In Vitro Cytotoxic Activity and Phytochemical Characterization (UPLC/T-TOF-MS/MS) of the Watermelon (Citrullus lanatus) Rind Extract
Abstract
:1. Introduction
2. Results and Discussion
2.1. Anticancer Activity Assays
2.1.1. Cytotoxic Screening of Watermelon Rind Extracts
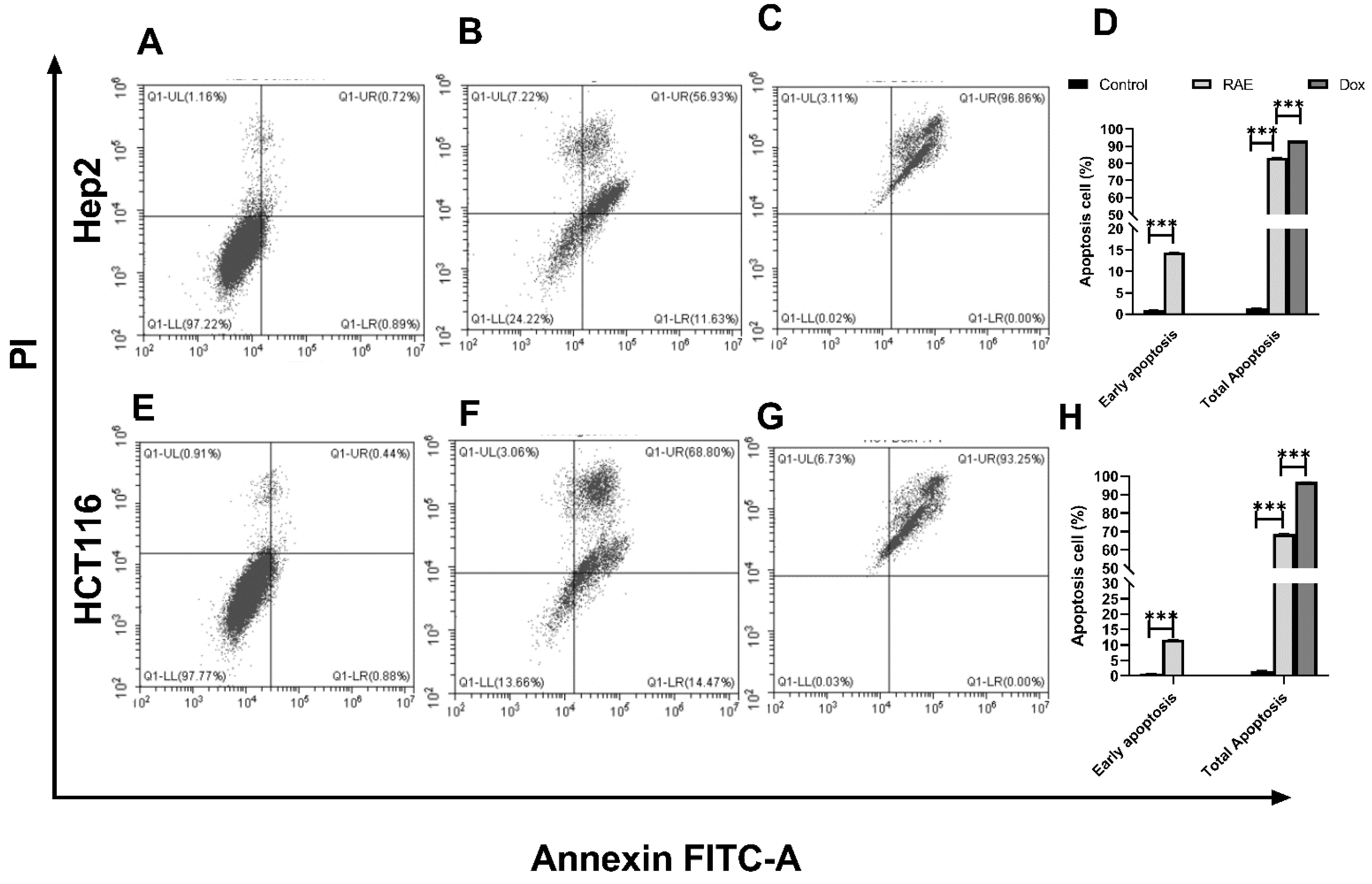
2.1.2. Annexin V-FITC/PI for Apoptosis Detection Using Flow Cytometry
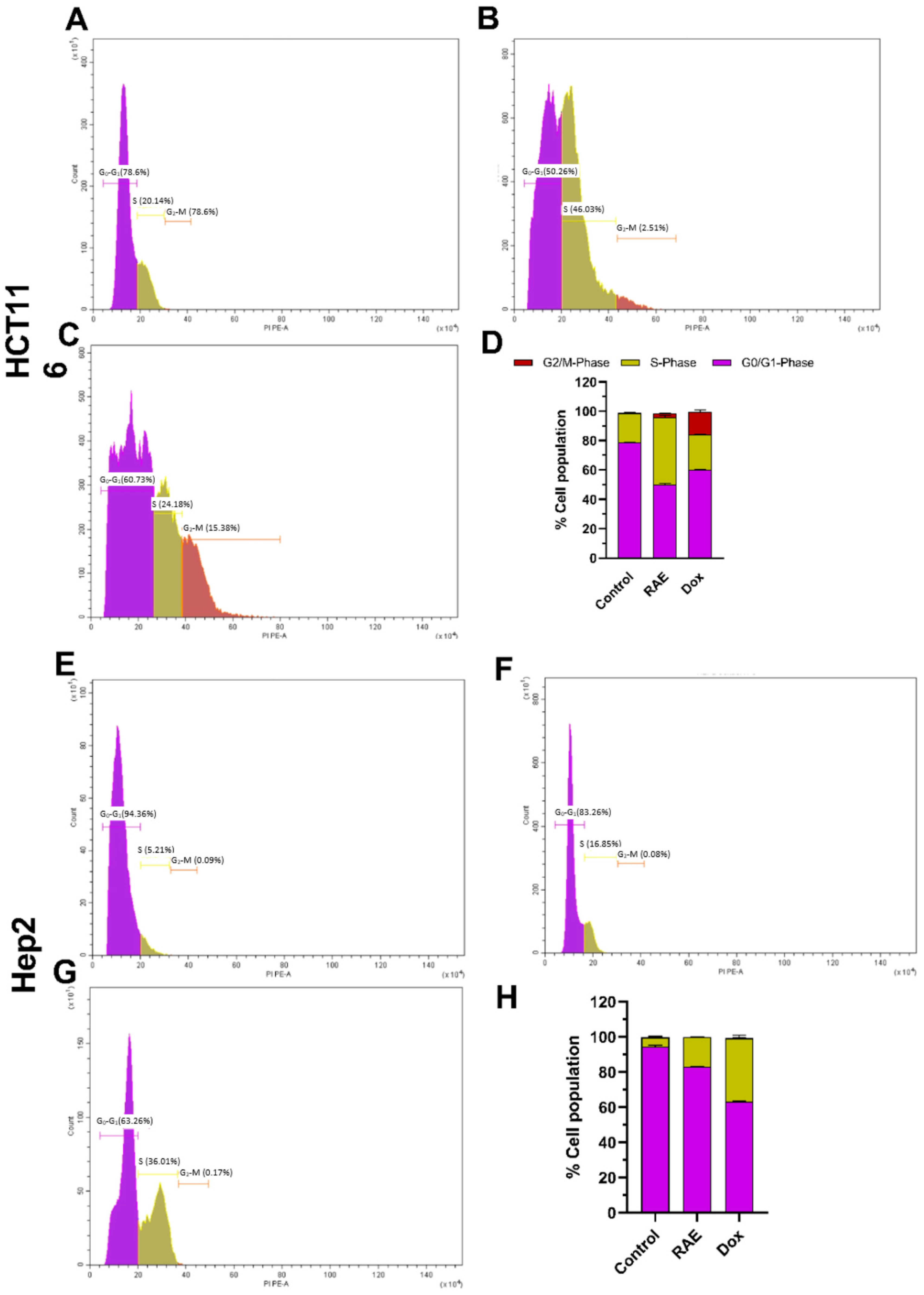
2.1.3. Cell Cycle Analysis
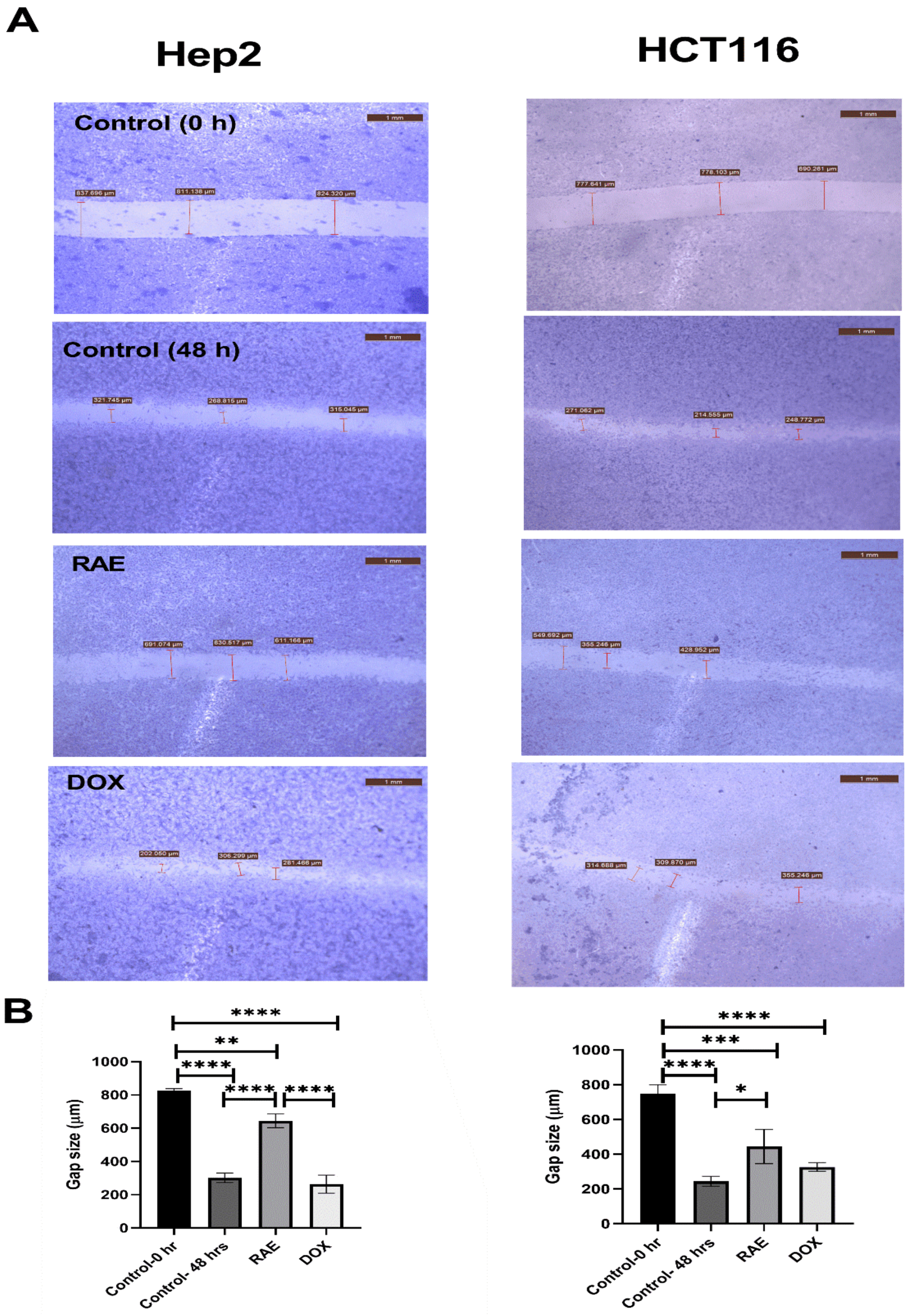
2.1.4. Wound Healing Assay
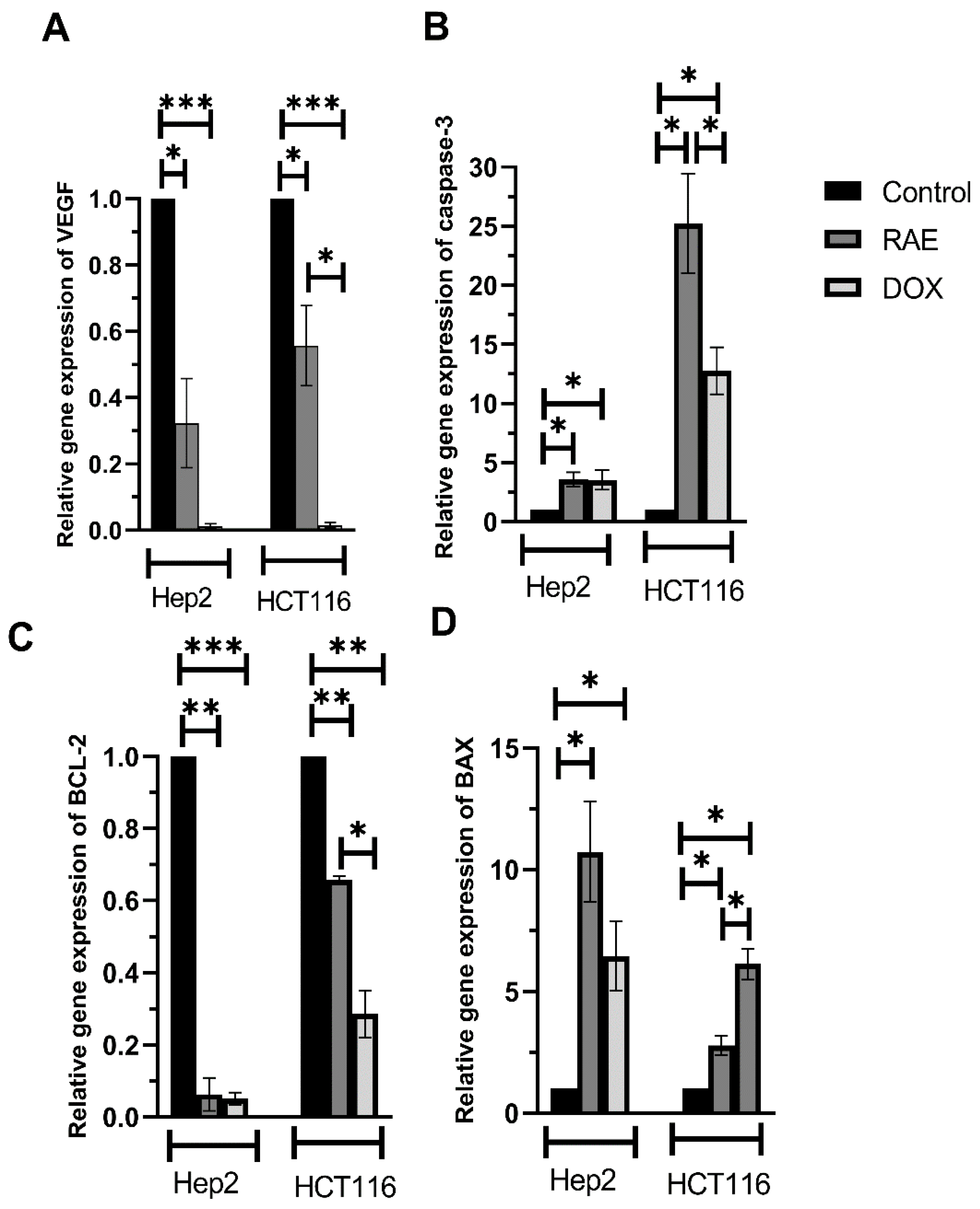
2.1.5. VEGF, Caspase-3, BAX, and BCL-2 mRNA Expression Levels
2.2. Antimicrobial Activity Assays
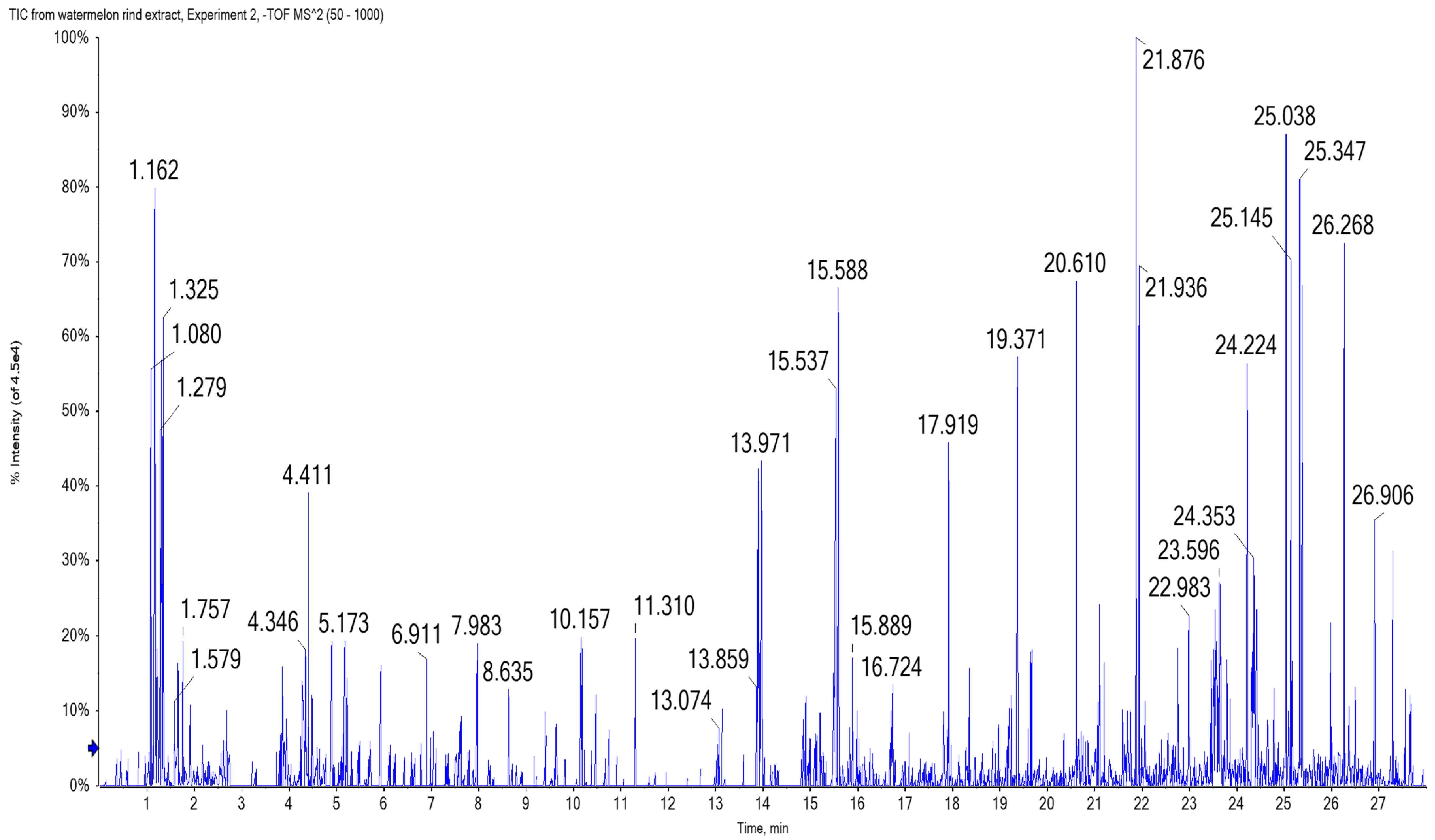
2.3. Chemical Characterization of Watermelon Rind Extract Using UPLC/T-TOF-MS/MS
- Hydroxybenzoic acid derivatives
- Hydroxycinnamic acid derivatives
- Flavonoid derivatives
- Amino and organic acid derivatives
- Miscellaneous metabolites
| Proposed Compounds | Formula | Rt | [M-H]− m/z | Ref. Mass | Diff. (ppm) | Ms2 (Characteristic Fragments) | Ref. | |
|---|---|---|---|---|---|---|---|---|
| Amino acid derivatives | ||||||||
| 1 | Oxoproline | C5H6NO3 | 1.19 | 128.0341 | 128.0342 | −0.8 | 84.0190 [M-H-CO2]− | -- |
| 2 | Citrulline-O-hexoside | C12H22N3O8 | 1.23 | 336.1411 | 336.1401 | 2.8 | 174.0882 [M-H-hexose]−, 131.0823 | -- |
| 3 | Citrulline | C6H12N3O3 | 1.28 | 174.0874 | 174.0873 | 0.8 | 131.0822, 113.0711, 70.0651 | [18] |
| 4 | 3,4-Dihydroxy-L-phenylalanine (DOPA) | C9H11NO4 | 1.45 | 196.0714 | 196.0615 | 2.9 | 160.8441, 151.0531 [M-H-COOH]−, 67.03075 | -- |
| 5 | Arginine | C6H13N4O2 | 1.72 | 173.1049 | 173.1033 | 9.2 | 131.0827, 89.0190 | [44] |
| 6 | Phenylalanine | C9H10NO2 | 2.28 | 164.0704 | 164.0706 | −1.3 | 147.0470 [M-H-OH]−, 119.0494 [M-H-COOH]−, 103.0607 | [43] |
| Organic acid derivatives | ||||||||
| 7 | Citric acid / Isocitrate | C6H7O7 | 1.08 | 191.0195 | 191.0186 | 4.3 | 173.0114 [M-H-H2O]−, 129.0236 [M-H-H2O-CO2]−, 111.0089 | [43] |
| 8 | Malic acid | C4H5O5 | 1.08 | 133.0136 | 133.0132 | 3.3 | 115.0030 [M-H-H2O]−, 71.0140 [M-H-H2O-CO2]− | [43] |
| 9 | Tartaric acid | C4H5O6 | 1.13 | 149.0092 | 149.0081 | 7.3 | 105.0021 [M-H-CO2]− | -- |
| 10 | Shikimic acid | C7H10O5 | 1.17 | 173.0115 | 173.0455 | −11.8 | 155.0439, 136.9845, 129.0206 [M-H-CO2]− | |
| 11 | Maleic acid | C4H3O4 | 1.19 | 115.0015 | 115.0026 | −9.2 | 71.0121 [M-H-CO2]− | -- |
| 12 | Citramalic acid / Citramalate | C5H7O5 | 1.33 | 147.0291 | 147.0288 | 2.0 | 129.0176 [M-H-H2O]−, 103.0401 [M-H-CO2]−, 87.0087, 61.9883 | -- |
| 13 | Citraconic acid | C5H6O4 | 1.70 | 128.9589 | 129.0193 | 2.1 | 101.0132, 84.9877 [M-H-CO2]−, 55.0225 | -- |
