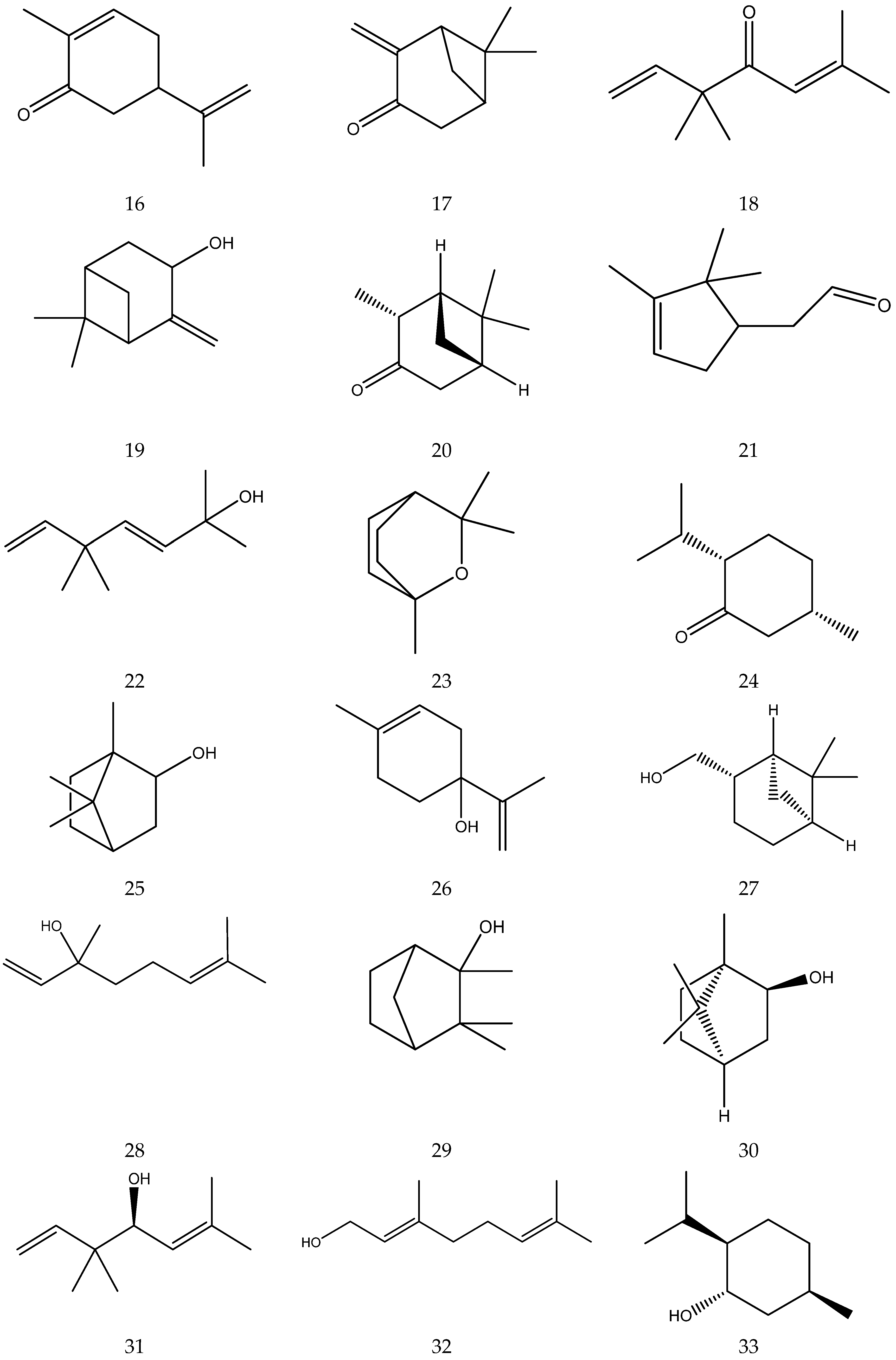
The flavonoids from Chamomile are shown in Table 3, and their chemical structures are shown in Figure 3.

Table 3.
Flavonoids from Chamomile.
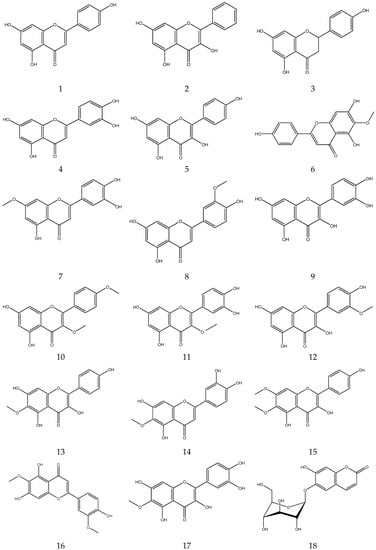
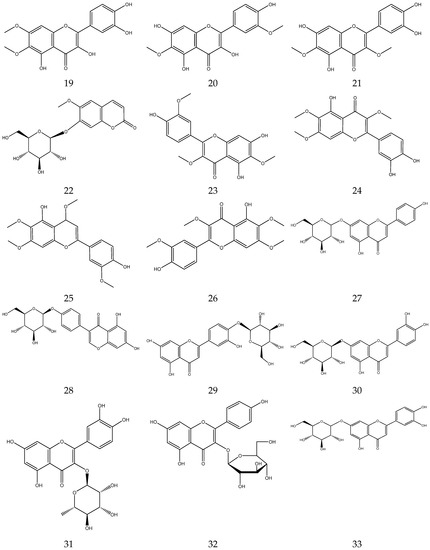
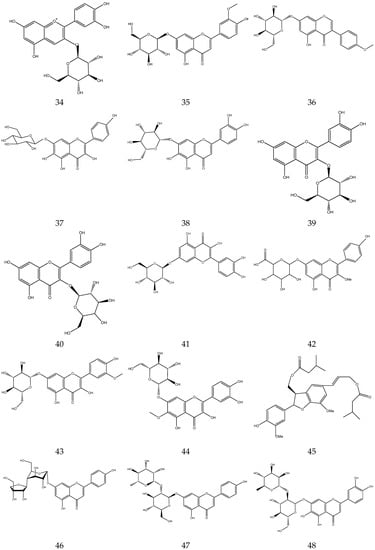
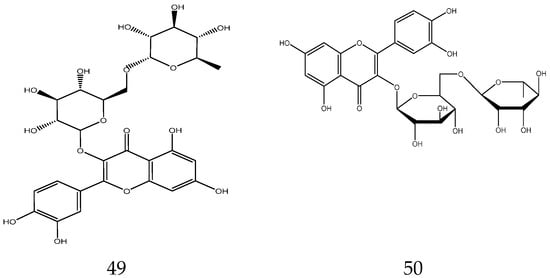
Figure 3.
The chemical structures of flavonoids from Chamomile.
2.3.3. Coumarins
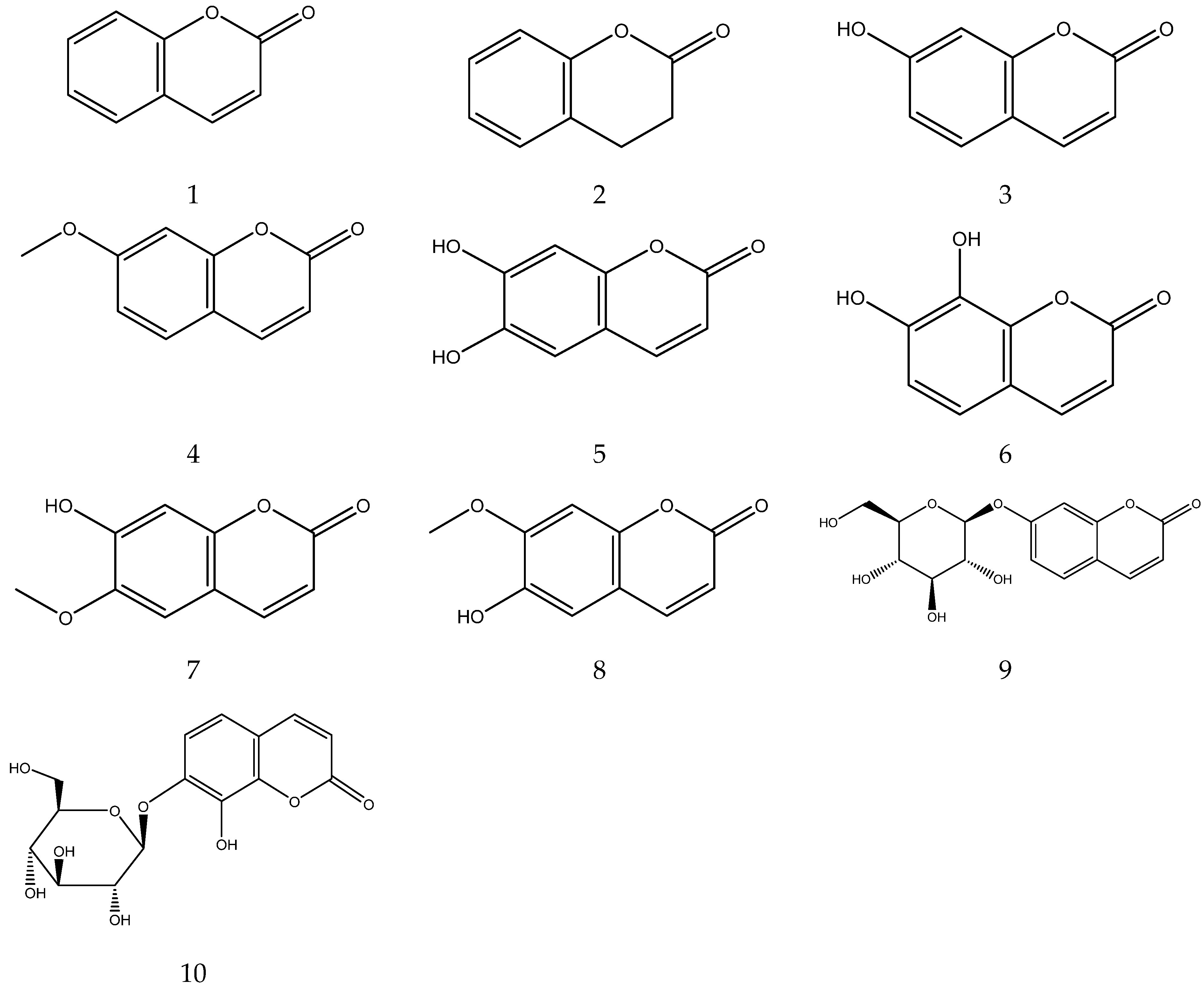
The parent nucleus of coumarin is benzopyrone. A total of 10 coumarins, including coumarin, 7-methoxycoumarin, esculetin, skimmin, daphnin, daphnetin, umbelliferone, scopoletin, isoscopoletin, and 3,4-Dihydrocoumarin, have been identified from Chamomile [33,34,38,43]. Li et al. established a method of simultaneous quantitative analysis for multi-components by single marker (QAMS) to determine the content of 7-methoxycoumarin, which uses an external standard method to determine apigenin, and uses a relative correction factor to determine 7-methoxycoumarin and other components [44].
The coumarins from chamomile are shown in Table 4 and their chemical structures are shown in Figure 4.

Table 4.
Coumarins from Chamomile.
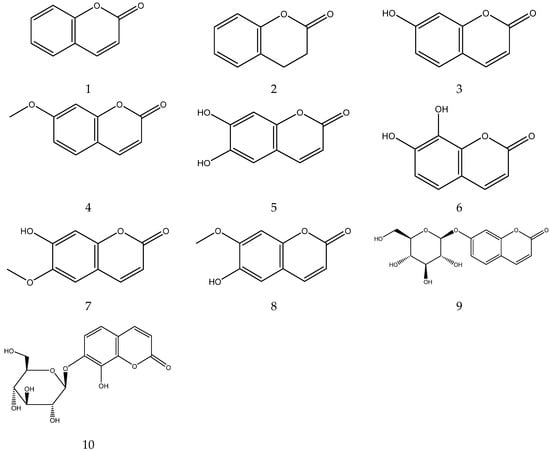
Figure 4.
The chemical structures of coumarin from Chamomile.
