Abstract
In order to rapidly and precisely identify the volatile compounds in Chinese chive (Allium tuberosum Rottler), seven key parameters of headspace solid-phase micro-extraction conditions (HS-SPME) from Chinese chive were optimized. A total of 59 volatile compounds were identified by using the optimized method, including 28 ethers, 15 aldehydes, 6 alcohols, 5 ketones, 2 hydrocarbons, 1 ester, and 2 phenols. Ethers are the most abundant, especially dimethyl trisulfide (10,623.30 μg/kg). By calculating the odor activity values (OAVs), 11 volatile compounds were identified as the major aroma-active compounds of Chinese chive. From the analysis of the composition of Chinese chive aroma, the “garlic and onion” odor (OAV = 2361.09) showed an absolute predominance over the other 5 categories of aroma. The results of this study elucidated the main sources of Chinese chive aroma from a chemical point of view and provided the theoretical basis for improving the flavor quality of Chinese chive.
1. Introduction
Chinese chive (Allium tuberosum Rottler) is a perennial herb plant in the Allium and lily family. It originated in China but is commonly cultivated in Asia and a few areas in Europe. Allium vegetables are widely regarded as traditional medicines [1], which can be used to prevent and treat certain human diseases such as cancer [2], cardiovascular [3], inflammatory diseases [4], and the like. These special qualities of Allium vegetables are primarily due to the organic sulfur compounds. Chinese chive is a perennial plant, and it can be harvested many times in the course of a year. The leaves are the main edible part of Chinese chive in daily life, containing a lot of nutrients and active substances, such as protein, saccharides, vitamins, mineral substances, S-containing compounds, N-containing compounds, flavonoids, steroidal saponins, and vegetable oil [5,6,7,8,9]. In addition, flower sauce prepared from Chinese chive is also an indispensable condiment in Asian regions.
Chinese chive has a distinctive odor, which is released after Chinese chive is crushed or cut [10]. The reason for this phenomenon is that alliinase is normally separated with S-alk(en)yl cysteine sulphoxide (CSO), the reaction substrates of the reaction. When the cells are damaged, they undergo a series of reactions to produce this special odor [11,12]. According to previous studies, the main components of this odor are sulphur-containing compounds, which are the secondary metabolites of Chinese chive [9,13]. These sulphur-containing compounds are derived from the same precursor, but different reaction pathways cause diversities in their chemical structures. These diversities are mainly reflected in the number of S atoms, methyl, and carbon–carbon double bonds and their relative positions. Besides, these volatile compounds differ in content and aroma characteristics. However, there are few studies that have interpreted the composition of Chinese chive aroma from the perspective of odor activity values (OAVs) and odor description. Therefore, it is necessary to interpret the composition mechanism of Chinese chive aroma from the perspective of odor activity values and find the major aroma-active compounds (OAVs great than 1).
Nowadays, there are many detection methods for volatile components, including simultaneous distillation extraction (SDE), solvent-assisted flavor evaporation (SAFE), solid-phase micro-extraction (SPME), and supercritical fluid extraction (SFE). SPME is a sample pretreatment method invented by Pawliszyn in 1990 [14]. Compared with the traditional solvent extraction method for extracting volatile compounds, the advantages of this method are that it does not require organic solvents, requires fewer analysis samples, has a simpler and faster operation, and is lower in cost [13,15,16]. It integrates sampling, extraction, concentration, and injection. At the same time, it can avoid the loss of aroma components and can also be used in conjunction with gas chromatography-mass spectrometry (GC-MS) [17,18]. The core device of HS-SPME technology is the SPME fiber. Different SPME fibers adsorb different substances according to the coating material and thickness. In addition to the SPME fiber, other experimental parameters including sample weight, Na2SO4 weight, extraction temperature, equilibration time, extraction time, and desorption time will also affect the ultimate extraction effect. However, these parameters in the process of HS-SPME of volatile compounds from Chinese chive have not been optimized at present. Therefore, it is necessary for us to optimize these parameters in order to obtain more accurate test results.
The aim of this study was to optimize seven important parameters in the process of HS-SPME of volatile compounds from Chinese chive by the univariate analysis method, to determine the qualitative and quantitative analysis of volatile compounds in Chinese chive by using an optimized method, and to seek major aroma-active compounds of Chinese chive by OAV. This study can provide the corresponding theoretical basis for the source of Chinese chive aroma and also provide a direction for the in-depth study of Chinese chive flavor quality.
2. Results and Discussion
2.1. The Optimization of HS-SPME
2.1.1. Selection of SPME Fiber
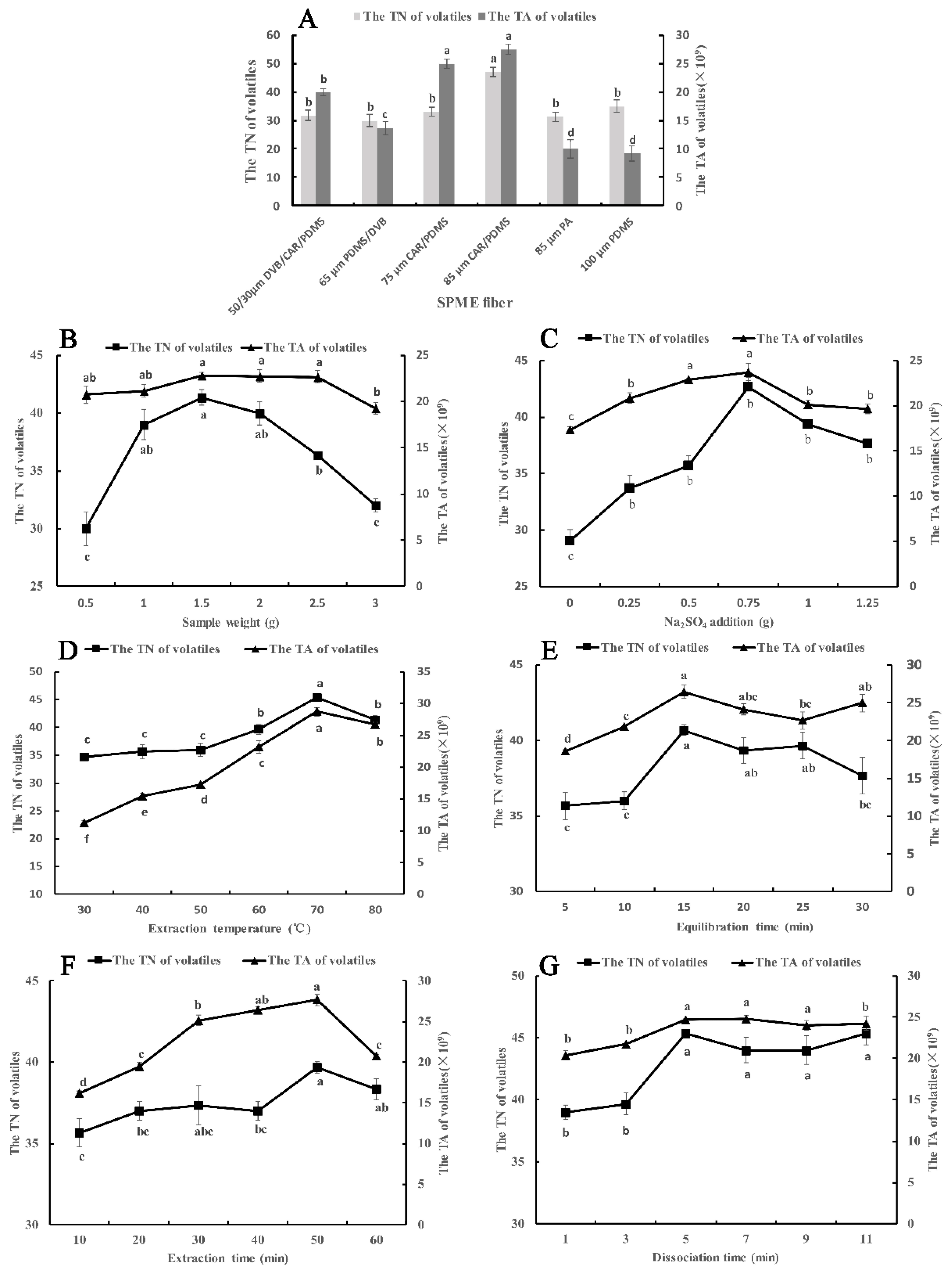
The difference between SPME fibers depends on the type of coating material and thickness. Six types of SPME fibers were optimized according to the total number (TN) and the total area (TA) of components. As shown in Figure 1A, the 85 μm CAR/PDMS fiber had a better extraction effect in TN and TA than the other five examined fibers. This may be because the 85 μm CAR/PDMS fiber is coated with mixed-phase polymeric film, carboxen, and polydimethylsiloxane, which is preferred for the adsorption of volatile low-molecular-mass and polar analytes [17]. In terms of TN, 47 volatile compounds were adsorbed by the 85 μm CAR/PDMS fiber, significantly higher than the other five types of fibers. At the same time, the thickness of the coating material can also affect the adsorption effect. Among the four fibers of mixed-phase coatings, the 85 μm CAR/PDMS fiber is coated with the thickest polymeric film. This means that more analytes can be adsorbed in it [19]. In addition, compared with the other four types of SPME fibers, the 85 μm PA fiber and 100 μm PDMS fiber have a single polymeric film, which is separately preferential for the adsorption of polar volatiles and nonpolar volatiles, so they can just adsorb one type of volatile compound according to its polarity. From the actual extraction effect, the TN and TA of the 85 μm PA fiber and 100 μm PDMS fiber were significantly lower than 85 μm CAR/PDMS fiber. In conclusion, the 85 μm CAR/PDMS fiber was chosen to adsorb the volatile compounds of Chinese chive and used in the following optimization tests.
