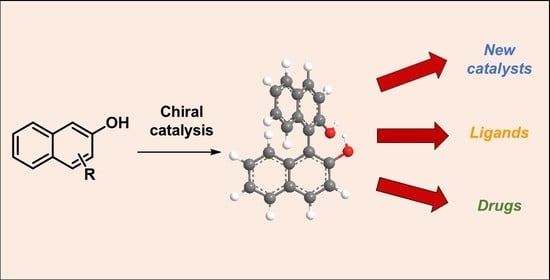
Advances in the Asymmetric Synthesis of BINOL Derivatives
Abstract
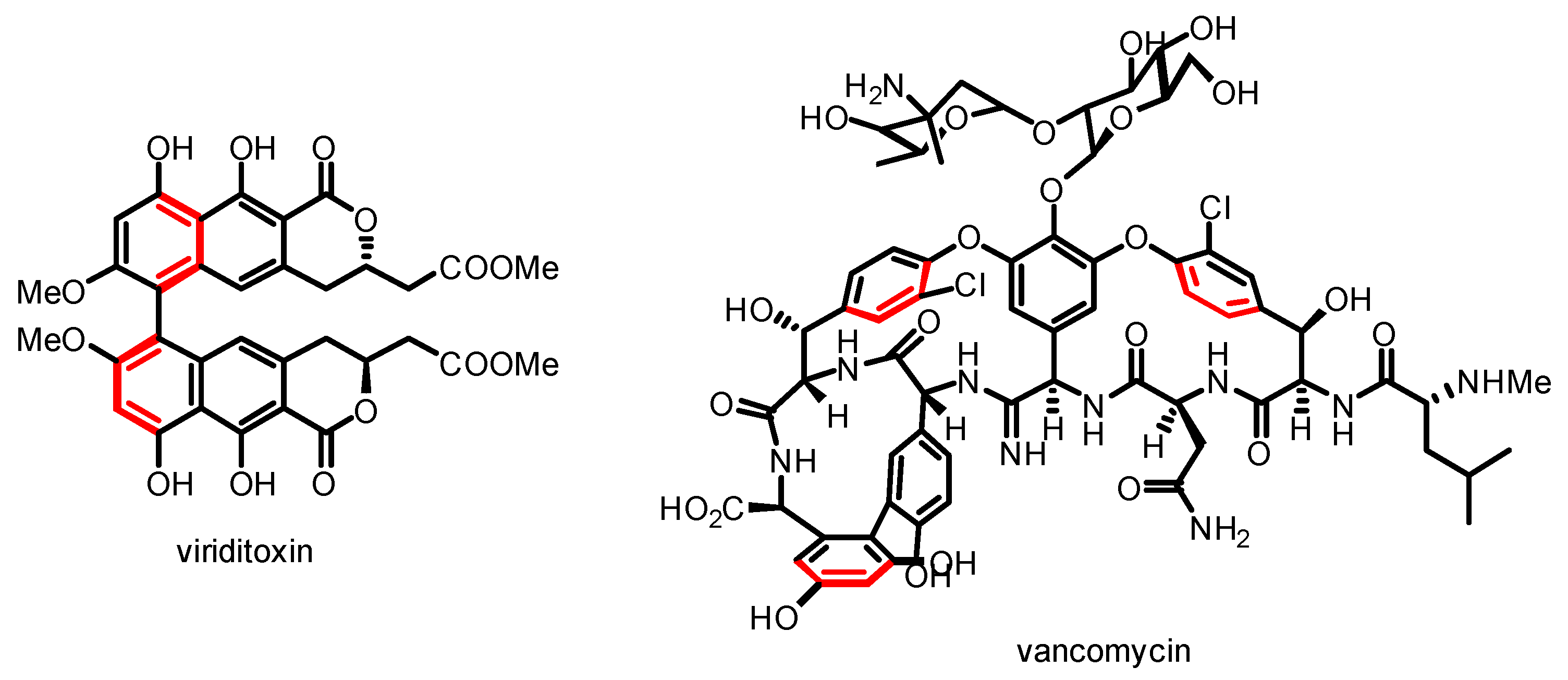
1. Introduction
2. Synthesis of BINOLs Skeleton
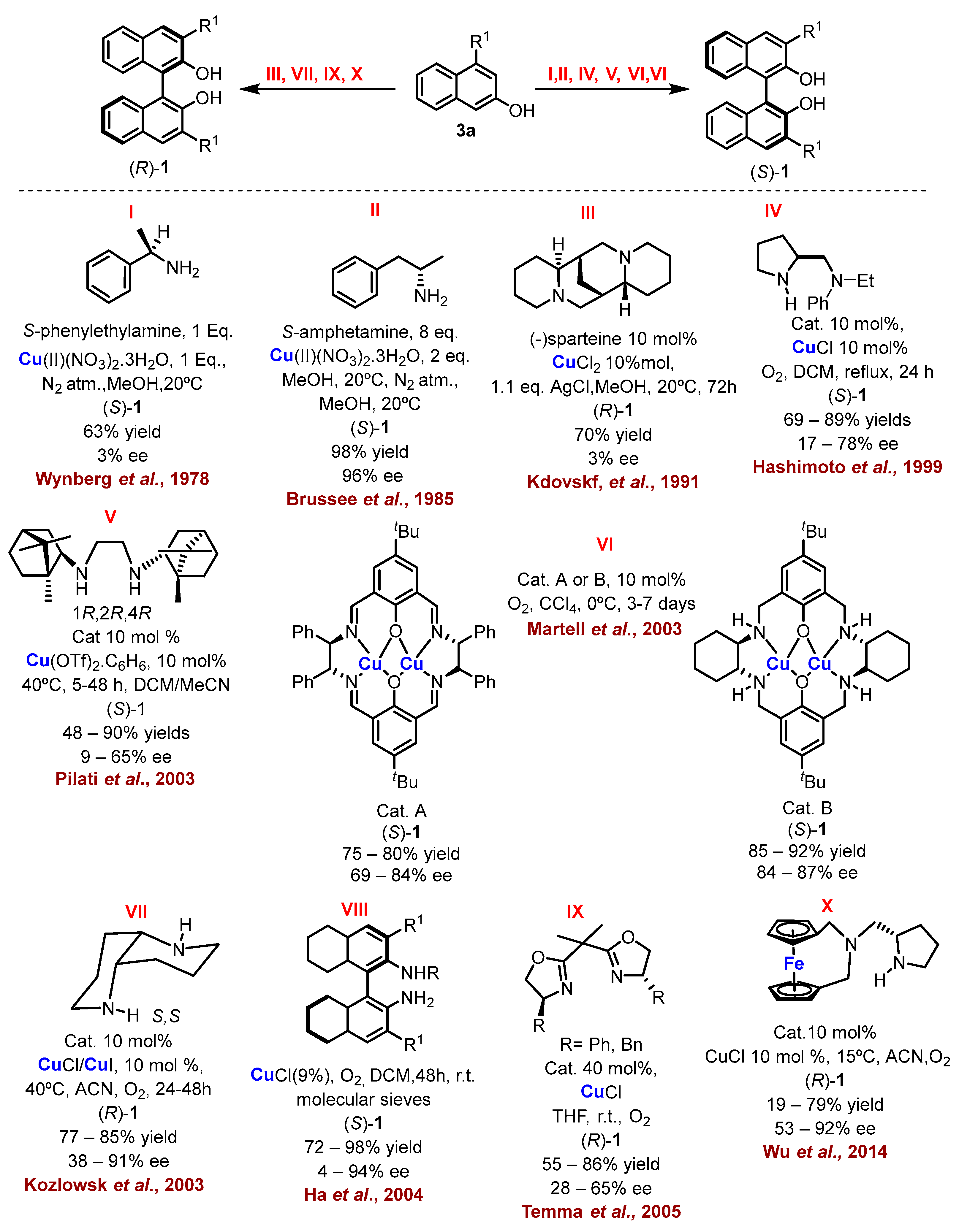
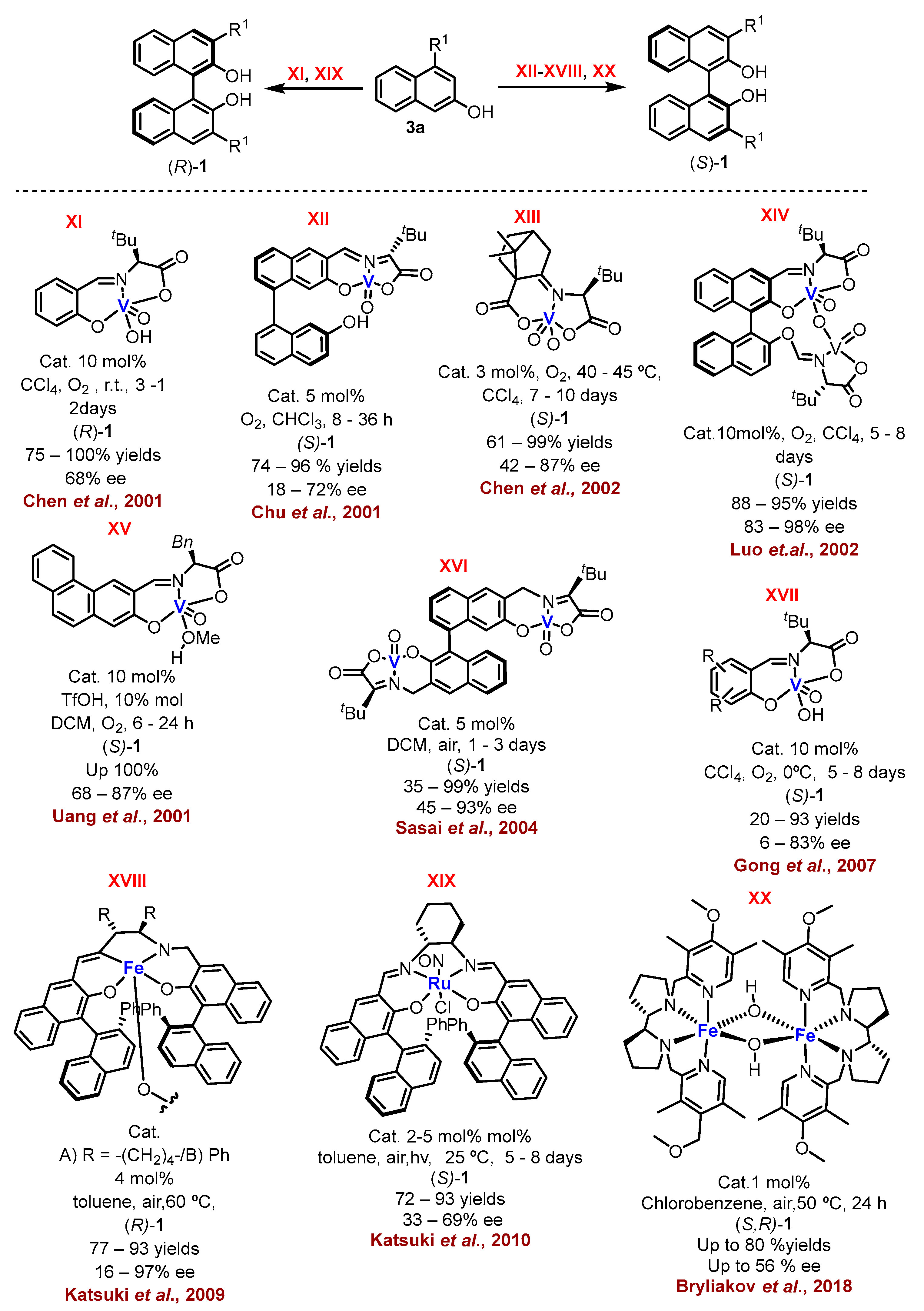
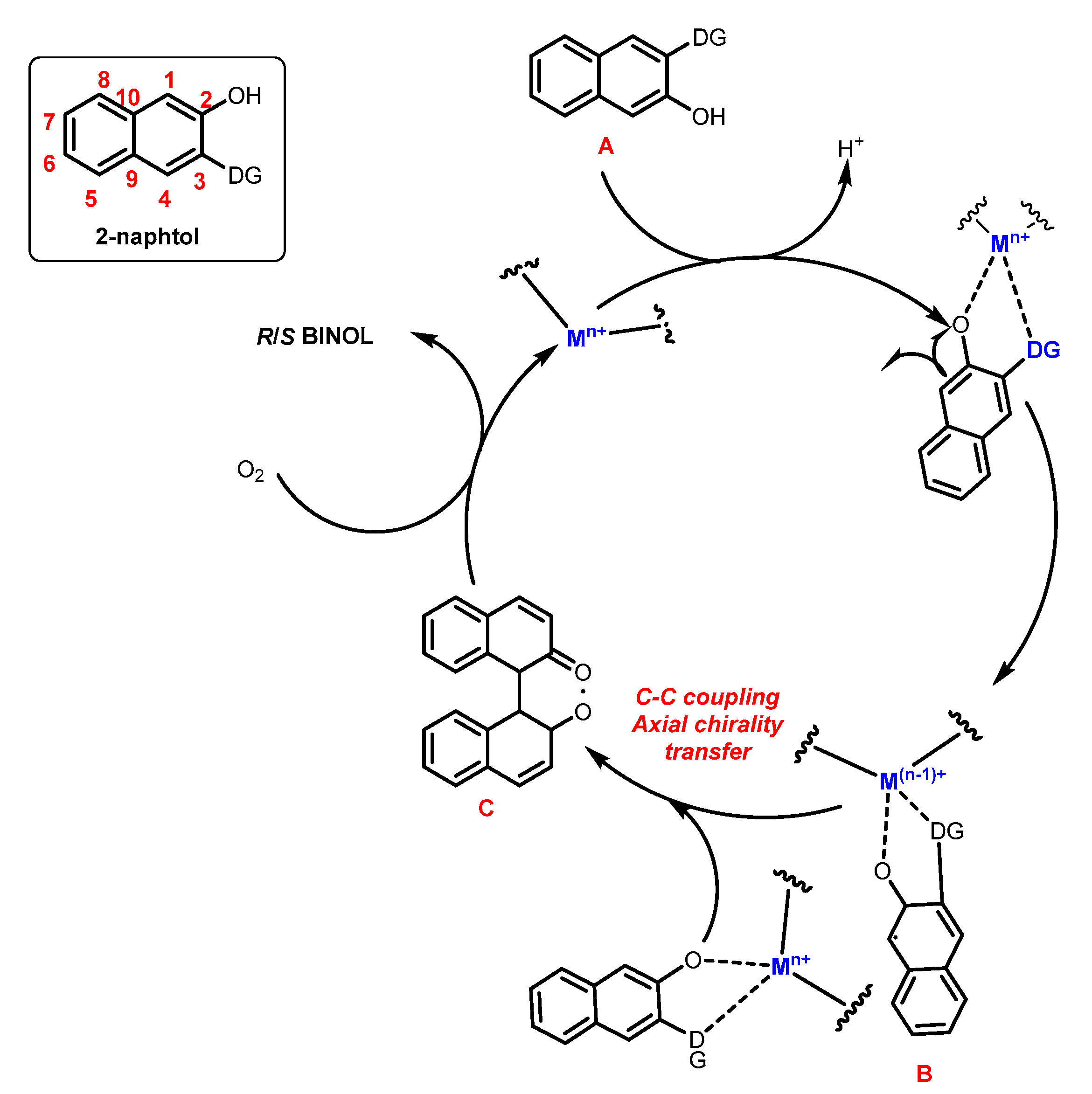
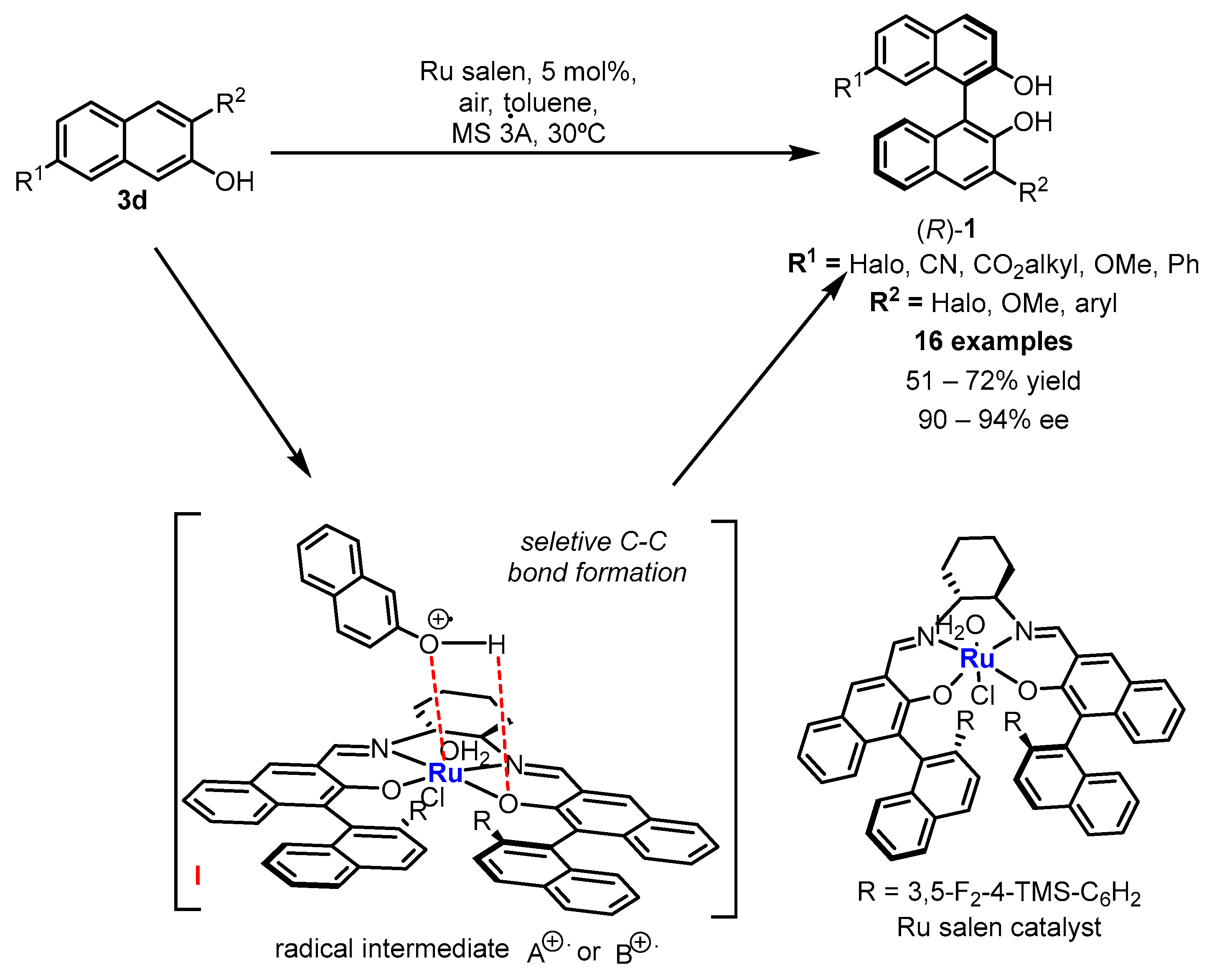
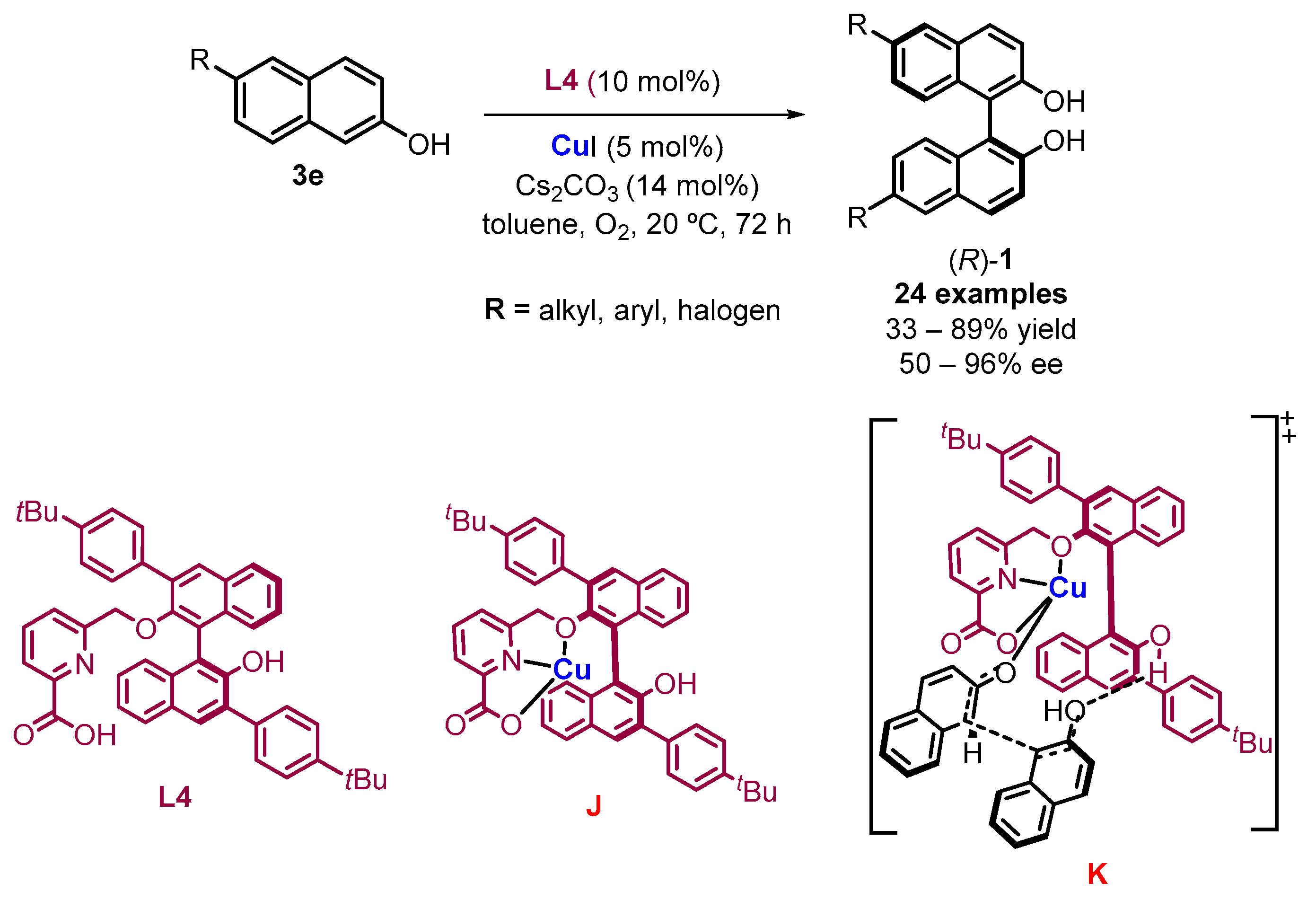
2.1. Metal-Mediated Oxidative Enantioselective Coupling
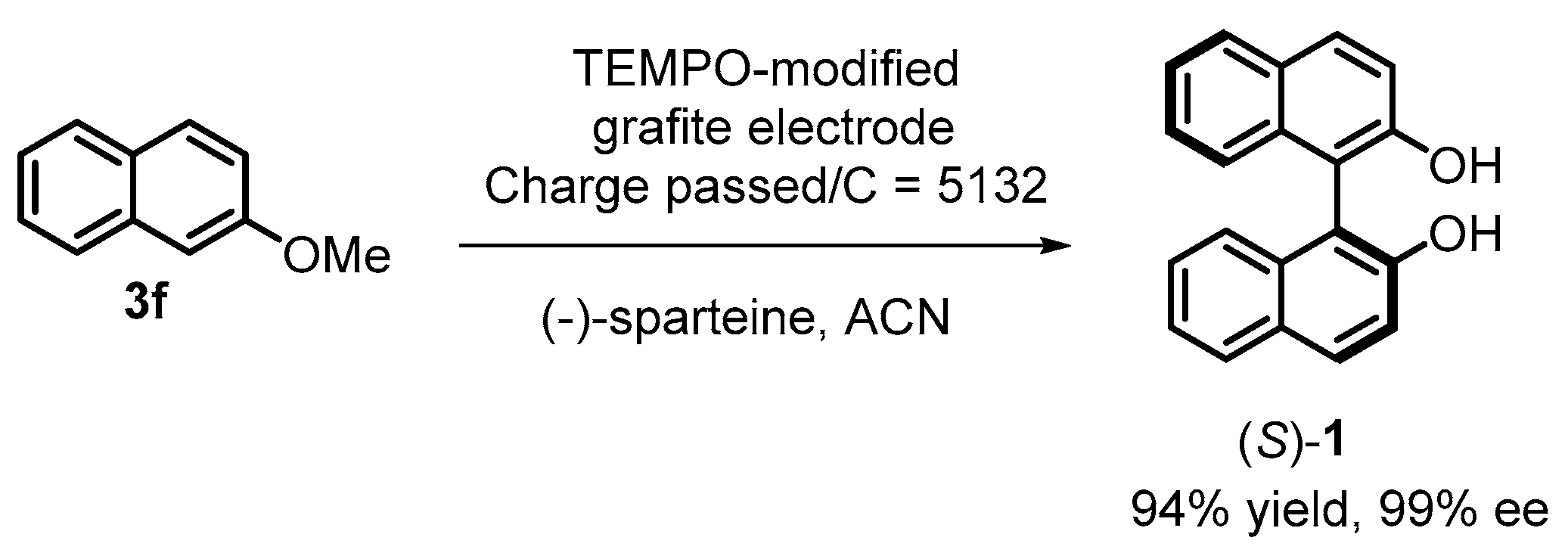
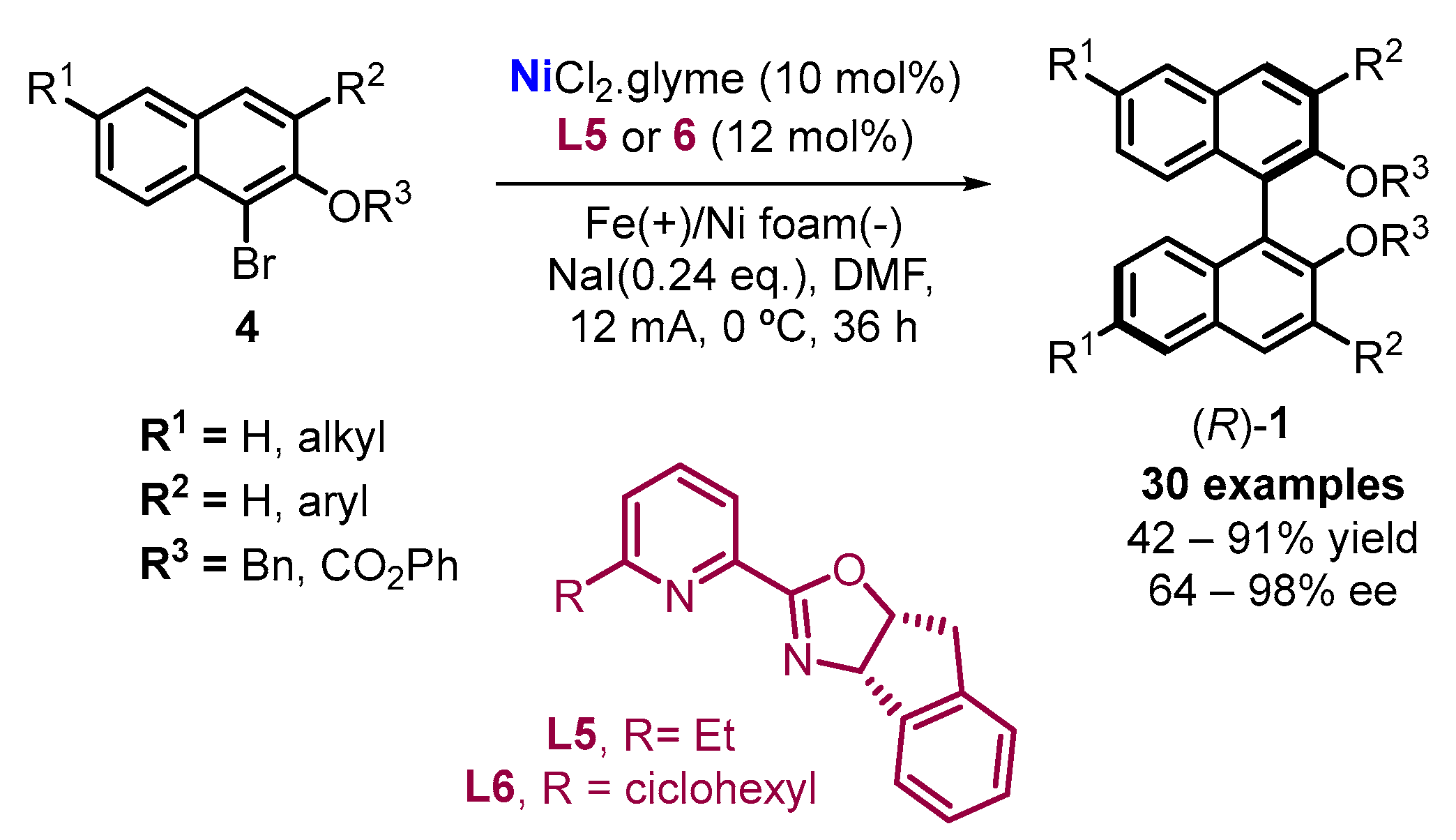
2.2. Electrochemical Synthesis
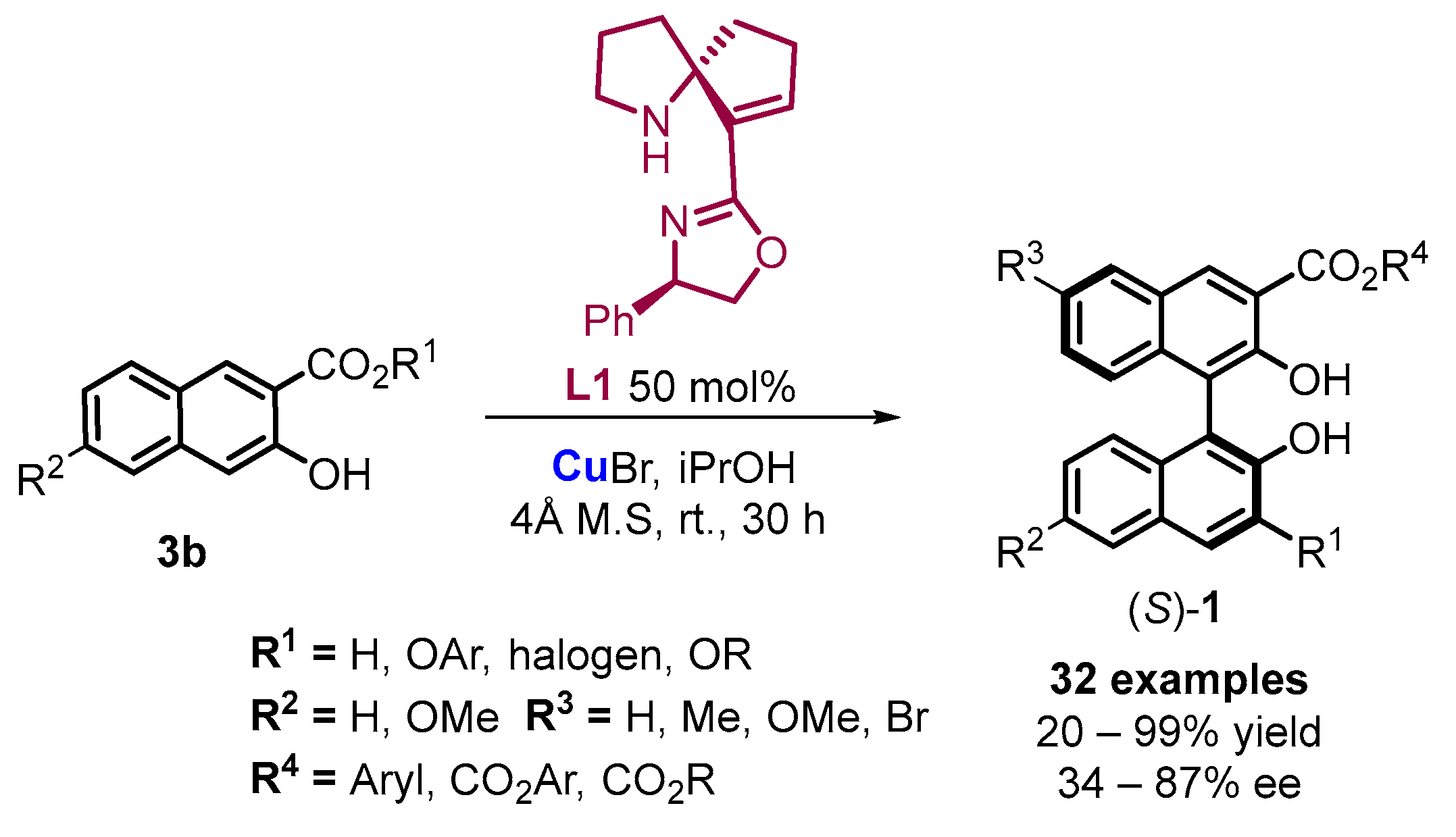
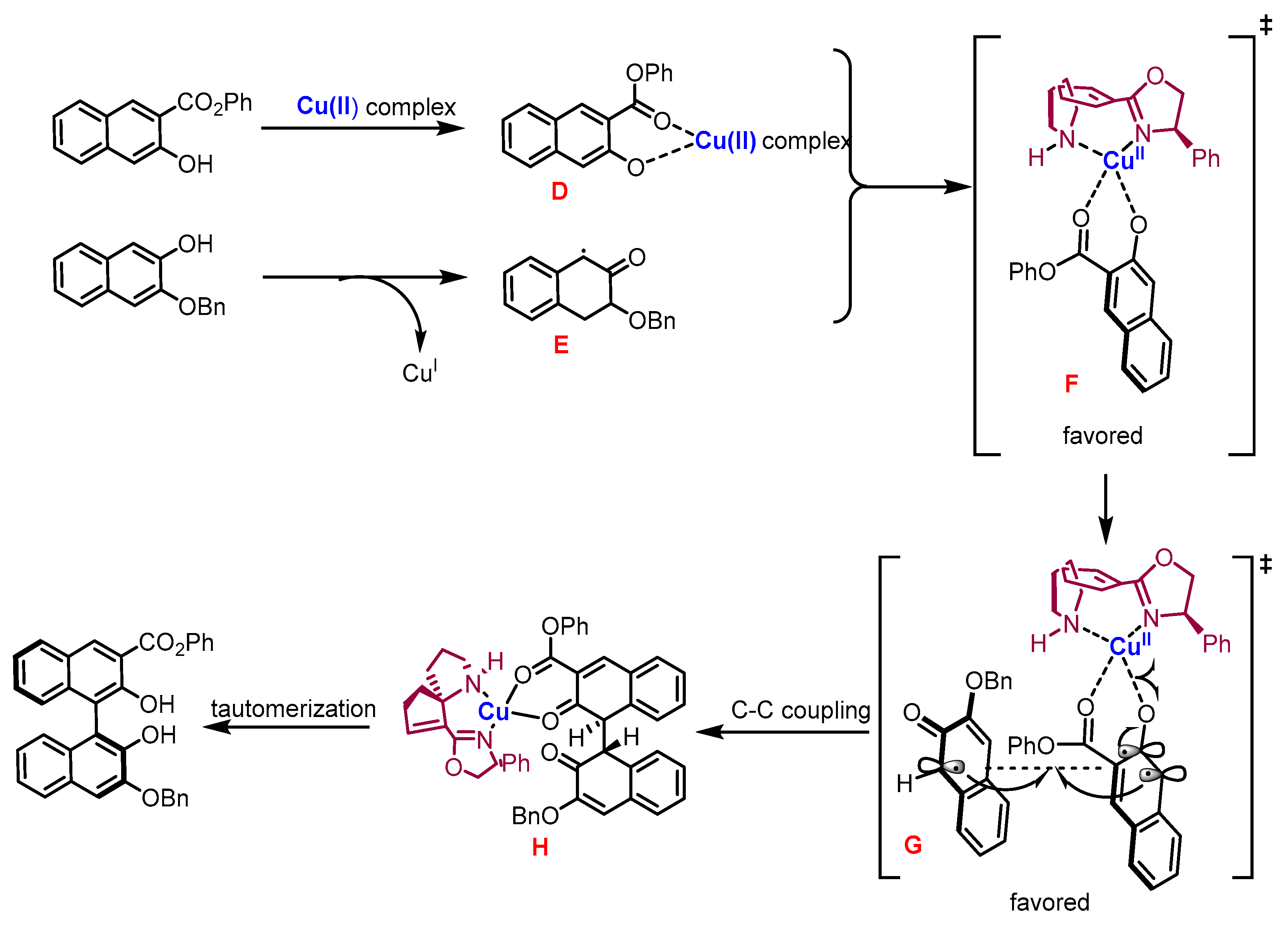
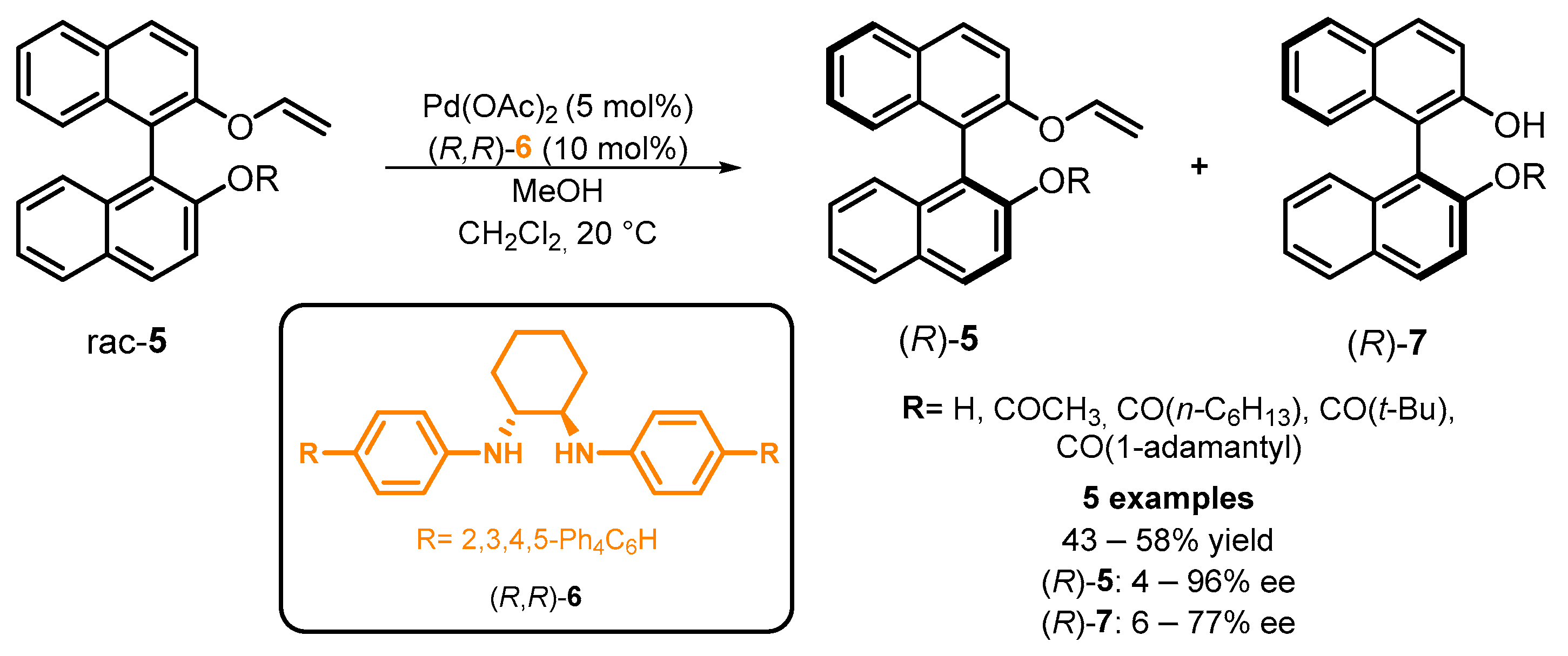
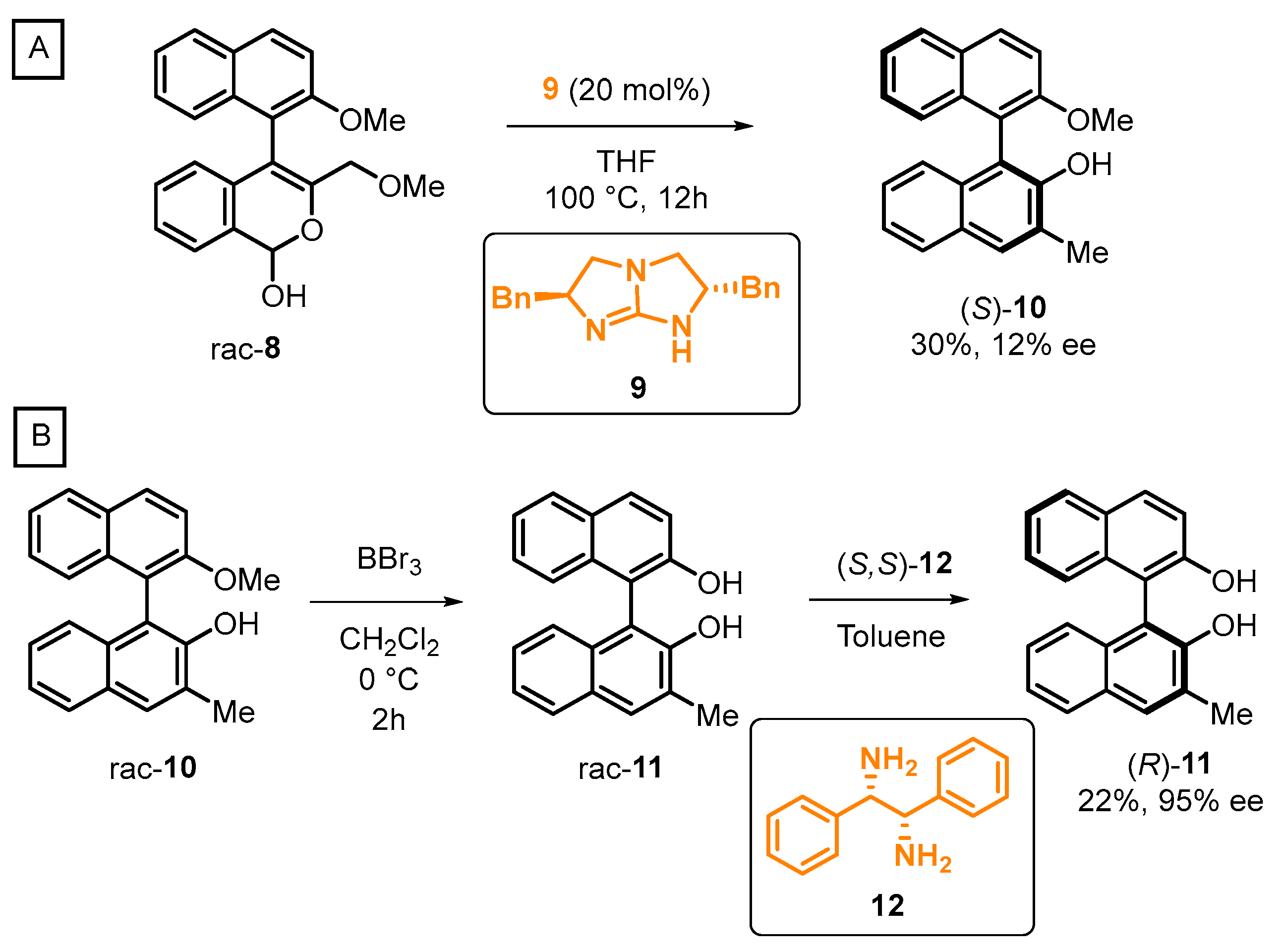
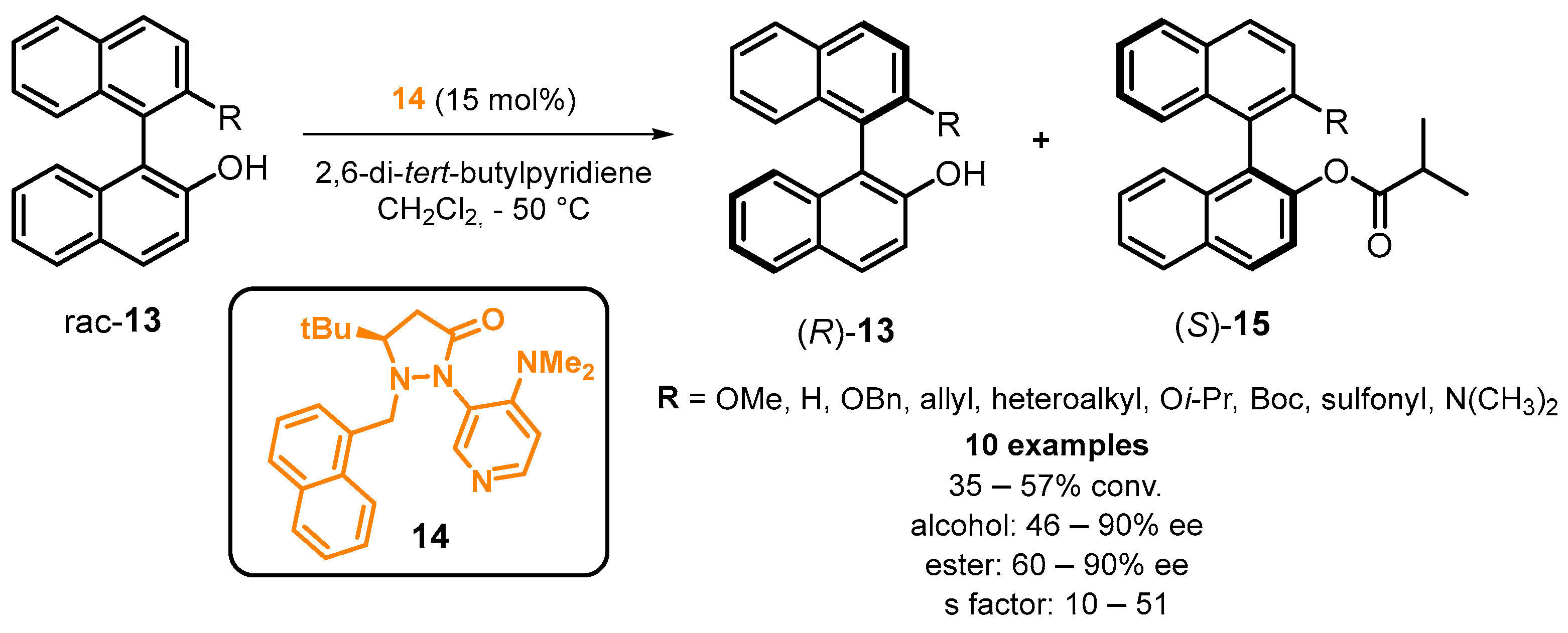
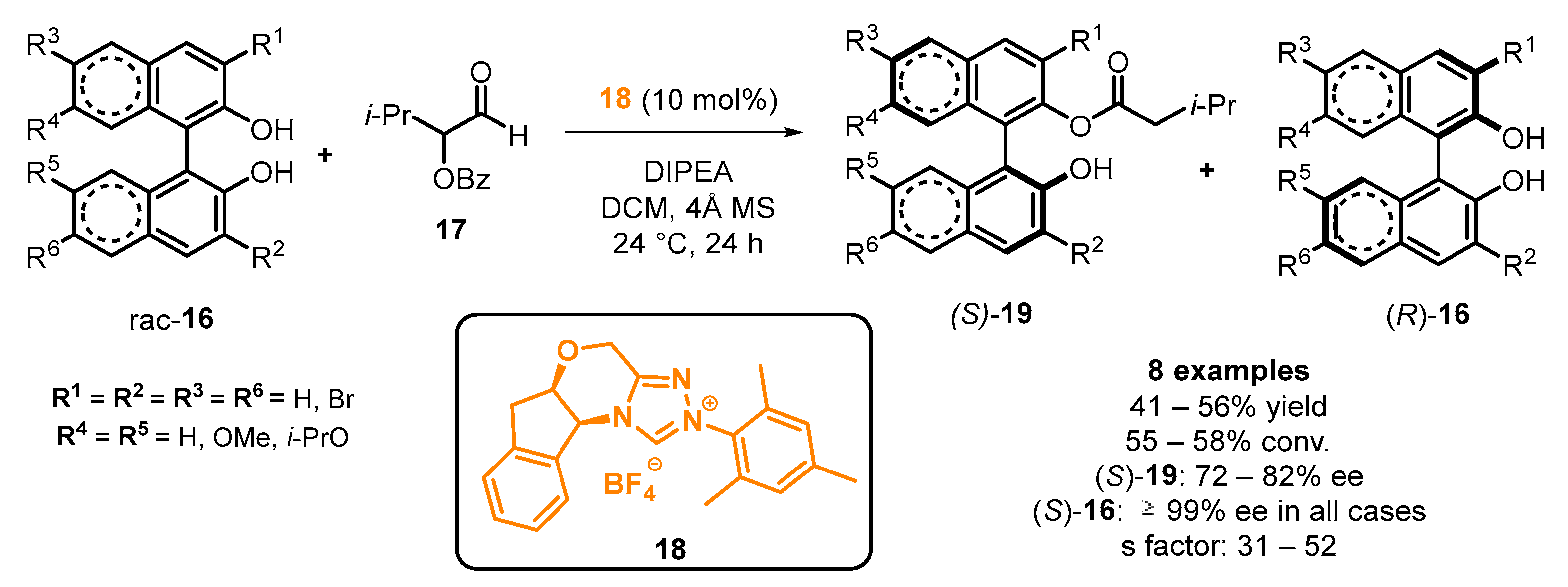
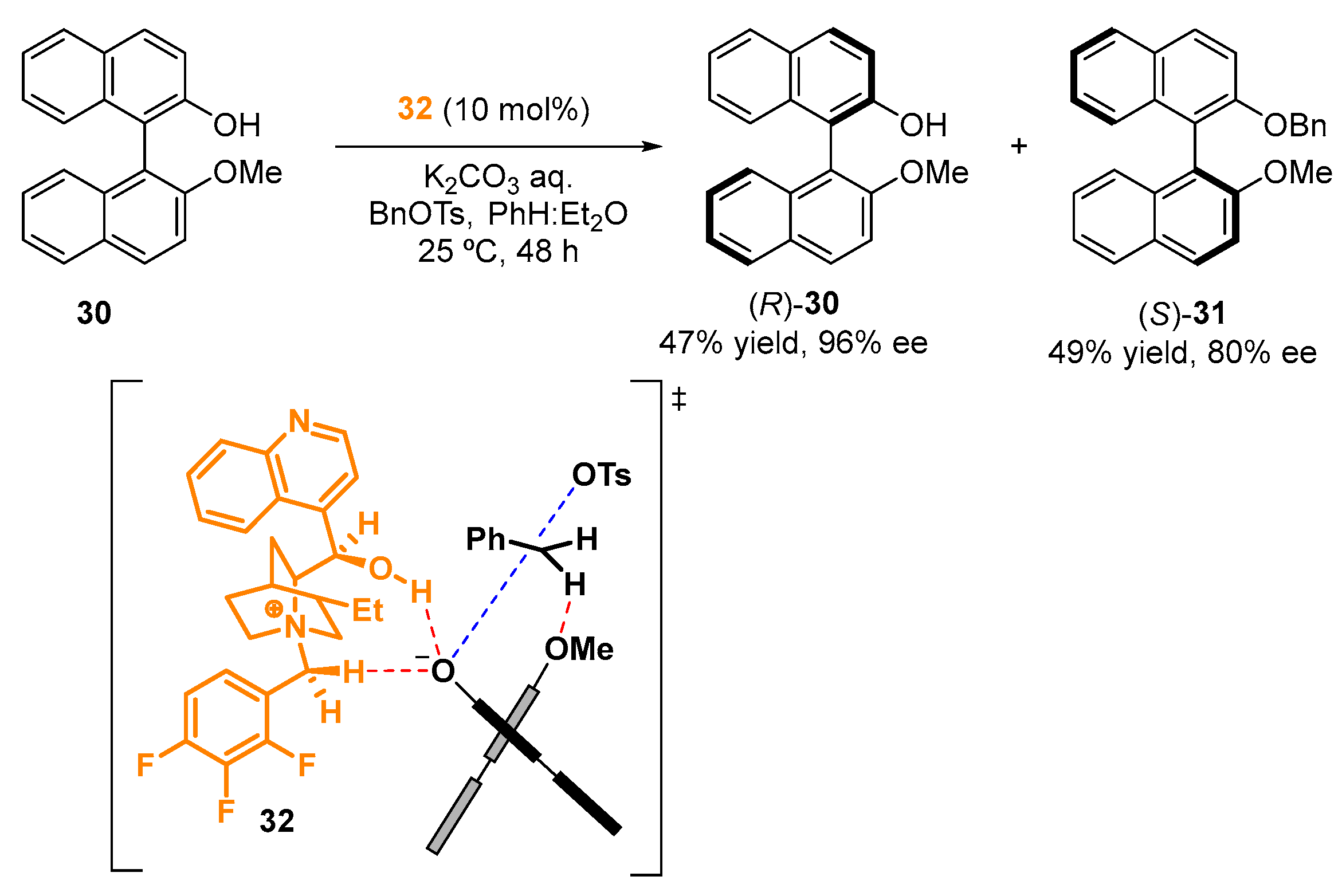
2.3. Organocatalyzed Synthesis/Kinetic Resolution of BINOLs/BINAPs
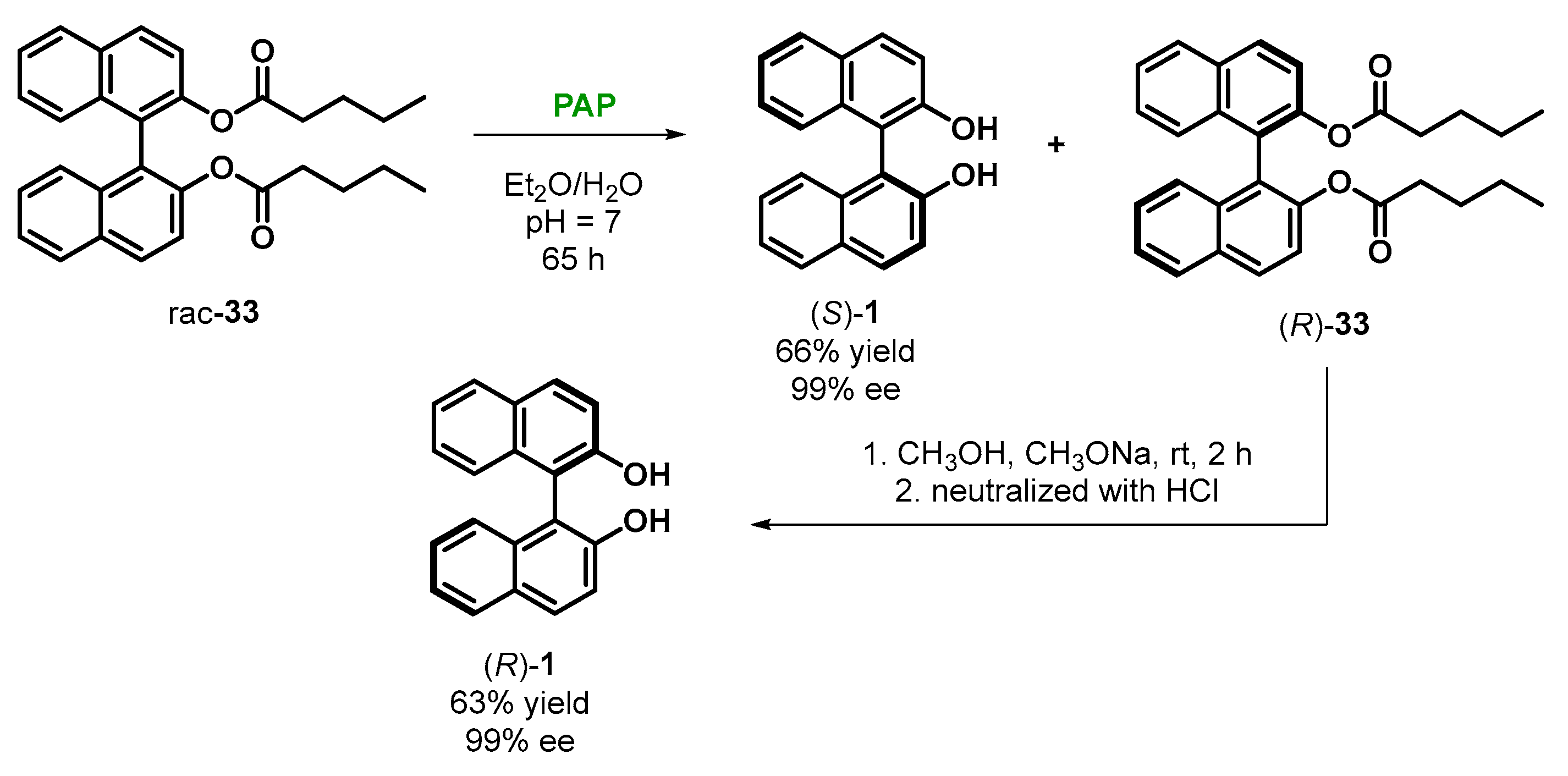
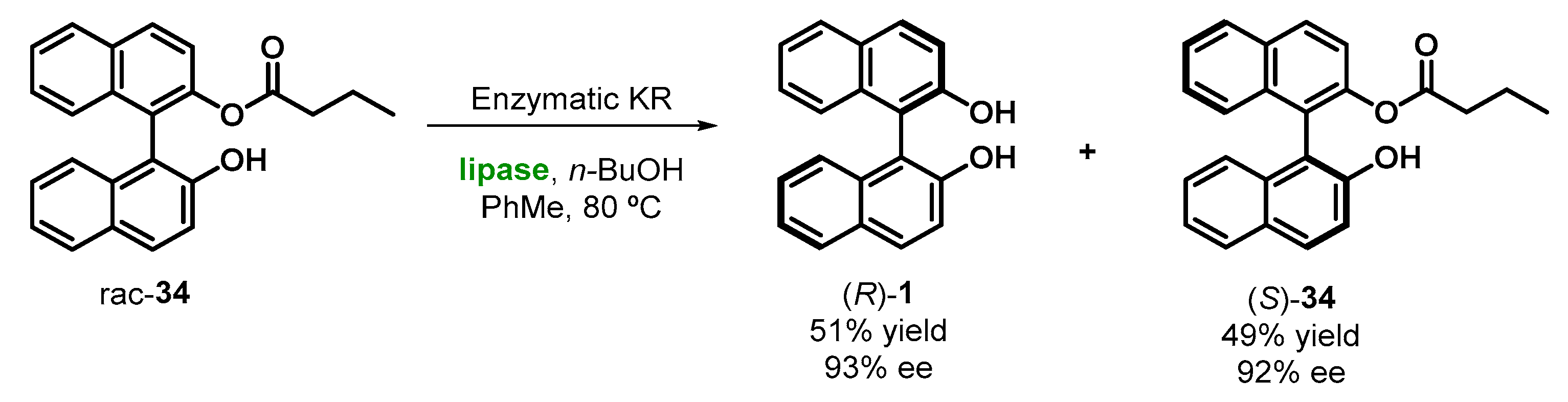
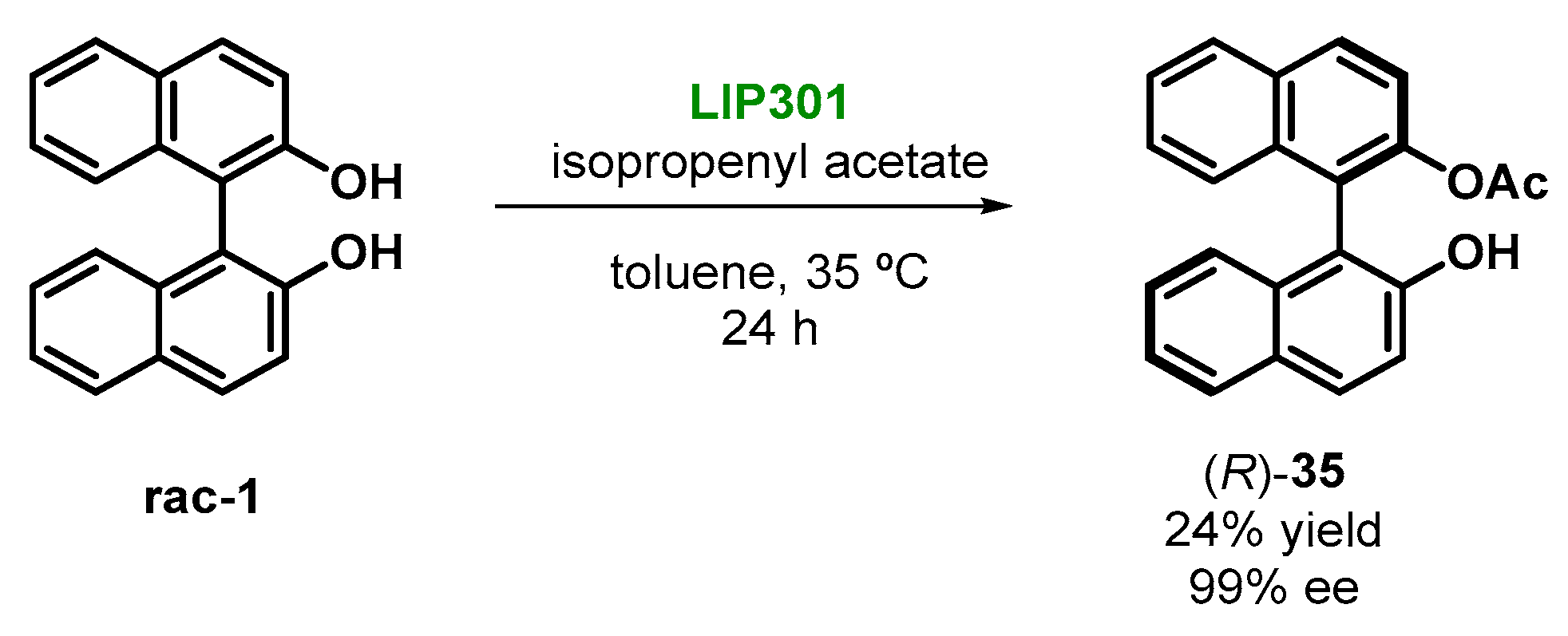
2.4. Enzymatic Kinetic Resolution of BINOL
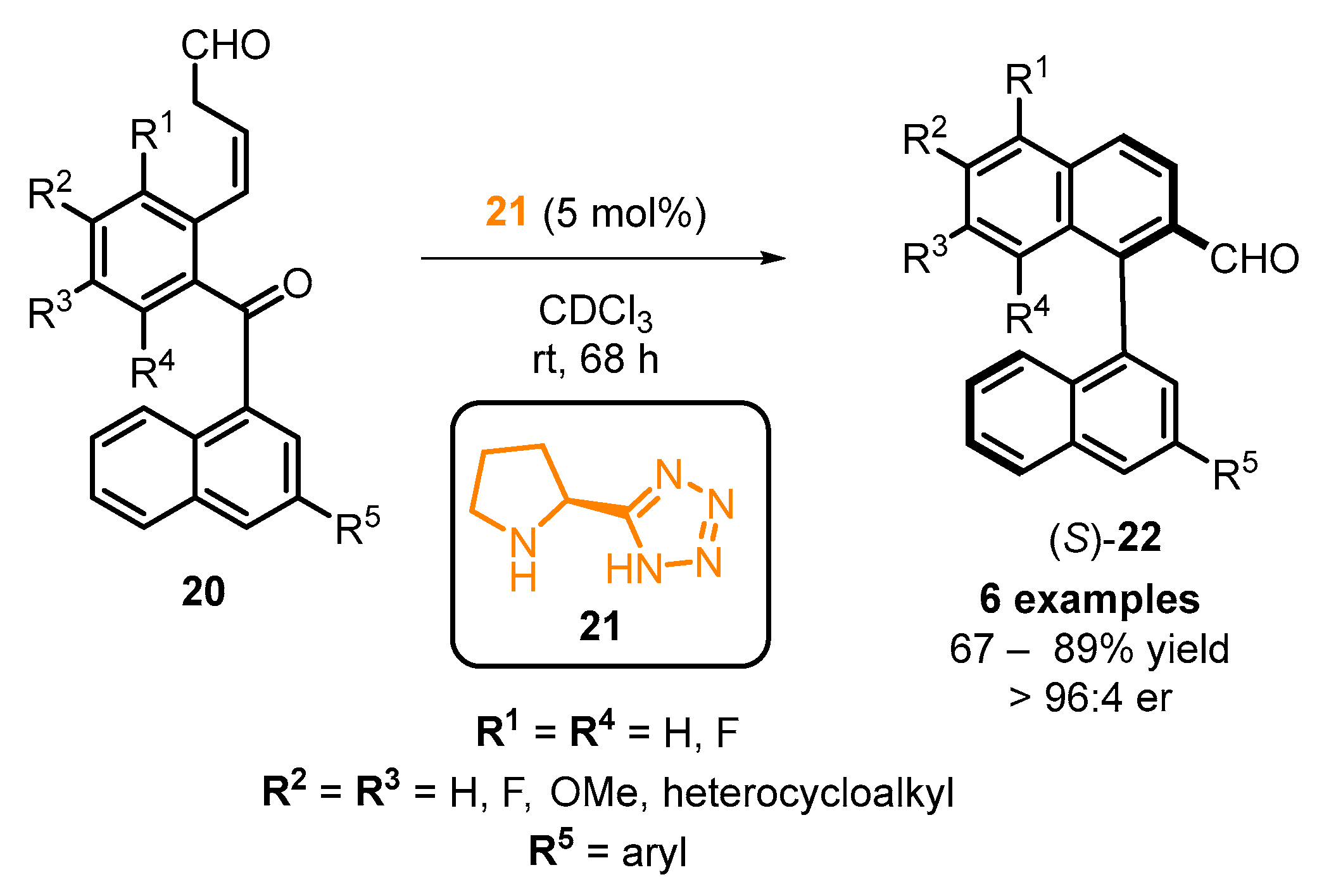
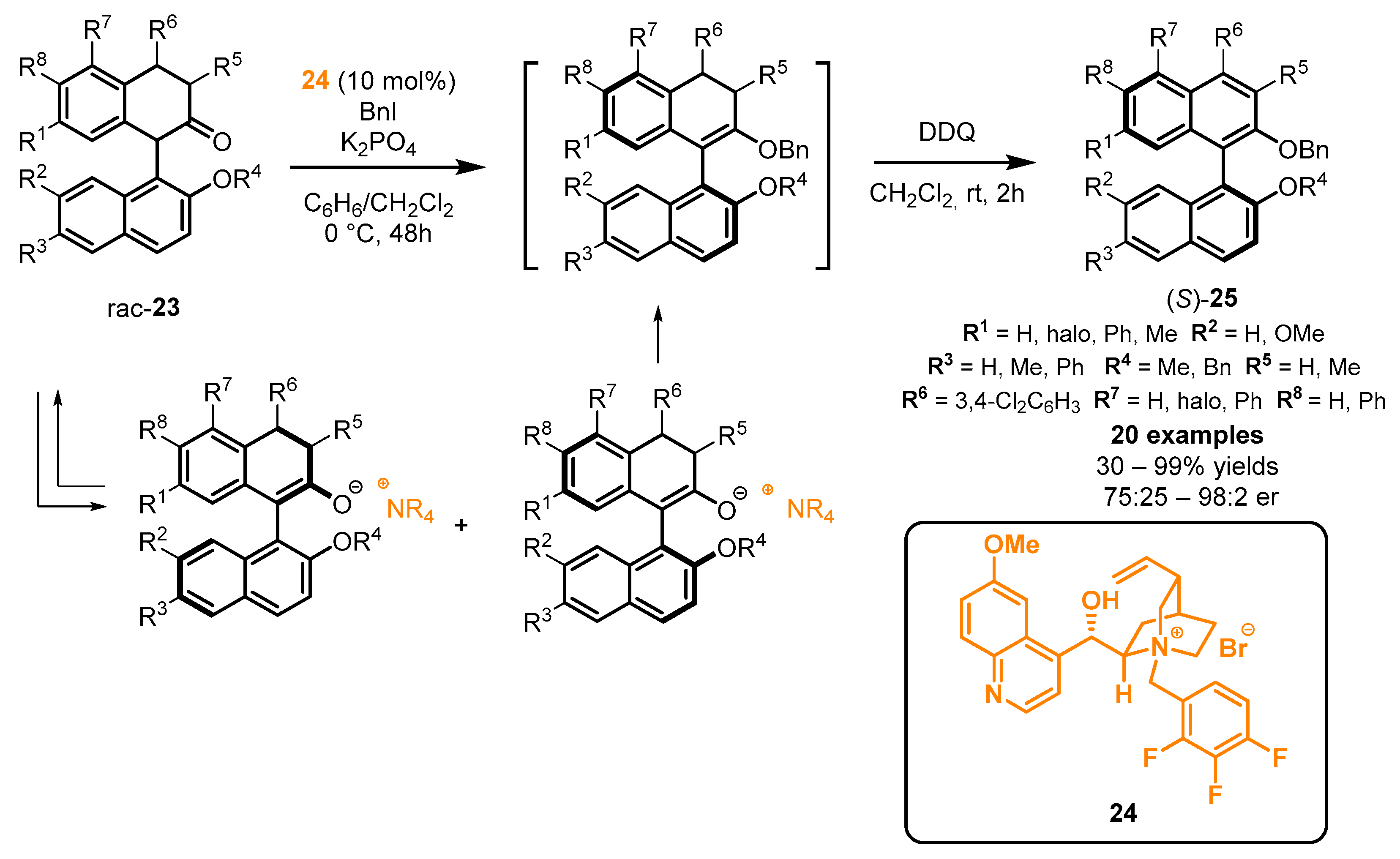
2.5. Chemical Derivatizations on the BINOL Skeleton
3. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
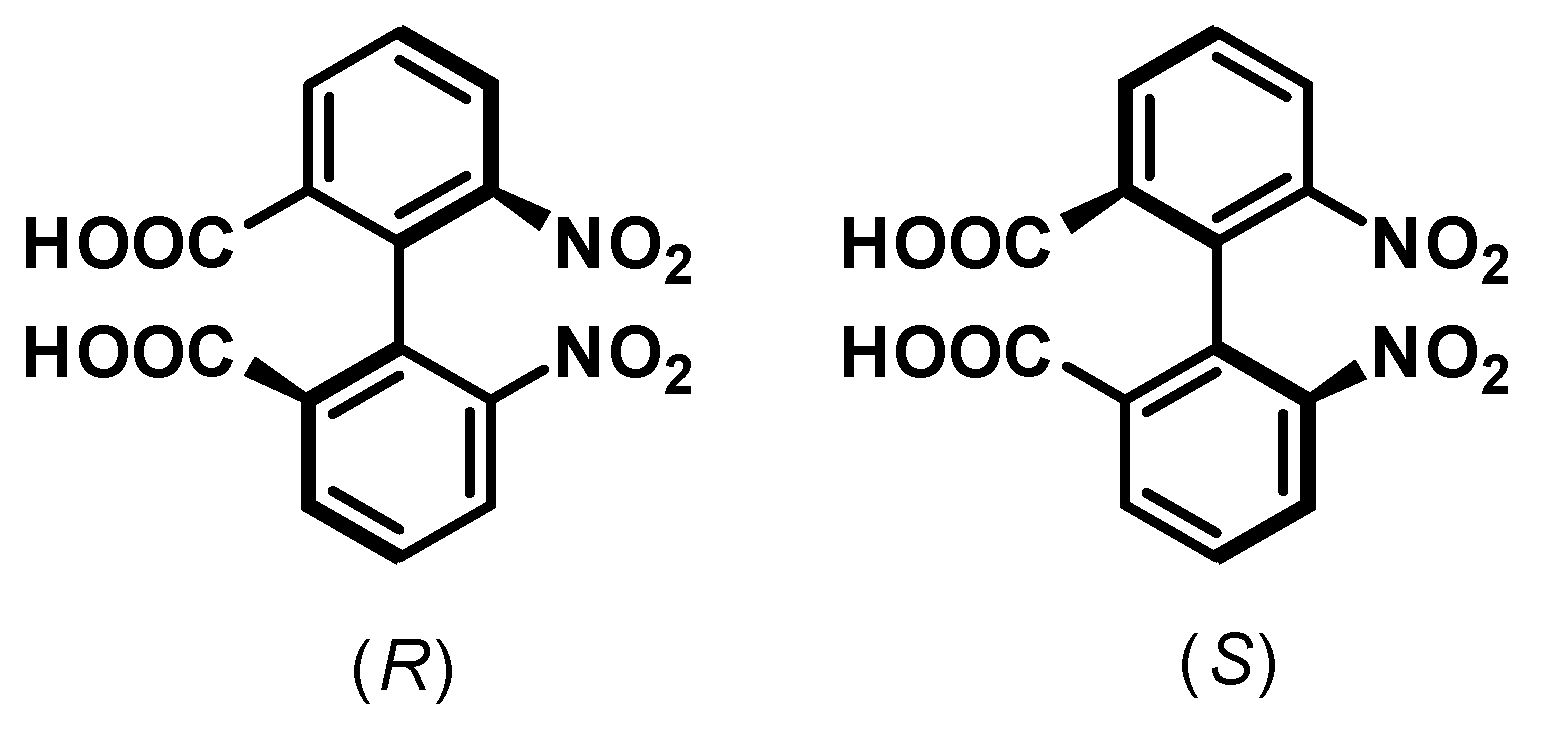
- Christie, G.H.; Kenner, J. LXXI.—The Molecular Configurations of Polynuclear Aromatic Compounds. Part I. The Resolution of γ-6 : 6′-Dinitro- and 4 : 6 : 4′ : 6′-Tetranitro-Diphenic Acids into Optically Active Components. J. Chem. Soc. Trans. 1922, 121, 614–620. [Google Scholar] [CrossRef]
- Kumarasamy, E.; Raghunathan, R.; Sibi, M.P.; Sivaguru, J. Nonbiaryl and Heterobiaryl Atropisomers: Molecular Templates with Promise for Atropselective Chemical Transformations. Chem. Rev. 2015, 115, 11239–11300. [Google Scholar] [CrossRef]
- Bringmann, G.; Gulder, T.; Gulder, T.A.M.; Breuning, M. Atroposelective Total Synthesis of Axially Chiral Biaryl Natural Products. Chem. Rev. 2011, 111, 563–639. [Google Scholar] [CrossRef]
- Zhang, X.; Zhao, K.; Gu, Z. Transition Metal-Catalyzed Biaryl Atropisomer Synthesis via a Torsional Strain Promoted Ring-Opening Reaction. Acc. Chem. Res. 2022, 55, 1620–1633. [Google Scholar] [CrossRef]
- Wang, Y.-B.; Tan, B. Construction of Axially Chiral Compounds via Asymmetric Organocatalysis. Acc. Chem. Res. 2018, 51, 534–547. [Google Scholar] [CrossRef]
- Bringmann, G.; Menche, D. Stereoselective Total Synthesis of Axially Chiral Natural Products via Biaryl Lactones. Acc. Chem. Res. 2001, 34, 615–624. [Google Scholar] [CrossRef] [PubMed]
- Rosini, C.; Franzini, L.; Raffaelli, A.; Salvadori, P. Synthesis and Applications of Binaphthylic C2-Symmetry Derivatives as Chiral Auxiliaries in Enantioselective Reactions. Synthesis 1992, 1992, 503–517. [Google Scholar] [CrossRef]
- Wencel-Delord, J.; Panossian, A.; Leroux, F.R.; Colobert, F. Recent Advances and New Concepts for the Synthesis of Axially Stereoenriched Biaryls. Chem. Soc. Rev. 2015, 44, 3418–3430. [Google Scholar] [CrossRef] [PubMed]
- Mei, G.-J.; Koay, W.L.; Guan, C.-Y.; Lu, Y. Atropisomers beyond the C–C Axial Chirality: Advances in Catalytic Asymmetric Synthesis. Chem 2022, 8, 1855–1893. [Google Scholar] [CrossRef]