2.3.4. Volatile Oil
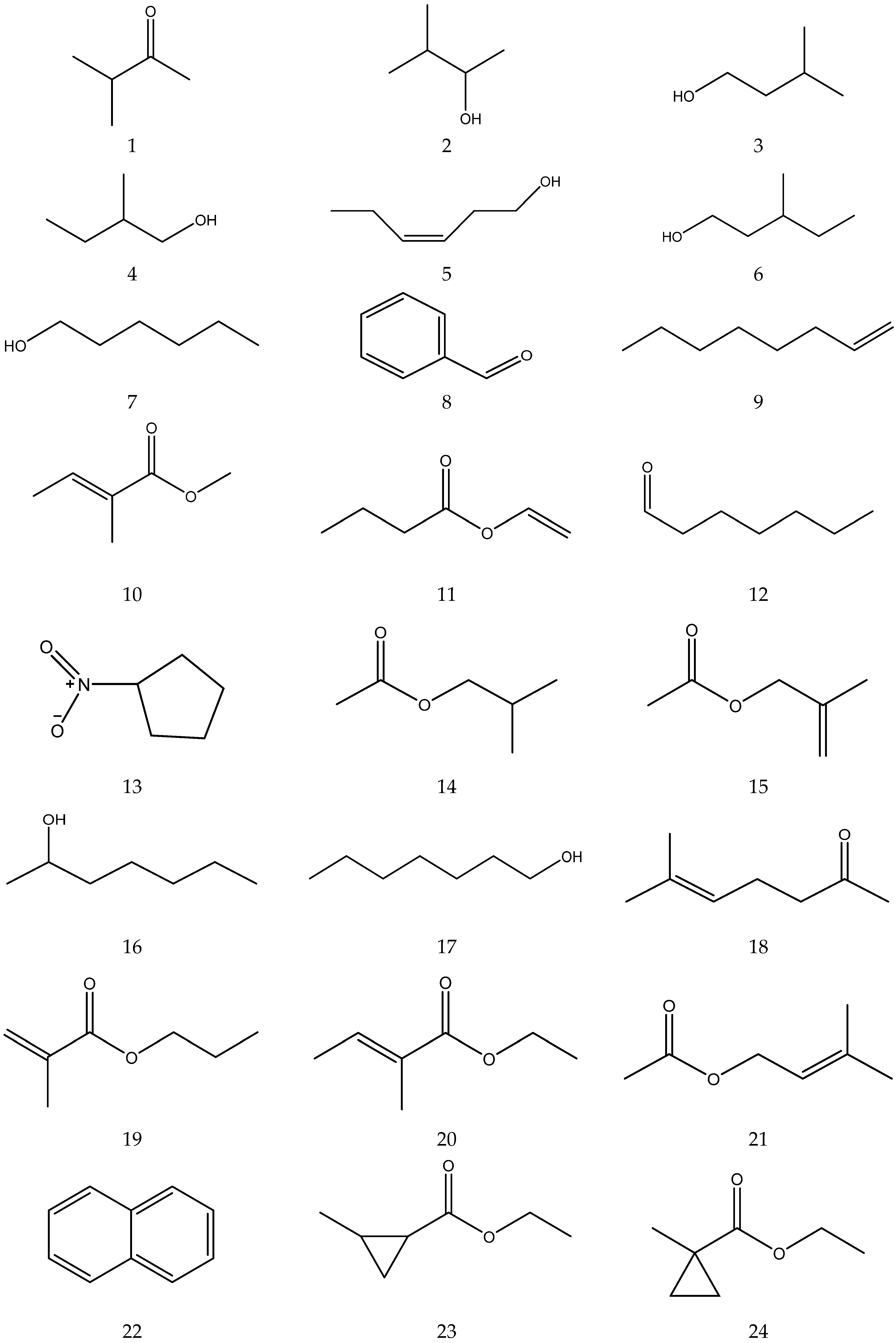
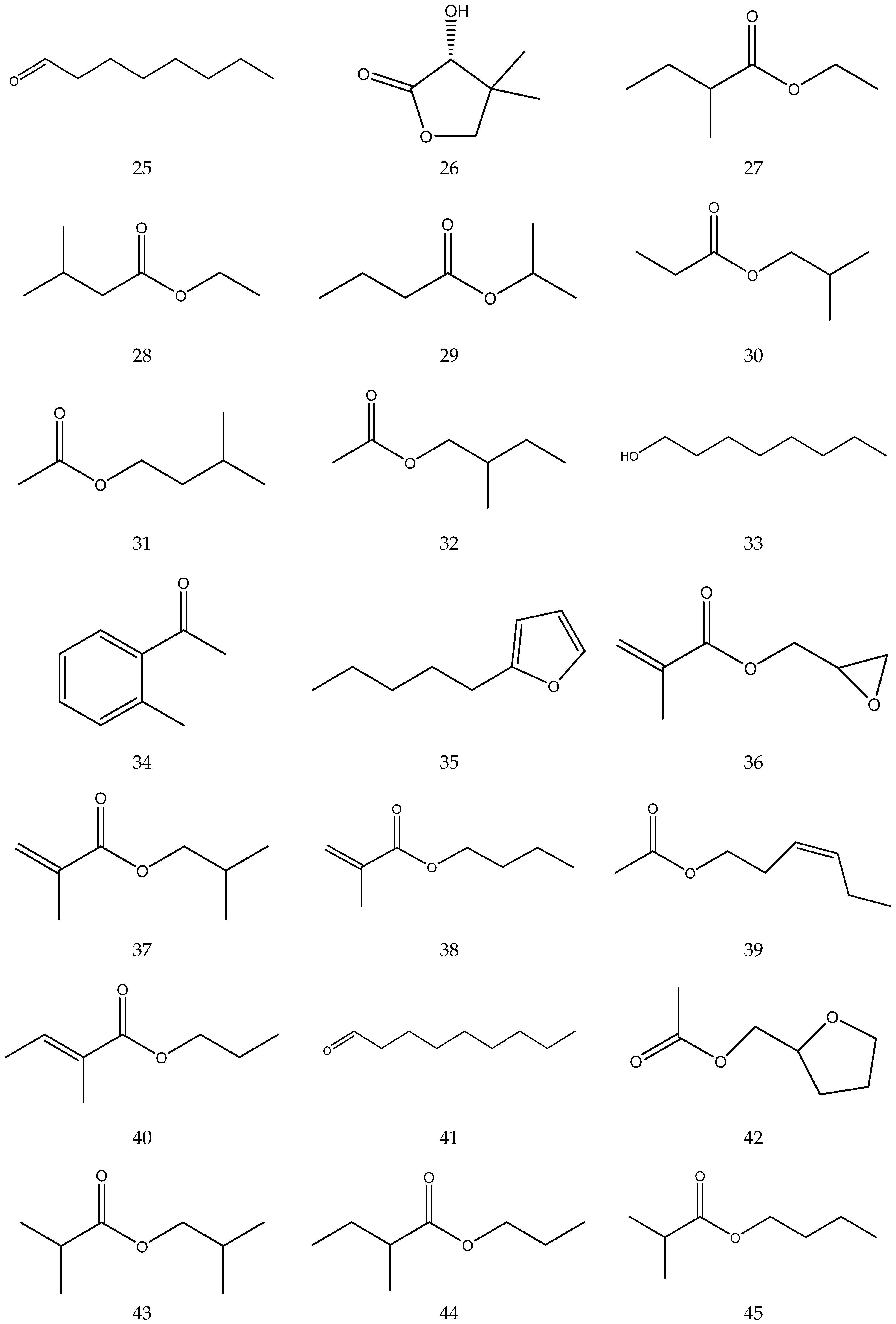
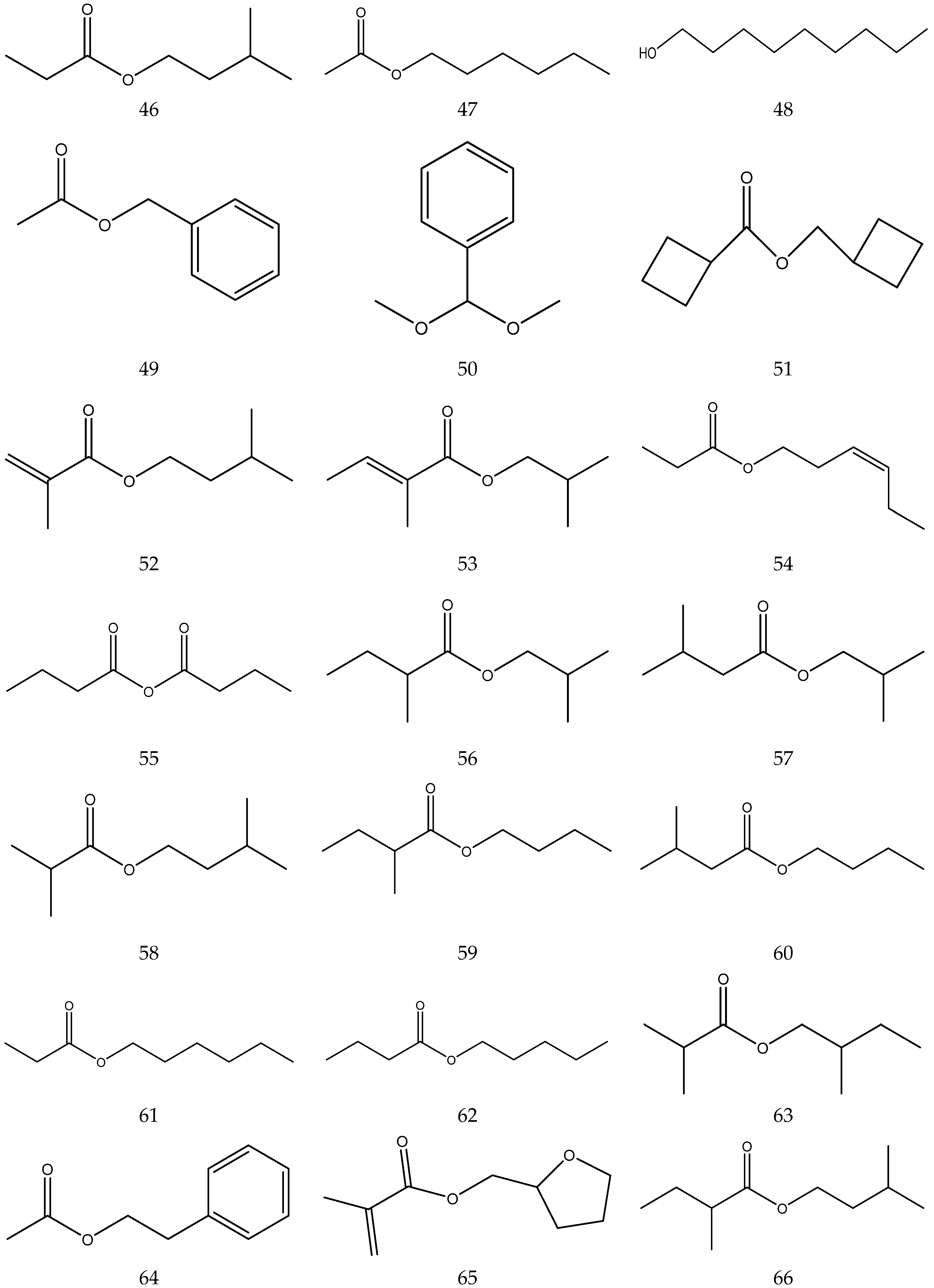
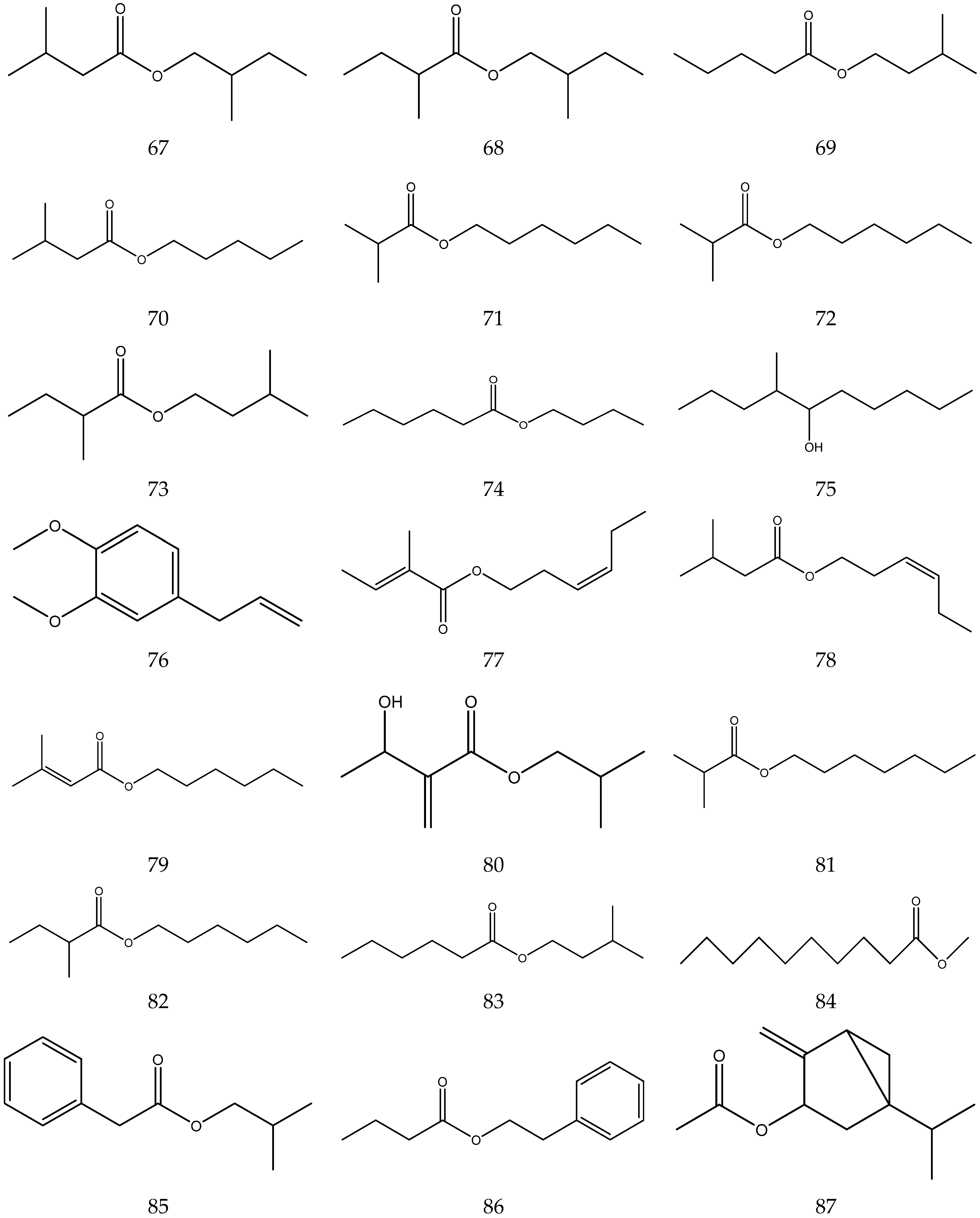
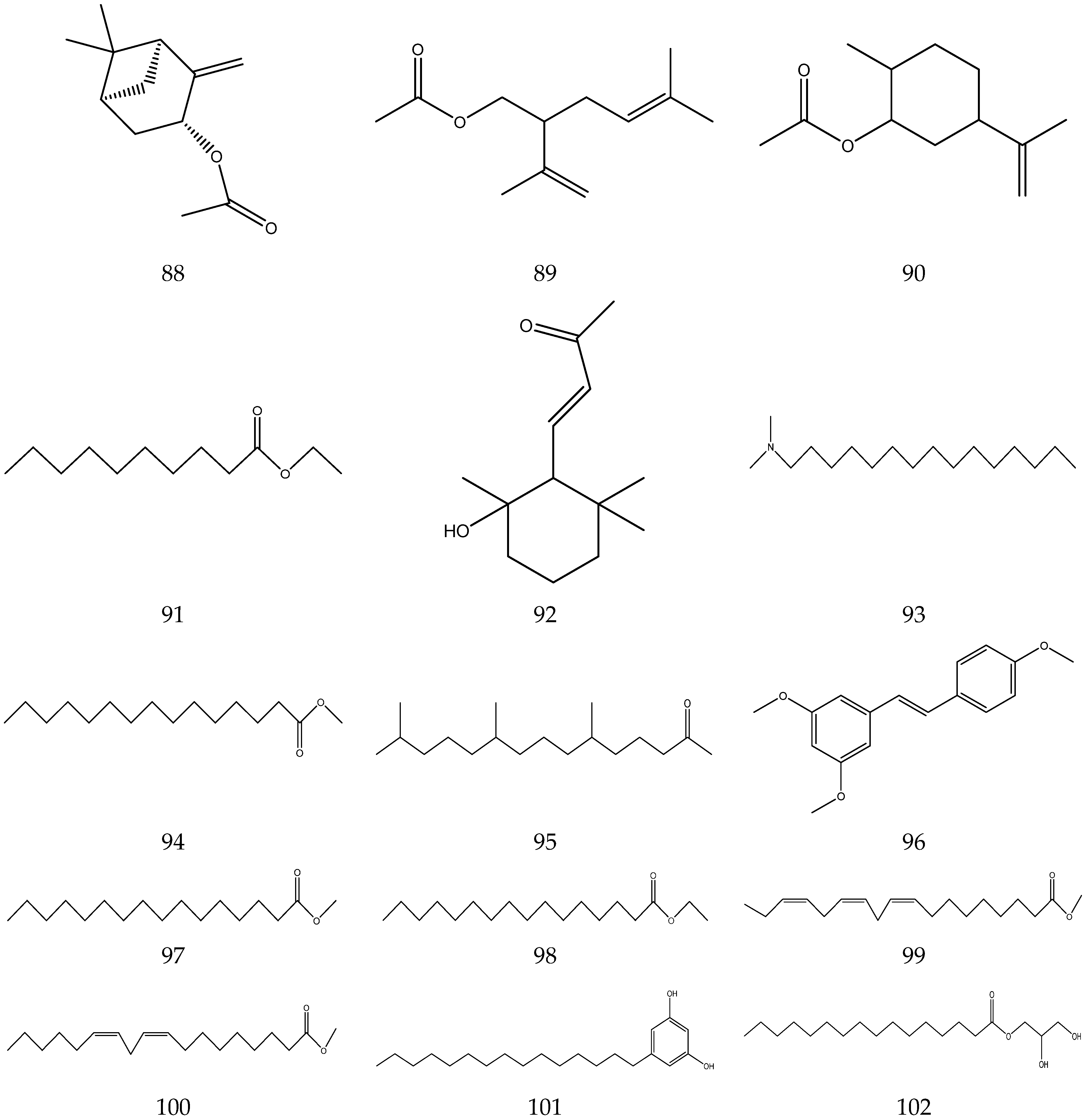
The volatile oil of Chamomile has been prepared using a water distillation method, and its components have been identified using gas chromatography-mass spectrometry (GC-MS). A total of 102 components are reported in the volatile oil [33,34,36,37]. Volatile components, such as isopentyl isobutyrate, isobutyl isobutyrate, and others, have been reported to exhibit sedative and calming effects [45].