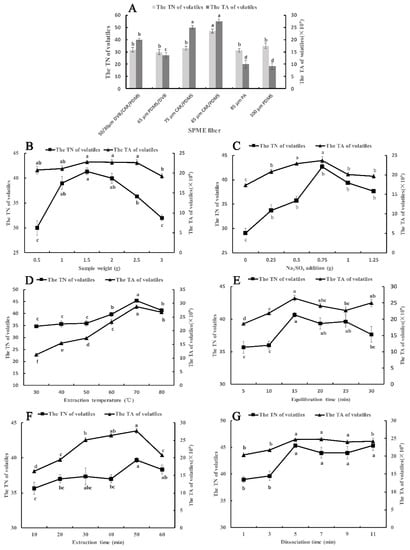
Figure 1.
Effects of different parameters and levels of SPME on the TA and TN of volatile compounds in Chinese chive. (A) SPME fiber; (B), sample weight; (C) Na2SO4 weight; (D) extraction temperature; (E) equilibration time; (F) extraction time; (G) desorption time. Different lowercase letters indicated that the significant differences between treatments according to the Duncan test (p < 0.05).
2.1.2. Effect of Sample Weight
When we performed the SPME experiment, a certain amount of material was put into a headspace vial, so we needed to optimize the sample weight of Chinese chive to reach the best extraction effect since the capacity volume of the SPME fiber to adsorb the analytes is confined [20]. We selected 0.5 g to 3.0 g of Chinese chive to optimize and the result is shown in Figure 1B. From 0.5 g to 3 g, the TN and TA of volatile compounds increased at first and decreased later on, reaching the maximum at 1.5 g. The reason for this phenomenon may be that more volatile compounds were released from the Chinese chive and adsorbed by the fiber with an increase in sample weight from 0.5 g to 1.5 g. However, the adsorption capacity of the fiber was saturated when the amount of sample increased to 1.5 g because the total capacity volume of the SPME fiber was occupied. Therefore, the TN and TA of volatile compounds will not increase with an increase in sample weight. On the contrary, when the sample weight exceeded 1.5 g, the extraction effect began to decline. This is due to the competition for adsorption sites between gas molecules. Hence, 1.5 g of sample weight was selected as the optimum sample weight.
2.1.3. Effect of Na2SO4 Amount
The addition of small amounts of inorganic salt to the liquid sample can enhance the ionic strength, reduce the solubility of polar organic compounds in water so that the SPME fiber can adsorb more analytical components, and improve the response value of fragrance substances [21,22]. Based on data from previous studies, we chose sodium sulfate as the inorganic salt. Sodium sulfate amounts of 0, 0.25, 0.5, 0.75, 1.0 and 1.25 g were designed in this part. Figure 1C shows that the addition of Na2SO4 could significantly improve the extraction effect of volatile compounds. When the amount of Na2SO4 was increased to 0.75 g, the TN and TA reached the maximum, because of what is called the salting-out effect [23]. However, the salting-out effect disappeared when the amount of Na2SO4 exceeded 0.75 g. This was probably because excessive salt ions may have electrostatic interactions with molecular substances and reduce their diffusion rate and the response values of some aroma components [24]. Therefore, an Na2SO4 amount of 0.75 g was used for the next experiments.
2.1.4. Effect of Extraction Temperature
Heating the sample can accelerate the speed of molecular motion, release the analytical components from the sample as soon as possible, increase the vapor pressure, and improve the sensitivity [25]. This is particularly important for headspace analysis. However, too high of a temperature will reduce the adsorption capacity of the fixed relative components of the fiber, so it is very imperative to choose an appropriate temperature. The effects of different extraction temperatures on volatile compounds are shown in Figure 1D. As the temperature kept rising, the TN and TA increased at first and then decreased, reaching the maximum at 70 °C. From 30 °C to 70 °C, heating provided energy for molecules to overcome the energy barrier. Then, it enhanced the mass transfer process, increased the vapor pressure of analytes, and promoted the release of analytes to the headspace. This resulted in a dramatic increase in TN and TA of volatile components, especially from 50 °C to 70 °C. However, the adsorption of volatile compounds on the fiber coating is an exothermic process, which means that high temperature is conducive to the release of analytes from the matrix. However, it will lead to a decrease in the partition coefficient, thus affecting the adsorption of analytes by the coating [20]. Therefore, as the temperature increased, the volatile compounds extracted at the equilibrium state were less, indicating that the inhibition effect of high temperatures above 70 °C on the adsorption process was greater than the promotion effect on the volatiles release process. On the other hand, excessive temperature increased the proportion of water vapor in the headspace vial, resulting in water vapor entering the extraction fiber or forming a water film on the surface, which affected the extraction and GC-MS analysis of the compounds. In summary, selecting an extraction temperature of 70 °C can better extract the volatile compounds of Chinese chive.
2.1.5. Effect of Equilibration Time
In order to extract more volatile compounds from samples, we need to keep the sealed headspace vial at the extraction temperature for a period of time. In the sealed headspace vial, the volatile components of Chinese chive will tardily volatilize to the top space of the vial, which will lead to an increase in the density of volatile compounds. However, the density of volatile compounds will slowly decrease after the molecular density of the headspace is in equilibrium with that of the sample. That means there is a dynamic equilibrium between the sample (liquid phase) and the headspace (gas phase), so the key to extracting more volatile compounds is to find the time point with the highest molecular density [20]. We elected six time points to optimize the best time, and the result is shown in Figure 1E. From 5 min to 15 min, the TN and TA rose rapidly, because a growing number of volatile components began to volatilize under the action of high temperature, especially those small molecular substances [26]. When the equilibration time exceeded 15 min, the results showed a downward trend, which was due to the volatile gas molecules returning to the sample being more than that volatilizing from the sample. Consequently, the optimum equilibration effect can be obtained in 15 min.