- LaPlante, S.R.; Edwards, P.J.; Fader, L.D.; Jakalian, A.; Hucke, O. Cover Picture: Revealing Atropisomer Axial Chirality in Drug Discovery. ChemMedChem 2011, 6, 381. [Google Scholar] [CrossRef]
- McCarthy, M.; Guiry, P.J. Axially Chiral Bidentate Ligands in Asymmetric Catalysis. Tetrahedron 2001, 57, 3809–3844. [Google Scholar] [CrossRef]
- Hashimoto, T.; Sakata, K.; Tamakuni, F.; Dutton, M.J.; Maruoka, K. Phase-Transfer-Catalysed Asymmetric Synthesis of Tetrasubstituted Allenes. Nat. Chem. 2013, 5, 240–244. [Google Scholar] [CrossRef]
- Li, X.; Sun, J. Organocatalytic Enantioselective Synthesis of Chiral Allenes: Remote Asymmetric 1,8-Addition of Indole Imine Methides. Angew. Chem. Int. Ed. 2020, 59, 17049–17054. [Google Scholar] [CrossRef]
- Woldegiorgis, A.G.; Han, Z.; Lin, X. Organocatalytic Asymmetric Dearomatization Reaction for the Synthesis of Axial Chiral Allene-Derived Naphthalenones Bearing Quaternary Stereocenters. Org. Lett. 2021, 23, 6606–6611. [Google Scholar] [CrossRef]
- Bai, H.-Y.; Tan, F.-X.; Liu, T.-Q.; Zhu, G.-D.; Tian, J.-M.; Ding, T.-M.; Chen, Z.-M.; Zhang, S.-Y. Highly Atroposelective Synthesis of Nonbiaryl Naphthalene-1,2-Diamine N-C Atropisomers through Direct Enantioselective C-H Amination. Nat. Commun. 2019, 10, 3063. [Google Scholar] [CrossRef]
- Chen, M.; Qian, D.; Sun, J. Organocatalytic Enantioconvergent Synthesis of Tetrasubstituted Allenes via Asymmetric 1,8-Addition to Aza- Para -Quinone Methides. Org. Lett. 2019, 21, 8127–8131. [Google Scholar] [CrossRef]
- Li, F.; Liang, S.; Luan, Y.; Chen, X.; Zhao, H.; Huang, A.; Li, P.; Li, W. Organocatalytic Regio-, Diastereo- and Enantioselective γ-Additions of Isoxazol-5(4 H )-Ones to β,γ-Alkynyl-α-Imino Esters for the Synthesis of Axially Chiral Tetrasubstituted α-Amino Allenoates. Org. Chem. Front. 2021, 8, 1243–1248. [Google Scholar] [CrossRef]
- Tap, A.; Blond, A.; Wakchaure, V.N.; List, B. Chiral Allenes via Alkynylogous Mukaiyama Aldol Reaction. Angew. Chem. Int. Ed. 2016, 55, 8962–8965. [Google Scholar] [CrossRef]
- Wang, J.; Chen, M.-W.; Ji, Y.; Hu, S.-B.; Zhou, Y.-G. Kinetic Resolution of Axially Chiral 5- or 8-Substituted Quinolines via Asymmetric Transfer Hydrogenation. J. Am. Chem. Soc. 2016, 138, 10413–10416. [Google Scholar] [CrossRef]
- Zhu, W.-R.; Su, Q.; Diao, H.-J.; Wang, E.-X.; Wu, F.; Zhao, Y.-L.; Weng, J.; Lu, G. Enantioselective Dehydrative γ-Arylation of α-Indolyl Propargylic Alcohols with Phenols: Access to Chiral Tetrasubstituted Allenes and Naphthopyrans. Org. Lett. 2020, 22, 6873–6878. [Google Scholar] [CrossRef]
- Mori, K.; Itakura, T.; Akiyama, T. Enantiodivergent Atroposelective Synthesis of Chiral Biaryls by Asymmetric Transfer Hydrogenation: Chiral Phosphoric Acid Catalyzed Dynamic Kinetic Resolution. Angew. Chem. 2016, 128, 11814–11818. [Google Scholar] [CrossRef]
- Wang, Z.; Lin, X.; Chen, X.; Li, P.; Li, W. Organocatalytic Stereoselective 1,6-Addition of Thiolacetic Acids to Alkynyl Indole Imine Methides: Access to Axially Chiral Sulfur-Containing Tetrasubstituted Allenes. Org. Chem. Front. 2021, 8, 3469–3474. [Google Scholar] [CrossRef]
