Binding Interaction of Betulinic Acid to α-Glucosidase and Its Alleviation on Postprandial Hyperglycemia
Abstract
1. Introduction
2. Results and Discussion
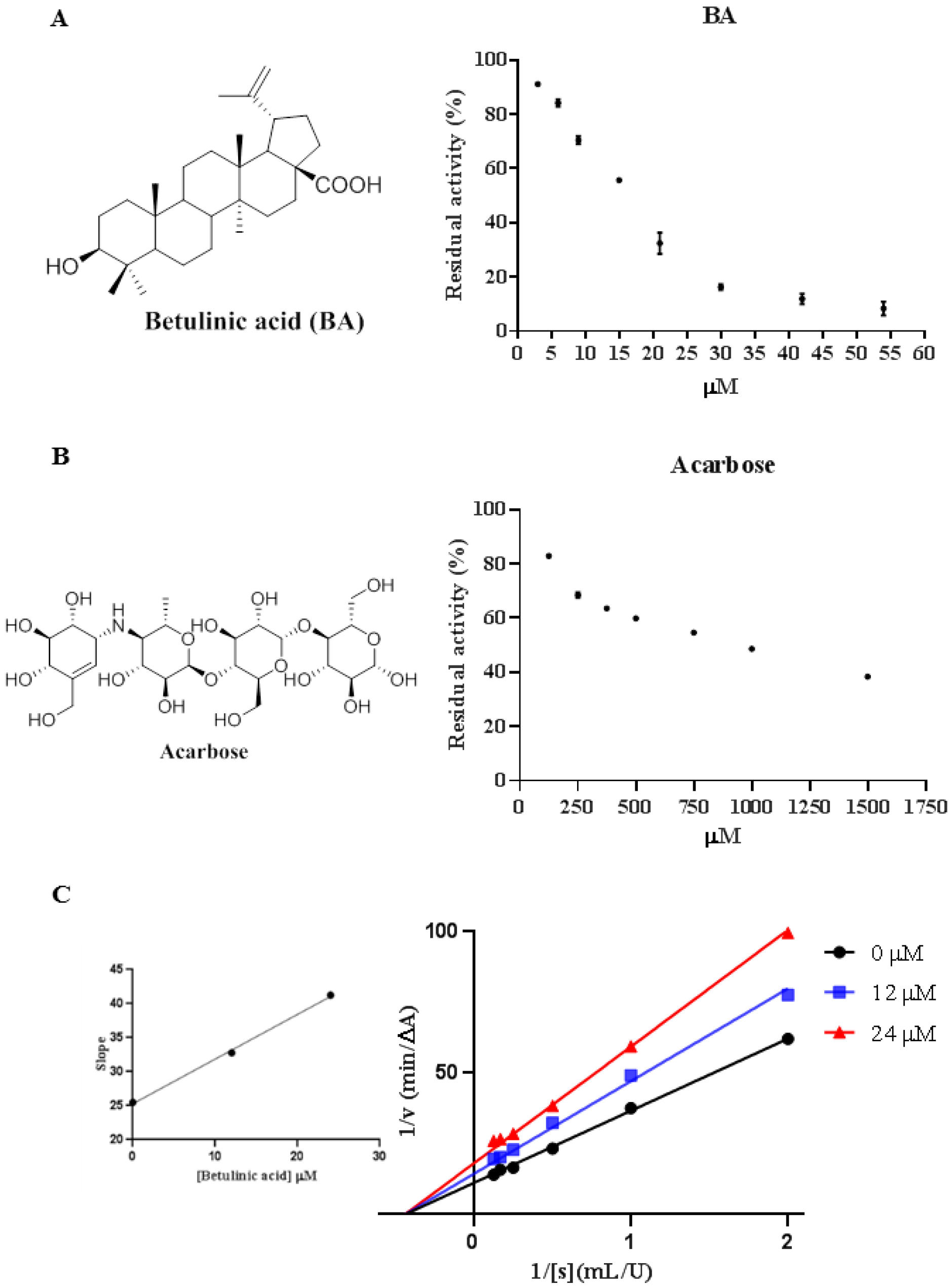
2.1. Inhibitory Effect of BA against α-Glucosidase
2.2. Inhibition of Acarbose Combined with BA against α-Glucosidase
2.3. Inhibition Types of BA on α-Glucosidase
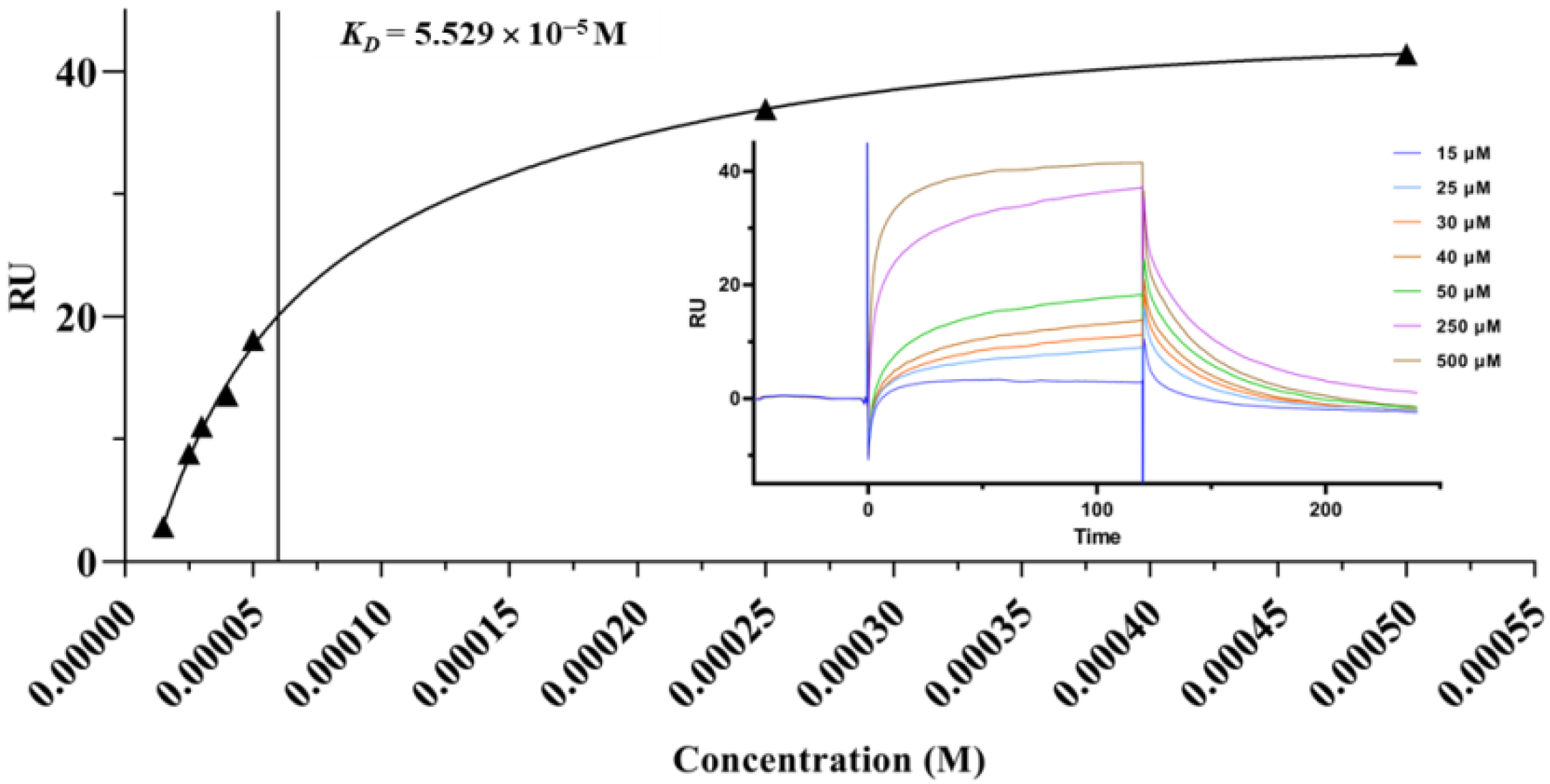
2.4. Surface Plasmon Resonance (SPR) Analysis
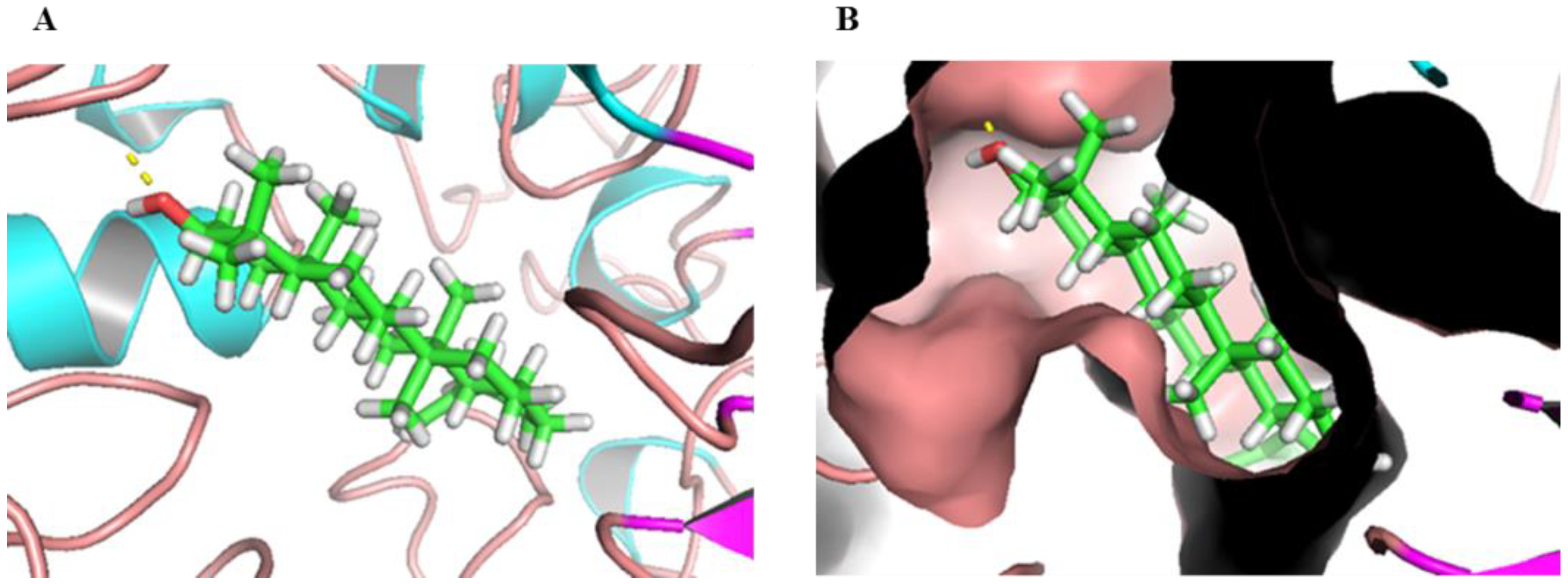
2.5. Molecular Docking
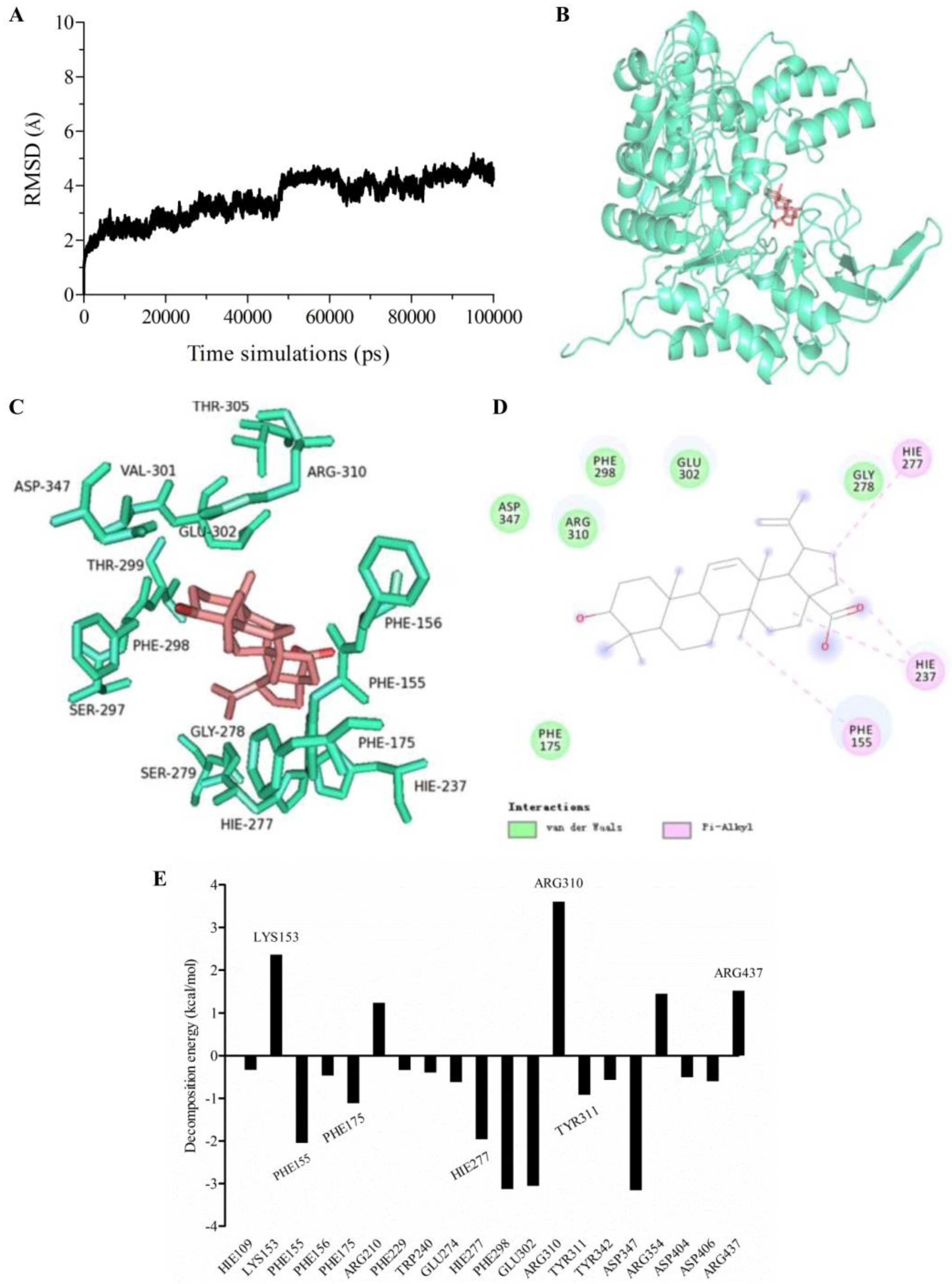
2.6. Molecular Dynamics (MD) Simulation and Calculation of Binding Free Energy
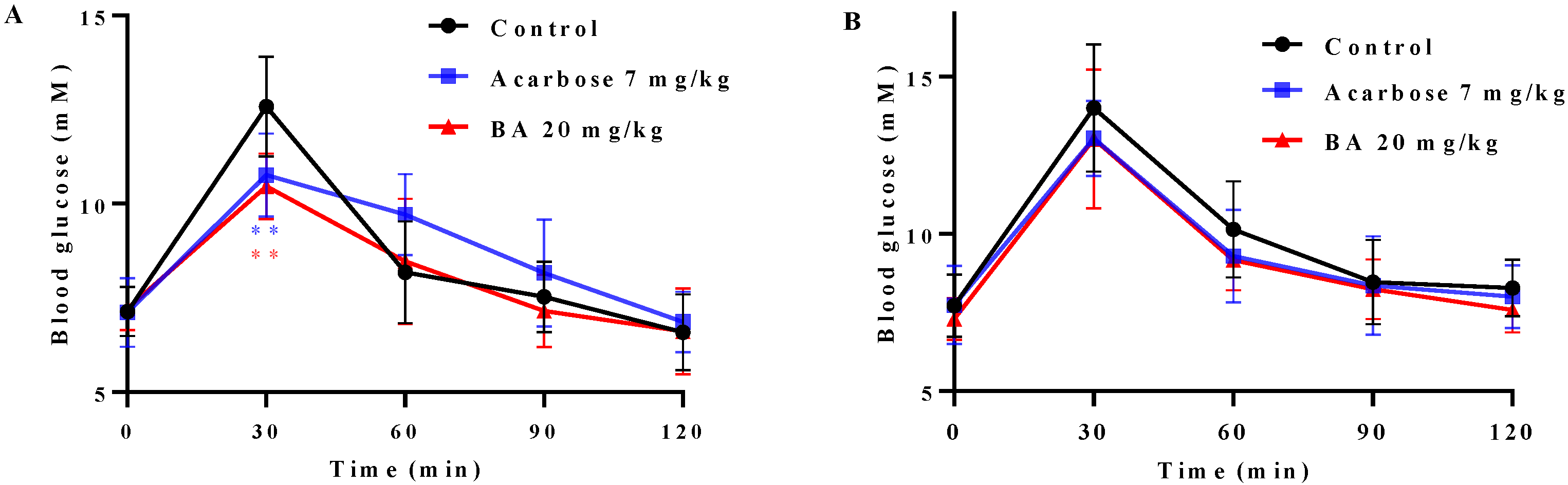
2.7. Effects of BA on Oral Saccharides Tolerance in Normal Mice
3. Materials and Methods
3.1. Materials
3.2. Inhibition Assay against α-Glucosidase
3.3. Combination between BA and Acarbose
3.4. Enzyme Inhibition Kinetics
3.5. SPR Assay
3.6. Molecular Docking
3.7. Molecular Dynamic (MD) Simulation and Free Energy Calculation and Decomposition
3.8. Oral Disaccharide Tolerance Test (ODTT) and Oral Glucose Tolerance Test (OGTT)
3.9. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Khursheed, R.; Singh, S.K.; Wadhwa, S.; Gulati, M.; Awasthi, A. Therapeutic potential of mushrooms in diabetes mellitus: Role of polysaccharides. Int. J. Biol. Macromol. 2020, 164, 1194–1205. [Google Scholar] [CrossRef] [PubMed]
- Ding, H.; Hu, X.; Xu, X.; Zhang, G.; Gong, D. Inhibitory mechanism of two allosteric inhibitors, oleanolic acid and ursolic acid on α-glucosidase. Int. J. Biol. Macromol. 2018, 107, 1844–1855. [Google Scholar] [CrossRef] [PubMed]
- Kato, A.; Hayashi, E.; Miyauchi, S.; Adachi, I.; Imahori, T.; Natori, Y.; Yoshimura, Y.; Nash, R.J.; Shimaoka, H.; Nakagome, I.; et al. α-1-C-butyl-1,4-dideoxy-1,4-imino-ι-arabinitol as a second-generation iminosugar-based oral α-glucosidase inhibitor for improving postprandial hyperglycemia. J. Med. Chem. 2012, 55, 10347–10362. [Google Scholar] [CrossRef] [PubMed]