| Sugar derivatives | ||||||||
| 14 | Mucate (Galactarate) | C6H9O8 | 1.05 | 209.0289 | 209.0292 | −1.4 | 191.0227 [M-H-H2O]−, 165.3369 [M-H-CO2]−, 147.0334 [M-H-H2O-CO2]−, 133.0136 | -- |
| 15 | Gluconic acid | C6H11O7 | 1.14 | 195.0503 | 195.0499 | 2.0 | 129.0198, 75.0085 | -- |
| 16 | Tagatose | C6H11O6 | 1.37 | 179.0553 | 179.0550 | 1.3 | 89.0244, 71.0155, 59.0144 | -- |
| 17 | Trehalose | C12H22O11 | 1.44 | 341.1078 | 341.1089 | 0.4 | 305.0897, 179.0544, 163.0495, 129.0205 | -- |
| 18 | Ribitol (xylitol) | C5H11O5 | 1.53 | 151.0607 | 151.0601 | 3.7 | 101.0217, 89.0241, 71.0148 | -- |
| 19 | Iditol | C6H13O6 | 1.53 | 181.0725 | 181.0707 | 10.1 | 163.0557 [M-H-H2O]−, 101.0251, 96.9682 | -- |
| Hydroxybenzoic acid derivatives | ||||||||
| 20 | Phloroglucinol glycuronide | C12H13O9 | 1.23 | 301.1045 | 301.0565 | 1.2 | 175.0781 [M-H-phloroglucinol]−, 125.0360 [M-H-glycuronic acid]− | [36] |
| 21 | Salicin benzoate | C20H21O8 | 1.41 | 389.1216 | 389.1231 | −3.8 | 343.1081 | [36] |
| 22 | 3-Phenyllactic acid | C9H10O3 | 1.23 | 165.0391 | 165.0557 | 5.1 | 147.0340, 129.0155, 89.0291, 72.9876 | -- |
| 23 | Salicylic acid | C7H5O3 | 3.30 | 137.0237 | 137.0233 | 2.8 | 93.0346 [M-H-CO2]− | [37] |
| Hydroxycinnamic acid derivatives | ||||||||
| 24 | O-Feruloylquinide | C17H17O8 | 1.28 | 349.0899 | 349.0918 | −5.5 | 193.0502 [ferulic acid]− | [38] |
| 25 | Caffoyl hexoside | C15H18O9 | 1.23 | 341.0731 | 341.0878 | −1.1 | 179.0557 [M-H-hexose]− | [38] [37] |
| 26 | Sinapic acid hexoside (isomer 1) | C17H21O10 | 1.86 | 385.1158 | 385.1129 | 7.4 | 223.0546 [M-H-hexose]− | [37] |
| 27 | Sinapic acid hexoside (isomer 2) | C17H21O10 | 1.97 | 385.1339 | 385.1129 | 5.0 | 223.0631 [M-H-hexose]− | [37] |
| 28 | O-feruloyl-pentosyl-deoxyhexose (isomer 1) | C21H27O12 | 4.65 | 471.1469 | 471.1497 | 6.7 | 193.0516 [M-H-sugar]− | [39] |
| 29 | O-feruloyl- pentosyl-deoxyhexose (isomer 2) | C21H27O12 | 4.78 | 471.1467 | 471.1497 | −6.0 | 193.0555 [M-H-sugar]− | [39] |
| 30 | Decaffeoyl acetoside/ Descaffeoyl verbascoside | C20H29O12 | 5.47 | 461.1656 | 461.1654 | 0.5 | 309.1171 [M-H-hydroxytyrose]−, 147.0672 [M-H-hydroxytyrose- hexose]− | [36] |
| 31 | Ferulic acid | C10H9O4 | 8.91 | 193.0499 | 193.0495 | 2.0 | 178.0285, 134.0360 | [40] |
| 32 | Chlorogenic acid | C16H18O9 | 15.55 | 353.1937 | 353.0878 | 5.6 | 353.1898, 352.0573 | -- |
| 33 | p-Coumaric acid hexoside | C15H18O8 | 15.89 | 325.1853 | 325.1753 | −5.4 | 325.1642, 325.1770 | -- |
| Flavonoid derivatives | ||||||||
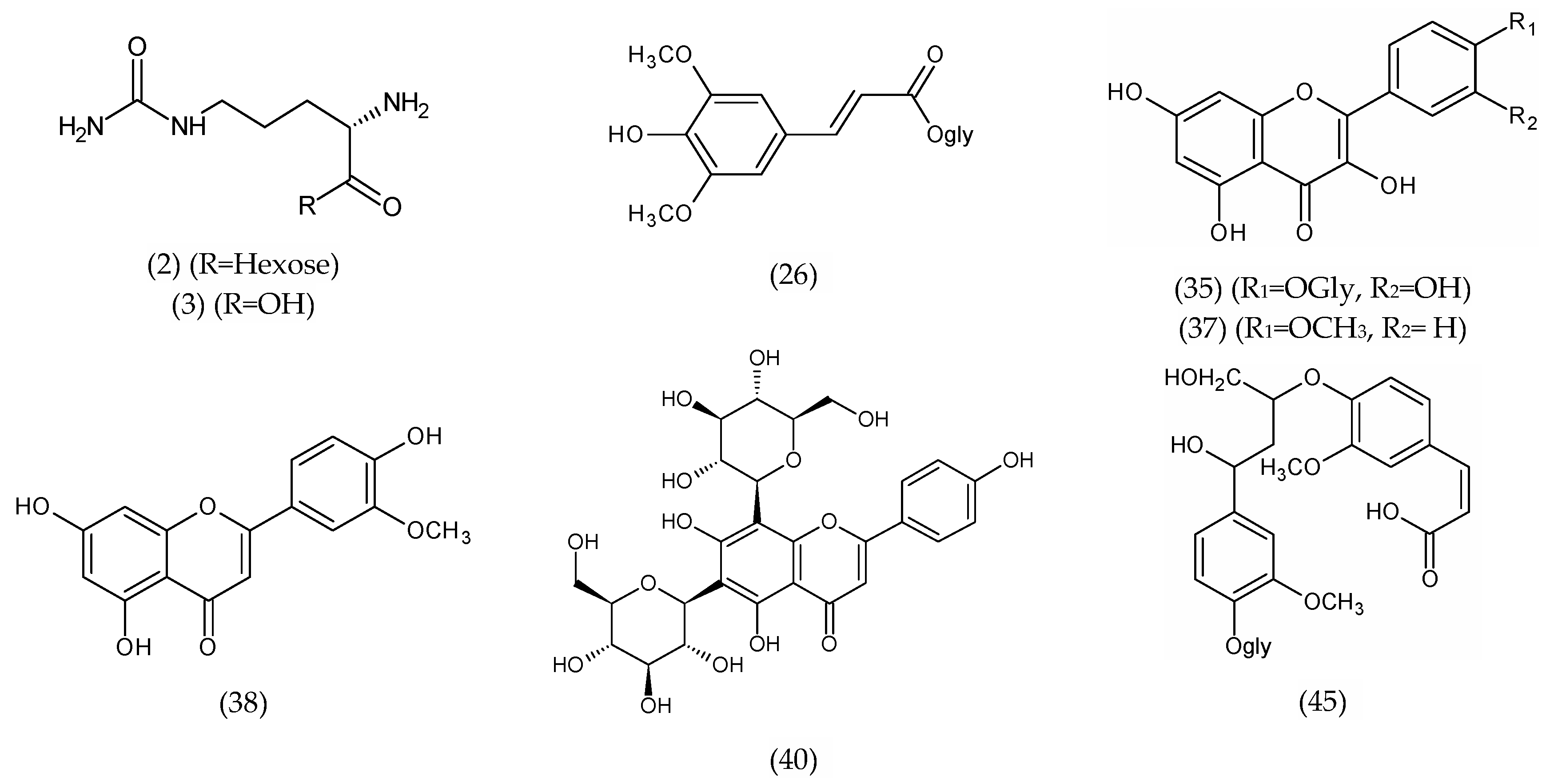
| 34 | Baicalein-O-glycuronide | C21H18O11 | 1.42 | 445.0681 | 445.0776 | 3.5 | 445.1562 | -- |
