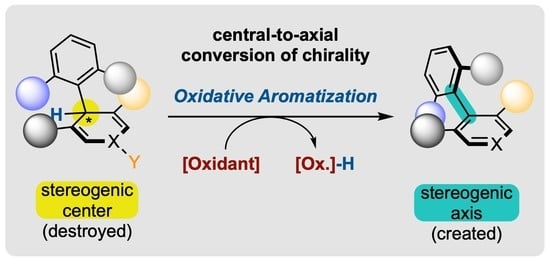
Enantioselective Synthesis of Atropisomers by Oxidative Aromatization with Central-to-Axial Conversion of Chirality
Abstract
1. Introduction
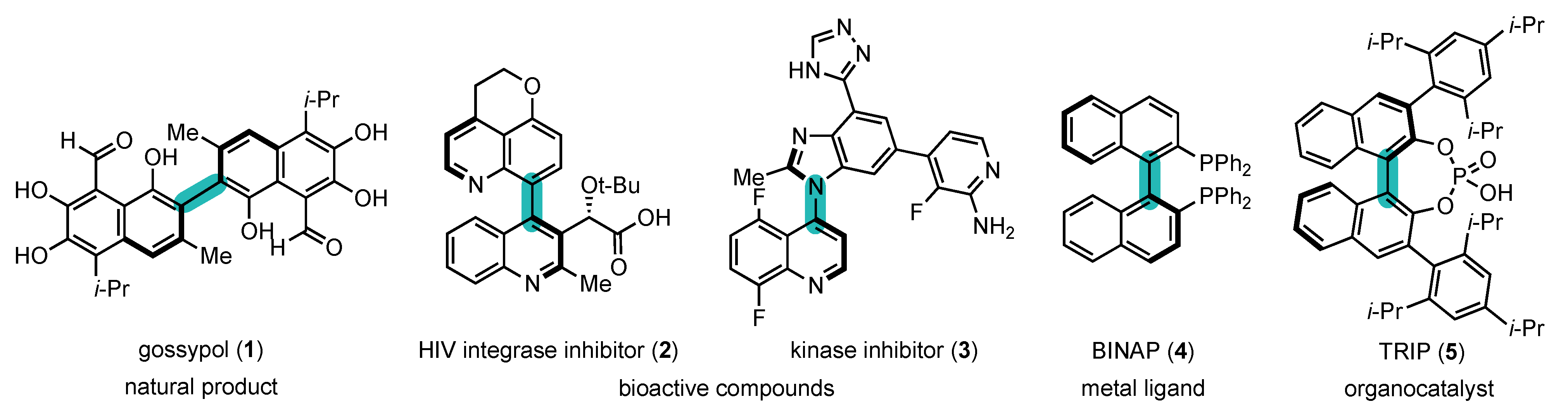
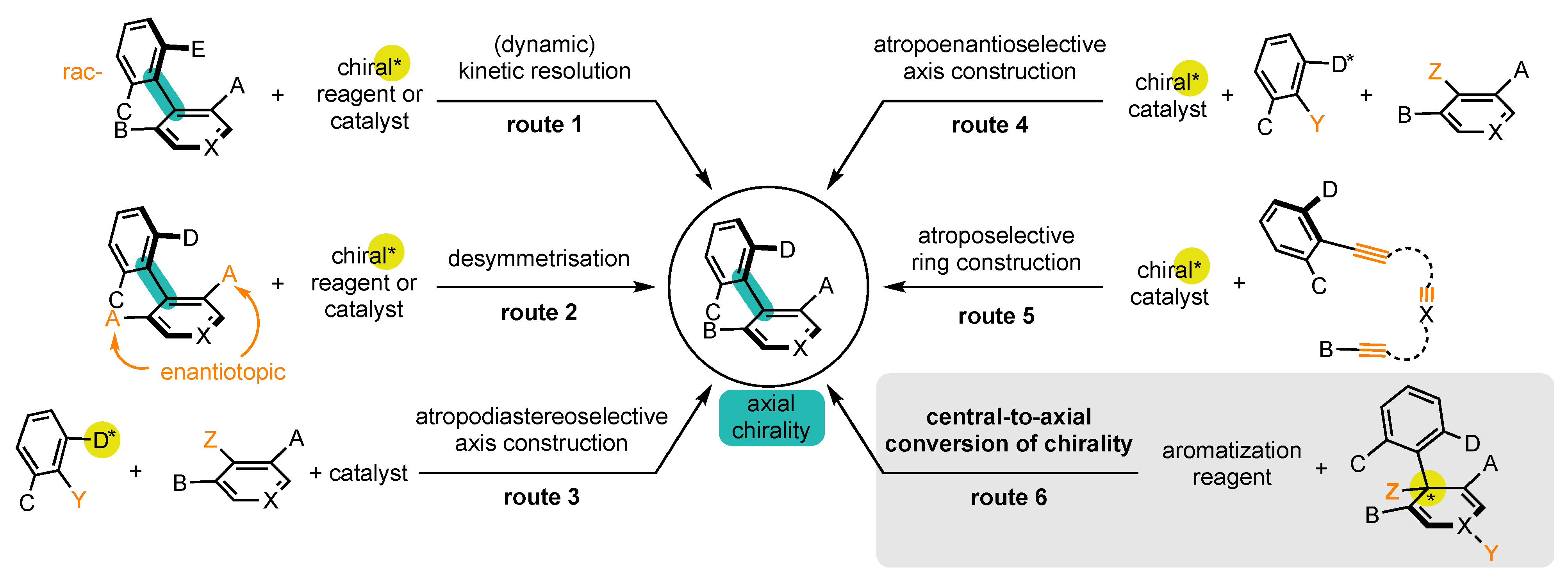
1.1. Interest of Atropisomers and Synthetic Methods to Prepare Them
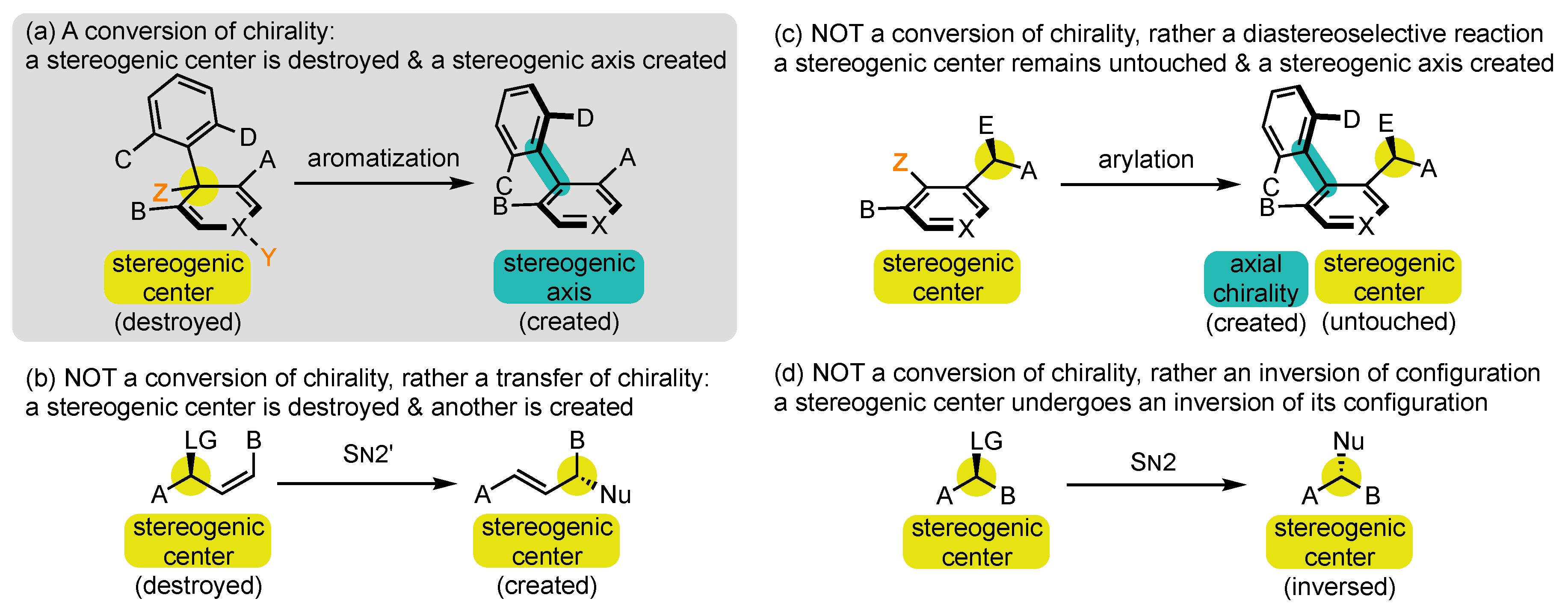
1.2. Defining Conversion of Chirality
- At first, chirality is used in a quite improper sense, as it is normally applied to a molecule or an object in its entirety, while the process discussed here falls within local stereochemistry discussions [52]. Conversion of stereogenicity should, in fact, be recommended, but it seems completely absent from the literature and perhaps more difficult to comprehend for those who are not specialists of this field.
- Conversions of chirality are also referred to in the literature by several other denominations, which all appear to us as less suited. Transfer has long been the most frequently used term [53,54,55], but it brings the idea of relocation rather than transformation. For us, it should be restricted to reactions where the stereogenic element that is destroyed and the one that is created are of the same nature, such as in SN2′ reactions with allylic leaving groups (Scheme 2b) [56]. Exchange [49,57] and interconversion [47] can also be encountered, but they evoke the idea of reversibility, which is generally not the case in those transformations.
2. Historical Background
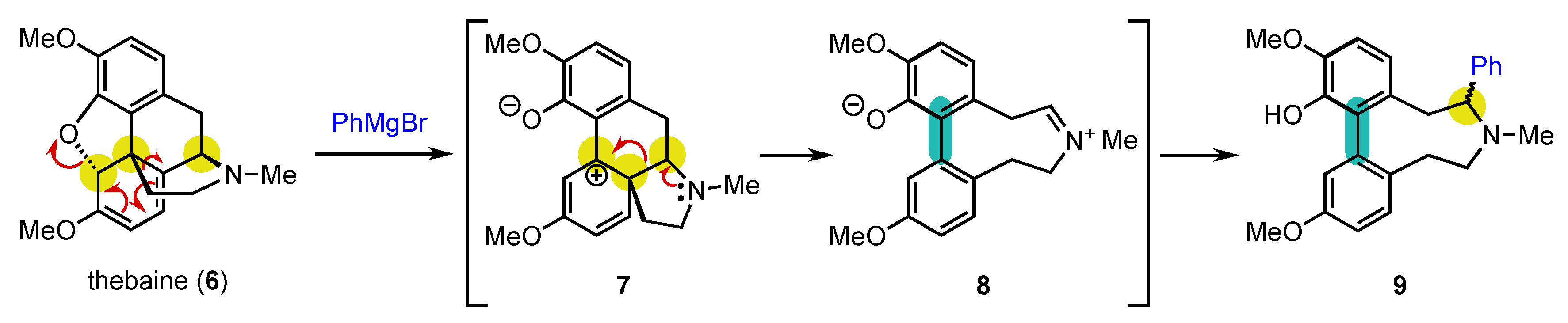
2.1. A surprisingly Early First Example!
2.2. Formulating Research Hypotheses to Achieve Conversion of Chirality
- Centrally chiral non-aromatic precursors should be accessible in enantioenriched form;
- Steric hindrance around the stereogenic axis should be sufficient to ensure its configurational stability;
- Aromatization should occur with a preservation of the enantiopurity.