- Esposito, K.; Giugliano, D.; Nappo, F.; Marfella, R.; Campanian Postprandial Hyperglycemia Study Group. Regression of carotid atherosclerosis by control of postprandial hyperglycemia in type 2 diabetes mellitus. Circulation 2004, 110, 214–219. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.; Yang, C.; Yang, R.; Zhao, W. Magnetically anchored antibody-coupled nanocomposite as α-Amylase inhibitor for long-time protection against glycemic variability. Chem. Eng. J. 2022, 430, 132984. [Google Scholar] [CrossRef]
- Ma, Y.Y.; Zhao, D.G.; Zhang, R.Q.; He, X.; Li, B.Q.; Zhang, X.Z.; Wang, Z.J.; Zhang, K. Identification of bioactive compounds that contribute to the α-glucosidase inhibitory activity of rosemary. Food Funct. 2020, 11, 1692–1701. [Google Scholar] [CrossRef]
- Ding, H.; Wu, X.; Pan, J.; Hu, X.; Gong, D.; Zhang, G. New insights into the inhibition mechanism of betulinic acid on α-glucosidase. J. Agric. Food Chem. 2018, 66, 7065–7075. [Google Scholar] [CrossRef]
- Shah, M.A.; Khalil, R.; Ul-Haq, Z.; Panichayupakaranant, P. α-Glucosidase inhibitory effect of rhinacanthins-rich extract from Rhinacanthus nasutus leaf and synergistic effect in combination with acarbose. J. Funct. Foods 2017, 36, 325–331. [Google Scholar] [CrossRef]
- Ali-Seyed, M.; Jantan, I.; Vijayaraghavan, K.; Bukhari, S.N. Betulinic Acid: Recent Advances in Chemical Modifications, Effective Delivery, and Molecular Mechanisms of a Promising Anticancer Therapy. Chem. Biol. Drug Des. 2016, 87, 517–536. [Google Scholar] [CrossRef]
- Luis Rios, J.; Manez, S. New pharmacological opportunities for betulinic acid. Planta Med. 2018, 84, 8–19. [Google Scholar] [CrossRef]
