A Review of Potential Use of Amazonian Oils in the Synthesis of Organogels for Cosmetic Application
Abstract
1. Introduction
2. Methodology
3. Organogel
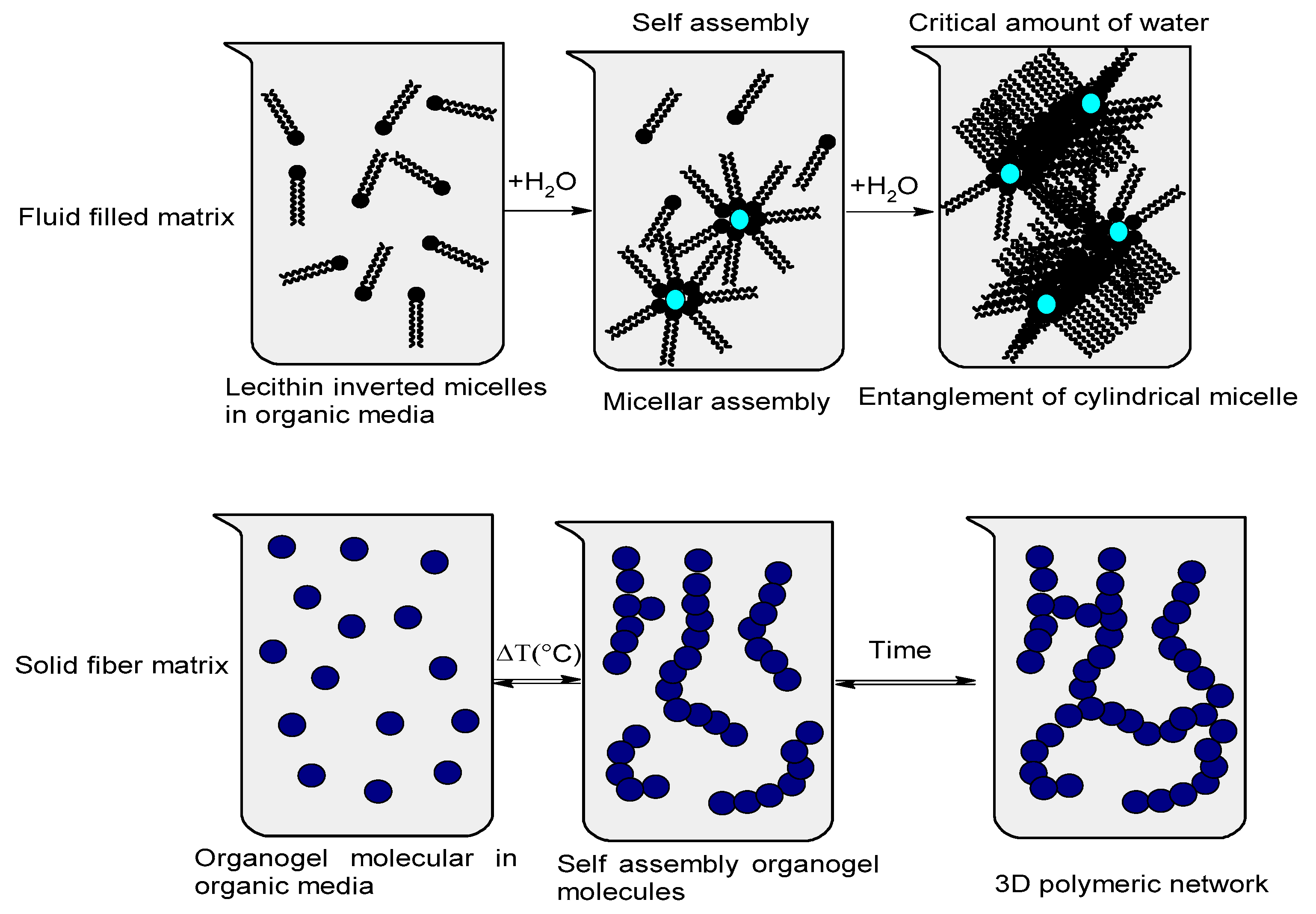
3.1. The Selection of the Organogel
3.2. General Considerations of Lipids (Natural Oils)
4. Influences of Solvent Polarity on the Formation of Organogel
5. Amazonian Oil and Their Use in the Formation of Organogels
| Vegetal Oil | Appearance (25 °C) | Color | Smell | Acidity Index | Peroxide Index | Iodine Value | Saponificatio Index | Refractive Index | Density | Unsaponified Material (Bioactive) | Fusion Point | Reference |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Acai | Líquid | Green | Chara | 1.20–1.60 mg KOH/g | 1.26 meq H2O2/kg | 70 g I2/100 g | 175.69 mg KOH/g | 1.481 (40 °C) | 0.893 g/mL | 2–3% | - | [72,73] |
| Andiroba | Líquid | Yellow to brown | Chara | 3.89 mg NaOH/g | 1.96 meq O2/kg | 89.77 g I2/100 g | 232.84 mg KOH/g | 1.4611 (40 °C) | 0.98 g/mL | 3–5% | 22 °C | [74] |
| Babassu | Solid | - | Chara | 3.47 mg KOH/g | 2.40 meq O2/kg | 14.0 g I2/100 g | 265 mg KOH/g | 1.451 (40 °C) | 0.9280 g/mL | 0.40% | 22–26 °C | [75] |
| Buriti | Liquid | Red | Chara | 3.12 mg NaOH/g | 14.12 meq O2/kg | 74.64 g I2/100 g | 192.88 mg KOH/g | 1.4610 (40 °C) | 0.909 g/mL | 0.5% | - | [76] |
| Cumaru | Liquid | Green | Chara | 0.22 mg KOH/g | <10 meq H2O2 kg | 67 g I2/100 g | 212.3 mg KOH/g | 1.460 (40 °C) | 0.935 g/mL | 4.9% | 69–73 °C | [73] |
| Inchi | Liquid | Translucent yellow | Chara | 3 g NaOH/g | 7.16 meq O2/kg | 136.53 g I2/100 g | 176.93 mg KOH/g | 1.4734 (25 °C) | 0.9065 g/mL | 1.0% | −14.33 °C | [77] |
| Patawa | Liquid | Green | Chara | 2 mg NaOH/g | <10.0 meq O2/kg | 84 g I2/100 g | 192 mg KOH/g | 1.468 (40 °C) | 0.9140 g/mL | 1.30% | 16 °C | [78] |
| Pequi | Liquid | Yellow | Chara | 5.4 g NaOH/g | 7.94 meq O2/kg | 50 g I2/100 g | 206.8 mg KOH/g | - | - | - | - | [79] |
| Pracaxi | Liquid | Translucent yellow | Chara | 3 mg NaOH/g | 5 meq O2/kg | 68 g I2/100 g | 170–180 mg KOH/g | 1.461 (40 °C) | 0.9173 g/mL | <1.5% | 18.5 °C | [73] |
| Sacha inchi | Liquid | Translucent yellow | Chara | 0.10 mg NaOH/g | 2.77 meq O2/kg | 189.16 g I2/100 g | 189.60 mg KOH/g | 14.816 (20 °C) | 0.9255 g/mL | - | - | [80] |
| Tucuma | Liquid | Green | Chara | 5.47 mg NaOH/g | 2.99 meq O2/kg | 12.7 g I2/100 g | 202.71 mg KOH/g | 1.461 (40 °C) | 0.9100 g/mL | <1.8% | 27 °C | [81,82] |
| Acai | Andiroba | Babassu | Buriti | Cumaru | Inchi | Patawa | Pequi | Pracaxi | Sacha Inchi | Tucumã | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Acids | Composition | ||||||||||
| Caprylic Acid (C 8: 0) | - | - | 6.21% | - | - | - | - | - | - | - | 1.94% |
| Capric Acid (C 10: 0) | - | - | 5.78% | - | - | - | - | - | - | - | 0.80% |
| Lauric acid (C 12: 0) | 0.07% | - | 47.40% | 0.03% | - | - | 1.37% | - | 1.20% | - | - |
| Miristic acid (C 14: 0) | 0.13% | - | 15.64% | 0.08% | - | 0.1% | 0.94% | 0.36% | 0.71% | - | - |
| Palmitic acid (C 16: 0) | 21.78% | 31.40% | 8.01% | 16.78% | 6.70% | 10.3% | 11.04% | 29.48% | 1.95% | 6.30% | 22.99% |
| Palmitoleic acid (C 16: 1) | 3.26% | 0.26% | 0.02% | 0.32% | - | 0.1% | 0.41% | 0.66% | - | - | - |
| Margaric Acid (C 17: 0) | - | - | 0.02% | 0.08% | - | 0.2% | - | - | - | - | - |
| Stearic acid (C 18: 0) | 2.17% | 10% | 3.15% | 1.77% | 4.53% | 3.4% | 5.09% | 2.44% | 2.92% | 3.81% | 2.95% |
| Oleic acid (C 18: 1-Omega 9) | 57.42% | 50.6% | 11.28% | 74.06% | 53.37% | 11.8% | 74.18% | 59.99% | 47.57% | 10.45% | 67.62% |
| Linoleic acid (C 18: 2-Omega 6) | 11.08% | 5.4% | 1.85% | 4.94% | 16.45% | 85.6% | 5.97% | 6.44% | 12.08% | 36.80% | 1.15% |
| Linolenic acid (C 18: 3-Omega 3) | 0.59% | - | 0.25% | 1.04% | 3.32% | - | 0.51% | - | 1.07% | 50.41% | 4.97% |
| Arachidonic acid (C 20: 4) | - | - | - | - | 0.70% | - | 0.5% | - | 1.34% | - | - |
| Arachidic acid (C 20: 0) | 0.11% | 0.62% | 0.05% | 0.12% | - | 0.5% | 0.60% | - | 1.05% | - | - |
| Behenic acid (C 22: 0) | - | 0.15% | 0.01% | 0.09% | 4.3% | - | - | - | 17.88% | - | - |
| Lignoceric acid (C 24: 0) | - | - | 0.04% | 0.09% | 3.9% | - | - | - | - | - | - |
| Saturated | 28.3% | 36.3% | 86.42% | 22.2% | 19.77% | 14.3% | 18.94% | 32.28% | 38.47% | 7.70% | 29.28% |
| Unsaturated | 68.1% | 63.7% | 13.58% | 77.8% | 80.23% | 85.7% | 81.07% | 67.71% | 61.54% | 95.2% | 68.77% |
| Reference | [83,84,85] | [86,87,88] | [75,89] | [76] | [90,91] | [77,92] | [1,93,94] | [79] | [95] | [96] | [81] |
| Traditional Name | Buriti | Inchi | Patawa | Pequi | Pracaxi | Sacha inchi | Tucuma |
|---|---|---|---|---|---|---|---|
| Carotenoids | - | - | - | 89.82 mg/kg | - | - | 16.37 mg/kg |
| α-Carotene | 76.8 mg/kg | - | - | - | - | - | - |
| β-Carotene | 8.8 mg/kg | - | 2.38 mg/kg | - | 8.84 mg/kg | - | - |
| γ-Carotene | 4.5 mg/kg | - | - | - | - | - | - |
| Aocarotenoids | 0.5 mg/kg | - | - | - | - | - | - |
| Total carotenoids | 1800 mg/kg | - | - | - | - | - | - |
| Squealene | 11.7 mg/kg | - | - | - | - | - | |
| Cholesterol | - | 0.8 mg/kg | 3.4 mg/kg | - | - | - | 3.0 mg/kg |
| Δ5-Avenasterol | - | 3.3 mg/kg | 27.8 mg/kg | - | - | - | 27.8 mg/kg |
| Cycloartenol | - | 1.3 mg/kg | 105 mg/kg | - | - | - | 86.0 mg/kg |
| Methylenecicloartenol | - | - | - | - | - | - | - |
| Citrostadienol | - | 0.8 mg/kg | - | - | - | - | - |
| Lanosterol | - | 1.2 mg/kg | - | - | - | - | - |
| Campestanol | - | - | 6 mg/kg | - | - | - | - |
| Campesterol | - | 12.2 mg/kg | 7.2 mg/kg | 42.82 mg/kg | - | 15.3 mg/kg | 16 mg/kg |
| Stigmasterol | - | 11.0 mg/kg | 19.2 mg/kg | 527.30 mg/kg | - | 34.61–58.7 mg/kg | 3 mg/kg |
| β-Sitosterol | - | 55.0 mg/kg | 34.2 mg/kg | 238.50 mg/kg | - | 43.46–127.4 mg/kg | 61 mg/kg |
| α-Tocopherol | 614 mg/kg | 175 mg/kg | 1.704 mg/kg | 91.49 mg/kg | - | 0.08 mg/kg | 96 mg/kg |
| β-Tocopherol | 687 mg/kg | 9 mg/kg | - | - | 72.92 mg/kg | 0.02 mg/kg | 2 mg/kg |
| γ-Tocopherol | 50 mg/kg | 575 mg/kg | - | 63.82 mg/kg | 416.13 mg/kg | 127.6–149.0 mg/kg | 1.8 mg/kg |
| δ-Tocopherol | 136 mg/kg | 57 mg/kg | - | - | 7.78 mg/kg | 60.0–84.0 mg/kg | - |
| Total tocopherol | 1517 mg/kg | 816 mg/kg | - | 155.31 mg/kg | - | 209–211.8 mg/kg | - |
| α-Tocotrienol | - | - | - | - | 93.53 mg/kg | - | - |
| γ-Tocotrienol | 12 mg/kg | - | 269 mg/kg | - | - | - | 55–59 mg/kg |
| δ-Tocotrienol | 18 mg/kg | - | - | - | - | - | - |
| Referencias | [76,93] | [77,92] | [1,93,94] | [79] | [95] | [96] | [81] |