| 35 | Quercetin hexoside | C21H20O12 | 1.47 | 463.0403 | 463.0882 | 0.1 | 301.1014 [M-H-hexose]−, 61.9916 | -- |
| 36 | Quercetin rhamnoside (Quercitrin) | C21H19O11 | 7.50 | 447.0911 | 447.0922 | −2.5 | 301.0372 [M-H-rhamnose]−, 174.9577 | [37] |
| 37 | Trihydroxy-methoxyflavone Kaempferide (isomer 1) | C16H11O6 | 15.67 | 299.0511 | 299.0550 | 8.2 | 284.0304 | -- |
| 38 | Trihydroxy-methoxyflavone (Chrysoeriol) (isomer 2) | C16H11O6 | 16.75 | 299.0564 | 299.0550 | 4.7 | 271.0251 | [36] |
| 39 | Trihydroxy-methoxyflavone (isomer 3) | C16H11O6 | 20.33 | 299.0547 | 299.0550 | −1.0 | 284.0340, 271.0251 | [36] |
| 40 | Apigenin-6,8-C-di-hexoside (Vicenin-2) (isomer 1) | C27H29O15 | 21.02 | 593.1573 | 593.1501 | 7.0 | -- | [37] |
| 41 | Apigenin-6,8-C-di-hexoside (Vicenin-2) (isomer 1) | C27H29O15 | 21.11 | 593.1505 | 593.1501 | 0.7 | -- | [37] |
| 42 | Kaempferol-7-neohesperidoside | C27H29O15 | 21.12 | 593.1442 | 593.1501 | −5.9 | -- | -- |
| Miscellaneous metabolites | ||||||||
| 43 | Esculetin hexoside | C15H16O9 | 1.36 | 339.0974 | 339.0721 | −2.2 | 303.1098, 296.0112, 213.0935, 134.0744 | -- |
| 44 | Leachianol G | C28H23O7 | 4.78 | 471.1469 | 471.1438 | 6.4 | 355.1081, 193.0555 | [36] |
| 45 | Glehlinoside C | C26H31O13 | 22.62 | 551.1824 | 551.1759 | 1.7 | -- | [36] |
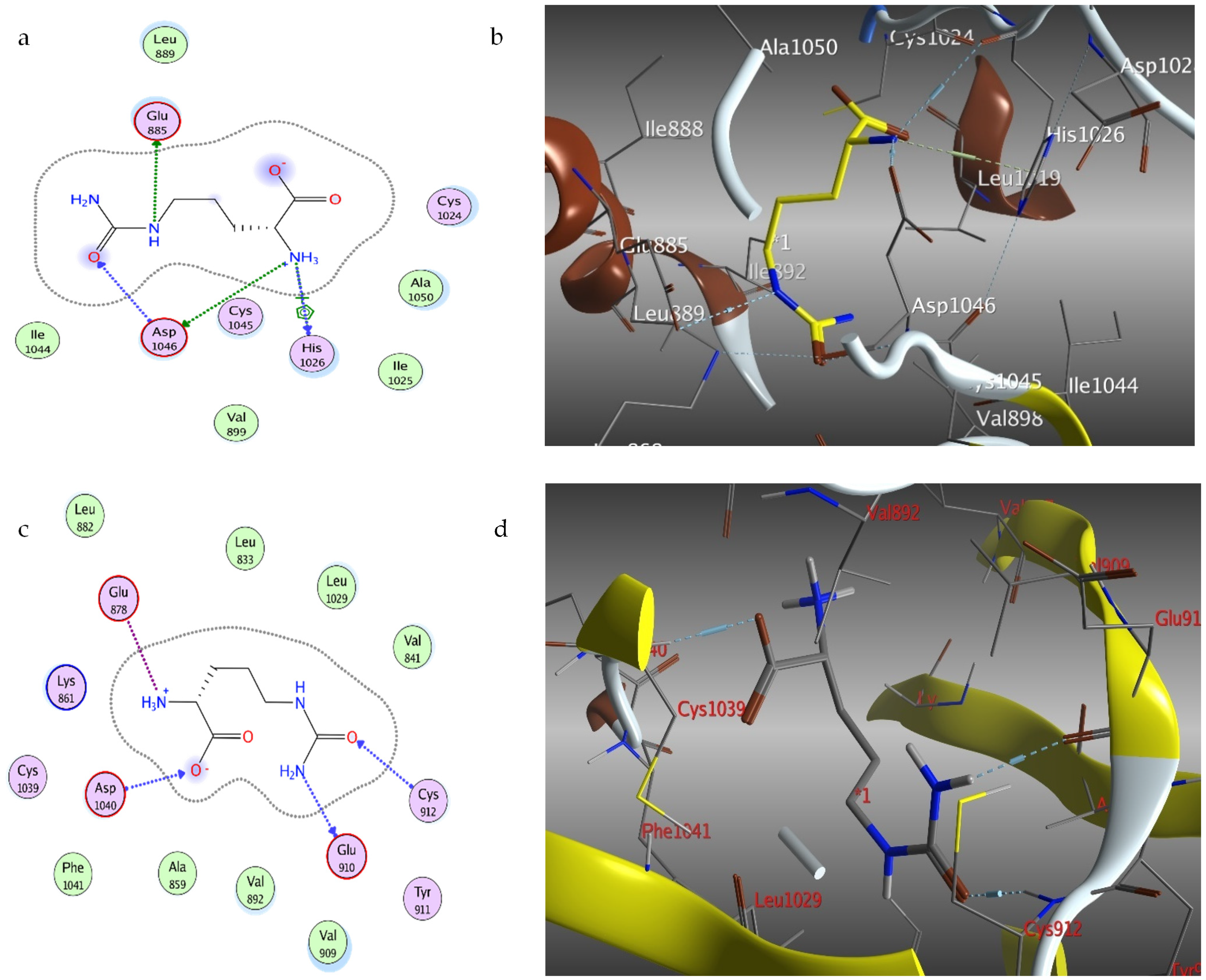
2.4. Docking of Citrulline on Caspase-3 and VEGF Kinase Proteins
3. Materials and Methods
3.1. Chemicals
3.2. Plant Material and Extraction Process
3.3. Experimental Design
3.4. Anticancer Activity
3.4.1. Cell Lines
3.4.2. Cytotoxicity Assay
3.4.3. Wound Healing Assay
3.4.4. Annexin V-FITC/PI for Apoptosis Detection
3.4.5. Cell Cycle Analysis
3.4.6. VEGF, BAX, BCL-2, and Caspase-3 mRNA Expression Levels
3.5. Antimicrobial Activity
3.5.1. Agar Well-Diffusion Assay
3.5.2. Antifungal Activity
3.6. Total Phenolic and Flavonoid Contents
3.7. UPLC/T-TOF-MS/MS Analysis
3.8. Molecular Docking Study
3.9. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries|Enhanced Reader. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhang, H.; Chen, X. Drug resistance and combating drug resistance in cancer. Cancer Drug Resist. 2019, 2, 141–160. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nurgali, K.; Jagoe, R.T.; Abalo, R. Editorial: Adverse effects of cancer chemotherapy: Anything new to improve tolerance and reduce sequelae? Front. Pharmacol. 2018, 9, 245. [Google Scholar] [CrossRef] [PubMed]
- Demain, A.L.; Vaishnav, P. Natural products for cancer chemotherapy. Microb. Biotechnol. 2011, 4, 687. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mitra, S.; Dash, R. Natural Products for the Management and Prevention of Breast Cancer. Evidence-Based Complement. Altern. Med. 2018, 2018, 8324696. [Google Scholar] [CrossRef] [PubMed]