- Melnikova, N.; Burlova, I.; Kiseleva, T.; Klabukova, I.; Gulenova, M.; Kislitsin, C.A.; Vasin, V.; Tanaseichuk, B. A practical synthesis of betulonic acid using selective oxidation of betulin on aluminium solid support. Molecules 2012, 17, 11849–11863. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Zhu, L.; Ou, Z.; Ma, C.; Kong, L.; Huang, Y.; Chen, Y.; Zhao, H.; Wen, L.; Wu, J.; et al. Betulinic acid protects against renal damage by attenuation of oxidative stress and inflammation via Nrf2 signaling pathway in T-2 toxin-induced mice. Int. Immunopharmacol. 2021, 101, 108210. [Google Scholar] [CrossRef] [PubMed]
- Harwansh, R.K.; Mukherjee, P.K.; Biswas, S. Nanoemulsion as a novel carrier system for improvement of betulinic acid oral bioavailability and hepatoprotective activity. J. Mol. Liq. 2017, 237, 361–371. [Google Scholar] [CrossRef]
- Serain, A.F.; Morosi, L.; Ceruti, T.; Matteo, C.; Meroni, M.; Minatel, E.; Zucchetti, M.; Salvador, M.J. Betulinic acid and its spray dried microparticle formulation: In vitro PDT effect against ovarian carcinoma cell line and in vivo plasma and tumor disposition. J. Photochem. Photobiol. B-Biol. 2021, 224, 112328. [Google Scholar] [CrossRef] [PubMed]
- Zeng, A.; Hua, H.; Liu, L.; Zhao, J. Betulinic acid induces apoptosis and inhibits metastasis of human colorectal cancer cells in vitro and in vivo. Bioorg. Med. Chem. 2019, 27, 2546–2552. [Google Scholar] [CrossRef] [PubMed]
- Liao, L.; Liu, C.; Xie, X.; Zhou, J. Betulinic acid induces apoptosis and impairs migration and invasion in a mouse model of ovarian cancer. J. Food Biochem. 2020, 44, e13278. [Google Scholar] [CrossRef]
- Kim, S.J.; Quan, H.Y.; Jeong, K.J.; Kim, D.Y.; Kim, G.W.; Jo, H.K.; Chung, S.H. Beneficial effect of betulinic acid on hyperglycemia via suppression of hepatic glucose production. J. Agric. Food Chem. 2014, 62, 434–442. [Google Scholar] [CrossRef]
- Ou, Z.; Zhao, J.; Zhu, L.; Huang, L.; Ma, Y.; Ma, C.; Luo, C.; Zhu, Z.; Yuan, Z.; Wu, J.; et al. Anti-inflammatory effect and potential mechanism of betulinic acid on lambda-carrageenan-induced paw edema in mice. Biomed. Pharmacother. 2019, 118, 109347. [Google Scholar] [CrossRef]