6. Oxidative Stability of Organogels
7. The Skin
7.1. Skin Permeation
7.2. Organogel Tecnology as a Controlled Release Mechanism
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bezerra, C.V.; da Rodrigues, A.M.C.; de Oliveira, P.D.; da Silva, D.A.; da Silva, L.H.M. Technological properties of amazonian oils and fats and their applications in the food industry. Food Chem. 2017, 221, 1466–1473. [Google Scholar] [CrossRef]
- Da Silva, M.J.F.; Rodrigues, A.M.; Vieira, I.R.S.; Neves, G.D.A.; Menezes, R.R.; Gonçalves, E.D.G.D.R.; Pires, M.C.C. Development and characterization of a babassu nut oil-based moisturizing cosmetic emulsion with a high sun protection factor. RSC Adv. 2020, 10, 26268–26276. [Google Scholar] [CrossRef]
- Zanatta, C.F.; Mitjans, M.; Urgatondo, V.; Rocha-Filho, P.A.; Vinardell, M.P. Photoprotective potential of emulsions formulated with Buriti oil (Mauritia flexuosa) against UV irradiation on keratinocytes and fibroblasts cell lines. Food Chem. Toxicol. 2010, 48, 70–75. [Google Scholar] [CrossRef]
- Barros, L.; Maria, E.; Lira, D.S.; Regina, S.; Lemos, A.; Izabelly, S.; Luis, T.; Rizo, S.; Paulo, S. Estudo do creme de buriti (Mauritia flexuosa L.) no processo de cicatrização. ConsSaude 2014, 13. [Google Scholar] [CrossRef]
- De Araújo, L.C.R.; Lins, M.A.; de Lima, G.R.; Moreschi, A.R.C.; Lima, E.S.; Hanan, S.A.; Toda, C.; Bandeira, M.F.C.L. Atividade Do Óleo De Copaíba Sobre Radicais Livres Formados Durante a Resposta Inflamatória. Braz. J. Dev. 2020, 6, 53538–53553. [Google Scholar] [CrossRef]
- Dong, W.; Chen, Q.; Wei, C.; Hu, R.; Long, Y. Ultrasonics Sonochemistry Comparison of the effect of extraction methods on the quality of green coffee oil from Arabica coffee beans: Lipid yield, fatty acid composition, bioactive components, and antioxidant activity. Ultrason. Sonochem. 2021, 74, 105578. [Google Scholar] [CrossRef]
- Martin-Franco, J. Comparison of two sesame oil extraction methods: Percolation and pressed extracción de aceite de ajonjolí: Percolación y prensado de extração de óleo de sésamo. Rev. Bio Agro 2016, 14, 10–18. [Google Scholar] [CrossRef]
- Nde, D.B.; Foncha, A.C. Optimization Methods for the Extraction of Vegetable Oils: A Review. Processes 2020, 8, 209. [Google Scholar] [CrossRef]
- Parker, T.D.; Adams, D.A.; Zhou, K.; Harris, M.; Yu, L. Fatty acid composition and oxidative stability of cold-pressed edible seed oils. J. Food Sci. 2003, 68, 1240–1243. [Google Scholar] [CrossRef]
- Aleksander Siger, M.N.-K.; Faculty, E.L.-S. The content and antioxidant activity of phenolic compounds in cold-pressed plant oils. System 2008, 15, 137–149. [Google Scholar]
- Bataglion, G.A.; da Silva, F.M.A.; Santos, J.M.; dos Santos, F.N.; Barcia, M.T.; de Lourenço, C.C.; Salvador, M.J.; Godoy, H.T.; Eberlin, M.N.; Koolen, H.H.F. Comprehensive characterization of lipids from Amazonian vegetable oils by mass spectrometry techniques. Food Res. Int. 2014, 64, 472–481. [Google Scholar] [CrossRef]
- Nascimento, G.O.; Souza, D.P.; Santos, A.S.; Batista, J.F.; Rathinasabapathi, B.; Gagliardi, P.R.; Gonçalves, J.F.C. Lipidomic profiles from seed oil of Carapa guianensis Aubl. and Carapa vasquezii Kenfack and implications for the control of phytopathogenic fungi. Ind. Crops Prod. 2019, 129, 67–73. [Google Scholar] [CrossRef]
- Marangon, C.A.; Martins, V.C.A.; Leite, P.M.F.; Santos, D.A.; Nitschke, M.; Plepis, A.M.G. Chitosan/gelatin/copaiba oil emulsion formulation and its potential on controlling the growth of pathogenic bacteria. Ind. Crops Prod. 2017, 99, 163–171. [Google Scholar] [CrossRef]
- Bovi, G.G.; Petrus, R.R.; Pinho, S.C. Feasibility of incorporating buriti (Mauritia flexuosa L.) oil nanoemulsions in isotonic sports drink. Food Sci. Technol. 2017, 57, 2201–2209. [Google Scholar] [CrossRef]
- Leão, K.M.M.; Reis, L.V.C.; Speranza, P.; Rodrigues, A.P.; Ribeiro, A.P.B.; Macedo, J.A.; Macedo, G.A. Physicochemical characterization and antimicrobial activity in novel systems containing buriti oil and structured lipids nanoemulsions. Biotechnol. Rep. 2019, 24, e00365. [Google Scholar] [CrossRef]
- Gomes, A.T.A.; Pereira, R.R.; Junior, A.P.D.; da Rodrigues, A.M.C.; Remédios, C.M.R.; do Brasil, D.S.B.; Morais, L.R.B.; Silva-Júnior, J.O.C.; Ribeiro-Costa, R.M. Tucumã (Astrocaryum vulgare) Fat: An Amazonian Material as a Pharmaceutical Input for Lipid Nanoparticle Production; Springer: Berlin/Heidelberg, Germany, 2020. [Google Scholar] [CrossRef]
- Patel, A.R. A colloidal gel perspective for understanding oleogelation. Curr. Opin. Food Sci. 2017, 15, 1–7. [Google Scholar] [CrossRef]
- Pernetti, M.; van Malssen, K.F.; Flöter, E.; Bot, A. Structuring of edible oils by alternatives to crystalline fat. Curr. Opin. Colloid Interface Sci. 2007, 12, 221–231. [Google Scholar] [CrossRef]
- Sullivan, C.M.O.; Barbut, S.; Marangoni, A.G. Edible oleogels for the oral delivery of lipid soluble molecules: Composition and structural design considerations. Trends Food Sci. Technol. 2016, 57, 59–73. [Google Scholar] [CrossRef]
- Davidovich-Pinhas, M. Oleogels. In Polymer Gels; Woodhead Publishing: Cambridge, UK, 2018; pp. 231–249. [Google Scholar] [CrossRef]
- Dassanayake, L.S.K.; Kodali, D.R.; Ueno, S. Formation of oleogels based on edible lipid materials. Curr. Opin. Colloid Interface Sci. 2011, 16, 432–439. [Google Scholar] [CrossRef]
- Tarun, G.; Ajay, B.; Bhawana, K.; Sunil, K.; Ravi, J. Organogels: Advanced and Novel Drug Delivery System. Int. Res. J. Pharm. 2011, 2, 15–21. [Google Scholar]
- Kirilov, P.; Le, C.A.K.; Denis, A.; Rabehi, H.; Rum, S.; Villa, C.; Haftek, M.; Pirot, F. Organogels for cosmetic and dermo-cosmetic applications: Classification, preparation and characterization of organogel formulations. Househ. Pers. Care Today 2015, 10, 16–21. [Google Scholar]
- Kirilov, P.; Tran, V.H.; Ducrotté-Tassel, A.; Salvi, J. Ex-Vivo percutaneous absorption of enrofloxacin: Comparison of LMOG organogel vs. pentravan cream. Int. J. Pharm. 2016, 498, 170–177. [Google Scholar] [CrossRef]
- Sagiri, S.S.; Behera, B.; Rafanan, R.R.; Bhattacharya, C.; Pal, K.; Banerjee, I.; Rousseau, D. Organogels as matrices for controlled drug delivery: A review on the current state. Soft Matter 2014, 12, 47–72. [Google Scholar] [CrossRef]