- Huang, M.; Lu, J.J.; Ding, J. Natural Products in Cancer Therapy: Past, Present and Future. Nat. Products Bioprospect. 2021, 11, 5–13. [Google Scholar] [CrossRef]
- Cragg, G.M.; Grothaus, P.G.; Newman, D.J. Impact of Natural Products on Developing New Anti-Cancer Agents. Chem. Rev. 2009, 109, 3012–3043. [Google Scholar] [CrossRef]
- Cranfill, R.; Vaughan, J.G.; Geissler, C.A. The New Oxford Book of Food Plants. Taxon 1998, 47, 225. [Google Scholar] [CrossRef]
- Egbuonu, A.C.C. Assessment of some Antinutrient Properties of the Watermelon (Citrullus lanatus) Rind and Seed. Res. J. Environ. Sci. 2015, 9, 225–232. [Google Scholar] [CrossRef] [Green Version]
- Rubatzky, V. World Vegetables: Principles, Production, and Nutritive Values, 2nd ed.; Chapman & Hall: New York, NY, USA, 1997; ISBN 9780412112218. [Google Scholar]
- Lin, C.S.K.; Pfaltzgraff, L.A.; Herrero-Davila, L.; Mubofu, E.B.; Abderrahim, S.; Clark, J.H.; Koutinas, A.A.; Kopsahelis, N.; Stamatelatou, K.; Dickson, F.; et al. Food waste as a valuable resource for the production of chemicals, materials and fuels. Current situation and global perspective. Energy Environ. Sci. 2013, 6, 426–464. [Google Scholar] [CrossRef]
- Nazeam, J.A.; AL-Shareef, W.A.; Helmy, M.W.; El-Haddad, A.E. Bioassay-guided isolation of potential bioactive constituents from pomegranate agrifood by-product. Food Chem. 2020, 326, 126993. [Google Scholar] [CrossRef] [PubMed]
- Pothitirat, W.; Chomnawang, M.T.; Supabphol, R.; Gritsanapan, W. Free radical scavenging and anti-acne activities of mangosteen fruit rind extracts prepared by different extraction methods. Pharm. Biol. 2010, 48, 182–186. [Google Scholar] [CrossRef] [PubMed]
- Naknaen, P.; Itthisoponkul, T.; Sondee, A.; Angsombat, N. Utilization of watermelon rind waste as a potential source of dietary fiber to improve health promoting properties and reduce glycemic index for cookie making. Food Sci. Biotechnol. 2016, 25, 415–424. [Google Scholar] [CrossRef] [PubMed]
- Al-Sayed, H.M.A.; Ahmed, A.R. Utilization of watermelon rinds and sharlyn melon peels as a natural source of dietary fiber and antioxidants in cake. Ann. Agric. Sci. 2013, 58, 83–95. [Google Scholar] [CrossRef] [Green Version]
- Mushtaq, M.; Sultana, B.; Bhatti, H.N.; Asghar, M. RSM based optimized enzyme-assisted extraction of antioxidant phenolics from underutilized watermelon (Citrullus lanatus Thunb.) rind. J. Food Sci. Technol. 2015, 52, 5048–5056. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zamuz, S.; Munekata, P.E.S.; Gullón, B.; Rocchetti, G.; Montesano, D.; Lorenzo, J.M. Citrullus lanatus as source of bioactive components: An up-to-date review. Trends Food Sci. Technol. 2021, 111, 208–222. [Google Scholar] [CrossRef]