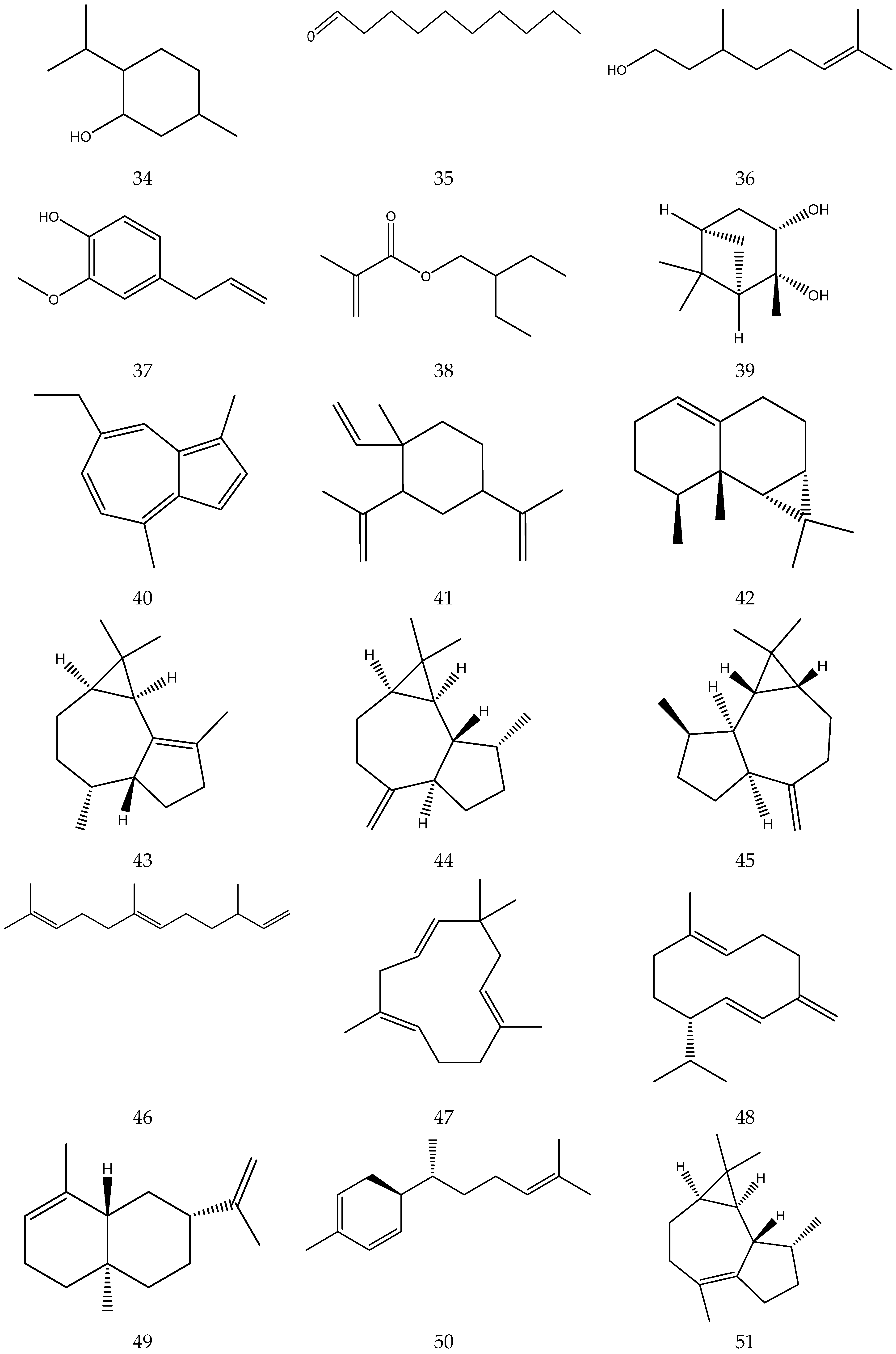
The chemical constituents of volatile oil from chamomile are shown in Table 5, and their chemical structures are shown in Figure 5.

Table 5.
The chemical constituents of volatile oil from Chamomile.
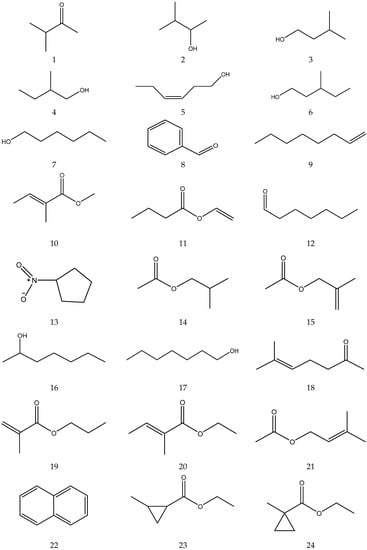
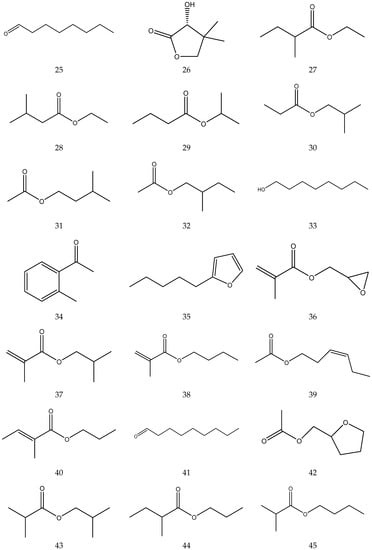
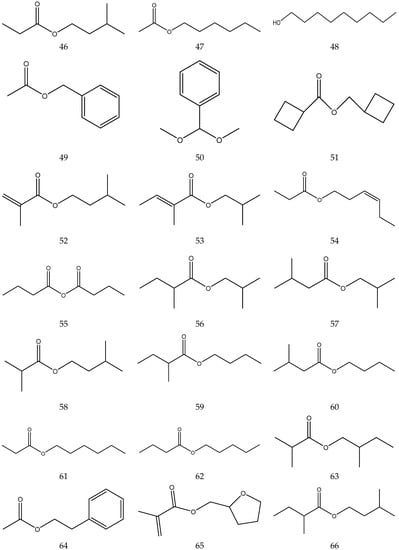
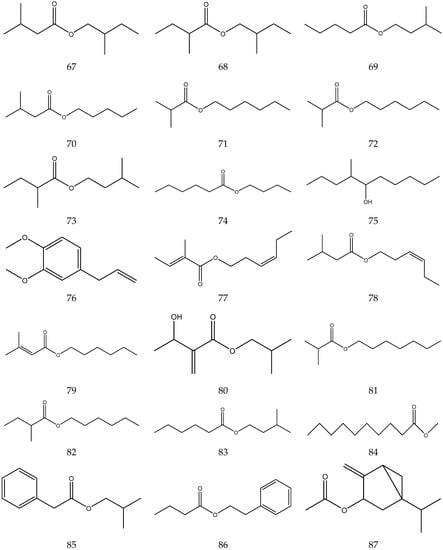
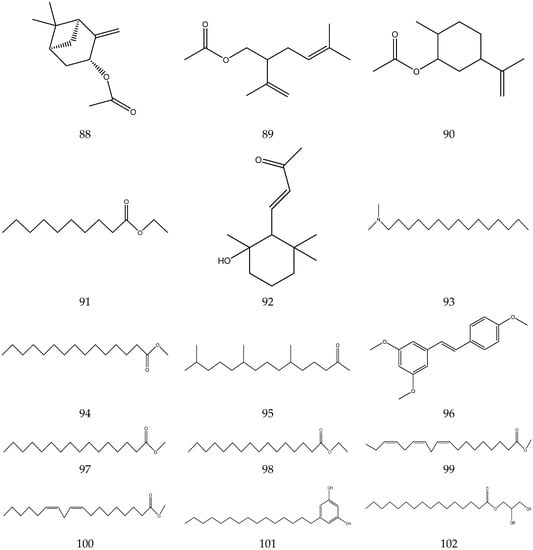
Figure 5.
The chemical structures of volatile oil from Chamomile.
2.3.5. Monoterpenes
Monoterpenes contain two isoprene molecules. Three types of monoterpenes are present in Chamomile. They are (1) chain monoterpenes (e.g., ocimene, geraniol, citronellol) (2) monocyclic monoterpenes (menthol, and others), and (3) bicyclic monoterpenes such as bornanol. A total of 39 monoterpenes have been reported so far [33,34,35,36,37,38].
2.3.6. Sesquiterpenes
Sesquiterpenes are polymerized from three molecules of isoprene. A total of 27 sesquiterpenes have been reported in chamomile so far [33,34,35,36,37,38]. Among them, α-bisabolol has anti-inflammatory activity, thereby protecting against acute liver injury caused by acetaminophen (APAP) [46].
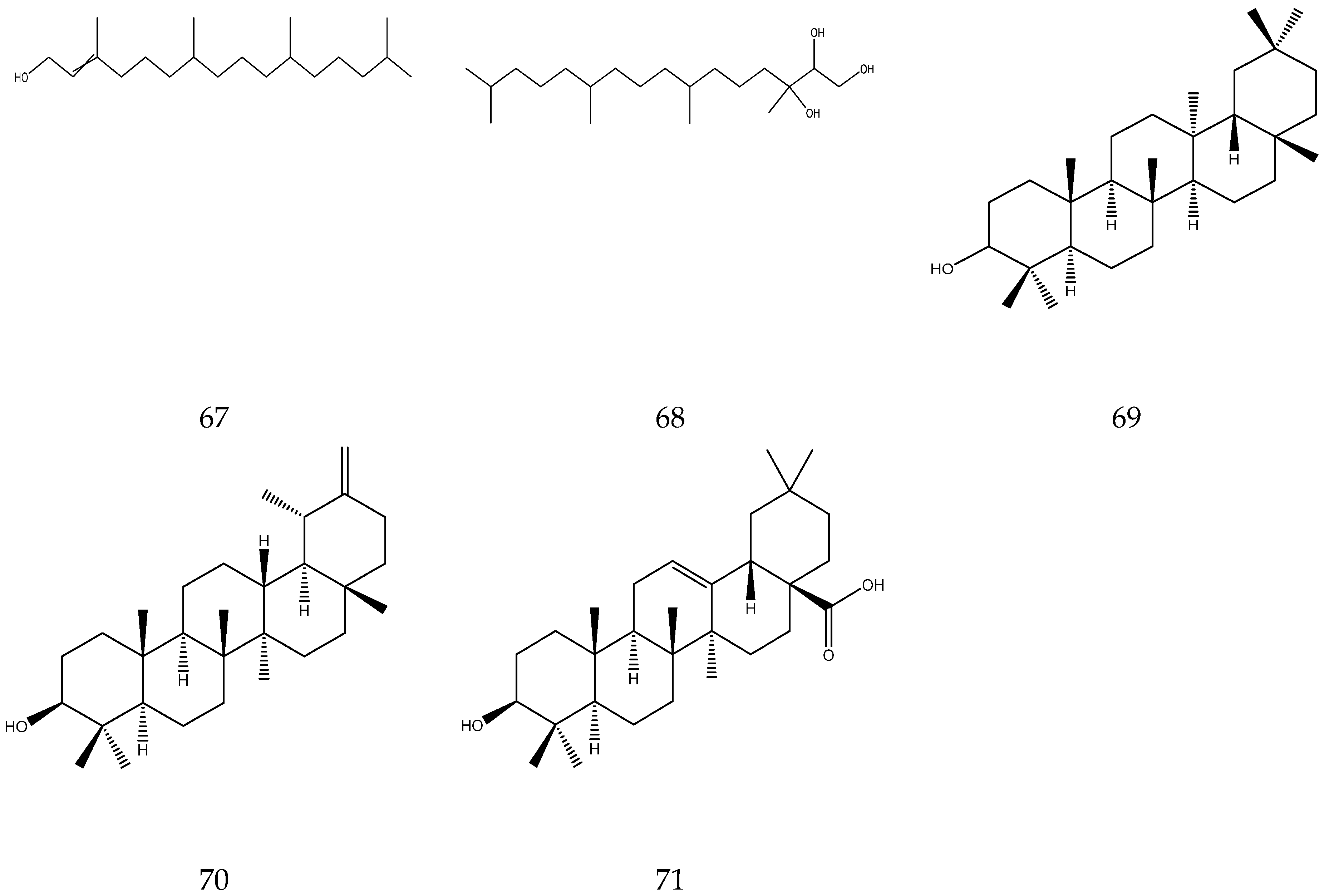
2.3.7. Diterpenes and Triterpenes
Diterpenes contain four isoprene units, whereas triterpenes contain six isoprene units. Up to now, two diterpenes (alcohol and phytanetriol) [33,36] and three triterpenes (oleanolic acid, taraxanol, and taraxasterol) have been reported in chamomile [7].
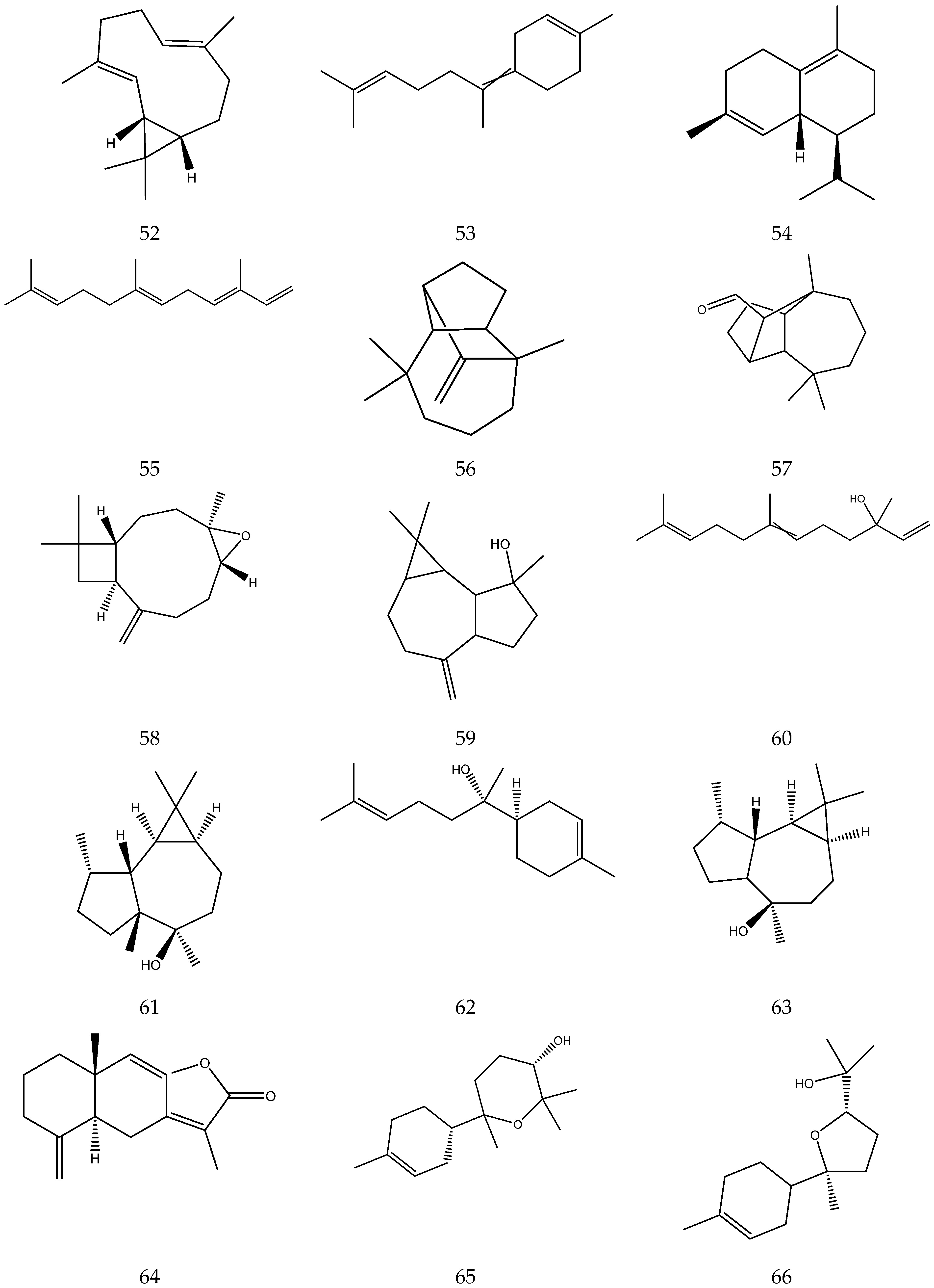
The terpenes (monoterpenes, sesquiterpenes, diterpenes and triterpenes) from chamomile are shown in Table 6, and their chemical structures are shown in Figure 6.