- Zhang, P.; Huang, Q.; Cheng, Y.; Li, R.; Li, P.; Li, W. Remote Stereocontrolled Construction of Vicinal Axially Chiral Tetrasubstituted Allenes and Heteroatom-Functionalized Quaternary Carbon Stereocenters. Org. Lett. 2019, 21, 503–507. [Google Scholar] [CrossRef] [PubMed]
- Li, G.-Q.; Gao, H.; Keene, C.; Devonas, M.; Ess, D.H.; Kürti, L. Organocatalytic Aryl–Aryl Bond Formation: An Atroposelective [3,3]-Rearrangement Approach to BINAM Derivatives. J. Am. Chem. Soc. 2013, 135, 7414–7417. [Google Scholar] [CrossRef] [PubMed]
- Qi, L.-W.; Mao, J.-H.; Zhang, J.; Tan, B. Organocatalytic Asymmetric Arylation of Indoles Enabled by Azo Groups. Nat. Chem. 2018, 10, 58–64. [Google Scholar] [CrossRef] [PubMed]
- Ma, C.; Sheng, F.-T.; Wang, H.-Q.; Deng, S.; Zhang, Y.-C.; Jiao, Y.; Tan, W.; Shi, F. Atroposelective Access to Oxindole-Based Axially Chiral Styrenes via the Strategy of Catalytic Kinetic Resolution. J. Am. Chem. Soc. 2020, 142, 15686–15696. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.-H.; Qi, L.-W.; Fang, F.; Tan, B. Organocatalytic Atroposelective Arylation of 2-Naphthylamines as a Practical Approach to Axially Chiral Biaryl Amino Alcohols. Angew. Chem. Int. Ed. 2017, 56, 16308–16312. [Google Scholar] [CrossRef]
- Hu, Y.-L.; Wang, Z.; Yang, H.; Chen, J.; Wu, Z.-B.; Lei, Y.; Zhou, L. Conversion of Two Stereocenters to One or Two Chiral Axes: Atroposelective Synthesis of 2,3-Diarylbenzoindoles. Chem. Sci. 2019, 10, 6777–6784. [Google Scholar] [CrossRef]
- Man, N.; Lou, Z.; Li, Y.; Yang, H.; Zhao, Y.; Fu, H. Organocatalytic Atroposelective Construction of Axially Chiral N-Aryl Benzimidazoles Involving Carbon–Carbon Bond Cleavage. Org. Lett. 2020, 22, 6382–6387. [Google Scholar] [CrossRef]
- Bisag, G.D.; Pecorari, D.; Mazzanti, A.; Bernardi, L.; Fochi, M.; Bencivenni, G.; Bertuzzi, G.; Corti, V. Central-to-Axial Chirality Conversion Approach Designed on Organocatalytic Enantioselective Povarov Cycloadditions: First Access to Configurationally Stable Indole–Quinoline Atropisomers. Chem. A Eur. J. 2019, 25, 15694–15701. [Google Scholar] [CrossRef]
- Kwon, Y.; Li, J.; Reid, J.P.; Crawford, J.M.; Jacob, R.; Sigman, M.S.; Toste, F.D.; Miller, S.J. Disparate Catalytic Scaffolds for Atroposelective Cyclodehydration. J. Am. Chem. Soc. 2019, 141, 6698–6705. [Google Scholar] [CrossRef]
- Lu, D.-L.; Chen, Y.-H.; Xiang, S.-H.; Yu, P.; Tan, B.; Li, S. Atroposelective Construction of Arylindoles by Chiral Phosphoric Acid-Catalyzed Cross-Coupling of Indoles and Quinones. Org. Lett. 2019, 21, 6000–6004. [Google Scholar] [CrossRef]
- Wang, C.; Li, T.; Liu, S.; Zhang, Y.; Deng, S.; Jiao, Y.; Shi, F. Axially Chiral Aryl-Alkene-Indole Framework: A Nascent Member of the Atropisomeric Family and Its Catalytic Asymmetric Construction. Chin. J. Chem. 2020, 38, 543–552. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, C.; Li, C.; Mei, G.; Li, Y.; Shi, F. Design and Enantioselective Construction of Axially Chiral Naphthyl-Indole Skeletons. Angew. Chem. 2017, 129, 122–127. [Google Scholar] [CrossRef]