2.3. Verification of Berson’s Hypotheses by Meyers
2.4. Transformations Other Than Oxidations for Conversion of Chirality
3. Synthesis of Pyridine Atropisomers
3.1. 4-Arylpyridineatropisomers
3.2. Nicotinamide Atropisomers
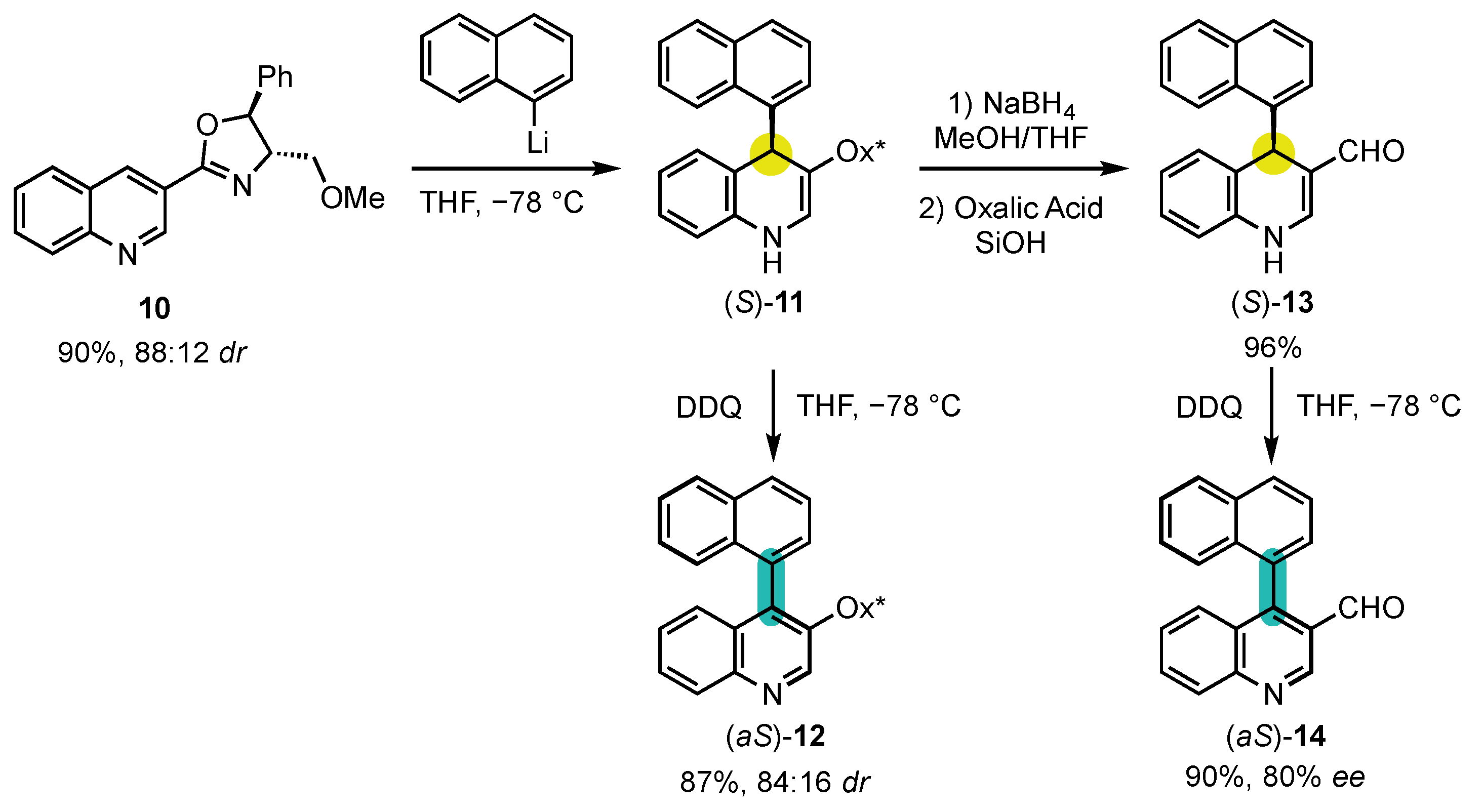
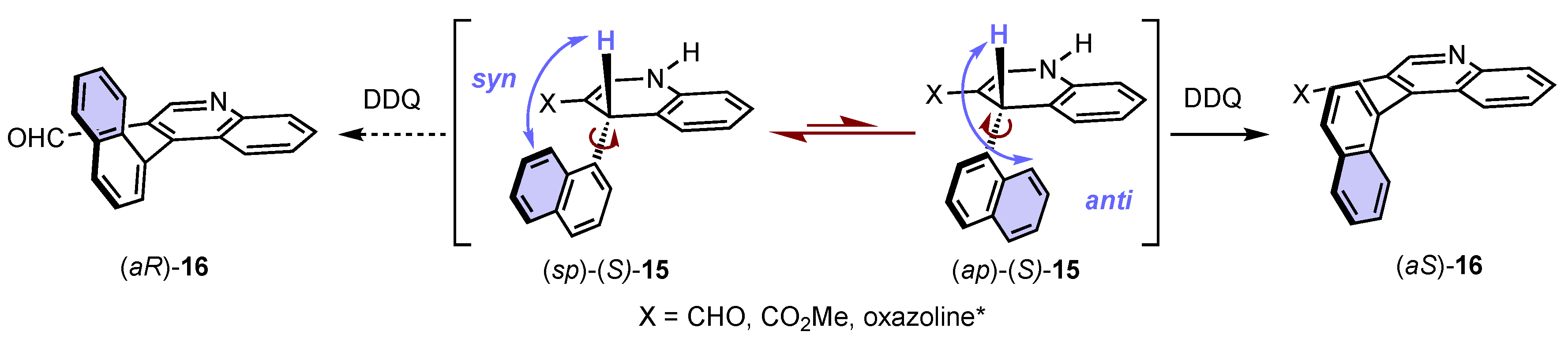
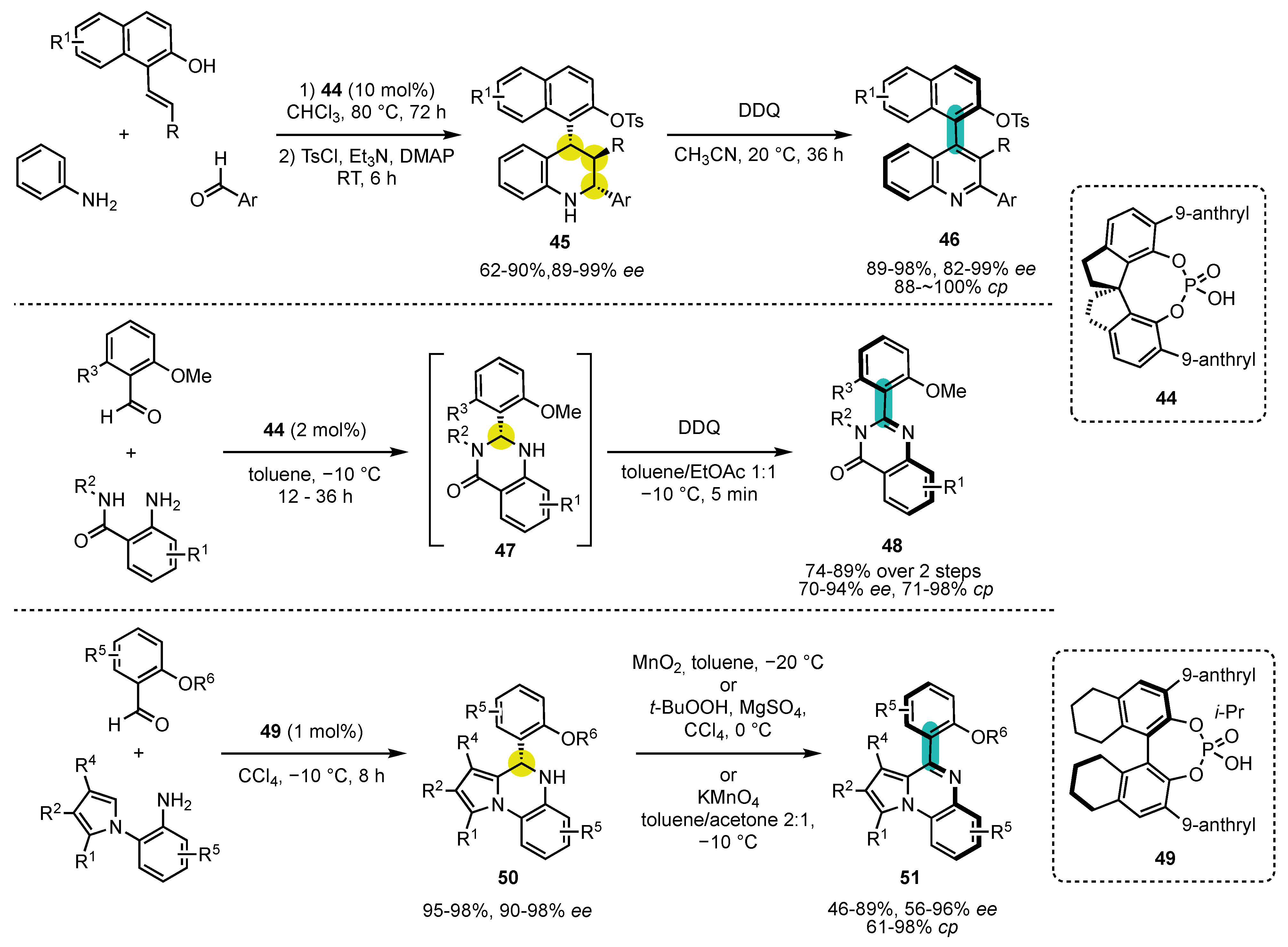
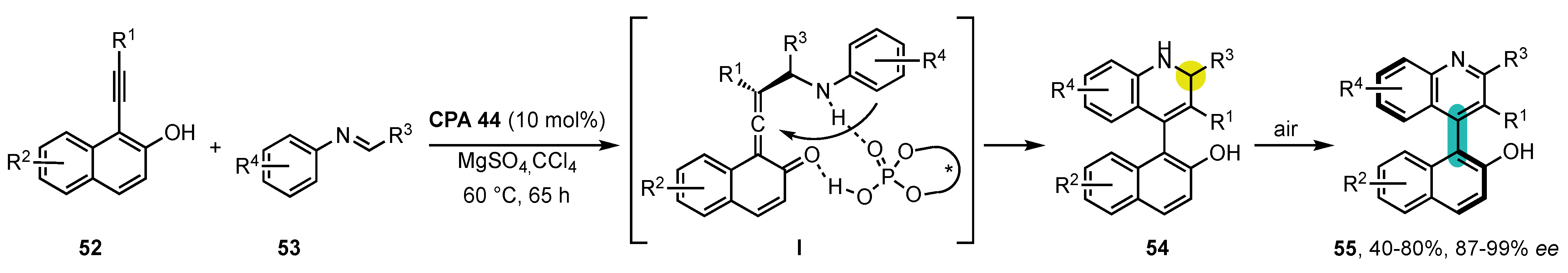
4. Synthesis of 4-Arylquinoline Atropisomers
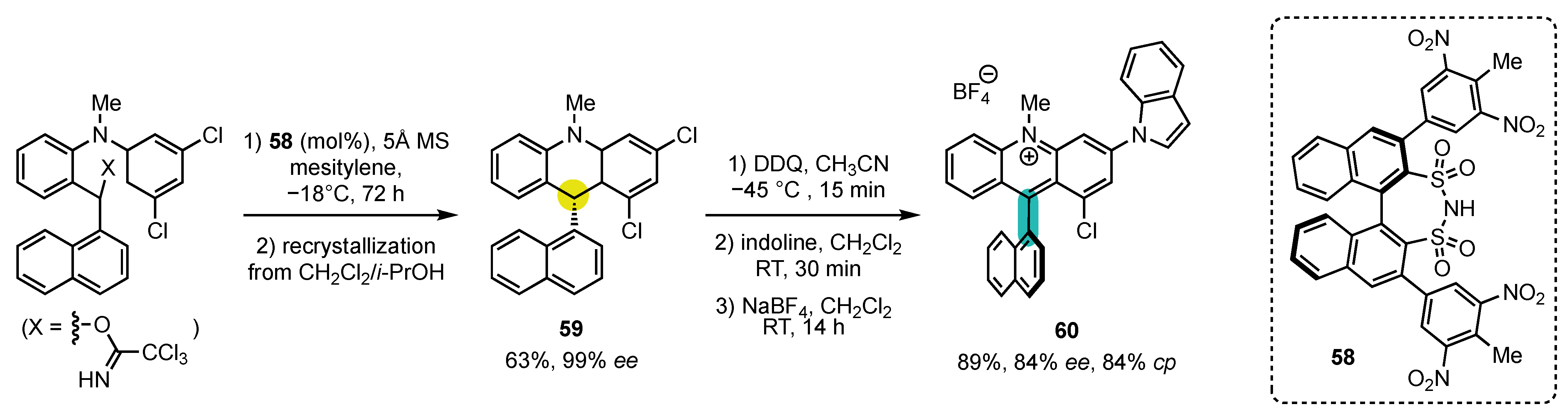
5. Synthesis of 4-Arylacridinium Atropisomers
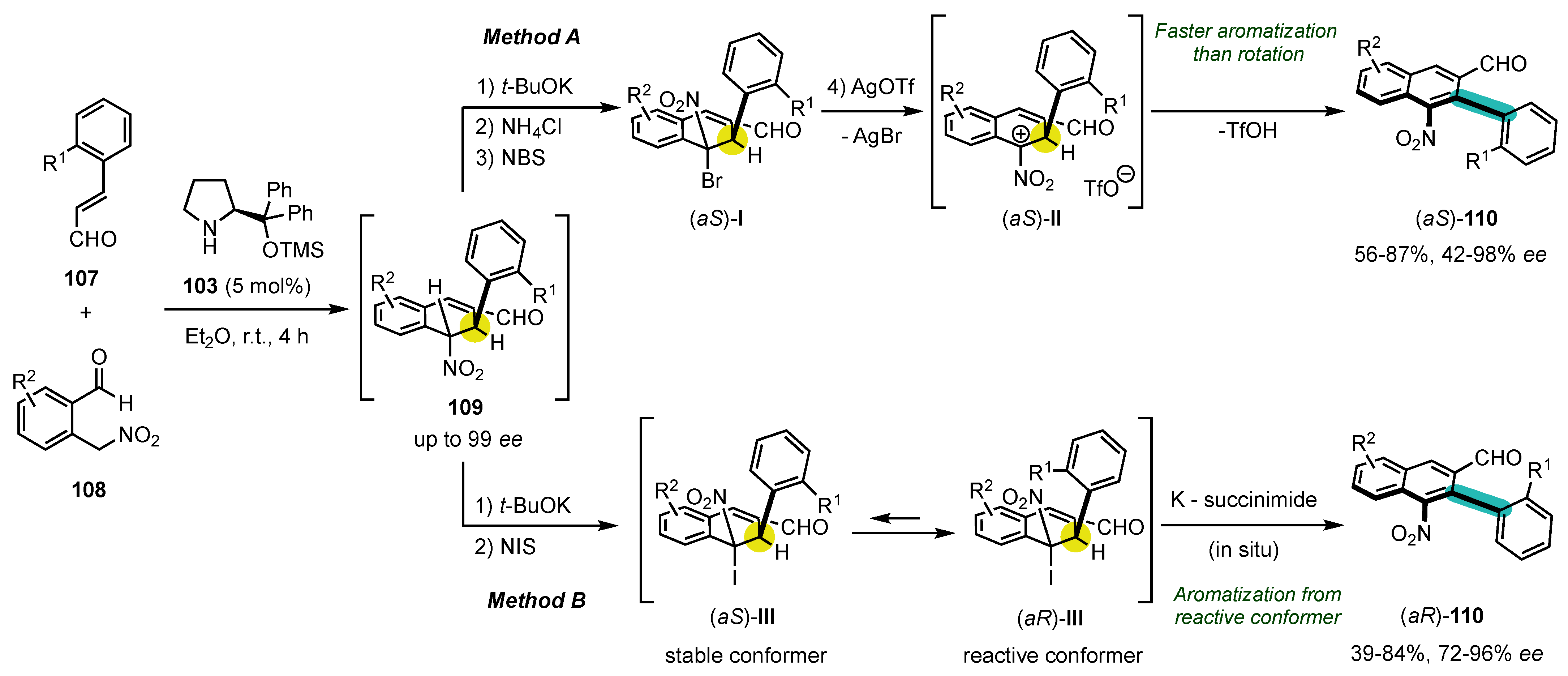
6. Synthesis of Five-Membered Heterocyclic Atropisomers
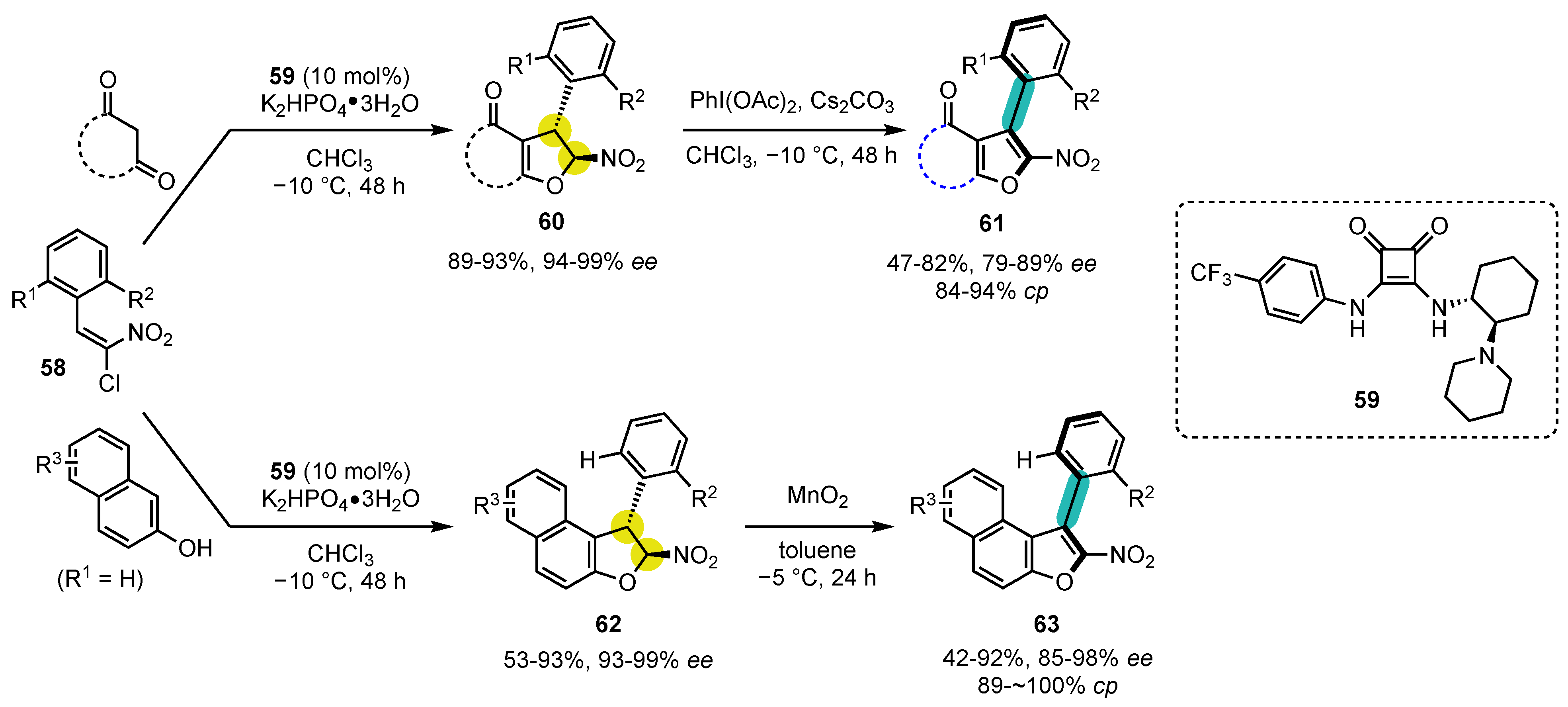
6.1. Oxygen-Containing Heterocycles
6.2. Nitrogen-Containing Heterocycles
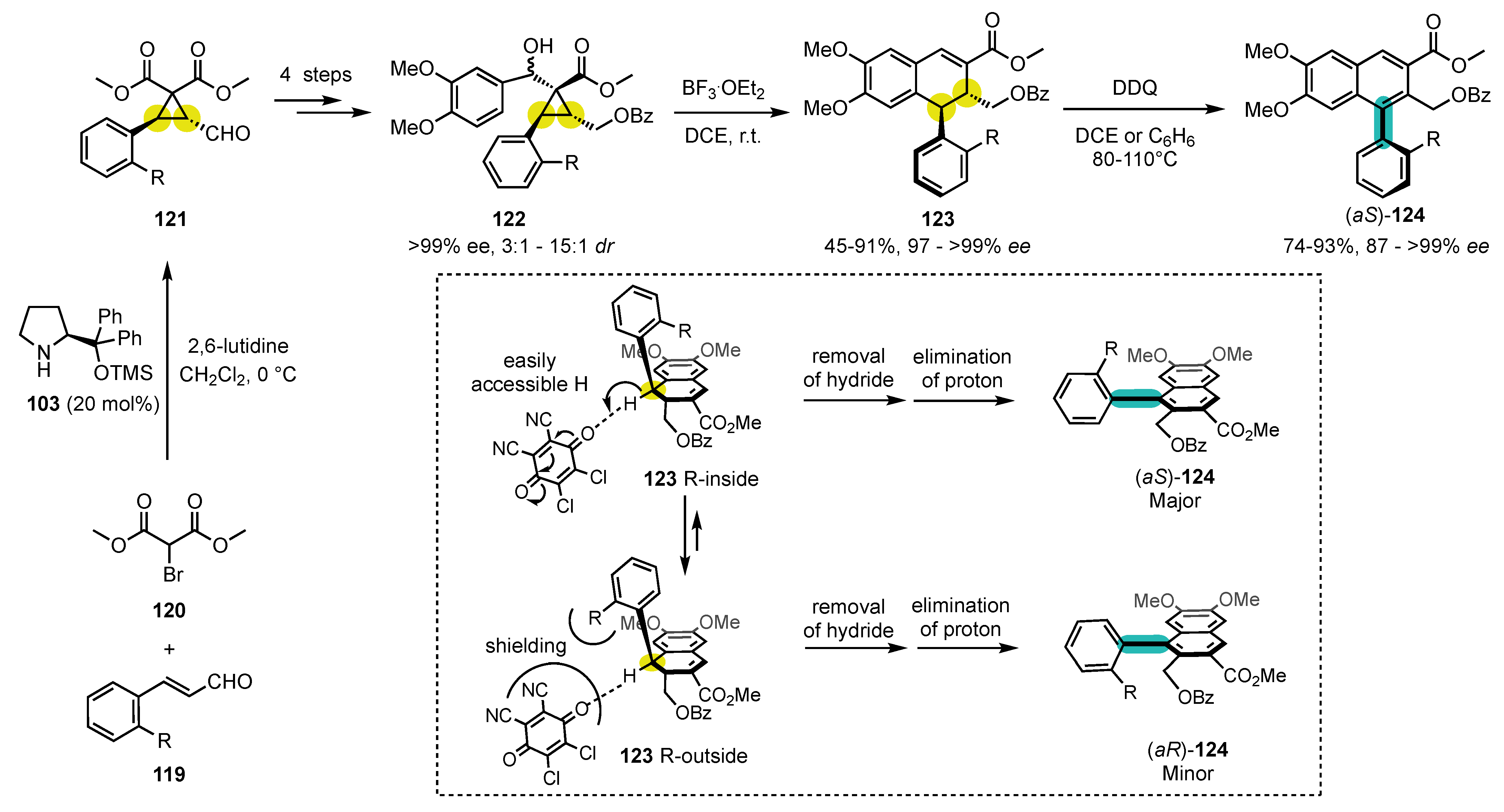
7. Synthesis of Carbocyclic Atropisomers
7.1. Seminal Results
7.2. Combining Enantioselective Organocatalysis with Conversion of Chirality
7.3. Miscellaneous
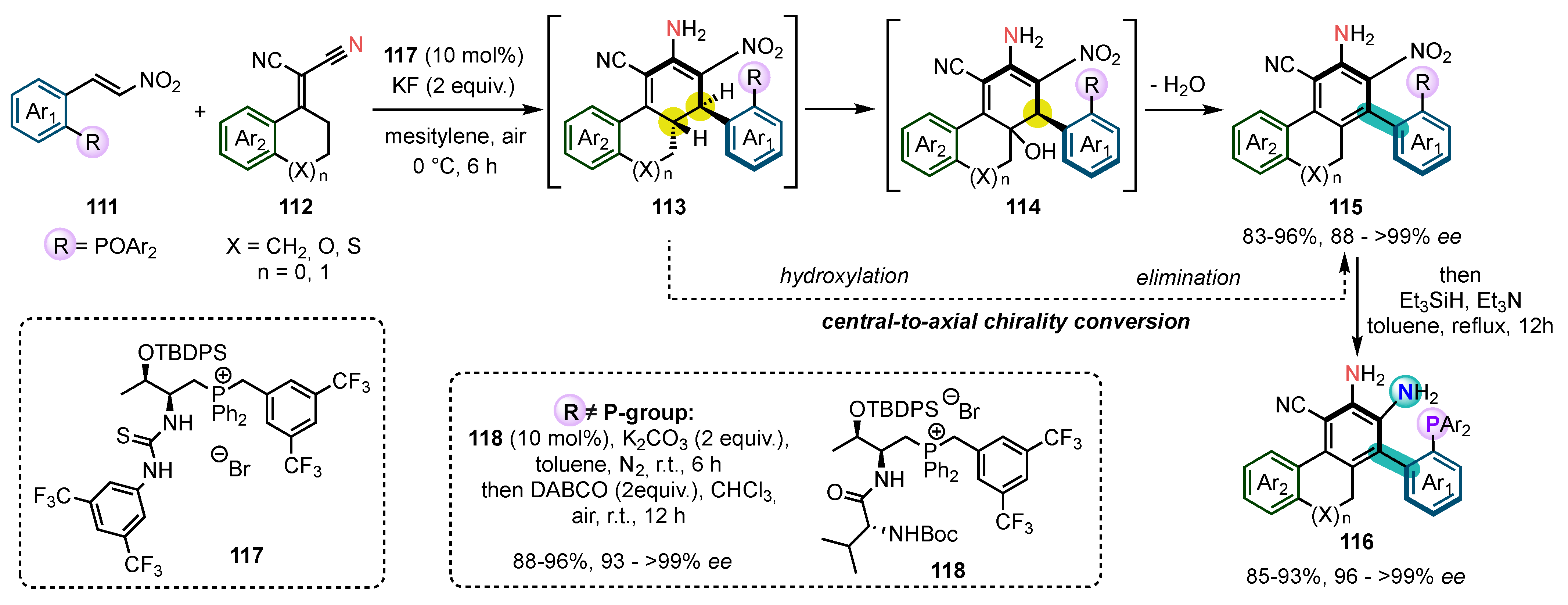
8. Control of C-N Atropisomerism
- With a very bulky tert-butyl group in ortho position (128a), the rotation around the C-N bond is already restricted before oxidative aromatization with very high diastereocontrol, so that this step is not really a conversion of chirality but rather a stereoablative process [114];
- With a smaller iodine atom (128b), the rotation is still possible around the C-N bond before aromatization, resulting in the production of equilibrating diastereomeric conformers, so that the oxidative aromatization fully meets the criteria to be considered a conversion of chirality;
- When a second ortho substituent is present (128c), the two diastereomers could be isolated, and each of them was oxidized separately, leading to the two enantiomers of the final atropisomeric product.
9. Conclusions and Perspective
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Christie, G.H.; Kenner, J. LX.—The molecular configurations of polynuclear aromatic compounds. Part V. The identity of the nitration products derived from 2:7- and 4:5-dinitrophenanthraquinones. J. Chem. Soc. Trans. 1922, 121, 614–620. [Google Scholar] [CrossRef]
- Ōki, M. Recent Advances in Atropisomerism. In Topics in Stereochemistry; Allinger, N.L., Eliel, E.L., Wilen, S.H., Eds.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2007; Volume 14, pp. 1–81. [Google Scholar] [CrossRef]
- Entropic factors are generally small for rotations around a bond, and can be neglected. As a consequence, barriers to rotation can be seen as temperature-independent.
- Kumarasamy, E.; Raghunathan, R.; Sibi, M.P.; Sivaguru, J. Nonbiaryl and Heterobiaryl Atropisomers: Molecular Templates with Promise for Atropselective Chemical Transformations. Chem. Rev. 2015, 115, 11239–11300. [Google Scholar] [CrossRef]
- Bonne, D.; Rodriguez, J. Enantioselective syntheses of atropisomers featuring a five-membered ring. Chem. Commun. 2017, 53, 12385–12393. [Google Scholar] [CrossRef] [PubMed]
- Bonne, D.; Rodriguez, J. A Bird’s Eye View of Atropisomers Featuring a Five-Membered Ring. Eur. J. Org. Chem. 2018, 2018, 2417–2431. [Google Scholar] [CrossRef]
- Lassaletta, J.M. Atropoisomerism and Axial Chirality; World Scientific: Singapore, 2019. [Google Scholar]
- Siegel, J.S. Prologue: Atropisomerism. Synlett 2018, 29, 2122–2125. [Google Scholar] [CrossRef]
- Mancinelli, M.; Bencivenni, G.; Pecorari, D.; Mazzanti, A. Stereochemistry and Recent Applications of Axially Chiral Organic Molecules. Eur. J. Org. Chem. 2020, 2020, 4070–4086. [Google Scholar] [CrossRef]
- Kozlowski, M.C.; Morgan, B.J.; Linton, E.C. Total synthesis of chiral biaryl natural products by asymmetric biaryl coupling. Chem. Soc. Rev. 2009, 38, 3193–3207. [Google Scholar] [CrossRef]
- Bringmann, G.; Gulder, T.; Gulder, T.A.M.; Breuning, M. Atroposelective Total Synthesis of Axially Chiral Biaryl Natural Products. Chem. Rev. 2011, 111, 563–639. [Google Scholar] [CrossRef]
- Heinstein, P.F.; Herman, D.L.; Tove, S.B.; Smith, F.H. Biosynthesis of Gossypol. J. Biol. Chem. 1970, 245, 4658–4665. [Google Scholar] [CrossRef]
- Clayden, J.; Moran, W.J.; Edwards, P.J.; LaPlante, S.R. The Challenge of Atropisomerism in Drug Discovery. Angew. Chem. Int. Ed. 2009, 48, 6398–6401. [Google Scholar] [CrossRef]
- LaPlante, S.R.; Fader, L.D.; Fandrick, K.R.; Fandrick, D.R.; Hucke, O.; Kemper, R.; Miller, S.P.F.; Edwards, P.J. Assessing Atropisomer Axial Chirality in Drug Discovery and Development. J. Med. Chem. 2011, 54, 7005–7022. [Google Scholar] [CrossRef] [PubMed]
- Zask, A.; Murphy, J.; Ellestad, G.A. Biological Stereoselectivity of Atropisomeric Natural Products and Drugs. Chirality 2013, 25, 265–274. [Google Scholar] [CrossRef] [PubMed]
- Smyth, J.E.; Butler, N.M.; Keller, P.A. A twist of nature—The significance of atropisomers in biological systems. Nat. Prod. Rep. 2015, 32, 1562–1583. [Google Scholar] [CrossRef] [PubMed]
- Toenjes, S.T.; Gustafson, J.L. Atropisomerism in medicinal chemistry: Challenges and opportunities. Future Med. Chem. 2018, 10, 409–422. [Google Scholar] [CrossRef]
- Fandrick, K.R.; Li, W.; Zhang, Y.; Tang, W.; Gao, J.; Rodriguez, S.; Patel, N.D.; Reeves, D.C.; Wu, J.-P.; Sanyal, S.; et al. Concise and Practical Asymmetric Synthesis of a Challenging Atropisomeric HIV Integrase Inhibitor. Angew. Chem. Int. Ed. 2015, 54, 7144–7148. [Google Scholar] [CrossRef]