- Gautam, R.; Jachak, S.M. Recent developments in anti-inflammatory natural products. Med. Res. Rev. 2009, 29, 767–820. [Google Scholar] [CrossRef]
- Kim, J.; Lee, Y.S.; Kim, C.S.; Kim, J.S. Betulinic acid has an inhibitory effect on pancreatic lipase and induces adipocyte lipolysis. Phytother. Res. 2012, 26, 1103–1106. [Google Scholar] [CrossRef]
- Silva, F.S.G.; Oliveira, P.J.; Duarte, M.F. Oleanolic, ursolic, and betulinic acids as food supplements or pharmaceutical agents for type 2 diabetes: Promise or illusion? J. Agric. Food Chem. 2016, 64, 2991–3008. [Google Scholar] [CrossRef] [PubMed]
- Vinayagam, R.; Xiao, J.; Xu, B. An insight into anti-diabetic properties of dietary phytochemicals. Phytochem. Rev. 2017, 16, 535–553. [Google Scholar] [CrossRef]
- Kumar, S.; Kumar, V.; Prakash, O. Enzymes inhibition and antidiabetic effect of isolated constituents from Dillenia indica. Biomed Res. Int. 2013, 2013, 382063. [Google Scholar] [CrossRef] [PubMed]
- de Melo, C.L.; Queiroz, M.G.R.; Arruda Filho, A.C.V.; Rodrigues, A.M.; de Sousa, D.F.; Almeida, J.G.L.; Pessoa, O.D.L.; Silveira, E.R.; Menezes, D.B.; Melo, T.S.; et al. Betulinic acid, a natural pentacyclic triterpenoid, prevents abdominal fat accumulation in mice fed a high-fat diet. J. Agric. Food Chem. 2009, 57, 8776–8781. [Google Scholar] [CrossRef]
- Gomes Castro, A.J.; Silva Frederico, M.J.; Cazarolli, L.H.; Bretanha, L.C.; Tavares, L.d.C.; Buss, Z.d.S.; Dutra, M.F.; Pacheco de Souza, A.Z.; Pizzolatti, M.G.; Mena Barreto Silva, F.R. Betulinic acid and 1,25(OH)2 vitamin D3 share intracellular signal transduction in glucose homeostasis in soleus muscle. Int. J. Biochem. Cell Biol. 2014, 48, 18–27. [Google Scholar] [CrossRef]
- Wen, X.; Sun, H.; Liu, J.; Cheng, K.; Zhang, P.; Zhang, L.; Hao, J.; Zhang, L.; Ni, P.; Zographos, S.E.; et al. Naturally occurring pentacyclic triterpenes as inhibitors of glycogen phosphorylase: Synthesis, structure-activity relationships, and X-ray crystallographic studies. J. Med. Chem. 2008, 51, 3540–3554. [Google Scholar] [CrossRef]