Table 6.
Terpenes from chamomile.
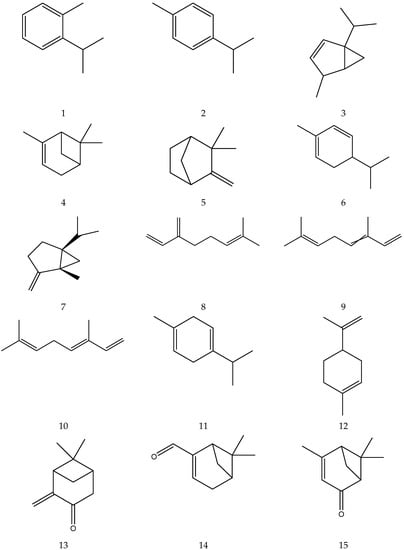
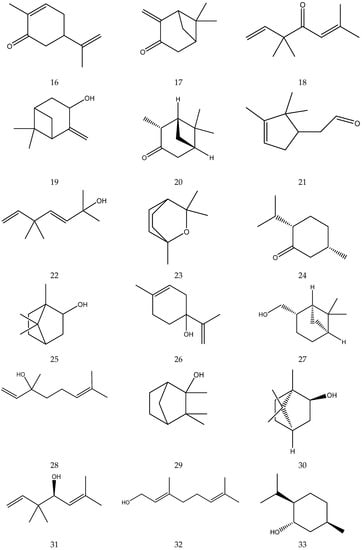
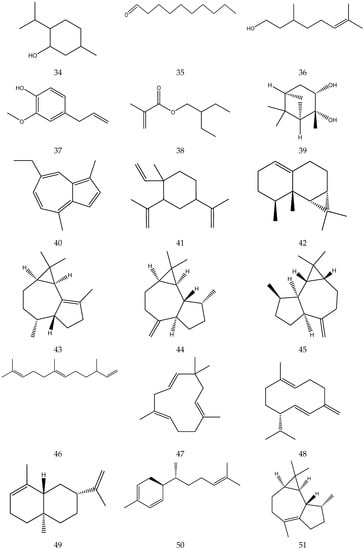
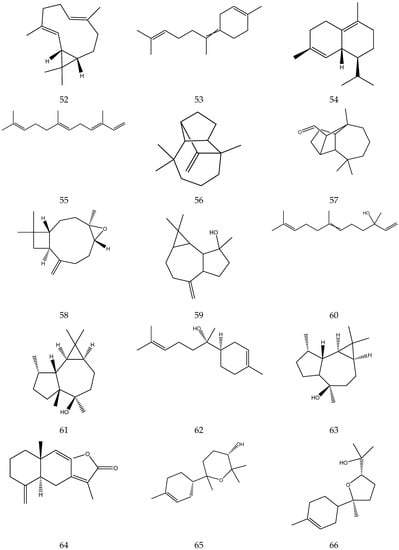
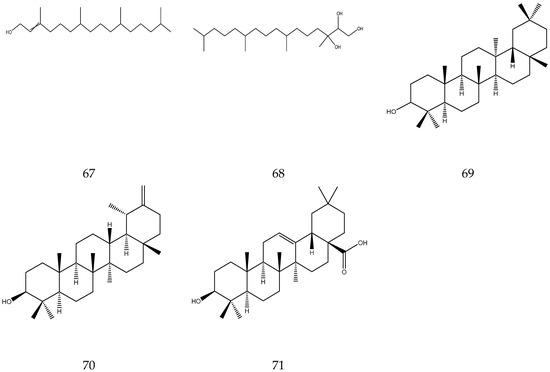
Figure 6.
The chemical structures of terpenes from Chamomile.
2.3.8. Other Phytochemicals
Chamomile contains 1.29–3.25% polysaccharides. Upon hydrolysis they yield 45% D-galacturonic acid, 20.8% D-xylose, 12.2% D-galactose, 10.2% L-arabinose, 5.3% L-rhamnose and 2.3% D-glucose [47]. A total of 16 sterols (e.g., stigmasterol, taraxasterol) and three guaiacolides (guaianolide, matricin and matricarin) have so far been reported by Zhao [7] as shown in Table 7 and Figure 7 below.

Table 7.
Sterols and guaiacolides from Chamomile.
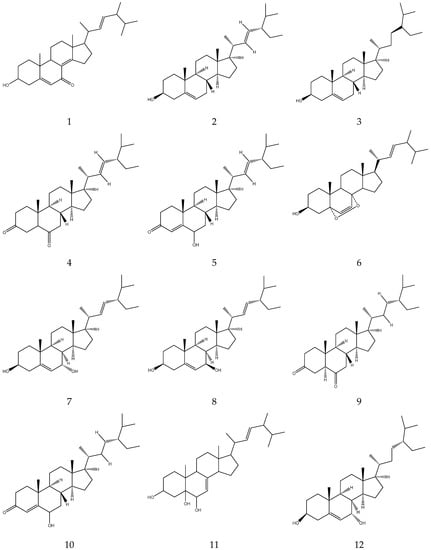
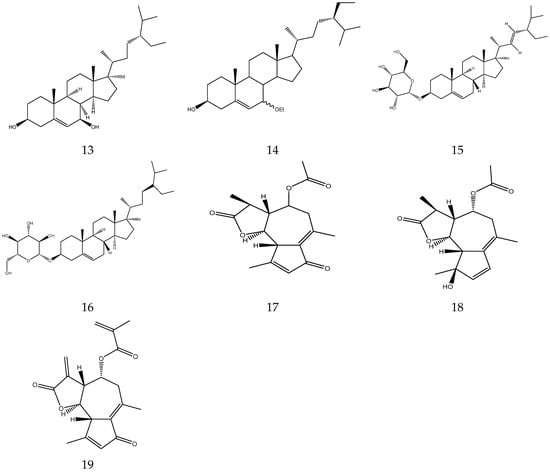
Figure 7.
The chemical structures of sterols and guaiacolides from Chamomile.
L(+)-ascorbic acid, adenosine, phenyl propionic acids, benzodiazepines, γ-aminobutyric acid (GABA), choline, bitter substances, gums, amino acids, and (Z,E)-en-yn-dicycloether [7,33,34], as well as trace elements, such as Ca, Zn, Fe, Mg, Mn, Na, As, also exists in Chamomile [48,49], also exist in Chamomile.
3. Pharmacological Activities
3.1. Anticancer Activity
Glioma is one of the common intracranial malignant tumors with high incidence, rapid growth, high recurrence rate, high mortality and poor prognosis. α-Bisabolol, a fat soluble sesquiterpene compound that is widely found in Chamomile essential oil, has been proven to possess the potential to affect glioma. Yan et al. tested the effect of α-bisabolol on human brain glioblastoma cells (U251 and U87) using the scratch assay. Its effect on migration and invasion was investigated. Protein expression studies have been conducted using Western blot. α-Bisabolol inhibited gliobla stoma cell migration and invasion by down regulating central mucoepidermoid tumor (c-Met) [50]. α-Bisabolol oxide A and apigenin-7-β-D-glucoside, obtained from chamomile flowers and stems, are reported to inhibit the migration of Caco-2 colon cancer cells and deactivate the vascular epidemal growth factor receptor-2 (VEGFR2) angiogenic enzymes [51]. Apigenin is a flavonoid component of this plant, which also shows a certain anticancer effect on the liver cancer cells (Hep G2) and leukaemia cells (HL-60) [52]. Additionally, Srivastava et al. confirmed that apigenin 7-O-glycoside obtained from chamomile extract had a good anticancer effect, but its effect was lower than that of apigenin [53].
In vitro studies confirmed the antiproliferative effect of this plant on cervical cancer cells (HeLa) [54]. The anticancer activity is mediated through the Wnt pathway in colon tissue, down-regulating the expression levels of factors such as wingless integration-5A (Wnt5A), β-catenin, transfer cell factor 4 (Tcf4), and up-regulating the expression levels antigen presenting cell (APC) and GSK-3β [55]. Hydroalcoholic extracts of chamomile (dose-and time-dependent) have been reported to increase apoptosis and necrosis, decrease cell proliferation or migration, colonization, invasion and attachment in Michigan cancer foundation-7 (MCF-7) and MDA-MB-468 cell lines [56]. Chamomile fermented with Lactobacillus plantarum for 72 h showed selective cytotoxicity on cancer cells compared to normal cells (medical research council cell strain 5 (MRC-5)) [57].
3.2. Anti-Infective Activity
Chamomile volatile oil has shown an anti-infective effect on the growth of fungi and bacteria [58]. Furthermore, it effectively reduces the protease in mites and can be used to treat urticaria [59]. Mean corpsular hemoglobin-ampicillin 1 (MCh-AMP1), a natural peptide from the plant, has broad-spectrum antifungal activity against human pathogenic moulds and yeasts. It kills Candida albicans by increasing cell membrane permeability and inducing reactive oxygen species (ROS) production [60]. Shikov et al. reported that the minimun inhibitory concentration 90 (MIC90) and MIC50 of chamomile extract against Helicobacter pylori were 125 and 62.5 mg/mL, respectively. In addition, this extract controls the production of urease via modulating the morphology of H. pylori and fermentation capacity [61]. At present, many mouthwashes and sprays made with chamomile are used for oral bacteriostasis in clinical products, such as the White Gold Medal Compositae essence Product [62].
3.3. Anti-Inflammatory Activity
The flavonoids in chamomile are reported to be responsible for its anti-inflammatory effects. The possible mechanism involves the suppression of nuclear factor kappa beta (NF-κB)-driven transcription [63]. Yuan et al. reported the potent anti-inflammatory activity of Chamomile volatile oil in animal models mediated by inhibiting the production of inflammatory mediators (tumor necrosis factor alpha (TNF-α) and interleukin-1β (IL-1β)) [64]. Because of this activity, chamomile is often used to treat inflammatory diseases such as mammitis, colitis, dermatitis, cystitis, and conjunctivitis [12]. Chamomile Jinshui, an essential oil mainly composed of Chamomile, can effectively relieve prickly heat in children caused by sweat gathering around sweat glands in summer [16].
3.4. Antithrombotic Activity
Cardiovascular diseases (CVD) are one of the leading causes of death worldwide. Chamomile extract exhibits antithrombotic activity by prolonging coagulation and hemostasis time [65]. Luteolin in this plant prevents the development of oxidative stress in adenosine diphosphate (ADP)-induced carotid artery thrombosis in rats [66]. Bijak et al. reported that polyphenol-polysaccharide conjugate obtained from chamomile exerts an antithrombotic effect by reducing platelet aggregation [67]. Bas et al. discovered that half-maximal inhibitory concentrations (IC50) of water and butanol extracts on angiotensin-converting enzyme (ACE) were 1.292 mg/mL and 0.353 mg/mL, respectively [68]. All of the above findings further validate the antithrombotic activity of chamomile.
3.5. Antioxidant Activity
Volatile oil [69], polysaccharides [70,71] and total flavonoids [72,73] of Chamomile have been confirmed to scavenge 1,1-diphenyl-2-picrylhydrazyl (DPPH) and hydroxyl free radicals. The antioxidant effect is dose-dependent [74]. In addition, chamomile ethanol extract increases the activities of superoxide dismutase (SOD) and glutathione peroxidase (GSH-PX) and reduces the malondialdehyde (MDA) content in mice [75]. These findings provide a scientific basis for the antioxidant effect of Chamomile.
3.6. Hypoglycaemic Activity
Chamomile extract reduces fasting blood glucose levels in diabetic mice [75,76] and normal mice and improves glucose tolerance [77]. In addition, the extract antagonizes the effect of exogenous glucose [65]. Yang et al. reported the hypoglycaemic effect of total flavonoids in this plant occurs by reducing fibrinogen (FBG), glycated haemoglobin, glucose tolerance and glycated serum protein (GSP) levels in diabetic mice, and promoting glucose tolerance and insulin secretion [78]. Cemek et al. investigated the hypoglycemic effect of chamomile extract in streptozotocin (STZ)-diabetic rats. The results showed that it protected islet cells and reduced the oxidative stress associated with hyperglycaemia [79].
3.7. Antihypertensive Activity
Studies have found an antihypertensive activity of chamomile extract in essential hypertensive rats [80]. At the same time, another study found that chamomile extract did not affect the systolic blood pressure (SBP) and diastolic blood pressure (DBP) in normotensive rats, suggesting it has no toxic side effects in normal blood pressure regulation [81]. Luo et al. reported that the antihypertensive activity of chamomile extract in N-omega-nitro-L-arginine (L-NNA)-induced hypertensive rats is mediated by reducing angiotension Ⅱ (Ang Ⅱ) content and oxidative stress, and increasing SOD content [82].
3.8. Hypolipidaemic Activity
Hyperlipidaemia (HLP) refers to a metabolic disorder syndrome in which lipid components in plasma are abnormally dysregulated (increased serum total cholesterol (TC), triglyceride (TG), and low-density lipoprotein cholesterol (LDL-C), with decreased high-density lipoprotein cholesterol (HDL-C)). Chamomile is an effective blood lipid-lowering herb. Lan et al. reported that a chamomile alcohol extract played a role in lowering lipids, reducing TC, TG and LDL-C values and elevating HDL-C values in the blood of experimental hyperlipidemic rats [83]. A compound in fuzhuan tea, a popular compound tea containing chamomile with a mellow taste and no toxic side effects, can also treat HLP by regulating the above various sterol indicators [84].
3.9. Antiallergy Activity
As a conventional medicine, chamomile is frequently used to relieve various allergic symptoms. Antiallergic tea, as an example, has positive anti-allergy activity and cosmetology when it is drunk for a long time [21]. The antiallergic activity of chamomile aqueous extract (1.0 mg/mL) was reported by measuring the β-hexosaminidase (β-Hex) release in rat basophil leukemia (RBL-2H3) cells. This was suggested to inhibit the β-Hex release by 21.42% [85]. A chamomile methanol extract could restrain compound 48/80-induced allergic reactions. The effect was dose-dependent and mediated via decreased histamine release and NO levels from mast cells [86].
3.10. Antidepressant Activity
Essential oil aromatherapy is considered an alternative treatment for depression. Chamomile is an excellent reliever when patients with depression have physical and psychological discomfort [8]. Chamomile tea made from chamomile flower heads can effectively relieve depressive symptoms and the sleep status of postpartum women, which provides a new idea for treatment of depression [87]. Some pharmacological experiments have suggested the antidepressant activity of Chamomile [88]. For example, α-pinene contained in this plant elevated protein expression related to oxidative phosphorylation and mRNA expression of parvalbumin in rat brain, as determined by isobaric tag for relative and absolute quantitation (iTRAQ) and polymerase chain reaction (PCR) analysis [89].