- Chandrasekhar, J.; Dick, R.; Van Veldhuizen, J.; Koditek, D.; Lepist, E.-I.; McGrath, M.E.; Patel, L.; Phillips, G.; Sedillo, K.; Somoza, J.R.; et al. Atropisomerism by Design: Discovery of a Selective and Stable Phosphoinositide 3-Kinase (PI3K) β Inhibitor. J. Med. Chem. 2018, 61, 6858–6868. [Google Scholar] [CrossRef]
- Noyori, R.; Takaya, H. BINAP: An efficient chiral element for asymmetric catalysis. Acc. Chem. Res. 1990, 23, 345–350. [Google Scholar] [CrossRef]
- Parmar, D.; Sugiono, E.; Raja, S.; Rueping, M. Complete Field Guide to Asymmetric BINOL-Phosphate Derived Brønsted Acid and Metal Catalysis: History and Classification by Mode of Activation; Brønsted Acidity, Hydrogen Bonding, Ion Pairing, and Metal Phosphates. Chem. Rev. 2014, 114, 9047–9153. [Google Scholar] [CrossRef]
- Kamer, P.C.J.; Cleij, M.C.; Nolte, R.J.M.; Harada, T.; Hezemans, A.M.F.; Drenth, W. Atropisomerism in polymers. Screw-sense selective polymerization of isocyanides by inhibiting the growth of one enantiomer of a racemic pair of helices. J. Am. Chem. Soc. 1988, 110, 1581–1587. [Google Scholar] [CrossRef]
- Lee, J.; Kalin, A.J.; Wang, C.; Early, J.T.; Al-Hashimi, M.; Fang, L. Donor-acceptor conjugated ladder polymer via aromatization-driven thermodynamic annulation. Polym. Chem. 2018, 9, 1603–1609. [Google Scholar] [CrossRef]
- Bringmann, G.; Price Mortimer, A.J.; Keller, P.A.; Gresser, M.J.; Garner, J.; Breuning, M. Atroposelective Synthesis of Axially Chiral Biaryl Compounds. Angew. Chem. Int. Ed. 2005, 44, 5384–5427. [Google Scholar] [CrossRef] [PubMed]
- Wallace, T.W. Biaryl synthesis with control of axial chirality. Org. Biomol. Chem. 2006, 4, 3197–3210. [Google Scholar] [CrossRef] [PubMed]
- Bencivenni, G. Organocatalytic Strategies for the Synthesis of Axially Chiral Compounds. Synlett 2015, 26, 1915–1922. [Google Scholar] [CrossRef]
- Wencel-Delord, J.; Panossian, A.; Leroux, F.R.; Colobert, F. Recent advances and new concepts for the synthesis of axially stereoenriched biaryls. Chem. Soc. Rev. 2015, 44, 3418–3430. [Google Scholar] [CrossRef] [PubMed]
- Renzi, P. Organocatalytic synthesis of axially chiral atropisomers. Org. Biomol. Chem. 2017, 15, 4506–4516. [Google Scholar] [CrossRef]
- Zilate, B.; Castrogiovanni, A.; Sparr, C. Catalyst-Controlled Stereoselective Synthesis of Atropisomers. ACS Catal. 2018, 8, 2981–2988. [Google Scholar] [CrossRef]
- Link, A.; Sparr, C. Stereoselective arene formation. Chem. Soc. Rev. 2018, 47, 3804–3815. [Google Scholar] [CrossRef]
- Bringmann, G.; Breuning, M.; Tasler, S. The Lactone Concept: An Efficient Pathway to Axially Chiral Natural Products and Useful Reagents. Synthesis 1999, 1999, 525–558. [Google Scholar] [CrossRef]
- Gustafson, J.L.; Lim, D.; Miller, S.J. Dynamic Kinetic Resolution of Biaryl Atropisomers via Peptide-Catalyzed Asymmetric Bromination. Science 2010, 328, 1251–1255. [Google Scholar] [CrossRef]
- Ma, G.; Sibi, M.P. Catalytic Kinetic Resolution of Biaryl Compounds. Chem. Eur. J. 2015, 21, 11644–11657. [Google Scholar] [CrossRef]
- Hayashi, T.; Niizuma, S.; Kamikawa, T.; Suzuki, N.; Uozumi, Y. Catalytic asymmetric synthesis of axially chiral biaryls by palladium-catalyzed enantioposition-selective cross-coupling. J. Am. Chem. Soc. 1995, 117, 9101–9102. [Google Scholar] [CrossRef]
- Di Iorio, N.; Crotti, S.; Bencivenni, G. Organocatalytic Desymmetrization Reactions for the Synthesis of Axially Chiral Compounds. Chem. Rec. 2019, 19, 2095–2104. [Google Scholar] [CrossRef] [PubMed]
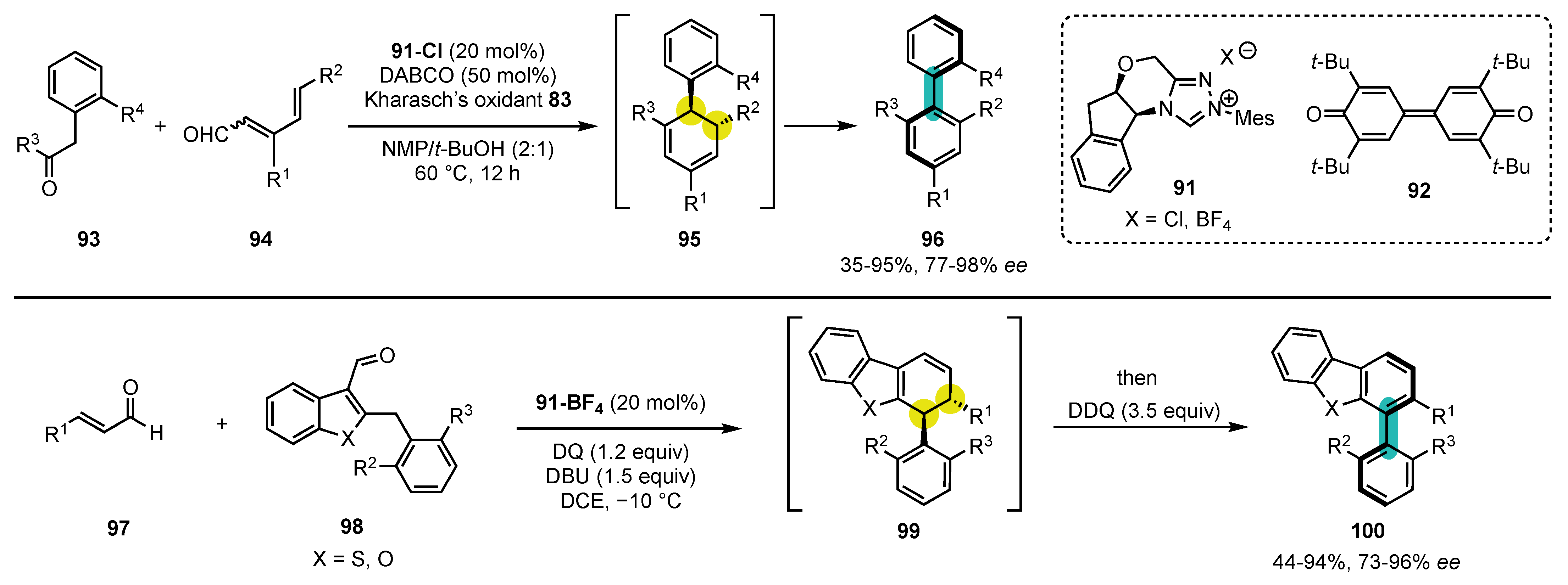
- Yang, G.; Guo, D.; Meng, D.; Wang, J. NHC-catalyzed atropoenantioselective synthesis of axially chiral biaryl amino alcohols via a cooperative strategy. Nat. Commun. 2019, 10, 3062. [Google Scholar] [CrossRef] [PubMed]
- Lipshutz, B.H.; Kayser, F.; Liu, Z.-P. Asymmetric Synthesis of Biaryls by Intramolecular Oxidative Couplings of Cyanocuprate Intermediates. Angew. Chem. Int. Ed. Engl. 1994, 33, 1842–1844. [Google Scholar] [CrossRef]
- Leermann, T.; Broutin, P.-E.; Leroux, F.R.; Colobert, F. Construction of the biaryl-part of vancomycin aglycon via atropo-diastereoselective Suzuki-Miyaura coupling. Org. Biomol. Chem. 2012, 10, 4095–4102. [Google Scholar] [CrossRef]
- Baudoin, O.; Décor, A.; Cesario, M.; Guéritte, F. A Novel 1,3-Central-to-Axial Chirality Induction Approach to Cyclooctadiene Lignans. Synlett 2003, 2003, 2009–2012. [Google Scholar] [CrossRef]
- Dherbassy, Q.; Djukic, J.-P.; Wencel-Delord, J.; Colobert, F. Two Stereoinduction Events in One C-H Activation Step: A Route towards Terphenyl Ligands with Two Atropisomeric Axes. Angew. Chem. Int. Ed. 2018, 57, 4668–4672. [Google Scholar] [CrossRef]
- Baudoin, O. The Asymmetric Suzuki Coupling Route to Axially Chiral Biaryls. Eur. J. Org. Chem. 2005, 2005, 4223–4229. [Google Scholar] [CrossRef]
- Loxq, P.; Manoury, E.; Poli, R.; Deydier, E.; Labande, A. Synthesis of axially chiral biaryl compounds by asymmetric catalytic reactions with transition metals. Coord. Chem. Rev. 2016, 308 Pt 2, 131–190. [Google Scholar] [CrossRef]