- Sahoo, S.; Kumar, N.; Bhattacharya, C.; Sagiri, S.S.; Jain, K.; Pal, K.; Ray, S.S.; Nayak, B. Organogels: Properties and Applications in Drug Delivery. Des. Monomers Polym. 2012, 5551, 95–108. [Google Scholar] [CrossRef]
- Luboradzki, R.; Gronwald, O.; Ikeda, A.; Shinkai, S. Sugar-Integrated “Supergelators” Which Can Form Organogels with 0.03–0.05% [g mL−1]. Chem. Lett. 2000, 29, 1148–1149. [Google Scholar] [CrossRef]
- Gelators, S.; Gronwald, O.; Shinkai, S. Sugar-Integrated Gelators of Organic Solvents. Chem. A Eur. J. 2001, 7, 4328–4334. [Google Scholar]
- Vintiloiu, A.; Leroux, J.C. Organogels and their use in drug delivery—A review. J. Control. Release 2008, 125, 179–192. [Google Scholar] [CrossRef]
- Zeng, C.; Wan, Z.; Xia, H.; Zhao, H.; Guo, S. Structure and Properties of Organogels Developed by Diosgenin in Canola Oil. Food Biophys. 2020, 15, 452–462. [Google Scholar] [CrossRef]
- Wang, F.C.; Gravelle, A.J.; Blake, A.I.; Marangoni, A.G. Novel trans fat replacement strategies. Curr. Opin. Food Sci. 2016, 7, 27–34. [Google Scholar] [CrossRef]
- Esposito, C.L.; Kirilov, P.; Roullin, V.G. Organogels, promising drug delivery systems: An update of state-of-the-art and recent applications. J. Control. Release 2018, 271, 1–20. [Google Scholar] [CrossRef]
- Toro-Vazquez, J.F.; Morales-Rueda, J.A.; Dibildox-Alvarado, E.; Charó-Alonso, M.; Alonzo-Macias, M.; González-Chávez, M.M. Thermal and textural properties of organogels developed by candelilla wax in safflower oil. J. Am. Oil Chem. Soc. 2007, 84, 989–1000. [Google Scholar] [CrossRef]
- Rocha, J.C.B.; Lopes, J.D.; Mascarenhas, M.C.N.; Arellano, D.B.; Guerreiro, L.M.R.; da Cunha, R.L. Thermal and rheological properties of organogels formed by sugarcane or candelilla wax in soybean oil. Food Res. Int. 2013, 50, 318–323. [Google Scholar] [CrossRef]
- Öğütcü, M.; Arifoğlu, N.; Yılmaz, E. Preparation and Characterization of Virgin Olive Oil-Beeswax. J. Am. Oil Chem. Soc. 2015, 92, 459–471. [Google Scholar] [CrossRef]
- Yi, B.R.; Kim, M.J.; Lee, S.Y.; Lee, J.H. Physicochemical properties and oxidative stability of oleogels made of carnauba wax with canola oil or beeswax with grapeseed oil. Food Sci. Biotechnol. 2017, 26, 79–87. [Google Scholar] [CrossRef]
- Saeed, R.; Naz, S. Effect of heating on the oxidative stability of corn oil and soybean oil. Grasas Y Aceites 2019, 70, 303. [Google Scholar] [CrossRef]
- Fasina, O.O.; Hallman, H.; Craig-Schmidt, M.; Clements, C. Predicting temperature-dependence viscosity of vegetable oils from fatty acid composition. J. Am. Oil Chem. Soc. 2006, 83, 899–903. [Google Scholar] [CrossRef]
- Sadoudi, R.; Ammouche, A.; Ali, A.D. Thermal oxidative alteration of sunflower oil. Afric. J. Food Sci. 2014, 8, 116–121. [Google Scholar] [CrossRef][Green Version]
- Hwang, H.S. A critical review on structures, health effects, oxidative stability, and sensory properties of oleogels. Biocatal. Agric. Biotechnol. 2020, 26, 101657. [Google Scholar] [CrossRef]
- Hwang, H.-S.; Fhaner, M.; Winkler-Moser, J.K.; Running, S.X.L. Oxidation of Fish Oil Oleogels Formed by Natural Waxes in Comparison with Bulk Oil. Eur. J. Lipid Sci. Technol. 2018, 120, 1700378. [Google Scholar] [CrossRef]
- Hansen, C.M. Hansen Solubility Parameters (HSP); Taylor and Francis Group: Abingdon, UK, 2007; Volume 118, ISBN 9780849372483. [Google Scholar]
- Gravelle, A.J.; Marangoni, A.G.; Davidovich-Pinhas, M. Ethylcellulose Oleogels; AOCS Press: Urbana, IL, USA, 2018; ISBN 9780128142707. [Google Scholar]
- Piasecka-Zelga, J.; Zelga, P.; Szulc, J.; Wietecha, J.; Ciechańska, D. An in vivo biocompatibility study of surgical meshes made from bacterial cellulose modified with chitosan. Int. J. Biol. Macromol. 2018, 116, 1119–1127. [Google Scholar] [CrossRef]
- Kumar, R.; Katare, O.P. Lecithin Organogels as a Potential Phospholipid-Structured System for Topical Drug Delivery: A Review. Aaps Pharmscitech 2005, 6, 298–310. [Google Scholar] [CrossRef] [PubMed]
- Mehta, C.; Bhatt, G.; Kothiyal, P. A Review on organogel for skin aging. Indian J. Pharm. Biol. Res. 2016, 4, 28–37. [Google Scholar] [CrossRef]
- Sehnem, D.; Benamor, L.; Jesus, L.; Valentim, R.; Souza, E.; Zambuzzi, W.; Takamori, E. Métodos alternativos para avaliação da citotoxidade de biomateriais. Rev. Rede Cuid. Saúde 2012, 6, 1–12. [Google Scholar]
- Lim, C.K.; Yaacob, N.S.; Ismail, Z.; Halim, A.S. In vitro biocompatibility of chitosan porous skin regenerating templates (PSRTs) using primary human skin keratinocytes. Toxicol. In Vitro 2010, 24, 721–727. [Google Scholar] [CrossRef]
- Macoon, R.; Robey, M.; Chauhan, A. In vitro release of hydrophobic drugs by oleogel rods with biocompatible gelators. Eur. J. Pharm. Sci. 2020, 152, 105413. [Google Scholar] [CrossRef] [PubMed]
- Raut, S.; Bhadoriya, S.S.; Uplanchiwar, V.; Mishra, V.; Gahane, A.; Jain, S.K. Lecithin organogel: A unique micellar system for the delivery of bioactive agents in the treatment of skin aging. Acta Pharm. Sin. B 2012, 2, 8–15. [Google Scholar] [CrossRef]
- Fahy, E.; Subramaniam, S.; Brown, H.A.; Glass, C.K.; Merrill, A.H.; Murphy, R.C.; Raetz, C.R.H.; Russell, D.W.; Seyama, Y.; Shaw, W.; et al. A comprehensive classification system for lipids. J. Lipid Res. 2005, 46, 839–861. [Google Scholar] [CrossRef]
- Fahy, E.; Cotter, D.; Sud, M.; Subramaniam, S. Lipid classification, structures and tools. Biochim. Biophys. Acta-Mol. Cell Biol. Lipids. 2011, 1811, 637–647. [Google Scholar] [CrossRef] [PubMed]
- Mcclements, D.J.; Decker, E.A.; Park, Y.; Weiss, J. Structural design principles for delivery of bioactive components in nutraceuticals and functional foods. Crit. Rev. Food Sci. Nutr. 2009, 49, 577–606. [Google Scholar] [CrossRef] [PubMed]
- Alves, A.; Sousa, E.; Kijjoa, A.; Pinto, M. Marine-Derived Compounds with Potential Use as Cosmeceuticals and Nutricosmetics. Molecules 2020, 25, 2536. [Google Scholar] [CrossRef] [PubMed]
- Gunstone, F.D.; Harwood, J.L.; Dijkstra, A.J. The Lipid Handbook; CRC Press: Boca Raton, FL, USA, 2007; ISBN 9780849396885. [Google Scholar]
- Gunstone, F.D. Fatty Acid and Lipid Chemistry; Springer: Berlin/Heidelberg, Germany, 1996; ISBN 9781461368526. [Google Scholar]
- Nollet, L.M.L.; Toldrá, F. Seafood and Seafood Products Analysis; CRC Press: Boca Raton, FL, USA, 2010; ISBN 9781420046335. [Google Scholar]
