Comprehensive Two-Dimensional Gas Chromatography as a Powerful Strategy for the Exploration of Broas Volatile Composition
Abstract
1. Introduction
2. Results and Discussion
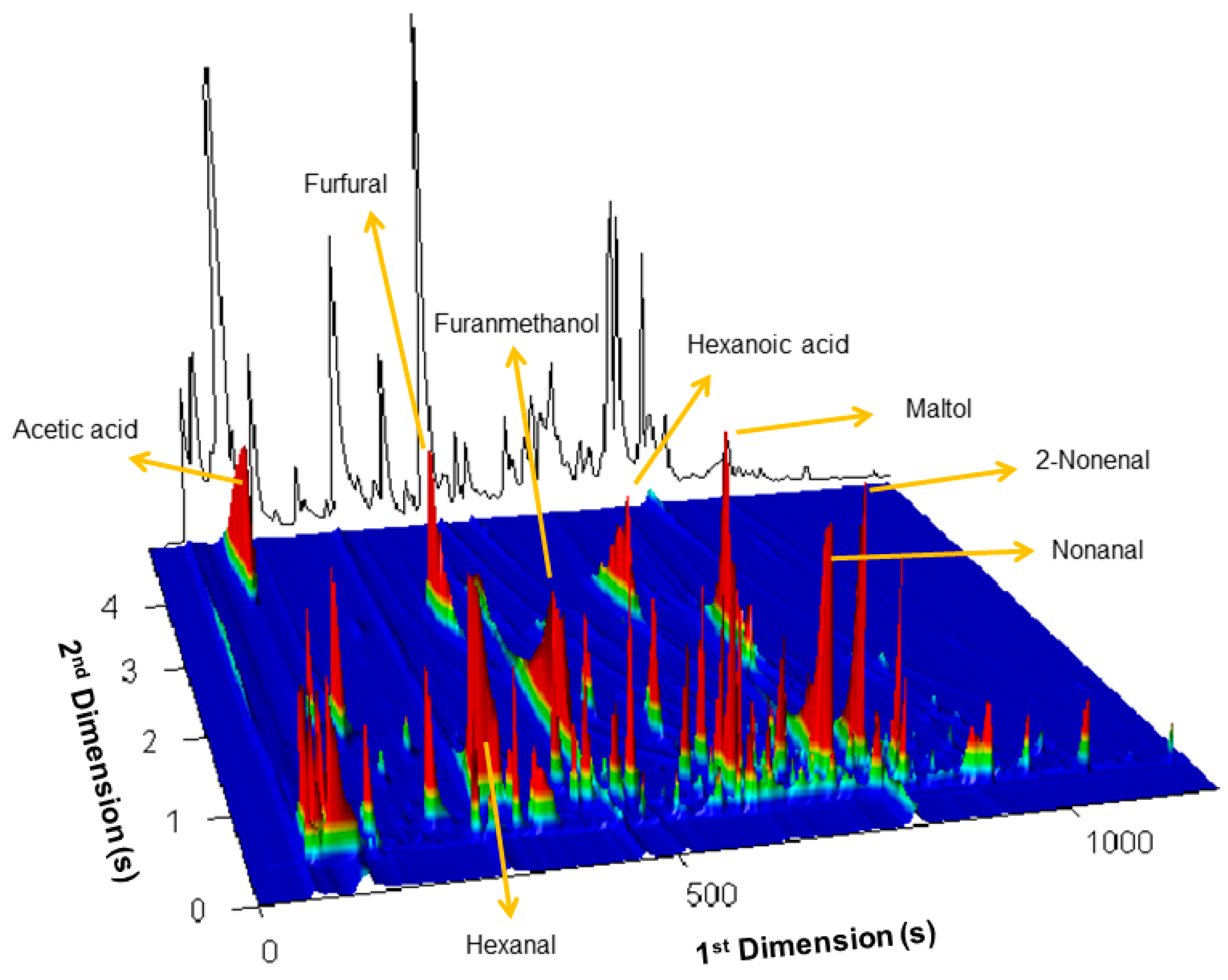
2.1. The potentialities of GC×GC–ToFMS in the Identification of Broas Volatile Compounds
2.2. Exploring the Volatile Compounds Associated with Baking
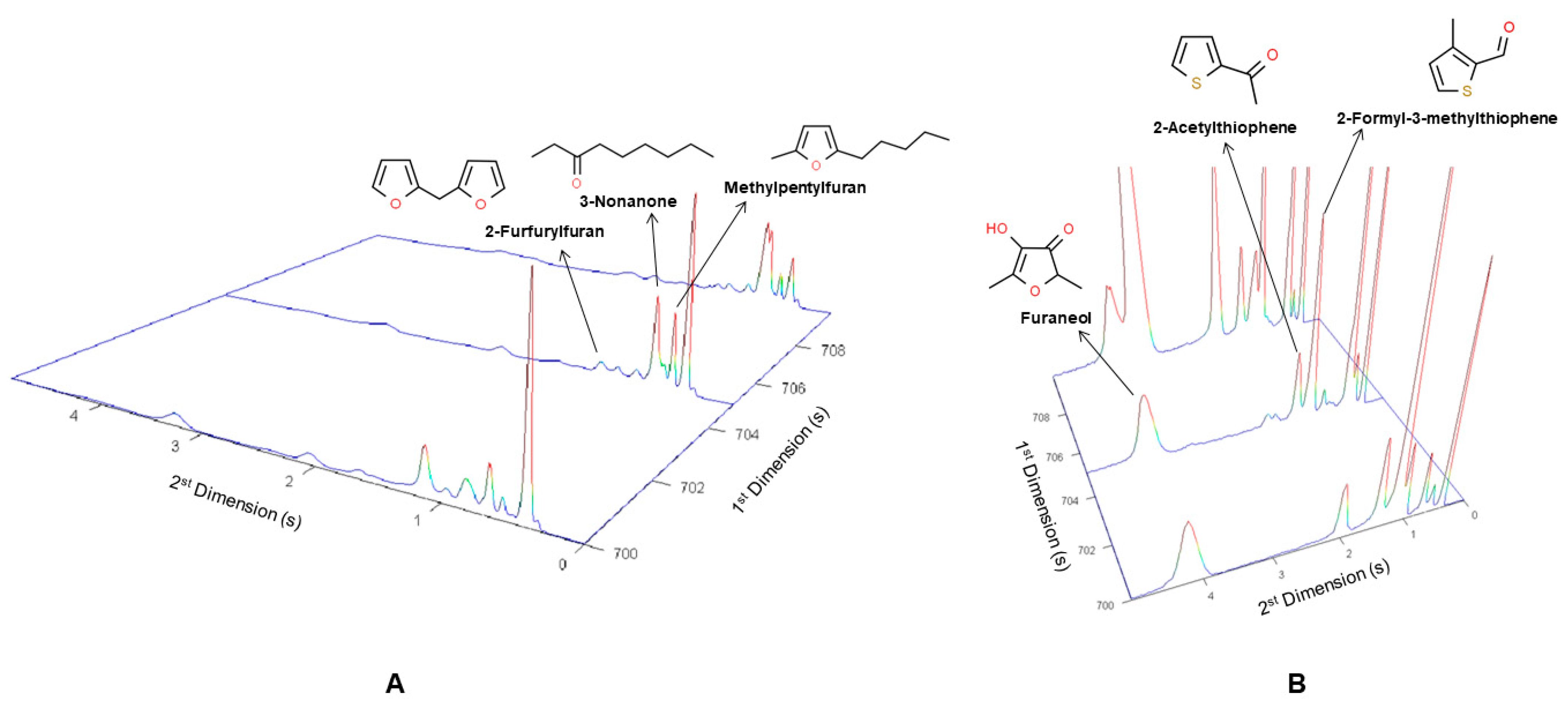
2.2.1. Furans and Furanones
2.2.2. Pyrans and Pyranones
2.2.3. Pyrazines
2.2.4. Pyridines and Pyrimidines
2.2.5. Pyrroles, Pyrrolines and Oxazoles
2.2.6. Sulfur-Containing Compounds
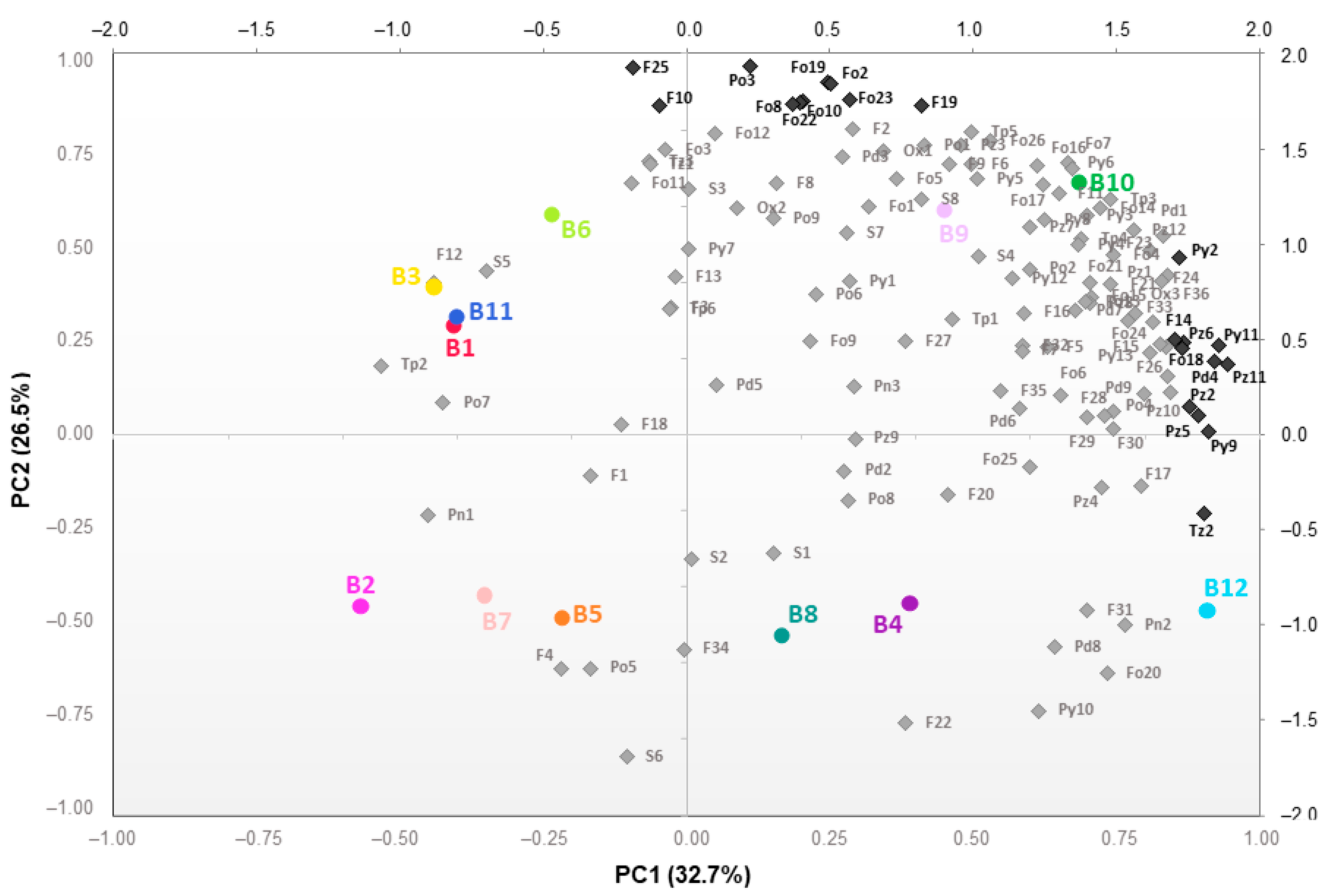
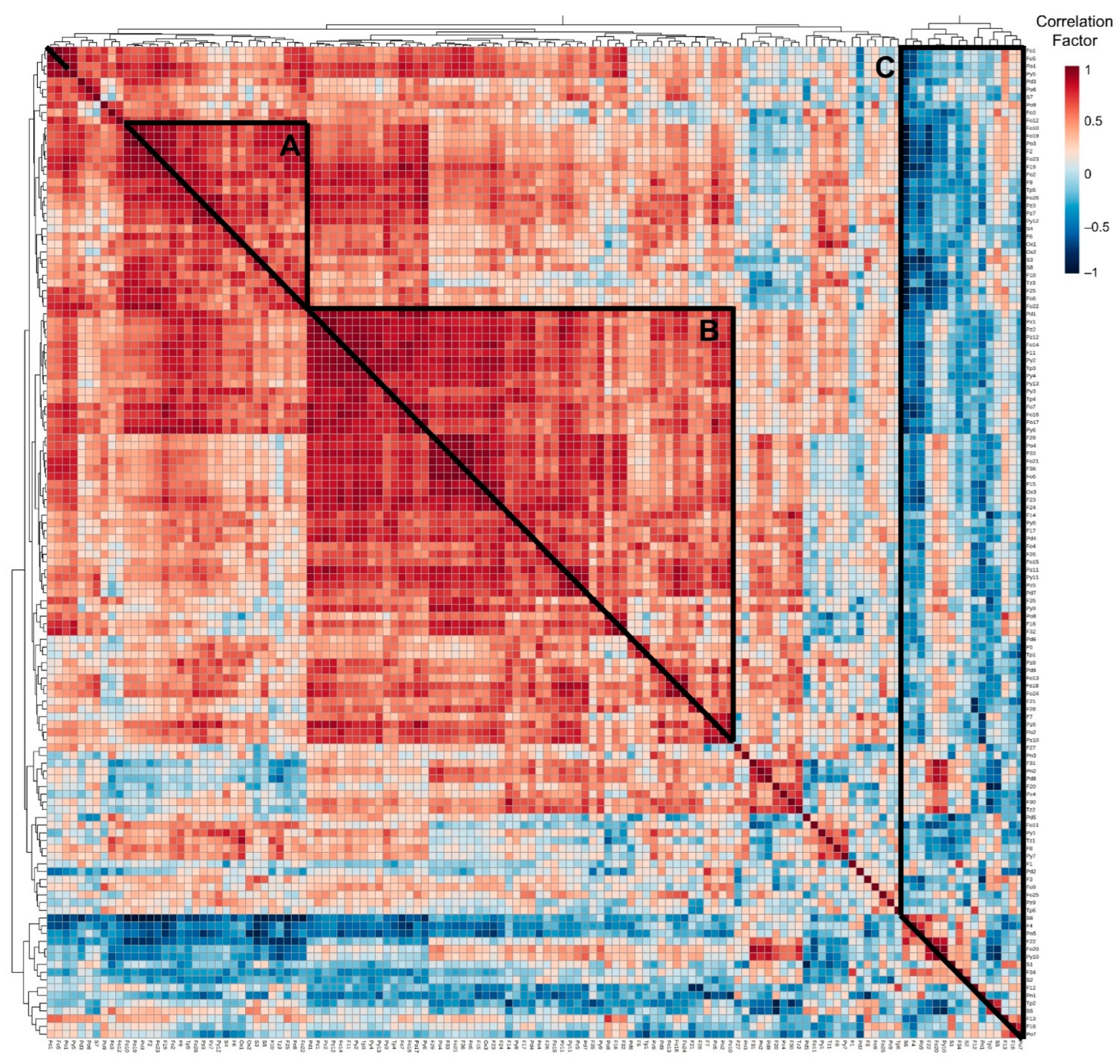
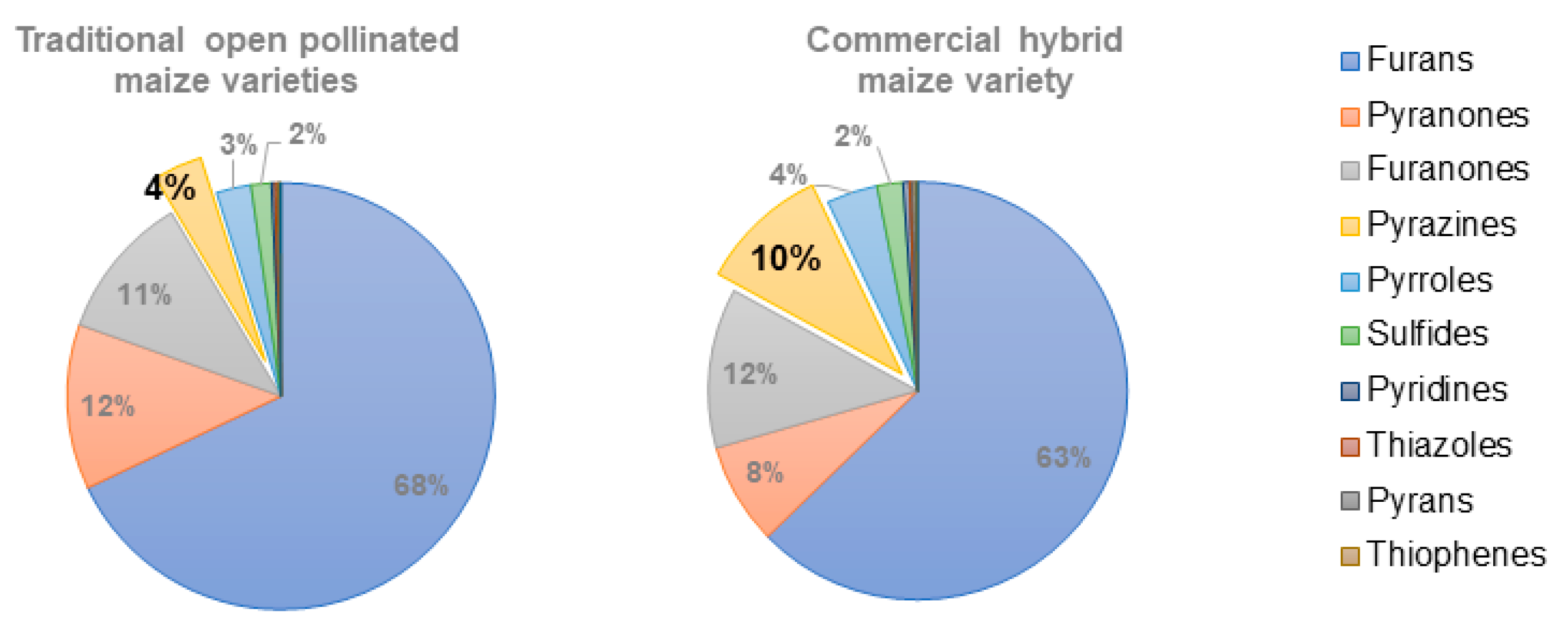
2.3. An Overall View of the Volatiles Associated with Baking in Broas
3. Materials and Methods
3.1. Samples
3.2. HS-SPME Methodology
3.3. GC×GC–ToFMS Analysis
3.4. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Pico, J.; Gómez, M.; Bernal, J.; Bernal, J.L. Analytical Methods for Volatile Compounds in Wheat Bread. J. Chromatogr. A 2016, 1428, 55–71. [Google Scholar] [CrossRef] [PubMed]
- Vaz Patto, M.C.; Alves, M.L.; Almeida, N.F.; Santos, C.; Moreira, P.M.; Satovic, Z.; Brites, C.; Patto, V.; Alves, N.F.; Almeida, C.S.; et al. Is the Bread Making Technological Ability of Portuguese Traditional Maize Landraces Associated with Their Genetic Diversity? Maydica 2009, 54, 297–311. [Google Scholar]
- Smith, J.R. 50 of the World’s Best Breads. Available online: https://edition.cnn.com/travel/article/world-50-best-breads/index.html (accessed on 26 March 2022).