- Zhan, B.-B.; Wang, L.; Luo, J.; Lin, X.-F.; Shi, B.-F. Synthesis of Axially Chiral Biaryl-2-amines by PdII-Catalyzed Free-Amine-Directed Atroposelective C-H Olefination. Angew. Chem. Int. Ed. 2020, 59, 3568–3572. [Google Scholar] [CrossRef]
- Tanaka, K. Cationic Rhodium(I)/BINAP-Type Bisphosphine Complexes: Versatile New Catalysts for Highly Chemo-, Regio-, and Enantioselective [2+2+2] Cycloadditions. Synlett 2007, 2007, 1977–1993. [Google Scholar] [CrossRef]
- Zhang, J.; Simon, M.; Golz, C.; Alcarazo, M. Gold-Catalyzed Atroposelective Synthesis of 1,1′-Binaphthalene-2,3′-diols. Angew. Chem. Int. Ed. 2020, 59, 5647–5650. [Google Scholar] [CrossRef] [PubMed]
- Wolf, C. Dynamic Stereochemistry of Chiral Compounds: Principles and Applications; RSC: Cambridge, UK, 2008; pp. 204–330. [Google Scholar]
- Quinonero, O.; Bressy, C.; Bugaut, X. Organocatalytic Enantioselective Construction of Polyaromatic Architectures. Angew. Chem. Int. Ed. 2014, 53, 10861–10863. [Google Scholar] [CrossRef] [PubMed]
- Pantaine, L.; Moreau, X.; Coeffard, V.; Greck, C. The impact of asymmetric organocatalysis in dearomatization and aromatization of carbocycles: Increasing molecular complexity and diversity. Tetrahedron Lett. 2016, 57, 2567–2574. [Google Scholar] [CrossRef]
- Nguyen, T.T. Traceless point-to-axial chirality exchange in the atropselective synthesis of biaryls/heterobiaryls. Org. Biomol. Chem. 2019, 17, 6952–6963. [Google Scholar] [CrossRef]
- Yang, H.; Chen, J.; Zhou, L. Construction of Axially Chiral Compounds via Central-to-Axial Chirality Conversion. Chem. Asian J. 2020, 15, 2939. [Google Scholar] [CrossRef]
- Berson, J.A.; Brown, E. Studies on Dihydropyridines. III. An Absolute Asymmetric Synthesis and an Attempted Conversion of Carbon Atom Asymmetry to Biphenyl Asymmetry. J. Am. Chem. Soc. 1955, 77, 450–453. [Google Scholar] [CrossRef]
- Mislow, K.; Siegel, J. Stereoisomerism and local chirality. J. Am. Chem. Soc. 1984, 106, 3319–3328. [Google Scholar] [CrossRef]
- Meyers, A.I.; Wettlaufer, D.G. The complete intramolecular transfer of a central chiral element to an axial chiral element. Oxidation of (S)-4-naphthyldihydroquinolines to (S)-4-naphthylquinolines. J. Am. Chem. Soc. 1984, 106, 1135–1136. [Google Scholar] [CrossRef]
- Baker, R.W.; Kyasnoor, R.V.; Sargent, M.V.; Skelton, B.W.; White, A.H. Formal Synthesis of Both Atropomers of Desertorin C and an Example of Chirality Transfer from a Biphenyl Axis to a Spiro Centre and its Reverse. Aust. J. Chem. 2000, 53, 487–506. [Google Scholar] [CrossRef]
- Campolo, D.; Gastaldi, S.; Roussel, C.; Bertrand, M.P.; Nechab, M. Axial-to-central chirality transfer in cyclization processes. Chem. Soc. Rev. 2013, 42, 8434–8466. [Google Scholar] [CrossRef] [PubMed]
- Alexakis, A.; Bäckvall, J.E.; Krause, N.; Pàmies, O.; Diéguez, M. Enantioselective Copper-Catalyzed Conjugate Addition and Allylic Substitution Reactions. Chem. Rev. 2008, 108, 2796–2823. [Google Scholar] [CrossRef] [PubMed]
- Guo, F.; Konkol, L.C.; Thomson, R.J. Enantioselective Synthesis of Biphenols from 1,4-Diketones by Traceless Central-to-Axial Chirality Exchange. J. Am. Chem. Soc. 2011, 133, 18–20. [Google Scholar] [CrossRef] [PubMed]
- Vorogushin, A.V.; Wulff, W.D.; Hansen, H.-J. Stereoselectivity of the Benzannulation Reaction: Efficient Central-to-Axial Chirality Transfer. J. Am. Chem. Soc. 2002, 124, 6512–6513. [Google Scholar] [CrossRef]
- Qin, T.; Skraba-Joiner, S.L.; Khalil, Z.G.; Johnson, R.P.; Capon, R.J.; Porco, J.A., Jr. Atropselective Syntheses of (−) and (+) Rugulotrosin A Utilizing Point-to-Axial Chirality Transfer. Nat. Chem. 2015, 7, 234–240. [Google Scholar] [CrossRef]
- Pizzolato, S.F.; Štacko, P.; Kistemaker, J.C.M.; van Leeuwen, T.; Otten, E.; Feringa, B.L. Central-to-Helical-to-Axial-to-Central Transfer of Chirality with a Photoresponsive Catalyst. J. Am. Chem. Soc. 2018, 140, 17278–17289. [Google Scholar] [CrossRef]
- Forkosh, H.; Vershinin, V.; Reiss, H.; Pappo, D. Stereoselective Synthesis of Optically Pure 2-Amino-2’-hydroxy-1,1’-binaphthyls. Org. Lett. 2018, 20, 2459–2463. [Google Scholar] [CrossRef]
- Bai, H.-Y.; Tan, F.-X.; Liu, T.-Q.; Zhu, G.-D.; Tian, J.-M.; Ding, T.-M.; Chen, Z.-M.; Zhang, S.-Y. Highly atroposelective synthesis of nonbiaryl naphthalene-1,2-diamine N-C atropisomers through direct enantioselective C-H amination. Nat. Commun. 2019, 10, 3063. [Google Scholar] [CrossRef]
- Watile, R.A.; Bunrit, A.; Margalef, J.; Akkarasamiyo, S.; Ayub, R.; Lagerspets, E.; Biswas, S.; Repo, T.; Samec, J.S.M. Intramolecular substitutions of secondary and tertiary alcohols with chirality transfer by an iron(III) catalyst. Nat. Commun. 2019, 10, 3826. [Google Scholar] [CrossRef]
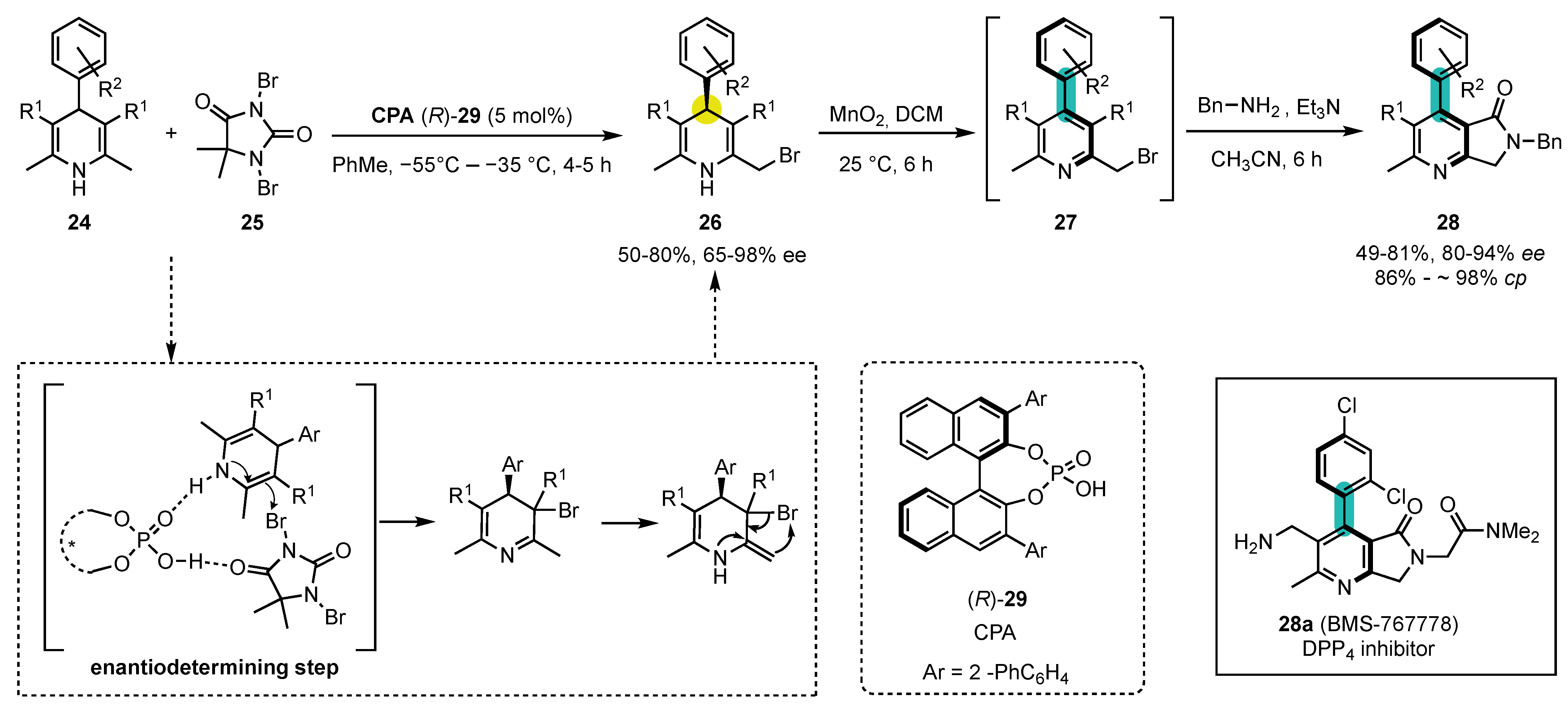
- Quinonero, O.; Jean, M.; Vanthuyne, N.; Roussel, C.; Bonne, D.; Constantieux, T.; Bressy, C.; Bugaut, X.; Rodriguez, J. Combining Organocatalysis with Central-to-Axial Chirality Conversion: Atroposelective Hantzsch-Type Synthesis of 4-Arylpyridines. Angew. Chem. Int. Ed. 2016, 55, 1401–1405. [Google Scholar] [CrossRef]
- Freund, M. Untersuchungen über das Thebaïn. Ber. Dtsch. Chem. Ges. 1905, 38, 3234–3256. [Google Scholar] [CrossRef]
- Small, L.; Sargent, L.J.; Bralley, J.A. The Phenyldhydrothebaines. J. Org. Chem. 1947, 12, 839–868. [Google Scholar] [CrossRef]