3.11. Organoprotective Effect
Chamomile has protective effects on organs such as the liver, lung, kidney, and stomach, among others.
3.11.1. Hepatoprotective and Pulmoprotective Effect
Chamomile flavonoids ameliorated mouse liver injury by restoring biochemical and molecular parameters in 1,2-Dimethyl hydrazine (DMH)-induced mice [90]. Zhang et al. ascertained that apigenin in this plant could treat APAP-induced liver injury by activating the adenosine monophosphate-activated protein kinase/GSK-3β (AMPK/GSK-3β) signaling pathway, promoting carnitine palmitoyl transferase 1A (CPT1A) activity and activating the nuclear factor erythroid 2-related factor 2 (Nrf2) antioxidant pathway [91]. Additionally, chamomile increases the total antioxidant capacity (TAC) and tissue transglutaminase (tTG) content in liver tissue, protecting against oxidative liver injury from paraquat (PQ) poisoning [92]. On the other hand, its extract protects oxidative lung damage from PQ poisoning, mainly by improving lipid peroxidation (LPO), SOD, glutathione peroxidase (GPx), and increasing the transporter associated with antigen processing (TAP) in plasma and lung tissue [93].
3.11.2. Nephroprotective Effect
According to records in the “Baidu Yi Medicine Book”, chamomile is able to resolve the threat posed by kidney stones [7]. Modern pharmacological studies confirm this plant is an alternative therapy for gastric protection. For example, Salama et al. evaluated the protective effect of chamomile against cisplatin nephrotoxicity by intraperitoneal injection in rats. The study demonstrated that it reduces oxidative stress markers, corrects hypocalcemia caused by cisplatin nephrotoxicity, and inhibits glutamyltransferase activity [94]. In addition, its extract also inhibits phenomena such as glomerular fibrosis, improves renal tissue structure, and protects against renal tissue damage resulting from hypertension [82].
3.11.3. Gastroprotective Effect
Chamomile is a promising gastroprotective herb to deal with stomach spasm, flatulence, stomachache, and decreased gastric secretion [95]. Its extract has shown antiulcer and antioxidant effects in ethanol-induced gastric mucosal injury in rats. Gastroprotective effects are mediated by reducing MDA levels, increasing GSH levels [96], protecting gastric sulfhydryl groups and the opposing effects of intracellular mediators such as free iron, hydrogen peroxide, and calcium [97].
3.12. Genitoprotective Effect
Chamomile extract improves reproductive function in polycystic ovary syndrome (PCOS) rats. Chamomile reduced the uterine and insulin resistance index, regulated sex hormones, leptin and blood lipids, and decreased inflammatory cells [77]. In addition, the interaction of chamomile with the GABA system can regulate luteinizing hormone (LH) secretion and increase dominant follicles for improving reproductive function in rats [98]. Soltani et al. performed surgical experiments on rats and treated the experimental group with chamomile extract. Histological characterization showed that the extract protected the testicular tissue from torsion/detorsion-induced damage by reducing MDA levels and inhibiting superoxide production [99]. Afrigan et al. injected formaldehyde and chamomile extract intraperitoneally into male Wistar rats to probe the hormonal status and sperm parameters of testicular tissue. It was found that this extract reduced the adverse effects of formaldehyde on the reproductive system in male rats [100].
3.13. Neuroprotective Effect
Chamomile has an excellent neuroprotective effect. Its extract restores scopolamine-decreased brain-derived neurotrophic factor (BDNF) expression, increases IL1β, and modulates cholinergic activity in the rat hippocampus [101]. It has been reported that a chamomile ethanol extract improves formaldehyde-induced memory impairment by reducing cell death and MDA content in the hippocampus, and increasing total antioxidant capacity [102]. Furthermore, Lim et al. found that apigenin in this plant inhibits H2O2-induced hippocampal cell (HT22) death [103]. Khan et al. reported the anti-Parkinson activity of chamomile extract by establishing an experimental animal model with chlorpromazine (CPZ). The extract showed vascular proliferation and increased the number of reactive glial cells [104].
3.14. Analgesic Activity
As early as hundreds of years ago, Chamomile was used as an analgesic remedy to relieve a variety of pains, such as arthralgia, stomach cramps, and neuralgia [13]. Nowadays, chamomile oil gel has been authenticated as an analgesic by reducing migraine pain without aura [105]. Saghafi et al. also reported the breast pain-relieving effect of chamomile (treated for 8 weeks) in patients using a visual analogue scale (VAS) and a breast pain scale (BPC) [106].
3.15. Antidiarrheal and Antispasmodic Activity
Chamomile is widely used in traditional Tunisian medicine and TCM against diarrhea and spasticity. In Germany, the extract of this plant is effective in treating children’s acute diarrhea by reducing symptoms and shortening the duration of disease [107]. Mehmood et al. reported the antidiarrheal and antispasmodic effects of chamomile using isolated rabbit jejunum. The chamomile extract activates K+ channels and reduces Ca2+ antagonism [108]. Hichem Sebai reported the beneficial effects of the extract in castor oil-induced diarrhea, which decreased MDA levels and antioxidant enzyme activity [109]. In addition, apigenin and apiin in Chamomile have a strong antispasmodic effect on smooth muscle [38].
3.16. Cosmetic Activity
Chamomile is useful in repairing sensitive skin, eliminating acne, and improving skin dehydration. It whitens the skin by inhibiting tyrosinase activity [110]. Therefore, it can be used as an ingredient in skincare products [38]. Ointments, creams and lotions containing chamomile active ingredients are used for the treatment of various skin infections and rashes in Europe and other places [111]. Chamomile Natural Milk Hand Soap, is widely popular for decontamination and sterilization, and can effectively moisturize the skin, enhance elasticity, and calm broken micro-blood vessels [112].
3.17. Other Activities
Chamomile alleviates muscle atrophy by suppressing muscle ring finger-1 (MuRF1) and increasing mitochondrial transcription factor A (TFAM), MyoD and myogenin-1 genes [113]. It relieves stiffness and pain in people suffering from knee osteoarthritis [114]. It also relieves general anxiety [115,116], with efficacy equivalent to conventional anti-anxiety drugs [117]. Its n-butanol extract showed a beneficial effect in relieving asthma in mice by reducing the eosinophils (EOS) and MDA levels and increasing IL-2, IL-10, IL-12 and SOD levels [118]. Studies report that Chamomile accelerates the healing of skin wounds by promoting fibroblast proliferation and migration [119]. Wan et al. indicated that apigenin in this plant inhibits transforming growth factor β1 (TGF-β1)-stimulated cardiac fibroblasts (CFs) differentiation and extracellular matrix (ECM) production by reducing microRNA-155-5p (miR-155-5p) expression, increasing c-Ski expression, and lowering Smad2/3 and p-Smad2/3 expression [120]. Studies report that chamomile extract abrogates withdrawal behavioural manifestations in morphine-dependent rats [121].
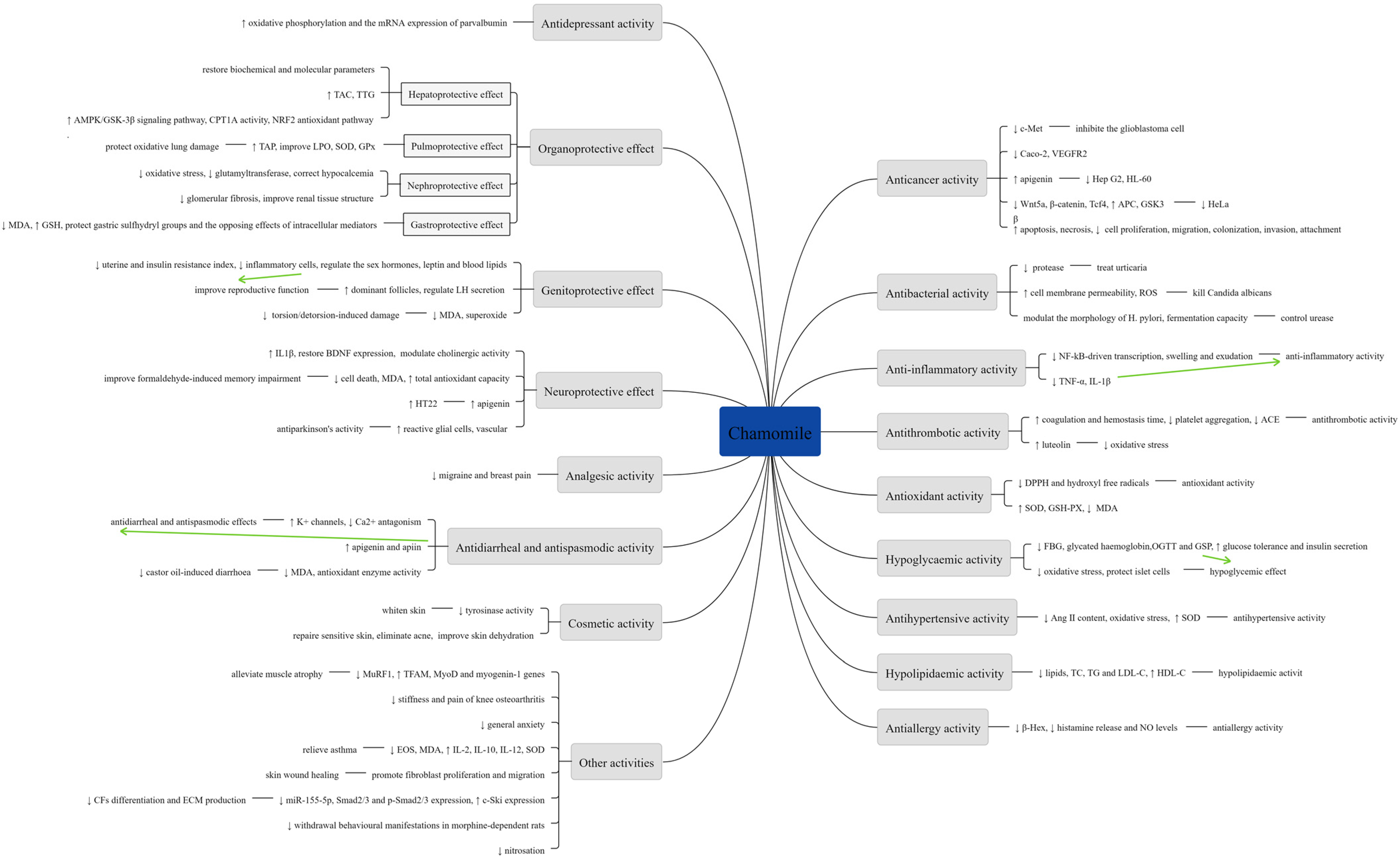
A summary of various pharmacological activities of Chamomile are shown in Figure 8.
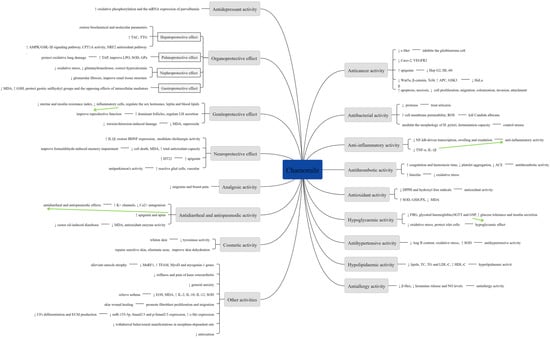
Figure 8.
Summary of various pharmacological activities of Chamomile.
4. Other Aspects
4.1. Adverse Reactions
According to records, the interaction between chamomile and some plants or drugs may cause adverse reactions. In theory, a combination of medicines that affect platelet aggregation may interfere with the effect of coagulation, thus increasing the risk of bleeding. Furthermore, high doses of Chamomile pose a teratogenic risk, affect the menstrual cycle, and cause vomiting [122]. Although this plant has antiallergic activity, a few studies have reported allergic reactions such as contact dermatitis and hypersensitivity, especially in people who are allergic to pollen or compositae [123,124]. There is a possibility to reduce follicular function and development, leading to premature birth in pregnant women when using chamomile [125,126].
4.2. Toxicity Studies
There have been reports of chamomile as a potential carrier of Clostridium botulinum spores [127]. Kalantari et al. reported the genotoxicity of Chamomile in mice using ultra-viable micronucleus assay of reticulocytes [128]. However, Wang et al. reported that the aqueous and alcohol extracts of this plant are safe for mice. It has been reported that the maximum tolerated dose of aqueous and alcohol extracts are 535 and 425 times higher than the usual adult dose in a clinic [129]. So far, only a few studies have been carried out to evaluate its toxicity, and more studies are required to confirm the safety of consuming chamomile.
4.3. Clinical Preparation
Chamomile has long been known as a medicinal and aromatic plant in Europe, the United States, Japan and other countries. At present, due to its multiple medicinal effects, many chamomile-containing herbal medicinal products have been developed, such as chamomile ointment, cream and lotion [111]. However, there are relatively few preparations in clinical products. Clinical preparations of this plant are mainly used in clinical research in the form of a mouth rinse [130,131]. For example, the White Gold Medal Compositae essence Gargle, which was listed in Japan in 2002, is able to be applied to oral bacteriostasis, clean care, and relieve oral discomfort [62]. In addition, three kinds of chamomile preparations can be retrieved from the database of Chinese patent medicine prescriptions “https://db.yaozh.com/chufang (accessed on 17 November 2022)”, including Fufang Munizi granules, Zukamu capsules and Zukamu granules [4]. The U.S. National Drug Code also lists more than two hundred kinds of preparations containing this plant, such as Allergies (pellet), SleepCalm (spray), OHM Fever Relief (spray), and Melatonin Pro (tablet) [6]
4.4. Quality Control Studies
High Performance Liquid Chromatography (HPLC) is a standard method for identifying and quantifying the components in herbs to assess their quality. Furthermore, evaluating chamomile quality is crucial to reproducing its clinical efficacy. You et al. reported the HPLC method for the simultaneous determination of luteolin-7-O-β-D glucoside and apigenin 7-O-β-D glucoside in chamomile extract and displayed the linear range of luteolin-7-O-β-D glucoside and apigenin-7-O-β-D glucoside was 5.11~409.00 and 12.56~1005.00 μg/mL, respectively [132]. Lan et al. reported the UPLC method to determine the content of apigenin-7-glucoside in chamomile, and the content was found to be 5.2 and 5.8 mg/g, in two batches [133]. Wu et al. adopted this method to quantify α-bisabolol, and the concentration of α-bisabolol in MC was 0.045 mg/mL, whereas it was not detected in CN [134]. Gas chromatography also plays a vital role in the quality control of pharmaceuticals. Zhao et al. reported a gas chromatographic method to determine the volatile oil components in this plant. α-Bisabolol, α-bisabolol oxide A and α-bisabolol oxide B were present in volatile oil of MC, whereas CN volatile oil contained only α-bisabolol oxide A. Therefore, the comprehensive quality evaluation of MC and CN could be qualitatively and quantitatively based on the content of bisabolol or the presence or absence of α-bisabolol oxide B [95].
Total saponins content in Chamomile was determined by utlraviolet (UV) spectrophotometry, and the detected content was 8.02% [135]. Jing et al. used a flame atomic absorption spectrometric method to measure the content of trace elements (Ca, Zn, Fe, Mg, Mn, and Na). The contents of Ca, Zn, Fe, Mg, Mn and Na were 2.016, 0.032, 0.684, 2.380, 0.044 and 1.235 mg/g, respectively [48]. In addition, Xin et al. reported the presence of arsenic in this plant using atomic fluorescence spectroscopy [49]. A thin-layer chromatography method can be used to identify luteolin in chamomile [136]. QAMS is a research tool for multi-component quality control, which is used to solve the bottleneck problem when the control substance is scarce and expensive in the qualitative evaluation of herbal medicine [137]. Li et al. utilized QAMS to determine cynaroside, apigenin-7-glucoside, 7-methoxycoumarin, luteolin and apigenin in chamomile. There was no significant difference in the contents calculated using either this method or an external standard method [44]. Han et al. reported a method in which polyamide was used as a stationary phase and microemulsion as a developing agent to identify above five compounds. This method is better than thin layer chromatography using silica gel [138].