- Rimando, A.M.; Perkins-Veazie, P.M. Determination of citrulline in watermelon rind. J. Chromatogr. A 2005, 1078, 196–200. [Google Scholar] [CrossRef]
- Gladvin, G.; Sudhaakr, G.; Swathi, V.; Santhisri, K.V. Mineral and Vitamin Compositions Contents in Watermelon Peel (Rind). Int. J. Curr. Microbiol. App. Sci. 2017, 5, 129–133. [Google Scholar]
- Abu-Hiamed, H.A.A. Chemical composition, flavonoids and β-sitosterol contents of pulp and rind of watermelon (Citrullus lanatus) fruit. Pakistan J. Nutr. 2017, 16, 502–507. [Google Scholar] [CrossRef] [Green Version]
- Patra, J.K.; Baek, K.H. Novel green synthesis of gold nanoparticles using Citrullus lanatus rind and investigation of proteasome inhibitory activity, antibacterial, and antioxidant potential. Int. J. Nanomed. 2015, 10, 7253–7264. [Google Scholar] [CrossRef] [Green Version]
- Manasanch, E.E.; Orlowski, R.Z. Proteasome inhibitors in cancer therapy. Nat. Rev. Clin. Oncol. 2017, 14, 417–433. [Google Scholar] [CrossRef] [PubMed]
- Boik, J. Natural Compounds in Cancer Therapy; Oregon Medical Press: Princeton, MN, USA, 2001; p. 521. [Google Scholar]
- Callahan, M.K.; Williamson, P.; Schlegel, R.A. Surface expression of phosphatidylserine on macrophages is required for phagocytosis of apoptotic thymocytes. Cell Death Differ. 2000, 7, 645–653. [Google Scholar] [CrossRef] [PubMed]
- Fesseha, M.; Hong, M.Y. Effects of Watermelon Consumption on Cellular Proliferation, and Apoptosis in Rat Colon (P05-019-19). Curr. Dev. Nutr. 2019, 3, nzz030. [Google Scholar] [CrossRef] [Green Version]
- Schwartz, G.K.; Shah, M.A. Targeting the cell cycle: A new approach to cancer therapy. J. Clin. Oncol. 2005, 23, 9408–9421. [Google Scholar] [CrossRef]
- Doan, P.; Musa, A.; Candeias, N.R.; Emmert-Streib, F.; Yli-Harja, O.; Kandhavelu, M. Alkylaminophenol induces G1/S phase cell cycle arrest in glioblastoma cells through p53 and cyclin-dependent kinase signaling pathway. Front. Pharmacol. 2019, 10, 330. [Google Scholar] [CrossRef]
- Guan, X. Cancer metastases: Challenges and opportunities. Acta Pharm. Sin. B 2015, 5, 402–418. [Google Scholar] [CrossRef] [Green Version]
- Dillekås, H.; Rogers, M.S.; Straume, O. Are 90% of deaths from cancer caused by metastases? Cancer Med. 2019, 8, 5574. [Google Scholar] [CrossRef] [Green Version]
- Chota, A.; George, B.P.; Abrahamse, H. Interactions of multidomain pro-apoptotic and anti-apoptotic proteins in cancer cell death. Oncotarget 2021, 12, 1615–1626. [Google Scholar] [CrossRef]