- Ha, D.T.; Dao Trong, T.; Nguyen Bich, T.; Nhiem, N.X.; Ngoc, T.M.; Yim, N.; Bae, K. Palbinone and triterpenes from Moutan Cortex (Paeonia suffruticosa, Paeoniaceae) stimulate glucose uptake and glycogen synthesis via activation of AMPK in insulin-resistant human HepG2 Cells. Bioorg. Med. Chem. Lett. 2009, 19, 5556–5559. [Google Scholar] [CrossRef]
- Heiss, E.H.; Kramer, M.P.; Atanasov, A.G.; Beres, H.; Schachner, D.; Dirsch, V.M. Glycolytic switch in response to betulinic acid in non-cancer cells. PLoS ONE 2014, 9, e115683. [Google Scholar] [CrossRef]
- Choi, C.I.; Lee, S.R.; Kim, K.H. Antioxidant and α-glucosidase inhibitory activities of constituents from Euonymus alatus twigs. Ind. Crops Prod. 2015, 76, 1055–1060. [Google Scholar] [CrossRef]
- Tri, M.D.; Phat, N.T.; Trung, N.T.; Phan, C.T.D.; Minh, P.N.; Chi, M.T.; Nguyen, T.P.; Dang, C.H.; Hong Truong, L.; Pham, N.K.T.; et al. A new 26-norlanostane from Phlogacanthus turgidus growing in Vietnam. J. Asian Nat. Prod. Res. 2021, 24, 196–202. [Google Scholar] [CrossRef]
- Thengyai, S.; Thiantongin, P.; Sontimuang, C.; Ovatlarnporn, C.; Puttarak, P. α-Glucosidase and α-amylase inhibitory activities of medicinal plants in Thai antidiabetic recipes and bioactive compounds from Vitex glabrata R. Br. stem bark. J. Herb. Med. 2020, 19, 100302. [Google Scholar] [CrossRef]
- Chukwujekwu, J.C.; Rengasamy, K.R.; de Kock, C.A.; Smith, P.J.; Slavetinska, L.P.; van Staden, J. α-glucosidase inhibitory and antiplasmodial properties of terpenoids from the leaves of Buddleja saligna Willd. J. Enzym. Inhib. Med. Chem. 2016, 31, 63–66. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kazakova, O.B.; Giniyatullina, G.V.; Mustafin, A.G.; Babkov, D.A.; Sokolova, E.V.; Spasov, A.A. Evaluation of Cytotoxicity and α-Glucosidase Inhibitory Activity of Amide and Polyamino-Derivatives of Lupane Triterpenoids. Molecules 2020, 25, 4833. [Google Scholar] [CrossRef] [PubMed]