2.1.6. Effect of Extraction Time
When the sample equilibration is completed, the fiber will be inserted into the headspace vial for a while for extraction. The volatile compounds in the top space of the sample will slowly transfer to the coating of the fiber membrane. After a while, the volatile compounds in the top space of the sample reach a dynamic equilibrium with molecules in the coating of the SPME fiber [27]. We set up six time points to observe the time when the gas molecules reach equilibrium with the SPME fiber (Figure 1F). The TN and TA showed an increasing trend first and then decreasing, reaching the highest value in 50 min. This was mainly because of competitive adsorption and desorption of gas molecules on the extraction coating [24]. When the extraction time exceeded 50 min, the number of desorbed molecules was larger than that of adsorbed molecules, so the extraction efficiency reduced. In addition, too long of an extraction time may cause some gases to diffuse to the outside due to the excessive gas pressure in the headspace vial. To sum up, more volatile substances can be extracted by extending the extraction time to 50 min.
2.1.7. Effect of Desorption Time
The volatile compounds will be desorbed out from the fiber coating with the high temperature in the injection port. The desorption time has an influence on the desorption efficiency of compounds, and ultimately affects the separation results of the chromatographic column. In order to ascertain the optimum desorption time, the fiber was desorbed for a different time at 260 °C (Figure 1G). The results indicated that partial components had not been desorbed out from fiber coating at 1 min and 3 min, which was due to some volatile compounds needing more time to desorb [28]. From 5 min to 11 min, there was no significant difference in TN and TA, suggesting that all components can be desorbed in 5 min. Furthermore, an excessive desorption time will accelerate the aging and affect the service life of the fiber membrane. In sum, 5 min was found to be the optimum desorption time.
2.2. Validation of the Analytical Reproducibility
After the above analysis, the optimum extraction conditions of volatile compounds from Chinese chive were as follows: weighing 1.5 g of sample and 0.75 g of Na2SO4 in a headspace vial, equilibrating at 70 °C for 15 min, extracting with the 85 μm CAR/PDMS fiber for 50 min, and finally desorbing at the injection port for 5 min. The optimization of the determination method is not only helpful to the rapid and accurate determination of samples but also can accurately find the differences between experimental treatments and reduce experimental error. At the same time, the precision of the ultimate optimized conditions shall be verified in order to determine the reliability of the method. To this end, we applied the above method to determine the TN and TA of the other two different varieties of Chinese chive named “Han Yu Zi Gen” and “Fu Jiu Bao F1”. The intraday precision was determined from three successive injections and the interday precision was determined on six different days. The RSD (relative standard deviation) of TN and TA of the intraday precision ranged from 1.67% to 5.68% and the interday precision ranged from 3.62% to 7.89%. Thus, the optimized method had high reproducibility and can precisely determine the volatile compounds of Chinese chive.
2.3. Analysis of Volatile Compounds of Chinese Chive
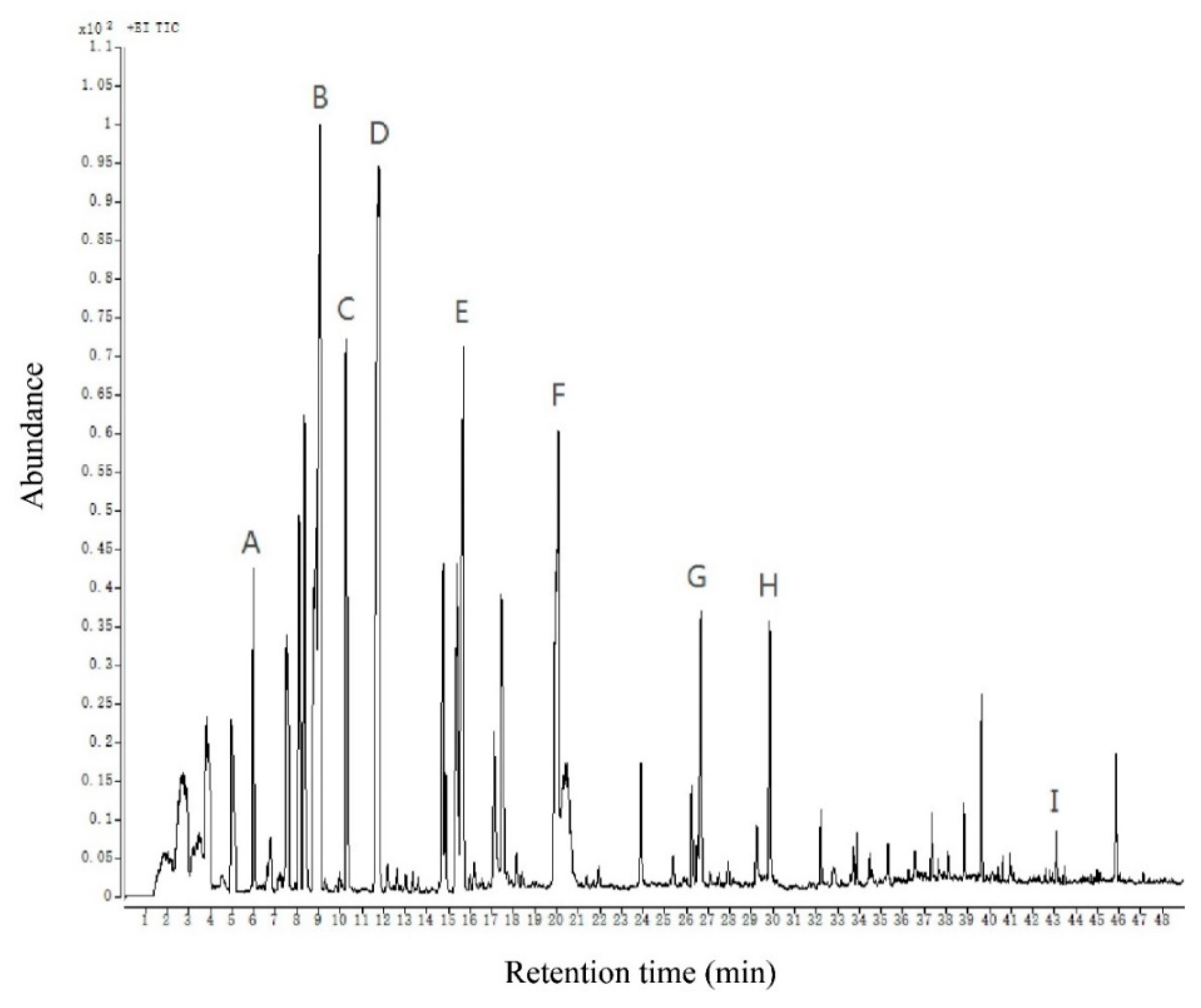
The gas chromatogram of volatile compounds in Chinese chive is shown in Figure 2. A total of 59 volatile compounds were detected by using the above-optimized method, including 28 ethers, 15 aldehydes, 6 alcohols, 5 ketones, 2 hydrocarbons, 1 ester, and 2 phenols. More substances were detected than in previous studies, particularly sulfur-containing compounds [9,13,15,29]. This means our method is more beneficial for detecting and analyzing volatile compounds of Chinese chive. The content of these 59 volatile compounds reached 56250.46 μg in 1 kg of Chinese chive, of which the highest content was ether compounds, reaching 50599.94 μg/kg, and the lowest content was phenols compounds, merely 21.44 μg/kg. This suggests that the content of each substance in the aroma of Chinese chive is quite different (Table 1).
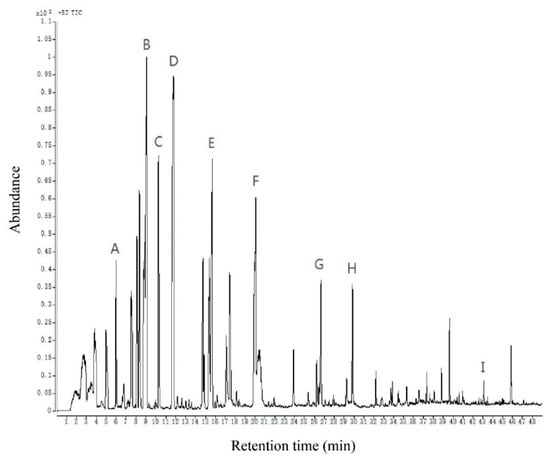
Figure 2.