- Vaz Patto, M.C.; Mendes-Moreira, P.M.; Alves, M.L.; Mecha, E.; Brites, C.; Bronze, R.; Pego, S. Participatory Plant Quality Breeding: An Ancient Art Revisited by Knowledge Sharing. The Portuguese Experience. In Plant Breeding from Laboratories to Fields; InTechOpen: London, UK, 2013; pp. 255–288. [Google Scholar]
- Pico, J.; Bernal, J.; Gómez, M. Wheat Bread Aroma Compounds in Crumb and Crust: A Review. Food Res. Int. 2015, 75, 200–215. [Google Scholar] [CrossRef] [PubMed]
- Lasekan, O.; Dabaj, F.; Muniandy, M.; Juhari, N.H.; Lasekan, A. Characterization of the Key Aroma Compounds in Three Types of Bagels by Means of the Sensomics Approach. BMC Chem. 2021, 15, 16. [Google Scholar] [CrossRef] [PubMed]
- Cho, I.H.; Peterson, D.G. Chemistry of Bread Aroma: A Review. Food Sci. Biotechnol. 2010, 19, 575–582. [Google Scholar] [CrossRef]
- Raffo, A.; Carcea, M.; Castagna, C.; Magrì, A. Improvement of a Headspace Solid Phase Microextraction-Gas Chromatography/Mass Spectrometry Method for the Analysis of Wheat Bread Volatile Compounds. J. Chromatogr. A 2015, 1406, 266–278. [Google Scholar] [CrossRef] [PubMed]
- Starowicz, M.; Zieliński, H. How Maillard Reaction Influences Sensorial Properties (Color, Flavor and Texture) of Food Products? Food Rev. Int. 2019, 35, 707–725. [Google Scholar] [CrossRef]
- Pétel, C.; Onno, B.; Prost, C. Sourdough Volatile Compounds and Their Contribution to Bread: A Review. Trends Food Sci. Technol. 2017, 59, 105–123. [Google Scholar] [CrossRef]
- Pozo-Bayón, M.A.; Guichard, E.; Cayot, N. Flavor Control in Baked Cereal Products; Taylor & Francis Group: Philadelphia, PA, USA, 2006; Volume 22, ISBN 8755912060. [Google Scholar]
- Chambers, E., IV; Koppel, K. Associations of Volatile Compounds with Sensory Aroma and Flavor: The Complex Nature of Flavor. Molecules 2013, 18, 4887–4905. [Google Scholar] [CrossRef]
- McGorrin, R.J. The Significance of Volatile Sulfur Compounds in Food Flavors. ACS Symp. Ser. 2011, 1068, 3–31. [Google Scholar] [CrossRef]
- Belitz, H.-D.; Grosch, W.; Schieberle, P. Cereals and Cereal Products. In Food Chemistry; Springer: Berlin/Heidelberg, Germany, 2009; pp. 670–745. [Google Scholar]
- Bastos, D.M.; Monaro, É.; Siguemoto, É.; Séfora, M.; Markowicz, D.; Monaro, E.; Siguemoto, E.; Sefor, M. Maillard Reaction Products in Processed Food: Pros and Cons. In Food Industrial Processes – Methods and Equipment; Benjamin, V., Ed.; InTechOpen: London, UK, 2012. [Google Scholar] [CrossRef]
- Buttery, R.G.; Ling, L.C.; Stern, D.J. Studies on Popcorn Aroma and Flavor Volatiles. J. Agric. Food Chem. 1997, 45, 837–843. [Google Scholar] [CrossRef]
- Martins, S.I.F.S.; Jongen, W.M.F.; Van Boekel, M.A.J.S. A Review of Maillard Reaction in Food and Implications to Kinetic Modelling. Trends Food Sci. Technol. 2000, 11, 364–373. [Google Scholar] [CrossRef]
- Birch, A.N.; Petersen, M.A.; Hansen, Å.S. REVIEW: Aroma of Wheat Bread Crumb. Cereal Chem. 2014, 91, 105–114. [Google Scholar] [CrossRef]
- Hellwig, M.; Henle, T. Baking, Ageing, Diabetes: A Short History of the Maillard Reaction. Angew. Chemie Int. Ed. 2014, 53, 10316–10329. [Google Scholar] [CrossRef]
- Berger, R.G. Flavours and Fragrances: Chemistry, Bioprocessing and Sustainability; Springer Science & Business Media: Berlin, Germany, 2007; ISBN 3540493395. [Google Scholar]
- Purlis, E. Browning Development in Bakery Products—A Review. J. Food Eng. 2010, 99, 239–249. [Google Scholar] [CrossRef]
- Cauvain, S.P. Bread Making: Improving Quality; Woodhead Publishing Limited: Cambridge, UK, 2003; ISBN 0-203-49500-4. [Google Scholar]
- Tamanna, N.; Mahmood, N. Food Processing and Maillard Reaction Products: Effect on Human Health and Nutrition. Int. J. Food Sci. 2015, 2015, 526762. [Google Scholar] [CrossRef]
- Bailey, M.E.; Gutheil, R.A.; Hsieh, F.-H.; Cheng, C.-W.; Gerhardt, K.O. Maillard Reaction Volatile Compounds and Color Quality of a Whey Protein Concentrate—Corn Meal Extruded Product; ACS Publications: Washington, DC, USA, 1994; ISBN 1947-5918. [Google Scholar]
- Seok, Y.-J.; Kim, Y.-G.; Jeong, S.Y.; Lee, J.; Yoon, H.-J. Furan in Thermally Processed Foods—A Review. Toxicol. Res. 2015, 31, 241. [Google Scholar] [CrossRef]
- Rizzi, G.P. The Strecker Degradation of Amino Acids: Newer Avenues for Flavor Formation. Food Rev. Int. 2008, 24, 416–435. [Google Scholar] [CrossRef]
- Bento-Silva, A.; Duarte, N.; Belo, M.; Mecha, E.; Carbas, B.; Brites, C.; Vaz Patto, M.C.; Bronze, M.R. Shedding Light on the Volatile Composition of Broa, a Traditional Portuguese Maize Bread. Biomolecules 2021, 11, 1396. [Google Scholar] [CrossRef]
- Adahchour, M.; Beens, J.; Brinkman, U.A.T. Recent Developments in the Application of Comprehensive Two-Dimensional Gas Chromatography. J. Chromatogr. A 2008, 1186, 67–108. [Google Scholar] [CrossRef]
- Tranchida, P.Q.; Purcaro, G.; Maimone, M.; Mondello, L. Impact of Comprehensive Two-Dimensional Gas Chromatography with Mass Spectrometry on Food Analysis. J. Sep. Sci. 2016, 39, 149–161. [Google Scholar] [CrossRef] [PubMed]
- Rocha, S.M.; Costa, C.P.; Martins, C. Aroma Clouds of Foods: A Step Forward to Unveil Food Aroma Complexity Using GC×GC. Front. Chem. 2022, 10, 820749. [Google Scholar] [CrossRef] [PubMed]
- Pacyński, M.; Wojtasiak, R.Z.; Mildner-Szkudlarz, S. Improving the Aroma of Gluten-Free Bread. Lebensm. -Wiss. Und-Technol. 2015, 63, 706–713. [Google Scholar] [CrossRef]
- Buttery, R.G.; Ling, L.C. Volatile Flavor Components of Corn Tortillas and Related Products. J. Agric. Food Chem. 1995, 43, 1878–1882. [Google Scholar] [CrossRef]
- Park, D.; Maga, J.A. Identification of Key Volatiles Responsible for Odour Quality Differences in Popped Popcorn of Selected Hybrids. Food Chem. 2006, 99, 538–545. [Google Scholar] [CrossRef]
- Linstrom, P.J.; Mallard, W.G. NIST Chemistry Webbook; NIST Standard Reference Database No. 69. Available online: http://webbook.nist.gov/chemistry/ (accessed on 26 March 2022).
- Lee, J.; Hong, S.J.; Cho, J.-J.; Boo, C.G.; And, D.-S.K.; Shin, E.-C. Peanut Coffee: Enhancement of Nutritional, Physicochemical, and Sensory Characteristics in Coffee Brewed with Conventional and High-Oleic Peanut Extracts. Foods 2020, 9, 1664. [Google Scholar] [CrossRef]
- Wang, Z.; Xiao, Q.; Zhuang, J.; Feng, T.; Ho, C.T.; Song, S. Characterization of Aroma-Active Compounds in Four Yeast Extracts Using Instrumental and Sensory Techniques. J. Agric. Food Chem. 2020, 68, 267–278. [Google Scholar] [CrossRef]
- Elmore, J.S.; Mottram, D.S.; Hierro, E. Two-Fibre Solid-Phase Microextraction Combined with Gas Chromatography-Mass Spectrometry for the Analysis of Volatile Aroma Compounds in Cooked Pork. J. Chromatogr. A 2001, 905, 233–240. [Google Scholar] [CrossRef]
- Mildner-Szkudlarz, S.; Różańska, M.; Gaca, A.; Jeleń, H.H. Changes in Volatile Compound Profiles of Cold-Pressed Berry Seed Oils Induced by Roasting. LWT 2021, 148, 111718. [Google Scholar] [CrossRef]
- Farag, M.A.; Otify, A.M.; El-sayed, A.M.; Michel, C.G.; ElShebiney, S.A.; Ehrlich, A.; Wessjohann, L.A. Sensory Metabolite Profiling in a Date Pit Based Coffee Substitute and in Response to Roasting as Analyzed via Mass Spectrometry Based Metabolomics. Molecules 2019, 24, 3377. [Google Scholar] [CrossRef]
- Bredie, W.L.P.; Mottram, D.S.; Guy, R.C.E. Aroma Volatiles Generated during Extrusion Cooking of Maize Flour. J. Agric. Food Chem. 1998, 46, 1479–1487. [Google Scholar] [CrossRef]
- El-Sayed, A.M. The Pherobase: Database of Insect Pheromones and Semiochemicals. Available online: http://www.pherobase.com (accessed on 22 March 2022).
- Paraskevopoulou, A.; Chrysanthou, A.; Koutidou, M. Characterisation of Volatile Compounds of Lupin Protein Isolate-enriched Wheat Flour Bread. Food Res. Int. 2012, 48, 568–577. [Google Scholar] [CrossRef]
- Buttery, R.G.; Ling, L.C. Additional Studies on Flavor Components of Corn Tortilla Chips. J. Agric. Food Chem. 1998, 46, 2764–2769. [Google Scholar] [CrossRef]
- Miller, G.H. Whisky Science—A Condensed Distillation; Springer Nature: Cham, Switzerland, 2019; ISBN 978-3-030-13732-8. [Google Scholar]
- Vázquez-Araújo, L.; Enguix, L.; Verdú, A.E.G.-G.; Carbonell-Barrachina, A.A. Investigation of Aromatic Compounds in Toasted Almonds Used for the Manufacture of Turrón. Eur. Food Res. Technol. 2008, 227, 243–254. [Google Scholar] [CrossRef]
- Ruth, J. Odor Thresholds and Irritation Levels of Several Chemical Substances: A Review. Am. Ind. Hyg. Assoc. J. 1986, 47, A142–A151. [Google Scholar] [CrossRef]
- Buttery, R.G.; Orts, W.J.; Takeoka, G.R.; Nam, Y. Volatile Flavor Components of Rice Cakes. J. Agric. Food Chem. 1999, 47, 4353–4356. [Google Scholar] [CrossRef]
- Leffingwell, J.C.; Leffingwell, D. GRAS Flavor Chemicals Detection Thresholds. Perfum. Flavorist 1991, 16, 1–13. [Google Scholar]
- Pu, D.; Zhang, H.; Zhang, Y.; Sun, B.; Ren, F.; Chen, H. Characterization of the Key Aroma Compounds in White Bread by Aroma Extract Dilution Analysis, Quantitation, and Sensory Evaluation Experiments. J. Food Process. Preserv. 2019, 43, e13933. [Google Scholar] [CrossRef]
- de Lerma, N.L.; Martínez, T.G.; Moreno, J.; Mauricio, J.C.; Peinado, R.A. Sweet Wines with Great Aromatic Complexity Obtained by Partial Fermentation of Must from Tempranillo Dried Grapes. Eur. Food Res. Technol. 2012, 234, 695–701. [Google Scholar] [CrossRef]
- The Good Scents Company. TGSC Information System. Available online: http://www.thegoodscentscompany.com/search2.html (accessed on 17 January 2022).
- Laukaleja, I.; Koppel, K. Aroma Active Compound Perception in Differently Roasted and Brewed Coffees by Gas Chromatography–Olfactometry. J. Sens. Stud. 2021, 36, e12708. [Google Scholar] [CrossRef]
- Oertling, H.; Gomann, C.; Vom Ende, M. Dihydrobenzofuran Derivatives as Fragrance and/or Flavoring Materials. U.S. Patent 2013024 3716A1, 2 June 2015. [Google Scholar]
- Acree, T.; Arn, H. Flavornet and Human Odor Space. Available online: http://www.flavornet.org/ (accessed on 5 March 2022).