- Lu, S.; Ng, S.V.H.; Lovato, K.; Ong, J.-Y.; Poh, S.B.; Ng, X.Q.; Kürti, L.; Zhao, Y. Practical Access to Axially Chiral Sulfonamides and Biaryl Amino Phenols via Organocatalytic Atroposelective N-Alkylation. Nat. Commun. 2019, 10, 3061. [Google Scholar] [CrossRef]
- Jiang, F.; Chen, K.; Wu, P.; Zhang, Y.; Jiao, Y.; Shi, F. A Strategy for Synthesizing Axially Chiral Naphthyl-Indoles: Catalytic Asymmetric Addition Reactions of Racemic Substrates. Angew. Chem. Int. Ed. 2019, 58, 15104–15110. [Google Scholar] [CrossRef]
- Mori, K.; Ichikawa, Y.; Kobayashi, M.; Shibata, Y.; Yamanaka, M.; Akiyama, T. Enantioselective Synthesis of Multisubstituted Biaryl Skeleton by Chiral Phosphoric Acid Catalyzed Desymmetrization/Kinetic Resolution Sequence. J. Am. Chem. Soc. 2013, 135, 3964–3970. [Google Scholar] [CrossRef]
- Vaidya, S.D.; Toenjes, S.T.; Yamamoto, N.; Maddox, S.M.; Gustafson, J.L. Catalytic Atroposelective Synthesis of N-Aryl Quinoid Compounds. J. Am. Chem. Soc. 2020, 142, 2198–2203. [Google Scholar] [CrossRef]
- Feringa, B.; Wynberg, H. Biomimetic Asymmetric Oxidative Coupling of Phenols. Bioorg. Chem. 1978, 7, 397–408. [Google Scholar] [CrossRef]
- Brussee, J.; Groenendijk, J.L.G.; te Koppele, J.M.; Jansen, A.C.A. On the Mechanism of the Formation of s(−)-(1, 1’-Binaphthalene)-2,2’-Diol via Copper(II)Amine Complexes. Tetrahedron 1985, 41, 3313–3319. [Google Scholar] [CrossRef]
- Nakajima, M.; Miyoshi, I.; Kanayama, K.; Hashimoto, S.; Noji, M.; Koga, K. Enantioselective Synthesis of Binaphthol Derivatives by Oxidative Coupling of Naphthol Derivatives Catalyzed by Chiral Diamine Copper Complexes. J. Org. Chem. 1999, 64, 2264–2271. [Google Scholar] [CrossRef]
- Caselli, A.; Giovenzana, G.B.; Palmisano, G.; Sisti, M.; Pilati, T. Synthesis of C2-Symmetrical Diamine Based on (1R)-(+)-Camphor and Application to Oxidative Aryl Coupling of Naphthols. Tetrahedron Asymmetry 2003, 14, 1451–1454. [Google Scholar] [CrossRef]
- Gao, J.; Reibenspies, J.H.; Martell, A.E. Structurally Defined Catalysts for Enantioselective Oxidative Coupling Reactions. Angew. Chem. Int. Ed. 2003, 42, 6008–6012. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Hewgley, J.B.; Mulrooney, C.A.; Yang, J.; Kozlowski, M.C. Enantioselective Oxidative Biaryl Coupling Reactions Catalyzed by 1,5-Diazadecalin Metal Complexes: Efficient Formation of Chiral Functionalized BINOL Derivatives. J. Org. Chem. 2003, 68, 5500–5511. [Google Scholar] [CrossRef]
- Kim, K.H.; Lee, D.-W.; Lee, Y.-S.; Ko, D.-H.; Ha, D.-C. Enantioselective Oxidative Coupling of Methyl 3-Hydroxy-2-Naphthoate Using Mono-N-Alkylated Octahydrobinaphthyl-2,2′-Diamine Ligand. Tetrahedron 2004, 60, 9037–9042. [Google Scholar] [CrossRef]
- Temma, T.; Habaue, S. Highly Selective Oxidative Cross-Coupling of 2-Naphthol Derivatives with Chiral Copper(I)–Bisoxazoline Catalysts. Tetrahedron Lett. 2005, 46, 5655–5657. [Google Scholar] [CrossRef]
- Zhang, Q.; Cui, X.; Chen, L.; Liu, H.; Wu, Y. Syntheses of Chiral Ferrocenophanes and Their Application to Asymmetric Catalysis. Eur. J. Org. Chem. 2014, 2014, 7823–7829. [Google Scholar] [CrossRef]
- Egami, H.; Katsuki, T. Iron-Catalyzed Asymmetric Aerobic Oxidation: Oxidative Coupling of 2-Naphthols. J. Am. Chem. Soc. 2009, 131, 6082–6083. [Google Scholar] [CrossRef]