- Robinson, R. An Essay in Correlation Arising from Alkaloid Chemistry. Nature 1947, 160, 815–817. [Google Scholar] [CrossRef] [PubMed]
- Berson, J.A. A Standard of Absolute Configuration for Optically Active Biphenyls. J. Am. Chem. Soc. 1956, 78, 4170. [Google Scholar] [CrossRef]
- Davidson, T.A.; Hollands, T.R.; Mayo, P.D.; Nisbet, M. Terpenoids: XII. The Structure of Chaparrin. Can. J. Chem. 1965, 43, 2996–3007. [Google Scholar] [CrossRef]
- Hollands, T.R.; Mayo, P.D.; Nisbet, M.; Crabbé, P. Terpenoids: XIII. The Stereochemistry of Chaparrin, Chaparrol, and Related Compounds. Can. J. Chem. 1965, 43, 3008–3017. [Google Scholar] [CrossRef]
- Warnhoff, E.W.; Lopez, S.V. A one-step conversion of carbon atom asymmetry into biphenyl asymmetry and its relation to the absolute configuration of caranine. Tetrahedron Lett. 1967, 8, 2723–2727. [Google Scholar] [CrossRef]
- Hofmann, H.J.; Cimiraglia, R. Conformation of 1,4-dihydropyridine—Planar or boat-like? FEBS Lett. 1988, 241, 38–40. [Google Scholar] [CrossRef] [PubMed]
- Straub, A.; Goehrt, A.; Born, L. 4-Biaryl-substituted dihydropyridines with an unusual antiperiplanar conformation. Bioorg. Med. Chem. Lett. 1997, 7, 2519–2522. [Google Scholar] [CrossRef]
- Bao, X.; Rodriguez, J.; Bonne, D. Enantioselective Synthesis of Atropisomers with Multiple Stereogenic Axes. Angew. Chem. Int. Ed. 2020, 59, 12623–12634. [Google Scholar] [CrossRef]
- Chenga, D.-J.; Shao, Y.-D. Advances in the Catalytic Asymmetric Synthesis of Atropisomeric Hexatomic N-Heterobiaryls. Adv. Synth. Catal. 2020, 362, 3081–3099. [Google Scholar] [CrossRef]
- Li, T.-Z.; Liu, S.-J.; Tan, W.; Shi, F. Catalytic Asymmetric Construction of Axially Chiral Indole-Based Frameworks: An Emerging Area. Chem. Eur. J. 2020, 26, 15779–15792. [Google Scholar] [CrossRef]
- Wencel-Delord, J.; Colobert, F. Challenging Atroposelective C–H Arylation. SynOpen 2020, 4, 107–115. [Google Scholar] [CrossRef]
- Corti, V.; Bertuzzi, G. Organocatalytic Asymmetric Methodologies towards the Synthesis of Atropisomeric N-Heterocycles. Synthesis 2020, 52, 2450–2468. [Google Scholar] [CrossRef]
- Wang, J.; Zhao, C.; Wang, J. Recent progress toward the construction of axially chiral molecules catalyzed by an N-heterocyclic carbene. ACS Catal. 2021, 11, 12520–12531. [Google Scholar] [CrossRef]
- Cheng, J.K.; Xiang, S.-H.; Li, S.; Ye, L.; Tan, B. Recent Advances in Catalytic Asymmetric Construction of Atropisomers. Chem. Rev. 2021, 121, 4805–4902. [Google Scholar] [CrossRef] [PubMed]
- Xu, K.; Wang, Z.; Zhu, T. Recent Progress towards Organocatalyzed Asymmetric (Hetero)- Arene Formation. Synthesis 2021, 53, 636–652. [Google Scholar] [CrossRef]
- Min, X.-L.; Zhang, X.-L.; Shen, R.; Zhang, Q.; He, Y. Recent advances in the catalytic asymmetric construction of atropisomers by central-to-axial chirality transfer. Org. Chem. Front. 2022, 9, 2280–2292. [Google Scholar] [CrossRef]
- Baker, R.W.; Kyasnoor, R.V.; Sargent, M.V. Chirality transfer from a biphenyl axis to a spiro centre and its reverse: Sequential self-immolation. Tetrahedron Lett. 1999, 40, 3475–3478. [Google Scholar] [CrossRef]
- Hattori, T.; Date, M.; Sakurai, K.; Morohashi, N.; Kosugi, H.; Miyano, S. Highly stereospecific conversion of C-centrochirality of a 3,4-dihydro-2H-1,1’-binaphthalen-1-ol into axial chirality of a 3,4-dihydro-1,1’-binaphthalene. Tetrahedron Lett. 2001, 42, 8035–8038. [Google Scholar] [CrossRef]
- Koyama, Y.; Kataoka, H.; Suzuki, K.; Matsumoto, T. Regioselective Remote Functionalization of Biaryl Framework via Tethered ortho-Quinol Intermediate. Chem. Asian J. 2011, 6, 355–358. [Google Scholar] [CrossRef] [PubMed]
- Link, A.; Sparr, C. Remote Central-to-Axial Chirality Conversion: Direct Atroposelective Ester to Biaryl Transformation. Angew. Chem. Int. Ed. 2018, 57, 7136–7139. [Google Scholar] [CrossRef]
- Zheng, S.-C.; Wang, Q.; Zhu, J. Catalytic Kinetic Resolution by Enantioselective Aromatization: Conversion of Racemic Intermediates of the Barton–Zard Reaction into Enantioenriched 3-Arylpyrroles. Angew. Chem. Int. Ed. 2019, 58, 9215–9219. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.-L.; Zhao, W.-M.; Zhang, R.-X.; Chen, J.; Zhou, L. Organocatalytic cycloaddition–elimination cascade for atroposelective construction of heterobiaryls. Chem. Sci. 2021, 12, 14920–14926. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Lu, K.; Dai, M.; Wang, K.; Wu, W.; Chen, J.; Quan, J.; Yang, Z. An Efficient One-Pot Asymmetric Synthesis of Biaryl Compounds via Diels-Alder/Retro-Diels-Alder Cascade Reactions. Org. Lett. 2007, 9, 805–808. [Google Scholar] [CrossRef]
- Zhao, C.; Guo, D.; Munkerup, K.; Huang, K.-W.; Li, F.; Wang, J. Enantioselective [3+3] atroposelective annulation catalyzed by N-heterocyclic carbenes. Nat. Commun. 2018, 9, 611. [Google Scholar] [CrossRef]
- Nishii, Y.; Wakasugi, K.; Koga, K.; Tanabe, Y. Chirality Exchange from sp3 Central Chirality to Axial Chirality: Benzannulation of Optically Active Diaryl-2,2-dichlorocyclopropylmethanols to Axially Chiral α-Arylnaphthalenes. J. Am. Chem. Soc. 2004, 126, 5358–5359. [Google Scholar] [CrossRef]