- Peinado, R.A.; Moreno, J.; Medina, M.; Mauricio, J.C. Changes in Volatile Compounds and Aromatic Series in Sherry Wine with High Gluconic Acid Levels Subjected to Aging by Submerged Flor Yeast Cultures. Biotechnol. Lett. 2004, 26, 757–762. [Google Scholar] [CrossRef] [PubMed]
- Drakula, S.; Mustač, N.Č.; Novotni, D.; Voučko, B.; Krpan, M.; Hruškar, M.; Ćurić, D. Optimization and Validation of a HS-SPME/GC–MS Method for the Analysis of Gluten-Free Bread Volatile Flavor Compounds. Food Anal. Methods 2021. [Google Scholar] [CrossRef]
- Feng, X.; Pan, L.; Wang, Q.; Liao, Z.; Wang, X.; Zhang, X.; Guo, W.; Hu, E.; Li, J.; Xu, J.; et al. Nutritional and Physicochemical Characteristics of Purple Sweet Corn Juice before and after Boiling. PLoS ONE 2020, 15, e0233094. [Google Scholar] [CrossRef] [PubMed]
- Galoburda, R.; Straumite, E.; Sabovics, M.; Kruma, Z. Dynamics of Volatile Compounds in Triticale Bread with Sourdough: From Flour to Bread. Foods 2020, 9, 1837. [Google Scholar] [CrossRef] [PubMed]
- Karahadian, C.; Johnson, K.A. Analysis of Headspace Volatiles and Sensory Characteristics of Fresh Corn Tortillas Made from Fresh Masa Dough and Spray-Dried Masa Flour. J. Agric. Food Chem. 1993, 41, 791–799. [Google Scholar] [CrossRef]
- Pico, J.; Martinez, M.M.; Bernal, J.; Gomez, M. Evolution of Volatile Compounds in Gluten-Free Bread: From Dough to Crumb. Food Chem. 2017, 227, 179–186. [Google Scholar] [CrossRef]
- Pico, J.; Antolín, B.; Román, L.; Bernal, J.; Gómez, M. Selection of the Most Suitable Mixture of Flours and Starches for the Improvement of Gluten-Free Breads through Their Volatile Profiles. Eur. Food Res. Technol. 2019, 245, 1755–1766. [Google Scholar] [CrossRef]
- Hussein, A.M.S.; Ibrahim, G.E. Effects of Various Brans on Quality and Volatile Compounds of Bread. Foods Raw Mater. 2019, 7, 42–50. [Google Scholar] [CrossRef]
- Rocha, S.M.; Coelho, E.; Zrostlíková, J.; Delgadillo, I.; Coimbra, M.A. Comprehensive Two-Dimensional Gas Chromatography with Time-of-Flight Mass Spectrometry of Monoterpenoids as a Powerful Tool for Grape Origin Traceability. J. Chromatogr. A 2007, 1161, 292–299. [Google Scholar] [CrossRef]
- Rocha, S.M.; Freitas, R.; Cardoso, P.; Santos, M.; Martins, R.; Figueira, E. Exploring the Potentialities of Comprehensive Two-Dimensional Gas Chromatography Coupled to Time of Flight Mass Spectrometry to Distinguish Bivalve Species: Comparison of Two Clam Species (Venerupis decussata and Venerupis philippinarum). J. Chromatogr. A 2013, 1315, 152–161. [Google Scholar] [CrossRef] [PubMed]
- Costa, C.P.; Bezerra, A.R.; Almeida, A.; Rocha, S.M. Candida Species (Volatile) Metabotyping through Advanced Comprehensive Two-Dimensional Gas Chromatography. Microorganisms 2020, 8, 1911. [Google Scholar] [CrossRef] [PubMed]
- Vranová, J.; Ciesarová, Z. Furan in Food—A Review. Czech J. Food Sci. 2009, 27, 1–10. [Google Scholar] [CrossRef]
- Mohsen, S.M.; Ammar, A.S.M. Total Phenolic Contents and Antioxidant Activity of Corn Tassel Extracts. Food Chem. 2009, 112, 595–598. [Google Scholar] [CrossRef]
- Starowicz, M. Analysis of Volatiles in Food Products. Separations 2021, 8, 157. [Google Scholar] [CrossRef]
- Pico, J.; Antolín, B.; Román, L.; Gómez, M.; Bernal, J. Analysis of Volatile Compounds in Gluten-Free Bread Crusts with an Optimised and Validated SPME-GC/QTOF Methodology. Food Res. Int. 2018, 106, 686–695. [Google Scholar] [CrossRef]
- Obuchowski, W.; Makowska, A.; Mildner-Szkudlarz, S.; Szwengiel, A.; Majcher, M.; Remiszewski, M. Effect of Triticale Grain Characteristics, Scouring, and Extrusion Conditions on Physico-Chemical Properties, Antioxidant Activity, and Volatile Compounds of Flat Bread. Acta Aliment. 2015, 44, 511–519. [Google Scholar] [CrossRef]
- Deblander, J.; Van Aeken, S.; Adams, A.; De Kimpe, N.; Abbaspour Tehrani, K. New Short and General Synthesis of Three Key Maillard Flavour Compounds: 2-Acetyl-1-Pyrroline, 6-Acetyl-1,2,3,4-Tetrahydropyridine and 5-Acetyl-2,3-Dihydro-4H-1,4-Thiazine. Food Chem. 2015, 168, 327–331. [Google Scholar] [CrossRef]
- Sanders, R.A.; Zyzak, D.V.; Morsch, T.R.; Rizzi, G.P. Elucidation of Tautomer Structures of 2-Acetyltetrahydropyridine Using Gas Chromatography/Mass Spectrometry and Gas Chromatography/Infrared Spectroscopy. Eur. J. Mass Spectrom. 2005, 11, 217–220. [Google Scholar] [CrossRef]
- Zhang, Q.; Wan, C.; Wang, C.; Chen, H.; Liu, Y.; Li, S.; Lin, D.; Wu, D.; Qin, W. Evaluation of the Non-Aldehyde Volatile Compounds Formed during Deep-Fat Frying Process. Food Chem. 2018, 243, 151–161. [Google Scholar] [CrossRef]
- Moskowitz, M.R.; Bin, Q.; Elias, R.J.; Peterson, D.G. Influence of Endogenous Ferulic Acid in Whole Wheat Flour on Bread Crust Aroma. J. Agric. Food Chem. 2012, 60, 11245–11252. [Google Scholar] [CrossRef] [PubMed]
- Bento-Silva, A.; Patto, M.C.V.; do Rosário Bronze, M.; Vaz Patto, M.C.; do Rosário Bronze, M.; Patto, M.C.V.; do Rosário Bronze, M. Relevance, Structure and Analysis of Ferulic Acid in Maize Cell Walls. Food Chem. 2017, 246, 360–378. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Capone, D.L.; Roland, A.; Jeffery, D.W. Chiral Analysis of Cis-2-Methyl-4-Propyl-1,3-Oxathiane and Identification of Cis-2,4,4,6-Tetramethyl-1,3-Oxathiane in Wine. Food Chem. 2021, 357, 129406. [Google Scholar] [CrossRef] [PubMed]
- Carbas, B.; Vaz-Patto, M.C.; Bronze, M.R.; Bento-Da-Silva, A.; Trigo, M.J.; Brites, C. Maize Flour Parameters That Are Related to the Consumer Perceived Quality of ‘Broa’ Specialty Bread. Food Sci. Technol. 2016, 36, 259–267. [Google Scholar] [CrossRef]
- Mariotti, M.; Granby, K.; Fromberg, A.; Risum, J.; Agosin, E.; Pedreschi, F. Furan Occurrence in Starchy Food Model Systems Processed at High Temperatures: Effect of Ascorbic Acid and Heating Conditions. J. Agric. Food Chem. 2012, 60, 10162–10169. [Google Scholar] [CrossRef]
- Van Lancker, F.; Adams, A.; Owczarek-Fendor, A.; De Meulenaer, B.; De Kimpe, N. Mechanistic Insights into Furan Formation in Maillard Model Systems. J. Agric. Food Chem. 2011, 59, 229–235. [Google Scholar] [CrossRef]
- Zhao, Q.; Yao, S.; Ou, S.Y. Maillard Volatiles in Baked Products as Affected by Feruloylated Oligosaccharides from Maize Bran. Int. J. Food Prop. 2017, 20, 3266–3273. [Google Scholar] [CrossRef]
- Frankel, E. Lipid Oxidation; Elsevier: Amsterdam, The Netherlands, 2014; ISBN 9780953194988. [Google Scholar]
- Bento-Silva, A.; Duarte, N.; Mecha, E.; Belo, M.; Serra, A.T.; Vaz Patto, M.C.; Bronze, M.R. Broa, an Ethnic Maize Bread, as a Source of Phenolic Compounds. Antioxidants 2021, 10, 672. [Google Scholar] [CrossRef]
- Ozolina, V.; Kunkulberga, D.; Cieslak, B.; Obiedzinski, M. Furan Derivatives Dynamic in Rye Bread Processing. Procedia Food Sci. 2011, 1, 1158–1164. [Google Scholar] [CrossRef]