- Magri, A.; Petriccione, M.; Cerqueira, M.A.; Gutiérrez, T.J. Self-assembled lipids for food applications: A review. Adv. Colloid Interface Sci. 2020, 285, 102279. [Google Scholar] [CrossRef] [PubMed]
- Ferro, A.C.; Okuro, P.K.; Badan, A.P.; Cunha, R.L. Role of the oil on glyceryl monostearate based oleogels. Food Res. Int. 2019, 120, 610–619. [Google Scholar] [CrossRef]
- Ghazani, S.M.; Marangoni, A.G. Minor components in canola oil and effects of refining on these constituents: A review. J. Am. Oil Chem. Soc. 2013, 90, 923–932. [Google Scholar] [CrossRef]
- Scharfe, M.; Ahmane, Y.; Seilert, J.; Keim, J.; Flöter, E. On the Effect of Minor Oil Components on β-Sitosterol/γ-oryzanol Oleogels. Eur. J. Lipid Sci. Technol. 2019, 121, 1800487. [Google Scholar] [CrossRef]
- Imai, T.; Nakamura, K.; Shibata, M. Relationship between the hardness of an oil-wax gel and the surface structure of the wax crystals. Colloids Surfaces a Physicochem. Eng. Asp. 2001, 194, 233–237. [Google Scholar] [CrossRef]
- Doan, C.D.; Tavernier, I.; Okuro, P.K.; Dewettinck, K. Internal and external factors affecting the crystallization, gelation and applicability of wax-based oleogels in food industry. Innov. Food Sci. Emerg. Technol. 2018, 45, 42–52. [Google Scholar] [CrossRef]
- Dassanayake, L.S.K.; Kodali, D.R.; Ueno, S.; Sato, K. Crystallization kinetics of organogels prepared by rice bran wax and vegetable oils. J. Oleo Sci. 2012, 61, 1–9. [Google Scholar] [CrossRef]
- Hwang, H.S.; Kim, S.; Evans, K.O.; Koga, C.; Lee, Y. Morphology and networks of sunflower wax crystals in soybean oil organogel. Food Struct. 2015, 5, 10–20. [Google Scholar] [CrossRef]
- Dragicevic, N.; Maibach, H.I. Percutaneous Penetration Enhancers Chemical Methods in Penetration Enhancement: Drug Manipulation Strategies and Vehicle Effects; Springer: Berlin/Heidelberg, Germany, 2015; pp. 1–341. [Google Scholar] [CrossRef]
- Shchipunov, Y.A.; Hoffmann, H. Growth, branching, and local ordering of lecithin polymer-like micelles. Langmuir 1998, 14, 6350–6360. [Google Scholar] [CrossRef]
- Hidalgo, P.S.P.; Nunomura, R.D.C.S.; Nunomura, S.M. Amazon oilseeds: Chemistry and antioxidant activity of patawa (Oenocarpus bataua Mart.). Rev. Virtual Quim. 2016, 8, 130–140. [Google Scholar] [CrossRef]
- Bart, J.C.J.; Palmeri, N.; Cavallaro, S. Oleochemical sources: Basic science, processing and applications of oils. Biodiesel Sci. Technol. 2010, 3, 62–113. [Google Scholar] [CrossRef]
- Kendall, A.C.; Nicolaou, A. Bioactive lipid mediators in skin inflammation and immunity. Prog. Lipid Res. 2013, 52, 141–164. [Google Scholar] [CrossRef] [PubMed]
- Rezaire, A.; Robinson, J.C.; Bereau, D.; Verbaere, A.; Sommerer, N.; Khan, M.K.; Durand, P.; Prost, E.; Fils-Lycaon, B. Amazonian palm Oenocarpus bataua (“patawa”): Chemical and biological antioxidant activity—Phytochemical composition. Food Chem. 2014, 149, 62–70. [Google Scholar] [CrossRef]
- Da Silva, J.J.M.; Rogez, H. Avaliação da estabilidade oxidativa do óleo bruto de açaí (Euterpe oleracea) na presença de compostos fenólicos puros ou de extratos vegetais amazônicos. Quim. Nova 2013, 36, 400–406. [Google Scholar] [CrossRef][Green Version]
- Morais, L.R.B.; Gutjahr, E. Química de Oleaginosas Chemistry of Vegetable Oils; Ekkehard Gutjahr: Belém, Brazil, 2011. [Google Scholar]
- Silva, L.R. Propriedades Físico-Químicas E Perfil Dos Ácidos Graxos Do Óleo Da Andiroba. Nativa 2018, 6, 147. [Google Scholar] [CrossRef]
- Melo, E.; Michels, F.; Arakaki, D.; Lima, N.; Gonçalves, D.; Cavalheiro, L.; Oliveira, L.; Caires, A.; Hiane, P.; Nascimento, V. First study on the oxidative stability and elemental analysis of babassu (Attalea speciosa) edible oil produced in Brazil using a domestic extraction machine. Molecules 2019, 24, 4235. [Google Scholar] [CrossRef] [PubMed]
- Silva, S.M.; Sampaio, K.A.; Taham, T.; Rocco, S.A.; Ceriani, Æ.R.; Meirelles, A.J.A. Characterization of Oil Extracted from Buriti Fruit (Mauritia flexuosa) Grown in the Brazilian Amazon Region. J. Am. Oil Chem. Soc. 2009, 86, 611–616. [Google Scholar] [CrossRef]
- De Padilla, F.C. Physico-chemical characteristics of the Barinas nut (Caryodendron orinocense Karst. Euphorbiaceae) crude oil. Arch. Latinoam. Nutr. 1994, 44, 172–175. [Google Scholar]
- Firestone, D. Physical and Chemical Characteristics of Oils, Fats, and Waxes; AOC Press: Washington, DC, USA, 2013; ISBN 9780983079194. [Google Scholar]
- Torres, L.R.D.O.; Santana, F.C.D.; Torres-Leal, F.L.; Melo, I.L.P.D.; Yoshime, L.T.; Matos-Neto, E.M.; Seelaender, M.C.L.; Araújo, C.M.M.; Cogliati, B.; Mancini-Filho, J. Pequi (Caryocar brasiliense Camb.) almond oil attenuates carbon tetrachloride-induced acute hepatic injury in rats: Antioxidant and anti-inflammatory effects. Food Chem. Toxicol. 2016, 97, 205–216. [Google Scholar] [CrossRef]
- Flores, S.; Flores, A.; Calderón, C.; Obregón, D. Synthesis and characterization of sacha inchi (Plukenetia volubilis L.) oil-based alkyd resin. Prog. Org. Coat. 2019, 136, 105289. [Google Scholar] [CrossRef]
- Bora, P.S.; Narain, N.; Rocha, R.V.M.; Monteiro, A.C.D.O. Characterisation of the oil and protein fractions of tucuma (Astrocaryum Vulgare Mart.) Fruit pulp and seed kernel caracterización de las fracciones protéicas y lipídicas de pulpa y semillas de tucuma (Astrocaryum Vulgare Mart.) . Cienc. Tecnol. Aliment. 2001, 3, 111–116. [Google Scholar] [CrossRef]
- De Souza Ferreira, E.; Georgina, L.V.; Siqueira, A.A.; da Silva, S.C. Physicochemical characterization of the fruit and oil extracted from tucuman (Astrocaryum vulgare Mart.). Braz. J. Food Nutr. 2008, 19, 427–433. [Google Scholar]
- Rosa, P.; Lago, M.; Rubert, D.; Librelotto, N.; Haselein, L.; Emanuelli, T.; de Bona, C.; Inês, A.; Adams, H.; de Pós-graduaç, P.; et al. Desonide nanoencapsulation with açai oil as oil core: Physicochemical characterization, photostability study and in vitro phototoxicity evaluation. J. Photochem. Photobiol. B Biol. 2019, 199, 111606. [Google Scholar] [CrossRef] [PubMed]
- Do Nascimento, R.J.S.; Couri, S.; Antoniass, R.; Freitas, S.P. Composição em ácidos graxos do óleo da polpa de açaí fatty acids composition of açaí pulp oil obtained by enzymatic. Rev. Bras. Frutic. 2008, 30, 498–502. [Google Scholar] [CrossRef]
- Loureiro, D.M.; Oliveira, E.; Ribeiro-Costa, R.M.; Silva-Júnior, O.C. Nanoemulsions of Acai Oil: Physicochemical Characterization for the Topical Delivery of Antifungal Drugs. Chem. Eng. Technol. 2020, 42, 1424–1432. [Google Scholar] [CrossRef]