The total ion chromatogram of volatile compounds of Chinese chive. All volatiles detected are listed in Table 1. The letters A–I are part of the volatile compounds of Chinese chive corresponding to the peaks. (A) 2-methylpent-4-enal; (B) allyl methyl disulfide; (C) 2,5-octanedione; (D) dimethyl trisulfide; (E) diallyl disulfide; (F) methyl allyl trisulfide; (G) 3-ethenyl-3,6-dihydrodithiine; (H) diallyl trisulfide; (I) 2,4-di-tert-butylphenol.

Table 1.
The composition and content of volatile compounds in Chinese chive.
2.3.1. Ethers
The aroma of Allium vegetables is mainly from sulfur-containing metabolites, which are called thioethers. These compounds are derived from the degradation of CSO with the action of alliinase. In the Chinese chive, we detected 28 thioethers, and the total content accounted for 89.95% of the volatile compounds. The proportion of absolute content of thioethers is consistent with the relative content of previous studies [9,13,30]. Because most of the previous studies on volatile compounds in Chinese chive are relatively quantitative, only the absolute contents of four thioethers (diallyl sulfide, methyl allyl di- sulfide, dimethyl trisulfide, diallyl disulfide) were calculated [29]. On the other hand, these 28 thioethers can be divided into the following groups: sulfides, disulfides, trisulfides, thiophenes, thiiranes, dithiane, sulfanes, disulfanes, disulphides, and so on. Among these ethers, the sum content of dimethyl trisulfide (10,623.30 μg/kg), (E)-1-methyl-2-(prop-1-en-1-yl) disulfane (8865.14 μg/kg), and allyl methyl disulfide (8146.24 μg/kg) accounted for 54.61%. This indicates that disulfides, trisulfides, and disulfanes are the main volatiles of thioethers, similar to the volatile components of onion and garlic [31,32].
2.3.2. Aldehydes
Aldehydes are another kind of main volatile compound found in Chinese chive. There were 15 aldehydes detected in Chinese chive, and the total content reached 4112.23 μg/kg, accounting for 7.31% of the total volatile compounds (Table 1). The aldehyde that was predominant in Chinese chive was trans-2-hexenal (2996.96 μg/kg), accounting for 72.88% of aldehydes. Trans-2-hexenal is a C6 volatile substance, which is released when plants are subjected to external stresses such as mechanical damage, insect feeding, pathogen infection, and so on [33]. At the same time, 2-hexenal (166.93 μg/kg) was also detected in Chinese chive, which can be transformed to trans-2-hexenal by isomerization. The aldehydes constituting volatile compounds in vegetables were derived from fatty acids through the action of related enzymes, and a moderately high temperature can promote their release [34]. Therefore, the extraction efficiency of aldehydes can be improved by heating.
2.3.3. Alcohols and Ketones
There were 6 alcohols and 5 ketones detected in Chinese chive, and their content was 169.76 μg/kg and 573.68 μg/kg (Table 1), respectively. Most of the alcohols reported in the literature could be authenticated in our sample, but the α-ionol was first reported, which may be due to different detection conditions or different genotypes [15]. The highest content of ketones was 2,5-octanedione (212.64 μg/kg), followed by β-ionone (167.56 μg/kg). In short, the total content of alcohols and ketones only accounted for 1.32%, and they contributed less to the composition of flavor substances in Chinese chive.
2.3.4. Hydrocarbons, Esters and Phenols
The hydrocarbons, esters, and phenols compounds contained in Chinese chive were also less, and their total content was 794.85 μg/kg, accounting for 1.41% of the total volatile compounds, not differing much from alcohols and ketones. The content of 4-methoxystyrene in hydrocarbons was absolutely dominant, up to 647.64 μg/kg. For esters, only one ester was detected, isopropyl myristate, which can be used to increase the skin permeation of massive drugs in medical science [35]. The two phenols compounds are butylated hydroxytoluene and 2,4-di-tert-butylphenol, in which butylated hydroxytoluene can be used to prevent the spoilage of foods [36]. Thus, it can be seen that there are many active substances in Chinese chive and they can be used in food science and medicine.
2.4. Odour Activity Values (OAVs) Analysis of Volatile Compounds
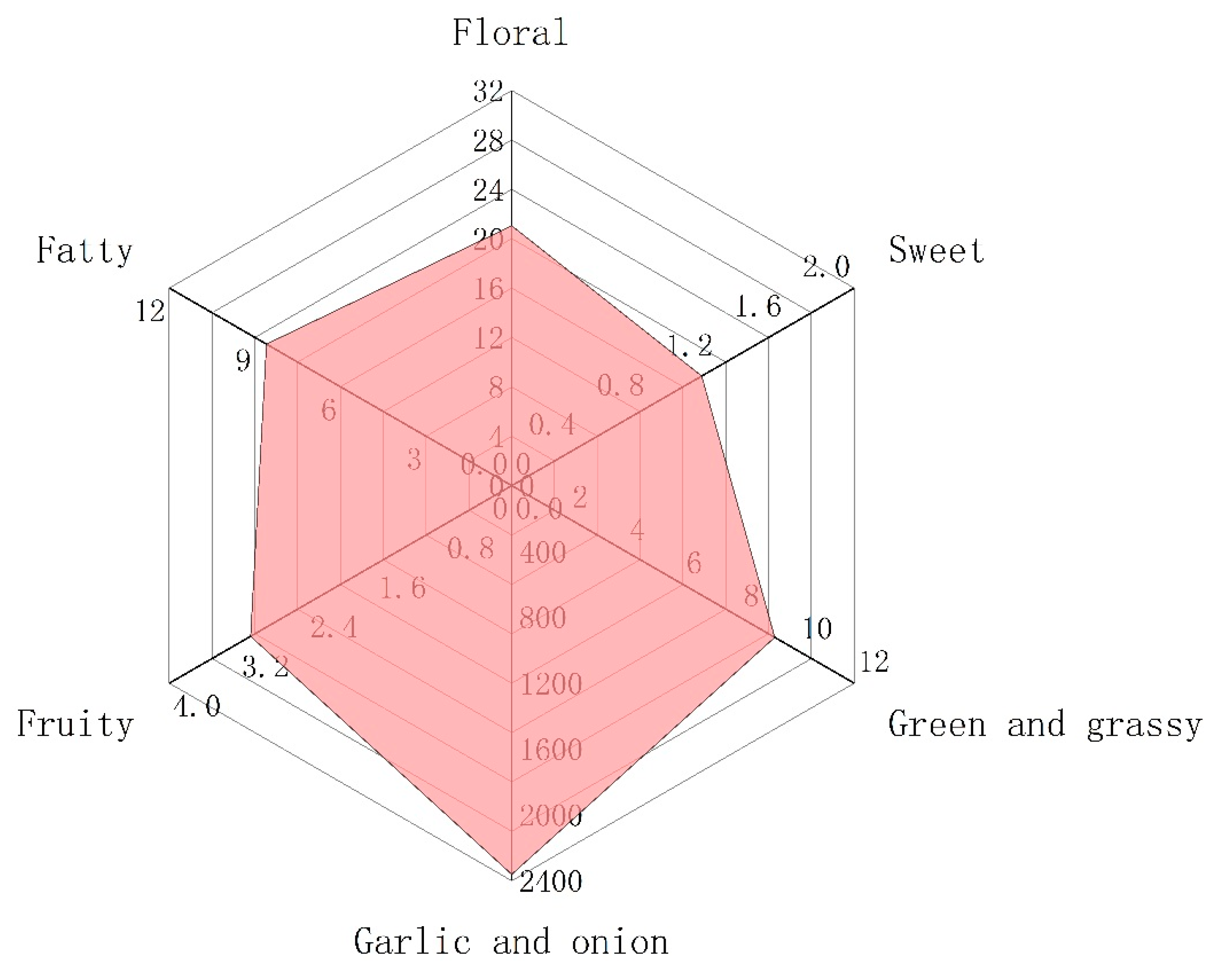
The generation of odor is due to the interaction between volatile compounds and human odor receptors, which is the result of the joint action of the whole set of volatiles. Among these volatiles, the contribution of a single substance to the overall flavor depends on two factors, the actual concentration and its odor threshold [37,38]. The ratio of these two factors is another significant parameter, odor activity values (OAVs). This is calculated by dividing the actual concentration by its odor threshold [39]. As a general rule, volatile compounds with OAVs greater than 1 are considered key contributors to flavor, i.e., the major aroma-active compounds. Table 2 shows that there were only 11 volatile components with OAVs greater than 1, mainly thioethers, but also aldehydes and ketones. Thus, these 11 compounds were recognized as major aroma-active compounds, which are essential for the aroma quality of Chinese chive (Figure 3). In addition, the substances with OAVs less than 1 also have a certain impact on the overall aroma. When OAVs are between 0.2 and 1, substances may affect the aroma of samples through an internal synergistic effect [40]. Across the board, the aroma of volatile compounds in Chinese chive can be divided into six categories, including “floral”, “fatty”, “garlic and onion”, “green and grassy”, “sweet”, and “fruity” (Figure 4). The odor of “garlic and onion” showed an absolute predominance in all odors, and OAVs reached 2361.09, of which dimethyl trisulfide contributed the most. Dimethyl trisulfide is a sulfur-containing compound widely existing in Allium, such as shallot, garlic, and onion, which has low sensory detection thresholds, so we can easily smell its existence [41]. Besides, these thioethers have a similar structure and smell because they were derived from common precursors. Therefore, increasing the content of these substances will be conducive to improving the flavor quality of Chinese chive.