- Van den Dool, H.; Kratz, P.D. A Generalization of the Retention Index System Including Linear Temperature Programmed Gas—Liquid Partition Chromatography. J. Chromatogr. A 1963, 11, 463–471. [Google Scholar] [CrossRef]
- Tetko, I.V.; Tanchuk, V.Y. Application of Associative Neural Networks for Prediction of Lipophilicity in ALOGPS 2.1 Program. J. Chem. Inf. Comput. Sci. 2002, 42, 1136–1145. [Google Scholar] [CrossRef] [PubMed]

| ID | 1tR | 2tR | Compound | Odor and Taste Descriptors | CAS | Formula | m/z | SI | LRIc | LRIlit | PA | OT (ppb) | Log10 PA/OT | Foods |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Furans | ||||||||||||||
| F1 | 95 | 0.38 | 2,3-Dihydrofuran | Pungent | 1191-99-7 | C4H6O | 70, 41, 39, 29 | 891 | 600 | n/f | 3,100,662 ± 2,605,740 | n/f | n/a | WRB |
| F2 | 100 | 0.40 | 2-Methylfuran or 3-Methylfuran (TI) | Ethereal, acetone, chocolate n/f | 534-22-5 930-27-8 | C5H6O | 82, 53, 39, 27 | 912 900 | 606 | 605 611 | 3,101,036 ± 1,363,010 | 90,450 n/f | 1.5 n/a | P, RSB, TB, WB, WRB RSB, WB |
| F3 | 130 | 0.43 | 2,3-Dihydro-5-methylfuran | n/f | 1487-15-6 | C5H8O | 43, 84, 39, 27, 69 | 872 | 641 | n/f | 1,630,190 ± 846,164 | n/f | n/a | n/i |
| F4 | 140 | 0.40 | Tetrahydrofuran | Ethereal | 109-99-9 | C4H8O | 42, 72, 27 | 942 | 647 | 633 | 15,225,039 ± 4,244,701 | 7375–177,000 | 1.9–3.3 | WB, WP |
| F5 | 155 | 0.47 | 2-Ethylfuran | Rubbery, pungent, acid, sweet | 3208-16-0 | C6H8O | 81, 53, 96, 39 | 975 | 662 | 689 | 2,595,043 ± 621,119 | n/f | n/a | P, RS, WB |
| F6 | 160 | 0.47 | 2,5-Dimethylfuran | Chemical, ethereal, meaty, gravy, juicy, bacon | 625-86-5 | C6H8O | 43, 96, 53, 81, 27, 39 | 916 | 667 | 667 | 843,893 ± 397,252 | n/f | n/a | WRB |
| F7 | 270 | 0.58 | 2-Propylfuran | n/f | 4229-91-8 | C7H10O | 81, 53, 110, 39, 27 | 973 | 780 | 792 | 613,397 ± 229,105 | n/f | n/a | WB |
| F8 | 295 | 0.59 | 2-Ethyl-5-methylfuran | Fresh, gassy, burnt | 1703-52-2 | C7H10O | 95, 110, 43 | 877 | 803 | 803 | 357,713 ± 172,466 | n/f | n/a | n/i |
| F9 | 325 | 0.60 | 2,3,5-Trimethylfuran | n/f | 10504-04-8 | C7H10O | 110, 109, 43, 95, 67 | 896 | 819 | 817 | 64,938 ± 50,557 | n/f | n/a | WB |
| F10 | 325 | 3.65 | 3-Furfural (3-furancarboxaldehyde) | Almond-like | 498-60-2 | C5H4O2 | 95, 39, 67, 29 | 942 | 820 | 832 | 4,539,412 ± 1,121,048 | n/f | n/a | GFB, NSB, P, RSB, WB |
| F11 | 355 | 0.80 | 2-Vinyl-5-methylfuran | n/f | 10504-13-9 | C7H8O | 108, 65, 43, 79, 56, 93 | 889 | 835 | 826 | 295,635 ± 120,061 | n/f | n/a | n/i |
| F12 | 360 | 3.43 | Furfural (2-furancarboxaldehyde) | Woody, almond, sweet, fruity, flowery | 98-01-1 | C5H4O2 | 96, 39, 29 | 966 | 839 | 843 | 200,344,626 ± 78,855,174 | 3000–23,000 | 3.9–4.8 | B, BAG, CWB, ME, GFB, NSB, P, T, TB, TC, S, WB, WSB |
| F13 | 425 | 1.70 | Furfuryl alcohol (2-furanmethanol) | Weak, fermented, creamy, caramel | 98-00-0 | C5H6O2 | 39, 41, 53, 81, 70, 98 | 949 | 872 | 865 | 404,124,163 ± 136,284,119 | 2000 | 5.3 | B, CWB, ME, GFB, NSB, P, RSB, T, TB, TC, TF, TS, WB, WP, WSB |
| F14 | 465 | 0.56 | 2-n-Butyl furan | Green | 4466-24-4 | C8H12O | 81, 53, 39, 27 | 951 | 892 | 898 | 5,012,989 ± 1,768,099 | 50,800 | 2.0 | B, RS, WB |
| F15 | 490 | 1.77 | Furfuryl formate | Ethereal | 13493-97-5 | C6H6O3 | 81, 53, 39, 44 | 918 | 909 | 902 | 643,862 ± 233,472 | n/f | n/a | WB |
| F16 | 495 | 1.77 | 2-Acetylfuran (1-(2-furanyl)-ethanone) | Smoky, roasty | 1192-62-7 | C6H6O2 | 95, 39, 43, 67 | 921 | 912 | 915 | 49,074,458 ± 10,718,656 | 10,000 | 3.7 | B, CWB, ME, GFB, P, RSB, T, TC, TF, WB, WP, WSB |
| F17 | 555 | 1.37 | 1-(2-Furyl)-2-propanone (2-furyl acetone) | Herbal, caramel, fruity, spicy, radish, green, burnt | 6975-60-6 | C7H8O2 | 81, 43, 53, 124 | 915 | 957 | 954 | 2,719,968 ± 2,692,555 | n/f | n/a | WB |
| F18 | 565 | 1.61 | 5-Methyl furfural (5-methyl-2-furancarboxaldehyde) | Almond, sweet, bitter | 620-02-0 | C6H6O2 | 110, 53, 39, 43, 81 | 979 | 964 | 966 | 33,471,223 ± 8,857,491 | 500 | 4.8 | B, ME, GFB, NSB, P, RSB, TB, WB |
| F19 | 565 | 2.98 | 5-methyl-2-furanmethanol (5-methylfurfuryl alcohol) | Bread-like, honey, sweet | 3857-25-8 | C6H8O2 | 95, 112, 43, 69, 53 | 913 | 965 | 966 | 8,379,336 ± 4,226,245 | 11.9 | 5.8 | BAG, CWB, WB |
| F20 | 600 | 0.53 | 2-Pentylfuran | Butter, green bean, floral, fruity, mushroom, raw nuts | 3777-69-3 | C9H14O | 81, 53, 39 | 934 | 989 | 993 | 55,139,336 ± 14,203,476 | 6 | 7.0 | B, BAG, ME, GFB, MF, MJ, NSB, P, RS, TC, TF, TS, WB, WP, WRB |
| F21 | 605 | 1.05 | Benzofuran | Styrene, aromatic | 271-89-6 | C8H6O | 118, 89, 63, 39, 51, 45 | 892 | 993 | 996 | 470,358 ± 286,276 | n/f | n/a | n/i |
| F22 | 605 | 1.53 | Isomaltol (2-acetyl-3-hydroxyfuran) | Caramel-like | 3420-59-5 | C6H6O3 | 111, 126, 43, 55, 84 | 918 | 994 | 989 | 392,890 ± 417,850 | n/f | n/a | n/i |
| F23 | 610 | 1.12 | Furfuryl acetate (2-furanmethanol acetate) | Sweet, fruity, banana-like, horseradish, ethereal, green | 623-17-6 | C7H8O3 | 81, 98, 43, 52, 140 | 920 | 997 | 998 | 5,202,352 ± 2,702,948 | n/f | n/a | WB |
| F24 | 625 | 1.21 | 1-(2-Furanyl)-1-propanone | Fruity | 3194-15-8 | C7H8O2 | 95, 39, 45, 74, 67, 57 | 872 | 1011 | 1016 | 2,589,462 ± 1,364,329 | n/f | n/a | WP |
| F25 | 645 | 1.22 | 2-Acetyl-5-methylfuran | Sweet, nutty with a caramel nuance, cocoa-like with a toasted bready nuance | 1193-79-9 | C7H8O2 | 109, 124, 43, 53 | 928 | 1030 | 1042 | 7,472,295 ± 7,428,702 | n/f | n/a | n/i |
| F26 | 655 | 1.13 | 2,2′-Bifuran (2-(2-furanyl)furan) | Vegetable, garlic | 5905-00-0 | C8H6O2 | 134, 78, 105, 51, 39 | 893 | 1039 | 1047 | 998,680 ± 662,678 | n/f | n/a | WB |
| F27 | 705 | 0.50 | Methylpentylfuran | n/f | - | C10H20O | 95, 152, 43, 67 | 892 | 1086 | 1083 | 3,099,292 ± 2,322,514 | n/f | n/a | B |
| F28 | 705 | 0.95 | 2-Furfurylfuran (2,2′-Methylenebisfuran) | Rich, roasted, aromatic | 1197-40-6 | C9H8O2 | 91, 148, 120, 39, 65 | 863 | 1087 | 1086 | 451,285 ± 232,810 | n/f | n/a | n/i |
| F29 | 710 | 3.24 | 5-Formylfurfural (2,5-furandicarboxaldehyde) | n/f | 823-82-5 | C6H4O3 | 124, 77 | 879 | 1094 | 1084 | 5,731,606 ± 2,639,042 | n/f | n/a | WB |
| F30 | 715 | 0.51 | 2-Hexylfuran | n/f | 3777-70-6 | C10H16O | 81, 53, 39, 41, 95, 123 | 905 | 1096 | 1096 | 463,776 ± 178,720 | n/f | n/a | n/i |
| F31 | 715 | 0.77 | 2-Butyl-tetrahydrofuran | n/f | 1004-29-1 | C8H16O | 71, 41, 55 | 864 | 1096 | 1096 | 3,919,696 ± 3,110,078 | n/f | n/a | n/i |
| F32 | 715 | 3.29 | Furyl hydroxymethyl ketone (1-(2-furanyl)-2-hydroxyethanone) | n/f | 17678-19-2 | C6H6O3 | 95, 39, 126, 29, 67 | 948 | 1098 | 1088 | 21,691,749 ± 9,667,403 | n/f | n/a | n/i |
| F33 | 805 | 1.32 | 1-(5-Methyl-2-furanyl)-2-hydroxyethanone | n/f | - | C7H8O3 | 109, 56, 69, 43, 140 | 833 | 1191 | n/f | 1,550,219 ± 734,005 | n/f | n/a | n/i |
| F34 | 865 | 4.97 | 2,3-Dihydrobenzofuran | Musky notes | 496-16-2 | C8H8O | 120, 91, 65, 51 | 911 | 1265 | 1222 | 2,807,760 ± 735,738 | n/f | n/a | B, BMJ |