- Sarquis, S.F.R.; Marinho, V.H.S.; Neves, F.B.; Sarquis, I.R.; Araújo, I.F.; Damasceno, L.F.; Ferreira, R.M.A.; Souto, R.N.P.; Carvalho, C.T.; Ferreira, I.M. Carapa guianensis Aubl. (Meliaceae) oil associated with silk fibroin, as alternative to traditional surfactants, and active against larvae of the vector Aedes aegypti. Ind. Crops Prod. 2020, 157, 112931. [Google Scholar] [CrossRef]
- Soares, A.D.S.; Wanzeler, A.M.V.; Cavalcante, G.H.S.; da Barros, E.M.S.; de Carneiro, R.C.M.; Tuji, F.M. Therapeutic effects of andiroba (Carapa guianensis Aubl) oil, compared to low power laser, on oral mucositis in children underwent chemotherapy: A clinical study. J. Ethnopharmacol. 2021, 264, 113365. [Google Scholar] [CrossRef]
- Iha, O.K.; Alves, F.C.S.C.; Suarez, P.A.Z.; Silva, C.R.P.; Meneghetti, M.R.; Meneghetti, S.M.P. Potential application of Terminalia catappa L. and Carapa guianensis oils for biofuel production: Physical-chemical properties of neat vegetable oils, their methyl-esters and bio-oils (hydrocarbons). Ind. Crops Prod. 2014, 52, 95–98. [Google Scholar] [CrossRef]
- Santos, J.A.A.; da Silva, J.W.; dos Santos, S.M.; Rodrigues, M.D.F.; Silva, C.J.A.; da Silva, M.V.; Correia, M.T.S.; Albuquerque, J.F.C.; Melo, C.M.L.; Silva, T.G.; et al. In Vitro and in Vivo Wound Healing and Anti-Inflammatory Activities of Babassu Oil (Attalea speciosa Mart. Ex Spreng., Arecaceae). Evid.-Based Complement. Altern. Med. 2020, 2020, 8858291. [Google Scholar] [CrossRef]
- Oliveros-bastidas, A.D.J.; Demuner, A.J.; de Química, D.; de Viçosa, U.F.; Rolf, A.P.H.; Mg, V. Chemical characterization by gc-ms and phytotoxic potential of non-polar and polar fractions of seeds of Dioteryx odorata (Aubl.) Willd. from venezuelan regions. Quim. Nova 2013, 36, 502–506. [Google Scholar] [CrossRef]
- Fetzer, D.L.; Hamerski, F.; Errico, M.; Corazza, M.L. Extraction of cumaru seed oil using compressed propane as solvent. J. Supercrit. Fluids 2021, 169, 105123. [Google Scholar] [CrossRef]
- Radice, M.; Viafara, D.; Neill, D.; Asanza, M.; Sacchetti, G.; Guerrini, A.; Maietti, S. Chemical Characterization and Antioxidant Activity of Amazonian (Ecuador) Caryodendron orinocense Karst. and Bactris gasipaes Kunth Seed Oils. J. Oleo Sci. 2014, 1250, 1243–1250. [Google Scholar] [CrossRef] [PubMed]
- Darnet, S.H.; da Silva, L.H.M.; da Rodrigues, A.M.C.; Lins, R.T. Nutritional composition, fatty acid and tocopherol contents of buriti (Mauritia flexuosa) and patawa (Oenocarpus bataua) fruit pulp from the amazon region. Food Sci. Technol. 2011, 31, 488–491. [Google Scholar] [CrossRef]
- Montúfar, R.; Laffargue, A.; Pintaud, J.C.; Hamon, S.; Avallone, S.; Dussert, S. Oenocarpus bataua Mart. (Arecaceae): Rediscovering a source of high oleic vegetable oil from amazonia. J. Am. Oil Chem. Soc. 2010, 87, 167–172. [Google Scholar] [CrossRef]
- Dos Santos Costa, M.N.F.; Marcos, C.; Nı, M.; Alberto, C.; Negra, B.; Maria, R.; Costa, R. Characterization of Pentaclethra macroloba oil. J. Therm. Anal. Calorim. 2013, 115, 2269–2275. [Google Scholar] [CrossRef]
- Wang, S.; Zhu, F.; Kakuda, Y. Sacha inchi (Plukenetia volubilis L.): Nutritional composition, biological activity, and uses. Food Chem. 2018, 265, 316–328. [Google Scholar] [CrossRef]
- De Magalhães, T.S.S.A.; de Macedo, P.C.O.; Pacheco, S.Y.K.; da Silva, S.S.; Barbosa, E.G.; Pereira, R.R. Development and Evaluation of Antimicrobial and Modulatory Activity of Inclusion Complex of Euterpe oleracea Mart Oil and β-Cyclodextrin or HP β-Cyclodextrin. Int. J. Mol. Sci. 2020, 21, 942. [Google Scholar] [CrossRef]
- Yuyama, O.; Kiyoko, L.; Paiva, J.; Aguiar, L.; Fernandes, D.; Filho, S.; Varejão, M.D.J.; Inês, D.; Fávaro, T.; Agostini, M.B.; et al. Caracterização físico-química do suco de açaí de Euterpe precatoria Mart. oriundo de diferentes ecossistemas amazônicos. Acta Amaz. 2011, 41, 545–552. [Google Scholar] [CrossRef]
- Kazumy, K.; Yamaguchi, D.L.; Felipe, L.; Pereira, R.; Victor, C.; Silva, E.; Florêncio, V. Amazon acai: Chemistry and biological activities: A review. Food Chem. 2015, 179, 137–151. [Google Scholar] [CrossRef]
- Ambrozin, A.R.P.; Leite, A.C.; Bueno, F.C.; Vieira, P.C. Limonoids from Andiroba Oil and Cedrela fissilis and their Insecticidal Activity. J. Braz. Chem. Soc. 2006, 17, 542–547. [Google Scholar] [CrossRef]
- Costa-Silva, J.H.; Lima, C.R.; Silva, E.J.R.; Araújo, A.V.; Fraga, M.C.C.A.; Ribeiro e Ribeiro, A.; Arruda, A.C.; Lafayette, S.S.L.; Wanderley, A.G. Acute and subacute toxicity of the Carapa guianensis Aublet (Meliaceae) seed oil. J. Ethnopharmacol. 2008, 116, 495–500. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, V.P.; Oliveira, R.R.; Figueiredo, M.R. Isolation of limonoids from seeds of Carapa guianensis aublet (meliaceae) by high-speed countercurrent chromatography. Phytochem. Anal. 2009, 20, 77–81. [Google Scholar] [CrossRef] [PubMed]
- Júnior, R.N.C.M.; Dolabela, M.F.; Da Silva, M.N.; Póvoa, M.M.; Maia, J.G.S. Antiplasmodial activity of the andiroba (Carapa guianensis Aubl., Meliaceae) oil and its limonoid-rich fraction. J. Ethnopharmacol. 2012, 142, 679–683. [Google Scholar] [CrossRef] [PubMed]
- Pinheiro, C.U.B.; Frazão, J.M.F. Integral processing of babassu palm (Orbignya phalerata, arecaceae) fruits: Village level production in maranhāo, Brazil. Econ. Bot. 1995, 49, 31–39. [Google Scholar] [CrossRef]
- Ribeiro, B.D.; Coelho, M.A.Z.; Barreto, D.W. Production of concentrated natural beta-carotene from buriti (Mauritia vinifera) oil by enzymatic hydrolysis. Food Bioprod. Process. 2012, 90, 141–147. [Google Scholar] [CrossRef]
- Comunian, T.A.; Silva, M.P.; Moraes, I.C.F.; Favaro-Trindade, C.S. Reducing carotenoid loss during storage by co-encapsulation of pequi and buriti oils in oil-in-water emulsions followed by freeze-drying: Use of heated and unheated whey protein isolates as emulsifiers. Food Res. Int. 2020, 130, 108901. [Google Scholar] [CrossRef] [PubMed]
- Batista, J.S.; Olinda, R.G.; Medeiros, V.B.; Rodrigues, C.M.F.; Oliveira, A.F.; Paiva, E.S.; Freitas, C.I.A.; da Medeiros, A.C. Atividade antibacteriana e cicatrizante do óleo de buriti Mauritia flexuosa L. Ciência Rural 2012, 42, 136–141. [Google Scholar] [CrossRef]
- Barros, E.M.L.; de Lira, S.R.S.; Lemos, S.I.A.; Barros, T.L.; Rizo, M.D.S. Estudo do creme de buriti (Mauritia flexuosa L.) no processo de cicatrização. ConSci. Saúde 2015, 13, 503–610. [Google Scholar] [CrossRef]