5. Discussion and Conclusions
A lot of research has been carried out on chamomile in recent years. This article reviews the latest research progress on this plant, including botanical characteristics, traditional uses, chemical constituents, pharmacological effects, and quality evaluation. A total of 301 compounds have been reported in Chamomile, including 26 organic acids, 50 flavonoids, 10 coumarins, 102 volatile oil constituents, 39 monoterpenes, 27 sesquiterpenes, 2 diterpenes, 3 triterpenes, 16 sterols, 6 polysaccharides, 3 guaiacolides, 7 trace elements and 10 other components. Flavonoids represented by apigenin have significant anti-inflammatory effects. Esters in volatile oils have sedative and anxiolytic effects. α-Bisabolol in sesquiterpenes can protect against APAP-induced acute liver injury. Chamomile also plays anticancer, anti-infective, antioxidant, hypoglycaemic, hypotensive, hypolipidaemic, antiallergic, antidepressant, organ protective, genitoprotective, and neuroprotective effects. In addition, chamomile is used as an ingredient in skin whitening products and relieves muscle atrophy, speeds up skin wound healing, and treats asthma. There are many scientific studies on it, but there are still numerous research gaps. For example, more toxicity tests should be carried out to confirm the safe use of chamomile. Its clinical effects depend on chemical composition and the amount of active components. HPLC is an effective analytical method for identifying and quantifying components in herbs. The content of luteolin-7-O-glucoside, apigenin-7-O-glucoside, chlorogenic acid, apigenin, apigenin-7-glucose, α-bisabolol and other substances have been quantified using various HPLC methods. In addition, volatile components such as α-bisabolol and α-bisabolol oxide B have been identified and quantified using gas chromatography. This method is useful to differentiate MC and CN.
In summary, chamomile is a widely used herb in the traditional medicine of Greece, Rome, China, Germany and other countries. It has great economic value due to its numerous pharmacological effects and wide uses. This paper summarizes its various aspects systematically. The information in this review will be a good scholarly resource for its further development and utilization. However, there is a need for further research to provide concrete scientific evidence and validate the medicinal uses of chamomile.
Author Contributions
Y.-L.D., Y.L. and Q.W. collected the literature, drew structures and wrote the manuscript; F.-J.N., K.-W.L., Y.-Y.W. and J.W. checked the tables and figures; C.-Z.Z. and L.-N.G. revised the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This review received no external funding.
Acknowledgments
We would like to express our gratitude to the Shandong University of Traditional Chinese Medicine, which provided us with precious, ancient books to complete the section “Traditional Uses”.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
| Abbreviations | Full name |
| TCM | Traditional Chinese medicine |
| MC | Matricaria chamomilia L. |
| CN | Chamaemelum nobile (L.) All. |
| 4-MUO | 4-Methylumbelliferone oleate |
| LPS | lipopolysaccharide |
| BV2 | a microglia |
| GSK-3β/Nrf2 | Glycogen Synthase Kinase 3β/Nuclear factor E2 related factor 2 |
| QAMS | quantitative analysis for multi-components by single marker |
| GC-MS | gas chromatography-mass spectrometry |
| APAP | acetaminophen |
| MyoD | myogenic differentiation |
| GABA | γ-aminobutyric acid |
| c-Met | central mucoepidermoid tumor |
| Caco-2 | a colon cancer cell |
| VEGFR2 | vascular epidemal growth factor receptor-2 |
| Hep G2 | a liver cancer cell |
| HL-60 | a leukaemia cell |
| HeLa | a cervical cancer cell |
| Wnt5A | wingless integration-5A |
| Tcf4 | transfer cell factor 4 |
| APC | antigen presenting cell |
| MCF-7 | michigan cancer foundation-7 |
| MDA-MB-468 | a triple-negative breast cancer cell line |
| MRC-5 | medical research council cell strain 5 |
| MCh-AMP1 | Mean corpsular hemoglobin—ampicillin 1 |
| ROS | reactive oxygen species |
| MIC90 | minimun inhibitory concentration 90 |
| NF-κB | nuclear factor kappa beta |
| TNF-α | tumor necrosis factor alpha |
| IL-1β | interleukin-1β |
| CVD | cardiovascular disease |
| ADP | adenosine diphosphate |
| IC50 | half-maximal inhibitory concentrations |
| ACE | angiotensin-converting enzyme |
| DPPH | the 1,1-diphenyl-2-picrylhydrazyl |
| SOD | superoxide dismutase |
| GSH-PX | glutathione peroxidase |
| MDA | malondialdehyde |
| FBG | fibrinogen |
| GSP | glycated serum protein |
| STZ | streptozotocin |
| SBP | systolic blood pressure |
| DBP | diastolic blood pressure |
| L-NNA | N-omega-nitro-L-arginine |
| Ang Ⅱ | angiotension Ⅱ |
| HLP | hyperlipidaemia |
| TC | serum total cholesterol |
| TG | triglyceride |
| LDL-C | low-density lipoprotein cholesterol |
| HDL-C | high-density lipoprotein cholesterol |
| β-Hex | β-hexosaminidase |
| RBL-2H3 | rat basophil leukemia |
| iTRAQ | isobaric tag for relative and absolute quantitation |
| PCR | polymerase chain reaction |
| DMH | 1,2-Dimethyl hydrazine |
| TAC | total antioxidant capacity |
| tTG | tissue transglutaminase |
| PQ | paraquat |
| AMPK/GSK-3β | adenosine monophosphate-activated protein kinase/glycogen synthase kinase 3β |
| CPT1A | carnitine palmitoyl transferase 1A |
| Nrf2 | nuclear factor erythroid 2-related factor 2 |
| LPO | lipid peroxidation |
| GPx | glutathione peroxidase |
| TAP | transporter associated with antigen processing |
| PCOS | polycystic ovary syndrome |
| LH | luteinizing hormone |
| BDNF | brain-derived neurotrophic factor |
| HT22 | a hippocampal cell |
| CPZ | chlorpromazine |
| VAS | visual analogue scale |
| BPC | breast pain scale |
| MuRF1 | muscle ring finger-1 |
| TFAM | mitochondrial transcription factor A |
| EOS | eosinophils |
| TGF-β1 | transforming growth factor β1 |
| CFs | cardiac fibroblasts |
| ECM | extracellular matrix |
| miR-155-5p | microRNA-155-5p |
| HPLC | High Performance Liquid Chromatography |
| UV | Utlraviolet |
References
- Tian, L.J.; Huang, T.K. Research on the development history of traditional Chinese medicine. Chin. Arch. Tradit. Chin. Med. 2007, 4, 753–755. [Google Scholar] [CrossRef]
- Zhou, P. Traditional Chinese medicine. Comb. Chem. High Throughput Screen 2010, 13, 836. [Google Scholar] [CrossRef] [PubMed]
- Ubessi, C.; Tedesco, S.B.; de Bona da Silva, C.; Baldoni, M.; Krysczun, D.K.; Heinzmann, B.M.; Rosa, I.A.; Mori, N.C. Antiproliferative potential and phenolic compounds of infusions and essential oil of chamomile cultivated with homeopathy. J. Ethnopharmacol. 2019, 239, 111907. [Google Scholar] [CrossRef]
- Wan, W.T.; Song, Y.J.; Xu, L.J.; Xiao, P.G.; Miao, J.H. Research Review and Application Prospect Analysis of Matricaria. Mod. Chin. Med. 2019, 21, 260–265. [Google Scholar] [CrossRef]
- Singh, O.; Khanam, Z.; Misra, N.; Srivastava, M.K. Chamomile (Matricaria chamomilla L.): An overview. Pharm. Rev. 2011, 5, 82–95. [Google Scholar] [CrossRef] [PubMed]
- Zhao, D.S.; Han, S.L.; Yi, Y.J.; Qiu, L.; Ren, L.J.; Li, X.X. Standard operating procedure for standardized planting of local medicinal herb German chamomile. J. Anhui Agric. Sci. 2015, 43, 70+85. [Google Scholar] [CrossRef]
- Zhao, Y.F. Study on Chemical Composition and Quality Standard of Uyghur Chamomile. Master’s Thesis, China Academy of Chinese Medical Sciences, Beijing, China, 2018. [Google Scholar]
- Fen, M.Y. Deciphering Chamomile Essential Oil. Chin. Cosmet. 2021, 12, 120–122. [Google Scholar]
- Petronilho, S.; Maraschin, M.; Delgadillo, I.; Coimbra, M.A.; Rocha, S.M. Sesquiterpenic composition of the inflorescences of Brazilian chamomile (Matricaria recutita L.): Impact of the agricultural practices. Ind. Crops Prod. 2011, 34, 1482–1490. [Google Scholar] [CrossRef]
- Liu, X.M.; Meng, X.X.; Zhang, W.W.; Liao, Y.L.; Chang, J.; Xu, F. Tissue Culture Technique of Chamomile. North. Hortic. 2018, 2, 72–76. [Google Scholar] [CrossRef]
- Ren, L.J.; Yuan, J.; Han, S.L.; Li, X.X.; Yao, J. Digital Microscopic Identification of Two Kinds of Chamomile Medicinal Materials in Xinjiang and Preliminary Study on Pharmacology. J. Xinjiang Med. Univ. 2016, 39, 724–726. [Google Scholar]
- Wang, Y.; Zhou, C.; Wu, Q.; Wang, Y.H. An Analysis of the Medicines for Treating Heart Diseases in Shennong’s Herbal Classic. J. Tradit. Chin. Med. Lit. 2021, 39, 26–30. [Google Scholar] [CrossRef]
- Srivastava, J.K.; Shankar, E.; Gupta, S. Chamomile: A herbal medicine of the past with bright future. Mol. Med. Rep. 2010, 3, 895–901. [Google Scholar] [CrossRef] [PubMed]
- Mazokopakis, E.E.; Vrentzos, G.E.; Papadakis, J.A.; Babalis, D.E.; Ganotakis, E.S. Wild chamomile (Matricaria recutita L.) mouthwashes in methotrexate-induced oral mucositis. Phytomedicine. 2005, 12, 25–27. [Google Scholar] [CrossRef] [PubMed]
- Xiao, W.H.; Yang, C.H.; Xu, Q.L.; Zhu, B.H.; Li, D.Y.; Xiao, X. Development of Compound Artemisia annua Low Alcohol Mosquito Repellent Liquid. Jiangxi Chem. Ind. 2017, 2, 91–92. [Google Scholar] [CrossRef]
- Wang, Z.Y.; Jiang, M.Y. Observation on the curative effect of traditional Chinese medicine chamomile Jinshui in the treatment of heat prickly heat in children. Clin. J. Chin. Med. 2016, 8, 117+119. [Google Scholar] [CrossRef]
- Wang, S.D.; Jin, Y.L.; Li, D.P. Clinical application of traditional Chinese medicine bathing agent. Res. Tradit. Chin. Med. 2000, 2, 60–61. [Google Scholar]
- McKay, D.L.; Blumberg, J.B. A review of the bioactivity and potential health benefits of chamomile tea (Matricaria recutita L.). Phytother. Res. 2006, 20, 519–530. [Google Scholar] [CrossRef]
- Huang, D.J. Development and Utilization of Herb Plants. North. Hortic. 2006, 3, 113–114. [Google Scholar] [CrossRef]
- Orav, A.; Raal, A.; Arak, E. Content and composition of the essential oil of Chamomilla recutita (L.) Rauschert from some European countries. Nat. Prod. Res. 2010, 24, 48–55. [Google Scholar] [CrossRef]
- Lv, Y.; Guo, W.F. Originating from Western “tea culture”—Vanilla and vanilla tea. China Tea Process. 1999, 4, 34–38. [Google Scholar] [CrossRef]
- Hu, Y.Z. Honey paste tea drink. J. Bee 2020, 40, 38. [Google Scholar]
- Gould, L.; Reddy, C.V.; Gomprecht, R.F. Cardiac effects of chamomile tea. J. Clin. Pharmacol. 1973, 13, 475–479. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.Y. Analysis of volatile components of chamomile essential oil and its application in beverages. Chin. Agric. Sci. Bull. 2021, 37, 138–145. [Google Scholar] [CrossRef]
- Guzelmeric, E.; Ristivojević, P.; Vovk, I.; Milojković-Opsenica, D.; Yesilada, E. Quality assessment of marketed chamomile tea products by a validated HPTLC method combined with multivariate analysis. J. Pharm. Biomed. Anal. 2017, 132, 35–45. [Google Scholar] [CrossRef] [PubMed]
- Tang, H.; Zhang, T.J.; Wang, S.; Qiao, A.N.; Cao, L.; Cui, W.Y. Preparation of traditional Chinese medicinal incense for preventing and treating insomnia. Sci. Technol. Ecnony Mark. 2014, 2, 90–91. [Google Scholar] [CrossRef]
- Zhang, Q.H. Pharmacological Experiment Study on Qingdan Capsule of Traditional Chinese Medicine. J. Guangdong Pharm. Univ. 2001, 3, 181–183. [Google Scholar] [CrossRef]
- Xu, R.T. The effect of crude drug extract bath in the treatment of rheumatoid arthritis. World Phytomedicines 1995, 5, 15. [Google Scholar]
- Ma, D.W. A Traditional Chinese Medicine Composition for Sunscreen Cosmetics Containing Marigold. CN105796448A, 8 June 2016. [Google Scholar]
- Wang, Z.Z. Application of natural ingredients in cosmetics. Proceedings of The 6th Southeast Asia Regional Medical Aesthetics Conference, Dalian, China, 8 August 2001; pp. 161–162. [Google Scholar]
- Lu, H.M. A Kind of Compound Plant Extract Repair Liquid and Preparation Method Thereof. CN112754979A, 3 February 2021. [Google Scholar]
- Chen, G.R.; Xin, Y.H. Antiviral Traditional Chinese Medicine Composition and Its Application. CN113797266A, 11 June 2020. [Google Scholar]
- Zhao, Y.F.; Zhang, D.; Yang, L.X.; Wang, K.; Ma, Y.; Yang, L. Determination of Volatile Components in Different Parts of Chamomile by HS-SPME-GC-MS. Chin. J. Exp. Tradit. Med. Formulae 2018, 24, 69–73. [Google Scholar] [CrossRef]
- Maimedy, M.; Wang, Y.; Zhao, S.J.; Lan, W. Analysis of total flavonoids of chamomile and its inhibitory effect on pancreatic lipase. Chem. Bioeng. 2021, 38, 62–67. [Google Scholar] [CrossRef]