| F35 | 905 | 1.17 | Difurfuryl ether (2,2′-[oxybis(methylene)]bis-furan) | Coffee, mushroom-like, nutty, earthy | 4437-22-3 | C10H10O3 | 81, 56, 27, 39, 97, 110 | 944 | 1308 | 1305 | 240,009 ± 95,353 | n/f | n/a | n/i |
| F36 | 935 | 4.45 | Hydroxymethylfurfural (5-(Hydroxymethyl)furfural) | Fatty, buttery, musty, waxy, caramel, herbal, tobacco | 67-47-0 | C6H6O3 | 97, 126, 41, 69, 53 | 907 | 1325 | 1266 | 22,784,687 ± 12,185,059 | 1,000,000 | 1.4 | P, RSB, WSB |
| Furanones | ||||||||||||||
| Fo1 | 245 | 3.32 | 2-Furanone (TI) (2(3H)-furanone) | n/f | 20825-71-2 | C4H4O2 | 55, 84, 27, 53, 39, 44 | 873 | 757 | 914 (DB-1) | 2,079,421 ± 625,998 | n/f | n/a | n/i |
| Fo2 | 305 | 1.58 | Dihydro-2-methyl-3-(2H)-furanone (2-methyltetrahydro-3-furanone) | Spicy, rancid, butter | 3188-00-9 | C5H8O2 | 43, 72, 100 | 974 | 809 | 812 | 19,616,932 ± 16,891,970 | n/f | n/a | WB, WP |
| Fo3 | 420 | 3.11 | 5-Methyl-2(3H)-furanone | Sweet, oily, coconut, tobacco, creamy, vanilla, hay | 591-12-8 | C5H6O2 | 55, 98, 43, 27, 70 | 851 | 870 | 869 | 235,195 ± 69,497 | n/f | n/a | n/i |
| Fo4 | 480 | 0.83 | 5-Methyl-5-furfuryl-2(5H)-furanone (5-(2-furanylmethyl)-5-methyl-2(5H)-furanone) (TI) | n/f | 31969-27-4 | C10H10O3 | 81, 53, 39, 69 | 751 | 901 | n/f | 1,263,633 ± 1,567,577 | n/f | n/a | WB |
| Fo5 | 505 | 4.88 | 2(5H)-Furanone (2,5-dihydrofuranone) | n/f | 497-23-4 | C4H4O2 | 55, 84, 27, 29, 39 | 940 | 922 | 918 | 43,176,086 ± 16,137,613 | n/f | n/a | WB, CWB |
| Fo6 | 540 | 2.77 | 5-Methyl-2(5H)-furanone | n/f | 591-11-7 | C5H6O2 | 69, 41, 39, 98 | 942 | 943 | 938 | 6,155,177 ± 2,897,005 | n/f | n/a | WB |
| Fo7 | 550 | 1.31 | 3-Methyl-2,5-furandione or 2,5-Dimethyl-3(2H)-furanone (TI) | n/f n/f | 616-02-4 14400-67-0 | C5H4O3 | 39, 68, 40, 28, 53, 112 | 706 926 | 953 | 949 924 (DB-1) | 1,818,359 ± 629,312 | n/f | n/a | WB n/i |
| Fo8 | 555 | 1.93 | γ-Valerolactone (dihydro-5-methyl-2(3H)-furanone) | Herbal, sweet, warm, tobacco, cocoa, woody, coconut | 108-29-2 | C5H8O2 | 56, 85, 41, 43 | 958 | 956 | 956 | 3,864,613 ± 1,759,554 | n/f | n/a | WB, CWB |
| Fo9 | 555 | 1.91 | 5,5-Dimethyl-2(5H)-furanone | n/f | 20019-64-1 | C6H8O2 | 97, 69, 43, 54, 26, 112 | 891 | 957 | 958 | 250,199 ± 71,863 | n/f | n/a | n/i |
| Fo10 | 560 | 1.90 | Dihydro-3-methyl-2(3H)-furanone or Dihydro-4-methyl-2(3H)-furanone (3-methylbutyrolactone) (TI) | n/f n/f | 1679-47-6 1679-49-8 | C5H8O2 | 41, 56, 27, 100 | 910 917 | 961 | 958 919 | 2,130,675 ± 842,009 | n/f n/f | n/a n/a | n/i n/i |
| Fo11 | 565 | 1.84 | Dihydro-3-methyl-2(3H)-furanone or Dihydro-4-methyl-2(3H)-furanone (3-methylbutyrolactone) (TI) | n/f n/f | 1679-47-6 1679-49-8 | C5H8O2 | 56, 85, 41, 43, 100 | 895 891 | 964 | 958 919 | 502,907 ± 283,902 | n/f n/f | n/a n/a | n/ n/i |
| Fo12 | 570 | 1.87 | n/i | n/a | - | C6H8O2 | 97, 69, 43, 26, 54 | n/a | 968 | n/a | 183,534 ± 115,610 | n/a | n/a | n/a |
| Fo13 | 575 | 1.24 | 5-Ethyl-(3H)-furan-2-one (2-ethylbutenolide) | Spicy | 2313-01-1 | C6H8O2 | 55, 112, 83, 97 | 910 | 971 | 954 | 612,612 ± 196,297 | n/f | n/a | WB |
| Fo14 | 615 | 1.64 | 2,5-Dihydro-3,5-dimethyl 2-furanone | n/f | 5584-69-0 | C6H8O2 | 69, 41, 115, 97 | 884 | 1002 | 993 | 973,653 ± 442,005 | n/f | n/a | n/i |
| Fo15 | 655 | 1.95 | 5-Ethyl-2(5H)-Furanone (TI) | Spicy | 2407-43-4 | C6H8O2 | 28, 83, 18, 55, 44 | 897 | 1040 | 984 (DB-1) | 5,650,222 ± 2,621,703 | n/f | n/a | WB |
| Fo16 | 655 | 1.79 | 3,4-Dimethyl-2,5-furandione (2,3-dimethyl maleic anhydride) | n/f | 766-39-2 | C6H6O3 | 39, 54, 82, 126 | 882 | 1040 | 1038 | 3,343,389 ± 1,471,465 | n/f | n/a | n/i |
| Fo17 | 665 | 4.90 | R-Pantolactone | Cotton candy, licorice, smoky, toasted bread | 599-04-2 | C6H10O3 | 71, 43, 29, 57 | 951 | 1047 | 1043 | 2,268,814 ± 1,125,826 | 50 | 4.7 | MF |
| Fo18 | 670 | 2.82 | 4-Methyl-2(5H)-furanone | n/f | 6124-79-4 | C5H6O2 | 69, 41, 39, 98 | 924 | 1055 | n/f | 2,855,095 ± 885,123 | n/f | n/a | n/i |
| Fo19 | 675 | 1.45 | γ-N-Caprolactone (γ-hexalactone, 5-ethyldihydro-2(3H)-furanone) | Coumarin-like, sweet | 695-06-7 | C6H10O2 | 85, 42, 56, 70, 114 | 942 | 1059 | 1058 | 15,427,139 ± 6,696,866 | 50 | 5.5 | B, CWB |
| Fo20 | 705 | 4.02 | Furaneol (2,5-dimethyl-4-hydroxy-3(2H)-furanone) | Caramel, strawberry | 3658-77-3 | C6H8O3 | 43, 57, 128, 85 | 918 | 1090 | 1090 | 3,801,345 ± 2,827,164 | 60 | 4.8 | WB, BAG, CWB, GFB, NSB, P, RS, TC |
| Fo21 | 765 | 3.00 | Solerone (TI) (5-acetyldihydro-2(3H)-furanone) | n/f | 29393-32-6 | C6H8O3 | 85, 29, 43, 57, 128 | 937 | 1151 | 1299 (SE-54) | 2,040,510 ± 929,704 | n/f | n/a | n/i |
| Fo22 | 775 | 1.26 | γ-Heptalactone (dihydro-5-propyl-2(3H)-furanone) | Sweet, coconut, nutty, caramel, creamy, milky, tobacco | 105-21-5 | C7H12O2 | 85, 29, 56, 41 | 948 | 1159 | 1163 | 833,364 ± 298,059 | 499 | 3.2 | n/i |
| Fo23 | 870 | 1.17 | γ-Octalactone (5-butyldihydro-2(3H)-furanone) | Sweet, coconut, waxy, creamy, milky, soapy, fruity | 104-50-7 | C8H14O2 | 85, 41, 56, 100 | 955 | 1266 | 1264 | 1,318,130 ± 558,277 | 8 | 5.2 | CWB, TC |
| Fo24 | 875 | 0.89 | 5-Pentyl-2(3H)-furanone (3-nonen-4-olide) | Tropical, fruity, milky, dairy | 51352-68-2 | C9H14O2 | 98, 111, 55, 83, 70, 154 | 840 | 1272 | 1273 | 3,499,672 ± 1,292,082 | n/f | n/a | n/i |
| Fo25 | 940 | 1.29 | 5-Pentyl-2(5H)-furanone (4-hydroxy-2-nonenoic acid lactone) | Minty, fruity | 21963-26-8 | C9H14O2 | 29, 28, 45, 57, 100, 113, 126, 85, 72 | 823 | 1352 | 1358 | 10,012,186 ± 4,179,765 | n/f | n/a | n/i |
| Fo26 | 955 | 1.10 | γ-Nonalactone (dihydro-5-pentyl-2(3H)-furanone) | Coconut-like, sweet, fruity | 104-61-0 | C9H16O2 | 85, 114, 41, 55, 99, 137 | 946 | 1370 | 1363 | 9,304,844 ± 4,182,379 | 9.7–27 | 5.5–6.0 | B, BMJ, CWB, MJ, WSB |
| Pyrans | ||||||||||||||
| Pn1 | 240 | 0.53 | 3,4-Dihydro-6-methyl-2H-pyran | n/f | 16015-11-5 | C6H10O | 43, 55, 98, 83 | 913 | 749 | n/f | 134,400 ± 30,229 | n/f | n/a | n/i |
| Pn2 | 675 | 1.50 | 5,6-Dihydro-2H-pyran-2-carboxaldehyde or 3,4-Dihydro-2H-pyran-2-carboxaldehyde (TI) | n/f n/f | 53897-26-0 100-73-2 | C6H8O2 | 83, 55, 29, 112, 39 | 867 860 | 1059 | n/f 853 (OV-101) | 1,444,173 ± 653,900 | n/f n/f | n/a n/a | n/i |
| Pn3 | 915 | 1.02 | 2-(1-Butenyl)-tetrahydropyran | n/f | 95652-24-7 | C9H16O | 111, 140, 83, 98, 125 | 801 | 1320 | n/f | 129,456 ± 54,493 | n/f | n/a | n/i |
| Pyranones | ||||||||||||||
| Po1 | 450 | 1.47 | Dihydro-2H-pyran-3(4H)-one (TI) | n/f | 23462-75-1 | C5H8O2 | 42, 27, 71, 55 | 947 | 885 | 1439 (HP-Wax) | 54,028 ± 25,761 | n/f | n/a | n/i |
| Po2 | 585 | 3.05 | 2H-Pyran-2-one (α-pyrone) | Herbal | 504-31-4 | C5H4O2 | 39, 68, 96 | 877 | 980 | 978 | 34,118 ± 13,808 | n/f | n/a | WP |
| Po3 | 650 | 1.58 | n/i | n/a | n/a | n/a | 68, 39, 98, 53 | 801 756 | 1035 | n/a | 5,592,787 ± 4,970,053 | n/a n/a | n/a n/a | n/i |
| Po4 | 655 | 2.74 | 5,6-Dihydro-2H-pyran-2-one (TI) | n/f | 3393-45-1 | C5H6O2 | 68, 39, 98, 53 | 929 | 1041 | 1838 (DB-Wax) | 1,621,497 ± 558,094 | n/f | n/a | n/i |