- Tkachenko, N.V.; Lyakin, O.Y.; Samsonenko, D.G.; Talsi, E.P.; Bryliakov, K.P. Highly Efficient Asymmetric Aerobic Oxidative Coupling of 2-Naphthols in the Presence of Bioinspired Iron Aminopyridine Complexes. Catal. Commun. 2018, 104, 112–117. [Google Scholar] [CrossRef]
- Hon, S.-W.; Li, C.-H.; Kuo, J.-H.; Barhate, N.B.; Liu, Y.-H.; Wang, Y.; Chen, C.-T. Catalytic Asymmetric Coupling of 2-Naphthols by Chiral Tridentate Oxovanadium(IV) Complexes. Org. Lett. 2001, 3, 869–872. [Google Scholar] [CrossRef]
- Chu, C.-Y.; Hwang, D.-R.; Wang, S.-K.; Uang, B.-J. Chiral Oxovanadium Complex Catalyzed Enantioselective Oxidative Coupling of 2-Naphthols. Chem. Commun. 2001, 11, 980–981. [Google Scholar] [CrossRef]
- Barhate, N.B.; Chen, C.-T. Catalytic Asymmetric Oxidative Couplings of 2-Naphthols by Tridentate N-Ketopinidene-Based Vanadyl Dicarboxylates. Org. Lett. 2002, 4, 2529–2532. [Google Scholar] [CrossRef]
- Luo, Z.; Liu, Q.; Gong, L.; Cui, X.; Mi, A.; Jiang, Y. The Rational Design of Novel Chiral Oxovanadium(IV) Complexes for Highly Enantioselective Oxidative Coupling of 2-Naphthols. Chem. Commun. 2002, 8, 914–915. [Google Scholar] [CrossRef]
- Somei, H.; Asano, Y.; Yoshida, T.; Takizawa, S.; Yamataka, H.; Sasai, H. Dual Activation in a Homolytic Coupling Reaction Promoted by an Enantioselective Dinuclear Vanadium(IV) Catalyst. Tetrahedron Lett. 2004, 45, 1841–1844. [Google Scholar] [CrossRef]
- Guo, Q.-X.; Wu, Z.-J.; Luo, Z.-B.; Liu, Q.-Z.; Ye, J.-L.; Luo, S.-W.; Cun, L.-F.; Gong, L.-Z. Highly Enantioselective Oxidative Couplings of 2-Naphthols Catalyzed by Chiral Bimetallic Oxovanadium Complexes with Either Oxygen or Air as Oxidant. J. Am. Chem. Soc. 2007, 129, 13927–13938. [Google Scholar] [CrossRef]
- Tanaka, H.; Nishikawa, H.; Uchida, T.; Katsuki, T. Photopromoted Ru-Catalyzed Asymmetric Aerobic Sulfide Oxidation and Epoxidation Using Water as a Proton Transfer Mediator. J. Am. Chem. Soc. 2010, 132, 12034–12041. [Google Scholar] [CrossRef] [PubMed]
- Brunel, J.M. BINOL: A Versatile Chiral Reagent. Chem. Rev. 2005, 105, 857–898. [Google Scholar] [CrossRef] [PubMed]
- Wang, H. Recent Advances in Asymmetric Oxidative Coupling of 2-Naphthol and Its Derivatives. Chirality 2010, 22, 827–837. [Google Scholar] [CrossRef]
- Tkachenko, N.V.; Bryliakov, K.P. Transition Metal Catalyzed Aerobic Asymmetric Coupling of 2-Naphthols. Mini. Rev. Org. Chem. 2019, 16, 392–398. [Google Scholar] [CrossRef]
- Liao, G.; Zhou, T.; Yao, Q.-J.; Shi, B.-F. Recent Advances in the Synthesis of Axially Chiral Biaryls via Transition Metal-Catalysed Asymmetric C–H Functionalization. Chem. Commun. 2019, 55, 8514–8523. [Google Scholar] [CrossRef]
- Matsushita, M.; Kamata, K.; Yamaguchi, K.; Mizuno, N. Heterogeneously Catalyzed Aerobic Oxidative Biaryl Coupling of 2-Naphthols and Substituted Phenols in Water. J. Am. Chem. Soc. 2005, 127, 6632–6640. [Google Scholar] [CrossRef]
- Egami, H.; Matsumoto, K.; Oguma, T.; Kunisu, T.; Katsuki, T. Enantioenriched Synthesis of C1-Symmetric BINOLs: Iron-Catalyzed Cross-Coupling of 2-Naphthols and Some Mechanistic Insight. J. Am. Chem. Soc. 2010, 132, 13633–13635. [Google Scholar] [CrossRef] [PubMed]