- Shibuya, M. Vascular Endothelial Growth Factor (VEGF) and Its Receptor (VEGFR) Signaling in Angiogenesis: A Crucial Target for Anti- and Pro-Angiogenic Therapies. Genes Cancer 2011, 2, 1097. [Google Scholar] [CrossRef]
- Gautam, K.A.; Singh, A.N.; Srivastav, A.N.; Sankhwar, S.N. Angiogenesis in prostate cancer and benign prostatic hyperplasia assessed by VEGF and CD-34 IHC: A comparative clinico-pathological study. Afr. J. Urol. 2018, 24, 98–103. [Google Scholar] [CrossRef]
- Ajani, R.; Olorunnisola, O. Citrullus lanatus Rind and Juice Extracts as Probable Therapeutics in the Prevention and Management of Induced Benign Prostatic Hyperplasia. Eur. J. Med. Plants 2016, 15, 1–11. [Google Scholar] [CrossRef]
- Augustia, V.A.S.; Oktaviani, I.I.; Setyati, W. Anthocyanin and flavonoid extracted from watermelon rind (Citrullus lanatus) with two different colors of watermelon flesh: Yellow and red. In Proceedings of the Materials Science Forum; Trans Tech Publications Ltd.: Bäch, Switzerland, 2020; Volume 998, pp. 261–265. [Google Scholar] [CrossRef]
- El-Haddad, A.E.; Saadeldeen, A.M.; El-Emam, S.Z. Anti-angiogenic Activity of Major Phenolics in Tamarind Assessed with Molecular Docking Study on VEGF Kinase Proteins. Pakistan J. Biol. Sci. 2019, 22, 502–509. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abu-Reidah, I.M.; Arráez-Román, D.; Segura-Carretero, A.; Fernández-Gutiérrez, A. Profiling of phenolic and other polar constituents from hydro-methanolic extract of watermelon (Citrullus lanatus) by means of accurate-mass spectrometry (HPLC-ESI-QTOF-MS). Food Res. Int. 2013, 51, 354–362. [Google Scholar] [CrossRef]
- Abu-Reidah, I.M.; Arráez-Román, D.; Quirantes-Piné, R.; Fernández-Arroyo, S.; Segura-Carretero, A.; Fernández-Gutiérrez, A. HPLC-ESI-Q-TOF-MS for a comprehensive characterization of bioactive phenolic compounds in cucumber whole fruit extract. Food Res. Int. 2012, 46, 108–117. [Google Scholar] [CrossRef]
- Bellés, J.M.; López-Gresa, M.P.; Fayos, J.; Pallás, V.; Rodrigo, I.; Conejero, V. Induction of cinnamate 4-hydroxylase and phenylpropanoids in virus-infected cucumber and melon plants. Plant. Sci. 2008, 174, 524–533. [Google Scholar] [CrossRef]
- Hosny, M.; Rosazza, J.P.N. Structures of ferulic acid glycoside esters in corn hulls. J. Nat. Prod. 1997, 60, 219–222. [Google Scholar] [CrossRef]
- Fadimu, G.J.; Ghafoor, K.; Babiker, E.E.; Al-Juhaimi, F.; Abdulraheem, R.A.; Adenekan, M.K. Ultrasound-assisted process for optimal recovery of phenolic compounds from watermelon (Citrullus lanatus) seed and peel. J. Food Meas. Charact. 2020, 14, 1784–1793. [Google Scholar] [CrossRef]