- Veiga, V.F.; Rosas, E.C.; Carvalho, M.V.; Henriques, M.G.M.O.; Pinto, A.C. Chemical composition and anti-inflammatory activity of copaiba oils from Copaifera cearensis Huber ex Ducke, Copaifera reticulata Ducke and Copaifera multijuga Hayne—A comparative study. J. Ethnopharmacol. 2007, 112, 248–254. [Google Scholar] [CrossRef]
- Da Trindade, R.; da Silva, J.K.; Setzer, W.N. Copaifera of the neotropics: A review of the phytochemistry and pharmacology. Int. J. Mol. Sci. 2018, 19, 1511. [Google Scholar] [CrossRef]
- Simões, C.A.C.G.; de Conde, N.C.O.; Venâncio, G.N.; Milério, P.S.L.L.; Bandeira, M.F.C.L.; da Veiga Júnior, V.F. Antibacterial Activity of Copaiba Oil Gel on Dental Biofilm. Open Dent. J. 2016, 10, 188–195. [Google Scholar] [CrossRef] [PubMed]
- Bandeira, M.F.C.L.; Freitas, A.L.; de Menezes, M.S.C.; dos Silva, J.S.; Sombra, G.A.D.; Araújo, E.A.M.; Toda, C.; Moreschi, A.R.C.; de Conde, N.C.O. Adhesive resistance of a copaiba oil-based dentin biomodifier. Braz. Oral Res. 2020, 34, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Vasconcelos, K.R.F.; da Junior, V.F.V.; Rocha, W.C.; Bandeira, M.F.C.L. Avaliação in vitro da atividade antibacteriana de um cimento odontológico à base de óleo-resina de Copaifera multijuga Hayne. Rev. Bras. Farmacogn. 2008, 18, 733–738. [Google Scholar] [CrossRef]
- Lisboa, M.C.; Wiltshire, F.M.S.; Fricks, A.T.; Dariva, C.; Carrière, F.; Lima, Á.S.; Soares, C.M.F. Oleochemistry potential from Brazil northeastern exotic plants. Biochimie 2020, 178, 96–104. [Google Scholar] [CrossRef]
- Da Silva, J.O.; Coppede, J.S.; Fernandes, V.C.; Sant’Ana, C.D.; Ticli, F.K.; Mazzi, M.V.; Giglio, J.R.; Pereira, P.S.; Soares, A.M.; Sampaio, S.V. Antihemorrhagic, antinucleolytic and other antiophidian properties of the aqueous extract from Pentaclethra macroloba. J. Ethnopharmacol. 2005, 100, 145–152. [Google Scholar] [CrossRef]
- Leal, I.C.R.; Júnior, I.I.; Pereira, E.M.; da Laport, M.S.; Kuster, R.M.; dos Santos, K.R.N. Pentaclethra macroloba tannins fractions active against methicillin-resistant staphylococcal and Gram-negative strains showing selective toxicity. Rev. Bras. Farm. 2011, 21, 991–999. [Google Scholar] [CrossRef][Green Version]
- Gutiérrez, L.-F.; Rosada, L.-M.; Jiménez, Á. Chemical composition of Sacha Inchi (Plukenetia volubilis L.) seeds and characteristics of their lipid fraction. Grasas Aceites 2011, 62, 76–83. [Google Scholar] [CrossRef]
- Silva, K.F.C.E.; da Silva Carvalho, A.G.; Rabelo, R.S.; Hubinger, M.D. Sacha inchi oil encapsulation: Emulsion and alginate beads characterization. Food Bioprod. Process. 2019, 116, 118–129. [Google Scholar] [CrossRef]
- Vicente, J.; de Carvalho, M.G.; Garcia-Rojas, E.E. Fatty acids profile of Sacha Inchi oil and blends by 1H NMR and GC-FID. Food Chem. 2015, 181, 215–221. [Google Scholar] [CrossRef]
- Gutiérrez, L.F.; Quiñones-Segura, Y.; Sanchez-Reinoso, Z.; Díaz, D.L.; Abril, J.I. Physicochemical properties of oils extracted from γ-irradiated Sacha Inchi (Plukenetia volubilis L.) seeds. Food Chem. 2017, 237, 581–587. [Google Scholar] [CrossRef]
- Santos, M.F.G.; Alves, R.E.; Ruíz-Méndez, M.V. Minor components in oils obtained from Amazonian palm fruits. Grasas Aceites 2013, 64, 531–536. [Google Scholar] [CrossRef]
- Bony, E.; Boudard, F.; Brat, P.; Dussossoy, E.; Portet, K.; Poucheret, P.; Giaimis, J.; Michel, A. Awara (Astrocaryum vulgare M.) pulp oil: Chemical characterization, and anti-inflammatory properties in a mice model of endotoxic shock and a rat model of pulmonary inflammation. Fitoterapia 2012, 83, 33–43. [Google Scholar] [CrossRef] [PubMed]
- Abd, E.; Yousef, S.A.; Pastore, M.N.; Telaprolu, K.; Mohammed, Y.H.; Namjoshi, S.; Grice, J.E.; Roberts, M.S. Skin models for the testing of transdermal drugs. Clin. Pharmacol. Adv. Appl. 2016, 8, 163–176. [Google Scholar] [CrossRef] [PubMed]
- Neupane, R.; Boddu, S.H.S.; Renukuntla, J.; Babu, R.J.; Tiwari, A.K. Alternatives to biological skin in permeation studies: Current trends and possibilities. Pharmaceutics 2020, 12, 152. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Stephanie, I.; Ramirez, A.; Yang, J.; Ciftci, O.N. Evaluation of oil-gelling properties and crystallization behavior of sorghum wax in fish oil. Food Chem. 2020, 309, 125567. [Google Scholar] [CrossRef]
- Tian, Y.; Acevedo, N.C. Kinetic study on photostability of retinyl palmitate entrapped in policosanol oleogels. Food Chem. 2018, 255, 252–259. [Google Scholar] [CrossRef]
- Da Silva, T.L.T.; Chaves, K.F.; Fernandes, G.D.; Rodrigues, J.B.; Bolini, H.M.A.; Arellano, D.B. Sensory and Technological Evaluation of Margarines with Reduced Saturated Fatty Acid Contents Using Oleogel Technology. J. Am. Oil Chem. Soc. 2018, 95, 673–685. [Google Scholar] [CrossRef]
- Ferrer-González, B.M.; García-Martínez, I.; Totosaus, A. Textural properties, sensory acceptance and fatty acid profile of cooked meat batters employing pumpkin seed paste or soybean oil oleogel as fat replacers. Grasas Aceites 2019, 70, 1–11. [Google Scholar] [CrossRef]
- Cui, M.; Mao, L.; Lu, Y.; Yuan, F.; Gao, Y. Effect of Monoglyceride Content on the Solubility and Chemical Stability of β-Carotene in Organogels. LWT 2019, 106, 83–91. [Google Scholar] [CrossRef]
- Zheng, H.; Mao, L.; Cui, M.; Liu, J.; Gao, Y. Development of food-grade bigels based on κ-carrageenan hydrogel and monoglyceride oleogels as carriers for β-carotene: Roles of oleogel fraction. Food Hydrocoll. 2020, 105, 105855. [Google Scholar] [CrossRef]
- Öğütcü, M.; Arifoğlu, N.; Yilmaz, E. Storage stability of cod liver oil organogels formed with beeswax and carnauba wax. Int. J. Food Sci. Technol. 2015, 50, 404–412. [Google Scholar] [CrossRef]
- Gray, J.I. Measurement of Lipid Oxidation: A Review. J. Am. Oil Chem. Soc. 1978, 55, 539–546. [Google Scholar] [CrossRef]
- Pignitter, M.; Somoza, V. Critical Evaluation of Methods for the Measurement of Oxidative Rancidity in Vegetable Oils. J. Food Drug Anal. 2012, 20, 772–777. [Google Scholar] [CrossRef]
- Hwang, J.L.H. Oil-structuring characterization of natural waxes in canola oil oleogels: Rheological, thermal, and oxidative properties. Appl. Biol. Chem. 2017, 60, 17–22. [Google Scholar] [CrossRef]
- Spellberg, B. The cutaneous citadel. Life Sci. 2000, 67, 477–502. [Google Scholar] [CrossRef]