- Narute, S.; Parnes, R.; Toste, F.D.; Pappo, D. Enantioselective Oxidative Homocoupling and Cross-Coupling of 2-Naphthols Catalyzed by Chiral Iron Phosphate Complexes. J. Am. Chem. Soc. 2016, 138, 16553–16560. [Google Scholar] [CrossRef] [PubMed]
- Libman, A.; Shalit, H.; Vainer, Y.; Narute, S.; Kozuch, S.; Pappo, D. Synthetic and Predictive Approach to Unsymmetrical Biphenols by Iron-Catalyzed Chelated Radical–Anion Oxidative Coupling. J. Am. Chem. Soc. 2015, 137, 11453–11460. [Google Scholar] [CrossRef] [PubMed]
- Shibasaki, M.; Matsunaga, S. Design and Application of Linked-BINOL Chiral Ligands in Bifunctional Asymmetric Catalysis. Chem. Soc. Rev. 2006, 35, 269. [Google Scholar] [CrossRef] [PubMed]
- Tian, J.; Wang, A.; Yang, J.; Zhao, X.; Tu, Y.; Zhang, S.; Chen, Z. Copper-Complex-Catalyzed Asymmetric Aerobic Oxidative Cross-Coupling of 2-Naphthols: Enantioselective Synthesis of 3,3′-Substituted C1 -Symmetric BINOLs. Angew. Chem. 2019, 131, 11139–11143. [Google Scholar] [CrossRef]
- Zang, C.; Liu, Y.; Xu, Z.-J.; Tse, C.-W.; Guan, X.; Wei, J.; Huang, J.-S.; Che, C.-M. Highly Enantioselective Iron-Catalyzed Cis -Dihydroxylation of Alkenes with Hydrogen Peroxide Oxidant via an Fe III -OOH Reactive Intermediate. Angew. Chem. 2016, 128, 10409–10413. [Google Scholar] [CrossRef]
- Wu, L.Y.; Usman, M.; Liu, W.B. Enantioselective Iron/Bisquinolyldiamine Ligand-catalyzed Oxidative Coupling Reaction of 2-naphthols. Molecules 2020, 25, 852. [Google Scholar] [CrossRef]
- Hayashi, H.; Ueno, T.; Kim, C.; Uchida, T. Ruthenium-Catalyzed Cross-Selective Asymmetric Oxidative Coupling of Arenols. Org. Lett. 2020, 22, 1469–1474. [Google Scholar] [CrossRef]
- Chinnaraja, E.; Arunachalam, R.; Pillai, R.S.; Peuronen, A.; Rissanen, K.; Subramanian, P.S. One-Pot Synthesis of [2+2]-Helicate-like Macrocycle and 2+4-Μ4-Oxo Tetranuclear Open Frame Complexes: Chiroptical Properties and Asymmetric Oxidative Coupling of 2-Naphthols. Appl. Organomet. Chem. 2020, 34, e5666. [Google Scholar] [CrossRef]
- Horibe, T.; Nakagawa, K.; Hazeyama, T.; Takeda, K.; Ishihara, K. An Enantioselective Oxidative Coupling Reaction of 2-Naphthol Derivatives Catalyzed by Chiral Diphosphine Oxide-Iron(II) Complexes. Chem. Commun. 2019, 55, 13677–13680. [Google Scholar] [CrossRef]
- Wang, P.; Cen, S.; Gao, J.; Shen, A.; Zhang, Z. Novel Axially Chiral Ligand-Enabled Copper-Catalyzed Asymmetric Oxidative Coupling of 2-Naphthols for the Synthesis of 6,6′-Disubstituted BINOLs. Org. Lett. 2022, 24, 2321–2326. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Sasai, H.; Takizawa, S. Atroposelective Synthesis of C-C Axially Chiral Compounds via Mono- and Dinuclear Vanadium Catalysis. Acc. Chem. Res. 2022, 55, 2949–2965. [Google Scholar] [CrossRef]
- Qiu, H.; Shuai, B.; Wang, Y.Z.; Liu, D.; Chen, Y.G.; Gao, P.-S.; Ma, H.X.; Ma, H.X.; Chen, S.; Mei, T.S. Enantioselective Ni-Catalyzed Electrochemical Synthesis of Biaryl Atropisomers. J. Am. Chem. Soc. 2020, 142, 9872–9878. [Google Scholar] [CrossRef] [PubMed]
- Osa, T.; Kashiwagi, Y.; Yanagisawa, Y.; Bobbitt, J.M. Enantioselective, Electrocatalytic Oxidative Coupling of Naphthol, Naphthyl Ether and Phenanthrol on a TEMPO-Modified Graphite Felt Electrode in the Presence of (-)-Sparteine (TEMPO = 2,2,6,6-Tetramethylpiperidin-1-Yloxyl). J. Chem. Soc. Chem. Commun. 1994, 1, 2535–2537. [Google Scholar] [CrossRef]