- Herrmann, K. Review on nonessential constituents of vegetables. I. Cumcumbers, melons, squashes and pumpkins, bell peppers, eggplants, peas, beans, and broad beans (author’s transl). Zeitschrift Leb. Und-Forsch 1977, 165, 87–98. [Google Scholar] [CrossRef]
- Fang, Y.-Z.; Yang, S.; Wu, G. Free radicals, antioxidants, and nutrition. Nutrition 2002, 18, 872–879. [Google Scholar] [CrossRef]
- Sulaiman, F.; Azam, A.A.; Bustamam, M.S.A.; Fakurazi, S.; Abas, F.; Lee, Y.X.; Ismail, A.A.; Faudzi, S.M.M.; Ismail, I.S. Metabolite profiles of red and yellow watermelon (Citrullus lanatus) cultivars using a 1H-NMR metabolomics approach. Molecules 2020, 25, 3235. [Google Scholar] [CrossRef]
- Jimoh, T.O.; Ademiluyi, A.O.; Oboh, G.; Boligon, A.A. Phenolic extracts and amino acids content from Cucumeropsis mannii naudin and Citrullus lanatus inhibit relevant enzymes of erectile dysfunction in rat’s penile tissue. Biochem. Biophys. Rep. 2017, 12, 5–11. [Google Scholar] [CrossRef] [PubMed]
- Boshra, S.A.; El-Haddad, A.E. The protective effects of MPLC isolated glycyrrhizin and mangiferin against brain toxicity in rats. Med. Chem Res. 2018, 27, 1449–1459. [Google Scholar] [CrossRef]
- Vichai, V.; Kirtikara, K. Sulforhodamine B colorimetric assay for cytotoxicity screening. Nat. Protoc. 2006, 1, 1112–1116. [Google Scholar] [CrossRef] [PubMed]
- Stamm, A.; Reimers, K.; Strauß, S.; Vogt, P.; Scheper, T.; Pepelanova, I. In vitro wound healing assays—State of the art. BioNanoMaterials 2016, 17, 79–87. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2−ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Howe, K.L.; Achuthan, P.; Allen, J.; Allen, J.; Alvarez-Jarreta, J.; Ridwan Amode, M.; Armean, I.M.; Azov, A.G.; Bennett, R.; Bhai, J.; et al. Ensembl 2021. Nucleic Acids Res. 2021, 49, D884–D891. [Google Scholar] [CrossRef]
- Valgas, C.; De Souza, S.M.; Smânia, E.F.A.; Smânia, A. Screening methods to determine antibacterial activity of natural products. Braz. J. Microbiol. 2007, 38, 369–380. [Google Scholar] [CrossRef] [Green Version]
- Magaldi, S.; Mata-Essayag, S.; Hartung De Capriles, C.; Perez, C.; Colella, M.T.; Olaizola, C.; Ontiveros, Y. Well diffusion for antifungal susceptibility testing. Int. J. Infect. Dis. 2004, 8, 39–45. [Google Scholar] [CrossRef] [Green Version]
- Attard, E. A rapid microtitre plate Folin-Ciocalteu method for the assessment of polyphenols. Open Life Sci. 2013, 8, 48–53. [Google Scholar] [CrossRef]
- Kiranmai, M.; Mahendra Kumar, C.B.; Ibrahim, M. Comparison of total flavanoid content of Azadirachta indica root bark extracts prepared by different methods of extraction. Res. J. Pharm. Biol. Chem. Sci. 2011, 2, 254–261. [Google Scholar]