- Afsar, F.S. Skin care for preterm and term neonates. Clin. Exp. Dermatol. 2009, 34, 855–858. [Google Scholar] [CrossRef]
- Prausnitz, M.R.; Elias, P.M.; Franz, T.J.; Schmuth, M.; Tsai, J.-C.; Menon, G.K.; Holleran, W.M.; Feingold, K.R. Skin Barrier and Transdermal Drug Delivery Structure and Origin of the Skin Barrier Stratum Corneum Structure and Organization. Med. Ther. 2012, 2065–2073. [Google Scholar]
- Petry, T.; Bury, D.; Fautz, R.; Hauser, M.; Huber, B.; Markowetz, A.; Mishra, S.; Rettinger, K.; Schuh, W.; Teichert, T. Review of data on the dermal penetration of mineral oils and waxes used in cosmetic applications. Toxicol. Lett. 2017, 280, 70–78. [Google Scholar] [CrossRef]
- Carrer, V.; Alonso, C.; Pont, M.; Zanuy, M.; Córdoba, M.; Espinosa, S.; Barba, C.; Oliver, M.A.; Martí, M.; Coderch, L. Effect of propylene glycol on the skin penetration of drugs. Arch. Dermatol. Res. 2020, 312, 337–352. [Google Scholar] [CrossRef]
- El Maghraby, G.M.; Barry, B.W.; Williams, A.C. Liposomes and skin: From drug delivery to model membranes. Eur. J. Pharm. Sci. 2008, 34, 203–222. [Google Scholar] [CrossRef]
- Zhang, X.; Yu, Y.; Gu, Y.; Li, X.; Zhang, X.; Yu, Y. In vitro determination of transdermal permeation of synthetic musks and estimated dermal uptake through usage of personal care products. Chemosphere 2017, 173, 417–424. [Google Scholar] [CrossRef] [PubMed]
- Roberts, M.S.; Mohammed, Y.; Pastore, M.N.; Namjoshi, S.; Yousef, S.; Alinaghi, A.; Haridass, I.N.; Abd, E.; Leite-Silva, V.R.; Benson, H.A.E.; et al. Topical and cutaneous delivery using nanosystems. J. Control. Release 2017, 247, 86–105. [Google Scholar] [CrossRef] [PubMed]
- Zhai, Y.; Zhai, G. Advances in lipid-based colloid systems as drug carrier for topic delivery. J. Control. Release 2014, 193, 90–99. [Google Scholar] [CrossRef] [PubMed]
- Escobar-Chavez, J.; Diaz-Torres, R.; Rodriguez-Cruz, I.M.; Dominguez-Delgado, C.L.; Morales, R.S.; Angeles-Anguiano, E.; Melgoza-Contreras, L.M. Nanocarriers for transdermal drug delivery. Res. Rep. Transdermal Drug Deliv. 2012, 1, 3–17. [Google Scholar] [CrossRef]
- Bolzinger, M.A.; Briançon, S.; Pelletier, J.; Chevalier, Y. Penetration of drugs through skin, a complex rate-controlling membrane. Curr. Opin. Colloid Interface Sci. 2012, 17, 156–165. [Google Scholar] [CrossRef]
- Moser, K.; Kriwet, K.; Naik, A.; Kalia, Y.N.; Guy, R.H. Passive skin penetration enhancement and its quantification in vitro. Eur. J. Pharm. Biopharm. 2001, 52, 103–112. [Google Scholar] [CrossRef]
- Baby, A.R.; Haroutiounian-Filho, C.A.; Sarruf, F.D.; Tavante-Júnior, C.R.; de Pinto, C.A.S.O.; Zague, V.; Arêas, E.P.G.; Kaneko, T.M.; Velasco, M.V.R. Estabilidade e estudo de penetração cutânea in vitro da rutina veiculada em uma emulsão cosmética através de um modelo de biomembrana alternativo. Rev. Bras. Ciências Farm. 2008, 44, 233–248. [Google Scholar] [CrossRef]
- Trommer, H.; Neubert, R.H.H. Overcoming the stratum corneum: The modulation of skin penetration. A review. Ski. Pharmacol. Physiol. 2006, 19, 106–121. [Google Scholar] [CrossRef]
- Pham, Q.D.; Topgaard, D.; Sparr, E. Tracking solvents in the skin through atomically resolved measurements of molecular mobility in intact stratum corneum. Proc. Natl. Acad. Sci. USA 2017, 114, E112–E121. [Google Scholar] [CrossRef]
- Subongkot, T.; Pamornpathomkul, B.; Rojanarata, T.; Opanasopit, P.; Ngawhirunpat, T. Investigation of the mechanism of enhanced skin penetration by ultradeformable liposomes. Int. J. Nanomed. 2014, 9, 3539–3550. [Google Scholar] [CrossRef]
- Van Smeden, J.; Bouwstra, J.A. Stratum Corneum Lipids: Their Role for the Skin Barrier Function in Healthy Subjects and Atopic Dermatitis Patients. Ski. Barrier Funct. 2016, 49, 8–26. [Google Scholar] [CrossRef]
- Lu, F.; Wang, C.; Zhao, R.; Du, L.; Fang, Z.; Guo, X.; Zhao, Z. Review of stratum corneum impedance measurement in non-invasive penetration application. Biosensors 2018, 8, 31. [Google Scholar] [CrossRef] [PubMed]
- Law, R.M.; Ngo, M.A.; Maibach, H.I. Twenty Clinically Pertinent Factors/Observations for Percutaneous Absorption in Humans. Am. J. Clin. Dermatol. 2020, 21, 85–95. [Google Scholar] [CrossRef] [PubMed]
- Vater, C.; Hlawaty, V.; Werdenits, P.; Cichoń, M.A.; Klang, V.; Elbe-Bürger, A.; Wirth, M.; Valenta, C. Effects of lecithin-based nanoemulsions on skin: Short-time cytotoxicity MTT and BrdU studies, skin penetration of surfactants and additives and the delivery of curcumin. Int. J. Pharm. 2020, 580, 119209. [Google Scholar] [CrossRef]
- Chaulagain, B.; Jain, A.; Tiwari, A.; Verma, A.; Jain, S.K. Passive delivery of protein drugs through transdermal route. Artif. Cells, Nanomed., Biotechnol. 2018, 46, 472–487. [Google Scholar] [CrossRef]
- Mohyeldin, S.M.; Mehanna, M.M.; Elgindy, N.A. Superiority of liquid crystalline cubic nanocarriers as hormonal transdermal vehicle: Comparative human skin permeation-supported evidence. Expert Opin. Drug Deliv. 2016, 13, 1049–1064. [Google Scholar] [CrossRef]
- Parhi, R.; Suresh, P.; Pattnaik, S. Pluronic lecithin organogel (PLO) of diltiazem hydrochloride: Effect of solvents/penetration enhancers on ex vivo permeation. Drug Deliv. Transl. Res. 2016, 6, 243–253. [Google Scholar] [CrossRef]
- Mishra, D.K.; Pandey, V.; Maheshwari, R.; Ghode, P.; Tekade, R.K. Cutaneous and Transdermal Drug Delivery: Techniques and Delivery Systems. In Basic Fundamentals of Drug Delivery; Academic Press: Cambridge, MA, USA, 2018; ISBN 9780128179093. [Google Scholar]
- Talaat, S.M.; Elnaggar, Y.S.R.; Abdalla, O.Y. Lecithin Microemulsion Lipogels Versus Conventional Gels for Skin Targeting of Terconazole: In Vitro, Ex Vivo, and In Vivo Investigation. AAPS PharmSciTech 2019, 20. [Google Scholar] [CrossRef]
- Gelker, M.; Müller-Goymann, C.C.; Viöl, W. Permeabilization of human stratum corneum and full-thickness skin samples by a direct dielectric barrier discharge. Clin. Plasma Med. 2018, 9, 34–40. [Google Scholar] [CrossRef]
- Szunerits, S.; Boukherroub, R. Heat: A highly efficient skin enhancer for transdermal drug delivery. Front. Bioeng. Biotechnol. 2018, 6, 1–13. [Google Scholar] [CrossRef]
- Singh, A.; Auzanneau, F.I.; Rogers, M.A. Advances in edible oleogel technologies—A decade in review. Food Res. Int. 2017, 97, 307–317. [Google Scholar] [CrossRef] [PubMed]