- Cheng, J.K.; Xiang, S.-H.; Tan, B. Organocatalytic Enantioselective Synthesis of Axially Chiral Molecules: Development of Strategies and Skeletons. Acc. Chem. Res. 2022, 55, 2920–2937. [Google Scholar] [CrossRef]
- Aoyama, H.; Tokunaga, M.; Kiyosu, J.; Iwasawa, T.; Obora, Y.; Tsuji, Y. Kinetic Resolution of Axially Chiral 2,2‘-Dihydroxy-1,1‘-biaryls by Palladium-Catalyzed Alcoholysis. J. Am. Chem. Soc. 2005, 127, 10474–10475. [Google Scholar] [CrossRef]
- Terada, M.; Dan, K. Synthesis of unsymmetrically substituted 2,2′ -dihydroxy-1,1′-biaryl derivatives using organic-base-catalyzed Ferrier-type rearrangement as the key step. Chem. Commun. 2012, 48, 5781–5783. [Google Scholar] [CrossRef]
- Kawashima, M.; Hirata, R. Epimerization-Crystallization Method in Optical Resolution of 2,2′-dihydroxy-1,1′-binaphthyl, and Kinetic Study. Bull. Chem. Soc. Jpn. 1993, 66, 2002–2005. [Google Scholar] [CrossRef]
- Ma, G.; Deng, J.; Sibi, M.P. Fluxionally Chiral DMAP Catalysts: Kinetic Resolution of Axially Compounds. Angew. Chem. Int. Ed. 2014, 53, 11818–11821. [Google Scholar] [CrossRef]
- Lu, S.; Poh, S.B.; Zhao, Y. Kinetic Resolution of 1,1′-Biaryl-2,2′-Diols and Amino Alcohols through NHC-Catalyzed Atroposelective Acylation. Angew. Chem. Int. Ed. 2014, 53, 11041–11045. [Google Scholar] [CrossRef] [PubMed]
- Link, A.; Sparr, C. Organocatalytic Atroposelective Aldol Condensation: Synthesis of Axially Chiral Biaryls by Arene Formation. Angew. Chem. Int. Ed. 2014, 53, 5458–5461. [Google Scholar] [CrossRef]
- Shirakawa, S.; Maruoka, K. A New Strategy for Organocatalyzed Asymmetric Synthesis of BINOL Derivatives. Chem 2017, 2, 329–331. [Google Scholar] [CrossRef][Green Version]
- Witzig, R.M.; Fäseke, V.C.; Häussinger, D.; Sparr, C. Atroposelective Synthesis of Tetra-Ortho-Substituted Biaryls by Catalyst-Controlled Non-Canonical Polyketide Cyclizations. Nat. Catal. 2019, 2, 925–930. [Google Scholar] [CrossRef]
- Jones, B.A.; Balan, T.; Jolliffe, J.D.; Campbell, C.D.; Smith, M.D. Practical and Scalable Kinetic Resolution of BINOLs Mediated by a Chiral Counterion. Angew. Chem. Int. Ed. 2019, 58, 4596–4600. [Google Scholar] [CrossRef] [PubMed]
- Kazlauskas, R.J. Resolution of Binaphthols and Spirobiindanols Using Cholesterol Esterase. J. Am. Chem. Soc. 1989, 111, 4953–4959. [Google Scholar] [CrossRef]
- Aoyagi, N.; Ogawa, N.; Izumi, T. Effects of Reaction Temperature and Acyl Group for Lipase-Catalyzed Chiral Binaphthol Synthesis. Tetrahedron Lett. 2006, 47, 4797–4801. [Google Scholar] [CrossRef]
- Moustafa, G.A.I.; Oki, Y.; Akai, S. Lipase-Catalyzed Dynamic Kinetic Resolution of C1- and C2-Symmetric Racemic Axially Chiral 2,2′-Dihydroxy-1,1′-Biaryls. Angew. Chem. Int. Ed. 2018, 57, 10278–10282. [Google Scholar] [CrossRef]
- Ooi, T.; Kameda, M.; Maruoka, K. Molecular Design of a C2-Symmetric Chiral Phase-Transfer Catalyst for Practical Asymmetric Synthesis of α-Amino Acids. J. Am. Chem. Soc. 1999, 121, 6519–6520. [Google Scholar] [CrossRef]