- Konkol, L.C.; Guo, F.; Sarjeant, A.A.; Thomson, R.J. Enantioselective Total Synthesis and Studies into the Configurational Stability of Bismurrayaquinone A. Angew. Chem. Int. Ed. 2011, 50, 9931–9934. [Google Scholar] [CrossRef]
- Candish, L.; Levens, A.; Lupton, D.W. N-Heterocyclic carbene catalysed redox isomerisation of esters to functionalised benzaldehydes. Chem. Sci. 2015, 6, 2366–2370. [Google Scholar] [CrossRef]
- Feringa, B.; Wynberg, H. Biomimetic asymmetric oxidative coupling of phenols. Bioorg. Chem. 1978, 7, 397–408. [Google Scholar] [CrossRef]
- Brussee, J.; Jansen, A.C.A. A highly stereoselective synthesis of s(-)-[1,1’-binaphthalene]-2,2’-diol. Tetrahedron Lett. 1983, 24, 3261–3262. [Google Scholar] [CrossRef]
- Meyers, A.I.; Lutomski, K.A. Enantioselective synthesis of binaphthyls via nucleophilic aromatic substitution on chiral oxazolines. J. Am. Chem. Soc. 1982, 104, 879–881. [Google Scholar] [CrossRef]
- Wilson, J.M.; Cram, D.J. Chiral leaving groups induce asymmetry in syntheses of binaphthyls in nucleophilic aromatic substitution reactions. J. Am. Chem. Soc. 1982, 104, 881–884. [Google Scholar] [CrossRef]
- Shindo, M.; Koga, K.; Tomioka, K. A catalytic method for asymmetric nucleophilic aromatic substitution giving binaphthyls. J. Am. Chem. Soc. 1992, 114, 8732–8733. [Google Scholar] [CrossRef]
- Armstrong, R.J.; Smith, M.D. Catalytic Enantioselective Synthesis of Atropisomeric Biaryls: A Cation-Directed Nucleophilic Aromatic Substitution Reaction. Angew. Chem. Int. Ed. 2014, 53, 12822–12826. [Google Scholar] [CrossRef]
- Castanet, A.-S.; Boussonniere, A.; Mortier, J. Asymmetric Nucleophilic Aromatic Substitution. In Arene Chemistry: Reaction Mechanisms and Methods for Aromatic Compounds; Mortier, J., Ed.; John Wiley & Sons: Hoboken, NJ, USA, 2015; pp. 195–217. [Google Scholar]
- Brandes, S.; Bella, M.; Kjærsgaard, A.; Jørgensen, K.A. Chirally Aminated 2-Naphthols—Organocatalytic Synthesis of Non-Biaryl Atropisomers by Asymmetric Friedel–Crafts Amination. Angew. Chem. Int. Ed. 2006, 45, 1147–1151. [Google Scholar] [CrossRef] [PubMed]
- Terada, M.; Dan, K. Synthesis of unsymmetrically substituted 2,2’-dihydroxy-1,1’-biaryl derivatives using organic-base-catalyzed Ferrier-type rearrangement as the key step. Chem. Commun. 2012, 48, 5781–5783. [Google Scholar] [CrossRef]
- De, C.K.; Pesciaioli, F.; List, B. Catalytic Asymmetric Benzidine Rearrangement. Angew. Chem. Int. Ed. 2013, 52, 9293–9295. [Google Scholar] [CrossRef]
- Li, G.-Q.; Gao, H.; Keene, C.; Devonas, M.; Ess, D.H.; Kürti, L. Organocatalytic Aryl-Aryl Bond Formation: An Atroposelective [3,3]-Rearrangement Approach to BINAM Derivatives. J. Am. Chem. Soc. 2013, 135, 7414–7417. [Google Scholar] [CrossRef]
- Link, A.; Sparr, C. Organocatalytic Atroposelective Aldol Condensation: Synthesis of Axially Chiral Biaryls by Arene Formation. Angew. Chem. Int. Ed. 2014, 53, 5458–5461. [Google Scholar] [CrossRef]
- Moliterno, M.; Cari, R.; Puglisi, A.; Antenucci, A.; Sperandio, C.; Moretti, E.; Di Sabato, A.; Salvio, R.; Bella, M. Quinine-Catalyzed Asymmetric Synthesis of 2,2′-Binaphthol-Type Biaryls under Mild Reaction Conditions. Angew. Chem. Int. Ed. 2016, 55, 6525–6529. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Zhang, J.; Ma, J.; Cheng, D.-J.; Tan, B. Highly Atroposelective Synthesis of Arylpyrroles by Catalytic Asymmetric Paal-Knorr Reaction. J. Am. Chem. Soc. 2017, 139, 1714–1717. [Google Scholar] [CrossRef] [PubMed]
- Qi, L.-W.; Mao, J.-H.; Zhang, J.; Tan, B. Organocatalytic asymmetric arylation of indoles enabled by azo groups. Nat. Chem. 2018, 10, 58–64. [Google Scholar] [CrossRef] [PubMed]
- Witzig, R.M.; Fäseke, V.C.; Häussinger, D.; Sparr, C. Atroposelective synthesis of tetra-ortho-substituted biaryls by catalyst-controlled non-canonical polyketide cyclizations. Nat. Catal. 2019, 2, 925–930. [Google Scholar] [CrossRef]
- Straub, A.; Goehrt, A. Inversion of Optically Active Dihydropyridines by Oxidation and Electroreduction. Angew. Chem. Int. Ed. Engl. 1996, 35, 2662–2664. [Google Scholar] [CrossRef]
- Koop, B.; Straub, A.; Schäfer, H.J. Stereoselective oxidation of 4-aryl-1,4-dihydropyridines to axially chiral 4-arylpyridines with 2,2,6,6-tetramethyl-1-oxopiperidinium tetrafluoroborate (TEMPO+BF4-). Tetrahedron Asymmetry 2001, 12, 341–345. [Google Scholar] [CrossRef]
- Han, M.; Zhang, S.-q.; Cui, X.; Wang, Q.-w.; Li, G.-x.; Tang, Z. Chiral Phosphoric Acid Catalyzed Enantioselective Desymmetrization of 1,4-Dihydropyridines by C(sp3)-H Bromination. Angew. Chem. Int. Ed. 2022, 61, e202201418. [Google Scholar] [CrossRef]
- Ohno, A.; Kashiwagi, M.; Ishihara, Y.; Ushida, S.; Oka, S. NAD(P)+-NAD(P)h models. 57: Stereochemistry in (net) hydride transfer from and to nad(p)+-nad(p)h models: Chirality sink. Tetrahedron 1986, 42, 961–973. [Google Scholar] [CrossRef]
- Mohr, J.T.; Ebner, D.C.; Stoltz, B.M. Catalytic enantioselective stereoablative reactions: An unexploited approach to enantioselective catalysis. Org. Biomol. Chem. 2007, 5, 3571–3576. [Google Scholar] [CrossRef]
- Ohno, A.; Ohara, M.; Oka, S. NAD(P)+-NAD(P)H models. 61. An interconversion between central and axial chiralities as evidence for a functional model of chemical evolution of an enzyme. J. Am. Chem. Soc. 1986, 108, 6438–6440. [Google Scholar] [CrossRef]