Table 2.
The odor activity values (OAVs) and odor description of volatile compounds in Chinese chive.
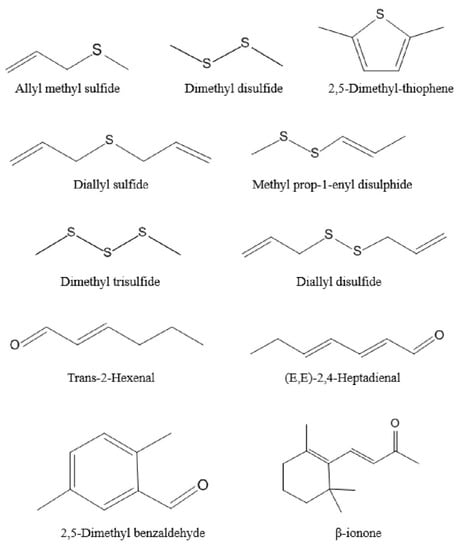
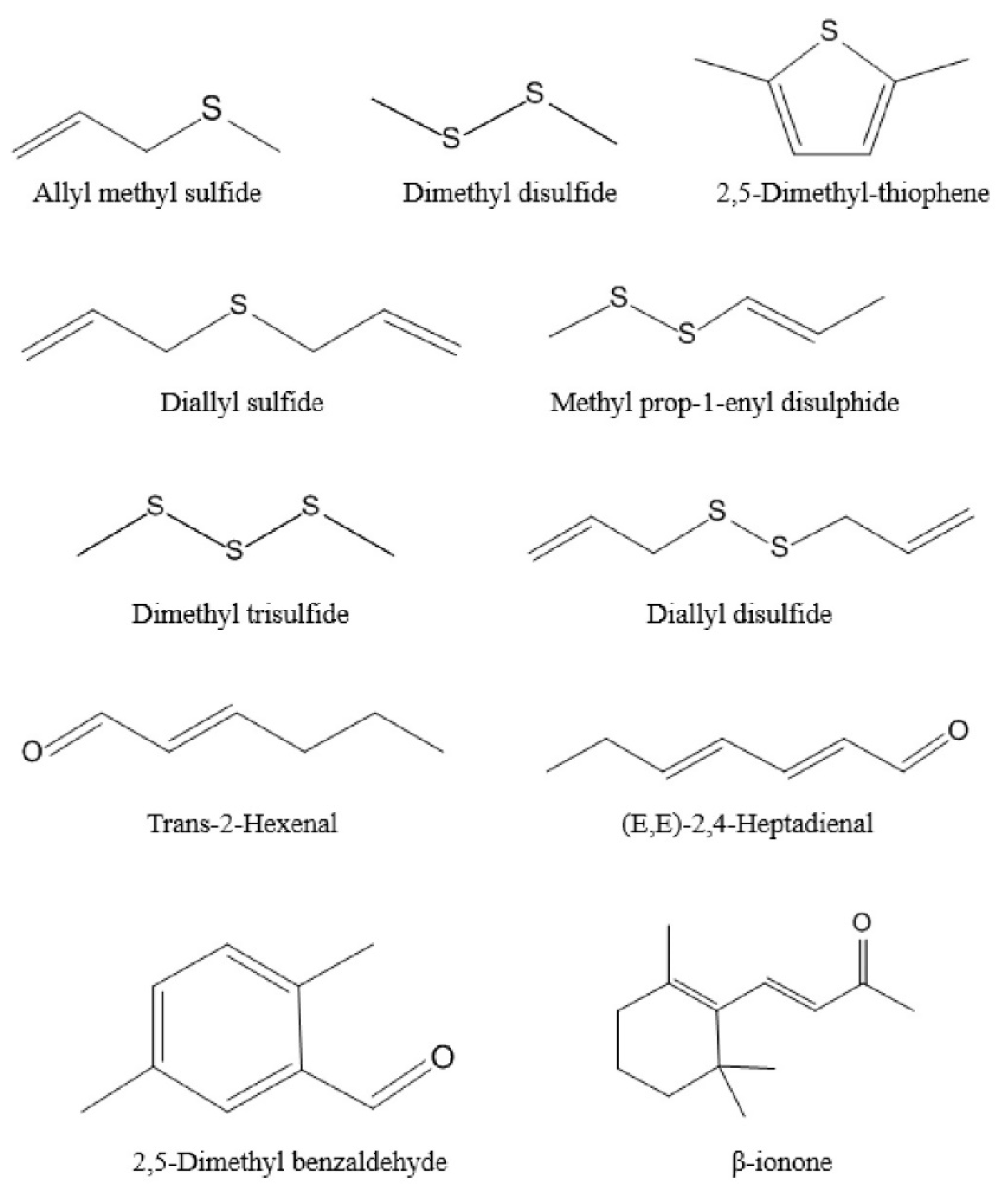
Figure 3.
The 11 major aroma-active compounds identified by OAV calculations in Chinese chive.
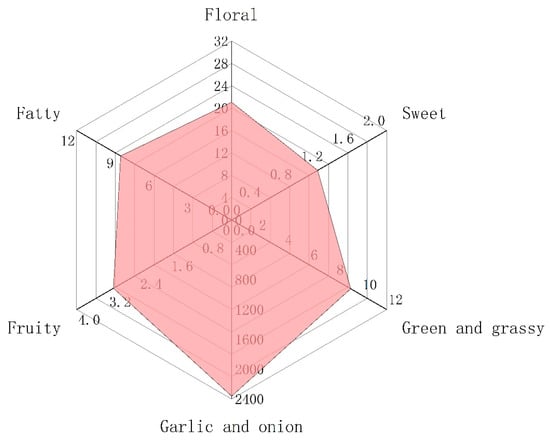
Figure 4.
The radar fingerprint chart of aroma composition of Chinese chive.