- Ooi, T.; Kameda, M.; Maruoka, K. Design of N-Spiro C2-Symmetric Chiral Quaternary Ammonium Bromides as Novel Chiral Phase-Transfer Catalysts: Synthesis and Application to Practical Asymmetric Synthesis of r -Amino Acids. J. Am. Chem. Soc. 2003, 125, 5139–5151. [Google Scholar] [CrossRef]
- Jiang, Y.; Diagne, A.B.; Thomson, R.J.; Schaus, S.E. Enantioselective Synthesis of Allenes by Catalytic Traceless Petasis Reactions. J. Am. Chem. Soc. 2017, 139, 1998–2005. [Google Scholar] [CrossRef] [PubMed]
- Nakashima, D.; Yamamoto, H. Design of Chiral N-Triflyl Phosphoramide as a Strong Chiral Brønsted Acid and Its Application to Asymmetric Diels-Alder Reaction. J. Am. Chem. Soc. 2006, 128, 9626–9627. [Google Scholar] [CrossRef] [PubMed]
- Rueping, M.; Nachtsheim, B.J.; Koenigs, R.M.; Ieawsuwan, W. Synthesis and Structural Aspects of N-Triflylphosphoramides and Their Calcium Salts’highly Acidic and Effective Brønsted Acids. Chem. A Eur. J. 2010, 16, 13116–13126. [Google Scholar] [CrossRef] [PubMed]
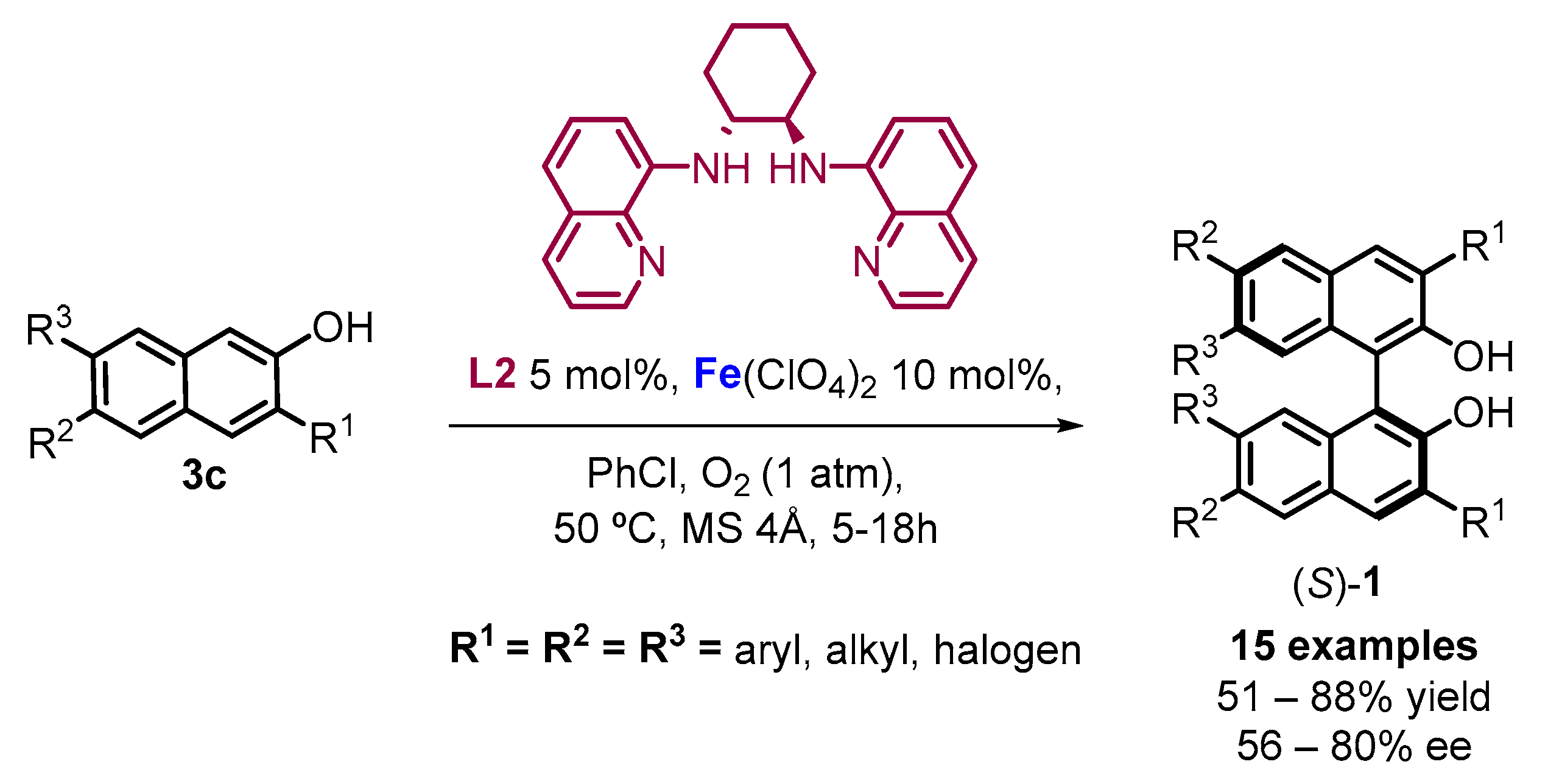

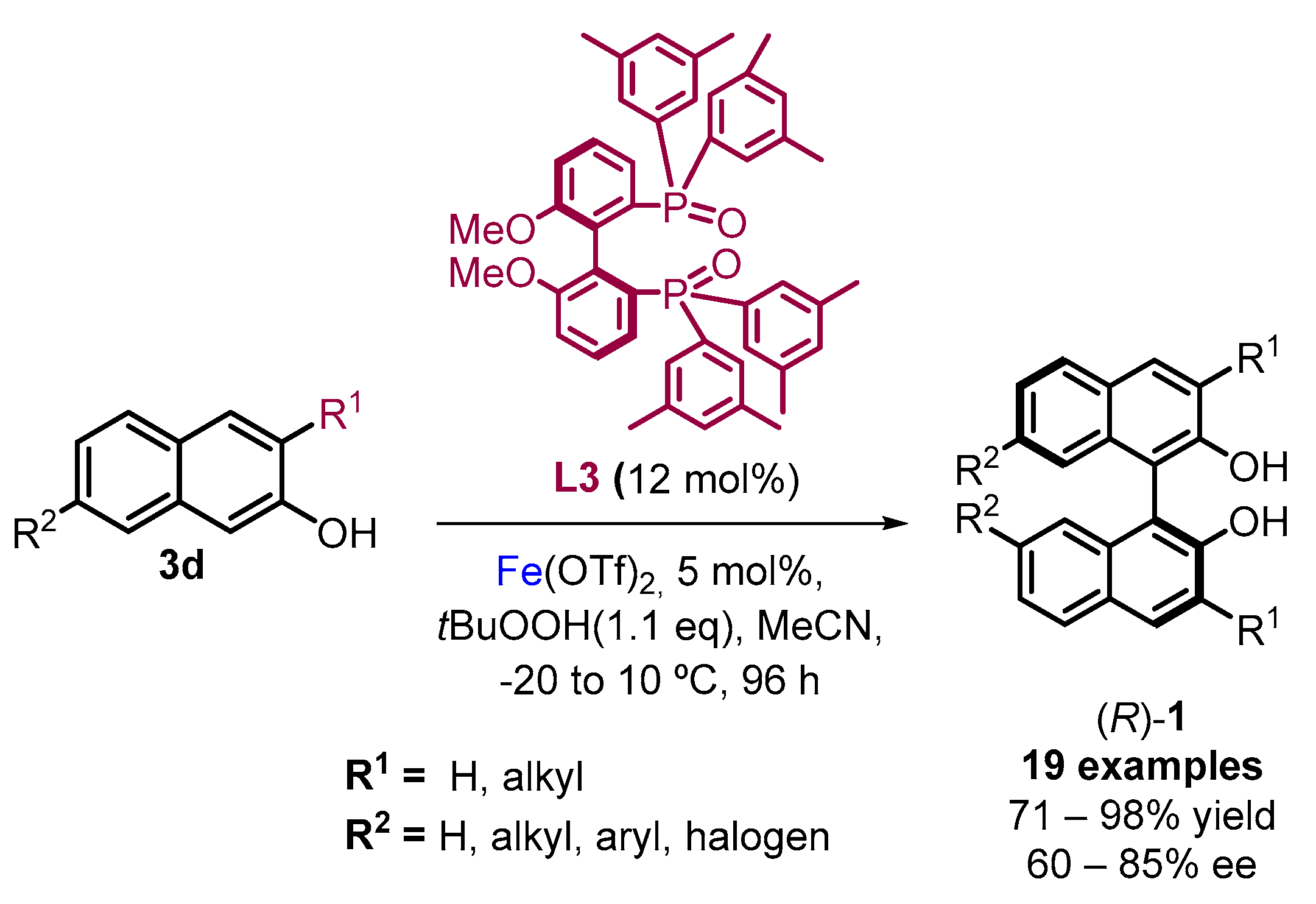


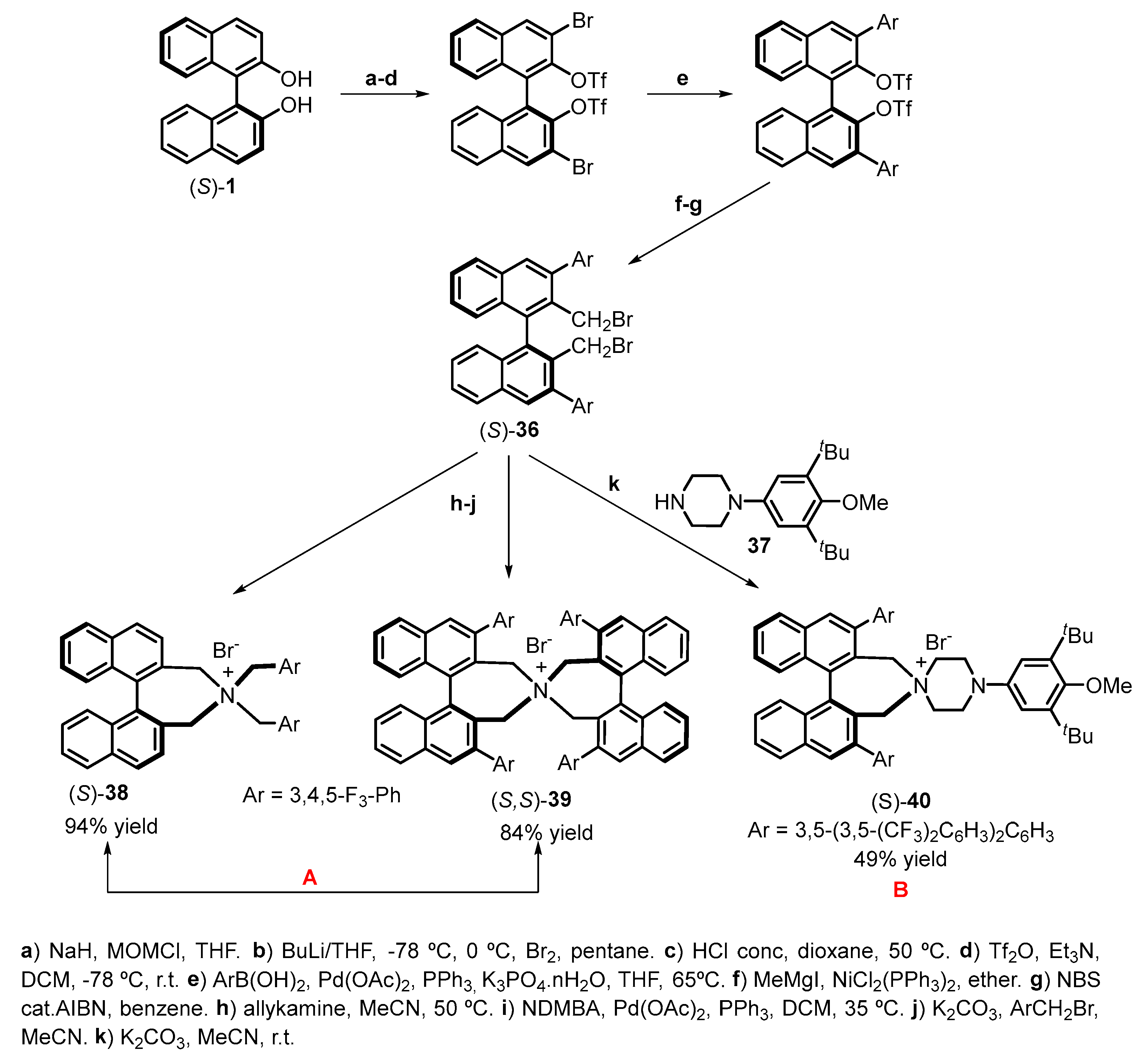
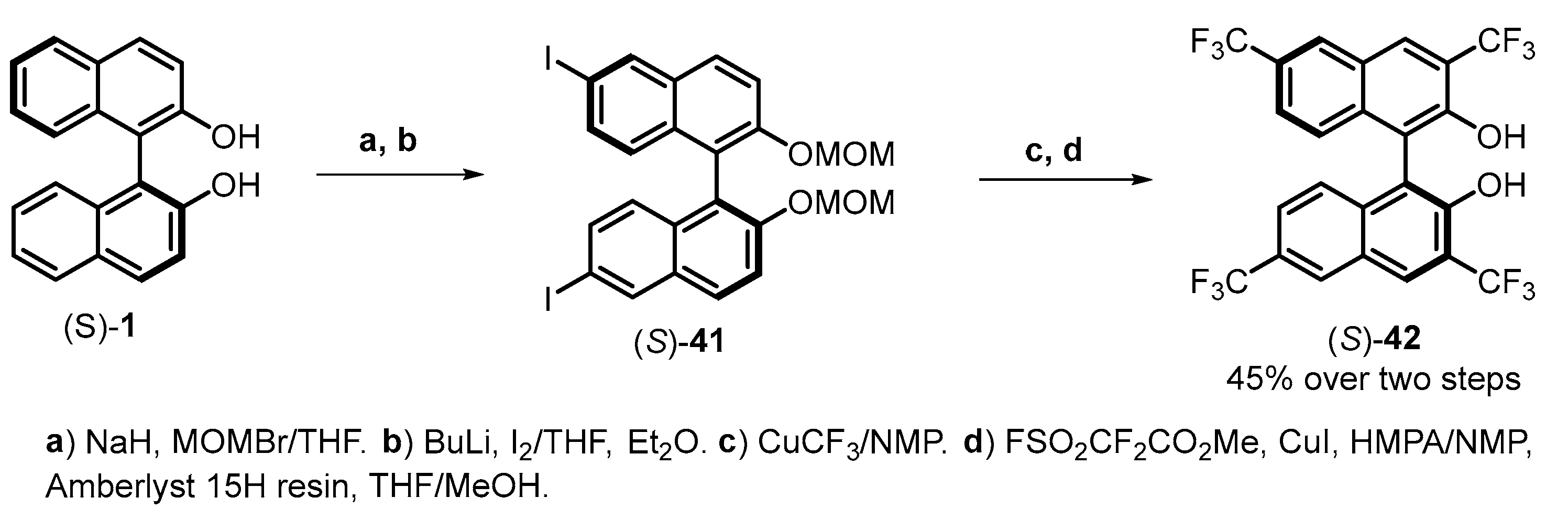
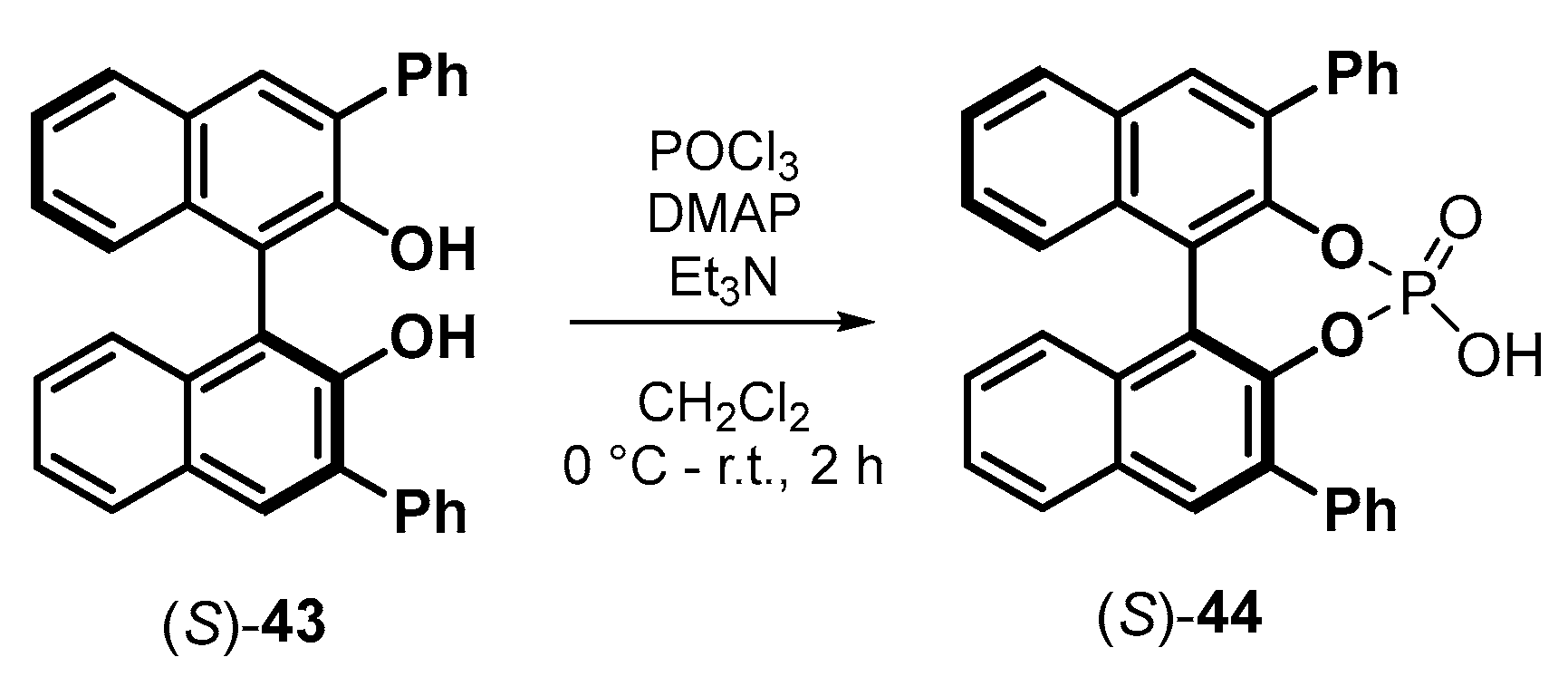
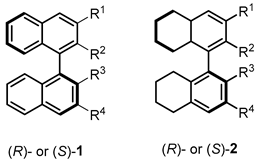 | |||||||
| Entry | R1/R4 | R2/R3 | Ref. | Entry | R1/R4 | R2/R3 | Ref. |
| (R)-1 | 3,5-(3,5-(CF3)2C6H3)2C6H3 |  | [12] | (R)-1 | 9-anthryl | 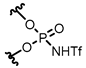 | [13] |
| (R)-1 | 3,5-(3,5-(CF3)2C6H3)2C6H3 | 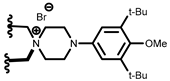 | [12] | (R)-1 | 9-phenanthryl |  | [14,15] |
| (R)-1 | 1-pyrenyl |  | [16] | (R)-1 | 2,4,6-(i-Pr)3C6H2 |  | [17] |
| (R)-1 | 9-anthryl |  | [16] | (S)-1 |  | 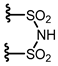 | [18] |
| (S)-1 | 2,5-Me2-4-t-BuC6H2 |  | [13] | (S)-1 | 2,4,6-(CH3)3C6H2 |  | [19] |
| (S)-1 | 1-naphthyl |  | [20] | (S)-1 | Si(3-F-C6H4)3 |  | [21] |
| (S)-1 | 1-pyrenyl |  | [20,22,23] | (S)-1 | 3,5-(CF3)2C6H3 |  | [24] |
| (S)-1 | C6F5 |  | [25] | (S)-1 | 2-naphthyl |  | [26] |
| (S)-1 | 2,4,6-(i-Pr)3C6H2 |  | [27,28,29,30] | (S)-1 | 9-anthryl |  | [23,31,32] |
| (R)-2 | 2-naphtyl |  | [21,33] | (S)-2 | 2,4,6-Me2C6H2 |  | [34] |
| (R)-2 | 2,4,6-(i-Pr)3C6H2 |  | [35] | (S)-2 | 2-naphtyl |  | [36] |
| (S)-2 | 9-anthryl |  | [37] | (S)-2 | 1-naphthyl |  | [38] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
da Silva, E.M.; Vidal, H.D.A.; Januário, M.A.P.; Corrêa, A.G. Advances in the Asymmetric Synthesis of BINOL Derivatives. Molecules 2023, 28, 12. https://doi.org/10.3390/molecules28010012
da Silva EM, Vidal HDA, Januário MAP, Corrêa AG. Advances in the Asymmetric Synthesis of BINOL Derivatives. Molecules. 2023; 28(1):12. https://doi.org/10.3390/molecules28010012
Chicago/Turabian Styleda Silva, Everton Machado, Hérika Danielle Almeida Vidal, Marcelo Augusto Pereira Januário, and Arlene Gonçalves Corrêa. 2023. "Advances in the Asymmetric Synthesis of BINOL Derivatives" Molecules 28, no. 1: 12. https://doi.org/10.3390/molecules28010012
APA Styleda Silva, E. M., Vidal, H. D. A., Januário, M. A. P., & Corrêa, A. G. (2023). Advances in the Asymmetric Synthesis of BINOL Derivatives. Molecules, 28(1), 12. https://doi.org/10.3390/molecules28010012