- Ohno, A.; Ogawa, M.; Oka, S. NAD(P)+-NAD(P)H models. 67. Stereospecific conversion of central chirality into axial chirality as a model for chemical evolution of a dehydrogenase. Tetrahedron Lett. 1988, 29, 3079–3082. [Google Scholar] [CrossRef]
- Ohno, A.; Ogawa, M.; Mikata, Y.; Goto, M. NAD(P)+-NAD(P)H Models. 69. Mechanism of Stereospecific (NET) Hydride Transfer Controlled by Electronic Effect. Bull. Chem. Soc. Jpn. 1990, 63, 813–818. [Google Scholar] [CrossRef]
- Ohno, A.; Mikata, Y.; Goto, M.; Kashiwagi, T.; Tanaka, T.; Sawada, M. NAD(P)H-NAD(P)+ Models. 73. Structure-Stereochemistry Relationship in the Reaction of NAD Analog. Bull. Chem. Soc. Jpn. 1991, 64, 81–86. [Google Scholar] [CrossRef]
- Okamura, M.; Kashiwagi, T.; Mikata, Y.; Maruyama, T.; Ohno, A. Stereospecificity observed in base-catalyzed electrochemical oxidation. Tetrahedron Lett. 1991, 32, 1475–1478. [Google Scholar] [CrossRef]
- Mikata, Y.; Mizukami, K.; Hayashi, K.; Matsumoto, S.; Yano, S.; Yamazaki, N.; Ohno, A. NAD/NADH Models with Axial/Central Chiralities: Superiority of the Quinoline Ring System. J. Org. Chem. 2001, 66, 1590–1599. [Google Scholar] [CrossRef]
- De Kok, P.M.T.; Bastiaansen, L.A.M.; Van Lier, P.M.; Vekemans, J.A.J.M.; Buck, H.M. Highly reactive and stereoselective (R)- and (S)-3-(N,N-dimethylcarbamoyl)-1,2,4-trimethyl-1,4-dihydropyridines for NADH-NAD+ mimicry. J. Org. Chem. 1989, 54, 1313–1320. [Google Scholar] [CrossRef]
- Beijer, N.A.; Vekemans, J.A.J.M.; Buck, H.M. Stereoselective reduction of benzoin by the NADH model 3-(dimethylcarbamoyl)-1,2,4-trimethyl-1,4-dihydropyridine. Recl. Trav. Chim. Pays-Bas 1990, 109, 434–436. [Google Scholar] [CrossRef]
- Vekemans, J.A.J.M.; Versleijen, J.P.G.; Buck, H.M. NADH model mediated reduction of C=N substrates: Enantioselective synthesis of D- and L-phenylglycinates. Tetrahedron Asymmetry 1991, 2, 949–952. [Google Scholar] [CrossRef]
- Versleijen, J.P.; Sanders-Hovens, M.S.; Vanhommerig, S.A.; Vekemans, J.A.; Meijer, E.M. Enantioselective reduction of C=O and C=N compounds with NADH model N,N,1,2,4-pentamethyl-1,4-dihydronicotinamide. Tetrahedron 1993, 49, 7793–7802. [Google Scholar] [CrossRef]
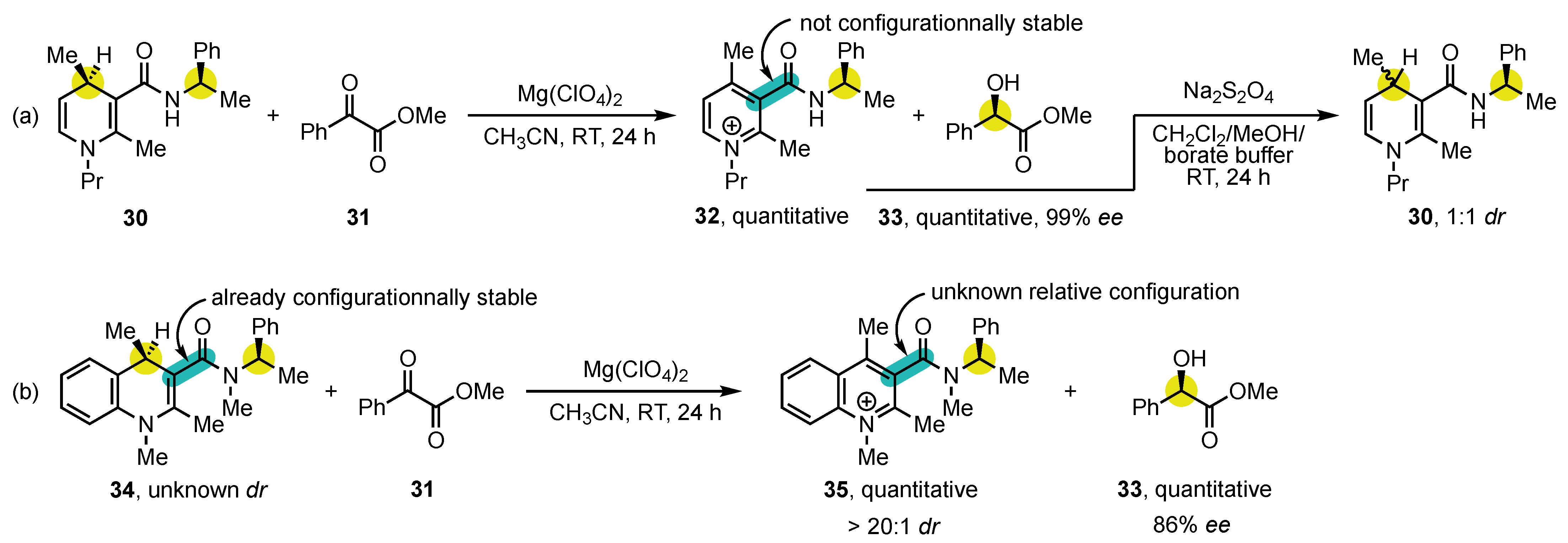
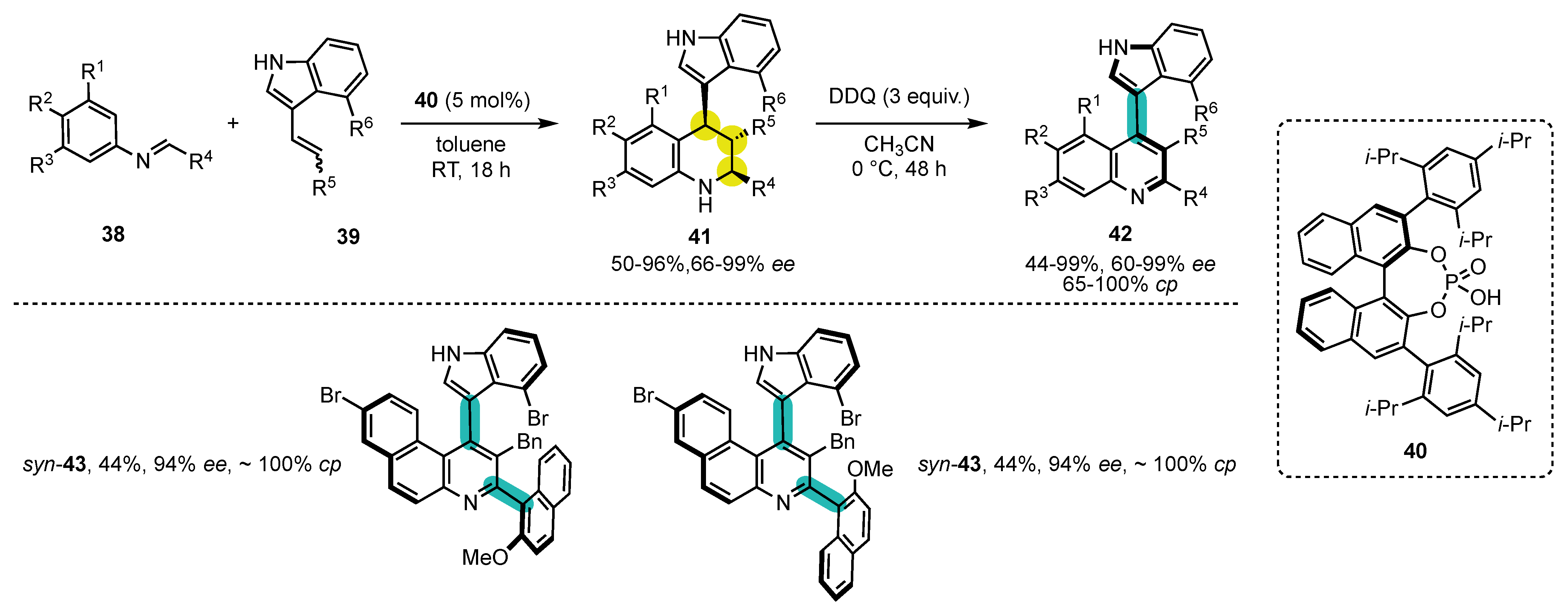
- Bisag, G.D.; Pecorari, D.; Mazzanti, A.; Bernardi, L.; Fochi, M.; Bencivenni, G.; Bertuzzi, G.; Corti, V. Central-to-Axial Chirality Conversion Approach Designed on Organocatalytic Enantioselective Povarov Cycloadditions: First Access to Configurationally Stable Indole-Quinoline Atropisomers. Chem. Eur. J. 2019, 25, 15694–15701. [Google Scholar] [CrossRef]
- Wang, S.-J.; Wang, Z.; Tang, Y.; Chen, J.; Zhou, L. Asymmetric Synthesis of Quinoline-Naphthalene Atropisomers by Central-to-Axial Chirality Conversion. Org. Lett. 2020, 22, 8894–8898. [Google Scholar] [CrossRef] [PubMed]
- Gao, Z.; Qian, J.; Yang, H.; Zhang, J.; Jiang, G. Enantioselective Construction of C-C Axially Chiral Quinazolinones via Chirality Exchange and Phase-Transfer Catalysis. Org. Lett. 2021, 23, 1731–1737. [Google Scholar] [CrossRef]
- Gao, Z.; Wang, F.; Qian, J.; Yang, H.; Xia, C.; Zhang, J.; Jiang, G. Enantioselective Construction of Quinoxaline-Based Heterobiaryls and P,N-Ligands via Chirality Transfer Strategy. Org. Lett. 2021, 23, 1181–1187. [Google Scholar] [CrossRef] [PubMed]
- Gou, B.-B.; Tang, Y.; Lin, Y.-H.; Yu, L.; Jian, Q.-S.; Sun, H.-R.; Chen, J.; Zhou, L. Modular Construction of Heterobiaryl Atropisomersand Axially Chiral Styrenesvia All-Carbon Tetrasubstituted VQMs. Angew. Chem. Int. Ed. 2022, 61, e202208174. [Google Scholar] [CrossRef] [PubMed]
- Fischer, C.; Sparr, C. Configurationally Stable Atropisomeric Acridinium Fluorophores. Synlett 2018, 29, 2176–2180. [Google Scholar] [CrossRef]
- Wu, X.; Sparr, C. Stereoselective Synthesis of Atropisomeric Acridinium Salts by the Catalyst-Controlled Cyclization of ortho-Quinone Methide Iminiums. Angew. Chem. Int. Ed. 2022, 61, e202201424. [Google Scholar] [CrossRef]
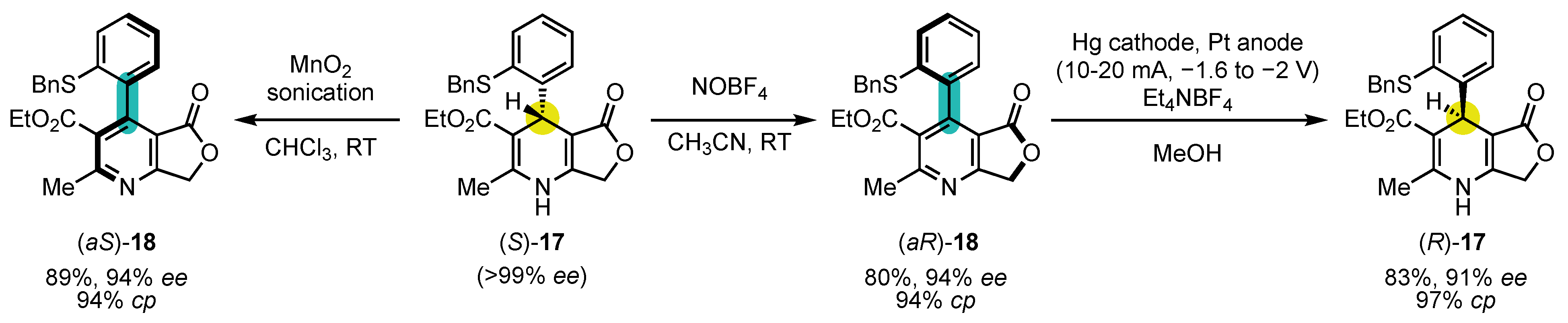
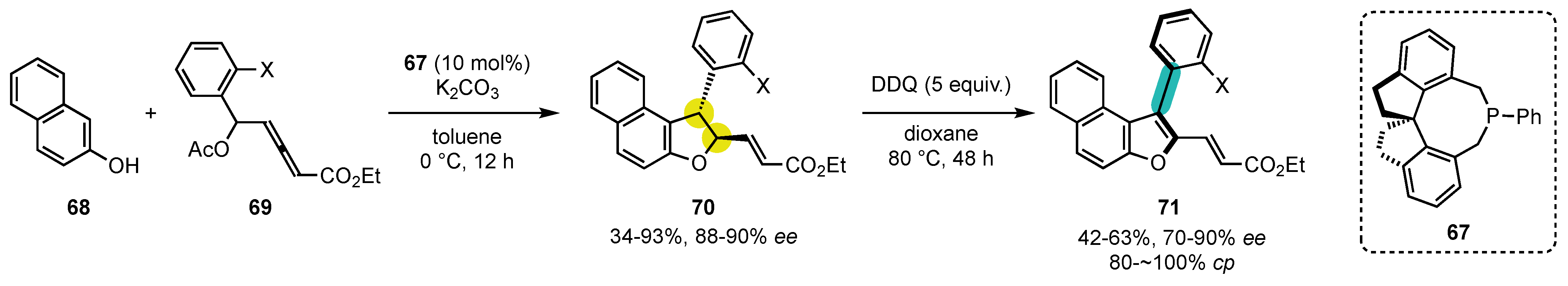
- Raut, V.S.; Jean, M.; Vanthuyne, N.; Roussel, C.; Constantieux, T.; Bressy, C.; Bugaut, X.; Bonne, D.; Rodriguez, J. Enantioselective Syntheses of Furan Atropisomers by an Oxidative Central-to-Axial Chirality Conversion Strategy. J. Am. Chem. Soc. 2017, 139, 2140–2143. [Google Scholar] [CrossRef]
- Wang, D.; Tong, X. Phosphine-Catalyzed Asymmetric (3+2) Annulations of δ-Acetoxy Allenoates with 2-Naphthols. Org. Lett. 2017, 19, 6392–6395. [Google Scholar] [CrossRef]
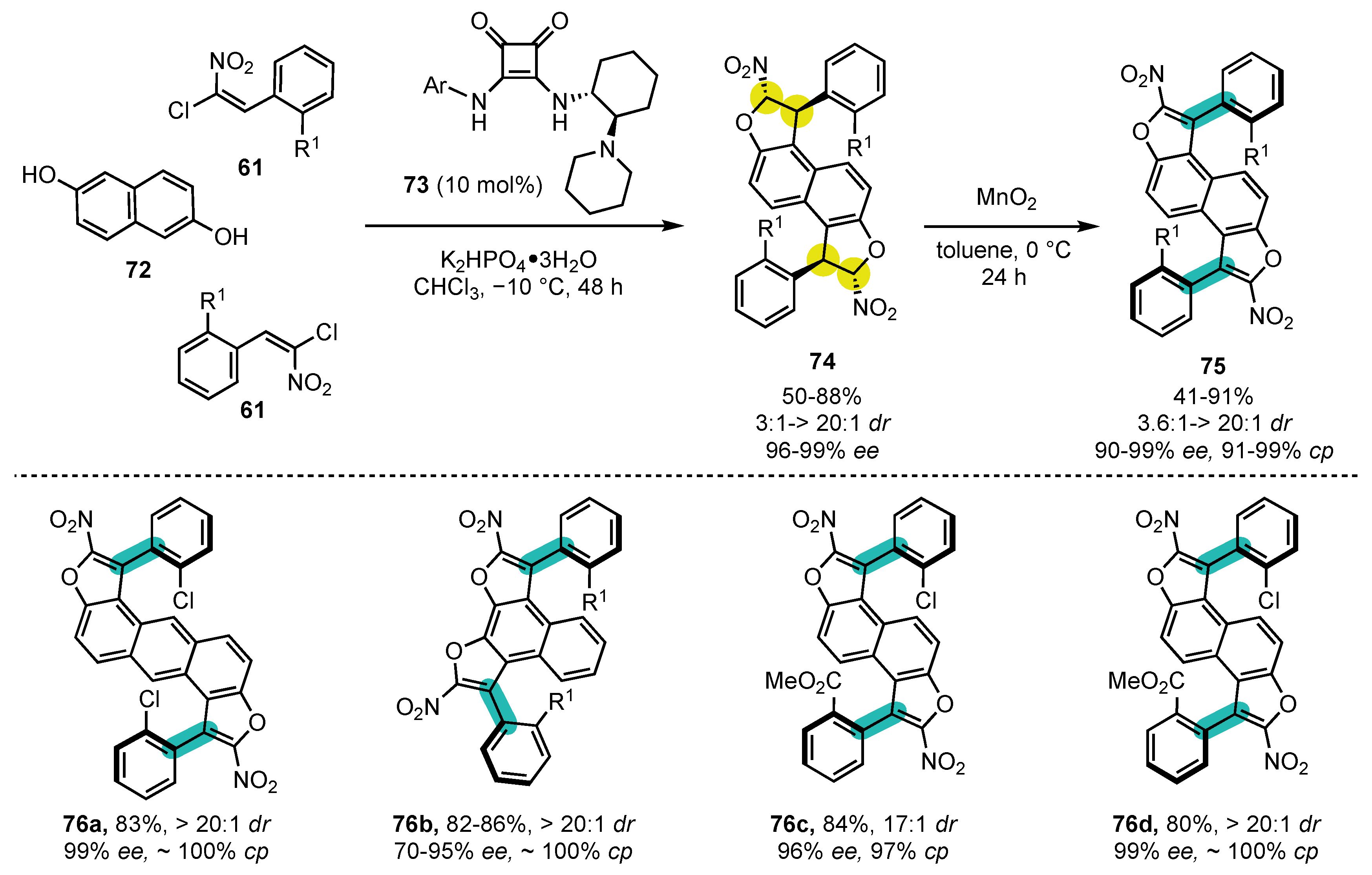
- Bao, X.; Rodriguez, J.; Bonne, D. Bidirectional enantioselective synthesis of bis-benzofuran atropisomeric oligoarenes featuring two distal C-C stereogenic axes. Chem. Sci. 2020, 11, 403–408. [Google Scholar] [CrossRef]
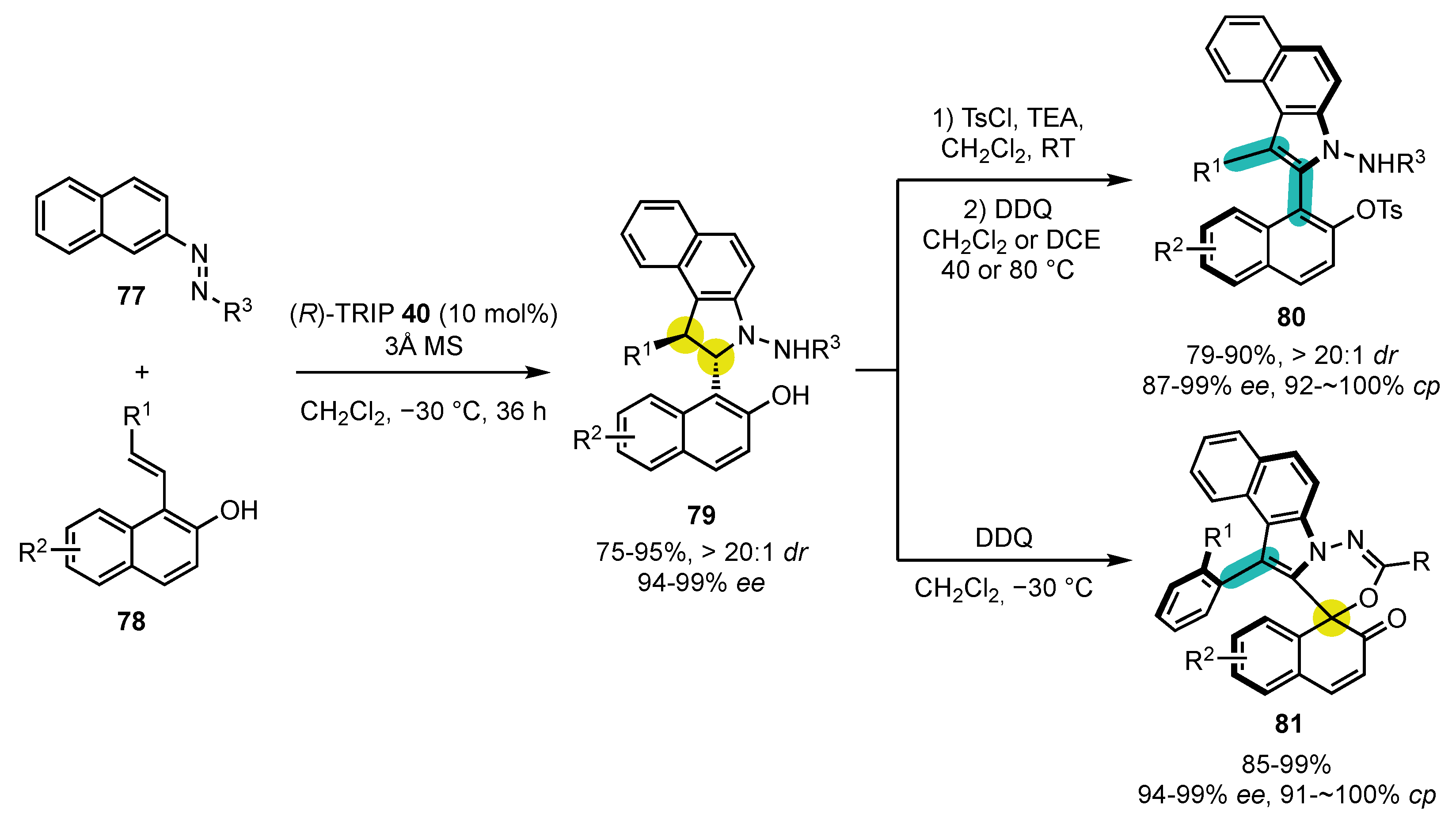
- Hu, Y.-L.; Wang, Z.; Yang, H.; Chen, J.; Wu, Z.-B.; Lei, Y.; Zhou, L. Conversion of two stereocenters to one or two chiral axes: Atroposelective synthesis of 2,3-diarylbenzoindoles. Chem. Sci. 2019, 10, 6777–6784. [Google Scholar] [CrossRef]