The “floral” odor was principally comprised of β-ionone (OAV = 19.95), a type of cyclized isoprene widely distributed in fruits and vegetables. It is also the major aroma-active compound in some plants and juices, such as tea (OAV = 20,496), mandarins (OAV = 655), grape juice (OAV = 233), and so on [45,46,47]. The trans-2-hexenal and (E,E)-2,4-heptadienal endow Chinese chive “fatty” and “green and grassy” odor, while trans-2-hexenal also has a “fruity” odor, such as banana. The “sweet” odor is the weakest aroma of all, and the OAVs of these three substances are less than 1. Therefore, the contribution of “sweet” odor to the aroma of Chinese chive cannot be taken into account. Although the aroma of Chinese chive can be divided into six categories, it seems that we can only perceive the “garlic and onion”. This is because the odor intensity of “garlic and onion” is so strong that it has a certain masking effect on other odors. Through the above analysis, we could ascertain that the aroma of Chinese chive is mainly composed of thioethers, which is conducive to the in-depth study of the aroma of Chinese chive and can also provide theoretical guidance for the high-quality breeding of Chinese chive.
3. Materials and Methods
3.1. Plant Material
The cultivar of Chinese chive used for this study was “Jiuxing 22” and was harvested on 22 November 2020, in Lanzhou, Gansu, China (36°03′ N, 103°73′ E). After harvesting, the same size, similar maturity, and health of Chinese chive were selected. The leaf surfaces were cleaned with distilled water, chopped into small segments about 2 cm in length, mixed well, and then immediately frozen in liquid nitrogen and stored at −80 °C until analysis.
3.2. Reagents and Instruments
Anhydrous sodium sulfate (Na2SO4) was purchased from Sinopharm Group Chemical Reagent Co., Ltd. (Shanghai, China); ultrapure water was prepared by Milli-Q (Burlington, MA, USA); difurfuryl sulfide was purchased from TCI (Shanghai) Development Co., Ltd. (Shanghai, China), as the internal standard for quantitative analysis.
An Agilent 7890B gas chromatograph coupled with an Agilent 7000D quadrupole mass spectrometric detector (Agilent, Santa Clara, CA, USA) and standard mass spectrometry library (NIST 2014) workstation was used for the separation and identification of the volatile compounds. A DB-WAX elastic quartz capillary column (30 m × 0.25 mm, 0.25 μm) was used as the stationary phase (Agilent, Santa Clara, CA, USA). Six types of SPME fibers, a manual SPME fiber holder, and n-alkanes (C7–C40) were purchased from Sigma-Aldrich (St. Louis, MO, USA). The 15 mL screw cap headspace vial fitted with PTFE/silicone septa and a magnetic stirring rotor was purchased from ANPEL Laboratory Technologies (Shanghai) Inc. (Shanghai, China).
3.3. Optimization of HS-SPME
Univariate analysis was used to optimize 7 parameters and 6 levels (42 schemes) of HS-SPME technology. The 7 parameters included SPME fiber, sample weight, Na2SO4 weight, extraction temperature, equilibration time, extraction time, and desorption time. The first parameter is a type of SPME fiber. Six types of SPME fiber were optimized in this experiment as follows: 50/30 μm divinylbenzene-carboxen-polydimethylsiloxane (DVB/CAR/PDMS), 65 μm polydimethylsiloxane-divinylbenzene (PDMS/DVB), 75 μm carboxen-polydimethylsiloxane (CAR/PDMS), 85 μm polyacrylate (PA), 85 μm carboxen-polydimethylsiloxane (CAR/PDMS), and 100 μm polydimethylsiloxane (PDMS). All of the SPME fibers were conditioned at different temperatures for different times in the GC injector port according to the conditioning guidelines and a blank test was performed to desorb the possible carry-over before being used.
The Chinese chive sample was removed from the −80 °C ultra-low temperature freezer into the mortar and quickly ground to a homogenate. Then, the homogenate was weighed (0.5–3.5 g) into the 15 mL screw cap headspace vial fitted with the PTFE/silicone septa containing 2 mL of ultrapure water, a certain amount of Na2SO4 (0–1.25 g), 4 μL of difurfuryl sulfide (21.4 mg/L), and a magnetic stirring rotor, and then the headspace vial fitted with the PTFE/silicone septa was quickly fastened to prevent gas leakage. After that, the headspace vial was heated to the extraction temperature (30–80 °C) for 5–30 min on a metal-heating agitation platform at 1000 rpm. After equilibration, the SPME fiber was inserted into the headspace vial to extract for 10–60 min with consecutive heating and agitation. Afterwards, the SPME fiber was pulled out lightly and then inserted into the injection port of the GC to desorb for 1–11 min with splitless mode. The previously optimized parameter was used to optimize the next parameter. The parameters and levels optimized in this study are shown in Table 3. All samples were analyzed in triplicate.

Table 3.
The optimized parameters and levels of the HS-SPME technology.
3.4. GC-MS Analysis
The volatile compounds of Chinese chive were analyzed using an Agilent 7890B/7000D GC-MS Agilent, Santa Clara, CA, USA) under the following conditions: capillary column, DB-WAX (30 m × 0.25 mm, 0.25 μm) with He (≥99.999% purity) as the carrier gas at a flow rate of 1 mL/min and splitless mode; initial temperature 40 °C held for 1 min, raised to 80 °C at 8 °C/min, then raised to 130 °C at 2 °C/min, and finally raised to 220 °C at 6 °C/min held for 3 min; total analysis time, 49 min; MS ionization, EI, 70 eV; MS source, 230 °C, scan area, 30–660 amu.
3.5. Qualitative and Quantitative Analysis of Volatile Compounds
After the program started, the volatile compounds were separated and identified by GC-MS with an automatic integration system and mass spectrometry library (NIST 2014, Standard Spectrum Library of the National Institute of Standards and Technology of the United States, https://www.nist.gov/srd). Compared with the mass spectrometry library, only those with a matching score of more than 70 were identified. The retention index (RI) was calculated using a series of n-alkanes (C7–C40) as the external references on a DB-WAX column under the same chromatographic conditions. The calculation formula is as follows (Equation (1)):
where T (n) is the retention time of n-alkane with a carbon number of n; T (t) is the retention time of measured substance; T (n+1) is the retention time of n-alkane with a carbon number of n+1; the retention time: T (n) < T (t) <T (n+1).
The concentration of volatile compounds was analyzed by the internal standard method, using the following formula (Equation (2)):
where A1 and A2 are the peak areas of determinand and the internal standard, respectively; M1 and M2 are the amount of the internal standard (μg) and sample (g), respectively.
3.6. Statistical Analysis
Excel 2010 and Origin 2018 software (OriginLab, Northampton, MA, USA) were used for statistical analysis and charting of data. SPSS 22.0 software (SPSS Inc., Chicago, IL, USA) was employed for analyzing data using Duncan’s multiple range tests of variance (p < 0.05) and significance test.
4. Conclusions
This study optimized seven parameters of HS-SPME by using univariate analysis and obtained the optimum extraction conditions of volatile compounds in Chinese chive. The optimum parameters were: 85 μm CAR/PDMS fiber, 1.5 g sample, 0.75 g Na2SO4, 70 °C extraction temperature, 15 min equilibrating time, 50 min extracting time, and 5 min desorbing time. The results of the reproducibility test showed that this method can accurately determine the volatile compounds in Chinese chive. Moreover, a total of 57 volatile compounds were identified through the optimized method, including 28 ethers, 15 aldehydes, 6 alcohols, 5 ketones, 2 hydrocarbons, 2 phenols, and 1 ester, of which the highest content was ethers, especially dimethyl trisulfide. Through the OAV calculation, 11 volatile compounds were detected as the major aroma-active compounds, which were allyl methyl sulfide, dimethyl disulfide, diallyl sulfide, 2,5-dimethyl-thiophene, methyl prop-1-enyl disulphide, dimethyl trisulfide, diallyl disulfide, trans-2-hexenal, (E,E)-2,4-heptadienal, 2,5-dimethyl benzaldehyde, and β-ionone. The “garlic and onion” (OAV = 2361.09) was the strongest odor in six categories of aroma. The results of this study not only clarified the main source of Chinese chive aroma but also provided a theoretical and methodological basis for the development and research of food with Chinese chive aroma.