- Eissa, M.A.; Hashim, Y.Z.H.Y.; El-Kersh, D.M.; Abd-Azziz, S.S.S.; Salleh, H.M.; Isa, M.L.M.; Warif, N.M.A. Metabolite profiling of Aquilaria malaccensis leaf extract using liquid chromatography-Q-TOF-mass spectrometry and investigation of its potential antilipoxygenase activity in-Vitro. Processes 2020, 8, 202. [Google Scholar] [CrossRef] [Green Version]

| Proteins | Energy Score (kcal/mol) | No. of Interactions | H-bonding Residues |
|---|---|---|---|
| VEGF proteins | |||
| 3HNG | −5.09 | 4 | GLU910, CYS912, ASP1040 |
| 2QU5 | −4.51 | 4 | GLU885, HIS1026 |
| 1YWN | −4.33 | 6 | ASP1044, GLU883, HOH123 |
| 2P2I | −4.79 | 6 | HIS1026, ASP1046, GLU885 |
| 3EWH | −4.19 | 4 | PHE1047, ASP1046 |
| Caspase-3 protein | |||
| IGFW | −4.24 | 8 | MET61, GLY122, ARG207 |
| Gene | Accession No. | Primer Sequence | Amplicon(bp) | Melting Temperature (°C) | Annealing Temperature (°C) |
|---|---|---|---|---|---|
| VEGF | NM_001025366.3 | F-5’-TCCTCACACCATTGAAACCA-3’ R-5’-GATCCTGCCCTGTCTCTCTG-3’ | 131 | 56.6 59.3 | 59.3 |
| Bax | NM_001291430.2 | F-5’-ATGGACGGGTCCGGGGAG-3’ R-5’-ATCCAGCCCAACAGCCGC-3’ | 256 | 65.6 62.8 | 62.8 |
| Bcl-2 | NM_000657.3 | F-5’-AAGCCGGCGACGACTTCT-3’ R-5’-GGTGCCGGTTCAGGTACTCA -3’ | 258 | 61.1 61.6 | 61.1 |
| Casp-3 | NM_001354783.2 | F-5′-TGGATTATCCTGAGATGGGTTT-3′ R-5′-TTGCTGCATCGACATCTGTA-3′ | 102 | 58 55.3 | 55.3 |
| GAPDH | NM_001357943.2 | F-5′-ACCCACTCCTCCACCTTTGA-3′ R-5′-CTGTTGCTGTAGCCAAATTCGT-3′ | 101 | 60.8 59.9 | 59.9 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
El Gizawy, H.A.; El-Haddad, A.E.; Attia, Y.M.; Fahim, S.A.; Zafer, M.M.; Saadeldeen, A.M. In Vitro Cytotoxic Activity and Phytochemical Characterization (UPLC/T-TOF-MS/MS) of the Watermelon (Citrullus lanatus) Rind Extract. Molecules 2022, 27, 2480. https://doi.org/10.3390/molecules27082480
El Gizawy HA, El-Haddad AE, Attia YM, Fahim SA, Zafer MM, Saadeldeen AM. In Vitro Cytotoxic Activity and Phytochemical Characterization (UPLC/T-TOF-MS/MS) of the Watermelon (Citrullus lanatus) Rind Extract. Molecules. 2022; 27(8):2480. https://doi.org/10.3390/molecules27082480
Chicago/Turabian StyleEl Gizawy, Heba A., Alaadin E. El-Haddad, Yasmin M. Attia, Sally A. Fahim, Mai M. Zafer, and Amr M. Saadeldeen. 2022. "In Vitro Cytotoxic Activity and Phytochemical Characterization (UPLC/T-TOF-MS/MS) of the Watermelon (Citrullus lanatus) Rind Extract" Molecules 27, no. 8: 2480. https://doi.org/10.3390/molecules27082480
APA StyleEl Gizawy, H. A., El-Haddad, A. E., Attia, Y. M., Fahim, S. A., Zafer, M. M., & Saadeldeen, A. M. (2022). In Vitro Cytotoxic Activity and Phytochemical Characterization (UPLC/T-TOF-MS/MS) of the Watermelon (Citrullus lanatus) Rind Extract. Molecules, 27(8), 2480. https://doi.org/10.3390/molecules27082480