- Zhao, Y.F.; Zhang, D.; Liang, C.X.; Yang, L.X.; Sun, P.; Ma, Y.; Wang, K.; Chang, X.Q.; Yang, L. Study on chemical constituents of Uyghur chamomile I. J. Chin. Pharm. Sci. 2018, 27, 324–331. [Google Scholar]
- Xu, Y.B.; Tang, H.; Zhu, S.Y.; Zhe, W.; Wang, K.; Mao, D.s.; Fu, L.; Chen, R.K. Analysis of volatile components in chamomile oil from different origins by gas chromatography time-of-flight mass spectrometry. Sci. Technol. Food Ind. 2015, 36, 69–74. [Google Scholar] [CrossRef]
- Li, B.; Zhou, W. Analysis of Chamomile Essential Oil by MassWorks~(TM) and Gas Chromatography-Mass Spectrometry. J. Chin. Mass Spectrom. Soc. 2011, 32, 241–245. [Google Scholar]
- Wang, G.L. Study on Active Ingredients of Chamomile for Whitening and Moisturizing. Master’s Thesis, Jiangnan University, Wuxi, China, 2016. [Google Scholar]
- Tvrzicka, E.; Kremmyda, L.S.; Stankova, B.; Zak, A. Fatty acids as biocompounds: Their role in human metabolism, health and disease—A review. Part 1: Classification, dietary sources and biological functions. Biomed. Pap. Med. Fac. Univ. Palacky Olomouc. Czech Repub. 2011, 155, 117–130. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.S.; Pan, L.S. Isolation and Structure Determination of Flavonoids in Chamomile. Appl. Chem. Ind. 2008, 6, 697–698. [Google Scholar] [CrossRef]
- Chen, P.; Huo, X.; Liu, W.; Li, K.; Sun, Z.; Tian, J. Apigenin exhibits anti-inflammatory effects in LPS-stimulated BV2 microglia through activating GSK3β/Nrf2 signaling pathway. Immunopharmacol. Immunotoxicol. 2020, 42, 9–16. [Google Scholar] [CrossRef]
- Asadi, Z.; Ghazanfari, T.; Hatami, H. Anti-inflammatory Effects of Matricaria chamomilla Extracts on BALB/c Mice Macrophages and Lymphocytes. Iran J. Allergy Asthma Immunol. 2020, 19, 63–73. [Google Scholar] [CrossRef] [PubMed]
- Petruľová-Poracká, V.; Repčák, M.; Vilková, M.; Imrich, J. Coumarins of Matricaria chamomilla L.: Aglycones and glycosides. Food Chem. 2013, 141, 54–59. [Google Scholar] [CrossRef]
- Li, J.G.; Han, S.L.; Zhao, D.S.; Zhang, H.B.; Geng, J.; Li, X.X. Determination of five chemical constituents in two kinds of chamomile in Xinjiang by one test and multiple evaluation method. Her. Med. 2014, 33, 1491–1495. [Google Scholar] [CrossRef]
- Setzer, W.N. Essential oils and anxiolytic aromatherapy. Nat. Prod. Commun. 2009, 4, 1305–1316. [Google Scholar] [CrossRef]
- Zhang, Q.S.; Wang, X.P.; Wu, L.L.; Xiang, Y. Protective effect of bisabolol on acetaminophen-induced acute liver injury in mice. J. Hubei Minzu Univ. 2018, 35, 5–7. [Google Scholar] [CrossRef]
- Chen, L.; He, X.M.; Meng, L.; Ma, X.L. Comparison of Determination of Monosaccharide Components in Chamomile Polysaccharides by High Performance Liquid Chromatography and High Performance Capillary Electrophoresis. Food Drug 2019, 21, 347–351. [Google Scholar] [CrossRef]
- Jing, Y.X.; Guo, Y.T.; Lan, W. Determination of 6 kinds of trace elements in chamomile. J. Xinjiang Med. Univ. 2013, 36, 1282–1283. [Google Scholar] [CrossRef]
- Xin, L.D.; Li, Q.; Wang, J.L.; Lao, F.; Tian, S.G. Determination of Arsenic in Chamomile by Microwave Digestion-Atomic Fluorescence Spectrometry. J. Xinjiang Med. Univ. 2014, 37, 860–861. [Google Scholar] [CrossRef]
- Yan, H.B.; Xu, R.X. Effects of α-bisabolol on migration and invasion of glioblastoma cells. Acad. J. Pla Postgrad. Med. Sch. 2018, 39, 699–706. [Google Scholar] [CrossRef]
- Shaaban, M.; El-Hagrassi, A.M.; Osman, A.F.; Soltan, M.M. Bioactive compounds from Matricaria chamomilla: Structure identification, in vitro antiproliferative, antimigratory, antiangiogenic, and antiadenoviral activities. Z Nat. C J. Biosci. 2021, 77, 85–94. [Google Scholar] [CrossRef]
- Hu, J.S.; Chen, J.P.; Chen, L.; Wu, X.F.; Wang, K.Q.; Liu, Z.H. Screening of celery flavonoids for antihypertensive, lipid-lowering and antitumor pharmacological activities. Hunan Agric. Sci. 2014, 11, 9–12. [Google Scholar] [CrossRef]
- Srivastava, J.K.; Gupta, S. Antiproliferative and apoptotic effects of chamomile extract in various human cancer cells. J Agric Food Chem. 2007, 55, 9470–9478. [Google Scholar] [CrossRef]
- Lan, W.; Guo, Y.T.; Chen, Y.; Duan, M.H.; Geng, Z.; Ni, J. Study on the effect of Uygur chamomile on inhibiting the proliferation of cervical cancer Hela cells in vitro. Yunnan J. Tradit. Chin. Med. Mater. Med. 2016, 37, 54–55. [Google Scholar] [CrossRef]
- El Joumaa, M.M.; Taleb, R.I.; Rizk, S.; Borjac, J.M. Protective effect of Matricaria chamomilla extract against 1,2-dimethylhydrazine-induced colorectal cancer in mice. J. Complement Integr. Med. 2020, 17, 20190143. [Google Scholar] [CrossRef]
- Nikseresht, M.; Kamali, A.M.; Rahimi, H.R.; Delaviz, H.; Toori, M.A.; Kashani, I.R.; Mahmoudi, R. The Hydroalcoholic Extract of Matricaria chamomilla Suppresses Migration and Invasion of Human Breast Cancer MDA-MB-468 and MCF-7 Cell Lines. Pharmacogn. Res. 2017, 9, 87–95. [Google Scholar] [CrossRef]
- Park, E.H.; Bae, W.Y.; Eom, S.J.; Kim, K.T.; Paik, H.D. Improved antioxidative and cytotoxic activities of chamomile (Matricaria chamomilla) florets fermented by Lactobacillus plantarum KCCM 11613P. J Zhejiang Univ. Sci. B 2017, 18, 152–160. [Google Scholar] [CrossRef] [PubMed]
- Felšöciová, S.; Kačániová, M.; Horská, E.; Vukovič, N.; Hleba, L.; Petrová, J.; Rovná, K.; Stričík, M.; Hajduová, Z. Antifungal activity of essential oils against selected terverticillate penicillia. Ann. Agric. Environ. Med. 2015, 22, 38–42. [Google Scholar] [CrossRef] [PubMed]
- Abd El-Moneim, M.R.; Fatma, S.A.; Turky, A.F. Control of Tetranychus urticae Koch by extracts of three essential oils of chamomile, marjoram and Eucalyptus. Asian Pac. J. Trop Biomed. 2012, 2, 24–30. [Google Scholar] [CrossRef] [PubMed]
- Seyedjavadi, S.S.; Khani, S.; Eslamifar, A.; Ajdary, S.; Goudarzi, M.; Halabian, R.; Akbari, R.; Zare-Zardini, H.; Imani Fooladi, A.A.; Amani, J.; et al. The Antifungal Peptide MCh-AMP1 Derived From Matricaria chamomilla Inhibits Candida albicans Growth via Inducing ROS Generation and Altering Fungal Cell Membrane Permeability. Front. Microbiol. 2019, 10, 3150. [Google Scholar] [CrossRef] [PubMed]
- Shikov, A.N.; Pozharitskaya, O.N.; Makarov, V.G.; Kvetnaya, A.S. Antibacterial activity of Chamomilla recutita oil extract against Helicobacter pylori. Phytother. Res. 2008, 22, 252–253. [Google Scholar] [CrossRef] [PubMed]
- He, H.; Jiang, X.J.; Wang, X.T.; Wang, S.J.; Xia, X.Y. Clinical effect of white gold medallion compositae essence product on various oral discomfort and mucosal diseases. Electron. J. Gen. Stomatol. 2018, 5, 77–80. [Google Scholar] [CrossRef]
- Bulgari, M.; Sangiovanni, E.; Colombo, E.; Maschi, O.; Caruso, D.; Bosisio, E.; Dell’Agli, M. Inhibition of neutrophil elastase and metalloprotease-9 of human adenocarcinoma gastric cells by chamomile (Matricaria recutita L.) infusion. Phytother. Res. 2012, 26, 1817–1822. [Google Scholar] [CrossRef]
- Yuan, Y.; Long, Z.J.; Yang, J.J.; Yuan, C.H. Study on the anti-inflammatory effect of Chamomile Volatile Oil. Pharm. Biotechnol. 2011, 18, 52–55. [Google Scholar] [CrossRef]
- Wang, Y.; Ma, H.M.; Lan, W.; Zhao, F.C. Effects of chamomile on blood sugar and antithrombotic effect of type 1 diabetes model mice. Chem. Bioeng. 2020, 37, 28–32. [Google Scholar] [CrossRef]
- Memariani, Z.; Moeini, R.; Hamedi, S.S.; Gorji, N.; Mozaffarpur, S.A. Medicinal plants with antithrombotic property in Persian medicine: A mechanistic review. J. Thromb. Thrombolysis 2018, 45, 158–179. [Google Scholar] [CrossRef]
- Bijak, M.; Saluk, J.; Tsirigotis-Maniecka, M.; Komorowska, H.; Wachowicz, B.; Zaczyńska, E.; Czarny, A.; Czechowski, F.; Nowak, P.; Pawlaczyk, I. The influence of conjugates isolated from Matricaria chamomilla L. on platelets activity and cytotoxicity. Int. J. Biol. Macromol. 2013, 61, 218–229. [Google Scholar] [CrossRef] [PubMed]
- Bas, Z.; Turkoglu, V.; Goz, Y. Investigation of inhibition effect of butanol and water extracts of Matricaria chamomilla L. on angiotensin-converting enzyme purified from human plasma. Biotechnol. Appl. Biochem. 2021, 69, 273–280. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.; Wang, D.; Sun, B.X.; Zhang, H.G.; Zhang, Y.F. Study on the application of chamomile volatile oil. J. Anhui Agric. Sci. 2020, 48, 211–213. [Google Scholar] [CrossRef]
- Lu, J.A.; Xie, D.X.; He, L.Y.; Wang, Y.; Zheng, Z.Y. Isolation, purification, properties, structure and antioxidant activity of chamomile polysaccharides. Food Ferment. Ind. 2021, 47, 72–78. [Google Scholar] [CrossRef]
- Lu, J.; Chang, Q.Q.; Xie, D.X.; Tan, L.; Gao, J.X. Optimization of ultrasonic extraction process of chamomile polysaccharide and study on its free radical scavenging ability. China Food Addit. 2018, 3, 124–130. [Google Scholar] [CrossRef]
- Ye, Q.; Wang, Y.; Li, S.C.; Mei, Y. Study on chemical constituents and DPPH free radical scavenging activity of Uygur chamomile. Natural Product Research and Development 2019, 31, 1907–1911. [Google Scholar] [CrossRef]
- Chu, B.Q.; Fang, R.S.; Li, L.; Xiao, G.N.; Yuan, H.N.; Gong, J.Y. Antioxidant activity and active components analysis of each extract phase of chamomile. Sci. Technol. Food Ind. 2019, 40, 1–6. [Google Scholar] [CrossRef]
- Al-Dabbagh, B.; Elhaty, I.A.; Elhaw, M.; Murali, C.; Al Mansoori, A.; Awad, B.; Amin, A. Antioxidant and anticancer activities of chamomile (Matricaria recutita L.). BMC Res. Notes 2019, 12, 3. [Google Scholar] [CrossRef]
- Ma, H.M.; Wang, Y.; Lan, W. Effects of chamomile extract on blood glucose and antioxidant activity in type 2 diabetic mice. Shanghai J. Tradit. Chin. Med. 2020, 54, 77–82. [Google Scholar] [CrossRef]
- Chen, S.C.; Chen, J.; Li, T.J.; Zheng, L.Y.; Yao, Y.; Wang, C.X. Effects of chamomile extract on endometrial tissue, insulin resistance, leptin and blood lipid levels in rats with polycystic ovary syndrome. Pract. Pharm. Clin. Remedies 2021, 24, 400–404. [Google Scholar] [CrossRef]
- Lan, W.; Guo, Y.T.; Chen, Y.; Duan, M.H.; Geng, Z.; Ni, J. Effects of Uygur chamomile on blood glucose and glucose tolerance in normal mice. J. Yunnan Univ. Tradit. Chin. Med. 2016, 39, 10–12. [Google Scholar] [CrossRef]
- Yang, Y.L.; Wang, Y.; Ma, H.M.; Lan, W. Hypoglycemic effect of German chamomile total flavonoids on diabetic mice. J. Food Saf. Qual. 2020, 11, 1524–1528. [Google Scholar] [CrossRef]
- Cemek, M.; Kağa, S.; Simşek, N.; Büyükokuroğlu, M.E.; Konuk, M. Antihyperglycemic and antioxidative potential of Matricaria chamomilla L. in streptozotocin-induced diabetic rats. J. Nat. Med. 2008, 62, 284–293. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.N.; Qin, X.L.; Huang, Z.Y.; Yang, J.Y.; Akorem, A.; Luo, J.J. Protective effect of chamomile extract on kidney in hypertensive rats. Biotechnology. 2022, 32, 350–354. [Google Scholar] [CrossRef]
- Awaad, A.A.; El-Meligy, R.M.; Zain, G.M.; Safhi, A.A.; Al Qurain, N.A.; Almoqren, S.S.; Zain, Y.M.; Sesh Adri, V.D.; Al-Saikhan, F.I. Experimental and clinical antihypertensive activity of Matricaria chamomilla extracts and their angiotensin-converting enzyme inhibitory activity. Phytother. Res. 2018, 32, 1564–1573. [Google Scholar] [CrossRef]
- Luo, J.J.; Yao, X.P.; Xuan, R.; Liu, B.; Yu, Y.Z.; Sun, Z. Effects of chamomile extract on blood pressure of L-nitroarginine-induced hypertensive rats and its mechanism. J. Food Saf. Qual. 2020, 11, 5719–5723. [Google Scholar] [CrossRef]
- Lan, W.; Wang, Y.; Hao, Y.W.; Liu, J.Y.; An, D.Q. Lipid-lowering effect of German chamomile on experimental hyperlipidemia rats. J. Xinjiang Med. Univ. 2018, 41, 208–210. [Google Scholar] [CrossRef]
- Zhou, X.L.; Chen, C.J.; Ran, L.S.; Yang, Y.; Song, J.Y.; Zhou, C.B.; Fu, D.H. Formula optimization and safety evaluation of compound poria brick tea bag. Mod. Food Sci. Technol. 2020, 36, 210–219. [Google Scholar] [CrossRef]
- Han, J.X. Anti-Allergic Activity of Natural Chinese Herbal Medicine Extracts. Master’s Thesis, Jiangnan University, Wuxi, China, 2021. [Google Scholar]
- Chandrashekhar, V.M.; Halagali, K.S.; Nidavani, R.B.; Shalavadi, M.H.; Biradar, B.S.; Biswas, D.; Muchchandi, I.S. Anti-allergic activity of German chamomile (Matricaria recutita L.) in mast cell mediated allergy model. J. Ethnopharmacol. 2011, 137, 336–340. [Google Scholar] [CrossRef]
- Chang, S.M.; Chen, C.H. Effects of an intervention with drinking chamomile tea on sleep quality and depression in sleep disturbed postnatal women: A randomized controlled trial. J. Adv. Nurs. 2016, 72, 306–315. [Google Scholar] [CrossRef]