Author Contributions
Conceptualization, X.X. and J.Y.; data curation, B.X., S.W. and J.Y.; formal analysis, B.X., Q.W. and H.L.; methodology, J.W.; software, J.L.; validation, Z.L.; funding acquisition, X.X. and J.Y.; investigation, B.X. and M.H.; project administration, X.X. and J.Y.; supervision, X.X. and J.Y.; writing—original draft, B.X. and J.Y.; writing—review and editing, B.X. and X.X. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Education science and technology innovation project of Gansu Province (GSSYLXM-02); China agriculture research system (CARS-23-C-07); Special project of central government guiding local science and technology development (ZCYD-2020-5) and General project of scientific research in colleges and universities in Gansu Province (2021B-141).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All data are contained within the article.
Conflicts of Interest
The authors declare no conflict of interest.
Sample Availability
Samples of the Chinese chive are available from the authors.
References
- Cecchi, L.; Ieri, F.; Vignolini, P.; Mulinacci, N.; Romani, A. Characterization of Volatile and Flavonoid Composition of Different Cuts of Dried Onion (Allium cepa L.) by HS-SPME-GC-MS, HS-SPME-GCxGC-TOF and HPLC-DAD. Molecules 2020, 25, 408. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Asemani, Y.; Zamani, N.; Bayat, M.; Amirghofran, Z. Allium vegetables for possible future of cancer treatment. Phytother. Res. PTR 2019, 33, 3019–3039. [Google Scholar] [CrossRef] [PubMed]
- Hiyasat, B.; Sabha, D.; Grotzinger, K.; Kempfert, J.; Rauwald, J.W.; Mohr, F.W.; Dhein, S. Antiplatelet activity of Allium ursinum and Allium sativum. Pharmacology 2009, 83, 197–204. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, M.; Shibamoto, T. Chemical Compositions and Antioxidant/Anti-inflammatory Activities of Steam Distillate from Freeze-Dried Onion (Allium cepa L.) Sprout. J. Agric. Food Chem. 2008, 56, 10462–10467. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Kim, D.-B.; Lee, S.; Park, J.; Shin, D.; Yoon, M. Profiling of organosulphur compounds using HPLC-PDA and GC/MS system and antioxidant activities in hooker chive (Allium hookeri). Nat. Prod. Res. 2016, 30, 2798–2804. [Google Scholar] [CrossRef]
- Sobolewska, D.; Michalska, K.; Podolak, I.; Grabowska, K. Steroidal saponins from the genus Allium. Phytochem. Rev. 2016, 15, 1–35. [Google Scholar] [CrossRef] [Green Version]
- Han, S.H.; Suh, W.S.; Park, K.J.; Kim, K.H.; Lee, K.R. Two new phenylpropane glycosides from Allium tuberosum Rottler. Arch. Pharm. Res. 2015, 38, 1312–1316. [Google Scholar] [CrossRef]
- Zhang, W.N.; Zhang, H.L.; Lu, C.Q.; Luo, J.P.; Zha, X.Q. A new kinetic model of ultrasound-assisted extraction of polysaccharides from Chinese chive. Food Chem. 2016, 212, 274–281. [Google Scholar] [CrossRef]
- Pino, J.A.; Fuentes, V.; Correa, M.T. Volatile Constituents of Chinese Chive (Allium tuberosum Rottl. ex Sprengel) and Rakkyo (Allium chinense G. Don). J. Agric. Food Chem. 2001, 49, 1328–1330. [Google Scholar] [CrossRef]
- Rose, P.; Whiteman, M.; Moore, P.K.; Zhu, Y.Z. Bioactive S-alk(en)yl cysteine sulfoxide metabolites in the genus Allium: The chemistry of potential therapeutic agents. Nat. Prod. Rep. 2005, 22, 351–368. [Google Scholar] [CrossRef]
- Yoshimoto, N.; Saito, K. S-Alk(en)ylcysteine sulfoxides in the genus Allium: Proposed biosynthesis, chemical conversion, and bioactivities. J. Exp. Bot. 2019, 70, 4123–4137. [Google Scholar] [CrossRef] [PubMed]
- Liu, N.; Tong, J.; Hu, M.; Ji, Y.; Wang, B.; Liang, H.; Liu, M.; Wu, Z. Transcriptome landscapes of multiple tissues highlight the genes involved in the flavor metabolic pathway in Chinese chive (Allium tuberosum). Genomics 2021, 113, 2145–2157. [Google Scholar] [CrossRef] [PubMed]
- Yabuki, Y.; Mukaida, Y.; Saito, Y.; Oshima, K.; Takahashi, T.; Muroi, E.; Hashimoto, K.; Uda, Y. Characterisation of volatile sulphur-containing compounds generated in crushed leaves of Chinese chive (Allium tuberosum Rottler). Food Chem. 2010, 120, 343–348. [Google Scholar] [CrossRef]
- Pawliszyn, J. Solid Phase Microextraction: Theory and Practice; VCH: New York, NY, USA, 1997. [Google Scholar]
- Mnayer, D.; Fabiano-Tixier, A.S.; Petitcolas, E.; Hamieh, T.; Nehme, N.; Ferrant, C.; Fernandez, X.; Chemat, F. Chemical composition, antibacterial and antioxidant activities of six essentials oils from the Alliaceae family. Molecules 2014, 19, 20034–20053. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Beltran Sanahuja, A.; Ponce Landete, M.; Domingo Martinez, M.I.; Prats Moya, M.S.; Valdes Garcia, A. Optimization of Volatile Compounds Extraction from Industrial Celery (Apium graveolens) By-Products by Using Response Surface Methodology and Study of Their Potential as Antioxidant Sources. Foods 2021, 10, 2664. [Google Scholar] [CrossRef]
- Risticevic, S.; Lord, H.; Gorecki, T.; Arthur, C.L.; Pawliszyn, J. Protocol for solid-phase microextraction method development. Nat. Protoc. 2010, 5, 122–139. [Google Scholar] [CrossRef]
- de Fatima Alpendurada, M. Solid-phase microextraction: A promising technique for sample preparation in environmental analysis. J. Chromatogr. A 2000, 889, 3–14. [Google Scholar] [CrossRef]
- Balasubramanian, S.; Panigrahi, S. Solid-Phase Microextraction (SPME) Techniques for Quality Characterization of Food Products: A Review. Food Bioprocess Technol. 2010, 4, 1–26. [Google Scholar] [CrossRef]
- Ho, C.W.; Wan Aida, W.M.; Maskat, M.Y.; Osman, H. Optimization of headspace solid phase microextraction (HS-SPME) for gas chromatography mass spectrometry (GC-MS) analysis of aroma compound in palm sugar (Arenga pinnata). J. Food Compos. Anal. 2006, 19, 822–830. [Google Scholar] [CrossRef]
- Câmara, J.S.; Alves, M.A.; Marques, J.C. Development of headspace solid-phase microextraction-gas chromatography–mass spectrometry methodology for analysis of terpenoids in Madeira wines. Anal. Chim. Acta 2006, 555, 191–200. [Google Scholar] [CrossRef]
- Manousi, N.; Zachariadis, G.A. Determination of Volatile Compounds in Nut-Based Milk Alternative Beverages by HS-SPME Prior to GC-MS Analysis. Molecules 2019, 24, 3091. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Monteiro, M.; Carvalho, M.; Henrique, R.; Jeronimo, C.; Moreira, N.; de Lourdes Bastos, M.; de Pinho, P.G. Analysis of volatile human urinary metabolome by solid-phase microextraction in combination with gas chromatography-mass spectrometry for biomarker discovery: Application in a pilot study to discriminate patients with renal cell carcinoma. Eur. J. Cancer 2014, 50, 1993–2002. [Google Scholar] [CrossRef]