- Khusnutdinova, E.F.; Petrova, A.V.; Thu, H.N.T.; Tu, A.L.T.; Thanh, T.N.; Thi, C.B.; Babkov, D.A.; Kazakova, O.B. Structural modifications of 2,3-indolobetulinic acid: Design and synthesis of highly potent α-glucosidase inhibitors. Bioorg. Chem. 2019, 88, 102957. [Google Scholar] [CrossRef]
- Cardullo, N.; Floresta, G.; Rescifina, A.; Muccilli, V.; Tringali, C. Synthesis and in vitro evaluation of chlorogenic acid amides as potential hypoglycemic agents and their synergistic effect with acarbose. Bioorg. Chem. 2021, 117, 105458. [Google Scholar] [CrossRef]
- Zhang, B.; Xing, Y.; Wen, C.; Yu, X.; Sun, W.; Xiu, Z.; Dong, Y. Pentacyclic triterpenes as α-glucosidase and α-amylase inhibitors: Structure-activity relationships and the synergism with acarbose. Bioorg. Med. Chem. Lett. 2017, 27, 5065–5070. [Google Scholar] [CrossRef]
- Yang, J.; Wang, X.; Zhang, C.; Ma, L.; Wei, T.; Zhao, Y.; Peng, X. Comparative study of inhibition mechanisms of structurally different flavonoid compounds on α-glucosidase and synergistic effect with acarbose. Food Chem. 2021, 347, 129056. [Google Scholar] [CrossRef]
- Mabate, B.; Daub, C.D.; Malgas, S.; Edkins, A.L.; Pletschke, B.I. A Combination Approach in Inhibiting Type 2 Diabetes-Related Enzymes Using Ecklonia radiata Fucoidan and Acarbose. Pharmaceutics 2021, 13, 1979. [Google Scholar] [CrossRef]
- Martin, A.; Montgomery, P. Acarbose: An α-glucosidase inhibitor. Am. J. Health-Syst. Pharm. 1996, 53, 2277–2290. [Google Scholar] [CrossRef]
- Oboh, M.; Govender, L.; Siwela, M.; Mkhwanazi, B.N. Anti-Diabetic Potential of Plant-Based Pentacyclic Triterpene Derivatives: Progress Made to Improve Efficacy and Bioavailability. Molecules 2021, 26, 7243. [Google Scholar] [CrossRef]