- Belgamwar, V.S.; Pandey, M.S.; Chauk, D.S.; Surana, S.J. Pluronic lecithin organogel. Asian J. Pharm. 2008, 2, 134–138. [Google Scholar] [CrossRef]
- Charoensumran, P.; Ajiro, H. Controlled release of testosterone by polymer-polymer interaction enriched organogel as a novel transdermal drug delivery system: Effect of limonene/PG and carbon-chain length on drug permeability. React. Funct. Polym. 2020, 148, 104461. [Google Scholar] [CrossRef]
- Kang-Mieler, J.J.; Mieler, W.F. Thermo-responsive hydrogels for ocular drug delivery. Dev. Ophthalmol. 2016, 55, 104–111. [Google Scholar] [CrossRef]
- Davis, J.T.; Spada, G.P. Supramolecular architectures generated by self-assembly of guanosine derivatives. Chem. Soc. Rev. 2007, 36, 296–313. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharyya, T.; Saha, P.; Dash, J. Guanosine-Derived Supramolecular Hydrogels: Recent Developments and Future Opportunities. ACS Omega 2018, 3, 2230–2241. [Google Scholar] [CrossRef]
- Uchida, J.; Yoshio, M.; Sato, S.; Yokoyama, H.; Fujita, M.; Kato, T. Self-Assembly of Giant Spherical Liquid-Crystalline Complexes and Formation of Nanostructured Dynamic Gels that Exhibit Self-Healing Properties. Angew. Chem. Int. Ed. 2017, 56, 14085–14089. [Google Scholar] [CrossRef]
- Buerkle, L.E.; Von Recum, H.A.; Rowan, S.J. Toward potential supramolecular tissue engineering scaffolds based on guanosine derivatives. Chem. Sci. 2012, 3, 564–572. [Google Scholar] [CrossRef]
- Daniels, R.; Knie, U. Galenics of dermal products—Vehicles, properties and drug release. J. Ger. Soc. Dermatol. 2007, 5, 367–383. [Google Scholar] [CrossRef]
- Alsaab, H.; Bonam, S.P.; Bahl, D.; Chowdhury, P.; Alexander, K.; Boddu, S.H.S. Organogels in drug delivery: A special emphasis on organogels pluronic lecithin. J. Pharm. Pharm. Sci. 2016, 19, 252–273. [Google Scholar] [CrossRef]
- Kirilov, P.; Rum, S.; Gilbert, E.; Roussel, L.; Salmon, D.; Abdayem, R.; Serre, C.; Villa, C.; Haftek, M.; Falson, F.; et al. Aqueous dispersions of organogel nanoparticles—Potential systems for cosmetic and dermo-cosmetic applications. Int. J. Cosmet. Sci. 2014, 36, 336–346. [Google Scholar] [CrossRef] [PubMed]
- Główka, E.; Wosicka-Frąckowiak, H.; Hyla, K.; Stefanowska, J.; Jastrzębska, K.; Klapiszewski, Ł.; Jesionowski, T.; Cal, K. Polymeric nanoparticles-embedded organogel for roxithromycin delivery to hair follicles. Eur. J. Pharm. Biopharm. 2014, 88, 75–84. [Google Scholar] [CrossRef] [PubMed]
- Willimann, H.; Walde, P.; Luisi, P.L.; Gazzaniga, A.; Stroppolo, F. Lecithin organogel as matrix for transdermal transport of drugs. J. Pharm. Sci. 1992, 81, 871–874. [Google Scholar] [CrossRef] [PubMed]
- Mitura, S.; Sionkowska, A.; Jaiswal, A. Biopolymers for hydrogels in cosmetics: Review. J. Mater. Sci. Mater. Med. 2020, 31, 14. [Google Scholar] [CrossRef] [PubMed]
- Simões, A.; Veiga, F.; Vitorino, C.; Figueiras, A. A Tutorial for Developing a Topical Cream Formulation Based on the Quality by Design Approach. J. Pharm. Sci. 2018, 107, 2653–2662. [Google Scholar] [CrossRef]
- Rai, V.K.; Mishra, N.; Yadav, K.S.; Yadav, N.P. Nanoemulsion as pharmaceutical carrier for dermal and transdermal drug delivery: Formulation development, stability issues, basic considerations and applications. J. Control. Release 2018, 270, 203–225. [Google Scholar] [CrossRef]
- Zsikó, S.; Cutcher, K.; Kovács, A.; Budai-Szűcs, M.; Gácsi, A.; Baki, G.; Csányi, E.; Berkó, S. Nanostructured lipid carrier gel for the dermal application of lidocaine: Comparison of skin penetration testing methods. Pharmaceutics 2019, 11, 310. [Google Scholar] [CrossRef]
- Alonso, C.; Carrer, V.; Espinosa, S.; Zanuy, M.; Córdoba, M.; Vidal, B.; Domínguez, M.; Godessart, N.; Coderch, L.; Pont, M. Prediction of the skin permeability of topical drugs using in silico and in vitro models. Eur. J. Pharm. Sci. 2019, 136, 104945. [Google Scholar] [CrossRef]
- Supe, S.; Takudage, P. Methods for evaluating penetration of drug into the skin: A review. Ski. Res. Technol. 2020, 27, 299–308. [Google Scholar] [CrossRef]
- Simon, A.; Amaro, M.I.; Healy, A.M.; Cabral, L.M.; de Sousa, V.P. Comparative evaluation of rivastigmine permeation from a transdermal system in the Franz cell using synthetic membranes and pig ear skin with in vivo-in vitro correlation. Int. J. Pharm. 2016, 512, 234–241. [Google Scholar] [CrossRef]
- Zhang, Q.; Song, Y.; Page, S.W.; Garg, S. Evaluation of Transdermal Drug Permeation as Modulated by Lipoderm and Pluronic Lecithin Organogel. J. Pharm. Sci. 2018, 107, 587–594. [Google Scholar] [CrossRef] [PubMed]
- Simsolo, E.E.; Eroğlu, İ.; Tanrıverdi, S.T.; Özer, Ö. Formulation and Evaluation of Organogels Containing Hyaluronan Microparticles for Topical Delivery of Caffeine. AAPS PharmSciTech 2018, 19, 1367–1376. [Google Scholar] [CrossRef] [PubMed]
- Ba, W.; Li, Z.; Wang, L.; Wang, D.; Liao, W.; Fan, W.; Wu, Y.; Liao, F.; Yu, J. Optimization and evaluation of pluronic lecithin organogels as a transdermal delivery vehicle for sinomenine. Pharm. Dev. Technol. 2016, 21, 535–545. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mosquera Narvaez, L.E.; Ferreira, L.M.d.M.C.; Sanches, S.; Alesa Gyles, D.; Silva-Júnior, J.O.C.; Ribeiro Costa, R.M. A Review of Potential Use of Amazonian Oils in the Synthesis of Organogels for Cosmetic Application. Molecules 2022, 27, 2733. https://doi.org/10.3390/molecules27092733
Mosquera Narvaez LE, Ferreira LMdMC, Sanches S, Alesa Gyles D, Silva-Júnior JOC, Ribeiro Costa RM. A Review of Potential Use of Amazonian Oils in the Synthesis of Organogels for Cosmetic Application. Molecules. 2022; 27(9):2733. https://doi.org/10.3390/molecules27092733
Chicago/Turabian StyleMosquera Narvaez, Luis Eduardo, Lindalva Maria de Meneses Costa Ferreira, Suellen Sanches, Desireé Alesa Gyles, José Otávio Carréra Silva-Júnior, and Roseane Maria Ribeiro Costa. 2022. "A Review of Potential Use of Amazonian Oils in the Synthesis of Organogels for Cosmetic Application" Molecules 27, no. 9: 2733. https://doi.org/10.3390/molecules27092733
APA StyleMosquera Narvaez, L. E., Ferreira, L. M. d. M. C., Sanches, S., Alesa Gyles, D., Silva-Júnior, J. O. C., & Ribeiro Costa, R. M. (2022). A Review of Potential Use of Amazonian Oils in the Synthesis of Organogels for Cosmetic Application. Molecules, 27(9), 2733. https://doi.org/10.3390/molecules27092733