| Po5 | 675 | 2.03 | δ-Valerolactone (TI) (tetrahydro-2H-pyran-2-one) | n/f | 542-28-9 | C5H8O2 | 42, 41, 27, 56, 100, 70 | 947 | 1059 | 965 | 916,387 ± 142,550 | n/f | n/a | n/i |
| Po6 | 715 | 1.60 | δ-Hexalactone (tetrahydro-6-methyl-2H-pyran-2-one, δ-caprolactone) | Creamy, fruity, coconut, spicy | 823-22-3 | C6H10O2 | 42, 70, 55, 99 | 944 | 1097 | 1084 | 840,593 ± 226,566 | n/f | n/a | WB |
| Po7 | 740 | 2.64 | Maltol (3-hydroxy-2-methyl-4H-pyran-4-one) | Warmy-fruity, caramel-sweet | 118-71-8 | C6H6O3 | 126, 71, 43, 55, 97 | 974 | 1124 | 1113 | 117,672,149 ± 40,223,031 | 2500 | 4.7 | B, P, TB, TC, WB |
| Po8 | 795 | 0.20 | 3-Hydroxy-2,3-dihydromaltol (2,3-dihydro-3,5-dihydroxy-6-methyl-4H-pyran-4-one) | Caramelized | 28564-83-2 | C6H8O4 | 43, 144, 101, 73, 55 | 972 | 1179 | 1144 | 29,961,549 ± 16,035,481 | n/f | n/a | B, P, WB |
| Po9 | 975 | 2.40 | 2-Hydroxy-3-methyl-4H-pyran-4-one | n/f | 61892-88-4 | C6H6O3 | 126, 71, 43, 55, 97 | 818 | 1397 | n/f | 37,421 ± 25,172 | n/f | n/a | n/i |
| Pyrazines | ||||||||||||||
| Pz1 | 190 | 1.25 | Pyrazine | Roasted | 290-37-9 | C4H4N2 | 80, 26, 53 | 960 | 704 | 739 | 1,631,354 ± 797,679 | 180,000 | 1.0 | WB, ME, GFB, NSB, P, TC, TS, WP |
| Pz2 | 345 | 1.34 | 2-Methylpyrazine | Roasted, burnt, sweet | 109-08-0 | C5H6N2 | 94, 67, 39, 26, 53 | 974 | 830 | 840 | 15,773,787 ± 11,169,354 | 60–105,000 | 2.2–5.4 | B, WB, BAG, ME, GFB, NSB, P, T, TC, TS, WFB, WP, WRB |
| Pz3 | 495 | 0.90 | 2,6-Dimethylpyrazine | Roasted | 108-50-9 | C6H8N2 | 42, 108, 39, 40, 28, 18 | 972 | 912 | 915 | 23,375,159 ± 12,540,414 | 200–9000 | 3.4–5.1 | WB, CWB, GFB, NSB, P, T, TC, WFB, WP, WRB |
| Pz4 | 500 | 0.91 | 2-Ethylpyrazine | Popcorn, nutty | 13925-00-3 | C6H8N2 | 107, 108, 80, 53, 39, 28 | 926 | 915 | 915 | 2,149,106 ± 1,864,070 | 6000–22,000 | 2.0–2.6 | WB, ME, GFB, NSB, P, RSB, T, TC, TS, WRB |
| Pz5 | 505 | 0.93 | 2,3-Dimethylpyrazine | Popcorn, roasted | 5910-89-4 | C6H8N2 | 108, 67, 42 | 929 | 919 | 919 | 2,413,964 ± 1,493,485 | 2500–35,000 | 1.8–3.0 | WB, ME, GFB, NSB, P, T, TC, TS |
| Pz6 | 615 | 0.75 | 2-Ethyl-3-methylpyrazine or 2,3,5-trimethylpyrazine (TI) | Potato-like, earthy Nutty, roasted, sweet | 15707-23-0 14667-55-1 | C7H10N2 | 42, 122, 81, 39 | 954 936 | 1001 | 1001 1005 | 5,256,433 ± 2,326,845 | 400–1800 130 | 4.6 3.5–4.1 | WB, ME, GFB, NSB, P, RSB, TC, WFB, WP, WRB WB, BAG, ME, GFB, TC |
| Pz7 | 630 | 0.90 | 2-Ethenyl-6-methylpyrazine (2-methyl-6-vinylpyrazine) | Coffee | 13925-09-2 | C7H8N2 | 120, 52, 39, 94 | 834 | 1015 | 1023 | 918,205 ± 550,329 | n/f | n/a | WB, ME, P, T, WP |
| Pz8 | 640 | 1.35 | Acetylpyrazine | Biscuit, cracker-like, crust-like, sweet, roasted | 22047-25-2 | C6H6N2O | 43, 52, 80, 122, 94, 28, 15 | 965 | 1025 | 1031 | 418,788 ± 223,595 | 62 | 3.8 | WB, GFB, NSB, RSB, WRB |
| Pz9 | 700 | 0.65 | 2-Ethyl-3,6-dimethylpyrazine (3-ethyl-2,5-dimethyl-pyrazine) or 2-Ethyl-3,5-dimethyl-pyrazine (3-ethyl-2,6-dimethylpyrazine) | Potato, cocoa, roasted, nutty Burnt, roasted, nutty, coffee, caramel, cocoa, maize | 13360-65-1 13925-07-0 | C8H12N2 | 135, 136, 42, 39, 56, 108 | 918 885 | 1082 | 1082 1082 | 749,792 ± 503,023 | 8.6 0.04–1 | 4.9 5.9–7.3 | WB, ME, P, RSB WB, ME, P, T, TC, WRB, WSB |
| Pz10 | 740 | 1.07 | 2-Acetyl-3-methylpyrazine | Nutty, roasted, hazelnut, corn chip, caramel, potato chip | 23787-80-6 | C7H8N2O | 43, 93, 136, 94, 42, 67, 52 | 864 | 1122 | 1128 | 207,115 ± 107,124 | n/f | n/a | n/i |
| Pz11 | 760 | 0.83 | 5H-5-Methyl-6,7-dihydrocyclopentapyrazine | Earthy, baked potato, sweet, roasted, corn with savory | 23747-48-0 | C8H10N2 | 119, 134 | 907 | 1143 | 1149 | 511,503 ± 176,711 | n/f | n/a | n/i |
| Pz12 | 875 | 1.40 | 2-(2′-Furyl)-pyrazine (TI) (2-(furan-2-yl)pyrazine) | n/f | 32736-95-1 | C8H6N2O | 146, 93, 63, 38, 39 | 816 | 1272 | 1255 (DB-1) | 293,650 ± 139,715 | n/f | n/a | n/i |
| Pyridines and Pyrimidines | ||||||||||||||
| Pd1 | 350 | 1.05 | 2-Methylpyridine | Sweat, astringent, hazelnut, nutty | 109-06-8 | C6H7N | 93, 66, 39, 78, 51 | 958 | 832 | 824 | 349,470 ± 195,878 | n/f | n/a | n/i |
| Pd2 | 420 | 0.98 | 4-Methylpyrimidine | n/f | 3438-46-8 | C5H6N2 | 94, 40, 53, 67, 79 | 802 | 869 | 853 | 9105 ± 6947 | n/f | n/a | n/i |
| Pd3 | 460 | 0.79 | 2,6-Dimethylpyridine (2,6-lutidine) | Nutty, ammoniacal, woody, bready, cocoa, coffee, musty | 108-48-5 | C7H9N | 107, 106, 39, 66, 79, 93 | 924 | 890 | 890 | 306,301 ± 109,246 | n/f | n/a | n/i |
| Pd4 | 490 | 0.79 | 2-Ethylpyridine (TI) | Green, grassy | 100-71-0 | C7H9N | 106, 107, 79, 52, 66, 39 | 702 | 908 | 906 | 135,706 ± 64,908 | n/f | n/a | T |
| Pd5 | 550 | 0.83 | 2,3-Dimethylpyridine (2,3-lutidine) | Coffee, caramel | 583-61-9 | C7H9N | 107, 106, 39, 66, 79, 92 | 937 | 952 | 952 | 29,778 ± 9134 | n/f | n/a | n/i |
| Pd6 | 605 | 0.71 | 2,4,6-Trimethylpyridine | Aromatic odor | 108-75-8 | C8H11N | 121, 79, 39, 106 | 842 | 993 | 993 | 167,322 ± 96,358 | n/f | n/a | n/i |
| Pd7 | 650 | 1.18 | 2-Acetylpyridine (1-(2-pyridinyl)-ethanone) | Biscuit-like, toasted, cracker-like, crust-like, roasted | 1122-62-9 | C7H7NO | 79, 121, 93, 43, 51 | 939 | 1035 | 1050 | 1,141,684 ± 1,282,175 | 19 | 4.8 | ME, P, T, TC |
| Pd8 | 810 | 1.13 | 1-Acetyl-1,2,3,4-tetrahydropyridine | Nutty | 19615-27-1 | C7H11NO | 85, 125, 83, 68, 43, 54 | 882 | 1196 | 1189 | 1,504,556 ± 534,232 | n/f | n/a | n/i |
| Pd9 | 815 | 0.65 | 2-Pentylpyridine (2-propylpyridine) | Green, fatty, roasted, tobacco, nutty | 622-39-9 | C8H11N | 93, 106, 120, 79, 65, 39 | 850 | 1201 | 1202 | 89,596 ± 34,399 | 0.6 | 5.2 | n/i |
| Pyrroles | ||||||||||||||
| Py1 | 315 | 1.04 | 1-Ethyl-1H-pyrrole | Burnt | 617-92-5 | C6H9N | 80, 95, 67, 39, 27, 53 | 879 | 814 | 815 | 68,416 ± 27,880 | n/f | n/a | ME |
| Py2 | 615 | 1.48 | N-Methyl-2-formylpyrrole (1-methyl-1H-pyrrole-2-carboxaldehyde) | Roasted, nutty | 1192-58-1 | C6H7NO | 109, 108, 53, 80, 39 | 920 | 1004 | 1010 | 848,932 ± 340,434 | 37 | 4.4 | P |
| Py3 | 665 | 1.14 | 1-Ethyl-2-formyl-1H-pyrrole (1-ethyl-1H-pyrrole-2-carboxaldehyde) | Burnt, roasted, smoky | 2167-14-8 | C7H9NO | 123, 94, 39, 108, 66, 53, 80 | 816 | 1047 | 1046 | 664,513 ± 343,483 | n/f | n/a | ME |
| Py4 | 665 | 0.14 | 2-Formyl-1H-pyrrole (1H-pyrrole-2-carboxaldehyde) | Musty | 1003-29-8 | C5H5NO | 95, 94, 66, 39 | 949 | 1048 | 1047 | 2,142,059 ± 1,055,032 | n/f | n/a | ME, P, WB |
| Py5 | 670 | 1.38 | 1-Methyl-2-pyrrolidinone | Fishlike | 872-50-4 | C5H9NO | 44, 42, 99, 98, 28 | 849 | 1057 | 1046 | 331,816 ± 211,791 | n/f | n/a | n/i |
| Py6 | 690 | 3.46 | 2-Acetylpyrrole (1-(1H-pyrrol-2-yl)-ethanone) | Musty | 1072-83-9 | C6H7NO | 94, 109, 66, 39, 43, 53 | 954 | 1076 | 1069 | 21,389,485 ± 7,698,915 | 170,000 | 2.1 | B, BAG, CWB, GFB, NSB, P, TC, WB |