- Kan, L.; Capuano, E.; Fogliano, V.; Verkerk, R.; Mes, J.J.; Tomassen, M.M.M.; Oliviero, T. Inhibition of α-glucosidases by tea polyphenols in rat intestinal extract and Caco-2 cells grown on Transwell. Food Chem. 2021, 361, 130047. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Qi, Q.; Wang, C.; Qian, Y.; Liu, G.; Wang, Y.; Fu, L. Surface plasmon resonance (SPR) biosensors for food allergen detection in food matrices. Biosens. Bioelectron. 2019, 142, 111449. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Xu, H.; Wang, M.; Zhan, R.; Chen, W.; Zhang, R.; Kuang, Z.; Zhang, F.; Wang, K.; Gu, J. Molecular docking and molecular dynamics studies on selective synthesis of α-Amyrin and β-Amyrin by oxidosqualene cyclases from Ilex asprella. Int. J. Mol. Sci. 2019, 20, 3469. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, P.; Yang, Z.; Du, F.; Li, Z.; Wu, C.; Fang, A.; Xu, X.; Zhou, G. Molecular dynamics simulation exploration of the interaction between curcumin and myosin combined with the results of spectroscopy techniques. Food Hydrocoll. 2020, 101, 105455. [Google Scholar] [CrossRef]
- Ni, M.; Hu, X.; Gong, D.; Zhang, G. Inhibitory mechanism of vitexin on α-glucosidase and its synergy with acarbose. Food Hydrocoll. 2020, 105, 105824. [Google Scholar] [CrossRef]
- Chen, S.D.; Yong, T.Q.; Xiao, C.; Su, J.Y.; Zhang, Y.F.; Jiao, C.W.; Xie, Y.Z. Pyrrole alkaloids and ergosterols from Grifola frondosa exert anti-α-glucosidase and anti-proliferative activities. J. Funct. Foods 2018, 43, 196–205. [Google Scholar] [CrossRef]
- Chen, S.D.; Yong, T.Q.; Xiao, C.; Gao, X.; Xie, Y.Z.; Hu, H.P.; Li, X.M.; Chen, D.L.; Pan, H.H.; Wu, Q.P. Inhibitory effect of triterpenoids from the mushroom Inonotus obliquus against α-glucosidase and their interaction: Inhibition kinetics and molecular stimulations. Bioorg. Chem. 2021, 115, 105276. [Google Scholar] [CrossRef] [PubMed]
- Cai, S.; Wang, O.; Wang, M.; He, J.; Wang, Y.; Zhang, D.; Zhou, F.; Ji, B. In vitro inhibitory effect on pancreatic lipase activity of subfractions from ethanol extracts of fermented Oats (Avena sativa L.) and synergistic effect of three phenolic acids. J. Agric. Food Chem. 2012, 60, 7245–7251. [Google Scholar] [CrossRef]