- Yang, H.; Xu, W.-L.; Zeng, X.-Y.; Chen, J.; Yu, L.; Zhou, L. Hydrogen Bond Assisted Central-to-Spiro Chirality Transfer and Central-to-Axial Chirality Conversion: Asymmetric Synthesis of Spirocycles. Org. Lett. 2021, 23, 9315–9320. [Google Scholar] [CrossRef] [PubMed]
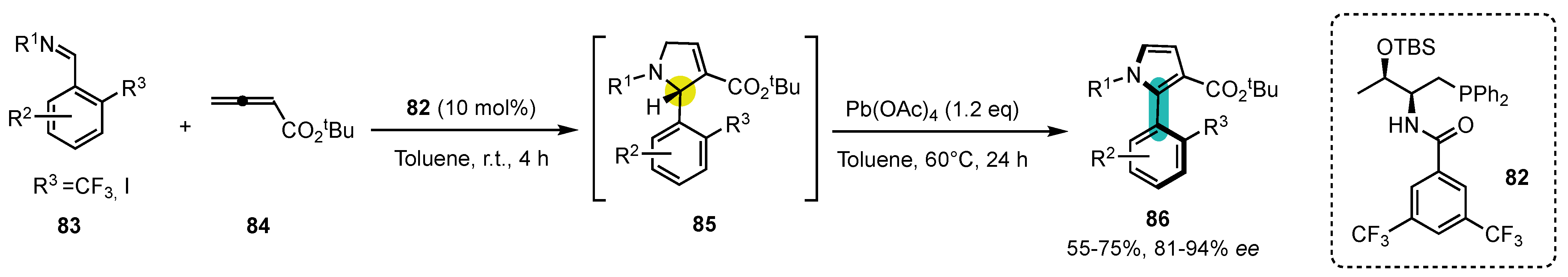
- Xiang Alvin Tan, C.; Li, R.; Zhang, F.; Dai, L.; Ullah, N.; Lu, Y. Synthesis of Axially Chiral CF3-Substituted 2-Arylpyrroles by Sequential Phosphine-Catalyzed Asymmetric [3+2] Annulation and Oxidative Central-to-Axial Chirality Transfer. Angew. Chem. Int. Ed. 2022, 61, e202209494. [Google Scholar] [CrossRef] [PubMed]
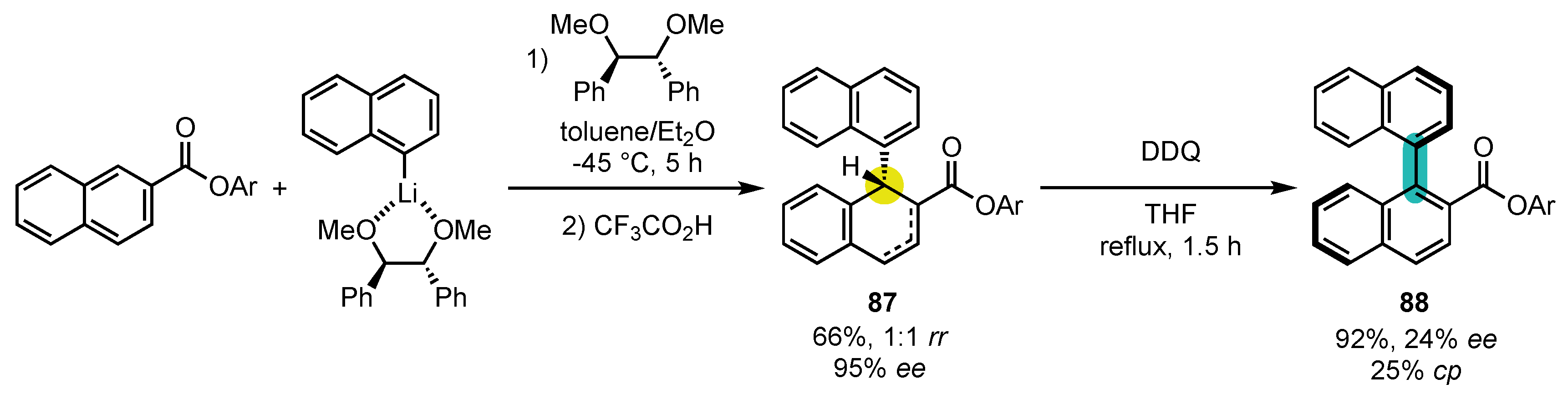
- Shindo, M.; Yamamoto, Y.; Yamada, K.-i.; Tomioka, K. Asymmetric Construction of Binaphthyl by the Chiral Diether-Mediated Conjugate Addition of Naphthyllithium to Naphthalenecarboxylic Acid 2,6-Di-t-butyl-4-methoxyphenyl Ester. Chem. Pharm. Bull. 2009, 57, 752–754. [Google Scholar] [CrossRef] [PubMed]
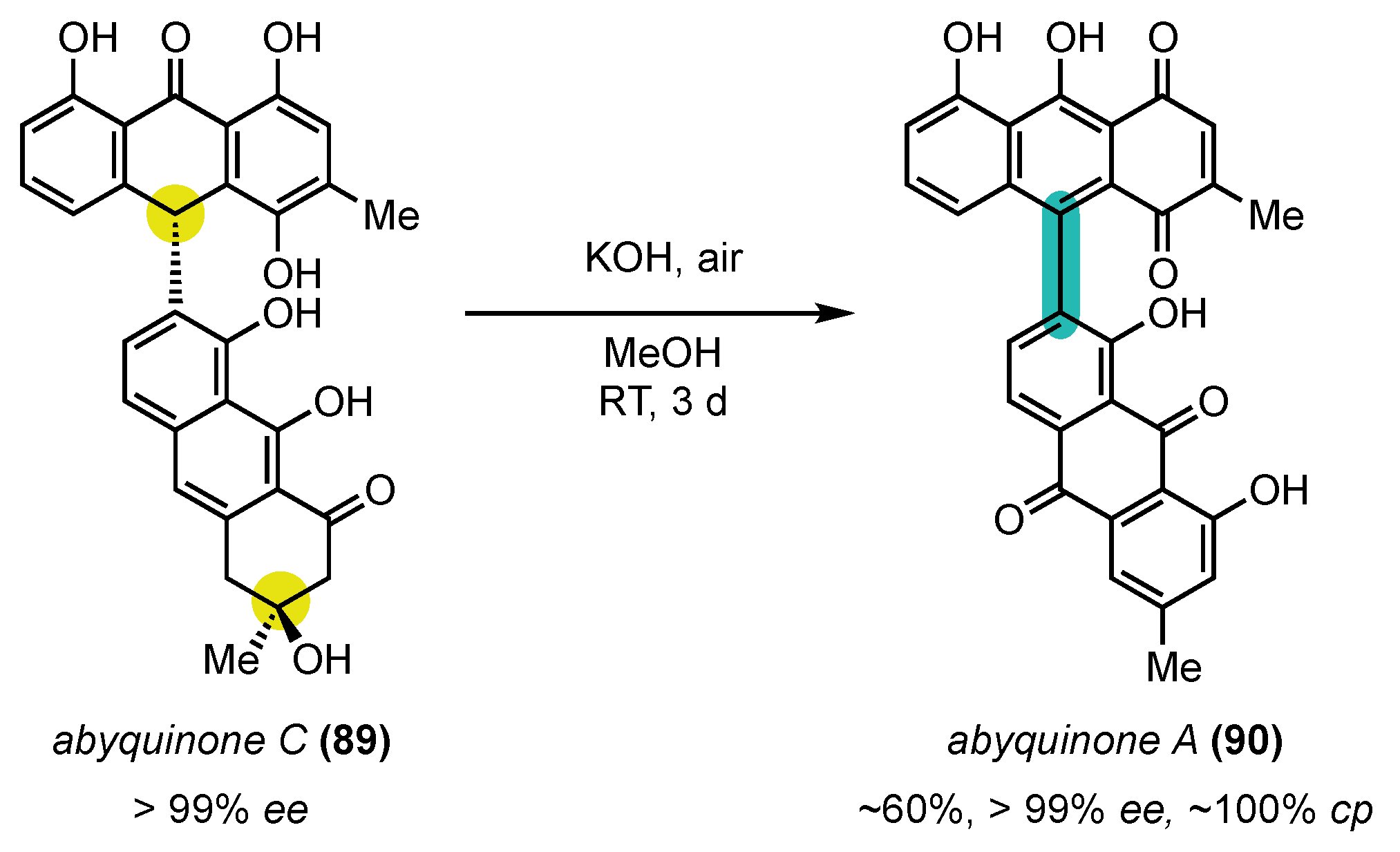
- Wanjohi, J.M.; Yenesew, A.; Midiwo, J.O.; Heydenreich, M.; Peter, M.G.; Dreyer, M.; Reichert, M.; Bringmann, G. Three dimeric anthracene derivatives from the fruits of Bulbine abyssinica. Tetrahedron 2005, 61, 2667–2674. [Google Scholar] [CrossRef]
- Xu, K.; Li, W.; Zhu, S.; Zhu, T. Atroposelective Arene Formation by Carbene-Catalyzed Formal [4+2] Cycloaddition. Angew. Chem. Int. Ed. 2019, 58, 17625–17630. [Google Scholar] [CrossRef]
- Zhang, C.-L.; Gao, Y.-Y.; Wang, H.-Y.; Zhou, B.-A.; Ye, S. Enantioselective Synthesis of Axially Chiral Benzothiophene/Benzofuran-Fused Biaryls by N-Heterocyclic Carbene Catalyzed Arene Formation. Angew. Chem. Int. Ed. 2021, 60, 13918. [Google Scholar] [CrossRef]
- Hayashi, Y.; Takikawa, A.; Koshino, S.; Ishida, K. Asymmetric Synthesis of Biaryl Atropisomers Using an Organocatalyst-Mediated Domino Reaction as the Key Step. Chem. Eur. J. 2019, 25, 10319–10322. [Google Scholar] [CrossRef]
- Koshino, S.; Takikawa, A.; Ishida, K.; Taniguchi, T.; Monde, K.; Kwon, E.; Umemiya, S.; Hayashi, Y. Inversion of the Axial Information during Oxidative Aromatization in the Synthesis of Axially Chiral Biaryls with Organocatalysis as a Key Step. Chem. Eur. J. 2020, 26, 4524–4530. [Google Scholar] [CrossRef]
- Koshino, S.; Taniguchi, T.; Monde, K.; Kwon, E.; Hayashi, Y. Enantiodivergent One-Pot Synthesis of Axially Chiral Biaryls Using Organocatalyst-Mediated Enantioselective Domino Reaction and Central-to-Axial Chirality Conversion. Chem. Eur. J. 2021, 27, 15786–15794. [Google Scholar] [CrossRef]
- Zhu, L.; Peng, H.; Guo, Y.; Che, J.; Wu, J.-H.; Su, Z.; Wang, T. Enantioselective Synthesis of Atropisomeric Biaryl Phosphorus Compounds by Chiral-Phosphonium-Salt-Enabled Cascade Arene Formation. Angew. Chem. Int. Ed. 2022, 61, e202202467. [Google Scholar] [CrossRef]
- Tan, J.-P.; Li, K.; Shen, B.; Zhuang, C.; Liu, Z.; Xiao, K.; Yu, P.; Yi, B.; Ren, X.; Wang, T. Asymmetric synthesis of N-bridged [3.3.1] ring systems by phosphonium salt/Lewis acid relay catalysis. Nat. Commun. 2022, 13, 357. [Google Scholar] [CrossRef] [PubMed]
- Fang, S.; Tan, J.-P.; Pan, J.; Zhang, H.; Chen, Y.; Ren, X.; Wang, T. Enantiodivergent Kinetic Resolution of 1,1′-Biaryl-2,2′-Diols and Amino Alcohols by Dipeptide-Phosphonium Salt Catalysis Inspired by the Atherton–Todd Reaction. Angew. Chem. Int. Ed. 2021, 60, 14921–14930. [Google Scholar] [CrossRef] [PubMed]
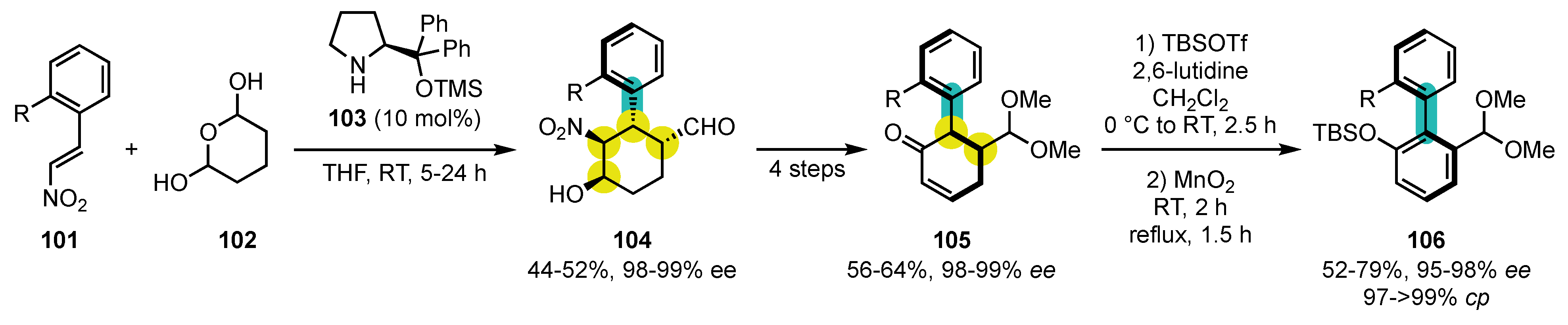
- Saito, T.; Shimizu, Y.; Araki, Y.; Ohgami, Y.; Kitazawa, Y.; Nishii, Y. From Enantioenriched Donor-Acceptor Cyclopropylcarbinols to Axially Chiral Arylnaphthalenes through Aryldihydronaphthalenes: Central-to-Axial Chirality Exchange. Eur. J. Org. Chem. 2022, 2022, e202101213. [Google Scholar] [CrossRef]
- Wang, Y.-B.; Zheng, S.-C.; Hu, Y.-M.; Tan, B. Brønsted acid-catalysed enantioselective construction of axially chiral arylquinazolinones. Nat. Commun. 2017, 8, 15489. [Google Scholar] [CrossRef] [PubMed]
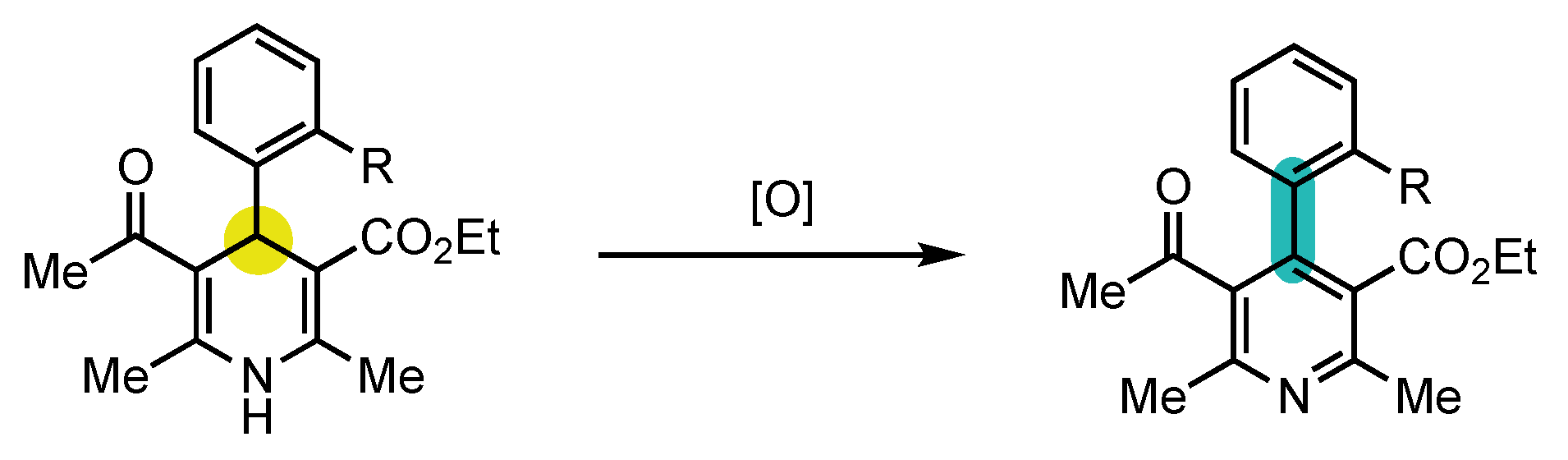




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lemaitre, C.; Perulli, S.; Quinonero, O.; Bressy, C.; Rodriguez, J.; Constantieux, T.; García Mancheño, O.; Bugaut, X. Enantioselective Synthesis of Atropisomers by Oxidative Aromatization with Central-to-Axial Conversion of Chirality. Molecules 2023, 28, 3142. https://doi.org/10.3390/molecules28073142
Lemaitre C, Perulli S, Quinonero O, Bressy C, Rodriguez J, Constantieux T, García Mancheño O, Bugaut X. Enantioselective Synthesis of Atropisomers by Oxidative Aromatization with Central-to-Axial Conversion of Chirality. Molecules. 2023; 28(7):3142. https://doi.org/10.3390/molecules28073142
Chicago/Turabian StyleLemaitre, Clément, Stefania Perulli, Ophélie Quinonero, Cyril Bressy, Jean Rodriguez, Thierry Constantieux, Olga García Mancheño, and Xavier Bugaut. 2023. "Enantioselective Synthesis of Atropisomers by Oxidative Aromatization with Central-to-Axial Conversion of Chirality" Molecules 28, no. 7: 3142. https://doi.org/10.3390/molecules28073142
APA StyleLemaitre, C., Perulli, S., Quinonero, O., Bressy, C., Rodriguez, J., Constantieux, T., García Mancheño, O., & Bugaut, X. (2023). Enantioselective Synthesis of Atropisomers by Oxidative Aromatization with Central-to-Axial Conversion of Chirality. Molecules, 28(7), 3142. https://doi.org/10.3390/molecules28073142