- Wei, S.; Xiao, X.; Wei, L.; Li, L.; Li, G.; Liu, F.; Xie, J.; Yu, J.; Zhong, Y. Development and comprehensive HS-SPME/GC-MS analysis optimization, comparison, and evaluation of different cabbage cultivars (Brassica oleracea L. var. capitata L.) volatile components. Food Chem. 2021, 340, 128166. [Google Scholar] [PubMed]
- Robbat, A., Jr.; Liu, T.-Y.; Abraham, B.M. On-Site Detection of Polycyclic Aromatic Hydrocarbons in Contaminated Soils by Thermal Desorption Gas Chromatography/Mass Spectrometry. Anal. Chem. 1992, 64, 1477–1483. [Google Scholar] [CrossRef]
- Ma, Q.L.; Hamid, N.; Bekhit, A.E.D.; Robertson, J.; Law, T.F. Optimization of headspace solid phase microextraction (HS-SPME) for gas chromatography mass spectrometry (GC–MS) analysis of aroma compounds in cooked beef using response surface methodology. Microchem. J. 2013, 111, 16–24. [Google Scholar] [CrossRef]
- Souza Silva, E.A.; Saboia, G.; Jorge, N.C.; Hoffmann, C.; Dos Santos Isaias, R.M.; Soares, G.L.G.; Zini, C.A. Development of a HS-SPME-GC/MS protocol assisted by chemometric tools to study herbivore-induced volatiles in Myrcia splendens. Talanta 2017, 175, 9–20. [Google Scholar] [CrossRef] [PubMed]
- Machiels, D.; Istasse, L. Evaluation of two commercial solid-phase microextraction fibres for the analysis of target aroma compounds in cooked beef meat. Talanta 2003, 61, 529–537. [Google Scholar] [CrossRef]
- Zhang, S.; Du, Z.; Li, G. Graphene-supported zinc oxide solid-phase microextraction coating with enhanced selectivity and sensitivity for the determination of sulfur volatiles in Allium species. J. Chromatogr. A 2012, 1260, 1–8. [Google Scholar] [CrossRef]
- Iida, H.; Hashimoto, S.; Miyazawa, M.; Kameoka, H. Volatile Flavor Components of Nira (Allium tuberosum Rottl.). J. Food Sci. 1983, 48, 660–661. [Google Scholar] [CrossRef]
- Molina-Calle, M.; Priego-Capote, F.; Luque de Castro, M.D. Headspace−GC–MS volatile profile of black garlic vs fresh garlic: Evolution along fermentation and behavior under heating. Lwt 2017, 80, 98–105. [Google Scholar] [CrossRef]
- Choi, S.M.; Lee, D.J.; Kim, J.Y.; Lim, S.T. Volatile composition and sensory characteristics of onion powders prepared by convective drying. Food Chem. 2017, 231, 386–392. [Google Scholar] [CrossRef]
- Noordermeer, M.A.; Goot, W.V.D.; Kooij, A.J.V.; Veldsink, J.W.; Veldink, G.A.; Vliegenthart, J.F.G. Development of a Biocatalytic Process for the Production of C6-Aldehydes from Vegetable Oils by Soybean Lipoxygenase and Recombinant Hydroperoxide Lyase. J. Agric. Food Chem. 2002, 50, 4270–4274. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rodrigo, D.; Jolie, R.; Loey, A.V.; Hendrickx, M. Thermal and high pressure stability of tomato lipoxygenase and hydroperoxide lyase. J. Food Eng. 2007, 79, 423–429. [Google Scholar] [CrossRef]
- Zhao, C.; Quan, P.; Liu, C.; Li, Q.; Fang, L. Effect of isopropyl myristate on the viscoelasticity and drug release of a drug-in-adhesive transdermal patch containing blonanserin. Acta Pharm. Sin. B 2016, 6, 623–628. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, W.; Kannan, K. Quantitative identification of and exposure to synthetic phenolic antioxidants, including butylated hydroxytoluene, in urine. Environ. Int. 2019, 128, 24–29. [Google Scholar] [CrossRef]
- Czerny, M.; Christlbauer, M.; Christlbauer, M.; Fischer, A.; Granvogl, M.; Hammer, M.; Hartl, C.; Hernandez, N.M.; Schieberle, P. Re-investigation on odour thresholds of key food aroma compounds and development of an aroma language based on odour qualities of defined aqueous odorant solutions. Eur. Food Res. Technol. 2008, 228, 265–273. [Google Scholar] [CrossRef]
- Tian, P.; Zhan, P.; Tian, H.; Wang, P.; Lu, C.; Zhao, Y.; Ni, R.; Zhang, Y. Analysis of volatile compound changes in fried shallot (Allium cepa L. var. aggregatum) oil at different frying temperatures by GC-MS, OAV, and multivariate analysis. Food Chem. 2021, 345, 128748. [Google Scholar] [CrossRef]
- Chen, S.; Tang, J.; Fan, S.; Zhang, J.; Chen, S.; Liu, Y.; Yang, Q.; Xu, Y. Comparison of Potent Odorants in Traditional and Modern Types of Chinese Xiaoqu Liquor (Baijiu) Based on Odor Activity Values and Multivariate Analyses. Foods 2021, 10, 2392. [Google Scholar] [CrossRef]
- Anon, A.; Lopez, J.F.; Hernando, D.; Orriols, I.; Revilla, E.; Losada, M.M. Effect of five enological practices and of the general phenolic composition on fermentation-related aroma compounds in Mencia young red wines. Food Chem. 2014, 148, 268–275. [Google Scholar] [CrossRef] [Green Version]
- Pan, X.; Zhang, W.; Lao, F.; Mi, R.; Liao, X.; Luo, D.; Wu, J. Isolation and identification of putative precursors of the volatile sulfur compounds and their inhibition methods in heat-sterilized melon juices. Food Chem. 2021, 343, 128459. [Google Scholar] [CrossRef]
- Van Gemert, L.J. Odour Thresholds: Compilations of Odour Threshold Values in Air, Water and Other Medhia; Oliemans Punter & Partners BV: Utrecht, The Netherlands, 2011. [Google Scholar]
- Alenyorege, E.A.; Ma, H.; Aheto, J.H.; Agyekum, A.A.; Zhou, C. Effect of sequential multi-frequency ultrasound washing processes on quality attributes and volatile compounds profiling of fresh-cut Chinese cabbage. Lwt 2020, 117, 108666. [Google Scholar] [CrossRef]
- Burdock, G.A. Fenaroli’s Handbook of Flavor Ingredients; CRC Press LLC: Boca Raton, FL, USA, 2010. [Google Scholar]
- Wang, M.Q.; Ma, W.J.; Shi, J.; Zhu, Y.; Lin, Z.; Lv, H.P. Characterization of the key aroma compounds in Longjing tea using stir bar sorptive extraction (SBSE) combined with gas chromatography-mass spectrometry (GC-MS), gas chromatography-olfactometry (GC-O), odor activity value (OAV), and aroma recombination. Food Res. Int. 2020, 130, 108908. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Z.; Wu, Q.; Niu, Y.; Wu, M.; Zhu, J.; Zhou, X.; Chen, X.; Wang, H.; Li, J.; Kong, J. Characterization of the Key Aroma Compounds in Five Varieties of Mandarins by Gas Chromatography-Olfactometry, Odor Activity Values, Aroma Recombination, and Omission Analysis. J. Agric. Food Chem. 2017, 65, 8392–8401. [Google Scholar] [CrossRef] [PubMed]
- Vilanova, M.; Siebert, T.E.; Varela, C.; Pretorius, I.S.; Henschke, P.A. Effect of ammonium nitrogen supplementation of grape juice on wine volatiles and non-volatiles composition of the aromatic grape variety Albariño. Food Chem. 2012, 133, 124–131. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).