- Zhu, B.; Li, M.Y.; Lin, Q.; Liang, Z.; Xin, Q.; Wang, M.; He, Z.; Wang, X.; Wu, X.; Chen, G.G.; et al. Lipid oversupply induces CD36 sarcolemmal translocation via dual modulation of PKC zeta and TB1CD1: An early event prior to insulin resistance. Theranostics 2020, 10, 1332–1354. [Google Scholar] [CrossRef]
| Acarbose-600 μM | Acarbose-800 μM | Acarbose-1000 μM | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Value | Interaction | Value | Interaction | Value | Interaction | ||||
| Vab | Vc | Vab − Vc | Vab | Vc | Vab − Vc | Vab | Vc | Vab − Vc | |
| BA-5 μM | 0.47 | 0.59 | −0.12SY | 0.42 | 0.56 | −0.15SY | 0.41 | 0.53 | −0.13SY |
| BA-10 μM | 0.39 | 0.57 | −0.18SY | 0.36 | 0.54 | −0.18SY | 0.34 | 0.51 | −0.17SY |
| BA-15 μM | 0.32 | 0.50 | −0.18SY | 0.29 | 0.48 | −0.18SY | 0.28 | 0.45 | −0.16SY |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, S.; Lin, B.; Gu, J.; Yong, T.; Gao, X.; Xie, Y.; Xiao, C.; Zhan, J.Y.; Wu, Q. Binding Interaction of Betulinic Acid to α-Glucosidase and Its Alleviation on Postprandial Hyperglycemia. Molecules 2022, 27, 2517. https://doi.org/10.3390/molecules27082517
Chen S, Lin B, Gu J, Yong T, Gao X, Xie Y, Xiao C, Zhan JY, Wu Q. Binding Interaction of Betulinic Acid to α-Glucosidase and Its Alleviation on Postprandial Hyperglycemia. Molecules. 2022; 27(8):2517. https://doi.org/10.3390/molecules27082517
Chicago/Turabian StyleChen, Shaodan, Bing Lin, Jiangyong Gu, Tianqiao Yong, Xiong Gao, Yizhen Xie, Chun Xiao, Janis Yaxian Zhan, and Qingping Wu. 2022. "Binding Interaction of Betulinic Acid to α-Glucosidase and Its Alleviation on Postprandial Hyperglycemia" Molecules 27, no. 8: 2517. https://doi.org/10.3390/molecules27082517
APA StyleChen, S., Lin, B., Gu, J., Yong, T., Gao, X., Xie, Y., Xiao, C., Zhan, J. Y., & Wu, Q. (2022). Binding Interaction of Betulinic Acid to α-Glucosidase and Its Alleviation on Postprandial Hyperglycemia. Molecules, 27(8), 2517. https://doi.org/10.3390/molecules27082517