| Py7 | 735 | 1.14 | 1-Ethyl-2-pyrrolidinone (TI) | Slight amine | 2687-91-4 | C6H11NO | 98, 113, 70, 41, 28 | 907 | 1117 | 1856 (FFAP) | 209,715 ± 147,230 | n/f | n/a | n/i |
| Py8 | 740 | 1.42 | Ethyl pyrrole 1-acetate (TI) | n/f | 5145-67-5 | C8H11NO2 | 80, 153, 53, 57, 71 | 718 | 1123 | n/f | 995,860 ± 1,013,831 | n/f | n/a | n/i |
| Py9 | 800 | 1.20 | N-Furfurylpyrrole (1-(2-furanylmethyl)-1H-pyrrole) | Vegetable, plastic, waxy, fruity, cereal, bready, potato | 1438-94-4 | C9H9NO | 81, 147, 53, 27, 39 | 955 | 1186 | 1179 | 775,590 ± 358,385 | 100 | 3.9 | B, ME, P, WB, WRB |
| Py10 | 910 | 4.10 | Indole (benzopyrrole) | Animal, naphthyl, fecal, pungent, musty; in low concentrations: powerful floral notes and pleasant radiation | 120-72-9 | C8H7N | 117, 90, 63, 39, 50 | 954 | 1318 | 1295 | 6,532,212 ± 1,981,606 | 140 | 4.7 | B, WB |
| Py11 | 980 | 1.18 | 5-Acetyl-2,3-dihydro-1H-pyrrolizine (TI) | Amine, grass, hay, smoky | 55041-85-5 | C9H11NO | 134, 149, 106, 79, 51 | 910 | 1402 | 1382 (DB-1) | 807,263 ± 424,045 | n/f | n/a | P |
| Py12 | 985 | 3.01 | Skatole (3-methyl-1H-indole) | Animal, fecal, warm, sweet, over-ripe fruit; in low concentrations: may give a note of ‘overmature flower’ | 83-34-1 | C9H9N | 130, 131, 77, 51, 65, 103, 39 | 940 | 1411 | 1410 | 50,749 ± 33,736 | 0.2 | 5.4 | P |
| Py13 | 1000 | 1.49 | 1-Furfuryl-2-formyl pyrrole (1-methyl-1H-pyrrole-2-carboxaldehyde) | Slightly burnt taste, acid n/f | 19377-82-3 | C10H9NO2 | 81, 175, 53, 39, 147 | 851 | 1429 | 1384 | 284,459 ± 165,711 | 97 | 3.5 | P, WRB |
| Oxazoles | ||||||||||||||
| Ox1 | 235 | 0.93 | 4,5-Dimethyloxazole | n/f | 7064-40-6 | C5H7NO | 97, 43, 55 | 821 | 745 | 750 | 29,777 ± 23,570 | n/f | n/a | n/i |
| Ox2 | 410 | 0.77 | Trimethyloxazole | Nutty, roasted, shellfish, mustard burnt, oily, mushroom | 20662-84-4 | C6H9NO | 111, 43, 68, 55 | 868 | 864 | 863 | 109,136 ± 176,271 | n/f | n/a | WP |
| Ox3 | 735 | 1.34 | Benzoxazole (TI) | n/f | 273-53-0 | C7H5NO | 119, 64, 91 | 853 | 1117 | 1067 (DB-1) | 182,454 ± 98,420 | n/f | n/a | n/i |
| Thiazoles | ||||||||||||||
| Tz1 | 195 | 1.49 | Thiazole | Fishy, nutty, meaty | 288-47-1 | C3H3NS | 85, 58, 45 | 967 | 704 | 694 | 294,112 ± 230,369 | n/f | n/a | WB |
| Tz2 | 635 | 1.46 | 2-Acetylthiazole | Roasty | 24295-03-2 | C5H5NOS | 43, 127, 99, 58, 85 | 944 | 1020 | 1018 | 1,155,525 ± 300,637 | 10 | 5.1 | WB, ME |
| Tz3 | 840 | 1.45 | Benzothiazole | Sulfurous | 95-16-9 | C7H5NS | 135, 108, 69, 45 | 956 | 1231 | 1231 | 1,124,234 ± 601,368 | 80 | 4.1 | WB |
| Thiophenes | ||||||||||||||
| Tp1 | 235 | 0.72 | 3-Methylthiophene | Fatty, winey | 616-44-4 | C5H6S | 97, 98, 45 | 968 | 744 | 770 | 22,399 ± 9459 | n/f | n/a | n/i |
| Tp2 | 600 | 1.97 | 3-Thiophenecarboxaldehyde | n/f | 498-62-4 | C5H4OS | 111, 112, 83, 39 | 847 | 990 | 1003 | 252,769 ± 141,357 | n/f | n/a | n/i |
| Tp3 | 610 | 1.98 | 2-Thiophenecarboxaldehyde (2-formylthiophene, thenaldehyde) | Sulfurous, almond, bitter, cherry | 98-03-3 | C5H4OS | 111, 112, 83, 58 | 952 | 998 | 1001 | 762,146 ± 260,839 | n/f | n/a | ME |
| Tp4 | 705 | 1.31 | 2-Formyl-3-methylthiophene (3-methyl-2-thiophenecarboxaldehyde) | Saffron, camphoreous | 5834-16-2 | C6H6OS | 125, 126, 97, 45 | 845 | 1087 | 1109 | 123,821 ± 40,906 | n/f | n/a | n/i |
| Tp5 | 705 | 1.56 | 2-Acetylthiophene (1-(2-thienyl)-ethanone) | Sulfurous, nutty, hazelnut, onion | 88-15-3 | C6H6OS | 111, 126 | 829 | 1087 | 1085 | 70,861 ± 22,816 | n/f | n/a | WB |
| Tp6 | 745 | 1.48 | 2-Formyl-5-methylthiophene (TI) (5-methyl-2-thiophenecarboxaldehyde) | Rancid, fatty, grass | 13679-70-4 | C6H6OS | 125, 126, 97, 45 | 760 | 1128 | 1124 | 90,000 ± 35,587 | n/f | n/a | WP, ME |
| Other sulfur-containing compounds | ||||||||||||||
| S1 | 70 | 0.32 | Methanethiol | Rotting cabbage; in low concentrations: tropical fruit, may contribute for the aroma of sweet maize | 74-93-1 | CH4S | 47, 45, 48 | 991 | 575 | <500 | 6,115,559 ± 1,459,463 | 0.04–82 | 4.9–8.2 | WB, CB |
| S2 | 80 | 0.35 | Dimethyl sulfide | Cabbage-like, in low concentrations: canned maize | 75-18-3 | C2H6S | 47, 62, 35 | 978 | 585 | 565 | 1,689,726 ± 724,639 | 0.3–1 | 6.2–6.8 | WB, CB, P, RS |
| S3 | 195 | 0.69 | Dimethyl disulfide | Garlic; in low concentrations: contributes to maize flavor | 624-92-0 | C2H6S2 | 94, 45, 79, 61 | 973 | 703 | 718 | 9,175,781 ± 4,840,433 | 12 | 5.9 | WB, CB, ME, GFB, P, RS, TC, WP |
| S4 | 220 | 1.56 | 1-Methylthiopropane (TI) (methyl propyl sulfide) | Alliceous, creamy, green, leek | 3877-15-4 | C4H10S | 61, 90, 48 | 714 | 730 | 715 | 100,912 ± 2,3581 | n/f | n/a | n/i |
| S5 | 375 | 1.58 | Methylthio-2-propanone (acetonyl methyl sulfide) | Melon, cabbage, garlic | 14109-72-9 | C4H8OS | 43, 61, 104 | 923 | 846 | 863 | 17,904 ± 16,085 | n/f | n/a | n/i |
| S6 | 480 | 1.10 | 1,4-Oxathiane, or 1,3-Oxathiane, or 1,2-Oxathiane (thioxane) (TI) | Characteristic n/f Green, grassy, leafy, cortex, foliage, aromatic, vegetable, floral, juicy mango, tropical | 15980-15-1 646-12-8 57917-36-9 | C4H8OS | 46, 104, 74, 61 | 811 863 n/a | 901 | 885 n/f n/f | 57,599 ± 47,389 | n/f n/f n/f | n/a n/a n/a | n/i n/i n/i |
| S7 | 485 | 1.52 | Methional (3-methylthiopropanal) | Boiled-potato, cooked-potato, malty, waxy | 3268-49-3 | C4H8OS | 48, 104, 61, 76 | 930 | 905 | 903 | 806,522 ± 660,313 | 0.2 | 6.6 | WB, CB, CWB, NSB, P, T, TC, TS, WP |
| S8 | 565 | 0.85 | Dimethyl trisulfide | Cabbage-like, in low concentrations: tropical fruit/grapefruit | 3658-80-8 | C2H6S3 | 126, 79, 45, 111 | 947 | 964 | 964 | 2,195,136 ± 1,508,373 | 0.01 | 8.3 | WB, ME, GFB, P, TC, WFB |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bento-Silva, A.; Duarte, N.; Santos, M.; Costa, C.P.; Vaz Patto, M.C.; Rocha, S.M.; Bronze, M.R. Comprehensive Two-Dimensional Gas Chromatography as a Powerful Strategy for the Exploration of Broas Volatile Composition. Molecules 2022, 27, 2728. https://doi.org/10.3390/molecules27092728
Bento-Silva A, Duarte N, Santos M, Costa CP, Vaz Patto MC, Rocha SM, Bronze MR. Comprehensive Two-Dimensional Gas Chromatography as a Powerful Strategy for the Exploration of Broas Volatile Composition. Molecules. 2022; 27(9):2728. https://doi.org/10.3390/molecules27092728
Chicago/Turabian StyleBento-Silva, Andreia, Noélia Duarte, Magda Santos, Carina Pedrosa Costa, Maria Carlota Vaz Patto, Sílvia M. Rocha, and Maria Rosário Bronze. 2022. "Comprehensive Two-Dimensional Gas Chromatography as a Powerful Strategy for the Exploration of Broas Volatile Composition" Molecules 27, no. 9: 2728. https://doi.org/10.3390/molecules27092728
APA StyleBento-Silva, A., Duarte, N., Santos, M., Costa, C. P., Vaz Patto, M. C., Rocha, S. M., & Bronze, M. R. (2022). Comprehensive Two-Dimensional Gas Chromatography as a Powerful Strategy for the Exploration of Broas Volatile Composition. Molecules, 27(9), 2728. https://doi.org/10.3390/molecules27092728