- Amsterdam, J.D.; Li, Q.S.; Xie, S.X.; Mao, J.J. Putative Antidepressant Effect of Chamomile (Matricaria chamomilla L.) Oral Extract in Subjects with Comorbid Generalized Anxiety Disorder and Depression. J. Altern. Complement. Med. 2020, 26, 813–819. [Google Scholar] [CrossRef] [PubMed]
- Kong, Y.Y.; Wang, T.; Wang, R.; Ma, Y.C.; Song, S.S.; Liu, J.; Hu, W.W.; Li, S.T. Smell of Roman chamomile essential oil alleviates depression-like behavior in WKY rats. Sci. Sin. 2017, 47, 377–385. [Google Scholar]
- Shebbo, S.; El Joumaa, M.; Kawach, R.; Borjac, J. Hepatoprotective effect of Matricaria chamomilla aqueous extract against 1,2-Dimethylhydrazine-induced carcinogenic hepatic damage in mice. Heliyon 2020, 6, e04082. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Liang, X.; Li, J.; Yin, H.; Liu, F.; Hu, C.; Li, L. Apigenin Attenuates Acetaminophen-Induced Hepatotoxicity by Activating AMP-Activated Protein Kinase/Carnitine Palmitoyltransferase I Pathway. Front Pharmacol. 2020, 11, 549057. [Google Scholar] [CrossRef] [PubMed]
- Tavakol, H.S.; Farzad, K.; Fariba, M.; Abdolkarim, C.; Hassan, G.; Seyed-Mostafa, H.Z.; Akram, R. Hepatoprotective effect of Matricaria chamomilla.L in paraquat induced rat liver injury. Drug Res. 2015, 65, 61–64. [Google Scholar] [CrossRef]
- Ranjbar, A.; Mohsenzadeh, F.; Chehregani, A.; Khajavi, F.; Zijoud, S.M.; Ghasemi, H. Ameliorative effect of Matricaria chamomilla. L on paraquat: Induced oxidative damage in lung rats. Pharmacogn. Res. 2014, 6, 199–203. [Google Scholar] [CrossRef]
- Salama, R.H. Matricaria chamomilla attenuates cisplatin nephrotoxicity. Saudi. J. Kidney Dis. Transpl. 2012, 23, 765–772. [Google Scholar] [CrossRef]
- Zhao, D.S.; Song, B.L.; Han, S.L.; Qiu, L.; Li, X.X. Evaluation of two kinds of chamomile commonly used in Xinjiang with multiple factors. Life Sci. Res. 2016, 27, 107–109. [Google Scholar]
- Cemek, M.; Yilmaz, E.; Büyükokuroğlu, M.E. Protective effect of Matricaria chamomilla on ethanol-induced acute gastric mucosal injury in rats. Pharm. Biol. 2010, 48, 757–763. [Google Scholar] [CrossRef]
- Jabri, M.A.; Aissani, N.; Tounsi, H.; Sakly, M.; Marzouki, L.; Sebai, H. Protective effect of chamomile (Matricaria recutita L.) decoction extract against alcohol-induced injury in rat gastric mucosa. Pathophysiology 2017, 24, 1–8. [Google Scholar] [CrossRef]
- Farideh, Z.Z.; Bagher, M.; Ashraf, A.; Akram, A.; Kazem, M. Effects of chamomile extract on biochemical and clinical parameters in a rat model of polycystic ovary syndrome. J. Reprod. Infertil. 2010, 11, 169–174. [Google Scholar] [PubMed]
- Soltani, M.; Moghimian, M.; Abtahi-Eivari, S.H.; Shoorei, H.; Khaki, A.; Shokoohi, M. Protective Effects of Matricaria chamomilla Extract on Torsion/ Detorsion-Induced Tissue Damage and Oxidative Stress in Adult Rat Testis. Int. J. Fertil. Steril. 2018, 12, 242–248. [Google Scholar] [CrossRef] [PubMed]
- Afrigan, L.; Jafari Anarkooli, I.; Sohrabi, D.; Abdanipour, A.; Yazdinezhad, A.; Sayyar, Z.; Ghorbanlou, M.; Arianmanesh, M. The effect of hydroethanolic extract of Matricaria chamomilla on the reproductive system of male rats exposed to formaldehyde. Andrologia 2019, 51, e13362. [Google Scholar] [CrossRef] [PubMed]
- Ionita, R.; Postu, P.A.; Mihasan, M.; Gorgan, D.L.; Hancianu, M.; Cioanca, O.; Hritcu, L. Ameliorative effects of Matricaria chamomilla L. hydroalcoholic extract on scopolamine-induced memory impairment in rats: A behavioral and molecular study. Phytomedicine 2018, 47, 113–120. [Google Scholar] [CrossRef]
- Sayyar, Z.; Yazdinezhad, A.; Hassan, M.; Jafari Anarkooli, I. Protective Effect of Matricaria chamomilla Ethanolic Extract on Hippocampal Neuron Damage in Rats Exposed to Formaldehyde. Oxid. Med. Cell. Longev. 2018, 2018, 6414317. [Google Scholar] [CrossRef]
- Lim, H.S.; Kim, O.S.; Kim, B.Y.; Jeong, S.J. Apigetrin from Scutellaria baicalensis Georgi Inhibits Neuroinflammation in BV-2 Microglia and Exerts Neuroprotective Effect in HT22 Hippocampal Cells. J. Med. Food 2016, 19, 1032–1040. [Google Scholar] [CrossRef]
- Khan, S.S.; Ikram, R.; Naeem, S.; Khatoon, H.; Anser, H.; Sikander, B. Effect of M. chamomilla L. tea on chlorpromazine induced catalepsy: A neuroprotective study. Pak. J. Pharm. Sci. 2020, 33, 1945–1953. [Google Scholar]
- Zargaran, A.; Borhani-Haghighi, A.; Salehi-Marzijarani, M.; Faridi, P.; Daneshamouz, S.; Azadi, A.; Sadeghpour, H.; Sakhteman, A.; Mohagheghzadeh, A. Evaluation of the effect of topical chamomile (Matricaria chamomilla L.) oleogel as pain relief in migraine without aura: A randomized, double-blind, placebo-controlled, crossover study. Neurol. Sci. 2018, 39, 1345–1353. [Google Scholar] [CrossRef]
- Shoorei, H.; Khaki, A.; Ainehchi, N.; Hassanzadeh Taheri, M.M.; Tahmasebi, M.; Seyedghiasi, G.; Ghoreishi, Z.; Shokoohi, M.; Khaki, A.A.; Abbas Raza, S.H. Effects of Matricaria chamomilla Extract on Growth and Maturation of Isolated Mouse Ovarian Follicles in a Three-dimensional Culture System. Chin. Med. J. 2018, 131, 218–225. [Google Scholar] [CrossRef]
- Biller, A. [Time and again it hits the little ones: Herbal therapy for childhood diarrhea]. Wien. Med. Wochenschr. 2007, 157, 308–311. [Google Scholar] [CrossRef]
- Mehmood, M.H.; Munir, S.; Khalid, U.A.; Asrar, M.; Gilani, A.H. Antidiarrhoeal, antisecretory and antispasmodic activities of Matricaria chamomilla are mediated predominantly through K(+)-channels activation. BMC Complement. Altern. Med. 2015, 15, 75. [Google Scholar] [CrossRef] [PubMed]
- Sebai, H.; Jabri, M.A.; Souli, A.; Rtibi, K.; Selmi, S.; Tebourbi, O.; El-Benna, J.; Sakly, M. Antidiarrheal and antioxidant activities of chamomile (Matricaria recutita L.) decoction extract in rats. J. Ethnopharmacol. 2014, 152, 327–332. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.L.; Dolma, D.; Xu, D.P. Study on the active components of chamomile inhibiting tyrosinase. J. Food Sci. Biotechnol. 2018, 37, 191–194. [Google Scholar] [CrossRef]
- Xia, Q.X.; Bai, H.T.; Sun, L.C.; Gao, T.G.; Jiang, C.D.; Shi, L. Research Progress on the Composition and Function of Chamomile. Acta Hortic. Sin. 2012, 39, 1859–1864. [Google Scholar] [CrossRef]
- Rao, L.; Xie, H.; Wang, T.L.; Shi, G.F. Preparation of Chamomile Milk Hand Soap. Ind. Sci. Trib. 2022, 21, 44–46. [Google Scholar] [CrossRef]
- Park, S.H.; Kim, D.S.; Oh, J.; Geum, J.H.; Kim, J.E.; Choi, S.Y.; Kim, J.H.; Cho, J.Y. Matricaria chamomilla (Chamomile) Ameliorates Muscle Atrophy in Mice by Targeting Protein Catalytic Pathways, Myogenesis, and Mitochondrial Dysfunction. Am. J. Chin. Med. 2021, 49, 1493–1514. [Google Scholar] [CrossRef]
- Shoara, R.; Hashempur, M.H.; Ashraf, A.; Salehi, A.; Dehshahri, S.; Habibagahi, Z. Efficacy and safety of topical Matricaria chamomilla L. (chamomile) oil for knee osteoarthritis: A randomized controlled clinical trial. Complement. Clin. Pr. 2015, 21, 181–187. [Google Scholar] [CrossRef]
- Mao, J.J.; Xie, S.X.; Keefe, J.R.; Soeller, I.; Li, Q.S.; Amsterdam, J.D. Long-term chamomile (Matricaria chamomilla L.) treatment for generalized anxiety disorder: A randomized clinical trial. Phytomedicine 2016, 23, 1735–1742. [Google Scholar] [CrossRef]
- Can, O.D.; Demir Özkay, U.; Kıyan, H.T.; Demirci, B. Psychopharmacological profile of Chamomile (Matricaria recutita L.) essential oil in mice. Phytomedicine 2012, 19, 306–310. [Google Scholar] [CrossRef]
- Keefe, J.R.; Mao, J.J.; Soeller, I.; Li, Q.S.; Amsterdam, J.D. Short-term open-label chamomile (Matricaria chamomilla L.) therapy of moderate to severe generalized anxiety disorder. Phytomedicine 2016, 23, 1699–1705. [Google Scholar] [CrossRef]
- Li, Q.; Lu, J.; Lu, J. Mechanism of action of n-butanol extract of galaxanthin on asthma model mice. Chin. Tradit. Pat. Med. 2017, 39, 2603–2606. [Google Scholar] [CrossRef]
- Pazyar, N.; Yaghoobi, R.; Rafiee, E.; Mehrabian, A.; Feily, A. Skin wound healing and phytomedicine: A review. Skin. Pharmacol. Physiol. 2014, 27, 303–310. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Fan, K.; Zhao, Y.; Xie, M.L. Apigenin attenuates TGF-β1-stimulated cardiac fibroblast differentiation and extracellular matrix production by targeting miR-155-5p/c-Ski/Smad pathway. J. Ethnopharmacol. 2021, 265, 113195. [Google Scholar] [CrossRef] [PubMed]
- Gomaa, A.; Hashem, T.; Mohamed, M.; Ashry, E. Matricaria chamomilla extract inhibits both development of morphine dependence and expression of abstinence syndrome in rats. J. Pharmacol. Sci. 2003, 92, 50–55. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X. German mother chrysanthemum. World Phytomedicines 2008, 2, 92. [Google Scholar]
- Avonto, C.; Rua, D.; Lasonkar, P.B.; Chittiboyina, A.G.; Khan, I.A. Identification of a compound isolated from German chamomile (Matricaria chamomilla) with dermal sensitization potential. Toxicol. Appl. Pharmacol. 2017, 318, 16–22. [Google Scholar] [CrossRef]
- Paulsen, E.; Otkjaer, A.; Andersen, K.E. The coumarin herniarin as a sensitizer in German chamomile [Chamomilla recutita (L.) Rauschert, Compositae]. Contact Dermat. 2010, 62, 338–342. [Google Scholar] [CrossRef]
- Saghafi, N.; Rhkhshandeh, H.; Pourmoghadam, N.; Pourali, L.; Ghazanfarpour, M.; Behrooznia, A.; Vafisani, F. Effectiveness of Matricaria chamomilla (chamomile) extract on pain control of cyclic mastalgia: A double-blind randomised controlled trial. J. Obstet. Gynaecol. 2018, 38, 81–84. [Google Scholar] [CrossRef]
- Trabace, L.; Tucci, P.; Ciuffreda, L.; Matteo, M.; Fortunato, F.; Campolongo, P.; Trezza, V.; Cuomo, V. “Natural” relief of pregnancy-related symptoms and neonatal outcomes: Above all do no harm. J. Ethnopharmacol. 2015, 174, 396–402. [Google Scholar] [CrossRef]
- Bianco, M.I.; Lúquez, C.; de Jong, L.I.; Fernández, R.A. Presence of Clostridium botulinum spores in Matricaria chamomilla (chamomile) and its relationship with infant botulism. Int. J. Food Microbiol. 2008, 121, 357–360. [Google Scholar] [CrossRef]
- Kalantari, H.; Dashtearjandi, A.A.; Kalantar, E. Genotoxicity study of Hypiran and Chamomilla herbal drugs determined by in vivo supervital micronucleus assay with mouse peripheral reticulocytes. Acta Biol. Hung. 2009, 60, 177–183. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Guo, Y.T.; Chen, Y.; Duan, M.H.; Hao, Y.W.; Liu, J.Y.; Lan, W. Experimental study on acute toxicity of Uygur chamomile. Jilin J. Tradit. Chin. Med. 2016, 36, 1036–1038. [Google Scholar] [CrossRef]
- Goes, P.; Dutra, C.S.; Lisboa, M.R.; Gondim, D.V.; Leitão, R.; Brito, G.A.; Rego, R.O. Clinical efficacy of a 1% Matricaria chamomile L. mouthwash and 0.12% chlorhexidine for gingivitis control in patients undergoing orthodontic treatment with fixed appliances. J. Oral. Sci. 2016, 58, 569–574. [Google Scholar] [CrossRef] [PubMed]
- Seyyedi, S.A.; Sanatkhani, M.; Pakfetrat, A.; Olyaee, P. The therapeutic effects of chamomilla tincture mouthwash on oral aphthae: A Randomized Clinical Trial. J. Clin. Exp. Dent. 2014, 6, e535–e538. [Google Scholar] [CrossRef] [PubMed]
- You, L.T.; Dong, X.X.; Leng, X.; Wang, M.L.; Yin, X.B.; Ni, J. Simultaneous determination of luteolin-7-O-β-D-glucoside and apigenin-7-O-β-D-glucoside in chamomile by HPLC-DAD. Res. Pract. Chin. Med. 2017, 31, 23–25. [Google Scholar] [CrossRef]
- Lan, W.; Guo, Y.T.; Geng, Z.; Ni, J. Determination of apigenin-7-glucoside in chamomile. J. Xinjiang Med. Univ. 2014, 37, 303–305. [Google Scholar] [CrossRef]
- Wu, S.L. Determination of α-bisabolol in Chamomile by High Performance Liquid Chromatography. J. China Prescr. Drug 2017, 15, 25–26. [Google Scholar] [CrossRef]
- Lan, W.; Guo, Y.T.; Geng, Z.; Ni, J. Determination of total saponins in German chamomile inflorescence. J. Xinjiang Med. Univ. 2014, 37, 315–316. [Google Scholar] [CrossRef]
- Xuanyuan, H.; Dong, W.J.; Cai, M.J. Study on quality standard of Uygur medicinal material chamomile. Pharm. Biotechnol. 2017, 24, 46–49. [Google Scholar] [CrossRef]
- Shi, L.Y.; Fang, Y.G.; Chen, L.M.; Li, Z.Y.; Wang, R.; Wang, Z.M.; Gao, H.M. Application of QAMS for quality evaluation and control of Chinese patent medicines:taking Bufonis Venenum-contained preparations as examples. Zhongguo Zhong Yao Za Zhi 2021, 46, 2931–2941. [Google Scholar] [CrossRef]
- Han, S.L.; Li, X.X.; Zhao, D.S.; He, X.W.; Yang, M.; Geng, J. Identification of 5 Compounds in Two Kinds of Chamomile in Xinjiang by Microemulsion Thin Layer Chromatography. Life Sci. Res. 2014, 25, 1643–1645. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).